Translate this page into:
Magnetically actuated graphene coated polyurethane foam as potential sorbent for oils and organics
⁎Corresponding author. nkrenu@gmail.com (N.K. Renuka)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Herein, we present Graphene-meso Iron Oxide composite incorporated Polyurethane foam (GPUF) as an elite sorbent for organic contaminants and oils. Anchoring Graphene-meso Iron Oxide composite switches Polyurethane foam to a super hydrophobic and super oleophilic moiety. Detailed structural, microscopic and wettability studies point the cooperative effect of the 3D porous polyurethane scaffold and the effect of Graphene-mesoporous Iron oxide composite on the adsorption dynamics. Benefitting from the hierarchical porous structure and heterogeneity, the as-fabricated sponge manifested superior selective adsorption capacity for a wide variety of oils and organic pollutants in the range 90–316 gg−1. Application of GPUF was demonstrated by the quick and selective removal of oils from water under magnetic field. In addition to this, the adsorbate can be released after sorption by simple squeezing without any deterioration in structure and performance, underlining the recyclability of the sorbent for over 150 cycles. Moreover, GPUF manifests appealing adsorption capacity in complex environments also. The results of the study promise a novel sorbent which can be easily scaled up for large scale treatment of oil spills.
Keywords
Graphene
Oil adsorbent
Magnetic actuation
1 Introduction
The quest for highly efficient, simple and benign materials to decontaminate water is of high demand among the scientific community. A broad spectrum of pollutants (Dyes, oil, heavy metals etc) adds to water pollution which is one of the most pervasive problems throughout the world. Oils and chemicals released from sinking ships and industrial accidents are catastrophic to marine and aquatic ecosystems. To cope up with these grievous environmental issues, techniques like flocculation, coagulation, bioremediation and adsorption are practiced, the latter one being highly efficient owing to its ease of operation, cost effectiveness, recyclability etc (Adebajo et al., 2003; Aziz et al., 2010; Bayat et al., 2005; Li et al., 2008). An ideal oil adsorbent must possess high surface area, fair chemical stability and cost effectiveness, in addition to synergistic hyrophobicity and oleophilicity. Sorbents like wool, straw, clay, saw dust, activated carbon have all been employed as adsorbents from ancient times due to their micro porosity. However, most of these sorbents are marked by low selectivity, low adsorption capacity and poor reusability which restrict their widespread application (Wang et al., 2008; Radetić et al., 2003; Ceylan et al., 2009; Suni et al., 2004). Apart from the afore said sorbents, commercially available 3D porous materials like Polyurethane sponges (PU) and Melamine sponges have emerged as desirable candidates for oil adsorption due to their high surface area (Wu et al., 2013; Yu et al., 2015). However, the presence of amino and carboxyl groups on its surfaces renders them hydrophilic which inhibits their selectivity and overall performance. These sponges must be tuned to exhibit hydrophobic nature for availing their maximum adsorption efficiency. For adsorbing oil selectively from water, substances must possess both oleophilic and hydrophobic nature. In this regard, inorganic nanowire membranes (Yuan et al., 2008), carbon based materials, like carbon nanotubes (Huaiyuan et al., 2015) and carbon fibers (Zhen-Yu et al., 2014) have drawn considerable attention and have been extensively used as they selectively separate oil from water. However, these substances have practical limitations on account of their cost along with complex fabrication procedures. Currently, membranes that selectively separate oil and water have enthused interest among researchers. However, they are vulnerable to surface and internal fouling which deteriorates their performance in practical operations (Merkel et al., 2002).
Graphene (GN), the novel 2D nanocarbon with astounding properties has touched the nook and corner of scientific and technological applications. GN has been taken to lime light in versatile fields namely in energy storage, Lithium ion batteries, solar cells, environmental remediation, Photocatalysis, sensing etc (Zhang et al., 2015; Quan et al., 2017; Yanhui et al., 2013; Min-Quan et al., 2017, 2014). On account of its hydrophobic and oleophilic nature, GN has emerged as a staple adsorbent in oil spills (Zhu et al., 2010). Several reports suggest the application of 3D graphene foams and graphene coated polymer foams as oil adsorbents. A few representative studies include reports by Singh et al. who fabricated GN sponges which displayed high adsorption efficacy for petroleum products and organic pollutants (Singh et al., 2013); GN coated melamine sponges designed by Nguyen et al. (2012) and GN coated PU foams were reported by Yue et al. (2013). Though these entities portrayed fair adsorption efficacy, few issues are to be addressed and optimized for their widespread application. The major hurdle in their practical application is the collection and reuse of substrates saturated with oil and pollutants. Imparting magnetism onto these adsorbents stands as a promising remedy to address this issue. Once these adsorbents are magnetically actuated, they can be easily driven to the target zone (spill areas) and can be fleetly removed after adsorption. In addition to this, the recyclability of the adsorbent is greatly enhanced and the adsorbents can be scaled up for quick and selective decontamination of water bodies (Bourlinos et al., 2001; Gross et al., 2003).
Keeping all these facts in mind, we have attempted to fabricate a simple GN coated magnetic PU sponge that could serve as an excellent platform for oil/organic chemicals separation from aqueous environment. PU sponge characterized by its micrometer scale reticulate network is switched from hydrophilic to super hydrophobic moiety by the inclusion of GN sheets and super paramagnetic mesoporous iron oxide respectively. To the best of our knowledge, this is the first report on the fabrication of magnetically actuated PU sponge with GN realized via an inexpensive dip coating method. The as prepared sponge, referred to as GPUF, portrayed impressive adsorption capacity towards oil and other organic liquids. It exhibits long time stability, outstanding hydrophobicity, elasticity as well as fair magnetic responsiveness, all these assuring GPUF as an elite pick among other established sorbents.
