Translate this page into:
Nature spermidine and spermine alkaloids: Occurrence and pharmacological effects
⁎Corresponding author. liu5308@sina.com (Jianqun Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Spermidine and spermine are special polyamines in organisms, and produced in vivo by putrescine and S-adenosylmethionine catalyzed by a variety of enzymes. Spermidine and spermine possess multiple amino groups, and are closely related to cell division, growth and survival. Spermidine and spermine alkaloids are widely distributed in plants, bacteria and marine organisms, and can be divided into macrocyclic and open chain according to the skeletons. Spermidine and spermine alkaloids exhibited numerous pharmacological effects such as anti-inflammatory, antibiotics, anti-tumor, anti-Alzheimer and anti-virus. However, up to now, there are few systematic reviews on spermidine and spermine alkaloids. In this review, based on the number of atoms in the ring, we summarized the distributions and pharmacological effects of spermidine and spermine alkaloids. Spermidine and spermine alkaloids have special chemophenetic significances in the plant kingdom, especially the macrocyclic spermidine and spermine alkaloids. Spermidine alkaloids are much more abundant in nature than spermine alkaloids. The pharmacological activities of the open chain spermidine and spermine alkaloids are studied in depth. Polycyclic guanidine spermidine alkaloids, isolated from marine sponge, exhibit great potential in various cancer cells. However, pharmacological studies of macrocyclic spermidine and spermine alkaloids are scarce. Synthesis is an effective way to get more spermidine and spermine alkaloids and their analogues for further study.
Keywords
Spermidine
Spermine
Alkaloids
Macrocyclic
Pharmacological effects
1 Introduction
Polyamines are a class of compounds containing two or more amino groups. The most common polyamines with important physiological functions are putrescine, spermidine and spermine (Fig. 1). Spermidine (N-C3-N-C4-N) is widely distributed in organisms and synthesized by putrescine and S-adenosylmethionine. Spermine (N-C3-N-C4-N-C3-N) is a kind of polyamine containing two primary and two secondary amines in its structure (Mude et al., 2022). It is produced in vivo by putrescine and S-adenosylmethionine catalyzed by a variety of enzymes. Spermidine and spermine are essential for cell viability, proliferation, function and differentiation, which possess biological activities such as anti-aging and anti-cancer (Pegg 2014; Madeo et al., 2018).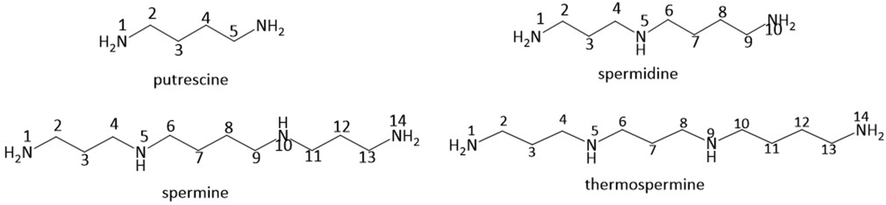
Chemical structures of putrescine, spermidine, spermine and thermospermine.
Spermidine and spermine alkaloids are widely distributed in plants, bacteria and marine organisms, which can be classified into macrocyclic and open chain according to the skeletons (Da Silva and Soengas 2017). Thermospermine is the isomer of spermine converted from spermidine (Fig. 1). Thermospermine alkaloids are also distributed in plants (Park et al., 2017). The spermidine and spermine alkaloids exhibit numerous pharmacological effects such as anti-inflammatory, antibiotics, anti-tumor, anti-Alzheimer and anti-virus. Although a great deal of spermidine and spermine alkaloids have been found during these years, the chemophenetic significances of the spermidine and spermine alkaloids are not clear. Therefore, it is meaningful to find the distribution characteristics of the spermidine and spermine alkaloids, especially macrocyclic spermidine and spermine alkaloids, which are less reported compared with open chain spermidine and spermine alkaloids. Meanwhile, up to now, there are few systematic reviews summarizing the pharmacological effects of the spermidine and spermine alkaloids.
Herein, in this review, we conclude the occurrence and pharmacological effects of the spermidine and spermine alkaloids. The macrocyclic spermidine and spermine alkaloids are classified based on the number of atoms in the ring.
2 Spermidine and spermine alkaloids
2.1 8-membered ring of spermidine alkaloids
Dovyalicin-type alkaloids are a class of amide alkaloids having a spermidine nucleus (Fig. 2, Table.1). Till now, this type of alkaloids was exclusively isolated from the family salicaceae, genus Dovyalis (D. abyssinica, D. macrocalyx, D. hebecarpa and D. caffra) (Stærk et al., 2003; Rasmussen et al., 2006; Zaki et al., 2019) and genus Homalium (H. cochinchinensis) (Addo et al., 2021), and nine members were identified named dovyalicin A, B, C, D, E, F, G, H and I. The dovyalicins have three types of skeletons, with dovyalicin A, B, C, E, G, H and I possessing spermidine as part of a perhydro-1,5-diazocine moiety to form an 8-membered heterocyclic ring, dovyalicin D possessing spermidine as part of a perhydro-1,4-diazepine moiety to form a 7-membered heterocyclic ring, while dovayalicin F is an open-chain spermidine alkaloid. Dovyalicin A, B, E, H and I all have a C-4 phenyl group, with the absolute configuration S. They differ in N-5 and C-4′ groups, with dovyalicin A/N-5(−|-) methyl analogue of dovyalicin E, dovyalicin B C-4′ methyl analogue of dovyalicin A, and dovyalicin H and dovyalicin I C-4′ trans and cis cinnamoyl analogues of dovyalicin A, respectively. Dovyalicin C is 3-benzoyl analogue of dovyalicin A, with no C-4 substituted group. Dovayalicin F has no substituted group in the 8-memberd perhydro-1,5-diazocine ring, with a long side chain at C-4′ position. Dovyalicin D possesses a perhydro-1,4-diazepine ring oxygenated at C-3 rather than the perhydro-1,5-diazocine ring, and is devoid of optical rotation and presumably racemic. At ambient temperature, dovayalicin F exists as a mixture of cis and trans conformers.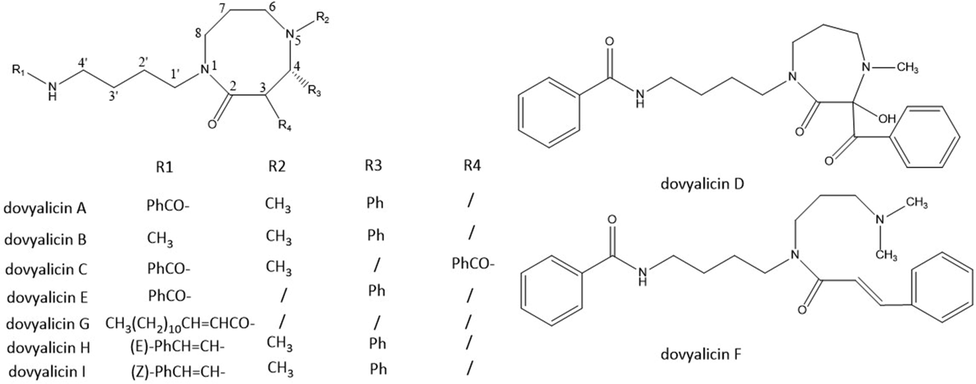
Chemical structures of 8-membered ring spermidine alkaloids.
Compound
plant
part
genus
family
ref
8-membered ring
dovyalicin A
Dovyalis abyssinica
leaf, twig
Dovyalis
salicaceae
(Rasmussen et al., 2006)
Dovyalis hebecarpa
leaf, twig
(Rasmussen et al., 2006)
Dovyalis macrocalyx
twig
(Stærk et al., 2003; Rasmussen et al., 2006)
dovyalicin B
Dovyalis abyssinica
leaf
(Rasmussen et al., 2006)
Dovyalis macrocalyx
leaf
(Stærk et al., 2003)
dovyalicin C
Dovyalis macrocalyx
leaf
(Stærk et al., 2003; Rasmussen et al., 2006)
dovyalicin D
Dovyalis macrocalyx
leaf
(Stærk et al., 2003)
dovyalicin E-F
Dovyalis abyssinica
leaf, twig
(Rasmussen et al., 2006)
dovyalicin G
Dovyalis caffra
leaf, twig
(Zaki et al., 2019)
dovyalicin H-I
Homalium cochinchinensis
aerial
homalium
salicaceae
(Addo et al., 2021)
2.2 13-membered ring of spermidine alkaloids
The spermidine lactam alkaloids with a 13-membered ring have been found mainly in the families of celastraceae, lamiaceae and fabaceae (Fig. 3, Table.2). Most of the spermidine alkaloids with a 13-membered ring differ in substituent groups of C-1 and C-8. Benzene and its derivatives are common substituents at C-8.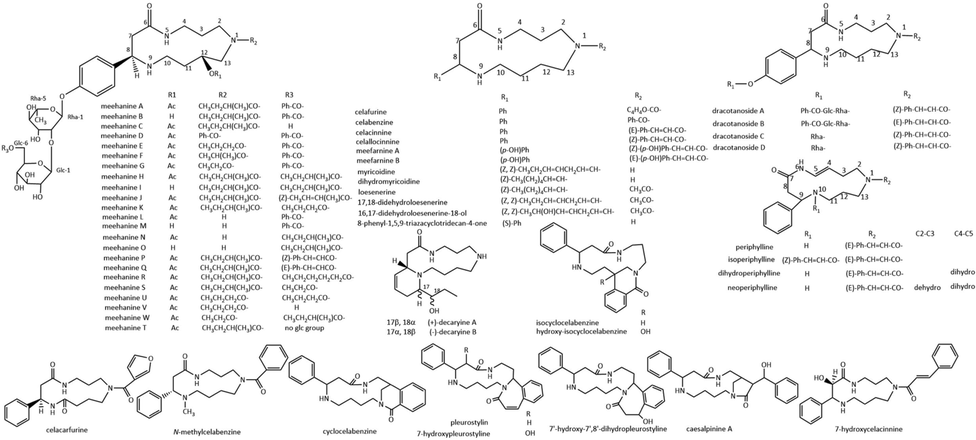
Chemical structures of 13-membered ring spermidine alkaloids.
Compound
plant
part
genus
family
ref
13-membered ring
celacinnine
Maytenus arbutifolia
twig
maytenus
celastraceae
(Kupchan et al., 1974)
Maytenus serrata
(Kupchan et al., 1977)
Tripterygium wilfordii
root
tripterygium
(Kupchan et al., 1977)
Pleurostylia opposita
leaf
pleurostylia
(Seguineau et al., 1992)
Pleurostylia africana
(Wagner and Burghart 1981)
celallocinnine
Maytenus arbutifolia
twig
maytenus
celastraceae
(Kupchan et al., 1974)
Maytenus serrata
(Kupchan et al., 1977)
Pleurostylia opposita
leaf
pleurostylia
celastraceae
(Seguineau et al., 1992)
Caesalpinia digyna
caesalpinia
fabaceae
(Mahato et al., 1985)
Pleurostylia africana
pleurostylia
celastraceae
(Wagner and Burghart 1981)
celafurine
Tripterygium wilfordii
root
tripterygium
celastraceae
(Kupchan et al., 1977)
celabenzine
Tripterygium wilfordii
root
tripterygium
celastraceae
(Kupchan et al., 1977)
Maytenus mossambicensis
leaf, twig
maytenus
(Wagner and Burghart 1982)
7-hydroxypleurostyline, 7-hydroxycelacinnine, 7′-hydroxy-7′,8′-dihydropleurostyline
Pleurostylia opposita
leaf
pleurostylia
celastraceae
(Seguineau et al., 1992)
pleurostylin
Pleurostylia opposita
leaf
pleurostylia
celastraceae
(Seguineau et al., 1992)
Pleurostylia africana
(Wagner and Burghart 1981)
caesalpinine A
Caesalpinia digyna
leaf
caesalpinia
fabaceae
(Mahafo et al., 1983; Mahato et al., 1985)
cyclocelabenzine, isocyclocelabenzine, hydroxyisocyclocelabenzine
Maytenus mossambicensis
leaf, twig
maytenus
celastraceae
(Wagner and Burghart 1982)
meefarnine A-B
Meehania fargesii
whole
meehania
lamiaceae
(Murata et al., 2010)
N-methylcelabenzine
Gymnosporia arenicola
leaf
gymnosporia
celastraceae
(Da Silva et al., 2015)
celecarfurine
Tripterygium wilfordii
root
tripterygium
celastraceae
(Liu et al., 2020)
myricoidine, dihydromyricoidine
Clerodendrum myricoides
whole
clerodendrum
lamiaceae
(Bashwira and Hootele 1988)
decaryine A, decaryine B, (2S)-2-phenyl-1,5,9-triazacyclotridecan-4-one
Androya decaryi
leaf
androya
scrophulariaceae
(Le Lamer et al., 2013)
loesenerine
Maytenus loeseneri
leaf
maytenus
celastraceae
(Díaz et al., 1987)
Euonymus fortunei
aerial
euonymus
(Wang et al., 2018a)
17,18-didehydroloesenerine, 16,17-didehydroloesenerin-18-ol
Maytenus loeseneri
leaf
maytenus
celastraceae
(Preiss et al., 1988)
dracotanoside A-D
Dracocephalum tanguticum
whole
dracocephalum
lamiaceae
(Wang et al., 2009)
meehanine A-K
Meehania urticifolia
whole
meehania
lamiaceae
(Murata et al., 2009a; Murata et al., 2009b)
meehanine L-w
Meehania urticifolia
periphylline, isoperiphylline, dihydroperiphylline, neoperiphylline, perimargine, dihydroperimargine
Peripterygia marginata
leaf
peripterygia
celastraceae
(Hocquemiller et al., 1977)
Celallocinnine, celacinnine, celafurine and celabenzine differ only in the acyl side chain (Kupchan et al., 1974; Kupchan et al., 1977; Wagner and Burghart 1982; Mahato et al., 1985; Seguineau et al., 1992). Celallocinnine and celacinnine are characterized by the presence of a 13-membered ring reflecting spermidine and cinnamoyl precursorial units at C-1. Celallocinnine and celacinnine are cis-and trans-isomers, the cinnamoyl double bond has cis configuration for celallocinnine and trans configuration for celacinnine. Meefarnine A and B were isolated from Meehania fargesii (Murata et al., 2010), with celallocinnine-type and celacinnine-type skeleton, individually. Similar to celallocinnine and celacinnine, they are a pair of cis-and trans-isomers. Pleurostylin (Wagner and Burghart 1981; Seguineau et al., 1992), isolated from genus pleurostylia, represents a structure which spermidine is incorporated in a 13-membered lactam ring to which an additional cinnamoyl residue is fused to yield a 7-membered ring. Two pleurostylin-type alkaloids 7-hydroxypleurostyline, 7′-hydroxy-7′,8′-dihydropleurostyline, along with one celacinnine-type alkaloid 7-hydroxycelacinnine, which exhibit unusual OH substitutions in the macrocycle or the 7-membered ring, were isolated from pleurostylia opposite (Seguineau et al., 1992). Caesalpinine A (Mahafo et al., 1983; Mahato et al., 1985), the only known 13-membered spermidine alkaloid isolated from fabaceae family, represents a structure which an hydroxylated cinnamoyl residue is incorporated in the macrocyclic to yield a 5-membered ring.
Celabenzine contains a spermidine unit N1-linked to a benzoyl group instead of cinnamoyl group. Da silva (Da Silva et al., 2015) found a N9-methylated celabenzine from Gymnosporia arenicola leaf. The three alkaloids cyclocelabenzine, isocyclocelabenzine and hydroxyl-isocyclocelabenzine (Wagner and Burghart 1982) show the 13-membered lactam ring of celabenzine being linked to the benzoyl residue within the spermidine unit. Hydroxyisocyclocelabenzine is the first known spermidine alkaloid with a hydroxy function at the macrocycle. Celafurine has a furan formate moiety, which was only found in Tripterygium wilfordii (Kupchan et al., 1977). Recently, a new 13-membered spermidine macrocyclic alkaloid celecarfurine was isolated by our group from the same plant (Liu et al., 2020). Celecarfurine is in the 2R-configuration and contains two amide carbonyls in the macrocycle.
Several spermidine alkaloids with no group linked to N-1 were found in Clerodendrum myricoides (Bashwira and Hootele 1988) and Androya decaryi (Le Lamer et al., 2013), which belong to family lamiaceae and scrophulariaceae, respectively. Myricoidine and dihydromyricoidine, with alkenyl linked to C-8, were isolated from Clerodendrum myricoides. Myricoidine has two carbon–carbon double bonds (both cis-configuration) whereas dihydromyricoidine only has one. Two optical isomers (+)-decaryine A and (-)-decaryine B, along with (-)-(2S)-2-phenyl-1,5,9-triazacyclotridecan-4-one, were isolated from the leaves of Androya decaryi. Decaryine A/B represent a structure which a 13-membered lactam ring is fused to a 6-membered ring. Loesenerine, N1- acetylated dihydromyricoidine, along with two loesenrine-type spermidine alkaloids 17,18-didehydroloesenerine and 16,17-didehydroloesenerin-18-ol were isolated from Maytenus loeseneri (Díaz et al., 1987; Preiss et al., 1988).
Several 13-membered cyclic spermidine alkaloidal glycosides have been isolated from leguminous plants Dracocephalum tanguticum (Wang et al., 2009) and Meehania urticifolia (Murata et al., 2009a; Murata et al., 2009b). Dracotanoside A-D are glycosides of celacinnine and celallocinnine, consisting of two pairs of cis-and trans-isomers dracotanoside A-B and dracotanoside C-D. The glycosyl moiety of dracotanoside A-B consists of a benzoyl group and two sugar units l-rhamnose and d-glucose, linked to the para position of N-8 benzene ring. Dracotanoside C-D differ with dracotanoside A-B only in the absence of the benzoylated glucopyranosyl unit. 23 spermidine alkaloidal glycosides named meehanine A-W were found in Meehania urticifolia. Unlike other 13-membered cyclic spermidine alkaloids, the glycosides have a C-12 hydroxy group or O-acetyl group. Several moieties such as benzamide, 2-methylbutyramide, butyramide, isobutyramide and propanamide are linked to N-1, respectively. All meehanines except meehanine T are diglycosides, possessing two monosaccharide moieties l-rhamnose and β-glucopyranose, with only l-rhamnose in meehanine T. Benzoate, 2-methybutyrate, trans-2-methyl-2-butenoate, butyrate, propionate, caproate, cis- and trans-cinnamoyl group are linked to C-6 of the glucopyranose unit, respectively.
