Translate this page into:
Nanocomposites of gold nanoparticles with pregabalin: The future anti-seizure drug
⁎Corresponding authors. amajlouni@uqu.edu.sa (Abdul-Wali Ajlouni), sfadil@ksu.edu.sa (Syed Farooq Adil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Application of nanoparticles in drug delivery has become an emerging phenomenon. This is typically achieved either via custom made nanoparticles or through the functionalization of pre-synthesized nanoparticles with the pharmaceutically active ingredients. In this study, Pregabalin, which is the active pharmaceutical ingredient of commercially available drug Lyrica, is used to functionalize pre-synthesized gold nanoparticles (AuNPs). The work was divided into two parts. The first part determined by synthesis of AuNPs. The second part was achieving conjugation of the AuNPs with Pregabalin to obtain nanocomposites (AuNPs-PGN). AuNPs formed were nanosized, spherical in shape, with a particle size ∼35 nm. The probable nanocomposite formation takes place by conjugation between AuNPs and the carboxyl group (COOH) of Pregabalin.
Keywords
AuNPs
Pregabalin
Nanocomposites
Drug delivery
1 Introduction
Recent advancements in the field of nanotechnology, a scientific multi-disciplinary field (Nasrollahzadeh et al., 2019), for the development of nanomaterials for various applications, including the competent transportation of drug molecules led to the development of nanomedicines (Majeed et al., 2019). Generally, nanomedicines deals with drug delivery applications with an emphasis on the applications and physio-chemical properties of the primary organic nanocarriers (dendrimers and liposomes) and inorganic nanocarriers (quantum dots, carbon nanotubes and gold nanoparticles) (Paul and Sharma, 2020). The inorganic nanocarriers, mostly nanoparticles (NPs) that have distinctive properties of displaying higher surface area to size, volume ratio, structure like rod or spherical etc. owing to which they are being extensively used (Menon et al., 2017), which is clearly evident by the gradual increase in the number of nanoparticles based drugs over the last couple of decades (Fan et al., 2016; Raza et al., 2019; Sahoo et al., 2017). They have made huge impact on drug delivery, as these materials have unique ability to enhance various properties of therapeutic carriers including bioavailability, solubility and immunogenicity etc. due to their physicochemical properties that enhance their interactions with various bio-macromolecules and cellular moieties (Gao et al., 2018; Shi et al., 2017).
However, among the various nanoparticles that have been reported for the medicinal applications, the Au NPs have attracted the spotlight particularly due to the combination of unique physical, chemical and optical properties, which has been tremendously exploited in various fields including medicine, and advanced nano-biomedical applications (Blanco et al., 2015). In particularly, the AuNPs are applied to imaging, photo thermal therapy, sensing, antimicrobials and drug delivery (Küünal et al., 2018). Mainly, AuNPs possess great potential in drug delivery, as they enhance the drug concentration at the infected sites. Moreover, they are capable of delivering several types of drugs molecules, recombinant proteins, vaccines, or nucleotides into their targets with control release mechanisms via biological stimuli (internal) or light activation (external) (Kong et al., 2017). They can be specifically engineered to reduce the toxicity of the drug and also lower the dose-limiting side effects of various active pharmaceutical ingredients (APIs) (Dreaden et al., 2012; Pardo et al., 2018; Han et al., 2007). Nevertheless, only few studies have been reported on the direct conjugation of NPs with commercial drugs for enhanced drug delivery. For instance, Saha et al., directly conjugated AuNPs with various antibiotics such as ampicillin, streptomycin and kanamycin and in vitro evaluation of the nanocomposites for their antimicrobial activities was carried out (Saha et al., 2007).
