Translate this page into:
Nanofluids application in enhanced oil recovery process-opportunities and challenges
⁎Corresponding authors. houjinjianhn@163.com (Jinjian Hou), lijiacheng@hainanu.edu.cn (Jiacheng Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Enhanced oil recovery (EOR) remains paramount for tapping into residual oil post primary and secondary recovery stages. While conventional methods hold their ground, the rise of chemical EOR, especially utilizing nanofluids, stands out due to its economic viability, enhanced recovery efficiency, and adaptability. The allure of nanofluid-enhanced oil recovery (N-EOR) has grown amongst researchers, albeit with underlying mechanisms that still harbor inconsistencies and ambiguities. This review meticulously examines the micro-mechanics of nanofluid interactions with heavy oil molecules, particles, and surfaces, the methodologies underpinning nanofluid-assisted EOR, multiphase displacement within pores and cores, and the fluid–solid coupling during such flows. Key findings show that nanofluids alter mineral wettability, adjust oil/water interfacial tension, shift structural disjoining pressure, and curtail viscosity. The prowess of N-EOR largely hinges on nanoparticle adsorption. Their affinity for mineral surfaces induces a shift towards water-wet states, while their interplay at oil/water boundaries can tweak interfacial tensions, fostering emulsification. One standout revelation is the adsorption of asphaltenes on nanoparticles, which mitigates asphaltene concentrations in heavy oil, thereby diminishing viscosity and amplifying oil extraction. Contrary to previous studies that merely spotlighted outcomes, our review delves deep into the complexities of nanoparticle adsorption, spotlighting the harmonious interplay between nanoparticle adsorption features and N-EOR operations. We unravel the intricacies of nanofluid behaviors during multiphase core displacement and provide a detailed overview of pertinent simulations. To encapsulate, this study demystifies potential N-EOR techniques and charts fresh research trajectories. Our revelations aim to enrich the comprehension of N-EOR phenomena, accentuating the pivotal role of nanofluids in multiphase core transitions and computational simulations. Furthermore, we highlight lingering challenges, directing the scientific community towards continued nanoparticle innovations and exploration. The novelty of this study is as follows: Nanofluids are mainly used in the third oil recovery process, and are not used in the first and second oil recovery processes. In addition, during the third oil recovery, some nanofluids may remain in the mine due to the sedimentation characteristics of nanomaterials. Currently, we are also committed to developing new processes to reduce the deposition of nanomaterials in the mine. At present, it is not clear how nanofluids enhance the oil recovery mechanism, and the oil recovery mechanism is relatively chaotic. In this article, we analyzed these mechanisms.
Keywords
Nanofluid
EOR mechanism
Nanoparticle adsorption
Interfacial properties
Intrinsic properties
1 Introduction
With the rapid development of the global economy and the continuous growth of energy demand, the supply situation of crude oil, as an important strategic resource, has attracted much attention. As a result, increasing the extraction rate of crude oil has become particularly important. Primary and secondary oil recovery technologies have now improved dramatically, but 50 % of the crude oil is still trapped in reservoir pipelines and cracks (Peng et al., 2017) Enhanced oil recovery (EOR), commonly known as “tertiary recovery”, is the key to solving this problem (Hussain et al., 2022). Thus, the intricacies of oil-solid interactions during the EOR have attracted much attention (Wei et al., 2020).
Nanofluids are mainly used in the third oil recovery process, and are not used in the first and second oil recovery processes. In addition, during the third oil recovery, some nanofluids may remain in the mine due to the sedimentation characteristics of nanomaterials. Currently, we are also committed to developing new processes to reduce the deposition of nanomaterials in the mine.
Traditionally, EOR methodologies are bucketed into three classes: miscible flooding, thermal flooding (Joonaki et al., 2014), and chemical flooding (Lv et al., 2018). However, miscible flooding calls for special solvents and gases, and thermal flooding requires high temperature, which is not the best way to EOR (Li et al., 2018).
Chemical EOR (C-EOR) stands out among these three. Its multifunctional tool kit includes surfactant (Lu et al., 2021), polymer (Sagala et al., 2020), alkali, foam and recently entered nanofluid (Esmaeilzadeh et al., 2015), which has won wide industry praise. The cost of nanoparticles and surfactents were shown in Table 1.
Polymer
Price (Yuan/g)
Nanoparticles
Price (Yuan/ml)
Acetatodicarbonylruthenium, polymer
2,215.9
Tungsten oxide nanoparticle ink
106.98
Bis-MPA-COOH dendrimer
11331.6
Gold nanoparticles
102.90
Bis-MPA-OH dendrimer
8164.5
Amino functionalized silver nanoparticles
74.18
Acetatodicarbonylruthenium, polymer
2,215.90
Cyanuric acid functionalized silver nanoparticles
148.38
Surfactants are common in the EOR series, but they face challenges such as low adsorption efficiency, high prices, and environmental issues. Synthetic surfactants can disrupt aquatic microbial populations, damage fish and other aquatic organisms, reduce the photochemical energy conversion efficiency of plants, and have adverse effects on wastewater treatment processes. The global usage of surfactants exceeds 15 million tons annually, with an estimated 60 % of surfactants ultimately entering aquatic environments and causing environmental impacts (Johnson et al., 2021). Excessive adsorption of surfactants can lead to cost overruns when changing wettability. Other factors that affect the adsorption rate of surfactants include surfactant structure, rock surface charge, and fluid interface. In porous systems, surfactant adsorption typically occurs through various complex phenomena, namely mass transfer and reaction. Therefore, the adsorption of surfactants is a physicochemical process. (Shahrabadi et al., 2022). However, nanofluids have opposite characteristics to surfactants, with high adsorption rates, low prices, and environmentally friendly properties. The reasons for the high conversion rate of nanofluids in improving oil recovery (EOR) mainly include their high specific surface area, high chemical reactivity, and the ability to change the wettability of oil wet sandstone surfaces. These characteristics enable nanofluids to more effectively reduce the interfacial tension between oil and water, alter the wettability of rock surfaces, and thus improve the recovery rate of crude oil. The application of nanofluids in EOR has environmental reasons, mainly because it can improve oil recovery and reduce negative impacts on the environment. Nanofluid is a stable dispersion system with small and uniform particle size obtained by dispersing nanoparticles into a liquid medium. Table 2 showed the application of nanofluids in EOR. Its application in EOR can be achieved through various mechanisms, and it also has environmental advantages. Polymers, while supporting enhanced oil extraction by amplifying displacement efficiency (Wang et al., 2023), cannot completely avoid environmental and budget constraints.
Types of nanoparticles
The EOR mechanism involved
Al2O3
Reduce oil viscosity, alter wettability, decrease IFT, alter wettability
TiO2
Reduce IFT, alter wettability, enhance water viscosity, decrease IFT, improve nanofluid stability
Fe3O4
Wettability changes, IFT decreases
ZrO2
Wettability alteration
ZnO
IFT reduces and lowers viscosity
CaCO3
Improve the stability and wettability of oil in water lotion
SiO2
Reduce the adsorption of nanoparticles at high temperatures
MgO
Thermal conductivity enhancement, IFT reduction, wetting modification, permeability reduction
CuO
Reduce viscosity
Fe2O3
Reduce viscosity
Ferro nanofluids
IFT reduction
Cobalt Ferrite
Reduce oil viscosity
Carbon Nanoparticles
Wettability modification
Carbonate nanotubes (CNT)
Reduce oil viscosity
Alumina Coated
Wettability modification
Hydrophobic oxide NP
Wettability modification
Spherical fumed silica NP
Wettability modification
Core/polymer-shell nano composite
IFT reduction
Polysilicon NP
IFT reduction, wetting modification
CDGs
Sweep improvement
Polymer coated NP
Reduce viscosity, sweet improvement
For surfactant or polymer enhanced oil recovery, research is very traditional, and the current oil recovery rate is still low. However, the addition of nanofluids can effectively improve oil recovery rate. In addition, studies have shown that nanomaterials have synergistic effects with surfactants and polymers. The mechanism of nanofluid enhanced separation of planting oil is not fully clear and differs significantly from other enhanced oil recovery agents such as surfactants and polymers. In addition, polymers and surfactants have poor resistance to high temperature and high salt during actual oil recovery processes, resulting in low recovery and utilization rates; some nanofluids have good resistance to high temperature and high salt, while some magnetic nanofluids have recyclability.
Recent academic research has begun to focus on the application of nanoparticles, particularly non-metallic oxides (such as SiO2) and metal oxides (such as ZrO2, NiO, TiO2, Al2O3). Due to their advantages in size, stability, mechanical robustness, and cost-effectiveness, these nanoparticles have become popular choices in the field of EOR (Bahraminejad et al., 2019). When nanoparticles are uniformly dispersed in water, after being stirred, stable nanoparticles can be formed. These innovative mixtures have opened up new prospects for improving oil recovery (Davoodi et al., 2022) and have received numerous honors, ranging from honing oil/water interface dynamics (Bollineni et al., 2021), reducing surface tensio (Ferreira et al., 2022), lowering interfacial tension (Sharma and Sangwai, 2017), to increasing viscosity and ultimately improving oil recovery (Cao et al., 2021).
Recently, scholars have made a profound study on the improvement of crude oil recovery (N-EOR) by nanofluids, and have acquired a series of important achievement. For example, through experiments using silica nanoparticles, it was discovered that the distribution and spontaneous delamination of the nanofluid film in the wedge-shaped region formed between the oil droplets and the solid surface, providing an intuitive understanding of the oil displacement mechanism of the nanofluid. Therefore, our review embarks on a meticulous odyssey through the warren of N-EOR mechanisms. The research on the role of nanofluid in enhanced oil recovery is discussed in depth. And the influence of factors such as temperature, salinity, and pH on nanofluids was explored. First, from the perspective of surface micromechanics of heavy oil molecules and particles, we analyze how nanofluids can promote the separation of oil droplets from the rock surface by reducing interfacial tension and changing wettability, thereby improving the fluidity and recovery of crude oil (Fig. 1) showed the different contact angle, and the results indicated different wettability). In addition, it also reveals the valuable contribution of nanofluids in the displacement of multiphase cores, which can significantly improve the distribution of oil and water in the pores and reduce the residual oil saturation, thereby improving the recovery efficiency of reservoirs at the macro scale. After that, we summarize the research progress of multiphase fluid–structure interaction simulation. These simulation studies provide an in-depth understanding of the mechanism of action of nanofluids, particularly how fluid–structure interaction effects affect oil–water flow and the mechanical behavior of rocks under multiphase flow conditions. It involves the combination of surfactants and polymers with nanofluids to improve the stability of nanofluids, thereby increasing oil recovery. This review is mainly based on a detailed analysis of the above questions, which can lead to the understanding of the great potential of nanofluid technology in enhanced oil recovery.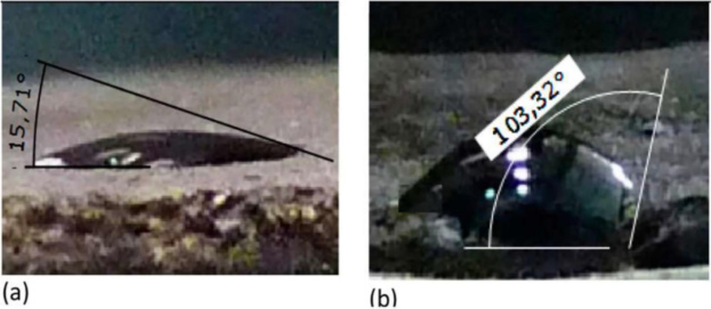
Diagram of Wettability Changes. Reprinted with permission from Lashari, N. & Ganat, T. Emerging applications of nanomaterials in chemical enhanced oil recovery: Progress and perspective. Chinese Journal of Chemical Engineering 28, 1995–2009, https://doi.org/10.1016/j.cjche.2020.05.019 (2020). Lashari et al. (2020), Copyright 2020, Chemical Industry Press Co., Ltd.
The difference between our research and previous studies lies in: in previous studies, nanomaterials were mostly applied under mild conditions, and there was relatively little research on nanomaterials under high temperature, high salt, and high pressure. In the previous research process, the mechanism of using nanomaterials for oil recovery was unclear, and the coupling relationship between various mechanisms was not thoroughly studied. In addition, previous research has mostly focused on laboratories and has been limited in industry; and we have conducted research in industry. We also studied the connections between various mechanisms of nanooil recovery and found that nano-oil recovery is not the result of a single process, but rather the result of a comprehensive effect.
We acknowledge our scope's limitations: the nuances of bespoke nanoparticle synthesis for harsh environments elude us, and we abstain from the less-charted industrial applications of N-EOR.
2 Heavy oil molecules/particle/surface micromechanics
In the field of oil extraction, nanofluidic technology has opened up a new way to enhance oil recovery with its unique interface properties. Starting from the basic concept of interfacial properties, this section discusses the key parameters such as wettability, structural separation pressure, and interfacial tension, which directly affect the flow behavior of fluids in the reservoir and the stability of the oil–water interface. This prologue is succeeded by a dive into intrinsic property shifts, encapsulating viscosity modulation and fluid mobility ratio alteration
2.1 Wettability alteration
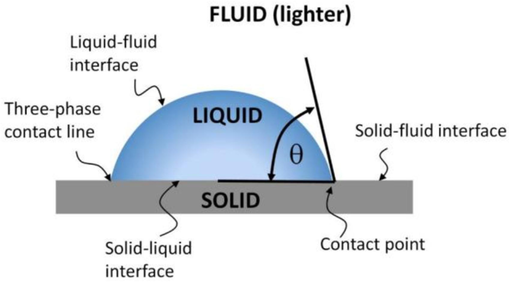
In the process of EOR, wettability is a key factor that determines the efficiency of the recovery. Its intricacies can be deciphered by examining the tripartite contact angles arising between water, a typical solid substrate (often mineral surfaces), and air or oil. Fig. 2 illustrates the measurement of the wettability conditions of rock-brine. The interfaces include solid–liquid interface, solid–gas interface, and liquid–gas interface. The contact angle is fitted by the three phases (Sadeghinezhad et al., 2020). Denoted as θ, this angle provides important insights into the wettability characteristics of the mineral surface:
When θ < 90°, the surface is predominantly hydrophilic.
At θ = 90°, a state of wettability equilibrium is achieved.
For θ > 90°, the mineral surface exhibits hydrophobic tendencies.
- Wettability conditions of rock-brine. Reprinted with permission from ref. Sadeghinezhad, E., Siddiqui, M. A. Q., Roshan, H. & Regenauer-Lieb, K. On the interpretation of contact angle for geomaterial wettability: Contact area versus three-phase contact line. Journal of Petroleum Science and Engineering 195, https://doi.org/10.1016/j.petrol.2020.107579 (2020), copyright 2020, ELSEVIER.
Yet, in airy environments, the swift evaporation of liquid droplets muddies the waters for precise equilibrium contact angle measurements. Here, an aqueous setting offers reprieve. Introducing an oil droplet onto water-immersed mineral surfaces renders the equilibrium contact angle observable: Angles below 90° denote a lipophilic inclination of the minerals. Angles surpassing 90° hint at their oleophobic tendencies.
