Translate this page into:
Novel Spiro-pyrrolizidine-Oxindole and Spiropyrrolidine-Oxindoles: Green synthesis under Classical, Ultrasonic, and microwave conditions and Molecular docking simulation for antitumor and type 2 diabetes
⁎Corresponding author. tamohamed@uqu.edu.sa (Thoraya A. Farghaly) thoraya-f@hotmail.com (Thoraya A. Farghaly) thoraya-f@cu.edu.eg (Thoraya A. Farghaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Novel spiropyrrolizine / pyrrolidineoxindole moieties were synthesized chemo-, and regio-selectively in high yields from knoevenagel reaction of bis[arylmethylidene]piperidin-4-ones, isatin and L-proline or sarcosine under classical, ultrasonic, and microwave conditions. Seven derivatives of the synthesized dispiro-pyrrolizidine-oxindole and spiropyrrolidine were screened for their antitumor activity against two cell lines MCF-7 (breast cancer) and HEPG2 (liver cancer). The results of biological activity indicated that the tested derivatives showed potent activity against breast cancer cell MCF-7. Molecular docking simulation screening studies of the synthesized products with each of the receptors of (3hb5) for breast cancer and (4k9g) for liver cancer and their interaction with 1H5U of glycogen phosphorylase B, type 2 diabetes drug were examined. The docking study of dispiro-pyrrolizidine-oxindole and spiropyrrolidine showed promising results with several derivatives.
Keywords
Spiropyrrolizineoxindole
Spiropyrrolidine oxindole
Glycogen metabolism
Antidiabates
Dispiroheterocyclic
1 Introduction
In full swing, scientists are searching day and night for anti-cancer drugs because of the side effects of the drugs circulating in the market, in addition to the body's resistance to these drugs (Richardson et al., 1988). Cancer of all kinds is one of the terrifying diseases whose name is associated with death. The number of deaths from this disease is increasing due to the increase in its causes and the side effects of chemotherapy (Sung et al., 2021). On the other hand, diabetes is a well-established disease that occurs when the insulin produced by the pancreas is not sufficiently produced or when the body cannot effectively use the insulin produced. Type 2 diabetes that is not dependent on insulin, or appears in adults, results from the body's ineffective use of insulin. The majority of diabetic patients have type 2 diabetes (Diabetes, 2021). Glycogen phosphorylase (GP) inhibition has been proposed as a therapeutic strategy for type 2 diabetes treatment (Oikonomakos et al., 2005; Somsák et al., 2008). Inhibition of this enzyme by potent and selective inhibitors, will lead to antihyperglycemic drugs. Glycogen phosphorylase is a typical allosteric protein with five different ligand-binding sites, providing multiple opportunities to modulate enzyme activity. Spiro heterocycles are naturally occurring substances with biological activities (James et al., 1990). Also, hybrid molecules like pyrrolidine, pyrrolizine and oxindole pharmacophores which had a wide spectrum of biological activities like antimicrobial and anticancer (Hassaneen et al, 2017; Hati et al., 2016; Freeman-Cook et al., 2007; Engen et al., 2014; Almansour et al., 2014; Mohareb et al., 2014; Khan et al., 2014; Kumar et al., 2021; Wei et al., 2012; Chen et al., 2009; Dandia et al., 2017; Arumugam et al., 2018; Nesi et al., 2013; Hanna et al., 2012). One method from the several methods to generate pyrrolidine ring (Poomathi et al., 2015; Hemamalini et al., 2011; Ghandi et al., 2009; Shahrestani et al., 2019; Altowyan et al., 2022) is 1,3-dipolar cycloaddition of azomethineylides which used to synthesize many dispiroheterocyclic systems with the definite dipolarophiles (Hassaneen et al., 2017; Barakat et al., 2021a; Barakat et al., 2021b; Islam et al., 2021a;Islam et al., 2021b; Islam et al., 2022; Al-Majid et al., 2021; Nájera and Sansano, 2019; Rajkumar et al., 2016). Scientists are making an extensive effort to improve the yield of synthesized heterocyclic compounds by using nano-catalysts or different methods of heating such as microwave, ultrasound or reflux and compare the percentage yield of these methods to get the best results. Nano-catalysts and a variety of heating techniques, such as microwave, ultrasound, and reflux, are among the many tools scientists are using to increase the yield of synthetic heterocyclic compounds, with the goal of determining which approach produces the highest percentage yield. Ultrasound form hot spots during acoustic cavitations which serve to increase reaction rates and yields without using rough conditions (Rezaei et al., 2012; Jadidi et al., 2008; Alaoui et al., 2018; Dandia et al., 2020). Microwave (MW) method has used for synthesis spiropyrrolidine oxindole derivatives, a lot of advantages in using this method than conventional heating (Kefayati et al., 2015; Hoz et al., 2005; Buriol et al., 2010; Engen et al., 2014; Jandourek et al., 2014; Kidwai et al., 1997; Dandia et al., 2021a; Dandia et al., 2021b). Where small volumes and solvent-free MW methods are used that have a high level of chemo-, regio-, and stereoselectivity in them Spiro-oxindole derivatives were made under safe conditions using catalysts in aquas medium (Dandia et al., 2021c; Dandia et al., 2011). There were only a few reports on how to make spirooxindole with each of pyrrolidines and pyrrolizidines under ultrasound, Microwave irradiations, and free solvents.
Following on from the foregoing and in continuation of our research into the synthesis of novel biologically active hybrid heterocyclic compounds using green heating methods, (Shaaban et al, 2022; Alnaja et al., 2021; Bumander et al., 2019; Abbas et al., 2014; Farghaly and Riyadh, 2009), we are interested in synthesising new series of pyrrolidineoxindole and spiropyrrolizidine moieties under microwave irradiation and ultrasonic conditions in order to increase their yields and decrease their reaction time.
Furthermore, we will test the anticancer efficacy of newly synthesised spiropyrrolizidine / pyrrolidineoxindole derivatives for their antitumor activity. In addition, they will utilise the docking research to examine their interaction with two proteins (3hb5) that are associated with breast cancer and (4k9g) that are associated with liver cancer. Additionally, via docking studies of these derivatives with the 1H5U domain of glycogen phosphorylase B, a type 2 diabetes medication, evaluate the capacity of the produced compounds to suppress the development of type 2 diabetes.
2 Results and discussion
We previously established in our earlier study (Hassaneen et al, 2017) that condensation reaction of isatin (1), amino acids as L-proline (2), or sarcosine (3), and 3,5-bis[phenylmethylidene]-4-oxopiperidine-N-carboxylate (4a-j), was carried out in methanol at 60 °C in 120 min Scheme 1 to produce dispiro-pyrrolizidine-oxindoles (5), or spiropyrrolidine (6) (Hassaneen et al, 2017).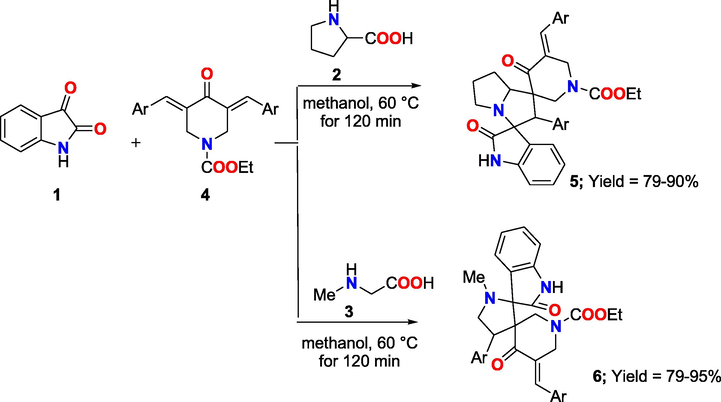
Synthesis of dispiro-pyrrolizidine-oxindoles (5) and spiropyrrolidine(6).
The innovative dispiro-pyrrolizidine-oxindole (9) and spiropyrrolidine (11) series were produced in this work under solvent-free conditions employing ultrasonic and MW irradiation.