Lyrica is a pharmaceutical compound, consisting of pregabalin (PGN), an anti-seizure drug that binds to alpha2 delta auxiliary subunits of voltage-gated calcium channels. The drug slows down impulses in the brain and affects the chemicals that send pain signals across the nervous system. In a chemical sense, it has the structure (as shown in Scheme 1) that is an alpha-2-delta ligand, that is believed to work by calming hyper-excited neurons. Hence, in this study, we have demonstrated the synthesis of AuNPs using tri sodium citrate as reducing agents and directly conjugated it with PGN to yield AuNPs-PGN nanocomposites (Scheme 1).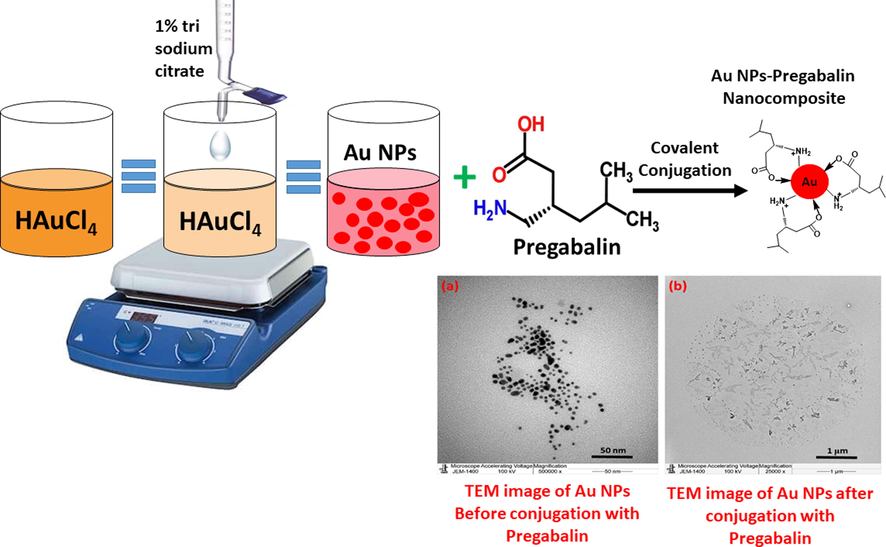
Schematic representation of the synthesis of AuNPs using tri sodium citrate as reducing agents and their use in preparation of AuNPs-PGN nanocomposites.
2 Materials and methods
2.1 Materials
Tetrachloroauric(III) acid trihydrate(HAuCl4·3H2O, 99%) was purchased from Sigma-Aldrich (USA), Tri-Sodium citrate dehydrate (Na3C6H5O7·2H2O, 99%) was obtained from BDH-AnalaR limited poole (England), Sodium hydroxide (NaOH, 98%) was purchased from Loba Chemie (India), High purity pregabalin powder was supplied from Jazeera Pharmaceutical Industries (KSA) and deionized (DI) water from a Millipore Milli-Q system which was used in all experiments.
2.2 Methods
2.2.1 Synthesis of AuNPs by using trisodium citrate
An approach used in this experiment depends on AuNPs dispersion in water is prohibited by electrostatic stabilization. The work has been implemented as follows, 10 mL of a 1 mM HAuCl4 aqueous solution in a round bottom flask and heated to boiling point. A 1 mL of a 1% solution of trisodium citrate dihydrate was added to the boiling solution of HAuCl4. The reaction done under constant stirring with a magnetic bar. The nanoparticles formation is achieved when the solution turns deep red color. The color transformation clearly indicates the formation of gold nanoparticles.
2.2.2 Preparation of AuNPs-PGN nanocomposites
The functionalization of the AuNPs was carried out using the active molecules of Lyrica, which is Pregabalin to form nanocomposites. The nanocomposite was prepared using AuNPs synthesized using Trisodium Citrate. The nanocomposite was prepared, at room temperature by dispersing 1.9 mg of AuNPs in 2.0 mL DI water. The sodium salt of PGN (PGN-Na) was prepared by preparing 98% NaOH by dissolving 0.05 g NaOH in 4.0 mL distilled water, to which 0.2 g of pregabalin (PGN) was added gradually to the solution until the whole PGN amount disappeared, which indicates the formation of PGN-Na. Later, 2.0 mL of (PGN-Na) sample was added to 2.0 mL of dispersed AuNPs. The obtained solution was put on magnetic stirrer, and covered with aluminum foil. The solution was stirred until the complete consumption of AuNPs in the solution, and the progress of the same was monitored by UV-Vis spectroscopy.
2.3 Characterization
UV-Vis spectrophotometer (Perkin Elmer lambda 35, Waltham, MA, USA) was used to measure the optical measurements of the as-synthesized AuNPs-PGN nanocomposite. FT-IR measurements were performed on a Perkin Elmer, 1000 FT-IR spectrometer (Waltham, MA, USA). The surface morphology and the elemental composition of the samples was determined by scanning electron microscopy (SEM) and Energy Dispersive X-ray Analysis (EDX), which were carried out using Jeol, JED-2200 series (Japan). Transmission electron microscopy (TEM) measurement was carried out using Jeol TEM model JEM-1011 (Japan) at 100 keV to determine the shape and size of nanoparticles and confirm the formation of nanocomposites.
