Microalgae-based green approach for effective chromium removal from tannery effluent: A review
⁎Corresponding author at: Animal Bi-Product Research Division, Leather Research Institute, Bangladesh Council of Scientific and Industrial Research, Nayarhat, Savar, Dhaka 1350, Bangladesh. asad2306@gmail.com (S. M. Asaduzzaman Sujan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Tannery effluent can cause serious environmental pollution due to its high levels of chromium, chemical oxygen demand (COD), biochemical oxygen demand (BOD) and other pollutants like nitrogen, phosphorus, dyes, sulphur etc. The problem of chromium in tannery effluent is that it is highly toxic and can be hazardous to aquatic life, wildlife, and human health. High levels of chromium can disrupt the ecological balance of a water body, leading to problems such as reduced aquatic life diversity. Additionally, chromium can enter the food chain from water sources and can potentially have negative effects on humans. Therefore, comprehensive waste management plans are highly required to reduce the environmental impact of tannery effluent. The conventional physicochemical methods for removing Cr from wastewater may generate a vast amount of sludge leading to sludge disposal issues. Besides, these techniques require huge capital investments, and the applied chemicals may cause secondary pollution. On the other hand, the biological removal of Cr from wastewater is sustainable, environmentally friendly, and cost-effective. Biological means of Cr remediation involve plant-based and microorganism-based approaches, including bacteria, fungi, and microalgae. This review emphasizes the adverse effects of chromium on human health and its remediation processes based on microalgae. Microalgal biomass can remove heavy metals, including Cr, in either a living (bioaccumulation) or non-living (biosorption) state. There are a number of microalgae species that can remove chromium from wastewater. Among them several algae species, including Chlorella vulgaris, Scenedesmus sp., Consortium of Phormidium sp. and Chlorella sp., Chlorella sorokiniana, and Arthrospira platensis (Spirulina), have found successful in removing Cr in the range of 100–73.5% from tannery wastewater. Additionally, it can mitigate CO2, can remove phosphorus, nitrogen, COD and BOD from tannery effluent, improving its quality to be discharged into the environment. Thereby, the use of microalgae is thought to be a viable substitute for traditional techniques to mitigate the problem of environmental pollution caused by tannery effluent. However, treatment efficiency can be depending on several factors such as nature and amount of effluent to be treated, environmental conditions, contact time, and amount of optimal algal biomass.
Keywords
Tannery effluent
Heavy metals
Bioremediation
Microalgae
Biosorption
Bioaccumulation
1 Introduction
The top most significant resource of nature is water. Every day, anthropogenic activities such as population growth, urbanization, and industrialization that eventually lead to environmental pollution are contaminating this natural resource (Islam, 2013). In the tannery industry, the tanning process utilizes a substantial amount of water, and nearly 30,000–35,000 L of water/ton of raw hides processed are discharged into the environment as effluent around the world (Sharaf et al., 2013). The exact number of hides and skins processed in tanneries around the world each year is not known. However, a study suggests that in 2006, about 6.5 million tons of raw hides and skins were processed in tanneries worldwide (Kanagaraj et al., 2006). Nevertheless, the exact figure may vary from year to year depending on the rate of leather production and tanning processes, which are highly variable. Around the world, tanneries produce more than 40 million tonnes of waste annually (Dabai and Mohammed, 2020). Among this waste, solid waste consists of about 56–60% fleshing, 35–40% is chrome shaving, chrome split, or chrome buffing dust, 5% is skin trims, and 2% is hair (Kanagaraj et al., 2006). In the world, chrome-tanned leather accounts for 80–90% of production. For chrome tanning, hides and skins need at least 2% chromium based on their weight, and only 60–70% of that chromium is fixed and the rest is released as waste (Mia, 2020). Most tanneries worldwide release between 25 and 40 percent of the basic chromium sulfate used in tanning (Sharaf et al., 2013). Therefore, approximately 0.5 kg chromium/ 1000 kg tanned hides may present in the utilized tanning solutions and untreated waste water contain 1500–3000 mg.L-1 of chromium (Mia, 2020; Rahaman, 2016). The wastewater produced by tanneries contains dirt, hair, blood, animal fats, dissolved and suspended hide proteins, greasers, leftover amounts of different kinds of inorganic and organic process chemicals, such as ammonium sulfate, sodium chloride, sodium sulfide, vegetable tanning, resin dyes, syntans, chromium, enzymes, fat, and bactericides among other things. They tend to have high biological oxygen demand (BOD) and chemical oxygen demand (COD). Besides, tannery effluents also contain total Kjeldahl nitrogen (TKN) (Chowdhury, 2013; Khan, 2014). Composition of the leather industry's wastewater with pollution load is given in Table 1.
| Parameter | Concentration (mg.L-1) | |
|---|---|---|
| BOD5 | 3000–4000 | (Zhao and Chen, 2019) |
| COD | 1500–2000 | |
| Suspended solids | 2000–3000 | |
| Sulphide (S2-) | 50–100 | |
| Total Cr | 60–100 | |
| Sulphates (SO42-) | 1400 (On average) | (Buljan et al., 2011) |
| Total nitrogen (TKN) | 160 (On average) | |
| Chlorides (Cl-) | 5000 (On average) | |
| Oil and grease | 130 (On average) | |
| pH value | 6–9 | |
A number of heavy metals, including cadmium, chromium, copper, lead, nickel, and zinc are released into various environments, including the aquatic environment, by industries. Tannery industries produce more toxic effluent than most other industries, putting stress on various ecosystems both directly and indirectly. Among the toxic heavy metals, chromium is of particular concern because it puts human health at risk and negatively affects human health and the health of other living things. Apart from tannery industries, chromium is also extensively used in the textile, metal finishing, electroplating, and chromium preparation industries (Mia, 2020; Alayi, 2021; Han, 2007), hence chromium wastewater can come from these sources.
The leather and leather products sector contributes significantly to global economic growth, with annual international trade valued at about US$100 billion. The leather industries are growing faster in many countries, such as the Republic of Korea, China, Taiwan, Viet Nam, Indonesia, India, Ethiopia, Pakistan, and Bangladesh because the majority of tanning companies in Japan, Europe, and the USA have shut down their factories (Organization, 2010). The growing concern about environmental pollution, notably water pollution, is among the most crucial factors for establishing tannery industries. The release of highly contaminated wastewater containing Cr is an environmental issue in the leather industry (Khan, 2014). Chromium wastewater can be hazardous to human health and the environment. In the water, fish are exposed to chromium, which concentrates its hexavalent form in their tissue and transports it to the human body through the food chain (Abbas and Ali, 2007). Fish gills accumulate a higher concentration of chromium. In contrast to their gills, the digestive systems of fish that consume heavy metals have the highest and maximum concentrations of heavy metal. However, other organs, such as muscles, skin, and bones, also accumulate chromium (Ali, 2021). Chromium can also be transported to the human body through crops such as rice, wheat, pulse crops, etc. (Kormoker, 2022; Nawaz, 2021; Kar and Prasad, 2020). Crops cultivated on polluted lands may acquire more toxic metals than those produced on uncontaminated ones. Chromium accumulation disrupts plant metabolism, which eventually affects morphological and physiological growth. Besides, toxic metals can affect seed germination, which is the first physiological step (Kormoker, 2022; Kar and Prasad, 2020). The list of superfund priorities of hazardous elements has listed chromium as one of the top 20 contaminants for the last fifteen years. As Cr has mutagenic, carcinogenic, and teratogenic characteristics, many countries, such as the United States, have prioritized chromium as a pollutant (Elahi, 2020). Among several oxidation states of chromium, the most enduring and prevalent types are trivalent Cr [Cr3+] and hexavalent Cr [Cr6+] in the range of 2 to 6 (Bishnoi, 2007). Hexavalent chromium compounds have enhanced water solubility, mobility, and ease of transport across biological membranes. These things lead to damage to nucleic acids and intracellular proteins. Therefore, Cr6+ compounds are substantially more hazardous than their trivalent counterparts (Elahi, 2020). Hexavalent chromium is a potent oxidizer, and produces Cr5+ and Cr4+ as intermediates when it reacts with various reducing substances within the cells. These interim products finally transformed into Cr3+ as the outcome. Various reactive species, including superoxide ions (O2–), nascent oxygen (O), peroxo ions (O2H–), hydroxyl ions (OH–) and free radicals are formed within cells when hexavalent Cr is reduced to trivalent Cr. The concentration of Cr6+ influences the generation of intracellular reactive species because the generation of free radicals rises with increased chromium exposure (Pradhan, 2017). The metabolism of hexavalent chromium within cells can cause DNA damage, such as oxidative and non-oxidative DNA damage. Cr-DNA binding is the most prominent and distinctive form of DNA damage. In-vitro reduction reactions and several types of cultivated cells have shown this, resulting in both chromosomal breaks and mutations (Zhitkovich, 2011).
When DNA-proteins react with reactive substances produced by intracellular Cr6+ reduction, several intermediate products known as oxidative DNA damage occurs. Stable Cr3+ species interact electrostatically with DNAs negatively charged phosphate groups to create cytotoxic and mutagenic Cr3+-DNA complexes. These complexes can result in mutagenesis by interfering with normal DNA replication and transcription. Besides the generation of metal complexes, DNA single-strand breaks have also been linked to Cr6+ metabolism (Messer, 2006). These can cause changes in cell function, leading to the development of lung cancer, liver fibrosis, liver cancer, kidney damage, and stomach cancer. Likewise, when hexavalent Cr comes into direct contact with the skin, it results in dermal necrosis, dermatitis, and dermal corrosion (Kimbrough, 1999; Yan, 2020; Massardier, 2020). Exposure to Cr6+ through breathing at work has been implicated in raising the risk of developing respiratory cancer (EPA, 1998). It has been found that Cr6+ also reduces neuronal cell numbers resulting in neurotoxicity, causing brain damage, hallucination, visual disturbance, etc. (Singh and Chowdhuri, 2017; Ma, 2019; Wise, 2022). Similar findings were observed for Pb, Cd, Hg, Cu, Ni, Ar, and Zn (Ma, 2019; Mason et al., 2014; Ekinci, 2014; Council, 2000; Genchi, 2020; Naje, 2020; Plum et al., 2010). The effects of Cr and other heavy metals on human health have been illustrated in Fig. 1. Hence, the U.S. EPA recommends regulating the concentration of hexavalent Cr to surface water under 0.05 mg/L, and total chromium, including trivalent chromium, hexavalent chromium, and its different other forms, is regulated under 2 mg.L-1. Therefore, Cr6+ must be reduced to satisfy the discharge requirements, and recycling and reuse are encouraged. As a result, there is a lot of interest in removing hexavalent chromium from industrial wastewater (Park et al., 2004). Trivalent chromium (Cr3+) has a low solubility and mobility, contributing to its lower toxicity (Elahi, 2020). Cr3+ is regarded as a crucial micronutrient that is required to keep the human body working normally, and plays a crucial role when sugar, amino acids, and lipids are metabolized normally (Cefalu and Hu, 2004). The classification of Cr3+ as an “essential trace element” is currently under doubt, according to the European Union for Food Safety, which stated in 2014 that there was little evidence to support this. But the United States and Canada still consider the element an essential one (Vincent, 2017). Trivalent chromium was not recommended for daily dietary intake by the EFSA due to insufficient evidence of its health benefits; nevertheless, an acceptable weekly dosage of 300 μg.kg−1 of body weight/ week has been released (Elahi, 2020). Therefore, the beneficial effects of this element should be considered with caution. Doses above the adequate level can cause intoxication and several diseases (Gutterres and Mella, 2014). Prolonged exposure to trivalent chromium also causes skin allergies and cancer (Yun, 2001).
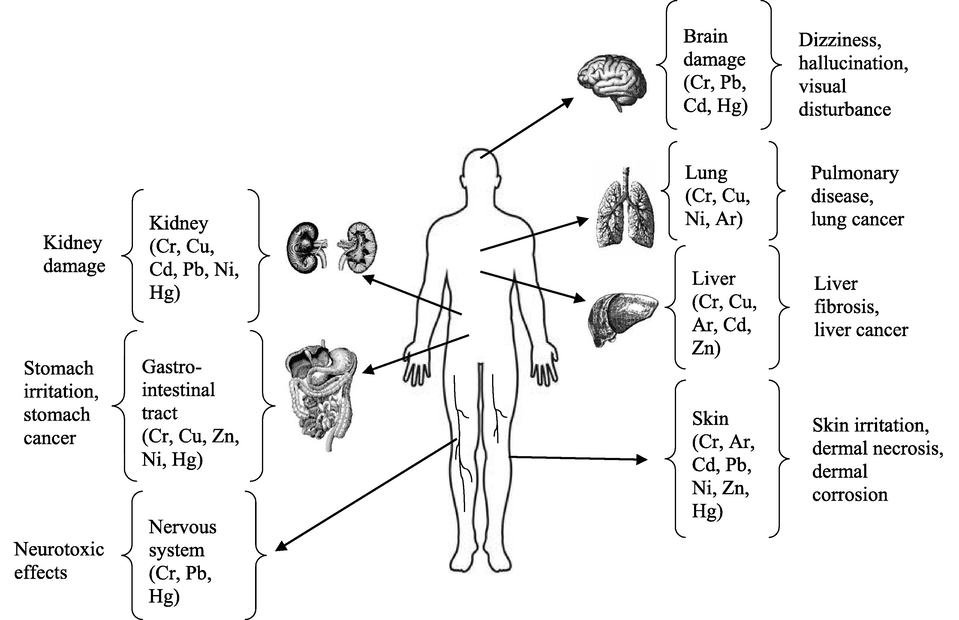
- Health effects due to exposure of the human body to toxic metals, including Cr (Pavithra, 2020).
