Translate this page into:
Nutritional content and the activities of l-Dopa (L-3,4-dihydoxyphenyalanine) from Mucuna pruriens L. DC seeds of Central Java accession
⁎Corresponding author. ukun@unpad.ac.id (Ukun M.S. Soedjanaatmadja)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mucuna pruriens (L.) DC (Velvet beans) is a tropical bean containing large amounts of l-Dopa (L-3,4-dihydroxyphenylalanine) and is the most important natural remedy for Parkinson’s disease. This plant has been used in Ayurveda medicine because it has a pharmacological activity, one of which is an antibacterial activity obtained from its seed extract. M. pruriens seeds fulfill the basic nutritional requirements, so they can be consumed. However, their utilization as food is limited due to the presence of some anti-nutritional factors. The objective of the research was to determine the nutritional content of seeds of M. pruriens Central Java accession by proximate analysis, isolation, and characterization of l-Dopa, determine the toxicity value ( LC50), against Artemia salina L. larvae by BSLT (brine shrimp lethality test), and determine its antibacterial activity against Streptococcus mutans ATCC 35668, and Escherichia coli ATCC 25,922 by agar disk diffusion method. The isolate of l-Dopa was prepared by maceration using methanol 80%, followed by extraction using water, then, the isolated product was analyzed by analytical TLC to determine the suitable mobile phase. The sample was then run on preparative TLC. The spot that corresponds to the l-Dopa standard was collected and further purified using adsorption column chromatography. Then, the isolate was characterized by HPLC and FTIR. The antioxidant activity was determined by the DPPH radical method. Finally, anti-Parkinson's activity was determined to the rotenone-induced behavior of white rats (Rattus norvegicus) by the classic labyrinth test method. The result showed that the strongest antibacterial activity against S. mutans ATCC 35,668 was shown by crude extract. Based on the HPLC analysis, the isolated l-Dopa showed a retention time of 5.345 min that is relatively similar to the l-Dopa standard and had a high purity. l-Dopa content in M. pruriens seed of Central Java accession was 0.49 mg/g dry weight. The l-Dopa isolate from M. pruriens Central Java accession has antioxidant activity with IC50 of 8.92 ± 0.03 ppm (highly active) and could significantly reduce the non-motoric Parkinsonism symptoms (p < 0.001) compared to the negative control.
Keywords
Antibacterial
Antioxidant
Anti-Parkinson
l-Dopa
M. pruriens (L.) DC
1 Introduction
Mucuna pruriens (L.) DC (Velvet beans) is a tropical bean containing large amounts of l-Dopa and is the most important natural remedy for Parkinson’s disease (Maldonado, 2018). M. pruriens are legumes that belong to the Fabaceae family. This plant is widely distributed in tropical and subtropical regions of the world,easily found in Southeast Asia, including Indonesia (Yadav et al., 2017; Pangestiningsih et al., 2017). M. pruriens seeds are quite popular in Central and East Java and have been traditionally used in the form of fermented foods, known as “Tempe Benguk” (Gandjar et al., 1973). M. pruriens is an edible legume because it fulfills the basic nutritional requirements rich in protein, essential minerals, unsaturated fatty acids, and vitamins (Bhat et al., 2008). Based on the proximate analysis, M. pruriens seeds from Indonesia, especially from the Yogyakarta area, contain proteins, water, ash, lipids, and carbohydrates, reaching 25.2, 12.9, 3.81, 2.44, and 55.7%, respectively (Susanti et al., 2014). Even so, the use of M. pruriens seeds in the food sector is still limited due to some anti-nutritional factors like free phenol, tannins, protease inhibitor, and hydrogen cyanide that can reduce its utilization (Daffodil et al., 2016). Mucuna seeds are benefificial, since seeds are high in levodopa (l-Dopa) which is used to maintain healthy cholesterol and blood sugar levels. The seeds of kewachh are used in traditional, Ayurvedic and Indian system of medicine for many diseases including Parkinsonism (Khanuja et al., 2007). It is also used as a cover crop for tropical areas, and in some parts of India green bean seeds are used as a vegetable (Lal, 2015).