3 Results and discussion
3.1 UV-Vis analysis
Initially, the formation of AuNPs was confirmed using UV-Vis spectroscopy. Fig. 1 shows the UV-Vis spectra of AuNPs using trisodium citrate. UV-Vis spectrometry measures the light absorbed by a medium at wavelengths in the ultraviolet and visible bands. The sample is irradiated with light of identified wavelength and intensity and the intensity of the transmitted light is measured. In present study, the absorption of AuNPs was measured using a single beam spectrophotometer and absorption maxima was noted at different wavelength (400–600 nm). Metallic nanoparticles manifest absorption peaks at the wavelengths consistent to plasmon excitation. The surface plasmon resonance of AuNPs showed a single absorption peak between 500 and 550 nm in the visible light, and show dense absorption of visible light around 522 nm. This provides bright red color of AuNPs, this color varies depending on their size.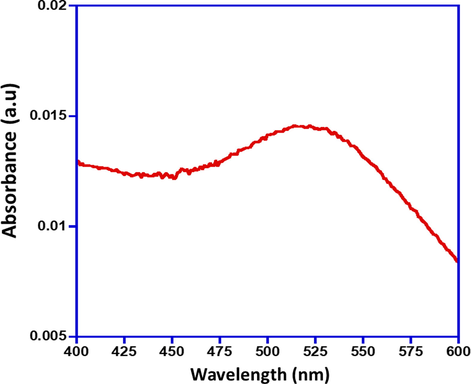
UV-Vis spectra of the as-synthesized AuNPs using trisodium citrate.
The AuNPs functionalization was carried out using the active molecules of Lyrica, which is Pregabalin (PGN) to form a nanocomposite. Primarily the AuNPs conjugation with Pregabalin was monitored by UV–Vis absorption spectroscopy. The UV–visible absorption spectra for PGN-Na yields λmax = 200 nm shown in Fig. 2. The UV–Vis absorption spectras obtained after the addition of PGN-Na to AuNPs are given in Fig. 3, and the readings are recorded at regular intervals, which depicts the gradual changes in the absorption spectra upon complexation of PGN-AuNPs. The absorption spectra immediately measured after adding PGN-Na to AuNPs and the reading is recorded as 0 h, which gives an absorption max at around 522 nm depicting the presence of un-complexed AuNPs in the sample.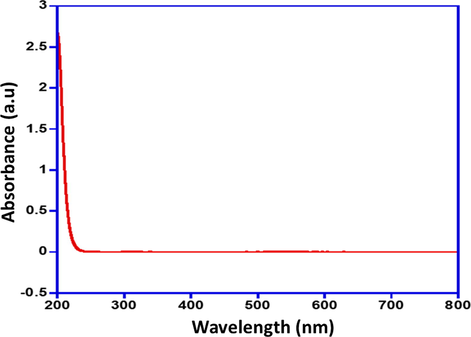
UV-Vis absorption spectra of PGN-Na.
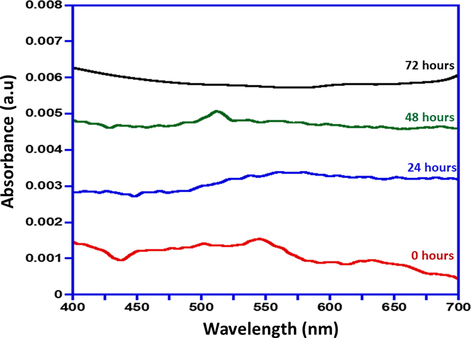
UV-Vis absorption spectra of gradual formation of AuNPs-PGN nanocomposites.
However, the absorption spectra of the sample measured after 24 h and 48 h of adding PGN-Na to AuNPs, shows that is a slump in the intensity of absorption max i.e. ∼522 nm indicating that the progress of conjugation of PGN-AuNPs nanocomposite and the presence of AuNPs in the sample. The absorption spectra obtained after 72 h of adding PGN-Na to AuNPs reveals that AuNPs have completely complexed with PGN-Na, that is evident from the disappearance of peak at absorption max of ∼522 nm completely. Fig. 3 signifies the comparative absorption spectra recorded at intervals i.e. 0 h, 24 h, 48 h and 72 h of adding PGN-Na to AuNPs and formation of AuNPs-PGN nanocomposites.
