Translate this page into:
Novel fluoroquinolone derivatives bearing N-thiomide linkage with 6-substituted-2-aminobenzothiazoles: Synthesis and antibacterial evaluation
⁎Corresponding author. Mobile: +91 9416025460. sharma_prabodh@rediffmail.com (Prabodh Chander Sharma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A series of novel fluoroquinolone derivatives bearing N-thiomide linkage with 6-substituted-2-aminobenzothiazole substituents at the C-7 position were synthesized to obtain potent analogs active against bacterial strains. Some compounds exhibited excellent antibacterial activity against Staphylococcus auerus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa bacterial strains. Among all the synthesized compounds 6-nitro substituted benzothiazole along with norfloxacin (4b) and gatifloxacin (4l) showed MIC 05 μg/ml when tested against S. auerus. Moreover, compounds 4d, 4f and 4l showed superior MIC (15, 10, and 15 μg/ml respectively) against B. subtilis. The results of the present study reveal that the compounds have significant antibacterial potential and are suitable candidates for further exploration.
Keywords
Fluoroquinolone
Antibacterial
Benzothiazole
Synthesis
1 Introduction
Since the introduction of fluoroquinolones in late 1970s, they have generated great excitement, opportunities and applications in the antibacterial chemotherapeutic world, as these agents potentially offer all the general attributes of ideal antibacterial agents. Over the last 15 years, researchers have attempted and proved these attributes as reality (Andriole, 1988; Sharma and Jain, 2008). Initially, great improvement in potency, spectrum and in vivo efficiency was achieved as a result of intensive structural modifications. Further, the systematic and well defined structure–activity relationships were established which reveal the optimal groups for each position in terms of size, shape and electronic properties. Once the multitude of new quinolones reached advanced toxicology and clinical trials, it was realized that quinolones like all other agents, do show some undesirable side effects in human and the laboratory testings. Their adverse reactions and toxicological profiles have been reviewed extensively (Lv et al., 2012). The major concern has been the growing incidences of resistance especially to Staphylococci and Enterococci. Some of the side effects of quinolone antibacterials are unacceptable, for example, grepafloxacin withdrawn from the market, due to increased cases of heart problems in clinical findings. Similarly trovafloxacin was removed from the market due to liver toxicity (Graul et al., 1999). The current phase of quinolone design is aimed at reducing or eliminating these side effects and overcome the emerging resistance through additional synthetic manipulations while further enhancing or maintaining potency.
Synthesis and evaluation of over 10,000 quinolone derivatives resulted in extensive knowledge of the structure–activity relationship for many quinolone substituents. The most intensive structural variation has been carried out on amines at the 7-position (Foroumadi et al., 2006; Foroumadi et al., 2005), partially due to the ease of their aromatic substitution reactions. Piperazine, aminopyrrolidine and their substituted derivatives have been the most successfully employed side chains, as evidenced by the compounds currently in the market (Sharma et al., 2009; Sharma et al., 2010; Domagala et al., 1988; Chu et al., 1985). Originally, the newer fluoroquinolones arose with the development of 7- piperazinyl quinolones, such as norfloxacin 1, ciprofloxacin 2 and gatifloxacin 3 (Fig. 1). The site near the C-7 substituent is regarded as drug enzyme interaction domain (Foroumadi et al., 2005).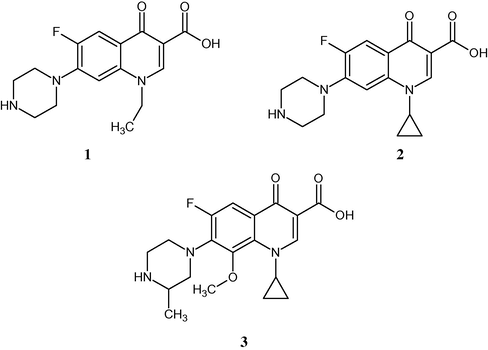
Some commercially available fluoroquinolones.
These facts motivated our concern to prepare C-7 substituents of quinolones. The piperazine moiety of fluoroquinolones possesses enough structural flexibility to allow product optimization. Thus, we anticipated that safer and superior antibacterial compounds can be developed by attaching an appropriate moiety through N-atom of the substituents at C-7 position. In this communication, we report the synthesis, characterization and antibacterial activity of a number of N-substituent piperazinyl quinolones by introducing specific substituents in the piperazine unit of 7-piperazinyl quinolones.
