Translate this page into:
Nanostructured materials based on g-C3N4 for enhanced photocatalytic activity and potentials application: A review
⁎Corresponding author. asifncp11@yahoo.com (Asif Hayat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Semiconductor-based photocatalytic technology is regarded as an efficient pathway for resolving the energy scarcity across the globe. In this regard, graphitic carbon nitride (g-C3N4)-based materials could be alternatively employed in photochemical applications such as photovoltaic energy generation via CO2 photoreduction and water splitting, along with natural resource purification via organic/inorganic pollutant degradation. Indeed, this kind of assertion has been made by considering the intrinsic physicochemical properties of g-C3N4 nanomaterials, owing to their increased surface area, quantum yield, surface charge isolation, distribution, and ease of modification through material configuration or incorporation of preferred interfacial capabilities. This review article has been designed to provide the most up-to-date information regarding the further assessment of the important advancements in fabrication along with photochemical applications of various g-C3N4 nanomaterials, while specifically focusing on the scientific reason behind its success in each assessment. The discovery of interventions to alleviate such restrictions and boost photocatalytic performance has gained substantial interest. Following photo-excitation fundamentals, this work explains two distinct photoexcitation mechanisms, the carrier and charge transfer techniques, wherein the significant exciting state impact of g-C3N4 has still not been widely focused on in past studies. In this regards, we cautiously introduce the updated advances and associated functions of the alteration techniques, including morphological features, elemental dopants, deficiency engineering, and heterojunction implemented in photocatalytic performance, which are equated from the carrier and charge transport perceptions. The future perspectives in designing and properly tuning the highly active hierarchical or copolymer g-C3N4 nanoparticles in a photocatalytic system, which may improve the renewable energy cultivation and reduction efficiency are critically deciphered in detail and outlined thoroughly.
Keywords
g-C3N4
Photocatalytic water splitting
Photocatalytic CO2 reduction
Various dimensions
Physiochemical properties
1 Introduction
1.1 Background
The utilization of clean and renewable energy is critical for fulfilling the growing global energy demands and resolving the fiscal energy problems caused by excessive consumption of fossil fuels (Child, 2018; Wang, 2018; Wei, 2018; Sohail, 2017; Sohail, 2018; Qadeer, 2020; Wageh, 2021; Wageh, 2021). With the development of science and technology, solar energy is an inexhaustible source of renewable energy on the earth, and has the extensive potential to overcome all of these limitations precincts (Faunce, et al., 2013; Mills et al., 1993; Qadeer, 2022; Hao, 2018; Wageh and Zhao, 2019; Swelm, 2018; Wageh, 2018; Wageh et al., 2016; Wageh, 2016) and its further use for the attended scientific consensus, since Honda and Fujishima used semiconductor based materials for water splitting in 1972 (Fujishima and Honda, 1972; Inoue, 1979). One such discovery, sparked an extensive research interest into the titanium dioxide (TiO2) based photocatalysts, which are being widely used for the efficient photocatalytic CO2 reduction, water splitting, pollutants degradation, sensors, photoelectronics, photobacterial agents, etc. under solar light irridiation (Yi, 2014; Gaya and Abdullah, 2008; Naciri, 2022; Naciri, 2020; Naciri, 2021; Huang, 2021; Zhang, 2016; Wageh, 2015; Wageh, 2014). Nonetheless, the efficient photocatalytic utilization of visible light (key constituent of photovoltaic irradiance as it produces the Earth texture) in order to accelerate the oxidation process, which is still a challenging task for scientists (Asahi, 2001; Kumar and Devi, 2011; Naciri, 2020; Qadeer, 2019), due to huge difficulty of identifying the compounds with narrow band gaps, an enhanced electronic structure, sufficient electron transfer mobility, and low level of replication, along with excellent consistency. Various materials have been used in multiple research fields (Ajmal, 2020; Ahmed, 2021; Hsini, 2021; Hsini, 2020; Hou, 2014; Hou, 2013; Wageh et al., 2013; Wageh et al., 2013; Wageh, 2013). Indeed, numerous outstanding studies on fabrication and modification of graphitic carbon nitride (g-C3N4)-based photocatalysts, as well as their uses in energy and ecological challenges, are already accessible. Only a few papers, however, have discussed on the varied features of g-C3N4-based photocatalyst rational design and its impact on the activity of the catalyst. As a result, it is relevant to provide a complete review of the current advancements in g-C3N4-based catalysts for photocatalysis and environmental remediation. We focus on the basics, diverse characteristics, design, and possible uses of g-C3N4 based photocatalysts in this review. We assume that this review will encourage the synthesis of state-of-the-art g-C3N4 based photocatalysts and designs that improve light utilization and photocatalytic performance, but will also assist with confronting the difficulties that g-C3N4-based photocatalysts face in manufacturing and storage of renewable and sustainable energy. The development of such unique photocatalysts will pave the way for their extensive use to complement the sustainable energy and the contaminant monitoring methods. The main postulates of our review are manifested in the fellowing graphical abstract as shown in Fig. 1.
Scheme exhibiting the applicability of g-C3N4-based nanomaterials in photocatalysis research and the accompanying methodologies for photocatalytic efficiency improvement.
1.2 Semiconductor photocatalysis
Heterogeneous photocatalysis accelerates the chemical reactions (oxidations and reductions) caused by the stimulation of a photocatalyst, that combines with metals, non-metals, and metal oxides to facilitate the optical absorptions with consecutive energy-conversion cultivator sources. Notably, the photocatalysis process is concerned with the considerable stimulation of reagents and derivatives by optical absorption and assessing the photocatalytic conversion. Additionally, the reaction process shows, that the photocatalyst assimilates the electrons rather than the sorbent (Rochkind, 2014; Pallavi, 2010; Ullah, 2016; Sohail, 2020; Wageh, 2007). The photoinduced charged particles basically stimulate the photoconversion of water and CO2 into associated renewable compounds in a photocatalytic production known as “fossil energy” (Styring, 2012; Chen et al., 2015). Such chemical reactions are comparable to natural photosynthesis (in which chloroplasts assimilate natural light in plants to encourage carbohydrate and oxygen fabrication from carbon dioxide and water) and are thus referred as artificial photosynthesis (Fig. 2). Moreover, the photoinduced electrons could also promote the total combustion (calcification) of environmental pollutants in marine ecosystems, either via a direct or indirect pathway, thereby, resulting into the formation of potential oxidizing agents such as a hydroxyl source (Nosaka and Nosaka, 2017; Wang and Domen, 2019).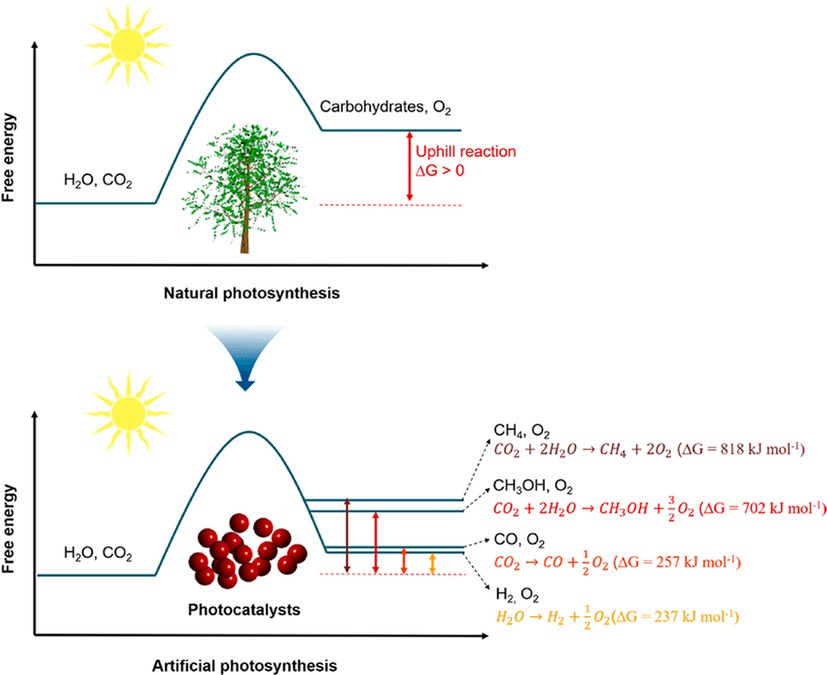
(a) Natural and (b) artificial photosynthesis processes. Reprinted with permission from Wang and Domen (2019).
Copyright © 2020, American Chemical Society.
1.3 Methodologies of photocatalysis
The photocatalysis process is initiated with the absorption of light from the ground state (valance band) toward the excited state (conduction band) (Step I) (Wageh, 2016). The subsequent clusters of electrons and holes could be thoroughly combined within or at the photocatalyst surface, with associated fuel released by luminescence or radiative activation of the crystalline structure (Step II). The charge recombination is critical, as it limits the photocatalytic performance for photoelectron absorption. Similarly, the photoinduced electrons/holes are deflected away from the material interface without charge recombination. Various photooxidation/reduction reactions with adsorbed species, including water, oxygen, and other organic or inorganic species (Steps III and IV) (Karamian and Sharifnia, 2016; White, 2015; Aleksandra, 2014), are presented in Fig. 3. These processes, which are essential for photocatalytic environmental remediation and solar fuel production, are ultimately limited by the reduction potential of photoexcited electrons in the conduction band (CB) and the oxidation potential of photogenerated holes in the valence band (VB). Therefore, the redox potential, band energies, and band gap of semiconductors (SC) play a significant role in determining the possibility and rate of charge transfer, which are considered to be the critical design parameters for selecting suitable photocatalysts (Sakata and Kawai, 1983; Zou, 2001; Qadeer, 2022). The semiconductor photocatalysis, whose fundamental configuration and surface-electronic structure differ between materials and applications, is an interfacial interaction between electrons and holes caused by band gap stimulation.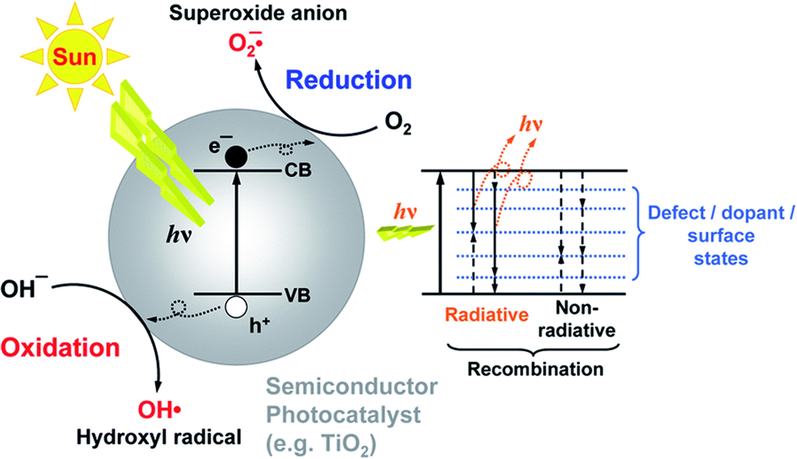
Principal photophysical processes for semiconductors (SC) under light irradiation. Reprinted with permission from Aleksandra (2014).
Copyright © 2014, Royal Society of Chemistry.
1.4 Photocatalytic materials
The use of titanium-based electrodes under UV irradiation for the overall water splitting (OWS) process has led to an extensive investigation for the evolution of H2 energy (Park et al., 2006). TiO2 powder was proven to be an effective catalyst for the photocatalytic decomposition of cyanide ions in water (Frank and Bard, 1977), and in the field of environmental purification. However, due to the wide band gap, recent research has focused on finding alternative semiconductors, which should provide better photocatalytic performance under solar (rather than UV) irradiation (Kubacka et al., 2016). According to the band gap of a semiconductor, a photocatalyst can be used in a variety of ways, including ZnO (Lee, 2016), Fe2O4 (Li, 2007), WO3 (Wang, 2012), SrTiO3 (Kato and Kudo, 2001), NaTaO3 (Yu et al., 2004), CdS (Jing and Guo, 2006), Ag3PO4 (Wang, 2012), BiPO4 (Pan and Zhu, 2010), and g-C3N4 (Venkatathri and Kumar, 2017) (Fig. 4). Despite substantial literature on the subject, it is still difficult to use of photocatalysts in order to generate solar fuel or the decomposition of organic pollutants owing to poor light-harvesting or ineffective light energy conversion.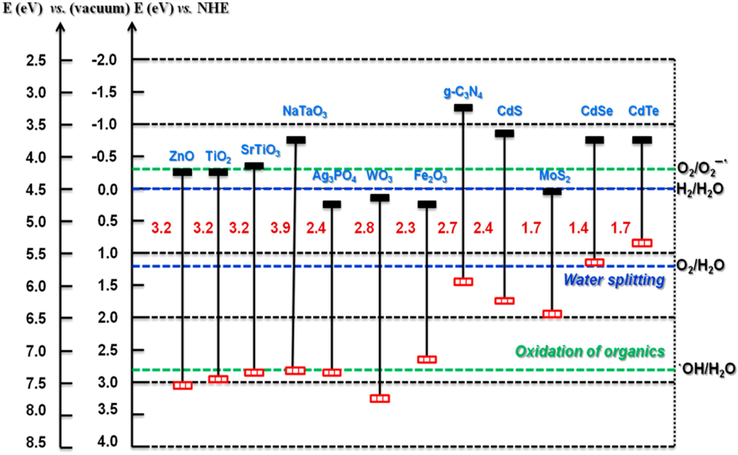
Band gap energy and band edge energies of different semiconductors. Reprinted with permission from Venkatathri and Kumar (2017).
1.5 Graphitic carbon nitride (g-C3N4)
The use of visible light photocatalysis provides the best possible way to capture the maximal solar energy owing to the dominance of three sections of the electromagnetic spectrum, UV (5 %), visible light (45 %), and IR (50 %), as manifested in (Fig. 5) respectively, (Wu, 2011). Several photocatalysts, such as TiO2 (3.0–3.2 eV), have broad band gaps and are, therefore, only active under UV irradiation (385 nm) (Yi, 2014).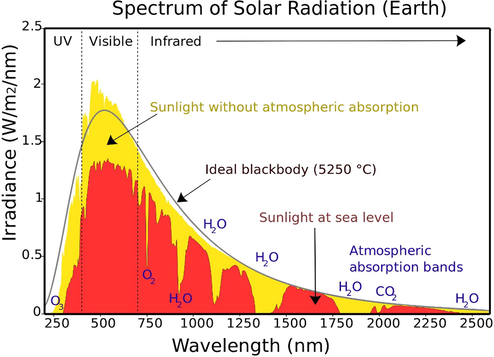
Spectral irradiance of solar light. Reprinted with permission from Wu (2011). .
Copyright © 2011, Elsevier
There has been a rapid advancement in the number of scientific papers and patents (Kumar and Devi, 2011) dealing with photocatalytic OWS process, CO2 reduction, and pollutants degradations (Hernández-Alonso, 2009; Sohail, 2021; Hayat, 2022). Fig. 6a shows that g-C3N4 is a promising metal-free polymeric semiconductor with a low band gap (Fig. 6b) (Wang et al., 2009), which is ideal for visible light absorption and is versatile for large-scale application. Unlike many other organic semiconductors, the g-C3N4 has a high thermal and chemical resistance to oxidation, even at temperatures of 500 °C, which makes it an ideal material for photovoltaic applications. The synthesis of g-C3N4 and its derivatives for diverse applications has been extensively documented (Vhna, 2021; Santosh, 2013; Qadeer, 2022). The photocatalytic applications in environmental remediation and solar fuel generation, which emphasize emerging synthetic strategies in order to improve the photoactivity of g-C3N4 nanostructures by controlling their size, morphology, light absorption, charge separation, and ultimately their surface reactions are covered in this review. In addition, future research directions are also highlighted in this review.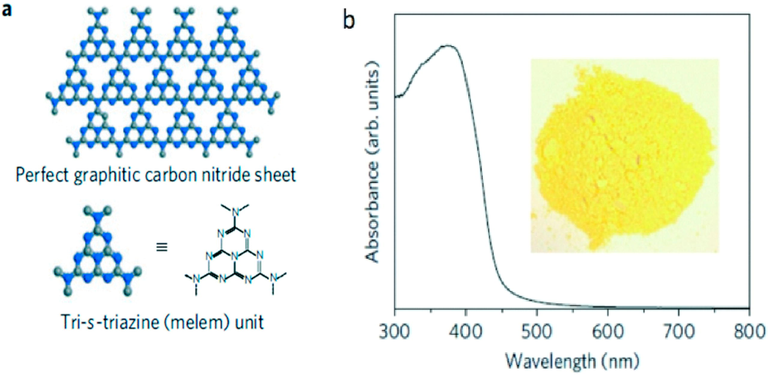
(a) g-C3N4 structure comprising melem units, and (b) UV–vis diffuse reflectance (DRS) spectrum and image (inset) of g-C3N4 (Wang et al., 2009).
1.5.1 g-C3N4 nanostructures: size and shape
There has been an increased interest in the application of nanoparticles, including electronics (Lewis and Nocera, 2006), catalysis (Rhind, 2009), biomedical (Faunce, 2013), sensing (Mills et al., 1993), and smart materials (Fujishima and Honda, 1972) throughout the science and engineering fields. The nanomaterials are distinct from their bulk precursors in some essential ways. It is due to their high surface-to-bulk atom ratio, as nanomaterials have a lower melting point and a higher solid–solid phase transition temperature. The electrical and optical properties of nanomaterials are greatly influenced by quantum confinement effects, which originate from the evolution of their band structure and the formation of atomistic-like behavior and several heterogeneous catalysts with strong size-dependencies due to quantum confinement (Inoue, 1979). For example, Gold (Fujishima et al., 2008; Ma, 2014) has a large surface area characterized by low coordination, and high energy sites exposed. Charge separation transfer from the photocatalyst surface to an adsorbed species may be accelerated, if these variables act synergistically (Gaya and Abdullah, 2008; Schneider, 2014; Habisreutinger et al., 2013; Qadeer, 2021). Mo et. al. investigated the effect of calcination factor onto the melamine-derived g-C3N4 crystalline lattice, morphological progression, and energy band construction (Mo et al., 2015). The XRD diffraction patterns revealed that g-C3N4 could be completely justified as the temperature was increased >500 °C (Fig. 7a). Significantly, the peaks observed of the material synthesized at 450 °C differed from another sample, indicating that the melem analogues were present. At 600 and 650 °C, the framework of g-C3N4 grew extremely loose, shorter, and plump, with the formation of numerous holes on the g-C3N4 interface (Fig. 7b, d). Conversely, Fig. 7e shows a significant red-shift in the sharp absorption edges stretching from 470 to 570 nm, as well as a color transition from yellow to deep oranges (Fig. 7e), thereby, signifying the higher light absorption for materials with rising temperatures (Mo et al., 2015). The red shift was caused by an increase in the π-plane conjugated and increased in the degree of crystallinity. According to the transitory current density responses (Fig. 7f), the g-C3N4 produced at 650 °C had the maximum current density with excellent repeatability and consistency, thereby, making it more suitable for photocatalytic performance.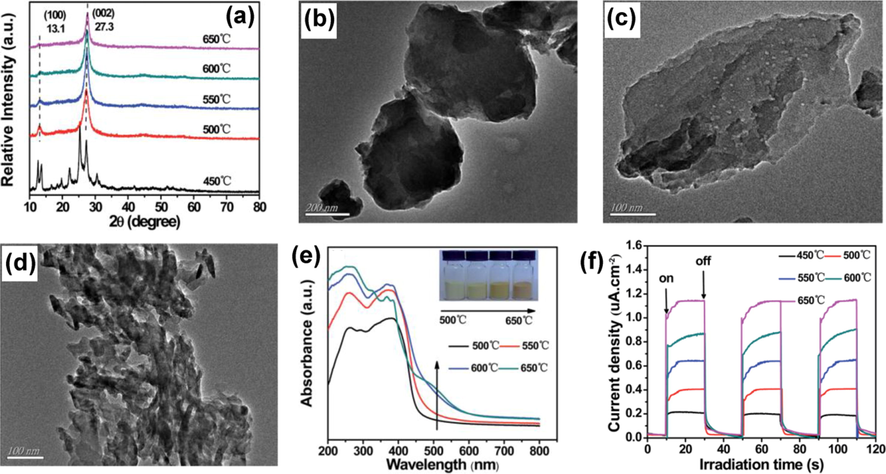
(a) XRD patterns, TEM at (b) 550 °C, (c) 600 °C, and (d) 650 °C. (e) UV–vis DRS and (f) transitory current densities responses of g-C3N4 produced at various annealing temp. The color of the g-C3N4 photocatalysts produced at distinct temps is shown in the inset of (e). Reprinted with permission from Mo et al. (2015).
Copyright © 2015, RSC Publishing.
Despite, several efforts have been made in order to exfoliate the bulk g-C3N4 into sheets morphology, but yet there is still a challenging task for achieving a good yield, precise morphology, and ultrathin nanosheets, thereby, necessitating further research in this field (Ong et al., 2016). The g-C3N4 has transformed into nanosheets (three to two dimensional), which results in the transformation of various morphologies like nanorods/nanotubes (one dimensional) and quantum dots (zero-dimensional) aimed at industrial applications and energy harvesting (Ong et al., 2016). In this context, the g-C3N4 was obtained using a complicated, soft, and self-templating technique, and quantum dots were generated using a hydrothermal etching technique. From a salt-template assisted process, the honeycomb-structured g-C3N4 remained effectively organized (Ong et al., 2016; Naseri, 2017). Zhao et. al. proposed the current trends in merging 0D with 2D g-C3N4 to form 0D/2D composites with enhanced photocatalytic performance (Zhao, 2018). Wang et. al. (Wang, 2017) compared 2D/2D interface of g-C3N4 with other 2D nanomaterials. Unquestionably, heterojunctions such as type II, Schottky, and Z-schemes improve photo-driven reactions by reducing the charge diffusion paths, and decreasing photogenerated charge recombination rates, along with increasing the surface area of the photocatalyst (Wang, 2018).
1.6 Elemental doping
In additional to the structural regulation outlined previously, elemental doping (both non-metal and metals) is another efficient approach of alteration (Khan, 2020; Raziq, 2020; Ullah, 2020; Shaishta, 2020; Rehman, et al., 2019; Khan, 2020; Hayat, 2019; Hayat, 2020; Hayat, 2019; Majeed, 2021; Rahman and Hayat, 2019; Hayat and Li, 2019; Hayat, 2019; Khan, 2019; Hayat, 2019; Hayat, 2019; Khan, 2018). By inserting heteroatoms, the Eg values of the compound materials may be modified to enhance the photo response range (Fig. 8a), and the resistance between the layers could be reduced to speed up the separation of charge, thereby enhancing the photocatalytic performance of g-C3N4 (Hasija, 2019; Liu et al., 2021; Hayat, 2022; Kongto, 2022; Alenad, 2022; Hayat, 2021; Hayat, 2021; Rehman, 2021). Fig. 8b depicts the proportions of distinct elemental doping (Hasija, 2019; Li, 2022). Notably, metal and nonmetal doping have markedly differing impacts on the corresponding photoexcitation processes.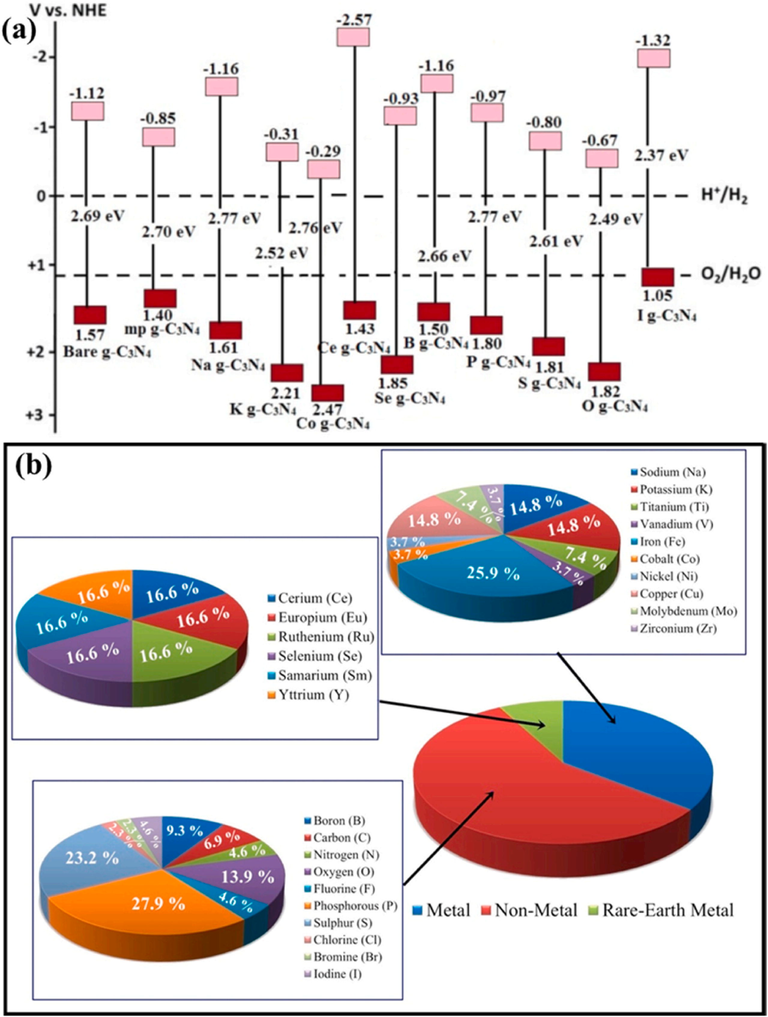
(a) Band configurations for pristine and modified g-C3N4 and (b) a description of the ratios of several modified g-C3N4 photocatalysts. Reprinted with permission from Hasija (2019)). Copyright © 2019, Elsevier.
1.6.1 Metal doping
Metal doping consists of precious metals/non-metals (such as alkali elements and transition elements) co-doping and single doping (Hayat, 2022; Pan, 2022; Taha et al., 2021; Sohail, 2021; Hayat, 2022). In particular, metals could be incorporated within g-C3N4 framework by calcining or hydrothermally method of their main precursors. The doping positions of the dopants are determined by the size of the heteroatoms and their length from or within the substrate plane. For metal doping, orbital hybridization could happen among the g-C3N4 doped orbital and molecular orbital, producing additional energy levels to reduce the Eg value and increase the optical absorption. Furthermore, additional defective phases could be incorporated into electronic frameworks, resulting in greater active sites and a stronger e–/h+ separation and transport. Wang and coworker synthesized K-doped ultrathin porous g-C3N4 by hydrothermal crystallization for degradation of TC (Wang et al., 2018). X-ray photoelectron spectroscopy (XPS) measurements revealed that K has been effectively added into the g-C3N4 structure by creating K-N bonds (Fig. 9a). Furthermore, K-doped g-C3N4 demonstrated improved separation and transfer rates, that resulted in increased the photocatalytic activity. Moreover, porous design can supply additional active sites, that can reduce the charge recombination even more. As a result, K-doped g-C3N4 had the greatest degradation performance, which was roughly 2.88 times greater than pure g-C3N4. Yan et. al. studied the impact of Na, K, Ca and Mg doping on the morphology and photocatalytic activity of g-C3N4 (Yan et al., 2018). The metal doping discussed earlier caused by the modifications in the electronic structure of g-C3N4 (Fig. 9b), that were ascribed due to electrons supplied by metal to the nitrogen atom or those escaping from the surface of g-C3N4. Additionally, Na, Ca, Mg and K were introduced into g-C3N4 framework by creating cationic covalent bonds or by producing oxides with g-C3N4. This finally increased the degradation rate of TC. Metal doping impacts not just carrier mechanisms as well as the exciton behaviors of g-C3N4. For instance, Shu and coworkers inserted Na+ as impurities into mesoporous g-C3N4 nanosheets (referred as Na-MCN) by calcining a solution of sodium chloride and dicyandiamide and examined the carriers and excitons within the resulting material. The lower fluorescence intensity of the above material suggested a lower charge recombination rate. In addition, the strong photocurrent response and low impedance circle size of Na-MCN validated the enhanced exciton dissolution and carrier transfer performance. The aforementioned findings were ascribed to the production of cyano groups in the fundamental g-C3N4 structure by sodium ion doping, the insertion of sodium sites, and morphological alteration inside and among the unit layers.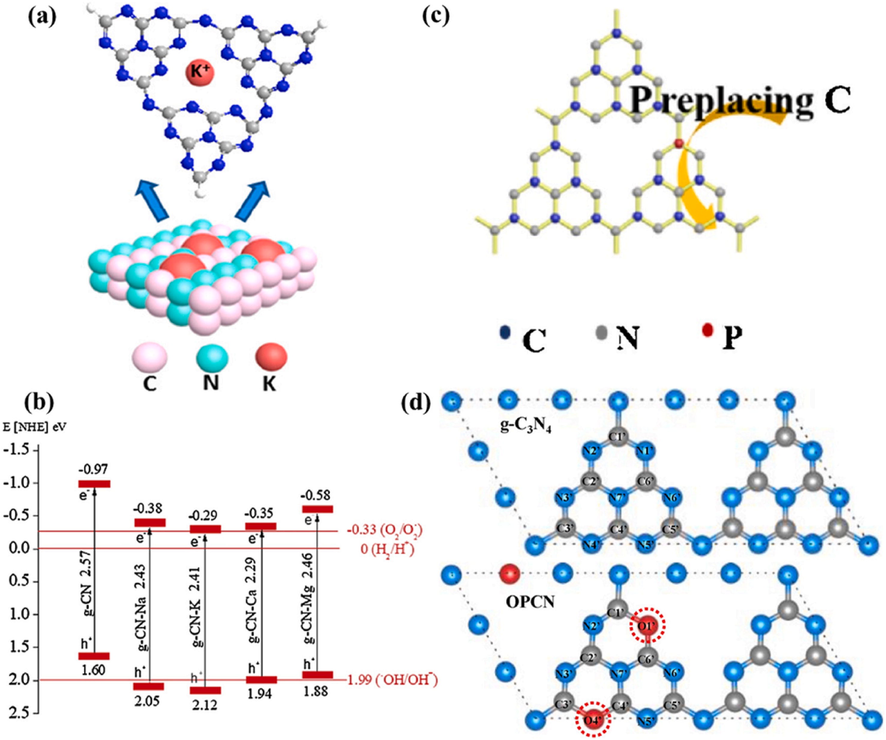
(a) Illustration of modified K ions in the g-C3N4 layer. Reprinted with permission from Wang et al. (2018). ; (b) Band configuration of modified g-C3N4. Reprinted with permission from Zhang (2021). Copyright © 2021, Elsevier; (c) Proposed P loaded sites within the g-C3N4 skeleton. Reprinted with permission from Mao (2018). Copyright © 2019, Elsevier; (d) framework of pristine g-C3N4 and O/g-C3N4 after activation. Reprinted with permission from Yuejie (2019). Copyright © 2019, Elsevier.
