Translate this page into:
Multicomponent reactions for synthesis of bioactive polyheterocyclic ring systems under controlled microwave irradiation
1st Heterocyclic Update
*Corresponding author. Tel.: +20 235676608 thoraya-f@hotmail.com (Thoraya A. Farghaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 25 November 2013

Peer review under responsibility of King Saud University.
Abstract
The multi-component reaction of 1-benzothiopyran-4-ones with heterocyclic amines and dimethylformamide-dimethylacetal (DMFDMA) in DMF at 150 °C under controlled microwave heating afforded novel poly-heterocyclic ring systems. Also, reaction of 3-dimethylaminomethylene-1-benzothiopyran-4-one with activemethylene derivatives was investigated. The structure of all products was established on the bases of spectral data and elemental analyses and alternative synthesis if possible. The prepared compounds were screened for their antitumor activity against HCT-116 “colon” cancer cell line and some derivatives showed promising activity.
Keywords
Multi-component reactions (MCR)
Microwave irradiation
Enaminone
1-Benzothiopyran-4-one
Antitumor activity
1 Introduction
Multi-component reactions (MCR), an important class of organic tandem reactions, are one-pot processes with at least three components to form a single product, which incorporates most or even all of the starting materials (Hulme and Gore, 2003; Orru and De Greef, 2003; Domling and Ugi, 2000; Nair et al., 2003; Zhu, 2003; Domling, 2002). The huge interest for such multi-component reactions during the last years has been oriented toward developing combinatorial chemistry procedures, because of their high efficiency and convenience of these reactions in comparison with multistage procedures. Hence, most of the scientific efforts have been focused on the development of multi-component procedures to prepare diverse heterocyclic compound libraries (Orru and De Greef, 2003). Also, the utility of MCR under microwave irradiation in the synthesis of heterocyclic compounds enhanced the reaction rates and improved the regioselectivity (Andrade et al., 2008; Lidstrom et al., 2001; Bortolini et al., 2008; El Ashry and Kassem, 2006; Sadek et al., 2012). 1-Benzothiopyran-4-ones, also known as thiochromen-4-one, are an important class of heterocycles. They serve as key intermediate for the synthesis of bioactive heterocyclic ring systems (Dawood et al., 2001; Al-Nakib et al., 1990, 1991; Ares et al., 1996; Wang et al., 1996). Recently, we have concentrated much of our recent work in the preparation of bioactive nitrogen-containing heterocycles (Farghaly et al., 2012; Abdel Hafez et al., 2010; Farghaly and Abdalla, 2009; Riyadh and Farghaly, 2012; Farghaly and Hassaneen, 2013; Farghaly and Mahmoud, 2013; Gomha et al., 2013; Gomha and Abdel-Aziz, 2013; Gomha and Khalil, 2012), and have already described simple and efficient procedures to prepare interesting molecules with biological properties. We describe here the preparation, through multi-component reactions under microwave heating, of new poly-heterocyclic ring systems incorporating of benzothiopyrane moiety that have not been reported hitherto in addition to the study of the effects of the newly synthesized compounds on the growth of human colon cancer cell line (HCT-116).
2 Experimental
2.1 Chemistry
Melting points were measured on an Electrothermal IA 9000 series digital melting point apparatus. IR spectra were recorded in potassium bromide disks on Pye Unicam SP 3300 and Shimadzu FTIR 8101 PC infrared spectrophotometers. NMR spectra were recorded on a Varian Mercury VX-300 NMR spectrometer operating at 300 or 400 MHz for 1H-NMR and run in deuterated dimethylsulfoxide (DMSO-d6). Chemical shifts were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer at 70 eV. Elemental analyses were measured by using a German made Elementar vario LIII CHNS analyzer. 3-Dimethylaminomethylene-1-benzothipyran-4-one 9 was prepared as previously reported in the respective literature (Bruno et al., 1999).
2.1.1 Reaction of 1-benzothiopyran-4-one (1) with heterocyclic amines 2, 10–14 and DMF-DMA
Method A: A solution of appropriate heterocyclic amine derivatives 2, 10–14 (1 mmol), 1-benzothiopyran-4-one (1) (1 mmol) and dimethylformamide dimethylacetal (DMF-DMA) (1 mmol) in dry dimethylformamide (10 mL) was heated under reflux in a Milestone Microwave Labstation at 150 °C for 10–15 min (TLC). After concentration and cooling to room temperature, the resulting solid product so formed was collected by filtration, washed well with EtOH, dried and recrystallized from EtOH.
