Translate this page into:
New potential and characterization of Andrographis paniculata L. Ness plant extracts as photoprotective agent
⁎Corresponding author. fredy@chem.its.ac.id (Fredy Kurniawan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The species Andrographis paniculata L. Ness, family Acanthaceae grows in many parts in the island of Java in Indonesia. In this research, new potentials of photoprotective agents from A. paniculata plant extracts, along with their characteristics, were investigated. The phytochemical test, infrared analysis, ultraviolet characterization, fluorescence identification, flavonoid content by thin layer chromatography, high performance liquid chromatography-mass spectrometry (LCMS/MS) and photoprotective analysis were performed in the extracts. The new potentials of A. paniculata plant extracts were studied at various concentrations of extracts and shown by the value of the sun protection factor (SPF). Based on phytochemical test, A. paniculata plant extracts contained flavonoids, alkaloids, tannins, triterpenoids and polyphenols. A. paniculata plant extracts have 2 maximum wavelengths at 230 nm and 362 nm. The excitation peak at 300 nm (552.11 a.u) and the emission peak at 605 nm (516.02 a.u). The flavonoid content of the extracts was 0.022 µg/mL quercetin with Rf value of 0.61. The chromatogram of the LCMS/MS is similar to quercetin-3-glycoside standard at a retention time of 2.77. The photoprotective activity of A. paniculata plant extracts had ≥ 15 SPF. Its shows the possibility to use this extract as photoprotective agent in pharmaceutical preparations.
Keywords
Herbal plant
Sambiloto
Ethanolic extracts
UV protector
SPF
1 Introduction
Andrographis paniculata L. Ness (sambiloto) plant thrives and is commonly found in Indonesia, in particular on the island of Java. A. paniculata is an important medicinal plant species which belong to the Acanthaceae family which grows wild in the open. Sambiloto leaves have a physical appearance similar to a green colour and appear bright in the sun. A. paniculata plants have been widely used to solve various health problems. Medicinal plants are important sources of valuable therapeutic agents, both in traditional and modern medicine. When compared to other medicinal plants, A. paniculata is one of the most potential herbs to be used as an alternative treatment for treating various deadly diseases. This is due to A. paniculata plant extracts have antihypertensive effect and have the ability of reducing the conversion of plasma angiotensin into enzyme (ACE) and lipid peroxidation in kidney (Akbar, 2011). In addition, this plant contains antimicrobial activity (Singha et al., 2003), antioxidants and anti-inflammatory (Chandrasekaran et al., 2010), antihyperglycemic and hypoglycemic activity (Zhang and Tan, 2000). The abilities of antioxidant, antimicrobial, anticancer and antimalarial activities of A. paniculata plants have been previously researched by Chuah et al. (2017), Kumar et al. (2004), Mishra et al. (2009).
Based on the results of the previous studies (Ismail et al., 2017; Jarukamjom and Nemoto, 2008; Praveen et al., 2014), it is known that A. paniculata plants contain flavonoid and phenolic compounds. Flavonoid compound is one of the organic compounds found in many types of plants. This compound helps biological processes that occur in plants, in particular processes that involve antimicrobial activity (Kurniawan et al., 2014), antioxidants and photoprotective. Photoprotective activity is related to radiation emitted by sunlight, known as ultraviolet radiation. Continuous ultraviolet radiation exposure can have positive and negative effects on human life (Cooley and Quale, 2013; Jain and Jain, 2010). The negative effects of ultraviolet radiation include photoaging and photocarcinogenic. Photoaging can cause sagging and wrinkling of the skin, whilst photocarcinogenic causes damage to cells and DNA. Continuous sun exposure can be a risk factor for nonmelanoma skin cancer (Moloney et al., 2002). Various chromophores in the skin, such as melanin, DNA, RNA, protein, amino acids and others can absorb ultraviolet radiation. DNA is an important macro molecule that can mutate and cause malignant transformation of skin cancer cells (Narayanan et al., 2010). Photochemical reactions due to the absorption of ultraviolet radiation by chromophores involve reactive oxygen species (ROS). This species has a dangerous effect if ultraviolet radiation is emitted continuously (Balogh et al., 2011; Diffey, 2007).
The use of UV filter types chemical sunscreen generally involves organic compounds which works by absorbing ultraviolet radiation (Salvador and Chisvert, 2007). Natural organic compounds are found in plant extracts. The need for plant extracts which contain a lot of flavonoid compounds become an important component in the discovery of new active molecules for human photoprotective (Costa et al., 2015; Lin et al., 2016). Plant extracts that are rich in flavonoids are able to absorb ultraviolet radiation in two regions of maximum wavelength. Hence in general, they generate two maximum peaks of absorption of ultraviolet radiation in the regions of UV A (320–400 nm) and UV-B (290–320 nm) (Baron and Suggs, 2014; Costa et al., 2015). The content of organic compounds in A. paniculata plants can be used as a natural active agent in sunscreen formulations. The performance of this active agent depends on the capacity of the active ingredient in absorbing ultraviolet radiation. The effectiveness of the active agents is measured by its absorption value at ultraviolet wavelengths and is expressed as the value of the sun protection factor (SPF) (Dutra et al., 2004; Olayemi et al., 2017; Patil et al., 2015). According to the Food and Drug Administration of the United States and European Union (FDA), the recommended value of sun protection factor is SPF-15 or higher so that it is able to reduce the risk of skin cancer and premature aging (Gabros et al., 2020). Thus, active agents that can provide high SPF values and increase filtering efficiency against ultraviolet radiation exposure are needed.