3.2 FT-IR analysis
Fig. 4 shows the FT-IR spectrum of as-synthesized AuNPs. The evidence of the surface functionalization of AuNPs by citrate ions was obtained by FT-IR measurements. The characteristic peaks at 1385 and 1595 cm−1 (Fig. 4) associated to the symmetric and anti-symmetric stretching of COO−. These characteristic peaks confirm the interaction between the AuNPs and citrate ions. The peak at 3427 cm−1 corresponds to hydroxy group and the peak at ∼2933 cm−1 associated to asymmetrical stretch of CH. The formation of AuNPs, reveals that the citrate ions play a dual role of reducing the Au(+3) to Au(0) as well as stabilizing/capping it as confirmed by its existence on the surface of AuNPs.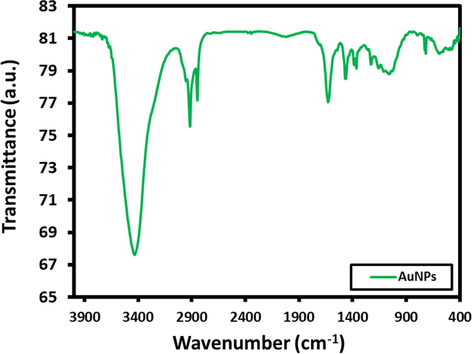
FT-IR spectra of the as-synthesized AuNPs.
FT-IR spectra of PGN (Fig. 5) was carried out to find out the functional groups of presents, it indicated the existence of carboxyl group (—COOH), with strong signal at 2700 cm−1, and amine group (NH2) with a weak signal at 1600 cm−1. However, the PGN is dissolved in NaOH in order to prepare sodium salt of PGN, wherein the carboxyl group (—COOH) gets converted to (—COONa), and amine group, is more free in the medium, so they can absorb IR more effectively, so the absorbance is enhanced, and became more effective at the two wavenumber values, i.e. signals at 2700 cm−1 and 1600 cm−1, as shown in Fig. 6.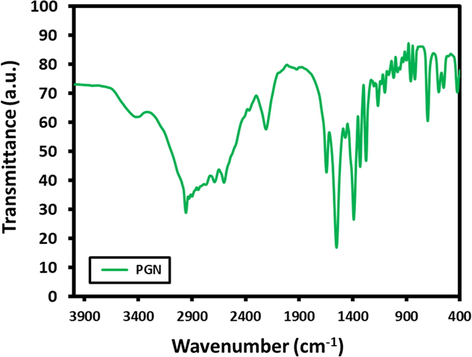
FTIR spectra of Pregabalin (PGN).
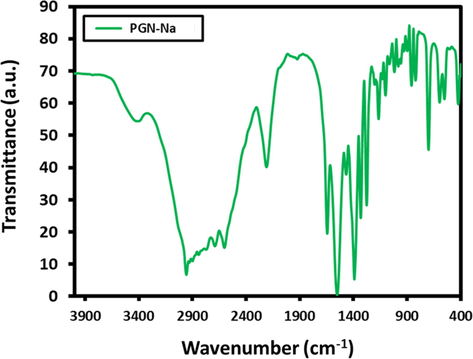
FTIR spectra of PGN-Na.
From the FTIR spectra of AuNPs-PGN nanocomposite (Fig. 7), it is clear that there is no presence of the 2700 cm−1, functional groups of carboxyl group totally, and amine group (—NH2) signal became very weak at 1600 cm−1. This indicates the nanocomposite formation takes place by the conjugation between AuNPs and the carboxyl group (—COONa). The decline in the signal intensity of amine group (—NH2) could be attributed to the additional conjugation of PGN-Na with the lone pair of electron from ‘N’ atom of the –NH2 group to the vacant orbitals of Au0 to yield a more stable covalent linkage, yielding a nanocomposite with enhanced stability.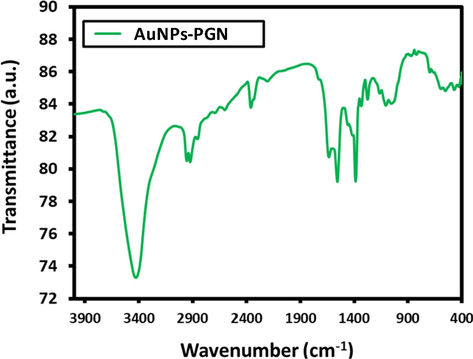
FTIR spectra of AuNPs-PGN nanocomposites.