Method B: To a solution of 9 (1.095 g, 0.005 mol) in acetic acid (20 ml) was added (0.005 mol) of the appropriate heterocyclic amines (2, 10–14 or 20). The mixture was heated under reflux in a Milestone Microwave Labstation at 150 °C for 5 min. After concentration and cooling to room temperature, the precipitated product was collected by filtration, washed well with EtOH, dried and recrystallized from EtOH to give compounds 5, 15–19 or 21, respectively. The products 5, 15–19 and 21 together with their physical constants are listed below.
2.1.1.1 6H-Thiochromeno[3,4-e][1,2,4]triazolo[1,5-a]pyrimidine (5)
Yellow solid, (82% yield); mp 185–187 °C; IR (KBr) υ 3057, 2970, 1595, 1496, 1263, 1234 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.20 (s, 2H, CH2), 7.45–7.64 (m, 4H, Ar-H), 8.75 (s, 1H, pyrimidine-H), 8.96 (s, 1H, triazole-H); MS, m/z (%) 240 (M+, 100), 239 (83), 200 (63), 69 (58); Anal. calcd for C12H8N4S (240.28): C, 59.98; H, 3.36; N, 23.32; found: C, 59.84; H, 3.23; N, 23.15%.
2.1.1.2 10-Phenyl-6H-thiochromeno[3,4-e]pyrazolo[1,5-a]pyrimidine (15)
Yellow solid, (83% yield); mp 168–170 °C; IR (KBr) υ 3047, 2901, 1593, 1499, 1463, 1234 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.12 (s, 2H, CH2), 7.35 (s, 1H, pyrazole-H), 7.41–8.10 (m, 9H, Ar-H), 8.62 (s, 1H, pyrimidine-H); MS, m/z (%) 316 (M++1, 24), 315 (M+, 100), 314 (89), 211 (20), 185 (17), 145 (14), 140 (17), 102 (12), 77 (66), 76 (27), 75 (13); Anal. calcd for C19H13N3S (315.39): C, 72.36; H, 4.15; N, 13.32; found: C, 72.19; H, 4.01; N, 13.09%.
2.1.1.3 10-Cyano-6H-thiochromeno[3,4-e]pyrazolo[1,5-a]pyrimidine (16)
Yellow solid, (76% yield); mp 218–220 °C; IR (KBr) υ 3052, 2974, 2220 (CN), 1602, 1514, 1446, 1372, 1239 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.17 (s, 2H, CH2), 7.43–7.65 (m, 4H, Ar-H), 8.85 (s, 1H, pyrimidine-H), 8.91 (s, 1H, pyrazole-H); MS, m/z (%) 265 (M++1, 18), 2264 (M+, 92), 263 (100), 262 (12), 236 (11), 171 (7), 140 (9), 105 (11), 92 (6), 69 (15); Anal. calcd for C14H8N4S (264.31): C, 63.62; H, 3.05; N, 21.20; found: C, 63.48; H, 2.98; N, 21.07%.
2.1.1.4 6H-thiochromeno[3,4-e]tetrazolo[1,5-a]pyrimidine (17)
Yellow solid, (80% yield); mp 138–140 °C; IR (KBr) υ 3052, 2968, 1594, 1498, 1261, 1230 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.31 (s, 2H, CH2), 7.39–8.49 (m, 4H, Ar-H), 8.71 (s, 1H, pyrimidine-H); MS, m/z (%) 242 (M++1, 21), 241 (M+, 100), 240 (55), 212 (33),186 (87), 159 (39), 103 (33), 102 (25), 84 (63), 76 (25), 75 (28); Anal. calcd for C11H7N5S (241.27): C, 54.76; H, 2.92; N, 29.03; found: C, 54.58; H, 2.76; N, 28.94%.
2.1.1.5 6H-thiochromeno[3,4-e]benzimidazo[1,2-a]pyrimidine (18)
Orange crystals, (88% yield); mp 255–256 °C; IR (KBr) υ 3051, 2968, 1450, 1299 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.06 (s, 2H, CH2), 7.18–8.15 (m, 8H, Ar-H), 8.85 (s, 1H, pyrimidine-H); MS, m/z (%) 290 (M++1, 36), 289 (M+, 100), 288 (92), 287 (61), 145 (30), 144 (35), 102 (22), 77 (22), 76 (20), 75 (22); Anal. calcd for C17H11N3S (289.36): C, 70.56; H, 3.83; N, 14.52; found: C, 70.38; H, 3.64; N, 14.37%.
2.1.1.6 6H-9,11-dimethyl-thiochromeno[3,4-e]pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (19)
Yellow solid, (82% yield); mp 227–229 °C; IR (KBr) υ 3072, 2965, 1624, 1577, 1503, 1406, 1289, 1225 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 1.91 (s, 3H, CH3), 2.64 (s, 3H, CH3), 4.27 (s, 2H, CH2), 7.0–7.36 (m, 4H, Ar-H), 7.58 (s, 1H, pyridine-H), 8.91 (s, 1H, pyrimidine-H); MS, m/z (%) 319 (M++1, 30), 318 (M+, 100), 317 (62), 316 (32), 289 (8), 158 (16), 140 (6), 115 (11), 75 (7); Anal. calcd for C18H14N4S (318.40): C, 67.90; H, 4.43; N, 17.60; found: C, 67.78; H, 4.39; N, 17.45%.
