Translate this page into:
MXene: A two-dimensional material in selective water separation via pervaporation
⁎Address: Gdansk University of Technology, Faculty of Chemistry, Department of Process Engineering and Chemical Technology, 11/12 Narutowicza St., 80-233 Gdansk, Poland. food.biotechnology88@gmail.com (Roberto Castro-Muñoz) castromr@tec.mx (Roberto Castro-Muñoz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
MXene, well-identified as Ti3C2TX, belongs to the family of two-dimensional (2D) materials, which have been currently explored in various applications. Very recently, such materials have been pointed out as potential nanomaterials for advanced solute separations when introduced in membranes, such as ion separation, gas separation, nanofiltration, chiral molecular separation, and solvent separation. This latter separation, generally named Pervaporation (PV), is identified as a highly selective technology for water separations. To date, few pieces of research have been released but providing interesting insights into several solvent (including water) separations. Hence, this brief review aims to analyze and discuss the latest advances for utilizing MXenes for PV membranes. Particular emphasis has been devoted to the relevant outcomes in the field, along with the strategies followed by researchers to tailor membranes. Based on the current findings, the perspectives in the field are also stated.
Keywords
MXene
Pervaporation
2D material
Membrane fabrication
Water transport
Solvent separations
1 Introduction
Since the graphene discovery in 2004, two-dimensional (2D) materials are currently explored due to their impressive features. Beyond graphene and graphene oxide, MXene, well-known as Ti3C2TX, is also an emerging 2D material (Naguib et al., 2011). These transition metal carbides/nitrides/carbonitrides have a general formula Mn+1XnTx (n = 1…3), where M corresponds to the transition metal, X corresponds to C or N atom, and Tx represents the functional groups (e.g., —F, ⚌O, —OH) (Naguib et al., 2014). MXene preparation implies the fine grinding of MAX phase precursor to powder, A-layer chemical etching from the precursor powder in the acidic fluoride solution, and finally, the washing and removing the products and by-products of the reactions (Pazniak et al., 2019). Since the first introduction of MXene DES in 2011, the exploration of this material has been exponentially increased as reflected in the growing number of publications on the topic (see Fig. 1), demonstrating an increasing research interest in catalysis (photo, electro and chemical catalysis), ion batteries, gas storage, Schottky barriers, thermo, ferro and piezoelectric applications, nanomedicine, biotechnology, among others (Khazaei et al., 2017, 2019; Sinopoli et al., 2019; Szuplewska et al., 2020).
Publications related to the applications of MXenes over the last decade (until May 29th, 2021; source: Scopus). Studies in red are those related to the application of MXenes in membranes.
Among their multiple properties, MXenes own unique surface chemistry, large surface hydrophilicity, adsorption ability (Khazaei et al., 2019; Soomro et al., 2020), large interlayer spacing, easily tunable structure (Chaudhari et al., 2017), along with tremendous mechanical and chemical stability (Naguib et al., 2011), which may render unprecedented separation performance in selective molecular separation for membrane technologies. In fact, MXenes recently opened their new avenue of application in membranes, as shown in Fig. 1. Their application includes gas separation (L. Ding et al., 2018; F. Shi et al., 2021), wastewater treatment (Al-Hamadani et al., 2020), desalination (Castro-Muñoz, 2020; Manawi et al., 2016), organic solvent purification via nanofiltration (X. Wu et al., 2016) and pervaporation (PV) (Y. Wu et al., 2019). This latter membrane technology may profit from MXenes for tailoring advanced separation membranes. PV generally uses defect-free membranes for the selective separation of solvents, where the hydrophilicity of MXenes and their defined interlayer spacing could be determinants for outperforming molecular separation. Additionally, thanks to the excellent chemical and thermodynamical properties of the MXene, the resulting membranes may render the possibility of more stable long-term operation. To date, several reviews have slightly addressed the use of MXene in liquid and gas separations using different membrane technologies (Ahmed et al., 2021; Karahan et al., 2020; P. Liu et al., 2020; Qu et al., 2021), however, there is still a need to comprehensively analyzing the findings and outcomes for water separation using MXene-based PV membranes. In this context, this paper extensively compiles the recent approaches using MXenes for PV, discussing the latest breakthroughs. In addition to the relevant outcomes in the field, we describe and extensively discuss the main strategies followed by researchers to implement MXenes in membranes and the associated interactions for unprecedented performances. To finalize, recommendations and research gaps for the new researchers are also declared.
2 MXene membranes for solvent separations
2.1 MXene fabrication, properties, transport and application in membranes
Many studies report the synthesis of multilayered MXene sheets (Anasori et al., 2017), including wet etching with HF (Ren et al., 2016), HCl-LiF (Chen et al., 2018) and HCl-NaF (F. Liu et al., 2017). In general. there are three fabrication strategies for MXene-based membranes: (i) MXenes are used as skeleton materials to prepare a lamellar-structure; (ii), distinct additives or other nanomaterials are incorporated to fabricate mixed matrix membranes with MXenes; and (iii) MXenes are applied as coating materials to modify a membrane support layer. To some extent, the preparation protocol of MXene sheets dictates the final structural properties of the material and thus the separation performance of the resulting membranes (Ahmed et al., 2021; L. Ding et al., 2017). For instance, Fig. 2 graphically illustrates a typical preparation protocol of an MXene membrane (a), together with the schematic representation of d-spacing and interlayer spacing between the two adjacent MXene nanosheets (b) and the hypothetic water permeation mechanism across the material (c). As mentioned previously, MXene surfaces present abundant oxygen-containing groups (e.g., O, OH,) and/or F groups that confer a hydrophilic nature to the material (Fig. 2b) (Naguib et al., 2011). In the PV process, such hydrophilic surface, together with the fast and selective water transport channels given by the MXene laminates, contribute to the high-water transport following the solution-diffusion model, as observed in Fig. 2c.
