Translate this page into:
Metabolites identification of oil palm roots infected with Ganoderma boninense using GC–MS-based metabolomics
⁎Corresponding authors at: Laboratory of Natural Products, Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor Malaysia (A. Isha); Functional Devices Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor Malaysia (N.A. Yusof). azizul_isha@upm.edu.my (Azizul Isha), azahy@upm.edu.my (Nor Azah Yusof)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An approach to metabolomics profiling of non-infected and Ganoderma boninense (G. boninsense) infected oil palm roots crude extracts that utilize gas chromatography-mass spectrometry (GC–MS) and multivariate statistics of principal component analysis (PCA) have been tested. This combination has provided a rapid approach in investigating the changes in the metabolite variations of non-infected and infected oil palm roots at 14 and 30 days post-infection. The extracts were prepared by using 80% (v/v) of methanol. In identifying the metabolites responsible for each differentiation, PCA model was generated in loading bi-plot. Dimethyl benzene-1,4-dicarboxylate, methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate, ergost-5-en-3-ol, (3β), stigmast-5-en-3-ol, (3β), stigmasterol, methyl hexadecanoate, methyl (9Z,12Z)-octadeca-9,12-dienoate, methyl octadecanoate, 2-(hydroxymethyl)-2-nitropropane-1,3-diol, methyl (Z)-octadec-6-enoate and (E)-icos-5-ene were found more abundant in G. boninense infected roots than in non-infected roots. Steroidal compounds and fatty acid derivatives which has been determined in the non-infected and G. boninense infected roots regulate a variety of responses to the G. boninense. The abundant of these metabolites in G. boninense infected roots are due to the crucial roles in pathogen defence.
Keywords
Ganoderma boninense
GC–MS
Metabolomics
Oil palm roots
Principal component analysis
1 Introduction
Ganoderma boninense (G. boninsense), is as a major threat to the lucrative palm oil industry which causes both basal stem rot and upper stem rot of oil palm (Elaeis Guineensis Jacq.) It affects the direct loss of stand and reduction of yield of diseased palms (Flood et al. 2002, Hushiarian et al., 2013). The problem and challenge to control the disease come from the characteristics of G. boninense. It has been reported that the fungus is soil borne. The fungicides may be ineffective due to degradation in the soil before they can get to their target. It also has varies mode of resting stages such as resistant mycelium, basidiospores, chlamydospores and pseudosclerotia (Muniroh et al., 2019; Susanto et al., 2005).
Plant metabolomics, an important tool for investigations of system biology. It has been used to identify the entire profile of detectable metabolites contained in a biological system. (Rozali et al., 2017)has studied the G. boninense infected oil palm leaf using two-dimensional gas chromatography coupled with time-of-flight mass spectrometry (GCxGC-TOF-MS) based on metabolomics approach. Their finding revealed that, mannose, xylose, glucopyranose, myo-inositol and hexadecanoic acid were found higher in partially tolerant oil palm whereas cadaverine and turanose were more abundant in susceptible oil palm after conducted partial least squares-discriminant analysis (PLS) and orthogonal partial least squares-discriminant analysis (OPLS-DA).
Determination of aqueous methanolic extract of parental palms root tissues that are partially tolerant and susceptible to G. boninense using liquid chromatography-mass spectrometry (LC-MS) has been done by (Zain et al., 2013). Nine metabolites which are broad range of sugars and phenolics derivatives such as sedoheptulase, procyanidin B1, procyanidin B isomer, pinocembrin malonylhexoside, hydroxy-dimethoxybenzoyl-sulfo-hexoside, dimethoxyphenyl-O-hexose-O-pentoside, trimethoxyphenyl-O-hexose-O-pentoside, dimethoxybenzyl-O-hexose-O-rhamnoside and dimethoxyphenylethyl-O-hexose-O-rhamnoside were determined in their study.
Gas chromatography-mass spectrometry (GC–MS) is an effective system with a highly sensitive and high-throughput analytical platform which has been demonstrated a beneficial tool for untargeted studies of primary metabolism in a several of fields (Durak & Genel, 2020; Durak et al., 2019; Zhou et al., 2020). Application of metabolomics approach in GC–MS requires a multistep procedure which include standardization of an untargeted GC–MS metabolomics protocol such as integrated optimization of pre-analytical, analytical and computational steps.
Metabolites in plants have a critical role in the interaction between host and pathogens (Hu et al., 2019; Karre et al., 2017; Kumar et al., 2016; Yogendra et al., 2015). The changes in metabolism caused by the interaction between G. boninense and oil palm roots are not yet clear. In this work, a comparative metabolome method based on GC–MS was applied to non-infected and G. boninense-infected oil palm roots to determine for biomarkers associated with pathogen infection and to setup a diagnostic PCA method. The aim was to further understand the interaction mechanism between G. boninense and oil palm roots and provide technical support for the early diagnosis of G. boninense disease in oil palm.