Consumption of anti-nutritional factors excessively can cause inhibition of the action of enzymes that play a role in the digestion system. And also can reduce the availability of vitamins and minerals, damage the digestive tract mucosa, damage the integrity of the intestinal epithelium thereby altering nutrient absorption and utilization, hallucinations, nausea, poisoning, etc. (Mohan et al., 2016). One of the efforts to it can be determined by studying the concentration or dose that can cause toxicity effects. Therefore, it is necessary to know the data regarding the toxicity value of M. pruriens seeds.
The determination of toxicity can be performed by the brine shrimp lethality test (BSLT) method. This method is one of the initial screening methods for the evaluation of toxicity, especially the toxicity of plant extracts (Wu, 2014). The BSLT method is based on the mortality rate of A. salina L. shrimp larvae caused by the test extract. The results are calculated as the value of LC50 (lethal concentration), namely the concentration of the test extract that can cause the death of shrimp larvae in the amount of 50% of the population after a 24 h incubation period (Lisdawati et al., 2006).
Apart from being a food ingredient, M. pruriens has pharmacological benefits such as antimicrobials (Lampariello et al., 2012). Plant-derived antimicrobials have enormous therapeutic potential; they are effective in treating of infectious diseases (Iwu et al., 1999). One of the most common infectious diseases that infect humans is urinary tract infection (UTI) caused by E. coli (Sharma et al., 2009). Also, the bacteria S. mutans plays a role in the formation of biofilms on the teeth surface and gums, known as dental caries, where this condition can lead to infection of the oral cavity (Krzyściak et al., 2014). Dhawan et al. (2011) had already done of comparative genetic analysis of trichome-less and normal pod genotypes of Mucuna pruriens (Fabaceae).
Based on the explanation above, the present study will investigate the nutritional value of the seeds of M. pruriens Central Java accession, toxicity test using the BSLT method, antibacterial activity with agar disc diffusion method, the l-Dopa content, and anti-Parkinson activity of l-Dopa isolate.
2 Materials and methods
2.1 Materials
M. pruriens seeds were purchased from M. pruriens farmers in Wonogiri, Central Java, Indonesia. A. salina L. larvae eggs (Supreme Plus: Golden West Artemia) were purchased from Golden Westindo Artajaya Company. S. mutans ATCC 35,668 was purchased from the Microbiology Laboratory, Department of Pharmacy, Universitas Padjadjaran. E. coli ATCC 25,922 was purchased from Microbiology Laboratory, Biology Department of Padjadjaran University. Levodopa (l-Dopa) standard was purchased from Sigma–Aldrich, Co, St. Louis USA. Chloramphenicol was purchased from PT Bernofarm Indonesia. All the chemicals used in this study are pro-analytical grade. The instruments used in this study were glass tools, Kjeldahl distillation apparatus, Soxhltation apparatus, thin layer chromatography silica gel 60 F254 (Merck), rotary vacuum evaporator (Buchi), HPLC (Allech 8011/2), glass column, C-18 nucleosil ODS column, and furnace (Thermolyne).
2.2 Proximate analysis
Proximate analysis of M. pruriens seeds is intended to determine the nutrient content in the seeds. This analysis includes the determination of protein, fat, ash, moisture, and carbohydrate content. Protein content was determined by the Kjeldahl method according to AOAC 955.04C with a correction factor of 6.25 (AOAC, 1990). Determination of fat content is determined by continuous extraction to use the Soxhlet apparatus with n-hexane as a solvent for 6 h at 80°C. The ash content was determined by the gravimetric method according to AOAC 923.03 and the water content according to AOAC 925.09 (AOAC, 1990). Then, the carbohydrate content is determined by the difference method.