Traditional fabrication of a MXene membrane (a) (L. Ding et al., 2017), illustration of d-spacing and interlayer spacing in MXene sheets (b) (Y. Sun et al., 2019), and hypothetic water permeation mechanism (c) (G. Liu et al., 2018).
It is worth mentioning that such interlayer spacing of MXene can be fine-tuned by creating pillared structures based on the spontaneous intercalation of surfactants. Of course, the nature of the surfactant used and the alkyl chain lengths (together with the temperature of the solution) play an important role in controlling and tuning the interlayer spacing between layers (Bhat et al., 2021; Simon, 2017). For instance, Luo et al. (2017) successfully fabricated pillared Ti3C2 MXenes with 10 nm Sn particles between the MXene layers. Fig. 3 illustrates such a preparation method of Ti3C2 MXene containing Sn(+IV)-complexed ions, labeled as CTAB-Sn(+IV) Ti3C2, in which the synthesis was reached in two distinct steps. Initially, the authors synthesized a prepillared Ti3C2 MXene by intercalating cationic surfactants with various lengths of hydrophobic alkyl chains. Once Ti3C2 was immersed in the cationic surfactant solution, the cationic surfactant was self-assembled and intercalated between the negatively charged layers of Ti3C2 by electrostatic interactions, provoking an interlayer spacing increase between Ti3C2 layers. Even if the interlayer spacing did not vary linearly with the alkyl chain length and the temperature of the surfactant solution, they successfully tailored Ti3C2 with controllable interlayer spacings between 1 and 2.708 nm, which were lower than the original interlayer spacing for the pristine Ti3C2 MXene (approximately 0.977 nm) (Luo et al., 2017). After this, the authors intercalated Sn(+IV) ions between the Ti3C2 layers prepillared with cetyltrimethylammonium bromide (CTAB@Ti3C2) showing a spacing of 2.23 nm.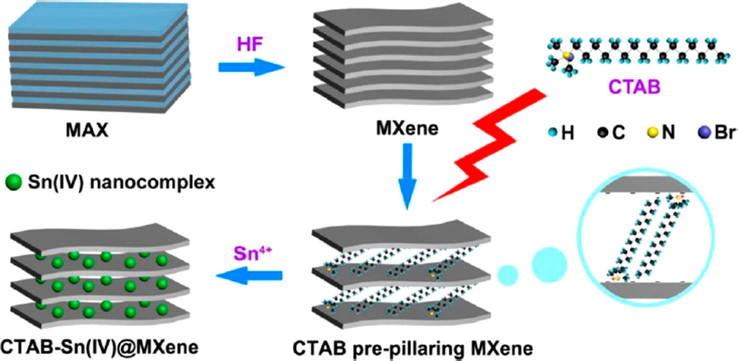
Graphical depiction of preparation of CTAB − Sn(IV)@Ti3C2 via HF etching, CTAB prepillaring, and Sn4+ pillaring methods (Luo et al., 2017).
MXene sheets can be assembled into films (also known as supports) by means of different strategies due to the strong Van der Waals forces. Unfortunately, MXene sheets can easily be aggregated, or restack, due to the strong interaction, which may result in the loss of surface area and active sites. To face such an issue, additional phases, such as polymers, carbon materials, among other inorganic materials, are incorporated into the MXene layer to increase the layer spacing. Additionally, reducing some fluoride and oxygen groups on the surface of Ti3C2Tx sheets could be contributed by annealing. Regarding the membrane preparation methods, several methods have been utilized by the research community, such as vacuum-assisted filtration, spin-coating, interfacial self-assembly, electrostatic self-assembly, atomic layer deposition, and chemical vapor deposition, among others (X. Li et al., 2021). Vacuum-assisted filtration is the most suitable method for fabricating ultrathin and controlled thickness in membranes ranging from 20 to 500 nm (Huang et al., 2021), but electrostatic self-assembly, atomic layer deposition and chemical vapor deposition can also tailor ultrathin membranes (X. Li et al., 2021).
2.2 Solvent dehydration
The primary application of PV deals with solvent dehydration aimed for solvent upgrading; the process is basically devoted to removing traces of water from organic solvents including ethanol, isopropanol, etc. (R. Castro-Muñoz et al., 2020). Experimentally, the nature of a PV membrane is fundamental for the separation of solvent molecules; for example, a hydrophilic membrane facilitates the transport (diffusion) of polar molecules (including water) while hindering the transport of less polar ones (R. Castro-Muñoz, 2019). On the contrary, a hydrophobic membrane transports preferentially less polar (and non-polar) molecules while hindering the more polar ones. The mass transport in PV membranes is given by the solution-diffusion model (Wijmans and Baker, 1995). In water transport, the water molecules are initially absorbed into the selective layer of the membranes based on chemical affinity; subsequently, they diffuse in vapor phase across the membrane, importantly, water vapor molecules are transported as a result of concentration gradient, and finally, the molecules are desorbed at the permeate side (Baker et al., 2010). The application and commercialization of PV depend on the design of highly permeable and selective membranes. To date, polymers have rendered good enough selectivity for such proposes while their low permeation constricts their commercial establishment, while commercial ceramic membranes based on silica and zeolites display high selective and permeable properties. As part of the researcher’s effort, specific inorganic materials with superior physicochemical properties are intentionally merged into polymers to produce the new generation of membranes that may show enhanced separation efficiency and productivity. MXene has been certainly proposed as filling material into a polymer phase, as reported in Table 1. For example, Xu et al. (2018) embedded the 2D material into a biopolymer, like chitosan (CS), to dehydrate ethanol, ethyl acetate and dimethyl carbonate. The MXene was easily incorporated into the MXene/CS dope solution, which was subsequently coated onto a porous PAN substrate by means of the spinning technique. The resulting membranes had a sub-micron thick layer displaying an average membrane thickness of ∼500 nm for both unfilled CS and MXene MMMs, as shown in Fig. 4a. Interestingly, the MMMs found their optimal filler content at 3 wt% MXene showing a total permeation over 1.4 kg m−2h−1 and separation factor of 1,421 for ethanol dehydration (see Fig. 4b). This separation performance seemed to be much better than the one offered by the well-explored graphene oxide (GO) used for ethanol dehydration, e.g., GO/CS (Suhas et al., 2015) and cross-linked GO PVA membranes (R. Castro-Muñoz et al., 2019), which exhibited lower permeation rates (lower than 0.14 kg m−2h−1) and separation efficiency of 1093 and 263, respectively. The performance was a result of both sorption and diffusion processes; however, even if the contribution of MXene into CS was not clear in terms of hydrophilic enhancement according to similar hydrophilic nature of both materials, MXene conducted to an improved selective diffusion of water compared to ethanol (Cao et al., 2015). Also, it was believed that MXene sheets were particularly aligned into laminates possessing interlayer channels suitable for water transport. The same membrane formulation (i.e., 3 wt% MXene) was further explored for the dehydration of ethyl acetate and dimethyl carbonate, as represented in Fig. 4c and d, respectively. Similarly, the performance of the MMM was better than that of the pristine CS membrane; but a greater separation efficiency was observed when dehydrating ethyl acetate thanks to variable molecular interactions between water and the solvent (Xia et al., 2011). These MMMs displayed a stable performance in terms of permeation (up to 1.4 kg m−2h−1) and high water content (as a result of high separation factor = 4,898) in permeate over the continuous ethyl acetate dehydration for 1800 min (Xu et al., 2018). Such outcomes are competitive with the ones from other types of composite membranes, e.g., PVA/PAN (with separation factor of 54 and permeation of 0.34 kg m−2h−1) (Yuan et al., 2011) and PBI/PEI dual-layer membranes (with separation factor of 99, 900 and permeation of 0.82 kg m−2h−1) (Wang, 2015).