2.1.1.7 1,2,3,4,7-Pentahydro-3-thioxo-thiochromeno[4′,3′:4,5]pyrido[2,3-d]pyrimidine-1-one (21)
Yellow solid, (88% yield); mp 352–354 °C; IR (KBr) υ 3423 (br, 2NH), 3058, 2978, 1714 (C = O), 1643, 1579, 1430, 1259 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.19 (s, 2H, CH2), 7.36–7.46 (m, 4H, Ar-H), 8.31 (s, 1H, pyrimidine-H), 12.0 (s, 1H, NH), 12.60 (s, 1H, NH),; MS, m/z (%) 300 (M++1, 24), 299 (M+, 100), 298 (95), 297 (26), 239 (21), 211 (28), 210 (15), 185 (12), 140 (16), 120 (12), 106 (16), 105 (12), 92 (12), 76 (10); Anal. calcd for C14H9N3OS2 (299.37): C, 56.17; H, 3.03; N, 14.04; found: C, 56.39; H, 3.15; N, 14.24%.
2.1.2 Reaction of enaminone (9) with activemethylene
To a solution of 9 (1.10 g, 0.005 mol) in acetic acid (20 ml) was added activemethylene derivatives (24–27) (0.005 mol) in the presence of ammonium acetate anhydrous (0.5 g). The mixture was heated under reflux in a Milestone Microwave Labstation at 150 °C for 2 min. After concentration and cooling to room temperature, the precipitated product was collected by filtration, washed well with EtOH, dried and recrystallized from EtOH to give compounds (28–31). The products (28–31) together with their physical constants are listed below.
2.1.2.1 3-Cyano-1H-thiochromeno[4,3-b]pyridin-2(5H)-one (28a)
Yellow solid, (88% yield); mp 140–142 °C; IR (KBr) υ 3353 (NH), 3055, 2891, 2211 (CN), 1639 (C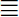 O), 1584, 1429, 1406, 1358, 1258 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.09 (s, 2H, CH2), 6.88–7.60 (m, 4H, Ar-H), 7.87 (s, 1H, pyridine-H), 12.40 (s, 1H, NH); MS, m/z (%) 240 (M+, 53), 239 (53), 238 (60), 151 (40), 149 (87), 105 (33), 104 (60), 83 (53), 77 (27), 71 (100); Anal. calcd for C13H8N2OS (240.28): C, 64.98; H, 3.36; N, 11.66; found: C, 64.73; H, 3.18; N, 11.43%.
O), 1584, 1429, 1406, 1358, 1258 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.09 (s, 2H, CH2), 6.88–7.60 (m, 4H, Ar-H), 7.87 (s, 1H, pyridine-H), 12.40 (s, 1H, NH); MS, m/z (%) 240 (M+, 53), 239 (53), 238 (60), 151 (40), 149 (87), 105 (33), 104 (60), 83 (53), 77 (27), 71 (100); Anal. calcd for C13H8N2OS (240.28): C, 64.98; H, 3.36; N, 11.66; found: C, 64.73; H, 3.18; N, 11.43%.
2.1.2.2 3-Benzoyl-1H-thiochromeno[4,3-b]pyridin-2(5H)-one (28b)
Yellow solid, (84% yield); mp 153–155 °C; IR (KBr) υ 3428 (NH), 3053, 2892, 1642 (C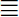 O), 1582, 1437, 1359, 1325, 1256 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.18 (s, 2H, CH2), 7.22–7.84 (m, 9H, Ar-H), 7.93 (s, 1H, pyridine-H), 9.48 (s, 1H, NH); MS, m/z (%) 320 (M++1, 14), 319 (M+, 31), 318 (24), 317 (45), 285 (52), 164 (35), 147 (59), 104 (48), 85 (41), 77 (51), 76 (45), 73 (100); Anal. calcd for C19H13NO2S (319.38): C, 71.45; H, 4.10; N, 4.39; found: C, 71.28; H, 4.01; N, 4.18%.
O), 1582, 1437, 1359, 1325, 1256 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.18 (s, 2H, CH2), 7.22–7.84 (m, 9H, Ar-H), 7.93 (s, 1H, pyridine-H), 9.48 (s, 1H, NH); MS, m/z (%) 320 (M++1, 14), 319 (M+, 31), 318 (24), 317 (45), 285 (52), 164 (35), 147 (59), 104 (48), 85 (41), 77 (51), 76 (45), 73 (100); Anal. calcd for C19H13NO2S (319.38): C, 71.45; H, 4.10; N, 4.39; found: C, 71.28; H, 4.01; N, 4.18%.