Keeping in view the diverse biological activity profile of 2-amino-6-substituted benzothiazoles such as cytotoxic, antibacterial, fungicidal, analgesic, antiinflammatory, antioxidant activities and pharmacological interventions of quinolone derivatives such as antibacterial, antimycobacterial, antimalarial, antioxidant, analgesic and antiviral activities, (Chu et al., 1985; Senthilkumar et al., 2009; Jayashree et al., 2009; El-Gazzar et al., 2009; Dinakaran et al., 2008; Winter et al., 2008; German et al., 2008; Jazayeri et al., 2009) clubbing of these scaffolds was considered to be of significant relevance. Moreover, due to our ongoing endeavors (Sharma et al., 2010, 2011; Kharb et al., 2011, 2011a, b) and research program on the design and synthesis of novel antimicrobial agents, a number of potent fluoroquinolone derivatives(Lv et al., 2012; Sharma and Jain, 2008; Sharma et al., 2011) have been synthesized. Recently, we have reported the synthesis of novel fluoroquinolone derivatives annulated with benzothiazoles with promising antibacterial activity (Sharma et al., 2011a, 2015). It was clearly indicated that clubbing of fluoroquinolone with benzothiazoles is worthwhile and the synthesized compounds were of promising pharmacological significance. Thus, the present study was aimed to achieve better antimicrobial profile at lower concentrations, by preparing N-substituted piperazinyl quinolone derivatives carrying benzothiazolyl substituents.
2 Experimental
2.1 General
All the chemicals and solvents used in this study were of laboratory grade and procured from E. Merck (Germany) and S. D. Fine Chemicals (India). Melting points were determined on a Labindia MR-VIS visual melting point apparatus and are uncorrected. The thin layer chromatography (TLC) plates (Silica Gel G) were used to confirm the purity of commercial reagents used, compounds synthesized and to monitor the reactions as well. Absorbance values against wavelength were taken on a Systronic double beam UV-166 spectrophotometer. The IR spectra were obtained on a Perkin Elmer IR spectrophotometer (KBr pellet). 1H NMR spectra were recorded using Bruker 400 spectrometer and chemical shifts are expressed as δ (ppm) using tetramethylsilane as an internal standard in DMSO-d6. Mass spectra of some selected compounds were obtained using a micromass-Q-TOF-micro spectrometer.
2.2 Chemistry
2.2.1 General procedure for the synthesis of 2-amino-6-substituted benzothiazole [26] (2a–2e)
The 2-amino-6-substituted benzothiazoles (2a–2e) were prepared by mixing p-substituted aniline (0.05 mol) (1a–1e) and potassium thiocyanate (0.2 mol) in 90 ml of 96% acetic acid. To this was added drop wise, with stirring, a solution of bromine (0.05 mol) in glacial acetic acid (37.5 ml) and temperature was maintained below 35 °C. After all the bromine solution was added, the mixture was stirred for another 10 h at room temperature and was filtered, the residue so obtained was washed with water. The combined filtrate and washings were neutralized with ammonium hydroxide solution. The precipitate thus obtained was collected and dried. Further purification was carried out by crystallization from benzene. Adopting the above procedure, five different 2-amino-6-substituted benzothiazoles (2a–2e) have been synthesized. The purity of the benzothiazoles was checked by TLC.
2.2.2 General procedure for the synthesis of fluoroquinolone derivatives (4a–4n)
For the synthesis of target fluoroquinolone derivatives, a mixture of 2-amino-6-substituted benzothiazole (0.05 mmol) (2a–2e), fluoroquinolone (0.05 mmol) (3a–3c) and carbon disulfide (0.05 mmol) in basic medium composed of sodium bicarbonate (NaHCO3) (0.05 mmol) and N,N-dimethyl formamide (10 ml), was refluxed for 10 h. After complete consumption of fluoroquinolone in the reaction (monitored by thin layer chromatography), the reaction mixture was poured into ice cold water and the precipitate was filtered, washed with water to yield the crude product. The precipitate was collected and dried. Further purification was carried out by crystallization from N,N-dimethyl formamide (DMF). The purity of title compounds was ascertained by thin layer chromatography. Employing the captioned procedure, fourteen novel target compounds (4a–4n) were synthesized. Structural and physicochemical data of synthesized compounds are presented in Table 1.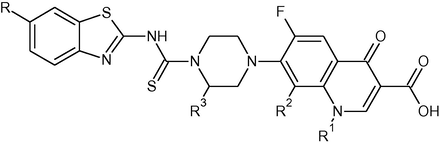
