Translate this page into:
Modified layered bimetallic compound used as a novel adsorbent for the solid-phase extraction of monoaromatic hydrocarbons from water samples prior to gas chromatography–mass spectrometry
⁎Corresponding author at: 1#, Dongsanlu, Erxianqiao, Chengdu 610059, Sichuan, PR China. zhuxiaping@cdut.edu.cn (Xiaping Zhu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study reports a promising method of solid-phase extraction for determining the toluene, ethylbenzene, p-xylene, m-xylene, o-xylene, 1,3,5-trimethylbenzene, and 1,2,4-trimethylbenzene in water samples by gas chromatography–mass spectrometry (GC–MS). Prior to this procedure, the magnesium–aluminum bimetallic hydroxides modified with sodium dodecylbenzenesulfonate (Mg/Al-SDBS-LDH) were prepared and served as the novel solid-phase extractant. The Mg/Al-SDBS-LDH has advantage of good hydrophobicity and larger spacing which facilitates the monoaromatic hydrocarbons (MAHCs) into the interlayer for adsorption. As a result, the seven MAHCs in 500 mL water samples were enriched greatly, and the theoretical enrichment factor reached to 125 times. Under the optimized conditions of solid-phase extraction (SPE) and GC–MS, the mass concentration of each MAHC (0.005–10, 0.01–10, or 0.05–10 ng/mL) had a fine linear relationship with peak area. The correlation coefficients were more than 0.995. The detection limits were between 0.001 and 0.01 ng/mL, and the RSD were between 3.1% and 6.6%. The method had been applied to determine the seven MAHCs in the Dongfengqu river water and laboratory wastewater of Chengdu University of Technology successfully.
Keywords
Solid-phase extraction
Monoaromatic hydrocarbons
Gas chromatography–mass spectrometry
SDBS-modified layered bimetallic hydroxide
1 Introduction
Monoaromatic hydrocarbons (MAHCs) are common pollutants that result from human activities. If not controlled, they will cause serious harm to the natural environment and human health. At present, they have been identified as a strong carcinogen by the World Health Organization (Lovreglio et al., 2016). The integrated wastewater discharge standard [GB 8978–1996] in China stipulates that the maximum residue limits of toluene, ethylbenzene, and xylene are 0.1, 0.4, and 0.4 μg/mL, respectively. In addition, the environmental quality standard for surface water [GB3838-2002] stipulates that the standard limits of toluene, ethylbenzene, and xylene are 0.7, 0.3, and 0.5 μg/mL, respectively. Consequently, effective monitoring of MAHCs in water samples is important to control MAHCs pollution. However, the separation and enrichment of MAHCs from the actual sample also are bottlenecks in the monitoring process.
The rapid collection, separation, and enrichment techniques of MAHCs from water samples mainly include headspace solid-phase microextraction (HS-SPME) (Arambarri et al., 2004; Fustinoni et al., 1999; Pavón et al., 2007; Bernardo et al., 2009; Bianchin et al., 2012; Ridgway et al., 2006; Přikryl et al., 2006; Gholivand et al., 2014; Li et al., 2010; Yazdi et al., 2011; Lee et al., 2007), liquid-phase microextraction (LPME) (Sarafraz-Yazdi et al., 2009; Przyjazny and Kokosa, 2002; Aguilera-Herrador et al., 2008), liquid–liquid microextraction (LLME) (Hashemi et al., 2012; Khezeli et al., 2015; Lan et al., 2017; Faraji et al., 2013), solid-phase extraction (SPE) (Xu et al., 2019; Li et al., 2016; Fernández et al., 2016; Kang et al., 2019) and so on. HS-SPME utilizes fiber head coated with a solid-phase adsorbent to adsorb volatile or semi-volatile substances exposed to the headspace. In the present study, the extraction efficiencies of MAHCs in water samples were improved by optimizing the extraction conditions (Arambarri et al., 2004; Fustinoni et al., 1999; Pavón et al., 2007; Bernardo et al., 2009; Bianchin et al., 2012; Ridgway et al., 2006; Přikryl et al., 2006), preparing cobalt oxide nanoparticles and improving carbon nanotube fibers (Gholivand et al., 2014; Li et al., 2010; Yazdi et al., 2011). LPME realizes solute extraction by equilibrating the target analyte between the samples and the micro-upgraded extraction solvent. Direct suspension droplet (Sarafraz-Yazdi et al., 2009) and headspace single droplet (Przyjazny and Kokosa, 2002) could effectively separate and enrich MAHCs in water samples. Aguilera-Herrador et al. (2008) solved the interface problem of gas phase system and the applicability of device had been verified. LLME is achieved through the dispersion and emulsification of a few organic solvents in the water phase. Some studies (Hashemi et al., 2012; Khezeli et al., 2015; Lan et al., 2017) used ultrasound to enhance emulsification and dispersion. Faraji et al. (2013) enriched MAHCs by coagulating floating droplets. SPE is a promising sample preparation techniques, there are many new reports about SPE adsorbents with high thermostability and chemical stability. Porous covalent organonitridic frameworks (PCONFs) were applied for rapid extraction of eight sulfonamide antibiotics from complex samples (Xu et al., 2019), Li et al. reported a magnetic metal-organic nanotubes for solid-phase extraction of seven polychlorinated biphenyls from environmental and biological samples (Li et al., 2016). However, fewer papers related to solid phase extraction of MAHCs. The zeolite/iron oxide composite was a promising adsorbent material for magnetic solid-phase extraction of benzene, toluene, ethylbenzene and xylenes from water samples (Fernández et al., 2016). The graphene oxide/magnesium–aluminium bimetallic hydroxides composite (Kang et al., 2019) (GO/Mg-Al-LDH) was a solid phase extractant that we developed earlier. It had used to determination of toluene, ethylbenzene, p-xylene, m-xylene, o-xylene, 1,3,5-trimethylbenzene and 1,2,4-trimethylbenzene in wastewater, the method detection limit range were 0.0005–0.005 ng/mL, respectively.