2.1.2.3 Ethyl 2-methyl-5H-thiochromeno[4,3-b]pyridine-3-carboxylate (29)
Yellow solid, (82% yield); mp 120–122 °C; IR (KBr) υ 3056, 2893, 1732 (C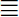 O), 1584, 1429, 1406, 1359, 1258 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 1.35 (t, J = 7 Hz, 3H, CH3), 2.78 (s, 3H, CH3), 4.14 (s, 2H, CH2), 4.36 (q, J = 7 Hz, 2H, CH2), 7.32–7.43 (m, 9H, Ar-H), 8.17 (s, 1H, pyridine-H); MS, m/z (%) 286 (M++1, 19), 285 (M+, 57), 284 (55), 256 (38), 149 (60), 104 (15), 84 (30), 77 (38), 57 (100); Anal. calcd for C16H15NO2S (285.36): C, 67.34; H, 5.30; N, 4.91; found: C, 67.17; H, 5.21; N, 4.74%.
O), 1584, 1429, 1406, 1359, 1258 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 1.35 (t, J = 7 Hz, 3H, CH3), 2.78 (s, 3H, CH3), 4.14 (s, 2H, CH2), 4.36 (q, J = 7 Hz, 2H, CH2), 7.32–7.43 (m, 9H, Ar-H), 8.17 (s, 1H, pyridine-H); MS, m/z (%) 286 (M++1, 19), 285 (M+, 57), 284 (55), 256 (38), 149 (60), 104 (15), 84 (30), 77 (38), 57 (100); Anal. calcd for C16H15NO2S (285.36): C, 67.34; H, 5.30; N, 4.91; found: C, 67.17; H, 5.21; N, 4.74%.
2.1.2.4 Indeno[2,1-e]thiochromeno[4,3-b]pyridin-8(6H)-one (30)
Yellow solid, (82% yield); mp 152–154 °C; IR (KBr) υ 3059, 2972, 1707 (C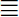 O), 1582, 1440, 1315, 1262 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 3.92 (s, 2H, CH2), 7.23–8.05 (m, 8H, Ar-H), 8.83 (s, 1H, pyridine-H); MS, m/z (%) 301 (M+, 44), 300 (25), 283 (31), 282 (44), 255 (56), 190 (56), 162 (50), 135 (69), 105 (69), 85 (56), 77 (69), 55 (100); Anal. calcd for C19H11NOS (301.36): C, 75.72; H, 3.68; N, 4.65; found: C, 75.54; H, 3.56; N, 4.42%.
O), 1582, 1440, 1315, 1262 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 3.92 (s, 2H, CH2), 7.23–8.05 (m, 8H, Ar-H), 8.83 (s, 1H, pyridine-H); MS, m/z (%) 301 (M+, 44), 300 (25), 283 (31), 282 (44), 255 (56), 190 (56), 162 (50), 135 (69), 105 (69), 85 (56), 77 (69), 55 (100); Anal. calcd for C19H11NOS (301.36): C, 75.72; H, 3.68; N, 4.65; found: C, 75.54; H, 3.56; N, 4.42%.
2.1.2.5 Chromeno[3,4-e]thiochromeno[4,3-b]pyridin-6(8H)-one (31)
Yellow solid, (86% yield); mp 144–146 °C; IR (KBr) υ 3052, 2971, 1727 (C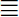 O), 1595, 1490, 1412, 1328, 1264 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.32 (s, 2H, CH2), 7.30–7.72 (m, 8H, Ar-H), 8.57 (s, 1H, pyridine-H); MS, m/z (%) 318 (M++1, 22), 317 (M+, 80), 316 (100), 315 (49), 136 (21), 75 (11); Anal. calcd for C19H11NO2S (317.36): C, 71.91; H, 3.49; N, 4.41; found: C, 71.75; H, 3.22; N, 4.29%.
O), 1595, 1490, 1412, 1328, 1264 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 4.32 (s, 2H, CH2), 7.30–7.72 (m, 8H, Ar-H), 8.57 (s, 1H, pyridine-H); MS, m/z (%) 318 (M++1, 22), 317 (M+, 80), 316 (100), 315 (49), 136 (21), 75 (11); Anal. calcd for C19H11NO2S (317.36): C, 71.91; H, 3.49; N, 4.41; found: C, 71.75; H, 3.22; N, 4.29%.