A. paniculata plant extracts which are rich in organic compounds have the potential to have high SPF values and are expected to provide effective protection against ultraviolet radiation exposure. The presence of flavonoid and phenolic compounds in A. paniculata plant extracts causes it to have photoprotective ability against ultraviolet radiation. However, research on this subject has never been taken further in previous studies. On this basis, new potentials of A. paniculata plant extracts as a protector for ultraviolet radiation and its characteristics were developed and analyzed using various methods. The results show some possibilities to use of A. paniculata plant extracts as a photoprotective agent in pharmaceutical preparations.
2 Experimental work
2.1 Reagents solutions and instruments
Andrographis paniculata L. Ness plant material obtained from Materia Medica Batu Technical Implementation Unit, Department of Health, East Java Province of Indonesia, with the key to plant determination1b-2b-3b-4b-6b-7b-9b-10b-11b-12b-13b-14b-16a-239b-244b-248b-249b-250a-251b-253b-254b-255a-256a-257b-259a-2b. Ethanol 96% E.Merck, reagent for phytochemical tests, all chemicals used in this study have a high degree of purity (pro analyst). All glassware’s used in this experiment were washed and rinsed with demineralised water before being used. Instrumentation used for characterization included FTIR spectrophotometer (Agilent 5500 FTIR) used to determine the specific wavelength of A. paniculata plant extracts through functional group detection, UV–Vis spectrophotometer (Thermo Sciencetific-Genesys 10 s UV Visible) to determine the maximum wavelength of A. paniculata plant extracts and to analyze the SPF value data from A. paniculata plant extracts. Other instruments used include, Fluorescence spectrophotometer (Perkin Elmer LS 55 fluorescence spectrometer connected with FL Winlab software) to determine the wavelength of excitation and specific emissions from A. paniculata plant extracts, High Performance Thin Layer Chromatography (HPTLC) to determine the flavonoid as quercetine and Liquid Chromatography Mass Spectrometry/Mass Spectrometry (LCMS/MS).
2.2 Preparation of plant extracts
The fresh leaves of A. paniculata was washed using aquadest, then dried in the open air for 5 days while protected from direct sunlight. The dry leaves were crushed using a grinder. The obtained powder called simplicia was stored in a dry tightly closed container. This container was stored at room temperature before used (Napagoda et al., 2016; Patil et al., 2015). 250 g of simplicia powder from the leaves of A. paniculata plant was weighed using the analytical balance of the Ohauss brand and extracted using an ethanol 96% E Merck E solvent in a ratio of 1:10. The extraction process used a maceration method for 72 h. Maceration results are filtered using Whatman filter paper No. 41 with a vacuum pump. The filtrate obtained was then concentrated with a vacuum rotary evaporator at 50 °C until a thick extract was obtained. The obtained extracts was kept at 4 °C in the refrigerator before used (Mishra et al., 2012; Thibane et al., 2019)
2.3 Phytochemical test
Phytochemical tests were carried out to determine the presence of flavonoid compounds, polyphenols, alkaloids, terpenoids, tannins and saponins in A. paniculata plant extracts. In principle, the procedure is performed by dissolving 2 mL of A. paniculata plant extracts in 8 mL demineralised water. The solution is subsequently filtered, and the filtrate is put into several test tubes. Next, a few drops of different reagents were added in accordance into each of the test tubes, where changes that occurred were consequently observed. The reagents used for the flavonoid test were concentrated HCl and Mg powder. Whereas the FeCl3 reagent was used for polyphenol test and tannin test, Meyer reagent and Dragendroff reagent were used for alkaloid test, and Bouchardate reagent was used for alkaloid test and terpenoid test. whilst the hot demineralised water was used for saponin test (Yadav and Agarwala, 2011).
2.4 Infrared analysis
Previously, the sampling device (DialPath) was opened by rotating the arm and the bottom window had to be seen. Droplets of a small sample of A. paniculata plant extracts were put in the sample window located on the DialPath base plate. The sample window is a 2 mm diameter yellow material held by a metal disk around it. The sample is confirmed to have covered the entire surface area of the lower window. A. paniculata plant extracts are ready for infrared analysis with a transportable diamond crystal attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectrometer (Agilent Technologies 5500a). This instrument was used to collect spectra in the 650–4000 cm−1 region. No sample preparation was required directly analysed on the diamond window. Intimate contact of the sample with the crystal window was required to give good quality spectra (Mitchell et al., 2013).
2.5 UV characterization
A. paniculata plant extracts are dissolved in a particular volume of ethanol solvent. The solution is measured at wavelengths of 200 nm to 400 nm (Napagoda et al., 2016). The wavelengths with maximum absorbance peaks are recorded as maximum wavelengths of A. paniculata plant extracts. The maximum absorbance peak in the spectra obtained shows the specific character of A. paniculata plant extracts.
2.6 Fluorescence identification
A. paniculata plant extracts were identified by excitation wavelength and emission using the Perkin Elmer LS 55 fluorescence spectrophotometer. Prior to measurement, fluorescence instrumentation was adjusted at scan speed of = 500, SlitEx/Em = 10 nm/10 nm. The initial step taken is to determine the wavelength at maximum intensity using a fluorescent spectrometer. Subsequently, pre-scan the sample at λex = 200–800, λem = 200–900 nm where these are then noted as excitation wavelength and emission wavelength at maximum intensity. The sample is measured at the wavelength that has been obtained previously. Sample measurements were carried out 5 times. The excitation and the emission monochromator slits were set to 10 nm. The FL WinLab software was used to register the fluorescent signals (Casale et al., 2018).
