Translate this page into:
Magnetite nanoparticles with surface modification for removal of methyl violet from aqueous solutions
⁎Corresponding author at: Department of Chemistry, Faculty of Sciences, Rasht Branch, Islamic Azad University, Rasht, Iran. Tel.: +98 9113314086; fax: +98 1314224949. Shariaty@iaurasht.ac.ir (Shahab Shariati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this research, the potential of Fe3O4 magnetic nanoparticles (MNPs) for efficient removal of methyl violet as a cationic dye from aqueous solutions was investigated. For this purpose, Fe3O4 MNPs were synthesized via chemical precipitation method. The synthesized MNPs were characterized by XRD and SEM techniques. To remove methyl violet, the surface of the MNPs was modified with sodium dodecyl sulfate (SDS) as an anionic surfactant. Also, the various parameters affecting dye removal were investigated and optimized. The kinetic studies for methyl violet adsorption showed rapid sorption dynamics by a second-order kinetic model, suggesting chemisorption mechanism. Dye adsorption equilibrium data were fitted well to the Langmuir isotherm rather than Freundlich isotherm. The maximum monolayer capacity, (qmax), was calculated from the Langmuir as 416.7 mg g−1. The results show that, SDS-coated magnetic nanoparticles, can be used as a cheap and efficient adsorbent for removal of cationic dyes from aqueous solutions.
Keywords
Magnetite nanoparticles
Methyl violet
Sodium dodecyl sulfate
1 Introduction
Dyes are used in large amount in many industries to color the products. Many dyes are toxic in nature with suspected carcinogenic and mutagenic effects that influence aquatic biota and also human beings (O’Mahony et al., 2002; Ozcan and Ozcan, 2004). Dyes usually have complex aromatic structures which make them stable and difficult to decompose (Kiran et al., 2006). Due to their good solubility, the discharge of dye-bearing wastewater into natural streams and rivers leads to a perilous problem, as dyes impart toxicity to aquatic life and, therefore, damage the esthetic nature of the environment (Mohan et al., 2002).
However, wastewater containing dyes is very difficult to treat, since the dyes are resistant to aerobic digestion and are stable to light, heat and oxidizing agents due to their structure and molecular size (Sun and Yang, 2003; Ravi Kumar et al., 1998). There are several methods for dyes removal, including membrane separation, flocculation, sonolysis, anaerobic biological treatments, oxidative destruction via UV/ozone treatment, photocatalytic degradation, which have certain efficiency, but their initial and operational costs are too high (Crini, 2006; Ramesh et al., 2007; Zaharia et al., 2006).
Adsorption has been discovered to be superior to other techniques for wastewater treatment in terms of initial cost, simplicity of design and ease of operation (Garg et al., 2004; McKay, 1981; Jumasiah et al., 2005). Most commercial systems currently use activated carbon as a sorbent to remove dyes in wastewater by reason of its excellent adsorption power. Activated carbon is structurally homogeneous material with high surface area, and has microporous structure and radiation stability, which are important in its use as adsorbent (Leyva-Ramos, 1989; Tsai et al., 2001; Pendleton and Wu, 2003), catalyst and catalyst support (Gerald and Russel, 1991), so it is extensively applied for industrial process, but activated carbon has not been able to reduce the concentration of contaminants at ppb levels (Pillay et al., 2009) and its use is usually limited due to its high cost. In order to decrease the cost of treatment, attempts have been made to find inexpensive alternative adsorbents. Recently, many approaches have been studied for the development of cheaper and more effective adsorbents. Some of these absorbents are perlite (Alkan and Dogan, 2001; Bereket et al., 1997; Gupta et al., 2000; Mohan et al., 2002), bentonite (Demirbas and Dogan, 2002), silica gels (Mohamed, 1996), fly ash (Dogan and Alkan, 2003), lignite (Allen et al., 1989), peat (Ho and Mckay, 1998), silica (Masciangioli and Zhang, 2003) etc. In most cases, these adsorbents are highly porous substances, providing sufficient surface area for adsorption.