2 Materials and methods
2.1 Plant materials and sample preparation
Plant materials used and preparation of samples were conducted according to our previous work reported in detection of G. boninense infected oil palm leaf (Isha et al., 2019). Three months old Commercial DxP GH500 germinated seedlings from Sime Darby Seeds & Agricultural Services Sdn. Bhd., Banting were used in this study.
Ten germinated seedlings were treated with G. boninense inoculated rubber wood blocks (RWBs) whereas ten germinated seedlings without any treatments of RWBs were used as control. RWB method was prepared based on the method described by Idris et al., 2006. G. boninense PER 71 cultures were provided by stock collection of Department of Plant Protection, Faculty of Agriculture, Universiti Putra Malaysia. They were maintained onto potato dextrose agar (Difco Laboratories, Detroit, Michigan, USA) and incubated at 27 ± 1 °C for 7 days. RWBs of 6 cm × 6 cm × 6 cm in size were used as a source of inoculum for fungal colonization. The RWBs were sterilized and autoclaved at 121 °C for 15 min. Each RWB was placed in a heat-resistant polypropylene bag containing 120 mL of malt extract broth and autoclaved at 121 °C for 15 min. Then the bags were left to solidify. Mature G. boninense plate cultures (cut into smaller pieces) were inoculated into the RWBs and incubated in the dark until the blocks were fully colonized.
For treatment seedlings, germinated seedlings were placed on top of the RWB and filled with more soil. Root sample were harvested at 14 and 30 days post-infection. Each root sample was quenched with liquid nitrogen and ground into powder form. Freeze-dry system (Scanvac Labogene) was used to remove the water content in the samples. Cultivation took place at the Transgenic Green House, Institute of Plantation Studies, UPM under control conditions.
The freeze-dried roots powder of non-infected and G. boninense infected oil palm (150 mg) were extracted in 250 mL 80% commercial-grade methanol (30 min, 40 °C) using sonicator. The residue was reextracted twice with the same procedure and the total combined supernatant was filtered through Whatman filter paper (125 mm). The supernatant was evaporated to dryness in a rotary evaporator. The crude extracts obtained were stored at −80 °C until further use.
2.2 GC–MS and data analysis
The solvent-free root extracts of non-infected and G. boninense infected oil palm were dissolved in HPLC-grade methanol. The samples were analyzed using gas chromatography equipped with mass spectrometry (GC–MS-2010 Plus-Shimadzu). The column temperature was set to 50 °C (4 min), then increased to 320 °C at the rate of 7 °C/min, and then held for 20 min. The injector temperature was set at 280 °C (split mode = 20:1; injection volume = 0.1 μL). The flow rate of the helium carrier gas was set to 1 mL/min (total run time = 60 min). Mass spectra were set from the range m/z 40 to 700 whereas the electron ionization at 70 eV. The chromatograms of the sample were examined by comparing their mass spectra with the database library of National Institute of Standards and Technology (NIST11). The GC–MS chromatograms were aligned using The XCMS package in R version 2.13.0. PCA using scaling based on Variance was analyzed by SIMCA 13.0 software.
3 Results and discussion
3.1 GC–MS analysis and metabolites identification
The GC–MS analysis of 80% (v/v) methanol crude extracts resulted in identification of 15 metabolites in non-infected roots (control of 14 days post-infection) and G. boninense infected roots at 14 days post-infection whereas 14 metabolites were identified in non-infected roots (control of 30 days post-infection) and 15 metabolites were identified in G. boninense infected roots at 30 days post-infection.
The metabolites and the percentage values of composition of metabolites present in non-infected roots (control of 14 days post-infection) are shown in Table 1. The obtained results indicated that the main compounds were 2,2-dimethoxybutane (1.02%), dimethyl 2-methoxybutanedioate (1.38%), 5-(hydroxymethyl)furan-2-carbaldehyde (3.81%), 2,3-dihydroxypropyl acetate (4.80%), 2-(hydroxymethyl)-2-nitropropane-1,3-diol (2.96%), dimethyl benzene-1,4-dicarboxylate (3.67%), methyl hexadecanoate (0.98%), methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate (9.00%), hexadecanoic acid (3.62%), methyl (9Z,12Z)-octadeca-9,12-dienoate (0.77%), methyl (Z)-octadec-6-enoate (1.25%), methyl octadecanoate (0.80%), (E)-icos-5-ene (0.85%), stigmasterol (1.65%) and stigmast-5-en-3-ol, (3β) (4.53%). *M.W. - molecular weight.