2.7 Flavonoids content
A total of 5 µL of A. paniculata plant extracts was injected into the Camag Linomat HPTLC column 5. The stationary phase used was GF254 silica gel with a mobile phase which was a mixture of chloroform, formic acid, and methanol mixed at a ratio of 3.3: 0.8: 0.2 respectively (Kshirsagar et al., 2008). This method is validated by an external calibration curve using a standard solution of quercetin, prepared in ethanol with five different concentrations.
2.8 LCMS/MS quantification
A total of 1 mL of A. paniculata plant extracts was injected into the LCMS/MS column. The effluent used consisted of 2 types of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). The column used was the Phenomenex type. This method is validated by an external calibration curve using a standard solution of quercetin, prepared in ethanol with four different concentrations. The standard quercetin-3-glycoside solution is injected, and line graphs are obtained on Microsoft Office Excel © by calculating the average. The accuracy is expressed as a similarity between the value measured experimentally and the reference value specified. The values of accuracy are calculated according to the formula RSD (%) = (SD × 100)/C, where RSD (%) is precision, SD is the standard deviation and C is the average concentration calculated. As for the accuracy, it is calculated by using Accuracy (%) = (Cexp × 100)/TC, where Cexp is the total quercetin concentration of the extract and TC is the theoretical standard reference concentration. The detection limit (LOD) and the quantification limit (LOQ) are estimated by the slope whereas the mean and the standard deviation of the concentration are used to construct the analytical curve (Costa et al., 2015; Santhanam et al., 2017)
2.9 Preparation of photoprotective analysis
A. paniculata plant extract was dissolved in 100 mL of ethanol solvent. The solution was ultrasonified for 5 min, and subsequently filtered by rejecting 10 mL of the first filtrate out so that the first solution was obtained. 10 mL aliquots from the first solution are dissolved in 100 mL ethanol and a second solution is obtained. 10 mL aliquots from the second solution are dissolved in 50 mL ethanol. The sample solution is ready to be measured using an ultraviolet spectrophotometer. Measurements were made at wavelengths of 290 nm to 320 nm with ethanol as a blank solution. The absorbance readings were performed in triplicate (Patil et al., 2015).
2.10 Sun protection factor (SPF) from extracts
Photoprotective analysis of A. paniculata plant extracts was performed by calculating the value of the sun protection factor or SPF. SPF value is obtained by calculating the absorption of ultraviolet radiation in the wavelength region of UV B (290–320 nm) using the Mansur equation. These equations can be written as follows:
The SPF value is calculated by applying the Mansur equation where CF is a correction factor of 10; EE (λ) is the erythemal efficiency spectrum; I (λ) is the spectrum of the sun's intensity; and abs (λ) is the absorbance of the solution (Costa et al., 2015; Patil et al., 2015). The value of EE (λ) × I (λ) is a constant. A. paniculata plant extracts were dissolved in ethanol to a final concentration of 4, 10, 12, 16, 20 µL/mL. The SPF model used in this study is in accordance with the methodology described by Mansur et al. (1986). Absorbance samples were measured in the UV-B wavelength range of 290–320 nm, with an increase of 5 nm and three determinations were made at each point.
2.11 Statistical analysis
SPF data were calculated as mean ± SD for five independent measurements (n = 5). Microsoft Excel 265 was used to perform the statistical analysis using one-way ANOVA with alpha = 0.05 followed by Least Significant Difference (LSD) test. Means were significantly different when P values < 0.05 for multiple range and compared each other with LSD test.
3 Results and discussion
In this work, the selection of ethanol as an organic solvent in the maceration process is based on the results of previous study conducted by Fardiyah et al. (2018). The results showed that in the maceration process of A. paniculata plants using 3 types of solvent. The obtained result showed that ethanol has optimum fluorescence intensity value in comparation with 2 others solvents (ethyl acetate and n-hexane). Different types of solvent extraction will result in difference pharmacokinetic profile of the bioactive components present in the A. paniculata plant extracts. The type, method of preparation and composition of prescribed herbal preparation can influence the pharmacokinetic behavior of the constituents present in herbal preparations (Mehta et al., 2017).
3.1 Phytochemical test
From the phytochemical test results of the A. paniculata plant extracts, it contains secondary metabolite compounds including flavonoids, alkaloids, triterpenoids, tannins and polyphenols, all of which are listed in Table 1. The compound is a plant defence chemical compound produced in plant tissues (Hsieh et al., 2016; Okhuarobo et al., 2014; Rajagopal et al., 2003; Sudhakaran, 2012). The presence of flavonoids is known to inhibit tumor growth and protect against gastrointestinal infections. It is also pharmacognostically important to provide evidence for the plants to be utilized in the field of ethnomedicine. Some of these bioactive compounds are formed as secondary metabolites along with the growth of the plants, which also function as antimicrobials to protect plants against microbial invasion. Alkaloids have been well researched and developed for several pharmacological properties, including antiprotozoal, cytotoxic, antidiabetic properties (Zhang and Tan, 2000), as well as anti-inflammatory properties (Appiah et al., 2017). The presence of alkaloids is crucial as some amounts of it can be used as antimalarial, analgesic and stimulant (Churiyah et al., 2015). Tannin compounds in A. paniculata plant extracts are reported to be able to selectively inhibit HIV replication in addition to being used as a diuretic (Hossain et al., 2014). In addition, the content of phenolic compounds, alkaloids and flavonoids in A. paniculta plant extracts is water-soluble anti-oxidants which functions as an anti-free radical that prevents oxidative cell damage (Sheeja et al., 2006). Other advantageous properties of A. paniculta plant extracts are they have a strong anticancer (Kumar et al., 2004) and anti-rheumatic activity (Hidalgo et al., 2013).