The layered bimetallic hydroxides (LDH) are important inorganic functional materials. They have a good prospect in the field of adsorption due to their tunable porosity and ion exchangeability (Wu et al., 2016). Zhou et al. (2017) synthesized Nickel-aluminum layered bimetallic hydroxides by co-precipitation method and packed into a micro pipette tip for the extraction of three non-steroidal anti-inflammatory drugs including ketoprofen, naproxen and flurbiprofen from aqueous samples. Rocha et al. (2018) showed a flow system designed with solenoid valves for preconcentration of fluoride using a mini-column coated with layered bimetallic hydroxides as adsorbent in water samples. Especially in recent years, the modified layered bimetallic hydroxides had been sequentially developed in many fields, such as modified electrodes (Fernánde et al., 2013), anti-aging materials (Xu et al., 2015), flame retardants (Lu et al., 2015).
In this paper, we proposed a promising method of using magnesium–aluminum bimetallic hydroxides modified with sodium dodecylbenzenesulfonate as an SPE absorbent (denoted as Mg/Al-SDBS-LDH) for enriching toluene, ethylbenzene, p-xylene, m-xylene, o-xylene, 1,3,5-trimethylbenzene, and 1,2,4-trimethylbenzene in water samples. The Mg/Al-SDBS-LDH has advantage of good hydrophobicity and larger spacing due to SDBS entering the material interlayer. In addition, we discussed the extraction conditions for seven MAHCs. The established method was proven to be rapid, simple, and sensitive for the determination of ultra-trace MAHCs in water samples.
2 Experimental
2.1 Instruments and reagents
MAHCs were isolated and determined by gas chromatography–mass spectrometry (GC–MS) coupled with a capillary column (7890A-5975C, DB-5MS, Agilent Technologies, USA). In the process of Mg/Al-SDBS-LDH preparation, a hydrothermal reactor (YZ-HRL, Yanzheng, China), a digital thermostat magnetic stirrer (85-2, Putian, China), and an electric blast oven (DHG-9035A, KeXue, China) were also used. The enrichment of MAHCs was carried out by using a SPE device (YGC-6, YaYuan, China) equipped with sieve plates and column tubes (AS150-A, AZ150, Agilent Technologies, USA). The flow rate was adjusted by using a vacuum pump (2511C-75, Welch, USA).
All reagents and solvents were of analytical grade and used with no further purification. The standard substances, including toluene, ethylbenzene, o-xylene, p-xylene, m-xylene, 1,3,5-trimethylbenzene, and 1,2,4-trimethylbenzene were obtained from Kelong Chemical Reagent Factory (≥99.7%, Chengdu, China). Methanol, n-hexane, chloroform, phenixin and isopropanol, SDBS, magnesium nitrate, aluminum nitrate, anhydrous sodium carbonate, and sodium hydroxide were purchased from Jinshan Chemical Reagent Factory (≥99.5%, Chengdu, China). Ultrapure water was used throughout the experiment. Mixed MAHC standard stock solution (1000 mg/L) was prepared by using methanol and stored at 3 °C in the dark. Mixed MAHC standard working solution was prepared by diluting the standard stock solution to 10 mg/L with methanol and to the desired concentration with ultrapure water.