Metabolites
Structure
Healthy roots
G. boninense infected roots
Concentration (%)
Retention Time (RT)
Concentration (%)
Retention Time (RT)
2,2-Dimethoxybutane
(C6H14O2)
M.W. = 118.176 g/mol
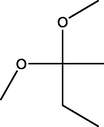
1.02
3.591
0.84
3.593
Dimethyl 2-methoxybutanedioate
(C7H12O5)
M.W. = 176.168 g/mol
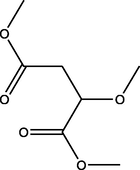
1.38
17.695
1.77
17.7
5-(hydroxymethyl)furan-2-carbaldehyde
(C6H6O3)
M.W. = 126.111 g/mol
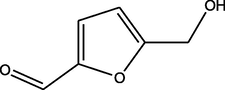
3.81
22.128
2.71
22.272
2,3-Dihydroxypropyl acetate
(C5H10O4)
M.W. = 134.131 g/mol
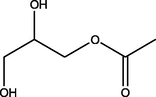
4.80
22.816
2.31
22.822
2-(Hydroxymethyl)-2-nitropropane-1,3-diol
(C4H9NO5)
M.W. = 151.118 g/mol
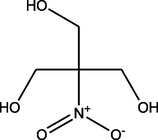
2.96
32.96
1.68
33.065
Dimethyl benzene-1,4-dicarboxylate
(C10H10O4)
M.W. = 194.186 g/mol
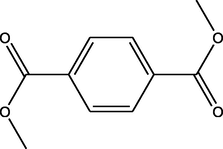
3.67
34.828
4.66
34.838
Methyl hexadecanoate
(C17H34O2)
M.W. = 270.457 g/mol
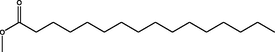
0.98
50.078
1.24
50.088
Methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate
(C18H28O3)
M.W. = 292.419 g/mol
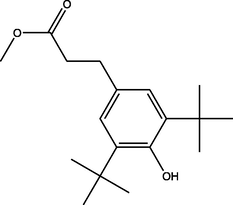
9.00
50.385
10.31
50.398
Hexadecanoic acid
C16H32O2
M.W. = 256.43 g/mol
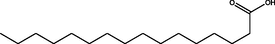
3.62
51.423
2.30
51.424
Methyl (9Z,12Z)-octadeca-9,12-dienoate
(C19H34O2)
M.W. = 294.479 g/mol
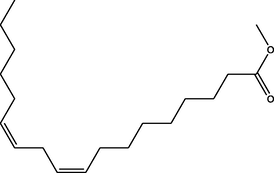
0.77
55.679
1.27
55.688
Methyl (Z)-octadec-6-enoate
(C19H36O2)
M.W. = 296.495 g/mol
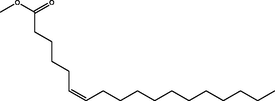
1.25
55.866
1.79
55.88
Methyl octadecanoate
(C19H38O2)
M.W. = 298.511 g/mol
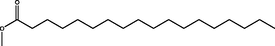
0.80
56.683
1.08
56.691
(E)-icos-5-ene
(C20H40)
M.W. = 280.54 g/mol

0.85
57.892
0.58
57.901
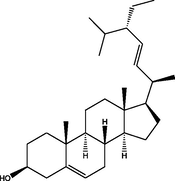
Stigmasterol
(C29H48O)
M.W. = 412.702 g/mol
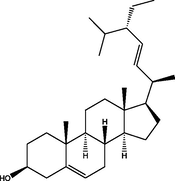
1.65
86.22
1.55
86.233
Stigmast-5-en-3-ol, (3β)
(C29H50O)
M.W. = 414.718 g/mol
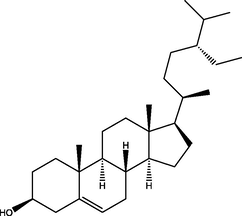
4.53
87.909
5.23
87.933
The metabolites that were characterized in G. boninense infected roots (14 days post-infection) (Table 1) were 2,2-dimethoxybutane (0.84%), dimethyl 2-methoxybutanedioate (1.77%), 5-(hydroxymethyl)furan-2-carbaldehyde (2.71%), 2,3-dihydroxypropyl acetate (2.31%), 2-(hydroxymethyl)-2-nitropropane-1,3-diol (1.68%), dimethyl benzene-1,4-dicarboxylate (4.66%), methyl hexadecanoate (1.24%), methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate (10.31%), hexadecanoic acid (2.30%), methyl (9Z,12Z)-octadeca-9,12-dienoate (1.27%), methyl (Z)-octadec-6-enoate (1.79%), methyl octadecanoate (1.08%), (E)-icos-5-ene (0.58%), stigmasterol (1.55%) and stigmast-5-en-3-ol, (3β) (5.23%).