Substituted pyrrolizidines (9) were synthesised in high yields using 1,3-dipolar cycloaddition of azomethineylides (8) to the adduct (7) using standard heating and ultrasonic irradiation (comparative results are shown in Scheme 2, Table 1). a: isolated yield based on isatin.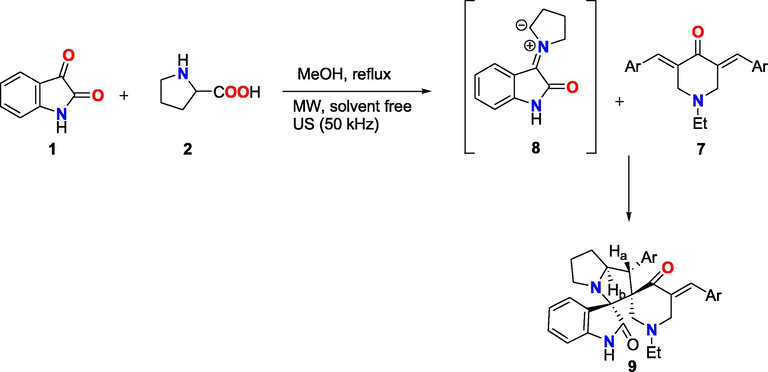
A proposed mechanism for the synthesis of compound 9a-e.
Ar
Classical
Ultrasonic
Time (min)
Yield%
Time(min)
Yield%a
9a
C6H5
120
80
30
96
9b
4-MeC6H4
120
83
30
88
9c
4-ClC6H4
120
82
30
84
9d
2,4-Cl2C6H3
120
85
30
90
9e
2-Thionyl
120
70
30
82
One-pot, three component reaction of isatin (1), L-proline (2), and adduct of type (7) in methanol under reflux. As seen in Scheme 1, the reaction began as a condensation reaction between isatin 1 and L-proline 2, which was then decarboxylated to generate an in-situ azomethine ylide intermediate (8). Then, a new form of spiropyrrolizidine-oxinidole (9) was synthesised in high quantities by cycloaddition of 3,5-bis[arylmethylidene]-4-oxipiperidine-N-ethyl (7a-e) with non-stabilized azomethine ylides (8). Highly diastereoselective and regioselective reaction as a single product was produced which confirmed by TLC and GC–mass analysis. Under mild-conditions and low-intensity ultrasound US (cleaning bath) and methanol as a solvent, cycloaddition reactions were carried. It’s note that single product 9 was obtained in each reaction condition. So, it can be suggested that the pathway of this homogeneous reaction suggested from a concerted mechanism in a thermolytic method to a US method (Scheme 1). Increase in the rate of decarboxylation and the formation of ylide (8) might be the reason in increasing the reaction rate, and cause the frequency of collisions between ylide (8) and 3,5-bis[arylmethylidene]-4-oxipiperidine-N-ethyl (7) to increase. Localized hot spots formed during the cavitational collapse due to the energy of these clashes and formation of ylide (8) which provided by short-lived. Next, we decided to change from conventional thermolytic condition for 120 min. to MW heating in a reaction vessel to reduce the reaction time. When heated to 150 °C with a dedicated MW oven, the reaction was completed in 10 min under solvent-free condition, resulting in a 95% yield of synthesized compound (9a) Table 2. Moreover, we confirmed that however method of the conventional thermal-heating affords only moderate yields of the predictable products of the cyclic-condensation reaction, MW irradiation through solvent-free conditions reveals the same products with significantly reduced reaction time and improved yields Table 2. Use of MW equipment for the synthesis allowed conditions Safe and repeatable interaction. a: isolated yield based on isatin.
Time (min)
Temp(oC)
Yield%a
10
200
85
8
200
85
5
200
86
10
150
95
5
150
87
10
80
90
10
65
87
Similar to this, preparation of spiropyrrolidine-oxindoles (11a-e) in high yields by a three-component reaction of 3,5-bis[arylmethylidene]-4-oxipiperidine-N-ethyl (7a-e) with two equivalents of both of isatin 1 and sarcosine 3 in methanol at reflux and in ultrasonic condition or microwave under solvent-free conditions to obtain the single product (11) Scheme 3, Table 3. a: isolated yield based on isatin.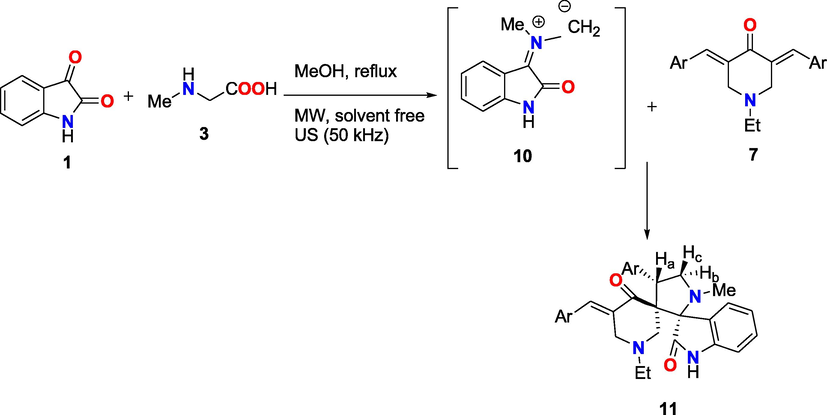
A proposed mechanism for the synthesis of compounds 11a-e.
Ar
Classical
Ultrasonic
Microwave
Time (min)
Yield%
Time (min)
Yield%a
Time (min)
Yield%a
11a
C6H5
120
84
30
95
10
95
11b
4-MeC6H4
120
79
30
84
10
90
11c
4-ClC6H4
120
80
30
93
10
94
11d
2,4-Cl2C6H3
120
87
30
88
10
93
11e
2-Thionyl
120
87
30
91
10
94
The structure of dispiro heterocyclic ring products 11a-e was proved based on the spectral data that extracted from their NMR, IR, Mass spectra as well as their elemental analysis. The IR spectra of all novel spiropyrrolizidines / pyrrolidineoxindole derivatives 9a-e and 11a-e revealed one NH and two C = O at ν = 3421–3251, 1720–1701 and 1716–1678 cm−1, respectively. The 1H NMR spectrum of compound 11c (Fig. 1) showed the characteristic signals for all protons in the skeleton of this derivative as follows: δ 0.75 triplet signal for CH3 of ester group, 1.95 singlet signal for N-CH3 group, 2.94 doublet of doublet for Hb, 3.14 multiplet signal for the two CH2 groups of pipridinone ring, 3.34 doublet of doublet for Hc, 3.76 quartet signal for CH2 of ester group, 4.61 doublet of doublet for Ha, 6.56–7.41multiplet with integration of 12 protos for the aromatic protons, 7.54 singlet signal for = CH group and one NH at 10.38 ppm. The regio-chemistry of the products spiropyrrolizidine / pyrrolidineoxindole derivatives 9a-e and 11a-e was confirmed through checking their 13C NMR data as for example, 13C NMR spectra of derivatives 9a and 11a have the characteristic carbon signals at δ 52.7, 54.2 ppm and 83.4, 88.5 ppm for the two spiro carbons in each derivative (Hassaneen et al, 2017). Also, the two downfield carbon signals at δ172.2, and 204.2 ppm in each derivative 9a and 11a were due to the C = O of oxindole and keto C = O carbons of piperidinone ring system, respectively. The mass spectra of all products spiropyrrolizidine / pyrrolidineoxindole derivatives 9a-e and 11a-e showed expected molecular ion peak for each derivative which confirms the formation of mono-adduct (Fig. 2).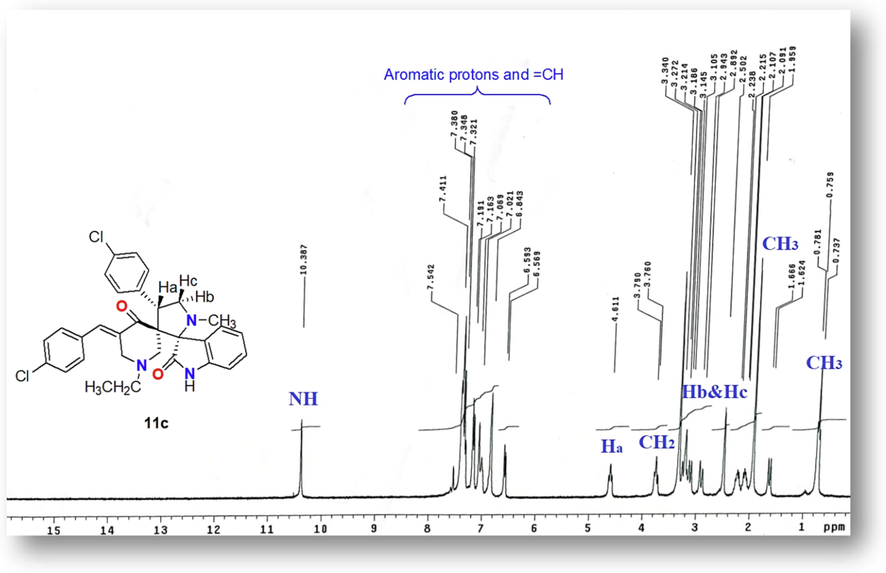
The 1H NMR (DMSO‑d6, 300 MHz) of derivative 11c.
