Microwave assisted preparation of TiO2/Al-pillared saponite for photocatalytic phenol photo-oxidation in aqueous solution
*Corresponding author. Tel.: +62 818273001 isfatimah@fmipa.uii.ac.id (Is Fatimah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 28 August 2011
Abstract
In present work, titania dispersed in aluminium pillared saponite was prepared by using microwave irradiation technique as a more efficient calcination procedure instead of conventional calcination by slow heating. Several instrumental techniques consist of XRD, N2 adsorption (BET), and diffuse reflectance UV–Visible spectrophotometry (DRUV–Vis) were employed to study physicochemical character of synthesized material and for photocatalytic activity evaluation, phenol photo-oxidation reaction in aqueous solution was conducted. The results of XRD showed that proposed method showed anatase formation in material whereas by BET analyses, higher specific surface area was gained compared to the conventional calcined. Prepared material demonstrates photocatalytic activity as evaluated from the kinetic study of phenol photo-oxidation.
Keywords
Pillared clays
Saponite
Titania
Photo-oxidation
Photocatalyst
1 Introduction
Application of titania and its supported form in some inorganic matrix for photocatalytic application is gaining interest in recent years. Photocatalytic application is not only in environmental application such as in water disinfection and dye degradation as reported before, but also in some organic synthesis (Li and Wang, 1997; Bunsho, 2006; Ohtani et al., 2003; Herrmann et al., 2007; Valenzuela et al., 2010; Palmisano et al., 2007). Titania (TiO2) is the most attractive and popular material having photocatalytic efficiency under UV-A illumination. However, several modifications were required for targeting developed efficiency and reusable properties. Among many approaches, some modifications aimed to improve the stability of titania material by supporting the inorganic matrix. Titania immobilization on coating glass, metal, ceramics, carbon, zeolite and clay and pillared clays were reported to be more efficient compared to the utilization of bulk-titania (Lajfah et al., 2011; Uğurlu and Karaoğlu, 2011; Li et al., 2008). In case of pillared clays, modifiable properties of clay is an interesting modifiable structure since clay’s pore size, properties, and structure can be created depending on the metal oxide for modification. Aluminium pillared clays from montmorillonite have been reported as an effective support for TiO2 photocatalyst due to the enhancement of photocatalytic reaction rates exhibited from higher porosity and surface area (Fatimah et al., 2010a,b).
Photoactivity improvement from titania was achieved due to the specific surface area and the pore volume of material that dominantly affect the photocatalysis mechanism. In order to obtain porous and crystalline form of dispersed titania, there are two steps of calcination that should be done in the preparation; first is the calcination process for aluminium pillars formation, and the second is the calcination process to form titanium oxides from titania precursor. Both process contributed to influence the porous structure and are the important steps in determining the metal oxide distribution and the formation of titania phase in material. Since the process is commonly time consuming, an alternative way associated with shorter time and reproducible properties is an innovative technique required to make preparation process simple. Referring to some researches on metal oxide formation engaging microwave irradiation (Tao et al., 2006; Suprabha et al., 2009; de Andrés et al., 1999; Jia et al., 2007), this research presents a new technique of microwave irradiation process as an alternative procedure for slow heating calcination or called as conventional calcination. Research deals with comparitive study to the use of microwave irradiation as an alternative for conventional calcination procedure for both aluminium pillarization and for titanium oxide formation from titania precursor. In order to minimize the effect of impurities from utilizing natural clay, synthetic saponite was used as clay model in this research. Characterization was studied by several instrumental analyses consisting of X-ray diffraction (XRD) analysis, N2 BET isotherm analysis, diffuse reflectance UV–Visible spectrophotometer (DRUV–Vis) and also photocatalytic activity test of material in phenol photo-oxidation.
2 Experimental
The starting material; synthetic saponite with the trade name of Sumecton SA was supplied from Kuninime industrial company, Japan. Some chemicals of AlCl3·6H2O, isopropanol, phenol, and titanium isopropoxide were purchased from E. Merck.
Samples were prepared following the method described in previous research for titania–aluminium pillared montmorillonite composite (Fatimah et al., 2010b) but the different condition specified by the mole content of Al used in pillared clay that was 10 mmol/g and calcination steps for aluminium oxide formation and titania formation were carried out under microwave irradiation. Aluminium pillaring solution was made by slow titration of AlCl3·6H2O solution with NaOH under the condition of strong stirring and a molar ratio of −OH/Al = 2.2 was achieved. Pillarization process was conducted by dispersing pillaring solution into saponite suspension (5% wt.) in water followed by continuous stirring for 24 h. Filtration, neutralization, and drying were the nest steps before dry solid was obtained and then calcined. The conventional calcination procedure was conducted in tubular furnace in N2 gas flow, with a heating rate of 5°/min until the final temperature of 500 °C was reached and then the temperature was hold for 6 h.