Membrane
Fabrication method
Solvent separation
Operating parameters
Permeate flux (kg m−2h−1)
Separation factor
Reference
MXene/CS (3 wt%)
Spin-coating
Water/ethanol
10 wt% water, 50 °C, 200 Pa
1.4
1,421
(Xu et al., 2018)
Spin-coating
Water/dimethyl carbonate
2 wt% water, 50 °C, 200 Pa
1.4
906
Spin-coating
Water/ethyl acetate
2 wt% water, 50 °C, 200 Pa
1.4
4, 898
MXene/SA (0.12 wt%)
Normal casting
Water/ethanol
10 wt% water, 70 °C, 100 Pa
0.5
9,946
(S. Li et al., 2020)
MXene/PVA (3 wt%)
Normal casting
Water/ethanol
7 wt% water, 37 °C, 200 Pa
0.074
2,585
(Cai et al., 2020)
MXene/SSA/PVA (2 wt%)
Casting process
Water/methanol
4 wt% water, 30 °C, 130 Pa
Water: 1.5
968
(Yang et al., 2020)
Casting process
Water/ethanol
4 wt% water, 30 °C, 130 Pa
Water: 1.4
4,738
Casting process
Water/IPA
4 wt% water, 30 °C, 130 Pa
Water: 1.1
7,913
Casting process
Water/tert-butanol
4 wt% water, 30 °C, 130 Pa
Water: 1.0
23,786
Thin MXene membrane (∼100 nm)
Interfacial polymerization/vacuum filtration
Water/IPA
10 wt% water, 50 °C
1.092
∼1,200
(G. Liu et al., 2019)
PDDA functionalized MXene membrane
Facile vacuum filtration
Water/IPA
10 wt% water, 50 °C
1.23
1,932
(G. Liu et al., 2020)
MXene membrane (∼2µm)
Vacuum-assisted filtration
Water/ethanol
5 wt% water, 50 °C, −0.1 MPa
0.263
135
(Y. Wu et al., 2019)

a) Cross-section view of the MXene/CS membrane containing 3 wt% MXene; b) PV performance of the MMMs as a function of MXene loading for ethanol dehydration (10 wt% water, at 50 °C); c) PV performance of the membrane with 3 wt% MXene for dehydration of ethyl acetate (2 wt% water, at 50 °C) and d) dimethyl carbonate (2 wt% water, at 50 °C). Adapted from Xu et al. (2018).
Li et al. (2020) also utilized multilayered MXene as a filling material into sodium alginate (SA) to generate MMMs. In comparison with MXene/CS membrane (Xu et al., 2018), MXene/SA MMMs are likely to be more selective to water in ethanol dehydration displaying a separation factor of 994; where MXene stands out as an enhancer of the preferential sorption of water in SA while hindering ethanol molecules. Unfortunately, a poor permeation rate of 0.50 kg m−2h−1 was noted in the resulting membranes.
Currently, biopolymers, such as CS and SA, are gaining notable attention as a more environmentally friendly way of membrane fabrication (Galiano et al., 2018). Apart from their swelling issues, both CS and SA are excellent candidate materials for water removal due to their high hydrophilicity. However, several chemically synthesized polymers have been widely investigated including polyimides (Kudasheva et al., 2015), polybenzimidazole (PBI) (G. M. Shi et al., 2012), polyacrylonitrile (PAN) (Okumuş et al., 2003) and poly(vinyl alcohol) (PVA) (Amirilargani and Sadatnia, 2014). This latter polymer is the only one to be consolidated and commercialized at industrial level. For example, international supplier, such as DeltaMem AG (http://www.deltamem.ch), currently manufactures and commercializes cross-linked PVA membranes for PV applications. At this point, current research is still focused on enhancing the separation performance of such hydrophilic polymer. For instance, Cai et al. (2020) proposed MMMs based on cross-linked PVA and MXene for ethanol dehydration. The membrane with 3 wt% MXene displayed enhanced separation factor values over 2,500 with permeation fluxes of 0.074 kg m−2h−1 when testing for ethanol solution (7 wt% water at 37 °C). Even though the permeation does not look attractive enough compared to other high-performance membranes, the selective properties of the pristine PVA membrane were substantially improved. In general, the total flux of MMMs decreased by increasing MXene loading, which was associated with a resistance increase towards water molecules to pass through the membrane, as a contribution of the cross-linking density increase, along with aggregation of MXene.
