Translate this page into:
Natural pigment zeaxanthin ameliorates lipopolysaccharides induced acute lung inflammation in both in vitro and in vivo models
⁎Corresponding author at: The Emergency Department, The Air Force Military Medical University Second Affiliated Hospital (Tangdu Hospital), Xi'an 710038, China. tdwangjianyu@outlook.com (Jianyu Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Natural pigments obtained from plants, animals, and microbes are not only a potent alternative to synthetic dyes in the food, textile, and cosmetic industries, but they also possess immense pharmacological properties. Carmine acids, carotenoids, flavonoids, indigo, anthocyanin, melanins, and curcumin are such natural pigments that have proven pharmacological properties. Zeaxanthin is one such natural water-soluble antioxidant pigment that belongs to the xanthophyll family and is predominantly accumulated in the retina of the eyes. The potency of zeaxanthin against LPS-induced inflammation in in vitro and in vivo conditions was examined. RAW264.7 cells were treated with zeaxanthin and challenged with LPS. The LPS-induced zeaxanthin-treated cells were subjected to assessments of cytotoxicity and inflammatory markers. For the in vivo study, BALB/c male mice were induced to have acute lung inflammation by LPS. The ALI-induced mice were treated with 25 and 50 mg/kg concentrations of zeaxanthin. BALF was collected from zeaxanthin-treated ALI-induced mice and was sacrificed for the excision of lung tissue. Pulmonary edema was examined in the lung tissues. Immune cell infiltration and protein content were examined in the BALF collected. Oxidative stress induction was analyzed in the lung tissue of ALI-induced mice. The inflammatory markers iNOS, COX-2, and PGE-2 were quantified in the lung tissues of zeaxanthin-treated ALI-induced mice. Zeaxanthin effectively prevented lung edema and immune cell infiltration. Oxidative stress and inflammatory cytokine synthesis induced by LPS in the lungs were significantly decreased with zeaxanthin treatment. Histopathological analysis also confirms our in vitro and in vivo biochemical analyses. Overall, our findings corroborate that zeaxanthin is a potent anti-inflammatory agent that effectively inhibits LPS-induced ALI in both in vitro and in vivo conditions.
Keywords
Natural pigments
Zeaxanthin
RAW264.7 cells
BALB/c mice
Inflammation
Oxidative stress
1 Introduction
Natural pigments are bioactive compounds extracted from various sources, such as minerals, plants, animals, microbes, and marine organisms. In recent decades, studies on the utilization of natural pigments instead of synthetic dyes have drastically increased (Martins et al., 2023). Even though synthetic dyes are cheap, easy to manufacture, and long-lasting their bioavailability and biosafety are questionable. Hence, at present, the usage of natural dyes in food, cosmetic and clothing sectors has increased (Di Salvo et al., 2023; De Faria et al., 2022). Recently, the utility of natural dyes has extended to the pharmaceutical industry. Natural pigments are not only non-hazardous; they possess immense antioxidant, antidiabetic, antitumor, anti-obesity, anti-microbial, and anti-inflammatory properties (Li et al., 2022; Fayez et al., 2022). Indigo, curcumin, β-carotene, anthocyanins, flavonoids, lycopene, lutein, and betalains are some of the most commonly used natural pigments in the food industry, which also have incredible pharmacological properties (Mohammad Azmin et al., 2022).
Zeaxanthin (β,β-Carotene-3,3′-diol), a carotenoid with 11 conjugate double bonds and 568.8 Daltons of molecular weight, is a natural lipid-soluble antioxidant belonging to the xanthophyll family (Sajilata et al., 2008). Zeaxanthin is not biosynthesized by humans; hence, the dietary source is the only option (Delgado-Pelayo and Hornero-Méndez, 2012). Zeaxanthin is present in oranges, squash, papaya, corn, egg yolk, nectarines, and orange pepper, which was found to contain the highest level of zeaxanthin (Sommerburg et al., 1998; Mozaffarieh et al., 2003; Sparrow and Kim, 2009). Zeaxanthin predominantly accumulates in the retina of the eyes and macula (Billsten et al., 2003). Zeaxanthin has been reported to prevent cataracts and other age-related eye problems (Moeller et al., 2000; Arunkumar et al., 2018). It is also proven to be a potent antioxidant and ameliorates systemic inflammation (Murillo et al., 2019). Studies with animal and human models suggest zeaxanthin treatment is effective against curing stroke, coronary heart disease, and macular degeneration stroke (Ribaya-Mercado and Blumberg, 2004).
Acute lung injury is a life-threatening infection that worsens acute respiratory distress, increases the severity of morbidity, and also causes mortalities in patients with septic shock, pneumonia infection, and ischemia (Cross and Matthay, 2011). ALI-induced mortality is more commonly reported in patients in intensive care units (Zhang et al., 2015). ALI is caused by the uncontrolled triggering of inflammatory cells, leading to airway dysfunction, hypoxemia, and lung tissue damage (Shin et al., 2015). At present, corticosteroids are the first-line treatment given for subsiding ALI-induced symptoms. There is no drug available for a complete cure; hence, the long-term usage of these steroids causes comorbidities in patients (Ayodele et al., 2022). Natural pigments may be a potent alterative to the currently available steroids to treat ALI and ADRS.
The lipopolysaccharide-induced ALI/ARDS mouse model is a well-recognized mouse model for studying the mechanism of action. It mimics the ALI pathogenesis exactly with the induction and infiltration of macrophages and neutrophils, thereby inducing inflammation in the lungs (Liu et al., 2010; Wei et al., 2012). In this study, we analyzed the ameliorative effect of the natural pigment zeaxanthin against LPS-induced acute lung inflammation.
2 Materials and methods
2.1 Chemicals
Zeaxanthin (molecular weight: 568.87; molecular formula: C40H56O2; purity: ≥95.0 %) and other chemicals were purchased from Sigma Aldrich, USA.
2.2 Cell culture
RAW 264.7 cells were utilized for the current study to analyze the cytotoxic nature of zeaxanthin. Upon arrival, the cells were examined for contamination and cultured in DMEM medium. The medium containing 10 % FBS and 1 % antibiotic cocktail was used to avoid contamination. The cells were cultured in a 5 % CO2-supplied incubator at 37˚C. Upon attaining 80 % confluency, the cells were subjected to trypsinization with a Trypsin-EDTA solution and subcultured.