The chirality of the 13-membered cyclic spermidine alkaloids mostly depends on the configuration of C-8. All known macrocyclic spermidine alkaloids such as dracotanosides, meehanines and N-methylcelabenzine are generally in the 8S-configuration, except that celecarfurine is in the 8R-configuration. In addition, meehanines have a 12R-configuration.
Periphylline and its analogues isoperiphylline, dihydroperiphylline, neoperiphylline, perimargine and dihydroperimargine, which resemble the difference that the spermidine moiety is attached in the opposite manner, were isolated from the leaves of Peripterygia marginata (Hocquemiller et al., 1977). Periphylline, isoperiphylline, dihydroperiphylline and neoperiphylline all possess a double bond in the 13-membered ring, while the chemical structure of perimargine and dihydroperimargine had not been fully clarified perhaps due to the limited experiment conditions at that time.
The pharmacological effects of the above spermidine alkaloids with 13-membered ring are less studied and reported. Tang (Tang et al., 2017) utilized a high-throughput in silico virtual screening method to find novel medicine formula as matrix metalloproteinase-9 inhibitors. Celacinnine and celallocinnine were qualified to interact with zinc-binding site of matrix metalloproteinase-9. Besides, celacinnine could interact with matrix metalloproteinase-9 related protein that identified by drug-target interaction network analysis. Celafurine could obviously inhibit denosine deaminase activity in HL-60 cell at concentrations of 10, 1 and 0.1 mg/L, with the inhibited activity proportional to the concentration (Wang et al., 2007). Loesenerine activated AMP-activated protein kinase pathway through increasing ADP/ATP ratio by inhibiting mitochondrial respiration, induced increment of glucose uptake in C2C12 cells, and increased glucose consumption in a dose-dependent manner, which exhibited potential hypoglycemic activity (Wang et al., 2018a). N-methylcelabenzine was more cytotoxic in cancer-derived cells, although not enough to be considered a cytotoxic agent (Da Silva et al., 2015). In our recent research (Liu et al., 2020), celecarfurine showed remarkable anti-inflammatory effects on IL-1β secretion in LPS-induced rat primary synovial fibroblasts at 10 μM.
2.3 17-membered ring of spermine alkaloids
The lactam alkaloids with a 17-membered ring are a class of macrocyclic alkaloids having a spermine nucleus (Fig. 4, Table.3). Protoverbine (Guggisberg et al., 2000), which possessed a C-11 phenyl group at the unique 17-membered spermine ring, along with its N-6, N-10-methylene-bridged derivative protomethine, were isolated from Verbascum pseudonobile. Several nature products which possess protoverbine-type skeleton had been isolated mainly from genus verbascum (V. pseudonobile, V. phoeniceum and V. nigrum) and incarvillea (I. sinensis). Prelandrine (Nezbedova et al., 2001), with a hydroxyl group linked to the para position of C-11 benzene ring, was isolated from Aphelandra squarrosa. Buchnerine (Lumbu and Hootele 1993), with a methoxy group linked to the para position of C-11 benzene ring, along with its derivative N-1-p-methoxycinnanoylbuchnerine have been isolated from Clerodendrum buchneri. Several pairs of trans–cis isomers such as verbacine-verballocine (Drandarov 1995; Chi et al., 1997; Drandarov et al., 1999; Govindan et al., 2019), verbamedine-isoverbamedine (Drandarov and Hesse 2002), verbascenine-verballoscenine (Seifert et al., 1982; Drandarov 1997), verbasitrine-isoverbasitrine (Drandarov et al., 1999) and incasine A-incasine A’ (Chi et al., 1997) were isolated, with a cinnamoyl or dimethoxy cinnamoyl group linked to N-1. Moreover, verbamedine-isoverbamedine, verbascenine-verballoscenine and incasine A-incasine A’ have a N-6 formyl, acetyl and imine group, respectively. Verbaskine (Koblicova et al., 1983), also isolated from Verbascum pseudonobile, is a N-6, N-10-carbonyl-bridged derivative of verbacine. Verbametrine and isoverbametrine (Drandarov et al., 1999) are N-6, N-10-methylene-bridged derivatives of verbasitrine and isoverbasitrine, respectively. Verbamethine (incasine C’) and isoverbamethine (incasine C) (Chi et al., 1997; Drandarov et al., 1998; Drandarov et al., 1999; Chi et al., 2007), the N-6, N-10-methylene-bridged derivatives of verbacine and verballocine, along with incasine B’ and incasine B (Chi et al., 1997), the amidinium salts of verbacine and verballocine, were isolated from Incarvillea sinensis. The above compounds all have a S-configuration at C-11, except incasine C and C’ were in the 11R-configuration. Verbacine exhibited significant cytotoxicity against C6 cells with IC50 of 15.09 μg/mL, and was a promising inhibitor of acetylcholinesterase with IC50 values of 16.01 μg/mL (Govindan et al., 2019).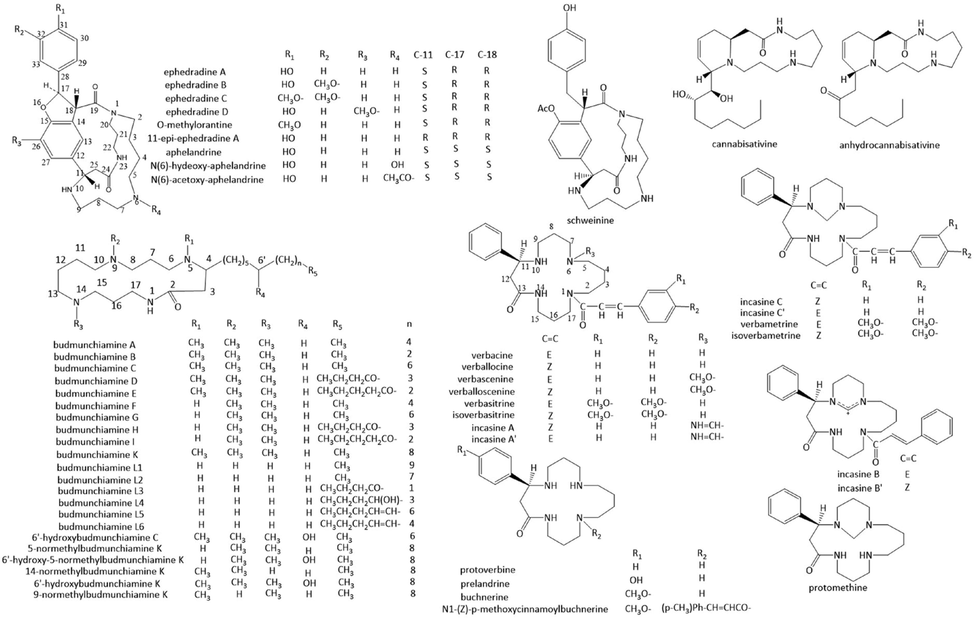
Chemical structures of 17-membered ring spermine alkaloids.
Compound
plant
part
genus
family
ref
17-membered ring
protoverbine, protomethine
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Guggisberg et al., 2000)
prelandrine
Aphelandra squarrosa
root
aphelandra
acanthaceae
(Nezbedova et al., 2001)
buchnerine, N-1-(Z)-p-methoxycinnamoylbuchnerine
Clerodendrum buchneri
leaf
clerodendrum
lamiaceae
(Lumbu and Hootele 1993)
verbacine
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Drandarov 1995; Drandarov et al., 1999)
Melocanna baccifera
fruit, leaf
melocanna
poaceae
(Govindan et al., 2019)
verballocine
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Drandarov 1995; Drandarov et al., 1999)
Incarvillea sinensis
whole
incarvillea
bignoniaceae
(Chi et al., 1997)
verbasitrine, isoverbasitrine, verbametrine, isoverbametrine
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Drandarov et al., 1999)
incasine A, incasine A’, incasine B, incasine B’
Incarvillea sinensis
whole
incarvillea
bignoniaceae
(Chi et al., 1997)
incasine C’
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Drandarov et al., 1998; Drandarov et al., 1999)
Incarvillea sinensis
whole
incarvillea
bignoniaceae
(Chi et al., 2007)
incasine C
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Drandarov et al., 1998; Drandarov et al., 1999)
Incarvillea sinensis
whole
incarvillea
bignoniaceae
(Chi et al., 1997; Chi et al., 2007)
verbamedine, isoverbamedine
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Drandarov and Hesse 2002)
verbascenine
Verbascum phoeniceum
aerial
verbascum
scrophulariaceae
(Seifert et al., 1982)
Verbascum nigrum
verballoscenine
Verbascum phoeniceum
leaf
verbascum
scrophulariaceae
(Drandarov 1997)
verbaskine
Verbascum pseudonobile
leaf
verbascum
scrophulariaceae
(Koblicova et al., 1983)
aphelandrine
Aphelandra squarrosa
root
aphelandra
acanthaceae
(Dätwyler et al., 1978; Youhnovski et al., 1999)
Aphelandra fuscopunctata
N-6-hydroxy-aphelandrine, N-6-acetoxy-aphelandrine
Aphelandra fuscopunctata
root
aphelandra
acanthaceae
(Youhnovski et al., 1999)
O-methylorantine
Chaenorhinum minus
whole
chaenorhinum
plantaginaceae
(Zhu and Hesse 1988)
Chaenorhinum villosum
(Dätwyler et al., 1979)
ephedradine A
Chaenorhinum minus
whole
chaenorhinum
plantaginaceae
(Zhu and Hesse 1988)
NG
aerial
ephedra
ephedraceae
(Tamada et al., 1979)
Schweinfurthia papilionacea
whole
schweinfurthia
plantaginaceae
(Ahmad and Viqar 1990)
ephedradine B
Chaenorhinum minus
whole
chaenorhinum
plantaginaceae
(Zhu and Hesse 1988)
NG
aerial
ephedra
ephedraceae
(Hikino et al., 1979)
ephedradine C
Chaenorhinum minus
whole
chaenorhinum
plantaginaceae
(Zhu and Hesse 1988)
NG
aerial
ephedra
ephedraceae
(Hikino et al., 1980)
ephedradine D
NG
aerial
ephedra
ephedraceae
(Hikino et al., 1982)
11-epi-ephedradine A, schweinine
Schweinfurthia papilionacea
whole
schweinfurthia
plantaginaceae
(Ahmad and Viqar 1990)
chaenorpine
Chaenorhinum minus
whole
chaenorhinum
plantaginaceae
(Zhu and Hesse 1988; Zhu et al., 1988)
chaenorhine
Chaenorhinum origanifolium
aerial
chaenorhinum
plantaginaceae
(Bernhard et al., 1973)
budmunchiamine A
Albizia lebbek
seed
albizia
fabaceae
(Dixit and Misra 1997)
Albizia schimperana
stem bark
(Rukunga and Waterman 1996b)
Albizia amara
seed
(Pezzuto et al., 1991)
budmunchiamine B-C
Albizia lebbek
seed
(Dixit and Misra 1997)
Albizia amara
(Pezzuto et al., 1991)
budmunchiamine F
Albizia lebbek
seed
(Dixit and Misra 1997)
Albizia amara
(Pezzuto et al., 1992)
budmunchiamine G
Albizia gummifera
stem bark
(Rukunga and Waterman 1996a)
Albizia amara
seed
(Pezzuto et al., 1992)
budmunchiamine D-E, H-I
Albizia amara
seed
(Pezzuto et al., 1992)
budmunchiamine L1-L6
Albizia lebbek
seed
(Misra et al., 1995; Dixit and Misra 1997)
6′-hydroxybudmunchiamine C, 5-normethylbudmunchiamine K, 6′-hydroxy-5-normethylbudmunchiamine K, 14-normethylbudmunchiamine K
Albizia schimperana
stem bark
(Rukunga and Waterman 1996b)
budmunchiamine K, 6′-hydroxybudmunchiamine K, 9-normethylbudmunchiamine K
Albizia gummifera
stem bark
(Rukunga and Waterman 1996a)
cannabisativine, anhydrocannabisativine
Cannabis sativa
root
cannabis
cannabaceae
(Lotter et al., 1975; Elsohly et al., 1978)
Several nature products possessing two special macrocycles and a furan ring have been found in the families of acanthaceae, ephedraceae, and plantaginaceae, among which hydroxylated coumaroyl residue is fused with the C-11 benzene ring of the 17-membered lactam ring to yield another 13-membered ring and a furan ring. Aphelandrine and two macrocyclic alkaloids of the aphelandrine type, namely N-6-hydroxy-aphelandrine and N-6-acetoxy-aphelandrine, were isolated from Aphelandra fuscopunctata (Dätwyler et al., 1978; Youhnovski et al., 1999). The alkaloids O-methylorantine from Chaenorhinum minus (Zhu and Hesse 1988) and Chaenorhinum villosum (Dätwyler et al., 1979) have the same constitution as aphelandrine, except that of O-methylorantine has a different substituent at C-31 (OCH3 instead of OH), and both compounds have the inverse configuration at C-17 and C-18. The other alkaloids with a similar backbone to aphelandrine are ephedradine A (Tamada et al., 1979; Zhu and Hesse 1988; Ahmad and Viqar 1990), 11-epi-ephedradine A (Ahmad and Viqar 1990), ephedradine B (Hikino et al., 1979; Zhu and Hesse 1988), ephedradine C (Hikino et al., 1980; Zhu and Hesse 1988), and ephedradine D (Hikino et al., 1982), with different substituents at C-26, C-31 and C-32, and 11-epi-ephedradine A has an inverse configuration at C-11 with ephedradine A. Ephedradine A, B, C and D elicited hypotensive effects in Wistar rats. Ephedradine B was the most potent hypotensive agent among these alkaloids, the hypotensive activity of ephedradine B was exerted mainly by the ganglion blocking action (Hikino et al., 1983). Schweinine (Ahmad and Viqar 1990), similar to ephedradine A, possesses a structure with no furan ring. Chaenorpine (Zhu and Hesse 1988; Zhu et al., 1988) and chaenorhine (Bernhard et al., 1973) represent another structure which caffeoyl residue is fused with the C-11 benzene ring of the 17-membered lactam ring to yield a 19-membered ring.
Budmunchiamines, possessing an amide macrocycle invariably substituted by two or three N-methyl groups and a homologous side-chain, were isolated from genus albizia, fabaceae family (Pezzuto et al., 1991; Pezzuto et al., 1992; Misra et al., 1995; Rukunga and Waterman 1996b; Rukunga and Waterman 1996a; Dixit and Misra 1997). N-demethyl derivatives budmunchiamine L1-L6 were isolated as well (Misra et al., 1995; Dixit and Misra 1997). Besides N-methyl, the budmunchiamines also differ in the group and length of the aliphatic side chain at C-11. 6′-hydroxybudmunchiamine C (Rukunga and Waterman 1996b), 6′-hydroxy-5-normethylbudmunchiamine K (Rukunga and Waterman 1996b) and 6′-hydroxybudmunchiamine K (Rukunga and Waterman 1996a) possessed a hydroxyl substitution in the long side chain. Budmunchiamines exhibited significant antibacterial activities. Budmunchiamine A significantly inhibited the growth and fumonisin B1 production by F. verticillioides in a dose dependent manner, with the minimum inhibitory concentrations 0.125 mg/mL and minimum fungicidal concentrations 0.25 mg/mL (Thippeswamy et al., 2014). Rukunga (Rukunga and Waterman 1996a) studied the structure–activity relationships of the budmunchiamines, nine budmunchiamines were all active against two gram-positive (Bacillus subtilis, Staphylococcus aureus) and two gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacteria at MIC levels below 80 μg/mL, and showed toxicity to brine shrimp larvae with LC50 values below 100 μg/mL. The presence of the hydroxyl in the side chain leads to an appreciable reduction in both antibacterial activity and cytotoxicity. Reduction of the degree of methylation on the macrocycle nitrogens from three to two did not cause a significant loss of antibacterial activity. A mixture of budmunchiamine A-C in the ratio 4: 1: 1 was bactericidal against Salmonella typhimurium strain TM677, and found to inhibit the catalytic activity of DNA polymerase, RNA polymerase, and HIV-1 reverse transcriptase (Mar et al., 1991). In addition, two lactam alkaloids cannabisativine and anhydrocannabisativine, with a 17-membered ring spermidine nucleus, were isolated from Cannabis sativa (Lotter et al., 1975; Elsohly et al., 1978).
2.4 24-membered ring of spermidine alkaloids
A spermidine moiety is linked with coumaroyl/caffeoyl/feruloyl groups to form a 24-membered lactam ring, containing two amide carbonyls in the macrocycle (Fig. 5, Table.4). Cadabicine, with two trans double bonds, along with cadabicine diacetate, was isolated from Cadaba farinose (Ahmad et al., 1985a), Crataeva nurvala (Ahmad et al., 1987a) and Capparis spinose (Khanfar et al., 2003). Cadabicine totally inhibited ADP, epinephrine-induced platelet aggregation and the plasma clotting at 0.82 mg/mL (Al Kury et al., 1999). Isocodonocarpine (Ahmad et al., 1989), monomethoxy analogue of cadabicine, was isolated from Capparis decidua. Monomethoxy and dimethoxy analogues capparisine (Ahmad et al., 1986), capparisinine (Ahmad et al., 1987b) and capparidisine (Ahmad et al., 1985b) were isolated from the same plant, while resemble the difference that the spermidine moiety is attached in the opposite manner. In addition, two N-acetylation analogues 14-N-acetylisocodonocarpine and 15-N-acetylcapparisine were isolated either (Ahmad et al., 1992). Analogue codonocarpine (Doskotch et al., 1971) was isolated from plant Codonocarpus australis of gyrostemonaceae family. Capparidisine possessed cardiovascular activity, exhibiting a dose-dependent depressant effect on heart rate and coronary flow in the isolated rabbit's heart (Rashid et al., 1989).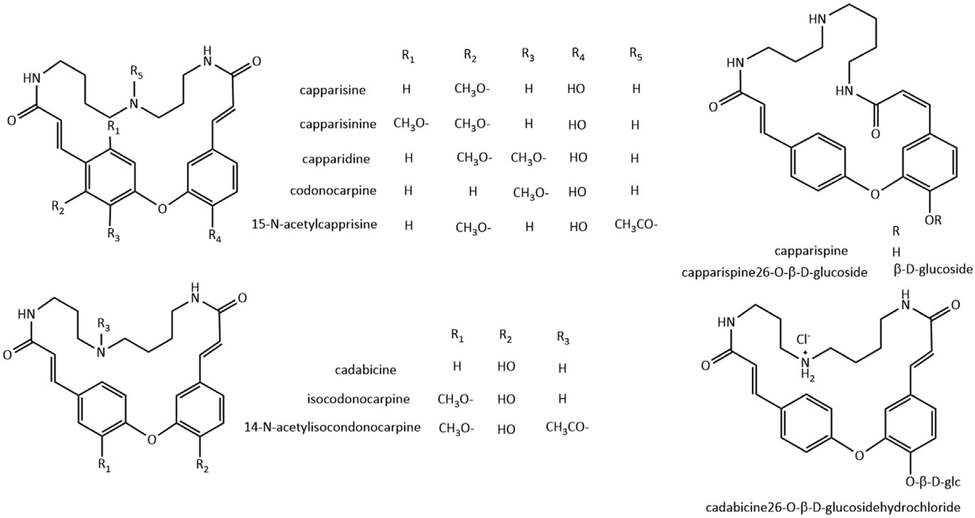
Chemical structures of 24-membered ring spermine alkaloids.