Therefore, before being released into water bodies or rivers, the Cr metal in the tannery effluent needs to be managed (Adetya et al., 2021). Wastewater can be treated using various techniques to remove heavy metals (Ayele and Godeto, 2021). These include traditional physicochemical as well as biological techniques. Among conventional methods, adsorption is a process in which specific adsorptives are adsorbed to the surface of suspended particles with preference from the fluid phase. A liquid stream is passed through a porous adsorbent material during the adsorption process. The soluble contaminants adhere to the surface of the adsorbent media while the liquid effluent passes through because the adsorbent media attracts the soluble contaminants more than the water in the stream does. Chromium and other heavy metals can be effectively separated from and removed from streams with low levels of heavy metal pollutants using the process of adsorption. A variety of natural and synthetic materials, including activated carbon, zeolite, peat moss, chitosan and bio-materials such as sawdust, rice husks or walnut shells, can be used for chromium separation applications. The sorption capacity is limited and can become saturated after a relatively short period of time. As a result, this approach requires higher expenses at higher contaminant concentrations due to the need for more frequent medium change. Therefore, adsorption is not effective in streams with higher chromium contents (SAMCO, 2020; Owlad, 2009). Biological means of Cr removal include the utilization of aquatic plants, bacteria, fungi and microalgae. Efficiency of plant based remediation largely depends on the kind of aquatic plant employed to accumulate heavy metals. Plants’ efficacy is dependent on the broad root systems, and cultivating and producing aquatic plants takes a lot of time (Lanka and Murari, 2022). In case of bacteria and fungi, additional sources of nutrients should be supplemented for their growth (Chojnacka, 2010). However, microalgae can be an eco-friendly remediation options. The utilization of microalgae, which is a photosynthetic organism, can convert sunlight, water, and carbon dioxide to chemical energy, can use nutrients that present in wastewater to produce biomass, can lessen the additional nutrients requirement (Hannon, 2010; Liu and Hong, 2021). They have high surface-to-volume ratio, high binding affinity to pollutants, and metal sorption can occur in both living (active biomass) and dead algal cells (inactive biomass) (Chong et al., 2000; Kim, 2011; Kumar, 2015). Therefore, they can be a suitable Cr remediation approach.
2 Traditional methods to remove chromium
Heavy metals like chromium can be removed from contaminated environments such as wastewater using traditional physicochemical techniques. These include chemical precipitation (sulfide precipitation, hydroxide precipitation, chelating precipitation), ion exchange (using resins), reverse osmosis, electrodialysis (Kumar, 2015), adsorption (using activated carbon, carbon nanotube as adsorbents), membrane filtration, photocatalysis (Elahi, 2020), resin adsorption, activated carbon adsorption (Pradhan, 2017), ultrafiltration, microfiltration, nanofiltration (Kanamarlapudi et al., 2018), floatation (using sodium dodecyl sulfate and hexadecyl trimethyl ammonium bromide as collector and ethanol and methyl isobutyl carbinol as frothers), coagulation and flocculation (using aluminium sulphate, polyaluminium chloride, aluminium hydroxide oxides, magnesium chloride (MgCl2)) (Renu and Singh, 2017). Figs. 2–6 summarizes some of the physiochemical techniques that could be used to help get rid of heavy metals including Cr toxicity.
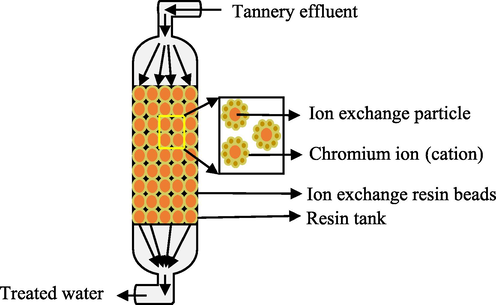
- Ion exchange.
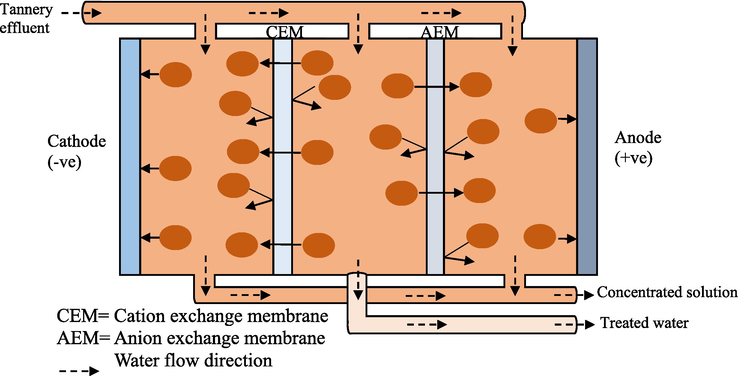
- Electrodialysis.
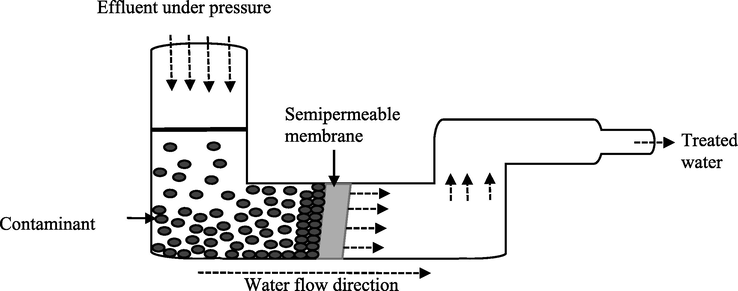
- Reverse osmosis.
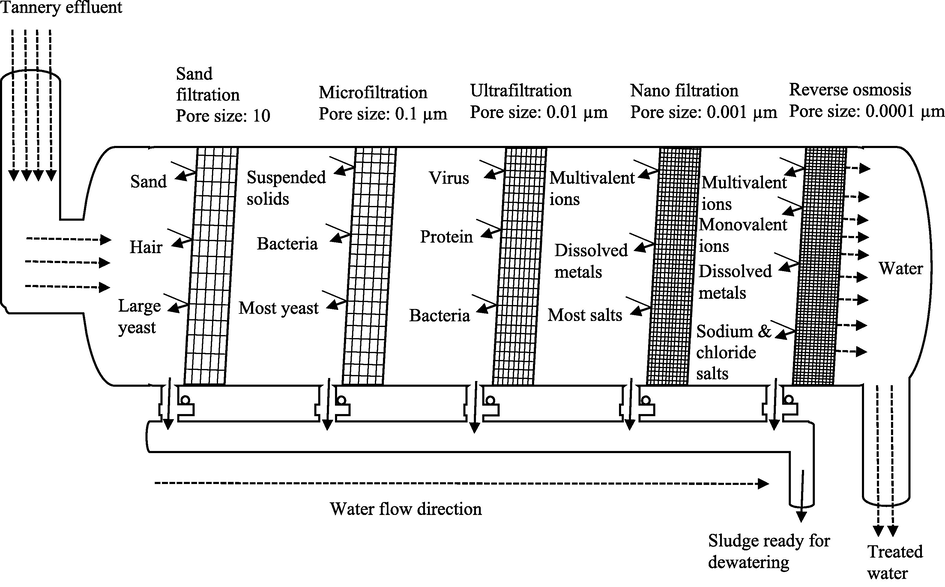
- Membrane filtration.
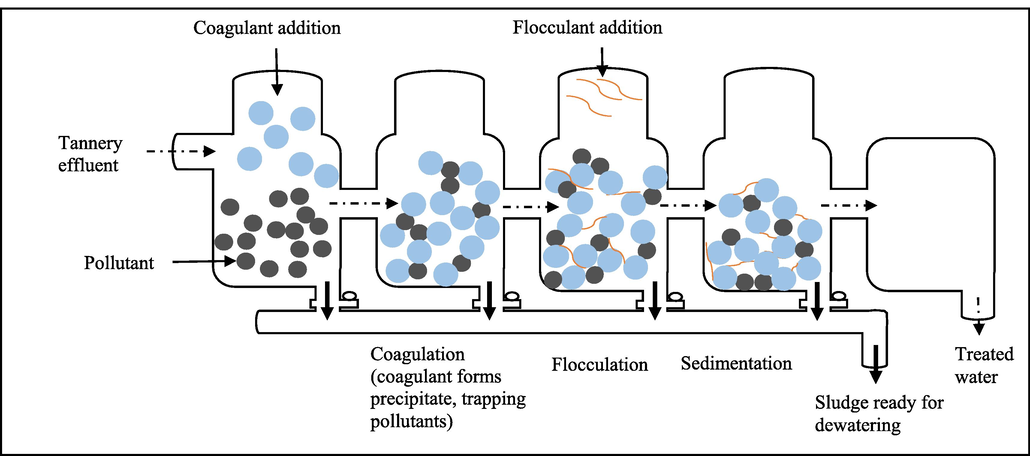
- Coagulation and flocculation.
Figs. 2–6, Traditional physiochemical techniques used for heavy metal bioremediation (Elahi, 2020; Renu and Singh, 2017).
However, these conventional approaches have several drawbacks, including inadequate metal removal, sludge production, high reagent and energy needs, metal precipitate aggregation, and membrane fouling (EPA, 1998). As a result, the scientific community is attempting to develop novel, creative, practical, affordable, effective, ecologically friendly, and sustainable ways to remove harmful compounds from wastewater and aquatic environments (Kumar, 2015).
3 Chromium bioremediation
Bioremediation technologies offer a promising chance to help achieve this goal sustainably. Generally, remediation techniques using living organisms can either include: i) biosorption, an inactive metabolic process in which the pollutant may adhere to the cellular composition; or ii) bioaccumulation, a metabolically active process that relies on energy from living things to operate and involves respiration, transferring pollutants onto and inside the surface of cells. They are inexpensive, highly effective biological technologies to remove heavy metals from diluted solutions, as well as the potential for metal recovery. The term “bio-removal” refers to the use of biological resources, including animal polymers (such as tannins and chitosan), plant biomass, or microorganisms like fungi, bacteria, and microalgae to accumulate and concentrate contaminants from aqueous solutions, assisting in the recovery and/or disposal of the contaminant in an ecologically responsible manner (Kumar, 2015). In biosorption, pollutants are attached to the surface of the cell wall; however, during bioaccumulation, they also accumulate inside the cell (Table 2, and Figs. 7, 8). When equilibrium is reached during biosorption, it can be moved in either direction: left for wastewater treatment or right for the removal and recovery of sorbate (Fig. 9) (Chojnacka, 2010). In general, biosorption can be broadly defined as biological ion exchange with various binding groups, including amido, amino, carboxyl, imidazole, phosphoryl, sulfhydryl, sulfate, etc., which are present on the cell wall surface, in the cytoplasm, and in the vacuoles of microorganisms (Fig. 10) (Chojnacka, 2010; Salama, 2019; Sibi, 2019). Since cationic metal ions are more common in water, they adhere to the surface of cells (Sibi, 2019). The process is extended in bioaccumulation. Thus, biosorption constitutes the initial stage, followed by the subsequent stages involving pollutant migration (mostly through energy-intensive active transportation systems) toward cells and ultimately increasing the concentration within cells. As a result of bioaccumulation, more pollutant binding sites are available, allowing for lower residual concentrations. If biosorption and bioaccumulation are to be carried out under laboratory conditions, it is first necessary to suspend the biomass in a sorbate-containing solution. After a few hours, the equilibrium would be attained, and biosorption would take place if metal-laden biomass were to be separated at this point. If the solution is nutrient-rich and suitable for the organism's minimal growth medium, the organism starts up its internal transport systems and metabolic functions. However, the effluent should be supplemented with an organic carbon source if heterotrophic organisms such as bacteria or fungi are being used. This is a significant limitation because sorbate and organic carbon sources are rarely present in wastewater that is to be treated by bioaccumulation. This relates to metallurgical industry effluent. Organic source supplementation is not advantageous (Chojnacka, 2010). The utilization of photosynthetic organisms, such as algae or aquatic plants, which have low nutritional requirements and need an inorganic carbon source, such as carbon dioxide from flue gasses, could be a solution to this issue (Chojnacka, 2010; Prasanna et al., 2008; Rose, 1996; Kara, 2004). Microalgae have a number of benefits over other biological treatments, such as CO2 bio-mitigation, the ability to use effluent as a source of nutrients (N, P, and carbon), the ability to produce products with added value, a flexible cultivation system, and the ability to adapt to environmental stressors like pH, salinity, temperature, the presence of heavy metals, etc. (Saranya and Shanthakumar, 2019). As a result, it is regarded as the perfect biological material for comprehensive wastewater treatment.
| Biosorption | Bioaccumulation |
|---|---|
| Passive process | Active process |
| Biomass is not alive | Biomass is alive |
| Metals are bound with cellular surface | Metals are bound with cellular surface and migrate inside the cell |
| Adsorption | Absorption |
| Reversible process | Partially reversible process |
| Nutrients are not required | Nutrients are required |
| Single-stage process | Double stage process |
| The rate is quick | The rate is slow |
| Not controlled by metabolism | Controlled by metabolism |
| No danger of toxic effect | Danger of toxic effects caused by contaminants |
| No cellular growth | Cellular growth occurs |
| Intermediate equilibrium concentration of metal ions | Very low equilibrium concentration of metal ions |
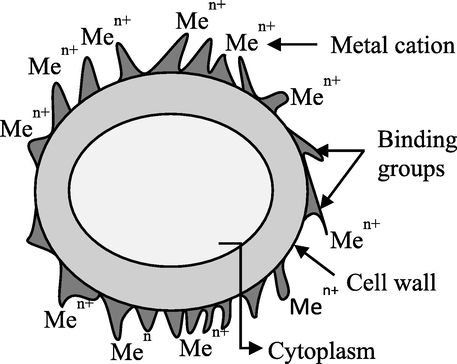
- Biosorption mechanism (Chojnacka, 2010).