2 Experimental
2.1 Materials and methods
PolyUrethane sponge was procured from a local market and sponges was cut into blocks with required dimensions. Graphite powder and Ascorbic Acid were purchased from Acros organics and Hi-Media respectively. General chemicals in chemical reagent grade were procured from Merck and were used as received. Hummers’ method was adopted for the preparation of Graphene Oxide (Hummers and Offeman, 1958). Iron oxide was prepared by a modified route as reported by Poyraz et al. (2013) Prior to all experiments, PU sponges were ultrasonically cleaned in water and ethanol, followed by vacuum drying for 12 h.
2.2 Preparation of graphene iron oxide composite coated poly
2.2.1 Urethane sponge (GPUF)
0.1 g of Fe3O4 and 0.01 g of GO were dispersed in 100 ml of water. The pH of metal oxide solution was adjusted in the range 5–6 using HCl. The pH of GO dispersion was kept 7–8 using NaOH. These two solutions were mixed together and stirred for an hour. Consequently, 50 mg ascorbic acid was added followed by the immersion of the treated PU sponges. After 24 h, the sponges were taken out and washed with copious amount of deionised water and was dried at 30 °C. As a control, graphene coated PU sponges (GPU) and Iron Oxide coated PU(FPU) sponges were prepared separately following the same procedure, without the addition of iron oxide and graphene respectively.
The adsorption efficiency was evaluated in sets of batch adsorption systems; In (i) pure organic solvent and (ii) organic solvent/water. For sorption tests, 40 ml of organic liquid/oil was poured into a 100 ml beaker and then pre weighed GPUF was immersed in it for 15 min. Ddiesel oil and chloroform were taken as the representative pollutants. Its weight was recorded after draining for a few minutes. The adsorption capacity was calculated using the equation: Q = (Wt − W0)/W0 (Wt is the weight of the sorbent after adsorption and W0 is the initial weight of the sorbent). For organic solvent/water system, sponges were immersed in a mixture of solvent and water. Typically, 4 g of diesel oil/chloroform was added to 50 ml of deionised water and the weight of the sponge before and after immersion were noted as mentioned above. Investigations on reusability were carried out by squeezing the saturated sponges manually and drying for 15 min and performing the adsorption process. The cycle was repeated for around 150 times to examine the reusability of GPUF.
2.3 Characterisation techniques
The X-ray diffraction pattern of the sponges was obtained with Brucker-Model Advance D8 Diffractometer in the 2θ range of 10–80° with Cu Kά1 radiation. The presence of functional groups and the successful incorporation of Iron oxide were analysed using Jasco FTIR-4100 spectrometer. The micro structure and the morphology of the samples were characterized using Scanning Electron Microscopy FE-SEM (JEOL, Model JSM-7600F) and SEM (JEOL, Model JSM-6390LV) and Transmission electron Microscopy (FEI TECNAI 30 G2). Contact angle measurements were carried out in Digidrop contact angle goniometer (GBX Digidrop). The magnetic measurements were done in Lake Shore model 7404 VSM at room temperature. The Thermogravimetric analysis was carried out in TGA Q50 (TA instrument).
3 Results and discussion
The XRD patterns of PU, GPU and GPUF are shown in Fig. 1(a). The PU foam displayed a characteristic diffraction peak at 19.3°, which can be ascribed to the presence of short range ordered structure, and both soft and hard domains of amorphous PU. The XRD pattern of GPU and GPUF obliviously displayed the peak assigned to PU, indicating that the PU framework remained intact after modification (Hu et al., 2017). Moreover, there is no characteristic peak of graphene oxide at 10°, assuring the reduction of graphene oxide to graphene. The presence of Iron Oxide cannot be deciphered from the XRD pattern as it is uniformly dispersed in the highly amorphous PU 3D network.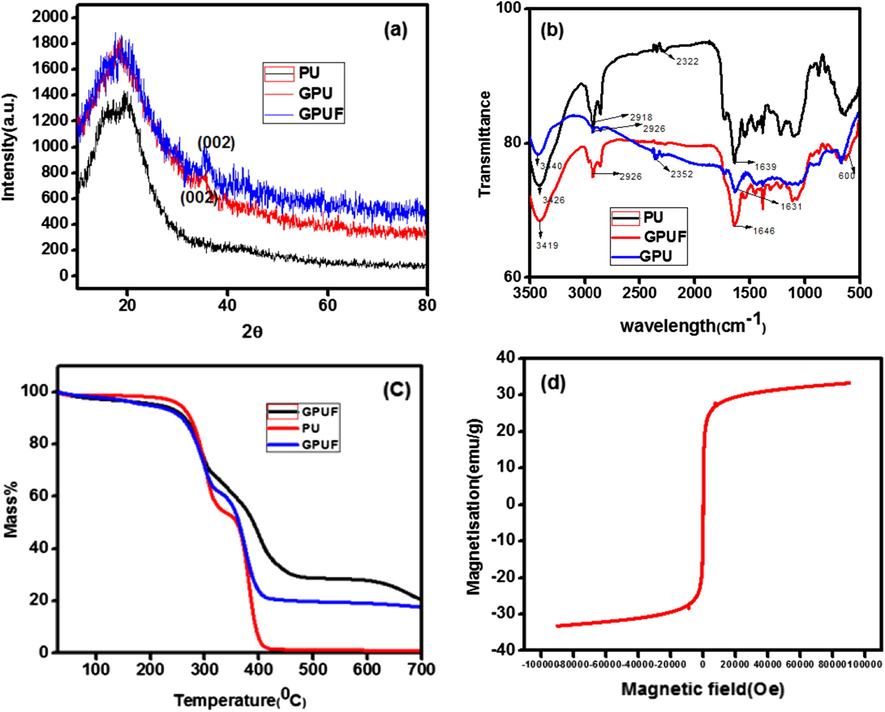
XRD patterns (a), FTIR spectra (b) and TG curves (c) of PU, GPU and GPUF. VSM analysis of Graphene-Iron Oxide composite (d).