Compound
R1
R2
R3
R
mp (°C)
Formula
Yield (%)
4a
–C2H5
–H
–H
–OCH3
247–250
C25H24FN5O4S2
67
4b
–C2H5
–H
–H
–NO2
278–281
C24H21FN6O5S2
60
4c
–C2H5
–H
–H
–CH3
254–257
C25H24FN5O3S2
58
4d
–C2H5
–H
–H
–F
245–248
C24H21F2N5O3S2
72
4e
–C2H5
–H
–H
–Br
256–259
C24H21BrFN5O3S2
69
4f
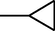
–H
–H
–OCH3
326–329
C26H24FN5O4S2
66
4g
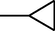
–H
–H
–NO2
332–335
C25H21FN6O5S2
64
4h
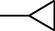
–H
–H
–CH3
319–322
C26H24FN5O3S2
59
4i
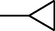
–H
–H
–F
329–331
C25H21F2N5O3S2
64
4j
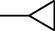
–H
–H
–Br
327–330
C25H21BrFN5O3S2
63
4k
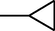
–OCH3
–CH3
–OCH3
229–232
C28H28FN5O5S2
68
4l
–OCH3
–CH3
–NO2
199–202
C27H25FN6O6S2
63
4m
–OCH3
–CH3
–CH3
197–200
C28H28FN5O4S2
63
4n
–OCH3
–CH3
–F
211–214
C27H25F2N5O4S2
64
2.2.2.1 1-Ethyl-6-fluoro-7-(4-(N-(6-methoxy-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4a)
M.p. 247–250 °C (67% yield). 1H NMR (DMSO-d6): δ/ppm = 1.38 (3H, J = 6.7, t, CH3 ethyl), 2.49 (2H, J = 6.7, q, CH2 ethyl), 3.02–3.76 (8H, m, piperazine), 3.93 (3H, s, OCH3 benzothiazole), 6.76–7.93 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.90 (1H, s, H2-quinolone), 9.36 (1H, s, NH), 15.14 (1H, s, COOH); IR (KBr): cm−1 = 1585 (C = C), 1705 (C = O), 1211 (C–O), 1157 (C–N), 3186–2854 (C–H), 3302 (COOH); MS: m/z = 541.6 (M+).
2.2.2.2 1-Ethyl-6-fluoro-7-(4-(N-(6-nitro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4b)
M.p. 278–281 °C (60% yield). 1H NMR (DMSO-d6): δ/ppm = 1.39 (3H, J = 7.2, t, CH3 ethyl), 2.19 (2H, J = 7.2, q, CH2 ethyl), 3.29–3.64 (8H, m, piperazine), 7.18–8.15 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.63 (1H, s, H2-quinolone), 9.35 (1H, s, NH), 15.24 (1H, s, COOH); IR (KBr): cm−1 = 1586 (C = C), 1718 (C = O), 1258 (C–O), 1052 (C–N), 2932 (C–H), 3415 (COOH); MS: m/z = 556.6 (M+).
2.2.2.3 1-Ethyl-6-fluoro-7-(4-(N-(6-methyl-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4c)
M.p. 254–257 °C (58% yield). 1H NMR (DMSO-d6): δ/ppm = 1.41 (3H, J = 6.6, t, CH3 ethyl), 2.29–2.49 (2H, J = 6.6, q, CH2 ethyl), 3.07–3.59 (8H, m, piperazine), 2.89 (3H, s, CH3 benzothiazole), 6.98–7.93 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.10 (1H, s, H2 quinolone), 8.90 (1H, s, NH), 15.19 (1H, s, COOH); IR (KBr): cm−1 = 1583 (C = C), 1717 (C = O), 1262 (C-O), 1098 (C-N), 2922 (C-H), 3415 (COOH).
2.2.2.4 1-Ethyl-6-fluoro-7-(4-(N-(6-fluoro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4d)
M.p. 245–248 °C (72% yield). 1H NMR (DMSO-d6): δ/ppm = 1.37 (3H, J = 6.8, t, CH3 ethyl), 1.89 (2H, J = 6.8, q, CH2 ethyl), 3.31–4.28 (8H, m, piperazine), 6.97–8.10 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.89 (1H, s, H2-quinolone), 9.35 (1H, s, NH), 15.02 (1H, s, COOH); IR (KBr): cm−1 = 1586 (C = C), 1715 (C = O), 1255 (C–O), 1062 (C–N), 2933 (C–H), 3423 (COOH).
2.2.2.5 7-(4-(N-(6-bromo-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1-ethyl-6-fluoro-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4e)
M.p. 256–259 °C (69% yield). 1H NMR (DMSO-d6): δ/ppm = 1.38 (3H, J = 6.2, t, CH3 ethyl), 1.89 (2H, J = 6.2, q, CH2 ethyl), 2.95–3.93 (8H, m, piperazine), 7.17–8.10 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.92 (1H, s, H2-quinolone), 9.56 (1H, s, NH), 15.26 (1H, s, COOH); IR (KBr): cm−1 = 1585 (C = C), 1715 (C = O), 1227 (C–O), 1050 (C–N), 2932 (C–H), 3409 (COOH).