2.1.3 Synthesis of compounds 32a,b
To a solution of the enaminone 9 (0.219 g, 1 mmol) in EtOH (10 mL) was added hydrazine hydrate (1 mL) or phenylhydrazine (1 mL) and the mixture was heated under reflux in a Milestone Microwave Labstation at 150 °C for 2 min. The reaction mixture was acidified by HCl/ice mixture and the formed product was filtered and crystallized from EtOH to give the respective 2,4-dihydrothiochromeno[4,3-c]pyrazole (32a) [mp 168–169 °C, lit. mp 168–170 °C] (Ramalingam et al., 1977) and 2-phenyl-2,4-dihydrothiochromeno[4,3-c]pyrazole (32b) [mp 169–171 °C, lit. mp 169–171 °C] (Ramalingam et al., 1977) in excellent yields.
2.1.4 Synthesis of 4H-thiochromeno[4,3-c]isoxazole (33)
Hydroxylamine hydrochloride (0.189 g, 1 mmol) was added to a mixture of enaminone (3) (0.219 g, 1 mmol) and sodium ethoxide solution [prepared from sodium metal (0.023 g, 1 mmole) in ethanol (10 mL)]. The mixture was heated under reflux in a Milestone Microwave Labstation at 150 °C for 3 min. After cooling the solution to room temperature, it was poured onto water. The solid product was filtered and crystallized from ethanol to give isoxazole 33 [mp 71–73 °C, lit. mp 71–73 °C] (Ramalingam et al., 1977).
2.1.5 Evaluation of the antitumor activity
The antitumor activity was evaluated on carcinoma cell lines, namely HCT-116 cell. The cell line was grown as monolayers in growth medium supplemented with 10% inactivated fetal calf serum and 50 μg/ml gentamycin. The monolayers of 10,000 cells adhered at the bottom of the wells in a 96-well microtiter plate (Falcon, NJ, USA) incubated for 24 h at 37 °C in a humidified incubator with 5% CO2. The monolayers were then washed with sterile phosphate buffered saline (0.01 M pH 7.2) and simultaneously the cells were treated with 100 μl from different dilutions of tested compound in a fresh maintenance medium and incubated at 37 °C. A control of untreated cells was made in the absence of the tested compound. Three wells were used for each concentration of the test sample. Every 24 h the observation under the inverted microscope was made. The number of the surviving cells was determined by staining the cells with crystal violet followed by cell lysing using 33% glacial acetic acid and read the absorbance at 590 nm using ELISA reader after well mixing. The absorbance values from untreated cells were considered as 100% proliferation and the percentage of viability was calculated as [1−(ODt/ODc)] × 100% where ODt is the mean optical density of wells treated with the tested compounds and ODc is the mean optical density of untreated cells.
3 Results and discussion
3.1 Chemistry