FTIR spectra of PU, GPU and GPUF are shown in Fig. 1(b). The the bands at 2322 cm−1 is due to the asymmetric stretching of NCO group in PU. The bands at 1734 cm−1, 1639 cm−1 and 1153 cm−1 can be ascribed to the C⚌O stretching vibration in amide, urea and ether respectively. The broad band near 3400 cm−1 corresponds to the OH stretching vibration, while the band at 2918 cm−1 can be related to the C—H stretching in —CH3 and —CH2 groups (Yue et al., 2013; Anju and Renuka, 2015). FTIR spectra of GPU, GPUF and PU displayed similar characteristic bands between 1000 cm−1 and 1800 cm−1. The characteristic bands of PU underwent a marginal shift/variation in intensity in GPU and GPUF which may be due to the interaction between the additives and PU. No new bands are formed in GPU and GPUF ruling out the formation of chemical bonds among the additives and PU. The band at 600 cm−1 corresponds to the Fe—O stretching vibration in Fe3O4.
The thermo grams of PU, GPU and GPUF are displayed in Fig. 1(C). The trio depicted similar TG curves and have a substantial weight loss in the range of 200–350 °C. This notable drop in mass is due to the combustion of carbon skeleton. TG curves point a slightly higher stability for GPUF which displayed a mass loss of 30% near 600 °C due to the decomposition of Fe3O4. Fig. 1(d) displays the magnetic behavior of GN-Fe3O4 measured at room temperature. The saturation magnetization is found to be 30.07 emu/g which is sufficient enough for magnetic removal of the sorbent after adsorption (Thanikaivelan et al., 2010). The hybrid exhibited super paramagnetic nature with low remnant magnetization and low coercivity at room temperature, assuring that the GPUF can be manipulated in an external magnetic field as depicted in Fig. 2.
A piece of GPUF manipulated by an external magnet.
The digital images of water droplets on GPUF sponge portrays quasi spherical shaped droplets hinting to the hydrophobic nature while oil droplet was instantaneously sucked into the GPUF depicting its oleophilic trait (Fig. 3a). The super hydrophobicity and the super oleophilic feature of the as prepared GPUF were established using Contact angle measurements. The apparent contact angle of water and oil were 151° (Fig. 3(b)) and 0° (Fig. 3(c)). The oil was instantaneously adsorbed into FUG confirming its super oleophilic nature, while it repelled water with high contact angle asserting its super hydrophobic nature. This super hydrophobic and super oleophilic nature of GPUF assure its application in selective removal of oil and organic contaminants from water. The super hydrophobic stability of GPUF in presence of corrosive media was studied by immersing GPUF in aqueous solutions in the pH range from 2 to 10 for 12 h (Fig. 3d). The GPUF displayed super hydrophobic behavior in near neutral pH (6–8). Though the contact angle for water was decreased slightly, the hydrophobic behavior (contact angle >120°) was still maintained in acidic and alkaline pH. This can be endorsed to the erosion of GN-Fe3O4 from the GPUF surface.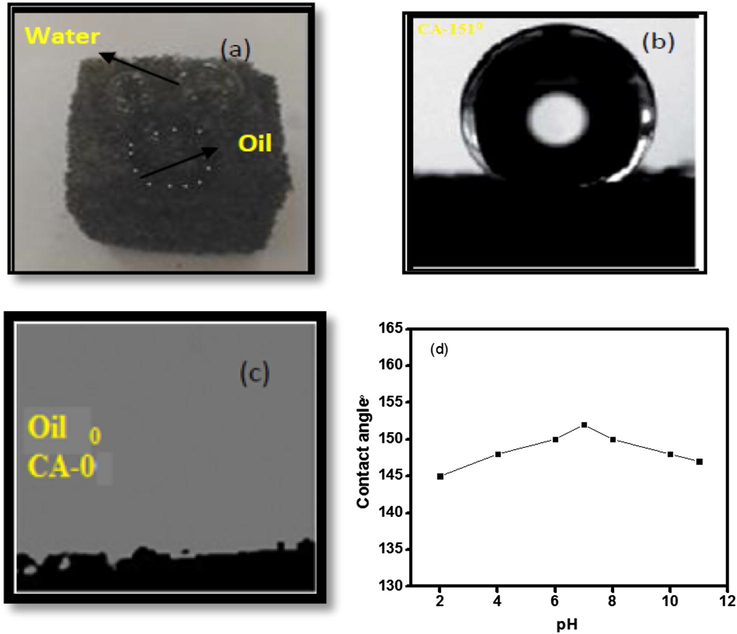
Digital images of water droplet and diesel oil droplet on GPUF (a); Water contact angle of GPUF (b); Oil contact angle of GPUF (c); variation of water contact angle with pH (d).