Compound
plant
part
genus
family
ref
24-membered ring
cadabicine
Cadaba farinosa
stem bark
cadaba
capparaceae
(Ahmad et al., 1985a)
Crataeva nurvala
stem bark
crateva
(Ahmad et al., 1987a)
Capparis spinosa
aerial
capparis
(Khanfar et al., 2003)
cadabicine diacetate
Cadaba farinosa
stem bark
cadaba
(Ahmad et al., 1987a)
Crataeva nurvala
stem bark
crateva
isocodonocarpine
Capparis decidua
root bark
capparis
(Ahmad et al., 1989)
capparisine
Capparis decidua
root bark
capparis
(Ahmad et al., 1986)
capparisinine
Capparis decidua
root bark
capparis
(Ahmad et al., 1987b)
capparidisine
Capparis decidua
root bark
capparis
(Ahmad et al., 1985b)
14-N-acetylisocodonocarpine, 15-N-acetylcapparisine
Capparis decidua
root bark
capparis
(Ahmad et al., 1992)
capparispine, capparispine26-O-b-d-glucoside, cadabicine26-O-b-d-glucosidehydrochloride
Capparis spinosa
root
capparis
(Fu et al., 2008)
codonocarpine
Codonocarpus australis
bark
codonocarpus
gyrostemonaceae
(Doskotch et al., 1971)
Capparispine, possessing both trans and cis configuration of the double bonds, along with two glycosides capparispine26-O-β-d-glucoside and cadabicine26-O-β-d-glucosidehydrochloride, were isolated from Capparis spinose (Fu et al., 2008). A cyclic spermidine amide was isolated from Brassica napus (Baumert et al., 2005), and the structure is similar to hydroxymethyl derivative of codonocarpine or isocodonocarpine.
2.5 Other membered ring of spermidine and spermine alkaloids
Other membered macrocyclic spermidine and spermine alkaloids have been found in plants (Fig. 6, Table.5). A pair of new macrocyclic spermidine alkaloids, (+)-(S)-scocycamide and (−)-(R)-scocycamide, featured a unique 6/18 fused bicyclic framework with spermidine and catechol units, were isolated from the roots of Scopolia tangutica (Wang et al., 2020a). (+)-(S)-scocycamide and (−)-(R)-scocycamide exhibited butyrylcholinesterase inhibition of 18.11 % and 37.83 % at 800 μM, respectively. In addition, they also showed potent antioxidant activity with the oxygen radical absorbance capacity.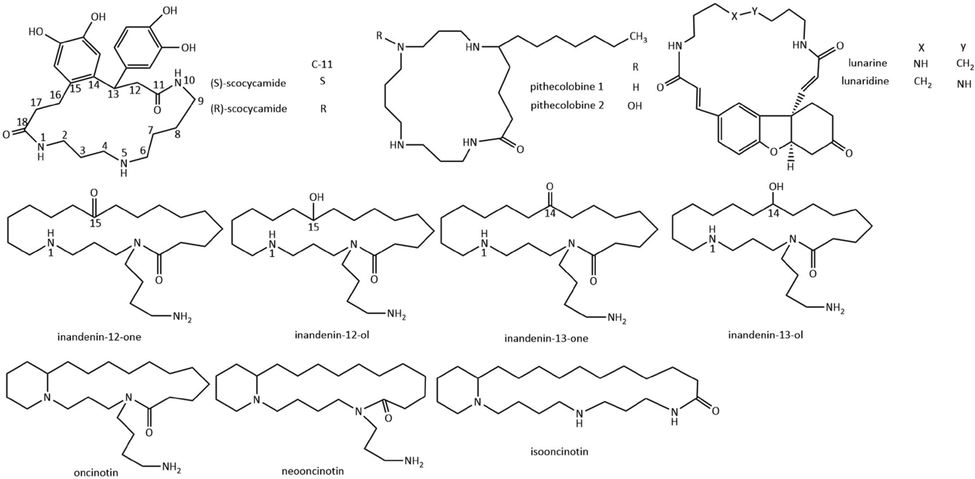
Chemical structures of other membered ring spermidine and spermine alkaloids.
Compound
plant
part
genus
family
ref
18-membered ring
(S)-scocycamide, (R)-scocycamide
Scopolia tangutica
root
scopolia
solanaceae
(Wang et al., 2020a)
19-membered ring
pithecolobine 1
Pithecolobium saman
bark
pithecolobium
fabaceae
(Wiesner et al., 1952)
Albizia saman
leaf
albizia
(Thippeswamy et al., 2014)
pithecolobine 2
Pithecolobium saman
bark
pithecolobium
(Wiesner et al., 1952)
20-membered ring
lunarine
Lunaria biennis
seed
peripterygia
celastraceae
(Potier et al., 1963)
Lunaria rediviva
lunaridine
Lunaria biennis
(Poupat et al., 1972)
21-membered ring
inandenin-12-one, inandenin-13-one, inandenin-12-ol, inandenin-13-ol
Oncinotis tenuiloba
leaf
oncinotis
apocynaceae
(Doll et al., 1995)
oncinotin
Oncinotis nitida
stem bark
(Guggisberg et al., 1974)
22-membered ring
neooncinotin
Oncinotis nitida
stem bark
oncinotis
apocynaceae
(Guggisberg et al., 1974)
26-membered ring
isooncinotin
Oncinotis nitida
stem bark
oncinotis
apocynaceae
(Guggisberg et al., 1974)
Macrocyclic spermine alkaloids pithecolobine 1 and pithecolobine 2 composing of a 19-membered ring were isolated from Pithecolobium saman (Wiesner et al., 1952) and Albizia saman (Thippeswamy et al., 2014). Pithecolobine 1 completely inhibited the fumonisin B1 production by F. verticillioides at 0.5 mg/mL in vitro, while in vivo evaluation showed complete inhibition at 0.5 g/kg in vivo (Thippeswamy et al., 2014).
Spermidine alkaloids lunarine (Potier et al., 1963) and lunaridine (Poupat et al., 1972) with a 20-membered ring were isolated from Lunaria biennis and Lunaria rediviva. The pharmacology of lunarine was first investigated by Henderson in the 1950 s (Henderson and Chen 1950). Lunarine exhibited pharmacological effects on the cardiovascular system, smooth muscle, carbohydrate metabolism, and glandular secretions. Lunarine is a competitive, time-dependent inhibitor of the protozoan oxidoreductase trypanothione reductase, a promising target in drug design against tropical parasitic diseases (Hamilton et al., 2006).
Spermidine alkaloids inandenin-12/13-one and the corresponding alcohols, inandenin-12/13-ol, with two nitrogen atoms in the 21-membered ring, were isolated from Oncinotis tenuiloba (Doll et al., 1995). Spermidine alkaloids oncinotin, neooncinotin, and isooncinotin, which possessed a 21-membered, 22-membered, and 26-membered ring, respectively, have been isolated from Oncinotis nitida (Guggisberg et al., 1974). The macro ring is naturally divided into two linked rings.
2.6 Open-chain spermidine and spermine alkaloids
Open-chain spermidine and spermine alkaloids are a class of alkaloids with no ring linked to the nitrogen atoms. Several mono-, di-, and tri-substituted spermidines/spermines and tetra-substituted spermines have been found. Open-chain spermidine alkaloids, in which p-coumaric acid, caffeic acid, ferulic acid, sinapic acid and benzoic acid are conjugated with spermidine via amide bonds at N-1, N-5 and N-10, are primarily dispersed in the family of solanaceae, and other families fagaceae, pandaceae and asteraceae.
Mono-substituted open chain spermidines such as N5-benzoylspermidine was isolated from Oncinotis tenuiloba (Doll et al., 1994). N1- and N8-coumaroyl and feruloyl spermidines were detected in Solanum dulcamara (Panagabko et al., 2000). Di-substituted open chain spermidines such as N1, N5-di-dihydrocaffeoylspermidine or N5, N10-di-dihydrocaffeoylspermidine (Gancel et al., 2008; Rodrigues et al., 2013), N1, N10-di-dihydrocaffeoylspermidine (Sattar et al., 1990; Gancel et al., 2008; Narvaez-Cuenca et al., 2013; Long et al., 2014), N1, N10-di-benzoylspermidine (Alemayehu et al., 1988), N1-methoxycaffeoyl, N10-dihydrocaffeoylspermidine (scotanamine B) (Long et al., 2014), N1, N10-di-dihydroferuloylspermidine (scotanamine C) (Long et al., 2014), N1-(E)-caffeoyl, N10-dihydrocaffeoylspermidine (scotanamine D) (Long et al., 2014; Yahia et al., 2020; Chen et al., 2021), N1-(E)-caffeoyl, N10-dihydrocaffeoylspermidine (Long et al., 2014; Zhao et al., 2014), and N1, N10-ditigiloylspermidine (Schimming et al., 2005) have been found. Scotanamine B exhibited analgesic effects, with moderate agonist activity at the µ-opioid receptor (EC50 = 7.3 µM) and induced analgesia in mice (Long et al., 2014). Scotanamine D could inhibit NO production in RAW 264.7 cells stimulated by lipopolysaccharide at 3 µM (Chen et al., 2021). Several N, N’-di-caffeoyl spermidine isomers were detected from Solanum melongena (Whitaker and Stommel 2003), Nicotiana tabacum (Camacho-Cristobal et al., 2004) and Physalis alkekengi (Wen et al., 2019) by HPLC/MS/MS. However, the structures were not determined because no single compound was obtained. N1-((4′-O-glycosyl)-sinapoyl), N10-(E, E)-sinapoylspermidine and N1, N10-di-(E, E)-sinapoylspermidine were isolated from Arabidopsis thaliana (Luo et al., 2009). N1, N10-di-(E, E)-coumaroylspermidine and N1, N10-di-(E, E)-sinapoylspermidine could modulate plant growth and development (Takahashi et al., 2021). N1, N5-di-(E, E)-p-coumaroylspermidine was found from Coix lacryma-jobi (Xu et al., 2018) and whole grain cereals (Zhang and Peterson 2018), which could improve the content of T-AOD and the activity of SOD, CAT and GPx and decrease the content of MDA in HepG2 cells, thus exhibited strong antioxidant activity (Xu et al., 2018). N1, N10-di-(E, E)-caffeoylspermidine was found from whole grain cereals (Drawbridge et al., 2021).
Certain tri-substituted open chain spermidines have been found in the plants and can be classified by different caffeic acid derivatives either. N1, N5, N10-tri-p-(E, E, E)-coumaroylspermidine was isolated from Artemisia caruifolia (Ma et al., 2001) and Orostachys japonicas (Lee et al., 2011). N1-feruloyl-N5, N10-di-p-(E, E, E)-coumaroyl (keayannidine A), N1, N10-di-feruloyl-N5-p-(E, E, E)-coumaroyl (keayannidine B), N1, N5, N10-tri-(E, E, E)-feruloyl spermidine (keayannidine C) were isolated from Microdesmis keayana, pandaceae family. The radical-scavenging activities of keayannidine A-C were found to be significant, although weaker than the positive control quercetin (Zamble et al., 2006). N1, N5, N10-tri-dihydrocaffeoylspermidine was isolated from genus solanum (Parr et al., 2005; Gancel et al., 2008; Rodrigues et al., 2013), which exhibited a strong angiotensin I-converting enzyme inhibitory activity with IC50 value of 9.55 ppm (Forero et al., 2016). Besides caffeic acid derivatives, tri-substituted open chain spermidines with other substituents have been found. Lyrium spermidine A, with carboxyl group substituted at N-5, was isolated from the fruits of Lycium ruthenicum (Zhao et al., 2014). N1, N10-di-benzoyl, N5-acetamidespermidine, isolated from the leaves of Banara parviflora (Moritz et al., 2016), could be biosynthesized by an acetylation using acetyl-CoA with N1, N10-di-benzoylspermidine. N1-methylthiocarbonyl-N5-(E)-cinnamoyl-N10-formylspermidine (chisitine 1) and N1-benzoyl-N5-(E)-cinnamoyl-N10-formylspermidine (chisitine 2) were isolated from the leaves of Chisocheton weinlandii (Tzourosa et al., 2004).
Open chain spermines which possess four nitrogen atoms, are substituted at N1, N5, N10 and N14. Mono-substituted N1-p-coumaroyl spermine was detected in Solanum dulcamara (Panagabko et al., 2000). N1, N10-di-dihydrocaffeoylspermine and N1, N5, N14-tri-dihydrocaffeoylspermine were found from Solanum tuberosum (Narvaez-Cuenca et al., 2013). N, N’-di-dihydrocaffeoylspermine isomers were detected from Physalis alkekengi (Wen et al., 2019) by HPLC/MS/MS as well. N1, N14-di-dihydrocaffeoylspermine (kukoamine A) (Funayama et al., 1980; Parr et al., 2005) and N1, N10-di-dihydrocaffeoylspermine (kukoamine B) (Funayama et al., 1995) were isolated from Lycium chinense and Solanum tuberosum.
Kukoamines exerted various pharmacological effects such as antioxidant, anti-inflammatory, antidiabetic and neuroprotective. Kukoamines protected bone marrow-derived mesenchymal stem cells from fenton-induced damage via antioxidant pathways such as electron-transfer, proton-transfer, hydrogen atom transfer, radical-adduct-formation, and Fe2+-chelating. kukoamine B exhibited higher antioxidant levels than kukoamine A (Li et al., 2018a). Kukoamine A showed inhibitory activity on soybean lipoxygenase with IC50 9.5 μM (Hadjipavlou-Litina et al., 2009). Kukoamine A significantly inhibited the production of reactive oxygen species (nitric oxide, prostaglandin E2, cyclooxygenase-2) and inflammatory factors (tumor necrosis factor-α, interleukin-1β, and interleukin-6) in lipopolysaccharide-treated RAW 264.7 macrophage cells (Yang et al., 2016; Wang et al., 2020b). In addition, kukoamine A significantly decreased inflammatory response to carrageenan induced paw edema of carrageenan-treated rats in vivo (Hadjipavlou-Litina et al., 2009; Wang et al., 2020b). Bacterial lipopolysaccharide and bacterial DNA/CpG DNA are important pathogenic molecules and drug targets for sepsis. Kukoamine B is a potent dual inhibitor for both LPS and CpG DNA and inhibits their activity in vitro and in vivo, thus exhibiting anti-sepsis effect (Liu et al., 2011a; Liu et al., 2011b). In LPS-induced septic mice, kukoamine B could protect against lung injury through anti-inflammation, which is related to HMGB1/NF-κB signaling pathway (Ming et al., 2016), protect the function of the small intestine via the Toll-like receptor 4 signaling pathway (Lyu et al., 2015) and protect against liver injury via the activation of NF-κB signaling pathway (Qin et al., 2015). The anti-oxidation and acute inflammation bioactivities of kukoamines may be related to the anti-diabetes properties. Kukoamine B ameliorated high-fat diet/high-fructose-induced insulin resistance and obesity by downregulating lipid accumulation, oxidative stress, and inflammatory factors (Zhao et al., 2020), probably via regulating nuclear transcription factors NF-κB and/or PPAR (Li et al., 2018b). Kukoamine A exhibited similar activities against insulin resistance through inhibiting Srebp-1c and downregulating genes expression (Li et al., 2017). Oxidative-stress and over-activation of N-methyl-d-aspartate receptors are important mechanisms of brain injury. Kukoamines could protect against radiation-induced rat brain injury through inhibition of oxidative stress and neuronal apoptosis (Zhang et al., 2016), via activating the PI3-K/Akt/GSK3β pathway (Hu et al., 2015c; Li et al., 2015), and blocking N-methyl-d-aspartate receptors in SH-SY5Y cells (Hu et al., 2015a; Hu et al., 2015b). Kukoamine A could ameliorate the neuroinflammatory response and protect neurogenesis after whole-brain irradiation, partially through regulating the activation of NF-κB, AP-1, and PPARδ (Zhang et al., 2017). Kukoamine A was able to protect the brain against injury induced by permanent middle cerebral artery occlusion via mitochondria mediated apoptosis signaling pathway (Liu et al., 2017). Kukoamines have better protective effects on brain degenerative diseases. Kukoamine A protected against neurotoxin-induced Pakinson’s disease due to the apoptosis inhibition and iron homeostasis maintaining (Hu et al., 2017; Li et al., 2020b). Alzheimer’s disease is an age-related disease characterized by amyloid fibrillogenesis. Kukoamines inhibited aggregation of amyloid β and human islet amyloid polypeptide in a dose-dependent manner. Kukoamine B exhibited stronger inhibitory activities than kukoamine A, and the number of catechol moieties is essential for inhibition of amyloid aggregation (Jiang et al., 2020). Besides, kukoamines also possessed other activities. Kukoamine A showed inhibitory activity against angiotensin I-converting enzyme, thus exerting hypotensive activity (Funayama et al., 1980). Kukoamine A inhibited trypanothione reductase as a mixed inhibitor with Ki = 1.8 μM, Kii = 13 μM) (Ponasik et al., 1995). Kukoamine A also exhibited anti-tumor activity, and inhibited human glioblastoma cell growth and migration in vitro and in vivo through apoptosis induction and epithelial-mesenchymal transition attenuation by downregulating expressions of 5-Lipoxygenase and CCAAT/enhancer binding protein β (Wang et al., 2016). Kukoamine B exhibited anti-osteoporotic effects, increased the osteoblastic differentiation and mineralized nodule formation of osteoblastic MC3T3-E1 cells, but did not affect osteoclast differentiation, and significantly inhibited OVX-induced bone mineral density loss and restored the impaired bone structural properties in osteoporosis model mice (Park et al., 2019). These differences of kukoamine A and B could be attributed to positional isomeric effects.