Copyright © 2018, American Chemical Society
1.6.2 Non-metal doping
Despite metal doping could significantly enhance the efficiency of g-C3N4 by regulating its band gap, constraints its photo-corrosion, elevated the charge recombination but expensive cost were observed. Non-metallic atoms could be integrated within the skeleton of g-C3N4 by using thermal polymerization or the hydrothermal technique for precursors mixture. Due to their higher electronegativity and ionization potential, non-metallic atoms can readily form covalent bonds by gaining electrons (Nor, 2019; Hayat, 2022; Hayat, 2022; Hayat et al., 2022; Hayat, 2022; Hayat, 2022; Hayat, 2021; Hayat, 2021; Hayat, 2021). In comparison to metallic doping, non-metal doping could avoid the above drawbacks and achieve the identical results as of metal doping, while maintaining the non-metallic properties of g-C3N4. Furthermore, contrary to metallic doping, non-metal doping is mostly accomplished through interatomic displacement (i.e., C, H and N) (Li, 2009; Hamid, 2020; Raziq, 2020; Khan, 2021). This interatomic replacement could have a considerable effect on carbon and nitrogen hybridization, influencing the electrical and optical performances of doped g-C3N4 (Jh et al., 2020; Hayat, 2022; Ghufran, 2021). Zhou and coworkers developed a phosphorus (P) doped g-C3N4 by thermal copolymerization for degradation of RhB dye (Zhou, 2015). P was effectively doped into g-C3N4 structure by replacing two distinct carbon sites to generate P–N or P––N bonds, resulting in more defects and greater active sites. The addition of P can facilitate the separation of charges even more, leading to the formation of a P+ center, that acts as a Lewis acid site. As a result, the Rhodamine B degradation efficiency of P-doped g-C3N4 enhanced 2.9-fold, when compared to pure g-C3N4. Despite this, Wu and coworkers discovered that P was exclusively doped into g-C3N4 skeleton via P-N bonds (Fig. 9c), and no P-N bond was identified (Mao, 2018). This meant that the exact doping location of P might alter based on the synthesis conditions. Recent studies have shown that inserting an oxygen (O) atom, which has merely an extra electron than nitrogen atom, enables an extremely effective substitution process. Zhang and coworkers created the double oxygen doped g-C3N4 (OPCN) materials through easy thermal copolymerization (Yuejie et al., 2019). They used DFT to determine the optimum oxygen replacement sites and discovered that the inserted oxygen atoms preferred to simultaneously substitute both the sp2-hybridized nitrogen atoms at the para-position of melem unit (Fig. 9d). In this situation, a delocalized arrangement developed on the OPCN surface, allowing carriers to migrate, while efficiently preventing electrons/holes recombination. Furthermore, under VL, the removal performance of the optimized OPCN for several organic contaminants was enhanced, and the photocatalytic performance for BP was roughly 9 times than pure g-C3N4 under solar light illumination.
1.7 Defect engineering
In contrast to the approaches described earlier, adding vacancies defects in g-C3N4 structure is an excellent approach for modifying the thermodynamic properties, kinetics, and mechanism of photocatalytic processes (Taha et al., 2021; You, 2017; Hayat, 2021; Khan, 2021; Ullah, 2021). Vacancy defects have following impacts on g-C3N4 photocatalytic efficiency. (1) capturing and storing electrons, leading to separation of charge; (2) Improvement of the ability to capture light and extension of the light response edge into the near-IR region through defect state; (3) Modification of the adsorption properties of the specific contaminant, hence enabling the targeted adsorption of various contaminants by g-C3N4; (4) The concentration, geometry, and position of vacancy defects all have an impact on the characteristics of g-C3N4. As a result, investigating vacancy flaws, particularly N and C vacancies, is crucial.
1.7.1 N-vacancy defects
N-vacancies may develop the electronic structural distortions, resulting in disrupted energy areas and aiding the exciton dissociation and transmission (Yw et al., 2020). Hydrogen bonds serve as strands in polymeric melon units having amino groups (NH/NH2) thus, g-C3N4 having N vacancy might be generated by removing the amino groups by means of high calcination. Zhou and colleagues discovered that difference of energy at the disordered junction induced by a N vacancy is at least 0.35 eV, that is enough to split singlet excitons having Eb of 0.2 eV into free holes and electrons (Zhou, 2020). These available electrons are used to produce activate oxygen, resulting in a significant amount of OH ions, which gives superior photocatalytic performance of the modified g-C3N4. Wang and coworkers created an N vacancy containing g-C3N4 with increased crystalline phase by using oxalyl dihydrazide (ODH) as an atmospheric modifier, which they named ODH-CNX can be seen in Fig. 10a (Yw et al., 2020). DFT simulations revealed that lowest occupied molecular orbital (LUMO) of ODH-CNX tilted toward the C-C bond, when contrasted to the electronic distribution of pure g-C3N4, resulting in non-coplanar HOMO and LUMO, that were advantageous for carrier production (Fig. 10b). Furthermore, the insertion of N vacancy resulted in a 0.13 eV reduction in the Eg value. Furthermore, the experimental findings revealed that crystalline structure and N vacancies improved charge transfer, exciton dissociation and VL capture. Furthermore, N vacancy promotes charge separation and transfer. Cao et. al. synthesized g-C3N4 having rich N vacancies (NV-g-C3N4) by processing melamine in a nitrogen environment and used it for carbamazepine degradation. (Cao, 2019; Li, 2020). The findings indicated that the deterioration rate of NV-g-C3N4 enhanced towards 270 % in comparison with bulk g-C3N4, that was due to capturing of electrons by N vacancies, hence limiting charge recombination. Liang et. al., examined the relation between degradation efficiency and N-vacancies (Liang, 2019). The developed catalysts greatly improved photocatalytic efficiency, because negative shift occurs in CB region of g-C3N4 due to N vacancies and improved both photo response and separation of charges.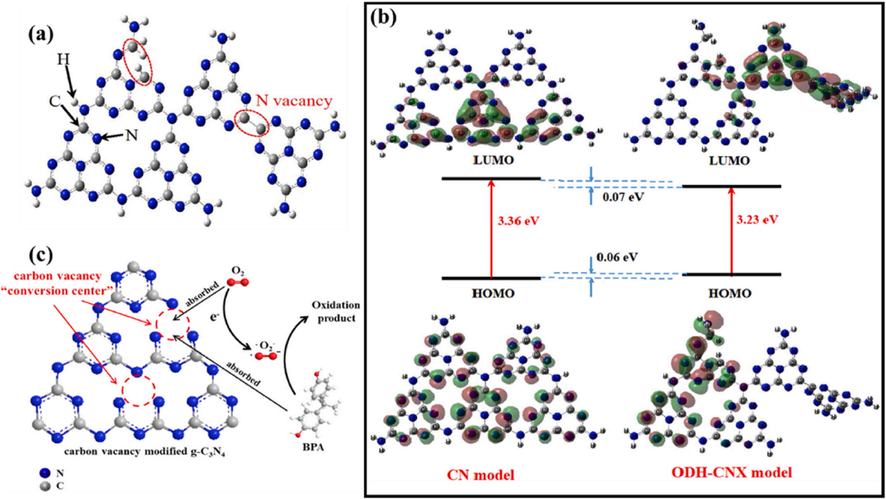
(a) Structures description of ODH-CNX with N defects, and (b) the best systemic electronics pattern of CN and ODH-CNX, and the predicted potential levels. Reprinted with permission from Yw et al. (2020) Copyright © 2020, Elsevier; (c) Pathway of g-C3N4 activation upon C vacancies alteration. Reprinted with permission from Liang et al. (2018).
Copyright © 2019, American Chemical Society.
1.7.2 C-vacancy defects
Multiple investigations have shown that introducing C-defects into g-C3N4 framework is a very attractive technique for optimizing carrier separation/transport (Dong, 2017). In general C vacancies could be produced by heating g-C3N4 in different environment such as ammonia (Liang, 2016; Ge, 2020), carbon dioxide (Ho, 2018), or argon atmosphere (Dong, 2016), etc. Liang and coworkers created C-vacancy modified g-C3N4 (VC–C3N4) using a simple two-step calcination procedure for increased photocatalytic performance (Liang, et al., 2018). C-vacancies can trap electrons, limit charges recombination, and transmit captured electrons to absorbed oxygen, boosting O2 production (Fig. 10c). Wang et. al. demonstrated that photocatalytic efficiency of C-vacancy integrated g-C3N4 was 1.65 times greater than pure g-C3N4 (Wang, 2021). The researchers created oxygen doped g-C3N4 having C-vacancy (VC-OCN) and utilized it to degrade atrazine (ATN) and p-nitrophenol (PNP). The C-vacancies concentration might be altered by adjusting the formaldehyde concentration. Likewise, the enhanced photocatalytic effectiveness of VC-OCN was correlated to the C vacancies, that suppressed the charge recombination and encouragement of adsorption of oxygen molecule. In the end, both C and N vacancies cause the Eg value of g-C3N4 to drop, because of its impurity state (Li, 2020). The CB of g-C3N4 is connected with the 2p orbital of carbon, and the C-vacancy causes the CB to shift positive (Li, 2016), so the intermediate gaps occur below the CB. In contrary, the VB of g-C3N4 is connected to the 2p orbital of nitrogen, and the N vacancy produces a negative shift in the VB, allowing the intermediate gap to form above the VB. Insufficient vacancies have little influence on the electronic configuration, while an excess of vacancies may act as carrier recombination centers, thus lowering its efficiency.
1.8 Heterojunction building
g-C3N4 has a multilayer structure that can be combined with other semiconductors substances. For carrier excited photocatalysis, heterostructures formed by intimate interaction between different substances have the following advantages: First, photoinduced electron hole pair could be spatially separated due to the of two distinct semiconductors heterojunction (Kongto, 2022; Zhang, 2021; Hayat, 2021; Rehman, 2021; Uddin, 2021; Hayat, 2021; Arif, 2021; Hayat, 2021). Second the spectral response could be enhanced or the oxidation/reduction ability could be increased by combining semiconductors of various band gaps (Wang, 2021). For exciton-generated photocatalysis, the construction of a heterostructure has been demonstrated to aid in the regulation of exciton energy state and binding energy (Zhou, 2020). Furthermore, the heterojunction internal electric field can cause the reverse flow of electrons and holes, resulting in exciton dissociation. Based on how charge carrier move, heterojunction can mostly be put into three groups: traditional/conventional heterojunction (types I-III), P-N heterojunction, and Z-scheme heterojunction, as seen in Fig. 11 (Zhang, 2021).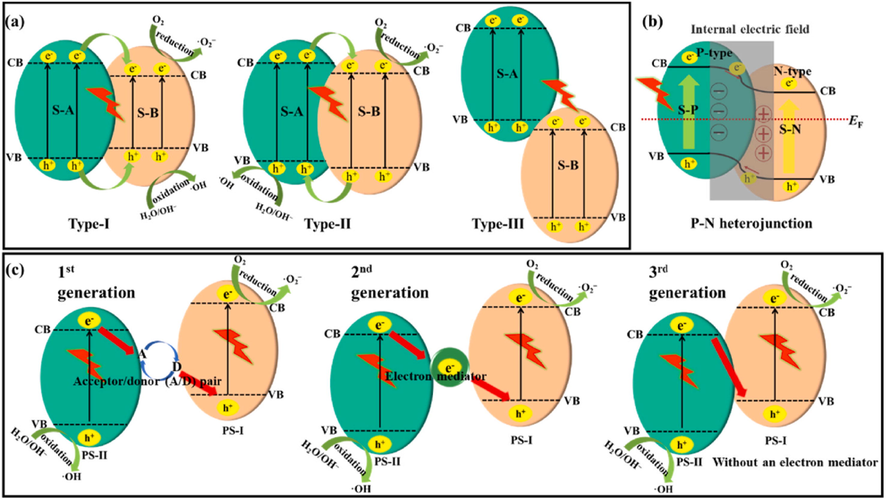
Electron transport diagram using (a) three kinds of traditional heterostructures, (b) P–N heterostructure, and (c) three phases of Z-scheme heterostructure. Reprinted with permission from Zhang (2021). Copyright © 2021, Elsevier.
1.8.1 Conventional/traditional heterojunctions
1.8.1.1 Type-I and Type-III heterojunctions
In accordance with band position of the two semiconductors that make up the heterojunction. There are usually 3 kinds of traditional heterojunctions (Fig. 11a) (Zhang, 2021). On photoexcitation, the electrons in VB of these 3 kinds of heterojunctions could be stimulated into the associated CB, generating positive holes in the VB. For type-I, semiconductor A (S-A) exceeds the semiconductor B (S-B), meaning that S-A has a much more substantial redox ability than S–B. Consequently, the electrons in the CB of S-A and holes from the VB of S–B move to the CB and VB of S-B. This form of charge transport channel could lower the Eg of the composite, extending the light response range, which is helpful for photocatalytic efficiency. Consequently, both electrons and holes are saturated on S–B, preventing effective separation of photoinduced carriers. Consequently, the redox ability of composites deteriorates dramatically. In type-III, the two semiconductors are incapable of forming a heterostructure, because their energy band locations do not overlap and the photoinduced electrons and holes cannot transfer to one another.
1.8.1.2 Type-II heterojunction
Type-II heterojunctions are the extensively kind of traditional heterojunctions (Fig. 11a) (Li, 2020). Two kinds of Type-II heterojunction formed by g-C3N4 and semiconductors. (1) Whenever a semiconductor has a high CB value (serving as S-A), g-C3N4 function as S–B, and electrons on S-A transfer to the CB of g-C3N4, while holes gather on the VB of S-B. Ehsan et. al. developed a g-C3N4/ZnSe hybrid photocatalyst for high photocatalytic performance. Analysis demonstrated that type-II heterojunction developed between ZnSe and g-C3N4. Due to its excellent surface charge transport pathway, the composite exhibited 1.81 and 1.57 times greater efficiency than g-C3N4, respectively (Ehsan, 2020). (2) When the CB of semiconductors is low (functioning as S–B), g-C3N4 functions as S–A. In this situation, the electrons in g-C3N4 can move to the CB of S–B, and this is also very rare type. Zhu and coworkers synthesized g-C3N4/BiPO4 heterostructure catalysts using varying concentrations of g-C3N4 for the degradation of TC using the ball milling technique. After 120 min of exposure to light, the optimum material had 97% degradation of the TC, and the greatly improved photocatalytic activity was closely linked to the efficient transport channel owing to the successful building of Type-II heterojunction. Hu and coworkers (Hu, 2019) obtained comparable findings when they developed carbon doped g-C3N4 (BCCN)/TiO2 composites (BCCNT) for improved photocatalytic performance. The improved photocatalytic efficiency was strongly linked to the creation of a type-II heterojunction between BCCN and TiO2. DFT simulations can be used to examine the viability of Type-II heterojunction fabrication. (Sun, 2021). Sun and coworkers used electrostatic self-assembly to create 0D SnO2 nanoparticle composite with 2D g-C3N4 for Rhodamine B degradation. (Sun, 2021). The direction of charge transport was identified using DFT simulations and 3D charge density. A positive mean of electron density change was discovered on SnO2 (1 1 0), whereas a negative mean of electron density variation was seen on g-C3N4 nanosheets (CNNSs), revealing that electrons concentrated on SnO2 (1 1 0). As a result, it was verified that a type-II heterojunction can occur among SnO2 (1 1 0) and CNNSs. The degrading performance of the material was 1.5 and 32.3 times greater than that of g-C3N4 and SnO2.
1.8.1.3 P–N heterojunction
Even Type-II can help in the separation of e−––h+ pairs, by preventing the charge separation due to electrostatic repulsion. In general, the CB and VB of N-type semiconductor (S-N) are greater than a P-type semiconductor (S-P). The fermi level (EF) of semiconductors is in the middle of CB, but it is in the band gap for semiconductors. Furthermore, the EF of S–N is near to CB, whereas the EF of S-P is near to VB. Work function (Wf) is inversely proportional to the fermi level (EF). Fig. 11b depicts the charge transfer mechanism. Once S-N and S-P together, trend is towards equilibrium, because the EF values are different. Consequently, electrons moved from P-type to N-type, until their EF values achieve equilibrium. The surface of N-type and P-type semiconductor are loaded with positive and negative charges, therefore generating the internal electric field from S-N to S-P direction. The photoinduced holes and electrons are propelled in the reverse way by the internal electric field, boosting charge separation ability under solar light illumination. The creation of P-N heterojunction could be verified by analyzing the compound semiconductor M S curve, which has both positive and negative slopes (Li, 2020). For example, Babu et. al. created P-N heterojunction in boron doped g-C3N4 composites with BiVO4 (BCN/BiVO4) by deposition of N-type BiVO4 on P-type 1 %wt boron doped g-C3N4 and used it to remove Cr(VI) metal (Babu, 2019). The highly efficient catalyst was 50% BiVO4/B-doped g-C3N4, for enhanced photocatalytic performance. Mass spectrometry studies found that the optimum material had an inverted U-shaped curve, suggesting the existence of negative and positive slopes, revealing the existence of P-N junction. The enhanced photocatalytic capabilities of the resultant material might be attributed to two reasons: (1) P-N junction facilitates the improvement of VL absorption; (2) P–N heterojunction can help electrons/holes pair separation while also decreasing their recombination likelihood.
1.8.1.4 Z-scheme heterojunction
The Z-scheme heterojunction system, that could be separated into three generations for addressing the problem of photocatalyst redox capacity caused by P-N and Type-II was created (Fig. 11c). Bard in 1979 introduced the concept of Z- scheme heterojunction (first generation), in which the transfer of charges was accomplished via acceptor–donor electron, that was mostly used in the aqueous phases (Bard, 1979). To expand the Z-scheme system uses, Tada and coworkers in 2006 studied Z-scheme system having all Solid state phase (second generation) in which the noble metal replaced by the electron mediator (Tada, 2006). But, due to the costly nature of valuable metals and its great light absorption capability, it is not suitable for practical uses. Vast studies on mediator free photocatalysis started in 2009 to develop more cost-effective photocatalysts (QingáLu, 2009), and Yu and colleagues introduced the direct Z-scheme system in 2013 (Third generation). Yu and his team made Ag3PO4/AgBr/g-C3N4 with dual Z-scheme system for improved the photocatalytic performance of Z-scheme heterostructure (Yu, 2020). Ag3PO4/AgBr-20%/g-C3N4 was indeed the best photocatalysts, because it was able to build a dual Z-scheme junction. The photoexcited electrons present in AgBr moved to the VB of g-C3N4, whereas the photoexcited holes in AgBr recombined with the photoexcited electrons in the CB of Ag3PO4 under solar light. In comparison to the binary Z-scheme, this ternary Z-scheme increased the separation of charges and composite material performance. Furthermore, unlike in a normal heterojunction (Fig. 12a), the oxidation and reduction reactions of dual Z-scheme system happened separately in the CB of g-C3N4 and the VB of Ag3PO4 (Fig. 12b), showing that the Eg of the composite rose, hence enhancing photocatalytic efficiency.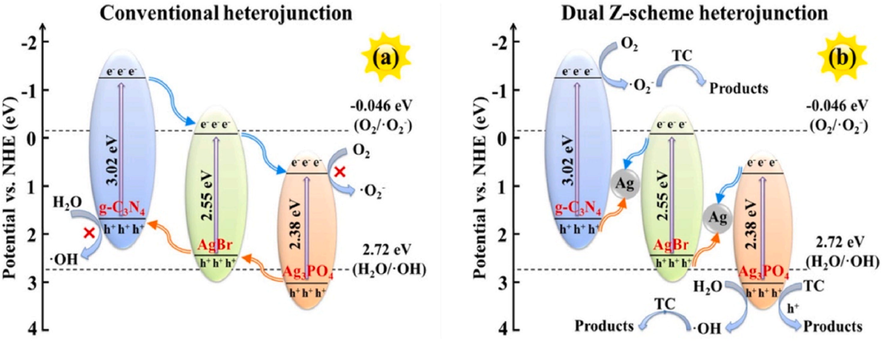
Proposed pathway employing Ag3PO4/AgBr-20 % g-C3N4: Traditional heterostructure (a) and double Z-scheme heterostructure (b). Reprinted with permission from Yu (2020). Copyright © 2020, Elsevier.
1.8.1.5 Isotype heterojunction
Furthermore, g-C3N4 may also produce an isotype heterojunction by forming heterojunctions with other semiconductors. It is widely recognized that g-C3N4 could be synthesized through condensation of precursors (urea, thiourea and melamine). g-C3N4 having varied band gap configurations could be synthesized from several precursors. The isotype heterostructure g-C3N4/g-C3N4 like Type-II (Wang, 2017), Type-I (Dong, 2015), P-N type (Liu, 2016), N-N type (Zhou, 2017), could be prepared by various precursors. For instance, Xu and coworkers used thermal copolymerization to synthesized 3D g-C3N4 heterojunction blocks by assembling nanowires and nanosheets building blocks (Fig. 13a) (Xu, 2019). The hierarchical porous framework created by combining nanowires and nanosheets boosted the utilization of light dramatically. Concurrently, the widespread Type-II isotype heterojunctions created an internal electric field, that aided separation of carrier and exciton dissociation (Fig. 13b). Consequently, the photocatalytic efficiency of generated 3D g-C3N4 units rose by >10 folds.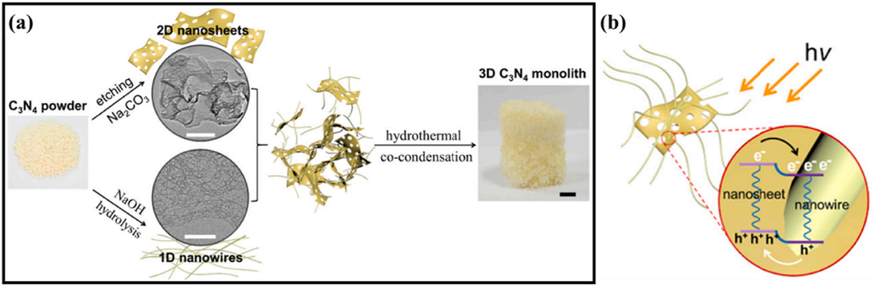
Schematics of (a) the fabrication of 3D microstructural g-C3N4 with isoform heterostructures and (b) the isoform heterostructure fabricated at the interface region of g-C3N4 nanostructure and nanorods. Reprinted with permission from Xu (2019).
In particular, the isotype heterostructure could also be created by altering the crystalline nature of g-C3N4. Wang and coworkers synthesized the semi crystalline g-C3N4 by chloride assisted hydrothermal treatment and they got Type-II isotype heterojunction at disordered-ordered interface (Fig. 14a–b) (Wang, 2017). The rapid passage of electrons at crystalline units and the blockage of holes in networks having poor crystallinity enabled for efficient rise in exciton dissociation at the junction.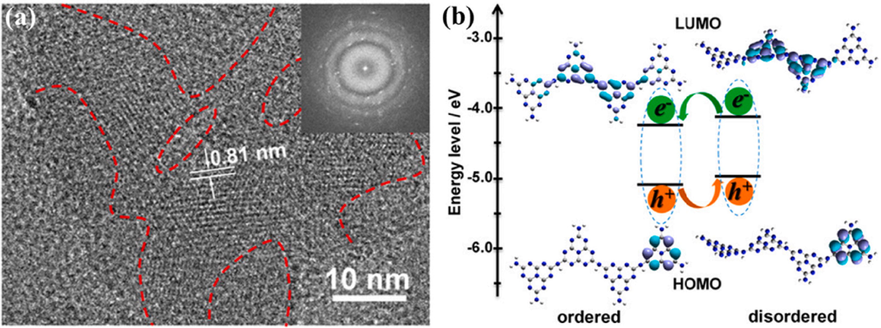
(a) HR-TEM photograph of semicrystalline g-C3N4, and (b) Type-II isoform heterostructure created at the orderly disorderly junction and modelled using DFT analysis. Reprinted with permission from Wang (2017).
Copyright © 2017, American Chemical Society.
2 2-Dimensional g-C3N4
2D-based materials have an extensive specific surface area, good crystallinity, numerous choices for host–guest interactions, maximum light absorption, and enhanced charge carrier separation due to their 3D analogues (Wang, 2012). Recently, g-C3N4 has become one of the most promising heterogeneous photocatalysts in the 2D nanomaterials field. To synthesized g-C3N4 nanosheets, the Ping and his colleagues proposed a simple approach called “etching” by annealing of bulk g-C3N4 in the air directly (Kato and Kudo, 2001). The bulk g-C3N4 can be reduced to the appropriate nanoscale thickness using this approach, which removes hydrogen-bonding interlayers through one oxidation, representing an easy, inexpensive and scalable synthesis. A blue shift in the intrinsic absorption edge of the UV–vis spectra is observed in resulting nanosheets compared to the bulk (Fig. 15) (Yu et al., 2004).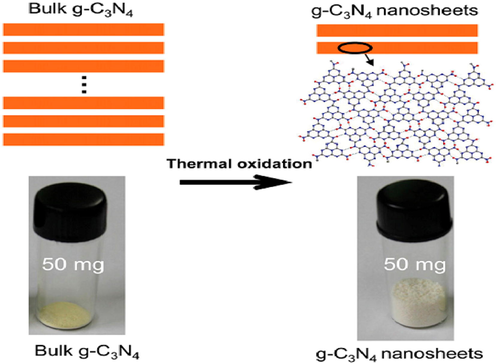
Thermal exfoliation is a low-cost and green method to prepare ultrathin g-C3N4 nanosheets from bulk g-C3N4 in water. Reprinted with permission from Niu (2012).
Copyright © 2012, John Wiley and Sons.
Similarly, the blue shift in the fluorescence emission spectra at 20 nm further confirms the increase in the band gap of nanosheets (2.97 eV), compared to their bulk intermediates (2.77 eV). The band gap has been larger, because of quantum confinement (Niu, 2012). It was found that the pristine g-C3N4 nanosheets exhibited semiconducting qualities, implying that the electron transport takes place within the nanosheet plane by analyzing their IV curves for related electronic properties. The bulk g-C3N4 had low electrical conductivity, as evidenced by the absence of any current, when a bias range of −10 to 10 V was applied. Furthermore, the lifetime of charge carriers in the nanosheets using time-resolved fluorescence decay spectra exceeded from that of bulk g-C3N4. According to Xiadong and co-workers, a new liquid exfoliation strategy has been proposed as a low-cost and environmentally friendly method in order to fabricate the ultrathin nanosheets from bulk g-C3N4 (Rochkind et al., 2015). Through the liquid exfoliation route, ultrathin nanosheets of g-C3N4 were designed, which may be due to their high polarity. The exfoliated g-C3N4 revealed free-standing nanosheets at 120 nm wide that were practically transparent and showed a well-defined Tyndall effect. The photoluminescence spectra (Pl) of these g-C3N4 nanosheets were highly stable, when exposed to an acidic or alkaline environment. However, it was dependent on PH of material. The g-C3N4 nanosheets exhibit a superior photo-absorption over the bulk counterpart, leading to an unusually high PL quantum yield of up to 19.6 %. Researchers found that liquid exfoliation of g-C3N4 led to nanosheets with better photocatalytic efficiency for the breakdown of organic contaminants than the bulk g-C3N4 (Fig. 16) (Barbero and Vione, 2016; Styring, 2012; Zhang, 2013).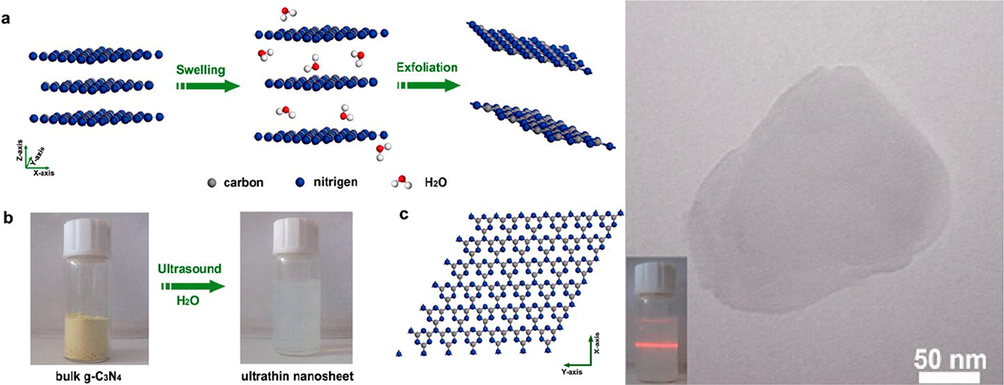
Liquid exfoliation route as a low-cost and green method to prepare the ultrathin g-C3N4 nanosheets from bulk g-C3N4 in water. Reprinted with permission from Zhang (2013).
Copyright © 2013, American Chemical Society.
2.1 1-Dimensional g-C3N4
One-dimensional nanostructures have recently gained immense interest as photocatalysts, due to their distinctive morphology and photophysical properties (Listorti et al., 2009; Chen et al., 2015; Bai, 2013). The nanorods with variable aspect ratios were synthesized by refluxing g-C3N4 nanoplates in different solvents and variations of time (Nosaka and Nosaka, 2017). An exfoliation process and subsequent re-growth of individual nanosheets resulted into the 'rolling up' nanorods from g-C3N4 nanoplates (Bai, 2013). This study investigated the photocatalytic activity of the as-prepared nanorods for the degradation of methylene blue (MB) under visible light (>420 nm). These nanorods were shown to be 50–100 % more active and stimulated under visible and solar light as compared to g-C3N4 nanoplates. Zhihong and co-workers demonstrated that the reactive thermolysis of mechanically activated molecular precursors, such as C3N6H6 and CN3Cl3, under heat treatment led to the large-scale production of well-aligned g-C3N4 nanorods (Kubacka et al., 2012). These PL emission and UV–vis absorption of nanorods demonstrate their unique optical characteristics. A template of monodispersed, chiral, mesostructured silica nanorods was easily generated via ammonia-catalyzed hydrolysis of tetraethyl orthosilicate with F127 cetyltrimethylammonium bromide (CTAB) surfactants in order to construct the uniform g-C3N4 nanorods. The hexagonal mesostructured porous silica nanorods allowed g-C3N4 condensation within the pores (Frank and Bard, 1977). The photocatalytic activity of the g-C3N4 nanorods was found to be significantly higher than that seen in the presence of triethanolamine and 1 % of Pt (Lee, 2016) as cocatalysts. Nanorods made from hydrous melamine nanofibers precipitated from an aqueous solution of melamine can also be synthesized by direct calcination (Li, 2007). The as-synthesized materials demonstrate high photocatalytic performance due to high interfacial area supplied by porosity. For hydrogen (H2) evolution, a visible light photocatalytic activity in the presence of triethanolamine was enhanced by adding oxygen atoms to the g-C3N4 matrix, which resulted in a more effective separation of electron/hole pairs. Similarly, a simple wet-chemical approach was also reported for the synthesis of nanofiber-like g-C3N4 structures with an average diameter of few nm and a length of 100 nm (Wang, 2012)., The nanofibers of g-C3N4 showed a slight blue shift of 0.13 eV compared to that of bulk g-C3N4, due to better packing, electrical coupling, and quantum confinement effects. The g-C3N4 nanofibers had a substantially greater photocatalytic activity than bulk g-C3N4 and were more stable for Rhodamine B photodegradation. The g-C3N4 nanotubes can be synthesized by heating melamine in a compact arrangement that favors tubular forms (Fig. 17a, d) (Wang, 2014). Commercial, low-cost, and large-scale applications were possibly made due to lack of organic templates in this method. There is potential for blue light fluorescence in this newly synthesized material, as it exhibits strong fluorescence at approximately 460 nm. The visible-light photocatalytic activity of these g-C3N4 nanotubes was superior to that of bulk g-C3N4 or P25 TiO2 as a reference (the latter is unsurprising since pure titania is a UV band gap material). Moreover, Muhammad and coworkers also used HNO3 pre-treatment of melamine in order to fabricate tubular g-C3N4 (Kato and Kudo, 2001). The g-C3N4 nanotubes once again became capable of degrading both MB and methylene orange (MO) and were found to be more stable than bulk g-C3N4 under visible light. The superior activity of the nanotubes was attributed to their larger surface area (182 m2/g1) and improved light absorption and charge separation/transfer. The g-C3N4 nanotubes can also be generated through rolling up nanosheets via an easy water-induced morphological transition, without the use of organic solvents and thus in support of green chemical principles (Jing and Guo, 2006).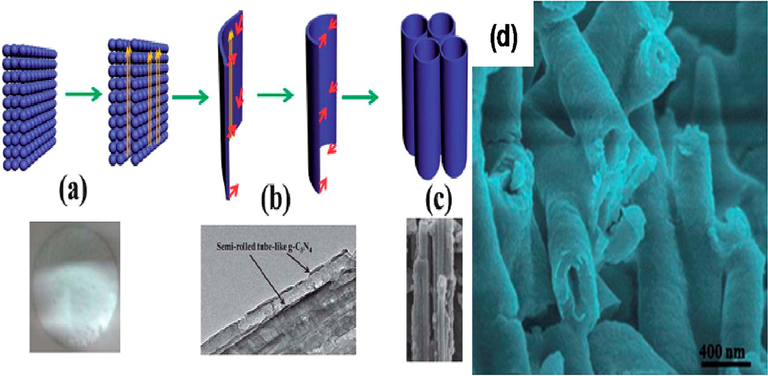
(a–c) Synthetic strategy and corresponding (d) TEM image of g-C3N4 nanotubes. Reprinted with permission from Wang (2014).