The metabolites in non-infected roots (control of 30 days post-infection) were identified (Table 2) as 2,2-dimethoxybutane (1.27%), 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one (5.28%), dimethyl 2-methoxybutanedioate (1.01%), 5-(hydroxymethyl)furan-2-carbaldehyde (9.83%), 2,3-dihydroxypropyl acetate (5.83%), dimethyl benzene-1,4-dicarboxylate (2.94%), methyl hexadecanoate (0.96%), methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate (6.69%), hexadecanoic acid (5.70%), methyl (Z)-octadec-6-enoate (0.95%), methyl octadecanoate (0.67%), (E)-icos-5-ene (1.32%), stigmasterol (2.87%) and stigmast-5-en-3-ol, (3β) (6.59%). *M.W. - molecular weight
Metabolites
Structure
Healthy roots
G. boninense infected roots
Concentration (%)
Retention Time (RT)
Concentration (%)
Retention Time (RT)
2,2-Dimethoxybutane
(C6H14O2)
M.W. = 118.176 g/mol
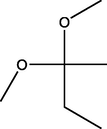
1.27
3.589
0.97
3.584
3,5-Dihydroxy-6-methyl-2,3-dihydropyran-4-one
(C6H8O4)
M.W. = 144.126 g/mol
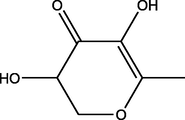
5.28
17.802
1.77
17.78
Dimethyl 2-methoxybutanedioate
(C7H12O5)
M.W. = 176.168 g/mol
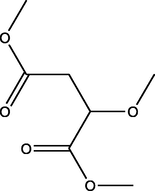
1.01
21.86
1.27
21.889
5-(Hydroxymethyl)furan-2-carbaldehyde
(C6H6O3)
M.W. = 126.111 g/mol
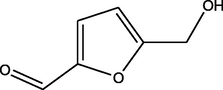
9.83
22.158
1.19
22.15
2,3-dihydroxypropyl acetate
(C5H10O4)
M.W. = 134.131 g/mol
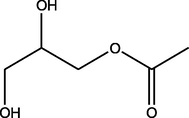
5.83
22.818
3.21
22.806
Dimethyl benzene-1,4-dicarboxylate
(C10H10O4)
M.W. = 194.186 g/mol
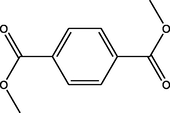
2.94
34.809
3.98
34.808
Methyl hexadecanoate
(C17H34O2)
M.W. = 270.457 g/mol
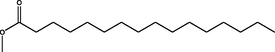
0.96
50.05
1.10
50.047
Methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate
(C18H28O3)
M.W. = 292.419 g/mol
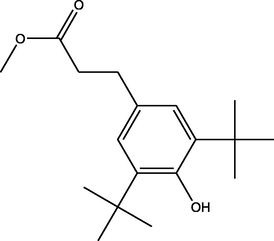
6.69
50.35
10.47
50.359
Hexadecanoic acid
(C16H32O2)
M.W. = 256.43 g/mol
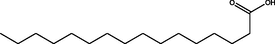
5.70
51.397
2.21
51.388
Methyl (Z)-octadec-6-enoate
(C19H36O2)
M.W. = 296.495 g/mol
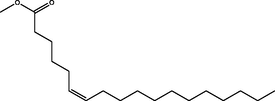
0.95
55.856
1.96
55.837
Methyl octadecanoate
(C19H38O2)
M.W. = 298.511 g/mol
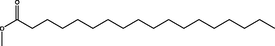
0.67
56.644
1.21
56.65
(E)-Icos-5-ene
(C20H40)
M.W. = 280.54 g/mol

1.32
57.868
0.51
57.859
Ergost-5-en-3-ol, (3β)
(C28H48O)
M.W. = 400.691 g/mol
Notdetected
Not detected
1.97
85.565
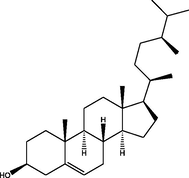
Stigmasterol
(C29H48O)
M.W. = 412.702 g/mol
2.87
86.165
2.07
86.162
Stigmast-5-en-3-ol, (3β)
(C29H50O)
M.W. = 414.718 g/mol

6.59
87.853
5.26
87.853
The G. boninense infected roots (30 days post-infection) contains 15 metabolites (Table 2) such as 2,2-dimethoxybutane (0.97%), 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one (1.77%), dimethyl 2-methoxybutanedioate (1.27%), 5-(hydroxymethyl)furan-2-carbaldehyde (1.19%), 2,3-dihydroxypropyl acetate (3.21%), dimethyl benzene-1,4-dicarboxylate (3.98%), methyl hexadecanoate (1.10%), methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate (10.47%), hexadecanoic acid (2.21%), methyl (Z)-octadec-6-enoate (1.96%), methyl octadecanoate (1.21%), (E)-icos-5-ene (0.51%), ergost-5-en-3-ol, (3β)- (1.97%), stigmasterol (2.07%) and stigmast-5-en-3-ol, (3β) (5.26%).