3.1 Adsorption capacity of GPUF and GPU in a variety of organic solvents
The above characterization results suggest the super hydrophobicity and super oleophilicity of GPUF, which can render it as a good sorbent for oil and other organic solvents. The response of GPUF in organic contaminant/water separation and its adsorption capacity were evaluated in a wide range of organic chemicals and oils. It was observed that the GPUF sponge settled instantaneously in organic solvents (≤10 s) and oil (≤30 s). The adsorption capacity of GPUF and GPU varied from 90 gg−1 to 316 gg−1 while that of GPU varied from 80 to 180 gg−1. The adsorption capacity followed the order: CHCl3>Diesel Oil > Lubricating oil > Bean Oil > Tetra Hydro Furan > Dimethyl Sulphoxide > Toluene > DimethylFormamide > Acetone (Fig. 4a and b). The swelling phenomenon was observed for a few solvents due to the diffusion of solvents onto GPUF. However, this did not alter the adsorption capacity of GPUF. The highest adsorption capacity was recorded for CHCl3, which is highest among several reported systems (Yue et al., 2013; Zhang et al., 2014; Bi et al., 2012). GPUF also have high adsorption capacity for oils which outshines the performance of many sorbents presented in literature (Tuncaboylu and Okay, 2009; Hayase et al., 2013; Carmody et al., 2007). The absorption for organic liquids was so instantaneous that the equilibrium time could not be measured. Viscosity played a central role in determining the equilibrium time of oils as the rate of adsorption varied linearly with the viscosity of oils (Fig. 4(c)). It is noteworthy that the sorption capacity does not follow a linear pattern with density as has been previously reported for other sorbents (Yue et al., 2013).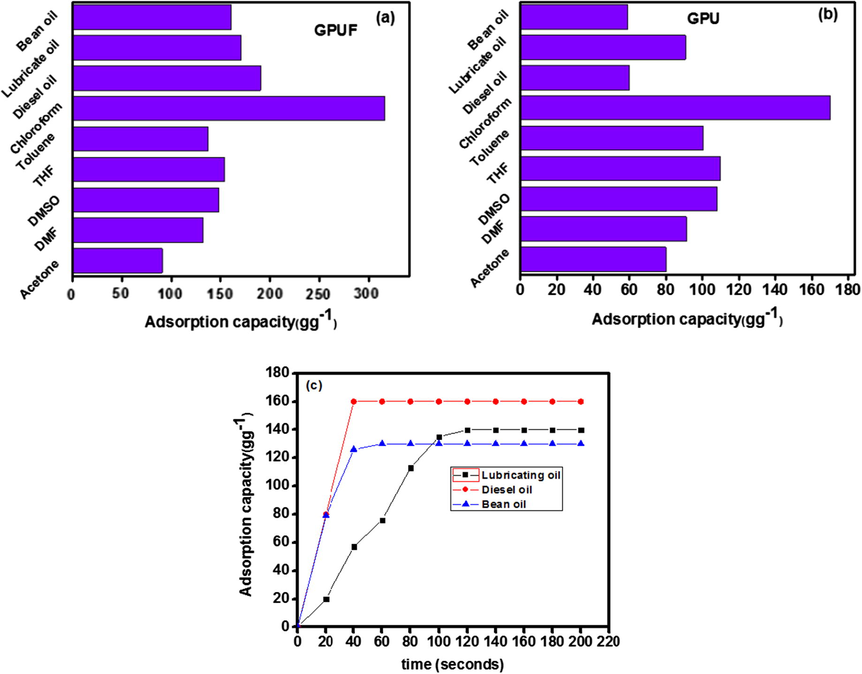
Adsorption capacity of GPUF (a) and GPU (b) in a variety of solvents and oils. Adsorption capacity of GPUF against contact time in a variety of oil (c).
The adsorption capacity of GPUF was also assessed in the mixture i.e. oil/organic solvent: water. As and when a small piece of GPUF was forced onto CHCl3 which sank into the beaker, CHCl3 was fleetly adsorbed (Fig. 5a). In addition to this, GPUF instantaneously adsorbed diesel oil within 10 s, leaving completely clear water without any pollutant (Fig. 5b). This affirms that density of the organic moiety is immaterial for the adsorption process. It was found that 80 mg of sorbent can readily remove 13 g of diesel oil of 0.5 cm thickness in 75 ml of DIW, which is highly imperative. The adsorbed oil can be completely recovered by simply squeezing the sorbent. There was a nominal decrease (less than 5%) in adsorption capacity in the studied system which can be accounted due to the competitive adsorption of water (which of course is negligible), presence of interfering ions and other foreign matter (Hua et al., 2013). However, the adsorption capacity was still higher when compared to several other reports available in literature (Table 1).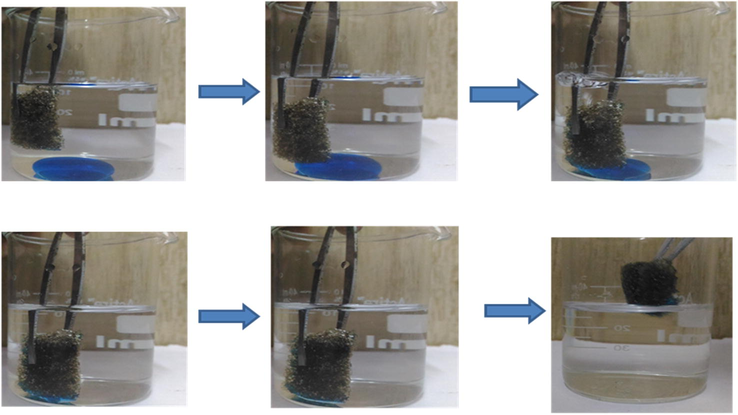
Adsorptive removal of CHCl3 (dyed blue) from water by GPUF within 5 s.
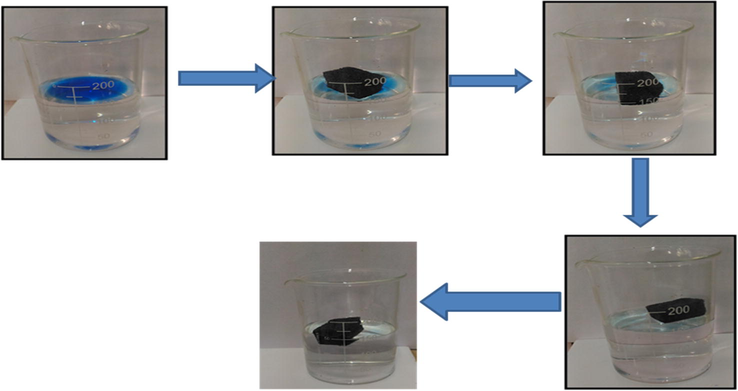
Progress in adsorptive removal of diesel oil (dyed blue) from water within 10 s.
