Translate this page into:
Nanoemulsion of Myrtus communis essential oil and evaluation of its larvicidal activity against Anopheles stephensi
⁎Corresponding authors. amani76@gmail.com (Amir Amani), sedaghat@hotmail.co.uk (Mohammad Mehdi Sedaghat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Purpose
Excessive use of chemical insecticides has caused environmental pollution and vector resistance. Herbal essential oils with larvicidal properties are good alternatives to synthetic insecticides.mIn this study, larvicide properties of Myrtus communis essential oil and its nanoemulsion against Anopheles stephensi were investigated.
Methods
Components of Myrtus communis essential oil were identified by GC–MS. Nanoemulsion of essential oil was made with Tween 80, Span 20, and water. Dynamic light scattering (DLS) and transmission electron microscopy (TEM) determined particle size and morphology of nanoemulsions. The larvicide activity compared with bulk essential oil.
Results
A total of 107 M. communis essential oil compounds were discovered. The morphology of a selected nanoemulsion was spherical. LC50 and LC90 of M. communis essential oil were calculated as 26.1 and 46.2 µg/ml, respectively. The larvicide activity of nanoemulsion increased by 40% compared to the bulk essential oil. The nanoemulsion's larvicide activity (100%) lasted up to 3 days, while the essential oil had larvicide properties only for up to 24 h.
Conclusions
Myrtus communis essential oil was found to be an effective larvicide and classified as an active larvicide. The residual efficacy of the nanoformulation of M. communis significantly increased compared with the bulk essential oil.
Keywords
Myrtus communis
Nanoemulsion
Larvicide
Anopheles stephensi
1 Introduction
Malaria remains a preventable cause of serious illness and death worldwide. The disease is caused by Plasmodium parasites, which are transmitted to other people through the bites of infected Anopheles mosquitoes (Ward 2008). Infants, children under 5, pregnant women, HIV / AIDS patients, migrant workers, and travelers are at higher risk for malaria (Osanloo et al., 2019a, 2019b, 2019c). Anopheles stephensi is a major vector of the disease. This species is the most abundant Anopheles species in Iran's malaria-prone areas (Osanloo et al., 2017a, 2017b, Firooziyan et al., 2021). According to the World Health Organization, until 2011, the distribution of A. stephensi was limited to certain countries in Southeast Asia and the Arabian Peninsula. Since then, this vector has expanded to Djibouti (2012), Ethiopia (2016), Sri Lanka (2017), and Sudan (2019). In the Horn of Africa, the vector appears to be expanding from neighboring countries (Djibouti) to neighboring countries (Osanloo et al., 2019a, 2019b, 2019c). Anopheles stephensi quickly adapts to the local environment. That is why it spreads to new lands (Osanloo et al., 2018). In addition, it resists several classes of insecticides and poses potential challenges to be controlled (Organization 2005, Fathian et al., 2015, Osanloo et al., 2019a, 2019b, 2019c). The danger of pesticides to ecosystems, human health, and other living organisms have become apparent, which has led to increased attention to herbal pesticides, especially plant essential oils, in recent years (Khanavi et al., 2013, Osanloo et al., 2019a, 2019b, 2019c).
The main problem of using essential oils in nature is their high volatility and consequent instability. To deal with this problem, nanoformulations should increase the stability and efficiency of the desired compound (Guan et al., 2010, Osanloo et al., 2017a, 2017b, Osanloo et al., 2018, Osanloo et al., 2019a, 2019b, 2019c, Firooziyan et al., 2021). Due to the small size of the droplets and the resulting transparency and long-term physical stability (without any apparent coagulation, precipitation, or biphasic), Nanoemulsions cause more adsorption and reduced material consumption (Naseema et al., 2021). The term nanocapsule with dimensions of 10 to 1000 nm is used (Hemingway et al., 2006). Nanoencapsulation of the active component prevents chemical reactions between the active substance and light, moisture, and oxygen. It also reduces side effects, prolongs the shelf- life, and controls the release of the active substance (Hemingway et al., 2006, Sell 2006, Bergeson 2010). For example, the controlled release of insecticides may provide several months of efficacy in vector control applications (Yadegarinia et al., 2006). Preparation and fabrication of plant-based nanoemulsions could be an interesting alternative to chemical pesticides for controlling mosquitoes. Nanoemulsions are also non-irritation to the skin, have minimal toxicity, and are not flammable (Messaoud et al., 2005, Gurpreet and Singh 2018).