2.2.2.6 1-Cyclopropyl-6-fluoro-7-(4-(N-(6-methoxy-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4f)
M.p. 326–329 °C (66% yield). 1H NMR (DMSO-d6): δ/ppm = 1.32 (4H, d, CH2-CH2- Cyclopropyl), 3.25 (1H, m, CH Cyclopropyl), 3.30–3.61 (8H, m, piperazine), 3.84 (3H, s, OCH3 benzothiazole), 7.43–8.11 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.60 (1H, s, H2-quinolone), 9.64 (1H, s, NH), 15.19 (1H, s, COOH); IR (KBr): cm−1 = 1581 (C = C), 1718 (C = O), 1257 (C-O), 1173 (C-N), 2924–2839 (C-H), 3294 (COOH).
2.2.2.7 1-Cyclopropyl-6-fluoro-7-(4-(N-(6-nitro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4g)
M.p. 332–335 °C (64% yield). 1H NMR (DMSO-d6): δ/ppm = 1.34 (4H, d, CH2-CH2- Cyclopropyl), 2.90 (1H, m, CH Cyclopropyl), 3.25–3.63 (8H, m, piperazine), 7.40–8.12 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.23 (1H, s, H2-quinolone), 9.09 (1H, s, NH), 14.87 (1H, s, COOH); IR (KBr): cm−1 = 1584 (C = C), 1715 (C = O), 1258 (C–O), 1062 (C-N), 2936 (C-H), 3448 (COOH); MS: m/z = 568.6 (M+).
2.2.2.8 1-Cyclopropyl-6-fluoro-7-(4-(N-(6-methyl-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4h)
M.p. 319–322 °C (59% yield). 1H NMR (DMSO-d6): δ/ppm = 1.18 (4H, d, CH2–CH2–Cyclopropyl), 2.87 (1H, m, CH Cyclopropyl), 3.33–3.80 (8H, m, piperazine), 2.49 (3H, s, CH3 benzothiazole), 7.38–8.21 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.65 (1H, s, H2-quinolone), 9.34 (1H, s, NH), 15.12 (1H, s, COOH); IR (KBr): cm−1 = 1584 (C = C), 1718 (C = O), 1265 (C–O), 1203 (C–N), 3094–2839 (C–H), 3294 (COOH).
2.2.2.9 1-Cyclopropyl-6-fluoro-7-(4-(N-(6-fluoro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4i)
M.p. 329–331 °C (64% yield). 1H NMR (DMSO-d6): δ/ppm = 1.17 (4H, d, CH2–CH2–Cyclopropyl), 3.12 (1H, m, CH Cyclopropyl), 3.20–3.95 (8H, m, piperazine), 7.00–8.11 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.64 (1H, s, H2-quinolone), 9.29 (1H, s, NH), 15.09 (1H, s, COOH); IR (KBr): cm−1 = 1583 (C = C), 1717 (C = O), 1260 (C–O), 1057 (C–N), 2909 (C–H), 3416 (COOH).
2.2.2.10 7-(4-(N-(6-bromo-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4j)
M.p. 327–330 °C (63% yield). 1H NMR (DMSO-d6): δ/ppm = 1.16 (4H, d, CH2–CH2–Cyclopropyl), 3.02 (1H, m, CH Cyclopropyl), 3.28–3.80 (8H, m, piperazine), 7.19–8.11 {5H, m, Aromatic proton (H5, H8-quinolone and H4', H5', H7'-benzothiazole)}, 8.60 (1H, s, H2-quinolone), 9.64 (1H, s, NH), 15.22 (1H, s, COOH); IR (KBr): cm−1 = 1585 (C = C), 1717 (C = O), 1260 (C–O), 1061 (C–N), 2917 (C–H), 3448 (COOH); MS: m/z = 602.5 (M+).
2.2.2.11 1-Cyclopropyl-6-fluoro-8-methoxy-7-(4-(N-(6-methoxy-1,3-benzothiazol-2-yl)amino-1-thio-methyl)-3-methylpiperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4k)
M.p. 229–232 °C (68% yield). 1H NMR (DMSO-d6): δ/ppm = 1.27 (4H, d, CH2–CH2–Cyclopropyl), 2.82 (1H, m, CH Cyclopropyl), 3.26–3.66 (7H, m, piperazine), 3.87 (3H, s, OCH3) 2.12–2.56 (3H, m, CH3 piperazine), 6.90–8.28 {4H, m, Aromatic proton (H5-quinolone and H4', H5', H7'-benzothiazole)}, 8.73 (1H, s, H2-quinolone), 9.03 (1H, s, NH), 15.06 (1H, s, COOH); IR (KBr): cm−1 = 1585 (C = C), 1717 (C = O), 1254 (C–O), 1073 (C–N), 2917 (C–H), 3448 (COOH).