2.3 Cytotoxicity assay
RAW 264.7 cells were cultured in a 96-well culture plate for cytotoxicity examination of zeaxanthin. The cells were treated with different concentrations of zeaxanthin ranging from 0 to 10 µM and incubated for 24 h in a 5 % CO2 incubator at 37˚C. To the 24 h zeaxanthin-treated cells, 5 mg/ml concentration of MTT was added (20 µl/well) and incubated further for 4 h. After the incubation period, the supernatant was removed from all the wells, and the formazan crystals formed were dissolved in DMSO solution (150 µl/well). The OD of the dissolved formazan crystals in each well was measured using a microplate reader at 570 nm. The experiments were repeated thrice, and each sample was examined in triplicate.
The cytoprotective property of zeaxanthin against immune cell activator LPS was assessed by challenging the zeaxanthin-treated RAW 264.7 cells with 1 µg/ml of LPS. The zeaxanthin-treated cells stimulated with LPS were then subjected to cytotoxicity assays to examine the viability of the cells.
2.4 Pro-inflammatory cytokines quantification
The pro-inflammatory cytokine levels in the LPS stimulated zeaxanthin challenged RAW 264.7 cells were quantified using the commercially available ELISA kits (MyBiosource, USA). The 5 and 7.5 µM zeaxanthin-treated cells were stimulated with LPS. The cells were trypsinized and subjected to sonication. The cell lysate was subjected to centrifugation at 2500 rpm for 5 min at 4˚C. The supernatant was collected and quantified for pro-inflammatory cytokines as per the instructions of the manufacturer provided in the kit.
2.5 Animals
The experimental procedures to be conducted on the mice were clearly explained to the ethical committee, and the same was followed during the study. Healthy, young, 3–4-week-old male BALB/c mice were housed in hygienic polypropylene cages with free access to food and water. Mice were fed standard laboratory rodent pellets. The mice were acclimatized for a period of a week at a temperature of 24 ± 2˚C with a humidity of 60 ± 10 % and a strict 12-hour light–dark cycle. The rodents were treated with the utmost care and concern; no mortality was observed throughout the experiment period.
2.6 Induction of acute lung inflammation model
An acute lung inflammation model was induced in mice by treating it with endotoxin lipopolysaccharides. The 5 mg/kg bwt of LPS was inoculated intratracheally to induce inflammation in mice. The LPS treatment was given for three successive days, and the animals were observed for any lethal abnormalities during the treatment period.
2.7 Grouping & treatment
The mice were randomly grouped into four as that each group consists of six.
Group I – Control mice treated with saline alone.
Group II – Acute lung inflammation induced mice.
Group III – 25 mg/kg bwt zeaxanthin was treated for 3 successive days to ALI induced mice.
Group IV – 50 mg/kg bwt zeaxanthin was treated for 3 successive days to ALI induced.
After 24 h of zeaxanthin treatment, BALF samples were collected, and the animals were anesthetized and further subjected to cervical dislocation. Blood samples and tissue samples were collected for further analysis.
2.8 BALF collection
Bronchoalveolar lavage fluid was collected from the anesthetized mice according to the protocol of Daubeuf et al. (Daubeuf and Frossard, 2012). The mice were subjected to tracheostomy, and a semi-excision was made on the trachea, which will allow them to pass a 21-gauge lavage tube. The tube and the needle were attached with cotton thread. 500 µl of saline was injected into the tube, and a chest massage was given for 10 s. The lavage was then aspirated back and stored in ice. The procedure was repeated five times, and the lavages collected were pooled together for further analysis.
2.9 Relative organ weight
The body weight of the mice was measured with a digital weighing machine, and the lung weight of each mouse was measured to calculate the relative organ weight. The dry weight of the lungs was measured by drying the tissue in an oven for 72 h at 70˚C, and the weight of the dried tissue was measured.
2.10 BALF total protein concentration
The concentration of total protein in BALF was quantified using the commercially available protein assay kit procured from ThermoScientific USA (Pierce BCA). The experiment was performed in triplicate as per the instructions provided by the manufacturer. The final absorbance of the sample was measured at 550 nm using a microplate reader. The concentration of protein was calculated using the standard curve plotted with different known concentrations of albumin.
2.11 BALF cell count
The erythrocytes in the BALF were removed by adding 1.5 ml of water to the cell pellet, followed by the addition of 0.5 ml of 0.6 M KCl, and the mixture was mixed gently. The suspension was centrifuged at 2000 rpm for 5 min, and the pellet was collected. To the pellet, 0.5 ml of saline-EDTA solution was added and mixed gently. The cell suspension was utilized for total and differential cell counts.
The 5 µl of cell suspension was loaded onto the hemocytometer, and the total number of cells was counted under the microscope manually. The differential cell count was counted by centrifuging 200 µl of cell suspension at 700 rpm for 5 min and then stained with Diffi-Quick stain.
2.12 Determination of lipid peroxidation
Lipid peroxidation in ALI-induced, untreated, and zeaxanthin-treated lung tissue was assessed according to the protocol of Devasagayam and Tarachand (1987) (Devasagayam and Tarachand, 1987). Thiobarbituric acid reacts with malondialdehyde, the end product of lipid peroxidation, to form a pink chromogen adduct. The final product absorbance was measured at 532 nm. The levels were expressed as nmoles/g tissue.
2.13 Determination of SOD
The superoxide dismutase concentration in the experimental animals was measured according to the protocol of Beauchamp and Fridovich (1971) (Beauchamp and Fridovich, 1971). SOD in the sample decreases the riboflavin-produced superoxide anions, thereby decreasing the formazan formation. The SOD concentration in the sample is directly proportional to the formazan formation. The concentration of SOD in the sample was expressed as Unit/g tissue.
2.14 Determination of GSH
The concentration of GSH in the lung tissue of experimental animals was measured according to the procedure of Beutler et al. (1963) (Beutler et al., 1963). The samples were titrated with 0.1 mmol/l of 5,5′ Dithibios (2-nitrobenzoic acid). The end product, reduced thionitrobenzene, was measured at 412 nm. The levels of GSH were expressed as µg/g tissue.
2.15 Quantification of inflammatory enzymes
The levels of proinflammatory enzymes iNOS, COX-2, and PGE-2 in the lung tissue of experimental mice were quantified using the ELISA kit procured from Sigma Aldrich, USA. The assay was performed according to the kit protocol provided by the manufacturer.
