Translate this page into:
Na/THF- Mediated cleavage of organic disulfides/diselenides. An efficient and one-pot regioselective method to the synthesis of β-hydroxy sulfides/selenides
⁎Corresponding author. soliemanbeigi@gmail.com (Mohammad Soleiman-Beigi) SoleimanBeigi@yahoo.com (Mohammad Soleiman-Beigi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new, facile, and efficient methodology for synthesizing β-hydroxy sulfides/selenides has been reported. In this study, 1,2-epoxide ring opening was used as the synthetic protocol, and this reaction was examined using metallic sodium with no additional catalyst. This lead to the synthesis of the desired ring-opened products. Relatively short reaction times, mild reaction conditions, a simple work-up, moderate to good yields of products, and remarkable regioselectivity of the nucleophilic attack were other important features of this study.
Keywords
Epoxides
Ring opening
Disulfides
Diselenides
Catalyst-free reaction
1 Introduction
1,2-Epoxies represent one of the most widely used functional groups in organic synthesis because of their susceptibility to reactions with numerous nucleophiles. This nucleophilic and regioselective cleavage yields valuable bifunctional compounds in a rather simple procedure (Lanke and Bhanage, 2013; Cossy et al., 2002; Tomioka et al., 2014). The ring opening of epoxides through nucleophilic attack has been studied extensively, and there are several references to the opening of epoxides with substances such as alcohols, thiols, amines and ethers (Trikittiwong et al., 2013; Shah et al., 2014; Aramesh et al., 2013; Ertürk et al., 2012; Thomas et al., 2014; Panchadhayee and Misra, 2009; Bandgar et al., 2007).
Since rigorous methodologies for asymmetric epoxidations are now available (Azizi and Saidi, 2006; Yang et al., 2008; Johnson and Sharpless, 2000; Katsuki, 2000) epoxide ring opening, if performed with rigid stereo- and regiocontrol, can be applied beneficially for constructing two adjacent stereochemically distinct sp3 chiral carbon centers in a one-step process.
Furthermore, β-hydroxy sulfides/selenides have emerged as two important classes of organic compounds and have found useful in medicinal chemistry (Tokareva et al., 2009; Jesse et al., 2008) and organic synthesis (Costa et al., 2004; Sala et al., 2002; Movassagh et al., 2003; Chimni et al., 2013; Tiecco et al., 2004). The best reported protocol for synthesizing these compounds is the ring opening of epoxides with nucleophilic sulfur and selenium species. However, the reported methods have some limitations such as the use of strong and nonselective catalysts, toxic and expensive reagents, low yield, long reaction times, and undesirable side reactions. Thus, the search for newer methodologies for epoxide ring opening is still an active area of research in organic chemistry (Sridhar et al., 2005; Khodaei et al., 2005; Azizi et al., 2009; Guo et al., 2009; Amantini et al., 2003; Fringuelli et al., 2003; Yadav et al., 2002; Polshettiwar and Kaushik, 2004; Sun et al., 2009; Movassagh and Soleiman-Beigi, 2007; Chen and Chen, 2007; Gao et al., 2008; Mojtahedi et al., 2012).
We were encouraged to search for a novel, mild, and efficient methodology for the ring opening of various epoxides in which some of the aforementioned limitations could be avoided. This study will report a novel catalyst-free procedure for synthesizing β-hydroxy sulfides/selenides including Na-mediated breaking of S—S and Se—Se bonds and nucleophilic epoxide ring opening with aryl thiolate or selenolate ions as nucleophiles.
2 Materials and methods
2.1 Chemicals and instruments
All solvents were purified by standard methods. Chemicals were purchased from commercial suppliers, Merck and Sigma-Aldrich, and used without further purification. Yields refer to isolated products. All the products are known compounds (Oil compounds) and were characterized by comparison of NMR and IR spectral data with those reported in the literature. The IR spectra were obtained on a FT-IR Hartman–Bomen spectrophotometer using KBr disks. The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Avance NMR spectrometer in CDCl3 solution. The progress of the reaction was monitored using TLC with silica gel SILG/UV 254 plates.