In the last decade, comprehensive investigations and developments were observed in the field of nano-sized magnetic particles. These materials often possess unique electrical, chemical, structural, and magnetic properties allowing for use in the field of novel applications including information storage, drug delivery, biosensors, chemical and biochemical separation and environmental remediation (Leslie-Pelecky and Rieke, 1996; Elliott and Zhang, 2001).
Magnetic nanoparticles (MNPs) are a class of nanoparticles which can be manipulated using a magnetic field. MNPs have the advantages of large surface area, high number of surface active sites and high magnetic properties, which cause high adsorption efficiency, high removal rate of contaminants, and easy and rapid separation of adsorbent from solution via magnetic field. After magnetic separation, the contaminants can be easily removed from nanoparticles by the desorbent agents, and the recovered MNPs can be reused (Oliveira et al., 2003).
The present work describes the chemical synthesis of Fe3O4 MNPs and the surface modification of synthesized MNPs with sodium dodecyl sulfate (SDS). After synthesis, the applicability of SDS-coated Fe3O4 MNPs as an efficient adsorbent for the removal of methyl violet as a cationic dye from aqueous samples was investigated.
2 Experimental
2.1 Reagents and materials
Methyl violet (C23H26N3Cl) (Fig. 1) as a cationic dye, ferric chloride (FeCl3·6H2O), ferrous chloride (FeCl2·4H2O), sodium hydroxide, sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB) and hydrochloric acid were purchased with high purity from Merck (Darmstadt, Germany). A stock standard solution of methyl violet at a concentration of 1000 mg L−1 was prepared in double distilled water. This standard solution was diluted with doubly distilled water to prepare stock solutions with the concentration of 5, 10 and 50 mg L−1 of methyl violet.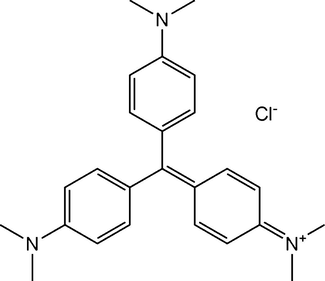
Chemical structure of methyl violet.
2.2 Apparatus
All absorbance measurements were obtained using a Milton Roy (Spectronic 601) UV–Vis spectrophotometer. For absorbance measurements, 585 nm was chosen as the maximum absorbance wavelength (λmax) of methyl violet. The pH of solutions was adjusted with a Jenway model 3320 pH meter (Staffordshire ST15 0SA, England) supplied with a combined glass electrode. A Stuart CB162 motor-stirrer (StaffordshireST15 0SA, England) was applied to stir dye solutions by a magnet. Magnetic separation was done by a strong super magnet with 1.4 T magnetic field (1 × 3 × 5 cm). A temperature controlled shaker was applied for shaking of the dye solutions in isotherm studies at constant temperature. X-ray powder diffraction (XRD) measurements were performed using a Philips diffractometer of X’pert company with mono chromatized Cu kα radiation. The morphology of synthesized samples was characterized with a scanning electron microscope (SEM) from Philips Company (XL30 ESEM).
2.3 Synthesis of Fe3O4 magnetic nanoparticles
Large scale synthesis of Fe3O4 MNPs with higher efficiency was carried out in a five-necked reactor that was designed in the laboratory as was written elsewhere (Faraji et al., 2010a,b,c,d). In the designed method, the synthesis of MNPs was done by introducing nitrogen gas through a sparger into the solution for oxygen removal. Sparger increases degassing efficiency of the solution by producing very small nitrogen bubbles. The bubbling of nitrogen gas through the solution protects MNPs against critical oxidation and reduces the particles size when compared to synthesis methods without oxygen removal (Kim et al., 2001).
For dropwise addition of ferrous and ferric chloride solutions, two dropping funnels were connected to two necks of the reactor. Slow and dropwise addition of stock solution helps to get high efficiency of the MNPs production. To prevent vaporization of the solution, a home-made condenser was connected to the central neck of the reactor to circulate cold water. Fifth neck was used in temperature measurement of the reactor solution and also in sampling of the reactor content in further studies. The glassware stirrer was applied to stir the reactor solution through the condenser and the central neck. An increase in the mixing rate tends to decrease the particle size.