For further, pillared saponite was used as matrix in titania dispersion section. Titanium isopropoxide in isopropanol solution was used as precursor and impregnation process was chosen at a titania theoretical concentration of 1.2% (wt.). Precursor solution was dropped into the pillared saponite powder dispersed in water followed by constant stirring for 6 h at room temperature. The solvent was then evaporated at 30 °C under vacuum and the solid was dried at 120 °C for 6 h. Titanium oxide formation in dispersed form was obtained after calcination process. In calcination by microwave irradiation procedure, irradiating solid for 15 min using a commercial microwave oven with a frequency of 2465 GHz was performed for both pillarization and titanium oxide formation. Material obtained from each step was designated as PILS-MW and Ti/PILS-MW respectively. In viewing the conventional procedure, conventional calcination by slow heating was also conducted by using a tubular furnace in N2 gas flow. Heating rate of 5°/min was set until the final temperature of 450 °C was reached and then the temperature was held for 4 h. PILS and Ti/PILS are encoded for the product of pillarization and titania dispersion.
For characterization purpose, a Shimadzu X6000 X-ray diffraction (XRD) with Ni-filtered Cu Kα radiation, N2 BET surface area analyzer NOVA 1200, and a JASCO V760 DRUV–Vis spectrophotometer with a BaSO4 standard were employed.
Photocatalytic activity of prepared material was tested in phenol photo-oxidation reaction. A 250 mL reactor walled with cooling water was placed under a UV-B Lamp in an isothermal box. Phenol solution (1 × 10−3 M) was reacted with H2O2 at a mole ratio of H2O2:phenol = 1:1 by stirring the mixture at room temperature under UV-B exposure. Kinetic study of phenol photo-oxidation was observed by analyzing liquid samples regularly obtained from withdrawing with syringe at a certain interval of time by using gas chromatography (Shimadzu). Phenol conversion was defined as mole of phenol converted into products divided by initial phenol concentration.
3 Results and discussion
The powder X-ray diffraction patterns of Ti/PILS-MW and PILS-MW compared with conventionally calcined samples are shown in Fig. 1.
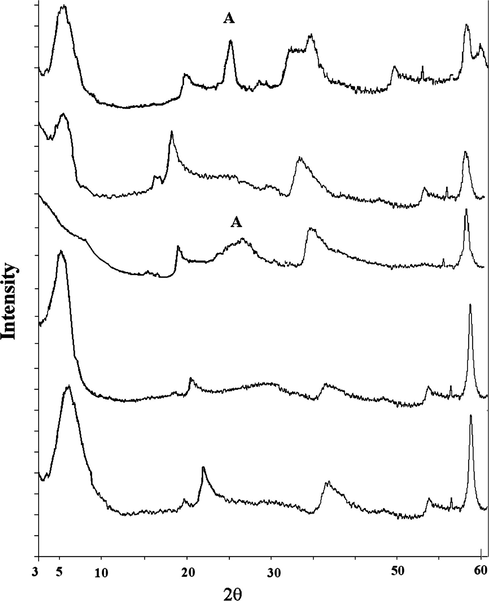
- From top XRD pattern of Ti/PILS, PILS, Ti/PILS-MW, PILS-MW, and saponite.
