Translate this page into:
Morphologically controlled synthesis of 1-dimensional selenium dioxide and study of its application as catalyst for diesel fuel additive
⁎Corresponding author. imrandin2007@gmail.com (Muhammad Imran Din)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
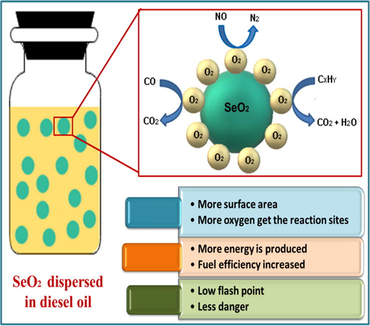
In this research work, selenium dioxide (SeO2) nanorods have been prepared by a solvothermal method in which a strong reducing agent (NaBH4) was used to reduce precursor salt into SeO2 nanorods. X-ray diffraction (XRD) technique was applied to observe the crystal structure which confirmed its tetragonal geometry. Moreover, morphology and particle size were studied by scanning electron microscopy (SEM). SEM fully described the 1-dimensional morphology of SeO2 nanorods which then arranged themselves to create a 3-dimensional flower-like structure with an average particle size of 50 nm. Also, the catalytic activity of SeO2 nanorods as diesel-additive was studied by defining different parameters such as fire and flash points, calorific value, cloud and pour points, specific gravity, and kinematic viscosity. Subsequently, SeO2 nanorods proved to be an excellent diesel additive due to higher total heat content and lower value of kinematic viscosity which enhances the better performance of the diesel engine.
Keywords
SeO2 Nanorods
Nano-additive
Diesel oil
Nanomaterial
Viscosity
Fuel
1 Introduction
Potential applications of transition metal derivatives have attracted the attention of various researchers worldwide. In particular, research on T, S, and Se based transition metal chalcogenides (TMCs) is of great interest. Among these TMCs, Se plays an important role due to its excellent properties in many areas of science (Ping et al., 2017). Selenium exhibits many linear and non-linear properties in various fields with good efficiency such as photocatalytic, electric, and surface properties (Nayak et al., 2021). Although selenium oxide behaves as a good catalyst in oxidation reactions, scientists still need to explore the influence of morphological variations in different applications (Zheng et al., 2020). Moreover, the role of stabilizing, oxidizing, and reducing agents is important in the synthesis of SeO2 because these factors affect the size and morphology of the particles (Chhabria and Desai, 2016). There are many reducing and stabilizing agents available e.g. citric acid and sodium borohydride, the latter being the strongest oxidizing agent (Filinchuk and Hagemann, 2008). Santos et al. (Santos and Sequeira, 2011) previously reported that sodium borohydride behaves as an excellent surfactant for the controlled synthesis of nanorods and nanotubes. SeO2 is of great importance nowadays due to the different chemical and physical properties and morphological variations of Se. Various methods have been used for the synthesis of selenium oxide but the solvothermal method is one of the user-friendly methods for nanoparticles fabrication (Medhi et al., 2020). The solvothermal method has gained huge importance due to desirable morphology and good crystallization which can be attained by controlling the pH, time and temperature conditions (Swathi et al., 2020).
Environmental pollution is generally increased by using fossil based fuels (Rogers and Seager, 2009). Several research studies have been carried out to reduce pollution caused by the incomplete and poor combustion of fuel which leads to the emission of harmful gases such as CO2 and NO (Sarıkoç, 2020). A fuel additive is a chemical compound or composite of various compounds which, when used in low concentrations, leads to improve the engine performance, heat content and fuel economy by optimizing the level of physicochemical properties of fuel through a certain mechanism (Li and Sun, 2012). Diesel additives are of three types according to the stages that they influence: (1) pre-flame additive (2) flame additive and (3) post-flame additive (Gopidesi and Rajaram, 2019; Lenin et al., 2013; Song, 2004). As catalysis is a surface phenomenon, the nanomaterials are considered to be a perfect additive due to their higher surface to volume ratio than conventional bulk powders (Ghanbari et al., 2020).