N1, N14-di-feruloyl-N5-(E, E, E)-p-coumaroylspermine (keayanine A), N1, N5, N14-tri-(E, E, E)-p-coumaroylspermine (keayanine B), N1-feruloyl, N5, N14-di-(E, E, E)-p-coumaroylspermine (keayanine C) and N1, N5, N14-tri-(E, E, E)-feruloylspermine (keayanine D) were isolated from genus Microdesmis (Zamble et al., 2007; Roumy et al., 2008). Keayanine A and keayanidine B had significant vasorelaxing properties, stimulating NO production in the vascular bed, probably due to their strong antioxidant activity versus superoxide anion and hydrogen peroxide and to their stimulation of eNOS mRNA expression (Zamble et al., 2009).
Tetra-substituted spermine N1, N5, N10, N14-tetra-(E, E, E, E)-p-coumaroylspermine was synthesis earlier (Ma et al., 2001) and later isolated from and Matricaria chamomilla (Yamamoto et al., 2002) and Tragopogon tommasinii (Granica et al., 2015). N1, N5, N10, N14-tetra-(E, E, E, E)-p-coumaroylspermine inhibited HIV-1 protease more potently than N1, N5, N10-tri-p-coumaroylspermidine (Ma et al., 2001). cis-trans isomers of N1, N5, N10, N14-tetra-p-coumaroylspermines (1, 5, 10, 14-(Z, Z, Z, Z), 1, 5, 10, 14-(E, Z, Z, E), and 1, 5, 10, 14-(E, E, E, E)) and N1, N5, N9, N14-tetra-p-coumaroyl thermospermines (1, 5, 9, 14-(Z, Z, Z, Z), 1, 5, 9, 14-(E, Z, Z, E), and 1, 5, 9, 14-(E, E, E, E)) were found in the flowers of Matricaria chamomilla (Park et al., 2017). These compounds are potent neurokinin-1 receptor antagonists, and competitively inhibited the binding of substance P and substance P-induced proliferation in breast cancer cell line MDA-MB-453, thus exerting positive effects on substance P/neurokinin-1 receptor-related diseases (Yamamoto et al., 2002; Park et al., 2017).
Compared with other parts of the plant, flowers are abundant in open chain spermidine alkaloids. Di-substituted open chain spermidines such as N5-caffeoyl, N10-(E)-dihydrocaffeoylspermidine was isolated from the flower of Lycium barbarum (Lopatriello et al., 2017). N1, N5-di-p-coumaroylspermidine and N5, N10-di-p-coumaroylspermidine were isolated from anthers of Aphelandra tetraffona and Aphelandra chamissoniana (Werner et al., 1995). Tri-coumaroyl spermidine derivatives, especially N1, N5, N10-tri-p-(E, E, E)-coumaroylspermidine, and its E-Z isomers were isolated or found in numerous plant flowers belonging to several families (Strack et al., 1990; Werner et al., 1995; Yamamoto et al., 2002; Jiang et al., 2008; Sobolev et al., 2008; Yang et al., 2012; Wiese et al., 2013; Xie et al., 2017; Chen et al., 2018; Mori et al., 2019; Zhou et al., 2021). There are many spermidine enantiomers with E (trans) or Z (cis) configurations of the double bonds. Eight E-Z isomers of N1, N5, N10-tri-p-coumaroylspermidine (EEE, EEZ, EZE, EZZ, ZEZ, ZEE, ZZE, and ZZZ) were isolated from the flower of Japanese apricot tree, Prunus mume (Mori et al., 2019). N1, N5, N10-tri-p-(E, Z, E)-coumaroyl spermidine and N1, N5, N10-tri-p-(E, Z, Z)-coumaroyl spermidine were named as safflospermidine A and B as well. These tri-coumaroyl spermidines are unstable and could show photoisomerization behavior under sunlight. N1, N5-N10-tri-p-(Z, Z, E)-coumaroylspermidine and safflospermidine A showed better inhibition effects on serotonin reuptake in rat brain synaptosomes, while N1, N5, N10-tri-p-(Z, Z, Z)-coumaroylspermidine showed weaker inhibition effect (Yuan et al., 2015). Safflospermidine A and B had a higher antityrosinase activity with IC50 of 13.8 and 31.8 μM, respectively (Khongkarat et al., 2020). N1, N5, N10-tri-p-(Z, Z, E)-coumaroylspermidine is a potent serotonin transporter inhibitor, which possess an inhibitory action on serotonin uptake in S6 cells or in synaptosomes, thus improve neuropsychological disorders (Zhao et al., 2009; Zhao et al., 2010). Both safflospermidine A and B had a higher antityrosinase activity with IC50 of 13.8 and 31.8 μM, respectively. N1, N5, N10-tri-p-(E, E, E) coumaroylspermidine exhibited remarkable hepatoprotective activity in HepG2 cells (Zhou et al., 2021). Coumaroylspermidine extracts from safflower, which included four coumaroylspermidine compounds, N1, N5, N10-tri-p-coumaroyl spermidine (ZZZ, ZZE, EZE and EEE), exhibited significant anti-depressant effects in rats (Li et al., 2020a). N1, N10-di-caffeoyl-N5-p-(E, E, E)-coumaroylspermidine, N1-caffeoyl-N5, N10-di-p-(E, E, E)-coumaroylspermidine and N1, N5-di-p-coumaroyl-N10-(E, E, E)-caffeoylspermidine were isolated from the bud of Capparis spinose (Lopatriello et al., 2017). Coumaroyl di-caffeoylspermidine, feruoyl di-coumaroylspermidine, coumaroyl di-feruoylspermidine and tri-feruoylspermidine were found in plant flowers as well (Yang et al., 2012; Sile et al., 2021). Acyl spermidine N1-acetyl, N5, N10-di-p-(E, E)-coumaroylspermidine was isolated from Arachis hypogaea flowers (Sobolev et al., 2008).
Pollen is the male germ cell of flowering plants and is abundant of spermidine alkaloids. In an early study, the chemical compositions of pollen from 67 species were investigated, N1, N10-di-(E, E)-feruloylspermidine, N5, N10-di-(E, E)-feruloylspermidine, N1, N10-caffeoyl-feruloylspermidine (E, E) and N, N’-di-p-coumaroylspermidine existed in the genera Alnus, Betula, Corylus, and Quercus (Meurer et al., 1988). N1-caffeoyl, N10-(E, E)-feruloylspermidine and N1, N10-di-(E, E)-feruloylspermidine were isolated from the pollen of Corylus avellana (Meurer et al., 1986). N1, N5-di-p-(E, E)-coumaroylspermidine was isolated from Brassica campestris pollen (Lv et al., 2013). Isomeric N, N'-dicoumaroyl, N, N'-diferuloyl, N, N'-disinapoyl, N-coumaroyl-N'-feruloyl, and N-feruloyl-N'-sinapoyl spermidine derivatives were found in the pollen of Hippeastrum × hortorum (Youhnovski et al., 1998). Tri-p-coumaroylspermidine, as the main antioxidant components in pollen, was found in numerous plants pollen (Bokern et al., 1995; Lin and Mullin 1999; Sugioka et al., 2018; Adler et al., 2020). N1, N10-di-p-coumaroyl, N5-(E, E, E)-caffeoylspermidine, N1, N5, N10-tri-(E, E, E)-caffeoylspermidine and N1, N5, N10-tri-p-(E, E, E)-coumaroylspermidine were isolated from the pollen of Quercus alba (Walters et al., 2001). N1, N10-di-p-coumaroyl, N5-(E, E, E)-caffeoylspermidine and N1, N5, N10-tri-p-(E, E, E)-coumaroylspermidine exhibited antifungal activity, which reduced mycelial growth of the oat leaf stripe pathogen Pyrenophora avenae and reduced infection of barley with the powdery mildew fungus Blumeria graminis when applied as a post-inoculation treatment (Walters et al., 2001). Human catechol-O-methyltransferase is a key neurotransmitter involved in Parkinson’s disease and depression. N1, N5, N10-tri-p-(E, E, E)- coumaroylspermidine competitively inhibited human catechol-O-methyltransferase activity with an IC50 value 16 μM (Miyata et al., 2022). Quercus dentata, belonging to the same genus of Quercus alba, contains other spermidines such as N1-p-coumaroyl-N5, N10-di-(E, E, E)-caffeoylspermidine, N1-feruloyl-N5, N10-di-(E, E, E)-caffeoylspermidine, N1-p-coumaroyl-N5-caffeoyl-N10-(E, E, E)-feruloylspermidine, and N1, N5-di-p-coumaroyl-N10-(E, E, E)-caffeoylspermidine in the pollen (Bokern et al., 1995; Nimtz et al., 1996). Acetyl spermidines were detected in the pollen of Sambucus nigra, including two obtained stereoisomers N1-acetyl-N5, N10-di-(Z, E)-feruloylspermidine and N1-acetyl-N5, N10-di-(E, E)-feruloylspermidine (Kite et al., 2013).
Bee pollen is produced by worker honey bees, which is composed of natural flower pollen mixed with nectar and bee secretions. The chemical components of bee pollen are complicated, and depend on the vegetation at the collection site, as honey bees collect pollen from target plants grown near bee hives. Therefore, the chemical diversity of bee pollen arises from differences in the botanical origins and collection sites resulting in differences in the biological activities and physicochemical properties of pollen, in addition to color, smell, and taste. Di- and tri- substituted hydroxycinnamic acid spermidines with the substituents of p-coumaroyl, caffeoyl and feruloyl have been found from been pollen, such as rape bee pollen (Wang et al., 2018b; Zhang et al., 2020), camellia bee pollen (Su et al., 2020), rose bee pollen (Yang et al., 2019) and several other species (Negri et al., 2011). Safflospermidine A-B were isolated from sunflower (Helianthus annuus) bee pollen as well (Khongkarat et al., 2020). Phenolamines, from the rape bee pollen, including several di-p-coumaroylspermidines and tri-p-coumaroylspermidines, showed better antioxidant activities, and protective effects on HepG2 cells injured by AAPH (Zhang et al., 2020). Tetra-substituted spermines N1, N5, N10, N14-tetra-p-(E, E, E, E)-coumaroylspermine and N5-caffeoyl-N1, N10, N14-tri-p-(E, E, E, E)-coumaroylspermine were isolated from a Brazilian bee pollen. N5-caffeoyl-N1, N10, N14-tri-p-(E, E, E, E)-coumaroylspermine showed the strong free radical-scavenging activity (Ohta et al., 2007).
Several glycosides of spermidine and spermine were found as well. N, N’-di-dihydrocaffeoylspermidine dihexoside, N1, N5 or N5, N10-di-dihydrocaffeoylspermidine hexoside, N1, N10-di-dihydrocaffeoylspermidine hexoside and N1, N5, N10-tri-dihydrocaffeoylspermidine hexoside were isolated from Solanum quitoense (Gancel et al., 2008). Twenty-three open-chain spermidine glycosides named lycibarbarspermidine A-T (Zhou et al., 2016a; Zhou et al., 2016b; Qian et al., 2020; Chen et al., 2021) and lyciamarspermidine A-C (Qian et al., 2020; Chen et al., 2021), along with two open-chain spermine glycosides lyciamarspermine A and B (Qian et al., 2020), have been isolated from Lycium barbarum. Lycibarbarspermidines and lyciamarspermidines are O-glycosylated products of dicaffeoylspermidine derivatives by one or two β-d-glucopyranose units. Lycibarbarspermidine N and O the cyclization products of dicaffeoylspermidine derivatives. The existing form of dicaffeoylspermidine derivatives depends on the pH value of the medium. The addition of CF3COOH or NH3·H2O was to isolate and purify these compounds. Thus, the compounds may exist as free base form or ionic form with other acids in the plant. Lycibarbarspermidine A-T all displayed antioxidant capacities. lycibarbarspermidine D, E, and G showed extraordinary potent antioxidant capacities, with the oxygen radical absorbance capacity values of 2.96, 2.71, and 3.07 μM TE/μM, respectively (Zhou et al., 2016b). Lycibarbarspermidines exhibited anti-inflammatory activities (Chen et al., 2021). Lycibarbarspermidine A, C, D, and T could inhibit NO production in RAW 264.7 cells stimulated by lipopolysaccharide. Among them, lycibarbarspermidine C and T could significantly inhibit the level of NO at 3 μM. According to the structure–activity relationship, the monosaccharide substitution decreased the anti-inflammatory activity. The sugar unit which occurred at the C-3′ position could affect the anti-inflammatory activity significantly. The double bond of the caffeic acid derivative part plays an important role in the anti-inflammatory activity either. Lycibarbarspermidine A-T exhibited different levels of anti-Alzheimer’s disease activity. Lycibarbarspermidine B, C, F, L, M and O exhibited the short-term memory enhancement capacity close to that of positive control memantine (Zhou et al., 2016b).
Acetyl-lycibarbarspermidine F, and tri-glucosyl derivatives glucosyl-lycibarbarspermidine F, hydroxy-glucosyl-lycibarbarspermidine F were detected in Lycium barbarum by UHPLC-QTOF-MS (Mocan et al., 2018). A number of different glycosides of spermidine derivatives were found in Lycium barbarum, including several isomers. Different types of spermidines were identified by distinctive MS/MS fragment ions. Despite the lack of structural details, a total of 41 out of 58 spermidines were tentatively characterized, including isomers of dicaffeoyl spermidines, cyclic dicaffeoyl spermidines, and their mono-, di-, tri- and spermidine hexosides (Ahad et al., 2020). Novel dicaffeoylspermine-glucosides isomers with approximately-one hundred different structures, which contains the spermine as polyamine core, rather than spermidine, and 1 to 4 β-d-glucopyranose units attached to different sites, were detected in Lycium barbarum (Dos Santos et al., 2022).
Open-chain spermidine and spermine alkaloids have a wide distribution in nature, not only in land plants, but also abundant in microorganism and marine organisms. N1, N8-bis(3,4-dihydroxybenzoyl) spermidine, also named pistillarin, was isolated form bacteria Clavariadelphus pistillaris and several Ramaria species (Steglich et al., 1984), fungus Penicillium bilaii (Capon et al., 2007) and Ramaria subaurantiaca (Choomuenwai et al., 2013). Pistillarin exhibited antimalarial activity against Plasmodium falciparum (3D7) parasites with IC50 0.23 μM (Choomuenwai et al., 2013). Pistillarin salt, isolated from the fruiting bodies of Gomphus floccosus, exhibited a significantly protective effect against DNA damage by hydroxyl radicals generated from the Fenton reaction via iron chelation as well as free radical-scavenging activity (Lee et al., 2010). N1, N5-di-dihydrocoumaroyl-N10-acetylspermine (JBIR-125) was isolated from a new species of Streptomyces (strain R56-07), which exhibited 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity with an IC50 value of 35.1 μM (Kawahara et al., 2012).
Polycyclic guanidine spermidine alkaloids ptilomycalin A (Kashman et al., 1989; Ohtani et al., 1992; Patil et al., 1995; Gallimore et al., 2005; Hua et al., 2007), crabescidin 800 (Jares-Erijman et al., 1991; Berlinck et al., 1993; Tavares et al., 1994; Patil et al., 1995; Chang et al., 2003; Hua et al., 2007), 816 (Jares-Erijman et al., 1991; Berlinck et al., 1993; Jares-Erijman et al., 1993; Patil et al., 1995), 826 (Chang et al., 2003), 830 (Jares-Erijman et al., 1991), 844 (Jares-Erijman et al., 1991), isocrabescidin 800 (Berlinck et al., 1993), 13,14,15-isocrambescidin 800 (Jares-Erijman et al., 1993), crambidine (Berlinck et al., 1993), and fromiamycalin (Chang et al., 2003) have been found in several classes of marine sponges. The structures differed in the number of methylene groups linked to N-5, functional groups and stereoisomerism of polycyclic guanidine ring and spermidine ring. Fromiamycalin, different from other ptilomycalin A analogues, which N-1 and N-5 of the spermidine formed a tetrahydropyrimidine ring. Later, one acyclic guanidine alkaloid, unguiculin A and four pentacyclic alkaloids, ptilomycalin E − H were isolated from the sponge Monanchora unguiculata (Campos et al., 2017). The polycyclic guanidine spermidine alkaloids possessed widely pharmacological effects such as anti-tumor, antiviral, and antifungal. Crabescidines exhibited attractive anti-tumor effects. Ptilomycalin A and crambescidine 800 showed significant growth inhibition of 11 different cancer cell lines with GI50 values of 0.04–0.19 mg/mL (Hua et al., 2007). Ptilomycalin A shows cytotoxicity against cancer cell lines P388, L1210, KB and MDA-MB-231 with IC50 of 0.1, 0.4, 1.3 μg/mL and 4.3 µM, respectively (Ohtani et al., 1992; Tabakmakher et al., 2013). Ptilomycalin E and the mixture of ptilomycalins G and H showed promising cytotoxicity against KB cells with IC50 of 0.85 and 0.92 μM, respectively (Campos et al., 2017). Crambescidin 816 was found to be active against HCT-16 human colon carcinoma cells with IC50 of 0.24 μg/mL (Berlinck et al., 1993). The anti-tumor mechanisms of crabescidines on cancer cells were investigated. Ptilomycalin A-like induced p53-independent programmed cell death and S-phase cell cycle arrest by activating JNK1/2 and ERK1/2, following AP-1 activation (Dyshlovoy et al., 2016a). Crambescidine 800 induced cell cycle arrest at the G2/M phase and apoptosis of triple negative breast cancer cells by the inhibition of phosphorylation of Akt, NF-κB, and MAPK pathways (Shrestha et al., 2018). Crambescidin 800 induced erythroid differentiation in K562 chronic myelogenous leukemia cells and neurite outgrowth in Neuro 2A neuroblastoma cells (Aoki et al., 2004). Crambescidin-816 inhibited HepG2 cell migration via inhibiting cell–cell adhesion, interfering with the formation of tight junctions, and cell-matrix adhesion (Rubiolo et al., 2014). Crambescidine 816, 830, and 800 strongly inhibited tumor cell proliferation, and disrupted tumor cell adhesion and cytoskeletal integrity promoting the activation of the intrinsic apoptotic signaling (Roel et al., 2016). In addition, ptilomycalin A exhibited antifungal activity against Candida albicans with MIC 0.8 μg/mL (Ohtani et al., 1992), and inhibited melanogenesis of Cryptococcus neoformans in vitro with an IC50 of 7.3 μM through inhibition of biosynthesis of laccase in the melanin biosynthetic pathway (Dalisay et al., 2011). Ptilomycalin F and fromiamycalin exhibited promising activity against Plasmodium falciparum with IC50 values of 0.23 and 0.24 μM, respectively (Campos et al., 2017). Crambescidine 800 strongly inhibited bacteria Acinetobacter baumannii, Klebsiella pneumoniae and Pseudomonas aeruginosa with MIC values of 2, 1, 1 μg/mL, respectively (Sun et al., 2015). Crambescidin 800 exhibited the comparable inhibition activity of Plasmodium falciparum strain 3D7 to quinine (Lazaro et al., 2006). Crambescidin-816 reduced cell viability in Saccharomyces cerevisiae inducing an increment in cell size and DNA content, and apoptosis (Rubiolo et al., 2013). Crambescidin 800 showed a potent protective effect on the cell death of HT22 and neuroblastoma induced by a hypoxic condition or nitric oxide (Suna et al., 2007). Calcium influx is considered the main mechanism responsible for neuronal cell death. Ptilomycalin A could interact with ATP at the ATP binding site of Na+, K+-ATPase or Ca2 +-ATPase with an IC50 of 2 μM and 10 μM, respectively (Ohizumi et al., 1996). Crambescidin 816 was found to have a potent Ca2+ antagonist effect and to inhibit the acetylcholine-induced contraction of guinea pig ileum at very low concentrations (Berlinck et al., 1993). Crambescidin 816 produced its main antagonist effect on l-type Ca2+ channels, and partially blocked voltage-gated calcium channels and voltage-dependent sodium channels in cortical neurons (Martin et al., 2013). Crambescidin 816 was proven to be cytotoxic against cortical neurons (Bondu et al., 2012). The cytotoxic effect of crambescidin 816 in cortical neurons may be related to an increase in the cytosolic calcium concentration elicited by the toxin, which is mediated by glutamate receptor activation (Mendez et al., 2017). Furthermore, Crambescidin 800, 816 and 844, fromiamycalin and ptilomycalin A strongly inhibited HSV-1 completely, with diffuse cytotoxicity (Jares-Erijman et al., 1991; Chang et al., 2003; Hua et al., 2007). Ptilomycalin A exhibited anti-HSV activity at a concentration of 0.2 μg/mL (Ohtani et al., 1992).