Myrtus communis is a small shrub that normally grows to a height of 1 to 3 m. This plant has an anti-hair loss, antifungal, antibacterial, anti-inflammatory, and anti-dandruff properties (Tuberoso et al., 2006, Akin et al., 2010).
The essential oils of M. communis showed different insecticidal activity against Culex pipiens, Aedes albopictus, Pediculus humanis capitis, Mediterranean flour moth Ephestia kuehniella Zeller, the Indian meal moth Plodia interpunctella Hubner and the bean weevil Acanthoscelides obtectus Say (Traboulsi et al., 2002, Ayvaz et al., 2010, Sumbul et al., 2011). In addition, the repellent properties of this essential oil have been reported against A. stephensi (Tavassoli et al., 2011, Kayedi et al., 2014).
In this study, the larvicidal activity of M. communis essential oil (MEO) against A. stephensi was investigated and for the first time, a nanoformulation of M. communis essential oil (MNE) was prepared. The larvicidal properties of MNE were compared with MEO according to the guidelines of the World Health Organization (Organization 2005).
2 Materials and methods
2.1 Materials
MEO (100% purity) was prepared from Green Plants of Life Company (Iran), Tween 80%, Span 20, and Ethanol were from Merck Chemicals (Germany).
2.2 Analysis of MEO compounds
NIST & Wiley libraries were used for the identification. The calculation was performed with a Flame ionization detector (FID). GC-MS analysis (Agilent Technologies, 7890A) was used to identify MEO compounds. The mass selection detector was 5975C VL MSD with a Triple-Axis detector and the Ion source was Electron Impact (EI) 70 eV. The column type was Rtx 5 MS with a length of 30 m and an inner diameter of 0.25 mm. The conditions and temperature program are given in Table 1.
Conditions
1. Injection port and ion source temperature
230 °C
2. Carrier gas
He 99.999%
3. Sample volume
0.2 μL
Temperature Program
Initial temperature (°C)
40
Initial time (min)
1
Program rate (°C/min)
3
Final temperature (°C)
270
Final time (min)
10
Split ratio (ml/min)
100
Septum purge (ml/min)
–
Flow rate (ml/min)
1
2.3 Mosquito raring
Anopheles stephensi mosquitoes were raised in the insectary at 29 ± 1 °C with a relative humidity of 70 ± 5% under 12 h lightness/12 h darkness. Mosquitoes were fed with sugar (10%) and defibrillated with sheep blood. The larvae were fed with fish flakes. Third and early fourth instar larvae were selected for larvicide tests that had been eaten the day before.
2.4 Bioassay test
Serial dilutions of the MEO were prepared using ethanol. According to the WHO guideline, water chlorine-free (room temperature, pH 7) was used for the bioassay of larvae. One ml of diluted MEO or MNE was added to 249 ml of water. Then 25 healthy larvae were added to the beaker. The number of live and dead larvae per beaker was counted after 24 h.
2.5 Residual effect
To test residual properties, 1 ml of MEO or MNE was added to 249 ml of water and 25 live larvae were added. After 24 h and reading the test results, without changing the solution, all larvae (dead or alive) were removed and 25 new live larvae were added to the beaker. The larvae were exchanged for up to 8 days, and the results were read each day.
All tests were repeated 4 times. In each replicate, two control groups were considered to have 1 ml of ethanol instead of MEO or MNE.