Nanomaterial oxide additives reportedly influence diesel properties and combustion. Different nanomaterial oxide additives have been used as fuel additives such as cerium oxide (Cassee et al., 2011), graphite oxide (Ooi et al., 2016), MnO2, MgO (Keskin et al., 2011), SnO2 (Khalid et al., 2018), and cobalt oxide (Kannan et al., 2011). The additive concentration limit depends on the type of engine and combustion properties. Most of the time low concentrations are favorable while high concentrations can be used for certain improvements. Mostly, fuel properties can be altered by the type and amount of fuel additives (Kegl et al., 2021; Varatharajan and Pushparani, 2018). Various properties such as fuel atomization and fuel injection are affected by changing the fuel properties such as density, gravity, sulfur content, volatility, fire and flash points (Pandey et al., 2012). Lubrication and atomization are significantly influenced by altering the viscosity of fuel. Moreover, low temperature handling for fuels is desirable in most cases for safety reasons. Therefore, an ideal level for physicochemical properties of fuel can also be achieved for optimization of the combustion process and safe handling of fuels (Gupta et al., 2010; Heuser et al., 2013).
SeO2 nanorods exhibit excellent catalytic activity because the large surface area in turn improves the fuel efficiency. In this present study, SeO2 nanorods were fabricated by a solvothermal method using sodium borohydride as a reducing agent and characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). Physicochemical properties of diesel with the addition of SeO2 nanorods are investigated by applying various parameters in the present work. To the best of our knowledge, this effect of SeO2 nanorods as a nano-fuel additive on diesel fuel performance has not been reported in the literature i.e. the novelty of this research work is that one-dimensional SeO2 nanorods have never been used before as a diesel fuel additive. The results obtained demonstrate that selenium oxide nanorods perfectly catalyze the diesel fuel and prove to be a good additive for diesel oil to enhance the efficiency of an engine.
2 Experimental
2.1 Materials
The following chemicals used in the experimental procedure were purchased from Sigma-Aldrich USA without any further modification: selenium powder (Se), sodium borohydride (NaBH4), distilled water, ethylene glycol (CH2OH)2, ethanol (C2H5OH), urea CO(NH2)2 and polyvinyl pyrrolidone ((C6H9NO)n).
2.2 Synthesis method
Solvothermal method was used to prepare SeO2 nanorods (Fig. 1). Initially, 1.5 g of selenium powder was dissolved in a mixture of distilled water (10 ml) and ethylene glycol (40 ml). This mixture was heated on a hot plate under the action of constant stirring for 30 min at 35 °C, with the addition of 1.5 g NaBH4. After this, the solution was sonicated for 1 hr followed by the addition of urea (4 g) and PVP (2 g). The solution was then transferred to a Teflon-lined stainless steel autoclave for 9 hr at 190 °C. After aging the solution for 2 days, the final product was centrifuged and washed with distilled water and ethanol until pH 7 was maintained. Finally, the precipitates of SeO2 nanorods were obtained after drying the mixture at 70 °C for 6 hr.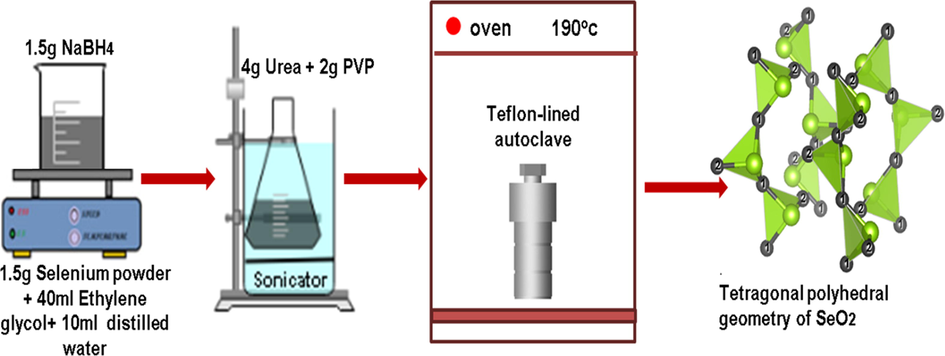
Flow sheet of fabrication of SeO2 nanorods.
2.3 Catalytic application of SeO2 nanorods as nano-fuel additive for diesel
Different concentrations of SeO2 nanorods (0.002, 0.004, 0.006, 0.008 ppm) were used to study the effect of their catalytic activity in diesel oil. Each solution of diesel (20, 40, 60, and 80 ppm) was sonicated for 5 min. before starting the catalytic study. Various parameters (kinematic viscosity, gravity, fire and flash point, calorific value, cloud and pour point) regarding the performance of diesel were studied.