Compounds identification
Parameter
Result
Flavonoid
Brick red, pink, dark red
+
Alkaloid
Meyer
White deposits
+
Dragendrof
Orange deposits
–
Bouchardhat
Chocolate deposits
–
Tannin
Dark green, dark blue, dark brown
+
Terpenoid
Steroid
A mix of blue and green
–
Triterpenoid
A mix of orange, brown, and orange
+
Polifenol
Dark green, dark blue, dark brown
+
Saponin
Permanent foam
–
Phytocompounds are effective in photoprotective activity because they contain phytoconstituents of natural active ingredients as a substitute for chemicals in photoprotection preparations. Phytocompounds are quite effective in their photoprotective action against solar radiation. Understanding the pharmacokinetic profile of phytocompounds is very important. These phytochemicals compounds show a more complex pharmacokinetic profile such as the study of time course of phytochemicals, absorption, distribution, metabolism, and excretion (Mehta et al., 2015). Most of the phytochemical like flavonoids, alkaloids, and terpenoids contain various subclasses and hence it is very essential to know specific bioavailability and metabolites associated with each subclass. The complexity of the chemical content in plant extracts is a major challenge (Mehta and Dhapte, 2016).
In this study, the photoprotective mechanism of phytoconstituents in A. paniculata plant extracts was carried out by absorbing photon energy from the sun at ultraviolet wavelengths (areas that are harmful to the skin) and then releasing that energy at visible light wavelengths, which are safe areas for skin protection. The presence of phytoconstituents in the ethanol extract of A. paniculata includes flavonoids, alkaloids, tannins, triterpenoids and polyphenols. The existence of these phytocompounds can increase the ability of the ethanol extract of A. paniculata to absorb photon energy so that it is quite effective to be used as a photoprotective agent.
3.2 Infrared spectra identification
Identification results of infrared spectra in A. paniculata plant extracts are shown in Fig. 1. Based on Fig. 1, it shows the presence of –OH group from the phenolic group at 3294.96 cm−1 and 905.74 cm−1. Phenolic compounds are compounds that are found in plants and have a unique structure that has one or more hydroxyl groups attached to one or more aromatic benzene rings (Lin et al., 2016). C-H bonds from alkanes appear at 2847.68 cm−1 and 2929.69 cm−1. A strain between C⚌O from carbonyl group or carboxyl group was also seen at 1640.03 cm−1. There is a –CH3 group at 1438.75 cm−1 and a -C-O-C- group at 1203.93 cm−1. The existence of a double bond in the gene alkene from an aromatic compound is shown at 1032.47 cm−1.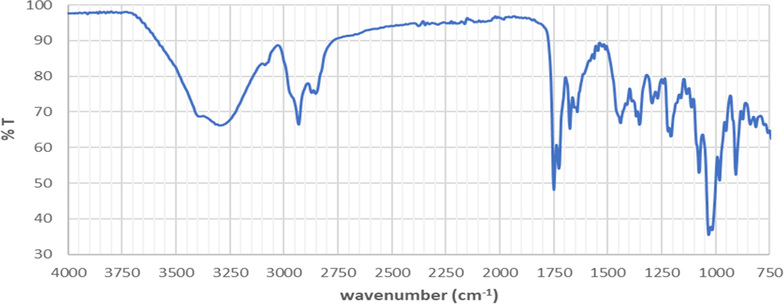
The infrared spectra of A. paniculata plant extracts.
3.3 UV characterization
Fig. 2 displays the ultraviolet characterization spectra of A. paniculata plant extracts. In Fig. 2, two maximum absorption peaks are shown. The first peak is seen at wavelength of 230 nm with an absorbance of 0.434 A, whereas the second peak is seen at wavelength of 362 nm with an absorbance of 0.952 A (see Table 2). The presence of these two maximum absorption peaks indicated that A. paniculata plant extracts has a rich content of flavonoids. This matches with the theory which states that plant extracts that are rich in flavonoids are efficient in absorbing ultraviolet rays, typically indicated by two maximum peaks of ultraviolet absorption. In this case, the two maximum peaks occurred between 230 and 280 nm and the other 300–550 nm (Costa et al., 2015).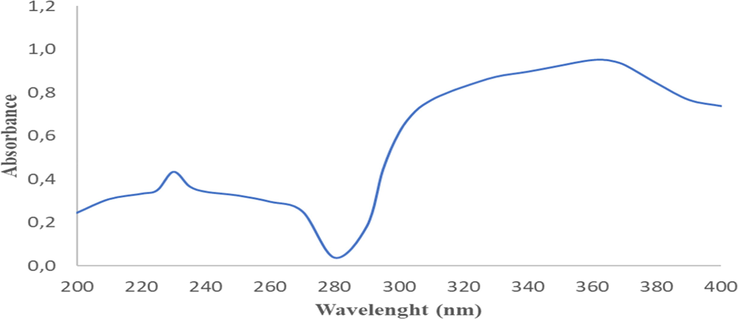
Ultraviolet spectra of A. paniculata plant extracts with 2 maximum wavelengths; 230 nm (0.434 A) and 362 nm (0.952 A).