2.3 Preparation and extraction of active compounds
The extraction of active compounds from M. pruriens seeds was adapted from the procedures performed by Eze & Ndukwe (2012) and Johnson et al. (2018). M. pruriens seeds powder was weighed as much as 200 g and macerated with 800 mL of methanol 80% (1:4 w/v) for 4 × 24 h using a separating funnel at room temperature. Then, the methanol extract of M. pruriens seeds was filtered using Whatman No. 1 filter paper. Then, the methanol extract was concentrated using a vacuum rotary evaporator at 40°C.
The concentrated methanol extract was weighed as much as 6.5 g and added with 250 mL of distilled water. After that, the extract was partitioned with ethyl acetate (3 × 250 mL) in a separating funnel. Then, the ethyl acetate fraction and the water fraction were separated. Furthermore, each fraction was concentrated using a rotary evaporator vacuum at a temperature of 40°C (for the ethyl acetate fraction) and 60–70°C (for the water fraction). Each concentrated extract was stored at 5°C in a tightly closed container until it was used for toxicity and antibacterial activity assay.
2.4 Brine shrimp lethality test (BSLT)
Toxicity of M. pruriens seed extract was performed according to the protocol described by Meyer et al. (1982), which was carried out on A. salina L. larvae. The test extract was diluted into various concentrations according to Weli et al. (2014) which was diluted with DMSO to reach concentrations of 1,000; 500; 250; 100; 10; and 0 ppm. The toxicity value was determined as LC50.
A. salina L. eggs were hatched in a saline solution (2% sodium chloride) in a large beaker glass. A saline solution is made by adding 2 g of sodium chloride and adds with water to give a final volume of 100 mL. Then, A. salina L. eggs were weighed out 50 mg and put into the saline solution that had been prepared. After 48 h, A. salina L. eggs were hatched and collected for the toxicity test. The shrimp larvae of A. salina L. were transferred using a pipette from the hatching medium into 5 mL of saline solution in the vial bottle, each vial contained ten A. salina L. larvae. Then, the various concentration of test extract (1,000; 500; 250; 100; 10; and 0 ppm) was added to the vial bottle containing the larvae of A. salina L. each as much as 50 μL and allowed to stand for 24 h. DMSO solvent without test extract was used as a negative control. After that, the mortality of A. salina L. was observed after 24 h. A. salina L. larvae mortality was calculated with a magnifying glass and the toxicity value was determined as the LC50 value by probit analysis. Then, the toxicity of the sample was valorized by comparison to Clarkson’s toxicity criteria. Clarkson’s toxicity criteria are shown in Table 1.
(LC50) ppm
Toxicity Criteria
0–100
Highly toxic
100–500
Medium toxic
500–1,000
Low toxic
>1,000
Non-toxic
The corrected mortality percentage was determined based on the Abbott formula as used by Kariuki et al. (2016) in the following equation:
2.5 Determination of antibacterial activity by agar disk-diffusion method
The antibacterial activity test was carried out on Gram-positive bacteria (S. mutans ATCC 35668) and Gram-negative bacteria (E. coli ATCC 25922). The test extract was diluted into various concentrations, which is diluted with DMSO to reach concentrations of 2,000; 1,000; 500; 250; and 0 ppm (Weli et al., 2014). The positive and negative controls used in this study were chloramphenicol and DMSO.
Inoculation or rejuvenation of bacteria begins with taking the test bacteria using sterile ose, and then the bacteria are implanted on the slant medium (nutrient agar, NA) using scratching. Furthermore, the media containing the test bacteria was incubated at 37°C for 24 h. Then, the test bacteria that have been inoculated on agar slant media was suspended into a tube containing 2 mL of 0.9% sodium chloride solution (0.18 g of sodium chloride dissolved with distilled water up to 20 mL) and the turbidity was compared to the McFarland standard 1.0.