As one of the latest works in the field, Yang et al. (2020) also assayed the embedding of MXene into PVA, followed by chemical in situ cross-linking with sulfosuccinic acid (SSA); the preparation protocol is represented in Fig. 5a. The resulting nanocomposite presented an ultrathin nature with thickness up to ≈230 nm, revealing an outperforming pervaporative separation for the removal of water from various solvents including ethanol, methanol, tert-butanol and isopropanol (IPA). As can be seen in Fig. 5b, the alcohols were easily hindered by the membrane while transporting water to permeate with water content over 97% (at 30 °C), which was a result of high separation factors of 968, 4738, 7913 and 23,786 for water/methanol, water/ethanol, water/IPA and water/tert-butanol, respectively. By maintaining the same concentration (4 wt% water in all alcohol mixtures), it is clear to visualize the greater effect of the sizes of the alcohol molecules, such as methanol (0.36 nm), ethanol (0.45 nm), IPA (0.47 nm), tert-butanol (0.46 nm), over water permeation flux. Moreover, Fig. 5c illustrates the stability of the cross-linked PVA/MXene membranes for 50 h operation in ethanol dehydration, basically, the water flux decreased from 1.46 to 1.31 kg m−2h−1 due to the reduction of water concentration in the feed tank. At this point, it could be thought that such membranes may find a stable long-term operation in continuous mode maintaining the concentration in the feed side.
a) Preparation strategy for the ultrathin PVA/MXene composite membrane; b) performance in desalination (left side) and organic solvent dehydration (right side); c) long-term operation for ethanol dehydration (Yang et al., 2020).
Towards IPA purification, Liu et al. (2019) developed ultrathin MXene membranes (approximately 100 nm) with highly ordered 2D nanochannels. In this work, a new strategy of membrane preparation was oriented to use positively charged hyperbranched polyethylenimine (HPEI), which was intercalated between negatively charged MXene nanosheets to fit out regular stacking structures via electrostatic interactions; after this, interfacial polymerization with trimesoyl chloride (TMC) was applied to seal possible non-selective defects. By separating IPA solution (10 wt% water, 50 °C), the resulting membrane (i.e., Ti2CTx–HPEI/TMC with d-pacing of 0.44 nm) was able to produce permeate fractions containing water content higher than 99 wt%, as a result of high separation factor (over 1,200). The permeation was also estimated as high as 1.092 kg m−2h−1, with stable long-term operation over 120 h. In the same research group, the authors explored a different protocol for the molecular separation of water from IPA using MXene membranes (G. Liu et al., 2020); at this point, they introduced various polyelectrolytes (such as PDDA, PEI, PAH) to control the membrane structure during the PV operation. After preliminary studies, the authors claimed that the poly (diallyldimethylammonium chloride) (PDDA) functionalized MXene membrane revealed the highest separation performance ascribed to the optimized electrostatic effect and hydrophilicity. When compared with Ti2CTx–HPEI/TMC membrane (G. Liu et al., 2019), this PDDA functionalized MXene demonstrated higher permeation (∼1.237 kg m−2h−1) and efficiency (separation factor of 1,932) (G. Liu et al., 2020). Here, MXene acquired plenty of oxygen groups and improved hydrophilic nature from PDDA, contributing to exceptional IPA dehydration. It is worth mentioning that PDDA functionalized MXene was fabricated in an easier way compared to Ti2CTx–HPEI/TMC, which is important from the feasibility and commercialization point of view.
The high permeation of 2D materials deals with their well-defined nanochannels and characteristic d-spacing; however, a specific 2D material, such as GO, has suffered from an interlayer d-spacing enlargement in the presence of water; as an example, the interlayer spacing in GO varied from ≈6.4 to 9.8 Å with relative humidity range from 0 to 100%, respectively (Abraham et al., 2017). This enlarged spacing can definitely affect the selective properties of the materials since water and other molecules can be indifferently transported across the channels. The phenomenon was investigated by Wu et al. (2019), who observed that the MXene channels of 1.37 nm exhibited an increase of 0.23 nm after immersing in water. Also, the authors monitored the d-spacing as a function of time showing an increase of 0.2 nm after immersed in water for 2 h. After immersion for 5 days, such an increase in d-spacing was still ∼0.2 nm in comparison with the dry membrane. This gives an idea that the increase in d-spacing is not infinite, noting enough stability in liquid solution. Here, MXene owns an advantage compared with its analogous GO since this latter material can easily be disintegrated in water due to the strong electrostatic repulsion forces among sheets, while MXene has demonstrated a stronger van der Waals attraction force and weaker electrostatic repulsion force. Thereby, MXene stands out as potential material for water separations and impressive stability in water (Lao et al., 2018), Additionally, MXene membranes also displayed an unchanged d-spacing when immersed in ethanol, suggesting a much better anti-swelling stability in polar organic solvents. When tested for ethanol dehydration (5 wt% water), MXene membranes (with 2 µm thick) eventually showed their best separation performance at room temperature with a separation factor and flux of 135 and 0.263 kg m−2h−1, respectively (Y. Wu et al., 2019). Regarding the flux improvement, the authors systematically increased the flux by decreasing the membrane thickness up to 0.5 µm with an average flux of 0.60 kg m−2h−1. The low permeation was attributed to tortuous 2D channels that produce a mass transfer resistance, which supports Cai’s statement, where an increase of MXene loading lowered the total flux by increasing the mass transport resistance (Cai et al., 2020).