λ (nm)
A1
A2
A3
Average
200
0.245
0.236
0.252
0.244
210
0.295
0.308
0.319
0.307
220
0.330
0.344
0.322
0.332
225
0.341
0.352
0.354
0.349
230
0.434
0.432
0.435
0.434
235
0.368
0.362
0.368
0.366
240
0.339
0.347
0.338
0.341
250
0.329
0.316
0.328
0.324
260
0.296
0.304
0.287
0.295
270
0.247
0.253
0.252
0.251
280
0.037
0.036
0.038
0.037
290
0.182
0.182
0.183
0.183
295
0.444
0.444
0.444
0.444
300
0.616
0.615
0.615
0.615
305
0.713
0.713
0.713
0.713
310
0.766
0.766
0.765
0.765
315
0.800
0.799
0.800
0.799
320
0.827
0.826
0.827
0.827
330
0.872
0.874
0.873
0.873
340
0.897
0.896
0.897
0.897
350
0.924
0.925
0.925
0.925
355
0.940
0.939
0.939
0.939
356
0.942
0.941
0.943
0.942
357
0.944
0.945
0.945
0.945
358
0.946
0.947
0.948
0.947
359
0.949
0.949
0.949
0.949
360
0.950
0.951
0.950
0.951
361
0.951
0.952
0.952
0.952
362
0.952
0.952
0.952
0.952
363
0.952
0.952
0.952
0.952
364
0.950
0.950
0.952
0.951
365
0.949
0.950
0.949
0.949
370
0.927
0.928
0.928
0.927
380
0.842
0.842
0.843
0.843
390
0.766
0.766
0.766
0.766
400
0.737
0.738
0.738
0.738
3.4 Fluorescence identification
Fluorescence identification of A. paniculata plant extracts produced two spectra. The obtained spectra are excitation spectra shown in Fig. 3 and emission spectra shown in Fig. 4. The ultraviolet radiation absorption by A. paniculata plant extracts was shown at an excitation wavelength of 300 nm with an absorption intensity of 552.11, whilst the emission of ultraviolet radiation by A. paniculata plant extracts occurred at an emission wavelength of 605 nm with an emission intensity of 516.02. The ability of absorption intensity of A. paniculata plant extracts was 36.09. In Fig. 3, it is shown that A. paniculata plant extracts absorb ultraviolet radiation in the UV B region of 290–320 nm. The presence of flavonoid and phenolic compounds in A. paniculata plant extracts causes this extract to absorb ultraviolet radiation in the UV B region. In Fig. 4, it shown that the emission of ultraviolet radiation by A. paniculata plant extracts occurred in visible areas of 400–800 nm. This indicates that the A. paniculata plant extracts can be used as a photoprotective agent with its potential to emit ultraviolet radiation in a safe area, which is the visible area.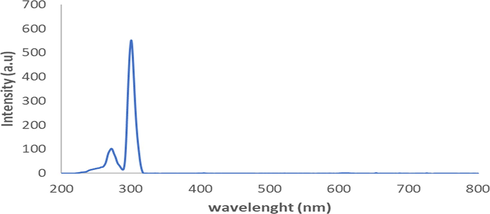
The excitation spectra of A. paniculata plant extracts at λexcitation 300 nm.
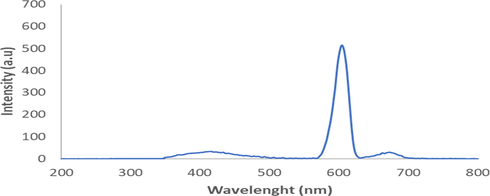
. The emission spectra of A. paniculata plant extracts at λemission 605 nm.
3.5 Flavonoid content
The presence of flavonoid content in A. paniculata plant extracts was analyzed using high performance thin layer chromatography (HPTLC) with a standard solution of quercetin. From the research results obtained, the HPTLC chromatogram of A. paniculata plant extracts showed a Retention factor (Rf) value of 0.61 (Fig. 5). The value of Rf is defined as the ratio of the distance traveled by a compound on the surface of the stationary phase divided by the distance traveled by the solvent as the mobile phase. The greater the Rf value of the sample, the greater the movement distance of these compounds on thin layer chromatography (TLC) plates. There are specific Rf values which suggest the characteristics of certain compounds in certain eluents. The value of Rf in a good TLC is typically between 0.2 and 0.8 (Cid-Hernández et al., 2018). The flavonoid content in A. paniculata plant extracts is shown in Fig. 6. The flavonoid content in the extract was calculated as quercetin equivalence. The calculation produced a result of 0.022 ± 0.002 μg/μL quercetin, which is equivalent to 10 mg per weight of ethanol extract.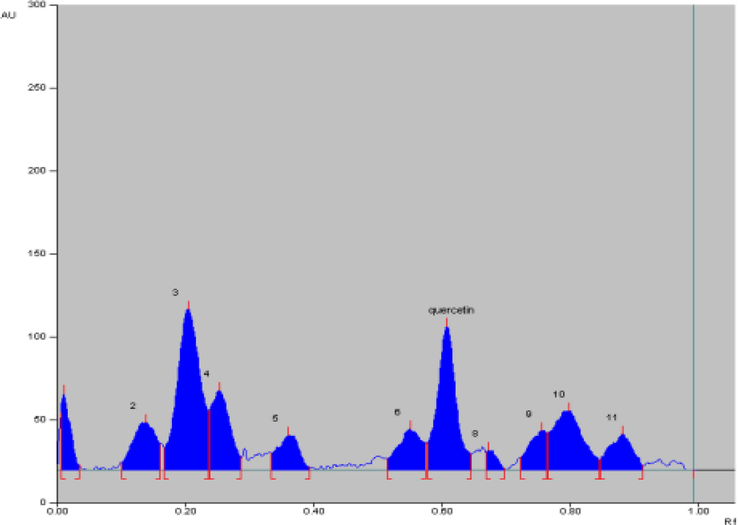
HPTLC chromatogram of A. paniculata plant extracts with Rf. 0,61.