2.2 Preparation of solid-phase sorbent
A total of 0.06 mol of Mg(NO3)2·6H2O, 0.02 mol of Al(NO3)3·9H2O, and 100 mL of ultrapure water were accurately added to a 250 mL beaker to obtain the magnesium–aluminum bimetallic salt solution. Meanwhile, 7.2 g of NaOH, 6.36 g of Na2CO3, and 100 mL of ultrapure water were added to a 250 mL beaker to form the alkali solution. Subsequently, the salt and alkali solutions were added dropwise to a 500 mL beaker with 50 mL of ultrapure water. Meanwhile, the mixed solution was stirred constantly at 60 °C with pH of 10 ± 0.5. After addition, the mixed solution was stirred for another 40 min and then aged at 80 °C for 24 h. Subsequently, the aged solution was filtrated, and the precipitate was washed to neutral. Finally, the magnesium–aluminum layered bimetallic hydroxides (Mg-Al-LDH) were obtained by drying and grinding to a mesh size of 200. Mg-Al-LDH was calcined in a muffle furnace at 400 °C for 2 h. Being cooled in air, 1.00 g of Mg-Al-LDH, 0.075 g of SDBS, and 50 mL of distilled water were added to a 100 mL beaker, and then the mixed solution was placed in a hydrothermal reactor and stood in an oven at 150 °C for 1 h. After cooling, filtrating, drying, and grinding to a mesh size of 200, Mg/Al-SDBS-LDH was obtained.
2.3 SPE
Dongfengqu river water and laboratory wastewater in Chengdu University of Technology were filtered through a 0.45 μm fiber membrane (Water filter membrane, mixed fiber resin microporous membrane). Subsequently, Dongfengqu river water were diluted 10 times, and laboratory wastewater were diluted 100 times. Exactly 1.00 g of Mg/Al-SDBS-LDH was compressed with two SPE sieves, which were filled into an extraction column and placed on a SPE device. The 500 mL mixed MAHC standard working solution or water samples were poured slowly into the extraction column. At the same time, the vacuum pump was used to adjust the extraction time for 5 min (pressure = 264 psi). After all water samples flowed through the extraction column, 50 mL of ultrapure water was used to wash the extraction column and discarded. Afterward, 4.00 mL of chloroform was added to elute the extraction column for 3 min (pressure = 207 psi). Finally, the MAHCs in the eluent were determined by GC–MS.
2.4 GC–MS conditions
Chromatography conditions: the inlet temperature was 220 °C, the carrier gas was helium with a flow rate of 1 mL/min, the split ratio was 30:1, and the injection volume was 1 μL. Considering the weak polarity of MACHs, the Agilent DB-5MS (30 m × 0.25 mm × 0.25 μm) nonpolar column was used. Toluene had the lowest boiling point and the earliest peak. Therefore, the lower initial temperature was chosen and remained for a period of time so that it could not be disturbed by the solvent peak. The slower temperature program was selected to optimize the peak shape and retention time of other MAHCs due to their boiling points being close. Considering the separation effect and peak shape of the seven kinds types of MAHCs, the temperature program was confirmed: the initial temperature was 45 °C and held for 3 min, then warmed up to 70 °C at 3 °C/min and continued until 200 °C at 30 °C/min, which was held for 2 min.
MS conditions: electron impact was used with an electron energy of 69.9 eV; the temperatures of the ion source and quadrupole were set to 240 °C and 150 °C, respectively; and the determination was taken with a solvent delay of 3 min. Full scan mode and selective ion scan mode were used for qualitative and quantitative analysis, respectively.
According to the optimized chromatographic and mass spectrometric conditions, the qualitative ion and quantitative ion of the seven MAHCs are selected as shown in Table 1. The standard solution containing 0.5 ng/mL of each of the seven MAHCs is determined to obtain the selective ion flow chart (Fig. 1). The seven MAHCs can be separated, and their peak shapes are symmetrical. The retention times are as follows: toluene is 4.36 min, ethylbenzene is 5.75 min, p-xylene is 6.09 min, m-xylene is 6.17 min, o-xylene is 6.77 min, 1,3,5-trimethylbenzene is 8.97 min, and 1,2,4-trimethylbenzene is 9.50 min.