2.3 Seawater desalination
One of the key contributions of MXene in PV membranes falls into seawater desalination, where impressive separation performances have been obtained. For example, Liu et al. (2018) created ultrathin MXene-based membranes (thickness ∼60 nm) onto polyacrylonitrile (PAN) ultrafiltration membranes using vacuum filtration, which reported high water flux (up to 85.4 kg m−2h−1) and salt rejection (∼99.5%) when separating a 3.5 wt% NaCl solution (at 65 °C). In this work, MXene conferred high hydrophilicity to the resulting membranes as a result of its plenty of e oxygen-containing groups. Additionally, such membranes were able to completely reject other ions including Mg2+, Ca2+, K+, SO4 2+, Cl−, and Br−, presenting a good long-term operation for desalination (over 100 h). However, unprecedented separation in seawater desalination has been reported by Ding et al. (2020), who designated ultrathin membranes (ca. 30 nm layer). Particularly, a maleic acid covalent-bridged MXene (MXMA) membrane supported onto nylon support using facile vacuum-assisted filtration synthesis. Here, adjacent MXene nanosheets were smartly fixed by the covalent bonding formed by the esterification reaction between the carboxyl groups in maleic acid and hydroxyl groups in MXene surface, as illustrated in Fig. 6a. The authors compared the performance of the MXMA membranes with the pristine MXene membranes, exhibiting both comparable salt rejection; however, MXMA membranes showed higher permeation values (ca. 22 kg m−2h−1) than MXene membranes (ca. 15 kg m−2h−1) (see Fig. 6b). It is important to point out that the performance in terms of water permeate flux (70 kg m−2h−1) and salt rejection (99%) was impressively achieved when operating at 65 °C. The study stated that the esterification reaction constructed well-defined nano-channels in MXMA membranes with a size of ca. 0.49 nm, which were larger in comparison with the MXene membrane (∼0.37 nm). Considering channels of 0.49 nm, they were still larger than the kinetic diameter of water molecules (∼0.29 nm) but smaller than both hydrated Cl– (∼0.66 nm) and Na+ (∼0.72 nm)(Joshi et al., 2014; Song et al., 2019), conducting to efficient sieving effect. Here, the high permeation was initially related to the still hydrophilic nature of MXMA that promotes water transportation, however, during the cross-linking protocol, Ding et al. (2020) speculated that the usage of hydrophilic hydroxyl groups and the production of hydrophobic ester groups in MXMA membranes resulted in more frictionless regions, introducing fast water flow within the channels. Fig. 6c also shows that MXMA membranes demonstrated a similar performance when treating real seawater while maintaining exceptional stability over 30 h operation. So far, these membranes are among the thinnest membranes with promising potential to compete with PVA/intercalated graphene oxide membranes (water permeation of 98 kg m−2h−1, at 85 °C) as the most efficient ultrathin membranes for seawater desalination, surpassing the performance of most of the ultrathin membranes reported in the literature (see Table 2). Once a selective and defect-free morphology is obtained, the ultrathin nature of membranes is pursued to break the trade-off between selectivity and permeance. Besides, the fabrication of ultrathin membranes needs a small amount to design the selective layer, contributing to material cost savings (Roberto Castro-Muñoz et al., 2020).
a) Hypothetical reaction between MXene and maleic acid via esterification reaction; b) desalination performance of both MXMA and MXene membranes with similar thickness (ca. 30 nm) (3.5 wt% NaCl solution, at 30 °C); c) and long-term operation of the MXMA membranes at the same operating conditions.
Membrane
Thickness (nm)
Operating parameters:
Permeate flux (kg m−2h−1)
Salt rejection
Reference:
PVA supported on PSF hollow fiber
100
30,000 ppm NaCl, 70 °C, vacuum
7.4
99.9%
(Chaudhri et al., 2015)
GO/PAN composite
100
35, 000 ppm NaCl, 90 °C, vacuum 0.1 kPa
65.1
99.8
(Liang et al., 2015)
PVA-SiO2 supported on PSF hollow fiber
220
30,000 ppm NaCl, 70 °C, vacuum
10.4
99.9
(Chaudhri et al., 2018)
Cross-linked PVA supported on PAN
800
35, 000 ppm NaCl, 70 °C, vacuum 0.1 kPa
46.3
99.8
(Liang et al., 2018)
Cross-linked PVA supported on PVDF hollow fiber
300
100 g L−1 NaCl, 80 °C, vacuum 1 kPa
9
99.9
(L. Li et al., 2017)
MXene-based membranes
60
3.5 wt% NaCl, 65 °C, vacuum 0.4 kPa
85.4
99.5
(G. Liu et al., 2018)
GO-based membranes
500
100 g L−1 NaCl, 70 °C, vacuum 3 kPa
42.4
99.9
(L. Li et al., 2019)
Maleic acid covalent-bridged MXene membranes
30
3.5 wt% NaCl, 65 °C, vacuum 0.1 kPa
70
99.0
(M. Ding et al., 2020)
PVA/intercalated GO membranes
66–669
10 wt% NaCl, 85 °C, vacuum 6 kPa
98
99.9
(J. Sun et al., 2020)
PVA/PAN nanofiber composite membranes
730
1.5 wt% NaCl, 75 °C, vacuum 0.1 kPa
243
99.7
(Xue et al., 2020)
Cross-linked PVA/MXene
230
3.5 wt% NaCl, 30 °C, vacuum 0.13 kPa
62.2
99.8
(Yang et al., 2020)
At almost room temperature (30 °C), Yang et al. (2020) reported that ultrathin cross-linked PVA/MXene membranes (ca. 230 nm) offer extremely high desalination performance (water permeation: 62.2 kg m−2h−1, salt rejection:99.8%), as shown previously in Fig. 5b. The authors claimed that such an impressive pervaporative performance was ascribed to the MXene incorporation. MXene composite membrane exhibited high water permeation flux due to the combination of several factors including a more amorphous region, increased free volume pore size, higher free volume, improved hydrophilicity and additional permeating paths through MXene and MXene-polymer interphase. Based on the excellent desalination performance towards NaCl, the authors also evaluated the rejection performance towards other salt ions obtaining high rejection values for KCl (99.81%), Na2SO4 (99.91%), MgCl2 (99.93%), CaCl2 (99.92%) and MgSO4 (99.93%). It was clear that this composite membrane owned a defined nanostructured, together with water affinity properties favouring high permeation and water selectivity; however, it is worth mentioning that the membranes also displayed a negative charge surface that is positive for ion rejection governed by the Donnan exclusion effect (Schaetzel et al., 1993), in which divalent ions tend to be more sensitive to charge exclusion than monovalent ions.