2.16 Lung histopathology
Excised lung tissue was fixed with 10 % neutral formalin for 48 h and then the tissue was subjected to dehydration with a series of alcohol. The processed lung tissue was then embedded in paraffin wax and cut into 5 µm. The tissue was then deparaffinized and stained with hematoxylin and eosin stains. The stained tissue sections were examined under a light microscope for histopathological changes. The lung injury induced by LPS and the protective effect of zeaxanthin were assessed by scoring the stained tissue sections from 0 to 5 according to the rate of inflammatory cell infiltration and dissemination.
2.17 Statistical analysis
The results were statistically analyzed with GraphPad Prism software. The difference among the group were examined with one way analysis of variance and the difference between the individual sample in a group was assessed with Student t test, p < 0.05 was considered as statistical significance.
3 Results
3.1 Cytoprotective effect of natural pigment zeaxanthin
Fig. 1a depicts the results of the MTT assay performed on zeaxanthin-treated RAW264.7 cells. Only a minimal percentage of cell death was observed in zeaxanthin-treated cells. 7.5 µM zeaxanthin-treated cells showed 9 ± 0.5 % of cell death, and it was further reduced to 4 ± 0.6 % of cell death in 5 µM zeaxanthin treated cells. The 5 and 7.5 µM zeaxanthin-treatments showed negligible percentages of cell death; hence, these two doses were utilized for the further examination of the zeaxanthin protective effect against LPS-induced inflammation.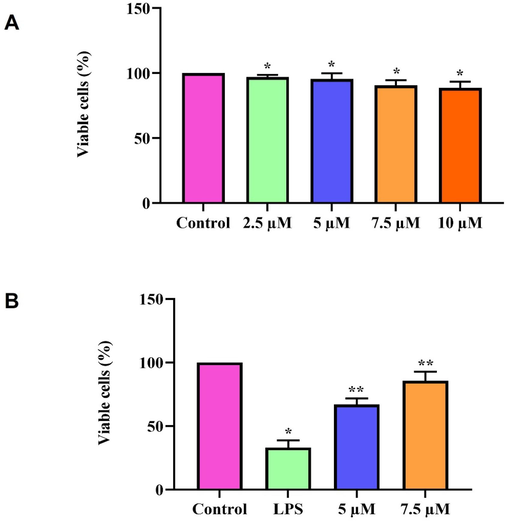
Cytoprotective effect of natural pigment zeaxanthin. A. RAW264.7 cells were treated with 0–10 µM for 24 h. MTT assay was performed. B) RAW264.7 cells were treated with zeaxanthin 5 &10 µM concentration challenged with 1 µg/ml LPS. MTT assay was performed. Results were statistically analyzed with GraphPad Prism software. One-way ANOVA followed by Student’s t test was done. Statistical significance p < 0.05.
3.2 Effect of natural pigment zeaxanthin against endotoxin LPS
The anti-inflammatory property of zeaxanthin was examined in RAW264.7 cells by inducing inflammation with LPS treatment. LPS treatment significantly increased cell death in RAW264.7 cells, whereas zeaxanthin prevented LPS-induced cell death. The percentage of cell death decreased in a dose-dependent manner. 5 µM zeaxanthin treatment showed 45 ± 3 % of cell death, whereas it was significantly reduced in 7.5 µM zeaxanthin treatment, which showed only 18 ± 2 % of cell death (Fig. 1b).
3.3 Anti-inflammatory effect of natural pigment zeaxanthin in vitro
The induction of pro-inflammatory cytokines by LPS treatment and the defensive role of zeaxanthin against LPS-induced inflammatory cytokines were measured using the ELISA technique (Fig. 2). LPS treatment significantly increased the proinflammatory cytokines TNF-α, IL-1β, and IL-6, whereas zeaxanthin treatment inhibited the production of LPS-induced pro-inflammatory cytokine synthesis. Zeaxanthin treatment decreased the levels of TNF-α, IL-1β, and IL-6 in a dose-dependent manner, which proves the anti-inflammatory property of zeaxanthin.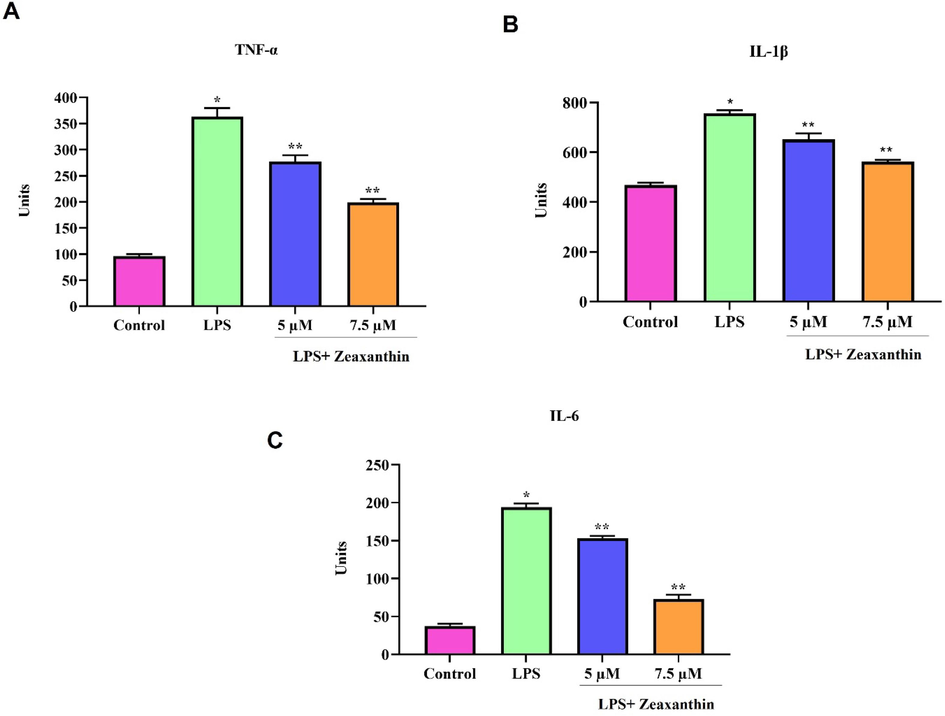
Anti-inflammatory effect of natural pigment zeaxanthin in vitro. RAW264.7 cells were treated with zeaxanthin 5 & 7.5 µM concentration challenged with 1 µg/ml LPS. ELISA technique was performed. A) TNF-α, B) IL-1β, C) IL-6. Results were statistically analyzed with GraphPad Prism software. One-way ANOVA followed by Student’s t test was done. Statistical significance p < 0.05.