2.1.1 Effect of nanofluidic on wettability
Nanoparticles are alter to change the surface properties of the minerals, changing the wettability of the minerals. Wettability refers to the ability of a liquid to spread on a solid surface, which determines the strength of the interaction between the liquid and the solid surface. In the process of EOR, wettability plays a crucial role (Li et al., 2022). A study has shown, nanoparticles can change the surface properties of minerals by reducing the contact angle, turning them from hydrophobic to hydrophilic, thereby increasing the oil recovery rate (Bhuvanesh and Kalaiselvam, 2023). Fig. 3 illustrates the changes in wettability caused by spontaneous imbibition with/without the dispersion of nanoparticles in different fluid components. When the concentration of SW (Smart Water) is consistent, the addition of SiO2 nanoparticles to SW can significantly reduce the contact angle (Rafiei and Khamehchi, 2021).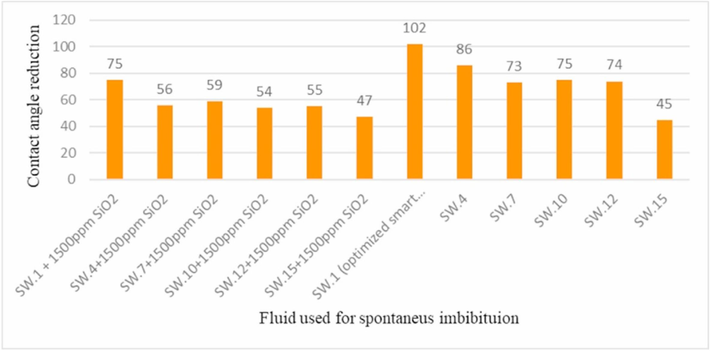
Wettability alteration (contact angle reduction) due to spontaneous imbibition with different fluid compositions with/without nanoparticle dispersion in fluids. Reprinted with permission from ref. Rafiei, A. & Khamehchi, E. Design of smart water composition based on scale minimization and its effect on wettability alteration in the presence of nanoparticles and mineral scales. Journal of Petroleum Science and Engineering 196, https://doi.org/10.1016/j.petrol.2020.107832 (2021), copyright 2021, ELSEVIER.
Nanofluids can drive the restructuring of wettability, mainly due to the nanoparticles' tendency to adhere to the mineral surface (Gbadamosi et al., 2019). Microfluidic flooding experiments have corroborated these adsorptive behaviors.
Abhishek et al conducted meticulous research and discovered that SiO2 nanoparticles exhibit a significant affinity for sandstone in the process of low-salinity oil displacement. Furthermore, through isothermal static adsorption experiments, the researchers observed that nanoparticles show a clear preference for quartz surfaces over kaolinite surfaces (Jia et al., 2018). Researchers have further revealed a tempting trend: the surge in water phase salinity reflects an increase in nanoparticle adsorption. It is worth noting that even at a concentration of 35 % by weight of dense nanoparticles, only 21.2 % by weight of nanoparticles were desorbed. Continuing the narrative, it emphasizes that pH value is an acceptable influencing factor, guiding the adsorption and desorption pathways – higher pH values will enhance the desorption of nanoparticles. The in-depth study of adsorption kinetics revealed that intra particle and thin film diffusion are the key factors for nanoparticle adsorption (He et al., 2021).
Li et al. made a profound study on the adsorption of nanoparticles on Berea sandstone and their impact on wettability (He and Liu, 2020). After injection, hydrophobic nanoparticles exhibit obvious adsorption within the core, in sharp contrast to hydrophilic nanoparticles, which exhibit reduced adsorption and enhanced desorption. Therefore, there is no doubt that the effectiveness of nanoparticle adsorption in regulating wettability. However, a salient asterisk surfaces: not every nanoparticle adsorption episode necessarily predicates a wettability metamorphosis.
2.1.2 Factors steering wettability in N-EOR processes
Within the realm of N-EOR, temperature variances, aqueous milieu peculiarities, oil and the inherent traits of minerals all affect the wettability of minerals. Recent scientific forays have illuminated nanofluids' prowess in reconfiguring mineral wettability. A succinct synthesis of pivotal discoveries includes:
Fluorinated SiO2 Nanoparticles: Mousavi et al. discerned a transformation in rock wettability proimal to the wellbore, transitioning from a predominantly liquid-wet to a quasi gas-wet state. They spotlighted a water/n-decane contact angle deviation from 147° to 61° (Hendraningrat et al., 2014).
Alumina-infused Nanofluids: Giraldo et al. demonstrated that in synergy with anionic surfactants, these nanofluids accentuated the hydrophilic disposition of sandstone cores, markedly so at concentrations sub-500 ppm (Hendraningrat et al., 2015). SiO2 Nanofluids in Saline Settings: Hendraningrat et al. observed a contact angle decrement from 54° to 22°, boosting oil recovery by an impressive 36.49 % (Hochang et al., 2018). TiO2 Nanoparticles: Ehtesabi et al. championed their application in enhancing heavy oil extraction from sandstone cores, documenting an uplift in oil retrieval from 49 % to 80 % post-treatment. SEM-energy dispersive X-ray spectroscopy attested to the homogenous nanoparticle distribution on the core plug facade (Hou et al., 2019). Thermal Influence on Oil Extraction: Bayat et al. identified a diminishing contact angle on limestone surfaces as temperatures ascended, leveraging nanofluids like Al2O3, TiO2, and SiO2 (Huang et al., 2020). γ-Al2O3 Nanofluids: Naseri applied them on calcite, capturing a contact angle descent from 119° to 38°, culminating in an 11.25 % enhancement in oil recovery (Huibers et al., 2017). Researchers such as Irfan et al. (2019), Ivanova et al. (2020), Jia et al. (2019), and Jia et al. (2018) have penned similar sanguine conclusions under an array of test conditions. However, in this bright mood. There is no inconsistency in the narrative of nanofluid EOR. In order to fully utilize the potential of nanofluids in EOR, having a detailed understanding of the underlying dynamics is necessary.
2.1.3 The influence of nanoparticle types on wettability
Each nanoparticle has its unique character, exhibiting a series of interactions with the mineral surface. These different interactions alter the wettability of mineral interfaces, which is a widely analyzed topic in scientific literature.
For example, Moghaddam et al. investigated the use of a wide range of nanofluids in the oil recovery process of carbonate rock formations (Ge and Wang, 2015). The research results indicate that SiO2 and CaCO3 nanoparticles can extraordinarily increase the recovery rate by 8 %–9 % and Transition from the wettability to hydrophilicity. By comparison, Bayat et al. proposed that nanofluids containing Al2O3 and TiO2 are superior to SiO2 nanofluids in improving oil recovery (Kazemzadeh et al., 2019).
Rodriguez et al. believe that in addition to nanoparticles being easy to pass through the pore throats in porous media due to their small volume, nanoparticles can also remain dispersed in solution due to their surface activity and high stability. Kanj et al. quantified the applicable size of nanoparticles that can be transported in porous media and reported that particles with a size of 200 μ m can be easily transported with high dispersion stability. In addition, Li and Torsaeter studied the transport and adsorption behavior of various types of silicon NPs through porous media. The results indicate that hydrophilic SiO2 unstructured particles (NSP) have better adsorption capacity than hydrophilic SiO2 colloidal nanoparticles (CNP). Aurand and Torsaeter believe that the adsorption efficiency and recovery performance of nanofluids using gas-phase SiO2 NPs are superior to those using colloidal SiO2 NPs. Recent studies have shown that different types of nanoparticles have a good effect on improving crude oil recovery, especially silicon-based nanoparticles, as they have the ability to alter wettability, reduce interfacial tension (IFT), and increase flowability ratio. In their attempt, Onyekonwu and Dogolo studied the ability of various polycrystalline silicon NPs (PSNPs), such as lipophilic hydrophilic (LHPN), hydrophilic oleophilic (LHPN), and neutral wet PSNP (NWPN), to enhance oil recovery; They found that all three types of NPs can effectively alter the wettability of reservoir rocks, forming a strong hydrophilic system. In various experimental studies, Hendraningratet et al. confirmed the role of silicon-based NPs in improving oil recovery by altering the surface forces of reservoir rocks. Their results indicate that in most nanofluid oil displacement processes, the ability of NPs is driven by important parameters such as NP size, initial wettability of the rock, NP concentration, injection rate, and temperature. In addition, Shahrabadi et al. investigated the effects of hydrophobic and lipophilic polycrystalline silicon (HLP) nanofluids on crude oil recovery. The results showed that the optimal wettability of HLP NP decreased from 123.34° to 95.44° contact angle, IFT decreased from 25.6 to 1.75mN/m, and the optimal concentration was 4 g/lit.
Recently, scholars have proposed that the ideal integration of nanoparticles (NPs) into polymer structures can enhance polymer properties such as rheology, thermal stability, and chemical resistance, surpassing traditional polymer materials to some extent The reported polymer solution contains NP silica (SiO2), titanium dioxide (TiO2), graphene oxide (GO), zinc oxide (ZnO), and aluminum oxide (Al2O3). In addition, the combination of nanoparticles and polymers reduces interfacial tension (IFT), alters wettability, improves organic pollutant adsorption from produced water, and enhances polymer viscoelasticity. All these mechanisms contribute to improving oil recovery.
There is no unified consensus on the optimal type of nanoparticles for different oil extraction processes. In other words, the optimal type of nanoparticles varies depending on the type, mineral deposit type, salinity, and temperature changes.
2.1.4 The effect of salinity on wettability
The effect of salinity on wettability may be remarkable, especially in N-EOR (Rebello et al., 2023). Salinity can affect the ionic strength of a solution, thereby altering the adsorption behavior of nanoparticles on mineral surfaces, leading to changes in wettability. For example, higher salinity levels can lead to nanoparticle aggregation, which may reduce reservoir permeability and affect the wetting state of rocks. Due to the fact that different nanofluids had different optimal salinity for remaining stable, the salinity would be altered according to nanoparticles species. The influence of different salt ions on wettability can be summarized as follows: the degree and mode of influence of different types of salt ions on wettability are different. Overall, Ca2+and Mg2+have a greater impact on wettability than Na+. Specifically, Ca2+and Mg2+ions significantly reduce the contact angle at lower concentrations, thereby increasing wettability; At higher concentrations, the influence of different types of salt ions on wettability tends to be consistent. Na+ ions are more effective in improving the wettability of rock surfaces in low salinity water, while Ca2+and Mg2+ions reduce the wettability to some extent. The mechanism of this phenomenon can be explained by the change in wetting angle. As time increases, the wetting angle gradually decreases, with a faster initial decrease rate and a slower later decrease rate. The salt ions in the aqueous phase increase the hydrophobicity of the sandstone surface and reduce the wetting rate of water on the sandstone surface. This wetting rate first increases and then decreases with the increase of mineralization. In addition, the specific effects of different types of salt ions on wettability also vary. For example, the contact angle between NaCl solution and KCl solution is basically the same, while CaCl2 solution reduces the three-phase contact angle more significantly at lower concentrations than NaCl solution.
Fig. 4 illustrates the average particle size at salt concentrations of 25 °C and 90 °C. At 90 °C, the salt concentration is directly proportional to the change in average particle size; when the salt concentration reaches 20 wt%, the average particle size is approximately 90 nm. At 25 °C, the relationship between salt concentration and average particle size is similar to that at 90 °C, and when the salt concentration is 20 wt%, the average particle size is about 50 nm. As the salt concentration increases, the nanoparticle size gradually increases. Larger nanoparticles may reduce the stability of the nanofluid, reduce the effectiveness of rock voids, affect wettability, and thus reduce oil recovery.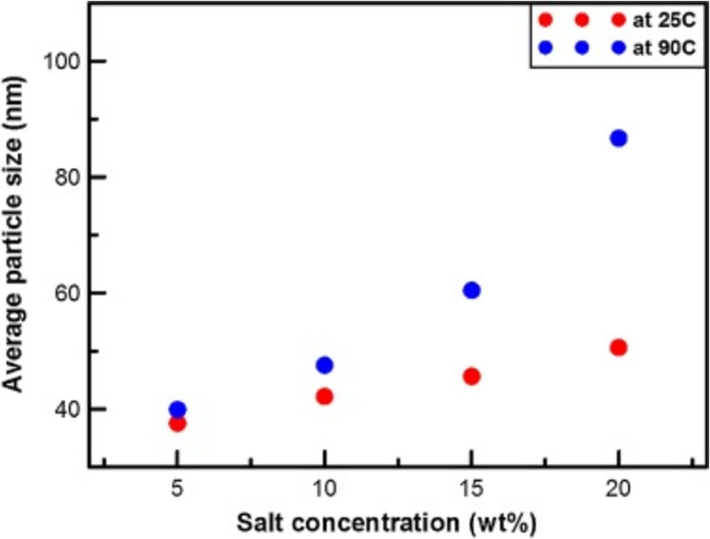
Average particle size for different salt concentrations at 25 °C and 90 °C. Reprinted with permission from ref. Jang, H., Lee, W. & Lee, J. Nanoparticle dispersion with surface-modified silica nanoparticles and its effect on the wettability alteration of carbonate rocks. Colloids and Surfaces a-Physicochemical and Engineering Aspects 554, 261–271, https://doi.org/10.1016/j.colsurfa.2018.06.045 (2018), copyright 2018, Elsevier.
Rostami et al. delved into the nuanced interplay of diverse salinities on mineral wettability (Keykhosravi et al., 2019). Their findings revealed that salt ions constrict the electric double layer, amplifying nanoparticle adsorption in salt-rich terrains, which in turn magnified the water-wet predisposition of sandstone. In a parallel narrative, Tabar et al. assessed the influence of SiO2 nanofluids on the wettability of carbonate rocks across the salinity spectrum. Their results marked a profound wettability pivot from an oil-wet stance (156°) to a water-centric one (41.7°), fostering oil recovery (Khademolhosseini et al., 2020).
Integrating the information above, it is clear that changes in salinity have a significant impact on wettability. The rational use of low-salinity water flooding and the adjustment of brine composition can serve as an effective strategy for improving oil and gas recovery rates. However, the relationship between salinity and wettability is complex and can be influenced by other factors such as temperature, the type of minerals present, and the specific chemistry of the formation water. Therefore, a comprehensive understanding of these interactions is crucial for optimizing EOR strategies.
2.1.5 The effect of temperature on wettability
The role of temperature in modifying mineral wettability is undeniable, affecting both the mobility and adsorption efficiency of nanoparticles on mineral interfaces.
As depicted in Fig. 5, prolonged immersion results in diminishing contact angles (Wang et al., 2022). Notably, when immersion duration remains constant, an increase in temperature sees a subsequent decrease in contact angle. This dynamic underscores that, to attain a specific contact angle, the immersion duration should be modulated in tandem with temperature variations.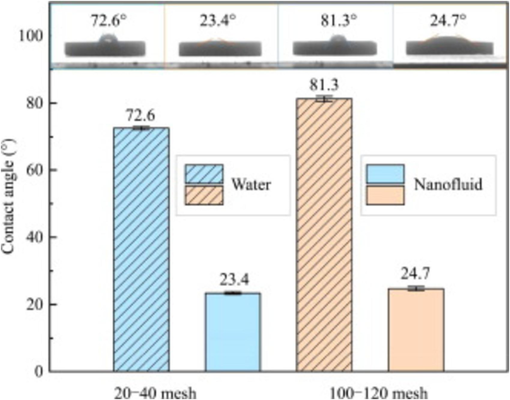
Measurement results of contact angle. Reprinted with permission from ref. Wang, G. et al. Experimental study on preparation of nanoparticle-surfactant nanofluids and their effects on coal surface wettability. International Journal of Mining Science and Technology 32, 387–397, https://doi.org/10.1016/j.ijmst.2021.12.007 (2022), copyright 2022, ELSEVIER.