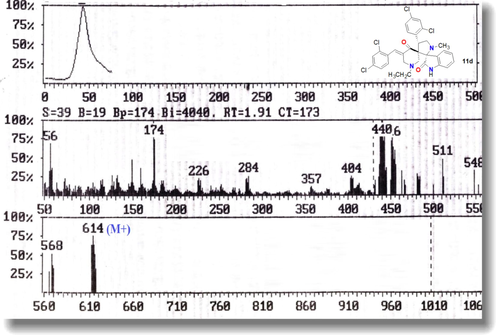
The mass spectrum of compound 11d (Mwt = 615.38).
The usage of methanol in the synthesis of new spiropyrrolizidine / pyrrolidineoxindole derivatives 9a-e and 11a-e by microwave irradiation or ultrasound demonstrated its effectiveness in terms of high yields for each derivative, a straightforward experimental approach, and ease of isolation.
2.1 Antitumor activity
Selected derivatives of the synthesized spiro compounds 9b,c,e and 11b-e were screened for their antitumor activity against two cell lines MCF-7 and HEPG2 (Table 4). We observed from the obtained data of IC50 in Table 4 that the tested derivatives displayed moderate to excellent cytotoxic activities against the breast cancer MCF-7 cell line with IC50 values ranging from 3.9 to 36.1 μg. 1′'-ethyl-1′-methyl-5′'-(4-methylbenzylidene)-4′-(p-tolyl)dispiro[indoline-3,2′-pyrrolidine-3′,3′'-piperidine]-2,4′'-dione (11b) is the most reactive derivative with IC50 = 3.9 μg in compared with the IC50 = 5.20 μg of the reference cisplatin drug. In addition to two derivatives 9b and 9c showed good activity with IC50 = 7.8 and 9.2 μg. The most potent derivatives as anti-breast cancer derivatives 11b and 9b carrying in their skeletons the methyl (EDG). Within the investigation the result for HEPG2 in Table 4, we noted that the most reactive spiro-derivative is 11b with IC50 = 5.7 μg that is near to the IC50 = 3.67 μg of the reference cisplatin drug. The other two spiro-derivatives 9c and 9b have good activity with IC50 = 9.7 and 10.6 μg. The other tested spiro-pyrazoles showed moderate activity with IC50 ranging from 24.8 to 46.2 μg.
Compd. No.
MCF-7
HEPG2
9b
7.8
10.6
9c
9.2
9.7
9e
21.0
28.7
11b
3.9
5.7
11c
35.9
37.2
11d
18.8
24.8
11e
36.1
46.2
Cisplatin
5.20
3.67
2.2 Docking study
2.2.1 Docking study with the receptors of (3hb5) for breast cancer and (4k9g) for liver cancer
To understand the obtained antitumor results depending on a structural bases, all synthesized spiro-derivatives 9a-e and 11a-e were evaluated through docking techniques (Table 5 and 6) with the receptors of (3hb5) for breast cancer and (4k9g) for liver cancer. Docking simulation was performed in this study using Molecular Operating Environment MOE-Dock 2014 software (MOE, 2014). All the interaction energies and different calculations were calculated. From the docking results of the spiro-synthesized derivatives 9a-e and 11a-e with the receptors of (3hb5) for breast cancer, they indicated that all derivatives were interacted with the active cites of (3hb5) with binding energies −6.5630 to −2.1721 Kcal/mol (Figs. 3 and 4) except derivative 9d. The most reactive derivative in the series of spiro-compounds 9a-e was found to be 9b with docking score − 5.7909 Kcal/mol with only H- acceptor from ARG 37 to the C = O of piperidone ring. In case of series 11a-e, spiro-derivative 11b is the most reactive compound with binding score = -6.5630 Kcal/mol which formed three types of interactions namely, H-acceptor, pi-cation and pi-pi with the amino acids GLY 92, ARG 37 and PHE 192, respectively. On the other hand, docking the spiro-compounds 9a-e didn’t show any interaction with the active sites of receptor 4k9g (Table 6 & Fig. 5). But, all derivatives 11a-e involved in the interaction with 4k9g with energy score = -5.5473 to −4.8955 Kcal/mol through H-acceptor, pi-cation and pi-H. The essential parts of the spiro-compounds 11a-d that involved in the interaction to the active site of the protein receptor 4k9g are C = O of indole, nitrogen atom of piperidone, the aryl ring of arylidene moieties.
Compd.
Ligand moiety
Receptor site
Interacting residues
(Type of interaction)
Distance (oA)
E (kcal/mol)
Docking score (kcal/mol)
9a
6-ring
CG LEU 93 (X)
pi-H
4.07
−0.6
− 5.0609
9b
O 38
NH2 ARG 37 (X)
H-acceptor
3.12
−1.3
− 5.7909
9c
CL 64
N 5
6-ring
6-ringO LEU 64 (X)
NH2 ARG 37 (X)
NE ARG 37 (X)
NZ LYS 195 (X)H-donor
H-acceptor
pi-cation
pi-cation3.23
2.99
4.02
4.23−0.5
−3.8
−1.0
−0.8−5.2109
9d
---
---
---
---
---
---
9e
N 13
O 41
6-ringNH2 ARG 37 (X)
N GLY 92 (X)
NE ARG 37 (X)H-acceptor
H-acceptor
pi-cation3.15
3.17
3.92−2.1
−1.3
−1.4−2.1721
11a
6-ring
6-ring PHE 192 (X)
pi-pi
3.98
−0.0
−5.7153
11b
O 39
6-ring
6-ringN GLY 92 (X)
NE ARG 37 (X)
6-ring PHE 192 (X)H-acceptor
pi-cation
pi-pi2.83
3.85
3.75−4.2
−1.5
−0.0−6.5630
11c
CL 60
O 39OD1 ASN 90 (X)
NH2 ARG 37 (X)H-donor
H-acceptor3.42
2.55−0.7
−2.4−5.3711
11d
CL 57
O 40O ALA 106 (X)
NZ LYS 195 (X)H-donor
H-acceptor3.59
2.79−1.1
−6.1−5.1334
11e
N 15
O 39
5-ringNH2 ARG 37 (X)
NH2 ARG 37 (X)
CA GLY 13 (X)H-acceptor
H-acceptor
pi-H3.22
2.76
4.09−1.1
−2.0
−0.6−6.1817
Compd.