Multi-component reaction of benzothiopyrane 1,3-aminotriazole 2 and dimethylformamide-dimethylacetal (DMF-DMA) in DMF under microwave irradiation at 150 °C for 10 min. afforded compound 5 rather than its isomeric structure 8 (Scheme 1). The conformation of compound 5 was established on the bases of spectral data (Ms, IR, 1H NMR) and elemental analyses. The mass spectrum of the reaction product 5 showed a molecular ion peak m/z = 240 (100%). Its 1H NMR spectrum revealed a singlet signal at δ 8.75 ppm corresponding to one proton at C7 (Farghaly et al., 2010) which was consistent with the isomeric structure 5, while chemical shift of the proton at C5 of the other isomer 8 is downfield at δ 9.09 (Al-Qalaf et al., 2009). Furthermore, alternative synthesis of compound 5 was achieved via condensation of benzothiopyrane 1 with dimethylformamide dimethylacetal (DMFDMF) to give compound 9 (Bruno et al., 1999), and treatment of the product 9 with 3-aminotriazole 2 under the same reaction condition to yield authentic product 5 (Scheme 1). In addition, literature reports explained that, enaminone of the active methylene compound was formed firstly then nucleophilic attack of the amino group of the heterocyclic amine on the double bond of the enaminone with concurrent elimination of the dimethylamine to form intermediate 4 rather than condensation of water molecule in intermediate 6 (Al-Saleh et al., 2005; Dawood, 2005). Also, this mechanism was established by X-ray crystallographic analysis in another report (Farghaly et al., 2010). On the basis of these findings, structure 8 was discarded and the isolated product from the studied reaction was assigned structure 5 (Scheme 1).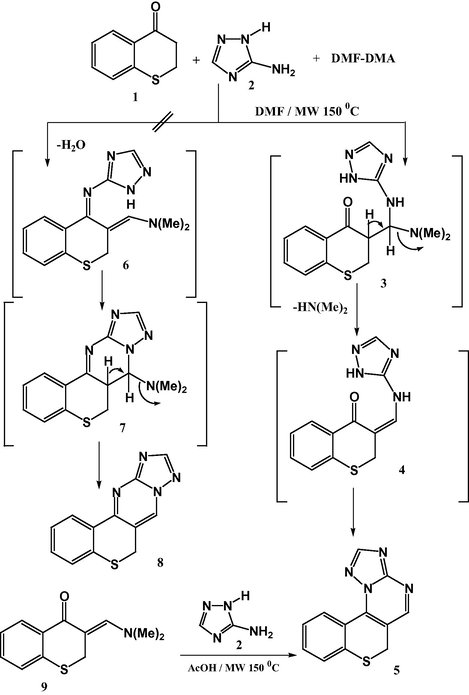
MCR of benzothiopyranone 1, aminotriazole 2 and DMF-DMA
With this result in hand, we went on to study the scope of such multi-component reactions with several heterocyclic amine derivatives 10–14 and 1-benzothiopyran-4-one 1 and dimethylformamide-dimethylacetal (DMF-DMA) under the same reaction conditions, afforded in each case the corresponding tetra-heterocyclic ring systems 15–17 and penta-heterocyclic ring systems 18 and 19, respectively (Scheme 2). The structure of compounds 15–19 was established on the bases of spectral data (Ms, IR, 1H NMR) and elemental analyses (see Experimental part).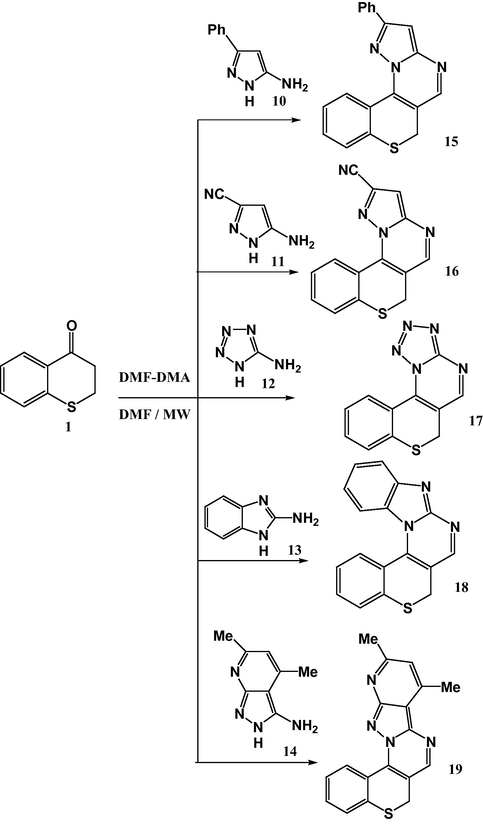
MCR of benzothiopyranone 1, heterocyclic amines 10-14 and DMF-DMA
By the same way reaction of 6-amino-2-thioxopyrimidin-4(3H)-one (20) with 1-benzothiopyran-4-one 1 and dimethylformamide-dimethylacetal (DMF-DMA) in DMF under microwave irradiation at 150 °C for 15 min. gave compound 21 or its isomeric structure 22. The 1H NMR spectrum of the product revealed a singlet signal at 8.31 ppm assigned for pyridine-2H proton not pyridine-4H proton (Sadek et al., 2012; Farghaly et al., 2010; Quiroga et al., 2002) which was consistent with the isomeric structure 21 rather than the isomeric structure 22. Compound 21 was also obtained by an alternative route: synthesis of compound 21 was achieved via condensation of 6-amino-2-thioxopyrimidin-4(3H)-one (20) with dimethylformamide dimethylacetal (DMFDMF) to give compound 23 (Hassaneen and Abdallah, 2003), and treatment of the product 23 with 1-benzothiopyran-4-one 1 to yield authentic product 21 (Scheme 3).