2.1.6 The effect of pH on wettability
The influence of solution pH on wettability is mainly reflected in the interaction between the acid-base properties of the solution and the surface of the liquid and solid. The pH value of a solution changes its wettability by affecting the surface charge and chemical composition of both liquids and solids.
Specifically, the pH value of a solution can effect the charge distribution on the surfaces of liquids and solids. For example, when the pH value of a solution changes, the ion concentration and charge distribution in the solution also change accordingly, which in turn affects the interaction force between the liquid and the solid surface, leading to a change in wettability.
In addition, the pH value of the solution can also affect the chemical composition of the liquid and solid surfaces. For example, acidic or alkaline solutions may react with certain chemical groups on the solid surface, altering the surface's chemical properties and thus affecting wettability. For example, after being treated with acidic or alkaline solutions, the wettability of lotus leaves will change. This is because the lipid components on the lotus leaf surface react with acidic or alkaline solutions, changing the microstructure and chemical composition of the surface.
In summary, the pH value of a solution has a significant impact on wettability by affecting the charge distribution and chemical composition of liquid and solid surfaces. This influence is of great significance in practical applications, for example, in the printing industry, controlling the pH value of the solution can ensure printing quality; In materials science, specific functions can be achieved by adjusting the wettability of materials.
2.1.7 Differential wettability dynamics in sandstone and carbonate minerals
The interactions between sandstone and carbonate minerals and petroleum are primarily influenced by their inherent characteristics. Sandstone formations are characterized by relatively weak oil-solid interactions, which facilitates the easier release of petroleum. By contrast, carbonate reservoirs exhibit stronger oil solid affinity, posing additional challenges to the oil extraction process.
Monhad et al. made a profound study on how SiO2 nanofluids affect the wettability of sandstone surfaces. Their research results (Li et al., 2019) indicate that SiO2 nanoparticles regulate wettability by changing the distribution of surface charges, and this effect is particularly evident when nanoparticles are immersed in deionized water. The assimilation of SiO2 nanoparticles in water increases the negative charge on the surface. Due to the continuous accumulation of negative charges, this can affect the wettability of minerals (Li et al., 2020).
Furthermore, it is important to recognize that although carbonate rock formations have a wide range of classifications, they have rich structural diversity. Compared to sandstone, many carbonate rock formations have unique Water conducting gaps that are placed with low permeability matrix area (Li et al., 2019; Lu et al., 2017). Therefore, the efficiency of extracting oil from carbonate formations is usually not as high as that from sandstone formations. Take the majority of in-situ oil is trapped in multi-hole frameworks into account, the best promising approach to enhance oil recovery is improving wettability (Lv et al., 2020).
Fig. 6 depicts the zeta potential of silica nanofluids, oil samples, and rock samples, and it can be found that rock samples are positively charged, and oil samples and nanofluids are negatively charged. The negatively charged nanofluids are attracted to the positive charge on the surface of the rock, which enhances the adhesion between them. This may help improve oil recovery or enhance the stability of the rock surface. In addition, Fig. 7 showed the PNP hydrogels form through the interactions of PEG-P PLA nanoparticles (NPs) and dodecyl-modified hydroxypropyl methylcellulose polymers (HPMC-C12). The research showed the relationship between nanoparticles and polymers.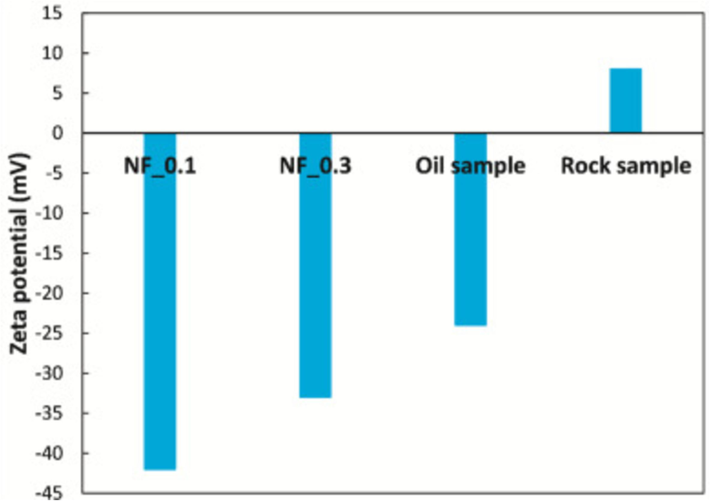
Zeta potential of silica nanofluids, oil sample and rock sample. Reprinted with permission from ref. Keykhosravi, A., Vanani, M. B., Daryasafar, A. & Aghayari, C. Comparative study of different enhanced oil recovery scenarios by silica nanoparticles: An approach to time-dependent wettability alteration in carbonates. Journal of Molecular Liquids 324, https://doi.org/10.1016/j.molliq.2020.115093 (2021), copyright 2021, ELSEVIER.
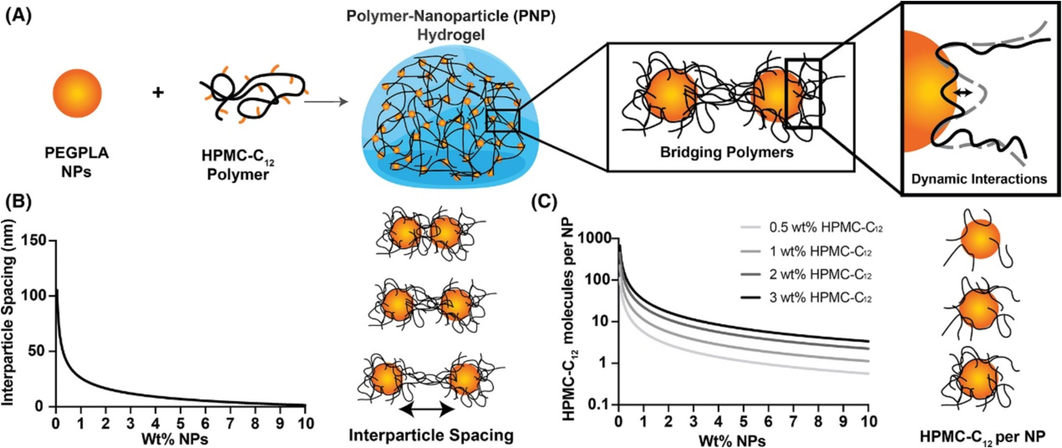
(A) PNP hydrogels form through the interactions of PEG-P PLA nanoparticles (NPs) and dodecyl-modified hydroxypropyl methylcellulose polymers (HPMC-C12). Polymers bridge between polymers and dynamically interact with the NP surface. (B)Average interparticle spacing of NPs as a function of the weight percent of NPs added. (C) Number of molecules of HPMC-C12 per N P as a function of the concentration of NPs and concentration of polymer. Reprinted with permission from ref. Grosskopf, A. K., Saouaf, O. A., Hernandez, H. L. & Appel, E. A. Gelation and yielding behavior of polymer-nanoparticle hydrogels. Journal of Polymer Science 59, 2854–2866, https://doi.org/10.1002/pol.20210652 (2021), copyright 2021, WILEY.
2.1.8 Potentiating wettability adjustments using polymer/nanoparticle blends
Polymer flooding, entailing the injection of polymers into reservoirs, is conceptualized to access untapped oil pockets. Although polymers, lauded for enhancing mobility ratios, potentially augment oil displacement, they bring along challenges, especially elevated operational costs. Integrating nanoparticles with polymers in EOR not only presents an avenue for cost mitigation but also promises amplified oil extraction rates (Zahiri et al., 2022; Liang et al., 2022).
Gbadamosi et al. embarked on exploring the cumulative effects of Al2O3 nanoparticles and polymers (e.g., PAM and HPAM) on mineral wettability and oil extraction, as portrayed. Post-application of HPAM on an initially oil-wet interface yielded a substantial contact angle drop to 55.7°, signifying a transition towards water-wet conditions. The underlying phenomena were identified as a combination of stripping and pulling mechanisms. The incorporation of nanoparticles into the polymer mix intensified these wettability transformations, primarily attributed to the nanoparticle adsorption onto the rock facades (Grosskopf et al., 2021).
Treading an innovative trajectory, Zhou et al. introduced a surfactant-enhanced SiO2 nanoparticle approach to bolster oil recovery, especially in high-temperature or salinity scenarios (Madhan et al., 2018). Similarly, in a seminal study by Yekeen et al., the combined influences of nanoparticles and surfactants on reshaping rock wettability were accentuated. The conjoint use of surfactants and nanoparticles outperformed standalone nanofluid applications in terms of wettability refinement. Different combinations of surfactants and nanoparticles have produced different results in regulating mineral wettability (Zhang et al., 2022).
2.1.9 Deciphering micro-mechanisms underpinning wettability alteration
Although previous studies have revealed nanofluids have played a crucial role in altering mineral wettability, many of these insights have emerged from a macroscopic perspective (Khosravi et al., 2021). In depth exploration of subtle microscopic mechanisms reveals the following:
At the fundamental level, nanoparticles form intrinsic bonds with mineral surfaces. This combination ultimately leads to an efficient adsorption process, causing the mineral surface to transition from oil saturation to a hydrophilic surface. However, this statement is not limited to the adhesion of nanoparticles. The introduction of nanofluids also alters the free energy trajectory of mineral surfaces, causing them to retreat. Closely related to surface morphology and chemical complexity, rougher mineral surfaces often imply a reduction in free energy. As this energy decreases, the surface's preference for water will also increase.
A study extended the influence of nanoparticles on energy dynamics by analyzing their effects on a range of factors, including electrostatic interaction energy, van der Waals attraction, hydration or structural energy, and overall combined interaction energy (Mousavi et al., 2013). In nanofluids, this aggregated interaction energy masks the energy in deionized water. Nanofluids exude a heightened repulsive force between rock and oil, compared to deionized water. The intensity of this repulsion inversely correlates with the contact angle, symbolizing the energy barrier impeding oil's affinity for rock surfaces. An uptick in this energy barrier cements the water layer's grip on the rock, leading to a sharp decrease in the contact angle.
Now, introducing the variable of salinity: with its increment, the stability of the protective water film becomes precarious (Hu et al., 2022). In this scenario, the electrostatic interaction energy component within the total interaction energy diminishes. Yet, nanofluids emerge as the hero, bolstering the water film's tenacity and resulting in a more water-receptive mineral interface. Such nanofluid-induced wettability shifts become especially pronounced in low salinity settings.
2.1.10 Wettability review
Although there are many impacts on the single aspect of nanofluid oil recovery, the interrelationships between various factors are not clear. For example, the mechanism by which a single factor such as pH, temperature, and salinity affects wettability has been clarified, but the interrelationships between these factors are not yet clear. In the previous research process, different oils have different effects on wettability. Different types of oil have different wettability and are influenced by many factors, such as the origin, density, and extraction cost of the oil. Therefore, there is currently no unified conclusion applicable to all types of oil. The types of mineral deposits are different, and the influence of wettability is not yet clear.
2.2 Interfacial tension
Interfacial tension, defining interface tension refers to the forces of interaction between molecules at the boundary between two different fluids, playing a pivotal role in the research of EOR (Zhao et al., 2022). The impact of this tension is particularly pronounced, especially between oil and water (Mustafa and Baojun, 2018).
Fig. 8 showed the schematic of the formed nano-film and competitive adsorption of ions and nanoparticles on water–oil interface. The ions and nanopaticles would adsorb onto oil/water interface. The adsorption was dynamics, in other words, nanoparticles (ions) would adsorb/desorb onto oil/water interface.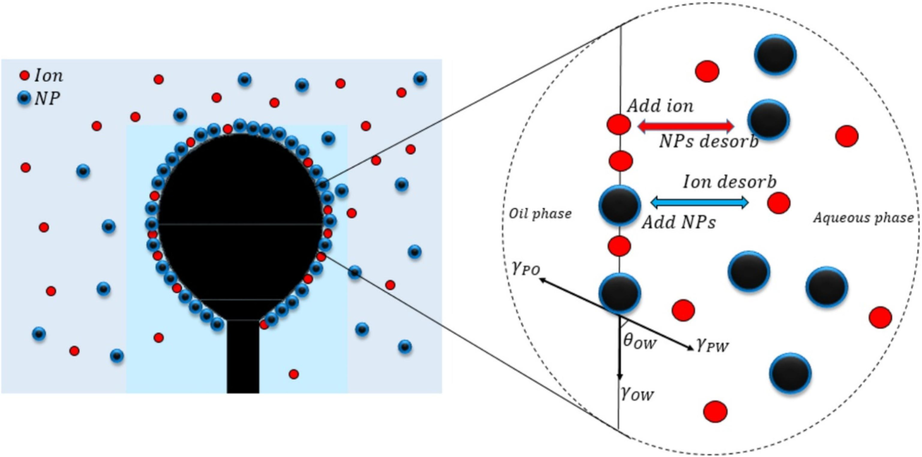
The schematic of the formed nano-film and competitive absorption of ions and nanoparticles on water–oil interface. Reprinted with permission from ref. Madhan, A., Kun, G. & Zhixin, Y. J. A. e. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. 8, 871 (2018), copyright 2019, Elsevier.
In general, when nanoparticles come into contact with oil and water, they typically cause a reduction in interfacial tension (Naseri, 2014). However, some studies have indicated that under specific conditions, the interfacial tension may unexpectedly increase (Negin et al., 2016), which contradicts the standard findings. In response, some scholars believe that nanoparticles alone may not be the key factor affecting interfacial tension and have noted the inconsistency in results obtained from different research avenues (Nwidee et al., 2017).
A landmark study by Wu et al. introduced an amphiphilic Janus nanofluid combined with SiO2 and hydrophobic chains. Experimental evidence indicated that a scant 0.05 wt-% of Janus nanoparticles brought the interfacial tension down to 2.28 mN m−1. Additionally, as the concentration of Janus nanofluid increased to 100 mg L−1, the oil recovery rate significantly improved, reaching 15.74 % (Olayiwola and Dejam., 2019). Further explorations in the field of nanoparticles by Joonaki et al. and Soleimani et al. have also yielded similar findings (Olayiwola and Dejam, 2020a, 2020b).
Saien et al. underscored that the hydrophilic or hydrophobic nature of nanoparticles has a profound impact on interfacial tension (Rezk and Allam (2019) Experimental data show that, compared with hydrophilic Al2O3 nanoparticles, hydrophobic Al2O3 nanoparticles significantly reduced the interfacial tension between toluene/water, partly due to the hydrophilic effect of hydrophilic Al2O3 nanoparticles in water.