3 Concluding remarks and perspectives
MXene is an emerging 2D nanostructured material widely explored in membranes (mainly nanofiltration) with great advances and outcomes in molecular separations (Zhu et al., 2018). Particularly, this review has found that MXene allows obtaining a rational design for nanohybrid membranes for different water, solvent and ion separations. According to the experts, MXene layers have a promising future in developing the next-generation of membranes for water separation (Ihsanullah, 2020b, 2020a). Even though the usage of this 2D material in membranes and membrane processes seem to be a relatively new field of application, its application in membranes deals with the selective separation of molecules including water purification, desalination, gas separation and pervaporation (Ahmed et al., 2021; L. Ding et al., 2017). This latter technology is one of the minimally explored but with great advances in specific water separations, in which ultrathin membranes (thinner than 800 nm) are the ones exhibiting exceptional performance (Roberto Castro-Muñoz et al., 2020). The ability of water selective transport of MXene has led to applying the material in water purification approaches especially seawater desalination (J. Li et al., 2020; P. Liu et al., 2020).
So far, pristine MXene and its composite polymer membranes in PV have demonstrated to offer unprecedented pervaporation performances for water removal from solvent mixtures and seawater desalination. Importantly, a low quantity between 0.1 and 3 wt% has been required for the improvement of polymer membranes (Xu et al., 2018). Of course, the limitation and commercialization of MXene in membranes rely on the extremely high cost of the material. At this point, the usage of mixed matrix membranes and ultrathin membranes may meet the requirement of using a minimal quantity of the material.
Vacuum-assisted filtration and mixed matrix membrane via dope polymer solution stand out as the primary protocols for the fabrication of MXene-containing membranes; in this way, there is still a long way of applying different strategies that may allow extend the feasibility and reducing the cost of membrane preparation. At this point, it is likely that cost-effective and time-saving protocols will be crucial for large-scale production and industrial use (P. Liu et al., 2020; Qu et al., 2021).
3.1 Research gaps and recommendations for the new researchers in the field
To finalize, according to the findings of this review, the following suggestions and research gaps for the new researchers in the field are given:
-
One of the bottlenecks of hydrophilic pervaporation deals with the swelling of polymer membranes by polar solvents, which conducts to compromising their selectivity. Even though MXenes have apparently enhanced the solvent resistance in polymer composites (P. Liu et al., 2020), there is still a large number of hydrophilic polymers (such as polyimide, polybenzimidazole, polyether ether ketone, among others) that suffer such a phenomenon. Therefore, precise studies on swelling issues should be analyzed in MXene-based nanocomposite declaring the mechanism or interaction for the successful swelling mitigation.
-
Until now, most of the research in pervaporation separation has pointed out the ability of MXene- based membranes for a long-term operation (up to 60 h) for binary solvent mixtures and aqueous salt solutions. To some extent, such evaluation is a starting point that opens the window of testing such membranes in harsh environments (real azeotropes, natural seawater, etc.); however, further research is needed to better understand the impact of MXene nanostructure on separation performance as well as the structural stability of selective layer (Zhu et al., 2018). Here, the evaluation of the membrane performance will give us a more realistic approximation of the feasibility of the membranes not only for real yield but also for their commercialization, where MXenes are still at an early stage of the investigation.
-
Molecular diffusion for understanding the water transport mechanism in MXenes has been explored by analyzing computer simulation (P. Liu et al., 2020). Unfortunately, parameters associated with the separation mechanism have been minimally investigated. In this context, simulation models should be studied in detail for a better understanding of water transport and separation (and other solvent molecules), along with new outperforming membrane designs (Karahan et al., 2020).
-
When dealing with mixed matrix membranes, the incorporation of inorganic materials (like 2D) contributes to higher fluxes compared with pristine polymer membranes. However, filler loading increase does not always guarantee the highest permeation. In particular, overuse of MXene can decrease the permeation rate due to resistance increase towards permeating molecules (Cai et al., 2020). At this point, researchers should optimize the MXene loading to compensate the distortion of the polymer chain, which can provoke the creation of non-selective gaps.
-
As widely investigated in its analogous GO, the influence of water content on the nanostructure of MXene, specially d-spacing, must be further evaluated since there is strong evidence of channel enlargement in 2D materials in the presence of water (Abraham et al., 2017), influencing the separation performance. Even if Wu et al. (2019) declared only ∼0.2 nm increase in respect to the initial d-spacing, researchers should be aware of any change in the structural properties of MXene membranes applied in polar separations.
Acknowledgments
Financial support from Polish National Agency for Academic Exchange (NAWA) under Ulam Programme (Agreement No. PPN/ULM/2020/1/00005/U/00001) is gratefully acknowledged. R. Castro-Muñoz also acknowledges the School of Engineering and Science and the FEMSA-Biotechnology Center at Tecnológico de Monterrey for their support through the Bioprocess (0020209I13) Focus Group.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol.. 2017;April:1-6.
- [CrossRef] [Google Scholar]
- Recent Advances in MXene-based Separation Membranes. ChemBioEng Rev.. 2021;8(2):110-120.
- [CrossRef] [Google Scholar]
- Applications of MXene-based membranes in water purification: A review. Chemosphere. 2020;254:126821.
- [CrossRef] [Google Scholar]
- Poly (vinyl alcohol)/ zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci.. 2014;469:1-10.
- [CrossRef] [Google Scholar]
- 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater.. 2017;2(2)
- [CrossRef] [Google Scholar]
- Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci.. 2010;348(1–2):346-352.
- [CrossRef] [Google Scholar]
- Prospects challenges and stability of 2D MXenes for clean energy conversion and storage applications. npj 2D Mater. Appl.. 2021;5(1):1-21.