Compound
Peak number
Relative molecular mass
Mass charge ratio(m/z)
Qualitative ion
Quantitative ion
Retention time
toluene
1
92.06
40, 91
91
4.37
ethylbenzene
2
106.08
91, 106
91
5.75
p-xylene
3
106.08
91, 106
91
6.09
m-xylene
4
106.08
91, 106
91
6.17
o-xylene
5
106.08
91, 106
91
6.77
1,3,5-trimethylbenzene
6
120.09
105, 120
105
8.98
1,2,4-trimethylbenzene
7
120.09
105, 120
105
9.50
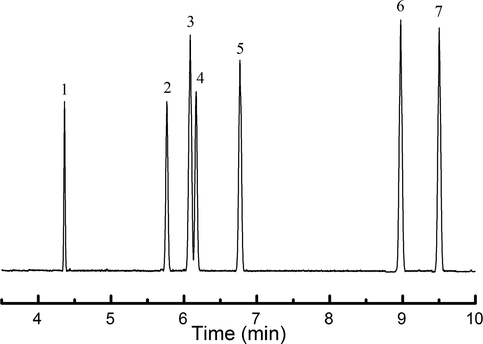
Selective ion flow chart of MACHs in mixed standard solution (peak numbers are the same as Table 1).
3 Results and discussion
3.1 Preparation and adsorption process of Mg/Al-SDBS-LDH
Fig. 2 shows the preparation of Mg/Al-SDBS-LDH and adsorption of MAHCs in water samples. Mg/Al-LDH is a layered structure material whose laminates consist of Mg(OH)2 and Al(OH)3, and NO3−, CO32−, OH−, and H2O exist in the interlayer. The interlayer anions and H2O are removed by calcining at 400 °C, and then the layered structure disappears without destroying the laminates. The layered structure of the material is restored by introducing the functional guest (SDBS) to the calcined material. The interlayer space increases from 0.74 nm to 1.16 nm due to SDBS entering the material interlayer (calculated by the Prague formula). At the same time, the SDBS replaces a large number of water molecules between interlayer so that the hydrophobicity of material is significantly improved. As a result, the SDBS are well hydrogen-bonded to the hydroxyl ions of laminates, which also apparently increases the stability of material. In the end, either the improvement of hydrophobicity or the increase of interlayer space facilitates the MAHCs into the interlayer for adsorption.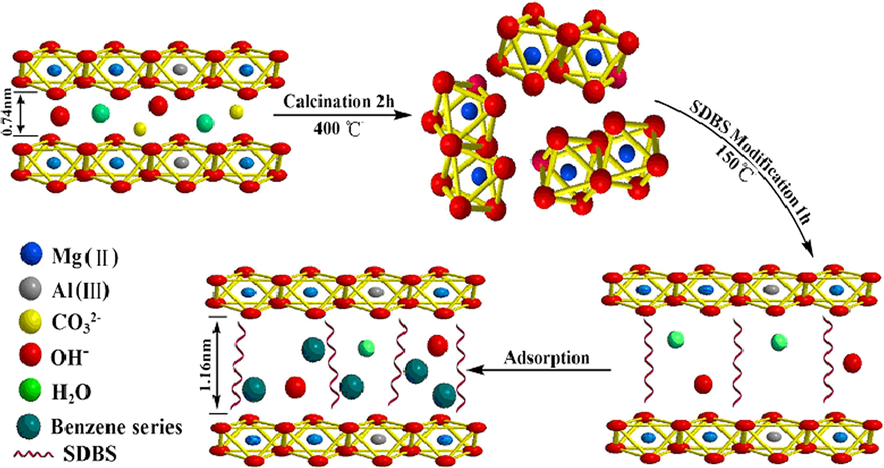
Preparation and adsorption process of Mg/Al-SDBS-LDH.