3.4 Ameliorative effect of natural pigment zeaxanthin against LPS induce pulmonary edema
Fig. 3A&B depict the relative organ weight and dry/wet ratio of lung tissue in LPS-induced zeaxanthin-treated mice. LPS treatment significantly increased the relative organ weight of the lungs, and it also caused pulmonary edema in mice, as evidenced by the increase in the dry/wet weight ratio compared to the control mice. Zeaxanthin treatment significantly repressed the pulmonary edema in ALI mice.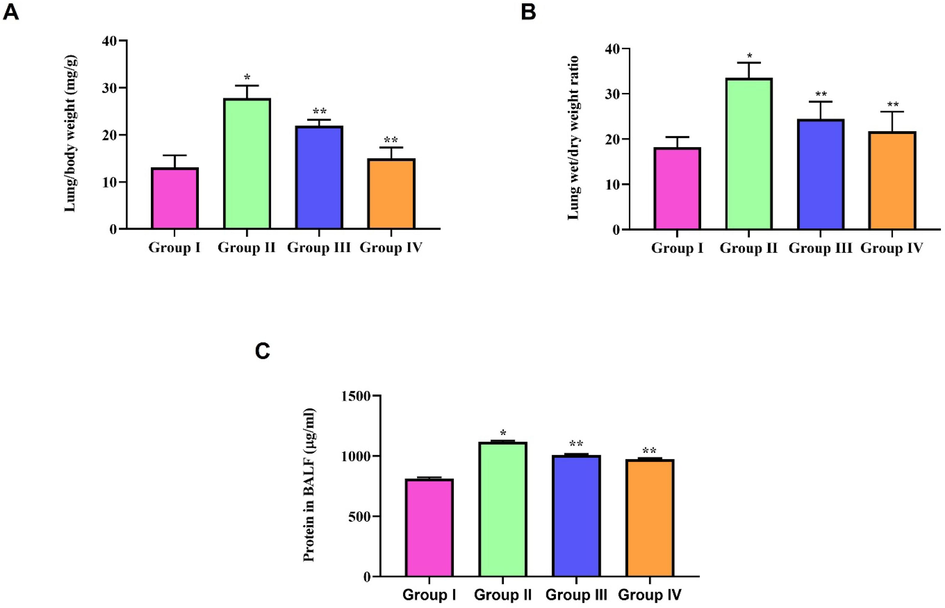
Ameliorative effect of natural pigment zeaxanthin against LPS induce pulmonary edema. BALB/c male mice were induced acute lung inflammation with LPS. A) Relative organ weight B) Dry/Wet weight ratio C) BALF Protein concentration. Results were statistically analyzed with GraphPad Prism software. One-way ANOVA followed by Student’s t test was done. Statistical significance p < 0.05. n = 6 mice.
3.5 Inhibitory effect of zeaxanthin against LPS induced infection
Fig. 3C illustrates the levels of protein concentration in the BALF of LPS-induced zeaxanthin-treated mice. LPS-induced respiratory infection leads to protein-rich fluid exudation into the alveolar space, whereas zeaxanthin treatment significantly prevented the infection, which was indicated by a reduction in BALF protein concentration compared to the LPS-only-treated cells.
3.6 Effect of natural pigment zeaxanthin against LPS activated immune cells
Endotoxin LPS treatment significantly increased the immune cells in BALF, whereas zeaxanthin decreased the total WBC count in BALF. The differential count results also prove that zeaxanthin treatment significantly decreased the levels of LPS-induced neutrophils, macrophages, and lymphocytes in the BALF of LPS-treated cells. The decrease in immune cells was observed in a dose-dependent manner (Fig. 4).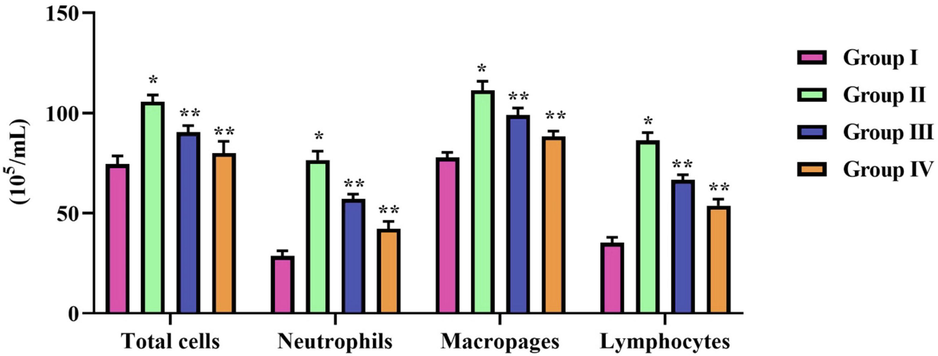
Effect of natural pigment zeaxanthin against LPS activated immune cells. BALB/c male mice were induced acute lung inflammation with LPS. Total cell count done manually with hematocytometer and Diffi-Quick stain was used to assess the differential count in BALF of LPS and zeaxanthin treated mice. Results were statistically analyzed with GraphPad Prism software. One-way ANOVA followed by Student’s t test was done. Statistical significance p < 0.05. n = 6 mice.