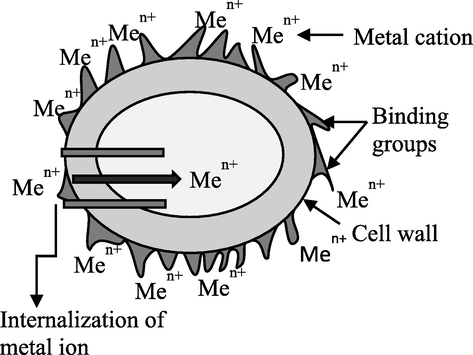
- Bioaccumulation mechanism (Chojnacka, 2010).
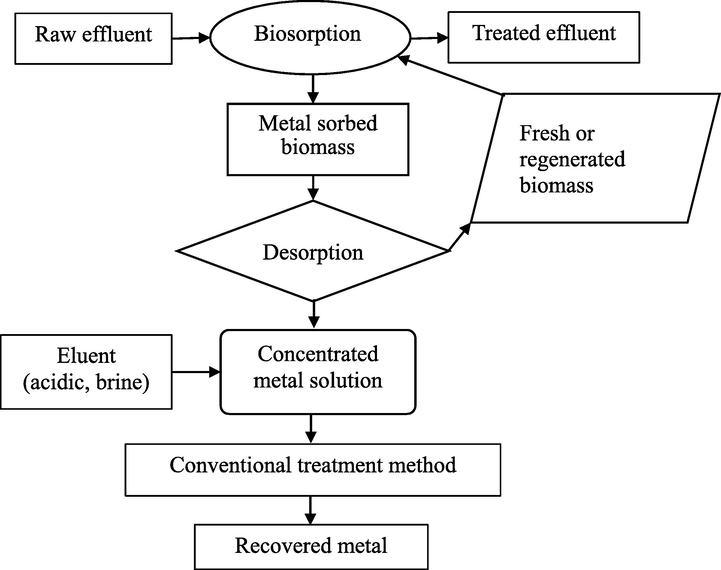
- Schematic diagram of biosorption (Chojnacka, 2010).
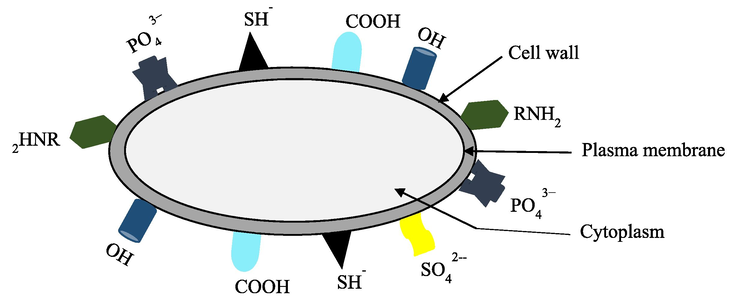
- Possible absorption sites contributing to metal excluding (avoidance) mechanisms (Richmond and Hu, 2013).
The species of bioaccumulating organisms that are resistant to high contamination loads should be chosen (Chen and Wilson, 1997). Effluent from the metalworking, electroplating, metal finishing and printed circuit board manufacturing, and mining operations industries, leachates, surface and ground waters are examples of effluents that can be dealt with using both biosorption and bioaccumulation. The various sorbates that can be eliminated through biosorption, as well as bioaccumulation, are extensive: Al, Au, Cd, Co, Cr, Cu, Fe, Pb, Mo, Ni, Pt, Ag, Hg, Se, V, U, and Zn (Chojnacka, 2010). In the presence of metal ions, proteins with low molecular weight, such as metallothioneins, are synthesized, which are abundant in thiol groups (e.g. cysteine). When metallic pollutants bind with those proteins, they become biologically inert. Therefore, they become excluded from metabolic reactions and are no longer toxic to cells. This process of inactivation supports bioaccumulation (Martín-González, 2006). This explains why numerous studies demonstrate that adapted microorganisms are more effective at bioaccumulation than non-adapted ones. Additionally, species isolated from polluted environments can bioaccumulate pollutants efficiently (Koçberber and Dönmez, 2007; de Silóniz et al., 2002).
3.1 Bioremediation through aquatic plants
Using aquatic plants in heavy metal cleanup is the most cost-effective method since they naturally absorb heavy metal pollutants. Aquatic plants are the best pollutant accumulators due to their broad root systems; however, cultivating and producing aquatic plants takes a lot of time (Lanka and Murari, 2022). Effective phytoremediation depends significantly on the kind of aquatic plant employed to accumulate heavy metals (Galal, 2018; Fritioff and Greger, 2003). Water hyacinth (Eicchornia crassipes) is a significant free-floating aquatic plant used in heavy metal removal, including hexavalent chromium (Lanka and Murari, 2022). It is the ideal plant to utilize in effluent water treatment due to its rapid growth rate, tolerance to high pollution, and capacity to absorb nutrients and heavy metals, including zinc and chromium (Chanakya, 1993; Singhal and Rai, 2003; Ingole and Bhole, 2003; Liao and Chang, 2004; Jayaweera and Kasturiarachchi, 2004; Swarnalatha and Radhakrishnan, 2015). A study showed that water hyacinth effectively removed 99.5% Cr6+ of the processed water of a chromite mine in 15 days (Saha et al., 2017). It was investigated that water hyacinth shoot powder (WHSP) efficiently removed 98.83% Cr from tannery effluent (TE) at an initial concentration of 10.4749 mg.L-1 Cr at 3 h period (Sarkar et al., 2017). In another report, with a starting Cr content in the TE sample of 12.0 mg.L−1, the maximum remediation of Cr6+ was 87.5% after 3 h using water hyacinth shoot powder (Shaibur, 2022). Studies on the efficacy of treating textile effluent with water hyacinth indicated that the plant could remove up to 94.78% of chromium (Priya and Selvan, 2014). Electrostatic forces may be the cause of the migration of Cr6+ ions to the surface of WHSP, and by this process, they are removed from a solution (Wu, et al., 2017). Ion exchange between WHSP and Cr6+ may also occur as the ion binds to the carboxyl, hydroxyl, and PO43− groups on the adsorbent. These functional groups might provide electrons to the Cr6+ ion, leading to the production of chelates. In this manner, the WHSP may absorb Cr6+ into its pores or onto its surface regions, resulting in a decrease in the solution's Cr6+ concentration (Shaibur, 2022). Additionally, free-floating aquatic plants like water lettuce (Pistia stratiotes L.) and duckweed are used in the removal of heavy metals (Lanka and Murari, 2022).
3.2 Bioremediation through bacteria
The removal of chromium using bacterial strains is described as relatively quick, economical, requiring less energy, and requiring little or no chemicals (Jobby, 2018). Species of bacteria recovered from habitats with metal pollution are much more tolerant to harmful heavy metals than those that are isolated from the unpolluted environment. For chromium biosorption, both Gram (+) and Gram (-) strains of bacteria isolated from water, soil, and other chromium-polluted environments, especially effluents from tanneries and electroplating industries, have been used (Vendruscolo et al., 2017; Thatoi, 2014). However, Gram-positive bacteria showed significantly greater resistance to dangerous Cr6+ at a reasonably high concentration compared to Gram-negative bacteria (Thatoi, 2014; Al-Battashi, 2016). There are many bacterial species that are used as biosorbents for chromium removal, as summarized in Table 3. Bacteria can reproduce in controlled settings and tolerate various environmental circumstances. Besides, they have high surface-to-volume ratios, and their cell walls contain an abundance of potent bioactive chemosorption sites such as teichoic acid. All these things make them excellent biosorbents (Zinicovscaia, 2012). Bacteria utilize two different processes for the remediation of chromium toxicity i) direct conversion using enzymes and ii) indirect reduction to reduce Cr6+ (Hwang, 2002). In the direct enzymatic conversion, intracellular chromate reductase converts Cr6+ into Cr3+ in the cell cytoplasm. Some of Cr6+ transforms into its intermediate species, such as Cr5+ and/or Cr4+, by cellular reductants (i.e. glutathione, ascorbic acid, and flavoenzymes). Cr5+ and Cr4+ are responsible for the generation of reactive oxygen species (ROS) (Elahi, 2020; Viti, 2014). Reactive species of oxygen such as superoxide radicals (O2∙−), hydroxyl radicals (∙OH), and hydrogen peroxide (H2O2) interact with lipids, proteins, and DNA to cause strand breaks, protein inactivation, and membrane lipid peroxidation (Krieg and Hoffman, 1986). In order to minimize damage from metal toxicity and preserve the reduced intracellular environment, the microorganisms' defense mechanisms are engaged to resist the intracellular oxidative stress caused by metals (Thatoi, 2014). The defense system is mainly composed of two mechanisms: (i) antioxidant enzymes and (ii) antioxidant molecules that cause scavenging of ROS and convert them into safe chemicals, and catalase, glutathione transferase, superoxide dismutase (SOD), etc. form the defense system (Ackerley, 2004; Khalid and Jin, 2013). SODs are the enzymes that turn O2 into hydrogen peroxide (H2O2) during the detoxification process, where catalases and peroxidases catalyze the conversion of H2O2 into innocuous water. SODs, thereby, act as the initial defense line against ROS species produced by metals. When Cr6+ accumulates to a particular amount, the efflux mechanism releases it (Elahi, 2020). In indirect reduction, iron- and sulfate-reducing bacteria generate metabolites including Fe2+ and HS that mediate the process (Hwang, 2002). After Cr6+ is reduced, the resulting product Cr3+ accumulates inside the cell, forming protein-chelating complexes. Microorganisms can produce a wide range of intracellular and extracellular proteins rich in electrons, which form chelating complexes with Cr3+ that aid in the accumulation of Cr3+ (Ksheminska, 2005). Fig. 11 shows the informative graphic on the chromium removal process using bacteria.