Contrastingly, some scholars opine that nanofluids don't necessarily modulate the interfacial tension (Rezvani et al., 2018; Rockey et al., 2018). Vignati et al. for instance, observed no discernible influence of SiO2 nanoparticles (both treated and untreated) on the iso-octane/octanol–water interfacial tension (Rostami et al., 2019).
Temperature is one of the key factors affecting interfacial tension (Neog, 2022). Some studies have shown a direct correlation between the increase in temperature and the reduction of interfacial tension (Wu et al., 2022), while other studies suggest the existence of a specific optimal temperature range. A study by Wen et al. using molecular dynamics simulations and another by Yekeen et al. emphasizes this direct relationship (Saha et al., 2018). Conversely, Ivanova et al. found a distinct “V” shaped trend, indicating that interfacial tension varies with temperature and is complexly related to salinity levels (Sharma et al., 2016).
Contemporary research points to a potential synergy between nanoparticles and surfactants in tempering interfacial tension (Wang et al., 2021). However, there are many differing opinions on this topic, and a unified consensus has not yet been formed.
Fig. 9 depicts the mechanism responsible for the stability of nanofluids in the presence of surfactants. At low con centrations, the surface of nanoparticles does not adsorb enough surfactants, which leads to unstable state due to the poor intermolecular affinity. With the increase in concentration, moderate surfactants are absorbed on the surface of nanoparticles to form a sta ble hydration film, which prevents the collision between particles. The stability of nanofluids is great at appropriate concentration. When the concentrations continue to increase, the surface of particles has reached a state of supersaturation, and nanoparticles agglomeration appears due to hinge winding, and the dispersion stability decreased (Qiao et al., 2021).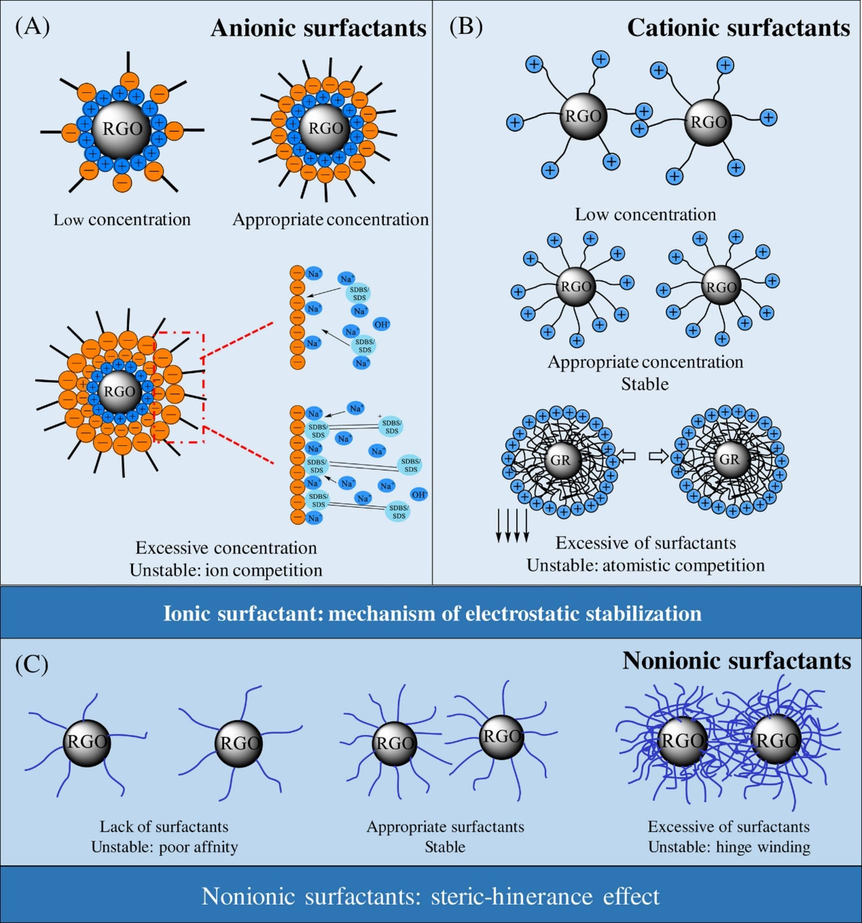
Mechanism responsible for the stability of nanofluids in the presence of surfactants. A, the mechanism of anionic surfactants. B, the mechanism of cationic surfactants. C, the mechanism of nonionic surfactants. Reprinted with permission from ref. Qiao, Y., Sheng, W., He, C., Liu, C. H. & Rao, Z. H. Experimental study on the effect of different surfactants on the thermophysical properties of graphene filled nanofluids. International Journal of Energy Research 45, 10043–10063, https://doi.org/10.1002/er.6497 (2021), copyright 2021, WILEY-HINDAWI.
Experiments by Saien and Bahrami illustrated the potent combined effect of modified SiO2 nanoparticles with an anionic surfactant, SDS (Soleimani et al., 2018). Yet, other researchers, such as Moghadam and Azizian and Biswal and Singh, showcased differing findings and potential reasons for these divergences (Songolzadeh and Moghadasi, 2016).
Overall, the combination of nanofluids and surfactants undoubtedly demonstrates great potential in EOR, which necessitates further exploration into the complex mechanisms of their interactions.
Surfactants are one of the chemical agents used in C-EOR process. They are substances that can form micelles between water and oil, and altering the intermolecular forces between oil and water, the pore structure of oil layer can be regulated (Zhou et al., 2022). so that the oil layer with small pores and poor connectivity can become more permeable (Lv et al., 2023), thereby increasing the recovery rate. In addition, surfactants can also change the wettability of rocks (Singh et al., 2023), reduce the interfacial tension between oil and water (Souayeh et al., 2021), and form viscous microemulsions. These viscous microemulsions create favorable lateral pressure gradients that promote the flow of crude oil (Sun et al., 2021), and enabling the production of otherwise difficult oil formations.
There are many researches on the mechanism of nanofluid oil recovery. Liang, T et al believe that IFT at oil–water interface is an important index affecting oil recovery (Liang et al., 2022).A mixture of surfactants and nanoparticles can further reduce interfacial tension, change wettability, reduce oil viscosity, improve foam/emulsion stability, and reduce capillarity. Zhao et al. (2022) believe that nanofluids can change the wettability of the rock surface, making the rock surface change from oil-wet to water-wet. The hydrophilic groups of the nanofluids adsorbed on the rock surface make the rock surface more hydrophilic. Their hydrophobic groups are adsorbed to the organic active components on the rock surface, making it easier for crude oil to peel off the rock surface. Moreover, due to Brownian motion and electrostatic repulsion between nanoparticles, strong diffusion force will be generated when the number of nanoparticles is large enough. However, the electrostatic repulsion force on the solid surface is unbalanced, resulting in larger oil-phase antenna and smaller water-phase antenna, thus forming a wedge structure in the three-phase contact region. The positive thrust generated by this wedge formation is the tectonic separation pressure. The wedge nanofluid film will push the oil droplets forward, while the contact area between the oil droplets and the rock surface will be further reduced, and the shape of the oil droplets will gradually change. The free surfactant molecules in the nanofluid system can enter smaller pore throats, and the oil droplets can be removed from the rock surface by reducing interfacial tension and wetting reversal (Asadzadeh et al., 2023; Shar et al., 2023), which greatly improves the oil absorption and drainage effect of the nanofluid system. In addition, there are many related literatures (Pan et al., 2023), which enrich the exploration process of nano oil recovery mechanism.
Many of the conclusions were made at very diluted nanoparticle concentrations (Bahraminejad et al., 2022). Therefore, whether the structural separation pressure generated by the confinement wedge membrane region in the N-EOR process can explain the removal process of residual oil on the rock surface when the nanoparticle concentration is low is still unknown. Moreover, we currently lack comprehensive simulation studies of EOR using nanoparticles agents. Previous numerical simulation studies using colloidal particle models have been proved to be wrong because they ignore quantum effects and the large area volume ratio of nanoparticles and cannot accurately characterize the behavior of nanoparticles.
In a word, nanofluids injected into oil Wells also have the effect of changing rock wettability (Asl et al., 2023) and reducing interface surface tension (Zhang e al., 2021). However, nanofluids have many functions that surfactants do not have in oil recovery. For example, reduce oil viscosity,control adsorption (Kakati et al., 2022), and generate structural disjoining pressure at the three-phase contact region. Akande et al. (2023) also found in the experiment that carbon-based nanofluids have significant viscosification and carbon fixation properties. However, nanomaterials are not omnipotent, because they tend to accumulate and deposit under harsh reservoir conditions. Unstable nanomaterials will reduce reservoir permeability and destroy pore throat structure.
2.3 Structural disjoining pressure
Structural disjoining pressure—the force inherent within the wedge film—has been comprehensively studied (Cui et al., 2022; Sun and Ge, 2022). For example, the work of Zhang et al. and Nikolov et al. investigated the profound impact of nanofluid driven structural separation pressure in EOR (Tangestani et al., 2019). Kondiparty et al.'s study (Tian et al., 2021) indicated the dispersion kinetics of nanofluids on solids and elucidated the relationship between nanoparticle concentration and the internal contact line velocity of propulsion. It is evident that the foundation of N-EOR lies in structural separation pressure (Chang et al., 2021), which provides explicit guidance for the extraction of light oil, yet the subtle mechanisms by which nanofluids enhance the recovery of heavy oils still require further in-depth exploration.
2.4 Viscosity
The viscosity of oil is one of the key factors in EOR (Eyinla et al., 2023). Contemporary research emphasizes the potential of nanoparticles in reducing the intrinsic viscosity of heavy oil.
Fig. 11 depicts effect of nanoparticles addition on crude oil viscosity at 25 °C. The addition of (Mg, Ni) O nanoparticles caused most significant increase in oil viscosity, which represents a 13.4 % increase in relation to crude oil. This result is attributed to the low density and large pore volume of these particles; therefore, they occupy a larger bulk volume than those heavier materials. The viscosity increases for the Al2O3-NiO and TiO2 particles were 9.8 % and 5.3 %, respectively (Parejas et al., 2021).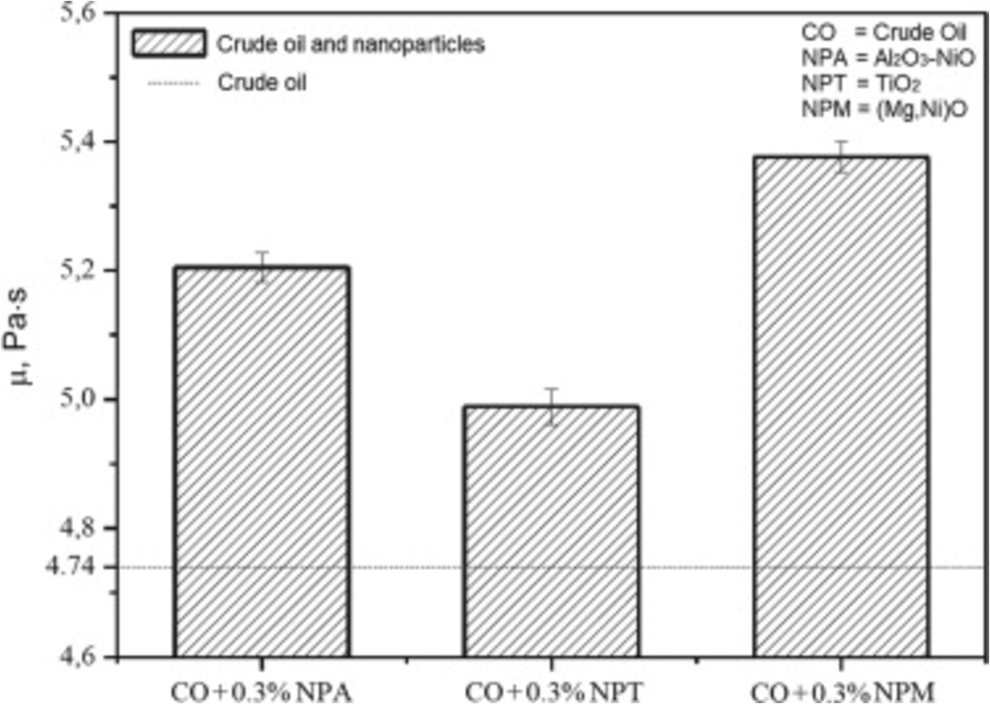
Effect of nanoparticles addition on crude oil viscosity at 25 °C. Reprinted with permission from ref. Parejas, R. D., Moura, F. J., de Avillez, R. R. & Mendes, P. R. D. Effects of Al2O3-NiO, TiO2 and (Mg,Ni)O particles on the viscosity of heavy oil during aquathermolysis. Colloids and Surfaces a-Physicochemical and Engineering Aspects 625, https://doi.org/10.1016/j.colsurfa.2021.126863 (2021),copyright 2021, ELSEVIER.
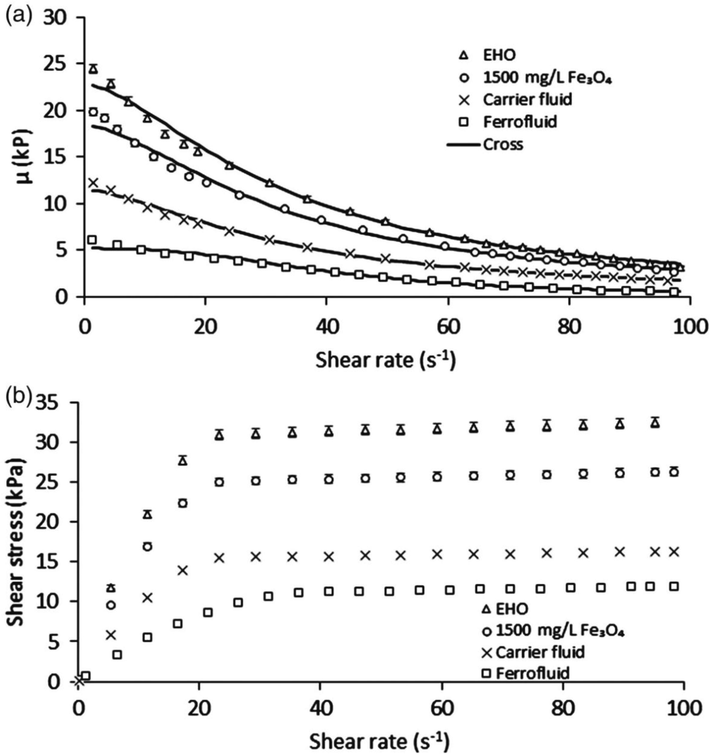
Effect of ferrofluid and each of its components on (a) viscosity and (b) shear stress of EHO at 298 K as a function of shear rate. The symbols are the experimental data, and the solid lines represent the Cross model. EHO: extra heavy crude oil. Reprinted with permission from ref. J.E. Aristizábal-Fontal, F.B. Cortés, C.A. Franco, Viscosity reduction of extra heavy crude oil by magnetite nanoparticle-based ferrofluids, Adsorption Science & Technology 36 (2018) 23–45 copyright 2017, Adsorption Science & Technology.