- [CrossRef] [Google Scholar]
- Poly(vinyl alcohol)-Modified Membranes by Ti3C2Txfor Ethanol Dehydration via Pervaporation. ACS Omega. 2020;5(12):6277-6287.
- [CrossRef] [Google Scholar]
- Highly water-selective hybrid membrane by incorporating g-C3N4 nanosheets into polymer matrix. J. Membr. Sci.. 2015;490:72-83.
- [CrossRef] [Google Scholar]
- Pervaporation: The emerging technique for extracting aroma compounds from food systems. J. Food Eng.. 2019;253
- [CrossRef] [Google Scholar]
- Towards the dehydration of ethanol using pervaporation cross-linked poly(vinyl alcohol)/graphene oxide membranes. J. Membr. Sci.. 2019;582:423-434.
- [Google Scholar]
- Recent advances in pervaporation hollow fiber membranes for dehydration of organics. Chem. Eng. Res. Des.. 2020;164
- [CrossRef] [Google Scholar]
- Breakthroughs on tailoring pervaporation membranes for water desalination: A review. Water Res.. 2020;187:116428.
- [CrossRef] [Google Scholar]
- Ultrathin permselective membranes: the latent way for efficient gas separation. RSC Adv.. 2020;10(21):12653-12670.
- [CrossRef] [Google Scholar]
- MXene: An emerging two-dimensional material for future energy conversion and storage applications. J. Mater. Chem. A. 2017;5(47):24564-24579.
- [CrossRef] [Google Scholar]
- Fabrication of efficient pervaporation desalination membrane by reinforcement of poly(vinyl alcohol)-silica film on porous polysulfone hollow fiber. J. Appl. Polym. Sci.. 2018;135(3):1-13.
- [CrossRef] [Google Scholar]
- Preparation of ultra-thin poly(vinyl alcohol) membranes supported on polysulfone hollow fiber and their application for production of pure water from seawater. Desalination. 2015;367:272-284.
- [CrossRef] [Google Scholar]
- Effect of glycine functionalization of 2D titanium carbide (MXene) on charge storage. J. Mater. Chem. A. 2018;6(11):4617-4622.
- [CrossRef] [Google Scholar]
- MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun.. 2018;9(1):1-7.
- [CrossRef] [Google Scholar]
- A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angewandte Chemie - Int. Ed.. 2017;56(7):1825-1829.
- [CrossRef] [Google Scholar]
- 2D laminar maleic acid-crosslinked MXene membrane with tunable nanochannels for efficient and stable pervaporation desalination. J. Membr. Sci.. 2020;600(January):117871.
- [CrossRef] [Google Scholar]
- Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci.. 2018;564(May):562-586.
- [CrossRef] [Google Scholar]
- MXene-Based Membranes for Separation Applications. Small Sci.. 2021;1(7):2100013.
- [CrossRef] [Google Scholar]
- MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. J.. 2020;388(February):124340.
- [CrossRef] [Google Scholar]
- Potential of MXenes in Water Desalination: Current Status and Perspectives. Nano-Micro Lett.. 2020;12(1):1-20.
- [CrossRef] [Google Scholar]
- Precise and ultrafast molecular sieving through graphene oxide membranes. Science. 2014;343(6172):752-754.
- [Google Scholar]
- MXene Materials for Designing Advanced Separation Membranes. Adv. Mater.. 2020;32(29):1-23.
- [CrossRef] [Google Scholar]
- Recent advances in MXenes: From fundamentals to applications. Curr. Opin. Solid State Mater. Sci.. 2019;23(3):164-178.
- [CrossRef] [Google Scholar]
- Electronic properties and applications of MXenes: a theoretical review. J. Mater. Chem. C. 2017;5(10):2488-2503.
- [CrossRef] [Google Scholar]
- Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol.. 2015;90(4):669-677.
- [CrossRef] [Google Scholar]
- Aqueous Stable Ti 3 C 2 MXene Membrane with Fast and Photoswitchable Nanofluidic Transport. ACS Nano. 2018;12(12):12464-12471.
- [CrossRef] [Google Scholar]
- An MXene-based membrane for molecular separation. Environ. Sci. Nano. 2020;7(5):1289-1304.
- [CrossRef] [Google Scholar]
- Pinning Down the Water Transport Mechanism in Graphene Oxide Pervaporation Desalination Membranes [Research-article] Ind. Eng. Chem. Res.. 2019;58(10):4231-4239.
- [CrossRef] [Google Scholar]
- Composite PVA/PVDF pervaporation membrane for concentrated brine desalination: Salt rejection, membrane fouling and defect control. Desalination. 2017;422(June):49-58.
- [CrossRef] [Google Scholar]
- Highly selective sodium alginate mixed-matrix membrane incorporating multi-layered MXene for ethanol dehydration. Separat. Purif. Technol.. 2020;235(October 2019)
- [CrossRef] [Google Scholar]
- Li, X., Ran, F., Yang, F., Long, J., Shao, L., 2021. Advances in MXene Films: Synthesis, Assembly, and Applications. In: Transactions of Tianjin University, vol. 27, Issue 3. Tianjin University. https://doi.org/10.1007/s12209-021-00282-y.
- Water permeance, permeability and desalination properties of the sulfonic acid functionalized composite pervaporation membranes. Desalination. 2018;433(November 2017):132-140.
- [CrossRef] [Google Scholar]
- High performance graphene oxide/polyacrylonitrile composite pervaporation membranes for desalination applications. J. Mater. Chem. A. 2015;3(9):5140-5147.
- [CrossRef] [Google Scholar]
- Preparation of High-Purity V 2 C MXene and Electrochemical Properties as Li-Ion Batteries. J. Electrochem. Soc.. 2017;164(4):A709-A713.
- [CrossRef] [Google Scholar]
- Polyelectrolyte functionalized ti2ct x mxene membranes for pervaporation dehydration of isopropanol/water mixtures. Ind. Eng. Chem. Res.. 2020;59(10):4732-4741.