Ligand moiety
Receptor site
Interacting residues
(Type of interaction)
Distance (oA)
E (kcal/mol)
Docking score (kcal/mol)
9a
---
---
---
---
---
---
9b
---
---
---
---
---
---
9c
---
---
---
---
---
---
9d
---
---
---
---
---
−5.8396
9e
---
---
---
---
---
---
11a
6-ring
N PRO 1 (A)
pi-cation
3.63
−1.4
−4.8955
11b
O 39
NE1 TRP 108 (A)
H-acceptor
2.84
−2.1
−5.5473
11c
6-ring
6-ringCA TYR 36 (A)
6-ring TRP 108 (A)pi-H
pi-pi4.68
3.88−0.6
−0.0−5.2285
11d
N 5
O 39NE2 GLN 35 (A)
NE2 GLN 35 (A)H-acceptor
H-acceptor3.44
3.02−1.1
−1.4−5.4566
11e
5-ring
CA TYR 36 (A)
pi-H
4.02
−0.6
−4.9266
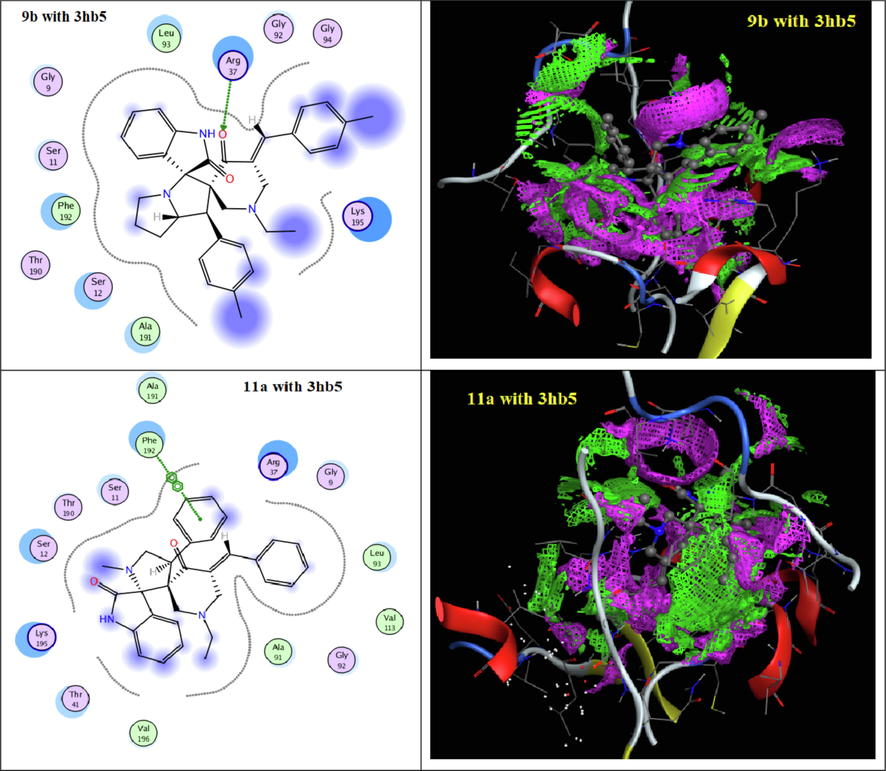
2D and contact performance of docked compounds 9b and 11a into the active site of 3hb5.
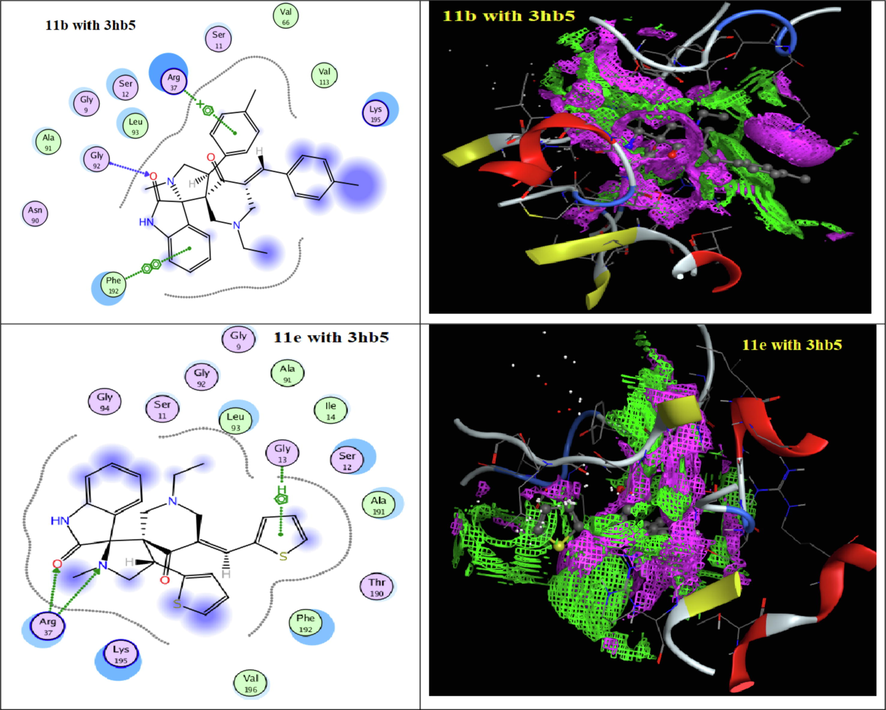
2D and contact performance of docked compounds 11b and 11e into the active site of 3hb5.
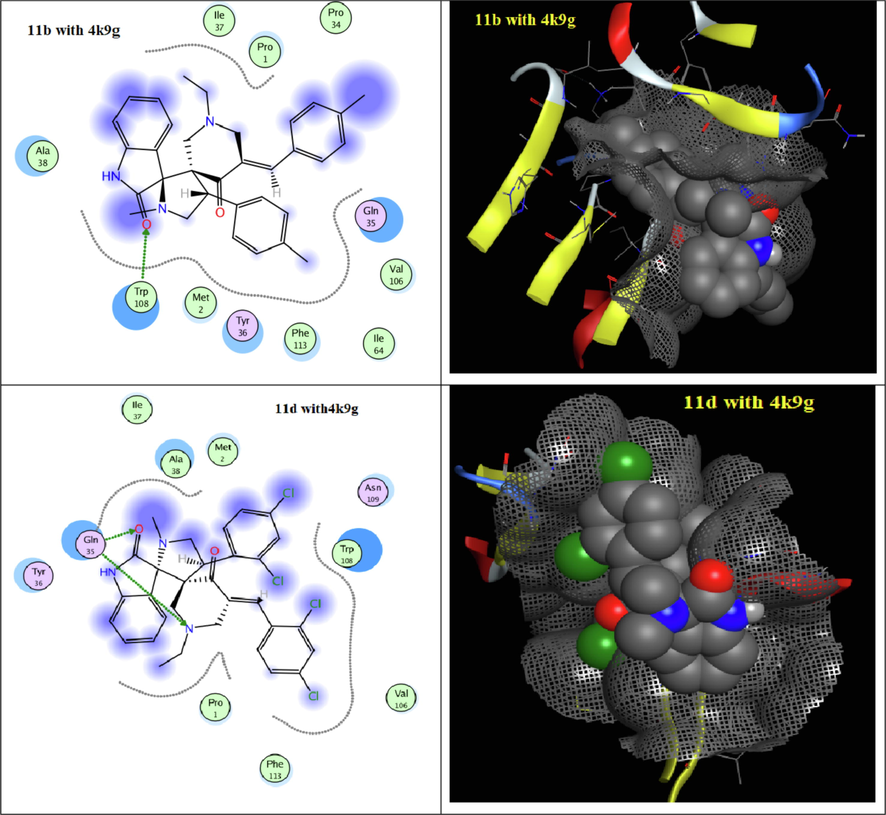
2D and surface interaction of docked compounds 11b and 11d into the active site of 4k9g.