2.2.2.12 1-Cyclopropyl-6-fluoro-8-methoxy-7-(3-methyl-4-(N-(6-nitro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4l)
M.p. 199–202 °C (63% yield). 1H NMR (DMSO-d6): δ/ppm = 0.93 (4H, d, CH2–CH2–Cyclopropyl), 3.15 (1H, m, CH Cyclopropyl), 3.28–3.85 (7H, m, piperazine), 2.18–2.65 (3H, m, CH3 piperazine), 3.96 (3H, s, OCH3), 7.16–8.00 {4H, m, Aromatic proton (H5-quinolone and H4', H5', H7'-benzothiazole)}, 8.44 (1H, s, H2-quinolone), 9.76 (1H, s, NH), 15.35 (1H, s, COOH); IR (KBr): cm−1 = 1586 (C = C), 1718 (C = O), 1249 (C–O), 1064 (C–N), 2947 (C–H), 3421 (COOH).
2.2.2.13 1-Cyclopropyl-6-fluoro-8-methoxy-7-(4-(N-(6-methyl-1,3-benzothiazol-2-yl)amino-1-thio-methyl)-3-methylpiperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4m)
M.p. 197–200 °C (63% yield). 1H NMR (DMSO-d6): δ/ppm = 1.22 (4H, d, CH2–CH2–Cyclopropyl), 2.92 (1H, m, CH Cyclopropyl), 3.04–3.76 (7H, m, piperazine), 2.19–2.76 (3H, m, CH3 piperazine), 3.88 (3H, s, OCH3), 7.02–8.20 {4H, m, Aromatic proton (H5-quinolone and H4', H5', H7'-benzothiazole)}, 8.78 (1H, s, H2-quinolone), 9.33 (1H, s, NH), 15.18 (1H, s, COOH); IR (KBr): cm−1 = 1587 (C = C), 1715 (C = O), 1268 (C–O), 1109 (C–N), 2917 (C–H), 3413 (COOH); MS: m/z = 581.7 (M+).
2.2.2.14 1-Cyclopropyl-6-fluoro-7-(4-(N-(6-fluoro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)-3-methylpiperazin-1-yl)-8-methoxy-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid (4n)
M.p. 211–214 °C (64% yield). 1H NMR (DMSO-d6): δ/ppm = 1.32 (4H, d, CH2–CH2–Cyclopropyl), 3.15 (1H, m, CH Cyclopropyl), 3.37–3.81 (7H, m, piperazine), 2.80–2.98 (3H, m, CH3 piperazine), 3.94 (3H, s, OCH3), 6.98–7.94 {4H, m, Aromatic proton (H5-quinolone and H4', H5', H7'-benzothiazole)}, 8.67 (1H, s, H2-quinolone), 9.28 (1H, s, NH), 15.17 (1H, s, COOH); IR (KBr): cm−1 = 1583 (C = C), 1705 (C = O), 1265 (C–O), 1157 (C–N), 3194–2854 (C–H), 3302 (COOH); MS: m/z = 585.6 (M+).
2.3 Biological evaluation
2.3.1 Antibacterial activity assay
The newly synthesized compounds (4a–4n) were evaluated for antibacterial activities using agar well diffusion method (McFarland, 1907) and by minimum inhibitory concentration (MIC) method (Kumar et al., 2009). Nutrient agar media and King's B media were used for the biological assay as per the following composition: Nutrient agar media (NAM) made up of peptone 5 g, beef extract 3 g, NaCl 5 g, nutrient agar 2% and the final volume of media was adjusted to 1000 ml with double distilled water (pH 7.0). King's B media containing peptone 2%, glycerol 1%, KH2PO4 0.15%, MgSO4 0.15%, agar 2% and the final volume of media was adjusted to 1000 ml with distilled water (pH 7.0). Synthesized compounds were screened for antibacterial activities against two Gram-positive bacteria i.e. Staphylococcus auerus (NCDC 110) and Bacillus subtilis (NCDC 71) and two Gram-negative bacteria i.e. E. coli (NCDC 134) and P. aeruginosa (NCDC 105). The bacterial cultures were revived as per the protocol provided by the National Collection of Dairy Cultures (NCDC) Karnal, India. P. aeruginosa culture was maintained on King's B media while all other cultures were maintained on nutrient agar media. Suspension of each test organism was prepared to evaluate antibacterial activity of the synthetic compounds. All stock cultures were stored at 4 °C.