3.2 Selection of SPE conditions
The effect of the amount of Mg/Al-SDBS-LDH on extraction efficiency is shown in Fig. 3a. With the increase of Mg/Al-SDBS-LDH amount, the peak area of seven MAHCs exhibits a tendency of rise–balance–decline. When the amount of the material is very small, extraction may be incomplete. On the contrary, the process will need more eluent if increase the amount of material. When the amount of material is 1.0–1.5 g, the extraction efficiency is the best.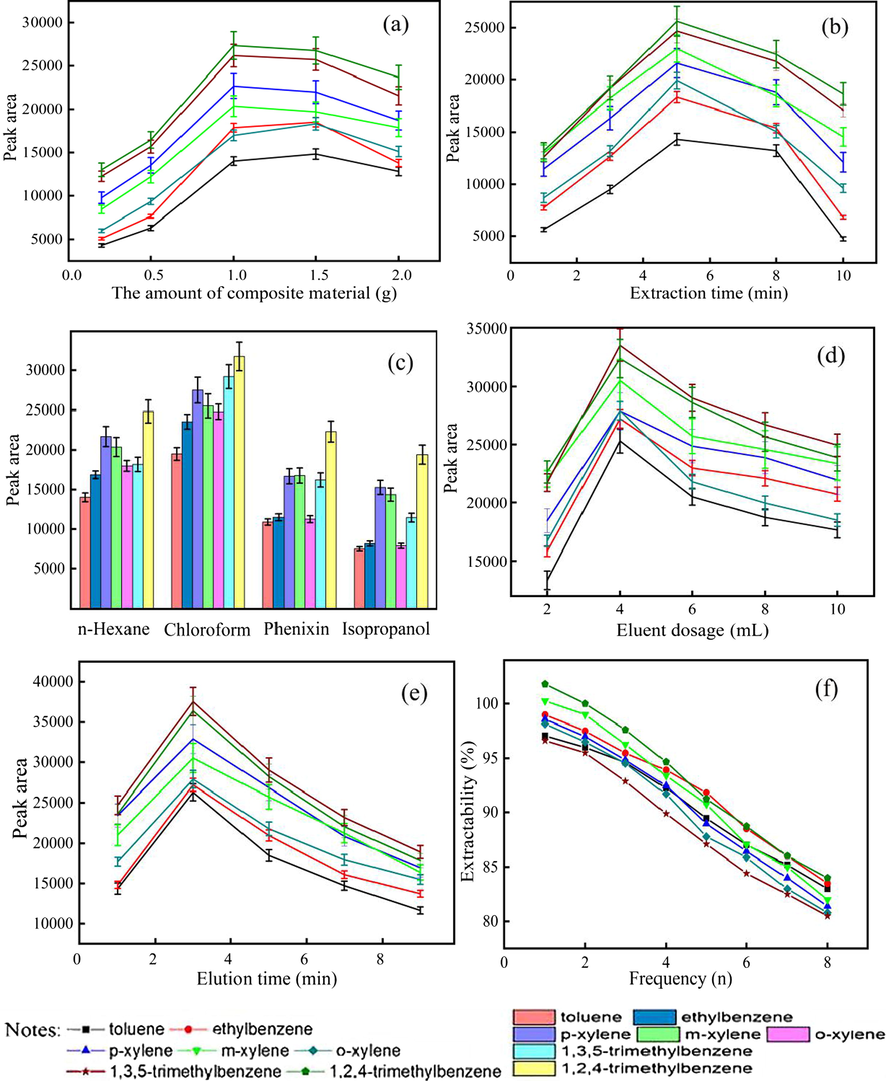
Selection of solid-phase extraction conditions. Mg/Al-SDBS-LDH amount (a); extraction time (b); eluent type(c); eluent amount (d); elution time (e); reusable property of Mg/Al-SDBS-LDH (f).
As illustrated in Fig. 3b, the peak areas of the seven MAHCs increase initially and then decrease with prolonged time. When the extraction time is less than 5 min, the effect of extraction is worse. By extending the time, volatilization of the MAHCs can bring some losses. When the extraction time is 5 min, the extraction efficiency is the highest.
In this study, organic solvents such as n-hexane, chloroform, phenixin, and isopropanol are selected as eluents (Fig. 3c). Chloroform has the best elution efficiency, followed by n-hexane and then phenixin and isopropanol. Hence, the effect of chloroform amount on elution efficiency was tested further as shown in Fig. 3d. With the increase of chloroform in amount, great changes have taken place in the peak area. However, no matter the amount of chloroform is too large or too small, the signals are significantly smaller. The MAHCs are diluted when the amount of chloroform is very large, which can affect the sensitivity. When the amount of chloroform is 4 mL, the elution efficiency is the highest. Fig. 3e shows the effect of elution time. When the elution time is 3 min, the elution efficiency is the highest. With the increase of elution time, the peak areas of MAHCs show a significant decline. This finding is probably due to the volatilization of MAHCs. Therefore, the elution time of 3 min selected in this experiment.
The used Mg/Al-SDBS-LDH was dried at 80 °C for 4 h to remove the MAHCs. Subsequently, extraction and elution were repeated in accordance with the method described in Section 2.3 to verify the reusability of the material. The result is shown in Fig. 3f. The extraction rates of the seven MAHCs are more than 80% within eight times under repeated use. Mg/Al-SDBS-LDH exhibits excellent reusability.
3.3 Calibration curve, precision, and detection limit of the proposed method
The standard working solution of 10 mg/L mixed MAHCs was diluted with ultrapure water and formulated as 0.005 ng/mL, 0.001 ng/mL, 0.05 ng/mL, 0.1 ng/mL, etc. SPE and determination were carried out under the optimized conditions. The calibration curve was drawn with the concentration of MAHCs as the abscissa and the peak area as the ordinate. The minimum concentration was determined for 11 times to calculated the standard deviation, and the detection limits of seven MAHCs were obtained in the way of dividing the slope of the calibration curve by triple standard deviation. Simultaneously, the standard solution of 0.5 ng/mL was determined 5 times in parallel to calculate the relative standard deviation (RSD). The linear range, linear regression equation, correlation coefficient, and detection limit of the seven MAHCs are shown in Table 2.