3.7 Antioxidant effect of natural pigment zeaxanthin on ALI induced mice
LPS treatment significantly increased the oxidative stress in mice, which was confirmed by increased lipid peroxidation and decreased antioxidant SOD and GSH levels. The antioxidant SOD and GSH levels were increased with zeaxanthin in a dose-dependent manner, which proves the antioxidant property of zeaxanthin. It also decreased LPS-induced lipid peroxidation in the lung tissue of mice (Fig. 5).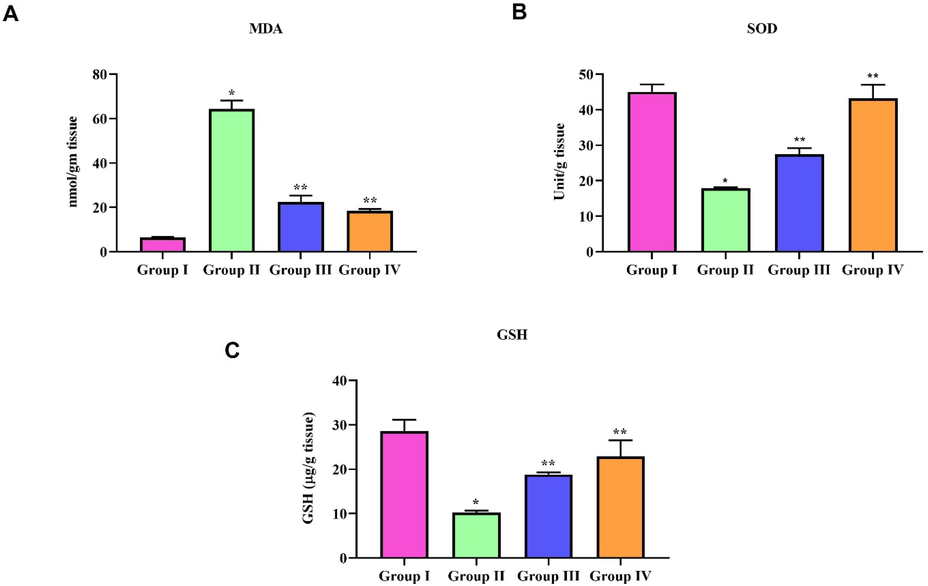
Antioxidant effect of natural pigment zeaxanthin on ALI induced mice. BALB/c male mice were induced acute lung inflammation with LPS. Oxidative stress markers were assessed in the lung tissue homogenate of LPS and zeaxanthin treated mice. A) MDA, B) SOD C) GSH. Results were statistically analyzed with GraphPad Prism software. One-way ANOVA followed by Student’s t test was done. Statistical significance p < 0.05. n = 6 mice.
3.8 Anti-inflammatory effect of natural pigment zeaxanthin on ALI induce mice
Fig. 6 represents the levels of the anti-inflammatory cytokine iNOS. COX-2 and PGE2 levels in LPS-induced and zeaxanthin-treated mice lung tissue. LPS treatment significantly triggered the inflammatory cytokine iNOS. COX-2 and PGE2 levels in the lungs of mice. Zeaxanthin treatment significantly prevented the pulmonary tissue from LPS-induced inflammation; it prevented the LPS-induced synthesis of the inflammatory cytokine iNOS. COX-2 and PGE2 levels in the lungs of LPS-treated mice. The reduction in inflammatory cytokine levels in ALI mice was observed in a dose-dependent manner.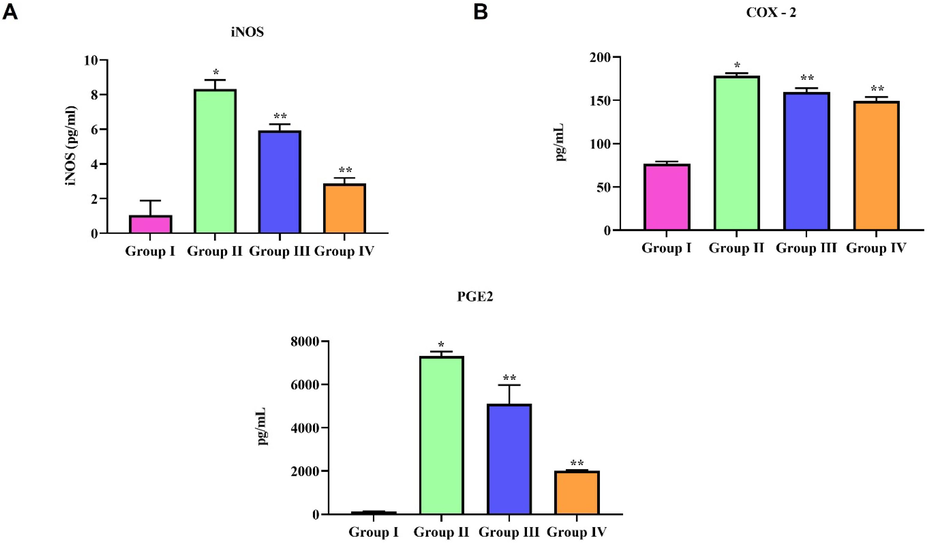
Anti-inflammatory effect of natural pigment zeaxanthin on ALI induce mice. BALB/c male mice were induced acute lung inflammation with LPS. Inflammatory cytokine levels in lung tissue homogenate of LPS and zeaxanthin treated mice were measured using ELISA technique. A) iNOS, B) COX-2 and C) PGE2. Results were statistically analyzed with GraphPad Prism software. One-way ANOVA followed by Student’s t test was done. Statistical significance p < 0.05. n = 6 mice.
3.9 Protective effect of natural pigment zeaxanthin against LPS induced lung histopathological changes in mice
Control group mice lung tissue showed no histopathological changes; the observed tissue section had a normal histoarchitecture of alveoli, bronchioles, and blood vessels (Fig. 7A). LPS-alone-treated mice lung tissue section (B) shows enlarged blood vessels with a thickened alveolar septa, and it shows an increased lung injury score compared to the control and zeaxanthin-treated mice. Zeaxanthin-treated mice showed few histopathological changes compared to LPS-alone-treated mice. The lung injury score was significantly reduced in 50 mg/kg zeaxanthin-treated mice (D) compared to the 25 mg/kg zeaxanthin-treated mice. The infiltration of cells and the alveolar septa thickness were considerably decreased in zeaxanthin-treated mice compared to LPS-treated mice lung tissue.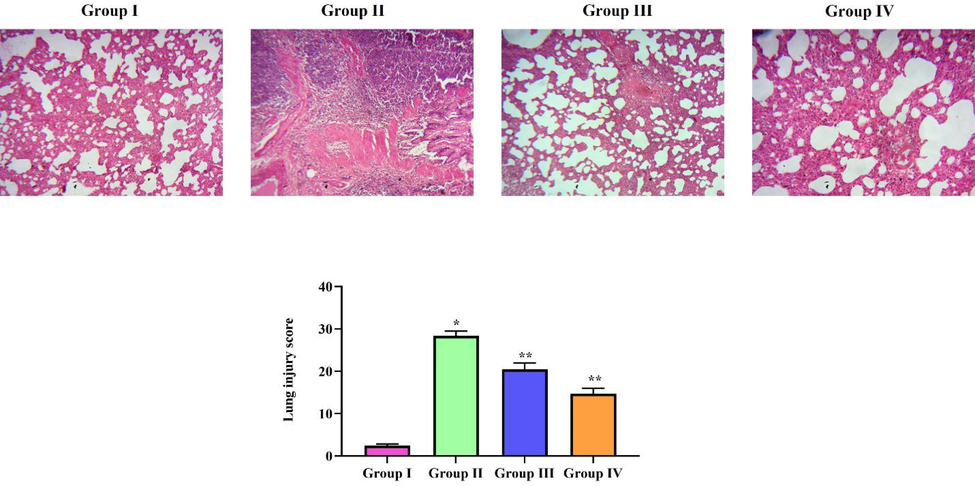
Protective effect of natural pigment zeaxanthin against LPS induced lung histopathological changes in mice. BALB/c male mice were induced acute lung inflammation with LPS. Lung tissue of LPS and zeaxanthin treated mice were processed and stained with H&E stain. The tissue section were subjected to lung injury scoring. A) Control B) ALI induced C) ALI induced treated with 25 mg/kg zeaxanthin D) ALI induced treated with 50 mg/kg zeaxanthin. Results were statistically analyzed with GraphPad Prism software. One-way ANOVA followed by Student’s t test was done. Statistical significance p < 0.05. n = 6 mice.