3 Results and discussion
3.1 X-ray diffraction analysis
The structure, purity, and crystallinity of the SeO2 nanorods were studied by the XRD pattern shown in Fig. 2. This characterization is based on Bragg’s law in which the beam of X-rays makes an angle 2-theta when it reflects through the crystal planes and provides information about various properties of nanomaterial. Diffraction peaks were spotted at 2-theta values of 12.71°, 23.43°, 27.51°, 29.59°, 41.27°, 43.55°, 45.31°, 51.71°, 55.97°, 61.05°, 65.03°, 71.43° having hkl planes (1 1 0), (1 2 0), (2 0 1), (2 1 1), (2 0 2), (2 1 2), (3 3 0), (4 2 1), (1 5 0), (4 2 2), (5 3 0), (5 2 2), respectively. The three peaks at 41.27°, 43.55° and 45.31° are for elemental selenium. No water peak in this data indicates that there is no moisture content in the final product of SeO2. The high intensity and broadening of peaks confirm the material’s high crystallinity and size in the range of nanoscale respectively.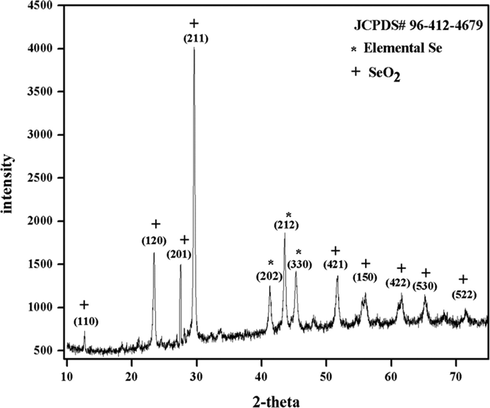
XRD pattern of SeO2 nanorods.
The SeO2 is one dimensional polymeric material forming tetrahedral geometry in solid form confirmed with the JCPDS# 96–412-4679 (Fig. 3a). The selenium atoms are alternately attached along with the polymeric chain. Hence, more unit cells are combined along the X, Y, Z axis to form long chain like polymeric geometry of SeO2 (Fig. 3b). The chain of this polymeric system consists of oxygen and selenium is alternately attached. Each selenium atom is attached to one terminal and two bridging oxide groups forming a pyramidal and the distance between two selenium atoms in the chain was calculated to be 5.1061 nm (Fig. 3c). The length of selenium with terminal oxide group (Se-O2) and bridging oxide group (Se-O1) is 0.175557 nm and 0.178994 nm respectively (Table.1).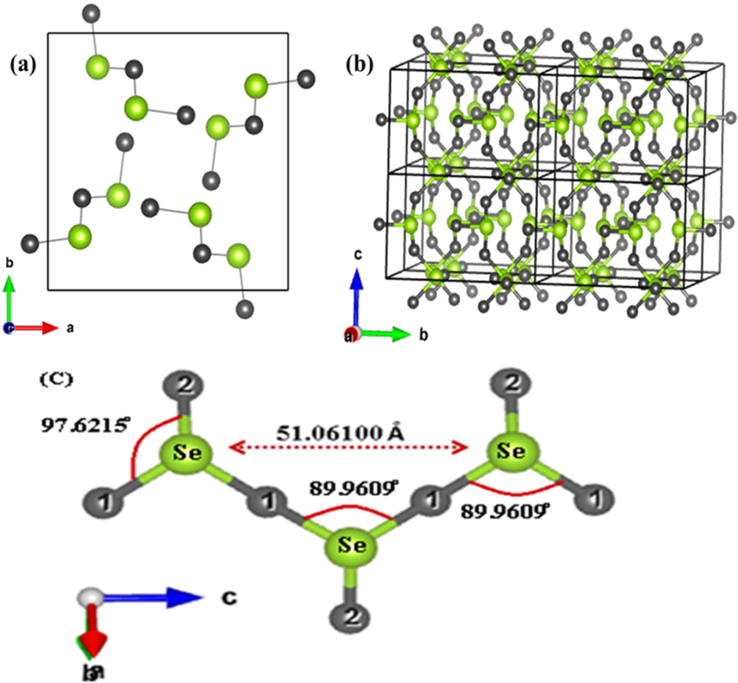
(a) Selenium oxide molecules within one unit cell (b) Basic structure of Selenium oxides molecules within 8 unit cells along X Y Z axis (c) Arrangement of Selenium and oxygen in chain form.