PCA was applied to understand the clustering features of the non-infected and infected sample and the metabolites contributing to the variability. A three-component model was generated with goodness of fit [R2X(cum) of 0.895 and Q2(cum) of = 0.393] and the first two principal component (PC1 and PC2) explaining 79.2%. A score plot was constructed using PC1 and PC2 [R2X(cum) of 0.792 and Q2(cum) of = 0.719; with 95% confidence level], showing two clear separated clusters were identified by PC1 without any notable outliers (Fig. 1).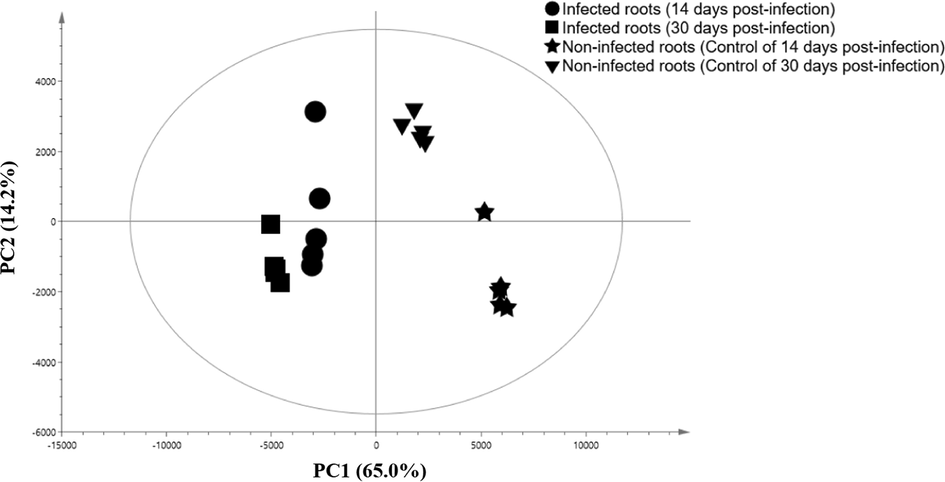
PCA score plots derived from the GC–MS chromatogram of healthy and G. boninense infected roots.
In identifying the root extract metabolites responsible for each differentiation, PCA models were generated in loading bi-pot (Fig. 2). 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one, dimethyl 2-methoxybutanedioate, 5-(hydroxymethyl)furan-2-carbaldehyde, 2,3-dihydroxypropyl acetate and hexadecanoic acid were found higher in non-infected roots whereas 2,2-dimethoxybutane, dimethyl benzene-1,4-dicarboxylate, methyl hexadecanoate, methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate, methyl (Z)-octadec-6-enoate, methyl octadecanoate, (E)-Icos-5-ene, 2-(hydroxymethyl)-2-nitropropane-1,3-diol, stigmasterol, stigmast-5-en-3-ol, (3β), ergost-5-en-3-ol, (3β) and methyl (9Z,12Z)-octadeca-9,12-dienoate were found more abundant in infected roots.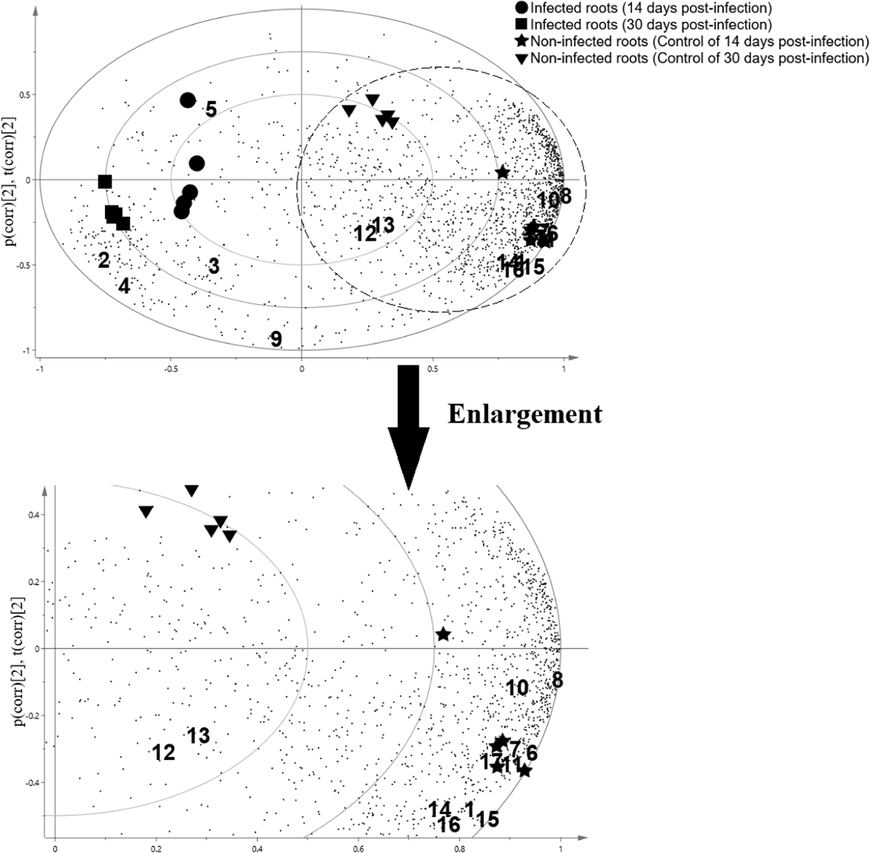
The PCA loadings bi-plot of the target compounds in oil palm roots and their correlation to the healthy and G. boninese infected. Abbreviations: 1: 2,2-Dimethoxybutane; 2: 3,5-Dihydroxy-6-methyl-2,3-dihydropyran-4-one; 3: Dimethyl 2-methoxybutanedioate; 4: 5-(Hydroxymethyl)furan-2-carbaldehyde; 5: 2,3-dihydroxypropyl acetate; 6: Dimethyl benzene-1,4-dicarboxylate; 7: Methyl hexadecanoate; 8: Methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate; 9: Hexadecanoic acid; 10: Methyl (Z)-octadec-6-enoate; 11: Methyl octadecanoate; 12: (E)-Icos-5-ene; 13: 2-(Hydroxymethyl)-2-nitropropane-1,3-diol; 14: Stigmasterol; 15: Stigmast-5-en-3-ol, (3β); 16: Ergost-5-en-3-ol, (3β); 17: Methyl (9Z,12Z)-octadeca-9,12-dienoate.