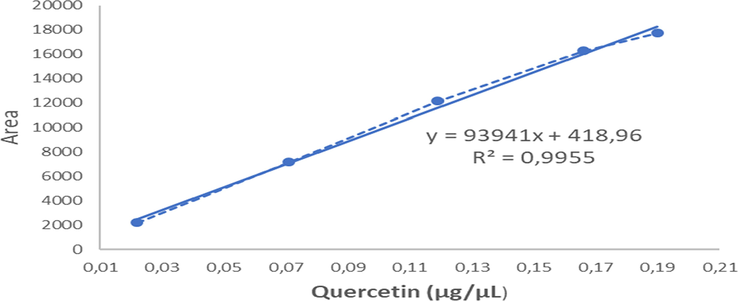
Quercetin standard curve.
3.6 LCMS/MS quantification
Quantification of A. paniculata plant extracts was analyzed by LCMS/MS, where the results are presented in Fig. 7A. The chromatogram showed the presence of glycosylated flavonoids at the retention peak of 2.77 min and non-glycosylated flavonoids found at the retention peak of 3.06 min. Based on the chromatogram results of A. paniculata plant extracts using LCMS/MS method shown in Fig. 7B, the peaks are seen at retention time of 2.77 min, similar to quercetin-3-glycoside (Que-3G) compounds. Therefore, que-3G compounds can be identified by comparing the retention times (Rt) using standard compounds.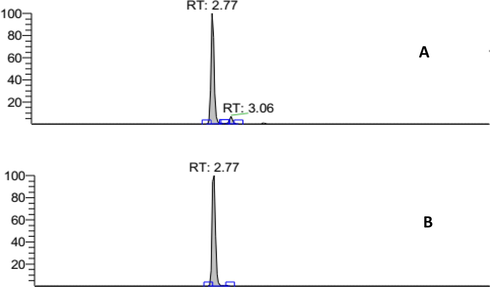
Quercetin 3 glycoside standard LCMS/MS chromatogram (Rt: 2.77 min) (A); LCMS/MS chromatogram of A. paniculata plant extracts: flavonoid glycoside area (Rt: 2.77 min) (B).
In Fig. 8, linearity is confirmed by preparing a standard solution of quercetin-3-glycoside in ethanol at four concentrations. Calibration curves are plotted and determined using standard data for quercetin-3-glycoside, y = 55874x + 2905.8 and R2 = 0.9997, which are linear for the specified concentration range. The detection limit was 0.1104 µg/mL and the quantification limit was 0.3679 µg/mL for que-3G. Quantification results indicate that the amount of que-3G present in the plant extracts of A. paniculata is quite high above the detection and quantification limits, which further emphasizes the reliability of this method. The accuracy of measurements is within the allowable value. The quercetin-3-glycoside concentration present in the plant extracts of A. paniculata was 9.17 µg/mL.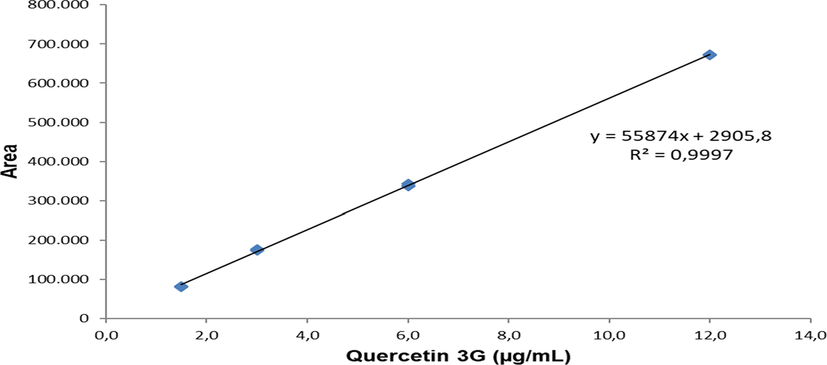
Quercetin-3-glycoside standard curve.
3.7 Sun protection factor (SPF) of A. paniculata plant extracts
The results of determining the SPF value from A. paniculata plant extracts are presented in Fig. 9. A. paniculata plant extracts have photoprotective abilities evaluated by methods developed by Mansur et al. (1986). According to the Food and Drug Administration of the United States and European Union (FDA), only SPF values that are greater than or equal to 15 are suitable for use in pharmaceutical preparations with photoprotective activity. A. paniculata plant extracts with different concentrations (12, 16 and 20 µL/mL) have satisfying photoprotective activity (15.42, 23.68 and 28.41, respectively), which are higher than those recommended by the FDA shown in Table 3. The SPF value of the A. paniculata plant extracts being tested depend on the concentration, whereby increasing the concentration results in an increase in SPF value. This is due to the flavonoid content found in the family Acanthaceae species. Plant extracts that are rich in flavonoids that are efficient in absorbing ultraviolet radiation usually show two maximum peaks of ultraviolet absorption. This is evident in A. paniculata plant extracts having two peaks of ultraviolet radiation absorption at 230 nm and 362 nm. Double-peak phenomenon is also one of the characteristics of Traditional Chinese Medicine (TCM) due to enterohepatic circulation and in vivo isomerization of chemical constituent. Interconversion of chemical structure of most of steroidal saponins and triterpenoid leads to the double-peak phenomenon (Mehta and Dhapte, 2016). Phytochemical studies described the presence of flavonoids, and other constituents such as tannins and alkaloids. A. paniculata plant extracts describe photoprotective activity that is significantly correlated with existing flavonoid compounds.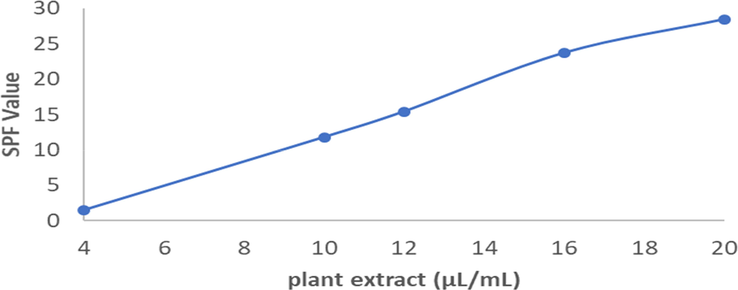
Sun Protection Factor (SPF) of A. paniculata plant extracts.