Parameters
Results
Name
Selenium dioxide
Formula
O2Se
Space group
P 42/m b c (1 3 5)
Crystal system
Tetragonal
Calculated density
4.15700 g/cm3
Cell parameter
a = 0.837 nm, b = 0.5061 nm
Number of formulas per unit cell
8
Number of different elements
2
Bond length
0.175557 nm
Se O1
0.178994 nm
Se O2
X Y Z
Atomic coordinates
O1
0.358 0.585 0.250
Se
0.133 0.207 0.000
O2
0.425 0.320 0.000
Bond angle (Fig. 2c)
O1 Se O1
89.9609°
O1 Se O2
97.6215°
Se Se Se
106.3751°
3.2 SEM analysis
The shape, particle size, morphology, crystallinity and porosity of nanoparticles were studied using SEM. The morphology of SeO2 nanorods was observed at two magnifications i.e. 2500 X and 5000 X as shown in Fig. 4a and b.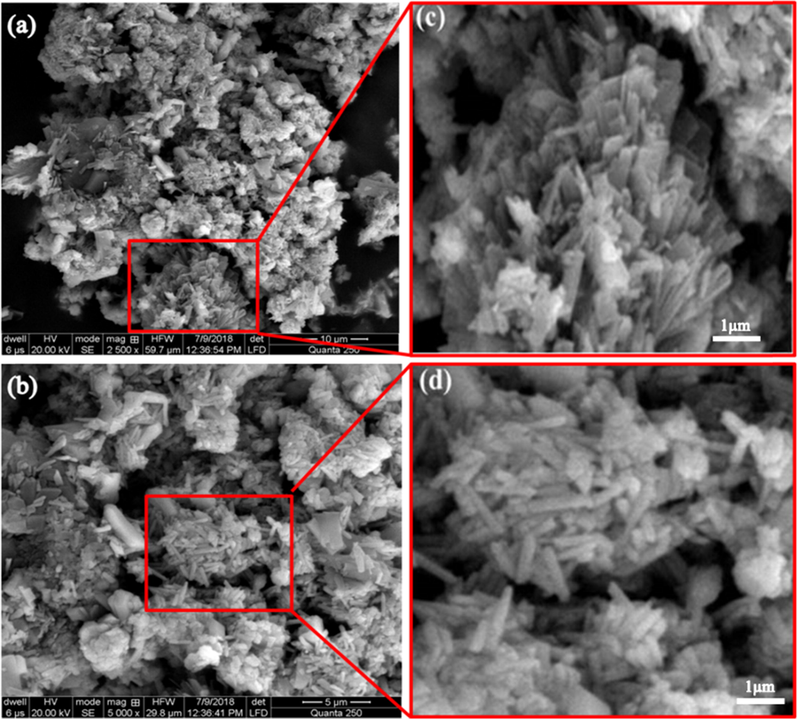
SEM images of SeO2 (a) 2500X (b) 5000X (c) flower orientation of SeO2 nanorods (d) magnified image of nanorods.
SEM images showed that SeO2 arranged themselves into controlled and defined rod like structures but the particles are not fully dispersed. Fig. 4d demonstrates some irregular small particles along with nanorods which may be attributed to the broken parts of rods. The overall aggregation in the final product may be due to the attraction between small particles during the process of nucleation and also the smaller particles attracted towards each other to become stable in the form of staggered bulk particles. Moreover, these 1-dimensional SeO2 nanorods further arrange themselves exhibiting 3-dimensional flower like morphology as illustrated in Fig. 4c. The average particle size of nanorods is calculated to be 50 nm along the minimum (39 nm) and maximum (60 nm). The calculated average nanorods length is 1670 nm with minimum (1258 nm) and maximum of 2004 nm.
3.3 Catalytic activity of SeO2 nanorods as nano-fuel additive
SeO2 nanorods as nano-fuel additive influence the performance of diesel by altering the physical (kinematic viscosity and specific gravity) and combustion properties (fire and flash points, calorific values surface tension, cloud and pour points) of fuel.