Fe3O4 MNPs were prepared by chemical coprecipitation method (Zhao et al., 2008). First, 10.4 g of FeCl3·6H2O, 4.0 g of FeCl2·4H2O and 1.7 mL of HCl (12 mol L−1) were dissolved in 50 mL of deionized water in a beaker which was degassed with nitrogen gas for 20 min before use, to prepare a stock solution of ferrous and ferric ions. On the other hand, 500 mL of 1.5 mol L−1 NaOH solution was degassed (for 15 min) and heated to 80 °C in the reactor. Then, the stock solution was added dropwise using a dropping funnel during 30 min under nitrogen gas protection and vigorous stirring (1000 rpm) by use of the glassware stirrer. During the whole process, the solution temperature was maintained at 80 °C and nitrogen gas was used to prevent the intrusion of oxygen. After the reaction, the obtained Fe3O4 MNPs were separated from the reaction medium by magnetic field (with 1.4 T magnetic strength), and then were washed with 500 mL deionized water four times. Finally, the obtained MNPs were resuspended in 500 mL of degassed deionized water. The pH of suspension after the washings was 11.0 and concentration of the generated MNPs in suspension was estimated to be about 10 mg mL−1. The obtained MNPs were stable in this condition up to one month.
3 Results and discussion
3.1 Characterization of the MNPs
Characterization of Fe3O4 MNPs was studied using XRD and SEM. Fig. 2 shows the XRD pattern of the synthesized MNPs, which was quite identical to pure Fe3O4 MNPs and matched well with the XRD pattern of it (JCPDS No. 19-629), indicating that the sample has a cubic crystal system. Also, we can see that no characteristic peaks of impurities were observed.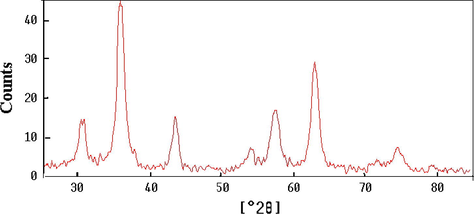
XRD pattern of synthesized Fe3O4 MNPs.
SEM image of the prepared MNPs was obtained as shown in Fig. 3. Fe3O4 surface morphology analysis demonstrated the agglomeration of many ultrafine particles with diameter of about 40 nm.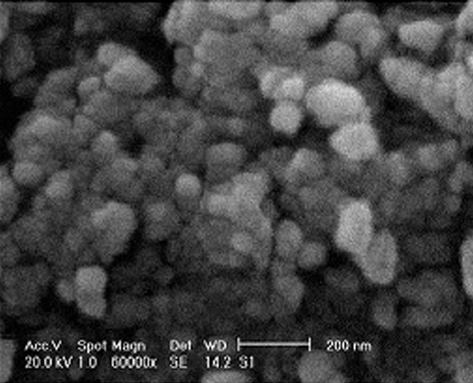
SEM image of synthesized MNPs.
In the proposed procedure, to achieve maximum adsorption efficiency, various parameters affecting the removal of methyl violet were studied and optimized with univariate method.
3.2 Removal of methyl violet using synthesized MNPs
In primary experiments the absorption behavior of methyl violet at various pHs was studied with measuring absorbance of solutions at 585 nm. To achieve maximum adsorption efficiency, various parameters affecting the dye removal were studied and optimized. Optimization studies were carried out according to the following procedure: (1) 25 mL aqueous solution of the dye (5 mg L−1) was poured in a 50 mL beaker, (2) 1.0 mL of the Fe3O4 MNPs suspension (containing 10 mg of Fe3O4 NPs) was added to the dye solution, (3) pH of the solution was adjusted to the desired value and then 1.0 mL of SDS solution was added into the dye solution, (4) the mixture was stirred for 10 min, (5) after dye adsorption; Fe3O4 MNPs were quickly separated from the sample solution using a magnet (1.4 T), (6) the residual dye concentration in the supernatant clear solution was determined spectrophotometrically using a calibration curve. The following equation was applied to calculate the dye removal efficiency:
3.2.1 Influence of pH
The pH value of the dye solution plays an important role in the whole adsorption process and particularly on the adsorption capacity. Most of the dyes are ionic and upon dissociation conferred dye ions into solution. The degree of adsorption of these ions onto the adsorbent surface is primarily influenced by the surface charge on the adsorbent, which in turn is influenced by the solution pH (Ramakrishna and Viraraghavan, 1997). So, adsorption of the dyes is highly pH dependent. For Fe3O4 MNPs, the surface charge is neutral at pHzpc, which is about 7.0 (Zhao et al., 2008).