2.6 Statistical analysis
Lethal concentrations of 50% and 90% (LC50 and LC90) were calculated using Minitab software (Pennsylvania State University by researchers Barbara F. Ryan, Thomas A. Ryan, Jr., Brian L. Joiner in 1972) and compared using an independent sample test by SPSS. The regression line was plotted using Excel 2007 software (the Microsoft Corporation in 1985).
2.7 Preparation of MNE
First, Tween 80, Span 20 and the MEO (10 min, 600 rpm) were mixed in colorless glass vials at room temperature. The water was then added dropwise and stirred for 38 min. The solutions were placed in a dark cupboard at room temperature for 24 h. Vials with signs of phase separation, precipitation, or creaminess were discarded. The particle size (PS) of homogeneous solutions was measured by dynamic light scattering (DLS) (K-ONE.LTD, Korea). Transmission electron microscopy (TEM) (Zeiss, Germany) was used to confirm PS and examine particle morphology.
3 Results
3.1 Chemical composition of MEO
A total of 107 compounds were identified in the MEO (Table 2). The major compounds identified were α-Pinene (34.199%), dl-Limonene (16.587%), 1,8-Cineole (8.301%), Linalool (8.223%), and Linalyl acetate (4.945%).
No.
Retention Index
Compound
%
No.
Retention Index
Compound
%
1
932
alpha Pinene
34.2
55
1423
1-Cyclohexyl-1-butyne
0.1
2
1137
dl-Limonene
16.6
56
1264
2,6-Octadienal, 3,7-dimethyl
0.1
3
1026
1,8-Cineole
8.3
57
1639
Aromadendrene
0.1
4
1214
Linalool
8.2
58
1188
Bicyclooct-1-ene, 7-exo-ethenyl
0.1
5
1373
Linalyl acetate
4.9
59
954
Camphene
0.1
6
1020
Benzene, 1-methyl-4-(1-methylethyl)-
3.4
60
1639
Phenacetic acid
0.1
7
1186
3-Cyclohexene-1-methanol
2.7
61
1365
2-Cyclohexen-1
0.1
8
1361
2,6-Octadien-1-ol, 3,7-dimethyl-, acetate, (Z)
2.7
62
1739
1H-Inden
0.1
9
1257
(+)-4-Carene
2.2
63
1844
2,5-Dihydro-5-methoxy-2-furanone
0.1
10
1001
Delta.3-Carene
0.9
64
1316
Camphene
0.1
11
1058
Bicyclo
0.9
65
1218
D-Fenchyl alcohol
0.1
12
1452
alpha-Humulene
0.8
66
1654
alpha-Farnesene
0.1
13
1570
trans-Caryophyllene
0.8
67
1407
Acetic acid
0.1
14
1590
Globulol
0.7
68
1578
1-Cycloheptene
0.1
15
1603
Propanoic acid
0.7
69
1065
Sabinene
0.0
16
1583
Caryophyllene oxide
0.6
70
1215
trans-Carveol
0.0
17
1324
Myrtenyl acetate
0.6
71
1740
alpha.-Bisabolene epoxide
0.0
18
1492
Benzene, 1,2-dimethoxy-4-(2-propenyl)
0.5
72
1653
trans-beta-Farnesene
0.0
19
1088
Bicyclo heptane, 6,6-dimethyl-2-methylene
0.5
73
1417
3-Methylenebicyclo octane-2-one
0.0
20
1141
Dimethyl ether
0.5
74
1632
2(1H)-Naphthalenone
0.0
21
1639
Aromadendrene
0.5
75
1474
exo-2-Hydroxycineole acetate
0.0