2.2.2 Docking study with glycogen phosphorylase B (PDB code: 1H5U)
Pyrrolidine and oxindole containing compounds are parts of antidiabetic α-glucosidase inhibitors (Trapero and Llebaria, 2012; Hamed, 2018; Zhu et al., 2021). This finding encourages us to test the interaction of all synthesized dispiro-pyrrolizidine-oxindoles (9a-e) and spiropyrrolidinebisoxindoles (11a-e) with glycogen phosphorylase B to inhibit the type 2 diabetes. The X- ray crystallography structure was reported for (PDB code: 1H5U). The behaviour of the synthesised products in binding site of the target protein was shown in Fig. 6 which predict the binding modes, affinities, and orientations of compounds (9a-e, 11a-e) at the active sites of the protein, docking scores were listed in Table 7. Dispiro-pyrrolizidine-oxindoles (9) showed more activity than spiropyrrolidinebisoxindoles (11). The carbonyl group of isatin has high activity as it bound with amino acids. In which 9a bounded with Arg 569 (bond length: 2.7 Å), in 9b Arg 589 (bond length: 2.4 Å), In 9d Lys 576 (bond length: 2.4 Å) and Arg 589 (bond length: 1.6 Å), and in 11b. Lys 680(bond length: 2.1 Å) and Thr 676 (bond length: 3.0 Å).The Nitrogen atom of N-ethylpiperidin-4-one also showed activity towards amino acids Lys 576 as in 9a (bond length: 2.3 Å), in 9b (bond length: 2.1 Å), in 9d (bond length: 2.78 Å), and Asn 284 (bond length: 2.5 Å), and in 11b (bond length: 2.4 Å). The carbonyl group of same rings piperidin-4-one showed high activity in binding with amino acids of (PDB code: 1H5U), in 9a Thr 676 (bond length: 2.8 Å), Gly 677 (bond length: 2.8 Å), in 9b Thr 676 (bond length: 2.7 Å), Gly 677 (bond length: 2.6 Å), and in 11b Thr 676 (bond length: 2.4 Å). The data showed that 9a, 9b, 9d, 11b have promising activity.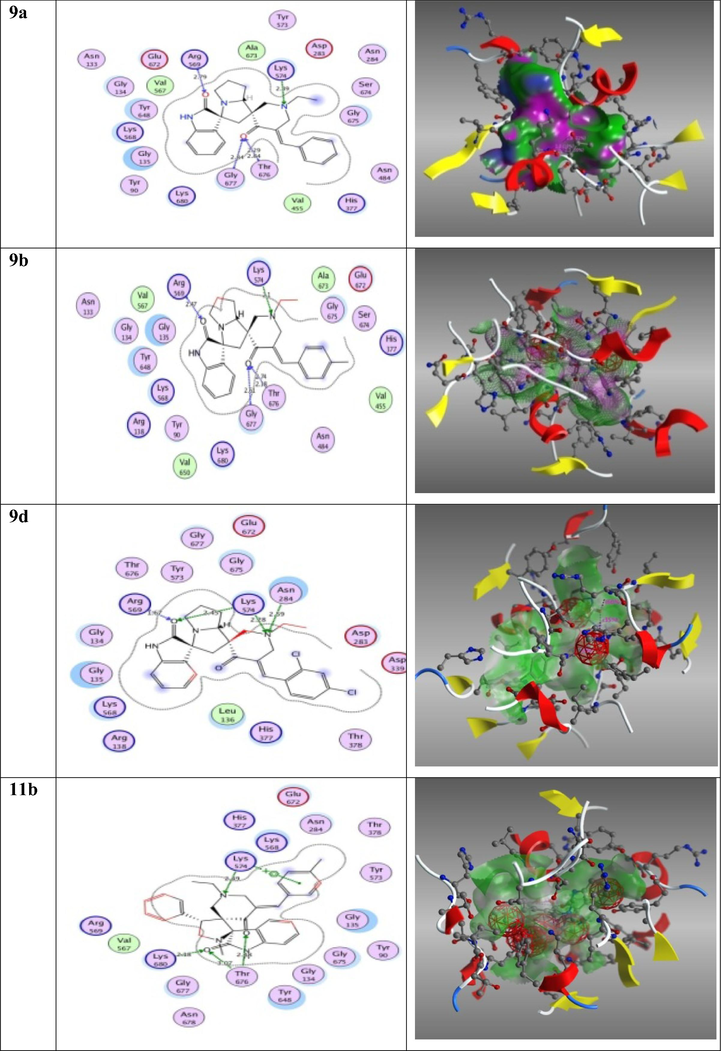
2D, 3D of the promising compounds against glycogen phosphorylase B (PDB code: 1H5U).
Compound
S (score)
9a
−10.61
9b
−12.37
9c
−12.41
9d
−12.40
11a
−8.79
11b
−6.14
11c
−9.96
11d
−12.00
3 Conclusion
Novel of spiropyrrolizidine / pyrrolidineoxindole moieties were synthesized under classical, ultrasonic, and microwave conditions in high yields. US method applied in methanol at 30 min and lower reaction temperature with high yield. In MW the reaction was completed in 10 min under solvent-free condition, resulting in high yield of synthesized compounds. Seven derivatives of the synthesized dispiro-pyrrolizidine-oxindoles and spiropyrrolidine were screened for their antitumor activity against two cell lines MCF-7 (breast cancer) and HEPG2 (liver cancer). The results of biological activity indicated that the tested derivatives have potent activity against breast cancer cell MF-6. Molecular docking simulation screening studies of the synthesized products with each of the receptors of (3hb5) for breast cancer and (4k9g) for liver cancer and their interaction with 1H5U of glycogen phosphorylase B, type 2 diabetes drug were examined. The docking study of dispiro-pyrrolizidine-oxindoles and spiropyrrolidine showed promising results with several derivatives.
4 Experimental section
4.1 Instrumentation
All instruments with their description were cited in supplementary file.
4.2 Classical heating
Under normal reflux, a mixture of 10 mmol of each of isatin, L-proline or sarcosine, and 5 mmol of 3,5-bis[arylmethylidene]-4-oxipiperidine-N-ethyl in 30 mL of methanol was heated (Table 1-3). When the reaction was completed by checking the progress of the reaction using TLC, the solid of 9a-e or 11a-e was collected and purified by recrystallization in ethanol. The physical and spectral data for 9a-e and 11a-e are listed below and their yields are tabulated in Table 1-3.
4.3 Ultrasound irradiation
Irradiation at 25–30 °C of a mixture of 1 mmol of each isatin, amino acids as L-proline, or sarcosine and 0.5 mmol of 3,5-bis[arylmethylidene]-4-oxipiperidine-N-ethyl in 10 mL for 30 min using ultrasound irradiation in a water-bath of an ultrasonic cleaner (to avert from the rising of the vapor pressure of methanol and to realize effective cavitation’s in this solvent, bath 50 kHz). The formed solid of 9a-e or 11a-e was filtered and recrystallized from ethanol. The physical and spectral data for 9a-e and 11a-e are listed below and their yields are tabulated in Table 1-3.
4.4 MW Irradiation
A 10 mL microwave vessel equipped with a standard cap (vessel commercially furnished by CEM Discover) was filled with isatin (2 equiv), the appropriate L-proline (2 equiv), or sarcosine (2 equiv) and 3,5-bis[arylmethylidene]-4-oxipiperidine-N-ethyl (1equiv) solvent-free at ambient temperature for 10 min, and the products 9a-e and 11a-e were separated in a pure form without further purification. The physical and spectral data for 9a-e and 11a-e are listed below and their yields are tabulated in Table 1-3.
4.4.1 5′'-benzylidene-1′'-ethyl-1′-phenyl-5′,6′,7′,7a'-tetrahydro-1′H-dispiro[indoline-3,3′-pyrrolizidine-2′,3′'-piperidine]-2,4′'-dione (9a)
M.p. 234–236 °C (Yellow crystals) IR (KBr) cm−1: 3400 (NH), 1720, 1712 (2CO);1H NMR (DMSO‑d6) δ 0.86 (CH2CH3, t, 3H, J = 7.2 Hz), 2.08 (2CH2, m, 4H), 2.13–2.16 (CH2, m, 2H), 2.14–2.20 (1Hb, m, 1H), 2.50 (1Ha, d, J = 10.2 Hz), 2.55–3.20 (m, 4H, 2CH2), 4.93 (CH2CH3, q, 2H, J = 7.2 Hz), 6.68 (Ar-H, d, J = 7.5 Hz, 2H), 6.94 (Ar-H, d, J = 7.5 Hz, 2H), 7.13–7.49 (Ar-H, m, 10H), 7.58 (=CH, s, 1H), 10.34 (NH, brs, 1H) ppm; 13C NMR (DMSO‑d6): δ 13.4 (CH3), 22.5, 26.4, 31.8, 52.7, 56.9, 60.1, 62.1, 68.2, 83.4, 114.5, 122.2, 126.1, 126.2, 127.9, 128.0, 128.6, 134.0, 134.9, 136.1, 137.5, 139.5, 152.5, 172.2 (C = O), 204.2 (C = O) ppm; MS, m/z (%): 503.26 (100.0%). Anal. calcd.(Found) for C33H33N3O2 (503.63): C, 78.70(78.83); H, 6.60(6.58); N, 8.34(8.23).