Monanchomycalin A, B and C, possessing similar structures with crambescidins, were isolated from the Far-Eastern marine sponge Monanchora pulchra (Makarieva et al., 2012; Tabakmakher et al., 2013). The anti-tumor activities of monanchomycalin A, B and C were investigated. Monanchomycalin A and B exhibited cytotoxic activities against HL-60 human leukemia cells with IC50 values of 120 and 140 nM, respectively (Makarieva et al., 2012). Monanchomycalin C exhibited potency to toxic activities against human breast cancer MDA-MB-231 cells with IC50 values of 8.2 µM (Tabakmakher et al., 2013). Monanchoxymycalin A, B and C were isolated from the marine sponge Monanchora pulchra either (Tabakmakher et al., 2016; Shubina et al., 2019). Monanchoxymycalin A, B and C exhibited potent cytotoxic activities against cervical epithelioid carcinoma HeLa cells with IC50 2.80 µM, 2.82 µM and 3.50 µM, respectively (Tabakmakher et al., 2016; Shubina et al., 2019). Monanchoxymycalin A and B exhibits cytotoxic activity against breast adenocarcinoma MDA-MB231 cells with IC50 values of 5.60 µM and 11.65 µM, respectively (Tabakmakher et al., 2016).
A new class of guanidine alkaloids monanchocidin A-E with an unprecedented skeleton system was isolated from the marine sponge Monanchora pulchra. The absolute configuration of the monanchocidin A was later fully determined as 5R, 8S, 10S, 13R, 14S, 15R, 19R, 23R, 37S, 42S, 43R (Shubina et al., 2018). Monanchocidin A-E showed potent cytotoxic activities against HL-60 human leukemia cells with IC50 values of 540, 200, 110, 830, and 650 nM, respectively (Guzii et al., 2010; Makarieva et al., 2011). Monanchocidin A showed very modest antibacterial, antifungal, and antiprotozoal activities, and exhibited potent selective activity for the melanoma panel in the NCI cancer cell screening panel (Gogineni et al., 2020). Monanchocidin A exerted anti-migratory activity, and was able to overcome cisplatin-resistance of the human germ cell tumor cell line (Dyshlovoy et al., 2014; Dyshlovoy et al., 2015; Dyshlovoy et al., 2016b). Two new indole spermidines didemnidines A and B, with an indole-3-glyoxylamide moiety linked to the N-1 position, were isolated from the New Zealand ascidian Didemnum sp. Didemnidine B exhibited mild in vitro growth inhibition of Plasmodium falciparum with IC50 of 15 μM (Finlayson et al., 2011). Ianthelliformisamines A–C were isolated from the marine sponge Suberea ianthelliformis, among which ianthelliformisamine A and C belong to spermine and Ianthelliformisamine B belongs to spermidine. Ianthelliformisamine A showed inhibitory activity against the Gram-negative bacterium Pseudomonas aeruginosa with an IC50 of 6.8 μM and MIC of 35 μM (Xu et al., 2012). Tokaradine C, a positional isomer of lanthelliformisamine B, was isolated from the Japanese marine sponge Pseudoceratina purpurea (Fusetani et al., 2001). Spermatinamine (Buchanan et al., 2007) and pseudoceramine A–C (Yin et al., 2011) were also isolated from genus Pseudoceratina. Spermatinamin inhibited the activity of isoprenylcysteine methyltransferase with an IC50 of 1.9 μM (Buchanan et al., 2007). Spermatinamine and pseudoceramine B significantly inhibited the secretion and enzyme activity of the Yersinia outer protein (Yin et al., 2011). Spermatinamine also exhibited antimalarial activity against Plasmodium falciparum (3D7) parasites with IC50 of 0.23 μM (Choomuenwai et al., 2013).
Petrobactin, composing of two spermidinyl moieties and one citryl moiety, was produced by Marinobacter hydrocarbonoclasticus, which could readily undergo a light-mediated decarboxylation reaction when bound to Fe(III) (Barbeau et al., 2002). The open-chain spermidine and spermine alkaloids from land plants and other sources are summarized in Table.6 and Table.7. The substituted groups, along with the structure of open chain alkaloids are included in Fig. 7.
Compound
plant
part
genus
family
ref
Open-chain
N5-benzoylspermidine
Oncinotis tenuiloba
leaf
oncinotis
apocynaceae
(Doll et al., 1994)
N1-coumaroylspermidine, N1-feruloylspermidine, N8-coumaroylspermidine, N8-feruloylspermidine, N1-coumaroylspermine
Solanum dulcamara
leaf, flower
solanum
solanaceae
(Panagabko et al., 2000)
N1, N5, N10-tri-dihydrocaffeoylspermidine
Solanum sessiliflorum
fruit
solanum
solanaceae
(Rodrigues et al., 2013)
Solanum quitoense
(Gancel et al., 2008)
Solanum tuberosum
tuber
solanum
(Parr et al., 2005)
N1, N5-di-dihydrocaffeoylspermidine
Solanum sessiliflorum
fruit
solanum
solanaceae
(Rodrigues et al., 2013)
Solanum quitoense
(Gancel et al., 2008)
scotanamine B-C
Scopolia tangutica
root
scopolia
solanaceae
(Long et al., 2014)
scotanamine D
Scopolia tangutica
root
scopolia
solanaceae
(Long et al., 2014)
Lycium barbarum
fruit
lycium
(Chen et al., 2021)
Hyoscyamus albus
leaf
hyoscyamus
(Yahia et al., 2020)
N1, N10-di-dihydrocaffeoylspermidine
Scopolia tangutica
root
scopolia
solanaceae
(Long et al., 2014)
Solanum tuberosum
tuber
solanum
(Narvaez-Cuenca et al., 2013)
Iochroma cyaneum
herb
iochroma
(Sattar et al., 1990)
Solanum quitoense
fruit
solanum
(Gancel et al., 2008)
N1-(E)-caffeoyl, N10-dihydrocaffeoylspermidine
Scopolia tangutica
root
scopolia
solanaceae
(Long et al., 2014)
Lycium ruthenicum
fruit
lycium
(Zhao et al., 2014)
N1, N10-di-benzoylspermidine
Cassia floribunda
leaf
cassia
fabaceae
(Alemayehu et al., 1988)
lyrium spermidine A
Lycium ruthenicum
fruit
lycium
solanaceae
(Zhao et al., 2014)
N1, N10-di-tigloylspermidine
Ipomoea nil
seed
ipomoea
convolvulaceae
(Schimming et al., 2005)
N, N’-di-caffeoylspermidine
Solanum melongena
fruit
solanum
solanaceae
(Whitaker and Stommel 2003)
Nicotiana tabacum
seed
nicotiana
(Camacho-Cristobal et al., 2004)
Physalis alkekengi
fruit
physalis
(Wen et al., 2019)
N, Ń-di-dihydrocaffeoylspermine
Physalis alkekengi
fruit
physalis
solanaceae
(Wen et al., 2019)
N1-((4′-O-glycosyl)-sinapoyl), N10-(E, E)-sinapoylspermidine, N1, N10-di-(E, E)-sinapoylspermidine
Arabidopsis thaliana
seed
arabidopsis
brassicaceae
(Luo et al., 2009)
N1, N5-di-p-(E, E)-coumaroylspermidine
Coix lacryma-jobi
whole
coix
poaceae
(Xu et al., 2018)
N1, N5, N10-tri-p-(E, E, E)-coumaroylspermidine
Artemisia caruifolia
aerial
artemisia
asteraceae
(Ma et al., 2001)
Orostachys japonicus
whole
orostachys
crassulaceae
(Lee et al., 2011)
Matricaria chamomilla
flower
matricaria
asteraceae
(Yamamoto et al., 2002)
Aphelandra tetraffona
anther
aphelandra
acanthaceae
(Werner et al., 1995)
Aphelandra chamissoniana
Carthamus tinctorius
flower
carthamus
asteraceae
(Jiang et al., 2008)
Capparis spinosa
bud
capparis
capparaceae
(Wiese et al., 2013)
Buddleja officinalis
flower
buddleja
scrophulariaceae
(Xie et al., 2017)
Crataegi flos
flower
crataegus
rosaceae
(Strack et al., 1990)
Rosa rugosa
flower
rosa
(Zhou et al., 2021)
Arachis hypogaea
flower
arachis
fabaceae
(Sobolev et al., 2008)
Helianthus annuus
pollen
helianthus
asteraceae
(Lin and Mullin 1999)
Quercus dentata
pollen
quercus
fagaceae
(Bokern et al., 1995; Walters et al., 2001)
Quercus alba
Prunus mume
flower
prunus
rosaceae
(Mori et al., 2019)
keayanidine A-B
Microdesmis keayana
root
microdesmis
pandaceae
(Zamble et al., 2006)
Microdesmis puberula
(Roumy et al., 2008)
Keayanidine C
Microdesmis keayana
root
microdesmis
pandaceae
(Zamble et al., 2006)
Microdesmis puberula
(Roumy et al., 2008)
Sambucus nigra
pollen
sambucus
adoxaceae
(Kite et al., 2013)
N1, N10-di-benzoyl-N5-acetamidespermidine
Banara parviflora
leaf
banara
salicaceae
(Moritz et al., 2016)
chisitine 1, chisitine 2
Chisocheton weinlandii
leaf
chisocheton
meliaceae
(Tzourosa et al., 2004)
N1, N14-di-dihydrocaffeoylspermine, N1,N5,N14-tri-dihydrocaffeoylspermine
Solanum tuberosum
tuber
solanum
solanaceae
(Narvaez-Cuenca et al., 2013)
kukoamine A
Solanum tuberosum
tuber
solanum
solanaceae
(Parr et al., 2005)
Lycium chinense
root bark
lycium
(Funayama et al., 1980)
kukoamine B
Lycium chinense
root bark
lycium
solanaceae
(Funayama et al., 1995)
keayanine A-D
Microdesmis puberula
root
microdesmis
pandaceae
(Zamble et al., 2007; Roumy et al., 2008)
Microdesmis keayana
N1, N5, N10, N14-tetra-p-(E, E, E, E)-coumaroylspermine
Matricaria chamomilla
flower
matricaria
asteraceae
(Yamamoto et al., 2002; Park et al., 2017)
Tragopogon tommasinii
aerial
tragopogon
(Granica et al., 2015)
N1, N5, N10, N14-tetra-p-(Z, Z, Z, Z)-coumaroylspermine, N1, N5, N10, N14-tetra-p-(E, Z, Z, E)-coumaroylspermine, N1, N5, N9, N14-tetra-p-(Z, Z, Z, Z)-coumaroylthermospermine, N1, N5, N9, N14-tetra-p-(E, E, E, E)coumaroylthermospermine, N1, N5, N9, N14-tetra-p-(E, Z, Z, E)coumaroylthermospermine
Matricaria chamomilla
flower
matricaria
asteraceae
(Park et al., 2017)
N5-(E)-caffeoyl, N10-dihydrocaffeoylspermidine
Lycium barbarum
flower
lycium
solanaceae
(Lopatriello et al., 2017)
N1, N5-di-(E, E)-p-coumaroylspermidine, N5, N10-di-(E, E)-p-coumaroylspermidine
Aphelandra tetraffona
anther
aphelandra
acanthaceae
(Werner et al., 1995)
Aphelandra chamissoniana
safflospermidine A-B
Carthamus tinctorius
flower
carthamus
asteraceae
(Jiang et al., 2008)
Prunus mume
flower
prunus
rosaceae
(Mori et al., 2019)
N1, N5-N10-tri-p-(Z, Z, E)-coumaroylspermidine
Carthamus tinctorius
flower
carthamus
asteraceae
(Jiang et al., 2008; Zhao et al., 2009)
Prunus mume
flower
prunus
rosaceae
(Mori et al., 2019)
N1, N5, N10-tri-p-(Z, Z, Z)-coumaroylspermidine
Carthamus tinctorius
flower
carthamus
asteraceae
(Jiang et al., 2008)
Prunus mume
flower
prunus
rosaceae
(Mori et al., 2019)
N1, N10-di-caffeoyl-N5-p-(E, E, E)-coumaroylspermidine, N1-caffeoyl-N5, N10-di-p-(E, E, E)-coumaroylspermidine
Capparis spinosa
bud
capparis
capparaceae
(Wiese et al., 2013)
N1, N5-di-p-coumaroyl-N10-(E, E, E)-caffeoylspermidine
Capparis spinosa
bud
capparis
capparaceae
(Wiese et al., 2013)
Quercus dentata
pollen
quercus
fagaceae
(Nimtz et al., 1996)
N1, N5, N10-tri-p-coumaroylspermidine (EEZ, ZEE, ZEZ)
Prunus mume
flower
prunus
rosaceae
(Mori et al., 2019)
N1-acetyl-N5, N10-di-(E, E)-p-coumaroylspermidine
Arachis hypogaea
flower
arachis
fabaceae
(Sobolev et al., 2008)
N1-caffeoyl, N10-(E, E)-feruloylspermidine, N1, N10-di-(E, E)-feruloylspermidine
Corylus avellana
pollen
corylus
betulaceae
(Meurer et al., 1986)
N1, N5-di-p-(E, E)-coumaroylspermidine
Brassica campestris
pollen
brassica
brassicaceae
(Lv et al., 2013)
N1, N5, N10-tri-(E, E, E)-caffeoylspermidine
Brassica campestris
pollen
brassica
brassicaceae
(Lv et al., 2013)
Quercus dentata
quercus
fagaceae
(Bokern et al., 1995; Walters et al., 2001)
Quercus alba
N1-p-coumaroyl, N5, N10-di-(E, E, E)-caffeoylspermidine
Quercus dentata
pollen
quercus
fagaceae
(Bokern et al., 1995)
N1, N10-di-p-coumaroyl, N5-(E, E, E)-caffeoylspermidine
Quercus dentata
pollen
quercus
fagaceae
(Bokern et al., 1995; Walters et al., 2001)
Quercus alba
N1-feruloyl-N5, N10-di-(E, E, E)-caffeoylspermidine, N1-p-coumaroyl-N5-caffeoyl-N10-(E, E, E)-feruloylspermidine
Quercus dentata
pollen
quercus
fagaceae
(Nimtz et al., 1996)
N5, N10-di-(E, E)-feruloylspermidine, N1-acetyl-N5, N10-di-(Z, E)-feruloylspermidine, N1-acetyl-N5, N10-di-(Z, E)-feruloylspermidine
Sambucus nigra
pollen
sambucus
adoxaceae
(Kite et al., 2013)
N, N’-di-dihydrocaffeoylspermidine dihexoside, N1, N5 or N5, N10-di-dihydrocaffeoylspermidine hexoside, N1, N10-di-dihydrocaffeoylspermidine hexoside, N1, N5, N10-tri-dihydrocaffeoylspermidine hexoside
Solanum quitoense
fruit
solanum
solanaceae
(Gancel et al., 2008)
lycibarbarspermidine A − T
Lycium barbarum
fruit
lycium
(Zhou et al., 2016a; Zhou et al., 2016b; Qian et al., 2020; Chen et al., 2021)
lyciamarspermidine A-C
Lycium barbarum
(Qian et al., 2020; Chen et al., 2021)
lyciamarspermine A-B
Lycium barbarum
(Qian et al., 2020)
acetyl-lycibarbarspermidine F, glucosyl-lycibarbarspermidine F, hydroxy-glucosyl-lycibarbarspermidine F
Lycium barbarum
(Mocan et al., 2018)
Compound
source
genus
family
ref
pistillarin
Clavariadelphus pistillaris
clavariadelphus
typhulaceae
(Steglich et al., 1984)
Penicillium bilaii
penicillium
trichocomaceae
(Capon et al., 2007)
Ramaria subaurantiaca
ramaria
thelephoraceae
(Choomuenwai et al., 2013)
pistillarin salt
Gomphus floccosus
gomphus
gomphidiaceae
(Lee et al., 2010)
JBIR-125
Streptomyces(strainR56-07)
streptomyces
streptomycetaceae
(Kawahara et al., 2012)
ptilomycalin A
Ptilocaulis spiculifer
ptilocaulis
axinellidae
(Kashman et al., 1989; Ohtani et al., 1992)
Hemimycale
hemimycale
hymedesmiidae
Monanchora unguifera
monanchora
crambeidae
(Gallimore et al., 2005; Hua et al., 2007)
Batzella
batzella
chondropsidae
(Patil et al., 1995)
crambescidin 800
Monanchora unguifera
monanchora
crambeidae
(Hua et al., 2007)
Batzella
batzella
chondropsidae
(Patil et al., 1995)
Crambe crambe
crambe
brassicaceae
(Berlinck et al., 1993)
Crambe crambe
crambe
brassicaceae
(Jares-Erijman et al., 1991)
Monanchora arbuscula
monanchora
crambeidae
(Tavares et al., 1994)
Monanchora
monanchora
crambeidae
(Chang et al., 2003)
Monanchora viridis
monanchora
crambeidae
(Shrestha et al., 2018)
Monanchora ungiculata
monanchora
crambeidae
(Aoki et al., 2004)
Clathria cervicornis
clathria
microcionidae