| Species | Source of Cr with concentration | % of removal efficiency or reduction | Reference |
|---|---|---|---|
| Bacteria | |||
| Bacillus subtilis | Synthetic K2Cr2O7 [Cr6+] solution, 50 mg.L-1 | 100 | (Mangaiyarkarasi, 2011) |
| Bacillus amyloliquefaciens | Synthetic K2Cr2O7 [Cr6+] solution, 100 mg.L-1 | 100 | (Pradhan, 2017) |
|
Alcaligenes faecalis and Pseudochrobactrum saccharolyticum |
Synthetic K2Cr2O7 [Cr6+] solution, 10 and 100 mg.L-1; industrial effluent, 10 mg.L-1 |
100 for both synthetic and effluent | (Pradhan, 2017) |
| Staphylococcus arlettae | Synthetic K2Cr2O7 [Cr6+] solution, 500 and 1000 mg.L-1 | For initial Cr6+ concentrations of 500 and 1000 mg.L-1, 98 and 75 were recorded respectively in 120 h | (Sagar, 2012) |
| Pseudomonas aeruginosa | Hard chrome plating industrial effluent, 2100 mg.L-1 | 84.85 | (Shukla et al., 2014) |
| Bacillus cereus | Synthetic K2Cr2O7 [Cr6+] solution, 100–500 mg.L-1 | 70 | (Tanu et al., 2016) |
| Pseudomonas sp. | Synthetic K2Cr2O7 [Cr6+] solution, up to 150 mg.L-1 | 60 | (Wani and Ayoola, 2015) |
| Fungi | |||
| Fusarium genus | Synthetic K2Cr2O7 [Cr6+] solution, 10 mg.L-1 | 100 | (Guria et al., 2014) |
| Trichoderma asperellum | Synthetic K2Cr2O7 [Cr6+] solution, 10 mg.L-1 | 100 | (Chang, 2016) |
|
Aspergillus niger |
Tannery wastewater, 18.125 mg.L-1 |
96.3 | (Sivakumar, 2016) |
| Aspergillus flavus | Tannery wastewater, 18.125 mg.L-1 |
92.8 | |
| A. fumigatus | Tannery wastewater, 18.125 mg.L-1 |
90.1 | |
| A. nidulans | Tannery wastewater, 18.125 mg.L-1 |
86.7 | |
| A. heteromorphus | Tannery wastewater, 18.125 mg.L-1 |
83.7 | |
| A. foetidus | Tannery wastewater, 18.125 mg.L-1 |
78.6 | |
| A. viridinutans | Tannery wastewater, 18.125 mg.L-1 |
74.4 | |
| Microalgae | |||
| Chlorella miniata | Synthetic K2Cr2O7 [Cr6+] solution, 100 mg.L-1 | 100 | (Han, 2007) |
| Chlorella vulgaris | Tannery wastewater, 50 % dilution, 0.88 mg.L-1 |
100 | (Das, 2017) |
| Spirulina platensis | Synthetic basic chromium sulphate (BCS) [Cr3+] solution, 100 mg.L-1 | 99.2 and 99.9 for fresh biomass 38 mg and 152 mg respectively 99.5 and 99.8 for dried biomass 38 mg and 152 mg respectively |
(Shashirekha et al., 2008) |
| Scenedesmus quadricauda | Synthetic K2Cr2O7 [Cr6+] solution, 10 mg.L-1 | 98 | (Daneshvar, 2019) |
| Scenedesmus sp. | Tannery wastewater, 0.613 mg.L-1 |
98 | (Ballén-Segura, 2016) |
| Consortium of Phormidium sp. and Chlorella sp. | Tannery wastewater, 9.57 mg.L-1 |
94.45 | (Das, 2018) |
| Chlorella sp. | Tannery wastewater, 9.57 mg.L-1 |
90.17 | (Das, 2018) |
| C. vulgaris | Synthetic [Cr3+] solution prepared by reduction of K2Cr2O7 [Cr6+], 20 mg.L-1 | 88.2 | (Dabai and Mohammed, 2020; Ardila et al., 2017) |
| Scenedesmus acutus | Synthetic [Cr3+] solution prepared by reduction of K2Cr2O7 [Cr6+], 20 mg.L-1 | 87.1 | (Dabai and Mohammed, 2020; Ardila et al., 2017) |
| Spirulina sp. | Synthetic K2Cr2O7 [Cr6+] solution, 50 mg.L-1 | 82.67 | (Rezaei, 2016) |
| Chlorella sorokiniana | Tannery effluent,100% (v/v) |
82.2 | (Gauje, 2022) |
| Spirogyra spp. | Synthetic chromic chloride (CrCl3) [Cr3+] solution, 50 mg.L-1 | 81.25 | (Bishnoi, 2007) |
| Arthrospira platensis (Spirulina) | Tannery effluent, 155 mg.L-1 |
73.5 (reduction) | (Balaji, 2015) |
| Chlorella miniata | Synthetic Cr3+ nitrate nonahydrate Cr(NO3)3·9H2O solution, 100 mg.L-1 | 70.0 | (Han, 2014) |
| Rhizoclonium hieroglyphicum | Tannery effluent, 8.26 mg.L-1 |
65 | (Onyancha, 2008) |
| Spirogyra condensata | Tannery effluent, 8.26 mg.L-1 |
55 | (Onyancha, 2008) |
|
Scenedesmus incrassalulus |
Synthetic K2Cr2O7 [Cr6+] solution, 1.2 mg.L-1 |
52.7 |
(Pena-Castro, 2004) |
| C. vulgaris | Tannery wastewater | 30 | (Dabai and Mohammed, 2020; Ardila et al., 2017) |
| Scenedesmus acutus | Tannery wastewater | 26 | (Dabai and Mohammed, 2020; Ardila et al., 2017) |
| Chlorella vulgaris | Synthetic K2Cr2O7 [Cr6+] solution, 50 mg.L-1 | Reduced to undetectable levels at the end of the biosorption experiment | (Shen, 2013) |
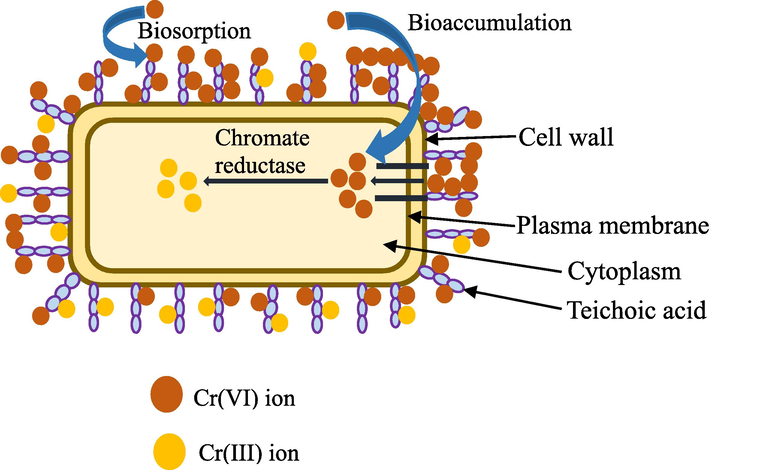
- Schematic chromium remediation mechanism by bacteria.
3.3 Bioremediation through fungi
As fungi produce high levels of biomass, they are employed as biosorbent for heavy metal removal from the environment. Since fungi are versatile, can adapt to severe environmental conditions, high temperature and pH levels, availability of nutrients, and can withstand elevated levels of hexavalent chromium (over 10,000 mg.L-1), they are most often utilized microorganisms for Cr6+ biosorption (Vendruscolo et al., 2017). Fungi utilize two methods for bioremediation of Cr6+: metabolism-independent and metabolism-dependent. The cell wall components of fungi, which have exceptional metal-binding properties, are largely responsible for their unique ability to act as an effective biosorbent (Elahi, 2020). The major chemical constituents that bind Cr6+ to the fungal cell are primarily lipids, proteins, polysaccharides such as chitin, galactosamine, and glycan, and a variety of functional groups like amine (–NH2), carboxyl (–COOH), hydroxide (–OH), phosphate (PO43-), and thiol (-SH) groups (García-Hernández et al., 2017). In fungi, biosorption is considered to be a more effective detoxifying technique than biotransformation (Ryan et al., 2005). A large number of the fungi groups listed in Table 3 were shown to have effective Cr6+ biosorption abilities. Chromate is moved into the cells via the chromate ion transporter (CHR) mediated by the CHR-1 protein and the sulfate transporter. Intracellular chromate reductase facilitates the reduction of Cr6+ to Cr3+ (Romo-Rodríguez and Gutiérrez-Corona, 2019). Enzymes are produced during metabolism-dependent biosorption to facilitate wastewater remediation activities. Fungi, unlike bacteria, can produce enzymes during their whole life cycle independent of pollutant concentrations (Ryan et al., 2005). Fungi detoxify metal pollution using various mechanisms, including extracellular and intracellular precipitation, redox reaction, and active uptake (Mala et al., 2006; Turnau, 2006; Cárdenas-González and Acosta-Rodríguez, 2010. 2010.). They also produce a range of metabolites, including phosphate, nitrogen-containing ligands, and proteins, which influence metal uptake (Bai, 2012; Chen, 2012).
Besides, metabolites produced by fungi cause extracellular reduction of Cr6+ to Cr3+ (Romo-Rodríguez and Gutiérrez-Corona, 2019). Fig. 12 shows the informative graphic on the chromium removal process using fungi.
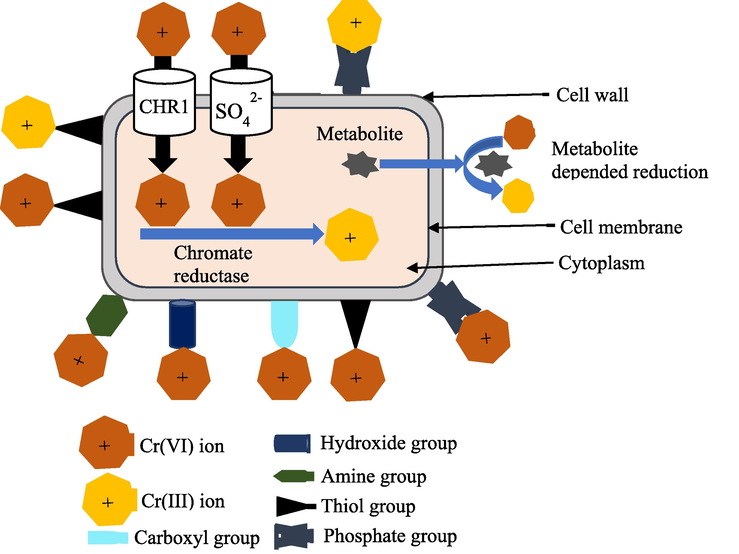
- Schematic chromium remediation mechanism by fungi.
3.4 Bioremediation through microalgae
Microalgae are microscopic algae that live in saline or freshwater habitats; they are photosynthetic microorganisms that can convert sunlight, water, and carbon dioxide to chemical energy. They are among the oldest living organisms. They can be unicellular or multicellular in structure and can endure harsh environments (Hannon, 2010). Since the 1970 s, algae has been grown to prevent eutrophication in wastewater ponds through secondary effluent treatment (Tam and Wong, 1989). Microalgae have been used to remediate wastewater for more than 50 years (Min, 2011). Algal's ability to biodegrade harmful pollutants indicates its role in cleaning wastewater contaminated with metals (Elahi, 2020). Several types of algae can store vast amounts of heavy metals. Still, green algae attract particular interest due to their unique ability to absorb substances, high surface-to-volume ratio, high binding affinity to pollutants, cost-effectiveness, and environmental compatibility, in particular, microalgae can perform over a wide range of pH such as pH between 1 and 10 to remove heavy metals (Chong et al., 2000; Kim, 2011; Goswami, 2021). The production cost of microalgae biomass is about 2.01 € kg−1 on dry weight basis in a 15.247 ha facility size for the production of biodiesel (Branco-Vieira, 2020), while 5 € kg−1 for raceway reactors. When employing freshwater, fertilizers, and CO2 as sources of nutrients for the biomass in raceway reactors, the cost of production was 4.5 € kg−1. Wastewater can be used in place of freshwater and nutrients (fertilizers, CO2) to reduce the cost of biomass production (Fernández et al., 2019). Since microalgae utilize nitrogen and phosphorus from waste resources and turn them into biomass, they are thought to be effective in the treatment of wastewater (Zabochnicka, 2022). By lowering nitrogen levels and producing goods with additional value such as β carotene (Fig. 13.a), astaxanthin (Fig. 13.b), fatty acids, peptides, phenolics, bio-fuels etc., algal biomass can potentially offer a double advantage (Gupta, 2016; Udayan, 2022; Rajalakshmi et al., 2021). Because microalgae are natural raw materials and less expensive to produce than filter membranes or ionites, they are utilized in wastewater technologies for the biosorption of heavy metals (Zabochnicka, 2022).
- Chemical structures of microalgal pigments. (a) β-Carotene, (b) astaxanthin.
Microalgal biomass can be used as the bio-adsorbent for removing Cr through biosorption and bioaccumulation (Kim, 2011; Salama, 2019; Pereira et al., 2013). Algae can remove both Cr6+ and Cr3+ from contaminated aqueous environments (Han, 2007; Han et al., 2006; Yen, 2017). Therefore, the algal removal of chromium has gained increasing attention (Sanjay, 2020). Metal sorption can occur in both living (active biomass) and dead algal cells (inactive biomass), but it involves different mechanisms. In most cases, metal ions are biosorbed onto the binding sites in the cellular structure after being entrapped there (Kumar, 2015). Previous studies have shown that microalgae can be employed in both living and non-living state for the bioremediation of heavy metals including chromium (Kumar, 2015; Pavithra, 2020; Daneshvar, 2019; Balaji, 2015; Shen, 2013; Saranya and Shanthakumar, 2019). The biosorption is divided into two categories: i) active biosorption (bioaccumulation), which uses live microalgae, and ii) passive biosorption (biosorption), which uses dead cells or materials (Goswami, 2021). Microalgae's cell wall is mostly made of proteins, lipids, and polysaccharides. There are numerous functional groups for these components, such as amino (–NH2), carboxyl (–COOH), hydroxyl (–OH), phosphate (–PO3), sulfate (–SO₄2-), sulfonate (–SO3−) and sulfhydryl (–SH) that give the surface of the cell overall negative charge. As a result, counter-ion interactions led to a high binding affinity for metal cations. The functional groups are essential for living and non-living microalgae to take up metal ions (Kumar, 2015). The hydroxyl, sulfonate, and carboxyl groups interact with Cr3+ ions (Zhadra, 2021; Leong and Chang, 2020). The negatively charged functional groups in the algal cells interact and bind with the positively charged metal ions. In contrast, the positively charged functional groups bind with the negatively charged metal ions (Goswami, 2021). Fig. 14 shows the informative graphic on the chromium removal process using microalgae.