Compound
LOD/ (ng/mL)
Linear range/(ng/mL)
Linear regression equation
Correlation coefficient/ (R)
RSD/ (%)
toluene
0.001
0.005–10
Y = 4 * 103x + 10649
0.997
4.5
ethylbenzene
0.003
0.01–10
Y = 6 * 103x + 2516
0.997
3.1
p-xylene
0.005
0.01–10
Y = 5 * 103x + 3659
0.997
6.6
m-xylene
0.003
0.01–10
Y = 5 * 103x + 5527
0.995
5.8
o-xylene
0.005
0.01–10
Y = 4 * 103x + 4851
0.998
4.1
1,3,5-trimethylbenzene
0.01
0.05–10
Y = 6 * 103x + 8557
0.995
5.4
1,2,4-trimethylbenzene
0.01
0.05–10
Y = 8 * 103x + 7333
0.996
6.2
The concentrations of the seven MAHCs (0.005–10, 0.01–10, or 0.05–10 ng/mL) demonstrate a good linear relationship with the peak area. The correlation coefficients are more than 0.995. The detection limits are between 0.001 and 0.01 ng/mL, and the RSD are between 3.1% and 6.6%. Table 3 shows information of the method previously reported for the determination of MAHCs. This work has some advantages: (1) the material is first used in solid phase extraction for MAHCs; (2) the detection limit is lower than that of the general SPE and equivalent to that of the solid-phase microextraction; (3) the seven MAHCs in the water samples can be determined at the same time; (4) the SPE technology used in this study is simple, reproducible, and easy to popularize. This method can meet the requirement of rapid analysis of the ultra-trace MAHCs in the water samples. Notes: a, b, c, d, e, f, g, h represent benzene, toluene, ethylbenzene, o-xylene, m-xylene, p-xylene, 1,3,5-trimethylbenzene, 1,2,4-trimethylbenzene,respectively. “+” indicates the total amount.
Method
Sample
Analyte
LOD/ (ng/mL)
Linear range/ (ng/mL)
Ref.
HS-PME-GC-MS
Well water
a, b, c, d, e+f
0.01–0.06
0–500
Fustinoni et al. (1999)
HS-PTV-GC-MS
River water
a, b, c, e
0.01–0.1
0.03–35
Pavón et al. (2007)
HS-GC-MS
Surface water
a, b, c, d+e, f
0.008–0.038
0.1–2
Bernardo et al. (2009)
HSSPME-GC-MS
Tap water
b, c, d, e, f
0.07–0.3
0.3–10
Bianchin et al. (2012)
SPDE-GC-MS
River water
a, b
0.17–0.48
1–50
Ridgway et al. (2006)
SDME-GC-MS
River water
a, b, c, d+e, f
0.02–0.091
0.07–5
Przyjazny and Kokosa (2002)
SPE-GC-MS
River water
a, b, c, d, e, f
0.3–3
1–100
Xu et al. (2019)
HSSPME-GC-MS
Ground water
a, b, c, d, e+f
0.01–0.05
0.0001–50
Li et al. (2016)
SPE-GC-MS
Waste water, River water
b, c, d, e, f, g, h
0.0005–0.005
0.001–10
This work
3.4 Analysis of real water samples
Water samples were collected from the Chengdu University of Technology’s laboratory and Dongfengqu river. The samples were pretreated and determined in accordance with the experimental method described in Sections 2.3 and 3.3. Simultaneously, 5 mL of 50 ng/mL mixed standard solution was added for the standard recovery experiment. The results are shown in Table 4. In the Dongfengqu river water, the amounts of toluene, ethylbenzene, p-xylene, m-xylene, o-xylene, 1,3,5-trimethylbenzene, and 1,2,4-trimethylbenzene are 2.74, 2.54, 0.82, 0.66, 1.08, 1.39, and 1.66 ng/mL, respectively. The recovery rates of the seven MAHCs are between 81.8% and 109.0%, and the RSD (n = 5) are between 3.5% and 9.6%. In the laboratory wastewater, the amounts of toluene, ethylbenzene, m-xylene and o-xylene are 25.70, 1.50, 2.30 and 7.50 ng/mL, respectively. However, p-xylene, 1,3,5-trimethylbenzene, and 1,2,4-trimethylbenzene are undetected. The recovery rates of the seven MAHCs are between 84.0% and 112.6%, and the RSD (n = 5) are between 4.5% and 8.8%.