(Sun et al., 2015)
crambescidin 816
Batzella
batzella
chondropsidae
(Patil et al., 1995)
Crambe crambe
crambe
brassicaceae
(Berlinck et al., 1993; Jares-Erijman et al., 1993)
Crambe crambe
crambe
brassicaceae
(Jares-Erijman et al., 1991)
isocrambescidin 800, crambidine
Crambe crambe
crambe
brassicaceae
(Berlinck et al., 1993)
crambescidin 830, 844
Crambe crambe
crambe
brassicaceae
(Jares-Erijman et al., 1991)
crambescidin 826, fromiamycalin
Monanchora
monanchora
crambeidae
(Chang et al., 2003)
13,14,15-isocrambescidin 800
Crambe crambe
crambe
brassicaceae
(Jares-Erijman et al., 1993)
unguiculin A, ptilomycalin E − H
Monanchora ungiculata
monanchora
crambeidae
(Campos et al., 2017)
monanchomycalin A-C
Monanchora pulchra
monanchora
crambeidae
(Makarieva et al., 2012; Tabakmakher et al., 2013)
monanchoxymycalin A-C
Monanchora pulchra
monanchora
crambeidae
(Tabakmakher et al., 2016; Shubina et al., 2019)
monanchocidin A-E
Monanchora pulchra
monanchora
crambeidae
(Guzii et al., 2010; Makarieva et al., 2011)
didemnidines A, didemnidines B
Didemnum lahillei
Didemnum
didemnidae
(Finlayson et al., 2011)
ianthelliformisamine A–C
Suberea ianthelliformis
suberea
aplysinellidae
(Xu et al., 2012)
tokaradine C
Pseudoceratina purpurea
pseudoceratina
pseudoceratinidae
(Fusetani et al., 2001)
spermatinamine
Pseudoceratina
pseudoceratina
pseudoceratinidae
(Buchanan et al., 2007)
pseudoceramine A–C
Pseudoceratina
pseudoceratina
pseudoceratinidae
(Yin et al., 2011)
petrobactin
Marinobacter hydrocarbonoclasticus
marinobacter
/
(Barbeau et al., 2002)
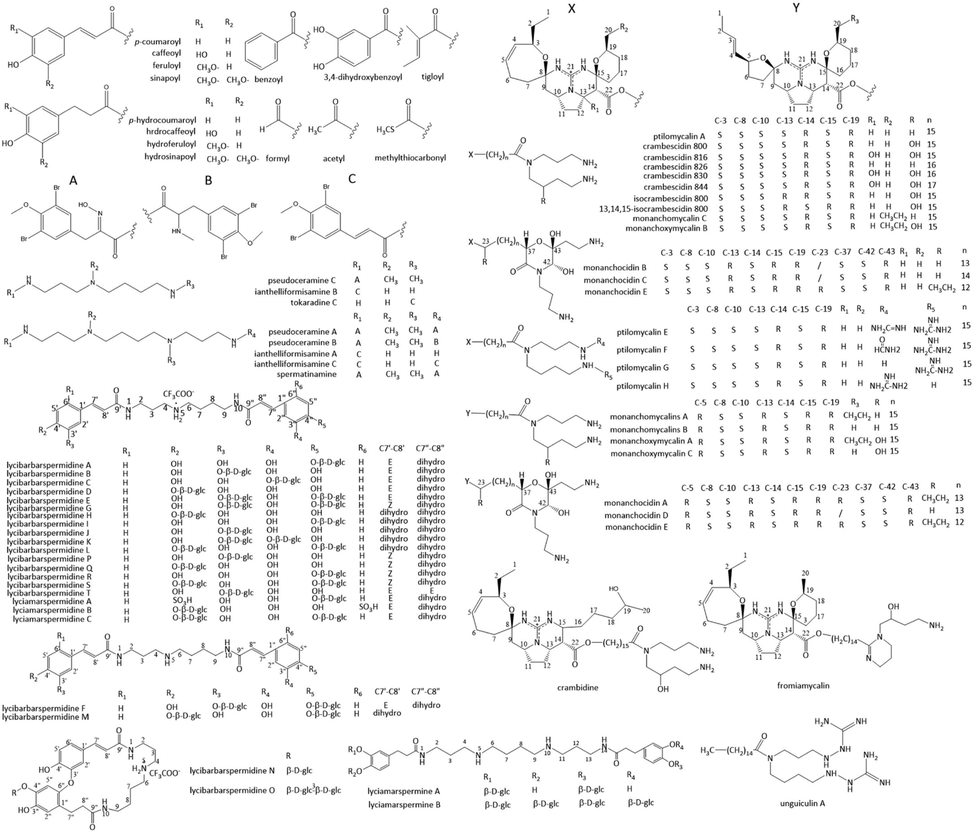
Chemical structures of open chain spermidine and spermine alkaloids.
3 Conclusions
In this review, we summarized the occurrence and pharmacological effects of spermidine and spermine alkaloids. Plants, seem to be the main sources of spermidine and spermine alkaloids, especially the macrocylic. Macrocylic spermidine alkaloids are much more abundant in nature than macrocylic spermine alkaloids. Dovyalicin-type alkaloids with an 8-membered ring were manly isolated from genus Dovyalis and Homalium, salicaceae family. The spermidine lactam alkaloids with a 13-membered ring have been found mainly in celastraceae family. Spermidine alkaloids with 24-membered lactam ring, containing two amide carbonyls in the macrocycle, were isolated from capparaceae family. Almost all the lactam alkaloids with a 17-membered ring are a class of macrocyclic alkaloids having a spermine nucleus.
Many mono-, di-, and tri-substituted spermidines/spermines and tetra-substituted spermines conjugated with hydroxycinnamic acids such as p-coumaric acid, caffeic acid, ferulic acid, sinapic acid were found from plants mainly belonging to family solanaceae, asteraceae and fagaceae. Compared with other parts of the plants, pollen is abundant in open chain spermidine and spermine alkaloids. In addition, numerous spermidine and spermine glycosides were detected and isolated from the fruit of Lycium barbarum. Marine sponges are abundant in open chain spermidine alkaloids. A great number of guanidine spermidine alkaloids with various skeletons were isolated from the marine sponges. The distribution characteristics of the spermidine and spermine alkaloids have guiding significance in finding new compounds, especially compounds with new skeletons.
A great quantity of the pharmacological effects of the open chain spermidine and spermine alkaloids are reported. anti-oxidative may be the main activity of the caffeic acid derivatives, probably due to the phenolic OH groups of the hydroxycinnamic acids moieties. Polycyclic guanidine spermidine alkaloids, which isolated from the marine sponge, show great potential in various cancer cells. However, pharmacological studies of macrocyclic spermidine and spermine alkaloids are scarce. One reason might be the low contents of the compound obtained. For instance, in our previous study (Zhang et al., 2022), the contents of celacinnine-type alkaloids are about 0.01 mg/g in dry power of Tripterygium wilfordii. In addition, some of the compounds were isolated several years ago, and the pharmacological effects were not investigated at that time. Interestingly, some macrocyclic and open chain spermine and spermidine alkaloids exhibited great potential in brain diseases, through inhibition of enzyme activity of acetylcholinesterase and butyrylcholinesterase, and showed protective effects on brain degenerative diseases. Therefore, considering remarkable pharmacological activities, further in-depth studies on the pharmacological effects of the macrocyclic spermidine and spermine alkaloids are required in the future.
In order to obtain more compounds for further study, numerous synthesis methods on the macrocyclic spermidine and spermine alkaloids and their analogues are reported. In the near future, we will summarize these synthesis methods. We hope this review will be helpful for phytochemistry research, especially in finding compounds with attractive pharmacological activities.
Acknowledgments
This research was financially supported by the Project for Academic and Technical Leaders of Major Disciplines in Jiangxi (No. 20182BCB22004), the National Natural Science Foundation of China (No. 81860686) and University Natural Science Foundation of Jiangsu province (No. 22KJD360001).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Spermidine alkaloid and glycosidic constituents of Vietnamese Homalium cochinchinensis. Phytochem. Lett.. 2021;43:154-162.
- [CrossRef] [Google Scholar]
- Assessing chemical mechanisms underlying the effects of sunflower pollen on a gut pathogen in bumble bees. J. Chem. Ecol.. 2020;46:649-658.
- [CrossRef] [Google Scholar]
- Chemical profiling of spermidines in goji berry by strong cation exchange solid-phase extraction (SCX-SPE) combined with ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS/MS) J. Chromatogr. B. 2020;1137:121923
- [CrossRef] [Google Scholar]
- Capparisinine, a New Alkaloid from Capparis decidua. Eur. J. Org. Chem.. 1987;1987:161-162.
- [CrossRef] [Google Scholar]
- Ahmad, V., Amber, A.u.R., Arif, S., et al., 1985a. Cadabicine, an alkaloid from Cadaba farinosa. Phytochemistry. 24, 2709–2711. https://doi.org/10.1016/S0031-9422(00)80700-1.
- Ahmad, V., Arif, S., Amber, A.u.R., et al., 1985b. A new spermidine alkaloid from Capparis decidua. Heterocycles. 23, 3015-3020. https://doi.org/10.3987/R-1985-12-3015.
- Ahmad, V., Arif, S., Amber, A.u.R., et al., 1986. A new alkaloid from root bark of Capparis decidua. Zeitschrift. für. Naturforschung. B. 41, 1033-1035. https://doi.org/10.1515/znb-1986-0818.
- Ahmad, V., Fizza, K., Amber, A.u.R., et al., 1987a. Cadabicine and Cadabicine Diacetate from Crataeva nurvala and Cadaba farinosa. J. Nat. Prod. 50, 1186-1186. https://doi.org/10.1021/np50054a038.
- Isocodonocarpine from Capparis decidua. Phytochemistry. 1989;28:2493-2495.
- [CrossRef] [Google Scholar]
- Two New N-acetylated spermidine alkaloids from Capparis decidua. J. Nat. Prod.. 1992;55:1509-1512.
- [CrossRef] [Google Scholar]
- Spermine Alkaloids from Schweinfurthia papilionacea. J. Nat. Prod.. 1990;53:1162-1167.
- [CrossRef] [Google Scholar]
- Effects of selected chemical ingredients from Capparis Spinosa L. on platelet aggregation and blood coagulation. Dirasat Pure Sci.. 1999;26:195-202.
- [Google Scholar]
- Bianthraquinones and a spermidine alkaloid from Cassia floribunda. Phytochemistry. 1988;27:3255-3258.
- [CrossRef] [Google Scholar]
- Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer Res.. 2004;24:2325-2330.
- [Google Scholar]
- Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J. Am. Chem. Soc.. 2002;124:378-379.
- [CrossRef] [Google Scholar]
- Myricoidine and dihydromyricoidine, two new macrocyclic spermidine alkaloids from Clerodendrum myricoides. Tetrahedron. 1988;44:4521-4526.
- [CrossRef] [Google Scholar]
- Formation of a complex pattern of sinapate esters in Brassica napus seeds, catalyzed by enzymes of a serine carboxypeptidase-like acyltransferase family? Phytochemistry. 2005;66:1334-1345.
- [CrossRef] [Google Scholar]
- Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca++ channel blocker activity of crambescidin 816. J. Nat. Prod.. 1993;56:1007-1015.
- [CrossRef] [Google Scholar]
- Chaenorhin, ein macrocyclisches spermin-Alkaloid. 149. mitteilung ber alkaloide. Helv. Chim. Acta. 1973;56:1266-1303.
- [CrossRef] [Google Scholar]
- Trisubstituted hydroxycinnamic acid spermidines from Quercus dentata pollen. Phytochemistry. 1995;39:1371-1375.
- [CrossRef] [Google Scholar]
- Additional bioactive guanidine alkaloids from the Mediterranean sponge Crambe crambe. Rsc Adv.. 2012;2:2828-2835.
- [CrossRef] [Google Scholar]
- Spermatinamine, the first natural product inhibitor of isoprenylcysteine carboxyl methyltransferase, a new cancer target. Bioorg. Med. Chem. Lett.. 2007;17:6860-6863.
- [CrossRef] [Google Scholar]
- Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves. J. Plant. Physiol.. 2004;161:879-881.
- [CrossRef] [Google Scholar]
- Unguiculin A and ptilomycalins E-H, antimalarial guanidine alkaloids from the Marine Sponge Monanchora unguiculata. J. Nat. Prod.. 2017;80:1404-1410.
- [CrossRef] [Google Scholar]
- Citromycetins and bilains A-C: new aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod.. 2007;70:1746-1752.
- [CrossRef] [Google Scholar]
- Crambescidin 826 and dehydrocrambine A: new polycyclic guanidine alkaloids from the marine sponge Monanchora sp. that inhibit HIV-1 fusion. J. Nat. Prod.. 2003;66:1490-1494.
- [CrossRef] [Google Scholar]
- Chemical constituents from Lycium barbarum (Solanaceae) and their chemophenetic significance. Biochem. Syst. Ecol.. 2021;97:104292-104297.
- [CrossRef] [Google Scholar]
- Insights into tissue-specific specialized metabolism in tieguanyin tea cultivar by untargeted metabolomics. Molecules. 2018;23:1817-1838.
- [CrossRef] [Google Scholar]
- Five novel macrocyclic spermine alkaloids from incarvillea sinensis. Tetrahedron Lett.. 1997;38:2713-2716.
- [CrossRef] [Google Scholar]
- A novel macrocyclic spermine alkaloid from Incarvillea sinensis. J. Asian Nat. Prod. Res.. 2007;9:115-118.
- [CrossRef] [Google Scholar]
- The discovery, synthesis and antimalarial product-based polyamine alkaloids. Tetrahedron Lett.. 2013;54:5188-5191.
- [CrossRef] [Google Scholar]
- A new spermidine macrocyclic alkaloid isolated from Gymnosporia arenicola leaf. Fitoterapia. 2015;106:7-11.
- [CrossRef] [Google Scholar]
- Natural occurrence, synthesis and biological applications of spermidine alkaloids. Curr. Org. Chem.. 2017;21:546-558.
- [CrossRef] [Google Scholar]
- Ptilomycalin A inhibits laccase and melanization in Cryptococcus neoformans. Bioorg. Med. Chem.. 2011;19:6654-6657.
- [CrossRef] [Google Scholar]
- Die struktur des spermin-alkaloides aphelandrin aus aphelandra squarrosa NEES. 170. mitteilung über organische naturstoffe. Helv. Chim. Acta. 1978;61:2646-2671.
- [CrossRef] [Google Scholar]
- Beitrag zur absoluten konfiguration der spermin-alkaloide O-methylorantin und Aphelandrin. 175. mitteilung über organische naturstoffe. Helv. Chim. Acta. 1979;62:2712-2723.
- [CrossRef] [Google Scholar]
- Loesenerine, an alkaloid from Maytenus loeseneri. Phytochemistry. 1987;26:1847-1848.
- [CrossRef] [Google Scholar]
- Macrocyclic Budmunchiamine Alkaloids from Albizia lebbek†. J. Nat. Prod.. 1997;60:1036-1037.
- [CrossRef] [Google Scholar]
- N4-Benzoylspermidine from Oncinotis tenuiloba: Analytical differentiation of the three isomeric N-benzoylspermidines. Helv. Chim. Acta. 1994;77:1229-1235.
- [CrossRef] [Google Scholar]
- Spermidine alkaloids type inandenine from Oncinotis tenuiloba. Phytochemistry. 1995;39:689-694.
- [CrossRef] [Google Scholar]
- Identification and fingerprint analysis of novel multi-isomeric Lycibarbarspermidines and Lycibarbarspermines from Lycium barbarum L. by liquid chromatography with high-resolution mass spectrometry (UHPLC-Orbitrap) J. Food Compos. Anal.. 2022;105:104194-104205.
- [CrossRef] [Google Scholar]
- Codonocarpine, a new lunaria-type alkaloid from Codonocarpus australis A. Cunn. J. Chem. Soc. Chem. Commun.. 1971;300–301
- [CrossRef] [Google Scholar]
- Verbacine and verballocine, novel macrocyclic spermine alkaloids from Verbascum pseudonobile Stoj. et Stef. (Scrophulariaceae) Tetrahedron Lett.. 1995;36:617-620.
- [CrossRef] [Google Scholar]
- Verballoscenine, the Z Isomer of verbascenine from Verbascum phoeniceum. Phytochemistry. 1997;44:971-973.
- [CrossRef] [Google Scholar]
- Chiroptical properties of the protoverbine class of macrocyclic spermine alkaloids. Helv. Chim. Acta. 1998;81:1773-1791.
- [CrossRef] [Google Scholar]
- Macrocyclic spermine alkaloids from verbascum: the (E/Z)-isomeric pairs (-)-(S)-verbasitrine/(-)-(S)-isoverbasitrine and (+)-(S)-verbametrine/(+)-(S)-isoverbametrine: isolation, structure elucidation, and synthesis. Helv. Chim. Acta. 1999;82:229-237.
- [CrossRef] [Google Scholar]
- C 1Derivatives of macrocyclic spermine alkaloids. Verbamedines versus incasines. Tetrahedron Lett.. 2002;43:5025-5027.