The patterns PILS-MW and PILS are consistent with the pattern of PILS reported in previous researches focused on saponite pillarization (Fatimah et al., 2008, 2010a,b). PILS shows reflections at 5.14°(d = 17.01 Å) and at 2θ = 19.80° (d = 4.50 Å) as specific reflection correspond to d001 and d004 respectively. Similar values are demonstrated by PILS-MW which are positioned at 5.48°(d = 16.55 Å) and 19.78°. The observed d001 of PILS-MW is slightly higher compared to those was reported indicate the higher aluminium oxide pillar formed between silica layers of saponite structure. The d001 value of both pillared materials is positioned around 16–18 Å and the higher value of d001 is derived from conventional calcination method. The value is comparable to that observed from previous research on Al-pillared saponite preparation (Vicente et al., 2004; Belver et al., 2004; Moreno et al., 1999; Ookaa et al., 2004). It is noted that the d001 value and the increased d001 value derived by pillarization were associated to surface characteristics and cation exchange capacity of raw saponite (Gil et al., 2008). Due to titania dispersion in Ti/PILS and Ti/PILS-MW there are new reflections at around 25.2° found at both samples which are responsible for the presence of titania formation in anatase phase. The significant difference in titania reflection of both samples is that conventional calcined sample gives the sharper peaks compared to microwave irradiated samples. Wider peak indicating lower crystallinity of anatase phase was demonstrated by Ti/PILS-MW. Since titania crystalline growth is influenced by hydrolysis rate from its titania precursor, this difference probably caused by matrix effect affecting the titania crystal growth (Grzmil et al., 2004). Referring to the previous thermal analysis towards pillared clay saponite indicating incomplete aluminium oxide transformation by using microwave, microwave radiation during calcination was consumed not only to transform titania precursor but also for the conversion of the rudimentary pillars converted into oxide (de Andrés et al., 1999). This difference in this sharpness anatase phase peak is also in line with result from DRUV–Vis spectrum (Fig. 2) in that the determined value of band gap energy from recorded edge wavelength for Ti/PILS-MW (3.21 eV) is lower than that of Ti/PILS (3.25 eV). It means that the titania particle size in Ti/PILS has homogeneous distribution or present smaller in size compared to in Ti/PILS-MW. However, both badgap energy values indicate the slight difference in semiconductance and titania mostly in anatase phase.
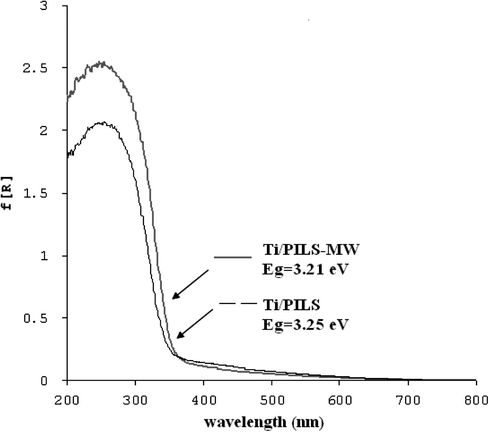
- DRUV–Vis spectrum of Ti/PILS-MW and Ti/PILS.
Furthermore, surface analysis towards material consists of specific surface area, pore volume, and pore radius measurement result in data listed in Table 1. Specific surface area of material was determined based on adsorption isotherm data by P/Po at the range 0–0.25 and pore radius was obtained from adsorption data utilize Barret–Joyner–Halenda (BJH) adsorption method.
| Photocatalyst | Specific surface area (m2/g) | Pore volume (cc/g) | Pore radius (Å) |
|---|---|---|---|
| Ti/PILS | 131.78 | 19.42 × 10−3 | 13.78 |
| Reused-Ti/PILS | 129.87 | 16.78 × 10−3 | 13.66 |
| Ti/PILS-MW | 275.89 | 19.19 × 10−3 | 13.39 |
| Reused-Ti/PILS-MW | 235.56 | 18.76 × 10−3 | 14.09 |
| PILS-MW | 190.66 | 42.11 × 10−3 | 13.77 |
Data in Table 1 exhibit that both titania dispersed material are having higher BET specific surface area and pore volume compared to the matrices (PILS and PILS-MW). This trend was also reported from previous researches dealing with the dispersion of ZrO2 in pillared montmorillonite and pillared saponite and also from titania dispersion in aluminium pillared montmorillonite (Fatimah et al., 2010a,b). The higher specific surface area due to the possibility of titania aggregation on pores and surface of matrix. With homogeneous distribution, aggregation created new space beneficially as adsorption site. Higher specific surface area observed for PILS-MW compared to PILS is also confirmed caused by the different thermal response during polycations conversion in that transformation occurred in calcination by microwave procedure which is in line with reports from XRD data. High intensity during thermal transformation in pillarization and titania formation by using conventional calcination results in susceptibility towards framework destruction. Linear comparision was also showed from Ti/PILS-MW and Ti/PILS. After were used, specific surface area of both Ti/PILS and Ti/PILS-MW samples are reduced, probably due to organic compounds residue deposited on catalyst surface.
Fig. 3 shows the kinetic of phenol concentration in phenol hydroxylation reaction by using photocatalysts and reused photocatalyst after various treatment times. According to the result, it can be seen that by using both Ti/PILS-MW and Ti/PILS photocatalyst, phenol concentration declined gradually on prolonged reaction time and this also occurred for the reused form. Kinetic examination towards the data of phenol concentration as function of reaction time informed that both photocatalyzed reaction follows second order respect to phenol concentration with initial rate values presented in Table 2.