3.3.1 Combustion properties
3.3.1.1 Fire and flash points
Fire and flash points of diesel oil were measured by open cup tester method. Fig. 5 represents the decrease in values of fire and flash points with the nano-additive concentration. It was observed that as the concentration of SeO2 nanorods (0, 20, 40, 60, 80 ppm) increased, the fire points (78, 63, 45, 42. 39 °C) and flash point values (87, 85, 53, 49, 47 °C) decreased, respectively. Results depict that the pure diesel oil has higher fire and flash points i.e. 78 °C and 87 °C, respectively and the decrease in fire and flash point with nano-additive concentration was non-linear and unsteady. Furthermore, the graph shows that there was a sudden decrease in fire points (63 and 45 °C) and flash points (85 and 53 °C) for 20 and 40 ppm concentration, respectively but this abrupt decrease was not observed when the concentration was further increased to 60 and 80 ppm. This is due to the reason that at high concentration the nano-additive becomes aggregated and less surface area is exposed to the diesel molecules for reaction as compared to lower concentrations.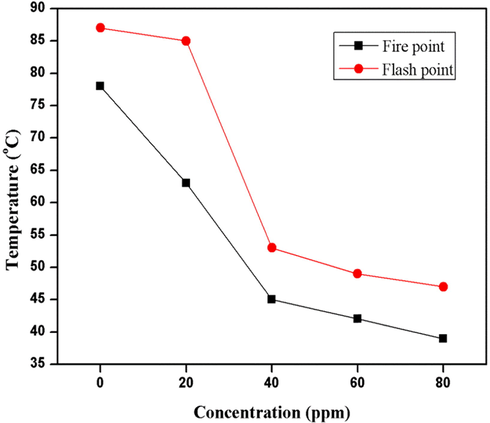
Graphical representation of fire and flash points.
The fire and flash points represent the volatility of diesel oil i.e. higher volatility is associated with lower fire and flash points. SeO2 nanorods adjust themselves within the layers of diesel oil due to their high surface area and small sized rods which leads to an increase in the volatility of fuel. As a result, surface tension of diesel increases which favors small droplet formation. Khan et al. reported that zinc oxide also increases the volatility and decreases the fire and flash points (Khan et al., 2019). Table 2 provides a summary of fuel properties with SeO2 and other different reported nano-additives. 68 64 60 71 68 62 −4.1 −4.5 −4.6 9.3 8.0 6.3 34,223 35,853 36,530 55 58 62 64 68 74 −15 −16 −17 −4 −4 −5 0.952 35.185 42.930 70 60 50 73 65 58 −18 −18 −18 8 4 2 952 2518 42,930 87 85 53 78 63 45 6 5 −4 15 9 8 814 11,004 31,862
Nano-Additives
Parameters at different concentrations
1) 0 ppm, 2) 20 ppm 3) 40 ppmReferences
Flash point(oC)
Fire point(oC)
Pour point
(oC)Cloud point(oC)
Calorific value (J/g)
Zinc oxide (ZnO)
(Khan et al., 2019)
Cobalt oxide (Co3O4)
(Kannan et al., 2011)
Tin oxide (SnO2)
(Khalid et al., 2018)
SeO2
This work
3.3.1.2 Calorific values
Calorific values are associated with the total heat content of fuel upon combustion. The results show that there is a linear escalation in calorific values as 814, 11004, 31862, 35983, 43772 J/g for nano-additive concentrations of 0, 20, 40, 60, 80 ppm, respectively. With an increase in additive concentration, the total heat content is increased whilst the highest calorific values (43772 J/g) are observed with 80 ppm solution of diesel oil (Fig. 6).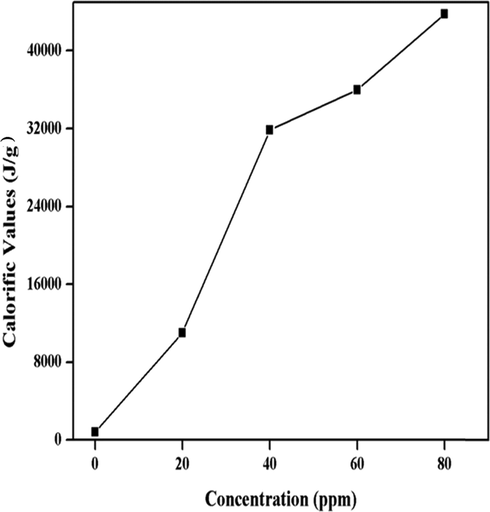
Graphical representation of calorific values.
In the case of SeO2 nano-additive, when added to diesel it acts as an oxygen buffer for instantaneous oxidation of hydrocarbon and causes simultaneous reduction of nitrogen thus heat content is increased accordingly. Moreover, higher surface to volume ratio of SeO2 nano-additive increases the efficiency of the diesel engine leading to decrease in emission of soot. As more surface area becomes available, more oxygen atoms and other groups adsorb onto the surface for oxidation. As a result, SeO2 nano-additive provides more oxygen to the diesel fuel hence the fuel becomes fully oxidized with greater amount of heat produced by complete combustion. Therefore, complete combustion has been achieved because of increased available reaction sites for oxidation of fuel hence, less soot is produced which is mostly caused by incomplete combustion. Subsequently, greater calorific value means ignition of fuel due to exothermic reaction thus reducing the consumption of fuel. The same has been reported by Sajith et al. (Sajith et al., 2010).