22
1709
1H-Cycloprop azulen
0.5
76
1792
Naphthalene
0.0
23
1608
12-Oxabicyclo
0.4
77
1186
3-Cyclohexene-1-methanol
0.0
24
1252
Benzene, 1-methoxy-4-(2-propenyl)
0.4
78
1559
Germacrene B (CAS)
0.0
25
1086
alpha Terpinolene
0.4
79
1590
1,2-Benzenedicarboxylic acid, 3-nitro
0.0
26
1299
3-Cyclohexen-1
0.4
80
961
Verbenene
0.0
27
1342
4,6-Diethyl-2-methoxypyrimidine
0.4
81
1880
Phosphonous dichloride
0.0
28
1577
1H-Cycloprop[e]azulene
0.3
82
1052
Bicyclo heptane, 7,7-dimethyl-2-methylene
0.0
29
1633
Benzamide, 3,4-fluoro
0.3
83
1674
10-Methyl-2,5;3,10-diepoxybicyclo decane
0.0
30
1434
Neryl acetete neryl
0.3
84
1444
Naphthalene
0.0
31
1370
Phenol, 2-methyl-5-(1-methylethyl)
0.3
85
1582
6,6-Dimethyl-3-oxatricyclo
0.0
32
1090
beta-Myrcene
0.3
86
1578
Spathulenol
0.0
33
1088
3-ethylpropadiene
0.3
87
1407
Longifolene-(V4)
0.0
34
1135
trans-Pinocarveol
0.2
88
1326
Limonene dioxide 2
0.0
35
1044
1,3,6-Octatriene, 3,7-dimethyl-, €
0.2
89
1489
beta-Selinene
0.0
36
1389
Neoisolongifolene
0.2
90
1283
Borneol
0.0
37
1280
CIS-Verbenol
0.2
91
1086
alpha-Terpinene
0.0
38
1310
2-Cyclohexen-1-one
0.2
92
1099
alpha-PINENE
0.0
39
1592
Veridiflorol
0.1
93
1122
3-Cyclopentene-1-acetaldehyde, 2
0.0
40
1590
Epiglobulol
0.1
94
1803
6,7-Dimethoxy-5-hydroxymethylbenzofuran
0.0
41
1294
l-Phellandrene
0.1
95
1871
Phosphorous acid, tributyl ester
0.0
42
1531
1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-
0.1
96
1350
Citronellyl acetate
0.0
43
1099
3-Oxatricyclo octane, 2,7,7-trimethyl-
0.1
97
1639
caryophylla-4(12),8(13)-dien-5beta-ol
0.0
44
1379
trans-Geraniol
0.1
98
650
2-Propanone (CAS)
0.0
45
1489
Eudesma-4(14),11-diene
0.1
99
1783
2-Naphthalenemethanol
0.0
46
1032
cis-Ocimene
0.1
100
1007
Acetic acid
0.0
47
1054
gamma Terpinene
0.1
101
1566
1H-Cyclopropa[a]naphthalene
0.0
48
1316
Camphene
0.1
102
1112
Bicyclo hept-2-ene, 3,7,7-trimethyl
0.0
49
1632
1-Ethyl-3,4,5,6,12,12b-hexahydro
0.1
103
1394
Benzene, 1-methyl-3-(1-methylethyl)
0.0
50
1170
cis-Linaloloxide
0.1
104
788
3-Pentanone, 2,4-dimethyl
0.0
51
1654
2-Octene, 2,3,7-trimethyl-
0.1
105
931
1,2,4,4-Tetramethylcyclopentene
0.0
52
1374
Isoledene
0.1
106
1008
Bicyclo hept-2-ene, 2,7,7-trimethyl
0.0
53
1319
Bicyclo hept-3-en-2-one
0.1
107
1008
Delta3-Carene
0.0
54
1496
Ledene
0.1
100
3.2 Determination of droplet size and morphology of MNE
Ten MNEs were prepared. From DLS results, a solution with d50 ∼ 200 nm, d90 ∼ 400 nm, and span < 1 was selected as the best nanoformulation (F4) for the next steps. Fig. 1 shows the DLS of the selected sample.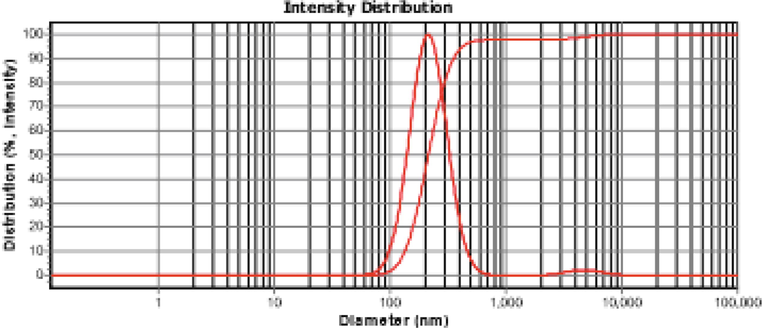
DLS results of MNE.