MCR of benzothiopyranone 1, 6-amino-2-thioxopyrimidine-4(3H)-one 20 and DMF-DMA
Also, we were interested to investigate the multi-component reactions of the activemethylene derivatives with 1-benzothiopyran-4-one and DMF-DMA, but all attempts to isolate pure single products failed. So, we carried the reaction of enaminone 9 with activemethylene under microwave irradiation. Reaction of enaminone 9 with activemethylene derivatives 24–27 in glacial acetic acid in the presence of ammonium acetate gave poly-heterocyclic ring systems 28–31, respectively (Scheme 4). The structure of the products was assigned based on the spectral data and elemental analyses. For example mass spectrum of compound 31 revealed molecular ion peak at m/z 317 (80%) and its 1H NMR spectrum showed a characteristic signal at δ 8.57 ppm assignable to pyridine-2H proton. Its IR spectra showed one carbonyl group band at 1727 cm−1.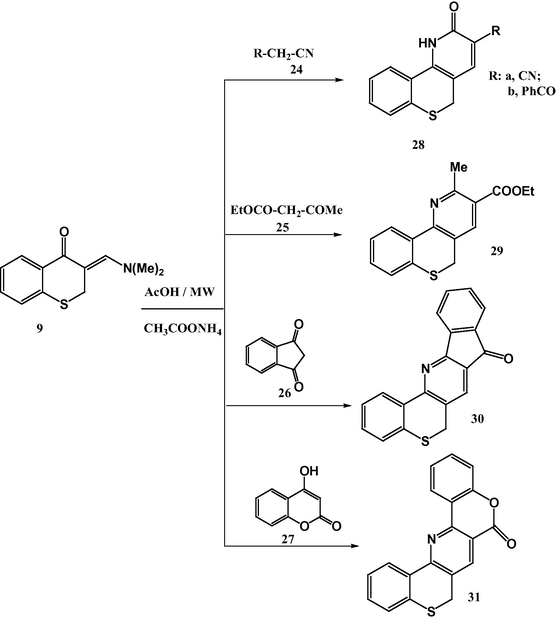
Reaction of enaminone 9 with activemethylene.
Finally, reaction of enaminone 9 with hydrazine derivatives or hydroxyl amine under microwave heating at 150 °C afforded thiochromeno[4,3-c]pyrazole derivatives 32a,b and thiochromeno[4,3-c]isoxazole 33, respectively (Scheme 5). It is worthily mentioned that compounds 32 and 33 had been prepared by Ramalingam et al. (Ramalingam et al., 1977) via a different method.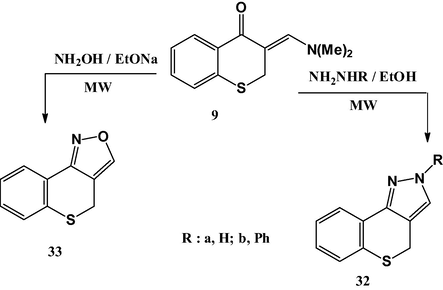
Synthesis of compounds 32 and 33.
3.2 Pharmacology
3.2.1 Anticancer activity
The in vitro anti-tumor activity of the tested compounds was evaluated at Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt. Eleven compounds were tested for their in vitro antitumor activity against HCT-116 “colon” cancer cell line. Doxorubicin was used as a reference drug and showed IC50 = 0.469 μg/ml. The most active compounds 28a, 15, 9, 28b, 5, 18, revealed IC50 < 20 μg/ml against HCT-116 (Table 1). 3-Dimethylaminomethylene-1-benzothipyran-4-one 9 showed good activity with IC50 = 4.6 μg/ml against HCT-116. Fused benzothiopyrane with the pyridine ring in compound 28a increases the activity (IC50 = 2.6 μg/ml) while, the replacement of the cyano group in compound 28a with the benzoyl group in compound 28b decreases the activity (IC50 = 5.3 μg/ml). On the other hand, fused benzothiopyrane with pyrazolo[1,5-a]pyrimidine in compound 15 increases the activity with IC50 = 3.6 μg/ml against HCT-116. The other derivatives have activity less than 3-dimethylaminomethylene-1-benzothipyran-4-one 9.
Compd. No
IC50 (μg/ml)
5
8.8
9
4.6
15
3.6
16
45.5
18
14.6
19
28.3
21
31.6
28a
2.6
28b
5.3
29
29.4
30
49.2
Doxorubicin
0.469
4 Conclusion
In this context, we describe an efficient MCR for the synthesis of polyheterocyclic ring systems under microwave irradiation. The anti-tumor activity of some of the prepared compounds against colon cancer cell line HCT-116 was evaluated. The results demonstrate that benzothiopyrane fused with pyridine or pyrazolopyrimidine derivatives showed good activity against colon cancer cell line (HCT-116 cell line).
References
- Synthesis of bioactive polyheterocyclic ring systems as 5α-reductase inhibitors. Eur. J. Med. Chem.. 2010;45:4838.
- [Google Scholar]
- Synthesis and antifungal activity of some 3-benzylidenechroman-4-ones, 3-benzylidenethiochroman-4-ones and 2-benzylidene-1-tetralones. Eur. J. Med. Chem.. 1990;25:455.
- [Google Scholar]
- The synthesis and antifungal activity of 2-amino-4-aryl-4H, 5H-[l]benzothiopyrano[4,3-b]pyran-3-carbonitriles and 2-alkoxy-4-aryl-5H-[l]benzothiopyrano[4,3-b]pyridine-3-carbonitriles. Eur. J. Med. Chem.. 1991;26:221.
- [Google Scholar]
- Synthesis of 5-substituted 3-amino-1H-pyrazole-4-carbonitriles as precursors for microwave assisted regiospecific syntheses of pyrazolo[1,5-a]pyrimidines. Molecules. 2009;14:78.