4 Discussion
Acute lung inflammation, a disease with significant global mortality and morbidity rates possesses a multifactorial etiology (Patel et al., 2019). Septic shocks, pneumonia, burns, and trauma are some of the factors that induce ALI progression (Wang et al., 2020). The pathogenesis of ALI includes pulmonary edema, depletion of antioxidant enzymes, pulmonary injury, an uncontrolled inflammatory response, epithelial dysfunction, and excessive infiltration of immune cells (Ware and Matthay, 2000; Butt et al., 2016). Corticosteroids, bronchodilators, leukotriene modifiers, and anti-inflammatory drugs are some of the medications prescribed to relieve symptoms in ALI patients. These medications provide only temporary relief, and long-term usage causes comorbidities; hence, a potent alternative for these medicines is needed today. In this study, we examined the ameliorative potency of the natural pigment zeaxanthin against LPS-induced acute lung inflammation.
We assessed the defensive effect of zeaxanthin against acute lung inflammation using an LPS-induced ALI model. Lipopolysaccharides are glycolipid proteins present on the cell walls of Gram-negative bacteria (Mayeux, 1997). These polysaccharides effectively trigger the immune cells, causing an inflammatory response. The administration of LPS via intratracheal or intranasal channel triggers the generation of reactive oxygen species and proinflammatory cytokines, which in turn lead to the immune cells migration to the alveolar space, and the uncontrolled disproportionate inflammatory response causes damage to lung tissue (Huang et al., 2015; Wang et al., 2021). The LPS-induced ALI model is globally accepted for assessing the pathophysiological and signaling mechanisms of action during acute lung inflammation (Philippakis et al., 2008; Xie et al., 2014; Ali et al., 2020).
In an in vitro model, we analyzed the efficacy of zeaxanthin in RAW264.7 macrophage cells by challenging them with LPS, since macrophages play a vital role in phagocytosis during physiological and infection conditions (Zhang and Wang, 2014). Zeaxanthin treatment did not show any considerable cytotoxic effect on RAW264.7 macrophage cells when treated with varied doses. It also prevented RAW264.7 cell death when challenged with lipopolysaccharides. Alveolar macrophages trigger the proinflammatory cytokines TNF-α, IL-1β, and IL-6 as an early response during exposure to toxicants such as LPS (Bhatia and Moochhala, 2004; Parsons et al., 2005; Chu et al., 2012; Yang et al., 2015). The uncontrolled synthesis of these inflammatory cytokines leads to acute lung inflammation. These cytokines were reported to be elevated in the cases of mortalities caused by acute lung inflammation (Neurath et al., 1997; Ma, 2001). Therefore, in our study, we treated RAW264.7 macrophage cells with zeaxanthin and challenged them with LPS to assess the levels of pro-inflammatory cytokines. The pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 levels were decreased in LPS-challenged macrophage cells treated with zeaxanthin.
ALI/ARDS are characterized by the unrestrained response of pulmonary immune cells, causing infiltration of immune cells in the alveolar space, epithelial cell disruption, interstitial edema, and lung tissue injury (Fan and Fan, 2018; Li et al., 2020). Inflammatory diseases of the lungs, such as deadly COVID-19, asthma, ARDS, ALI, pulmonary fibrosis, and chronic obstructive pulmonary disease, indicate that macrophage polarization induces the excessive synthesis of cytokines, chemokines, and transcription factors, which leads to lung tissue damage (Saradna et al., 2018 Jan; Booz et al., 2020; Chen et al., 2020). Macrophages and neutrophils work hand in hand to evacuate microbial and other allergens from the host. In conditions such as ALI, these alveolar macrophages polarize into M1/M2 macrophages to lead the exudative, rehabilitation, and fibrotic phases of pathogenicity (Lee et al., 2021). Excessive infiltration of neutrophils triggers cytokine synthesis, which causes lung tissue damage (Zemans and Matthay, 2017). The infiltration of neutrophils and macrophages into alveolar space, which was evidenced in the BALF, was decreased with zeaxanthin treatment. The inhibition of neutrophil influx further prevented LPS-challenged mice from pulmonary edema and lung tissue damage.
Neutrophil influx during ALI/ADRS causes the excessive release of oxidants and proteases (Abraham, 2003; Guo and Ward, 2007; Kantrow et al., 2009). LPS intensifies the inflammatory response in mice by inducing free radical generation and causing oxidative stress in lung tissue (El-Sayed et al., 2011). The host defense mechanism produces high levels of superoxide anions and other reactive oxygen species during the encounter of microbial invasion (Ong et al., 2003). During some conditions, this respiratory burst of inflammatory cells, in spite of guarding the host, suppresses the antioxidant system and causes tissue injury (Weiss, 1989). Zeaxanthin treatment in LPS-challenged had significantly scavenged free radicals and prevented lung tissue from lipid peroxidation. It also increased the levels of the prime antioxidants SOD and GSH, whose fluctuations were often reported in ALI patients (Khazri et al., 2016; Zhang et al., 2017).