2.2 Procedure for synthesizing β-hydroxy sulfides/selenides through epoxide ring opening
A mixture of diphenyl disulfide derivatives (1 mmol) and sodium metal (2 mmol) in dry THF (4 mL) was stirred for 30 min at room temperature and monitored using TLC (EtOAc:n-Hexane, 1:10). After complete transformation to thiolate/selenolate anions, 1,2-epoxide derivative (1.2 mmol) was added to the reaction mixture, and the mixture was stirred at 25 °C until the reaction completed (TLC analysis, EtOAc:n-Hexane, 1:4). The THF solvent was evaporated in vacuo, and the reaction mixture was acidified by 20% (v/v) HCl solution and stirred at 25 °C for 15 min. The mixture was then extracted using dichloromethane (3 × 30 mL), and the combined organic layer was collected, dried over Na2SO4, and evaporated in vacuo. The remaining oil in order to obtain the pure β-hydroxy sulfides/selenides was then purified by preparative TLC (Silica gel; EtOAc:n-Hexane, 1:4).
2.3 Spectroscopic data for selected β-hydroxy sulfides/selenides prepared using the above mentioned procedure
2.3.1 1-(4-Bromophenylthio)butan-2-ol (Table 2, entry 6)
Yield 95%; 1H NMR (400 MHz, DMSO-d6) δ in ppm: 0.98 (t, 3H, J = 7.6 Hz, CH3), 1.59 (m, 2H, CH2), 2.49 (br s, 1H, OH), 2.86 (dd, 1H, J = 13.8, 8.4 Hz, CH2), 3.13 (dd, 1H, J = 13.6, 3.6 Hz, CH2), 3.62 (m, 1H, CH), 7.4 (dd, 4H, J = 8.8 Hz, Ar–H) 13C NMR (100 MHz, DMSO-d6) δ in ppm: 9.98, 29.05, 41.47, 70.73, 120.40, 131.36, 132.10, 134.84.
2.3.2 1-(4-Bromophenylthio)propan-2-ol (Table 2, entry 10)
Yield 94%; 1H NMR (400 MHz, DMSO-d6) δ in ppm: 1.27 (d, 3H, J = 6.4 Hz, CH3), 2.67 (br s, 1H, OH), 2.87 (dd, 1H, J = 13.6, 8.4 Hz, CH2), 3.07 (dd, 1H, J = 13.6, 3.6 Hz, CH2), 3.86 (m, 1H, CH), 7.29 (dd, 4H, J = 8.4 Hz, Ar–H) 13C NMR (100 MHz, DMSO-d6) δ in ppm: 22.09, 43.4, 65.72, 120.41, 131.35,132.10, 134.76.
2.3.3 1-Phenoxy-3(phenylselenenyl)propan-2-ol (Table 2, entry 14)
Yield 97%; 1H NMR (400 MHz, DMSO-d6) δ in ppm: 3.01 (br s,1H, OH), 3.20 (dd,1H, J = 12.8, 6.8 Hz, CH2), 3.28 (dd, 1H, J = 12.8, 6 Hz, CH2), 4.08 (m, 2H, CH2), 4.20 (m, 1H, CH2), 6.94 (d, 2H, J = 8 Hz, Ar–H), 7.04 (t, 1H, J = 7.2 Hz, Ar–H), 7.32 (m, 5H, Ar–H), 7.61 (dd, 2H, J = 3.6, 7.2 Hz, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ in ppm: 31.88, 69.23, 70.58, 77.30, 114.66, 121.30, 127.38, 129.37, 129.61, 132.89, 158.45.
3 Results and discussion
Initially, we explored the optimized reaction conditions for the Na-mediated cleavage of diphenyl disulfide followed by the addition of phenoxymethyl epoxide at room temperature as our model reaction (Scheme 1).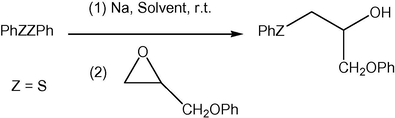
Reaction conditions optimization.
The results are summarized in Table 1. As indicated in Table 1, the cleavage process was performed with 2 mmol of Na in proportion to 1 mmol of diphenyl disulfide or diselenide and 1.2 mmol of phenoxymethyl epoxide (Table 1, entry 9). Using lower amounts of Na resulted in a dramatic decrease in the yield of ring-opened product (Table 1, entry 10). Moreover, the addition of more Na to the reaction interestingly lead to lower yields relative to the case of using 2 mmol of Na (Table 1, entries 7 and 8). Furthermore, the model reaction did not proceed at all in other solvents, such as CH2Cl2, DMF, Et2O, CHCl3, and n-hexane. Small amounts of reactivity were observed only in CH3CN (Table 1, entries 1–6). Based on these results, THF was chosen as the optimal reaction medium. These results show that both the type of solvent and the amount of sodium can affect the process of converting disulfide to the corresponding thiolate anion, as in the first step of the reaction. As expected, metallic sodium is able to react with disulfide in THF because the sodium ions formed because of the conversion of disulfide to thiolate anion can be better stabilized by coordination to THF molecules.