According to the Fig. 4, it was found that the adsorption efficiency of methyl violet is high in acidic solutions (pH = 2.0–3.0).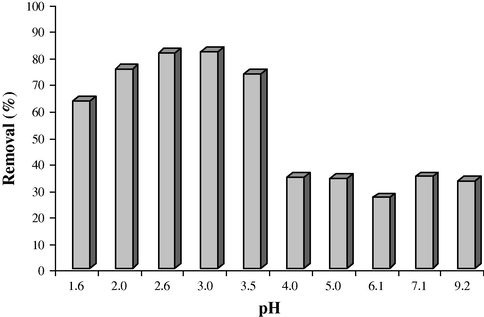
Effect of pH of solution on the methyl violet removal (Cdye = 5 mg L−1, V = 25 mL).
In acidic pHs, dye is in cationic form and can interact with the negatively charged surface of SDS-coated Fe3O4 MNPs Fig. 5. On the other hand, at alkaline pH, surface of Fe3O4 MNPs is negatively charged and the dye is negative too. So, the dye can’t directly interact with Fe3O4 MNPs and is adsorbed to the adsorbent surface. Therefore, for further works pH of solution was adjusted at pH = 3.0.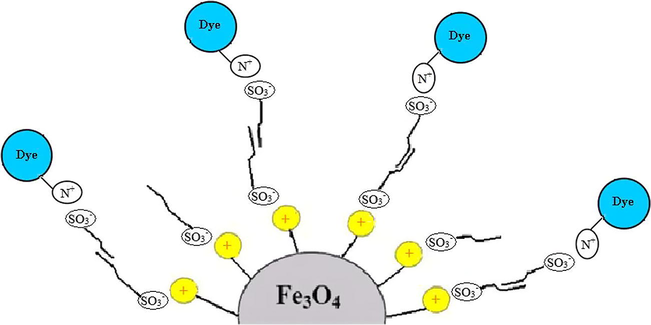
Schematic presentation of dye removal mechanism by SDS-coated Fe3O4 MNPs.
3.2.2 Influence of SDS and salt concentration
SDS was added to the solution at concentrations lower than its critical micelle concentration (CMC = 2.3 g L−1) to modify the surface of MNPs. At acidic pH, the surface of MNPs is positive and negative head group of SDS can interact with the surface of MNPs to form double layer on the surface of particles. In this condition, the cationic dye can be adsorbed to the surface of MNPs via electrostatic interactions. Fig. 6 shows the influence of SDS amount on the removal efficiency of methyl violet. The addition of SDS can facilitate the collection of magnetic particles via magnet. According to the results, with increase in SDS amount, the adsorption amount of the dye increased remarkably. The increase in adsorption can be explained by the gradual formation of SDS aggregates (hemimicelles, mixed hemimicelles or admicelles) on the Fe3O4 MNPs surface and the dyes are adsorbed gradually. Maximum adsorption was obtained when SDS amount was 2 mg. Therefore, this value was chosen as optimum value for further works. At high concentrations of SDS, the adsorption of dye decreased due to formation of SDS aggregates in solution.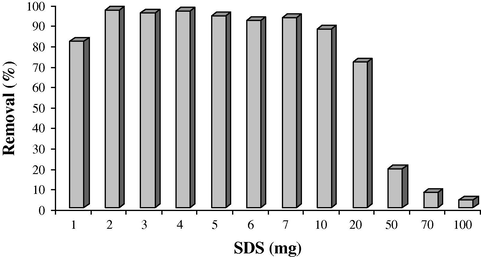
Effect of SDS amounts on the removal of methyl violet (Cdye = 5 mg L−1, V = 25 mL, pH = 3.0).