In an insightful exploration, Patel et al. delved into the nuanced interrelationships between nanoparticulate entities, temperature, and heavy oil viscosity. Utilizing a diverse array of nanoparticles like CuO, Fe2O3, and NiO, introduced at different concentrations, they observed a viscosity decrement in the range of 50 %–70 %. Such reductions, they surmised, emanate from intricate molecular interplays between the nanoparticles and the heavy oil matrix. Taborda et al, in their seminal work, emphasized the efficacy of nanoparticles, not just in heavy oils but also in their extra-heavy counterparts (Wu et al., 2019). Intriguingly, they reported a remarkable viscosity drop of 52 % upon integrating SiO2 nanoparticles at concentrations of 1000 mg/L, especially at shear rates subsuming 10 s−1.
On this basis, Franco Ariza et al. proposed an innovative viewpoint: the hypothesis that nanoparticles may adsorb asphaltene, ultimately leading to a decrease in crude oil viscosity (Zhang et al., 2016). In order to further investigate this theory, Parejas et al. conducted a series of studies analyzing the effects of various parameters, including temperature, type and concentration of nanoparticles, on the viscosity distribution of heavy oil (Rostami et al., 2019). Similarly, Liu et al. mixed nanoparticles with ethyl cellulose and heptadecenyl hydroxyethyl imidazolium quaternary ammonium salt (QASHI) to reduce asphalt viscosity (Tangestani et al., 2019). Their research not only emphasizes the synergistic effect between nanoparticles and surfactants, but also suggests that surfactants may further disperse aggregates of resin and asphaltene conglomerates. The results of these studies indicate that nanoparticles can reduce the viscosity of asphaltene and provide new ideas and methods for technological innovation to improve oil recovery in the future. El-hoshoudy et al. found that the viscosity of three polymers, HPAM-SiO2, HPAM-Al2O3, and HPAM-ZrO2, increased by 107 %, 45 %, and 12 %, respectively, at different temperatures.
Although it has made major progress in this research field, a comprehensive understanding of the underlying mechanisms, especially the hypothesis regarding the adsorption of asphaltenes on the surfaces of nanoparticles, still requires further clarification. The uniqueness and multifunctionality of nanoparticles and nanofluids require a more in-depth and systematic study of their impact on viscosity regulation of heavy crude oil. This exploration has important theoretical and practical significance for optimizing heavy oil extraction and improving recovery efficiency.
2.5 Shear stress
Shear stress, as one of the core concepts in fluid mechanics, refers to the frictional force between adjacent layers within a fluid and is a key factor determining the ease of fluid flow. The shear stress of crude oil can affect the purity and quality of oil recovery. For example, during the tertiary oil recovery process, the phenomenon of crude oil stratification can lead to a decrease in oil purity, thereby affecting oil recovery efficiency. Nanoparticles in nanofluids can enhance convective heat transfer and reduce interfacial thermal resistance, especially in high-temperature environments, which helps to reduce shear stress. Both an increase in pressure and a decrease in temperature will reduce the distance between molecules, leading to an increase in fluid viscosity and shear stress. Studies have shown that the load-bearing capacity and frictional surface shear stress of nanofluids are both greater than those without the addition of nanoparticles, further indicating that the addition of nanoparticles improves the load-bearing capacity of lubricants and demonstrates the lubrication characteristics of nanofluids. The shear stress increases with the increase of shear rate. At low shear rates, the shear stress significantly increases, reflecting the viscoelastic behavior of ultra heavy crude oil as follows: the shear stress significantly increases at low shear rates.
2.5.1 The influence of nanofluids on shear stress
Nanofluids as oil displacement agents can reduce shear stress during enhanced oil recovery processes. Nanoparticles form a regularly arranged adsorption layer on the friction surface, reducing shear stress during the friction process. In addition, the rolling of nanoparticles reduces the shear stress during the friction process, thereby lowering the friction coefficient. The reduction of shear stress can improve the flow performance of fluids, reduce flow resistance, and make the oil displacement agent flow into the formation more smoothly, thereby improving oil recovery efficiency. In addition, it can also make the oil displacement agent cover the oil layer more evenly, improve the sweep coefficient, ensure that more oil and gas are extracted, and thus improve the oil recovery effect.
The experimental and numerical simulation results indicate that the adsorption characteristics and distribution state of nanoparticles have a significant impact on reducing shear stress. For example, nano-SiO2 particles have stronger adsorption force on rock surfaces than TiO2 and ZnO particles, and the roughness of the micro pore walls in the storage layer has a significant impact on the adsorption characteristics. The larger the roughness, the thicker the nanoparticle adsorption layer. By adjusting the type, concentration, and particle size of particles in nanofluids, the shear stress level of the fluid can be finely controlled to better adapt to different reservoir conditions and production requirements. This flexible regulatory ability can enable nanofluids to demonstrate stronger applicability and superiority in handling different types of oil reservoirs, such as high-temperature and high-pressure reservoirs, deep-sea reservoirs, and unconventional oil and gas reservoirs.
2.5.2 Revealing the microscopic mechanism of shear stress reduction
When nanoparticles move in a fluid, they form a lubricating layer near the solid wall, reducing friction between the solid wall and the fluid, thereby reducing shear stress. This effect is particularly evident at the micro nano scale, resulting in fluid behavior that differs from that at the macroscopic scale. Specifically, factors such as the volume fraction of nanoparticles, shear velocity, and magnetic field strength can all affect the boundary slip effect. As the volume fraction of nanoparticles increases, the number density at the interface decreases and the slip velocity increases, thereby reducing the shear stress.
In addition, changes in shear velocity and magnetic field strength can also affect the formation of the lubricating layer, thereby affecting the magnitude of shear stress. Heavy oil typically behaves as a pseudoplastic fluid, where the rate of increase in shear stress is slower than the rate of increase in shear rate. Due to the faster destruction of the microstructure inside the fluid at high shear rates, the intermolecular interactions are reduced, making the fluid easier to flow.
Experimental and simulation studies have shown that boundary slip is related to shear rate, and only occurs when a certain ultimate shear rate is reached. Molecular dynamics simulations and finite element analysis have also revealed the behavior of nanoparticles in fluids and their influence on shear stress. The addition of nanoparticles can break the microstructure of the original fluid, such as disrupting weak intermolecular interactions and forming new networks or clusters at the nanoscale. This structural change directly leads to changes in shear stress. Cheraghian et al. used nanoscale gas-phase silica to enhance the viscosity of polymer solutions. The experimental results indicate that the overall viscosity of the nanosuspension increases gradually with the increase of solid content (by weight) in the nanofluid. This is because a higher concentration of nanoparticles can promote the shear stress intensity inside the fluid. Compared with the simple polymer injection method, the nano silica assisted oil recovery scheme can increase the additional crude oil recovery by up to 8.3 %. Further research on the specific impact of nanofluids on the efficiency of heavy oil recovery during polymer flooding has found that it is necessary to ensure that the concentration of nanofluids exceeds a certain critical value in order for polymer flooding technology to maximize its effectiveness. Taking titanium dioxide as an example, the threshold is set at 2.3 wt%. Empirical data shows that compared to traditional polymer flooding methods, following this concentration guidance principle for nanofluid flooding can significantly improve crude oil recovery by 3.9 %.
According to the study by Aristizábal-Fontaine et al. (Aristizábal-Fontal et al., 2018), ferromagnetic fluids based on magnetite nanoparticles can effectively reduce the shear stress of ultra heavy oil (by 78 %). The research results have opened up broader prospects for the use of nanotechnology in petroleum, achieving low-cost and cost-effective implementation.
2.6 Non-Newtonian characteristics
Non-Newtonian fluids refer to fluids that do not satisfy Newton's law of viscosity, and the relationship between shear stress and shear strain rate is not linear. Due to the unique properties of nanoparticles and base fluids, certain nanofluids may exhibit non-Newtonian fluid characteristics such as shear thinning, viscoelasticity, and yield stress (Zhang et al., 2016). Experiments have shown that nanofluids can alter their rheological properties and exhibit shear thinning behavior, as well as non-Newtonian shear thinning characteristics. This characteristic enables nanofluids to exhibit different flow behaviors under stress, thus better adapting to complex flow environments during oil recovery. In EOR, the use of fluids with shear thinning characteristics can improve the flow performance of fluids, making them easier to inject into oil reservoirs and better displacing crude oil during the injection process. Thickeners with shear thinning and viscoelasticity can displace more oil than water or surfactant solutions, increasing oil recovery. Heavy oil reservoirs exhibit non-Newtonian flow characteristics in their rheological properties due to the abundance of high molecular weight polymers. During the EOR process, heavy oil may also transition from Newtonian rheological behavior to non-Newtonian rheological behavior.
2.6.1 Application of non-Newtonian characteristics in EOR
As a type of non-Newtonian fluid, polymers can effectively improve crude oil recovery due to their viscoelastic effect. The oil displacement efficiency of polymer solution increases with the increase of its mass concentration, and the contribution of elasticity to oil displacement efficiency is about 40 %. According to experiments, the viscosity range of polymer solutions that can improve heavy oil displacement efficiency through their elastic response does not exceed 5486 cp. When the efficiency of crude oil polymer flooding drops to 31.68 %, the contribution of its elastic flooding is only 1.5 %. The enhanced oil displacement effect of polymers in heavy oil can be achieved by improving the in-situ elastic properties of polymer solutions in porous media (Xue et al., 2024).
2.6.2 Application of nanofluid shear dilution effect in EOR
The shear thinning effect helps to improve the flow performance of nanofluids in oil reservoirs, which is beneficial for oil recovery. Through experimental research, it was found that the thermal conductivity of nanofluids is significantly higher than that of the base liquid, and it increases with the increase of particle volume fraction and temperature. In addition, the shear thinning characteristic exhibited by nanofluids in shear flow helps to reduce the flow resistance of fluids in reservoirs, further improving the recovery rate of crude oil. For example, nanocomposites based on graphene oxide and clay nanosheets have a tensile strength of up to 1215 ± 80 MPa and a Young's modulus of 198.8 ± 6.5 GPa. This high-strength and high modulus material has great potential in EOR applications.
2.6.3 Comparison between shear thickening liquid and nanofluid
According to experiments on some non-Newtonian EOR fluids (Nilsson et al., 2013), it can be concluded that commercially available fluid thickeners with shear thinning and viscoelasticity have higher oil recovery efficiency than surfactant solutions and water, while shear thickeners containing nanoparticle mixtures have higher oil recovery rates compared to water, surfactants, and commercially available fluid thickeners. Unlike nanofluids used directly for EOR, shear thickeners are mainly used to improve the shear strength and wear resistance of materials. Although shear thickeners can improve fluid flowability in certain situations, their effectiveness in enhancing oil recovery is still not as significant as that of nanofluids. The rheological properties of lotion will be changed from Newtonian fluid to non-Newtonian fluid due to nano particles. When emulsifying retained oil, the formation of non-Newtonian fluids with higher viscosity in situ may result in better sweep efficiency than the injected fluid, and the resulting fluid can replace the oil in a better form.
2.6.4 Nanofluid shear thinning enhanced oil recovery
The study found that (Alade et al., 2019), the rheological properties of heavy oil aqueous lotion will show a non-Newtonian shear thinning behavior due to the presence of silica nanoparticles. Regardless of the silica content, it is easier to observe shear thinning (pseudoplastic) non-Newtonian behavior of the original oil at lower shear rates. Exhibiting non-Newtonian behavior at high shear rates. Alphonse et al. (Alphonse et al., 2009) reported the Newtonian behavior of TiO2 with water at low shear rates, which became shear thinning when the shear rate exceeded 100 s−1. Tseng and Chen (Tseng and Chen, 2006) studied nickel/terpineol in the shear rate range of 1 to 1000 s−1 and found that all suspensions of nickel containing nanofluids exhibited shear thinning behavior throughout the entire shear rate. Silver/diethylene glycol (DEG) nanofluids exhibit non-Newtonian (pseudoplastic) flow behavior in the shear rate range of 1–200 s−1, and viscosity increases with increasing fluid concentration. Duan et al. studied the flow behavior of graphite nanofluids in deionized water and found that the suspension exhibited shear thinning behavior when the shear rate ranged from 1 to 100 s−1, with viscosity increasing with increasing fluid concentration. The report also indicates that the enhancement effect of the nanofluid maintained for 3 days is higher than that of the newly prepared nanofluid. Moghaddam et al. studied graphene nanofluids in glycerol and found that at low shear rates, the nanofluids exhibited shear thinning behavior at all temperatures, but at high shear rates, the nanofluids exhibited Newtonian behavior.
Li et al. analyzed the impact of shear thinning effect on oil recovery efficiency by studying the case of Daqing Oilfield. Shear thinning fluids can maintain lower viscosity under high pressure and high-speed flow conditions, which is beneficial for better permeation and displacement of crude oil in porous media. For example, xanthan gum is a polymer that exhibits significant shear thinning behavior and can form a “piston like” displacement mode in porous media, effectively improving oil recovery. El-hoshoudy et al. found that the new composite material was characterized using Fourier transform infrared spectroscopy (FTIR). Compared with natural xanthan gum, the composite xanthan gum co polymerized with vinyl silane, vinyl monomer, and silica nanoparticles exhibited good tolerance to salinity, temperature, and pressure even under high shear rates and reservoir conditions. After adding silica nanoparticles, the composite viscosity of natural xanthan gum increased tenfold. Under reservoir conditions, both natural and composite xanthan gum exhibit shear thinning behavior, but simulation experiments have shown that xanthan gum composite materials are superior to natural composite materials. Composite xanthan gum can recover 48.13 % from crude oil, 39.37 % from natural xanthan gum, and 37.07 % from water flooding alone. These studies indicate that the shear thinning properties of nanofluids can improve oil recovery efficiency.
They also studied natural chitosan and composite chitosan, both of which exhibited shear thinning behavior under reservoir conditions of 135,000 ppm salinity, 196° F temperature, and 2200 psi pressure. Through the core displacement test of sandstone core plugs, it was found that in the third stage of oil displacement, composite chitosan increased the oil recovery rate by 8.67 %, while natural chitosan was 4.73 %.
El-hoshoudy et al. demonstrated through rheological analysis that the viscosity of two modified composite materials, XG-g-AM&MMA and XG-g-AM, MMA&TEVS, decreases with increasing shear rate, indicating that pseudoplastic fluids (a non-Newtonian fluid) exhibit shear thinning behavior characteristics. Especially modified compounds containing silica particles exhibit salt resistance, temperature resistance, and better viscosity characteristics. These new composite materials can become EOR candidates for solving problems such as high temperature and high salinity reservoirs.
3 Nanofluid-assisted EOR techniques
3.1 EOR nanoparticle surfactant foam system
The injection of gases such as CO2, N2, CH4, and H4S is very popular in traditional EOR methods because it can effectively displace oil from reservoir pores (Lu et al., 2022). Among these gases, carbon dioxide is usually the preferred choice because it is mild, non-toxic, and non-flammable. The essence of this technology lies in the ability of the gas to combat capillary and viscosity barriers in the reservoir, thereby increasing the efficiency of oil extraction (Zhang et al., 2022). Yet, gas injection isn't without its challenges.