Entry
Solvent
Naa (eq)
Time (h)
Yieldb (%)
1
DMF
2
10
N.R.
2
CH2Cl2
2
10
N.R.
3
CHCl3
2
10
N.R.
4
Et2O
2
10
N.R.
5
n-Hexane
2
10
N.R.
6
CH3CN
1
10
38
7
THF
2
3.5
76
8
THF
1.5
3.5
79
9
THF
1
3.5
86
10
THF
0.5
3.5
35
To evaluate the scope and generality of this reaction for the ring opening of epoxides, the procedure with optimized reaction conditions was conducted on various disulfides and diselenides as well as numerous epoxide derivatives (Scheme 2, Table 2).
β-hydroxysulfides/selenides derivatives synthesis through ring opening of epoxides.
Entry
R
Z
R′/epoxy
Time (h)
Yielda (%)
1
C6H5
S
CH2OPh
3:30
96b
2
C6H5CH2
S
CH2OPh
5
70c
3
4-CH3C6H5
S
CH2OPh
4:30
81d
4
4-BrC6H5
S
CH2OPh
3:45
89e
5
C6H5
S
C2H5
4
76b
6
4-BrC6H5
S
C2H5
5
95e
7
C6H5
S
CH3
4.50
86b
8
C6H5CH2
S
CH3
6
73c
9
4-CH3C6H5
S
CH3
6
71c
10
4-BrC6H5
S
CH3
4.50
94e
11
C6H5
S
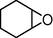
4
83b
12
4-CH3C6H5
S
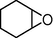
4.5
74g
13
C6H5
Se

3.5
80f
14
C6H5
Se
CH2OPh
4
97f
15
C6H5
Se
C2H5
4.5
75
16
C6H5
Se
CH3
4
81
17
C6H5
S
C6H5
5
33(A), 57(B)b
18
4-BrC6H5
S
C6H5
5.5
33(A), 52(B)e
19
C6H5
Se
C6H5
6
38(A), 51(B)h
All reactions were completed in a simple manner under air atmosphere with relatively short reaction times at ambient temperature (3.5–7 h) and with moderate to good yields of the products. The work-up stage of the processes was performed using preparative TLC. When common aliphatic epoxides were used with disulfides, the reactions proceeded with remarkable regioselectivity, and the β-hydroxy sulfides were obtained solely as regioisomers (A). No sign of regioisomer (B) was detected by PTLC and NMR analysis (Table 2, entries 1–15). This observation might be due to the fact that the nucleophilic attack by disulfides on aliphatic epoxides is performed in a pure SN2 manner exclusively on the less sterically hindered carbon atom of the ring. This means that the only governing factors in these cases would be steric. The same pattern was observed for the case of a diphenyl diselenide nucleophile in the ring opening process (Table 2, entries 13–16). The results were achieved using diphenyl disulfide/diselenide as the nucleophile for the ring opening of phenoxymethyl epoxide (Table 2, entries 1 and 14).
In the case of reactions involving epoxides with aryl oxacyclopropane ring, no strong regioselectivity was observed. This could be due to the fact that in these cases, the substituents can distort the electron distribution on the three-membered ring, which leads to the formation of some partial positive charge on the ring carbon atom bearing these substituents. No notable regioselectivity or yield was observed in these cases due to this partial charge formation on the more sterically hindered carbon atom of the epoxide ring, which somewhat facilitates the nucleophilic attack on the ring atom with more substituents (Table 2, entries 17–19). The competition between two carbon atoms of the ring to be the center for the nucleophilic attack by the thiolate or selenolate anions can be the reason for poor regioselectivity observed in these cases. However, this process still indicates a noticeable inclination for synthesizing the desired β-hydroxy sulfides and selenides with significant regioselectivity and high yields.