3.3.1.3 Cloud and pour points
Cloud point refers to a temperature at which the fuel starts to crystalize and pour point is that temperature at which fluidity of liquid ceases. From the Fig. 7 it can be depicted that cloud point values of the nano-additive are 15, 9, 8, 4, 1 °C and pour point values are 6, 5, −4, −6, −7°C for 0, 20, 40, 60, 70 ppm concentration, respectively. It was seen that pure diesel oil possesses the highest cloud and pour points i.e. 15 and 6 °C, respectively and decreases with increasing nano-additive concentration. After 20 ppm, the cloud point values linearly decreased from 40 to 80 ppm dosage concentrations as compared to pour point values which decreased in random manners.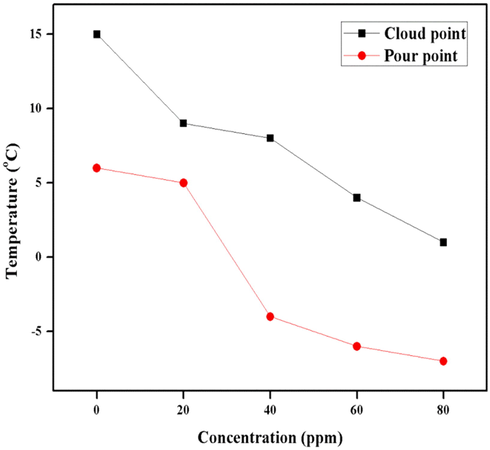
Graphical representation of cloud and pour points.
There are two possible mechanisms behind the decrease of cloud and pour points upon the addition of additive in fuel:- (1) the nano-additive is adsorbed on the surface of paraffin wax and prevents further crystallization by decreasing the freezing temperatures and (2) the nano-additive is incorporated with the crystal and alters the shape of the paraffin crystal therefore limiting the adhesiveness with each other (Danilov, 2015). The basic condition for better performance of nano-fuel additive is its releasing temperature which means that the nano-fuel additive must release at the same time with wax formation from the fuel under the range of its cloud and pour points. Closer releasing temperature of nano-fuel additive and wax crystal favors better performance of the cloud and pour point depressant. Nano-fuel additive does not inhibit wax crystallization but instead prevents the growth of wax crystals. Ultimately, these wax crystals sink towards the bottom and two layers start to form in then diesel containing nano-fuel additive. The lower layer comprises actual wax crystals whereas the upper layer contains almost transparent, smaller sized crystals. This occurs because additive changes the shape and structure of the crystal thus preventing freezing of the fuel. Moreover, it has been postulated that the nano-fuel additive acts as a wax dispersant. The mechanism behind this effect of the nano-fuel additive is that additive molecules impart an electrostatic layer of like charges on the wax particles to ensure repulsion among wax molecules which in turn prevents crystallization of the wax into bulk crystals and precipitation. Subsequently, this increases the conductivity of hydrocarbons and favors the flow of diesel oil by dispersing the wax molecules (Grishin, 2017).
Since it is necessary to operate transport in differing environmental and climatic conditions, there is a need to have a compatible fuel additive to facilitate the better performance of diesel oil especially during cold temperature conditions. Hence, SeO2 is considered to be the best nano-additive for lowering cold temperature conditions by efficiently catalyzing the diesel fuel. This allows the diesel to perform stably during extreme winter conditions (Gureev et al., 1993).
3.3.1.4 Surface tension
The effect of SeO2 nano-additive on the surface tension of diesel is shown in Fig. 8. The surface tension is a combustion property and its value increases (23.62, 28.52, 31.62, 33.97 and 38.73 mN/m) on increasing the addition of SeO2 nano-additive (0, 20, 40, 60, 80 ppm) in diesel oil. Additionally, surface tension affects the fuel atomization and spray characteristics. The amount of nano-additive is larger at liquid gas surface as compared to the droplet inside due to the aggregation of nano-additives on gas liquid interface. Among particles, some electrostatic and Van der Waal’s forces are present that change the surface free energy at liquid gas interface. The surface free energy lead to enhancing the surface tension value. Higher surface tension results in efficient combustion.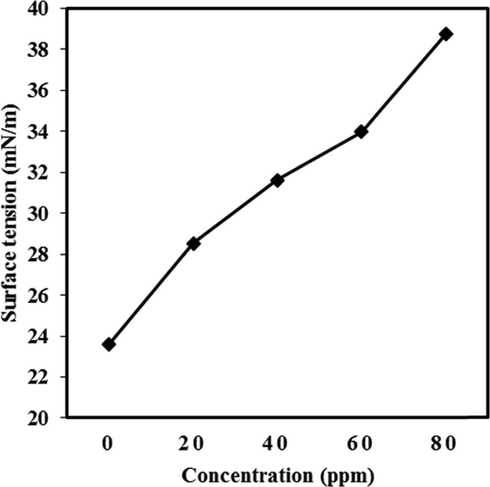
Graphical representation of Surface tension.