Issues like gas channelling can curtail sweep efficiency, undermining the technique's effectiveness (Monette and Nguyen, 2021). To overcome this limitation, researchers have introduced foam flooding technology. Experiments involve co-injection of gas and surfactants to induce foam formation, which can strategically control the flow of gas and thus enhance oil displacement efficiency (Zheng et al., 2021). However, Conventional foams often exhibit poor thermodynamic stability, especially under harsh reservoir conditions or in large-scale applications.
The selection of surfactants (Liu et al., 2021), reservoir attributes (Xu et al., 2022a), temperature (Xu et al., 2022b), and the physicochemical conditions of the aqueous phase (Phukan and Saha, 2022) are all factors that can affect the effectiveness of foam flooding. But it is worth noting that when surfactants can craft a stable interface with residual oils, foam stability is enhanced.
Nanoparticles, with their petite dimensions, can easily pass through rock pores and anchor at the oil–water interface, exhibit behavior similar to that of surfactants (Rattanaudom et al., 2021). Nanoparticles such as SiO2, Al2O3, TiO2, and CuO can significantly strengthen the foam's intensity (Gu et al., 2022; Chaturvedi et al., 2021), with Al2O3 showing particularly outstanding performance. Fundamental scientific research indicates that nanoparticles adhere to the gas–liquid interface, preventing the coalescence of foam (Kim et al., 2022). To optimize foam stability and enhance oil recovery efficiency, adjustments can be made to the hydrophobicity of nanoparticles or integrating agents like polyethylene glycol. Thus, nanoparticle-surfactant foam systems can maintain unparalleled stability even under extremely challenging conditions.
The integration of nanoparticles in EOR foam systems offers numerous benefits, such as: they not only enhance the adhesion energy of the foam film, minimizing the coalescence of bubbles (Lv et al., 2021), but also promote adsorption between foam bubbles, increasing the thickness of the lamellae and further strengthening the overall structure of the foam (Bhatt et al., 2023). Additionally, nanoparticles can reduce the drainage of the foam lamellae, lower the interfacial tension and capillary pressure between gas and liquid, and significantly improve the stability and tenacity of the foam (Sun and Ge, 2022).
3.2 Nanoemulsions for EOR
Emulsions are categorized into macro-emulsions, micro-emulsions, and nanoemulsions. Among these, nanofluid the petroleum industry, nanoemulsions are being explored for EOR applications due to their unique properties, such as high stability, large specific surface area, improved interface and wetting behavior, and tunable rheological properties (Fig. 10) (Jia et al., 2022; Naseri, 2014). Some studies position emulsions as storage mechanisms for heavy oil, while others emphasize the pivotal role of asphaltenes in emulsion stabilization (Rockey et al., 2018). Nanoparticles have emerged as key agents in enhancing emulsion tenacity by anchoring themselves at oil/water interfaces, thereby deterring droplet coalescence.
Amidst the myriad of nano-enhanced EOR strategies, Pickering emulsions, stabilized by nanoparticles, often steal the spotlight (Dibaji et al., 2022). Factors such as temperature, aqueous-phase conditions, and nanoparticle attributes (Li et al., 2022) critically shape emulsion stability. Emulsion development aids nanofluid flow through restrictive regions, catalyzing the liberation of entrapped oil.
For maintaining emulsion integrity, nanoparticle wettability is paramount. Deviations in the nanoparticle contact angle from 90° can intensify their adsorption, bolstering Pickering emulsion stability. Depending on whether the nanoparticles are hydrophilic or hydrophobic, the predominance of specific types of emulsions can be observed.
Sharma et al studied the effect of nanoparticles on oil–water emulsion in a polymer-rich settings. Their findings revealed a synergistic interplay between nanoparticles, surfactants, and polymers, manifesting in fortified Pickering emulsions, and hinted at potential oil recovery enhancements of 1 %–6 %.
3.3 Refining the injected fluid mobility ratio
The mobility ratio of the injected phase, defined by the balance between its relative permeability and viscosity, is crucial for oilfield extraction (Beteta et al., 2022). Optimal EOR outcomes are realized when the viscosity of the injected fluid surpasses that of the in-situ oil, ensuring commendable mobility management (Salmo et al., 2022). However, improper balance in viscosity may lead to issues like viscous fingering. There is a potential solution here to increase the viscosity of the injection medium.
Researchers have found that nanofluids significantly improve their performance by increasing the viscosity of the introduced reagents. This enhancement is due to the ability of nanoparticles to bind themselves to the oil/water interface, making the viscosity of the obtained foam or lotion reactivate. The accumulated research results support the transformative potential of nanoparticles in increasing viscosity (Li et al., 2022), which further ensures excellent flowability control and lays a solid foundation for improving oil recovery.
4 Multiscale multiphase pore/core displacement experiments
The flourishing development of multi-scale multiphase pore/core displacement experiments has made N-EOR technology a focus of advanced oil recovery research (Liang et al., 2023). A key focus is on identifying nanofluid dynamics during displacement processes (Santamaria et al., 2021) and evaluating their subsequent impact on oil extraction (Waqas et al., 2021). Factors such as changes in porosity (Huang et al., 2022), subtle differences in electrochemistry, microbial activity, diffusion rate, and permeation flux can all affect the behavior of porous media. In this complex environment, the action of nanofluids is closely regulated by the characteristics of aqueous solution, oil phase, and porous matrix (Shende et al., 2022).
In the displacement stage, a series of interaction forces, ranging from electrostatic to structural, and encompassing van der Waals forces, mold the trajectory of nanoparticles and the behavior of the oil phase (Son et al., 2022). Among them, wettability plays a central role in this process (Liu et al., 2022), affecting the efficiency, relative permeability (Kumar et al., 2021), and capillary dynamics (Song et al., 2021) of multiphase flow. For example, the distribution of reservoir fluids and subsequent oil phase conduction largely depend on the wettability of the reservoir.
The arrangement of immiscible multiscale multiphase displacement is supported by two main behaviors: imbibition, where non wetting phases penetrate into the pores; drainage, which is opposite to each other. As multiphase flow dances, wetting entities mask the rock skeleton and pores (Ji et al., 2023), while non wetting phases mainly carve out seamless channels (Shi et al., 2022). Cutting edge imaging technologies, such as micro computed tomography (micro-CT), have uncovered the mysteries of these processes, revealing the complex interactions between pore structure and fluid distribution.
Historical literature emphasizes the powerful role of nanofluids in regulating mineral wettability (Ali, 2023) and refining oil–water interfacial tension (Nasr et al., 2021). In this multiphase process, the dynamics guiding nanoparticle transport, such as nanoparticle concentration (Bijani et al., 2022), injection rate, salinity gradient (Hadia et al., 2021), and thermal conditions (Jafarbeigi et al., 2022), have become key research topics. It is worth noting that transition metal nanoparticles have demonstrated superb skills in the rock terrain of reservoirs. However, this journey is not without challenges (Liu et al., 2022).
Cheng et al. conducted in-depth research on the microscopic world of residual oil at the pore scale using micro-CT technology (Wu et al., 2019). Their meticulous exploration spanned the morphological deformation of retained oil, the microscopic symphony of oil within pores, and the EOR mechanism at different flow temperatures. This study ultimately demonstrated the synchronicity of rock texture and fluid properties, as well as the subtle arrangement of rock fluid interactions in shaping the process of oil extraction in multiphase fluid dynamics environments. Obviously, the interrelated indicators such as capillary force, relative permeability, and oil–water saturation gradient jointly determine the results that affect permeability (Al-Yaari et al., 2022).
5 Multiphase flow fluid-solid coupling simulation
In the current research field, there is a clear trend towards developing numerical models that can simulate multiphase flow in fluid structure coupling systems (as shown in Fig. 12). These models are powerful tools (Zafar et al., 2023) that can decipher the subtle interactions between capillary pressure, relative permeability, and water saturation. Therefore, the field of nanofluid EOR can focus on the ability of mathematical models to elucidate the complexity of nanoparticle transport in various terrains.
Viscoelasticity of polymer solution at different positions from the bottom of the pore. Reprinted with permission from ref. Enhancing heavy‐oil displacement efficiency through viscoelasticity of polymer solution by investigating the viscosity limit of crude oil: An experimental study, copyright 2024, SCI.
The retention behavior of nanoparticles plays a crucial role in shaping the prospects of oil extraction during their passage through porous media (Agi et al., 2023). The retention mode of these nanoparticles is coordinated by multiple processes, including surface adhesion (Miclotte et al., 2022), single particle blockage, and collective particle barriers (Ahmed et al., 2021). Given the severity of these processes on a wider ecosystem, it is crucial to design models that can reveal their potential impacts. In order to further investigate the EOR paradigm, researchers merged different simulation libraries. By absorbing variables such as gas, saltwater (Zhang et al., 2018), and chemical surges, these models elucidate potential breakthroughs in petroleum extraction technology. Through this lens, they gained a profound understanding of the intricate arrangement of N-EOR dynamics and multiphase fluid motion in porous backgrounds.
In a landmark study, Ali et al. delved into nanofluid dynamics in two-phase flow matrices (Zhu et al., 2017). By utilizing the combined force of micro-CT and molecular dynamics simulation, they emphasized the ability of nanofluids to alter the wettability of sandstone in synergy with saltwater. Their strategy is to utilize these nanofluids suspended in saltwater to enhance oil release in the maze like crevices of sandstone. Subsequently, the in-situ micro-CT protocol was used to track the Odyssey of oil micro lobules. The key parameters, including capillary pressure, relative permeability, viscosity, and density, were carefully recorded. A resounding conclusion drawn from their research is that BiFeO3 nanofluids have unparalleled efficacy in reshaping the wettability of porous media, outshining their peers. This unique nanofluid further exhibits excellent capillary pressure and relative permeability indicators.
Abdelfatah et al. constructed a mathematical building and carefully plotted the migration of nanoparticles within a porous framework. Their efforts are a microcosm of avant-garde art, and the advancements in methodology and continuous development have propelled our understanding of nanoparticle dynamics in these complex landscapes (Jafarbeigi et al., 2022).
6 Opportunities and challenges in N-EOR
In the field of EOR, nanofluids represent convincing juxtapositions, hopes for change, and complex obstacles (El-Masry et al., 2023; Zhou et al., 2023). When we delve into the subtle differences of N-EOR, we encounter a series of opportunities and challenges intertwined:
Stability and Synthesis: In the rigorous environment of reservoirs—marked by high pressures, temperatures, and salinity—nanoparticles tend to cluster, thus restricting their broader applicability. To resolve this problem, researchers can use cutting-edge methods to manufacture highly stable nanofluids. Furthermore, it is crucial to produce eco-friendly nanoparticles without reducing their efficacy (Kumar et al., 2021).
Environmental and health considerations: Some nanoparticles pose ecological risks due to their environmental footprint (Ghobashy et al., 2021). Their microscopic size can also pose potential health hazards due to inhalation (Zhang et al., 2018). The research trajectory must adopt a broad perspective to analyze the impact of these particles on the environment and health. In addition, thorough research on the economic impact and recovery of nanofluids is necessary before large-scale implementation, which is crucial to ensure their economic feasibility and environmental sustainability for integration into industry.
Theoretical exploration: The theoretical basis of N-EOR is still in the formation stage. Developing robust mathematical simulations will be the key to drawing the vast field of N-EOR. Especially for processes driven by surfactants or polymers, these frameworks can provide a deeper understanding of the mechanisms of oil extraction and the intersections of various processes.
Adsorption kinetics: The adsorption phenomenon generated by the interaction between nanofluids and minerals is influenced by various factors, such as rock composition, temperature, and salinity (Pan et al., 2023). However, the core mechanisms underlying these interactions are still enigmatic. Through Strict and intensive research on these dynamic processes, we can gradually reveal the complexity of adsorption phenomena and provide theoretical support for improving the efficiency of nanofluids in oil extraction.
From laboratory to field: Although laboratory tests have consistently shown the potential of nanofluids in improving oil recovery, this promise has not been fully translated into widespread field applications. In the real world, the exploration of nanoparticles traversing complex porous environments remains largely unknown. Future efforts need to highlight the journey of nanoparticles, surfactants, and polymers in these geological complexities. Future efforts must focus on the trajectories of nanoparticles, surfactants, and polymers in these complex geological environments. Molecular integrated dynamics simulation and mathematical modeling are key to the widespread deployment of N-EOR strategies.
In short, there is no doubt that nanofluids mark a new era of EOR, but it is not easy for them to transition from controlled laboratory environments to vast oil fields. Successfully embarking on this path can lead the industry into a new era characterized by improved operational efficiency and a commitment to environmental sustainability.
The insights that nanofluids bring to our current and future are: nanomaterials are of great significance in improving oil recovery efficiency. In recent years, the oil recovery process in oil fields has put forward higher requirements for the performance of oil displacement materials, and the use of nanomaterials is an important method to improve the performance of oil displacement agents from a microscopic perspective. The research shows that nano materials can promote the flow of crude oil by reducing the interfacial tension between oil and water, emulsifying crude oil to reduce the viscosity of crude oil, improving the wettability of rock surface, stabilizing foam, etc., so as to improve oil recovery.
The main mechanisms and methods of using nanomaterials to enhance oil recovery include the following aspects: (1) reducing oil–water interfacial tension: Nanomaterials can significantly reduce oil–water interfacial tension, making it easier for crude oil to peel off from the rock surface. (2) Improving the wettability of rock surface: By changing the wettability of rock surface, enhancing water infiltration and absorption, the recovery rate can be increased. (3) Enhancing the stability of lotion and foam: Nanoparticles can enhance the stability of lotion and foam, thereby improving the stability of the system. (4) Inhibition of Asphalt Deposition: Nanomaterials can inhibit the precipitation of asphaltene and reduce formation damage.
There is a close relationship between nanomaterials and sustainable energy development, which is reflected in multiple aspects, including improving energy conversion efficiency, optimizing energy storage, and promoting the development of renewable energy technologies.
7 Summary and conclusions
The review mainly studies the mechanism of nanofluid oil recovery and the relationship between mechanisms. This review delves into the complexity of N-EOR and emphasizes the broad prospects for future research. Through our in-depth exploration, the main findings and conclusions are:
Nanofluids in Oil Dynamics: Nanofluids demonstrate an impressive aptitude for interacting with the complex interplay of oil molecules, particles, and surfaces.They help to alter the wettability of minerals and reshape the micro mechanics of particles and surfaces. In addition, they are crucial in regulating oil–water interfacial tension and viscosity, especially in heavy oil environments.
Wettability Dynamics: Nanofluids exert adhesive forces on minerals and alter their wettability. As these minerals shift towards more hydrophilic behavior, the oil recovery rate also increases accordingly. The extent of altered wettability is influenced by many factors, including nanofluid concentration, nanoparticle size, temperature, and surfactant concentration. Intriguingly, when nanofluids position themselves at the oil–water interface, they can affect interfacial tension, which has implications for oil extraction. However, the exact role of nanoparticles in this dynamic is still somewhat ambiguous, as studies present a mix of confirmations, contradictions, and neutral findings. Additionally, the reduction in viscosity often correlates with the reduced affinity of asphaltenes to minerals. Lower asphaltene content correlates with reduced oil viscosity, leading to enhanced oil extraction.