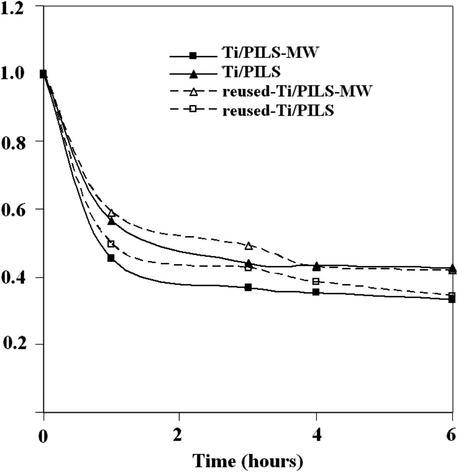
- Kinetic curve of phenol photo-oxidation using Ti/PILS-MW, Ti/PILS, and its reused form.
| Photocatalyst | Initial rate (M/h) | Reaction order |
|---|---|---|
| Ti/PILS | 4.28 × 10−5 | 2nd order |
| Reused-Ti/PILS | 2.57 × 10−5 | 2nd order |
| Ti/PILS-MW | 9.26 × 10−5 | 2nd order |
| Reused-Ti/PILS-MW | 6.28 × 10−5 | 2nd order |
The higher values of the initial rate for both Ti/PILS-MW and reused-Ti/PILS-MW indicate that the use of Ti/PILS-MW affect to decrease in phenol concentration more rapidly than the use of Ti/PILS. Referring to previous studies (Fatimah et al., 2010b), this is likely related to higher specific surface area in Ti/PILS-MW compared to Ti/PILS. The process of adsorption on Ti/PILS-MW which supports the mechanism of photocatalysis occurs more intensively than in Ti/PILS.
In the reaction system used, photocatalyst having specific surface area was dispersed and contact with phenol solution directly, so adsorption process was also involved and affect the reduction of phenol concentration. However, the results in the chromatographic analysis (Fig. 4) prove the involvement of chemical conversion as shown by reducing phenol peak along with the emergence of product peaks. Detected oxidation products in chromatography analysis are benzoquinones (BQ), hydroquinone, (HQ) and catechol (CAT) also in line with that reported in other phenol oxidation/hydroxylation researches regarding to the reaction mechanism discussed in previous research (Klaewkla et al., 2003; Choi et al., 2006; Yube et al., 2007).
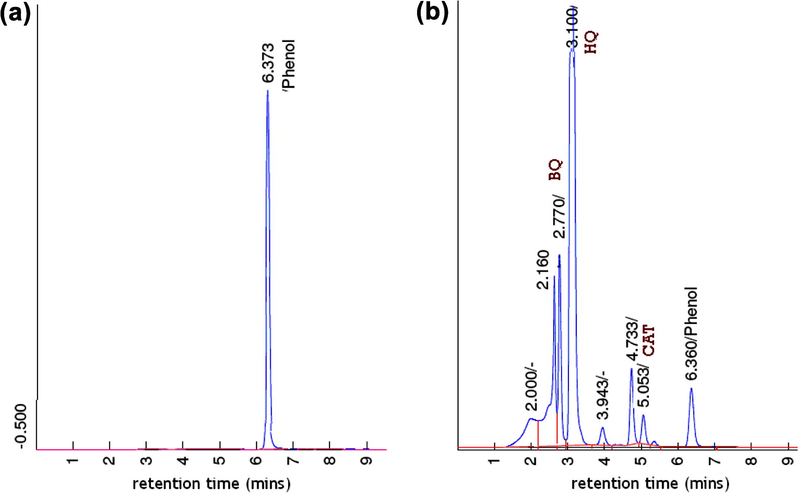
- Chromatogram of (a) phenol reactant (b) treated phenol using Ti/PILS-MW for 1 h.
4 Conclusion
The study of microwave irradiation assisted preparation of titania dispersed in aluminium pillared saponite (Ti/PILS-MW) has shown that microwave irradiation can be used to replace conventional calcination procedure. XRD, BET surface area analysis, and also DRUV–Visible spectrophotometry reveal that the physicochemical character is not too much different from the material produced by preparation using conventional calcination. The measurement of photocatalytic activity in phenol photo-oxidation has clearly shown that material having photoactivity based on the kinetic curve of phenol decrease as function of reaction time, even from reused material.
Acknowledgements
Authors are acknowledged for the financial support from research grant Hibah Penelitian Mahasiswa Doktor 2009, DP2M-DIKTI via Lembaga Penelitian dan Pengabdian pada Masyarakat, Gadjah Mada University.