Compound
Detected amount/(ng/mL)
Sample amount/(ng/mL)
Added amount/(ng/mL)
Amount recovered/(ng/mL)
Recovery/(%)
RSD/(%)
Dongfengqu river
toluene
0.274
2.74
0.50
0.698
84.8
3.5
ethylbenzene
0.254
2.54
0.50
0.775
104.2
8.6
p-xylene
0.082
0.82
0.50
0.519
87.4
5.7
m-xylene
0.066
0.66
0.50
0.475
81.8
9.0
o-xylene
0.108
1.08
0.50
0.585
95.4
4.2
1,3,5-trimethylbenzene
0.139
1.39
0.50
0.591
90.4
9.6
1,2,4-trimethylbenzene
0.166
1.66
0.50
0.711
109.0
6.1
Laboratory wastewater
toluene
0.257
25.70
0.50
0.731
94.8
7.8
ethylbenzene
0.015
1.50
0.50
0.578
112.6
4.8
p-xylene
–
–
0.50
0.551
110.2
5.5
m-xylene
0.023
2.30
0.50
0.515
98.4
5.9
o-xylene
0.075
7.50
0.50
0.532
91.4
8.2
1,3,5-trimethylbenzene
–
–
0.50
0.547
109.4
4.5
1,2,4-trimethylbenzene
–
–
0.50
0.420
84.0
8.8
4 Conclusion
The magnesium–aluminum layered bimetallic hydroxides modified with SDBS (Mg/Al-SDBS-LDH) were prepared, and it was first as adsorbent material for toluene, ethylbenzene, p-xylene, m-xylene, o-xylene, 1,3,5-trimethylbenzene, and 1,2,4-trimethylbenzene successfully. The solid-phase material is a layered compound with a large interlayer space and good hydrophobicity, which is conducive to MAHCs into the interlayer area. The established method is simple, economic, and environmentally friendly and has high theoretical enrichment factor, which can meet the determination requirements of ultra-trace MAHCs in water samples.
Acknowledgement
We gratefully acknowledge the financial support from Science & Technology Department of Guizhou Province ([2019]2833 and [2019]1424). We also gratefully acknowledge the financial support from Science & Technology Department of Sichuan Province (2015GZ0243).
References
- Ionic liquid-based single-drop microextraction/ gas chromatographic/mass spectrometric determination of benzene, toluene, ethylbenzene and xylene isomers in waters. J. Chromatogr. A. 2008;1201:106-111.
- [CrossRef] [Google Scholar]
- Determination of fuel dialkyl ethers and BTEX in water using headspace solid-phase microextraction and gas chromatography-flame ionization detection. J. Chromatogr. A. 2004;1033:193-203.
- [CrossRef] [Google Scholar]
- Determination of aromatic compounds in eluates of pyrolysis solid residues using HS-GC-MS and DLLME-GC-MS. Talanta. 2009;80:104-108.
- [CrossRef] [Google Scholar]
- Simultaneous determination of polycyclic aromatic hydrocarbons and benzene, toluene, ethylbenzene and xylene in water samples using a new sampling strategy combining different extraction modes and temperatures in a single extraction solid-phase microextraction-gas chromatography-mass Spectrometry procedure. J. Chromatogr. A. 2012;1233:22-29.
- [CrossRef] [Google Scholar]
- Modified dispersive Liquid-liquid microextraction for pre- concentration of benzene, toluene, ethylbenzene and xylenes prior to their determination by GC. Microchim. Acta. 2013;180:1141-1148.
- [CrossRef] [Google Scholar]
- Horseradish peroxidase modified electrode based on a film of Co–Al layered double hydroxide modified with sodium dodecylbenzenesulfonate for determination of 2-chlorophenol. Sen. Act. B. 2013;182:625-632.
- [CrossRef] [Google Scholar]
- Zeolite/iron oxide composite as sorbent for magnetic solid-phase extraction of benzene, toluene, ethylbenzene and xylenes from water samples prior to gas chromatography-mass spectrometry. J. Chromatogr. A. 2016;1458:18-24.
- [CrossRef] [Google Scholar]
- Headspace solid-phase microextraction for the determination of benzene, toluene, ethylbenzene and xylenes in urine. J. Chromatogr. B. 1999;723:105-115.
- [CrossRef] [Google Scholar]
- Cobalt oxide nanoparticles as a novel high-efficiency fiber coating for solid phase microextraction of benzene, toluene, ethylbenzene and xylene from aqueous solutions. Anal. Chim. Acta.. 2014;822:30-36.