4.4.2 1′'-ethyl-5′'-(4-methylbenzylidene)-1′-(p-tolyl)-5′,6′,7′,7a'-tetrahydro-1′H-dispiro-[indoline-3,3′-pyrrolizidine-2′,3′'-piperidine]-2,4′'-dione (9b)
M.p. 249–251 °C (Yellow crystals) IR (KBr) cm−1: 3421 (NH), 1715, 1710 (2CO);1H NMR (DMSO‑d6) δ 0.86 (CH2CH3, t, 3H, J = 7.2 Hz), 1.08 (2CH2, m, 4H), 2.08 (2CH, m, 2H), 2.13 (1Hb, m, 1H), 2.31 (CH3, s, 3H), 2.42 (CH3, s, 3H), 2.55 (1Ha, d, J = 10.2 Hz), 2.57 (m, 4H, 2CH2), 4.93 (CH2CH3, q, 2H, J = 7.2 Hz), 6.57 (Ar-H, d, J = 7.5 Hz, 2H), 6.78 (Ar-H, d, J = 7.5 Hz, 2H), 7.09 (Ar-H, d, J = 7 Hz, 2H), 7.16 (Ar-H, d, J = 7.5 Hz, 2H), 7.21–7.48 (Ar-H, m, 4H), 7.58 (=CH, s, 1H), 10.34 (NH, s, 1H) ppm; MS, m/z (%): 531.29 (100.0%). Anal. calcd.(Found) for C35H37N3O2 (531.69): C, 79.06(79.13); H, 7.01(6.94); N, 7.90(7.87).
4.4.3 5′'-(4-chlorobenzylidene)-1′-(4-chlorophenyl)-1′'-ethyl-5′,6′,7′,7a'-tetrahydro-1′H-dispiro[indoline-3,3′-pyrrolizidine-2′,3′'-piperidine]-2,4′'-dione. (9c)
M.p. 230–233 °C (Yellow crystals) IR (KBr) cm−1: 3251 (NH), 1720, 1710 (2CO); 1H NMR (DMSO‑d6) δ 0.74 (CH2CH3, t, 3H, J = 7.2 Hz), 1.91 (2CH2, m, 4H), 2.00 (2 CH, m, 2H), 2.20 (1Hb, m, 1H), 3.29 (2CH2, m, 4H), 3.34 (CH2CH3, q, 2H, J = 7.2 Hz), 4.42 (1Ha, d, J = 10.2 Hz), 6.83–7.33 (Ar-H, m, 12H), 7.36 (=CH, s, 1H), 10.43 (NH, s, 1H) ppm; MS, m/z: 571.18 (100.0%). Anal. calcd.(Found) for C33H31Cl2N3O2 (572.52): C, 69.23(69.16); H, 5.46(5.43); N, 7.34(7.57).
4.4.4 5′'-(2,4-dichlorobenzylidene)-1′-(2,4-dichlorophenyl)-1′'-ethyl-5′,6′,7′,7a'-tetrahydro-1′H-dispiro[indoline-3,3′-pyrrolizidine-2′,3′'-piperidine]-2,4′'-dione. (9d)
M.p. over 300 °C (Yellow crystals) IR (KBr) cm−1: 3255 (NH), 1710, 1681 (2CO); 1H NMR (DMSO‑d6) δ 0.67 (CH2CH3, t, 3H, J = 7.2 Hz), 1.92 (2 CH2, m, 4H), 2.00 (2 CH, m, 2H), 2.20 (1Hb, m, 1H), 3.34 (2CH2, m, 4H), 3.77 (CH2CH3, q, 2H, J = 7.2 Hz), 4.82 (1Ha, d, J = 10.2 Hz), 6.64 (Ar-H, d, J = 7.5 Hz, 1H), 6.83 (Ar-H, d, J = 7.5 Hz, 1H), 7.07–7.67 (ArH, m, 8H), 7.95 (=CH, s, 1H), 10.59 (NH, s, 1H) ppm;; MS, m/z (%): 639.10 (78.2%). Anal. calcd. (Found) for C33H29Cl4N3O2 (641.41): C, 61.79(61.69); H, 4.56(4.41); N, 6.55(6.49).
4.4.5 1′'-Ethyl-5′'-(2-thienyl)-1′-(2-thienyl)-5′,6′,7′,7a'-tetrahydro-1′H-dispiro[indoline-3,3′-pyrrolizidine-2′,3′'-piperidine]-2,4′'-dione (9e)
M.p over 300 °C (Yellow crystals) IR (KBr) cm−1: 3253 (NH), 1701, 1678 (2CO); 1H NMR (DMSO‑d6) δ 1.11 (CH2CH3, t, 3H, J = 7.2 Hz), 1.64 (2 CH2, m, 4H), 2.18 (2 CH, m, 2H), 2.20 (1Hb, m, 1H), 2.40 (2CH2, m, 4H), 3.78 (CH2CH3, q, 2H, J = 7.2 Hz), 4.50 (1Ha, d, J = 10.2 Hz), 6.81–7.93 (Ar-H and thiophene-H, m, 10H), 7.81 (=CH, s, 1H), 10.45 (NH, s, 1H,) ppm; MS, m/z: 517 (M++2, 27%), 515 (M+, 49%). Anal. calcd. (Found) for C29H29N3O2S2 (515.69): C, 67.54(67.32); H, 5.67(5.45); N, 8.15(8.03).
4.4.6 5′'-Benzylidene-1′'-ethyl-1′-methyl-4′-phenyldispiro[indoline-3,2′-pyrrolidine-3′,3′'-piperidine]-2,4′'-dione. (11a)
M.p. 232–234 °C (Colourless crystals) IR (KBr) cm−1: 3253 (NH), 1720, 1713 (2CO); 1H NMR (DMSO‑d6): δ 0.74 (CH2CH3, t, 3H, J = 7 Hz), 1.96 (CH3, s, 3H), 2.82 (Hb, dd, 1H, J = 9.0, 7.0 Hz), 3.15 (2CH2, m, 4H), 3.30 (1Hc, dd, 1H, J = 10.7, 7.0 Hz), 3.78 (CH2CH3, q, 2H, J = 7.2 Hz), 4.60 (1Ha, dd, 1H, J = 10.7, 9.0 Hz), 6.55–7.17 (Ar-H, m, 14H), 7.20 (=CH, s, 1H), 10.27 (NH, s, 1H) ppm; 13C NMR (DMSO‑d6) δ 13.7, 29.8, 40.1, 53.3, 54.2, 56.9, 57.2, 64.2, 88.5, 114.5, 122.2, 125.7, 126.3, 126.4, 127.4, 127.8, 128.2, 128.6, 134.2, 135.4, 137.0, 139.5, 140.2, 152.9, 172.2, 204.2 ppm; MS, m/z (%):477.24 (100.0%). Anal. calcd.(Found) for C31H31N3O2 (477.60): C, 77.96(77.87); H, 6.54(6.42); N, 8.80(8.74).
4.4.7 1′'-Ethyl-1′-methyl-5′'-(4-methylbenzylidene)-4′-(p-tolyl)dispiro[indoline-3,2′-pyrrolidine-3′,3′'-piperidine]-2,4′'-dione.(11b)
M.p. 242–245 °C (Yellow crystals) IR (KBr) cm−1: 3251 (NH), 1720, 1712 (2CO); 1H NMR (DMSO‑d6): δ 0.76 (CH2CH3, t, 3H, J = 7 Hz), 1.95 (CH3, s, 3H), 2.70 (CH3, s, 3H), 2.92 (Hb, dd, 1H, J = 9.0, 7.0 Hz), 3.14 (2CH2, m, 4H), 3.17 (CH3, s, 3H), 3.28 (CH2CH3, q, 2H, J = 7.2 Hz), 3.89 (1Hc, dd, 1H, J = 10.7, 7.0 Hz), 4.62 (1Ha, dd, 1H, J = 10.7, 9.0 Hz), 6.57–7.16 (Ar-H, m, 12H), 7.19 (=CH, s, 1H), 10.27 (NH, s, 1H) ppm; MS, m/z (%):505 (7.3%), 506 (1.5%). Anal. calcd. (Found) for C33H35N3O2 (505.65): C, 78.38(78.29); H, 6.98(6.82); N, 8.31(8.20).