4 Morphology of MNE
The TEM image of F4 is given in Fig. 2. The MNE droplets were well-formed, and the particles were almost spherical.
TEM image of the MNE particles.
4.1 MEO larvicide properties
The larvicide effect started from 12.5 µg/ml and increased with increasing concentration and at 100 µg/ml all larvae were killed. Based on probit analysis, calculated LC50 and LC90 values for the essential oil were 26.1 (17.56–39.44) and 46.2 (38.62–61.18) µg/ml, respectively (Fig. 3).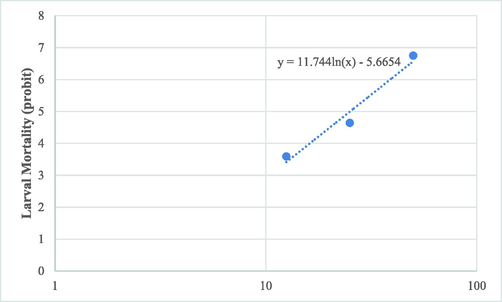
Probit regression line of A. stephensi larvae exposed to different interval concentrations of MEO.
4.2 Comparison of larvicidal properties MNE with MEO
To compare the larvicide properties of MNE and MEO against A. stephensi larvae, equal concentrations of MEO and MNE (F4) were used in the test. As shown in Fig. 4, after 24 h, MNE had 40% more efficacy compared with MEO. Based on the t-test, p < 0.001 was obtained.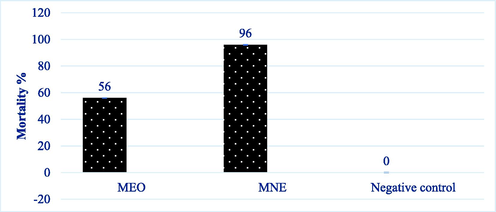
Comparison of larvicidal effects of MNE and MEO against A. stephensi larvae after 24 h.
The MNE showed better larvicidal properties in the 8-day test compared to its MEO and was able to kill all larvae in up to three days. After the third day, larval mortality decreased and reached 44%. MEO was able to control 100% of the larvae only for the first day, and the larval mortality rate decreased from 92% to 16% from the second day to the sixth day. MEO did not affect the larvae on the seventh and eighth days of the experiment (Fig. 5).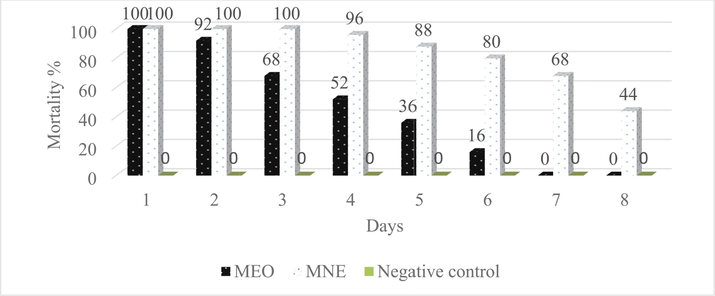
Comparison of larvicidal properties MNE and MEO against A. stephensi larvae in 8 days.
5 Discussion
Chemical pesticides cause serious damage to the environment and non-target animals (Guan et al., 2010). In addition, frequent use of pesticides has led to pests resistance. This has reduced the number of pesticides suitable for use in vector control programs, especially in the control of malaria and Aedes-borne diseases (Hemingway et al., 2006, Sell 2006). Nanoformulations, by providing faster and more absorption in the target pest, can increase the efficacy of pesticides (Bergeson 2010, Gurpreet and Singh 2018). Nano-formulations of plant pesticides could be an interesting alternative in the fight against agricultural pests and vector-borne diseases.