Fatty acids has been known as essential macromolecules exist in all living organisms which are composed of carboxylic acids attached to hydrocarbon chains. Saturated fatty acids can be defined as a compound in which hydrogen molecules occupy all bonding positions between the carbons, whereas unsaturated fatty acids contain one or more double bonds between their carbons. Different fatty acids can be identified by the length of their carbon chain and the number of double bonds between the carbons. Fatty acids and their methyl esters regulate a variety of responses to biotic and abiotic stresses in plant (Kachroo and Kachroo, 2009; McFarlane 1968).
Previous study reported that, fatty acids and their methyl esters such as methyl hexadecanoate, methyl octadecanoate, methyl (9Z,12Z)-octadeca-9,12-dienoate, dimethyl benzene-1,4-dicarboxylate, methyl (Z)-octadec-6-enoate, methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate and 5-eicosene could be acting as potent antimicrobial agent againts pathogen (Akpuaka et al., 2013; Bashir et al., 2012; Pu et al., 2010; Rozlianah et al., 2015). This can be indicated that, the abundance of these fatty acids and their methyl esters in G. boninense infected roots are due to the crucial roles in pathogen defence (Varns & Glynn, 1979; Alexander et al., 2017).
The highest concentration of the steroidal compounds in the G. boninense infected samples such as stigmasterol, stigmast-5-en-3-ol, (3β) and ergost-5-en-3-ol, (3β) in G. boninense infected samples are related to the act as plant defense metabolites apart from performing as plant growth regulators (Faure et al., 2009) and controlling the fluidity of plant membranes for alteration to changes in temperature (Piironen et al., 2000). The plant cell triggered plant defense response during the pathogen attack and embarks signaling events leading to increase expression of genes involved in sterol biosynthesis, leading to induction of stigmasterol (Fig. 3) (Wang et al., 2012; Aboobucker & Suza, 2019).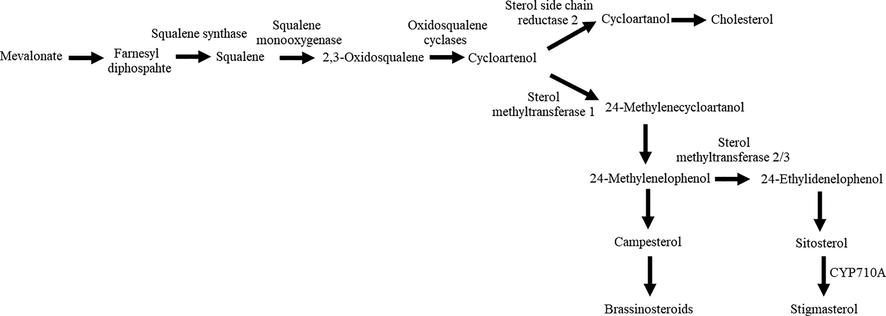
Biosynthetic pathway for plant sterols. Modified from Wang et al., 2012 and Aboobucker and Suza, 2019.