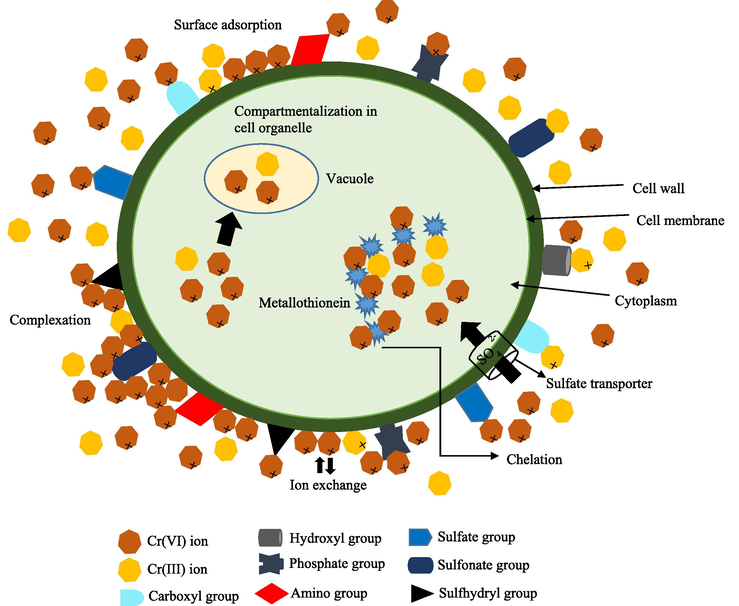
- Schematic chromium remediation mechanism by microalgae (Javanbakht et al., 2014; Monteiro et al., 2012).
Generally, metals like Fe, Co, Cd, Cu, Cr, Ni, Pb, Zn, Au, and U are bound by –COOH groups (Ting et al., 1995; Baldrian and Gabriel, 2003; Davis et al., 2003; Chojnacka et al., 2005; Uchimiya et al., 2012; Flouty and Estephane, 2012); whereas the –OH groups are responsible for binding Pb, Cr, Cd, Cu (Chojnacka et al., 2005; Flouty and Estephane, 2012; Sheng, 2004; Greene, 1986). The binding affinity ranking of the functional groups on biomass surface is considered in the following order for metal ion complexation: carboxylate > aromatic ring > hydroxyl > amine > phosphate > carbonyl > thiol > amide sulfonate (Nurchi and Villaescusa, 2011).
Metal affinity for ligands is proposed and shown in accordance with the classification of metals proposed by Pearson (Pearson, 1963) and Nieboer and Richardson (Nieboer and Richardson, 1980) in Table 4 (Remacle, 1990). Alkyl radicals, such as CH2−, CH3CH2−, etc., are represented by the symbol R. The ligands of I prefer to bind with class A metal ions via oxygen. Class B metal ions make a strong bond with the ligands of II while simultaneously having a high affinity for the ligands of III. All these three different types of ligands can be bound by borderline metal ions, each with a different preference (Wang and Chen, 2009). The first remediation process is passive removal in which metal ions are electrostatically attracted to functional groups on the surface of both living and dead cells. Chemisorption, chelation, physical adsorption, ion exchange, coordination, complexation, micro-precipitation, and polysaccharide entrapment are all components of this process (Monteiro et al., 2012; Jyoti and Awasthi, 2014). The second phase, which takes place inside the cell, wherein metal ion transport across the cell membrane barrier, accumulation within the cell, and eventual binding to intracellular molecules and/or organelle containment is a metabolism-dependent process. In addition, metal ions bind to chelating proteins (such as metallothioneins) and may compete for binding to multivalent ion carriers before entering the cell through endocytosis or may enter the cell through active transport after binding to low-molecular-weight thiols (such as cysteine) (Kumar, 2015). The movement of metal ions across membranes and into the cell's cytoplasm is an essential function.
| Ligand class | Ligands name | Metal classes | Reference |
|---|---|---|---|
| Ligand class I (Class A) |
OH−, H2O, O2−, F−, CO32−, NO3−, HPO42−, SO42-, C = O, ROSO3−, PO43−, ROH, RCOO−, ROR | Class A: Al, Ac, Ba, Ca, Cs, La, Li, Be, Na, Mg, K, Rb, Sc, Sr, Fr, Ra, Y Actinides, Lanthanides |
(Nieboer and Richardson, 1980; Pearson, 1963; Remacle, 1990) |
| Ligand class II (other important ligands) |
Br−, Cl−, SO32−, =N−, N2, NH3, N3−, NO2−, RNH2, R2NH, R3N,–CO–N– R, O2, O2−, O22− | Borderline ions: Ti, V, Cr, As Co, Cu, Cd, Mn, Fe, Ni, Zn, Ga, In, Sn, Sb | |
| Ligand class III (Class B) |
CO, H−, I−, CN−, S2−, R−, RS−, R2S, R3As | Class B: B, Au, Hg, Rh, Pd, Ag, Lr, Pt, Tl, Pb |
Metal ions are transported into cells by a number of different transporters. Members of the families of CTR (Cu TRansporter), FTR (Fe TRansporter), NRAMP (Natural Resistance Associated Macrophage Proteins), and ZIP (Zrt-, Irt-like Proteins) play an important role in cytoplasmic transportation of metal ions. The assimilative transporters found in the plasma membrane are also a part of this group. Similar to assimilative transporters, the vacuole membrane also contains group A transporters and serves a similar purpose. However, unlike the external environment, the intracellular storage compartment acts as the source of metal. Metal concentrations in the cytoplasm are reduced by Group B transporters. When present in the secretory pathway membranes, Group B transporters enhance the exocytosis of excess metal. Distributive transporters that transfer metal to proteins that are confined in organelles are included in this group. Members of the families of CDF (Cation Diffusion Facilitator), Ccc1 (Ca (II)-sensitive Cross-Complementer 1)/ VIT1 (Vacuolar Iron Transporter 1), P1B-type ATPases and FPN (FerroPortiN) are included in this group (Blaby-Haas and Merchant, 2012). Besides, sulfate transporters play a role in transferring chromate ion inside the algal cell (Ferrari, 2022). Metal ions eventually compartmentalize in specific subcellular organelles, such as vacuoles after entering the cell (Kumar, 2015).
3.4.1 Heavy metal tolerance mechanism in algae
The following stages make up the mechanism for algal resistance: metal ion binding at cell surfaces; metal complex formation and insoluble metal complex precipitation thereon; metal ion complexation with excreted metabolites that may extracellularly conceal a hazardous metal; energy-driven efflux pump development, which maintains the cell's hazardous element concentrations low; oxidation state modification, so that toxic metal may be enzymatically transformed to the less harmful state. The resistance mechanism, in addition, is composed of enzyme-based methylation, which stops a poisonous substance from interacting with clusters of ASH within the cell, and cytoplasmic binding of metal ions to polysaccharides or proteins that may reduce the toxicity of metal (Monteiro et al., 2012). Even though ion exchange predominates, complexation and microprecipitation are the most effective methods (Mehta and Gaur, 2005). Several studies found similar findings. Algae have used several approaches, including chelation, ion exchange, physical adsorption, and complexation, as intracellular and extracellular metal-binding strategies to reduce the toxicity of heavy metals (Priyadarshini et al., 2019). These mechanisms work well because they convert toxic metals into non-toxic forms (Mantzorou, 2018). Algae can detoxify metals through a variety of mechanisms, including binding to particular intracellular organelles or transporting them to particular cellular parts (like vacuoles or polyphosphate bodies), leaching them into the solution with an efflux system, and producing phytochelatins or metallothioneins (Perales-Vela et al., 2006). Tolerance is also aided by the cytoplasmic deposition of metal as well as within the vacuoles. In the cytoplasm, the concentrations of metal are reduced by complexing or phytochelatin binding of metal ions or as compounds of metallic sulfur, metallic iron, or metallic phosphate in the cytosol. These complexes are subsequently transported into the vacuoles. Once within the vacuoles, the acidic pH displaces the metal, enabling the peptide to return to the cytosol. In the vacuole, organic acids are typically found in high concentrations, which would trap the metal. The aforementioned technique may serve as a detoxification or cellular defense mechanism (Shanab et al., 2012).
3.4.2 Potential chromium-remediation microalgal species
The ability to bind metal ions may be influenced by the algae's division, genus, and species, algal characteristics of the structure, functional groups, and surface area, as well as the metal's ionic size, atomic weight, or reduction potential (Dönmez, 1999). Microalgae have been widely discussed as a means of removing heavy metals from effluents (Mallick, 2002). Cr bioremediation effectiveness varies between different microalgae species, including both Cr6+ and Cr3+ indicated in Table 3. Even within the same group, different algae may have distinct adsorption capacities. In fact, many species of algae from the same genus respond to heavy metals in different ways. For instance, divalent heavy metals, namely cadmium, mercury, lead, nickel, copper, and zinc are reportedly removed by the freshwater green microalgae C. miniata, C. vulgaris, and C. reinhardtii, whereas C. vulgaris and S. platensis were reported to remove trivalent metals such as iron and chromium. Contrarily, C. miniata and C. vulgaris were reported to eliminate the hexavalent cations (viz. Cr) (González, 2011). Microalgae can also remove other heavy metals, including Pb, Zn, Cu, As, Cd, Al, Au, Ni (Urbina-Suarez et al., 2021; Zeraatkar, 2016). Different algal strains have different metal ions biosorption abilities as a result of the variations in cell wall components' distribution and abundance (Sibi, 2019). Several studies have been conducted for the removal of chromium from water solution as well as tannery wastewater using microalgae which have been shown in Table 3. Microalgae Neochloris aquatica biosorption capacity for chromium from tannery effluent was found about Cr-93.66% in a pilot scale investigation. The biosorption capacity for other metals such as Nickel, Copper, Zink, Lead, Cobalt and Cadmium was 95.61%, 95.39%, 94.90%, 94.87%, 92.86%, and 89.44% respectively. The experiment was carried out in batch mode in an open tank with a working volume of 2,500 L over a period of 15 days in the direct sun (Tamil Selvan, 2020). Besides, a photo bioreactor study using tannery effluent found a maximum chromium removal efficiency of 95.59% by Chlorella sp. The experiment was conducted in batch operation manner for 20 days (Rajalakshmi et al., 2021). In another field trials with the effluent from a leather-processing chemical manufacturing facility, C. vulgaris was grown in a High-rate Algal Pond (HRAP) for 5 days. For enhanced aeration and efficient effluent dispersion, the pond's contents were manually mixed three times per day. The reduction of TDS, TKN, nitrites, nitrates, phosphate levels, BOD and COD was reported as 21%, 74%, 48%, 24%, 99%, nearly 50% for both BOD and COD respectively (Rao, 2011).
3.4.3 Microalgal potentiality in treating wastewater and reducing pollution to the surrounding environment
Besides removing heavy metals, microalgae utilize nitrogen and phosphate which present in the wastewater for their growth; hence, they can remove nitrogen and phosphate from wastewater. Thereby, they remove these biogenic compounds, and reduce nitrogen and phosphorous pollution in the surrounding environment (Kligerman and Bouwer, 2015). According to reports, conventional nitrogen and phosphorus removal costs $4.4 kg-1N and $3.05 kg-1P. On the other hand, it has been reported that a plant utilizing wastewater with a capacity of 70–110 ton ha−1 annum−1 microalgae can save $48 400–$74 800 ha−1 annum−1 for nitrogen removal and $4575–$7625 ha−1 annum−1 for phosphorus removal (Rawat, 2013). In addition, microalgae are able to lower chemical oxygen demand (COD) and biological oxygen demand (BOD) from tannery effluent (Das, 2018). Furthermore, the use of microalgae as flocculants can decrease the quantity of chemicals used in the flocculation process during the wastewater sedimentation stage (Nagi, 2020). Moreover, microalgae can use CO2 during photosynthesis, and to produce 100 tons of biomass, 183 tons of CO2 were utilized (Chisti, 2007). When microalgae are added to wastewater treatment plants during the primary or secondary stage, they provide oxygen through the photosynthetic cycle that supports the growth of aerobic microorganisms. This strategy is called photosynthetic oxygenation, which reduces mechanical aeration costs (Tsioptsias, 2016). The ability of microalgae to bioremediate effluent and to survive under hostile environments boosts their applicability in industrial wastewater or effluent treatment (Nagi, 2020). Because pesticides and other synthetic chemicals that can disrupt the equilibrium of an ecosystem, causing species extinctions and the buildup of xenobiotics in soil and water, are not necessary for the growth of algae, therefore, the production of algae does not affect the environment. Microalgal species such as species of Chlorella are able to survive at higher concentrations of CO2, thereby, can be used to sequestrate atmospheric CO2 to mitigate the problem of global warming. Algal biomass harvested from wastewater treatment plant can be used as a resource to produce biofuels and can also be used to generate bio-hydrogen and in biogas facilities. Thus, algal biomass could contribute to the circular economy and help the environment, the economy, and society (Zabochnicka, 2022). On the other hand, conventional methods do not have such simultaneous benefits of wastewater treatment. Moreover, some of the traditional processes produce huge amount of sludge which add more cost for disposal, others require additional cost for chemical agents, energy input and power supply, membrane filtration method causes membrane fouling, resins for ion exchangers are not suitable for all metal types, floatation needs for further treatments to improve the heavy metal removal yield, and coagulation and flocculation cannot work alone to remove the heavy metals (Goswami, 2021; Zhao, 2022; Manzoor et al., 2019).