Each petri plate was prepared by pouring 40 ml of appropriate agar media. A fixed volume (100 μl) of respective microorganism was spread on each petri plate with the help of a spreader. In each seeded agar plate, wells were bored using a borer of 6 mm diameter. Three concentrations (100, 50, and 10 μg/ml) of each compound reconstituted in dimethyl sulphoxide (DMSO) were added to the wells of seeded plates. DMSO was used as a control for all the experiments. The plates were kept in laminar air flow for 15 min to allow diffusion of the compounds into the agar. The plates were incubated at 37 °C for 16 h and antibacterial activity was determined by measuring the diameter of inhibition zone. Each test was performed in triplicates and mean diameter of zone of inhibition was calculated. The results obtained were compared against three standard drugs, i.e. ciprofloxacin, norfloxacin, and gatifloxacin.
MIC of the newly synthesized compounds was determined using the method described by Kumar et al. (2009). A stock solution of 3 mg/ml of each compound was prepared in DMSO and further diluted to get a final concentration ranging from 200–0.05 μg/ml. Optical density was measured at 600 nm using UV–visible spectrophotometer. The minimum concentration, where no microbial growth was observed is called as MIC of the compound.
3 Results and discussion
The synthetic route of desired compounds is presented in Scheme 1. The requisite 2-amino-6-substituted benzothiazoles (2a–2e) were prepared according to the method described by Stuckwisch (1949). Reaction between 2-amino-6-substituted benzothiazole, different fluoroquinolones and carbon disulfide in the presence of basic medium composed of sodium bicarbonate and N,N-dimethyl formamide, afforded the target compounds after a refluxing time of 10 h. The fluoroquinolone derivatives (4a–4n) were found in 58–72% yields. TLC analysis was done to confirm the purity of the compound. Analytical and spectral data (IR and 1H NMR) of all the newly synthesized compounds were found in full agreement with the proposed structures. In the 1H NMR spectra the signals of the respective proton of the compounds were verified on the basis of their chemical shifts, multiplicities. The spectra showed a characteristic singlet of one proton in DMSO-d6 at δ 8.8–9.4 ppm corresponding to NH group of thiomide linkage, proton at the second position of quinolone ring showed a characteristic singlet at a shift at δ 8.6–8.9 ppm and a broad singlet at δ 14.7–15.3 ppm of COOH group. The IR spectra of compounds revealed C = O, C = C, stretching at approximately 1710, 1600 cm−1, whereas in general C–O, O–H and C–H bands were revealed at nearby 1300, 3400, 2900 and 3000. It was found interesting that only two compounds among all were in lesser yield than 60% whereas all other compounds are in good yields. Compound 1-ethyl-6-fluoro-7-(4-(N-(6-fluoro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid was afforded in most effective yield of 72%. Melting range of various compounds of this series is found to be higher than 200 °C. Compound 4g showed the maximum melting temperature among all synthesized compounds which is 332–335 °C.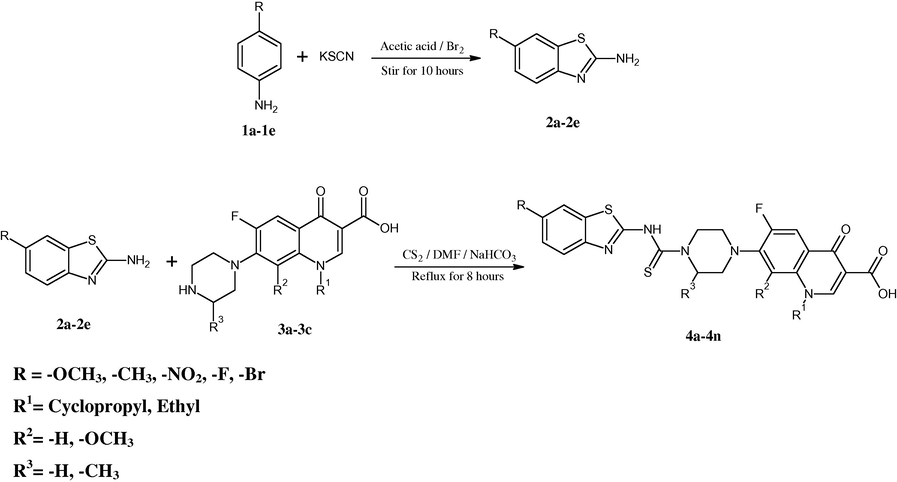
Synthetic route of target compounds.
All the synthesized fluoroquinolone derivatives were screened for antibacterial activities against two Gram positive bacterial strains i.e. S. auerus (NCDC 110) and Bacillus subtilis (NCDC 71) and two Gram negative strains i.e. E. coli (NCDC 134) and P. aeruginosa (NCDC 105). The antibacterial activity was determined by measuring the diameter of the zone of inhibition and minimum inhibitory concentration (MIC). The results of zone of inhibition and MIC screening are summarized in Tables 2 and 3 respectively.