Copyright © 2014, Royal Society of Chemistry.
Dicyandiamide (DCDA) and NaCl crystals have been used as structure-directing agents in order to generate the ribbon-like nanostructures of the g-C3N4. These ribbon-like g-C3N4 nanostructures have intriguing optical and electrical features, including a substantial blue shift in their absorption spectra corresponding to a rise in the band gap from 2.7 eV to 3.0 eV (Fig. 18) (Yuan, 2014). For that, a possible explanation is that the nitride pores are functionalized with cyano groups and Na+ ions. While bulk g-C3N4 emitted yellow-green light, ribbon-like g-C3N4 emitted blue light roughly at 400 nm under 365-nm excitation. Nevertheless, no research has been done on the potential photocatalytic properties of these ribbon-like g-C3N4 nanostructures.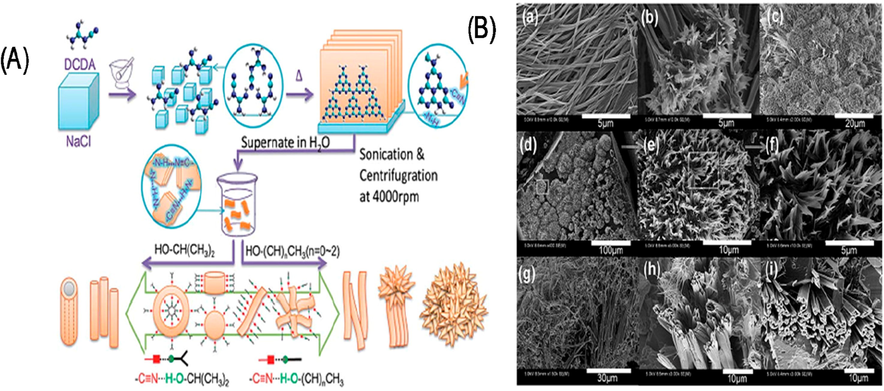
Synthesis strategy (A) Ribbon-like g-C3N4 nanostructures (B) TEM image. Reprinted with permission from Yuan (2014).
Copyright © 2014, Royal Society of Chemistry.
2.2 0-Dimensional g-C3N4
0D materials like quantum dots are of tremendous interest (Wang, 2014). As, thermochemical etching has been used in order to fabricate the g-C3N4 quantum dots. In this multi-step process, the g-C3N4 is thermally exfoliated into 2D nanosheets, and then etched with concentrated H2SO4 and HNO3 in order to form 1D nanoribbons. This process is adjustable. The functional groups, such as carboxylic acid, could be found at the margins and on the base of tri-s-triazine units, when some C–N linkages are oxidized. The preferential cleavage and the preferred orientation result in the formation of nanoribbons, that are approximately 10 nm in diameter and several hundred nanometers in length. The hydrothermal treatment converts nanoribbons into 0D quantum dots of 5–9 nm (Fig. 19) that are highly soluble in water and stable in solution for approximately eight months (Wang, 2014). For example, when 705–862 nm of light was irradiated onto a quantum dot, the emitted light was 350–600 nm in wavelength, thereby covering the whole visible spectrum. Anti-Stokes photoluminescence has been postulated as the mechanism for this up-conversion. The g-C3N4 quantum dots hold great promise as a photocatalytic component for long-wavelength solar energy harvesting, Since they can convert NIR to visible light. The quantum dots increased the photocatalytic H2 generation by platinized bulk g-C3N4 and P25. Remark rate-enhancements (up to 52-fold) were observed for the latter under visible light irradiation in the presence of a methanol sacrificial hole scavenger. Guoping and coworkers fabricated quantum dots for two-photon fluorescence imaging of cellular nucleus (Herron, 2015). A multi-step synthesis was used comprising the acid treatment of bulk g-C3N4 in order to generate a porous material and ultrathin nanosheets, followed by the addition of ammonia, hydrothermal treatment, and ultrasonication of the porous g-C3N4 nanosheets to liberate g-C3N4 quantum dots.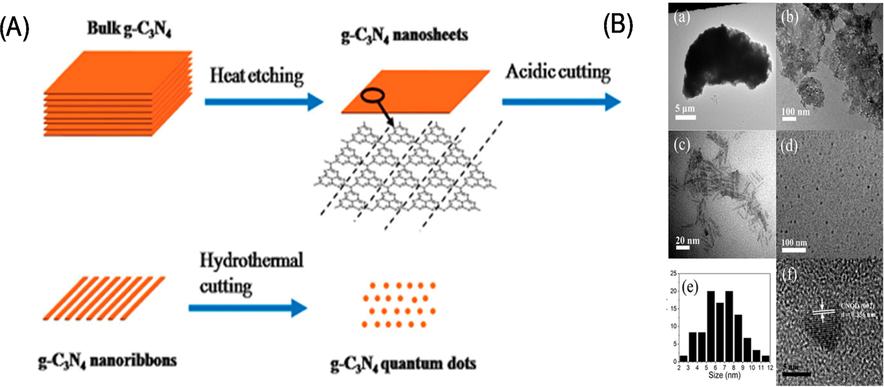
(a) Synthesis of g-C3N4 nanosheets, and (b) TEM images of g-C3N4 quantum dots. Reprinted with permission from Wang (2014).
Copyright © 2014, Royal Society of Chemistry.
2.3 3-Dimensional g-C3N4
The 3D nanomaterials can be used in order to create highly ordered 3D nanoporous g-C3N4 microspheres that can be synthesized without the need of templates, to create unique and highly efficient photochemical systems. The nano-porous g-C3N4 micro-spheres were first made from cyanuric chloride and melamine in acetonitrile, then thermally processed at 550 °C under argon in order to turn them into hierarchical g-C3N4 microspheres (Fig. 20) (Gu, 2015). These highly ordered g-C3N4 microspheres displayed a redshift compared to their bulk counterparts, due to a higher condensation and filling among different layers inside the micro-spheres. Hydraulic charge transfer in hierarchical g-C3N4 was suppressed by calcination, and the photoluminescence emission intensity of the bulk and uncalcined microspheres, respectively. A narrower band gap (2.42 eV), reduced electrical resistance, and increased photo-response of these microspheres enables highly effective harvesting and charge carriers separation generated by visible light. Jinshui and co-workers also generated highy ordered g-C3N4 nano-spheres, constituted of nano-sheet assemblies, using high surface area of silica nanospheres as sacrificial templates (Ong, 2016).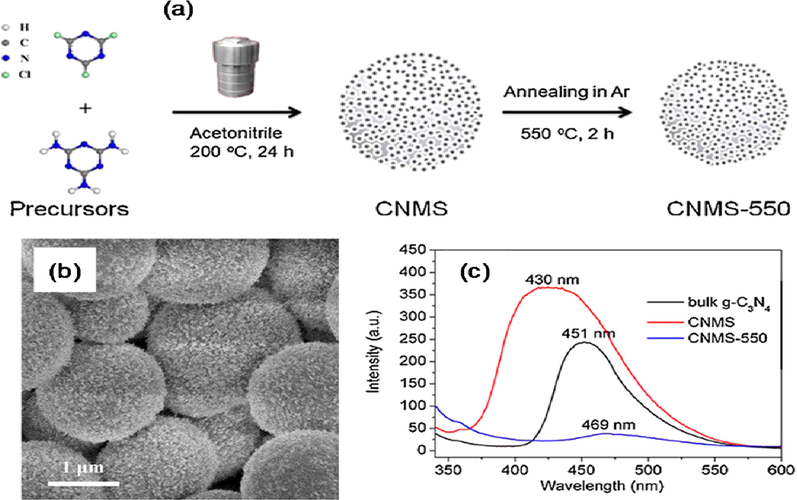
(a) Synthetic strategy, (b) TEM image, and (c) room-temperature photoluminescence spectra of porous g-C3N4 microspheres. Reprinted with permission from Gu (2015). Copyright © 2015, Elsevier.
The silica template provides excellent cyanamide adsorption and a framework for producing the interconnected 2D g-C3N4 nanosheets during self-polymerization of the g-C3N4 polymer after heating. As a result, the silicon spheres demonstrate an outstanding thermal and mechanical durability, elevated temperature based synthesis of highly ordered g-C3N4 was possible, and g-C3N4 nanospheres might be easily detached via etching with an (NH4HF2) solution, while maintaining their spherical morphology. Fig. 21 shows how the highly ordered nano-spheres are fabricated from flat nano-sheets that radiate from center (sphere surface) and formerly interconnect by forming a meso-porous shell (Zhang, 2014). This structure may be advantageous in photocatalysis, because it promotes both separation of charges and transport of mass (Fig. 21). Such kind of as-synthesized nano-spheres exhibited broader band gap as compared to bulk g-C3N4 owing to the effect of their quantum size. Also they have higher potential of light garnering toward whole optical range, particularly between (430 and 590 nm). It might be caused by several reflections (absorption) of incident light within the highly ordered structure and due to the existence of a higher number of defective sites linked through the revelation of low-coordination positions over strident ends of the integral nanosheets at the ends of the highly ordered meso-porous structure.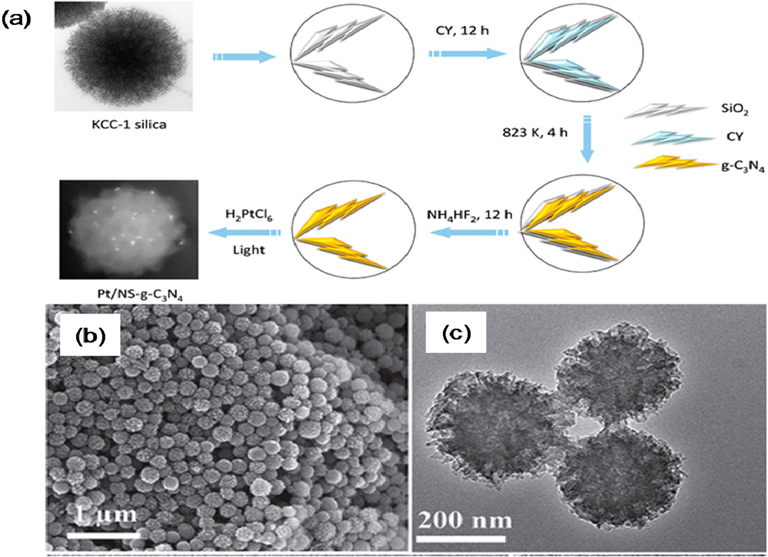
(a) Synthetic strategy, and (b, c) Scanning electron microscopy and TEM images of hierarchical g-C3N4 microspheres. Reprinted with permission from Zhang (2014).
Copyright © 2014, John Wiley and Sons.
2.4 Hollow g-C3N4
The researchers have focused on designing and synthesizing hollow nanostructures having high intricacy by deploying their interior architecture, building units, chemical composition, and its geometry for improving the as-prepared materials performance in a wide variety of fields (Fu, 2018; Wang et al., 2012; Kizito, 2017). The hollow nanostructures is considered additional auspicious morphology for energy conversion and storage applications. The silica nanoparticles were used as templates in order to fabricate the hollow g-C3N4 nanospheres (Dong, 2014; Zhu, 2014; Sun, 2012; Kizito, 2017). Such hollow nanospheres are made of polymeric g-C3N4 and can withstand at 400 °C processing without the core–shell configuration (Fig. 22) (Sun, 2012). For example, the co-polymerization process could extend the π-system by anchoring aromatic motifs, which could red-shift the optical absorption by improving the separation of charge in shell deprived of d-blocking. This technique has turned the shell of hollow g-C3N4 nanospheres in order to boost the photocatalytic performance towards H2 evolution in visible light. Young-Si et al. (Jariwala, 2013) demonstrate a direct facile chemical approach for assembling the triazine unit within the hollow g-C3N4 enabling the simultaneous textural tuning and photo-physical characteristics of g-C3N4.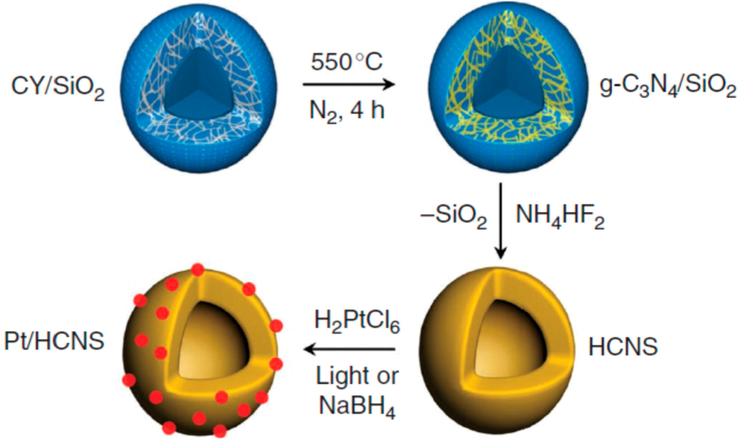
Synthetic strategy for fabricating hollow g-C3N4 nanospheres. Reprinted with permission from Sun (2012).
2.5 Mesoporous g-C3N4
Mesporous photocatalysts have gotten a lot of research interest, because of their (relatively) high molecular mass transport in-pore, superior surface areas, higher quantum efficacy and, prospects of improved light collecting via inner dispersion of incident light. The photocatalytic efficiency for H2 generation was demonstrated in a recently synthesized mesoporous network of g-C3N4 nano-sheets (Fig. 23) (Han, 2016).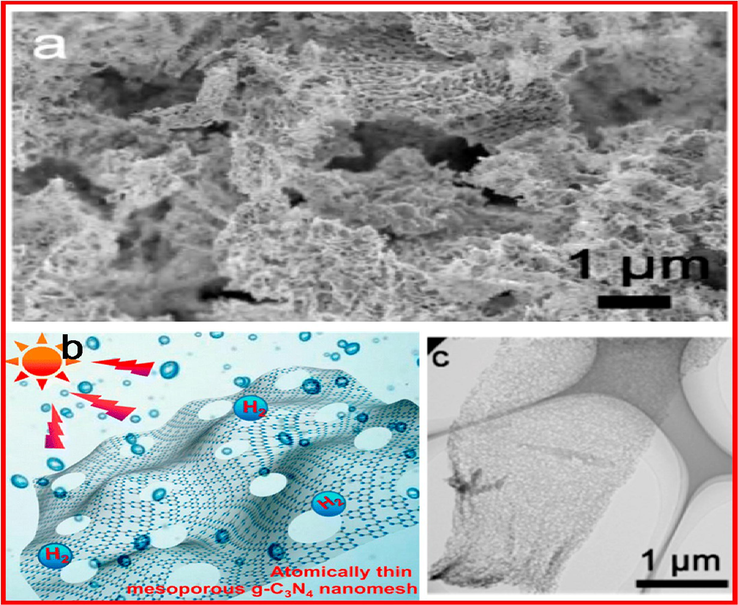
(a) SEM images, (b) Sketch of photocatalytic H2 from water splitting, and (c) TEM image of atomically thin, mesoporous g-C3N4 nanosheets. Reprinted with permission from Han (2016).
Copyright © 2016, American Chemical Society.
2.6 g-C3N4 based quantum dots
In recent years, quantum dots (QD, s) sensitized semiconductors have gotten a lot of attention, because their active sites are more visible. They have a quantum confinement effect, and have a special effect at the interface (Liu et al., 2020). Research findings have shown that quantum dots sensitized could effectively change the width of the band structure and increase the light response rate of semiconductors (Liang, 2020). Moreover, small size of quantum dots may lower the diffusion time of photoexcited electrons/holes from bulk to the surface, that enhance and boost their separation/isolation ability on catalyst surfaces, and significantly increase the quantum efficiency of the whole system (Liang, 2020; Yang, 2019). Liang and coworker (Liang, 2020) synthesized the P g-C3N4 (P-CNT) added with CdS QDs to make a new highly efficient (CdS QDs/P-CNT) using an in-situ oil bath method. The synthesized materials demonstrate a superior performance of photocatalytic water reduction (H2 evolution) and was more stable. It also demonstrated a significantly increased in the optical absorption of light (Fig. 24a), which was attributed to CdS broad photo absorption edge (560 nm) (Zhou, 2018), that significantly improved the photocatalytic efficiency. Wang and coworker (Wang, 2020) used ice-assisted ultrasonic approach to synthesized black phosphorus quantum dots (BPQDs) incorporated with tubular g-C3N4 (BPTCN) nanohybrid with a 1D tubular framework. BPQDs refelecting an average size of 3.32 nm were scattered on the tubular CN in this system (TCN). The addition of BPQDs has greatly increased the photon absorption of BPTCN (Fig. 24b). Alternatively, BPQDs (act as electron mediator) were successfully added on TCN surface to generate a unique 0D/1D structure (interfacial PeC bonds) that slowed charge recombination (Liu, 2019). The PeC bonds established a close association between BPQDs and TCN, thereby increasing cycle stability and electron transfer (Fig. 24c–d) (Wang, 2020). Carbon quantum dots (CQDs) could change the transfer of photoexcited electrons and holes by acting as electron acceptor. Moreover, CQDs/g-C3N4 band alignment can help the isolation of photogenerated electron-hole pairs (Liu, 2016; Qin and Zeng, 2017). Li and coworkers (Di, 2016) fabricated g-C3N4 nanosheets loaded with CQDs using a simple solvothermal process in ethanol and improved the photocatalytic HER. When g-C3N4 nanosheets (CNNS) stimulated in light, CQDs act as photosensitizer, causing more absorption of light in order to transfer electrons to the CB of CNNS. Liu and coworkers (Liu, 2019) also employed CQDs to adorn the g-C3N4 by a simple polymerized process without affecting its skeletal framework. CQDs expanded the absorbance spectra of g-C3N4 and acted as charge carriers for photogenerated electrons, leading to increased its photocatalytic performance. Qin (2020) created a seed-mediated hydrothermal g-C3N4 modified by NiS2QDs as a co-catalyst. The quantum confinement effect and small size of synthesized materials may give additional active sites and help in the formation of an intimate interface contact (Zhang, 2018; Chen, 2017). Furthermore, existence of NiS2QDs in a NiS2QDs/g-C3N4 might increase the quick transfer of photogenerated electrons from g-C3N4 to NiS2QDs, considerably enhancing the effective photoexcited electrons and holes separation (Lu, 2018). Lu and coworkers fabricated a 0D/2D heterostructure by modifying Ni2PQDs as a co-catalyst over the g-C3N4 surface, that could significantly boost HER and PEC activity due to improved light absorption and better photogenerated separation of electrons and holes.
(a). The optical absorption of synthesized materials (Zhou, 2018), (b) the BPQDs has greatly increased the photon absorption of BPTCN. (c, d) the increasing cycle stability and electron transfer between BPQDs and TCN materials. Reprinted with permission from Wang (2020). Copyright © 2020, Elsevier.
Shakeelur and coworkers (Ar, s.r., 2020) created new type of catalyst composed of CdSeQDs loaded on thiol added g-C3N4 (TF-g-C3N4) and investigated for H2 production. The fast rate of H2 generation could be ascribed to the broad wavelength absorption and excellent reduction in recombination of electrons-holes pair of photocatalyst. Yang (2019) used a two-step approach for the synthesis of highly dispersed Co3S4QDs/g-C3N4 (Co3S4/CNNS) nanocomposites (water bathing and deposition). Co3S4QDs supplied additional active sites for HER, considerably widened the visible light range of CNNS, eased carrier separation and migration, and reduced the photoinduced electron migration distance, resulting in high HER performance. Electrons from the VB of CNNS migrated to CB, and the sacrificial agent such as triethanolamine generated the holes on VB. Electrons were then transported to Co3S4QDs. Co3S4QDs served as electron storage sites and hindered the recombination of photoexcited holes and electrons, resulting in a large quantity of active sites for HER. Following, the electrons had transferred from the CNNS to the Co3S4QDs reacted with the H2O molecules to generate H2, that was collected on the photocatalysts surfaces. QDs is a form of semiconductor material, have been identified as one of the promising architectures for suppressing the photoexcited electron-hole pair recombination (Fang, 2016). The strong quantum confinement provided by QDs is advantageous for shortening the path length of photogenerated charge transfer (Yang, 2019). On the other hand, faster the charge transport can boost the photocatalytic rates (Cao, 2013). While, QD can be used as a source of photoelectric response for the optical excitation (Meng, 2018). The considerably increased performance of g-C3N4 is due to the quantum dots acting as electron sources to capture electrons for improved separation and better light absorption.
3 Use of g-C3N4 in photocatalysis
g-C3N4 is an outstanding photocatalyst, which has diverse applications in different research fields (Su, 2012; Mandal and Ganguly, 2011; Anpo, 1987; Chen and Mao, 2007; Lu et al., 2009), including water splitting for hydrogen evolution reaction (HER), oxygen evolution reaction (OER) (Daniel and Astruc, 2004; Watanabe, 2014; Claus, 2000), fuel cells (Bell, 2003), CO2 reduction to value-added products (Hu et al., 2008; Alenad, 2022; Hayat, 2021), organic synthesis (Kumar, 2016) and environmental remediation (Mclaren, 2009). Herein, we will specifically discuss the application of g-C3N4 in photocatalysis.
3.1 Solar fuel generation
During photocatalysis, solar fuel generation from water and carbon dioxide (C02) is the best way to produce H2, O2, hydrocarbons, and syngas as chemical feedstocks and sustainable energy (Li and Liu, 2011). For that purpose, g-C3N4 is considered as the best metal-free and excellent photocatalyst in the visible range. In the recent times, a lot of semiconductor materials were fabricated, for the photocatalytic water splitting (WS) under solar illumination. Feasibly, g-C3N4 photocatalyst have great potential for water splitting, since its CB and VB locations are in the right places (Yang, 2017). In general, there isn't much informative about photocatalytic oxygen progression and WS. This is even though the OER is quite complex than the HER, that only involves two electrons transfer. Fig. 25 is a simplest diagram of the photocatalytic water splitting (WS) into H2 and O2 over g-C3N4 (Tian, 2019). When g-C3N4 is exposed to illumination, the photoinduced electrons move from VB to CB, having left gaps in VB. Electron-hole pairs move individually to the activated and oxidation locations on the interface of g-C3N4 to turn water molecules into H2 and O2 in the mentioned expressions: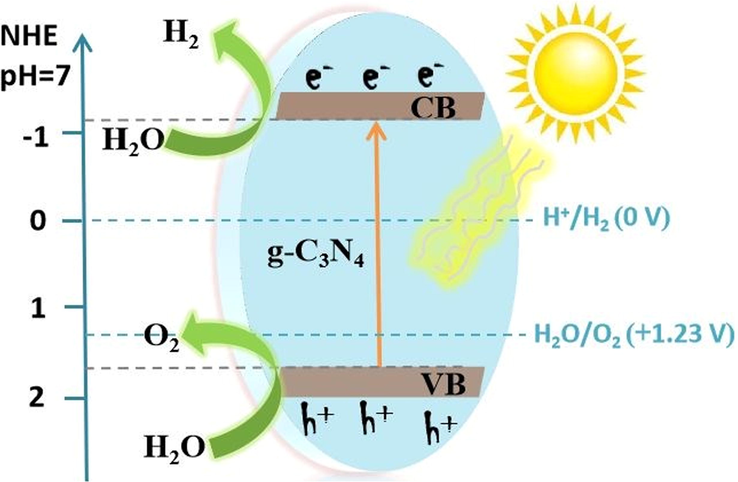
Depiction of photocatalytic WS employing pristine g-C3N4 for H2 and O2 energy sources under illumination. Reprinted with permission from Tian (2019).
Copyright © 2019, Royal Society of Chemistry.
Reduction reaction:
Oxidation reaction:
Overall water splitting reaction:
The photochemical H2 and O2 production, which existed at the g-C3N4/water surface is strongly based on the thickness, shape, and deficiencies of g-C3N4. The huge particular surface areas, powerful elevated optical absorbance, maximum charge isolation performance and plentiful surface reacting spots are all helpful for the photocatalytic H2 formation of the g-C3N4. Since g-C3N4 does have a comparable complex architecture with graphite, the surface area might potentially be enhanced up to 2500 m2/g1 for ideal multilayer g-C3N4 (Sano, 2013). Although it is generally approximately upto 10 m2/g1 for the pure g-C3N4 material owing to the compaction of polymer nanostructure. Here are numerous papers stated earlier emphasizing the advantages for photocatalytic H2 and O2 development by morphological manipulation of g-C3N4. The AQE of the 2D g-C3N4 nanostructure with a diameter of ∼1.2 nm, equivalent to ∼4 layer, that was synthesized utilizing urea as starting material by a sustainable steaming reformer technique, may obtain up to 11.3 % at 405 nm (Fig. 26) (Yang, 2017). A surface area of 210 m2/g1 is achieved for such ∼1.2 nm g-C3N4 nanostructure, that is 4.38 times greater than that of conventionally synthesized g-C3N4 by urea calcination. The produced nanostructure additionally promotes the charge transport, participating in the increased effective H2 generation function.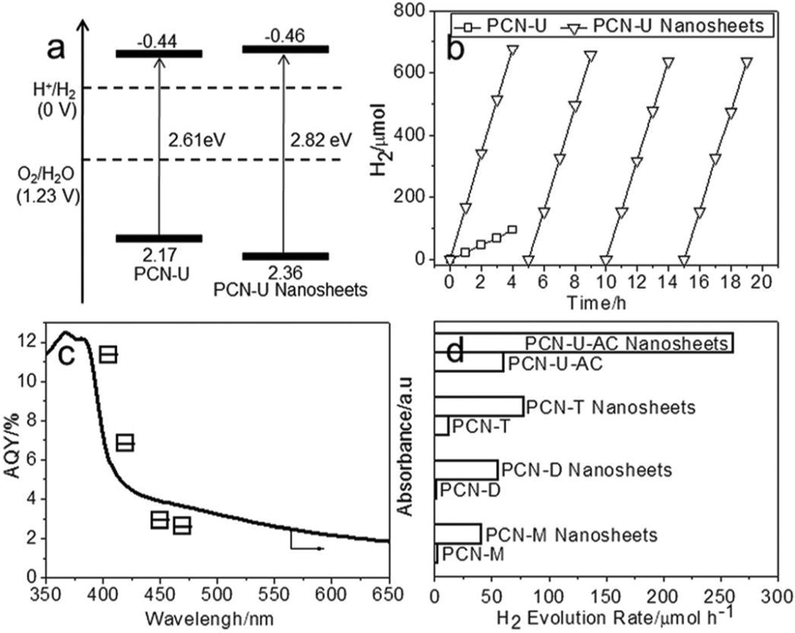
(a) Graphical depiction of the electronic framework. (b) Photocatalytic performance and durability of the PCN-U and PCN-U nanostructure. (c) Wavelength dependent of AQE on the H2 development in the PCN-U nanostructure. (d) Photocatalytic H2 production kinetics of the PCN and PCN nanostructure produced from various intermediates. Reprinted with permission from Yang (2017).
Copyright © 2017, John Wiley and Sons.
Basically, developing nanosized unique shaped nanomaterials may reduce the diffusing route of charged particles, enabling fast movement of charges over the interface of materials to contribute in the oxidation processes (Cui, 2018). The subsequent illustration is an effective spatially anisotropy charged isolation technique for acknowledging the benefit of morphological controlling on g-C3N4 (Liu, 2017). Using an NH4Br based intermediate-mediated technique, the researchers generated the numerous phenomena (porous/microporous/ultraportable/horn-like) incorporated g-C3N4 tubes (Fig. 27a–d) (Liu, 2017). The g-C3N4 tubes possessed larger surface area and aided electron transport, carrier concentrations and interface charge transport performance, therefore exhibiting an exceptional photocatalytic performance for H2 generation with an AQE of 14.3 percent at 420 nm (Fig. 27e, f). As seen in Fig. 27g, the selected photo-deposition findings reveal that the photoinduced electrons transfer to the external layer and holes might preferred to migrate onto the interior layer of the g-C3N4 tubes, offering a deep understanding into charges transport dynamics and increased photo performance.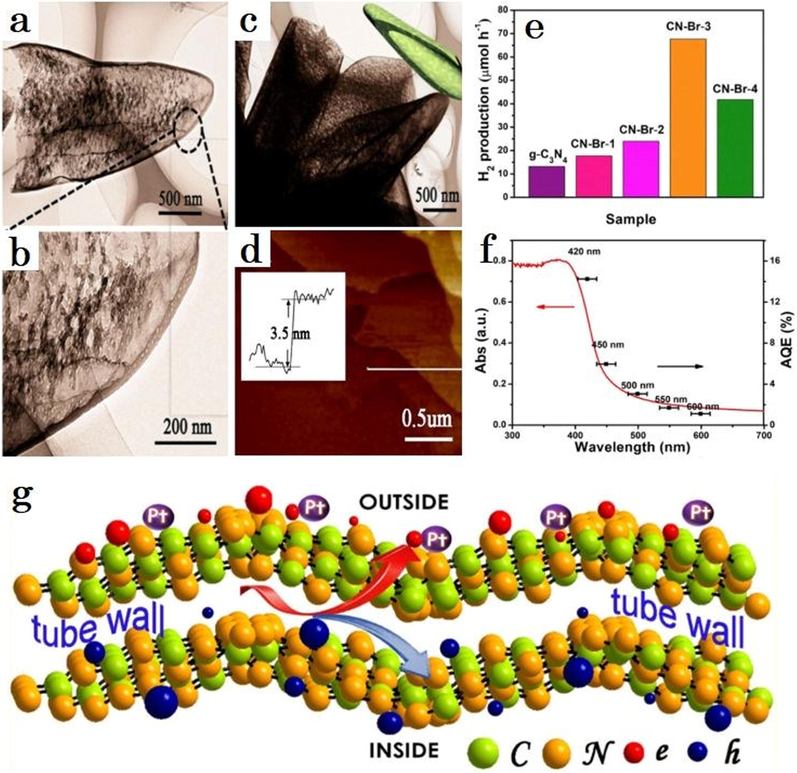
(a, b) The enlarged TEM pictures of a singular g-C3N4 tube. (c) TEM pictures of CN-Br-3, (d) AFM imaging and diameter of g-C3N4 tubes shell. (e) the estimated rate ratios for HER of pristine g-C3N4 and CN-Br-X. (f) AQE and DRS spectra of CN-Br-3. (g) Graphical representation for the peculiar spatially charges isolation of g-C3N4 tubes in the photocatalytic reaction. Reprinted with permission from Liu (2017). Copyright © 2017, Elsevier.