3.4.4 Metal desorption and recovery
Algae have been regarded as suitable biological adsorbents in a number of restoration methods because of their distinctive cell wall structure, high capacity to remove heavy metals, and ease of desorption (Lu et al., 2006). Additionally, the metal that has been adsorbed to the microalgal biomass can be removed with the help of an appropriate eluant or desorbing solution, allowing the biomass to be used again in additional sorption–desorption cycles (Kumar, 2015). Desorbing agent must prevent permanent chemical or physical changes and/or damage to the biomass in order to maintain the sorbent's ability for biosorption (Monteiro et al., 2012). According to reports, 0.5 M NaOH desorbed more Cr6+ from Chlorella miniata biomass than deionized water and 0.5 M HCl (Han, 2007). However, a batch system desorption study of Cr revealed that 0.1 M HNO3 effectively desorbed Cr from the biomass of Spirulina sp., removing 95% of the chromium ions (Rezaei, 2016). Nitric acid was superior to 0.1 M EDTA and deionized water for removing Cr3+, Cu2+ and Cd2+ ions bound with the biomass of Spirulina sp., and the desorbing percentage was 98%, 95% and 90% respectively. Additionally, this nitric acid concentration did not result in a reduction in biosorption capacity (the observed decrease in biosorption capacity did not surpass 4%) (Chojnacka et al., 2005). The inorganic acid HCl showed a potent ability to remove metals from biomass. However, it was reported that when applied in sequential cycles, HCl damages the metal binding sites of biosorbents, including hydrolyzing the cell wall’s polysaccharides, which reduces biosorbents’ ability to bind metal. A considerable reduction (about 26%) in adsorption capacity was observed after the first cycle when 0.1 M HCl was utilized as the desorbing agent to desorb Cd2+ from Spirulina platensis biomass that had been immobilized in alginate and silica gel. Furthermore, the effectiveness of Ni2+ removal from immobilized C. vulgaris biomass applying 0.1 M HCl increased during the first cycle but leveled out after that (Kumar, 2015; Monteiro et al., 2012). Although acidic solutions are occasionally employed, basic solutions are most frequently utilized as desorbents for Cr6+. Because Cr6+ is present in anionic form, bases like NaOH, Na2CO3, or NaHCO3 can be used to remove it from the loaded adsorbents (Mishra, 2014). Exchange of CrO42-, the predominant form of Cr6+ in alkaline solution, with OH– allows for desorption of Cr6+ at basic pH levels (Daneshvar, 2019). After desorption, Cr6+ is moved from the solid phase (saturated adsorbent) to the solution (desorbent). The disposal of Cr6+ containing solutions to the environment raises another issue because Cr6+ is so poisonous. This issue can be resolved by separating metal ion from the solution by membrane filtration, electrodialysis, ion exchange and employing chemical precipitation methods such as barium chloride precipitation of Cr6+. When the barium chloride salt is dissolved in a solution that has a lot of Cr6+, the barium ions (Ba2+) interact with the chromate ions (CrO42-). As a result, bright yellow barium chromate is precipitated according to the following equation (Han, 2007; Gupta and Babu, 2009).
Ba2++ CrO42- → BaCrO4
The precipitated chromate ions can be collected in the solid phase by filtration. Smaller amounts of barium chromate are simpler to handle than greater quantities of Cr6+ solution. Industries can use barium chromate, which has a higher market value than barium chloride, which is one of the benefits of barium chromate (Mikhaylov, 2018). Thus, desorbed and recovered Cr6+ can be recycled in industries, and the leftover water can be reused in tanning processes. After Cr6+ has been desorbed from microalgal biomass, it can be utilized again to adsorb Cr6+, can be used as a resource for the production of biofuel or disposed away safely.
3.4.5 Algal biosorbent technologies and commercial application of biosorption
To treat complex wastewater with a high volume and little heavy metal content, biosorption is thought to be a potentially cost-effective technology (Wang and Chen, 2006). There are some effective natural biosorbents that can be prepared with little modification. Few studies have been done to determine whether the biosorbent is compatible with actual industrial effluents (Wang and Chen, 2009). Several commercially accessible technologies are available that use microalgae either alone or in combination with other organisms (Kumar, 2015). The EPA (EPA/540/S5-90/005) approved the commercialization of the biosorption technique. AlgaSORB, BIO-FIX, and B. V. SORBEX are a few commercial biosorbents that are on the market. Biorecovery Systems is the company that produces AlgaSORB (Singh and Prasad, 2000). With the help of algae, the AlgaSORB sorption method removes heavy metal ions from aqueous solutions. This technique traps dead algae cells in a silica gel polymer (Kuyucak and Volesky, 1990). There are various types of AlgaSORB available, such as the algae-silica preparation (AlgaSORB-scy), which is used to remove As3+. It is purposefully manufactured from Scytonema dead cells, which is naturally occurring blue-green cyanobacterial algae (Prasad et al., 2006). Additionally, the sorbent also consists of the biofilm of the filamentous multicellular green alga Spirogyra that has been immobilized in silica gel (Singh and Prasad, 2000). Adsorption of metals from diluted solutions with concentrations between 1 and 100 mg.L-1 is accomplished by the AlgaSORB technique, which uses C. vulgaris biomass immobilized in silica and polyacrylamide gels. Heavy metal biosorption is unaffected by light metals like calcium and magnesium. The biosorbent can go through more than 100 cycles of biosorption and desorption and resembles an ion-exchange resin (Wang and Chen, 2009). Metal removal from industrial effluents has been achieved commercially by using bio-traps (algaSORBVR) made of inactive microalgal biomass (Monteiro et al., 2012). A study showed that carbon‑activated algae granules (CAAG) of Chlorella vulgaris and Scenedesmus obliquus were effective in chromium removal from tannery effluent, and for the technology to be sustainable for use in commercial applications, more research is required (Mirza, 2021). Another biosorbent, called BIO-FIX, has also gained popularity. It is composed of a range of biomasses, including algae (Ulva spp., and Spirulina), yeast (Saccharomyces cerevisiae), bacteria, Sphagnum peat moss, and/or aquatic flora (Lemna spp) by polysulfone immobilization with high density (Kumar, 2015). BIO-FIX has the following metal affinity: Al3+> Cd2+ > Zn2+> Mn2+. Besides, Mg2+ and Ca2+ have a far lower affinity for it. The biosorbent can be employed for more than 120 extraction-elution cycles, and metals can be eluted using HCl or HNO3 (Gupta, 2000). A number of biosorbents have been created by B. V. SORBEX, Inc., a Canadian company, using a variety of biomaterials, including the algae A. nodosum, Chondrus crispus, C. vulgaris, Halimeda opuntia, Palmyra pamata, and S. natans. The biosorbent was efficient at a variety of pH levels and solution conditions and can biosorb a wide range of metal ions. Organics had little impact on the metal biosorption, and could be easily regenerable (Wang and Chen, 2009). Two more commercial biosorbents are “MetaGeneR” and “RAHCO Bio-Beads”. They efficiently remove metal ions from waste streams through electroplating or mining. Despite numerous laboratory and field trials, there is still a lack of information regarding their industrial application (Chojnacka, 2010).
4 Limitation
Micro-algae are known to function effectively at low contamination levels; they don't produce hazardous sludge, are simple to manage, and have a good binding affinity (due to relatively high specific surface area and net negative charge). In addition to preventing the negative effects of heavy metals in the environment, microalgae are a key eco-friendly tool for reducing carbon dioxide. However, there are some limitations of microalgae based remediation technology. The major limitations of treating tannery wastewater with microalgae include:
-
Treatment efficacy of microalgae is influenced by a number of factors such as strain, growth and life stages, tolerance, metal type and concentration, contact time, light intensity, pH, temperature, salinity and hardness, etc. Selection of specific strain is necessary for the better remediation of particular heavy metal from wastewater.
-
Growth of microalgae requires a considerable time.
-
Optimizing and developing effective microalgae consortia that can grow faster, withstand higher concentrations of heavy metals, and remove nutrients and micro-pollutants even in trace quantities are necessary.
-
The existence of different contaminants reduces the effectiveness of microalgae for removing particular metals.
-
Tannery wastewater usually contains high concentrations of total suspended solids, which could clog or cover the surface area of the microalgae, limiting their ability to photosynthesize.
-
Tannery effluent needs to be pretreated or diluted for better efficiency of microalgae based remediation system.
-
Large-scale application for wastewater treatment is limited.
-
Microalgal technologies with the commercial success in removing and recovering heavy metals are still rare.
-
The system needs to be monitored regularly for optimal performance and changes in the environmental conditions in order to get the best results.
5 A look toward the future work
There are currently a number of unresolved problems with microalgal-based treatment systems, one of which is finding an effective species or the consortia that can remove heavy metals and other pollutants more effectively.
-
For tannery wastewater treatment using microalgal systems, more research is required for the optimization and development of effective microalgae consortia with faster growth rates, greater resistance to heavy metal concentrations, and the ability to remove nutrients and micro-pollutants even in trace amounts.
-
It is necessary to have a deeper understanding of several factors, such as metal ion concentrations, physico-chemical conditions, contact periods, biomass recovery, and spent biomass disposal, in order to utilize algal biosorption technology in industrial and environmental remediation.
-
Any utility-connected algae systems should have their life-cycle evaluation, techno-economic analysis, and energy intensity carefully assessed before being put into utilization.
-
The development of new algae strains through genetic engineering that have greater tolerance and affinity, stronger biosorption capacity, and selectivity for particular metal ions will be a new field of research.
-
For upstream heavy metal cleanup using microalgal cultivation and their downstream purification to produce value-added products as well as to produce biofuel from microalgae biomass, more research is required.
-
Understanding the function of algal-bacterial consortia for nutrient recycling and pollution absorption requires more research.
6 Conclusion and remarks
In developing countries, Cr is still used in tanning process which produces large volume of wastewater per tanning circle containing toxic heavy metals such as Cr. Traditional remediation technologies are typically expensive, sophisticated, energy-intensive, leads to formation of sludge, uneconomical when the concentration of chromium in the effluent is low, causes secondary pollution and require skilled employees to operate. Therefore, there is a need for the development of effective, economical, and environmentally friendly means of treatment. Microalgae are simple to cultivate, are cost-effective, less nutritional requirements, do not produce hazardous sludge, have a net negative charge with a relatively large particular surface area, and perform at low contaminant levels as well as function at a broad pH level to remove heavy metal. They can also be cultivated in wastewater using the nutrients present there, grow through photosynthesis, and take in heavy metals and other pollutants. In addition, microalgae are a key eco-friendly tool for reducing carbon dioxide from the atmosphere, and removing nitrogen and phosphorous from the aquatic environment. Microalgae have the necessary adsorption capabilities and the potential to be used as adsorbents to remove Cr from industrial wastewater, notably tannery effluent. Thus, microalgae can be an environmentally friendly remediation options for a better environment. This study's contribution has been to increase knowledge of the current situation and potential future growth of algal biosorbent derived from microalgal biomass.
-
The isolation and identification of certain algal species or microalgae consortium that can persist in sewage, and tannery effluents, suggests that they own several mechanisms to resist the noxious effect of heavy metals. Therefore, these algae can act as appropriate nominees for chromium bioremediation.
-
Several algal species, such as Chlorella vulgaris, Scenedesmus sp., Consortium of Phormidium sp. and Chlorella sp., Chlorella sorokiniana, Arthrospira platensis (Spirulina) found effective in removing Cr from tannery wastewater in the range of 100–73.5%.
-
Improving removal effectiveness can aid in the development of microalgae-based remediation technology.
-
For the use of biosorption, appropriate and affordable immobilization methods can play a crucial role in treating real industrial wastewater.
-
Applying desorbing agents in proper concentrations is required as some desorbing agents can damage the metal binding sites of biosorbents and limit their reuse.
-
Future study is still urgently needed to determine the viability and economics of microalgae-based technology under actual operating conditions because industrial scale performance may differ from that of a lab scale.
Author Contributions
S. M. Asaduzzaman Sujan and Sahana Parveen conceived the idea and designed the research. S. M. Asaduzzaman Sujan and Md. Tushar Uddin and Ajoy Kanti Mondal wrote the chemical part of the manuscript. Shashanka Shekhar Sarker and Taslima Akter wrote the microbiological part. Shashanka Shekhar Sarker and S. M. Asaduzzaman Sujan designed and illustrated the artwork.
Funding statement
No funding was received for this research.
Acknowledgement
The authors wish to acknowledge the efforts of the researchers whose works were cited.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Study the effect of hexavalent chromium on some biochemical, citotoxicological and histopathological aspects of the Orechromis spp. fish. Pakistan J. Biol. Sci.: PJBS. 2007;10(22):3973-3982.
- [Google Scholar]
- Chromate-reducing properties of soluble flavoproteins from Pseudomonas putida and Escherichia coli. Appl. Environ. Microbiol.. 2004;70(2):873-882.
- [Google Scholar]
- The removal of chromium (VI) from tannery waste using Spirulina sp. immobilized silica gel. Turk. J. Chem.. 2021;45(6):1854-1864.
- [Google Scholar]
- Investigating the capability of chromium heavy metal removal and biodiesel production by three species of algae: Scenedesmus acutus, Scenedesmus incrass atulus, Scenedesmus obliquus. Ann. Roman. Soc. Cell Biol. 2021:21127-21138.