Compound
S. auerus (NCDC 110)
B. subtilis (NCDC 71)
E. coli (NCDC 134)
P. aeruginosa (NCDC 105)
10
50
100
10
50
100
10
50
100
10
50
100
4a
–
2.5
5.0
–
8.0
8.5
–
5.8
6.5
2.5
3.3
5.3
4b
–
5.0
6.0
–
–
–
–
–
–
3.0
3.1
4.5
4c
–
–
–
–
–
3.8
–
–
–
2.5
2.8
3.0
4d
–
4.3
5.2
3.8
6.8
8.0
–
2.7
6.5
2.5
3.0
3.5
4e
–
2.0
6.0
–
–
4.3
–
–
–
3.0
3.3
3.4
4f
–
–
4.3
–
4.3
5.8
–
–
4.5
3.0
3.5
3.7
4g
9.0
14.0
15.0
7.3
10.0
10.5
–
8.0
10.0
2.8
3.5
5.0
4h
4.3
7.5
10.0
6.5
10.0
11.0
10.3
14.8
15.0
2.8
6.8
7.1
4i
8.5
10.2
10.7
–
5.5
7.3
–
3.0
5.0
1.3
2.3
2.5
4j
11.3
12.8
15.0
7.5
9.0
10.3
7.8
11.0
13.3
3.3
3.8
5.0
4k
–
4.2
5.7
–
2.8
3.0
–
–
3.8
–
-
3.8
4l
–
5.5
7.5
–
2.8
5.0
–
4.0
3.5
3.8
3.8
5.0
4m
–
2.7
5.0
–
3.8
4.5
–
–
–
1.5
3.3
4.0
4n
7.5
10.5
12.0
–
5.0
6.8
1.0
4.3
6.0
–
3.3
5.0
Norfloxacin
9.2
10.5
12.5
5.0
8.0
10.2
4.2
8.5
10.8
1.0
2.0
7.2
Ciprofloxacin
8.0
11.0
13.0
8.0
11.0
12.2
8.2
10.5
11.3
4.5
8.0
10.9
Gatifloxacin
8.0
11.0
11.3
6.5
10.2
11.5
6.0
9.7
9.8
2.7
4.2
4.7
Control
–
–
–
–
–
–
–
–
–
–
-
-
Compound
S. auerus (NCDC 110)
B. subtilis (NCDC 71)
E. coli (NCDC 134)
P. aeruginosa (NCDC 105)
4a
35
30
20
100
4b
05
120
140
120
4c
100
50
140
30
4d
40
15
05
30
4e
20
35
100
20
4f
30
10
30
110
4g
35
150
05
50
4h
10
30
175
20
4i
30
40
20
80
4j
15
25
35
35
4k
80
70
80
120
4l
05
15
20
35
4m
120
100
90
20
4n
25
55
05
20
Norfloxacin
10
05
70
15
Ciprofloxacin
50
20
25
60
Gatifloxacin
05
100
100
50
Control
–
–
–
–
These novel derivatives demonstrated varying antibacterial activity (zone of inhibition) against different strains. Compound 4j possessing bromo functionality at position-6 of benzothiazole nucleus, depicted significant zone of inhibition of 11.3, 12.8 and 15.0 mm at 10, 50 and 100 μg/ml concentrations respectively against S. auerus. Compounds bearing N-cyclopropyl functionality i.e. 4g, 4h, 4i and 4n also found to possess significantly potent antibacterial activities against S. auerus and the zone of inhibition depicted by ciprofloxacin analogs 4g, 4i and 4j was even better than the standard antibiotics ciprofloxacin, norfloxacin, and gatifloxacin when tested against S. auerus. While compound 1-cyclopropyl-6-fluoro-7-(4-(N-(6-fluoro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)-3-methylpiperazin-1-yl)-8-methoxy-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid showed moderate activity. Compounds 4g, substituted with the NO2 group at position-6 of benzothiazolyl moiety annulated with ciprofloxacin molecule, depicted zone of inhibition of 7.3 mm at concentration 10 μg/ml against Gram positive Bacillus subtilis bacterial strain exhibit even better activity than the standard drugs norfloxacin (5.0 mm) and gatifloxacin (6.5 mm). Compounds 4d, 4h and 4j exhibited good antibacterial activities against Bacillus subtilis while 4g, 4h and 4j showed moderate activity and the activities shown by 4h and 4j are significantly potent having a zone of inhibition of 10.3 and 7.8 mm respectively at concentration 10 μg/ml against the standard antibiotics norfloxacin (4.2 mm) and gatifloxacin (6.0 mm). Analogs 4h and 4j showed even better antibacterial activities against the standard antibiotics ciprofloxacin, norfloxacin and gatifloxacin when tested against E. coli and 1-cyclopropyl-6-fluoro-7-(4-(N-(6-nitro-1,3-benzothiazol-2-yl)amino-1-thio-methyl)piperazin-1-yl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid showed comparable activities at higher concentration (50 μg/ml and 100 μg/ml). Compounds 4b, 4e, 4f, 4g, 4h, 4j, 4l and 4m showed improved antibacterial activities against the standard antibiotics norfloxacin and gatifloxacin when tested against P. aeruginosa.