The bacterial suspension (1 mL) was transferred into sterile Petri dishes aseptically. Then, the liquid agar medium (nutrient agar, NA) was added to the bacterial suspension in the petri dish aseptically, up to 0.5 cm high. Furthermore, homogenization of the agar medium with a bacterial suspension was carried out by rotating the Petri dishes in a figure-eight pattern on a flat plane (about ten times) and allowed to stand until solidified. Next, disc paper that had been immersed in the test extract and control for 24 h at 37°C was transferred to the surface so that the NA was aseptically solid, and closed tightly. After that, the incubation was carried out at 37°C for 24 h and the formed inhibition zone was measured.
2.6 Liquid-liquid extraction for isolation of l-Dopa
The concentrated methanol extract was weighed as much as 6.5 g and added with 250 mL of distilled water. After that, the extract was partitioned with ethyl acetate (3 × 250 mL) in a separating funnel. Then, the ethyl acetate fraction and the water fraction were separated. Furthermore, water fraction was concentrated using a rotary evaporator vacuum at a temperature of 60–70°C.
2.7 Analytical thin layer chromatography of isolate
Analytical thin-layer chromatography was performed using aluminum plates coated with silica 60 GF-254. The plate was eluted with an isocratic mobile phase of butanol: acetic acid: water (6: 1: 2 v/v) until the eluent move to the upper limit. The result was air-dried and sprayed with ninhydrin reagent (100 mg of ninhydrin in 100 mL of methanol). The Rf value was determined.
2.8 Adsorption column chromatography of isolate
The 0.5 g of concentrated water fraction was impregnated with impreg silica gel (1:1 w/w) and added to the G-6- silica gel column then eluted with isocratic mobile phase butanol: acetic acid: water (6: 1: 2 v/v) Fraction of 1 mL was collected. The fraction was analyzed by analytical thin-layer chromatography and the Rf value of each fraction was compared to the Rf value of the l-Dopa standard. The same Rf with the standard were combined.
2.9 Preparative thin layer chromatography isolate
As much as 1 mL of the collected fractions were spotted on a glass plate (20 × 20 cm) that has been collected with silica gel GF-254 and 100 ppm l-Dopa was used as standard. The glass plate was eluted with isocratic mobile phase consisted of butanol: acetic acid: water (6: 1: 2 v/v) stains with the same Rf with the standard are scraped off and suspended in methanol and centrifuge. The supernatant was decanted and concentrated by a rotary vacuum evaporator. Then, the residues were dissolved in 1 mL methanol: water (1:1 v/v).
2.10 High-performance liquid chromatography condition of l-Dopa isolate
The condition is adapted and modified from Elbarbry et al. (2019). The Alltech 8011/2 reversed-phase consisting of an auto-sampler, a degasser, a binary pump, a UV and fluorescence detector. The chromatographic analysis was performed using the C-18 Nucleosil ODS column with a UV detector at λ = 230 nm. The isocratic mobile phase consisted of 20 mM potassium dihydrogen phosphate (pH 2.5) and methanol HPLC grades (97:3 v/v) was run at a flow rate of 1 mL/min for 10 min.
2.11 Fourier transform infrared (FTIR) of l-Dopa isolate
Identification of l-Dopa isolate was done by methods that have been developed as tools for the determination of organic components, including chemical bonds, functional groups, and organic content. IR radiation can impact the atomic vibration of a molecule in a sample, resulting in the absorption and/or transmission of certain energies. This makes FTIR useful for determining the vibration of specific molecules contained in the sample (Nandiyanto et al., 2019).
2.12 Determination of antioxidant activity of l-Dopa isolate
In vitro DPPH assay was done to estimate the antioxidant activity of the l-Dopa isolate and crude extract of M. pruriens seed using the standard method adapted to the research of Sreekala et al. (2020). A solution of 0.25 mM DPPH was prepared and 1 mL of this solution was added to 1 mL of five different concentrations 10, 15, 25, and 30 ppm of the l-Dopa isolate (and different concentrations of crude extract), and 1 mL of methanol. The reaction mixture was then incubated at room temperature in a dark chamber for 30 min. The change in colour from deep violet to light yellow was noted and the absorbance of the mixture was measured at 517 nm using ultra violet spectrophotometer. Ascorbic acid was used as reference and DPPH solution in methanol was taken as the control. Estimation of the ability of test drug to scavenge DPPH free radical was calculated using the following formula;
The percentage of DPPH radical scavenging activity = [(Abs control) – (Abs sample)]/(Abs control) × 100%.