Nitric oxide synthase catalyzes the L-arginine to L-citrulline conversion, which releases nitric oxide. Each type of NOS plays different roles in homeostasis maintenance, such as neuromodulators, neurotransmitters, blood vessel regulators, etc. Inducible nitric oxide synthase (iNOS), specifically elevated during inflammation, was reported in various inflammatory diseases (Cedergren et al., 2002; Donnelly and Barnes, 2002; Gao et al., 2022). In our study, iNOS levels were significantly increased with LPS treatment, whereas zeaxanthin treatment reduced the expression of LPS-induced iNOS in ALI mice. Most anti-inflammatory and analgesic drugs target the cyclooxygenase, specifically COX-2, to alleviate inflammation. COX-2 levels were significantly increased in the lungs during exposure to endotoxins (Amann et al., 1999). Stimulated alveolar macrophages trigger the expression of COX-2, which in turn increases prostanoids and pulmonary edema (Salih et al., 2023). COX-2 metabolizes arachidonic acid in alveolar epithelial cells to produce the cyclooxygenase metabolite PGE2, which further worsens the inflammation. Subsequent COX-2 activity prevents the synthesis of PGE-2, thereby protecting against inflammation-induced lung tissue damage (Vancheri et al., 2004; Lukkarinen et al., 2006). In our study, zeaxanthin treatment significantly inhibited COX-2 levels and diminished PGE-2 levels in the lung tissues of ALI-induced mice.
LPS-induced lung tissue damage was analyzed with histopathological analysis. Zeaxanthin treatment decreased endothelila permeability, alveolar thickening, and neutrophil infiltration in LPS-challenged mice (Yin et al., 2008). It correlates with our biochemical analysis, where zeaxanthin treatment scavenged free radicals and inhibited the synthesis of pro-inflammatory cytokines. Both our in vitro and in vivo results confirmed the anti-inflammatory potency of zeaxanthin against LPS-induced acute inflammation.
5 Conclusion
To summarize, our results support the ameliorative effect of zeaxanthin against LPS-induced acute lung inflammation. Zeaxanthin treatment is non-toxic to RAW264.7 macrophage cells, and it also significantly prevents the apoptosis of RAW264.7 macrophage cells from LPS-induced inflammatory cytokines. Zeaxanthin treatment increased the antioxidants and inhibited lipid peroxidation in the lung tissue of LPS-challenged mice. It also inhibited pro-inflammatory iNOS, COX-2, and PGE2 synthesis and prevented pulmonary edema and tissue damage in ALI-induced mice.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP2024R5) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Neutrophils and acute lung injury. Crit. Care Med.. 2003 Apr;31(4 Suppl):S195-S199.
- [Google Scholar]
- Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol. Toxicol.. 2020 Nov 25;21(1):81.
- [Google Scholar]
- Eicosanoid release in the endotoxin-primed isolated perfused rat lung and its pharmacological modification. Inflamm. Res.. 1999 Dec;48(12):632-636.
- [Google Scholar]
- What do we know about the macular pigment in AMD: the past, the present, and the future. Eye (Lond.). 2018 May;32(5):992-1004.
- [Google Scholar]
- Glucocorticoids and Risk of Venous Thromboembolism in Asthma Patients Aged 20–59 Years in the United Kingdom's CPRD 1995–2015. Clin. Epidemiol.. 2022 Jan;20(14):83-93.
- [Google Scholar]
- Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem.. 1971 Nov;44(1):276-287. PMID: 4943714
- [CrossRef] [Google Scholar]
- Improved method for the determination of blood glutathione. J. Lab. Clin. Med.. 1963;61(5):882-888.
- [Google Scholar]
- Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol.. 2004 Feb;202(2):145-156.
- [Google Scholar]
- Photophysical Properties of Xanthophylls in Carotenoproteins from Human Retina. Photochem. Photobiol.. 2003;78:138-145.
- [Google Scholar]
- Macrophage responses associated with COVID-19: A pharmacological perspective. Eur. J. Pharmacol.. 2020 Nov;15(887):173547
- [Google Scholar]
- Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med.. 2016 Apr;140(4):345-350.
- [Google Scholar]
- Inducible nitric oxide synthase is expressed in synovial fluid granulocytes. Clin. Exp. Immunol.. 2002;130:150-155.
- [Google Scholar]
- Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res.. 2020 Sep;69(9):883-895.
- [Google Scholar]
- Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J. Agric. Food Chem.. 2012 Apr 18;60(15):3947-3954.
- [Google Scholar]
- Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit. Care Clin.. 2011 Apr;27(2):355-377.
- [Google Scholar]
- Performing Bronchoalveolar Lavage in the Mouse. Curr Protoc Mouse Biol.. 2012 Jun 1;2(2):167-175. PMID: 26069010
- [CrossRef] [Google Scholar]
- Amaral De Faria Silva L, Ferreira Alves M, Florêncio Filho D, Aparecida Takahashi J, Soares Santos L, Almeida De Carvalho S. Pigment produced from Arcopilus aureus isolated from grapevines: Promising natural yellow colorants for the food industry. Food Chem. 2022 Sep 30;389:132967.
- Identification and quantitative analysis of carotenoids and their esters from sarsaparilla (Smilax aspera L.) berries. J. Agric. Food Chem.. 2012 Aug 22;60(33):8225-8232.
- [Google Scholar]
- Decreased lipid peroxidation in the rat kidney during gestation. Biochem. Biophys. Res. Commun.. 1987 May 29;145(1):134-138. PMID: 3593335
- [CrossRef] [Google Scholar]
- Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients. 2023 Apr 16;15(8):1923.
- [Google Scholar]
- Expression and regulation of inducible nitric oxide synthase from human primary airway epithelial cells. Am. J. Respir. Cell Mol. Biol.. 2002;26:144-151.
- [Google Scholar]
- Tempol, a membrane-permeable radical scavenger, ameliorates lipopolysaccharide-induced acute lung injury in mice: a key role for superoxide anion. Eur. J. Pharmacol.. 2011 Aug 1;663(1–3):68-73.
- [Google Scholar]
- Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res.. 2018 Mar 27;19(1):50.
- [Google Scholar]
- Carotegenic Virgibacillus halodenitrificans from Wadi El-Natrun Salt Lakes: Isolation, Optimization, Characterization and Biological Activities of Carotenoids. Biology. 2022;11(10):1407.