Owing to rising technical emphasis on attaining “ecofriendly” power conservation and, continued endeavors was already made to the WS reaction for the research of a conceptual modeling on g-C3N4 catalysts. Besides novel nanomaterial g-C3N4, metal and non-metal loading, deficiencies variations construction, noble metal coating etc. for boosting the WS performance were also thoroughly published and evaluated in the preceding (Xiao, 2015). Although, it is also tough and prospective to generate completely employment of non-metallic g-C3N4 as an OWS photocatalysts. Acquiring a profound acknowledgement of the chemistry underlying the association among water molecules and g-C3N4, since the photocatalytic WS reaction is an essential part to alleviate the aforementioned problem. Yu et. al. (Tian, 2017) demonstrated that the lower dimensional charge transfer and electron mediated by interfacial hydroxylation might increase the photocatalytic H2 evolution of g-C3N4. The plentiful interfacial hydroxyls (—OH) inserted on the —C≡N location, therefore enlarge the 2D coupling charged particle mechanism of g-C3N4 to 3D space to recognize multiple scales electron transport, and radicalize the adjacent N unit (C—N⚌C) to encourage the electron (H+) adsorbent and stimulation on them (Fig. 28). Incorporation of fundamental ammonium salts offers a pH-dependent hydroxylation-level mechanism and produces a ∼11-time boost in H2 productivity. These finding might alter a potential route for producing extremely active HER composites basis on surface polarization of ligands.
(a) Interactive localized functions, (b) charged variation at upper aspect and side angle; (c) charged variation of protons deposited on CN, O-CN. Charging production is in blue and dissipation in yellow. Eads and Δq represents for the absorbance and carrying charges for protons. Reprinted with permission from Tian (2017).
Copyright © 2019, Royal Society of Chemistry.
Many single compounds based photocatalysts for HER have weak photocatalytic activity under visible light. Moreover, combining the g-C3N4 with hole scavenger and metal, resulting into higher photocatalytic activity. Shubin et. al. (Niu, 2012) used thermal exfoliation approach, in order to synthesize the g-C3N4 nanosheets with outstanding HER in a triethanolamine/water solution, compared to bulk g-C3N4 with a thickness of 2 nm HER rates of 3 and 5.5 under visible and UV–vis, respectively. The activity of one layer g-C3N4 nanosheets for HER is better than that of bulk g-C3N4, which is being synthesized by exfoliation method (Alivisatos, 1996) due to better charge transport and separation. The mesoporous g-C3N4 thin films synthesized by the solvothermal method have an outstanding photocatalytic activity for HER (Zhang, 2013) about 8510 mol/h1 g1 (with a quantum efficiency of 5%, 420 nm), greater than the 1560 mol/h1 g1, which was observed for 2D non-porous g-C3N4 nanoparticles or the 350 mol/h1 g1, observed for bulk g-C3N4. The platinum (Pt) nanoparticles loaded with g-C3N4 nanorods with TEOA and 1% wt Pt co-catalyst (Yang, 2013). These materials also outperform their mesoporous counterparts (Pan, 2012). Even though the reason for this difference is unknown, and Jones et. al. suggested lone pairs in nitrogen are the reason for excellent performance (Tian, 2014). The TEOA is mainly used as a hole scavenger for g-C3N4 and gives methanol as an extra performance (a 14-fold rate boost). When TEOA is used instead of methanol (as hole scavenger), the P25 gives excellent performance for HER, but less than g-C3N4. The g-C3N4 nanomaterials synthesized by the rolling up method show perfect HER (Weng, 2014). When used with TEOA (as sacrificial agent) and Pt as co-catalyst, the g-C3N4 nanosheets or QDs as a catalyst give three times better performance than pristine g-C3N4. The band structure g-C3N4 QDs could be altered in order to make them more sensitive for visible and near-infrared light, allowing for even greater photocatalytic HER. Other metal-free g-C3N4 nanosheets have photocatalytic HER of 1596 mmol/h1 g1 (quantum efficiency 3.56% at 420 nm), while P-doped g-C3N4 nanosheets have photocatalytic HER of 1596 mmol/h1 g1 (quantum efficiency of 3.56%). Similarly, the WS reaction was done with 3D g-C3N4 nanoporous material, TEOA, and 3% wt Pt as co-catalyst (Bai, 2013) that gives 2.5 times better HER than pristine g-C3N4 and remains stable after five cycles under visible light. The hollow g-C3N4 having a quantum efficiency of 7.5 %, has also been significant photoactivity for WS (Zhang, 2001). The inclusion of a MoS2 co-catalyst considerably improved HER over hollow g-C3N4 spheres, forming a MOS2/g-C3N4 hybrid (Fig. 29), thereby, increasing its light absorption and charge transfer ability (Zheng, 2016).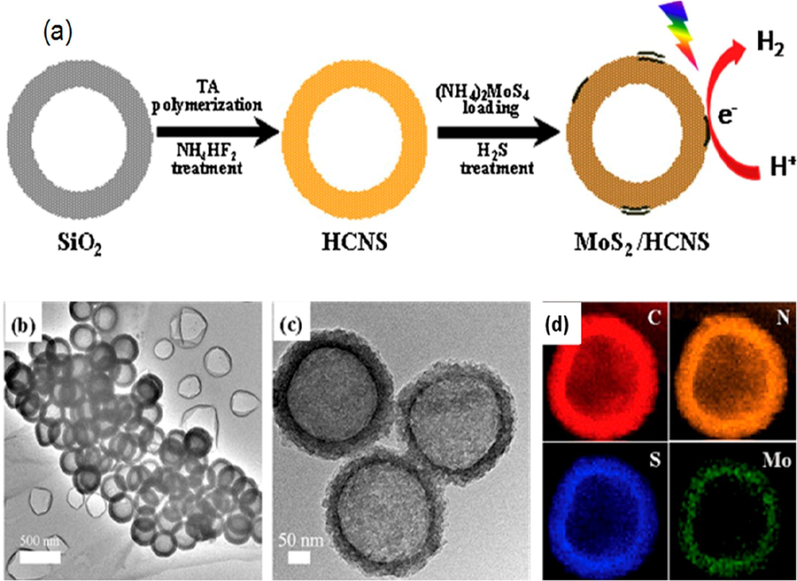
(a) Synthetic strategy and (b, c) TEM images and (d) Energy dispersive X-ray spectroscopy (EDX) elemental maps of MoS2@hollow g-C3N4. Reprinted with permission from Zheng (2016). Copyright © 2016, Elsevier.
In order to broaden the spectrum range of g-C3N4, other semiconductors and metal nanoparticles have been added, which further induce surface plasmon resonance. In such heterojunction materials, the photoexcited charge carriers are better segregated, reducing charge recombination and loss of energy for fluorescence (Li et al., 2012; Zeng, 2018; Ajmal, 2020). The metal doped g-C3N4 has several benefits, including high diffusion of molecules, better light absorption, and more active sites (Tahir, 2014; Wang, 2014; Tahir, 2013). For creating TiO2/g-C3N4 composite, the melamine was used for hydrothermal calcination, followed by solid-state reaction (Zeng, 2014). When compared the pristine g-C3N4 (108 mol/h1 g1) with TiO2/g-C3N4 heterostructures, the TiO2/g-C3N4 had low band gap and high photoactivity (556 mol/h1 g1) for HER under visible-light irradiation (130 mol/h1 g1). The core/shell heterojunction nanocomposites were found to be more beneficial, because of the high facial contact between core and shell components (Yuan, 2014). The core/shell nanowires of CdS/g-C3N4 with different g-C3N4 content were synthesized using the solvothermal and chemisorption method (Fig. 30) (Zhang, 2013). The g-C3N4 has absorbed on CdS nanowires by giving an excellent HER rate of 4152 mol/h1 g1 for 2 wt% g-C3N4. A one-step self-assembly approach for the synthesis of the core/shell nanomaterial of carbon spheres coated with g-C3N4 has recently been devised. These composites exhibit better light absorption, mechanical stability, and charge transfer conductivity (Weiss, 2017), resulting into maximum HER rates of 129 mol/h1, an 8-fold excellent over new g-C3N4 (16 mol/h1). Other nanocomposites of g-C3N4 (Gu, 2015; Zhang, 2014; Zheng, 2016; Fattakhova-Rohlfing et al., 2014; Cheng, 2017; Nguyen et al., 2015; Sun, 2012; Zheng, 2015; Jun 2013; Hong, 2014; Han, 2016; Lang et al., 2014; Zhang et al., 2015; Ye, 2015; Zheng, 2012; Maeda, 2011; Ahmad, 2015; Xu, 2013; Bandyopadhyay, 2017; Ran, 2015; Di, 2010; Ajmal, 2018) were studied using a variety of materials and morphologies in order to get a deeper insight into the possible charge transfer pathways between g-C3N4 and other materials. Examples of different g-C3N4 heterojunctions include Z-scheme heterojunction (Ou, 2017; Wang, 2017), the Type II (Shen, 2017), p-n type (Zhang, 2013; Zhang, 2017; Ge, 2012; Xiang et al., 2011; Sun, 2017; Fang, 2017; Naseri, 2017; Lu, 2017; Xu, 2017; Zeng, 2017; Mao, 2017; Yu, 2017; Wen, 2017; Zhao, 2017; Barrio et al., 2018; Jin, 2017; Pramoda, 2017), g-C3N4 with metal (Ye, 2016; Xu, 2015) and g-C3N4 with carbon (Yuan, 2013). Controlling the structure of g-C3N4 heterojunctions for better light absorption is an interesting strategy in recent times.
(a, b) TEM and (c) HR TEM image of core–shell CdS/g-C3N4 heterojunction nanocomposite. Reprinted with permission from Zhang (2013).
Copyright © 2013, American Chemical Society.
3.2 Carbon dioxide (CO2) reduction
The ongoing use of fossil fuels for chemical and energy production is causing severe problems (Tong, 2015; Martin, 2014). Among them, the photocatalytic CO2 reduction to sustainable hydrocarbons and oxygenates might be a long-term solution. Because CO2 reduction includes the multi-electron transfer, methanol, methane, formaldehyde, formic acid, and carbon monoxide formation kinetics are much slower than H2 formation kinetics. The CO2 reduction involves the molecule absorption onto the catalyst surface, where photoexcited electrons are transported across the semiconductor bandgap to form the anion radical. In an aqueous environment, the CO2 reduction is caused by photoexcited holes transferred to hydrogen atoms from water, and then the proton is shifted to the CO2 anion (Fig. 31) (Tian, 2019). The CO2 reduction gives various products, which are shown below (relative to NHE at pH = 7):
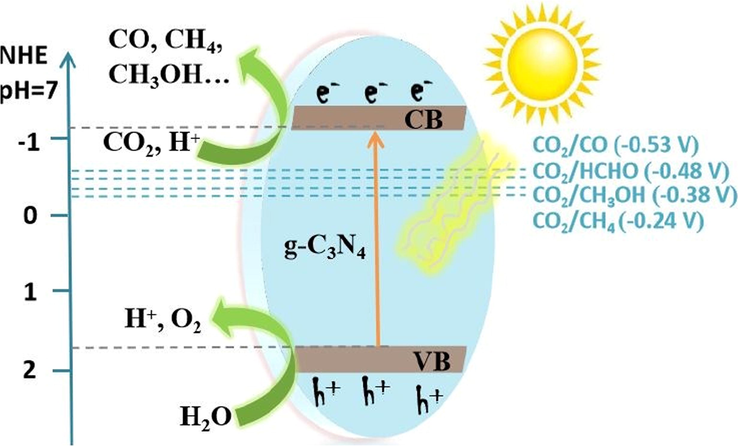
Graphical depiction of photocatalytic conversion of CO2 to fossil energy under photoexcitation upon pure g-C3N4. Reprinted with permission from Tian (2019).
Copyright © 2019, Royal Society of Chemistry.
The essential features of photocatalyst, CO2 absorption capacity, and strength are the main factors in the photocatalytic CO2 reduction process (Gu, 2015). Electron and hole transfer kinetics to CO2, charge separation, bandgap, and reductant are essential for photocatalytic CO2 reduction process. g-C3N4 material has been studied for CO2 reduction due to their negative CB, stability, and low bandgap (Li et al., 2015; Liang, 2015). The g-C3N4 activity is enhanced by doping of metal and nonmetal, heterojunction construction, and Z scheme hybrid (Han, 2015; Zhu et al., 2015; Huang, 2015; Wu, 2014; Han, 2015; Ajmal et al., 2021). Pengfei et. al. (Li, 2015) used ultrathin sheets of g-C3N4 for excellent CO2 reduction, which gives tremendous charge carrier potential, greater surface area for absorption, and improved light absorption with methane and methanol productivities of 1.39 and 1.87 mol/h1 g1, respectively. According to Jianguo and colleagues (Wu, 2014), the decline in overpotential of CO2 is due to the better charge separation of Pt nanoparticles on metal/semiconductor surface area. According to Qingqing et. al. (Wang, 2013) the Pd nanoparticles with double defect increase the reduction of CO2 into CH4 and carbon monoxide (CO) over g-C3N4 nanosheets. The 61.4 % conversion of CO2 achieved and average CO production was 4.3 mol/g1 h1, with an average methane (CH4) production rate of 0.45 mol/g1 h1, which showed reactive CO2 and many active sites. The g-C3N4 nanotubes doped with oxygen synthesized by thermal oxidation and condensation of pristine g-C3N4, were demonstrated to have considerable photocatalytic CO2 reduction capability under visible light (Zhang, 2012). The oxygen-doped g-C3N4 nanotubes have multi-walled tubes and have a diameter of 30 nm, and give methanol at the rate of 0.88 mol/g1 h1, which is five times greater than individual g-C3N4 nanotubes (0.17 mol/g1 h1). The ZnO/g-C3N4 heterojunction was synthesized by one-step calcination (Sui, 2013). The bulk g-C3N4 demonstrate 2.5-fold performance due to a direct Z-scheme mechanism indicating robust transportation of electrons from g-C3N4 towards ZnO surface. Zhong Xing et. al. showed that modified g-C3N4 with CeO2 representing an excellent photocatalytic activity for CO2 reduction to CH4, with an average production rate of 4.79 mmol/g1 h1, which is 3 times greater than g-C3N4. Wang et. al. did a situ redox reaction between MnSO4 and KMnO4 and then synthesized a 2D/2D-MnO2/g-C3N4 heterojunction photocatalyst for CO2 reduction to CO (9.6 mmol/g1), which gives excellent charge separation. The g-C3N4/SnS2 was used for photocatalytic CO2 reduction in which SnS2 electrons interact with holes of g-C3N4 to give CH3OH (2.3 mol/g1) and CH4 (2.3 mol/g1), respectively. A unique Z scheme method was used in MoO3/g-C3N4 heterojunction (Liu, 2015). TRyo et. al. used a different method in which they connected Ru(biby) complex into the structure of g-C3N4, which enhanced the performance of the reduction of CO2 to formic acid, having an average quantum yield of 5.7% at 400 nm (Fig. 32) (Kuriki, 2015). The active photocatalysts for CO2 reduction are generated, when polyoxometalate clusters are attached to g-C3N4 (Zhang et al., 2015). After 10 h of visible light irradiation, the Co4/g-C3N4 hybrid photocatalyst produced 107 mol/g1 h1 of CO and 94% selectivity for CO, significantly exceeding unpromoted g-C3N4.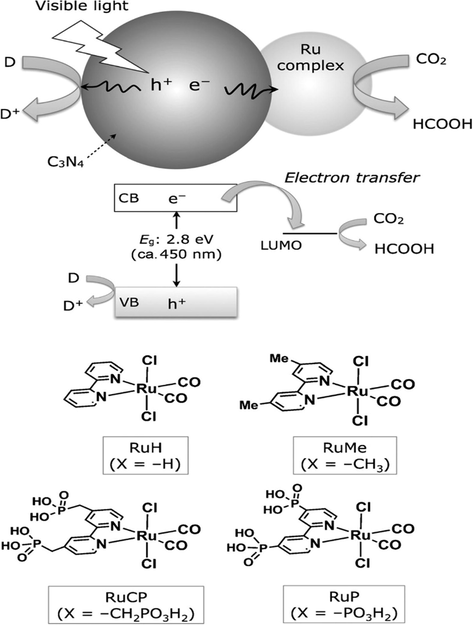
The photocatalytic reduction of CO2 via Ru complexes structure and Ru complex/C3N4 hybrid photocatalyst. Reprinted with permission from Kuriki (2015)).
Copyright © 2015, John Wiley and Sons.
An intercorrelated super hybrid formed of g-C3N4 with AgBr ornamented onto N-doped graphene (produced by chemical synthesis) was also employed to show photo-catalytic CO2 reduction to CH3OH and C2H5OH (Fig. 33) (Li, 2015). Using Cu/TiO2/g-C3N4 (Cao, 2013), nanocomposites fabricated by impregnation and pyrolysis, the Oluwatobi et. al. demonstrated an increased rate of photo-reduction of CO2 to CH3OH and HCOOH under UV–vis radiation, with peak rates of CH3OH at 2574 and HCOOH at 5069 mmol/g1, respectively. The increased performance was attributed to the nanocomposite photoexcited electrons distribution and metal position. The Hailong et. al. demonstrated that AG/TiO2/g-C3N4 heterojunction has excellent performance toward CO (28 mol/g1) and CH4 (19 mol/g1) production from CO2 reduction. However, depending upon lower Fermi level, the photoexcited electrons were transferred towards the TiO2/g-C3N4 homojunction and then after TiO2 to Ag nanoparticles, thereby, resulting into a significant reduction in electron-hole recombination onto the TiO2 surface-modified Ag nanoparticles.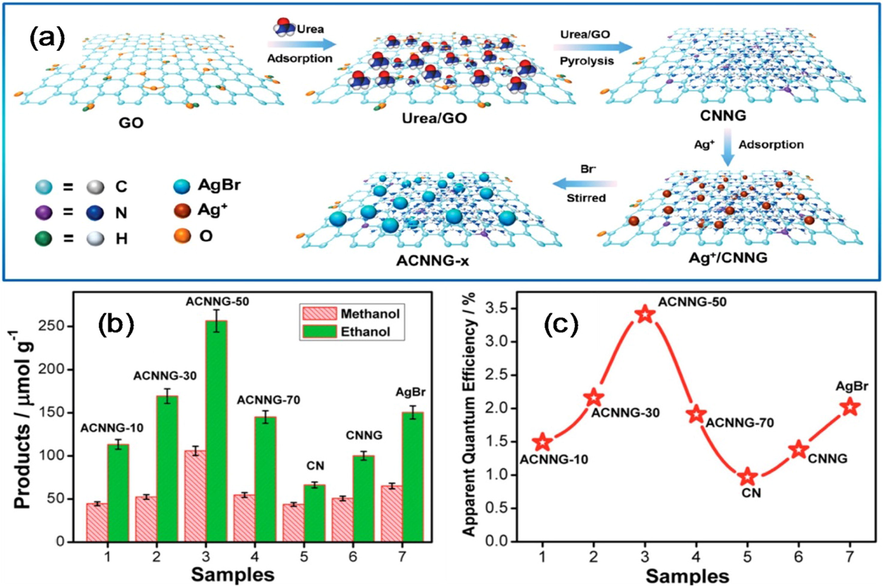
(a) Synthetic strategy, and (b, c) photocatalytic performance for CO2 reduction of intercorrelate super hybrid g-C3N4 nanocomposites under visible light and corresponding apparent quantum efficiencies. Reprinted with permission from Li (2015).
Copyright © 2015, John Wiley and Sons.
Yu et. al. (Fu, 2017) developed hierarchy permeable O incorporated g-C3N4 nanotubes (OCN-Tube) via serial heat oxidizing exfoliating and curling-condensation of pristine g-C3N4. The OCN-Tubes composed of interconnecting multi-walled nanotube with consistent sizes of 20–30 nm. Upon light irradiation, the photocatalytic methanol generation ratio of OCN-Tubes is 0.88 µmol/g1 h1, that is 5-fold greater that of pure g-C3N4 (0.17 µmol/g1 h1) (Fig. 34a). The improved photocatalytic performance of OCN-Tube is attributed to the synergism impact of hierarchy nanotube configuration and O coupled interaction. The hierarchy nanotubes design imbues OCN-Tube, increased particular surface area, increased light usage reliability, and enhanced mobility dynamics, owing to the numerous exposure energetic sides and various illumination dispersion pathways. Furthermore, the integration of O improves the electronic structure of g-C3N4, leading in a smaller absorption edge, stronger CO2 affinities and an improved isolation of photoinduced electrons transportation. As demonstrated in Fig. 34b, whenever 13CO2 was employed as the fuel, a significant pattern with the m/z value of 29 ascribed to 13CO was found (Ou, 2018). Consequently, it is established that CO is contributed from CO2 in the photocatalytic reaction.
Qin et. al. employed barbituric acid (BA) integrated g-C3N4 for the reduction of CO2 to CO and H2 as a biproduct (Qin, 2015). Despite alleviating the physical/chemical parameters of g-C3N4 with improved photostability, the molecular doping of urea and barbituric acid considerably simplified the charged transfer and isolation through the creation of interfacial chemical heterostructures. As demonstrated in Fig. 35a, the optimum CNU-BA0.03 displayed a substantially improved photocatalytic CO generation, surpassing 15 times (31.1 μmol/h1) more pristine g-C3N4 (2.1 μmol/h1). Furthermore, high loading of BA resulted to the reduction in the CO generation ratio, that was ascribed to the degradation of g-C3N4 polymer structure due to BA monomer. It obviously illustrates the necessity of specific handling of the level of co-polymerization in boosting the photoreduction performance. Furthermore, the tendency of the materials such as CO and H2 coincided effectively with the light absorbance of CNU-BA0.03 predicated on the reaction spectra (Fig. 35b) and also the material held a good reliability with no declination in the final products (Fig. 35c). To extensively reveal the involvement of molecular doping process, various monomers comprising ABN, ATCN and DAMN were also applied to combine with urea raw material. Surprisingly, an elevated photocatalytic efficiency in all comonomer integrated g-C3N4 materials was detected contrasted with the simple g-C3N4 (Fig. 35d). This signifies those plentiful options of organic monomers may be merged with g-C3N4 using the co-polymerization approach to boost the reduction reaction of CO2 source. To validate the formation of CO from the CO2 photoreduction, a 13C tagged isotopic tracing assay utilizing 13CO2 was demonstrated (Fig. 35e–f). Depending on the MS data, a fundamental signal pertaining to the 13CO (m/z value of 29) was depicted rather than 12CO (m/z value of 28). It contended the generation of CO exclusively resulted from the photocatalytic CO2 reduction instead of the breakdown of carbon contaminants from the surface of photocatalysts.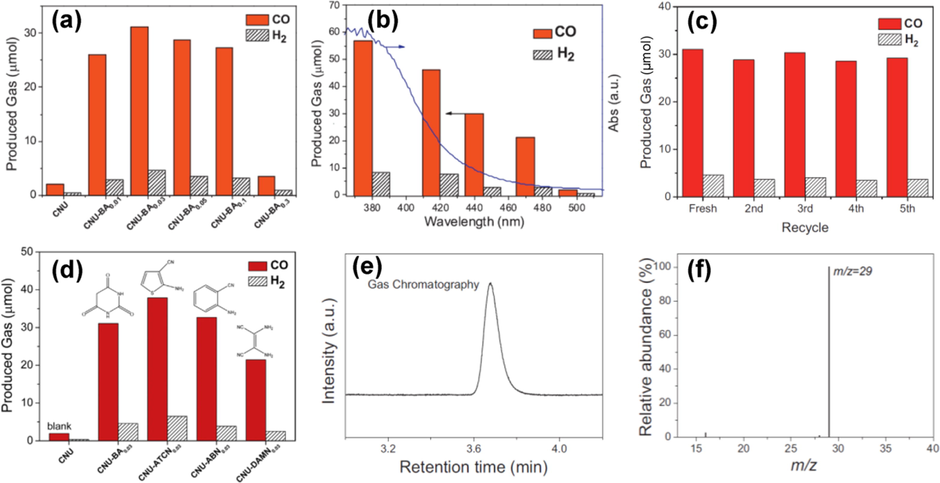
(a) Production of CO and H2 from the photocatalytic CO2 reduction employing CNU-BAx with varying quantities of barbituric acid embedded g-C3N4. (b) Wavelength based production photocatalytic CO2 reduction. (c) Durability analysis of the generation of CO and H2 ove CNU-BA0.03 material. (d) Influence of several monomers integrated g-C3N4 on the synthesis of CO and H2 under illumination. (e–f) GC–MS findings of the produced CO from the reduction reaction of 13CO2 over the CNU-BA0.03 material. Reprinted with permission from Qin (2015). Copyright © 2015, Elsevier.
The integration of metals with g-C3N4 is also an intriguing technique for the creation of noble metal-based g-C3N4 heterostructure materials for efficient CO2 reduction to sustainable compounds. Lately, a researcher team has showed an improved conversion of CO2 to CH4 using Pt integrated g-C3N4 composites, that has been synthesized via a chemical reduction technique in the solvent of ethylene glycol (Ong, 2014). This research enabled the photocatalytic reaction extra competitively possible and operationally practicable by employing a low-power of 15 W energy-saving daylight bulb as the illumination. As indicated in Fig. 36A–B, the photocatalytic CH4 generation over the 2Pt/g-C3N4 with an ideal 2 wt% level of Pt can be obtained up to 13.02 μmol/g1 after 10 h of solar illumination, that was 5.1 times larger than the pristine g-C3N4 (2.55 μmol/g1). Therefore, the considerably enhanced photoactivity of Pt/g-C3N4 was attributed to the enhanced optical absorbance and remarkable surface electron transport from g-C3N4 to Pt owing to its reduced Fermi level to restrict the replication of electrons/hole pairs (Fig. 36C). Correspondingly to another prior report by Yu and team utilized Pt/g-C3N4 mixture photocatalysts for the reduction of CO2 under visible illumination (Yu, 2014). With the ideal amount of Pt, CO2 was decreased to potential hydrocarbon products, i.e., CH4, CH3OH and HCHO in the liquid–liquid interactions (Fig. 36D). Following the use of Pt, the alternating voltage was drastically lowered, thereby enabling the electron transport the photoreduction of CO2. Table. 1 compares the performance of different g-C3N4 photocatalysts for photocatalytic CO2 reduction and H2 production.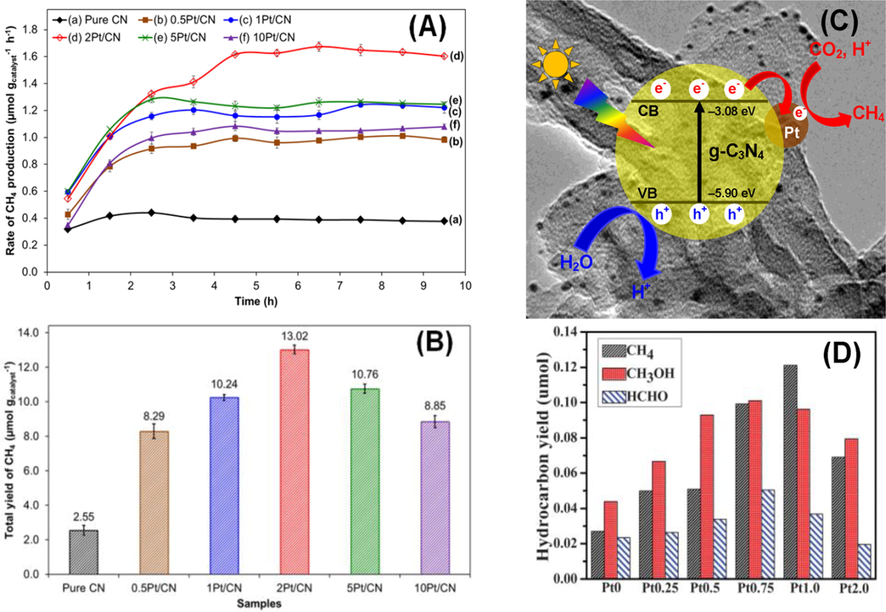
(A) Photoreduction time function, (B) overall CH4 output over g-C3N4 and Pt/g-C3N4 catalysts for 10 h of operation. (C) Charge transport process for CO2 reduction using H2O to CH4 using Pt/g-C3N4 composite. Reprinted with permission from Ong (2014). (D) Output rate of hydrocarbons with regard to various integration of Pt (0–2.0 wt%) coated on the g-C3N4 nanostructure under visible light for 4 h of operation. Reprinted with permission from Ref. (Yu, 2014).
Copyright © 2015, ROYAL SOCIETY OF CHEMISTRY.Copyright © 2014, ROYAL SOCIETY OF CHEMISTRY.
Serial No.