This is in accordance with the previously reported studies wherein it was observed that covalent conjugation is achievable through reaction between gold nanoparticles and hetero atoms (Zhao et al., 2013). Moreover, for an easily modifiable surface that can be selectivity geared towards application specific targets, carboxyl (—COOH) functionalized gold nanoparticles give researchers the power to maximize the utility of AuNPs (DeLong et al., 2010).
3.3 Scanning electron microscopy
The surface morphology of the as-synthesized AuNPs using trisodium citrate was investigated using scanning electron microscopy (Fig. 8). SEM micrograph of the AuNPs suggest that the morphology of the AuNPs are well defined spherical in shape. In addition, SEM micrograph also showed that the surface of the AuNPs were very homogeneous without any apparent phase separation.
SEM micrographs of the as-synthesized AuNPs.
SEM micrographs of the AuNPs-PGN nanocomposites are shown in Fig. 9. This figure suggests that the Pregabalin casings the AuNPs completely, indicating complete conjugation between them.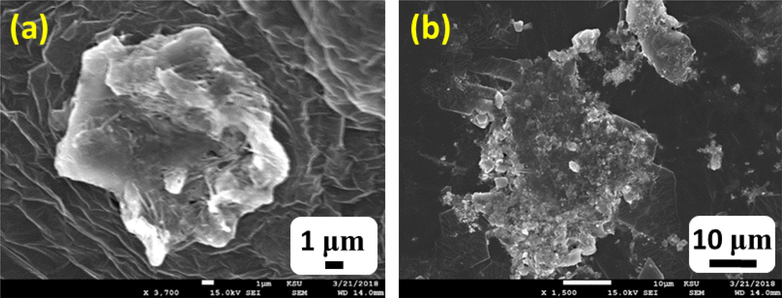
SEM micrographs of the AuNPs-PGN nanocomposites (a) 1 µm and (b) 10 µm.
3.4 Transmission electron microscopy and energy dispersive X-ray spectroscopy
The TEM images of the AuNPs synthesized using trisodium citrate exhibited size and shape as shown in Fig. 10(a). The TEM images evidently shows that the AuNPs are nanosized and spherical in shape with a particle size of ∼ 35 nm. Furthermore, the elemental composition of the as-synthesized AuNPs was also determined by energy-dispersive X-ray spectroscopy (EDX), which discloses the elemental composition of the AuNPs as shown in Fig. 10(b). The intense signal at 2.18, 9.6 and 11.5 keV strongly recommends that Au was the major element, which has an optical absorption in this range due to the surface plasmon resonance. It was also illustrious that other signals were also found in the range 0.0–0.5 keV, which represent the typical absorption of oxygen and carbon. This EDX analysis confirms the formation of AuNPs.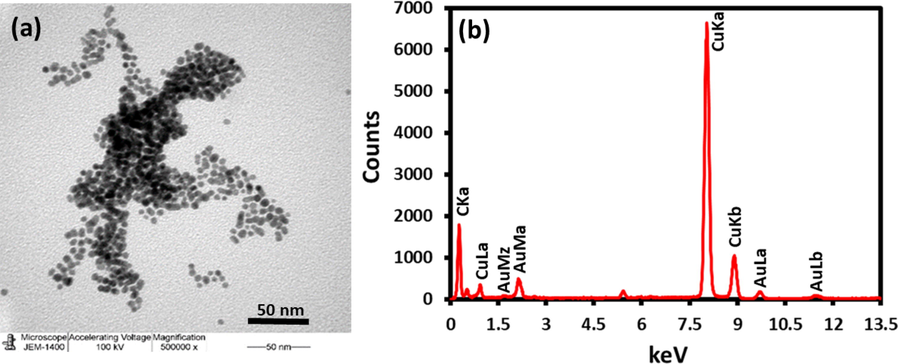
(a) TEM image of the as-synthesized AuNPs and (b) EDX of the AuNPs.