3.2.3 Influence of ionic strength and contact time
The effect of ionic strength on the adsorption of dye (Cdye = 5 mg L−1, V = 25 mL) was investigated by adding NaCl within the range of 0–1.0 mol L−1. The results showed that with increase in NaCl concentration, the adsorption capacity of the Fe3O4 MNPs was decreased significantly. This phenomenon can be explained by the competition of cationic dye and Na+ ion for the sorption sites. Thus, the strategy of no salt addition was performed for the kinetic and isotherm studies.
The study of contact time between adsorbent and dye within the range of 0–20 min showed that the adsorption capacity of the dye increased with contact time up to 10 min after that a maximum removal was attained. For this reason, the optimum contact time was selected as 10 min.
3.3 Adsorption kinetics
The study of kinetics of dye adsorption onto Fe3O4 MNPs is required for selecting optimum operating conditions for the full-scale bath processes. In the present study, kinetic studies were performed at concentrations of 5, 15, 25 and 75 mg L−1 of methyl violet. For this purpose, 1.0 mL of Fe3O4 MNPs (10 mg mL−1) and 1.0 mL of SDS solution (2 mg L−1) were added into 25 mL of the dye solutions at ambient temperature and the solutions were stirred in the time intervals ranged from 0 to 60 min. Then, the clear supernatant solutions were analyzed using spectrophotometer for residual methyl violet concentration in the solution. Fig. 7 shows the equilibrium concentrations of methyl violet at the adsorption time interval of 0–60 min. The removal rate was very fast during the initial stages of the adsorption processes.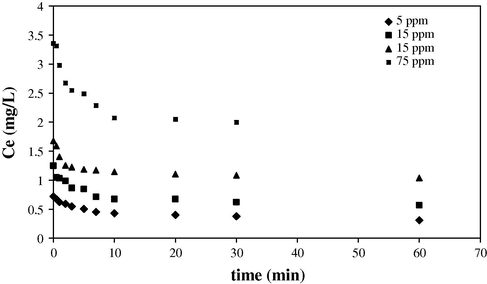
The kinetic curve of methyl violet adsorption on SDS-coated Fe3O4 MNPs for methyl violet concentration in the range of 5–75 mg L−1 (V = 25 mL, pH = 3.0, 10 mg SDS-coated Fe3O4 MNPs, 2 mg SDS).
The kinetic of adsorption onto SDS-coated Fe3O4 MNPs was analyzed using pseudo-first order (Lagergren, 1898), pseudo-second order (Chien and Clayton, 1980) and intra-particle diffusion models (Weber et al., 1963) to find out the adsorption rate expression. The conformity between experimental data and the model-predicted values was expressed by the correlation coefficients (R2). In four studied concentrations of methyl violet, fitting of kinetic data to pseudo-first order and intra-particle diffusion models obtained R2 values lower than 0.94 and 0.86, respectively. Fitting of kinetic data to pseudo-second order kinetic model obtained R2 values equal to 1.
The rate of pseudo-second order reaction may be dependent on the amount of solute adsorbed on the surface of adsorbent and the amount adsorbed at equilibrium. The kinetic rate equations for pseudo-second order reaction can be written as follows:
Fitting of kinetic data to pseudo-second order kinetic model was shown in Fig. 8. The best fit of the pseudo-second order kinetic model (R2 = 1) in the present system shows the adsorption of dye followed by chemisorption mechanism via electrostatic attraction.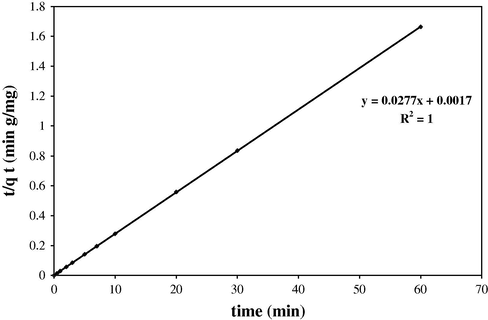
Fitting of kinetic data to pseudo-second order kinetic model (V = 25 mL, Cdye = 15 mg L−1, pH = 3.0, 10 mg SDS-coated Fe3O4 MNPs, 2 mg SDS).