Sorbent
Solvents
Adsorption capacity (gg−1)
Cost
Reference
RGO foam
Oils and Organic Liquids
5–40
High
Radetić et al. (2003)
Macroporous organogel
Oils and Solvents
50–102
Medium
Hayase et al. (2013)
PDMS
Organic Liquids
8
Medium
Choi et al. (2011)
KGPPY
Diesel
101
Medium
Gupta and Kulkarni (2011)
GN/CNT/Fe2O3
Diesel
27
High
Sudong et al. (2014)
Boron doped CNT
Oils and Organic Liquids
25–125
High
Hashim et al. (2012)
Graphene Sponge
Oils and Organic Liquids
60–160
High
Niu et al. (2012)
TCF aerogels
Oils and Organic Liquids
50–192
Low
Hengchang et al. (2013)
CNT sponge
Oils and Organics
50–102
High
Gui et al. (2010)
GPUF
Oils and solvents
90–316
Low
This work
The microstructure of the GPUF sponge was analysed by Scanning Electron Microscopy (SEM). A closer investigation of the SEM images revealed the porous nature and the surface heterogeneity of GPUF. Fig. 6(a) represents the SEM image of GPUF which portrays porous nature inherent to the PU framework. Adsorption occurs in voids of these porous materials. Fig. 6b represents the SEM images of walls of the reticulate polymer network of GPUF decorated with wrinkled GN sheets. Zooming in on the wall reveals wrinkled GN sheets (inset of 6b) adorned with iron oxide nanoparticles (Fig. 6c). It must be noted here that the structural roughness is of prior importance in wettability. The wide angle XRD pattern of GN-Fe3O4 composite established the amorphous nature of the composite and hints the presence of Fe3O4 nanoparticles of grain size of few tens of nanometer, ensuring a typical nano composite structure (Fig. 6d). Size of the iron oxide nanoparticles as obtained from the TEM images was found to be in the range of 18–20 nm. Mesoporous nature of the Iron Oxide system was also proved from the HRTEM image (Fig. inset of 6c) and small angle XRD pattern (Fig. inset of 6e), as evident from the porous character and the peak at low 2 theta value, respectively (Nikhila et al., 2017).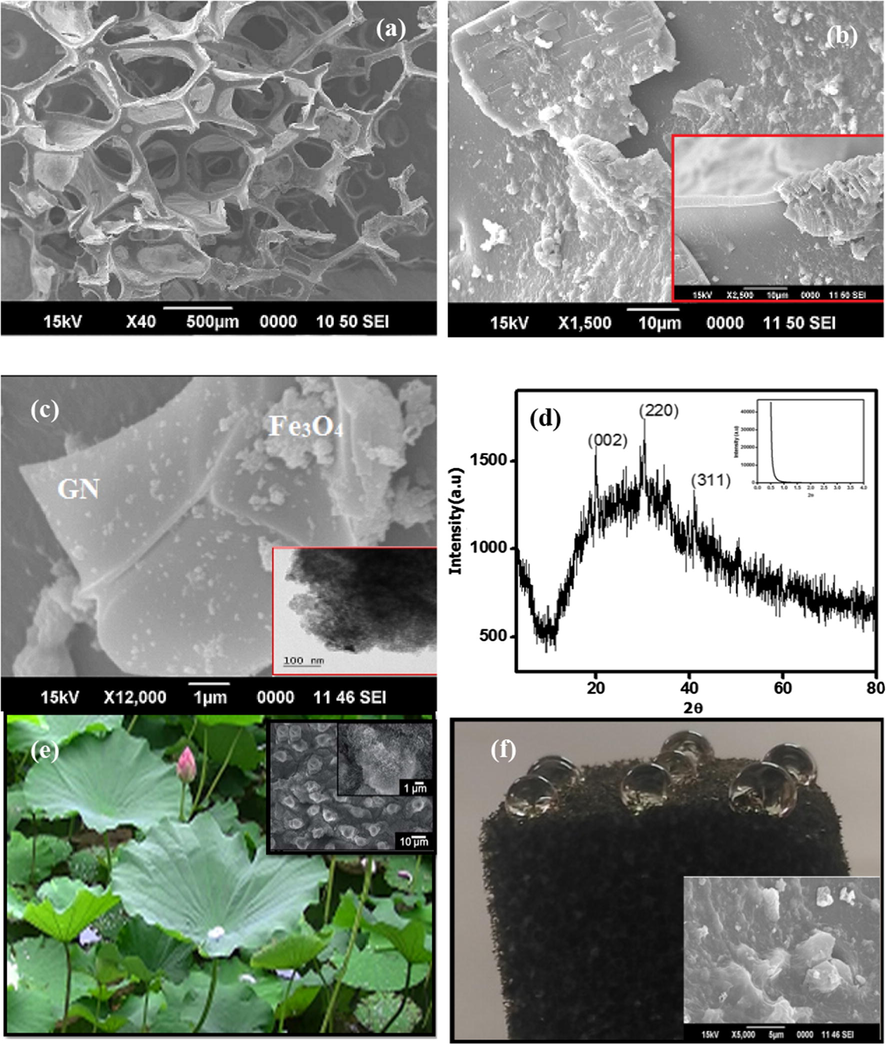
SEM images of GPUF depicting the porous nature (a); SEM images depicting the incorporation of wrinkled Graphene sheets and Iron oxide (b). Inset depict the walls of GPUF revealing the heterogeneity; SEM image confirming the incorporation of Iron oxide on GN sheets (c). TEM image in the inset represents the mesoporous nature of Iron oxide nanoparticles; XRD pattern of Graphene-iron oxide composite depicting reflections characteristic of planes in Graphene at 2theta values 21° and that of Fe3O4 at 30.5° and 41.1° (d) Inset shows the Low angle XRD of iron oxide nanoparticles. Comparison of surface roughness of lotus leaf and GPUF revealing the super hydrophobicity (e), (f). Images of water drops on the surface and the corresponding SEM images (inset).