Photocatalysts
Co-Catalysts (Loading)
Experimental details
H2/CO2 productivity/μmol·g−1·h−1
Enhancement Relative to Conventional g-C3N4
Refrences
1
g-C3N4 nanosheets
Pt (1 wt%)
10 vol% TEOA
full sunlight and λ > 400 nm1395
5.6
(Tong, 2015)
2
K-g-C3N4
Pt (0.5 wt%)
10 vol% TEOA
300 W Xe (λ > 400 nm1028
14
(Wu, 2014)
3
AuPd/g-C3N4
Au and Pd
10 vol% TEOA
300 W Xe (λ ≥ 400 nm)326
3.5
1.6(Han, 2015)
4
Cu2O@g-C3N4 core@shell
10 vol% TEOA
300 W Xe202.28
5.7
(Liu, 2015)
5
CdS/g-C3N4 core/shell
Pt (0.6 wt%)
0.35 M Na2S and 0.25 M Na2SO3 300 W Xe (λ ≥ 420 nm)
4152
(Ou, 2017)
6
MoS2/g-C3N4
N/A
10 vol% TEOA, 300 W Xe (λ > 400 nm
252
8
(Zhao, 2015)
7
CaIn2S4/g-C3N4
Pt (1 wt%)
0.5 M Na2S and 0.5 M Na2SO3 300 W Xe
102
3
(Jiang, 2015)
8
CdS nanorods/g-C3N4
NiS
10 vol% triethanolamine, 300 W Xe (λ ≥ 420 nm)
2563
1.6
(Yuan, 2015)
9
AgQCs/g-C3N4
Pt (1 wt%)
25 vol% methanol simulator AM 1.5 G
5.59
1.7
(Sridharan, 2015)
10
Thiourea and urea derived g-C3N4
300 W Xe/420 nm, 40 mg catalysts
Urea derived g-C3N4
CO: 0.56, CH3CHO: 0.44, CH4: 0.04
thiourea derived g-C3N4
CO: 0.36, CH3CHO: 0.26,
CH4 = 0.025N/A
(Wang, 2016)
11
Melamine & urea derived g-C3N4
300 W Xe (420 nm), 0.2 g and 1.0 M NaOH
solution (100 mL)Urea derived g-C3N4
CH3OH: 6.28, C2H5OH:
4.51, O2: 21.33
melamine derived g-C3N4
CH3OH: TRACE,
C2H5OH: 3.64, O2: 10.29N/A
(Mao, 2013)
12
Sulfur-doped g-C3N4
300 W simulated solar Xe and 200 mL Pyrex
reactor, 100 mg 1 wt% Pt co-catalyst, 0.12 g
NaHCO3 and 0.25 mL 4 M HCl solutionCH3OH: 0.37
1.37
(Wang, 2015)
13
Pt-loaded g-C3N4
15 W energy-saving daylight bulb, flow rate of CO2 fixed at 5 mL·min−1
CH4: 1.3
5.2
(Ong, 2015)
14
Ag3PO4/g-C3N4
500 W Xe/420 nm, stainless-steel reactor 132 mL,
10 mg in 4 mL H2O, 0.4 MPa CO2 at 80 °CCO: 44, CH3OH: 9, CH4:
0.2, C2H5OH: 0.1CO: 11
(He, 2015)
15
BiOI/g-C3N4
300 W Xe (400 nm), 0.10 g catalyst, CO2 bubbled
through waterCO: 3.58, O2: 1.96, H2: 0.4, CH4: 0.2
CO: 17.9
(Wang, 2016)
16
CeO2/g-C3N4
300 W Xe, reactor volume 500 mL, 50 mg catalyst, CO2 bubbled through water
2 wt%
CO: 11.8 and CH4: 9.08
3 wt%
CO: 10.16 and CH4: 13.88CH4: 69.4
(Li, 2016)
17
g-C3N4/NaNbO3
300 W Xe, reaction volume 230 mL, 50 mg catalyst, reactor purged with CO2, then 2
mL H2O injectedCH4: 6.4
8
(Shi, 2014)
3.3 Environmental remediation
Polluted water is being discharged into the aquatic environment by several large-scale petrochemical, textile, and food sectors (Yuan, 2015). The use of organic dyes are often is being oftently observed in the printing, textile, as well as photography sectors. A significant amount of such colors is released into the effluent wastewater streams during the dying process. These colors are toxic to people and animals even at low concentrations (Jiang, 2015; Yuan, et al., 2014). As a result, modern oxidation methods are being developed in order to treat the contaminated drinking water and wastewater containing hazardous or non-biodegradable compounds (Sridharan, 2015; Li, 2015). The photocatalysis via semiconductors (Hou, 2013; Wu, 2014; Wen, 2015; Pany and Parida, 2015), is regarded as a low-cost, high-efficiency technique towards efficient removal of refractory organic/inorganic compounds from wastewater. The procedure of photocatalytic degrading of contaminants during light treatment on g-C3N4 is depicted in Fig. 37 (Tian, 2019). The principal active components in photocatalytic ecosystem cleaning are photoexcited holes, which are effective oxidants in and of themselves or combine with water in order to form hydroxyl radicals (•OH), those are strong oxidants having oxidation potential about (2.8 eV). (NHE). Adsorbed pollutants on photocatalyst surfaces or in the solution can be attacked by reactively generated OH, causing them to mineralize as water and CO2. The literature has broadly premeditated the organic pollutants photocatalytic oxidation in H2O (Chen, 2015; Zhang, 2015; Kamat, 2007).
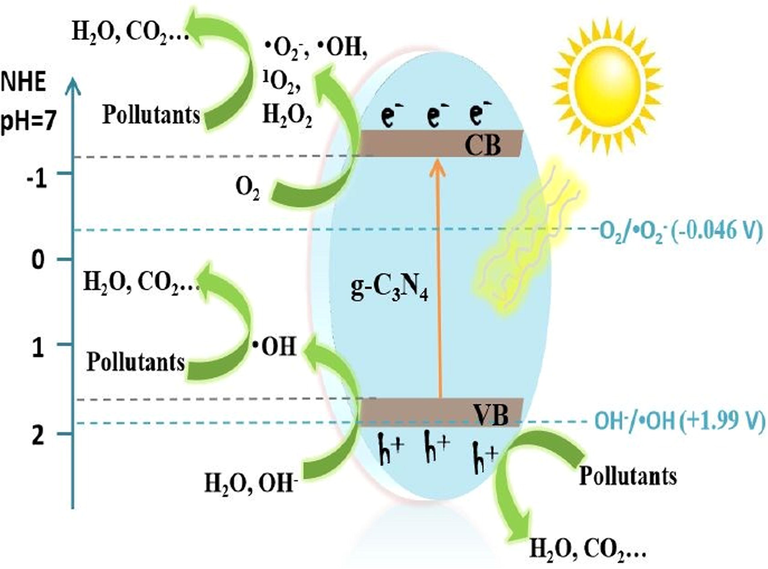
Scheme of photocatalytic degrading of different contaminants under solar illumination employing pristine g-C3N4. Reprinted with permission from Tian (2019).
Copyright © 2019, Royal Society of Chemistry.
Mineralization causing oxidants have been related to a number of reactive species, including OH, HO2, and H2O2, with •OH being the most probable candidate as illustrated in Eq. (22). The 'photo-Kolbe reaction,' in which photoexcited holes oxidize carboxylic acids, which directly produce the CO2, has also been shown. The VB and CB must be sited, so hydroxyl radicals E (NHE) and superoxide radicals E (NHE) oxidation and reduction potentials for semiconductor photocatalysts are well located within the band gap of materials. However, the requirement for photocatalysts thermodynamically dictate that the hydroxyl radicals E (NHE) and superoxide radicals E (NHE) oxidation and reduction potentials are well located within the band gap. To put it another way, holes must have a sufficiently positive redox potential to make OH• radicals, whereas photoexcited electrons must have a sufficiently negative redox potential to generate O2•. It has taken a long way to synthesize photocatalysts for water purification under the sunlight. The g-C3N4 materials physical properties are modified by doping some heteroatom and synthesizing heterojunction with the aim of increasing its surface area and porosity, thereby, making nanostructured based g-C3N4 as probable photo-catalysts for a wide variety of pollutants (Kumar, 2012; Yu et al., 2015). The ultrathin g-C3N4 nanosheets fabricated through methanol exfoliation from bulk g-C3N4 showed better photocatalytic activity towards methylene blue (MB) degradation (Kuriki, 2015). Under visible light, g-C3N4 nanotubes have a higher photoactivity towards MB degradation as compared to P25 or pristine g-C3N4 (Walsh, 2016). Tahir and colleagues discovered that tubular g-C3N4 displayed improved stability and activity than bulk g-C3N4 towards photocatalytic MB degradation and methyl orange (MO) under visible light due to larger surface area of (182 m2/g1), more excellent light absorption, and efficient separation/transfer of charges (Yu, 2014). Under visible light (>420 nm) and imitation sun light (>290 nm), one-dimensional g-C3N4 nanorods of varied ratios were studied for MB degradation (Gao, 2016). The g-C3N4 nanorods had 1.5–2.0 times greater photocatalytic activity and photocurrent responsiveness of g-C3N4 nanoplates. Aother effective but a facile chemical approach was employed in order to synthesize the nanofiber-like g-C3N4, which showed a probable photodegradation performance during Rhodamine B (RhB) degradation (Wang, 2015). One typical technique in order to drive the photo-generated charge carriers is the use of g-C3N4 doping to increase spectrum utilization and band alignment. In environmental pollution applications, metals like Fe and Cu (Fu, 2017; Mao, 2013; Shi, 2014), non-metal such as C, B, S or O (He, 2015; Li, 2015; Lin et al., 2014; Xia, 2017; Han, 2017), and Co doping (Wang, 2017; Di, 2017; Feng, 2017) all have been used. For the comparison purpose, with primeval g-C3N4 nanosheet, the O and S co-doped g-C3N4 were synthesized via melamine polymerization process followed by H2O2 activation subsequent to the functionalization of trithiocyanuric acid (Fig. 38a) which brought about the significant increased in the photo-catalytic degrading of RhB (Fig. 38b) up to 6-fold (You, 2017). It is due to the incremental increase in the total number of surface active sites, possibly leads to the enhanced separation and ultimate transfer of photo-generated electrons and holes, which in turn results into the high degree of delocalization in the HOMO and LUMO.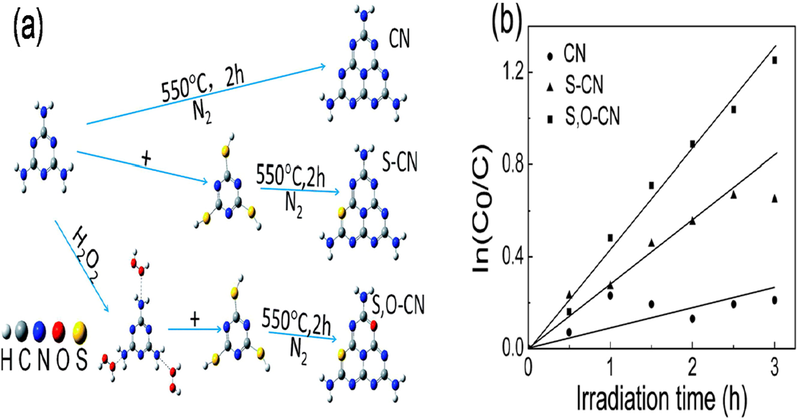
(a) Fabrication process, and (b) O and S co-doped g-C3N4 phtocaalytic performamce towards RhB degradation. Reprinted with permission from You (2017).
Copyright © 2017, RSC Publishing.
Similarly, a popular antibiotic photocatalytic degradation namely tetracycline chloride, whose uncontrolled release cause problem was examined (Li, 2017; Brini, 2021; Brini, 2021). The process containing the photodeposition of (7–15) nm of (Au and Pt) nanoparticles over g-C3N4 surface sites are good examples. The surface plasmon resonance of Au increases the optical adsorption range, but Pt sinks the photoexcited electrons. The multicomponent heterostructures like Ag3PO4/g-C3N4 for MO degradation (Huang, 2015; Niu, 2014) are viable for environmental pollution (Wang, 2016; Ong, 2015; Ni, 2016; Huang, 2015; Essekri, 2021). The coupling of g-C3N4 and metals provide adjustable heterojunctions with excellent charge transfer than typical nanocomposites (Wang, 2016; Li, 2016; Raziq, 2017; Maeda, 2014; Ong, 2016; Zhang, 2016; Wang, 2016), and multicomponent heterostructures like Ag3PO4/g-C3N4 systems for MO degradation (Ong, 2015; Zhou, 2014; Wu, 2017). The composite such as Ag3PO4/g-C3N4 core–shell converted MB at a rate of 97% in 30 min, compared to 79% Ag3PO4 and g-C3N4 components physical mixture and 69% untainted Ag3PO4. Actually, the g-C3N4 shell may make the composite more stable by preventing Ag3PO4 from dissolving. The core–shell g-C3N4@TiO2 were seven times more photoactive than pristine g-C3N4/TiO2, which was prepared via in situ sol–gel re-assembly pathway and then utilized to remove phenol during light source. Photo-degradation rate constant was fund to be enhanced from (0.0018–0.0386 h1) by increasing the thickness of g-C3N4 shell from (0 to 1 nm). However the bigger shells impeded transport of charge towards external surface of photocatalyst, and ultimately decreasing the overall performance. A simple, environmentally friendly, and low-cost ultrasonic dispersion approach was used in order to efficiently construct a Z-scheme N-doped g-C3N4/ZnO core–shell nanomaterials (Fig. 39a, b). The thickness of the g-C3N4 shell can be adjusted with g-C3N4. A novel energy level was generated into the N-doped ZnO band gap by creating straight interaction between g-C3N4 shell and N-doped ZnO core by significantly reducing it. Therefore, these such kind of composite based core–shell demonstrated a considerably higher visible-light photocatalytic efficiency towards RhB deterioration than simple g-C3N4 and N-doped ZnO counterparts (Fig. 39a, c) (Kumar, 2014). Magnetically detachable g-C3N4/Fe3O4 hybrid composite were made via simple, repeatable, template-free synthesis (Fig. 39b, a) (Kumar, 2014). The mono-dispersed Fe3O4 nanoparticles with an (8 nm) diameter were consistently produced on g-C3N4 sheets, displaying better charge separation and photocatalytic performance for RhB degradation (Fig. 39b, c). After six recycles, the photocatalytic activity of these g-C3N4/Fe3O4 nanocomposites remained unchanged, permitting magnetic catalyst recovery (Fig. 39b, d) (Kumar, 2013). The Xiao et. al. demonstrated that during organic pollutants presnece, the g-C3N4 photocatalytic oxidation stability is due to the last intense of hydroxyl radicals in normal conditions, thereby, ensuing into the preferential decomposition of the pollutants over g-C3N4 (Maeda et al., 2013).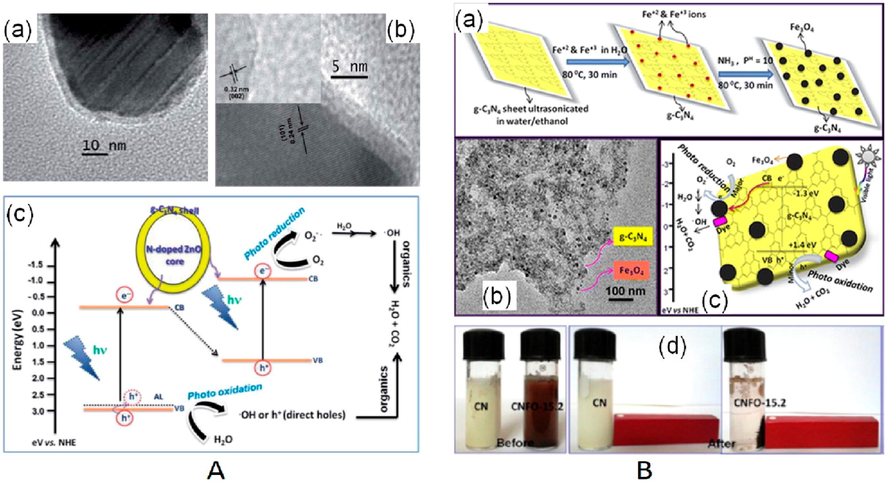
(a, b) As prepared N-ZnO-g-C3N4 core–shell nanoplates TEM pictures, along with (c) Z-scheme mechanism. Reprinted with permission from Kumar (2014). (B) (a) fabrication approach, (b) TEM photograph, and (c) g-C3N4–Fe3O4 nanocomposite based photodegradation mechanism along with (d) photocatalyst post-reactions magnetic separation. Reprinted with permission from Kumar (2013).
Copyright © 2014, RSC publishing.Copyright © 2013, American Chemical Society.
Similarly, Li (2018) devoted greater emphasis on addressing two interfaces of 2D layer g-C3N4, including the disorganized of electron transport and inefficient intercellular electrons propagation. Researchers created interior van der Waals heterojunction (IVDWHj) inside g-C3N4 using a straightforward synthetic method, greatly increasing the interfacial coulomb association and allowing the axially directed charged dissociation (Fig. 40a, b). The resulting g-C3N4 containing IVDWHs showed an improved protracted photocatalytic performance, with over 100 percent augmentation of NO effectiveness comparison with pristine g-C3N4 (Fig. 40c, d). NO purifying over CN premised materials is performed by NO oxidation to NO2− and NO3− by photoinduced superoxide radicals, including •O2– and •OH. The improved •O2– and •OH levels (Fig. 40e, f) clearly demonstrate the accelerated electrons activation and charge transport in O, K− integrated CN.
Graphical depiction of the interior van der Waals heterojunction (IVDWHj) (a) “cake Framework” configuration of OCN-K-CN; (b) Band design of the OCN-K-CN IVDWH; (c, d) Photocatalytic performance contrast of CN and OCN-K-CN against NO purifying and (c) stability assess of OCNK-CN (d); (e, f) DMPO ESR peaks of CN and OCNKCN in dark/light region (λ ≥ 420 nm). Reprinted with permission from Li (2018).
Copyright © 2018, American Chemical Society.
3.4 g-C3N4-based 3D hydrogels for waste water treatment
Direct discharge of wastewater containing organic contaminants is particularly hazardous for environment and human health (Zubair, 2021; Liang, 2019). These organic contaminants can be removed by absorption using porous materials (Tan, 2015), but degradation of these organic contaminants using photocatalyst (Hao, 2018), could help to solve this problem (Huang, 2021). In particular, polymeric hydrogels having high specific surface area might be effective contaminant adsorbents. To enhance the waste water treatment performance, g-C3N4-based hydrogels has shown to be great approach for degradation of organic contaminants photcatalyically. It is because photocatalyst-based hydrogels have greater ultra violet and light response rate, producing active species to convert contaminants into CO2 and water, but they have greater porous structure, large pore volume and surface area, enabling for effective contaminant adsorption. Self-assembled g-C3N4/graphene type and polymeric g-C3N4-based hydrogels are utilized for the photodegradation of hazardous organic and inorganic contaminants in wastewater. The 3D CNGA porous framework enhanced light absorption and boost the photoexcited electrons transfer from g-C3N4 to the surface of GO through interconnected pores (Tong, 2015; Hao, 2021). Furthermore, organic pollutants with high adsorption capacity (32 mgmg/1 for methyl orange (MO) dye) implies the availability of numerous active sites, so kinetically advantageous for photocatalytic reactions on surface. The degradation efficiency was significantly reduced after adding methanol and EDTA in a photocatalytic mechanism test, demonstrating that CO2@ and COH are species involve in methyl orange degradation utilizing CNGA materials (Fig. 41a).
(a) Degradation of MO dye with CNGA in the presence of scavengers and inset showing the cyclic test of CNGA, (b) Highly selective dye adsorption toward MB, azure B, acriflavine, safranin O, RhB, eosin Y, and MO using h-CN. Inset of (b) shows photographs of dyes. Reprinted with permission from Tong (2015).
Copyright © 2015, American Chemical Society.
Wu and colleagues found that g-C3N4/GO based aerogel can degrade MO and MB (20 mgL@1) >90% in just half an hour under solar light and active CO2 @ species were main element for MO degradation in oxygen environment (O2 + e@!CO2 @) (Tang, 2017). Zhang et. al. discovered self-assembled g-C3N4 hydrogel exhibited incredibly preferential dye adsorption due to its large surface area (190 m2 g−1) and induced by high negative or positive charge character (Zhang, 2016). Furthermore, the negative zeta potential of self-assembled hydrogels (h-CN), have strong electrostatic π-π interaction among self-assembled hydrogels and dye molecules having distinct charge states, determine the extraordinary capacity for photocatalytic reaction and selective adsorption of h-CN system (Fig. 41b). Shalom and team also demonstrated the adsorption of cationic dyes for MB using self-standing h-CN (Sun, 2017). The fact that the adsorbate and h-CN have strong chemical bonds suggests that dye adsorption on hydrogel is important for its removal as consistent with previous study. In addition, as a hybrid hydrogel composite comprising conductive material (PANI), synthetic polymer (PA) and biopolymer (Agar) were synthesized with g-C3N4 (Zhang, 2016; Jiang, 2016; Lamkhao and Randorn, 2017). Zhu and colleagues demonstrate a direct co-condensation process for the synthesis of g-C3N4/agar hydrogel with easy transfer of mass for adsorption and decontamination (Zhang, 2016). A PANI/h-CN having 3D framework demonstrates suitable HOMO and LUMO for good transmission of photoinduced electrons/holes pair, leading to increased the photocatalytic performance (Jiang, 2016). Zhu and colleagues discovered that a g-C3N4/SiO2 hybrid hydrogel had adsorption capabilities of 3.5 and 48 mgg@1 for phenol and MB, respectively (Zhang, 2016). The zeta potential of the SiO2 hydrogel got more negative as the g-C3N4 content increased, but the adsorption ability for organic contaminants don’t have any effect. Zhang and co-workers discover the idea that the zeta potentials and surface charge natures of g-C3N4/SiO2 hydrogels alter their adsorption abilities, especially for MB and phenol adsorption (Zhang, 2016). Among all, the coupling of BiOBr or Cu2O can result in more effective charge separation for g-C3N4-based hydrogels (Yu, 2017; Yan, 2017). The photocatalytic performance of these oxide loaded g-C3N4 based hydrogels depend on two factors. First one is of good surface area due to 3D porous structure, which allow for multiple surface-active sites, while the other is the p–n heterostructure interface among g-C3N4 and oxide materials, which allows for excellent separation of charges (Fig. 42). The performance of oxide loaded g-C3N4 hydrogel is increased by working together in both mechanisms.
Schemattic representation of heterostructure of the g-C3N4/GO hydrogel with (a) BiOBr. Reprinted with permission from Yu (2017). Copyright © 2017, Elsevier, and (b) Cu2O. Reprinted with permission from Yan (2017). Copyright © 2017, Elsevier.
3.5 Nitrogen photo-fixation
Nitrogen fixation is a revolutionary achievement by using g-C3N4 photocatalyst. Nitrogen could be transformed to ammonium, nitric acid, nitrous acid, and ammonia that are more valuable products than normal nitrogen. Fig. 43 depicts the nitrogen fixation procedure utilizing g-C3N4 as a photocatalyst (Ajiboye et al., 2020). In recent decades, photocatalytic N2 fixation technique has been attracted more attention in energy and fuel production for its easy reaction conditions, low utilization of energy, cheap, and sustainable method that relies solely on limitless clean sunlight energy (Chen, 2018; Hao, 2020). Especially metal-free g-C3N4 photocatalysts, having nitrogen vacancies and matched heterostructure surfaces, have attracted the attention of N2 fixation process (Li, 2018; Liang et al., 2017; Shi, 2018; Cao, 2018). Nitrogen vacancies are useful in chemical adsorption and activations of nitrogen, because the nitrogen atoms in dinitrogen have identical size and shape.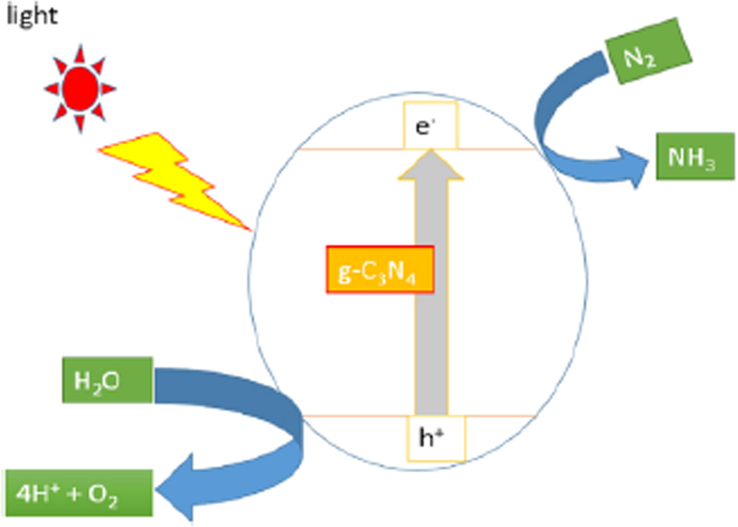
Nitrogen fixation using g-C3N4 mechanism. Reprinted with permission from Ajiboye et al. (2020). Copyright © 2020, Elsevier.
Dong and coworkers demonstrated integration of nitrogen vacancies within g-C3N4 framework that could photo reduced the nitrogen content and considerably improve isolation of charge carriers by capturing the photoexcited electrons and stimulating their transfer to the adsorbed nitrogen (Dong et al., 2015). Liu and coworkers demonstrated the fabrication of MXene derived TiO2@C/g-C3N4 heterostructure by calcination of melamine and Mxene Ti3C2Tx (Chen, 2015). The fabrication method yields carbon nanosheets (2D-Planer) supported on TiO2 with a high amount of Ti3+ entities, that are compactly enfolded by in situ synthesized g-C3N4 nanosheets. Surprisingly, as-prepared catalyst showed good photocatalytic performance for reduction of nitrogen to NH3 with an average rate of 250 mmol/g1 h1. Such high performance is owing to the presence of adequate surface defects, increased light capture, and remarkable charge separation and transfer ability of materials. For 2D graphene, Hu and coworkers created metal free 2D/2D nanocomposites of g-C3N4/rGO by connecting protonated g-C3N4 and rGO, in which reduced GO works as a conductive material (Hu, 2016). The as-prepared heterostructure produced five times more NH3 than bulk g-C3N4, owing to appropriate interaction of rGO and protonated g-C3N4 across the g-C3N4/rGO heterostructure, that effectively suppressed the radiative charge carrier recombination and improved charge separation and transfer ability. Other than 2D/2D heterostructure, multi metal oxides with tunable band structures suit well with g-C3N4 semiconductor photocatalyst through intact heterostructure. Wang and coworkers effectively demonstrated the fabrication of a g-C3N4/MgAlFeO nanorod heterostructure for excellent performance for nitrogen reduction (Wang, 2017). Similarly, in Fig. 44a, a Z scheme heterostructure was formed, in which the photoinduced electrons from the CB of MgAlFeO moved to VB of g-C3N4, leading in better separation of charge and higher interfacial charge transfer, resulting in strong N2 photofixation. Cao et. al. discovered a comparable Z-scheme heterostructure method for nitrogen photofixation, in which 3,4-dihydroxybenzaldehyde doped Ga2O3/g-C3N4 (Ga2O3-DBD/g-C3N4) was synthesized by introducing Ga2O3-DBD nanoparticles within the g-C3N4 structure through a simple post-grafting approach (Fig. 44b) (Cao, 2017). In the composites, the benzene ring act as a electron mediators that enhanced the separation of photogenerated charges by making well-matched interface among Ga2O3-DBD/g-C3N4 and making it easier to change nitrogen into ammonia with help of reducing ability of CO2 (Hao, 2021. 2021.), as seen in Fig. 44c. The preceding research effectively demonstrated that the metal free g-C3N4 photocatalyst with rational heterostructure fabrication can provide impressive performance towards ecofriendly nitrogen photofixation technology for global growth.
(a) g- C3N4/MgAlFeO possible mechanism, (b) The synthesis scheme and, (c) photocatalytic N2 reduction mechanism of Ga2O3-DBD/g-C3N4. Reprinted with permission from Cao (2017). Copyright © 2017, Elsevier.
3.6 Photoelectronic application
The addition of nitrogen into the carbon matrix brought an improvement in its electrical, structural, and mechanical properties, particularly its electronic properties, which in turn makes it a potential candidate to be used in rechargeable batteries (He, 2015; Li, 2017; Reli, 2016; Ohno, 2014; Hao, 2021), light-emitting devices (He, 2015; Zhao, 2017), fuel cells (Li, 2015; Bai, 2016; Li, 2017; Liu, 2017; Ye, 2016), solar cells, and other applications (Shi, 2015; Bai, 2016). Moreover, the g-C3N4 has been regarded as a most promising candidate in the overhead application domains. The sp2 hybridization of nitrogen and carbon results in forming a π-conjugated electronic structure, which has an excellent photoelectronic characteristic (Richardson and Ternes, 2014).
3.6.1 Light-emitting devices
Due to its semiconductor features, the researchers are now concentrating onto the luminescence of g-C3N4 (Tyborski, 2013). In general, the optical band gap of g-C3N4 dominates the photoluminescence area, and it has been shown that the band gap of g-C3N4 is tunable by adjusting the processing temperature. The optical band gap and synthesized temperature are thought to be efficiently connected in the tectonic unit of g-C3N4, as shown in Fig. 45 (Tyborski, 2012).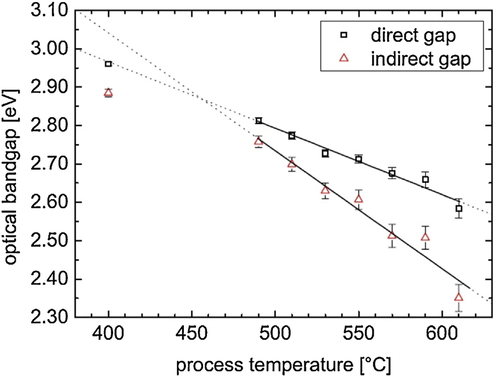
Values of direct (squares) and indirect bandgaps (triangles) for carbon nitride powders processed at different temperatures. The solid black lines denote linear fits in the range between 490 and 610 ◦C, the dotted lines being extrapolations. Reprinted with permission from Tyborski (2012).
Copyright © 1989, IOP Publishing.
The photoluminescence functionality of g-C3N4 was explored in depth by Dong's group. As shown in Fig. 46 (Zhang, 2013), the resultant emission area of g-C3N4 ranging from 400 nm to 510 nm, due to constant modulation of the processing temperature. On the basis of two-dimensional conjugated polymeric system and the lone pair of g-C3N4, a promising energy level mechanism diagram has been proposed, thereby, concluding that the narrow band gap was primarily instigated through the advancement of the conjugated polymeric structure and electron delocalization.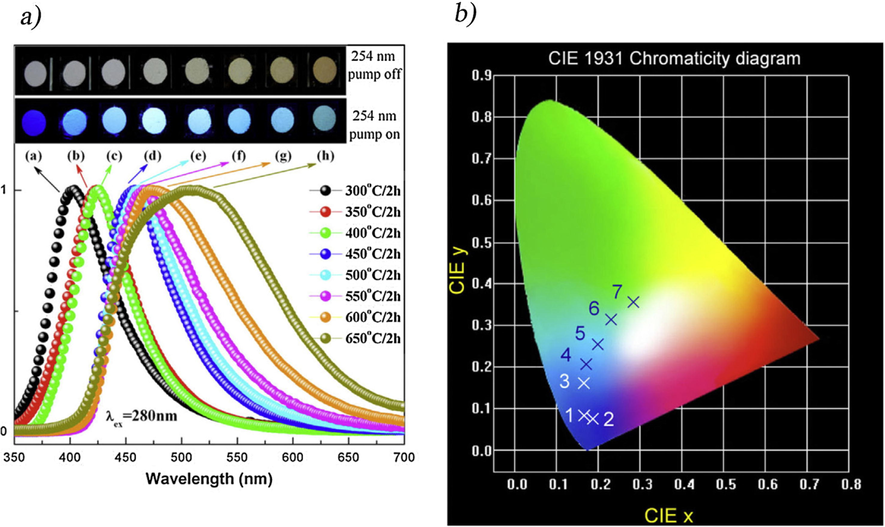
(a) The normalized PL emission spectra of the g-C3N4 products were synthesized via melamine's thermal condensation at different temperatures for 2 h (ex = 280 nm). The inset shows the digital photos of g-C3N4 products with (bottom) and without (upper) 254 nm excitation; (b) The Commission International de L’Eclairage (CIE) chromaticity diagram of the carbon nitride products synthesized at different temperatures for 2 h. (1) 350 ◦C, (2) 400 ◦C, (3) 450 ◦C, (4) 500 ◦C, (5) 550 ◦C, (6) 600 ◦C, (7) 650 ◦C. Reprinted with permission from Zhang (2013).
Copyright © 2013, Springer Nature.