3.4 Adsorption isotherm
The adsorption isotherm is important from both theoretical and practical points of view. In order to optimize the design of an adsorption system to remove a dye, it is important to establish the most appropriate correlations of the equilibrium data of each system. Equilibrium isotherm equations are used to describe the experimental sorption data. The parameters obtained from the different models provide important information on the sorption mechanisms and the surface properties and affinities of the adsorbent. In this study, the two most common isotherms, Langmuir and Freundlich models, were used to describe the experimental adsorption data. The equilibrium adsorption isotherms were determined using batch studies. 25 mL of the dye solution with various initial dye concentrations of 25–400 mg L−1 were poured into glass bottle and 1.0 mL of Fe3O4 MNPs (10 mg mL−1) and 1.0 mL of SDS solution (2 mg L−1) were added to the mixture. Then, the mixture was stirred for 60 min. The amount of dye uptake by the SDS-coated Fe3O4 MNPs, qe (mg g−1), was obtained as follows:
The Langmuir equation (4) and Freundlich equation (5) isotherms can be linearized into the following forms:
The obtained correlation coefficients (
,
) showed that dye adsorption equilibrium data were fitted well to the Langmuir isotherm rather than Freundlich isotherm (n = 3.33). Fig. 9 shows the Langmuir parameters. The maximum monolayer capacity qmax and KL the Langmuir constant (L mg−1) were calculated from the Langmuir model as 416.7 mg g−1 and 0.273 L mg−1, respectively. It is also evident from these data that the surface of the SDS-coated Fe3O4 is made up of homogenous adsorption patches than heterogeneous adsorption patches.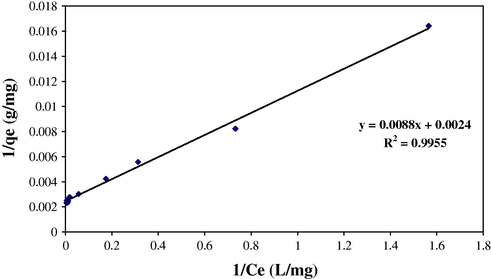
Fitting of isotherm data to the Langmuir model (V = 25 mL, pH = 3.0, 10 mg SDS-coated Fe3O4 MNPs, 2 mg SDS).
4 Conclusion
The sorption of pollutants from aqueous solutions plays a significant role in water pollution control. For this purpose, the utilization of the SDS-coated Fe3O4 MNPs as an efficient adsorbent was successfully carried out to remove the methyl violet dye from wastewater samples. The adsorption followed a pseudo-second order kinetic model, suggesting chemisorption. The fit of the Langmuir model in the present system shows the formation of a monolayer covering of the adsorbate at the outer surface of the adsorbent. The MNP sorbents are easy to synthesize and simple to regenerate. Adsorption on the SDS-coated Fe3O4 MNPs is a reversible process. Therefore, it is possible to regenerate or activate the MNPs by organic solvents in order to reuse them for successive removal processes with high removal efficiency. The data reported here should be useful for the design and fabrication of an economical treatment process for dye adsorption and for diluting industrial effluents. In addition, it should not be forgotten that the SDS-coated Fe3O4 MNPs adsorbent was magnetically recoverable. This function will be quite useful in their practical applications and will reduce time-consumption.
Acknowledgment
This work was supported by a grant from the Young Researchers club of Islamic Azad University and we thank for this valuable cooperation.
References
- Equilibrium adsorption isotherms for basic dyes onto lignite. J. Chem. Tech. Biotechnol.. 1989;45:291-302.
- [Google Scholar]
- Removal of Pb(II), Cd(II), Cu(II) and Zn(II) from aqueous solutions by adsorption on bentonite. J. Colloid Interf. Sci.. 1997;187:338-343.
- [Google Scholar]
- Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J.. 1980;44:265.
- [Google Scholar]
- Non-conventional low-cost adsorbents for dye removal. Bioresour. Technol.. 2006;97:1061-1085.
- [Google Scholar]
- The removal of Victoria blue from aqueous solution by adsorption on a low-cost material. Adsorption. 2002;8:341-349.
- [Google Scholar]
- Removal of methyl violet from aqueous solution by perlite. J. Colloid Interf. Sci.. 2003;267:32-41.
- [Google Scholar]
- Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ. Sci. Technol.. 2001;35:4922-4926.