Stigmasterol is produced via the mevalonate pathway following a series of enzyme-catalyzed reactions leading to the generation of 2,3-oxidosqualene (Schaller, 2003; Bach, 2016). This is followed by conversion of 2,3-oxidosqualene to cycloartenol by cycloartenol synthase (Benveniste, 2004; Griebel and Zeier, 2010; Gas-Pascual et al., 2014; Sonawane et al., 2016). Two phytosterol biosynthetic pathways has been identified by (Ohyama et al., 2009) after they identified lanosterol synthase 1 in Arabidopsis. The major part of the metabolic flux occurs via cycloartenol whereas a minor part takes place via LAS1 and lanosterol. Several enzymatic steps such as methyl transferases, reductases, isomerases, demethylases and desaturases are needed to convert cycloartenol or lanosterol to stigmast-5-en-3-ol (Schaller, 2004). Subsequently, stigmast-5-en-3-ol or known as β-sitosterol is converted to stigmasterol by the cytochrome P450 CYP710A1 via C22 desaturation (Griebel and Zeier, 2010).
Ergost-5-en-3-ol, (3β) or known as campesterol has been reported to be produced from 24-methylenecycloartanol in which 24-methylenecycloartanol is converted to 24-methylenecholesterol through several steps (Fig. 3). Then it is epimerized to 24-methyldesmosterol followed by hydrogenaration of the epimerized double bond to produce campesterol (Yokota, 1999). The production of these steroidal compounds that is triggered after pathogen infection could be suggested the higher level of stigmasterol and stigmast-5-en-3-ol in G. boninense infected roots.
4 Conclusion
The results showed that GC–MS chromatogram of non-infected and G. boninense infected roots (14 and 30 day post-infection) exhibited differences which were discriminated and clustered into groups through multivariate data analysis of PCA. Steroidal compounds (stigmasterol, stigmast-5-en-3-ol, (3β) and ergost-5-en-3-ol, (3β)) and fatty acid derivatives (methyl hexadecanoate, methyl octadecanoate, methyl (9Z,12Z)-octadeca-9,12-dienoate, dimethyl benzene-1,4-dicarboxylate, methyl (Z)-octadec-6-enoate, methyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate and 5-eicosene) which has been found more abundant in the G. boninense infected oil palm roots could be used as chemical marker for early detection of basal stem rot disease in oil palm in the future since there is no single chemical marker that could serve as marker in detection of G. boninense.
Acknowledgements
This work was supported by Ministry of Higher Education Malaysia, Long Term Research Grant Schemes (LRGS)-Nanomite and Universiti Putra Malaysia [grant numbers 5526301, 5526304, 9443101 & 9443104].
Declaration of Competing Interest
The author declare that there is no conflict of interest.
References
- Why do plants convert sitosterol to stigmasterol? Front. Plant Sci.. 2019;10(354):1-8.
- [CrossRef] [Google Scholar]
- Biological activities of characterized isolates of n-hexane extract of Azadirachta Indica A. Juss (Neem) leaves. Nat. Sci.. 2013;11(5):141-147.
- [Google Scholar]
- Secondary metabolism: high cholesterol in tomato. Nat. Plants. 2016;3:16213.
- [CrossRef] [Google Scholar]
- Chemical composition and antifungal, phytotoxic, brine shrimp cytotoxicity, insecticidal and antibacterial activities of the essential oils of Acacia modesta. J. Med. Plants Res.. 2012;6(31):4653-4659.
- [CrossRef] [Google Scholar]
- The changes of oil palm roots cell wall lipids during pathogenesis of Ganoderma boninense. IOP Conf. Ser. Earth Sci.. 2017;77:012014
- [Google Scholar]
- Biosynthesis and accumulation of sterols. Annu. Rev. Plant Biol.. 2004;55:429-457.
- [CrossRef] [Google Scholar]
- Catalytic Hydrothermal Liquefaction of Lactuca scariola with a heterogeneous catalyst: the investigation of temperature, reaction time and synergistic effect of catalysts. Bioresour. Technol.. 2020;309:123375
- [CrossRef] [Google Scholar]
- Pyrolysis of black cumin seed: Significance of catalyst and temperature product yields and chromatographic characterization. J. Liq. Chromatogr. Relat. Technol.. 2019;42(11–12):331-350.
- [CrossRef] [Google Scholar]
- Ganoderma diseases of oil palm-an interpretation from Bah Lias Research Station. Planter. 2002;78:689-710.