Although the exact reaction mechanism is not understood completely, thiolate/selenate appears to be generated first in the Red/Ox reaction between S—S/Se—Se bond and Na in THF. The generated thiolate/selenate is then reacted successfully with 1,2-epoxides to produce β-hydroxy sulfides/selenides (Scheme 3).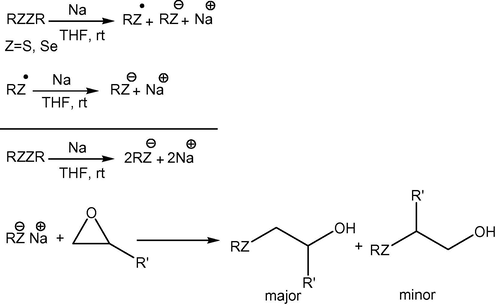
A proposed mechanism for cleavages of organic disulfide/diselenide by Na/THF.
4 Conclusion
In summary, a new efficient methodology for synthesizing β-hydroxy sulfides/selenides through the ring opening of epoxides has been reported. In this protocol, metallic sodium was used for the in situ conversion of disulfides/diselenides to their corresponding thiolate (selenolate) anions, which subsequently open the epoxide rings by nucleophilic attack. The major advantages of the reported methodology are: no use of any extra acidic catalyst for activating the epoxide ring, mild reaction conditions, simple work-up, moderate to good yield of products, and remarkable regioselectivity in most cases.
Acknowledgment
The authors acknowledge the partial financial support by Ilam University Research Council.
References
- ZnCl2 as an efficient catalyst in the thiolysis of 1,2-epoxides by thiophenol in aqueous medium. Synlett. 2003;15:2292-2296.
- [Google Scholar]
- Fe(III) substituted Wells–Dawson type polyoxometalate: an efficient catalyst for ring opening of epoxides with aromatic amines. Inorg. Chem. Commun.. 2013;28:37-40.
- [Google Scholar]
- LiClO4·3H2O promoted highly regioselective ring-opening of epoxides with thiols under neutral conditions. Catal. Commun.. 2006;7:224-227.
- [Google Scholar]
- Waste-free thiolysis of epoxide in water with high yield. J. Iran. Chem. Soc.. 2009;6:165-167.
- [Google Scholar]
- Regioselective ring opening of epoxides with thiols under solvent free and mild conditions using heterogeneous catalyst. Catal. Commun.. 2007;8:1065-1069.
- [Google Scholar]
- Enantioselective ring-opening reaction of meso-epoxides with ArSH catalyzed by a C2-symmetric chiral bipyridyldiol-titanium complex. Tetrahedron: Asymmetry. 2007;18:1313-1319.
- [Google Scholar]
- Highly enantioselective kinetic resolution of trans-2-(phenylthio)cyclohexanol derivatives by immobilized Candida antarctica B lipase. J. Mol. Catal. B: Enzyme. 2013;96:67-74.
- [Google Scholar]
- Regioselective ring opening of epoxides by nucleophiles mediated by lithium bistrifluoromethanesulfonimide. Tetrahedron Lett.. 2002;43:7083-7086.
- [Google Scholar]
- Enzymatic resolution of (RS) – β-hydroxy selenides in organic media. Tetrahedron: Asymmetry. 2004;15:3945-3954.
- [Google Scholar]
- Regioselective ring opening of epoxides with ortho-lithioanisoles catalyzed by BF3·OEt2. Tetrahedron. 2012;68:6463-6471.
- [Google Scholar]
- Zn(II)-catalyzed thiolysis of oxiranes in water under neutral conditions. J. Org. Chem.. 2003;68:8248-8251.
- [Google Scholar]
- Borax-catalyzed thiolysis of 1,2-epoxides in aqueous medium. Tetrahedron Lett.. 2008;49:6536-6538.
- [Google Scholar]
- Rongalite promoted highly regioselective synthesis of β-hydroxy sulfides by ring opening of epoxides with disulfides. Tetrahedron. 2009;65:5240-5243.
- [Google Scholar]
- Spinal mechanisms of antinociceptive effect caused by oral administration of bis-selenide in mice. Brain Res.. 2008;1231:25-33.
- [Google Scholar]
- Asymmetric Oxidations and Related Reactions: Catalytic Asymmetric Epoxidation of Allylic Alcohols (second ed.). Wiley-VCH; 2000.