Chen et. al. lately stated the ECL of g-C3N4 nanoflake films. The substantial ECL performance with (435 nm) blue light emission was estimated, under reductive-oxidative co-reactants environment (Jo, 2017). This is noteworthy that the ultrathin (g-C3N4) nanosheets might be fabricated via exfoliating bulk g-C3N4, and its PL quantum vintage could be high about 19.6% (Li et al., 2016). The optical characteristics and ECL activity of g-C3N4 demonstrate a superior performance in light-emitting devices. In light-emitting devices, the fluorescence powers of commercial nitrides via doped with transition ions or rare-earth ions are commonly employed. Although their luminescence qualities and quantum efficiency are good, metal toxicity, standard synthesis parameters, and their limit utilization (Tonda, 2014; Gao, 2015; Essekri, 2020). The Reyes et. al. further employed amorphous-CNx as a light-emitting material in order to develop a light maneuver, that exhibited a white–blue electro-luminescence at ambient temperature, which is presented in Fig. 47 (Yan et al., 2010; Ji, 2017). According to their investigations on the white light-emitting device, the C-N link of amorphous g-C3N4 emits green and blue light, whereas the C-H bond emits red light. However, it has been further proposed that g-C3N4 might be employed as a semiconductor layer in light-emitting devices, since it has a similar emission area to amorphous-CNx.
Emitting light device configuration (Ji, 2017).
3.6.2 Photocathodes
Semiconductor materials used in photoelectrochemical cells (PEC) for solar energy conversion into value-added chemicals are vital in developing sustainable energy to address current energy issues. The photoanodes for PEC water oxidation by employing n-type semiconductors i.e., WO3, Fe2O3, ZnO, and TiO2, are extensively investigated. Furthermore, few investigations have been conducted on the use of p-type semiconductors as photocathodes for photoelectrochemical cell reduction of water. The g-C3N4 is considered as a possible semiconductor for photocathodes, because of its high chemical durability and unusual electrical structure. The Zhang et. al. integrated active carbon sites to fabricate a long-lasting photocathode with three times more cathodic PEC performance as compared to pristine g-C3N4 (Panneri, 2017). Carbon inclusion is anticipated to significantly impact the electrical characteristcis of the g-C3N4 structure via the following mechanisms: (1) Charge separation efficiency is demonstrated by a decrease in photoemission intensity, which enhances charge mobility; (2) the π-conjugated framework is improved. Both of these elements help to increase the mobility of the charge. Furthermore, the nanostructure influences the free carrier diffusion, mass transfer, and active sites, impacting PEC efficiency. Fig. 48 depicting the differences in PEC activity amid pure and carbon-doped g-C3N4 (Zhang, 2013). The PEC activity is projected to increase as electrical structures, change-carrier mobility, and surface area are all balanced.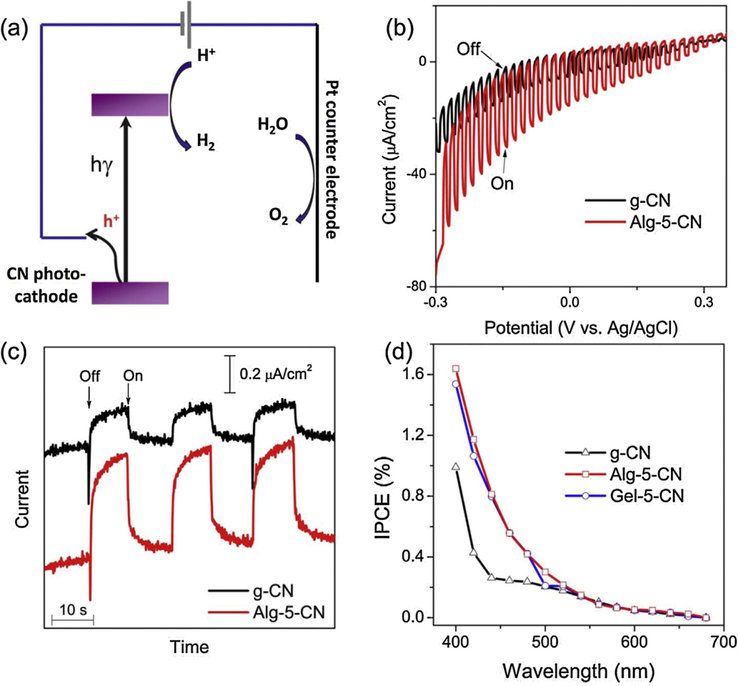
Photoelectrochemical (PEC) properties of carbon nitrides in 0.1 M KCl aqueous solution. (a) The PEC cell configuration and possible charge transfer (the reference electrode was omitted for clarity). (b) Characteristic photocurrents at g-CN (black) and Alg-5-CN (red) photocathode under a chopped sunlight simulator at a scan rate of 10 mV/s. (c) The transient current response of g-CN (black) and Alg-5-CN (red) at −0.2 V vs Ag/AgCl (sat. KCl) under chopped monochromatic light at 440 nm. (d) Photo action spectra of g-CN (black), Alg-5-CN (red), and Gel-5-CN (blue) biased at −0.2 V vs Ag/AgCl (sat. KCl). Reprinted with permission from Zhang (2013).
Copyright © 2013, Springer Nature.
3.6.3 Optical sensors
Optical sensor systems have attracted much research interest, because of their precise and reasonable detection limit and guaranteed biocompatibility and flexibility. Usually, the optical sensor is a molecule receptor with variable optical capabilities based on the specific visitors (Dai, 2013). Presently, fluorescent receptors are commonly used as optical sensors for detections by the interaction of fluorescent with adherent guests. The optical response is dominated by the transport of electrons from receptors to their binding guests. It is well known that g-C3N4 is an excellent catalyst to substantially absorb metal ions via chelation or redox reaction, since it has functions such as NH2/NH/N over surface site. Despite many sensors with an optical receptor attaches to permeable materials, the g-C3N4 is the receptor itself and has excellent sensitivity. Lee et. al. investigated the possibility of using g-C3N4 as an optical sensor to check the presence of metal ions in an aqueous solution by examining its photoluminescence quenching effects (You, 2017; Dai, 2013). The findings in Fig. 49 reveal that g-C3N4 has good Cu2+ sensitivity. The Cu2+ entirely quenches photoluminescence thru no visible interfering from other metals, because photo-generated electrons are collected by Cu2+ (Lee, 2010). Furthermore, the Stern–Volmer equation may be used in order to quantify the degree of quenching: I0/I = 1 + KSV[Q], where I0 and I indicate the luminescence intensity in the absence and presence of metal ions, respectively. The Stern–Volmer constant (You, 2017) is denoted by [Q], which is molar concentration of the metal ion. This comparation depicts a roughly linear relationship between the effect of Cu2+ concentration on g-C3N4 photoluminescence.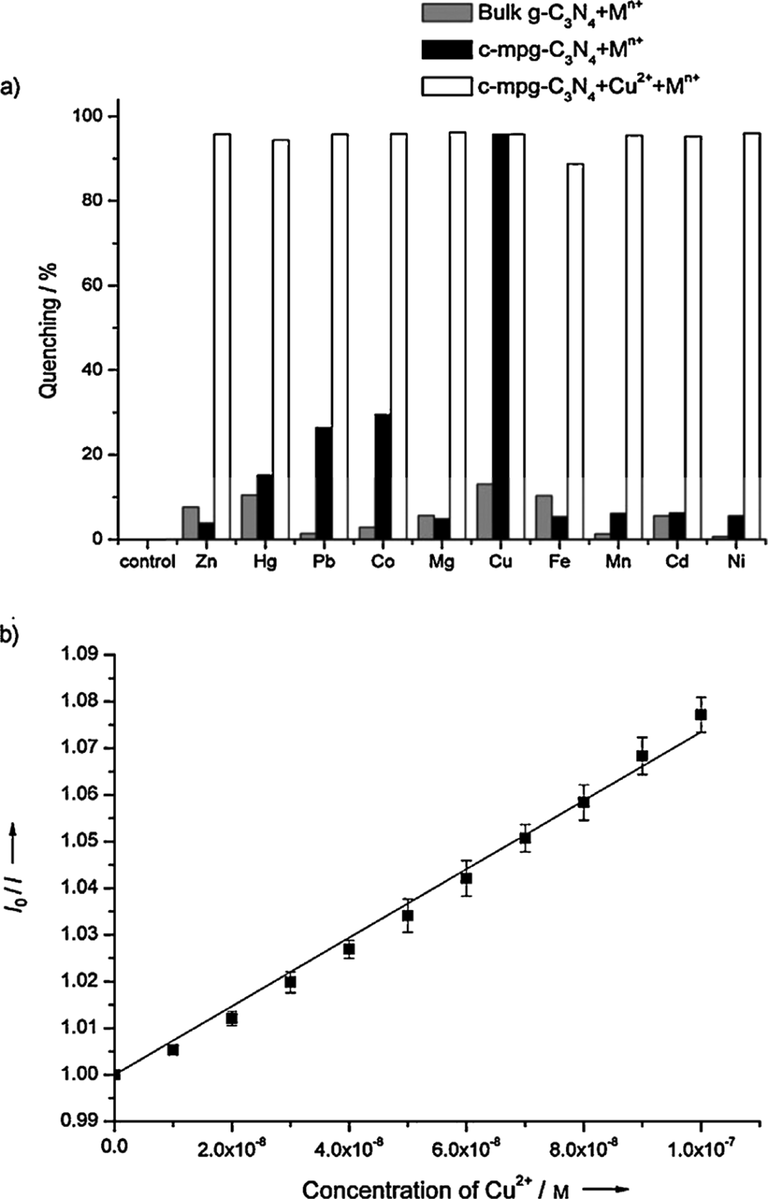
(a) PL spectra of both c-mpg-C3N4 (black bar) and bulk (gray bar) after 1 mm of metal ion solutions treatment, and (1 mm) of other metal ions intrusion with (1 mm) Cu2+ (white bar); (b) PL spectra of c-mpg-C3N4 to Cu2+ in 10–100 nm concentration range. Reprinted with permission from Lee (2010).
Copyright © 2010 John Wiley and Sons.
3.7 Bacterial disinfection
It is critical for human health to remove pathogenic bacteria from water. Although earlier disinfection processes such as chlorination and ozonation were highly successful for inactivation, as they frequently produced hazardous disinfection by-products having carcinogenic and mutagenic potential (Muellner, 2007; He, 2016; Malik, 2021). In 1985, the Matsunaga et. al. (Matsunaga, 1985), were the first to demonstrate photocatalytic sterilization of microbial cells in water. The first time Huang et. al. found the bactericidal effects of g-C3N4 on Escherichia coli (E.coli) K-12 in water, when irradiated with visible light (Huang et al., 2014). Mechanistic illustration showed that, the light-induced holes over g-C3N4 surface sites were shown to be a major source of the inactivation of bacteria. As a result, using a hole scavenger like OH had minimal influence on the inactivation efficiency (Huang et al., 2014). The use of single layer g-C3N4 was invetigated by Zhao et. al. in order to examined the photocatalytic disinfection of E. coli in visible-light (Zhao, 2014). The Fig. 50a displays the disinfection activity of several photocatalysts for E. coli, with single-layer g-C3N4 by providing the best results. As demonstrated in (Fig. 50b), adding isopropanol or Cr (VI) caused in killing around five log10 of E. coli cells in 4 h. Though, only about two log10 of E. coli cells were smashed, when KI was added. As a result of the findings above, the disinfection in visible light seems to be a hole oxidation process. The effects of disinfection on E Coli were investigated further using microstructure and morphology analysis earlier and afterward disinfection. Fig. 50c shows the E Coli cell wall, before photocatalytic disinfection, which is highly conserved. The cell wall of bacteria is destroyed after 12 h of irradiation, as seen in Fig. 50d, f.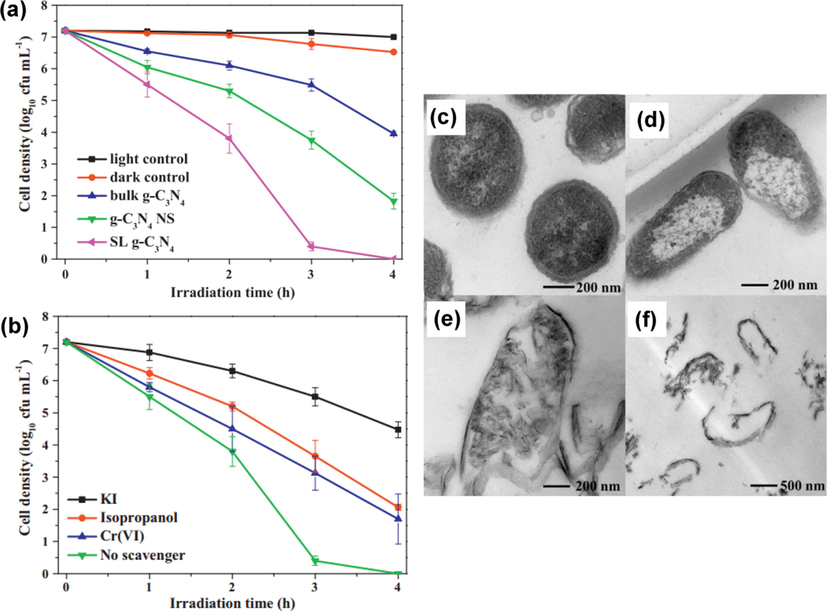
Photocatalytic disinfection performace of (a) various g-C3N4 and (b) single-layer g-C3N4 with diverse scavengers. g-C3N4 NS and SL g-C3N4 indicates g-C3N4 nanosheets and, single-layer g-C3N4, correspondingly. The TEM picture of E. coli before and after photocatalytic disinfection: (c) Before reaction and after irradiation for (d) 4 h, (e) 8 h, and (f) 12 h. Reprinted with permission from Zhao (2014). Copyright © 2014, Elsevier.
Wang et. al. developed a metal-free heterostructure of cow wrapping Sulfur (−S8) on rGO sheets and g-C3N4 for bacteria disinfection during visible light. They examined the disinfection of E. coli K-12 cells via two diverse heterostructures (Fig. 51a) and rGO was found between -S8 and g-C3N4 in the first structure (CNRGOS8), whereas g-C3N4 was found between -S8 and rGO in the second structure (CNRGOS8). The aforesaid synthesized heterostructures (CNRGOS8 and RGOCNS8) exhibit different photocatalytic performance towards E. coli K-12 in anaerobic and aerobic conditions (Fig. 51b, c). As shown in Fig. 51a, b, the CNRGOS8 has a more excellent photocatalytic activity for bacterial inactivation in an aerobic environment than RGOCNS8, whereas the RGOCNS8 works better in anaerobic situations (Fig. 51C). The photocatalytic route in an anaerobic environment was more reductive than the oxidation process. Therefore, such discovery unfastens inventive paths for studying the use of metal-free catalysts to improve bacterial photoinactivation effectiveness (Wang, 2013).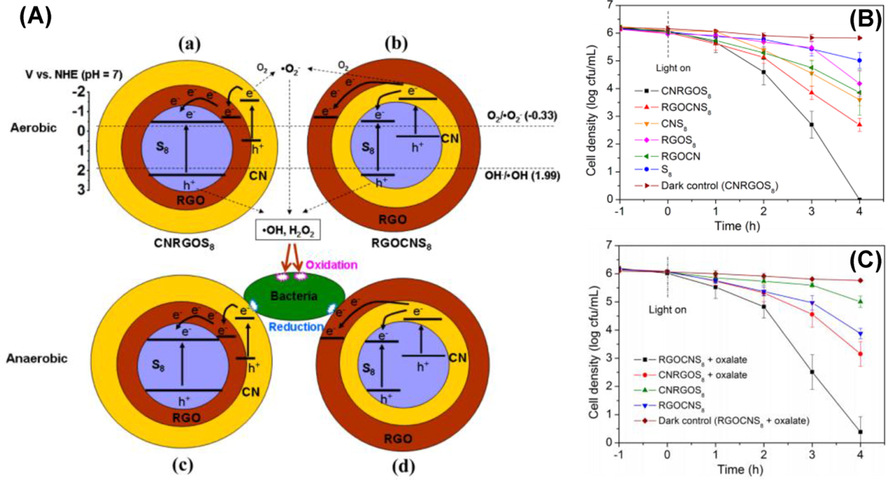
(A) Graphic represnetation of mechanism of photocatalytic bacterial inactivation (a) CNRGOS8 and (b) RGOCNS8 in aerobic and (c) CNRGOS8 and (d) anaerobic conditions of RGOCNS8. Photocatalytic inactivation activity vs E. Coli K-12 during as-prepared samples presence (B) aerobic and (C) anaerobic conditions under visible-light irradiation. Reprinted with permission from Wang (2013).
Copyright © 2013, American Chemical Society.
4 Future prospective
Despite tremendous advances in past years, the major challenges persist in rationally designed extremely effective g-C3N4-based photocatalyst in terms of the number of their uses and the full knowledge of the fundamental tailoring process of g-C3N4-based photocatalysts. Hence, several unresolved concerns and areas exist, where more research is needed. So far, deep studies are urgently needed to fully explore the structural and electrical characteristics of g-C3N4 composites. Conversely, the diverse features of g-C3N4-based photocatalysts also need to be thoroughly investigated. Owing to the challenges in developing extremely efficient and sustainable g-C3N4-based photocatalysts with decreased band gaps, the creation of low band gap conjugated polymers may yield additional concepts for speeding organic semiconductor-based photocatalysis advances. The g-C3N4 nanosheets further require accurate surface defect regulation and simple scale preparation techniques. Advanced photo-electrocatalysts must be employed to explore the applications of a g-C3N4-based catalysts for the scientific community. In constructing practical g-C3N4-based photocatalysts, dimension controll, pore-size modifications, heterostructure building, cocatalyst and catalyst loading, and nanocarbon loading appear to be the most promising design strategies. More research into this method is required at this time. In these photocatalytic areas, developing Earth-abundant co-catalysts is still a challenging task. Nanocarbons will forever play vital roles in the construction of highly effective g-C3N4-based photocatalysts. In addition, the uses of g-C3N4-based photocatalyst are primarily concentrated on H2 evolution and pollutant degradation. In contrast, during the photocatalytic CO2 reduction process, these catalysts have grown in popularity over the past few years. The fundamental character of the surface of g-C3N4 dictates its promising future in CO2 reduction fields. Moreover, study of OER from the water splitting half reaction, which involves both CO2 reduction and overall water splitting, should receive more attention in the coming years. Furthermore, the process's precise mechanism, especially CO2 reduction by g-C3N4-based catalyst, remains unclear and unexplored. Deep research in reaction pathways is mandatory for elucidating the fundamentals improvements of photocatalysis and designing effective g-C3N4-based photocatalysts in the future. Moreover, a few key concerns that are necessary for good photocatalytic activity, such as light absorption, charge separation and transfer, and band structure, must be thoroughly evaluated using both DFT and practical experiments to gain theoretical insights. Charge separation and transfer ability, substrate adsorption sites and molecular orbitals should all be comprehensively studied pertaining to experimental efforts. The collaborative efforts of researchers from many fields and countries should result in an exciting period for g-C3N4-based photocatalysts.
5 Conclusions
This review article mainly deals with recent progress in the improvement of photocatalytic performance of a wide variety of properly tuned highly active carbon based g-C3N4 photocatalysts in photocatalysis research field. However, due to electrical, optical, and mechanical properties, the g-C3N4 could be further tailored for solar energy use in environmental applications and other energy conversions. The g-C3N4 nanomaterials with their varying porosity and dimensionality and their incorporation in multi-functional nanocomposites, have brought about the significant enhancement in the solar light absorption, charge separation/transportation, quantum yields, and photocatalytic performance. At the same time, the main difficulty still being experienced is the lack of multidisciplinary actions, and the use of sacrificial reagents to improve the photoreactor design persists. The preliminary step in the photocatalytic reduction of CO2 is the utmost selection of the best specific product—either energy (e.g., CO, CH4, or formic acid) or chemicals (e.g., olefins or alkanes)—to improve the activity and the capacity as well. Although the quantitative comparisons are found to be difficult owing to the decoupling of adsorption from the reaction medium to the photocatalysis process as through photo-chemical stimulation of chromophores, the dearth of calibration in experimental protocols, and the design of the reactor during wastewater treatment using a g-C3N4-based photocatalyst appears to be remarkable.
Acknowledgments
We gratefully acknowledge the support of this research by funding from the Foundation of Yangtze Delta Region Institute (Huzhou) of UESTC, China, (Nos. U03210057). Authors acknowledge support and funding of King Khalid University through Research Center for Advanced Materials Science (RCAMS) under grant no: RCAMS/KKU/008/21.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hydrogen from photo-catalytic water splitting process: a review. Renew. Sustain. Energy Rev.. 2015;43:599-610.
- [Google Scholar]
- Solvent assisted synthesis of hierarchical magnesium oxide flowers for adsorption of phosphate and methyl orange: kinetic, isotherm, thermodynamic and removal mechanism. Surf. Interf.. 2021;23:100953.
- [Google Scholar]
- Graphitic carbon nitride-based catalysts and their applications: a review. Nano-Struct. Nano-Obj.. 2020;24:100577.
- [Google Scholar]
- Phosphate removal from aqueous solution using iron oxides: adsorption, desorption and regeneration characteristics. J. Colloid. Interf. Sci.. 2018;528:145-155.
- [Google Scholar]
- Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal. J. Environ. Manage.. 2020;253:109730.
- [Google Scholar]
- Use of nano-/micro-magnetite for abatement of cadmium and lead contamination. J. Environ. Manage.. 2020;264:110477.
- [Google Scholar]
- Prospects of photocatalysis in the management of nitrate contamination in potable water. In: Oladoja N.A., Unuabonah E.I., eds. Progress and Prospects in the Management of Oxyanion Polluted Aqua Systems. Cham.: Springer International Publishing; 2021. p. :185-217.
- [Google Scholar]
- Strategies for improving the efficiency of semiconductor metal oxide photocatalysis. Mater. Horiz 2014
- [Google Scholar]
- Selectivity, stability and reproducibility effect of Uric acid integrated carbon nitride for photocatalytic application. J. Photochem. Photobiol., A. 2022;423:113591.
- [Google Scholar]
- Semiconductor clusters, nanocrystals, and quantum dots. Science. 1996;271(5251):933-937.
- [Google Scholar]
- Photocatalytic hydrogenation of propyne with water on small-particle titania: size quantization effects and reaction intermediates. J. Phys. Chem.. 1987;91(16):4305-4310.
- [Google Scholar]
- CdSe quantum dots modified thiol functionalized g-C3N4: intimate interfacial charge transfer between 0D/2D nanostructure for visible light H2 evolution. Renew. Energy. 2020;158:431-443.
- [Google Scholar]
- Homogeneous iron-doped carbon-nitride-based organo-catalysts for sensational photocatalytic performance driven by visible light. Polym. Int.. 2021;70(9):1273-1281.
- [Google Scholar]
- Serendipitous assembly of mixed phase BiVO4 on B-doped g-C3N4: an appropriate p–n heterojunction for photocatalytic O2 evolution and Cr (VI) reduction. Inorg. Chem.. 2019;58(18):12480-12491.
- [Google Scholar]
- Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C. 2013;117(19):9952-9961.
- [Google Scholar]
- Size-dependent role of gold in g-C3N4/BiOBr/Au system for photocatalytic CO2 reduction and dye degradation. Sol. Energy Mater. Sol. Cells. 2016;157:406-414.
- [Google Scholar]
- g-C3N4/Bi4O5I2 heterojunction with I3−/I− redox mediator for enhanced photocatalytic CO2 conversion. Appl. Catal. B. 2016;194:98-104.
- [Google Scholar]
- Photocatalytic activity of g-C3N4 quantum dots in visible light: effect of physicochemical modifications. J. Phys. Chem. C. 2017;121(3):1982-1989.
- [Google Scholar]
- Why Dyes Should not be used to Test the Photocatalytic Activity of Semiconductor Oxides. ACS Publications; 2016.
- Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem.. 1979;10(1):59-75.
- [Google Scholar]
- Design of a unique energy-band structure and morphology in a carbon nitride photocatalyst for improved charge separation and hydrogen production. ACS Sustain. Chem. Eng.. 2018;6(1):519-530.
- [Google Scholar]
- The impact of nanoscience on heterogeneous catalysis. Science. 2003;299(5613):1688-1691.
- [Google Scholar]
- Synthesis and characterisation of PANI- coated Heliotrope Leaves (PANI@HL) with high clean-up capacity for Orange G dye from aqueous media. Int. J. Environ. Analyt. Chem. 2021:1-17.
- [Google Scholar]
- Synthesis and characterization of arginine-doped heliotrope leaves with high clean-up capacity for crystal violet dye from aqueous media. Water Sci. Technol.. 2021;84(9):2265-2277.
- [Google Scholar]
- In-situ growth of CdS quantum dots on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen generation under visible light irradiation. Int. J. Hydrogen Energy. 2013;38(3):1258-1266.
- [Google Scholar]
- All-solid-state Z-scheme 3, 4-dihydroxybenzaldehyde-functionalized Ga2O3/graphitic carbon nitride photocatalyst with aromatic rings as electron mediators for visible-light photocatalytic nitrogen fixation. Appl. Catal. B. 2017;218:600-610.
- [Google Scholar]
- Nitrogen photofixation by ultrathin amine-functionalized graphitic carbon nitride nanosheets as a gaseous product from thermal polymerization of urea. Appl. Catal. B. 2018;224:222-229.
- [Google Scholar]
- Photocatalytic activation of sulfite by nitrogen vacancy modified graphitic carbon nitride for efficient degradation of carbamazepine. Appl. Catal. B. 2019;241:18-27.
- [Google Scholar]
- Efficient visible-light-driven Z-scheme overall water splitting using a MgTa2O6− xNy/TaON heterostructure photocatalyst for H2 evolution. Angew. Chem.. 2015;127(29):8618-8621.
- [Google Scholar]
- Nitrogen-doped CeOx nanoparticles modified graphitic carbon nitride for enhanced photocatalytic hydrogen production. Green Chem.. 2015;17(1):509-517.
- [Google Scholar]
- Facile synthesis and enhanced photocatalytic H2-evolution performance of NiS2-modified g-C3N4 photocatalysts. Chin. J. Catal.. 2017;38(2):296-304.
- [Google Scholar]
- Photocatalytic fixation of nitrogen to ammonia: state-of-the-art advancements and future prospects. Mater. Horiz.. 2018;5(1):9-27.
- [Google Scholar]
- Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev.. 2007;107(7):2891-2959.
- [Google Scholar]
- Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J. Mater. Chem. A. 2015;3
- [Google Scholar]
- Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J. Mater. Chem. A. 2015;3(28):14487-14516.
- [Google Scholar]
- WO3/g-C3N4 composites: one-pot preparation and enhanced photocatalytic H2 production under visible-light irradiation. Nanotechnology. 2017;28(16):164002.
- [Google Scholar]
- Sustainability guardrails for energy scenarios of the global energy transition. Renew. Sustain. Energy Rev.. 2018;91:321-334.
- [Google Scholar]
- Supported gold nanoparticles from quantum dot to mesoscopic size scale: effect of electronic and structural properties on catalytic hydrogenation of conjugated functional groups. J. Am. Chem. Soc.. 2000;122(46):11430-11439.
- [Google Scholar]
- Constructing highly uniform onion-ring-like graphitic carbon nitride for efficient visible-light-driven photocatalytic hydrogen evolution. ACS Nano 2018 acsnano.8b01271
- [Google Scholar]
- Synthesis and characterization of graphitic carbon nitride sub-microspheres using microwave method under mild condition. Diam. Relat. Mater.. 2013;38:109-117.
- [Google Scholar]
- Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev.. 2004;104(1):293-346.
- [Google Scholar]
- Making metal carbon nitride heterojunctions for improved photocatalytic hydrogen evolution with visible light. ChemCatChem. 2010;2(7):834-838.
- [Google Scholar]
- Carbon-nanotube fibers for wearable devices and smart textiles. Adv. Mater.. 2016;28(47):10529-10538.
- [Google Scholar]
- A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J. Catal.. 2017;352:532-541.
- [Google Scholar]
- A fantastic graphitic carbon nitride (g-C3N4) material: electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol., C. 2014;20:33-50.
- [Google Scholar]
- A general method for type I and type II g-C3N4/g-C3N4 metal-free isotype heterostructures with enhanced visible light photocatalysis. New J. Chem.. 2015;39(6):4737-4744.
- [Google Scholar]
- Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B Environ. Int. J. Devot. Catal. Sci. Appl. 2016
- [Google Scholar]
- Carbon vacancy regulated photoreduction of NO to N2 over ultrathin g-C3N4 nanosheets. Appl. Catal. B. 2017;218:515-524.
- [Google Scholar]
- Selective photocatalytic N2 fixation dependent on g-C3N4 induced by nitrogen vacancies. J. Mater. Chem. A. 2015;3(46):23435-23441.
- [Google Scholar]
- Reactive oxygen species: New insights into photocatalytic pollutant degradation over g-C3N4/ZnSe nanocomposite. Appl. Surf. Sci.. 2020;532:147418.
- [Google Scholar]
- Enhanced adsorptive removal of crystal violet dye from aqueous media using citric acid modified red-seaweed: experimental study combined with RSM process optimization. J. Disper Sci. Technol. 2020:1-14.
- [Google Scholar]
- Novel citric acid-functionalized brown algae with a high removal efficiency of crystal violet dye from colored wastewaters: insights into equilibrium, adsorption mechanism, and reusability. Int. J. Phytorem.. 2021;23(4):336-346.
- [Google Scholar]
- Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B. 2016;185:225-232.
- [Google Scholar]
- Facile fabrication of large-aspect-ratio g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. ACS Sustain. Chem. Eng.. 2017;5(3):2039-2043.
- [Google Scholar]
- Three-dimensional titanium dioxide nanomaterials. Chem. Rev.. 2014;114(19):9487-9558.
- [Google Scholar]
- Artificial photosynthesis as a frontier technology for energy sustainability. Energy Environ. Sci.. 2013;6(4):1074-1076.
- [Google Scholar]
- Artificial Photosynthesis as a Frontier Technology for Energy Sustainability. Social Science Electronic Publishing; 2013.
- In situ preparation of Z-scheme MoO3/g-C3N4 composite with high performance in photocatalytic CO2 reduction and RhB degradation. J. Mater. Res.. 2017;32(19):3660-3668.
- [Google Scholar]
- Heterogeneous photocatalytic oxidation of cyanide and sulfite in aqueous solutions at semiconductor powders. J. Phys. Chem.. 1977;81(15):1484-1488.
- [Google Scholar]
- Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at titanium dioxide powder. Cheminform. 1977;8(14):303-304.
- [Google Scholar]
- Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small. 2017;13(15):1603938.
- [Google Scholar]
- g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater.. 2018;8(3):1701503.
- [Google Scholar]
- Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238(5358):37-38.
- [Google Scholar]
- TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep.. 2008;63(12):515-582.
- [Google Scholar]
- One-pot synthesis of copper-doped graphitic carbon nitride nanosheet by heating Cu–melamine supramolecular network and its enhanced visible-light-driven photocatalysis. J. Solid State Chem.. 2015;228:60-64.
- [Google Scholar]
- Single atom (Pd/Pt) supported on graphitic carbon nitride as an efficient photocatalyst for visible-light reduction of carbon dioxide. J. Am. Chem. Soc.. 2016;138(19):6292-6297.
- [Google Scholar]
- Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol., C. 2008;9(1):1-12.