The whole credit for the imperative adsorption efficiency of GPUF can be solely laid to the optimized combination of surface heterogeneity, super hydrophobicity and porosity. As drawn from the microscopic analysis, GPUF presents a highly roughened surface with the addition of GN-meso iron oxide composite. Hydrophobic surfaces can be designed by the introduction of heterogeneity onto surfaces. Graphene, persistently known for its hydrophobicity will introduce nanoscale roughness that alters the PU network to a super hydrophobic moiety. As seen from the SEM images, stacked GN sheets with crumpled edges and decorated with meso iron oxide nanoparticles create a fittingly roughened surface which is analogous to the lotus leaf leading to super hydrophobicity and super oleophilicity (Manekkathodi and Lih-Juann, 2011). It is noticed that the water droplets attain a quasi spherical shape on the GPUF surface with a contact angle of 151° (Fig. 6f). The sub micrometer roughness of the exposed GN layers renders hydrophobic nature to the foam, and hence water stays intrinsically at the exterior, while oleophilicity renders oil penetration to the material thus affecting separation of oil and water. Reports suggest that surface roughness can be correlated to hydrophobicity as the air trapped between the water droplet and solid surface minimizes the contact area (Herminghaus, 2000; McHale et al., 2004). Sorption of oil to magnetic particle can be explained with fine particle-oil flocculation which is associated with an electrostatic interaction between magnetic particles with charged surface and polar compounds in oil as well as in the polar solvents (Zhang et al., 2006). The loading of Fe3O4 nanoparticles onto GN sheets benefits in adding to the roughness of GPUF and also facilitates the oil adsorption (Wenzel, 1936; Liuhua et al., 2015). This novel GPUF is characterized by the presence of 3D structure with a wide range of micropores and mesopores within. The presence of mesoporous iron oxide nanoparticles further drives the oil adsorption. The oil is driven through the porous walls of the foam into the bulk via capillary action and the movement is further facilitated by the presence of oleophilic nanoparticles decorating the reticulate walls. The oils will be stored in the mesopores of the oleophilic iron oxide nano particles creating more volume for oil storage due to the π-π interaction between GN sheets and π cloud in oils, which accounts for the selectivity of GPUF (Su et al., 2010).
The intrinsic hydrophobicity of GN sheets clubbed with magnetic responsiveness of Fe3O4 as well as porosity and oleophilicity of PU template lead to very high sorption capacity. Of course, the mesoporosity of Fe3O4 is also has to be counted in this regard. Wenzel theory suggests that actual wetting area available on the surface of solid increases proportionately with roughness (Wenzel, 1936). The synergistic action of inherent porosity, the super hydrophobicity and the inbuilt heterogeneity greatly facilitate the diffusion of oil into the sponge and result in appreciable adsorption efficiency. Preparation of iron oxide incorporated PU sponge alone was also done as a control. Though at the first instance, it displayed notable adsorption efficiency, the leaching of Fe3O4 was noticed in course of time, which rendered the system considerably unstable. This clearly points to the structural stability on incorporation of graphene. Though a few systems displayed better adsorption capacity than GPUF, this foam competes and outshines those sorbents in terms of its cost effectiveness, simplicity and recyclability (Zhao et al., 2011; Xuchun et al., 2013; Zhao et al., 2012).
3.2 Recyclability of the sorbent
Reusability of a sorbent is very significant for practical application in oil removal. The GPUF can be reharvested by reclamation by magnetic force and manual squeezing after sorption. To further elucidate the practical applicability of GPUF, it was employed to magnetically remove oil from water. Diesel oil (dyed blue) was dropped in water (oil: water, 1:6 by volume) and was magnetically actuated to move under the influence of weak magnetic force. The progress of the removal is shown in Fig. 7. The oil was almost instantaneously removed by the magnetic sponge and it can serve as energy less strategy to allay the perils of collection of sorbent after adsorption.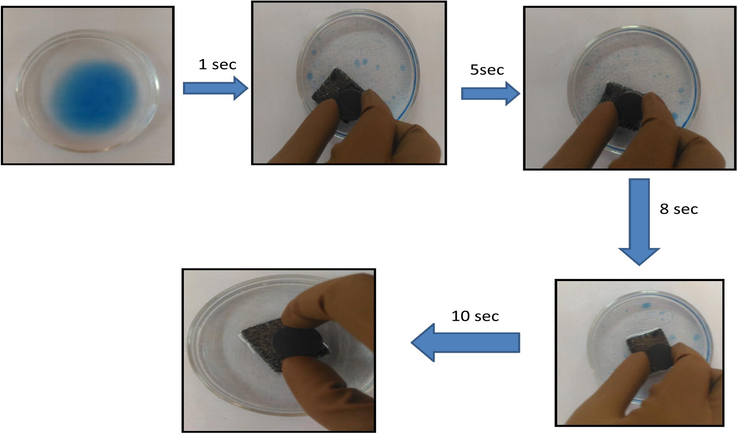
Progress in oil removal by magnetically driven GPUF.