In this study, the number of identified components (i.e. 107) in MEO was more than in previous studies (i.e. 16 to 70) (Vanhaelen and Vanhaelen-Fastré 1980, Chalchat et al., 1998, Asllani 2000, Messaoud et al., 2005, Tuberoso et al., 2006, Yadegarinia et al., 2006, Akin et al., 2010, Zomorodian et al., 2013, Rasooli et al., 2018). Comparison and study of ingredients of EO are very important since different numbers of biologically active substances affect the biological activity of EO (Koutsaviti et al., 2015). Various factors can affect the chemical composition of the studied EOs. They include geographical location, the plant parts studied, type and method of extraction and/ or analysis of the EOs, method of drying, storage conditions, fruit ripening stage, type of the plant studied (wild / lab), cultivar and genotype of the plants (Parra and Amo-Marco 1998). It is difficult to establish a relationship between the larvicidal efficiency and the components of the essential oil, as the interactions and synergistic effects between the components can affect the activity of the MEO (Felipe et al., 2008, Scotti et al., 2014). However, it is well known that multi-component formulations are less likely to slow resistance in vectors. This is the main advantage of using EOs as pesticides.
Although the effect of many substances is still unknown, what is certain is that the vector resistance to pesticides is more common to a formulation with an effective component rather than a multi-component formulation (Intirach et al., 2012, Worthington and Melander 2013, Araujo et al., 2016).
A few studies have been performed on the larvicidal properties of MEO against important health vectors. A comparable result was obtained by Koutsavitia et al. (2015). They collected various taxa of the M. communis from different parts of Greece and extracted essential oils. All samples of Greek essential oils were tested against Culex pipiens. They reported moderate to weak larvicide properties compared with our results (LC95 and LC50 were 160 and 76.6 mg / l, respectively). Such differences may be attributed to the different chemical compositions of EOs were prepared from plants, although there was not a significant relationship between the chemical composition of different taxa of this plant in this study and their larvicidal activity (Koutsaviti et al., 2015). In 2010, Conti and colleagues investigated the larvicide properties of essential oils against Aedes albopictus. The results were shown at a concentration of 300 µg/ml only 36.7% of larvae were killed (Conti et al., 2010) while our results indicated 100% mortality at 50 µg/ml.
Plants EOs have been categorized based on the LC50 into six categories (Vatandoost et al. 2012). According to their suggestion, plants EOs with the LC50 values of less than 1 consider as extremely-active, 1–5 very active, 5–50 active, 50–100 moderately active, 100–200 slightly active, and more than 200 non-active respectively. The larvicide properties of MEO are active in this classification and can compete with chemical larvicides, we used nanoemulsion to increase the stability of the EO in nature. From our findings, the MNE increased the larvicide properties against A. stephensi by ∼ 40% compared to the bulk EO (Osanloo et al., 2017a, 2017b). This is due to smaller particles which contribute to improved penetration into the larvae. Also, the residual effect test showed complete larvicide activity for 3 days for the MNE and 1 day for the MEO. A similar pattern of results has been obtained previously. Firooziyan et al. (2021) investigated the larvicide properties of cinnamon essential oil and nanoemulsion against A. stephensi larvae. The results were shown a 32% increase in the larvicide effects of nanoemulsion of cinnamon essential oil compared to cinnamon essential oil. Nanoemulsion of cinnamon essential oil had a high larvicide effect for up to 72 h, while after 24 h the residual effect of cinnamon essential oil decreased (Firooziyan et al., 2021). Osanloo et al. (2021) achieved similar results. Nanoemulsion of Artemisia dracunculus essential oil increased the larvicide properties of its essential oil against A. stephensi from two days to nine days (Osanloo et al., 2019a, 2019b, 2019c). A comparable result was obtained by Volpato et al. (2016). They investigated the effect of cinnamon essential oil and nanoemulsion against Alphitobius diaperinus. Nanoemulsion at a concentration of 5% caused 70% mortality of larvae after two days and had a threefold effect compared to treatment with essential oil (Volpato et al., 2016). Balasubramani et al. (2017) also showed increased larvicide activity of nanoformulation of Vitex negundo L.essential oil compared to its essential oil against Aedes aegypti (Balasubramani et al., 2017).