- [Google Scholar]
- Synthesis and efficient visible light photocatalytic hydrogen evolution of polymeric g-C3N4 coupled with CdS quantum dots. J. Phys. Chem. C. 2012;116(25):13708-13714.
- [Google Scholar]
- Nitrogen photofixation on holey g-C3N4 nanosheets with carbon vacancies under visible-light irradiation. Chin. Chem. Lett.. 2020;31(3):792-796.
- [Google Scholar]
- Biochemical analysis of root exudates of canola plant in response to chemical and physical abiotic stress. In: Other Conferences. 2021.
- [Google Scholar]
- Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light. RSC Adv.. 2015;5(61):49317-49325.
- [Google Scholar]
- Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability. Appl. Catal. B. 2015;165:503-510.
- [Google Scholar]
- Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed.. 2013;52(29):7372-7408.
- [Google Scholar]
- Probing the physio-chemical appraisal of green synthesized PbO nanoparticles in PbO-PVC nanocomposite polymer membranes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2020;235:118303.
- [Google Scholar]
- One-step preparation of iodine-doped graphitic carbon nitride nanosheets as efficient photocatalysts for visible light water splitting. J. Mater. Chem. A. 2015;3(8):4612-4619.
- [Google Scholar]
- AuPd bimetallic nanoparticles decorated graphitic carbon nitride for highly efficient reduction of water to H2 under visible light irradiation. Carbon. 2015;92:31-40.
- [Google Scholar]
- Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano. 2016;10(2):2745-2751.
- [Google Scholar]
- Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano. 2016;10(2):2745-2751.
- [Google Scholar]
- The band structure and photocatalytic mechanism for a CeO2-modified g-C3N4 photocatalyst. New J. Chem.. 2017;41(18):9724-9730.
- [Google Scholar]
- A separation-free polyacrylamide/bentonite/graphitic carbon nitride hydrogel with excellent performance in water treatment. J. Cleaner Prod.. 2018;197:1222-1230.
- [Google Scholar]
- Surface defect-abundant one-dimensional graphitic carbon nitride nanorods boost photocatalytic nitrogen fixation. New J. Chem.. 2020;44(47):20651-20658.
- [Google Scholar]
- A reusable, separation-free and biodegradable calcium alginate/g-C3N4 microsphere for sustainable photocatalytic wastewater treatment. J. Cleaner Prod.. 2021;314:128033.
- [Google Scholar]
- Emerging artificial nitrogen cycle processes through novel electrochemical and photochemical synthesis. Mater. Today. 2021;46:212-233.
- [Google Scholar]
- A green synthesis of Ru modified g-C3N4 nanosheets for enhanced photocatalytic ammonia synthesis. Energy Mater. Adv.. 2021;2021
- [Google Scholar]
- Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: a review. Appl. Mater. Today. 2019;15:494-524.
- [Google Scholar]
- Rational Ionothermal copolymerization of TCNQ with PCN semiconductor for enhanced Photocatalytic full water splitting. ACS Appl. Mater. Interf.. 2019;11(50):46756-46766.
- [Google Scholar]
- Visible-light enhanced photocatalytic performance of polypyrrole/g-C3N4 composites for water splitting to evolve H2 and pollutants degradation. J. Photochem. Photobiol., A. 2019;379:88-98.
- [Google Scholar]
- Fusion of conjugated bicyclic co-polymer within polymeric carbon nitride for high photocatalytic performance. J. Colloid Interface Sci.. 2019;554:627-639.
- [Google Scholar]
- Nickel oxide nano-particles on 3D nickel foam substrate as a non-enzymatic glucose sensor. J. Electrochem. Soc.. 2019;166(15):B1602-B1611.
- [Google Scholar]
- Synthesis and optimization of the trimesic acid modified polymeric carbon nitride for enhanced photocatalytic reduction of CO2. J. Colloid Interf. Sci.. 2019;548:197-205.
- [Google Scholar]
- Molecular engineering of polymeric carbon nitride based Donor-Acceptor conjugated copolymers for enhanced photocatalytic full water splitting. J. Colloid Interface Sci.. 2020;560:743-754.
- [Google Scholar]
- π-deficient pyridine ring-incorporated carbon nitride polymers for photocatalytic H2 evolution and CO2 fixation. Res. Chem. Intermed.. 2021;47(1):15-27.
- [Google Scholar]
- Molecular engineering of carbon nitride towards photocatalytic H2 evolution and dye degradation. J. Colloid Interface Sci.. 2021;597:39-47.
- [Google Scholar]
- Organic conjugation of polymeric carbon nitride for improved photocatalytic CO2 conversion and H2 fixation. Energy Technol.. 2021;9(10):2100091.
- [Google Scholar]
- Molecular grafting based polymeric carbon nitride for wondrous artificial photosynthesis. Int. J. Energy Res. 2021
- [Google Scholar]
- Organic heterostructure modified carbon nitride as apprehension for Quercetin Biosensor. Synth. Met.. 2021;278:116813.
- [Google Scholar]
- A superficial intramolecular alignment of carbon nitride through conjugated monomer for optimized photocatalytic CO2 reduction. Catalysts. 2021;11(8):935.
- [Google Scholar]
- A simplistic molecular agglomeration of carbon nitride for optimized photocatalytic performance. Surf. Interf.. 2021;25:101166.
- [Google Scholar]
- A butterfly shaped organic heterojunction photocatalyst for effective photocatalytic CO2 reduction. CrystEngComm. 2021;23(28):4963-4974.
- [Google Scholar]
- A molecular amalgamation of carbon nitride polymer as emphasized photocatalytic performance. Int. J. Energy Res.. 2021;45(14):19921-19928.
- [Google Scholar]
- Enhanced photocatalytic overall water splitting from an assembly of donor-π-acceptor conjugated polymeric carbon nitride. J. Colloid Interf. Sci.. 2022;624:411-422.
- [Google Scholar]
- Graphitic carbon nitride (g–C3N4)–based semiconductor as a beneficial candidate in photocatalysis diversity. Int. J. Hydrogen Energy. 2022;47(8):5142-5191.
- [Google Scholar]
- Biomass lignin integrated polymeric carbon nitride for boosted photocatalytic hydrogen and oxygen evolution reactions. Mole. Catal.. 2022;518:112064.
- [Google Scholar]
- Fabrication, characteristics, and applications of boron nitride and their composite nanomaterials. Surf. Interf.. 2022;29:101725.
- [Google Scholar]
- Recent advancement of the current aspects of g-C3N4 for its photocatalytic applications in sustainable energy system. Chem. Rec. 2022
- [Google Scholar]
- State of the art advancement in rational design of g-C3N4 photocatalyst for efficient solar fuel transformation, environmental decontamination and future perspectives. Int. J. Hydrogen Energy. 2022;47(20):10837-10867.
- [Google Scholar]
- Molecular grafting based polymeric carbon nitride for wondrous artificial photosynthesis. Int. J. Energy Res.. 2022;46(2):1882-1893.
- [Google Scholar]
- Molecular engineering control defects within carbon nitride for optimized co-catalyst Pt induced photocatalytic CO2 reduction and NO2 oxidation reaction. Int. J. Hydrogen Energy. 2022;47(31):14280-14293.
- [Google Scholar]
- A facile supramolecular aggregation of trithiocyanuric acid with PCN for high photocatalytic hydrogen evolution from water splitting. Int. J. Energy Res.. 2019;43(10):5479-5492.
- [Google Scholar]
- New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol.. 2015;49(1):649-656.
- [Google Scholar]
- Z-scheme SnO2− x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energy Mater. Sol. Cells. 2015;137:175-184.
- [Google Scholar]
- High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B. 2015;168:1-8.
- [Google Scholar]
- Treatment of alkaline stripped effluent in aerated constructed wetlands: feasibility evaluation and performance enhancement. Water. 2016;8(9):386.
- [Google Scholar]
- Development of alternative photocatalysts to TiO2. Challeng. Opport.. 2009;2(12):1231.
- [Google Scholar]
- A general framework for the assessment of solar fuel technologies. Energy Environ. Sci.. 2015;8(1):126-157.
- [Google Scholar]
- Carbon vacancy-induced enhancement of the visible light-driven photocatalytic oxidation of NO over g-C3N4 nanosheets. Appl. Surf. Sci. 2018
- [Google Scholar]
- Porous carbon nitride nanosheets for enhanced photocatalytic activities. Nanoscale. 2014;6(24):14984-14990.
- [Google Scholar]
- Layered nanojunctions for hydrogen-evolution catalysis. Angew. Chem. Int. Ed.. 2013;52(13):3621-3625.
- [Google Scholar]
- Layered nanojunctions for hydrogen-evolution catalysis. Angew. Chem.. 2013;125(13):3709-3713.
- [Google Scholar]
- N-doped graphene/porous g-C3N4 nanosheets supported layered-MoS2 hybrid as robust anode materials for lithium-ion batteries. Nano Energy. 2014;8:157-164.
- [Google Scholar]
- Synthesis and characterization of arginine-doped polyaniline/walnut shell hybrid composite with superior clean-up ability for chromium (VI) from aqueous media: equilibrium, reusability and process optimization. J. Mol. Liq.. 2020;316:113832.
- [Google Scholar]
- Facile synthesis and characterization of a novel 1,2,4,5-benzene tetracarboxylic acid doped polyaniline@zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water. J. Colloid. Interf. Sci.. 2021;585:560-573.
- [Google Scholar]
- Construction of a 2D/2D g-C3N4/rGO hybrid heterojunction catalyst with outstanding charge separation ability and nitrogen photofixation performance via a surface protonation process. RSC Adv.. 2016;6(31):25695-25702.
- [Google Scholar]
- Construction of carbon-doped supramolecule-based g-C3N4/TiO2 composites for removal of diclofenac and carbamazepine: a comparative study of operating parameters, mechanisms, degradation pathways. J. Hazard. Mater.. 2019;380:120812.
- [Google Scholar]
- Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J. Am. Chem. Soc.. 2008;130(48):16136-16137.
- [Google Scholar]
- Preparation of 2D hydroxyl-rich carbon nitride nanosheets for photocatalytic reduction of CO2. RSC Adv.. 2015;5(42):33254-33261.
- [Google Scholar]
- Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4. Appl. Surf. Sci.. 2015;358:350-355.
- [Google Scholar]
- Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy. 2015;12:646-656.
- [Google Scholar]
- Ultralight biodegradable 3D-g-C3N4 aerogel for advanced oxidation water treatment driven by oxygen delivery channels and triphase interfaces. J. Cleaner Prod.. 2021;288:125091.
- [Google Scholar]
- Metal-free disinfection effects induced by graphitic carbon nitride polymers under visible light illumination. Chem. Commun.. 2014;50(33):4338-4340.
- [Google Scholar]
- Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979
- [Google Scholar]
- Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature. 1979;277(5698):637-638.
- [Google Scholar]
- Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev.. 2013;42(7):2824-2860.
- [Google Scholar]
- Ultrathin Ag2WO4-coated P-doped g-C3N4 nanosheets with remarkable photocatalytic performance for indomethacin degradation-Science Direct. J. Hazardous Mater.. 2020;392
- [Google Scholar]
- Large scale synthesis of porous carbon-nitride microsphere for visible light harvesting. Mater. Sci. Forum 2017
- [Google Scholar]
- Two-dimensional CaIn2S4/g-C3N4 heterojunction nanocomposite with enhanced visible-light photocatalytic activities: interfacial engineering and mechanism insight. ACS Appl. Mater. Interf.. 2015;7(34):19234-19242.
- [Google Scholar]
- Polyaniline/carbon nitride nanosheets composite hydrogel: a separation-free and high-efficient photocatalyst with 3D hierarchical structure. Small. 2016;12(32):4370-4378.
- [Google Scholar]
- Efficient photocatalytic hydrogen evolution on band structure tuned polytriazine/heptazine based carbon nitride heterojunctions with ordered needle-like morphology achieved by an in situ molten salt method. J. Phys. Chem. C. 2017;121(39):21497-21509.
- [Google Scholar]
- A novel method for the preparation of a highly stable and active CdS photocatalyst with a special surface nanostructure. J. Phys. Chem. B. 2006;110(23):11139.
- [Google Scholar]
- Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B. 2017;201:159-168.
- [Google Scholar]
- From melamine-cyanuric acid supramolecular aggregates to carbon nitride hollow spheres. Adv. Funct. Mater.. 2013;23(29):3661-3667.
- [Google Scholar]
- Meeting the clean energy demand: nanostructure architectures for solar energy conversion. J. Phys. Chem. C. 2007;111(7):2834-2860.
- [Google Scholar]
- On the general mechanism of photocatalytic reduction of CO2. J. CO2 Utiliz.. 2016;16:194-203.
- [Google Scholar]
- Water splitting into H2 and O2 on alkali tantalate photocatalysts ATaO3 (A = Li, Na, and K) J. Phys. Chem. B 2001
- [Google Scholar]
- Water splitting into H2 and O2 on alkali tantalate photocatalysts ATaO3 (A= Li, Na, and K) J. Phys. Chem. B. 2001;105(19):4285-4292.
- [Google Scholar]
- Comparative study of the ball milling and acid treatment of functionalized nanodiamond composites. Int. J. Refract Metal Hard Mater.. 2018;73:46-52.
- [Google Scholar]
- Multi-dimensional anatase TiO2 materials: synthesis and their application as efficient charge transporter in perovskite solar cells. Sol. Energy. 2019;184:323-330.
- [Google Scholar]
- Functionalized nano diamond composites for photocatalytic hydrogen evolution and effective pollutant degradation. Int. J. Hydrogen Energy. 2020;45(53):29070-29081.
- [Google Scholar]
- Surface optimization of detonation nanodiamonds for the enhanced mechanical properties of polymer/nanodiamond composites. Diam. Relat. Mater.. 2020;107:107897.
- [Google Scholar]
- A concise review on the elastomeric behavior of electroactive polymer materials. Int. J. Energy Res.. 2021;45(10):14306-14337.
- [Google Scholar]
- Synergistic effect of nanodiamond and titanium oxide nanoparticles on the mechanical, thermal and electrical properties of pitch-derived carbon foam composites. Polym. Int.. 2021;70(12):1733-1740.
- [Google Scholar]
- Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: Role of media and tidal operation. Sci. Total Environ.. 2017;592:197-205.
- [Google Scholar]
- Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: dynamics of adsorption, desorption and regeneration. J. Environ. Manage.. 2017;201:260-267.
- [Google Scholar]
- Intensive exploration of the fuel characteristics of biomass and biochar from oil palm trunk and oil palm fronds for supporting increasing demand of solid biofuels in Thailand. Energy Rep.. 2022;8:5640-5652.
- [Google Scholar]
- Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev.. 2012;112(3):1555-1614.
- [Google Scholar]
- Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev.. 2016;112(3):1555-1614.
- [Google Scholar]
- Photochemical and photoelectrochemical reduction of CO2. Annu. Rev. Phys. Chem.. 2012;63:541-569.
- [Google Scholar]
- Synthesis of magnetically separable and recyclable g-C3N4–Fe3O4 hybrid nanocomposites with enhanced photocatalytic performance under visible-light irradiation. J. Phys. Chem. C. 2013;117(49):26135-26143.
- [Google Scholar]
- Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core–shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale. 2014;6(9):4830-4842.
- [Google Scholar]
- Facile synthesis of hierarchical Cu2O nanocubes as visible light photocatalysts. Appl. Catal. B. 2016;189:226-232.
- [Google Scholar]
- Review on modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A. 2011;115(46):13211-13241.
- [Google Scholar]
- Visible-light-driven CO2 reduction with carbon nitride: enhancing the activity of ruthenium catalysts. Angew. Chem. Int. Ed. Engl.. 2015;54(8):2406-2409.
- [Google Scholar]
- Self-initiated photocatalytic polymerization of tough and flexible polyacrylamide hydrogel/polymeric semiconductor g-C3N4 composites. J. Photopolym. Sci. Technol.. 2017;30(4):425-429.
- [Google Scholar]
- Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev.. 2014;43(1):473-486.
- [Google Scholar]
- Cubic mesoporous graphitic carbon (IV) nitride: an all-in-one chemosensor for selective optical sensing of metal ions. Angew. Chem.. 2010;122(50):9900-9904.
- [Google Scholar]
- Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res.. 2016;88(JAN.1):428-448.
- [Google Scholar]
- Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res.. 2016;88:428-448.
- [Google Scholar]
- Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci.. 2006;103(43):15729-15735.
- [Google Scholar]
- Template-free synthesis and photocatalytic properties of novel Fe2O3 hollow spheres. J. Phys. Chem. C. 2007;111(5):2123-2127.
- [Google Scholar]
- A simple template-free synthesis of nanoporous ZnS–In2S3–Ag2S solid solutions for highly efficient photocatalytic H2 evolution under visible light. Chem. Commun.. 2009;15:2020-2022.
- [Google Scholar]
- Intercorrelated superhybrid of AgBr supported on g-C3N4 decorated nitrogen-doped graphene: high engineering photocatalytic activities for water purification and CO2 reduction. Adv. Mater.. 2015;27(43):6906-6913.
- [Google Scholar]
- Structure modification function of g-C3N4 for Al2O3 in the in situ hydrothermal process for enhanced photocatalytic activity. Chem. – A Eur. J.. 2015;21(28):10149-10159.
- [Google Scholar]
- Hydrogenated defects in graphitic carbon nitride nanosheets for improved photocatalytic hydrogen evolution. J. Phys. Chem. C. 2015;119(27):14938-14946.
- [Google Scholar]
- Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A. 2015;3(9):5189-5196.
- [Google Scholar]
- Mesostructured CeO2/g-C3N4 nanocomposites: remarkably enhanced photocatalytic activity for CO2 reduction by mutual component activations. Nano Energy. 2016;19:145-155.
- [Google Scholar]
- Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B. 2016;190:26-35.
- [Google Scholar]
- Carbon nitride nanodots decorated brookite TiO2 quasi nanocubes for enhanced activity and selectivity of visible-light-driven CO2 reduction. Appl. Catal. B. 2017;203:910-916.
- [Google Scholar]
- Core-shell LaPO4/g-C3N4 nanowires for highly active and selective CO2 reduction. Appl. Catal. B. 2017;201:629-635.
- [Google Scholar]
- Fabrication of heterostructured g-C3N4/Ag-TiO2 hybrid photocatalyst with enhanced performance in photocatalytic conversion of CO2 under simulated sunlight irradiation. Appl. Surf. Sci.. 2017;402:198-207.
- [Google Scholar]
- The spatially oriented charge flow and photocatalysis mechanism on internal van der Waals heterostructures enhanced g-C3N4. ACS Catal.. 2018;8(9):8376-8385.
- [Google Scholar]
- Efficient photocatalytic fixation of N2 by KOH-treated g-C3N4. J. Mater. Chem. A. 2018;6(7):3005-3011.
- [Google Scholar]
- 2D g-C3N4 for advancement of photo-generated carrier dynamics: status and challenges. Mater. Today. 2020;41:270-303.
- [Google Scholar]
- C3N4 with engineered three coordinated (N3C) nitrogen vacancy boosts the production of 1O2 for Efficient and stable NO photo-oxidation. Chem. Eng. J.. 2020;389:124421.
- [Google Scholar]
- Recent progress in g–C3N4–Based materials for remarkable photocatalytic sustainable energy. Int. J. Hydrogen Energy. 2022;47(49):21067-21118.
- [Google Scholar]
- Visible photocatalytic water splitting and photocatalytic two-electron oxygen formation over Cu-and Fe-doped g-C3N4. J. Phys. Chem. C. 2016;120(1):56-63.
- [Google Scholar]
- Particle size, shape and activity for photocatalysis on titania anatase nanoparticles in aqueous surroundings. J. Am. Chem. Soc.. 2011;133(39):15743-15752.
- [Google Scholar]
- Photocatalytic hydrogen evolution from silica-templated polymeric graphitic carbon nitride–is the surface area important? ChemCatChem. 2015;7(1):121-126.
- [Google Scholar]
- Mesoporous g-C3N4 nanorods as multifunctional supports of ultrafine metal nanoparticles: hydrogen generation from water and reduction of nitrophenol with tandem catalysis in one step. Chem. Sci.. 2012;3(6):2170-2174.
- [Google Scholar]
- Macroscopic 3D porous graphitic carbon nitride monolith for enhanced photocatalytic hydrogen evolution. Adv. Mater.. 2015;27(31):4634-4639.
- [Google Scholar]
- Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater.. 2016;25(44):6885-6892.
- [Google Scholar]
- Synthesis and photo-catalytic activity of porous g-C3N4: Promotion effect of nitrogen vacancy in H2 evolution and pollutant degradation reactions. Int. J. Hydrogen Energy. 2019;44(31)
- [Google Scholar]
- A g-C3N4@ppy-rGO 3D structure hydrogel for efficient photocatalysis. Appl. Surf. Sci.. 2019;466:666-672.
- [Google Scholar]
- In situ growth of CdS quantum dots on phosphorus-doped carbon nitride hollow tubes as active 0D/1D heterostructures for photocatalytic hydrogen evolution. J. Colloid Interface Sci.. 2020;577:1-11.
- [Google Scholar]
- Preparation of the W 18 O 49/g-C3N4 heterojunction catalyst with full-spectrum-driven photocatalytic N 2 photofixation ability from the UV to near infrared region. New J. Chem.. 2017;41(17):8920-8926.
- [Google Scholar]
- Liang, X., et al., 2018. Graphitic Carbon Nitride with Carbon Vacancies for Photocatalytic Degradation of Bisphenol A.
- Photochemical reduction of CO2 by graphitic carbon nitride polymers. ACS Sustain. Chem. Eng.. 2014;2(3):353-358.
- [Google Scholar]
- Stable Cu2O@ g-C3N4 core@ shell nanostructures: efficient visible-light photocatalytic hydrogen evolution. Mater. Lett.. 2015;158:278-281.
- [Google Scholar]
- Ultrathin g-C3N4 nanosheets coupled with carbon nanodots as 2D/0D composites for efficient photocatalytic H2 evolution. Appl. Catal. B. 2016;193:248-258.
- [Google Scholar]
- In situ bond modulation of graphitic carbon nitride to construct p–n homojunctions for enhanced photocatalytic hydrogen production. Adv. Funct. Mater.. 2016;26(37):6822-6829.
- [Google Scholar]
- Novel visible-light-driven CdIn2S4/mesoporous g-C3N4 hybrids for efficient photocatalytic reduction of CO2 to methanol. Mole. Catal.. 2017;430:9-19.
- [Google Scholar]
- Intermediate-mediated strategy to horn-like hollow mesoporous ultrathin g-C3N4 tube with spatial anisotropic charge separation for superior photocatalytic H2 evolution. Nano Energy 2017:738-748.
- [Google Scholar]
- Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res.. 2019;151:8-19.
- [Google Scholar]
- In situ decoration of SnS quantum dots on the α-SnWO4 nanosheets for superior visible-light photocatalytic performance. Appl. Surf. Sci.. 2020;531:147379.
- [Google Scholar]
- Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Env. Sci. Technol.. 2021;51(8):751-790.
- [Google Scholar]
- Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen. ACS Sustain. Chem. Eng.. 2017;5(2):1436-1445.
- [Google Scholar]
- Construction 0D/2D heterojunction by highly dispersed Ni2P QDs loaded on the ultrathin g-C3N4 surface towards superhigh photocatalytic and photoelectric performance. Appl. Catal. B. 2018;237:919-926.
- [Google Scholar]
- One-dimensional composite nanomaterials: synthesis by electrospinning and their applications. Small. 2009;5(21):2349-2370.
- [Google Scholar]
- Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev.. 2014;114(19):9987-10043.
- [Google Scholar]
- Photocatalytic water splitting using semiconductor particles: history and recent developments. J. Photochem. Photobiol., C. 2011;12(4):237-268.
- [Google Scholar]
- The effect of the pore-wall structure of carbon nitride on photocatalytic CO2 reduction under visible light. J. Mater. Chem. A. 2014;2(36):15146-15151.
- [Google Scholar]
- A polymeric-semiconductor–metal-complex hybrid photocatalyst for visible-light CO2 reduction. Chem. Commun.. 2013;49(86):10127-10129.
- [Google Scholar]
- Chemical mismanagement and skin burns among hospitalized and outpatient department patients. Int. J. Occupat. Safe. Ergon.. 2021;27(3):817-830.
- [Google Scholar]
- Investigation of textile dyeing effluent using activated sludge system to assess the removal efficiency. Water Environ. Res.. 2021;93(12):2931-2940.
- [Google Scholar]
- Applications of nanomaterials in the different fields of photosciences. Indian J. Phys.. 2011;85(8):1229-1245.
- [Google Scholar]
- Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal. Sci. Technol.. 2013;3(5):1253-1260.
- [Google Scholar]
- Novel g-C3N4/CoO nanocomposites with significantly enhanced visible-light photocatalytic activity for H2 evolution. ACS Appl. Mater. Interf.. 2017;9(14):12427-12435.
- [Google Scholar]
- In-situ construction of coral-like porous P-doped g-C3N4 tubes with hybrid 1D/2D architecture and high efficient photocatalytic hydrogen evolution. Appl. Catal. B 2018 S0926337318308671
- [Google Scholar]
- Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew. Chem. Int. Ed.. 2014;53(35):9240-9245.
- [Google Scholar]
- Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett.. 1985;29(1–2):211-214.
- [Google Scholar]
- Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc.. 2009;131(35):12540-12541.
- [Google Scholar]
- Integrating both homojunction and heterojunction in QDs self-decorated Bi2MoO6/BCN composites to achieve an efficient photocatalyst for Cr (VI) reduction. Chem. Eng. J.. 2018;334:334-343.
- [Google Scholar]
- Water purification by semiconductor photocatalysis. Chem. Soc. Rev.. 1993;22(6):417-425.
- [Google Scholar]
- Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution. RSC Adv.. 2015;5
- [Google Scholar]
- Haloacetonitriles vs. regulated haloacetic acids: are nitrogen-containing DBPs more toxic? Environ. Sci. Technol.. 2007;41(2):645-651.
- [Google Scholar]
- Influence of Sr-doping on structural, optical and photocatalytic properties of synthesized Ca3(PO4)2. J. Colloid. Interf. Sci.. 2020;572:269-280.
- [Google Scholar]
- Recent progress on the enhancement of photocatalytic properties of BiPO4 using π–conjugated materials. Adv. Colloid. Interf. Sci.. 2020;280:102160.
- [Google Scholar]
- Photocatalytic oxidation of pollutants in gas-phase via Ag3PO4-based semiconductor photocatalysts: recent progress, new trends, and future perspectives. Criti. Rev. Environ. Sci. Technol. 2021:1-44.
- [Google Scholar]
- Recent advances of bismuth titanate based photocatalysts engineering for enhanced organic contaminates oxidation in water: a review. Chemosphere. 2022;300:134622.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4)-based photocatalysts for solar hydrogen generation: recent advances and future development directions. J. Mater. Chem. A. 2017;5(45):23406-23433.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4)-based photocatalysts for solar hydrogen generation: recent advances and future development directions. J. Mater. Chem. A. 2017;5
- [Google Scholar]
- Recent advances in the development of sunlight-driven hollow structure photocatalysts and their applications. J. Mater. Chem. A. 2015;3(36):18345-18359.
- [Google Scholar]
- New insights into how Pd nanoparticles influence the photocatalytic oxidation and reduction ability of g-C3N4 nanosheets. Catal. Sci. Technol.. 2016;6(16):6448-6458.
- [Google Scholar]
- Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater.. 2012;22(22):4763-4770.
- [Google Scholar]
- Switching the selectivity of the photoreduction reaction of carbon dioxide by controlling the band structure of a g-C3N4 photocatalyst. Chem. Commun.. 2014;50(74):10837-10840.
- [Google Scholar]
- Effects of hydrophilic surface macromolecule modifier loading on PES/O-g-C3N4 hybrid photocatalytic membrane for phenol removal. Appl. Surf. Sci. 2019
- [Google Scholar]
- Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017 acs.chemrev.7b00161
- [Google Scholar]
- Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev.. 2017;117(17):11302-11336.
- [Google Scholar]
- Photocatalytic reduction of CO2 over a hybrid photocatalyst composed of WO3 and graphitic carbon nitride (g-C3N4) under visible light. J. CO2 Utiliz.. 2014;6:17-25.
- [Google Scholar]
- Heterojunction engineering of graphitic carbon nitride (g-C3N4) via Pt loading with improved daylight-induced photocatalytic reduction of carbon dioxide to methane. Dalton Trans.. 2014;44(3):1249-1257.
- [Google Scholar]
- Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene–g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun.. 2015;51(5):858-861.
- [Google Scholar]
- Heterojunction engineering of graphitic carbon nitride (g-C3N4) via Pt loading with improved daylight-induced photocatalytic reduction of carbon dioxide to methane. Dalton Trans.. 2015;44(3):1249-1257.
- [Google Scholar]
- Heterostructured AgX/g-C3N4 (X= Cl and Br) nanocomposites via a sonication-assisted deposition-precipitation approach: emerging role of halide ions in the synergistic photocatalytic reduction of carbon dioxide. Appl. Catal. B. 2016;180:530-543.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev.. 2016;116(12):7159-7329.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev. 2016
- [Google Scholar]
- Single Pt atoms deposition on g-C3N4 nanosheets for photocatalytic H2 evolution or NO oxidation under visible light. Int. J. Hydrogen Energy. 2017;42(44):27043-27054.
- [Google Scholar]
- Amino-assisted anchoring of CsPbBr 3 perovskite quantum dots on porous g-C3N4 for enhanced photocatalytic CO2 reduction. Angew. Chem.. 2018;130(41):13758-13762.
- [Google Scholar]
- A comparative study of photocatalytic activity of some coloured semiconducting oxides. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed.. 2010;29(2):43-48.
- [Google Scholar]
- Dramatic activity of C3N4/BiPO4 photocatalyst with core/shell structure formed by self-assembly. Adv. Funct. Mater.. 2012;22(7):1518-1524.
- [Google Scholar]
- A facile molecular aggregation of isoquinoline based g-C3N4 for high photocatalytic performance under visible light illumination. Mater. Res. Bull.. 2022;152:111865.
- [Google Scholar]
- New type of BiPO(4) oxy-acid salt photocatalyst with high photocatalytic activity on degradation of dye. Environ. Sci. Technol.. 2010;44(14):5570-5574.
- [Google Scholar]
- Photoregenerable, bifunctional granules of carbon-doped g-C3N4 as adsorptive photocatalyst for the efficient removal of tetracycline antibiotic. ACS Sustain. Chem. Eng.. 2017;5(2):1610-1618.
- [Google Scholar]
- A facile in situ approach to fabricate N, S-TiO2/g-C3N4 nanocomposite with excellent activity for visible light induced water splitting for hydrogen evolution. PCCP. 2015;17(12):8070-8077.
- [Google Scholar]
- Photoelectrochemical water splitting at titanium dioxide nanotubes coated with tungsten trioxide. Appl. Phys. Lett.. 2006;89(16):735.
- [Google Scholar]
- Nanocomposites of C3N4 with layers of MoS2 and nitrogenated RGO, obtained by covalent cross-linking: synthesis, characterization, and HER activity. ACS Appl. Mater. Interf.. 2017;9(12):10664-10672.
- [Google Scholar]
- Historically linked residues profile of OCPs and PCBs in surface sediments of typical urban river networks, Shanghai: ecotoxicological state and sources. J. Clean. Prod.. 2019;231:1070-1078.