3.3.2 Physical properties
3.3.2.1 Kinematic viscosity and specific gravity
The effect of SeO2 nano-additive on the kinematic viscosity and specific gravity is shown in Fig. 9. The kinematic viscosity values (2.39, 2.37, 2.25, 2.03 and 1.97) × 10−6 m−2s−1 decrease and specific gravity values (0.8854, 0.8979, 0.9102, 0.9374, 0.9426 gcm−2) increase upon nano-additive (0, 20, 40, 60, 80 ppm) addition in diesel oil. Results depict that the lowest value of specific gravity (0.8854 gcm−2) and highest value for kinematic viscosity (2.39 × 10−6 m−2s−1) are encountered by pure diesel oil but when the concentration of nano-additive is increased to 80 ppm, the values of specific gravity and kinematic viscosity are found to be 0.9436 gcm−2 and 1.97 × 10−6 m−2s−1, respectively.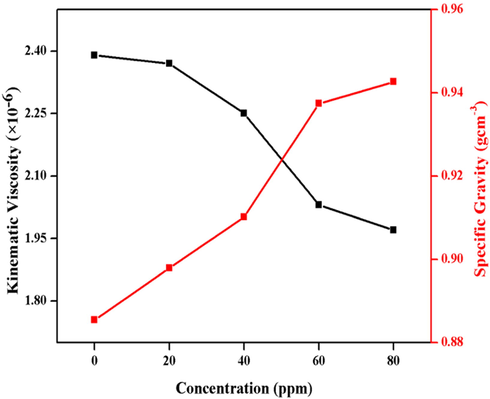
Graphical representation of kinematic viscosity and specific gravity.
Viscosity accounts for perfect lubrication of the engine. Higher viscosity hinders the flow of diesel as the nanoparticles incorporate themselves within the layer of diesel and act as dispersants by preventing them to attach. This makes the diesel oil more compact without being extremely viscous. Low viscosities favor enhancement of engine efficiency by reducing the turbulence flow of diesel with less lubrication as compared to pure diesel. Higher lubrication comes with poor and incomplete combustion. These two contradictions can be overcome by using a certain compromised amount of diesel-additive. On the other hand, higher gravity increases the van der Waals forces because the nanoparticles cover the interatomic distance between layers of fuel so energy content increases. The same trend, using zinc oxide. was reported by Khan et al. (Khan et al., 2019).
4 Conclusion
SeO2 nanorods were prepared by solvothermal method at 190 °C using NaBH4 as a reducing agent. SeO2 was characterized by XRD and SEM. The XRD identified that SeO2 is arranged in a polymeric chain with tetragonal geometry and SEM confirmed its rod like morphology which is arranged in a flower-like structure with average particle size of 50 nm. This research work mainly focused on the catalytic application of SeO2 as a nano-additive for diesel oil. Various combustion and physical parameters were studied to explore its catalytic activity. Combustion properties of diesel oil were improved by using SeO2 as a nano-additive. As calorific values were increased to 43,772 J/g, fire and flash points decreased to 39 °C and 47 °C, respectively when concentration of additive was increased to 80 ppm. This indicates the increased heat content and volatility of diesel. Moreover, physical properties such as kinematic viscosity and specific gravity were also found to enhance fuel efficiency.
5 Authors agreement
With this submission, I would like to confirm and take responsibility that this work is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere. Furthermore, this publication is approved by all the concerned co-authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit. Rev. Toxicol.. 2011;41:213-229.
- [Google Scholar]
- Selenium nanoparticles and their applications. Encycl. Nanosci. Nanotechnol.. 2016;20:1-32.
- [Google Scholar]
- Structure and properties of NaBH4· 2H2O and NaBH4. Eur. J. Inorg. Chem.. 2008;112:3127-3133.
- [Google Scholar]
- Experimental studying the effect of nano particles additives in diesel-biodiesel blends on the emission characteristics of a CI engine. Automot. Sci. Eng.. 2020;10:3408-3420.