- [Google Scholar]
- Plant oxidosqualene metabolism: cycloartenol synthase-dependent sterol biosynthesis in Nicotiana benthamiana. PLoS One. 2014;9:e109156
- [CrossRef] [Google Scholar]
- A role for β-sitosterol to stigmasterol conversion in plant–pathogen interactions. Plant J.. 2010;63(2):254-268.
- [CrossRef] [Google Scholar]
- Metabolic Profiling to Identify the Latent Infection of Strawberry by Botrytis cinerea. Evolutionary Bioinformatics. 2019;15:1-7.
- [CrossRef] [Google Scholar]
- Detection and control of Ganoderma boninense: strategies and perspectives. SpringerPlus. 2013;2(555):1-12.
- [CrossRef] [Google Scholar]
- Technique for inoculation of oil palm geminated seeds with Ganoderma. MPOB Inf. Ser.. 2006;314:1.
- [Google Scholar]
- An NMR metabolomics approach and detection of Ganoderma boninense-infected oil palm leaves using MWCNT-based electrochemical sensor. J. Nanomater.. 2019;2019:4729706
- [CrossRef] [Google Scholar]
- Fatty Acid-derived signals in plant defense. Annu. Rev. Phytopathol.. 2009;47:153-176.
- [CrossRef] [Google Scholar]
- Metabolo-transcriptome profiling of barley reveals induction of chitin elicitor receptor kinase gene (HvCERK1) conferring resistance against Fusarium graminearum. Plant Mol. Biol.. 2017;93:247-267.
- [CrossRef] [Google Scholar]
- WAX INDUCER1 (HvWIN1) transcription factor regulates free fatty acid biosynthetic genes to reinforce cuticle to resist Fusarium head blight in barley spikelets. J. Exp. Bot.. 2016;67:4127-4139.
- [CrossRef] [Google Scholar]
- Fatty acids, methyl esters and insect growth. Comp. Biochem. Physiol.. 1968;24(2):377-384.
- [CrossRef] [Google Scholar]
- Proficiency of biocontrol agents as plant growth promoters and hydrolytic enz, yme producers in Ganoderma boninense infected oil palm seedlings. Curr. Plant Biol.. 2019;20:100116
- [CrossRef] [Google Scholar]
- Antibacterial activity of 9-octadecanoic acid-hexadecanoic acid-tetrahydrofuran-3,4-diylester from neem oil. Agr. Sci. China. 2010;9(8):1236-1240.
- [CrossRef] [Google Scholar]
- Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis. Proc Natl Acad Sci USA. 2009;106(3):725-730.
- [CrossRef] [Google Scholar]
- Plant sterols: biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric.. 2000;80:939-966.
- [CrossRef] [Google Scholar]
- Metabolomics differentiation of oil palm (Elaeis guineensis Jacq.) spear leaf with contrasting susceptibility to Ganoderma boninense. Plant Omics J.. 2017;10(2):45-52.
- [CrossRef] [Google Scholar]
- Fatty acids and phenols involved in resistance of oil palm to Ganoderma boninense. Adv. Environ. Biol.. 2015;9(2):11-16.
- [Google Scholar]
- The role of sterols in plant growth and development. Prog. Lipid Res.. 2003;42(3):163-175.
- [CrossRef] [Google Scholar]
- New aspects of sterol biosynthesis in growth and development of higher plants. Plant Physiol. Biochem.. 2004;42:465-476.
- [CrossRef] [Google Scholar]
- Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants. 2016;3:16205.
- [CrossRef] [Google Scholar]
- Enhancing biological control of basal stem rot disease (Ganoderma boninense) in oil palm plantations. Mycopathologia. 2005;159(1):153-157.
- [CrossRef] [Google Scholar]
- Detection of disease in stored potatoes by volatile monitoring. Am. Potato J.. 1979;56:185-197.
- [CrossRef] [Google Scholar]
- Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast. Plant Physiol.. 2012;158(4):1789-1802.
- [CrossRef] [Google Scholar]
- Transcription factor StWRKY1 regulates phenylpropanoid metabolites conferring late blight resistance in potato. J. Exp. Bot.. 2015;66:7377-7389.
- [CrossRef] [Google Scholar]
- Yokota, T., 1999. Brassinosteroids in Hooykass P.J.J., Hall M.A., Libbenga K.R. (Eds.), Biochemistry and Molecular Biology of Plant Hormones. Elsevier Science, London, pp. 277-29.
- Metabolite profiling of oil palm towards understanding basal stem rot (BSR) disease. J. Oil Palm. Res.. 2013;25(1):58-71.
- [Google Scholar]
- Integrated LC–MS and GC–MS-based untargeted metabolomics studies of the effect of azadirachtin on Bactrocera dorsalis larvae. Sci. Rep.. 2020;10:1-11.
- [CrossRef] [Google Scholar]