2.13 Determination of anti-Parkinson activity of isolated l-Dopa using classical labyrinth test
The procedure of anti-Parkinson test activity of isolated l-Dopa was adapted the experimental protocol carried out by Gasmi (2018). The Classic Labyrinth Test (CLT) is a simple way to evaluate behaviors in rodents (white Wistar) such as learning ability, memory, and anxiety. In short, the CLT uses a square-shaped maze with a starting point and a stopping point. After the animal is trained, the animal is allowed to view and explore the labyrinth freely for 10 min. During this time, all of the animal's vertical and horizontal movements within the labyrinth are recorded. The CLT is performed in a square-shaped plastic enclosure (125 × 125 × 40 cm) with several labyrinth passages of identical width and height (25 × 35 cm), but with variable length. Usually, this labyrinth is placed on a table 90 cm high. Control rats can quickly move through the labyrinth between the starting and the ending point. When the animal explores the maze, it increases the time it spends in the maze's passages, which will be considered as aversive or anxiety-provoking for the stressed animal; while the leak behaviour will be observed when the animal spends more time in the starting point or corners, which will be associated with a refuge (Kracuter et al. 2019; Gasmi et al., 2017).
2.14 Data analysis
This study was conducted in three repetitions. The determination of significance and data grouping used Fisher's One Way- ANOVA method in Minitab 19 software.
3 Results and discussions
3.1 Proximate analysis of Central Java M. Pruriens seeds
The seeds of Central Java M. pruriens have a shape close to a square with an average thickness of 0.5 cm; light skin color (white); average length reaches 1.7 cm, and the average width reaches 1.2 cm. (Fig. 1).
M. pruriens seeds of Central Java accession.
Proximate analysis was carried out on whole M. pruriens seeds of Central Java accession. Obtained contents of protein, fat, ash, water, and carbohydrates are expressed in units of a percent (%). Protein content; fat; ash; water; and carbohydrates in the Central Java M. pruriens seeds, were 31.79 ± 1.64 %; 4.52 ± 0.12%; 3.38 ± 0.16%; 5.56 ± 0.21%; and 54.75 ± 1.71%. The proteins and fats content of Central Java M. pruriens seeds are lower than that contained in soybeans. However, the seeds of Central Java M. pruriens have a higher carbohydrate content than the carbohydrate content in soybeans. These results are in line with the results of the proximate analysis conducted by Susanti et al. (2014) on the seeds of Yogyakarta M. pruriens, where the nutritional content is shown in Table 2. Based on this proximate analysis, M. pruriens seeds have the potential to become an alternative source of vegetable proteins. Note: *present study, the data presented is the result of three repetitions.
Component
Variety
Central Java M. pruriens seeds*
Yogyakarta M. pruriens seeds (Susanti et al., 2014)
Soybean (Glycine max (L.) Merr) (Alghamdi et al., 2018)
Protein (%)
31.79 ± 1.64
25.2
35.63–43.13
Fat (%)
4.52 ± 0.12
2.44
16.92–22.94
Ash (%)
3.38 ± 0.16
3.81
4.55–6.28
Water (%)
5.56 ± 0.21
12.9
3.08–5.88
Carbohydrate (%)
54.75 ± 1.71
55.7
26.11–33.18
3.2 The toxicity (LC50) of Central Java M. Pruriens seeds
The toxicity of Central Java M. pruriens seeds was determined by the BSLT (Brine Shrimp Lethality Test) method. The BSLT method is a simple bioassay for the toxicity test of natural products (Meyer et al., 1982). The toxicity value obtained in this study is expressed as LC50. The LC50 value is determined through probit analysis. The LC50 values of the methanol extract, water fraction, and ethyl acetate fraction of M. pruriens seeds are shown in Table 3. The Negative control (DMSO without the test extract) showed 20% mortality. Note: The data presented is the result of three repetitions. Means with different superscript letters differ significantly according to LSD Fisher's post hoc test.