- [Google Scholar]
- The geomicrobiology of chromium (VI) pollution: microbial diversity and its bioremediation potential. Open Biotechnol. J.. 2016;10(1)
- [Google Scholar]
- Toxicity and bioaccumulation of manganese and chromium in different organs of common carp (Cyprinus carpio) fish. Toxicol. Rep.. 2021;8:343-348.
- [Google Scholar]
- Sorption capacity measurement of Chlorella vulgaris and Scenedesmus acutus to remove chromium from tannery waste water. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2017.
- [Google Scholar]
- Bioremediation of chromium by microorganisms and its mechanisms related to functional groups. J. Chem.. 2021;2021
- [Google Scholar]
- Biosorption of uranium by magnetically modified Rhodotorula glutinis. Enzyme Microb. Technol.. 2012;51(6–7):382-387.
- [Google Scholar]
- Bioremediation potential of Arthrospira platensis (Spirulina) against chromium (VI) CLEAN–Soil, Air Water. 2015;43(7):1018-1024.
- [Google Scholar]
- Adsorption of Heavy Metals to Microbial Biomass. In: The Utilization of Bioremediation to Reduce Soil Contamination: Problems and Solutions. Springer; 2003. p. :115-125.
- [Google Scholar]
- Using Scenedesmus sp. for the phycoremediation of tannery wastewater. Tecciencia. 2016;11(21):69-75.
- [Google Scholar]
- Biosorption of Cr (III) from aqueous solution using algal biomass spirogyra spp. J. Hazard. Mater.. 2007;145(1–2):142-147.
- [Google Scholar]
- The ins and outs of algal metal transport. Biochim. Biophys. Acta (BBA) – Mol. Cell Res.. 2012;1823(9):1531-1552.
- [Google Scholar]
- Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep.. 2020;6:325-332.
- [Google Scholar]
- Introduction to treatment of tannery effluents. Vienna: UNIDO; 2011.
- Hexavalent chromium removal by a Paecilomyces sp. fungal strain isolated from environment. Bioinorg. Chem. Appl. 2010
- [Google Scholar]
- Role of chromium in human health and in diabetes. Diabetes Care. 2004;27(11):2741-2751.
- [Google Scholar]
- Solid-phase biogas production with garbage or water hyacinth. Bioresour. Technol.. 1993;46(3):227-231.
- [Google Scholar]
- Discrepant hexavalent chromium tolerance and detoxification by two strains of Trichoderma asperellum with high homology. Chem. Eng. J.. 2016;298:75-81.
- [Google Scholar]
- Removal of Cd (II), Cu (II) and Zn (II) from aqueous solutions by live Phanerochaete chrysosporium. Environ. Technol.. 2012;33(23):2653-2659.
- [Google Scholar]
- Genetic engineering of bacteria and their potential for Hg2+ bioremediation. Biodegradation. 1997;8(2):97-103.
- [Google Scholar]
- Biosorption and bioaccumulation–the prospects for practical applications. Environ. Int.. 2010;36(3):299-307.
- [Google Scholar]
- Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere. 2005;59(1):75-84.
- [Google Scholar]
- Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere. 2000;41(1–2):251-257.
- [Google Scholar]
- Treatment of leather industrial effluents by filtration and coagulation processes. Water Resour. Ind.. 2013;3:11-22.
- [Google Scholar]
- Health effects of excess copper, in Copper in drinking water. National Academies Press (US); 2000.
- Chromium removal from tannery wastewater: a review. Platform: J. Sci. Technol.. 2020;3(1):63-73.
- [Google Scholar]
- Hexavalent chromium removal from water by microalgal-based materials: adsorption, desorption and recovery studies. Bioresour. Technol.. 2019;293:122064
- [Google Scholar]
- Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris. J. Appl. Phycol.. 2017;29(1):235-243.
- [Google Scholar]
- Efficient bioremediation of tannery wastewater by monostrains and consortium of marine Chlorella sp. and Phormidium sp. Int. J. Phytorem.. 2018;20(3):284-292.
- [Google Scholar]
- A review of the biochemistry of heavy metal biosorption by brown algae. Water Res.. 2003;37(18):4311-4330.
- [Google Scholar]
- Feasibility of copper uptake by the yeast Pichia guilliermondii isolated from sewage sludge. Res. Microbiol.. 2002;153(3):173-180.
- [Google Scholar]
- A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem.. 1999;34(9):885-892.
- [Google Scholar]
- Toxic effects of chronic mercury exposure on the retinal nerve fiber layer and macular and choroidal thickness in industrial mercury battery workers. Med. Sci. Monitor: Int. Med. J. Exp. Clin. Res.. 2014;20:1284.
- [Google Scholar]
- Successive use of microorganisms to remove chromium from wastewater. Appl. Microbiol. Biotechnol.. 2020;104(9):3729-3743.
- [Google Scholar]
- EPA, U., 1998. Toxicological Review of Trivalent Chromium (CAS No. 16065-83-1). National Center for Environmental Assessment, Office of Research and Development, Washington, DC. http://www.epa.gov/iris/toxreviews/0028-tr.pdf.
- Costs analysis of microalgae production. In: Biofuels From Algae. Elsevier; 2019. p. :551-566.
- [Google Scholar]
- Role of Sulfate Transporters in Chromium Tolerance in Scenedesmus acutus M. (Sphaeropleales) Plants. 2022;11(2):223.
- [Google Scholar]
- Bioaccumulation and biosorption of copper and lead by a unicellular algae Chlamydomonas reinhardtii in single and binary metal systems: a comparative study. J. Environ. Manage.. 2012;111:106-114.
- [Google Scholar]
- Aquatic and terrestrial plant species with potential to remove heavy metals from stormwater. Int. J. Phytorem.. 2003;5(3):211-224.
- [Google Scholar]
- Bioaccumulation and rhizofiltration potential of Pistia stratiotes L. for mitigating water pollution in the Egyptian wetlands. Int. J. Phytorem.. 2018;20(5):440-447.
- [Google Scholar]
- Metallophilic fungi research: an alternative for its use in the bioremediation of hexavalent chromium. Int. J. Environ. Sci. Technol.. 2017;14(9):2023-2038.
- [Google Scholar]
- Simultaneous phytoremediation of tannery effluent and production of fatty acids rich biomass by Chlorella sorokiniana. J. Appl. Phycol.. 2022;34(2):929-940.
- [Google Scholar]
- Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health. 2020;17(3):679.
- [Google Scholar]
- Algal biosorption and biosorbents. In: Microbial Biosorption of Metals. Springer; 2011. p. :159-178.
- [Google Scholar]
- Bioremediation of heavy metals from wastewater: a current perspective on microalgae-based future. Lett. Appl. Microbiol. 2021
- [Google Scholar]
- Interaction of gold (I) and gold (III) complexes with algal biomass. Environ. Sci. Technol.. 1986;20(6):627-632.
- [Google Scholar]
- Microbial biosorbents: meeting challenges of heavy metal pollution in aqueous solutions. Curr. Sci. 2000:967-973.
- [Google Scholar]
- Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J. Environ. Manage.. 2016;184:585-595.
- [Google Scholar]
- Removal of toxic metal Cr (VI) from aqueous solutions using sawdust as adsorbent: Equilibrium, kinetics and regeneration studies. Chem. Eng. J.. 2009;150(2–3):352-365.
- [Google Scholar]
- A green chemical approach for biotransformation of Cr (VI) to Cr (III), utilizing Fusarium sp. MMT1 and consequent structural alteration of cell morphology. J. Environ. Chem. Eng.. 2014;2(1):424-433.
- [Google Scholar]
- Chromium in tannery wastewater. Heavy Metals Water: Presence, Removal Safety. 2014;315:315-344.
- [Google Scholar]
- Biosorption and bioreduction of Cr (VI) by a microalgal isolate, Chlorella miniata. J. Hazard. Mater.. 2007;146(1–2):65-72.
- [Google Scholar]
- Cr (III) removal by a microalgal isolate, Chlorella miniata: effects of nitrate, chloride and sulfate. Ecotoxicology. 2014;23(4):742-748.
- [Google Scholar]
- Surface complexation mechanism and modeling in Cr (III) biosorption by a microalgal isolate, Chlorella miniata. J. Colloid Interface Sci.. 2006;303(2):365-371.
- [Google Scholar]
- Effects of ferrous iron and molecular oxygen on chromium (VI) redox kinetics in the presence of aquifer solids. J. Hazard. Mater.. 2002;92(2):143-159.
- [Google Scholar]
- Removal of heavy metals from aqueous solution by water hyacinth (Eichhornia crassipes) J. Water Supply: Res. Technol.—AQUA. 2003;52(2):119-128.
- [Google Scholar]
- Heavy metals concentration at different tannery wastewater canal of Chittagong city in Bangladesh. Int. J. Agric. Environ. Biotechnol.. 2013;6(3):355-361.
- [Google Scholar]
- Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci. Technol.. 2014;69(9):1775-1787.
- [Google Scholar]
- Removal of nitrogen and phosphorus from industrial wastewaters by phytoremediation using water hyacinth (Eichhornia crassipes (Mart.) Solms) Water Sci. Technol.. 2004;50(6):217-225.
- [Google Scholar]
- Biosorption and biotransformation of hexavalent chromium [Cr (VI)]: a comprehensive review. Chemosphere. 2018;207:255-266.
- [Google Scholar]
- Bioremediation of wastewater chromium through microalgae: a review. Int. J. Eng. Res.. 2014;3:1210-1215.
- [Google Scholar]
- Kanagaraj, J., et al., 2006. Solid wastes generation in the leather industry and its utilization for cleaner environment – a review.
- Application of biosorption for removal of heavy metals from wastewater. Biosorption. 2018;18(69):70-116.
- [Google Scholar]
- Adverse Effects of Chromium, Cadmium and Zinc on the Growth and Metabolic Activities of Pulse Crops and their Key Management Strategies: A Review. Int. J. Curr. Microbiol. App. Sci.. 2020;9(4):48-63.
- [Google Scholar]
- Bioaccumulation of copper from contaminated wastewater by using Lemna minor. Bull. Environ. Contam. Toxicol.. 2004;72(3):467-471.
- [Google Scholar]
- Heavy metal resistance of bacteria and its impact on the production of antioxidant enzymes. Afr. J. Microbiol. Res.. 2013;7(20):2288-2296.
- [Google Scholar]
- Leather Industry in Bangladesh: opportunities and challenges. Am. J. Trade Policy. 2014;1(3):119-126.
- [Google Scholar]
- Biosorption of chromium (Cr (III)/Cr (VI)) on the residual microalga Nannochloris oculata after lipid extraction for biodiesel production. Bioresour. Technol.. 2011;102(24):11155-11160.
- [Google Scholar]
- A critical assessment of chromium in the environment. Crit. Rev. Environ. Sci. Technol.. 1999;29(1):1-46.
- [Google Scholar]
- Prospects for biodiesel production from algae-based wastewater treatment in Brazil: a review. Renew. Sustain. Energy Rev.. 2015;52:1834-1846.
- [Google Scholar]
- Chromium (VI) bioaccumulation capacities of adapted mixed cultures isolated from industrial saline wastewaters. Bioresource Technol.. 2007;98(11):2178-2183.
- [Google Scholar]
- Presence of toxic metals in rice with human health hazards in Tangail district of Bangladesh. Int. J. Environ. Health Res.. 2022;32(1):40-60.
- [Google Scholar]
- Chromium (III) and (VI) tolerance and bioaccumulation in yeast: a survey of cellular chromium content in selected strains of representative genera. Process Biochem.. 2005;40(5):1565-1572.
- [Google Scholar]
- Microalgae–a promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf.. 2015;113:329-352.
- [Google Scholar]
- Aquatic Plants in Phytoextraction of Hexavalent Chromium and Other Metals from Electroplating Effluents. In: Advances in Bioremediation and Phytoremediation for Sustainable Soil Management. Springer; 2022. p. :129-139.
- [Google Scholar]
- Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol.. 2020;303:122886
- [Google Scholar]
- Heavy metal phytoremediation by water hyacinth at constructed wetlands in Taiwan. J. Aquatic 2004:Plan-t.
- [Google Scholar]
- Microalgae-based wastewater treatment and recovery with biomass and value-added products: a brief review. Curr. Pollut. Rep.. 2021;7:227-245.
- [Google Scholar]
- Characteristics of heavy metals enrichment in algae ano its application prospects. Ying Yong Sheng tai xue bao J. Appl. Ecol.. 2006;17(1):118-122.
- [Google Scholar]
- Association of typical toxic heavy metals with schizophrenia. Int. J. Environ. Res. Public Health. 2019;16(21):4200.
- [Google Scholar]
- Bioaccumulation and biosorption of chromium by Aspergillus niger MTCC 2594. J. Gen. Appl. Microbiol.. 2006;52(3):179-186.
- [Google Scholar]
- Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals. 2002;15(4):377-390.
- [Google Scholar]
- Bioreduction of Cr (VI) by alkaliphilic Bacillus subtilis and interaction of the membrane groups. Saudi J. Biol. Sci.. 2011;18(2):157-167.
- [Google Scholar]
- Microalgae: a potential tool for remediating aquatic environments from toxic metals. Int. J. Environ. Sci. Technol.. 2018;15(8):1815-1830.