Extracts concentration (µL/mL)
SPF value
4
1.52 ± 0.00
10
11.80 ± 0.18
12
15.42 ± 0.01
16
23.68 ± 0.08
20
28.41 ± 0,05
4 Conclusion
In summary, Andrographis paniculata L. ness plant extracts were characterized by various analytic methods. Specific characteristics of A. paniculata plant extracts were obtained from phytochemical test, infrared analysis, UV characterization, fluorescence identification and LCMS/MS characterization. The plant extract of A. paniculata displayed excellent photoprotective properties against UV-B and UV-A radiation due to the presence of flavonoid derivatives present in the plants, including quercetin. The crude plant extracts of A. paniculata showed a higher SPF value compared to the value recommended by the FDA. The plant extract of A. paniculata has great a potential for use as a photoprotective agent in pharmaceutical preparations.
Acknowledgement
The author is very grateful to the Directorate General of KEMENRISTEKDIKTI (Ministry of Research, Technology and Higher Education of the Republic of Indonesia) and Institut Teknologi Sepuluh Nopember for providing Domestic Postgraduate Education Scholarship (BPPDN) with grant number 2903.4/D3/PG/2017 and supporting the research funding of this work under project scheme of the Doctoral Dissertation Research (PDD) with grant number 1239/PKS/ITS/2020. This research is also partially funded by the Indonesian Ministry of Research, Technology and Higher Education under WCU Program, managed by Institut Teknologi Bandung.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Andrographis paniculata: a review of pharmacological activities and clinical effects. Alternative Med. Rev.: J. Clin. Therapeutic. 2011;16:66-77.
- [Google Scholar]
- Chapter Seven - Antiinflammatory and Hepatoprotective Medicinal Herbs as Potential Substitutes for Bear Bile. In: Zeng B.-.Y., Zhao K., eds. International Review of Neurobiology, Neurobiology of Chinese Herb Medicine. Academic Press; 2017. p. :149-180.
- [CrossRef] [Google Scholar]
- Ultraviolet radiation protection: Current available resources in photoprotection. Anais brasileiros de dermatologia. 2011;86:732-742.
- [CrossRef] [Google Scholar]
- Combining excitation-emission matrix fluorescence spectroscopy, parallel factor analysis, cyclodextrin-modified micellar electrokinetic chromatography and partial least squares class-modelling for green tea characterization. J. Pharm. Biomed. Anal.. 2018;159:311-317.
- [CrossRef] [Google Scholar]
- Effect of an extract of Andrographis paniculata leaves on inflammatory and allergic mediators in vitro. J. Ethnopharmacol.. 2010;129:203-207.
- [CrossRef] [Google Scholar]
- Comparison study of anti-microbial activity between crude extract of Kappaphycus alvarezii and Andrographis paniculata. Asian Pacific J. Tropical Biomed.. 2017;7:729-731.
- [CrossRef] [Google Scholar]
- Churiyah, Pongtuluran, O.B., Rofaani, E., Tarwadi, 2015. Antiviral and Immunostimulant Activities of Andrographis paniculata. HAYATI J. Biosci. 22, 67–72. https://doi.org/10.4308/hjb.22.2.67.
- Two-dimensional thin layer chromatography-bioautography designed to separate and locate metabolites with antioxidant activity contained on spirulina platensis [www Document] Int. J. Anal. Chem. 2018
- [CrossRef] [Google Scholar]
- Skin cancer preventive behavior and sun protection recommendations. Semin. Oncol. Nurs.. 2013;29:223-226.
- [CrossRef] [Google Scholar]
- In vitro photoprotective effects of Marcetia taxifolia ethanolic extract and its potential for sunscreen formulations. Revista Brasileira de Farmacognosia. 2015;25:413-418.
- [CrossRef] [Google Scholar]
- A method for broad spectrum classification of sunscreens. Int. J. Cosmet. Sci.. 2007;16:47-52.
- [CrossRef] [Google Scholar]
- Dutra, E.A., Oliveira, D.A.G. da C., Kedor-Hackmann, E.R.M., Santoro, M.I.R.M., 2004. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Revista Brasileira de Ciências Farmacêuticas 40, 381–385. https://doi.org/10.1590/S1516-93322004000300014.
- Gabros, S., Nessel, T.A., Zito, P.M., 2020. Sunscreens and Photoprotection, in: StatPearls. StatPearls Publishing, Treasure Island (FL).
- Fluorescence analysis of Andrographis paniculata L. ness medicinal plant extract as a potential protector of ultraviolet radiation. AIP Conf. Proc.. 2018;2049(1):0094-243X.