- [Google Scholar]
- Oridonin attenuates lung inflammation and fibrosis in silicosis via covalent targeting iNOS. Biomed. Pharmacother.. 2022 Sep;153:113532
- [Google Scholar]
- Role of oxidants in lung injury during sepsis. Antioxid. Redox Signal.. 2007 Nov;9(11):1991-2002.
- [Google Scholar]
- Anti-Inflammatory Effects of Monoammonium Glycyrrhizinate on Lipopolysaccharide-Induced Acute Lung Injury in Mice through Regulating Nuclear Factor-Kappa B Signaling Pathway. Evid. Based Complement. Alternat. Med.. 2015;2015:272474
- [Google Scholar]
- Neutrophil-mediated lung permeability and host defense proteins. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2009 Oct;297(4):L738-L745.
- [Google Scholar]
- Grape seed and skin extract protects against bleomycin-induced oxidative stress in rat lung. Biomed. Pharmacother.. 2016 Jul;81:242-249.
- [Google Scholar]
- The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells.. 2021 Apr 14;10(4):897.
- [Google Scholar]
- Hydrogen treatment prevents lipopolysaccharide-induced pulmonary endothelial cell dysfunction through RhoA inhibition. Biochem. Biophys. Res. Commun.. 2020 Feb 5;522(2):499-505.
- [Google Scholar]
- Insight into the Progress on Natural Dyes: Sources, Structural Features, Health Effects, Challenges, and Potential. Molecules. 2022 May 20;27(10):3291.
- [Google Scholar]
- Taraxacum officinale protects against lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol.. 2010 Jul 20;130(2):392-397.
- [Google Scholar]
- Inhibition of COX-2 aggravates neutrophil migration and pneumocyte apoptosis in surfactant-depleted rat lungs. Pediatr. Res.. 2006 Mar;59(3):412-417.
- [Google Scholar]
- TNF-α and IL-12: a balancing act in macrophage functioning. Microbes Infect.. 2001;3:121-129.
- [Google Scholar]
- Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: a comprehensive review. Molecules. 2023 Jul 11;28(14):5344.
- [Google Scholar]
- Pathobiology of lipopolysaccharide. J. Toxicol. Environ. Health 387. 1997;51(5):415-435.
- [Google Scholar]
- The potential role of dietary xanthophylls in cataract and age-related macular degeneration. J. Am. Coll. Nutr.. 2000 Oct;19(5 Suppl):522S-527S.
- [Google Scholar]
- A review on recent advances on natural plant pigments in foods: functions, extraction, importance and challenges. Appl. Biochem. Biotechnol.. 2022 Oct;194(10):4655-4672.
- [Google Scholar]
- The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: a review based on controversial evidence. Nutr. J.. 2003 Dec;11(2):20.
- [Google Scholar]
- Zeaxanthin: metabolism, properties, and antioxidant protection of eyes, heart, liver, and skin. Antioxidants (Basel). 2019 Sep 11;8(9):390.
- [Google Scholar]
- Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, KHM ZB, Strober W, Kollias G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur. J. Immunol. 1997;27:1743–1750.
- coli pneumonia induces CD18-independent airway neutrophil migration in the absence of increased lung vascular permeability. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2003 Oct;285(4):L879-L888.
- [Google Scholar]
- Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit. Care Med.. 2005;33:1-6.
- [Google Scholar]
- Extracorporeal membrane oxygenation as rescue therapy for severe hypoxemic respiratory failure. J. Thorac. Dis.. 2019 Sep;11(Suppl 14):S1688-S1697.
- [Google Scholar]
- Adrenaline attenuates the acute lung injury after intratracheal lipopolysaccharide instillation: an experimental study. Inhal. Toxicol.. 2008 Feb;20(4):445-453.
- [Google Scholar]
- Lutein and zeaxanthin and their potential roles in disease prevention. J. Am. Coll. Nutr.. 2004 Dec;23(6 Suppl):567S-587S.
- [Google Scholar]
- My the carotenoid pigment zeaxanthin—a review. Compr. Rev. Food Sci. Food Saf.. 2008;7:29-49.
- [Google Scholar]
- LincRNA-Cox2 Regulates Smoke-induced Inflammation in Murine Macrophages. Am. J. Respir. Cell Mol. Biol.. 2023 May;68(5):511-522.
- [Google Scholar]
- Callicarpa japonica Thunb. reduces inflammatory responses: a mouse model of lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol.. 2015;26:174-180.
- [Google Scholar]
- Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br. J. Ophthalmol.. 1998 Aug;82(8):907-910.
- [Google Scholar]
- The carotenoids of macular pigment and bisretinoid lipofuscin precursors in photoreceptor outer segments. In: Carotenoids: Physical, Chemical and Biological Functions and Properties. Boca Raton, FL, USA: CRC Press; 2009. p. :355-363.
- [Google Scholar]
- The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol.. 2004 Jan;25(1):40-46.
- [Google Scholar]
- NLRC5 negatively regulates inflammatory responses in LPS-induced acute lung injury through NF-κB and p38 MAPK signal pathways. Toxicol. Appl. Pharmacol.. 2020 Sep;15(403):115150
- [Google Scholar]
- EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep.. 2021 May 26;11(1):11014.
- [Google Scholar]
- The acute respiratory distress syndrome. N. Engl. J. Med.. 2000 May 4;342(18):1334-1349.
- [Google Scholar]
- Protocatechuic acid attenuates lipolysaccharide-induced acute lung injury. Inflammation. 2012 Jun;35(3):1169-1178.
- [Google Scholar]
- Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol.. 2014 Mar;19(1):103-109.
- [Google Scholar]
- Protective effect of Jolkinolide B on LPS-induced mouse acute lung injury. Int. Immunopharmacol.. 2015 May;26(1):119-124.
- [Google Scholar]
- Fructose-1,6-diphosphate attenuates 434 acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol.. 2008;435(8):1842-1847.
- [Google Scholar]
- Anti-inflammatory effects of novel curcumin analogs in experimental acute lung injury. Respir. Res.. 2015;16:43.
- [Google Scholar]
- Inflammatory response of macrophages in infection. Hepatobiliary Pancreat. Dis. Int.. 2014 Apr;13(2):138-152.
- [Google Scholar]
- Dexmedetomidine Alleviates Hyperoxia-Induced Acute Lung Injury via Inhibiting NLRP3 Inflammasome Activation. Cell. Physiol. Biochem.. 2017;42(5):1907-1919.
- [Google Scholar]