- [CrossRef] [Google Scholar]
- Bioaccessibility of phenolic acids in Canadian hulless barley varieties. Food Chem.. 2021;358:129905-129913.
- [CrossRef] [Google Scholar]
- Marine alkaloid Monanchocidin a overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget. 2015;6:17328-17341.
- [CrossRef] [Google Scholar]
- Marine alkaloid Monanchocidin A induces lysosome membrane permeabilization and overcomes cisplatin resistance in germ cell tumor cells. Onclo. Res. Treat.. 2014;37:4-5.
- [Google Scholar]
- Guanidine alkaloids from the Marine Sponge Monanchora pulchra show cytotoxic properties and prevent EGF-induced neoplastic transformation in Vitro. Mar. Drugs.. 2016;14:133-149.
- [CrossRef] [Google Scholar]
- Anti-migratory activity of marine alkaloid monanchocidin A-proteomics-based discovery and confirmation. Proteomics. 2016;16:1590-1603.
- [CrossRef] [Google Scholar]
- Anhydrocannabisativine, a new alkaloid from Cannabis sativa L. J. Pharm. Sci.. 1978;67:124.
- [CrossRef] [Google Scholar]
- Didemnidines A and B, indole spermidine alkaloids from the New Zealand ascidian Didemnum sp. J. Nat. Prod.. 2011;74:888-892.
- [CrossRef] [Google Scholar]
- Spermidine derivatives in lulo (Solanum quitoense Lam.) fruit: sensory (taste) versus biofunctional (ACE-Inhibition) properties. J. Agric. Food Chem.. 2016;64:5375-5383.
- [CrossRef] [Google Scholar]
- New spermidine alkaloids from Capparis spinosa roots. Phytochem. Lett.. 2008;1:59-62.
- [CrossRef] [Google Scholar]
- Structure of kukoamine A, a hypotensive principle of Lycium chinense root barks1. Tetrahedron Lett.. 1980;21:1355-1356.
- [CrossRef] [Google Scholar]
- Kukoamine B, a spermine alkaloid from Lycium chinense. Phytochemistry. 1995;38:1529-1531.
- [CrossRef] [Google Scholar]
- Three new bromotyrosine derivatives lethal to crab from the marine sponge, Pseudoceratina purpurea. Tetrahedron. 2001;57:7507-7511.
- [CrossRef] [Google Scholar]
- Alkaloids from the Sponge Monanchora unguifera. J. Nat. Prod.. 2005;68:1420-1423.
- [CrossRef] [Google Scholar]
- Identifying Carotenoids and Phenolic Compounds In Naranjilla (Solanum quitoense Lam. Var. Puyo Hybrid), an Andean Fruit. J. Agric. Food Chem.. 2008;56:11890-11899.
- [CrossRef] [Google Scholar]
- Monanchocidin A from subarctic sponges of the genus monanchora and their promising selectivity against melanoma in vitro. Front. Mar. Sci.. 2020;7:58-68.
- [CrossRef] [Google Scholar]
- Secondary metabolites from the unique bamboo, Melocanna baccifera. Nat. Prod. Res.. 2019;33:122-125.
- [CrossRef] [Google Scholar]
- Novel stilbenoids, including cannabispiradienone glycosides, from Tragopogon tommasinii (Asteraceae, Cichorieae) and their potential anti-inflammatory activity. Phytochemistry. 2015;117:254-266.
- [CrossRef] [Google Scholar]
- Über die struktur der makrocyclischen spermidin-alkaloide oncinotin, neooncinotin und isooncinotin. 151. mitteilung über alkaloide. Helv. Chim. Acta. 1974;57:414-434.
- [CrossRef] [Google Scholar]
- Protoverbine, the parent member of a class of macrocyclic spermine alkaloids. Helv. Chim. Acta. 2000;83:3035-3042.
- [CrossRef] [Google Scholar]
- Monanchocidin: a new apoptosis-inducing polycyclic guanidine alkaloid from the marine sponge Monanchora pulchra. Org. Lett.. 2010;12:4292-4295.
- [CrossRef] [Google Scholar]
- Kukoamine A analogues with lipoxygenase inhibitory activity. J. Enzym. Inhib. Med. Chem.. 2009;24:1188-1193.
- [Google Scholar]
- Time-dependent inhibitors of trypanothione reductase: analogues of the spermidine alkaloid lunarine and related natural products. Bioorg. Med. Chem.. 2006;14:2266-2278.
- [CrossRef] [Google Scholar]
- The pharmacology of lunarine, the alkaloid of Lunaria biennis. J. Am. Pharm. Assoc.. 1950;39:516-519.
- [CrossRef] [Google Scholar]
- Structure of ephedradine B, a hypotensive pronciple of ephedra Roots. Heterocycles. 1979;12:783-786.
- [CrossRef] [Google Scholar]
- Structure of ephedradine C, a hypotensive principle of ephedra Roots. Heterocycles. 1980;14:295-298.
- [CrossRef] [Google Scholar]
- Structure of ephedradine D, a hypotensive principle of ephedra roots. Heterocycles. 1982;17:155-158.
- [CrossRef] [Google Scholar]
- Hypotensive actions of ephedradines, macrocyclic spermine alkaloids of Ephedra roots. Planta. Med.. 1983;48:290-293.
- [CrossRef] [Google Scholar]
- Alcaloides du peripterygia marginata (baill.) lues. (célastracées)—I. Tetrahedron. 1977;33:645-651.
- [CrossRef] [Google Scholar]
- Neuroprotection by Kukoamine A against oxidative stress may involve N-methyl-D-aspartate receptors. Biochim. Biophys. Acta. 2015;1850:287-298.
- [CrossRef] [Google Scholar]
- Kukoamine B, an amide alkaloid, protects against NMDA-induced neurotoxicity and potential mechanisms in vitro. Neurochem. Int.. 2015;87:66-76.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ. Toxicol. Pharmacol.. 2015;40:230-240.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of Kukoamine A on neurotoxin-induced Parkinson's model through apoptosis inhibition and autophagy enhancement. Neuropharmacology. 2017;117:352-363.
- [CrossRef] [Google Scholar]
- Batzelladine alkaloids from the Caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron. 2007;63:11179-11188.
- [CrossRef] [Google Scholar]
- Polycyclic guanidine-containing compounds from the Mediterranean sponge Crambe crambe: the structure of 13,14,15-isocrambescidin 800 and the absolute stereochemistry of the pentacyclic guanidine moieties of the crambescidins. J. Org. Chem.. 1993;58:4805-4808.
- [CrossRef] [Google Scholar]
- Crambescidins: new antiviral and cytotoxic compounds from the sponge Crambe crambe. J. Org. Chem.. 1991;56:5712-5715.
- [CrossRef] [Google Scholar]
- New spermidines from the florets of Carthamus tinctorius. J. Asian Nat. Prod. Res.. 2008;10:447-451.
- [CrossRef] [Google Scholar]
- Inhibitory activities of kukoamines A and B from Lycii Cortex on amyloid aggregation related to Alzheimer's disease and type 2 diabetes. J. Nat. Med.. 2020;74:247-251.
- [CrossRef] [Google Scholar]
- Ptilomycalin A: a novel polycyclic guanidine alkaloid of marine origin. J. Am. Chem. Soc.. 1989;111:8925-8926.
- [CrossRef] [Google Scholar]
- JBIR-94 and JBIR-125, antioxidative phenolic compounds from Streptomyces sp. R56–07. J. Nat. Prod.. 2012;75:107-110.
- [CrossRef] [Google Scholar]
- The chemical constituents of Capparis spinosa of Jordanian origin. Nat. Prod. Res.. 2003;17:9-14.
- [CrossRef] [Google Scholar]
- Safflospermidines from the bee pollen of Helianthus annuus L. exhibit a higher in vitro antityrosinase activity than kojic acid. Heliyon. 2020;6:e03638.
- [Google Scholar]
- Acyl spermidines in inflorescence extracts of elder (Sambucus nigra L., Adoxaceae) and elderflower drinks. J. Agric. Food Chem.. 2013;61:3501-3508.
- [CrossRef] [Google Scholar]
- Verbaskine, a macrocyclic spermine alkaloid of a novel type from Verbascum pseudorobile Stoj. et Stef. (Scrophulariaceae) Tetrahedron Lett.. 1983;21:4381-4384.
- [CrossRef] [Google Scholar]
- Kupchan, S.M., Hintz, H., P. J, Smith, R., M, et al., 1974. Celacinnine, a novel macrocyclic spermidine alkaloid prototype. J. Chem. Soc. Chem. Commun. 5, 329-330. https://doi.org/10.1039/c39740000329
- Macrocyclic spermidine alkaloids from Maytenus serrata and Tripterygium wilfordii. J. Org. Chem.. 1977;42:3660-3664.
- [CrossRef] [Google Scholar]
- Antimalarial activity of crambescidin 800 and synthetic analogues against liver and blood stage of Plasmodium sp. J. Antibiot.. 2006;59:583-590.
- [CrossRef] [Google Scholar]
- Macrocyclic spermidine alkaloids from Androya decaryi L. Perrier. Molecules. 2013;18:3962-13671.
- [CrossRef] [Google Scholar]
- Pistillarin salt, a dicatecholspermidine family member from Gomphus floccosus, inhibits DNA single strand breakage by the fenton reaction. J. Korean Soc. Appl. Bl.. 2010;54:312-315.
- [CrossRef] [Google Scholar]
- Characterization of flavonoids in Orostachys japonicus A. Berger using HPLC-MS/MS: contribution to the overall antioxidant effect. Food Chem.. 2011;124:1627-1633.
- [CrossRef] [Google Scholar]
- Preventing H2O2-induced toxicity in primary cerebellar granule neurons via activating the PI3-K/Akt/GSK3β pathway by kukoamine from Lycii Cortex. J. Funct. Foods. 2015;17:709-721.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of kukoamine A on 6-OHDA-induced Parkinson's model through apoptosis and iron accumulation inhibition. Chin. Herb. Med.. 2020;13:105-115.
- [CrossRef] [Google Scholar]
- Antidepressant-like effects of coumaroylspermidine extract from safflower injection residues. Front. Pharmacol.. 2020;11:713-727.
- [CrossRef] [Google Scholar]
- Antioxidant and cytoprotective effects of kukoamines A and B: comparison and positional isomeric effect. Molecules. 2018;23:973-986.
- [CrossRef] [Google Scholar]
- A metabolomics approach to investigate kukoamine B-A potent natural product with anti-diabetic properties. Front. Pharmacol.. 2018;9:1575-1590.
- [CrossRef] [Google Scholar]
- Kukoamine A attenuates insulin resistance and fatty liver through downregulation of Srebp-1c. Biomed. Pharmacother.. 2017;89:536-543.
- [CrossRef] [Google Scholar]
- Lipid, polyamide, and flavonol phagostimulants for adult western corn rootworm from sunflower (Helianthus annuus L.) pollen. J. Agric. Food Chem.. 1999;47:1223-1229.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of Kukoamine A against cerebral ischemia via antioxidant and inactivation of apoptosis pathway. Neurochem. Int.. 2017;107:191-197.
- [CrossRef] [Google Scholar]
- A novel spermidine macrocyclic alkaloid from the roots of Tripterygium wilfordii. Chem. Nat. Compd+.. 2020;56:496-499.
- [CrossRef] [Google Scholar]
- Dual targets guided screening and isolation of Kukoamine B as a novel natural anti-sepsis agent from traditional Chinese herb Cortex lycii. Int. Immunopharmacol.. 2011;11:110-120.
- [CrossRef] [Google Scholar]
- Kukoamine B, a novel dual inhibitor of LPS and CpG DNA, is a potential candidate for sepsis treatment. Br. J. Pharmacol.. 2011;162:1274-1290.
- [CrossRef] [Google Scholar]
- Amide alkaloids from Scopolia tangutica. Planta. Med.. 2014;80:1124-1130.
- [CrossRef] [Google Scholar]
- NMR-based identification of the major bioactive molecules from an Italian cultivar of Lycium barbarum. Phytochemistry. 2017;144:52-57.
- [CrossRef] [Google Scholar]
- Cannabisativine, a new alkaloid from Cannabis sativa l. root. Tetrahedron Lett.. 1975;16:2815-2818.
- [CrossRef] [Google Scholar]
- Buchnerine and N1-(Z)-p-methoxycinnamoylbuchnerine, two new macrocyclic alkaloids from Clerodendrum buchneri. J. Nat. Prod.. 1993;56:1418-1420.
- [CrossRef] [Google Scholar]
- A novel polyamine acyltransferase responsible for the accumulation of spermidine conjugates in Arabidopsis seed. Plant Cell.. 2009;21:318-333.
- [CrossRef] [Google Scholar]
- Chemical constituents of the residue of pollen of Brassica campestris L. Chin. J. Pharm.. 2013;44:669-673.
- [Google Scholar]
- Inhibitory effects of Kukoamine B on the inflammatory response of small intestine in lipopolysaccharide- induced septic mice and its potential mechanisms. Chin. Crit. Care Med.. 2015;27:121-126.
- [Google Scholar]
- Inhibitory effects on HIV-1 protease of tri-p-coumaroylspermidine from Artemisia caruifolia and related amides. Chem. Pharm. Bull.. 2001;49:915-917.
- [CrossRef] [Google Scholar]
- Structure of Caesalpinine A“A Novel Spermidine Alkaloid”. J. Am. Chem. Soc.. 1983;105:4441-4445.
- [CrossRef] [Google Scholar]
- Stereochemistry of a macrocyclic spermidine alkaloid from Caesalpinia digyna Rottl. X-Ray determination of the structure of caesalpinine C (celallocinnine) J. Chem. Soc. Perkin. Trans.. 1985;16:193-196.
- [CrossRef] [Google Scholar]
- Monanchocidins B-E: polycyclic guanidine alkaloids with potent antileukemic activities from the sponge Monanchora pulchra. J. Nat. Prod.. 2011;74:1952-1958.
- [CrossRef] [Google Scholar]
- Monanchomycalins A and B, unusual guanidine alkaloids from the sponge Monanchora pulchra. Tetrahedron Lett.. 2012;53:4228-4231.
- [CrossRef] [Google Scholar]
- Biological activity of novel macrocyclic alkaloids (budmunchiamines) from Albizia amara detected on the basis of interaction with DNA. J. Nat. Prod.. 1991;54:1531-1542.
- [CrossRef] [Google Scholar]
- Differential effects of crambescins and crambescidin 816 in voltage-gated sodium, potassium and calcium channels in neurons. Chem. Res. Toxicol.. 2013;26:169-178.
- [CrossRef] [Google Scholar]
- The Marine Guanidine alkaloid crambescidin 816 induces calcium influx and cytotoxicity in primary cultures of cortical neurons through glutamate receptors. ACS. Chem. Neurosci.. 2017;8:1609-1617.
- [CrossRef] [Google Scholar]
- Hydroxycinnamic acid spermidine amides from pollen of Corylus avellana L. Phytochemistry. 1986;25:433-435.
- [CrossRef] [Google Scholar]
- Phenylpropanoid patterns in fagales pollen and their phylogenetic relevance. Phytochemistry. 1988;27:823-828.
- [CrossRef] [Google Scholar]
- Inhibitory effect of kukoamine B on high mobility group protein B1/nuclear factor-kappa B signaling pathway in lung injury of mice with sepsis. Chin. Crit. Care Med.. 2016;28:994-997.
- [CrossRef] [Google Scholar]
- N-demethyl budmunchiamines from Albizzia lebbek seeds. Phytochemistry. 1995;39:247-249.
- [CrossRef] [Google Scholar]
- Chemical profiles of Korean bee pollens and their catechol-O-methyltransferase inhibitory activities. J. Agric. Food Chem.. 2022;70:1174-1181.
- [CrossRef] [Google Scholar]
- UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol.. 2018;115:414-424.
- [CrossRef] [Google Scholar]
- The unusual conformational preference of N1, N5, N10-tri-p-coumaroylspermidine E-Z isomers from the Japanese apricot tree, Prunus mume, for the (ZZZ)-form. Phytochem. Lett.. 2019;31:131-139.
- [CrossRef] [Google Scholar]
- Spermidine alkaloid from Banara parviflora. Rev. Bras. Farmacogn.. 2016;26:759-762.
- [CrossRef] [Google Scholar]
- Enhanced antibacterial, antioxidant and anticancer activity of caffeic acid by simple acid-base complexation with spermine/spermidine. Nat. Prod. Res.. 2022;1–6
- [CrossRef] [Google Scholar]
- Meehanines A-K, spermidine alkaloidal glycosides from Meehania urticifolia. J. Nat. Prod.. 2009;72:1049-1056.
- [CrossRef] [Google Scholar]
- Meehanines L-W, spermidine alkaloidal glycosides from Meehania urticifolia. J. Nat. Prod.. 2009;72:1937-1943.
- [CrossRef] [Google Scholar]
- Cyclic spermidine alkaloids and flavone glycosides from Meehania fargesii. Chem. Pharm. Bull.. 2010;58:696-702.
- [CrossRef] [Google Scholar]
- Diversity of (dihydro) hydroxycinnamic acid conjugates in Colombian potato tubers. Food Chem.. 2013;139:1087-1097.
- [CrossRef] [Google Scholar]
- Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from Southeast Brazil. J. Agric. Food Chem.. 2011;59:5516-5522.
- [CrossRef] [Google Scholar]
- Prelandrine, the key-step intermediate in the biosynthesis of the macrocyclic spermine alkaloid aphelandrine. Helv. Chim. Acta. 2001;84:172-179.
- [CrossRef] [Google Scholar]
- Minor hydroxycinnamic acid spermidines from pollen of Quercus dentata. Phytochemistry.. 1996;43:487-489.
- [CrossRef] [Google Scholar]
- Ptilomycalin A, a novel Na+, K(+)- or Ca2(+)-ATPase inhibitor, competitively interacts with ATP at its binding site. Eur. J. Pharmacol.. 1996;310:95-98.
- [CrossRef] [Google Scholar]
- Antioxidant hydroxycinnamic acid derivatives isolated from Brazilian bee pollen. Nat. Prod. Res.. 2007;21:726-732.
- [CrossRef] [Google Scholar]
- Structure and chemical properties of ptilomycalin A. J. Am. Chem. Soc.. 1992;114:8472-8479.
- [CrossRef] [Google Scholar]
- Ion-pair HPLC determination of hydroxycinnamic acid monoconjugates of putrescine, spermidine and spermine. Phytochem. Anal.. 2000;11:11-17.