- [CrossRef] [Google Scholar]
- Andrographolide a new potential drug for the long-term treatment of rheumatoid arthritis disease. Innovative Rheumatol. 2013
- [CrossRef] [Google Scholar]
- Andrographis paniculata (Burm. f.) Wall. ex Nees: A Review of Ethnobotany, Phytochemistry, and Pharmacology [WWW Document] Sci. World J. 2014
- [CrossRef] [Google Scholar]
- Andrographis paniculata extract attenuates pathological cardiac hypertrophy and apoptosis in high-fat diet fed mice. J. Ethnopharmacol.. 2016;192:170-177.
- [CrossRef] [Google Scholar]
- Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Traditional Complementary Med.. 2017;7:452-465.
- [CrossRef] [Google Scholar]
- Jain, S.K., Jain, N.K., n.d. Multiparticulate carriers for sun-screening agents. International Journal of Cosmetic Science 32, 89–98. https://doi.org/10.1111/j.1468-2494.2010.00547.x.
- Pharmacological aspects of Andrographis paniculata on health and its major diterpenoid constituent andrographolide. J. Health Sci.. 2008;54:370-381.
- [Google Scholar]
- Kshirsagar, V., Deokate, U., Bharkad, V., Khadabadi, S., 2008. (PDF) HPTLC Method Development and Validation for the simultaneous Estimation of Diosgenin and Levodopa in marketed formulation [www Document]. ResearchGate. URL https://www.researchgate.net/publication/265822230_HPTLC_Method_Development_and_Validation_for_the_simultaneous_Estimation_of_Diosgenin_and_Levodopa_in_marketed_formulation (accessed 9.29.20).
- Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol.. 2004;92:291-295.
- [Google Scholar]
- New bacterial cellulose membranes from chayote fruit and bamboo shoots. Int. J. Appl. Chem.. 2014;10(2):101-112.
- [Google Scholar]
- An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21:1374.
- [CrossRef] [Google Scholar]
- A Comprehensive Review on Pharmacokinetic Profile of Some Traditional Chinese Medicines. New J. Sci. 2016:7830367.
- [CrossRef] [Google Scholar]
- Pharmacokinetic behaviour of clinically important TCM prescriptions. Orient. Pharm. Exp. Med.. 2017;17(3):171-188.
- [CrossRef] [Google Scholar]
- Pharmacokinetic profile of phytoconstituent(s) isolated from medicinal plants—A comprehensive review. J. Tradit. Complement. Med.. 2015;5(4):207-227.
- [CrossRef] [Google Scholar]
- Assessment of in vitro sun protection factor of Calendula Officinalis L. (Asteraceae) essential oil formulation. J. Young Pharm.. 2012;4:17-21.
- [CrossRef] [Google Scholar]
- Anti-malarial activities of Andrographis paniculata and Hedyotis corymbosa extracts and their combination with curcumin. Malar. J.. 2009;8:1-9.
- [Google Scholar]
- Assessment of historical polymers using attenuated total reflectance-Fourier transform infra-red spectroscopy with principal component analysis. Herit Sci.. 2013;1:28.
- [CrossRef] [Google Scholar]
- Sunscreens: safety, efficacy and appropriate use. Am. J. Clin. Dermatol.. 2002;3:185-191.
- [CrossRef] [Google Scholar]
- Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complement. Alternative Med.. 2016;16
- [CrossRef] [Google Scholar]
- Review: ultraviolet radiation and skin cancer: UVR and skin cancer. Int. J. Dermatol.. 2010;49:978-986.
- [CrossRef] [Google Scholar]
- Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: a review of its phytochemistry and pharmacology. Asian Pacific J. Tropical Disease. 2014;4:213-222.
- [CrossRef] [Google Scholar]
- Olayemi, O., Isimi, C., Ekere, K., Gbate, A., Emeje, M., n.d. Determination of sun protection factor number: an emerging in–vitro tool for predicting UV protection capabilities. Int. J. Herbal Med. 4.
- Determination of sun protection effect of herbal sunscreen cream. World J. Pharmacy Pharm. Sci.. 2015;4:12.
- [Google Scholar]
- Polyphenol composition and antioxidant activity of Andrographis paniculata L. Nees. Mapana J. Sci.. 2014;13:33.
- [Google Scholar]
- Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Therapeutic Oncol.. 2003;3:147-158.
- [Google Scholar]
- Analysis of Cosmetic Products. Elsevier; 2007.
- Santhanam, R. kumar, Akhtar, M.T., Ahmad, S., Abas, F., Ismail, I.S., Rukayadi, Y., Shaari, K., 2017. Utilization of the ethyl acetate fraction of Zanthoxylum rhetsa bark extract as an active ingredient in natural sunscreen formulations. Industrial Crops and Products 96, 165–172. https://doi.org/10.1016/j.indcrop.2016.11.058.
- Antioxidant and anti-inflammatory activities of the plant Andrographis paniculata Nees. Immunopharmacol. Immunotoxicol.. 2006;28:129-140.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of Andrographis paniculata. Fitoterapia. 2003;74:692-694.
- [CrossRef] [Google Scholar]
- Botanical pharmacognosy of Andrographis paniculata (Burm. F.) Wall. Ex. Nees. Pharmacognosy J.. 2012;4:1-10.
- [CrossRef] [Google Scholar]
- The cosmetic potential of plants from the Eastern Cape Province traditionally used for skincare and beauty. South African J. Botany, Ethnobotany. 2019;122:475-483.
- [CrossRef] [Google Scholar]
- Anti-diabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rats. Acta Pharmacol. Sin.. 2000;21:1157-1164.
- [Google Scholar]