Comparative TEM images of the AuNPs and AuNPs-PGN nanocomposites are shown in Fig. 11, from which it can be observed that the AuNPs surface is completely covered with PGN indicating an remarkable conjugation takes place between the AuNPs and PGN to form the AuNPs-PGN nanocomposites.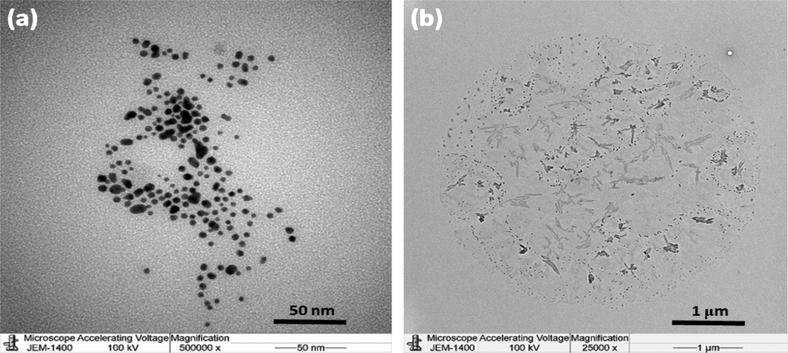
TEM images of (a) AuNPs and (b) AuNPs-PGN Nanocomposites.
4 Conclusion
Herein we report the successful preparation the nanocomposites of AuNPs-PGN, an anti-seizure drug. The UV–Vis absorption spectra of samples of AuNPs and PGN-Na revealed that a complete formation of nanocomposite takes place in 72 h. SEM and TEM micrographs of the nanocomposites of AuNPs-PGN suggested that the PGN completely covers the surface of the AuNPs indicating the excellent conjugation. Fourier transform infrared spectroscopy (FTIR) also confirmed the functional groups of PGN on the surface of AuNPs, also indicating the formation of nanocomposites. It also confirmed that the conjugation takes place by the interaction between the –COONa functional group of PGN-Na and additional stability is augmented to the nanocomposite by the donation lone pair of ‘N’ atom from the –NH2 group and AuNPs to yield a more stable covalent linkage in the nanocomposite, which was confirmed by the partial absence of amine group (NH2) peaks in the FT-IR spectra. Further studies into the employment of these nanocomposites for in vitro and in vivo studies will be carried out and the results will be reported later.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project No. RG-1436-032.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol.. 2015;33:941.
- [Google Scholar]
- Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl.. 2010;3:53.
- [Google Scholar]
- Size matters: gold nanoparticles in targeted cancer drug delivery. Therapeutic Deliv.. 2012;3:457-478.
- [Google Scholar]
- On The Latest Three-Stage Development of Nanomedicines based on Upconversion Nanoparticles. Adv. Mater.. 2016;28:3987-4011.
- [Google Scholar]
- Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev.. 2018;127:46-57.
- [Google Scholar]
- Han, G., Ghosh, P., Rotello, V.M., 2007. Functionalized gold nanoparticles for drug delivery. Nanomedicine (Lond). 2,113–123.
- Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1445.
- [Google Scholar]
- Emerging Applications of Nanoparticles and Architecture Nanostructures. Elsevier; 2018. p. :411-446. 10.1016/B978-0-323-51254-1.00014-2
- Nanobiotechnology: Applications of nanomaterials in biological research. Integrating Green Chem. Sustain. Eng. 2019:581-615.
- [Google Scholar]
- A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resource-Efficient Technol.. 2017;3:516-527.
- [Google Scholar]
- An Introduction to Nanotechnology. In: Interface science and technology. Elsevier; 2019. p. :1-27.
- [Google Scholar]
- Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Molecules. 2018;23:378.
- [Google Scholar]
- Inorganic nanoparticles for targeted drug delivery. In: Biointegration of Medical Implant Materials. Elsevier; 2020. p. :333-373.
- [Google Scholar]
- Solid nanoparticles for oral antimicrobial drug delivery: A review. Drug Discovery Today. 2019;24:858-866.
- [Google Scholar]
- In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett.. 2007;2:614.
- [Google Scholar]
- Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. In: Nanomedicine in cancer. Pan Stanford Publishing; 2017. p. :73-124.
- [Google Scholar]
- Cancer nanomedicine: progress, challenges and opportunities. Nature Rev. Cancer. 2017;17:20.
- [Google Scholar]
- The structural and bonding evolution in cysteine–gold cluster complexes. Phys. Chem. Chem. Phys.. 2013;15:1690-1698.
- [Google Scholar]