- [CrossRef] [Google Scholar]
- Anti-osteoporotic effects of kukoamine B isolated from Lycii radicis cortex extract on osteoblast and osteoclast cells and ovariectomized osteoporosis model mice. Int. J. Mol. Sci.. 2019;20:2784-2797.
- [CrossRef] [Google Scholar]
- Tetra-cis/trans-coumaroyl polyamines as NK1 receptor antagonists from Matricaria chamomilla. Planta. Med. Int. Open. 2017;4:e43-e51.
- [CrossRef] [Google Scholar]
- Dihydrocaffeoyl polyamines (kukoamine and allies) in potato (Solanum tuberosum) tubers detected during metabolite profiling. J. Agric. Food Chem.. 2005;53:5461-5466.
- [CrossRef] [Google Scholar]
- Novel alkaloids from the sponge Batzella sp.: inhibitors of HIV gp120-human CD4 binding. J. Org. Chem.. 1995;60:1182-1188.
- [CrossRef] [Google Scholar]
- DNA-based isolation and the structure elucidation of the Budmunchiamines, novel macrocyclic alkaloids from Albizia amara. Heterocycles. 1991;32:1961-1967.
- [CrossRef] [Google Scholar]
- Budmunchiamines D-I from Albizia amara. Phytochemistry. 1992;31:1795-1800.
- [CrossRef] [Google Scholar]
- Kukoamine A and other hydrophobic acylpolyamines: potent and selective inhibitors of Crithidia fasciculata trypanothione reductase. Biochem. J.. 1995;311:371-375.
- [CrossRef] [Google Scholar]
- Etudes récentes des alcaloïdes du Lunaria biennis Moench, Crucifères—II. Tetrahedron. 1972;28:3103-3111.
- [CrossRef] [Google Scholar]
- 17,18-Didehydroloesenerine and 16,17-didehydroloesenerin-18-ol, alkaloids from Maytenus loeseneri. Phytochemistry. 1988;27:589-593.
- [CrossRef] [Google Scholar]
- Dicaffeoyl polyamine derivatives from bitter goji: contribution to the bitter taste of fruit. Fitoterapia. 2020;143:104543-104549.
- [CrossRef] [Google Scholar]
- A novel role of kukoamine B: Inhibition of the inflammatory response in the livers of lipopolysaccharide-induced septic mice via its unique property of combining with lipopolysaccharide. Exp. Ther. Med.. 2015;9:725-732.
- [CrossRef] [Google Scholar]
- Preliminary cardiovascular activity evaluation of capparidisine, a spermidine alkaloid from Capparis decidua. Pak. J. Pharm.. 1989;6:61-66.
- [Google Scholar]
- Dovyalicin-type spermidine alkaloids from Dovyalis species. J. Nat. Prod.. 2006;69:1300-1304.
- [CrossRef] [Google Scholar]
- Carotenoids and phenolic compounds from Solanum sessiliflorum, an unexploited Amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J. Agric. Food Chem.. 2013;61:3022-3029.
- [CrossRef] [Google Scholar]
- Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget. 2016;7:83071-83087.
- [CrossRef] [Google Scholar]
- Characterisation and identification of spermine and spermidine derivatives in Microdesmis keayana and Microdesmis puberula roots by electrospray ionisation tandem mass spectrometry and high-performance liquid chromatography/electrospray ionisation tandem mass spectrometry. Eur. J. Mass. Spectrom.. 2008;14:111-115.
- [CrossRef] [Google Scholar]
- Crambescidin-816 acts as a fungicidal with more potency than crambescidin-800 and -830, inducing cell cycle arrest, increased cell size and apoptosis in Saccharomyces cerevisiae. Mar. Drugs. 2013;11:4419-4434.
- [CrossRef] [Google Scholar]
- Mechanism of cytotoxic action of crambescidin-816 on human liver-derived tumour cells. Br. J. Pharmacol.. 2014;171:1655-1667.
- [CrossRef] [Google Scholar]
- New macrocyclic spermine (budmunchiamine) alkaloids from Albizia gummifera “with some observations on the structure−activity relationships of the budmunchiamines”. J. Nat. Prod.. 1996;59:850-853.
- [CrossRef] [Google Scholar]
- Spermine alkaloids from Albizia schimperana. Phytochemistry. 1996;42:1211-1215.
- [CrossRef] [Google Scholar]
- Hydroxycinnamic acid amides from Iochroma cyaneum. Phytochemistry. 1990;29:3931-3933.
- [CrossRef] [Google Scholar]
- N1, N10-ditigloylspermidine, a novel alkaloid from the seeds of Ipomoea nil. Pharmazie. 2005;60:958-959.
- [Google Scholar]
- New Hydroxylated Spermidine Alkaloids from Pleurostylia opposita(WALL.) MERILL-METCALF. Helv. Chim. Acta. 1992;75:2283-2288.
- [CrossRef] [Google Scholar]
- Verbascenin, ein macrocyclisches spermin-alkaloid aus verbascum 184. mitteilung über organische naturstoffe. Helv. Chim. Acta. 1982;65:2540-2547.
- [CrossRef] [Google Scholar]
- Crambescidin 800, isolated from the Marine Sponge Monanchora viridis, induces cell cycle arrest and apoptosis in triple-negative breast cancer cells. Mar. Drugs. 2018;16:53-72.
- [CrossRef] [Google Scholar]
- Absolute configuration of the cytotoxic marine alkaloid monanchocidin A. J. Nat. Prod.. 2018;81:1113-1115.
- [CrossRef] [Google Scholar]
- Monanchoxymycalin C with anticancer properties, new analogue of crambescidin 800 from the marine sponge Monanchora pulchra. Nat. Prod. Res.. 2019;33:1415-1422.
- [CrossRef] [Google Scholar]
- Chemical composition of Prunus padus L. flower extract and its anti-inflammatory activities in primary bone marrow-derived macrophages. J. Ethnopharmacol.. 2021;268:113678-113685.
- [CrossRef] [Google Scholar]
- Spermidine and flavonoid conjugates from peanut (Arachis hypogaea) flowers. J. Agric. Food Chem.. 2008;56:2960-2969.
- [CrossRef] [Google Scholar]
- A new class of spermidine-derived alkaloids. Org. Lett.. 2003;5:2793-2796.
- [CrossRef] [Google Scholar]
- Pistillarin, a characteristic metabolite of Clavariadelphus pistillaris and several Ramaria species (basidiomycetes) Zeitschrift. Naturforschung. C. 1984;39:10-12.
- [CrossRef] [Google Scholar]
- Tricoumaroylspermidine in flowers of Rosaceae. Phytochemistry. 1990;29:2893-2896.
- [CrossRef] [Google Scholar]
- Antioxidant and anti-tyrosinase activities of bee pollen and identification of active components. J. Apicult. Res.. 2020;60:1-11.
- [CrossRef] [Google Scholar]
- A pollen diet confers ultraviolet-B resistance in phytoseiid mites by providing antioxidants. Front. Ecol. Evol.. 2018;6:1-13.
- [CrossRef] [Google Scholar]
- A potent antimicrobial compound isolated from Clathria cervicornis. Bioorg. Med. Chem. Lett.. 2015;25:67-69.
- [CrossRef] [Google Scholar]
- Crambescidin 800, a pentacyclic guanidine alkaloid, protects a mouse hippocampal cell line against glutamate-induced oxidative stress. J. Nat. Med.. 2007;61:288-295.
- [CrossRef] [Google Scholar]
- Monanchomycalin C, a new pentacyclic guanidine alkaloid from the far-eastern marine sponge Monanchora pulchra. Nat. Prod. Commun.. 2013;8:1399-1402.
- [Google Scholar]
- Monanchoxymycalins A and B, new hybrid pentacyclic guanidine alkaloids from the far-eastern Marine Sponge Monanchora pulchra. Nat. Prod. Commun.. 2016;11:1817-1820.
- [Google Scholar]
- Function of hydroxycinnamoyl spermidines in seedling growth of Arabidopsis. Biosci. Biotech. Bioch.. 2021;86:294-299.
- [CrossRef] [Google Scholar]
- Structure of ephedradine A, a hypotensive principle of roots. Tetrahedron Lett.. 1979;23:873-876.
- [CrossRef] [Google Scholar]
- Network pharmacology-based approach of novel traditional Chinese medicine formula for treatment of acute skin inflammation in silico. Comput. Biol. Chem.. 2017;71:70-81.
- [CrossRef] [Google Scholar]
- Isolation of Crambescidin 800 from Monanchora arbuscula (Porifera) Biochem. Syst. Ecol.. 1994;22:645-646.
- [CrossRef] [Google Scholar]
- Inhibitory effect of alkaloids of Albizia amara and Albizia saman on growth and fumonisin B1 production by Fusarium verticillioides. Int. Food. Res. J.. 2014;21:947-952.
- [Google Scholar]
- Two new spermidine alkaloids from Chisocheton weinlandii. Helv. Chim. Acta. 2004;87:1411-1425.
- [CrossRef] [Google Scholar]
- Macrocyclische Spermidinalkaloide aus Pleurostylia africana LOES. 9. Mitteilung über Celastraceen-Inhaltsstoffe. Helv. Chim. Acta. 1981;12:283-296.
- [CrossRef] [Google Scholar]
- Macrocyclische Spermidinalkaloide aus Maytenus mossambicensis. Helv. Chim. Acta. 1982;65:739-752.
- [CrossRef] [Google Scholar]
- Antifungal activity of three spermidine conjugates. FEMS Microbiol. Lett.. 2001;201:255-258.
- [CrossRef] [Google Scholar]
- Enhancement of glucose utilization by loesenerine through AMPK activation in myotubes. Chem. Pharm. Bull.. 2018;66:885-886.
- [CrossRef] [Google Scholar]
- Kukoamine A inhibits human glioblastoma cell growth and migration through apoptosis induction and epithelial-mesenchymal transition attenuation. Sci. Rep.. 2016;6:36543.
- [CrossRef] [Google Scholar]
- Dracotanosides A−D, spermidine glycosides from Dracocephalum tanguticum: structure and amide rotational barrier. J. Nat. Prod.. 2009;72:1006-1010.
- [CrossRef] [Google Scholar]
- Various monomers of Tripterygium wilfordii effecting adenosine deaminase activity and inducing HL-60 cell apopotosis. Fudan. Univ. J. Med. Sci.. 2007;34:107-110.
- [Google Scholar]
- Anti-Inflammatory Activities of Kukoamine A From the Root Bark of Lycium chinense Miller. Nat. Prod. Commun.. 2020;15:1-8.
- [CrossRef] [Google Scholar]
- Scocycamides, a pair of macrocyclic dicaffeoylspermidines with butyrylcholinesterase inhibition and antioxidation activity from the roots of Scopolia tangutica. Org. Lett.. 2020;22:8240-8244.
- [CrossRef] [Google Scholar]
- Wang, R.d., Su, G.h., Wang, L., et al., 2018b. Identification and mechanism of effective components from rape (Brassica napus L.) bee pollen on serum uric acid level and xanthine oxidase activity. J. Funct. Foods. 47, 241-251. https://doi.org/10.1016/j.jff.2018.05.064.
- Physicochemical characteristics and phytochemical profiles of yellow and red Physalis (Physalis alkekengi L. and P. pubescens L.) fruits cultivated in China. Food. Res. Int.. 2019;120:389-398.
- [CrossRef] [Google Scholar]
- Dicoumaroylspermidines in anthers of different species of the genus Aphelandra. Phytochemistry. 1995;40:461-465.
- [CrossRef] [Google Scholar]
- Distribution of hydroxycinnamic acid conjugates in fruit of commercial eggplant (Solanum melongena L.) cultivars. J. Agric. Food Chem.. 2003;51:3448-3454.
- [CrossRef] [Google Scholar]
- Coupling HPLC-SPE-NMR with a microplate-based high-resolution antioxidant assay for efficient analysis of antioxidants in food–validation and proof-of-concept study with caper buds. Food. Chem.. 2013;141:4010-4018.
- [CrossRef] [Google Scholar]
- Pithecolobine the alkaloid of Pithecolobium saman Benth. i. Can. J. Chem.. 1952;30:761-772.
- [CrossRef] [Google Scholar]
- Chemical constituents from Buddleja officinalis. Chin. Pharm. J.. 2017;52:1893-1898.
- [CrossRef] [Google Scholar]
- Ianthelliformisamines A-C, antibacterial bromotyrosine-derived metabolites from the marine sponge Suberea ianthelliformis. J. Nat. Prod.. 2012;75:1001-1005.
- [CrossRef] [Google Scholar]
- Effect of adlay polyphenols on antioxidant enzyme activity in HepG2 cells. Food Sci. Tech.. 2018;43:278-284.
- [Google Scholar]
- New Bioactive Molecules Isolated for the First Time from Hyoscyamus albus L. and their Mechanisms Underlying the Anticancer Effects. Indian J. Pharm. Educ.. 2020;54:s309-s315.
- [CrossRef] [Google Scholar]
- A new nonpeptide tachykinin NK1 receptor antagonist isolated from the plants of compositae. Chem. Pharm. Bull.. 2002;50:47-52.
- [CrossRef] [Google Scholar]
- Isolation and identification of spermidine derivatives in tea (Camellia sinensis) flowers and their distribution in floral organs. J. Sci. Food Agric.. 2012;92:2128-2132.
- [CrossRef] [Google Scholar]
- Effect of ultrasonic and ball-milling treatment on cell wall, nutrients, and antioxidant capacity of rose (Rosa rugosa) bee pollen, and identification of bioactive components. J. Sci. Food Agric.. 2019;99:5350-5357.
- [CrossRef] [Google Scholar]
- Kukoamine B promotes TLR4-independent lipopolysaccharide uptake in murine hepatocytes. Oncotarget. 2016;7:57498-57513.
- [CrossRef] [Google Scholar]
- Pseudoceramines A-D, new antibacterial bromotyrosine alkaloids from the marine sponge Pseudoceratina sp. Org. Biomol. Chem.. 2011;9:6755-6760.
- [CrossRef] [Google Scholar]
- On-Line coupling of high-performance liquid chromatography to atmospheric pressure chemical ionization mass spectrometry (HPLC/APCI-MS and MS/MS). the pollen analysis of Hippeastrum x hortorum (Amaryllidaceae) Helv. Chim. Acta. 1998;81:1654-1671.
- [CrossRef] [Google Scholar]
- Two macrocyclic spermine alkaloids from Aphelandra fuscopunctata (Acanthaceae) Phytochemistry. 1999;52:1717-1723.
- [CrossRef] [Google Scholar]
- Isolation and purification of coumaroylspermidines from Carthamus tinctorius L. and their inhibition effects on [3H]-5-HT reuptake. J. Shanxi. Med. Univ.. 2015;46:442-447.
- [Google Scholar]
- New dovyalicin-type spermidine alkaloid from Dovyalis caffra (warb.); family: salicaceae, cultivated in Egypt. Al-Azhar J. Pharm. Sci.. 2019;59:88-106.
- [CrossRef] [Google Scholar]
- N1, N5, N10-Tris(4-hydroxycinnamoyl)spermidines from Microdesmis keayana roots. Chem. Biodivers.. 2006;3:982-989.
- [CrossRef] [Google Scholar]
- Two new quinoline and tris(4-hydroxycinnamoyl)spermine derivatives from Microdesmis keayana roots. Chem. Pharm. Bull.. 2007;55:643-645.
- [CrossRef] [Google Scholar]
- Effects of Microdesmis keayana alkaloids on vascular parameters of erectile dysfunction. Phytother. Res.. 2009;23:892-895.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of kukoamine a against radiation-induced rat brain injury through inhibition of oxidative stress and neuronal apoptosis. Neurochem. Res.. 2016;41:2549-2558.
- [CrossRef] [Google Scholar]
- Zhang, L., Xu, W., Jiang, H., et al., 2022. A simple and sensitive HPLC method for simultaneous quantification of macrocyclic spermidine alkaloids in root, stem and leaf of Tripterygium wilfordii. Acta. Chromatographica. https://doi.org/10.1556/1326.2022.01014.
- Kukoamine A prevents radiation-induced neuroinflammation and preserves hippocampal neurogenesis in rats by inhibiting activation of NF-kappaB and AP-1. Neurotox. Res.. 2017;31:259-268.
- [CrossRef] [Google Scholar]
- Separation and characterization of phenolamines and flavonoids from rape bee pollen, and comparison of their antioxidant activities and protective effects against oxidative stress. Molecules. 2020;25:1264-1280.
- [CrossRef] [Google Scholar]
- Identification of bitter compounds in extruded corn puffed products. Food Chem.. 2018;254:185-192.
- [CrossRef] [Google Scholar]
- A novel compound N(1), N(5)-(Z)-N(10)-(E)-tri-p-coumaroylspermidine isolated from Carthamus tinctorius L. and acting by serotonin transporter inhibition. Eur. Neuropsychopharmacol.. 2009;19:749-758.
- [CrossRef] [Google Scholar]
- Kukoamine B ameliorate insulin resistance, oxidative Stress, inflammation and other metabolic abnormalities in high-fat/high-fructose-fed rats. Diabet. Metab. Synd. Ob.. 2020;13:1843-1853.
- [CrossRef] [Google Scholar]
- Structural identification of a new tri-p-coumaroylspermidine with serotonin transporter inhibition from safflower. Chem. Pharm. Bull.. 2010;58:950-952.
- [CrossRef] [Google Scholar]
- A new spermidine from the fruits of Lycium ruthenicum. Chem. Nat. Compd+.. 2014;50:880-883.
- [CrossRef] [Google Scholar]
- Four new dicaffeoylspermidine derivatives From Lycium barbarum. World J. Tradit. Chin. Med.. 2016;2:1-5.
- [CrossRef] [Google Scholar]
- Lycibarbarspermidines A-O, new dicaffeoylspermidine derivatives from wolfberry, with activities against Alzheimer's disease and oxidation. J. Agric. Food Chem.. 2016;64:2223-2237.
- [CrossRef] [Google Scholar]
- Tricoumaroylspermidine from rose exhibits inhibitory activity against ethanol-induced apoptosis in HepG2 cells. Food Funct.. 2021;12:5892-5902.
- [CrossRef] [Google Scholar]
- The spermine alkaloids of Chaenorhinum minus. Planta. Med.. 1988;54:430-433.
- [CrossRef] [Google Scholar]