- [Google Scholar]
- Extraction of trace amounts of mercury with sodium dodecyle sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta. 2010;81:831-836.
- [Google Scholar]
- Nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal. Chim. Acta. 2010;659:172-177.
- [Google Scholar]
- Cetyltrimethylammonium bromide-coated magnetite nanoparticles as highly efficient adsorbent for rapid removal of reactive dyes from the textile companies wastewaters. J. Iran. Chem. Soc.. 2010;7:S130-S144.
- [Google Scholar]
- Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization and applications. J. Iran. Chem. Soc.. 2010;7:1-37.
- [Google Scholar]
- Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis Cineraria. Dyes Pigments. 2004;62:1-10.
- [Google Scholar]
- Carbon molecular sieves as catalysts and catalyst supports. J. Am. Chem. Soc.. 1991;113:1636-1639.
- [Google Scholar]
- Removal of basic dye (Rhodamine B and Methylene blue) from aqueous solutions using bagasse fly ash. Sep. Sci. Technol.. 2000;35:2097-2113.
- [Google Scholar]
- Adsorption of basic dye onto palm kernel shell activated carbon: sorption equilibrium and kinetics studies. Desalination. 2005;186:57-64.
- [Google Scholar]
- Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J. Magn. Magn. Mater.. 2001;225:30-36.
- [Google Scholar]
- Biosorption kinetics and isotherm studies of Acid Red 57 by dried Cephalosporium aphidicola cells from aqueous solutions. Biochem. Eng. J.. 2006;31:197-203.
- [Google Scholar]
- Review: magnetic properties of nanostructured materials. Chem. Mater.. 1996;8:1770-1783.
- [Google Scholar]
- Effect of temperature and pH on the adsorption of an anionic detergent on activated carbon. J. Chem. Tech. Biotechnol. A. 1989;33:231-240.
- [Google Scholar]
- Environmental technologies at the nanoscale. Environ. Sci. Technol. A. 2003;102:102-108.
- [Google Scholar]
- Design models for adsorption systems in wastewater treatment. J. Chem. Tech. Biotechol.. 1981;81:717-731.
- [Google Scholar]
- Adsorption properties of ionic surfactants on molybdenum-modified silica gels. Colloid Surf. A: Physicochem. Eng. Aspects.. 1996;108:39-48.
- [Google Scholar]
- Ind. Eng. Chem. Res.. 2002;42:1965.
- Removal of dyes from waste water using flyash, alow cost adsorbant. Ind. Eng. Chem. Res.. 2002;41:3688-3695.
- [Google Scholar]
- Clay-iron oxide magnetic composites for the adsorption of contaminant in water. Appl. Clay Sci.. 2003;22:169-177.
- [Google Scholar]
- Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microb. Technol.. 2002;31:456-463.
- [Google Scholar]
- Adsorption of acid dyes from aqueous solutions onto acidactivated bentonite. J. Colloid Interf. Sci.. 2004;276:39-46.
- [Google Scholar]
- Kinetics of dodecanoic acid adsorption from caustic solution by activated carbon. J. Colloid Interf. Sci.. 2003;226:245-250.
- [Google Scholar]
- Multi-walled carbon nanotubes as adsorbents for the removal of parts per billion levels of hexavalent chromium from aqueous solution. J. Hazard. Mater.. 2009;166:1067-1075.
- [Google Scholar]
- Cotton textile processing: waste generation and effluent treatment. J. Cotton Sci.. 2007;11:141-153.
- [Google Scholar]
- The adsorption of basic dyes from aqueous solution on modified peat-resin particle. Water Res.. 2003;37:1535-1544.
- [Google Scholar]
- Adsorption of acid dyes onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere. 2001;45:51-58.
- [Google Scholar]
- Kinetics of adsorption on carbon from solution. Eng. Div. Am. Soc. Civil Eng.. 1963;89:31.
- [Google Scholar]
- Study of flocculation with PONILIT GT-2 anionic polyelectrolyte applied into a chemical wastewater treatment. Centr. Eur. J. Chem.. 2006;5:239-256.
- [Google Scholar]
- Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. J. Chromatogr. A. 2008;1188:140.
- [Google Scholar]