- [Google Scholar]
- Removal of heavy metal contaminants from wastewater by using Chlorella vulgaris beijerinck: a review. Curr. Environ. Manage. (Formerly: Curr. Environ. Eng.). 2019;6(3):174-187.
- [Google Scholar]
- Cytotoxicity and bioaccumulation of heavy metals by ciliated protozoa isolated from urban wastewater treatment plants. Res. Microbiol.. 2006;157(2):108-118.
- [Google Scholar]
- Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res. Int. 2014
- [Google Scholar]
- Metal-metal hip prosthesis and kidney cancer: assumed role of chromium and cobalt overload. Am. J. Case Rep.. 2020;21 e923416-1
- [Google Scholar]
- Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit. Rev. Biotechnol.. 2005;25(3):113-152.
- [Google Scholar]
- Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic. Biol. Med.. 2006;40(11):1981-1992.
- [Google Scholar]
- An overview of chromium removal techniques from tannery effluent. Appl. Water Sci.. 2020;10(9):1-22.
- [Google Scholar]
- Express Al/Fe oxide–oxyhydroxide sorbent systems for Cr (VI) removal from aqueous solutions. Chem. Eng. J.. 2018;350:344-355.
- [Google Scholar]
- Cultivating Chlorella sp. in a pilot-scale photobioreactor using centrate wastewater for microalgae biomass production and wastewater nutrient removal. Appl. Biochem. Biotechnol.. 2011;165(1):123-137.
- [Google Scholar]
- Biosorption of chromium from tannery effluent using carbon-activated algae granules of Chlorella vulgaris and Scenedesmus obliquus. Int. J. Environ. Sci. Technol.. 2021;18(10):3061-3070.
- [Google Scholar]
- Metal uptake by microalgae: underlying mechanisms and practical applications. Biotechnol. Prog.. 2012;28(2):299-311.
- [Google Scholar]
- Utilization of tannery wastewater for biofuel production: New insights on microalgae growth and biomass production. Sci. Rep.. 2020;10(1):1-14.
- [Google Scholar]
- The harmful effects of argon welding on human health. Int. J. Sci. Business. 2020;4(8):13-16.
- [Google Scholar]
- Cadmium, chromium, nickel and nitrate accumulation in wheat (Triticum aestivum L.) using wastewater irrigation and health risks assessment. Ecotoxicol. Environ. Saf.. 2021;208:111685
- [Google Scholar]
- The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. B.. 1980;1(1):3-26.
- [Google Scholar]
- The chemistry behind the use of agricultural biomass as sorbent for toxic metal ions: pH influence, binding groups, and complexation equilibria. Biomass-Detection Prod. Usage 2011:419-420.
- [Google Scholar]
- Studies of chromium removal from tannery wastewaters by algae biosorbents, Spirogyra condensata and Rhizoclonium hieroglyphicum. J. Hazard. Mater.. 2008;158(2–3):605-614.
- [Google Scholar]
- Future Trends in the World Leather and Leather Products Industry and Trade. UNIDO Vienna; 2010.
- Removal of hexavalent chromium-contaminated water and wastewater: a review. Water Air Soil Pollut.. 2009;200:59-77.
- [Google Scholar]
- Reduction of hexavalent chromium with the brown seaweed Ecklonia biomass. Environ. Sci. Tech.. 2004;38(18):4860-4864.
- [Google Scholar]
- Microalgae for biofuel production and removal of heavy metals: a review. Environ. Chem. Lett.. 2020;18(6):1905-1923.
- [Google Scholar]
- Heavy metals removal by the microalga Scenedesmus incrassatulus in continuous cultures. Bioresour. Technol.. 2004;94(2):219-222.
- [Google Scholar]
- Bioadsorption and bioaccumulation of chromium trivalent in Cr (III)-tolerant microalgae: a mechanisms for chromium resistance. Chemosphere. 2013;93(6):1057-1063.
- [Google Scholar]
- The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health. 2010;7(4):1342-1365.
- [Google Scholar]
- Recent bioreduction of hexavalent chromium in wastewater treatment: a review. J. Ind. Eng. Chem.. 2017;55:1-20.
- [Google Scholar]
- An AlgaSORB column for the quantitative sorption of arsenic (III) from water samples. Water Qual. Res. J.. 2006;41(2):190-197.
- [Google Scholar]
- Cyanobacteria as potential options for environmental sustainability—promises and challenges. Indian J. Microbiol.. 2008;48(1):89-94.
- [Google Scholar]
- Water hyacinth (Eichhornia crassipes)–an efficient and economic adsorbent for textile effluent treatment–a review. Arab. J. Chem.. 2014;2
- [Google Scholar]
- Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol.. 2019;103(8):3297-3316.
- [Google Scholar]
- A Study on removal of chromium from tannery effluent treatment of chrome tanning waste water using tannery solid waste. Int. J. Hum. Cap. Urban Manag.. 2016;1(4):237-242.
- [Google Scholar]
- Small scale photo bioreactor treatment of tannery wastewater, heavy metal biosorption and CO2 sequestration using microalga Chlorella sp.: a biodegradation approach. Appl. Water Sci.. 2021;11(7):108.
- [Google Scholar]
- Application of phycoremediation technology in the treatment of wastewater from a leather-processing chemical manufacturing facility. Water SA. 2011;37(1)
- [Google Scholar]
- Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl. Energy. 2013;103:444-467.
- [Google Scholar]
- The cell wall and metal binding. In: Biosorption of Heavy Metals. Boca Ranton: CRC Press; 1990. p. :83-92.
- [Google Scholar]
- Methodologies for removal of heavy metal ions from wastewater: an overview. Interdiscip. Environ. Rev.. 2017;18(2):124-142.
- [Google Scholar]
- Handbook of Microalgal Culture: Applied Phycology and Biotechnology. John Wiley & Sons; 2013.
- High rate algal oxidation ponding for the treatment of tannery effluents. Water Sci. Technol.. 1996;33(7):219-227.
- [Google Scholar]
- Fungal bioremediation of phenolic wastewaters in an airlift reactor. Biotechnol. Prog.. 2005;21(4):1068-1074.
- [Google Scholar]
- Hexavalent chromium reduction and plant growth promotion by Staphylococcus arlettae strain Cr11. Chemosphere. 2012;86(8):847-852.
- [Google Scholar]
- Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytorem.. 2017;19(1):87-96.
- [Google Scholar]
- Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol.. 2019;35(5):1-19.
- [Google Scholar]
- SAMCO, 2020. Which Technologies Are the Best at Removing Chromium from Industrial Water and Wastewater?
- Isolation and identification of chromium reducing bacteria from tannery effluent. J. King Saud Univ.-Sci.. 2020;32(1):265-271.
- [Google Scholar]
- Opportunities for phycoremediation approach in tannery effluent: A treatment perspective. Environ. Prog. Sustain. Energy. 2019;38(3):e13078.
- [Google Scholar]
- Green microalgae for combined sewage and tannery effluent treatment: Performance and lipid accumulation potential. J. Environ. Manage.. 2019;241:167-178.
- [Google Scholar]
- Remediation of chromium and copper on water hyacinth (E. crassipes) shoot powder. Water Resour. Ind.. 2017;17:1-6.
- [Google Scholar]
- Removal of Cr (VI) and Cu (II) from tannery effluent with water hyacinth and arum shoot powders: a study from Jashore, Bangladesh. J. Hazard. Mater. Adv. 2022100102
- [Google Scholar]
- Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates) Plant Signal. Behav.. 2012;7(3):392-399.
- [Google Scholar]
- Biosorption of trivalent chromium by free and immobilized blue green algae: kinetics and equilibrium studies. J. Environ. Sci. Health A. 2008;43(4):390-401.
- [Google Scholar]
- Hexavalent chromium detoxification by nonliving Chlorella vulgaris cultivated under tuned conditions. Chem. Eng. J.. 2013;228:993-1002.
- [Google Scholar]
- Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci.. 2004;275(1):131-141.
- [Google Scholar]
- Optimization of chromium (VI) detoxification by Pseudomonas aeruginosa and its application for treatment of industrial waste and contaminated soil. Biorem. J.. 2014;18(2):128-135.
- [Google Scholar]
- Factors influencing heavy metal removal by microalgae—A review. J. Crit. Rev. 2019;6:29-32.
- [Google Scholar]
- Environmental presence of hexavalent but not trivalent chromium causes neurotoxicity in exposed Drosophila melanogaster. Mol. Neurobiol.. 2017;54(5):3368-3387.
- [Google Scholar]
- Trace metal analysis: selective sample (copper II) enrichment on an AlgaSORB column. Process Biochem.. 2000;35(9):897-905.
- [Google Scholar]
- Biogas production from water hyacinth and channel grass used for phytoremediation of industrial effluents. Bioresour. Technol.. 2003;86(3):221-225.
- [Google Scholar]
- Biosorption of hexavalent chromium in a tannery industry wastewater using fungi species. Global J. Environ. Sci. Manage.. 2016;2(2):105-124.
- [Google Scholar]
- Studies on removal of Zinc and Chromium from aqueous solutions using water Hyacinth. Pollution. 2015;1(2):193-202.
- [Google Scholar]
- Wastewater nutrient removal by Chlorella pyrenoidosa and Scenedesmus sp. Environ. Pollut.. 1989;58(1):19-34.
- [Google Scholar]
- Pilot scale wastewater treatment, CO2 sequestration and lipid production using microalga, Neochloris aquatica RDS02. Int. J. Phytorem.. 2020;22(14):1462-1479.
- [Google Scholar]
- Bacterial tolerance and reduction of chromium (VI) by Bacillus cereus isolate PGBw4. Am. J. Environ. Prot.. 2016;5(2):35-38.
- [Google Scholar]
- Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J. Environ. Manage.. 2014;146:383-399.
- [Google Scholar]
- Enhancement of the performance of a combined microalgae-activated sludge system for the treatment of high strength molasses wastewater. J. Environ. Manage.. 2016;183:126-132.
- [Google Scholar]
- Role of mycorrhizal fungi in phytoremediation and toxicity monitoring of heavy metal rich industrial wastes in Southern Poland. In: Soil and Water Pollution Monitoring, Protection and Remediation. Springer; 2006. p. :533-551.
- [Google Scholar]
- Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agric. Food Chem.. 2012;60(7):1798-1809.
- [Google Scholar]
- Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2022:1-28.
- [Google Scholar]
- Advanced oxidation processes and biotechnological alternatives for the treatment of tannery wastewater. Molecules. 2021;26(11):3222.
- [Google Scholar]
- Biosorption of hexavalent chromium by microorganisms. Int. Biodeter. Biodegr.. 2017;119:87-95.
- [Google Scholar]
- New evidence against chromium as an essential trace element. J. Nutr.. 2017;147(12):2212-2219.
- [Google Scholar]
- Molecular mechanisms of Cr (VI) resistance in bacteria and fungi. FEMS Microbiol. Rev.. 2014;38(4):633-659.
- [Google Scholar]
- Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol. Adv.. 2006;24(5):427-451.
- [Google Scholar]
- Biosorbents for heavy metals removal and their future. Biotechnol. Adv.. 2009;27(2):195-226.
- [Google Scholar]
- Bioreduction of Cr (VI) by heavy metal resistant Pseudomonas species. J. Environ. Sci. Technol.. 2015;8(3):122-130.
- [Google Scholar]
- Current understanding of hexavalent chromium [Cr (VI)] neurotoxicity and new perspectives. Environ. Int.. 2022;158:106877
- [Google Scholar]
- Activated biochar prepared by pomelo peel using H3PO4 for the adsorption of hexavalent chromium: performance and mechanism. Water Air Soil Pollut. 2017:1-13.
- [Google Scholar]
- Hedgehog signaling pathway regulates hexavalent chromium-induced liver fibrosis by activation of hepatic stellate cells. Toxicol. Lett.. 2020;320:1-8.
- [Google Scholar]
- The use of autotrophic Chlorella vulgaris in chromium (VI) reduction under different reduction conditions. J. Taiwan Inst. Chem. Eng.. 2017;74:1-6.
- [Google Scholar]
- Biosorption of trivalent chromium on the brown seaweed biomass. Environ. Sci. Tech.. 2001;35(21):4353-4358.
- [Google Scholar]
- Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manage.. 2016;181:817-831.
- [Google Scholar]
- Analysis of Cr (III) Ions Adsorption on the Surface of Algae: Implications for the Removal of Heavy Metal Ions from Water. Eastern-Eur. J. Enterprise Technol.. 2021;4(10):112.
- [Google Scholar]
- Tannery wastewater treatment: conventional and promising processes, an updated 20-year review. J. Leather Sci. Eng.. 2022;4(1):10.
- [Google Scholar]
- A review for tannery wastewater treatment: some thoughts under stricter discharge requirements. Environ. Sci. Pollut. Res.. 2019;26:26102-26111.
- [Google Scholar]
- Chromium in drinking water: sources, metabolism, and cancer risks. Chem. Res. Toxicol.. 2011;24(10):1617-1629.
- [Google Scholar]
- A review on biosorption of chromium ions by microorganisms. Chem. J. Moldova. 2012;7(2):27-31.
- [Google Scholar]