Owing to the intrinsic elastic nature of GPUF, the adsorbate can be easily removed by manual squeezing (Fig. 8). The adsorption capacity as a function of adsorption cycle for diesel oil and CHCl3 is depicted in Fig. 8a and b. The adsorption capacity remained identical up to 120 cycles for diesel oil and 150 cycles for chloroform. Afterwards, there was a nominal decrease in adsorption capacity due to the presence of slight traces of adsorbate in the sorbent which cannot be just removed by squeezing. Less than 2% weight remained in GPUF even after 100 cycles for both oil and CHCl3, establishing the highly stable reusability of GPUF. Squeezing of the sorbent stands as a promising route for recyclability when compared to other techniques like burning, extraction etc. as it is does not lead to structural damage when subjected to heat treatment and can also be employed for organic solvents and oils. The recyclability behavior is nearly analogous for all the investigated solvents and oils. It is noteworthy that, the recyclability achieved is the highest among other reported polymeric sorbents and carbon based sorbents (Hengchang et al., 2014; Sun et al., 2013). Cyclic performance studies assert its easy to recover and reuse capacity.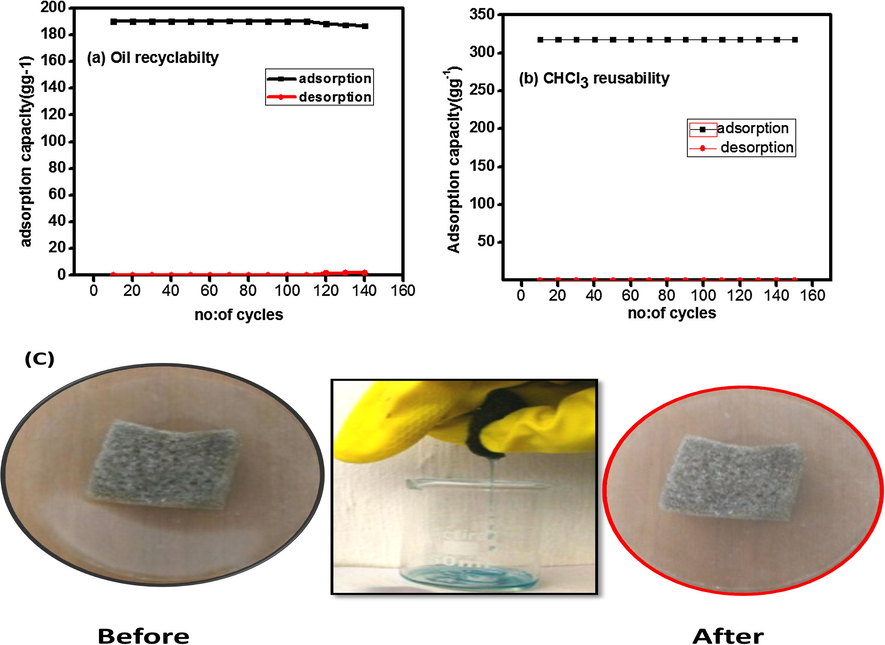
Adsorption capacity of GPUF in cycles of adsorption and desorption Diesel Oil (a), Chloroform (b). Digital images of GPUF before and after reusability studies using diesel oil (c).
We believe that GPUF presents itself as a feasible alternative over many of its other counterparts tabulated in Table 1. Though the sorbents based on Nitrogen doped Graphene aerogel, Graphene foam etc exhibited superior efficiency than the prepared GPUF foam, they fail in competing with the efficiency, simplicity, cost effectiveness, reusability and user friendliness of the system projected herein.
4 Conclusions
To brief up, a novel and cost effective functional hybrid(GPUF) based on polyurethane as 3D architecture and Graphene-mesoporous Iron oxide composite as reinforcement material is fabricated and its water/oil separation characteristics is investigated in detail. Decked by Graphene-mesoporous iron oxide composite, this hybrid foam depicted hierarchical roughness and heterogeneity paving the lane for its estimably high adsorption capacity. GN and iron oxide, well known for its hydrophobic and oleophilic nature respectively, is believed to introduce nano and micro scale roughness, altering the PU scaffold into a super hydrophobic but super oleophilic moiety. The as prepared foam exhibited impressive traits like super hydrophobicity, super oleophilicity, and selectivity culminating in its extra ordinary high adsorption efficiency for a broad spectrum of oils and organic contaminants. Being magnetically actuated, GPUF can be driven easily with the help of a magnet to target zones and can be recollected quickly after sorption. The recovered sponge can be reused for about 150 cycles maintaining its high adsorption capacity. To the best of our knowledge, this work is the first report on magnetically driven PU foams based on Graphene. We envision that the findings of our study projects a privileged sorbent, GPUF, that could be scaled up as a promising platform for large scale decontamination of pollutants in water.
Acknowledgements
M. Anju gratefully acknowledges the financial assistance received from UGC and also University of Calicut for providing research facilities.
References
- Porous materials for oil spill cleanup: a review of synthesis and absorbing properties. J. Porous Mater.. 2003;10:159-170.
- [Google Scholar]
- A novel template free synthetic strategy to graphene–iron oxide nanotube hybrid. RSC Adv.. 2015;5:78648-78654.
- [Google Scholar]
- Optimal conditions for bioremediation of oily seawater. Bioresour. Technol.. 2010;101:9455-9460.
- [Google Scholar]
- Oil spill cleanup from sea water by sorbent materials. Chem. Eng. Technol.. 2005;2:1525-1528.
- [Google Scholar]
- Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater.. 2012;21:4421-4425.
- [Google Scholar]
- Magnetic modification of the external surfaces in the MCM-41 porous silica: synthesis, characterization, and functionalisation. J. Phys. Chem. B. 2001;105:7432-7437.
- [Google Scholar]
- Adsorption of hydrocarbons on organo-clays—implications for oil spill remediation. J. Colloid Interface Sci.. 2007;305:17-24.
- [Google Scholar]
- Evaluation of butyl rubber as sorbent material for the removal of oil and polycyclic aromatic hydrocarbons from seawater. Environ. Sci. Technol.. 2009;43:3846-3852.
- [Google Scholar]
- A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl. Mater. Interfaces.. 2011;3:4552-4556.
- [Google Scholar]
- Controlling magnetic coupling between cobalt nanoparticles through nanoscale confinement in hexagonal mesoporous silica. J. Phys. Chem. B. 2003;107:5475-5482.
- [Google Scholar]
- Removal of organic compounds from water by using a gold nanoparticle–poly(dimethylsiloxane) nanocomposite foam. ChemSusChem. 2011;4:737-743.
- [Google Scholar]
- Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions. Sci. Rep.. 2012;2:363.
- [Google Scholar]
- Facile synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water. Angew. Chem. Int. Ed.. 2013;52:1986-1989.