- [CrossRef] [Google Scholar]
- Application of ultrasound-assisted emulsification microextraction for determination of benzene, toluene, ethylbenzene and o-xylene in water samples by gas chromatography. Desalination. 2012;288:93-97.
- [CrossRef] [Google Scholar]
- The graphene oxide/magnesium–aluminium bimetallic hydroxides composite used as solid-phase extractant for determination of monoaromatic hydrocarbons in waste water. Int. J. Environ. Anal. Chem.. 2019;99:878-891.
- [CrossRef] [Google Scholar]
- Emulsification liquid-liquid microextraction based on deep eutectic solvent: An extraction method for the determination of benzene, toluene, ethylbenzene and seven polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A. 2015;1425:25-33.
- [CrossRef] [Google Scholar]
- Ultrasound assisted-emulsification liquid liquid microextration-gas chmatography for determination of seven kinds of benzene series in water samples. Chin. Meas. Test.. 2017;43:50-54.
- [CrossRef] [Google Scholar]
- Determination of benzene, toluene, ethylbenzene, xylenes in water at sub-ng.L -1 levels by solid-phase microextraction coupled to cryo-trap gas chromatography-mass spectrometry. Chemosphere 2007:1381-1387.
- [CrossRef] [Google Scholar]
- Evaluation of the solid-phase microextraction fiber coated with single walled carbon nanotubes for the determination of benzene, toluene, ethylbenzene, xylenes in aqueous samples. J. Chromatogr. A. 2010;1217:2191-2196.
- [CrossRef] [Google Scholar]
- Magnetic metal-organic nanotubes: An adsorbent for magnetic solid-phase extraction of polychlorinated biphenyls from environmental and biological samples. J. Chromatogr. A. 2016;1449:39-47.
- [CrossRef] [Google Scholar]
- Study on synergistic dispersibility and flame retardance of nano-SiO2 and MgAl-SDBS-LDHs in polypropylene. Mater. Rev.. 2015;29:62-67.
- [CrossRef] [Google Scholar]
- Simultaneous determination of gasoline oxygenates and benzene, toluene, ethylbenzene and xylene in water samples using headspace-programmed temperature vaporization-fast gas chromatography-mass spectrometry. J. Chromatogr. A. 2007;1175:106-111.
- [CrossRef] [Google Scholar]
- Comparison of needle concentrator with SPME for GC determination of benzene, toluene, ethylbenzene, and xylenes in aqueous samples. Chromatographia. 2006;64:65-70.
- [CrossRef] [Google Scholar]
- Analytical characteristics of the determination of benzene, toluene, ethylbenzene and xylenes in water by headspace solvent microextraction. J. Chromatogr. A. 2002;977:143-153.
- [CrossRef] [Google Scholar]
- DNA damage and repair capacity in workers exposed to low concentrations of benzene. Mol. Mutagen.. 2016;57:151-158.
- [CrossRef] [Google Scholar]
- Comparison of in-tube sorptive extraction techniques for non-polar volatile organic compounds by gas chromatography with mass spectrometric detection. J. Chromatogr. A. 2006;1124:181-186.
- [CrossRef] [Google Scholar]
- A flow injection procedure using Layered Double Hydroxide for on line pre-concentration of fluoride. Talanta. 2018;178:102-108.
- [CrossRef] [Google Scholar]
- Separation and determination of benzene, toluene, ethylbenzene and o-xylene compounds in water using directly suspended droplet microextraction coupled with gas chromatography-flame ionization detector. Talanta. 2009;78:936-941.
- [CrossRef] [Google Scholar]
- A novel surface molecularly imprinted polymer as the solid-phase extraction adsorbent for the selective determination of ampicillin sodium in milk and blood samples. J. Pharm. Anal.. 2016;6:157-164.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of organic intercalated layered double hydroxides and their application in bitumen modification. Mater. Chem. Phys.. 2015;152:54-61.
- [CrossRef] [Google Scholar]
- Porous covalent organonitridic frameworks for solid-phase extraction of sulfonamide antibiotics. Microchim. Acta. 2019;186:1-7.
- [CrossRef] [Google Scholar]
- A novel solid-phase microextraction using coated fiber based sol-gel technique using poly(ethylene glycol) grafted multi-walled carbon nanotubes for determination of benzene, toluene, ethylbenzene and o-xylene in water samples with gas chromatography-flam ionization detector. J. Chromatogr. A. 2011;1218:5757-5764.
- [CrossRef] [Google Scholar]
- Layered double hydroxides based ion exchange extraction for high sensitive analysis of non-steroidal anti-inflammatory drugs. J. Chromatogr. A. 2017;1515:23-29.
- [CrossRef] [Google Scholar]