Structural Disjoining Pressure Paradigm: This principle underscores the role of structural disjoining pressure in liberating oil from mineral strongholds during N-EOR processes. Such a mechanism can significantly boost oil recovery rates, a sentiment echoed in both empirical and theoretical studies.
Multiscale, Multiphase Endeavors: Within the vast landscape of multiscale, multiphase core dislocation experiments, nanofluids stand out as crucial players, their significance further affirmed by simulation outcomes.
In conclusion, the fact that nanofluids showed excellent properties and high interaction role with heavy oil, nanofluids were optimum agent for improving heavy oil recovery. However, fully unraveling its potential, understand its underlying mechanisms, and interpret its role in the complex petroleum structure requires ongoing academic exploration to fully realize the promise of nanofluids in the realm of heavy oil recovery.
CRediT authorship contribution statement
Feifei Liang: Writing – original draft, Validation, Supervision, Resources, Project administration, Formal analysis, Data curation. Wenjuan Wang: Writing – original draft, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Siyu Zhu: Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Formal analysis, Data curation. Yuting Hu: Writing – original draft, Formal analysis, Data curation, Conceptualization. Ziyu Zhao: Writing – review & editing. Yuxing Tan: Investigation, Writing – review & editing. Gaobo Yu: Writing – original draft, Supervision, Conceptualization. Jinjian Hou: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization. Jiacheng Li: Visualization, Formal analysis, Data curation.
Funding
The work is supported by the foundation of the Hainan Provincial Natural Science Foundation of China (224QN187), Hainan University Startup Fund (XJ2300004011), Open Project Program of Shandong Engineering Research Center for Environmental Protection and Remediation on Groundwater (801KF2023-1), Anhui Province Key Laboratory of Pollution Sensitive Materials and Environmental Remediation (PSMER2023005).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dynamic stabilization of formation fines to enhance oil recovery of a medium permeability sandstone core at reservoir conditions. J. Mol. Liq.. 2023;371:121107
- [CrossRef] [Google Scholar]
- Recent advances in nanoparticles associated ecological harms and their biodegradation: global environmental safety from nano-invaders. J. Environ. Chem. Eng.. 2021;9:106093
- [CrossRef] [Google Scholar]
- A viscosity study of charcoal-based nanofluids towards enhanced oil recovery. J. Mol. Liq.. 2023;387
- [CrossRef] [Google Scholar]
- Investigation into the effect of silica nanoparticles on the rheological characteristics of water-in-heavy oil emulsions. Pet. Sci.. 2019;16:1374-1386.
- [CrossRef] [Google Scholar]
- Effect of Fe3O4/mineral-soil nanocomposites on wettability alteration and oil production under the spontaneous imbibition process. Arab. J. Sci. Eng.. 2023;48:9259-9268.
- [CrossRef] [Google Scholar]
- Effect of PEG on rheology and stability of nanocrystalline titania hydrosols. J. Colloid Interface Sci.. 2009;337:81-87.
- [CrossRef] [Google Scholar]
- Thermophysical properties of nanofluid in two-phase fluid flow through a porous rectangular medium for enhanced oil recovery. Nanomaterials. 2022;12
- [CrossRef] [Google Scholar]
- Viscosity reduction of extra heavy crude oil by magnetite nanoparticle-based ferrofluids. Adsorpt. Sci. Technol.. 2018;36:23-45.
- [CrossRef] [Google Scholar]
- Effects of temperature and nanofluid type on the oil recovery from a vertical porous media in antigravity fluid injection. Chem. Eng. Res. Des.. 2023;193:394-408.
- [CrossRef] [Google Scholar]
- CuO/TiO2/PAM as a Novel Introduced Hybrid Agent for Water-Oil Interfacial Tension and Wettability Optimization in Chemical Enhanced Oil Recovery. Energy Fuel. 2019;33(11):10547-10560.
- [CrossRef] [Google Scholar]
- NEOR mechanisms and performance analysis in carbonate/sandstone rock coated microfluidic systems. Fuel. 2022;309
- [CrossRef] [Google Scholar]
- The role of immiscible fingering on the mechanism of secondary and tertiary polymer flooding of viscous oil. Transp. Porous Media. 2022;143:343-372.
- [CrossRef] [Google Scholar]
- Perspectives of foam generation techniques and future directions of nanoparticle-stabilized CO2 foam for enhanced oil recovery. Energy Fuels. 2023;23
- [CrossRef] [Google Scholar]
- Enhanced oil recovery by polymer flooding using polyacrylamide stabilised with alumina/graphene oxide nanocomposite. J. Arab. J. Sci. Eng.. 2023;48(12):16819-16830.
- [CrossRef] [Google Scholar]
- Comprehensive experimental investigation of the effective parameters on stability of silica nanoparticles during low salinity water flooding with minimum scale deposition into sandstone reservoirs. Sci. Rep.. 2022;12
- [CrossRef] [Google Scholar]
- An experimental investigation of the viscosity behavior of solutions of nanoparticles, surfactants, and electrolytes. Phys. Fluids. 2021;33:26601.
- [CrossRef] [Google Scholar]
- Effects of surface oxides and nanostructures on the spontaneous wettability transition of laser-textured copper surfaces. Applied Surface Science: A Journal Devoted to the Properties of Interfaces in Relation to the Synthesis and Behaviour of Materials.. 2021;560:150021
- [CrossRef] [Google Scholar]
- Nanomechanical characteristics of trapped oil droplets with nanoparticles: a molecular dynamics simulation. J. Pet. Sci. Eng.. 2021;203:10.
- [CrossRef] [Google Scholar]
- Effect of single-step silica nanoparticle on rheological characterization of surfactant based CO2 foam for effective carbon utilization in subsurface applications. J. Mol. Liq.. 2021;341
- [CrossRef] [Google Scholar]
- New technique for enhancing residual oil recovery from low-permeability reservoirs: the cooperation of petroleum hydrocarbon-degrading bacteria and SiO2 nanoparticles. Microorganisms. 2022;10
- [CrossRef] [Google Scholar]
- Experimental and field applications of nanotechnology for enhanced oil recovery purposes: A review. Fuel. 2022;324:124669
- [CrossRef] [Google Scholar]
- Synthesizing CNT-TiO2 nanocomposite and experimental pore-scale displacement of crude oil during nanofluid flooding. Pet. Explor. Dev.. 2022;49:1430-1439.
- [CrossRef] [Google Scholar]
- Comprehensive review on utilizing nanomaterials in enhanced oil recovery applications. Energies. 2023;16
- [CrossRef] [Google Scholar]
- Wettability alteration of carbonate rocks from liquid-wetting to ultra gas-wetting using TiO2, SiO2 and CNT nanofluids containing fluorochemicals, for enhanced gas recovery. J. Nat. Gas Sci. Eng.. 2015;26:1294-1305.
- [CrossRef] [Google Scholar]
- A comprehensive review of the potential of rock properties alteration during CO2 injection for EOR and storage. Fuel. 2023;353:24.
- [CrossRef] [Google Scholar]
- Hyperbranched polyglycerols derivatives as cetyltrimethylammonium bromide nanocarriers on enhanced oil recovery processes.. 2022;139(9):51725.
- [CrossRef]
- Effect of aluminium oxide nanoparticles on oilfield polyacrylamide: Rheology, interfacial tension, wettability and oil displacement studies. J. Mol. Liquids. 2019;296
- [CrossRef] [Google Scholar]
- Surfactant enhanced oil recovery in a high temperature and high salinity carbonate reservoir. J. Surfact. Deterg. 2015
- [Google Scholar]
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment. Nanotechnol. Rev.. 2021;10(1):954-977.
- [CrossRef] [Google Scholar]
- Gelation and yielding behavior of polymer-nanoparticle hydrogels. J. Polym. Sci.. 2021;59:2854-2866.
- [CrossRef] [Google Scholar]
- Experimental investigation on the SiO2 nanoparticle foam system characteristics and its advantages in the heavy oil reservoir development. J. Pet. Sci. Eng.. 2022;214:18.
- [CrossRef] [Google Scholar]
- High salinity and high temperature stable colloidal silica nanoparticles with wettability alteration ability for EOR applications. J. Nanomater.. 2021;11
- [CrossRef] [Google Scholar]
- Treatment of oily wastewaters using magnetic Janus nanoparticles of asymmetric surface wettability. J. Colloid Interface Sci. 2020:568.
- [Google Scholar]
- Cellulose-coated magnetic Janus nanoparticles for dewatering of crude oil emulsions. J. Chem. Eng. Sci.. 2021;230:116215
- [CrossRef] [Google Scholar]
- Effects of the initial rock wettability on silica-based nanofluid-enhanced oil recovery processes at reservoir temperatures. Energy Fuels. 2014;28:6228-6241.
- [Google Scholar]
- Metal oxide-based nanoparticles: revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci.. 2015;5:181-199.
- [Google Scholar]
- Nanoparticle dispersion with surface-modified silica nanoparticles and its effect on the wettability alteration of carbonate rocks. Colloids Surf. A: Physicochem. Eng. Aspects. 2018;554:261-271.
- [CrossRef] [Google Scholar]
- Wettability alteration of oil-wet carbonate surface induced by self-dispersing silica nanoparticles: mechanism and monovalent metal ion's effect. J. Mol. Liquids. 2019;294:111601
- [Google Scholar]
- Thickness of adsorbed water film in underground gas reservoir. Desalin. Water Treat.. 2022;269:212-220.
- [CrossRef] [Google Scholar]
- Designing novel high-performance shale inhibitors by optimizing the spacer length of imidazolium-based bola-form ionic liquids. Energy Fuels 2020
- [Google Scholar]
- Experimental research on seepage law and migration characteristics of core-shell polymeric nanoparticles dispersion system in porous media. Polymers. 2022;14
- [CrossRef] [Google Scholar]
- Wettability alteration of sandstones by silica nanoparticle dispersions in light and heavy crude oil. J. Nanopart. Res.. 2017;19:323.
- [Google Scholar]
- Fundamental mechanisms and factors associated with nanoparticle-assisted enhanced oil recovery. Ind. Eng. Chem. Res.. 2022;61:17715-17734.
- [CrossRef] [Google Scholar]
- Mathematical modeling and simulation of nanoparticle-assisted enhanced oil recovery—a review. Energies 2019
- [Google Scholar]
- Ivanova, A.A. et al., 2020. Effect of nanoparticles on viscosity and interfacial tension of aqueous surfactant solutions at high salinity and high temperature.
- A review on applications of nanoparticles in the enhanced oil recovery in carbonate reservoirs. J. Pet. Sci. Technol.. 2022;40:1811-1828.
- [CrossRef] [Google Scholar]
- Experimental study on conformance control using acidic nanoparticles in a heterogeneous reservoir by flue gas flooding. Energies. 2023;16
- [CrossRef] [Google Scholar]
- Systematic investigation of the effects of zwitterionic surface-active ionic liquids on the interfacial tension of a water/crude oil system and their application to enhance crude oil recovery. Energy Fuels. 2018;32:154-160.
- [Google Scholar]
- Synergistic effects of AlOOH and sodium benzenesulfonate on the generation of Pickering emulsions and their application for enhanced oil recovery. Colloid Surf. A-Physicochem. Eng. Asp.. 2022;638:10.
- [CrossRef] [Google Scholar]
- The improvement of the cationic/anionic surfactant interfacial activity via the selective host-guest recognition. J. Mol. Liquids. 2018;268:266-272.
- [CrossRef] [Google Scholar]
- The effects of surfactant/hydrocarbon interaction on enhanced surfactant interfacial activity in the water/hydrocarbon system. J. Mol. Liquids. 2019;293
- [Google Scholar]
- Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci.. 2021;288:102340
- [CrossRef] [Google Scholar]
- The application of nanofluids for enhanced oil recovery: effects on interfacial tension and coreflooding process. Pet. Sci. Technol.. 2014;32(21):2599-2607.
- [CrossRef] [Google Scholar]
- A review on advanced nanoparticle-induced polymer flooding for enhanced oil recovery. Chem. Eng. Sci.. 2022;262
- [CrossRef] [Google Scholar]
- Review on application of nanoparticles for EOR purposes: a critical review of the opportunities and challenges. Chin. J. Chem. Eng.. 2019;27:10.
- [Google Scholar]
- Insights into stability of silica nanofluids in brine solution coupled with rock wettability alteration: an enhanced oil recovery study in oil-wet carbonates. Aspects. 2019;583:124008
- [Google Scholar]
- Synthesis of silica nanoparticles with different morphologies and their effects on enhanced oil recovery. Appl. Nanosci.. 2020;10:1105-1114.
- [Google Scholar]
- A model for interpretation of nanoparticle-assisted oil recovery: Numerical study of nanoparticle-enhanced spontaneous imbibition experiments. Fuel. 2021;292
- [CrossRef] [Google Scholar]
- Spontaneous generation of stable CO2 emulsions via the dissociation of nanoparticle-aided CO2 hydrate. J. Pet. Sci. Eng.. 2022;208:8.
- [CrossRef] [Google Scholar]
- Performance evaluation of silica nanofluid for sand column transport with simultaneous wettability alteration: an approach to environmental issue. J. Clean. Prod.. 2021;303
- [CrossRef] [Google Scholar]
- Nanoemulsion flooding for enhanced oil recovery: theoretical concepts, numerical simulation and history match. J. Pet. Sci. Eng.. 2021;202
- [CrossRef] [Google Scholar]
- Effect of nanoparticle on rheological properties of surfactant-based nanofluid for effective carbon utilization: capturing and storage prospects. Environ. Sci. Pollut. Res.. 2021;28:53578-53593.
- [CrossRef] [Google Scholar]
- Formation, characteristics and oil industry applications of nanoemulsions: a review. J. Pet. Sci. Eng.. 2021;206:109042
- [CrossRef] [Google Scholar]
- Oxygen-deficient titanium dioxide supported cobalt nano-dots as efficient cathode material for lithium-sulfur batteries. J. Energy Chem. 2020
- [Google Scholar]
- Multi-molecular mixed polymer flooding for heavy oil recovery. J. Dispers. Sci. Technol.. 2022;43:1700-1708.
- [CrossRef] [Google Scholar]
- Fabrication of surface hydroxyl modified g-C3N4 with enhanced photocatalytic oxidation activity. Catal. Sci. Technol.. 2019;9
- [Google Scholar]
- A hybrid process for oil-solid separation by a novel multifunctional switchable solvent[. Fuel. 2018;221:303-310.
- [CrossRef] [Google Scholar]
- Application of nanomaterial for enhanced oil recovery. Pet. Sci.. 2022;19:882-899.
- [CrossRef] [Google Scholar]
- Mechanisms of nanofluid based modification MoS2 nanosheet for enhanced oil recovery in terms of interfacial tension, wettability alteration and emulsion stability. J. Dispers. Sci. Technol.. 2023;44:26-37.
- [CrossRef] [Google Scholar]
- Synergistic effects between anionic and sulfobetaine surfactants for stabilization of foams tolerant to crude oil in foam flooding. J. Surfactant Deterg.. 2021;24:683-696.