- [Google Scholar]
- A review on emulsified fuels and their application in diesel engine. Int. J. Ambient. Energy. 2019:1-9.
- [Google Scholar]
- Depressant, antiwear, and antioxidant additives to hydrotreated diesel fuels with low and ultralow sulfur content. Pet. Chem.. 2017;57:813-825.
- [Google Scholar]
- Bio-fuels for the gas turbine: A review. Renew. Sust. Energ. Rev.. 2010;14:2946-2955.
- [Google Scholar]
- Gureev, A., Azev, V., Kamfer, G., 1993. Diesel fuel. Properties and application. Khimiya Moscow.
- Optimization of diesel combustion and emissions with tailor-made fuels from biomass. SAE Int. J. Fuels Lubr.. 2013;6:922-934.
- [Google Scholar]
- Effect of metal based additive on performance emission and combustion characteristics of diesel engine fuelled with biodiesel. Appl. Energy. 2011;88:3694-3703.
- [Google Scholar]
- Nanomaterials as fuel additives in diesel engines: A review of current state, opportunities, and challenges. Prog. Energy Combust. Sci.. 2021;83:100897
- [Google Scholar]
- Influence of metallic based fuel additives on performance and exhaust emissions of diesel engine. Energy Convers. Manag.. 2011;52:60-65.
- [Google Scholar]
- Morphologically controlled synthesis of cubes like tin oxide nanoparticles and study of its application as photocatalyst for Congo red degradation and as fuel additive. J. Inorg. Organomet. Polym. Mater.. 2018;28:168-176.
- [Google Scholar]
- Layer by layer assembly of zinc oxide nanotubes and nanoflowers as catalyst for separate and simultaneous catalytic degradation of dyes and fuel additive. Chem. Select.. 2019;4:5548-5559.
- [Google Scholar]
- Performance and emission characteristics of a DI diesel engine with a nanofuel additive. Fuel. 2013;109:362-365.
- [Google Scholar]
- Li, T.L., Sun, Z.Y. (2012). Which alternative fuel is more suitable for vehicles in China: hydrogen gas or fossil-based fuels? In “Advanced Materials Research”, Vol. 512, pp. 1450-1455. Trans Tech Publ.
- Visible-light-active doped metal oxide nanoparticles: review of their synthesis, properties, and applications. ACS Appl. Nano Mater.. 2020;3:6156-6185.
- [Google Scholar]
- Potentialities of selenium nanoparticles in biomedical science. New J. Chem.. 2021;45:2849-2878.
- [Google Scholar]
- Graphite oxide nanoparticle as a diesel fuel additive for cleaner emissions and lower fuel consumption. Energy Fuels.. 2016;30:1341-1353.
- [Google Scholar]
- Impact of alternative fuel properties on fuel spray behavior and atomization. Renew. Sust. Energ. Rev.. 2012;16:1762-1778.
- [Google Scholar]
- Recent advances in sensing applications of two-dimensional transition metal dichalcogenide nanosheets and their composites. Adv. Funct. Mater.. 2017;27:1605817.
- [Google Scholar]
- Environmental decision-making using life cycle impact assessment and stochastic multiattribute decision analysis: a case study on alternative transportation fuels. ACS Publications 2009
- [Google Scholar]
- Experimental investigations on the effects of cerium oxide nanoparticle fuel additives on biodiesel. Adv. Mech. Eng.. 2010;2:581407
- [Google Scholar]
- Sodium borohydride as a fuel for the future. Renew. Sust. Energ. Rev.. 2011;15:3980-4001.
- [Google Scholar]
- Fuels of the Diesel-Gasoline Engines and Their Properties. Diesel Gasoline Engines. 2020;31
- [Google Scholar]
- Song, K.H. (2004). “Effects of oxygenated additives on soot and PAH in a diesel engine and premixed flame,” The Pennsylvania State University.
- Designing rational and cheapest SeO2 electrocatalyst for long stable water splitting process. J. Phys. Chem. Solids.. 2020;145:109544
- [Google Scholar]
- Screening of antioxidant additives for biodiesel fuels. Renew. Sust. Energ. Rev.. 2018;82:2017-2028.
- [Google Scholar]
- Excess Se-doped MoSe2 and nitrogen-doped reduced graphene oxide composite as electrocatalyst for hydrogen evolution and oxygen reduction reaction. J. Alloys Compd.. 2020;848:156588
- [Google Scholar]