6 Conclusion
The study showed that nanoemulsion of essential oil may play an important role in the control of larvae. The nanoemulsion also showed increased stability for the essential oils the main challenge when using essential oils as larvicides.
Acknowledgments
This research has been supported by Tehran University of Medical Sciences & Health Services grant no. IR. TUMS.VCR. REC. 1397. 584.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antibacterial activity and composition of the essential oils of Eucalyptus camaldulensis Dehn. and Myrtus communis L. growing in Northern Cyprus. Afr. J. Biotechnol.. 2010;9
- [Google Scholar]
- Larvicidal activity of Syzygium aromaticum (L.) Merr and Citrus sinensis (L.) Osbeck essential oils and their antagonistic effects with temephos in resistant populations of Aedes aegypti. Mem. Inst. Oswaldo Cruz.. 2016;111:443-449.
- [Google Scholar]
- Chemical composition of Albanian myrtle oil (Myrtus communis L.) J. Essent. Oil Res.. 2000;12:140-142.
- [Google Scholar]
- Insecticidal activity of the essential oils from different plants against three stored-product insects. J. Insect Sci.. 2010;10
- [Google Scholar]
- Development of nanoemulsion from Vitex negundo L. essential oil and their efficacy of antioxidant, antimicrobial and larvicidal activities (Aedes aegypti L.) Environ. Sci. Pollut. Res.. 2017;24:15125-15133.
- [Google Scholar]
- Nanosilver: US EPA's pesticide office considers how best to proceed. Environ. Qual. Manage.. 2010;19:79-85.
- [Google Scholar]
- Essential oils of myrtle (Myrtus communis L.) of the Mediterranean littoral. J. Essent. Oil Res.. 1998;10:613-617.
- [Google Scholar]
- Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae) Parasitol. Res.. 2010;107:1455-1461.
- [Google Scholar]
- Susceptibility of Culicidae mosquitoes to some insecticides recommended by WHO in a malaria-endemic area of southeastern Iran. Journal of arthropod-borne diseases.. 2015;9:22.
- [Google Scholar]
- Alterations in behavior and memory induced by the essential oil of Zingiber officinale Roscoe (ginger) in mice are cholinergic-dependent. Journal of Medicinal Plants Research.. 2008;2:163-170.
- [Google Scholar]
- Preparation of nanoemulsion of Cinnamomum zeylanicum oil and evaluation of its larvicidal activity against the main malaria vector Anopheles stephensi. Journal of Environmental Health Science and Engineering. 2021:1-10.
- [Google Scholar]
- Dynamics of residues from a novel nano-imidacloprid formulation in soyabean fields. Crop Prot.. 2010;29:942-946.
- [Google Scholar]
- Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci.. 2018;80:781-789.
- [Google Scholar]
- The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends in parasitology.. 2006;22:308-312.
- [Google Scholar]
- Chemical constituents and combined larvicidal effects of selected essential oils against Anopheles cracens (Diptera: Culicidae) Psyche (Stuttg.). 2012;2012
- [Google Scholar]
- Evaluation of repellency effect of essential oils of Satureja khuzestanica (Carvacrol), Myrtus communis (Myrtle), Lavendula officinalis, and Salvia sclarea using standard WHO repellency tests. J. Arthropod-Borne Diseas.. 2014;8:60.
- [Google Scholar]
- Larvicidal activities of some Iranian native plants against the main malaria vector Anopheles stephensi. Acta Med. Iran. 2013:141-147.