4.4.8 5′'-(4-chlorobenzylidene)-4′-(4-chlorophenyl)-1′'-ethyl-1′-methyldispiro[indoline-3,2′-pyrrolidine-3′,3′'-piperidine]-2,4′'-dione. (11c)
M.p. 253–255 °C (Yellow crystals) IR (KBr) cm−1: 3251 (NH), 1718, 1710 (2CO); 1H NMR (DMSO‑d6) δ 0.75 (CH2CH3, t, 3H, J = 7.2 Hz), 1.95 (CH3, s, 3H), 2.94 (Hb, dd, 1H, J = 9.0, 7.0 Hz), 3.14 (2CH2, m, 4H), 3.34 (1Hc, dd, 1H, J = 10.7, 7.0 Hz), 3. (CH2CH3, q, 2H, J = 7.2 Hz), 4.61 (1Ha, dd, 1H, J = 10.7, 9.0 Hz), 6.57 (Ar-H, d, J = 8 Hz, 2H), 6.84 (Ar-H, d, J = 8 Hz, 2H), 7.02–7.41 (Ar-H, m, 8H), 7.54 (=CH, s, 1H), 10.38 (NH, s, 1H) ppm; MS, m/z (%): 546.17 (33.5%). Anal. Calcd. (Found) for C31H29Cl2N3O2 (546.49): C, 68.13 (68.01); H, 5.35 (5.29); N, 7.69 (7.86).
4.4.9 5′'-(2,4-dichlorobenzylidene)-4′-(2,4-dichlorophenyl)-1′'-ethyl-1′-methyldispiro-[indoline-3,2′-pyrrolidine-3′,3′'-piperidine]-2,4′'-dione. (11d)
M.p. over 300 °C (Yellow crystals) IR (KBr) cm−1: 3340 (NH), 1716, 1710 (2CO); 1H NMR (DMSO‑d6): δ 0.69 (CH2CH3, t, 3H, J = 7.2 Hz), 1.93 (CH3, s, 3H), 2.51 (Hb, dd, 1H, J = 9.0, 7.0 Hz), 2.88 (2CH2, m, 4H), 2.94 (1Hc, dd, 1H, J = 10.7, 7.0 Hz), 3.77 (CH2CH3, q, 2H, J = 7.2 Hz), 4.96 (1Ha, dd, 1H, J = 10.7, 9.0 Hz), 6.65 (Ar-H, d, J = 7 Hz, 1H), 6.82 (Ar-H, d, J = 7 Hz, 1H), 7.07–7.87 (Ar-H, m, 8H), 7.99 (=CH, s, 1H), 10.55 (NH, s, 1H) ppm; MS, m/z (%):615.09 (4.3%). Anal. calcd. (Found) for C31H27Cl4N3O2 (615.38): C, 60.50 (60.78); H, 4.42 (4.31); N, 6.83 (6.70).
4.4.10 1′'-Ethyl-1′-methyl-5′'-(2-thienyl)-4′-(2-thienyl)-dispiro[indoline-3,2′-pyrrolidine-3′,3′'-piperidine]-2,4′'-dione (11e)
M.p. over 300 °C (Yellow crystals) IR (KBr) cm−1: 3400 (NH), 1720, 1716 (2CO); 1H NMR (DMSO‑d6): δ 0.86 (CH2CH3, t, 3H, J = 7.2 Hz), 1.94 (CH3, s, 3H), 2.72 (Hb, dd, 1H, J = 9.0, 7.0 Hz), 2.72 (2CH2, m, 4H), 3.26 (CH2CH3, q, 2H, J = 7.2 Hz), 3.68 (1Hc, dd, 1H, J = 10.7, 7.0 Hz), 4.82 (1Ha, dd, 1H, J = 10.7, 9.0 Hz), 6.61 (Ar-H, d, J = 7.5 Hz, 1H), 6.71–7.38 (Ar-H and thiophene-H, m, 7H), 7.46 (thiophene-H, d, J = 4.8 Hz, 1H), 7.62 (=CH, s, 1H), 7.83 (thiophene-H, d, J = 4.8 Hz, 1H), 10.40 (NH, s, 1H) ppm; MS, m/z (%):649 (64%). Anal. calcd. (Found) for C27H27N3O2S2 (489.65): C, 66.23 (66.07); H, 5.56 (5.38); N, 8.58 (8.61).
4.5 Antitumor assay
The method for antitumor assay were reported in the literature report (Mosmann, 1983).
4.6 Molecular docking studies
Molecular docking study was carried out for spiropyrrolizidines / pyrrolidineoxindoles 9a-e and 11a-e using MOE-Dock 2014 software (Inc, 2016). Chemical structures of spiropyrrolizidines / pyrrolidineoxindoles 9a-e and 11a-e were drawn by the MOE-builder and the program force field MMFF94x minimized them. After download the protiens 3hb5, 4k9g and 1H5U for breast cancer, liver cancer and type2 diabetes, respectively, all hydrogen atoms were aded followed by removal the water molecules. After that, docking of spiropyrrolizidines / pyrrolidineoxindoles 9a-e and 11a-e were done.
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4350477DSR04)
References
- The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J. Clin. Oncol.. 1988;6(11):1746-1752.
- [CrossRef] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L, Laversanne M., Soerjomataram I., Jemal A., Bray F. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin., 71(3), 209–249. doi: 10.3322/caac.21660.
- Diabetes, https://www.who.int/news-room/fact-sheets/detail/diabetes, (accessed 12 March 2021).
- Crystallographic studies on acyl ureas, a new class of glycogen phosphorylase inhibitors, as potential antidiabetic drugs. Protein Sci.. 2005;14(7):1760-1771.
- [Google Scholar]
- New inhibitors of glycogen phosphorylase as potential antidiabetic agents. Curr. Med. Chem.. 2008;15(28):2933-2983.
- [Google Scholar]
- Two new brominated tyrosine derivatives from the sponge Druinella (= Psammaplysilla) purpurea. J. Nat. Prod.. 1990;54:1137-1140.
- [Google Scholar]
- Hassaneen H. M. E., Eid E. M., Eid H. A., Farghaly T. A., Mabkhot Y. N. (2017) Facial Regioselective Synthesis of Novel Bioactive Spiropyrrolidine/Pyrrolizine-Oxindole Derivatives via a Three Components Reaction as Potential Antimicrobial Agents. Molecules, 22, 357; doi:10.3390/molecules22030357.
- Spiro[pyrrolidine-3, 3́-oxindole] as potent anti-breast cancer compounds: Their design, synthesis, biological evaluation and cellular target identification. Sci. Rep.. 2016;6:32213.
- [Google Scholar]
- Potent, selective spiropyrrolidine pyrimidinetrione inhibitors of MMP-13. Bioorg. Med. Chem. Lett.. 2007;17(23):6529-6534.
- [Google Scholar]
- Microwave heated flow synthesis of spiro-oxindole dihydroquinazolinone based IRAP inhibitors. Org. Process Res. Dev.. 2014;18(11):1582-1588.
- [Google Scholar]
- Facile, regio-and diastereoselective synthesis of spiro-pyrrolidine and pyrrolizine derivatives and evaluation of their antiproliferative activities. Molecules. 2014;19(7):10033-10055.
- [Google Scholar]
- New approaches for the synthesis of pyrazole, thiophene, thieno[2,3-b]pyridine, and thiazole derivatives together with their anti-tumor evaluations. Med. Chem. Res.. 2014;23:564-579.
- [Google Scholar]
- Discovery of novel oxindole derivatives as potent α-glucosidase inhibitors. Bioorg. Med. Chem.. 2014;22(13):3441-3448.
- [Google Scholar]
- Cholinesterase inhibitory activity of highly functionalized fluorinated spiropyrrolidine heterocyclic hybrids. Saudi J. Biol. Sci.. 2021;28(1):754-761.
- [Google Scholar]
- Antimycobacterial activity: A facile three-component [3+ 2]-cycloaddition for the regioselective synthesis of highly functionalised dispiropyrrolidines. Bioorg. Med. Chem. Lett.. 2012;22(15):4930-4933.
- [Google Scholar]
- Synthesis and antitumor activity evaluation of regioselective spiro [pyrrolidine-2, 3'-oxindole] compounds. Heterocycl. Commun.. 2009;15(5):355-360.
- [Google Scholar]
- Diversity-oriented sustainable synthesis of antimicrobial spiropyrrolidine/thiapyrrolizidine oxindole derivatives: New ligands for a metallo-β-lactamase from Klebsiella pneumonia. Bioorg. Med. Chem. Lett.. 2017;27(13):2873-2880.
- [Google Scholar]
- Spiropyrrolidine/spiroindolizino [6, 7-b] indole heterocyclic hybrids: stereoselective synthesis, cholinesterase inhibitory activity and their molecular docking study. Bioorg. Chem.. 2018;79:64-71.