- [Google Scholar]
- carbon fiber aerogel made from raw cotton: a novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater.. 2013;41:5916-5921.
- [Google Scholar]
- Highly enhanced performance of spongy graphene as an oil sorbent. J. Mater. Chem. A. 2014;2:1652-1656.
- [Google Scholar]
- Lightweight conductive graphene/thermoplastic polyurethane foams with ultrahigh compressibility for piezoresistive sensing. J. Mater. Chem. C. 2017;5:73-83.
- [Google Scholar]
- Covalent assembly of 3D graphene/polypyrrole foams for oil spill cleanup. J. Mater. Chem. A. 2013;1:3446-3453.
- [Google Scholar]
- A novel carbon nanotubes reinforced superhydrophobic and superoleophilic polyurethane sponge for selective oil–water separation through a chemical fabrication. J. Mater. Chem. A. 2015;3:266-273.
- [Google Scholar]
- Superoleophilic and superhydrophobic inverse opals for oil sensors. Adv. Funct. Mater.. 2008;18:3258-3264.
- [Google Scholar]
- Fe3O4/PS magnetic nanoparticles: Synthesis, characterization and their application as sorbents of oil from waste water. J. Magnetism Magnetic Mater.. 2015;394:14-21.
- [Google Scholar]
- Anomalous adhesive superhydrophobicity on aligned ZnO nanowire arrays grown on a lotus leaf. J. Mater. Chem.. 2011;21:18061-18066.
- [Google Scholar]
- Ultrapermeable, reverse-selective nanocomposite membranes. Science. 2002;296:519-522.
- [Google Scholar]
- Artificial photosynthesis over graphene–semiconductor composites. Are we getting better? Chem. Soc. Rev.. 2014;43:8240-8254.
- [Google Scholar]
- Metal-free, robust, and regenerable 3D graphene–organics aerogel with high and stable photosensitization efficiency. J. Catal.. 2017;346:21-29.
- [Google Scholar]
- Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci.. 2012;5:7908-7912.
- [Google Scholar]
- P123 and solvent-assisted synthesis of titania nanocuboids with co-exposed 101 and 001 planes. CrystEnggComm. 2017;19:511-518.
- [Google Scholar]
- A leavening strategy to prepare reduced graphene oxide foams. Adv. Mater.. 2012;24:4144-4150.
- [Google Scholar]
- A general approach to crystalline and monomodal pore size mesoporous materials. Nat. Commun.. 2013;4:2952.
- [Google Scholar]
- Graphene and its derivatives as versatile templates for materials synthesis and functional applications. Nanoscale. 2017;9:2398-2416.
- [Google Scholar]
- Recycled wool-based nonwoven material as oil sorbent. Environ. Sci. Technol.. 2003;37:1008-1012.
- [Google Scholar]
- Nano to micro structural hierarchy is crucial for stable superhydrophobic and water-repellent surfaces. Langmuir. 2010;26:4984-4989.
- [Google Scholar]
- Magnetic graphene foam for efficient adsorption of oil and organic solvents. J. Colloid Interface Sci.. 2014;430:337-344.
- [Google Scholar]
- Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv. Mater.. 2013;25:2554-2560.
- [Google Scholar]
- Use of a by-product of peat excavation cotton grass fibre as a sorbent for oil-spills. Mar. Pollut. Bull.. 2004;49:916-921.
- [Google Scholar]
- Collagen based magnetic nanocomposites for oil removal applications. Sci. Rep.. 2010;2:230.
- [Google Scholar]
- Preparation and characterization of single-hole macroporous organogel particles of high toughness and superfast responsivity. Eur. Polym. J.. 2009;45:2033-2042.
- [Google Scholar]
- Synthesis and dye separation performance of ferromagnetic hierarchical porous carbon. Carbon. 2008;46:1593-1599.
- [Google Scholar]
- Resistance of solid surfaces to wetting by water. Ind. Eng. Chem.. 1936;28:988-994.
- [Google Scholar]
- Mechanically flexible and multifunctional polymer-based graphene foams for elastic conductors and oil-water separators. Adv Mater.. 2013;25:5658-5662.
- [Google Scholar]
- Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Appl. Mater. Interfaces. 2013;5:5845-5850.
- [Google Scholar]
- Size effect induced activity enhancement and anti-photocorrosion of reduced graphene oxide/ZnO composites for degradation of organic dyes and reduction of Cr(VI) in water. Appl. Catal. B. 2013;140:598-607.
- [Google Scholar]
- Oil Absorbents Based on Melamine/Lignin by a Dip Adsorbing Method. ACS Sustain. Chem. Eng.. 2015;3:3012-3018.
- [Google Scholar]
- Superwetting nanowire membranes for selective absorption. Nat. Nanotechnol.. 2008;3:332-336.
- [Google Scholar]
- Cost-effective reduced graphene oxide-coated polyurethane sponge as a highly efficient and reusable oil-absorbent. ACS Appl. Mater. Interfaces.. 2013;5:10018-10026.
- [Google Scholar]
- A composite polymer film with both superhydrophobicity and superolephilicity. Macromol. Rapid. Commun.. 2006;27:804-808.
- [Google Scholar]
- Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem. Mater.. 2014;26:2659-2668.
- [Google Scholar]
- Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev.. 2015;115:10307-10377.
- [Google Scholar]
- A versatile, ultralight, nitrogen-doped graphene framework. Angew. Chem. Int. Ed.. 2012;51:11371-11375.
- [Google Scholar]
- Improvement of oil adsorption performance by a sponge-like natural vermiculite-carbon nanotube hybrid. Appl. Clay Sci.. 2011;53:1-7.
- [Google Scholar]
- Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions. Sci. Rep.. 2014;4:4079
- [Google Scholar]
- Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater.. 2010;22:3906-3924.
- [Google Scholar]