- [CrossRef] [Google Scholar]
- Two-dimensional Ti2CT: X MXene membranes with integrated and ordered nanochannels for efficient solvent dehydration. J. Mater. Chem. A. 2019;7(19):12095-12104.
- [CrossRef] [Google Scholar]
- Ultrathin two-dimensional MXene membrane for pervaporation desalination. J. Membrane Sci.. 2018;548(September 2017):548-558.
- [CrossRef] [Google Scholar]
- Two-dimensional material membranes for critical separations. Inorg. Chem. Front.. 2020;7(13):2560-2581.
- [CrossRef] [Google Scholar]
- Pillared Structure Design of MXene with Ultralarge Interlayer Spacing for High-Performance Lithium-Ion Capacitors. ACS Nano. 2017;11(3):2459-2469.
- [CrossRef] [Google Scholar]
- Can carbon-based nanomaterials revolutionize membrane fabrication for water treatment and desalination? Desalination. 2016;391:69-88.
- [CrossRef] [Google Scholar]
- Two-dimensional nanocrystals produced by exfoliation of Ti 3AlC 2. Adv. Mater.. 2011;23(37):4248-4253.
- [CrossRef] [Google Scholar]
- 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater.. 2014;26(7):992-1005.
- [CrossRef] [Google Scholar]
- Effect of fabrication and process parameters on the morphology and performance of a PAN-based zeolite-filled pervaporation membrane. J. Membr. Sci.. 2003;223(1–2):23-38.
- [CrossRef] [Google Scholar]
- Ti3C2Tx MXene characterization produced from SHS-ground Ti3AlC2. Mater. Des.. 2019;183:108143.
- [CrossRef] [Google Scholar]
- Recent progress in the design and fabrication of MXene-based membranes. Front. Chem. Sci. Eng. 2021
- [CrossRef] [Google Scholar]
- Porous Two-Dimensional Transition Metal Carbide (MXene) Flakes for High-Performance Li-Ion Storage. ChemElectroChem. 2016;3(5):689-693.
- [CrossRef] [Google Scholar]
- Mass transfer analysis of pervaporation through an ion exchange membrane. Desalination. 1993;90(1–3):259-276.
- [CrossRef] [Google Scholar]
- MXene versus graphene oxide: Investigation on the effects of 2D nanosheets in mixed matrix membranes for CO2 separation. Journal of Membrane Science. 2021;620(July 2020)
- [CrossRef] [Google Scholar]
- Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci.. 2012;415–416:577-586.
- [CrossRef] [Google Scholar]
- Two-Dimensional MXene with Controlled Interlayer Spacing for Electrochemical Energy Storage. ACS Nano. 2017;11(3):2393-2396.
- [CrossRef] [Google Scholar]
- Electrocatalytic/photocatalytic properties and aqueous media applications of 2D transition metal carbides (MXenes) Curr. Opin. Solid State Mater. Sci.. 2019;23(5):100760.
- [CrossRef] [Google Scholar]
- Mass transport mechanisms within pervaporation membranes. Front. Chem. Sci. Eng.. 2019;13(3):458-474.
- [CrossRef] [Google Scholar]
- A mini-review on MXenes as versatile substrate for advanced sensors. Chin. Chem. Lett.. 2020;31(4):922-930.
- [CrossRef] [Google Scholar]
- Hydrogen peroxide treated graphene as an effective nanosheet filler for separation application. RSC Adv.. 2015;5(122):100984-100995.
- [CrossRef] [Google Scholar]
- Tailoring the microstructure of poly(vinyl alcohol)-intercalated graphene oxide membranes for enhanced desalination performance of high-salinity water by pervaporation. J. Membr. Sci.. 2020;599(1045)
- [CrossRef] [Google Scholar]
- Adjustable interlayer spacing of ultrathin MXene-derived membranes for ion rejection. J. Membr. Sci.. 2019;591(May):117350.
- [CrossRef] [Google Scholar]
- Future Applications of MXenes in Biotechnology. Nanomed. Sensors Trends Biotechnol.. 2020;38(3):264-279.
- [CrossRef] [Google Scholar]
- Pervaporation dehydration of ethyl acetate via PBI/PEI hollow fiber membranes. Ind. Eng. Chem. Res.. 2015;54(11):3082-3089.
- [CrossRef] [Google Scholar]
- The solution-diffusion model: a review. J. Membr. Sci.. 1995;107(1–2):1-21.
- [CrossRef] [Google Scholar]
- Polymer-Ti3C2Tx composite membranes to overcome the trade-off in solvent resistant nanofiltration for alcohol-based system. J. Membr. Sci.. 2016;515:175-188.
- [CrossRef] [Google Scholar]
- Two-dimensional MXene membrane for ethanol dehydration. J. Membr. Sci.. 2019;590(April):1-8.
- [CrossRef] [Google Scholar]
- Dehydration of ethyl acetate-water mixtures using PVA/ceramic composite pervaporation membrane. Sep. Purif. Technol.. 2011;77(1):53-59.
- [CrossRef] [Google Scholar]
- Two-dimensional MXene incorporated chitosan mixed-matrix membranes for efficient solvent dehydration. J. Membr. Sci.. 2018;563(May):625-632.
- [CrossRef] [Google Scholar]
- Tailoring the molecular structure of crosslinked polymers for pervaporation desalination. Nat. Commun.. 2020;11(1):1461.
- [CrossRef] [Google Scholar]
- Ultrathin poly (vinyl alcohol)/MXene nanofilm composite membrane with facile intrusion-free construction for pervaporative separations. J. Membr. Sci.. 2020;614(August):118490.
- [CrossRef] [Google Scholar]
- Dehydration of ethyl acetate aqueous solution by pervaporation using PVA/PAN hollow fiber composite membrane. Desalination. 2011;280(1–3):252-258.
- [CrossRef] [Google Scholar]
- The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A. 2018;6(9):3773-3792.
- [CrossRef] [Google Scholar]