- [Google Scholar]
- Chemical Composition and Larvicidal Activity of Greek Myrtle Essential Oils against Culexpipiens biotype molestus. Nat. Prod. Commun.. 2015;10 1934578X1501001031
- [Google Scholar]
- Myrtus communis in Tunisia: variability of the essential oil composition in natural populations. Flavour Fragrance J.. 2005;20:577-582.
- [Google Scholar]
- A critical review of synthesis procedures, applications, and future potential of nanoemulsions. Adv. Colloid Interface Sci.. 2021;287 102318
- [Google Scholar]
- Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization 2005
- [Google Scholar]
- Preparation and optimization nanoemulsion of Tarragon (Artemisia dracunculus) essential oil as an effective herbal larvicide against Anopheles stephensi. Ind. Crops Prod.. 2017;109:214-219.
- [Google Scholar]
- Extraction and chemical composition of essential oil of Kelussia odoratissima and comparison of its larvicidal activity with Z-ligustilide (Major Constituent) against Anopheles stephensi. J. Entomol. Zool. Stud.. 2017;25:27.
- [Google Scholar]
- Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. J. Nanostruct.. 2019;9:723-735.
- [Google Scholar]
- Larvicidal activity of essential oil of Syzygium aromaticum (Clove) in comparison with its major constituent, eugenol, against Anopheles stephensi. J. Arthropod-Borne Dis.. 2018;12:361.
- [Google Scholar]
- Plant-derived essential oils; their larvicidal properties and potential application for control of Mosquito-Borne diseases. Galen Med. J.. 2019;8:e1532
- [Google Scholar]
- Nano-encapsulated tarragon (Artemisia dracunculus) essential oil as a sustained release nano-larvicide. J. Contemp. Med. Sci.. 2019;5
- [Google Scholar]
- Secondary somatic embryogenesis and plant regeneration in myrtle (Myrtus communis L.) Plant Cell Rep.. 1998;18:325-330.
- [Google Scholar]
- Rasooli, I., Moosavi, M., Rezaee, M.B., et al., 2018. Susceptibility of microorganisms to Myrtus communis L. essential oil and its chemical composition.
- Chemometric studies on potential larvicidal compounds against Aedes aegypti. Med. Chem.. 2014;10:201-210.
- [Google Scholar]
- The chemistry of fragrances: from perfumer to consumer. Royal Society of Chemistry; 2006.
- Sumbul, S., Ahmad, M.A., Asif, M., et al., 2011. Myrtus communis Linn.-A review.
- Repellency effects of essential oils of myrtle (Myrtus communis), Marigold (Calendula officinalis) compared with DEET against Anopheles stephensi on human volunteers. Iran. J. Arthropod Borne Dis.. 2011;5:10.
- [Google Scholar]
- Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae) Pest Manag. Sci.. 2002;58:491-495.
- [Google Scholar]
- Chemical composition of volatiles in Sardinian myrtle (Myrtus communis L.) alcoholic extracts and essential oils. J. Agric. Food Chem.. 2006;54:1420-1426.
- [Google Scholar]
- Identification of chemical constituents and larvicidal activity of Kelussia odoratissima Mozaffarian essential oil against two mosquito vectors Anopheles stephensi and Culex pipiens (Diptera: Culicidae) Exp. Parasitol.. 2012;132:470-474.
- [Google Scholar]
- Larvicidal and insecticidal effect of Cinnamomum zeylanicum oil (pure and nanostructured) against mealworm (Alphitobius diaperinus) and its possible environmental effects. J. Asia-Pac. Entomol.. 2016;19:1159-1165.
- [Google Scholar]
- Ward, R.D., 2008. Service MW: Medical entomology for students, BioMed Central.
- Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol.. 2013;31:177-184.
- [Google Scholar]
- Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry. 2006;67:1249-1255.
- [Google Scholar]
- Chemical composition and antimicrobial activities of the essential oil from Myrtus communis leaves. J. Essential Oil Bear. Plants. 2013;16:76-84.
- [Google Scholar]