- [Google Scholar]
- Concentrations, pollution indices and health risk assessment of heavy metals in road dust from two urbanized cities of Pakistan: comparing two sampling methods for heavy metals concentration. Sustain. Cities. Soc.. 2020;53:101959.
- [Google Scholar]
- Agricultural plastic mulching as a potential key source of microplastic pollution in the terrestrial ecosystem and consequences. Resour. Conserv. Recycl.. 2021;175:105855.
- [Google Scholar]
- Rebuttal to comment on “alternative plasticizers as emerging global environmental and health threat: another regrettable substitution?” Focus on DINCH as an example. Environ. Sci. Technol.. 2022;56(8):5294-5297.
- [Google Scholar]
- Sustainable development goals under threat? Multidimensional impact of COVID-19 on our planet and society outweigh short term global pollution reduction. Sustain. Cities. Soc. 2022:103962.
- [Google Scholar]
- Alternative plasticizers as emerging global environmental and health threat: another regrettable substitution? Environ. Sci. Technol.. 2022;56(3):1482-1488.
- [Google Scholar]
- Photocatalytic reduction of CO2 by graphitic carbon nitride polymers derived from urea and barbituric acid. Appl. Catal. B. 2015;179:1-8.
- [Google Scholar]
- 0D NiS2 quantum dots modified 2D g-C3N4 for efficient photocatalytic CO2 reduction. Colloids Surf., A. 2020;600:124912.
- [Google Scholar]
- Photocatalysts fabricated by depositing plasmonic Ag nanoparticles on carbon quantum dots/graphitic carbon nitride for broad spectrum photocatalytic hydrogen generation. Appl. Catal. B. 2017;209:161-173.
- [Google Scholar]
- Enhanced photocatalytic hydrogen evolution by prolonging the lifetime of carriers in ZnO/CdS heterostructures. Chem. Commun.. 2009;23:3452-3454.
- [Google Scholar]
- Green synthesis, properties, and catalytic application of zeolite (P) in production of biofuels from bagasse. Int. J. Energy Res.. 2019;43(9):4820-4827.
- [Google Scholar]
- Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci.. 2015;8(12):3708-3717.
- [Google Scholar]
- Synthesis of SnO2/BP codoped g-C3N4 nanocomposites as efficient cocatalyst-free visible-light photocatalysts for CO2 conversion and pollutant degradation. Appl. Catal. B. 2017;201:486-494.
- [Google Scholar]
- Promoting visible-light photocatalytic activities for carbon nitride based 0D/2D/2D hybrid system: beyond the conventional 4-electron mechanism. Appl. Catal. B. 2020;270:118870.
- [Google Scholar]
- Photocatalytic solar fuel production and environmental remediation through experimental and DFT based research on CdSe-QDs-coupled P-doped-g-C3N4 composites. Appl. Catal. B. 2020;270:118867.
- [Google Scholar]
- Characterisation of magnesium, zinc and iron sulfates for thermochemical storage. Proc. Inst. Civil Eng. – Energy. 2019;173(2):60-67.
- [Google Scholar]
- Thermochemical heat storage ability of ZnSO4·7H2O as potential long-term heat storage material. Int. J. Energy Res.. 2021;45(3):4746-4754.
- [Google Scholar]
- Paper title: thermochemical heat storage behavior of ZnSO4.7H2O under low-temperature. Heat Mass Transf.. 2021;57(5):765-775.
- [Google Scholar]
- Novel TiO2/g-C3N4 photocatalysts for photocatalytic reduction of CO2 and for photocatalytic decomposition of N2O. J. Phys. Chem. A. 2016;120(43):8564-8573.
- [Google Scholar]
- Anthropogenic pollutants: a threat to ecosystem sustainability? Philos. Trans. Royal Soc. B: Biol. Sci.. 2009;364(1534):3391-3401.
- [Google Scholar]
- Water analysis: emerging contaminants and current issues. Anal. Chem.. 2014;86(6):2813-2848.
- [Google Scholar]
- Using dyes for evaluating photocatalytic properties: a critical review. Molecules. 2014;20:88-110.
- [Google Scholar]
- Using dyes for evaluating photocatalytic properties: a critical review. Molecules. 2015;20(1):88-110.
- [Google Scholar]
- Photosynthesis and photocatalysis with semiconductor powders. Energy Resour. Photochem. Catal. 1983:331-358.
- [Google Scholar]
- Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A. 2013;1(21):6489-6496.
- [Google Scholar]
- Synthesis of a novel and stable g-C3N4-Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. J. Mater. Chem. A Mater. Energy Sustain. 2013
- [Google Scholar]
- Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev.. 2014;114(19):9919-9986.
- [Google Scholar]
- Red-emitting CaSc2O4:Eu3+ phosphor for NUV-based warm white LEDs: structural elucidation and Hirshfeld surface analysis. Int. J. Energy Res.. 2020;44(11):8328-8339.
- [Google Scholar]
- Black TiO2 nanobelts/g-C3N4 nanosheets laminated heterojunctions with efficient visible-light-driven photocatalytic performance. Sci. Rep.. 2017;7(1):1-11.
- [Google Scholar]
- Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel. ACS Catal.. 2014;4(10):3637-3643.
- [Google Scholar]
- Conversion of CO₂ into renewable fuel over Pt–g-C3N4/KNbO3 composite photocatalyst. RSC Adv. 2015
- [Google Scholar]
- Photocatalytic NH3 versus H2 evolution over g-C3N4/CsxWO3: O2 and methanol tipping the scale. Appl. Catal. B. 2018;235:197-206.
- [Google Scholar]
- Synthesis of well-dispersed TiO2@ reduced graphene oxide (rGO) nanocomposites and their photocatalytic properties. Mater. Res. Bull.. 2017;90:125-130.
- [Google Scholar]
- Synthesis of well-dispersed TiO2/CNTs@ CoFe2O4 nanocomposites and their photocatalytic properties. Mater. Res. Bull.. 2018;101:83-89.
- [Google Scholar]
- Synthesis of flower-like Co9S8/reduced graphene oxide nanocomposites and their photocatalytic performance. J. Inorg. Organomet. Polym Mater.. 2020;30(12):5168-5179.
- [Google Scholar]
- Nanostructure engineering via intramolecular construction of carbon nitride as efficient photocatalyst for CO2 reduction. Nanomaterials. 2021;11(12):3245.
- [Google Scholar]
- Silver quantum cluster (Ag9)-grafted graphitic carbon nitride nanosheets for photocatalytic hydrogen generation and dye degradation. Chem. – A Eur. J.. 2015;21(25):9126-9132.
- [Google Scholar]
- Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem.. 2012;22(35):18101-18110.
- [Google Scholar]
- Dispersed conductive polymer nanoparticles on graphitic carbon nitride for enhanced solar-driven hydrogen evolution from pure water. Nanoscale. 2013;5(19):9150-9155.
- [Google Scholar]
- Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun.. 2012;3(1):1139.
- [Google Scholar]
- Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun.. 2012;3(1):1-7.
- [Google Scholar]
- Self-standing carbon nitride-based hydrogels with high photocatalytic activity. ACS Appl. Mater. Interf.. 2017;9(3):2029-2034.
- [Google Scholar]
- Phase effect of NixPy hybridized with g-C3N4 for photocatalytic hydrogen generation. ACS Appl. Mater. Interf.. 2017;9(36):30583-30590.
- [Google Scholar]
- Synthesis of 0D SnO2 nanoparticles/2D g-C3N4 nanosheets heterojunction: improved charge transfer and separation for visible-light photocatalytic performance. J. Alloys Compd.. 2021;871:159561.
- [Google Scholar]
- Facile synthesis of ternary alloy of CdSe(1–x)S(x) quantum dots with tunable absorption and emission of visible light. Nanomaterials (Basel, Switzerland). 2018;8(12):979.
- [Google Scholar]
- All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system. Nat. Mater.. 2006;5(10):782-786.
- [Google Scholar]
- Dielectric relaxation studies on PVC-Pb3O4 polymer nanocomposites. J. Mater. Sci.: Mater. Electron.. 2021;32(23):27666-27675.
- [Google Scholar]
- Tubular graphitic-C3N4: a prospective material for energy storage and green photocatalysis. J. Mater. Chem. A. 2013;1(44):13949-13955.
- [Google Scholar]
- Multifunctional g-C3N4 nanofibers: a template-free fabrication and enhanced optical, electrochemical, and photocatalyst properties. ACS Appl. Mater. Interf.. 2014;6(2):1258-1265.
- [Google Scholar]
- Adsorption of dyes by nanomaterials: recent developments and adsorption mechanisms. Sep. Purif. Technol.. 2015;150:229-242.
- [Google Scholar]
- Fabrication of compressible and recyclable macroscopic g-C3N4/GO aerogel hybrids for visible-light harvesting: a promising strategy for water remediation. Appl. Catal. B. 2017;219:241-248.
- [Google Scholar]
- Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: a review. Chem. Soc. Rev.. 2014;43(20):6920-6937.
- [Google Scholar]
- Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy. 2017;38:72-81.
- [Google Scholar]
- Rational nanostructure design of graphitic carbon nitride for photocatalytic applications. J. Mater. Chem. A. 2019;7(19):11584-11612.
- [Google Scholar]
- Fe-doped and-mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A. 2014;2(19):6772-6780.
- [Google Scholar]
- Three-dimensional porous aerogel constructed by g-C3N4 and graphene oxide nanosheets with excellent visible-light photocatalytic performance. ACS Appl. Mater. Interf.. 2015;7(46):25693-25701.
- [Google Scholar]
- An efficient top-down approach for the fabrication of large-aspect-ratio g-C3N4 nanosheets with enhanced photocatalytic activities. PCCP. 2015;17(36):23532-23537.
- [Google Scholar]
- Tunable optical transition in polymeric carbon nitrides synthesized via bulk thermal condensation. J. Phys.: Condens. Matter. 2012;24(16):162201.
- [Google Scholar]
- Crystal structure of polymeric carbon nitride and the determination of its process-temperature-induced modifications. J. Phys.: Condens. Matter. 2013;25(39):395402.
- [Google Scholar]
- Conventional and cement-catalyzed co-pyrolysis of rice straw and waste polyethylene into liquid and gaseous fuels by using a fixed bed reactor. Biomass Convers. Biorefin. 2021
- [Google Scholar]
- Preparation, characterization and optoelectronic properties of nanodiamonds doped zinc oxide nanomaterials by a ball milling technique. Mater. Res. Express. 2016;3(7):075016.
- [Google Scholar]
- Fabrication of polymer carbon nitride with organic monomer for effective photocatalytic hydrogen evolution. J. Photochem. Photobiol., A. 2020;401:112764.
- [Google Scholar]
- Platinum-alumina modified SO42−-ZrO2/Al2O3 based bifunctional catalyst for significantly improved n-butane isomerization performance. Surf. Interf.. 2021;25:101227.
- [Google Scholar]
- Venkatathri, N., Kumar, A.A., 2017. Development of porous titanosilicate-based hybrid nanocomposites for photocatalytic applications under UV and solar light irradiation.
- Corrigendum to “Synthesis, characterization, and photocatalytic performance of Ag/AgFeO2 decorated on g-C3N4-nanosheet under the visible light irradiation” [Journal of the Taiwan Institute of Chemical Engineers 115 (2020) 279–292] - ScienceDirect. J. Taiwan Inst. Chem. Eng. 2021
- [Google Scholar]
- Raman and photoluminescence study of CdSe nanoparticles capped with a bifunctional molecule. Physica E. 2007;39(1):8-14.
- [Google Scholar]
- Preparation and characterization of a novel system of CdS nanoparticles embedded in borophosphate glass matrix. J. Alloys Compd.. 2013;555:161-168.
- [Google Scholar]
- Nano silver-anchored reduced graphene oxide sheets for enhanced dielectric performance of polymer nanocomposites. RSC Adv.. 2014;4(54):28426-28431.
- [Google Scholar]
- Synthesis and characterization of mercaptoacetic acid capped cadmium sulphide quantum dots. J. Nanosci. Nanotechnol.. 2015;15(12):9861-9867.
- [Google Scholar]
- Ternary ZnS: Te nanoparticles capped with 3-mercaptopropionic acid prepared in aqueous media. J. Mater. Sci.: Mater. Electron.. 2016;27(10):10877-10887.
- [Google Scholar]
- Photodiode based on Pb0.9Cd0.1S ternary alloy semiconductor for solar tracking systems. J. Mater. Sci.: Mater. Electron.. 2018;29(19):16880-16893.
- [Google Scholar]
- Digital printing of a novel electrode for stable flexible organic solar cells with a power conversion efficiency of 8.5%. Sci. Rep.. 2021;11(1):14212.
- [Google Scholar]
- Silver nanowires digital printing for inverted flexible semi-transparent solar cells. Adv. Eng. Mater.. 2021;23(4):2001305.
- [Google Scholar]
- Optical properties and thermal degradation of CdSe capped with 3-mercaptopropionic acid. J. Mater. Sci.: Mater. Electron.. 2013;24(8):3049-3057.
- [Google Scholar]
- Band edge emission of ZnS nanoparticles prepared by excess of thiourea as a source of sulfur. J. Sol-Gel Sci. Technol.. 2013;66(3):443-451.
- [Google Scholar]
- Ultra-violet electroluminescence of ZnO nanorods/MEH-PPV heterojunctions by optimizing their thickness and using AZO as a transparent conductive electrode. Materials. 2019;12:18.
- [Google Scholar]
- Effect of adding reducing agent on the structure and optical properties of one-pot preparation method of CdTe quantum dots. J. Mater. Sci.: Mater. Electron.. 2016;27(8):8384-8393.
- [Google Scholar]
- Photochemical CO2 reduction using structurally controlled gC 3 N 4. PCCP. 2016;18(36):24825-24829.
- [Google Scholar]
- Visible-light photocatalytic activity and deactivation mechanism of Ag3PO4 spherical particles. Chem. – Asian J.. 2012;7(8):1902-1908.
- [Google Scholar]
- Carbon nitride nanosheets for photocatalytic hydrogen evolution: remarkably enhanced activity by dye sensitization. Catal. Sci. Technol.. 2013;3(7):1703-1711.
- [Google Scholar]
- Graphene and g-C3N4 nanosheets cowrapped elemental α-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light. Environ. Sci. Technol.. 2013;47(15):8724-8732.
- [Google Scholar]
- Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A. 2014;2(9):2885-2890.
- [Google Scholar]
- g-C3N4 quantum dots: direct synthesis, upconversion properties and photocatalytic application. Chem. Commun.. 2014;50(70):10148-10150.
- [Google Scholar]
- Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B. 2015;176:44-52.
- [Google Scholar]
- Facile one-step synthesis of hybrid graphitic carbon nitride and carbon composites as high-performance catalysts for CO2 photocatalytic conversion. ACS Appl. Mater. Interf.. 2016;8(27):17212-17219.
- [Google Scholar]
- Surprisingly advanced CO2 photocatalytic conversion over thiourea derived g-C3N4 with water vapor while introducing 200–420 nm UV light. J. CO2 Utiliz.. 2016;14:143-151.
- [Google Scholar]
- Indirect Z-scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl. Mater. Interf.. 2016;8(6):3765-3775.
- [Google Scholar]
- 0D/2D Z-scheme heterojunctions of bismuth tantalate quantum dots/ultrathin g-C3N4 nanosheets for highly efficient visible light photocatalytic degradation of antibiotics. ACS Appl. Mater. Interf. 2017:43704-43715.
- [Google Scholar]
- 2D–2D MnO2/g-C3N4 heterojunction photocatalyst: in-situ synthesis and enhanced CO2 reduction activity. Carbon. 2017;120:23-31.
- [Google Scholar]
- Boosting hot-electron generation: exciton dissociation at the order–disorder interfaces in polymeric photocatalysts. J. Am. Chem. Soc.. 2017;139(6):2468-2473.
- [Google Scholar]
- In situ construction of Z-scheme g-C3N4/Mg1.1Al0.3Fe0.2O1.7 nanorod heterostructures with high N2 photofixation ability under visible light. Rsc Adv.. 2017;7(29):18099-18107.
- [Google Scholar]
- Facile gel-based morphological control of Ag/g-C3N4 porous nanofibers for photocatalytic hydrogen generation. ACS Sustain. Chem. Eng.. 2017;5(11):10633-10639.
- [Google Scholar]
- Unlocking the door to highly active ORR catalysts for PEMFC applications: polyhedron-engineered Pt-based nanocrystals. Energy Environ. Sci.. 2018;11(2):258-275.
- [Google Scholar]
- 0D/2D Au25(Cys)18 nanoclusters/g-C3N4 nanosheets composites for enhanced photocatalytic hydrogen production under visible light. ACS Sustain. Chem. Eng.. 2018;6(7)
- [Google Scholar]
- 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B. 2020;273:119051.
- [Google Scholar]
- Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal.. 2021;42(1):56-68.
- [Google Scholar]
- Controllable approach to carbon-deficient and oxygen-doped graphitic carbon nitride: robust photocatalyst against recalcitrant organic pollutants and the mechanism insight. Adv. Funct. Mater.. 2021;31(20):2010763.
- [Google Scholar]
- A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. mater.. 2009;8(1):76-80.
- [Google Scholar]
- Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal.. 2012;2(8):1596-1606.
- [Google Scholar]
- Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies. Chem. Rev.. 2019;120(2)
- [Google Scholar]
- Alkali metal-assisted synthesis of graphite carbon nitride with tunable band-gap for enhanced visible-light-driven photocatalytic performance. ACS Sustain. Chem. Eng.. 2018;6(11):15503-15516.
- [Google Scholar]
- Atomically precise cluster catalysis towards quantum controlled catalysts. Sci. Technol. Adv. Mater. 2014
- [Google Scholar]
- Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energy Environ. Sci.. 2018;11(9):2581-2589.
- [Google Scholar]
- Designing the surfaces of semiconductor quantum dots for colloidal photocatalysis. ACS Energy Lett.. 2017;2(5):1005-1013.
- [Google Scholar]
- Enhanced visible-light H2 evolution of g-C3N4 photocatalysts via the synergetic effect of amorphous NiS and cheap metal-free carbon black nanoparticles as co-catalysts. Appl. Surf. Sci.. 2015;358:204-212.
- [Google Scholar]
- Constructing multifunctional metallic Ni interface layers in the g-C3N4 nanosheets/amorphous NiS heterojunctions for efficient photocatalytic H2 generation. ACS Appl. Mater. Interf.. 2017;9(16):14031-14042.
- [Google Scholar]
- One-dimensional nanostructure based materials for versatile photocatalytic applications. RSC Adv.. 2014;4(25):12685-12700.
- [Google Scholar]
- Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chem. Rev. 2015
- [Google Scholar]
- Design and fabrication of an albedo insensitive analog sun sensor. Procedia Eng.. 2011;25:527-530.
- [Google Scholar]
- Synthesis of potassium-modified graphitic carbon nitride with high photocatalytic activity for hydrogen evolution. ChemSusChem. 2014;7(9):2654-2658.
- [Google Scholar]
- Synthesis of carbon black/carbon nitride intercalation compound composite for efficient hydrogen production. Dalton Trans.. 2014;43(31):12013-12017.
- [Google Scholar]
- High alkalinity boosts visible light driven H2 evolution activity of g-C3N4 in aqueous methanol. Chem. Commun.. 2014;50(98):15521-15524.
- [Google Scholar]
- Treatment of anaerobic digestate supernatant in microbial fuel cell coupled constructed wetlands: evaluation of nitrogen removal, electricity generation, and bacterial community response. Sci. Total Environ.. 2017;580:339-346.
- [Google Scholar]
- Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A. 2017;5(7):3230-3238.
- [Google Scholar]
- Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C. 2011;115(15):7355-7363.
- [Google Scholar]
- Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A. Mater. Energy Sustain.. 2015;3(6):2485-2534.
- [Google Scholar]
- Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A. 2013;1(46):14766-14772.
- [Google Scholar]
- Insights into enhanced visible-light photocatalytic hydrogen evolution of g-C3N4 and highly reduced graphene oxide composite: the role of oxygen. Chem. Mater.. 2015;27(5):1612-1621.
- [Google Scholar]
- Bi2O3 cocatalyst improving photocatalytic hydrogen evolution performance of TiO2. Appl. Surf. Sci.. 2017;400:530-536.
- [Google Scholar]
- Toward efficient preconcentrating photocatalysis: 3D g-C3N4 monolith with isotype heterojunctions assembled from hybrid 1D and 2D nanoblocks. ACS Appl. Mater. Interf.. 2019;11(35):31934-31942.
- [Google Scholar]
- Enhanced photocatalytic activity of Cu2O/g-C3N4 heterojunction coupled with reduced graphene oxide three-dimensional aerogel photocatalysis. Mater. Res. Bull.. 2017;96:18-27.
- [Google Scholar]
- Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir. 2010;26(6):3894-3901.
- [Google Scholar]
- Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: structure, photoactivity and degradation mechanisms. Environ. Appl. Catal. B 2018
- [Google Scholar]
- Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater.. 2013;25(17):2452-2456.
- [Google Scholar]
- A facile steam reforming strategy to delaminate layered carbon nitride semiconductors for photoredox catalysis. Angew. Chem. Int. Ed.. 2017;56(14):3992-3996.
- [Google Scholar]
- Constructing highly dispersed 0D Co3S4 quantum dots/2D g-C3N4 nanosheets nanocomposites for excellent photocatalytic performance. Sci. Bullet.. 2019;64(20):1510-1517.
- [Google Scholar]
- In-situ growth of Zn–AgIn5S8 quantum dots on g-C3N4 towards 0D/2D heterostructured photocatalysts with enhanced hydrogen production. Int. J. Hydrogen Energy. 2019;44(30):15882-15891.
- [Google Scholar]
- One-step low-temperature synthesis of 0D CeO2 quantum dots/2D BiOX (X= Cl, Br) nanoplates heterojunctions for highly boosting photo-oxidation and reduction ability. Appl. Catal. B. 2019;250:17-30.
- [Google Scholar]
- A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci.. 2015;358:15-27.
- [Google Scholar]
- Phosphorylation of g-C3N4 for enhanced photocatalytic CO2 reduction. Chem. Eng. J.. 2016;304:376-383.
- [Google Scholar]
- Fabrication of CoTiO3/g-C3N4 hybrid photocatalysts with enhanced H2 evolution: Z-scheme photocatalytic mechanism insight. ACS Appl. Mater. Interf.. 2016;8(22):13879-13889.
- [Google Scholar]
- Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev.. 2014;114(19):9987-10043.
- [Google Scholar]
- Graphitic carbon nitride with S and O codoping for enhanced visible light photocatalytic performance. RSC Adv.. 2017;7(26):15842-15850.
- [Google Scholar]
- Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4–Pt nanocomposite photocatalysts. PCCP. 2014;16(23):11492-11501.
- [Google Scholar]
- Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4-Pt nanocomposite photocatalysts. PCCP. 2014;16(23):11492-11501.
- [Google Scholar]
- Ternary-component reduced graphene oxide aerogel constructed by g-C3N4/BiOBr heterojunction and graphene oxide with enhanced photocatalytic performance. J. Alloys Compd.. 2017;729:162-170.
- [Google Scholar]
- Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production. Appl. Catal. B. 2017;219:693-704.
- [Google Scholar]
- Enhanced photocatalytic degradation of tetracycline under visible light by using a ternary photocatalyst of Ag3PO4/AgBr/g-C3N4 with dual Z-scheme heterojunction. Sep. Purif. Technol.. 2020;237:116365.
- [Google Scholar]
- Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism. J. Mater. Chem. A. 2015;3(39):19936-19947.
- [Google Scholar]
- Synthesis of nanosized NaTaO3 in low temperature and its photocatalytic performance. J. Solid State Chem.. 2004;177(11):3868-3872.
- [Google Scholar]
- Large impact of heating time on physical properties and photocatalytic H2 production of g-C3N4 nanosheets synthesized through urea polymerization in Ar atmosphere. Int. J. Hydrogen Energy. 2013;38(30):13159-13163.
- [Google Scholar]
- Water-soluble ribbon-like graphitic carbon nitride (g-C3N4): green synthesis, self-assembly and unique optical properties. J. Mater. Chem. C. 2014;2(39):8212-8215.
- [Google Scholar]
- Enhanced photocatalytic H2 evolution over noble-metal-free NiS cocatalyst modified CdS nanorods/g-C3N4 heterojunctions. J. Mater. Chem. A. 2015;3(35):18244-18255.
- [Google Scholar]
- Yuan, J., et al., 2014. Novel 3-D nanoporous graphitic-C3N4 nanosheets with heterostructured modification for efficient visible-light photocatalytic hydrogen production.
- Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: Mechanism exploration from both experimental and DFT studies. Appl. Catal. B: Environ.. 2019;248:1-10.
- [Google Scholar]
- Synthesis of nitrogen vacancies g-C3N4 with increased crystallinity under the controlling of oxalyl dihydrazide: visible-light-driven photocatalytic activity – ScienceDirect. Appl. Surf. Sci.. 2020;505
- [Google Scholar]
- Fabrication of carbon nitride nanotubes by a simple water-induced morphological transformation process and their efficient visible-light photocatalytic activity. RSC Adv.. 2014;4(103):59513-59518.
- [Google Scholar]
- Ni12P5 nanoparticles embedded into porous g-C3N4 nanosheets as a noble-metal-free hetero-structure photocatalyst for efficient H 2 production under visible light. J. Mater. Chem. A. 2017;5(31):16171-16178.
- [Google Scholar]
- Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B. 2018;224:1-9.
- [Google Scholar]
- High-pressure bulk synthesis of crystalline C6N9H3⊙ HCl: a novel C3N4 graphitic derivative. J. Am. Chem. Soc.. 2001;123(32):7788-7796.
- [Google Scholar]
- Co-monomer control of carbon nitride semiconductors to optimize hydrogen evolution with visible light. Angew. Chem. Int. Ed.. 2012;51(13):3183-3187.
- [Google Scholar]
- Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core/shell CdS/g-C3N4 nanowires. ACS Appl. Mater. Interf.. 2013;5(20):10317-10324.
- [Google Scholar]
- Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc.. 2013;135(1):18-21.
- [Google Scholar]
- Biopolymer-activated graphitic carbon nitride towards a sustainable photocathode material. Sci. Rep.. 2013;3(1):1-5.
- [Google Scholar]
- Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep.. 2013;3(1):1943.
- [Google Scholar]
- Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface. Adv. Mater.. 2014;26(24):4121-4126.
- [Google Scholar]
- A polymer–metal–polymer–metal heterostructure for enhanced photocatalytic hydrogen production. J. Mater. Chem. A. 2015;3(1):109-115.
- [Google Scholar]
- Photocatalytic CO2 reduction over B4C/C3N4 with internal electric field under visible light irradiation. J. Colloid Interface Sci.. 2016;464:89-95.
- [Google Scholar]
- Reversible assembly of graphitic carbon nitride 3D network for highly selective dyes absorption and regeneration. ACS Nano. 2016;10(9):9036-9043.
- [Google Scholar]
- Photodegradation of phenol via C3N4-agar hybrid hydrogel 3D photocatalysts with free separation. Appl. Catal. B. 2016;183:263-268.
- [Google Scholar]
- Separation free g-C3N4/SiO2 hybrid hydrogels as high active photocatalysts for TOC removal. Appl. Catal. B. 2016;194:105-110.
- [Google Scholar]
- High-efficiency broadband C3N4 photocatalysts: synergistic effects from upconversion and plasmons. ACS Catal.. 2017;7(9):6225-6234.
- [Google Scholar]
- Individual high-quality N-doped carbon nanotubes embedded with nonprecious metal nanoparticles toward electrochemical reaction. ACS Appl. Mater. Interf.. 2018;10(46):39757-39767.
- [Google Scholar]
- Research progress on g–C3N4–based photocatalysts for organic pollutants degradation in wastewater: from exciton and carrier perspectives. Ceram. Int.. 2021;47(22):31005-31030.
- [Google Scholar]
- Two-dimensional covalent carbon nitride nanosheets: synthesis, functionalization, and applications. Energy Environ. Sci.. 2015;8(11):3092-3108.
- [Google Scholar]
- Surface modification of carbon nitride polymers by core–shell nickel/nickel oxide cocatalysts for hydrogen evolution photocatalysis. ChemCatChem. 2015;7(18):2864-2870.
- [Google Scholar]
- Fabrication of atomic single layer g-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B. 2014;152–153:46-50.
- [Google Scholar]
- In situ light-assisted preparation of MoS2 on g-C3N4 nanosheets for enhanced photocatalytic H2 production from water. J. Mater. Chem. A. 2015;3(14):7375-7381.
- [Google Scholar]
- Noble-metal-free iron phosphide cocatalyst loaded graphitic carbon nitride as an efficient and robust photocatalyst for hydrogen evolution under visible light irradiation. ACS Sustain. Chem. Eng.. 2017;5(9):8053-8060.
- [Google Scholar]
- Co-porphyrin/carbon nitride hybrids for improved photocatalytic CO2 reduction under visible light. Appl. Catal. B. 2017;200:141-149.
- [Google Scholar]
- Nitrogen defects-rich 0D/2D α-Fe2O3/g-C3N4 Z-scheme photocatalyst for enhanced photooxidation and H2 evolution efficiencies. Nano brief reports and reviews. Nano. 2018;13(7)
- [Google Scholar]
- Graphitic carbon nitride materials: controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci.. 2012;5(5):6717-6731.
- [Google Scholar]
- Shell-engineering of hollow g-C3N4 nanospheres via copolymerization for photocatalytic hydrogen evolution. Chem. Commun.. 2015;51(47):9706-9709.
- [Google Scholar]
- Layering MoS2 on soft hollow g-C3N4 nanostructures for photocatalytic hydrogen evolution. Appl. Catal. A. 2016;521:2-8.
- [Google Scholar]
- Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B. 2014;158:20-29.
- [Google Scholar]
- Brand new P-doped g-C3N4: enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A. 2015;3(7):3862-3867.
- [Google Scholar]
- n/n junctioned g-C3N4 for enhanced photocatalytic H 2 generation. Sustain. Energy Fuels. 2017;1(2):317-323.
- [Google Scholar]
- Boron carbon nitride semiconductors decorated with CdS nanoparticles for photocatalytic reduction of CO2. ACS Catal.. 2018;8(6):4928-4936.
- [Google Scholar]
- Metal-free hydrophilic DA conjugated polyelectrolyte dots/g-C3N4 nanosheets heterojunction for efficient and irradiation-stable water-splitting photocatalysis. Appl. Catal. B. 2020;270:118852.
- [Google Scholar]
- Nitrogen vacancy mediated exciton dissociation in carbon nitride nanosheets: Enhanced hydroxyl radicals generation for efficient photocatalytic degradation of organic pollutants. J. Hazard. Mater.. 2020;387:122023.
- [Google Scholar]
- Graphitic carbon nitride: synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interf.. 2014;6(19):16449-16465.
- [Google Scholar]
- Mesoporous phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS Appl. Mater. Interf.. 2015;7(30):16850-16856.
- [Google Scholar]
- Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature. 2001;414(6864):625-627.
- [Google Scholar]
- Heavy metals occurrence, seasonal variation and enrichment in urban soils augmented with industrial waste. Polish J. Environ. Stud.. 2021;30(5):4871-4886.
- [Google Scholar]