- Asymmetric oxidations and related reactions: asymmetric epoxidation of unfunctionalized olefins and related reactions. In: Ojima I., ed. Catalytic Asymmetric Synthesis (second ed.). Wiley-VCH; 2000. p. :287-325.
- [Google Scholar]
- Bi(OTf)3 or Bi(OFA)3 catalyzed efficient, region and chemoselective synthesis of β-hydroxy thioethers from aryldisulfides in the presence of zinc powder. J. Braz. Chem. Soc.. 2005;16:673-676.
- [Google Scholar]
- Amberlyst-15: an efficient heterogeneous reusable catalyst for selective anti-Markovnikov addition of thiols to alkenes/alkynes and for thiolysis of epoxides. Catal. Commun.. 2013;41:29-33.
- [Google Scholar]
- Polyethylene glycol (PEG) 4000 catalysed regioselective nucleophilic ring opening of oxiranes a new convenient synthesis of β-hydroxyl sulfone and β-hydroxyl sulfide. Tetrahedron Lett.. 1994;50:10483-10490.
- [Google Scholar]
- Recyclable superparamagnetic Fe3O4 nanoparticles for efficient synthesis of thiolysis of epoxides. J. Mol. Catal. A: Chem.. 2012;361–362:68-71.
- [Google Scholar]
- Stereo- and regioselective thiolysis of 1,2-epoxides in water. Synth. Commun.. 2007;37:3239-3244.
- [Google Scholar]
- Regioselective reaction of epoxides with disulfides using Zn/AlCl3 system: a simple synthesis of β-hydroxy sulfides. Synth. Commun.. 2003;33:3103-3108.
- [Google Scholar]
- Odorless regioselective ring opening of epoxides with s-alkylisothiouronium salts as masked thiols in water. Arkivoc. 2009;2:298-307.
- [Google Scholar]
- CsF–Celite catalyzed regio- and chemoselective SN2 type ring opening of epoxides with thiol. Catal. Commun.. 2004;5:515-518.
- [Google Scholar]
- Diastereoselective oxidation of β-hydroxyl sulfides with TBHP: a comparative study of titanocenes and ti(oi-pr)4 as catalysts. Tetrahedron Lett.. 2002;58:6679-6683.
- [Google Scholar]
- Fe(OH)3 nano solid material: an efficient catalyst for regioselective ring opening of aryloxy epoxides with amines under solvent free condition. Original Res. Art.. 2014;469:442-450.
- [Google Scholar]
- Synthesis of-β-hydroxyselenides using benzeneselenol and oxiranes under supra molecular catalysis in the presence of β-cyclodextrin in water. Tetrahedron Lett.. 2005;46:8837-8839.
- [Google Scholar]
- Enantioselective ring-opening reaction of meso-epoxides with ArSH catalyzed by heterobimetallic Ti—Ga—Salen system. Tetrahedron Lett.. 2009;50:548-551.
- [Google Scholar]
- Quaternary ammoniums and a cationic sodium complex as supramolecular catalysts in ring-opening of epoxides by amines. Tetrahedron. 2014;70:1646-1650.
- [Google Scholar]
- Synthesis of enantiomerically pure substituted tetrahydrofurans from epoxides and phenylselenium reagents. Tetrahedron: Asymmetry. 2004;15:405-412.
- [Google Scholar]
- Reaction of 2-sulfanylbenzoic acid with 3,3-dibromobutane-2-thione as a route to benzoxathiepine derivatives. Russ. J. Org. Chem.. 2009;45:633-635.
- [Google Scholar]
- Regioselective ring-opening α-methylenation of aryl epoxides to conjugated allyl alcohols utilizing n-BuLi and Me2S⚌CH2 reagents. Tetrahedron Lett.. 2014;55:3443-3445.
- [Google Scholar]
- Regioselective epoxide ring opening mediated by iron oxide-pillared clay. J. Mol. Catal. A: Chem.. 2013;378(1):76-81.
- [Google Scholar]
- In Cl3-catalyzed highly regioselective ring opening of epoxides with thiols. Chem. Lett.. 2002;9:906-907.
- [Google Scholar]
- Regioselective ring-opening reactions of 1,2-epoxides with thiol and arylselenols directly promoted by [Bmim] Bf4. Tetrahedron Lett.. 2008;49:6471-6474.
- [Google Scholar]