- [Google Scholar]
- Enaminones in heterocyclic synthesis: synthesis and chemical reactivity of 3-anilino-1-substituted-2-propene-1-one. J. Heterocycl. Chem.. 2005;42:563.
- [Google Scholar]
- Microwave assisted solvent-, support- and catalyst-free synthesis of enaminones. ARKIVOC. 2008;Xii:226.
- [Google Scholar]
- Synthesis and biological evaluation of flavonoids and related compounds as gastroprotective agents. Bioorg. Med. Chem. Lett.. 1996;6:995.
- [Google Scholar]
- Solvent-free, microwave assisted 1,3-cycloaddition of nitrones with vinyl nucleobases for the synthesis of N,O-nucleosides. Tetrahedron. 2008;64:8078.
- [Google Scholar]
- Antiinflammatory agents: new series of N-substituted amino acids with complex pyrimidine structures endowed with antiphlogistic activity. Farmaco. 1999;54:95.
- [Google Scholar]
- Synthesis of spiro-pyrazole-3,3′-thiopyrano[2,3-b]pyridines and azolo[a]pyrido[2′,3′:5,6]thiopyrano[3,4-d]pyrimidines as new ring systems with antifungal and antibacterial activities. J. Heterocycl. Chem.. 2005;42:221.
- [Google Scholar]
- Recent advances in the synthesis of heterocyclic compounds in aqueous medium. J. Org. Chem.. 2001;66:7030.
- [Google Scholar]
- Recent advances in isocyanide-based multicomponent chemistry. Curr. Opin. Chem. Biol.. 2002;6:306.
- [Google Scholar]
- Account of microwave irradiation for accelerating organic reactions. ARKIVOC. 2006;ix:1.
- [Google Scholar]
- Synthesis, tautomerism, and antimicrobial, anti-HCV, anti-SSPE, antioxidant, and antitumor activities of arylazobenzosuberones. Bioorg. Med. Chem.. 2009;17:8012.
- [Google Scholar]
- Synthesis of pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5-ones as potential antimicrobial agents. Arch. Pharmacal Res.. 2013;36:564.
- [Google Scholar]
- Synthesis, tautomeric structures, and antitumor activity of new perimidines. Arch. Pharm. Chem. Life Sci.. 2013;346:392.
- [Google Scholar]
- Synthesis, anti-HCV, antioxidant, and peroxynitrite inhibitory activity of fused benzosuberone derivatives. Eur. J. Med. Chem.. 2010;45:492.
- [Google Scholar]
- Hydrazonoyl halides as precursors for new fused heterocycles of 5α-reductase inhibitors. Archiv der Pharmazie. 2012;345:117.
- [Google Scholar]
- An efficient synthesis of functionalized 2-(heteroaryl)-3 H-benzo[f]chromen-3-ones and antibacterial evaluation. J. Chem. Res. 2013:298.
- [Google Scholar]
- A Convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules. 2012;17:9335.
- [Google Scholar]
- A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazolines. Heterocycles. 2013;87(5):1109.
- [Google Scholar]
- New routes to pyridino[2,3-d]pyrimidin-4-one and pyridino-[2,3-d]triazolino[4,5-a]pyrimidin-5-one derivatives. Molecules. 2003;8:333.
- [Google Scholar]
- Multi-component reactions: emerging chemistry in drug discovery from xylocain to crixivan. Curr. Med. Chem.. 2003;10:51.
- [Google Scholar]
- Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes. Acc. Chem. Res.. 2003;36:899.
- [Google Scholar]
- Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003:1471.
- [Google Scholar]
- A novel product from the reaction of 6-aminopyrimidines and 3-formylchromone. Tetrahedron Lett.. 2002;43:9061.
- [Google Scholar]
- Synthesis and biological activity of some derivatives of thiochroman-4-one and tetrahydrothiapyran-4-one. J. Med. Chem.. 1977;20:847.
- [Google Scholar]
- Effect of solvent on the regioselective synthesis of spiropyrazoles. Tetrahedron. 2012;68(44):9056.
- [Google Scholar]
- Regioselectivity in the multicomponent reaction of 5-aminopyrazoles, cyclic 1,3-diketones and dimethylformamide dimethylacetal under controlled microwave heating. Beilstein J. Org. Chem.. 2012;8:18.
- [Google Scholar]
- Antitumor agents. 166. Synthesis and biological evaluation of 5,6,7,8-substituted-2-phenylthiochromen-4-ones. J. Med. Chem.. 1996;39:1975.
- [Google Scholar]
- Recent developments in the isonitrile-based multicomponent synthesis of heterocycles. Eur. J. Org. Chem.. 2003;7:1133.
- [Google Scholar]