- [Google Scholar]
- Synthesis of novel 3, 5-disubstituted-2-oxindole derivatives as antitumor agents against human nonsmall cell lung cancer. ACS Med. Chem. Lett.. 2013;4(12):1137-1141.
- [Google Scholar]
- Synthesis and antitumor evaluation of some novel pyrrolizine derivatives. Med. Chem. Res.. 2012;21(9):2349-2362.
- [Google Scholar]
- A facile access to novel spiroxindole fused pyrrolidine and thiazolo pyrrolidine benzimidazole derivatives via 1, 3-dipolar cycloaddition reaction. Tetrahedron Lett.. 2015;56(5):721-726.
- [Google Scholar]
- An easy access to novel sugar-based spirooxindole-pyrrolidines or-pyrrolizidines through [3+ 2] cycloaddition of azomethine ylides. Synthesis. 2011;2011(15):2495-2504.
- [Google Scholar]
- Synthesis of novel spiropyrrolidine/pyrrolizine-oxindole scaffolds through 1, 3-dipolar cycloadditions. Tetrahedron Lett.. 2009;50(33):4724-4726.
- [Google Scholar]
- Organocatalytic synthesis of enantiopure spiro acenaphthyl-pyrrolizidine/pyrrolidines: justifying the regioselectivity based on a distortion/interaction model. Org. Biomol. Chem.. 2019;17(29):7013-7024.
- [Google Scholar]
- [3+2] Cycloaddition Reaction for the Stereoselective Synthesis of a New Spirooxindole Compound Grafted Imidazo[2,1-b]thiazole Scaffold: Crystal Structure and Computational Study. Crystals. 2022;12(1):5.
- [CrossRef] [Google Scholar]
- Regio- and Stereoselective Synthesis of a New Series of Spirooxindole Pyrrolidine Grafted Thiochromene Scaffolds as Potential Anticancer Agents. Symmetry. 2021;13(8):1426.
- [CrossRef] [Google Scholar]
- Synthesis of Spirooxindole Analogs Tethered Pyrazole Scaffold as Acetylcholinesterase Inhibitors. Chem. Select. 2021;6:14039-14053.
- [Google Scholar]
- Barakat A., Haukka M., Soliman S. M., Ali M., Al-Majid A. M. , El-Faham A., Domingo L. R. (2021). Straightforward Regio- and Diastereoselective Synthesis, Molecular Structure, Intermolecular Interactions and Mechanistic Study of Spirooxindole-Engrafted Rhodanine Analogs. Molecules, 26(23), 7276; https://doi.org/10.390/molecules26237276.
- Stereoselective Synthesis of the Di-Spirooxindole Analogs Based Oxindole and Cyclohexanone Moieties as Potential Anticancer Agents. Molecules. 2021;26(20):6305.
- [CrossRef] [Google Scholar]
- Regio- and stereoselective synthesis of spiro-heterocycles bearing the pyrazole scaffold via [3+2] cycloaddition reaction. J. Mol. Struct.. 2022;1250:131711
- [Google Scholar]
- Islam, M. S., Al-Majid, A. M., Azam, M., Verma, V. P., Barakat, A., Haukka, M., Elgazar, A. A, Mira, A., Badria, F. A. (2021). Construction of Spirooxindole Analogues Engrafted with Indole and Pyrazole Scaffolds as Acetylcholinesterase Inhibitors. ACS Omega, 12; 6(47): 31539-31556. doi: 10.1021/acsomega.1c03978.
- Synthesis of pyrrolizidines and indolizidines by multicomponent 1, 3-dipolar cycloaddition of azomethine ylides. Pure Appl. Chem.. 2019;91(4):575-596.
- [Google Scholar]
- Regio-and diastereoselective construction of a new set of functionalized pyrrolidine, spiropyrrolidine and spiropyrrolizidine scaffolds appended with aryl-and heteroaryl moieties via the azomethine ylide cycloadditions. Tetrahedron. 2016;72(36):5578-5594.
- [Google Scholar]
- An efficient ultrasound-promoted one pot synthesis of spiroacenaphthylene pyrazolotriazole and pyrazolophthalazine derivatives. Tetrahedron Lett.. 2012;53(38):5123-5126.
- [Google Scholar]
- A facile synthesis of novel pyrrolizidines under classical and ultrasonic conditions. Ultrason. Sonochem.. 2008;15(2):124-128.
- [Google Scholar]
- Ultrasound-assisted facile one-pot sequential synthesis of novel sulfonamide-isoxazoles using cerium (IV) ammonium nitrate (CAN) as an efficient oxidant in aqueous medium. Ultrason. Sonochem.. 2018;40:289-297.
- [Google Scholar]
- Chapter 3 - Sonochemical protocol for stereoselective organic synthesis. In: Green Sustainable Process for Chemical and Environmental Engineering and Science Sonochemical Organic Synthesis. 2020. p. :71-93.
- [Google Scholar]
- Unexpected One-pot Synthesis of Novel 2-Aminopyrimidine-4-ones under Microwave Irradiation. J. Chin. Chem. Soc.. 2015;62(2):107-111.
- [Google Scholar]
- Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev.. 2005;34(2):164-178.
- [Google Scholar]
- Pyrazole synthesis under microwave irradiation and solvent-free conditions. J. Braz. Chem. Soc.. 2010;21:1037-1044.
- [Google Scholar]
- New potentially active pyrazinamide derivatives synthesized under microwave conditions. Molecules. 2014;19(7):9318-9338.
- [Google Scholar]
- Synthesis of novel fungicidal organomercurials using microwaves. Monatshefte für Chemie/Chemical Monthly. 1997;128(12):1291-1295.
- [Google Scholar]
- Chapter 8 - Microwave-assisted stereoselective organic synthesis Green Sustainable Process for. In: Chemical and Environmental Engineering and Science Microwaves in Organic Synthesis. 2021. p. :331-357.
- [Google Scholar]
- Dandia, A., Gupta, S. L., Sharma, R., Saini, P., Parewa, V., (2021) Chapter 13 - Microwave-assisted catalyst-free organic synthesis Green Sustainable Process for Chemical and Environmental Engineering and Science Microwaves in Organic Synthesis, Pages 539–622.
- Site-specific role of bifunctional graphitic carbon nitride catalyst for the sustainable synthesis of 3,3-spirocyclic oxindoles in aqueous media. RSC Adv.. 2021;11:28452-28465.
- [Google Scholar]
- Step-economic, efficient, ZnS nanoparticle-catalyzed synthesis of spirooxindole derivatives in aqueous medium viaKnoevenagel condensation followed by Michael addition. Green Chem.. 2011;13:2135-2145.
- [Google Scholar]
- Microwaves assisted synthesis of antitumor agents of novel azoles, azines, and azoloazines pendant to phenyl sulfone moiety and molecular docking for VEGFR-2 kinase. J. Mol. Struct.. 2022;1249:131657
- [Google Scholar]
- Cytotoxicity, Docking Study of New Fluorinated Fused Pyrimidine Scaffold: Thermal and Microwave Irradiation Synthesis. Med. Chem.. 2021;17(5):501-518.
- [Google Scholar]
- Comparative Study Between Thermal Heating and Microwave-Assisted Synthesis for New Series of Phenothiazine Derivatives. Russ. J. Org. Chem.. 2019;55(9):1407-1415.
- [Google Scholar]
- Multicomponent reactions for synthesis of bioactive polyheterocyclic ring systems under controlled microwave irradiation. Arabian J. Chem.. 2014;7(5):623-629.
- [Google Scholar]
- Microwave assisted synthesis of annelated benzosuberone as new penta-heterocyclic ring systems. Arkivoc. 2009;10:53-64.
- [Google Scholar]
- Molecular Operating Environment (MOE) 2014.09, Chemical Computing Group Inc., 1010 Sherbrooke Street West, Suite 910, Montréal, H3A 2R7, Canada, http://www.chemcomp.com.
- A Prospect for Pyrrolidine Iminosugars as Antidiabetic α–Glucosidase Inhibitors. J. Med. Chem.. 2012;55:10345-10346.
- [Google Scholar]
- Antidiabetic Activity of Medicinal Plants. Int. J. Pharm. Sci. Rev. Res.. 2018;51(1):151-165.
- [Google Scholar]
- Research progress of indole compounds with potential antidiabetic activity. Eur. J. Med. Chem.. 2021;223:113665
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55-63.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103930.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