References
- Fe-saponite pillared and impregnated catalysts: I. Preparation and characterisation,. Applied Catalysis B: Environmental. 2004;50(2):101-112.
- [Google Scholar]
- Photoreaction as a green chemistry process: photocatalytic organic syntheses. Chemical Industry. 2006;57(6):453-460.
- [Google Scholar]
- Synthesis of pillared clays assisted by microwaves. Materials Research Bulletin. 1999;34(4):641-651.
- [Google Scholar]
- A comparative study on aluminium pillared smectite synthesis from synthetic saponite and Indonesian montmorillonite. Asian Journal of Chemistry. 2008;8(1):69-74. <http://www.aseanjche.ugm.ac.id/ojs/index.php/jce/article/view/153>
- [Google Scholar]
- Preparation of ZrO2/Al2O3-pillared saponite and its spectroscopic investigation on NOX adsorption. Journal of Physical Science. 2010;21(1):53-65. <http://www.web.usm.my/jps/a-21-1-5.html>
- [Google Scholar]
- Composites of TiO2-aluminum pillared montmorillonite: Synthesis, characterization and photocatalytic degradation of methylene blue. Applied Clay Science. 2010;50(4):588-593.
- [CrossRef] [Google Scholar]
- Recent advances in the control and characterization of the porous structure of pillared clay catalysts. Catalysis Reviews. 2008;50(2):153-221.
- [Google Scholar]
- Inhibition of the anatase—rutile phase transformation with addition of K2O, P2O5, and Li2O. Chemicals Paper. 2004;58(6):410-414.
- [Google Scholar]
- Environmental green chemistry as defined by photocatalysis. Journal of Hazardous Materials. 2007;146(3):624-629.
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis of anatase TiO2 nanorods with mesopores. Nanotechnology. 2007;18(7):075602.
- [Google Scholar]
- Phenol hydroxylation using Ti- and Sn-containing silicalites. Chemical Communication. 2003;2003:1500-1501.
- [Google Scholar]
- Beta zeolite supported sol–gel TiO2 materials for gas phase photocatalytic applications. Journal of Hazardous Material. 2011;186(2-3):1218-1225.
- [Google Scholar]
- Photodegradation of an azo dye using immobilized nanoparticles of TiO2 supported by natural porous mineral. Journal of Hazardous Materials. 2008;152(3):1037-1044.
- [Google Scholar]
- Semiconductor-mediated photocatalysis for organic synthesis. Studies in Surface Science and Catalysis. 1997;103:391-415.
- [CrossRef] [Google Scholar]
- Al-, Al, Zr-, and Zr-pillared montmorillonites and saponites: preparation, characterization, and catalytic activity in heptane hydroconversion. Journal of Catalysis. 1999;182:174-185.
- [Google Scholar]
- Photocatalytic organic syntheses: selective cyclization of amino acids in aqueous suspensions. Catalysis Surveys from Asia. 2003;7(2–3):165-176.
- [CrossRef] [Google Scholar]
- Effect of surface hydrophobicity of TiO2-pillared clay on adsorption and photocatalysis of gaseous molecules in air. Applied Catalysis A: General. 2004;260:47-53.
- [Google Scholar]
- Photocatalysis: a promising route for 21st century organic chemistry. Chemical Communication 2007:3425-3437.
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis of titania nanocubes, nanospheres and nanorods for photocatalytic dye degradation. Nanoscale Research Letter. 2009;4:144-152.
- [CrossRef] [Google Scholar]
- Microwave-assisted preparation of TiO2/activated carbon composite photocatalyst for removal of methanol in humid air streams. Industrial Engineering Chemistry Research. 2006;45(14):5110-5116.
- [Google Scholar]
- TiO2 supported on sepiolite: preparation, structural and thermal characterization and catalytic behaviour in photocatalytic treatment of phenol and lignin from olive mill wastewater. Chemical Engineering Journal. 2011;166(3):859-867.
- [Google Scholar]
- Photocatalytic reduction of organic compounds. Journal of Advanced Oxidation Technologies. 2010;13(3):321-340.
- [Google Scholar]
- Preparation and characterisation of Mn- and Co-supported catalysts derived from Al-pillared clays and Mn- and Co-complexes. Applied Catalysis A: General. 2004;267(1–2):47-58.
- [Google Scholar]
- Control of selectivity in phenol hydroxylation using microstructured catalytic wall reactors. Applied Catalysis A: General. 2007;327:278-286.
- [Google Scholar]