- [CrossRef] [Google Scholar]
- Size effect of carnauba wax nanoparticles on water vapor and oxygen barrier properties of starch-based film. Carbohydr. Polym.. 2022;296
- [CrossRef] [Google Scholar]
- Experimental quantification of oil displacement efficiency in pores and throats with varied sizes in conglomerate oil reservoirs through CO2 flooding. Chem. Tech. Fuels Oils. 2022;57:933-940.
- [CrossRef] [Google Scholar]
- Enhanced oil recovery of low-permeability cores by SiO2 nanofluid. Energy Fuels. 2017;31:5612-5621.
- [Google Scholar]
- Enhancing oil-solid and oil-water separation in heavy oil recovery byCO(2)-responsive surfactants. AIChE J.. 2021;67(1):17033.
- [CrossRef] [Google Scholar]
- CO2 mobility control in porous media by using armored bubbles with silica nanoparticles. Ind. Eng. Chem. Res.. 2021;60:128-139.
- [CrossRef] [Google Scholar]
- Difference in step-wise production rules of SP binary flooding for conglomerate reservoirs with different lithologies. Polymers. 2023;15
- [CrossRef] [Google Scholar]
- The great improvement of the surfactant interfacial activity via the intermolecular interaction with the additional appropriate salt. Colloids and Surfaces A. Physicochemical and Engineering Aspects. 2018;554:142-148.
- [CrossRef] [Google Scholar]
- Study of Janus amphiphilic graphene oxide as a high-performance shale inhibitor and its inhibition mechanism. Front. Chem.. 2020;8:201.
- [Google Scholar]
- A state-of-the-art review of nanoparticles application in petroleum with a focus on enhanced oil recovery. Appl. Sci.. 2018;8:871.
- [Google Scholar]
- Thermoresponsive block copolymer core-shell nanoparticles with tunable flow behavior in porous media. ACS Appl. Mater. Interfaces. 2022;14:54182-54193.
- [CrossRef] [Google Scholar]
- Investigation of the effect of gas compositions on low-tension-gas flooding. J. Pet. Sci. Eng.. 2021;207:12.
- [CrossRef] [Google Scholar]
- Synthesis of fluorinated nano-silica and its application in wettability alteration near-wellbore region in gas condensate reservoirs. Appl. Surf. Sci.. 2013;273:205-214.
- [Google Scholar]
- The synergistic effects of nanoparticle-surfactant nanofluids in EOR applications. J. Pet. Sci. Eng.. 2018;171:196-210.
- [Google Scholar]
- An experimental investigation of wettability alteration in carbonate reservoir using γ-Al2O3 nanoparticles. Iran. J. Oil Gas Sci. Technol.. 2014;95:2863.
- [Google Scholar]
- Nitrogen-doped graphene quantum dot nanofluids to improve oil recovery from carbonate and sandstone oil reservoirs. J. Mol. Liq.. 2021;330
- [CrossRef] [Google Scholar]
- Application of nanotechnology for enhancing oil recovery – a review. Petroleum. 2016;2
- [Google Scholar]
- Effect of temperature on the pH of water flood effluents and irreducible water saturation: a study with reference to the Barail sandstone outcrop of the upper Assam Basin. J. Pet. Explor. Prod. Technol.. 2022;12:1129-1145.
- [CrossRef] [Google Scholar]
- Effect of fluid rheology on enhanced oil recovery in a microfluidic sandstone device. J. Nonnewton. Fluid Mech.. 2013;202:112-119.
- [CrossRef] [Google Scholar]
- Wettability alteration of oil-wet limestone using surfactant-nanoparticle formulation. J. Colloid Interf. Sci.. 2017;334
- [Google Scholar]
- Mathematical modelling of surface tension of nanoparticles in electrolyte solutions - ScienceDirect. Chem. Eng. Sci.. 2019;197:345-356.
- [Google Scholar]
- Experimental study on the viscosity behavior of silica nanofluids with different ions of electrolytes. Ind. Eng. Chem. Res.. 2020;59:3575-3583.
- [Google Scholar]
- Synergistic interaction of nanoparticles with low salinity water and surfactant during alternating injection into sandstone reservoirs to improve oil recovery and reduce formation damage. J. Mol. Liquids. 2020;317
- [Google Scholar]
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery. Nanotechnol. Rev.. 2023;12
- [CrossRef] [Google Scholar]
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery. Nanotechnol. Rev.. 2023;12:28.
- [CrossRef] [Google Scholar]
- Effects of Al2O3-NiO, TiO2 and (Mg, Ni)O particles on the viscosity of heavy oil during aquathermolysis. Colloids Surf. A-Physicochem. Eng. Aspects. 2021;625
- [CrossRef] [Google Scholar]
- A review of nanomaterials for nanofluid enhanced oil recovery. RSC Adv.. 2017;7:32246-32254.
- [CrossRef] [Google Scholar]
- Low salinity surfactant alternating gas/CO2 flooding for enhanced oil recovery in sandstone reservoirs. J. Pet. Sci. Eng.. 2022;212:16.
- [CrossRef] [Google Scholar]
- Experimental study on the effect of different surfactants on the thermophysical properties of graphene filled nanofluids. Int. J. Energy Res.. 2021;45:10043-10063.
- [CrossRef] [Google Scholar]
- Design of smart water composition based on scale minimization and its effect on wettability alteration in the presence of nanoparticles and mineral scales. J Journal of Petroleum Science and Engineering. 2021;196:107832
- [CrossRef] [Google Scholar]
- Effect of pH on silica nanoparticle-stabilized foam for enhanced oil recovery using carboxylate-based extended surfactants. J. Pet. Sci. Eng.. 2021;196:12.
- [CrossRef] [Google Scholar]
- Nanofluid formulation based on the combined effect of silica nanoparticles and low salinity water for Enhanced Oil Recovery in sandstones. Geoenergy Sci. Eng.. 2023;223:11.
- [CrossRef] [Google Scholar]
- Impact of nanotechnology on enhanced oil recovery: a mini-review. Ind. Eng. Chem. Res.. 2019;58
- [Google Scholar]
- Rezvani, H. et al., 2018. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4 @ chitosan nanocomposites. 15–27 (2018).
- Adsorption of silica nanoparticles and its synergistic effect on fluid/rock interactions during low salinity flooding in sandstones. Colloids Surf. A: Physicochem. Eng. Aspects. 2018;555:397-406.
- [Google Scholar]
- The effect of nanoparticles on wettability alteration for enhanced oil recovery: micromodel experimental studies and CFD simulation. Pet. Sci. 2019
- [Google Scholar]
- Enhanced oil recovery using silica nanoparticles in the presence of salts for wettability alteration. J. Dispers. Sci. Technol. 2019
- [Google Scholar]
- On the interpretation of contact angle for geomaterial wettability: Contact area versus three-phase contact line. J Journal of Petroleum Science and Engineering.. 2020;195:107579
- [CrossRef] [Google Scholar]
- Hydroxyl-functionalized silicate-based nanofluids for enhanced oil recovery. Fuel. 2020;269:117462
- [CrossRef] [Google Scholar]
- Silica nanoparticle assisted polymer flooding of heavy crude oil: emulsification, rheology, and wettability alteration characteristics. Ind. Eng. Chem. Res. 2018 acs.iecr.8b00540
- [Google Scholar]
- Immiscible viscous fingering: modelling unstable water-oil displacement experiments in porous media. Transp. Porous Media. 2022;145:291-322.
- [CrossRef] [Google Scholar]
- Phenomenological study of the micro- and macroscopic mechanisms during polymer flooding with SiO2 nanoparticles. J. Pet. Sci. Eng.. 2021;198
- [CrossRef] [Google Scholar]
- Experimental investigation of the adsorption process of the surfactant-nanoparticle combination onto the carbonate reservoir rock surface in the enhanced oil recovery (EOR) process. Chem. Thermodyn. Therm. Anal.. 2022;6:100036
- [CrossRef] [Google Scholar]
- Wettability alteration of limestone carbonate cores using methylene blue and alumina-based nanofluid: implications for EOR. Appl. Sci.-Basel. 2023;13
- [CrossRef] [Google Scholar]
- Silica nanofluids in polyacrylamide with and without surfactant: viscosity, surface tension, and interfacial tension with liquid paraffin. J. Pet. Sci. Eng.. 2017;152:575-585.
- [CrossRef] [Google Scholar]
- Silica nanofluids in an oilfield polymer polyacrylamide: interfacial properties wettability alteration, and applications for chemical enhanced oil recovery. Ind. Eng. Chem. Res. 2016 acs.iecr.6b03299
- [Google Scholar]
- Nanoparticle transport within non-Newtonian fluid flow in porous media. Phys. Rev. E. 2022;106
- [CrossRef] [Google Scholar]
- Effect of a nanoparticle on wettability alteration and wettability retainment of carbonate reservoirs. J. Pet. Sci. Eng.. 2022;215
- [CrossRef] [Google Scholar]
- Biosurfactant derived from fenugreek seeds and its impact on wettability alteration, oil recovery, and effluent treatment of a rock system of mixed composition. Energy Fuels. 2023;37:6683-6696.
- [CrossRef] [Google Scholar]
- Synthesis of ZnO nanoparticles for oil–water interfacial tension reduction in enhanced oil recovery. Appl. Phys. A. 2018;124:128.
- [Google Scholar]
- The effect of concentration of silica nanoparticles surface-modified by zwitterionic surfactants for enhanced oil recovery (EOR) Kor. J. Chem. Eng.. 2022;39:3286-3294.
- [CrossRef] [Google Scholar]
- Engulfing behavior of nanoparticles into thermoresponsive microgels: a mesoscopic simulation study. J. Phys. Chem. B. 2021;125:2994-3004.
- [CrossRef] [Google Scholar]
- Stabilizing silica nanoparticles in high saline water by using ionic surfactants for wettability alteration application. Colloid Polym. Sci. 2016
- [Google Scholar]
- Wettability alteration and oil recovery by surfactant assisted low salinity water in carbonate rock: the impact of nonionic/anionic surfactants. J. Pet. Sci. Eng.. 2021;197:108108
- [CrossRef] [Google Scholar]
- Synergism between lipophilic surfactant and lipophilic SiO2 nanoparticles enhances self-emulsification performance. J. Pet. Sci. Eng.. 2022;216
- [CrossRef] [Google Scholar]
- Experimental study on the mechanism of adsorption-improved imbibition in oil-wet tight sandstone by a nonionic surfactant for enhanced oil recovery. Pet. Sci.. 2021;18:1115-1126.
- [CrossRef] [Google Scholar]
- A review on the mechanisms of low salinity water/surfactant/nanoparticles and the potential synergistic application for c-EOR. Pet. Res.. 2023;8:324-337.
- [CrossRef] [Google Scholar]
- Performance of low-salinity water flooding for enhanced oil recovery improved by SiO2 nanoparticles. Pet. Sci.. 2019;16:9.
- [Google Scholar]
- A critical review of enhanced oil recovery by imbibition: theory and practice. Energy Fuels 2021
- [Google Scholar]
- Dispersion and rheology of nickel nanoparticle inks. J. Mater. Sci.. 2006;41:1213-1219.
- [CrossRef] [Google Scholar]
- New technique for enhancing oil recovery from low-permeability reservoirs: the synergy of silica nanoparticles and biosurfactant. Energy Fuels. 2021;35:318-328.
- [CrossRef] [Google Scholar]
- Experimental study on preparation of nanoparticle-surfactant nanofluids and their effects on coal surface wettability. Int. J. Min. Sci. Technol.. 2022;32:387-397.
- [CrossRef] [Google Scholar]
- Experimental Study on the Enhanced Oil Recovery Mechanism of an Ordinary Heavy Oil Field by Polymer Flooding. ACS Omega. 2023;8:14089-14096.
- [CrossRef] [Google Scholar]
- Chemically reactive transport of magnetized hybrid nanofluids through Darcian porous medium. Case Stud. Therm. Eng.. 2021;28
- [CrossRef] [Google Scholar]
- Alkyl polyglucosides for potential application in oil recovery process: adsorption behavior in sandstones under high temperature and salinity. J. Pet. Sci. Eng.. 2020;189:107057
- [CrossRef] [Google Scholar]
- Silica-based amphiphilic Janus nanofluid with improved interfacial properties for enhanced oil recovery. Colloids Surf. A-Physicochem. Eng. Aspects. 2019;586:124162
- [Google Scholar]
- Wetting and interfacial tension of molten CaO-Al2O3-MgO-FeO slag and BN substrate. J. Mater. Res. Technol.-JMRT. 2022;16:764-772.
- [CrossRef] [Google Scholar]
- Assessing the performance of foams stabilized by anionic/nonionic surfactant mixture under high temperature and pressure conditions. Colloid Surf. A-Physicochem. Eng. Asp.. 2022;651:12.
- [CrossRef] [Google Scholar]
- Flow characteristics and EOR mechanism of foam flooding in fractured vuggy reservoirs. J. Pet. Sci. Eng.. 2022;211:11.
- [CrossRef] [Google Scholar]
- Enhancing heavy-oil displacement efficiency through viscoelasticity of polymer solution by investigating the viscosity limit of crude oil: an experimental study. Energy Sci. Eng.. 2024;12:2493-2504.
- [CrossRef] [Google Scholar]
- A numerical investigation of mathematical modelling in 3D hexagonal porous prism on oil recovery using nanoflooding. Heliyon. 2023;9:e18676
- [CrossRef] [Google Scholar]
- Effect of polymer-graphene-quantum-dot solution on enhanced oil recovery performance. J. Mol. Liq.. 2022;349:11.
- [CrossRef] [Google Scholar]
- Comparison of nanomaterials for enhanced oil recovery in tight sandstone reservoir. Front. Earth Sci.. 2021;9
- [CrossRef] [Google Scholar]
- Experimental and numerical simulation study on the influence of fracture distribution on gas channeling in ultralow-permeability reservoirs. J. Energy Eng.-ASCE. 2022;148:11.
- [CrossRef] [Google Scholar]
- Synthesis and application of hydrophilically-modified Fe3O4 nanoparticles in oil sands separation. RSC Adv.. 2018;8
- [Google Scholar]
- Enhanced oil recovery driven by nanofilm structural disjoining pressure: flooding experiments and microvisualization. J. Fuels. 2016;30:2771-2779.
- [Google Scholar]
- In situ micro-emulsification during surfactant enhanced oil recovery: a microfluidic study. J. Colloid Interface Sci.. 2022;620:465-477.
- [CrossRef] [Google Scholar]
- Investigation of nanoclay-surfactant-stabilized foam for improving oil recovery of steam flooding in offshore heavy oil reservoirs. ACS Omega. 2021;6:22709-22716.
- [CrossRef] [Google Scholar]
- New insight to experimental study on pore structure of different type reservoirs during alkaline-surfactant-polymer flooding. Energy Sci. Eng.. 2022;10:2527-2539.
- [CrossRef] [Google Scholar]
- Nanoparticles for enhancing heavy oil recovery: recent progress, challenges, and future perspectives. J. Energy Fuels. 2023;37:8057-8078.
- [CrossRef] [Google Scholar]
- Zhu et al., 2017. Systematic investigation of the synergistic effects of novel biosurfactant ethoxylated phytosterol-alcohol systems on the interfacial tension of a water/model oil system.