When tested against S. auerus compounds having nitro substitution at position-6 of benzothiazole clubbed with norfloxacin (4b) and gatifloxacin (4l) showed MIC 05 μg/ml, which is 10 times more potent as compared to the standard drug ciprofloxacin (MIC value 50 μg/ml). While all the norfloxacin and ciprofloxacin derivatives showed comparable activity. Compounds 4a, 4c, 4e, 4h, 4i and 4j showed significant MIC against Bacillus subtilis when compared with standards. Moreover, compounds 4d, 4f and 4l showed superior MIC (15, 10, 15 μg/ml respectively) against Bacillus subtilis than the standard antibiotics ciprofloxacin (MIC value 20 μg/ml) and gatifloxacin (MIC value 100 μg/ml). Analogs 4g, 4i, 4l and 4n showed better MIC against the standard antibiotics ciprofloxacin, norfloxacin and gatifloxacin when tested against E. coli. Compounds 4c, 4d, 4e, 4g, 4h, 4j, 4l, 4m and 4n showed comparable MIC against the standard antibiotics norfloxacin and gatifloxacin when tested against P. aeruginosa.
4 Conclusion
In conclusion, we have synthesized fourteen novel derivatives of three fluoroquinolone drugs i.e. norfloxacin, ciprofloxacin and gatifloxacin. These drugs have been linked to different 2-amino-6-substituted benzothiazoles via thiomide bridge linkage. The derivatives are characterized by physicochemical and spectral analyses such as 1H NMR and IR. The spectral data obtained were in full agreement with the proposed structures. The in vitro evaluation of newly synthesized compounds revealed improved therapeutic effectiveness as compared to the parent drugs. Some derivatives showed more potent or equipotent antibacterial activities against different strains (S. auerus, B. subtilis, E. coli, P. aeruginosa). The experimental data of this investigation reveal that the synthesized novel derivatives of fluoroquinolones have remarkable antibacterial potential.
References
- The Quinolones. New York: Academic Press; 1988. pp. 1–55
- J. Med. Chem.. 1985;28:1558-1564.
- Bioorg. Med. Chem.. 2008;16:3408-3418.
- J. Med. Chem.. 1988;31:991-1001.
- Eur. J. Med. Chem.. 2009;44:609-624.
- Bioorg. Med. Chem. Lett.. 2005;15:4488-4492.
- Bioorg. Med. Chem. Lett.. 2006;16:3499-3503.
- Eur. J. Med. Chem.. 2008;43:2453-2463.
- Drug Fut.. 1999;24:1324-1331.
- Med. Chem. Res.. 2009;19:193-209.
- Eur. J. Med. Chem.. 2009;44:1205-1209.
- Curr. Med. Chem.. 2011;18:3265-3297.
- J. Enzyme Inhib. Med. Chem.. 2011;26:1-21.
- Mini Rev. Med. Chem. 2011;11:84-96.
- Eur. J. Med. Chem.. 2009;44:2260-2264.
- ChemMedChem.. 2012;7:1230-1236.
- J. Am. Med. Assoc.. 1907;14:1176-1178.
- Eur. J. Med. Chem.. 2009;44:345-358.
- Acta Pol. Pharm. Drug Res.. 2008;65:551-556.
- Acta Pharm. Sci.. 2008;50:35-40.
- Acta Pol. Pharm. Drug Res.. 2009;66:587-604.
- J. Enzyme Inhib. Med. Chem.. 2010;25:577-589.
- Sharma, O.P., Sharma, P.C., Jain, S. 2011. In: Proceedings of the International Conference on Bioscience, Biochemistry and Bioinformatics, held at Singapore, February 26–28, pp. 1–5.
- Curr. Med. Chem.. 2011;18:4786-4812.
- J. Enzyme Inhib. Med. Chem. 2011 (Accepted Manuscript)
- Arab. J. Chem.. 2015;8:671-677.
- J. Am. Chem. Soc.. 1949;71:3417.
- Exp. Parasitol.. 2008;118:487-497.