Sample
LC50 value (ppm)
Toxicity Criteria
Methanol extract
144.30 ± 32.8a
Medium toxic
Water fraction
130.97 ± 30.6a
Medium toxic
Ethyl acetate fraction
27.85 ± 17.9b
Highly toxic
The result of research showed that the LC50 of the methanol, water, and ethyl acetate extract (Table 3), and based on the Clarkson toxicity criteria, the methanol extract and water fractions were included in the “Medium toxic” criteria. Meanwhile, ethyl acetate fraction is included in the criteria for “Highly toxic”. In a previous study, the LC50 was also determined for the methanol extract of Bangladesh M. pruriens seeds and the LC50 value obtained (Chhabi et al., 2017). These results are similar to the LC50 values of methanol extract obtained in this study.
The difference in LC-50 values obtained from the methanol extract, water fraction, and ethyl acetate fraction of Central Java M. pruriens seeds can be influenced by different active compounds contained in each extract. This study showed that the ethyl acetate fraction was highly toxic. According to Jhariya & Kakkar (2016), ethyl acetate fraction of the M. pruriens seeds identified containing pyrrolidine, 1-(1-oxo-7, 10-Hexadecadienyl), which have the biological activity of cytotoxic. Therefore, this fraction is highly toxic. Also, this study showed that the total carbohydrate content of M. pruriens seeds was > 50 % (Table 2), some of which are known to be cyanogenic glycosides. This cyanogenic glycoside is a plant poison that releases cyanogenic hydrogen when hydrolized. Hydrogen cyanide is known to cause acute and chronic toxicity (Lampariello et al., 2012).
3.3 Antibacterial activity of Central Java M. Pruriens seeds against S. Mutans and E. Coli
The antibacterial activity of the seeds of Central Java M. pruriens was determined by the disc diffusion method against S. mutans ATCC 35,668 and E. coli ATCC 25922. The data from the results of the antibacterial activity test are shown in Table 4. Based on the results of this study, methanol extract has significant antibacterial activity against S. mutans and is not significantly different against E. coli. This is indicated by the p-value obtained from the methanol extract for S. mutans which is 0.030 (p < 0.05) while for E. coli is 0.177 (p > 0.05). The strongest antibacterial activity against S. mutans was shown at a concentration of 500 ppm with an inhibition zone of 11.97 ± 2.67 mm and against E. coli at the same concentration as an inhibition zone of 13.03 ± 2.92 mm. Note: The data presented are means of three repetitions. means with different superscript letters are significantly different according to LSD Fisher post hoc test with α = 0.05.
Test Sample
Concentration (ppm)
Inhibition Zone (mm)
S. mutans ATCC 35,668
E. coli ATCC 25,922
Positive control (Chloramphenicol)
10
10.25 ± 0.64
10.14 ± 0.39
Methanol extract
0
8.37 ± 0.64b
8.63 ± 1.12b
250
10.73 ± 0.53a,b
11.45 ± 0.44a,b
500
11.97 ± 2.67a
13.03 ± 2.92a
1,000
13.78 ± 1.81a
12.70 ± 2.86a
2,000
11.82 ± 1.73a
11.70 ± 2.23a,b
p-value
0.030
0.177
Water fraction
0
8.43 ± 0.39c
8.32 ± 0.48b
250
10.08 ± 0.14a,b
10.13 ± 1.59a
500
10.45 ± 0.18a
10.85 ± 1.03a
1,000
9.88 ± 0.44b
9.90 ± 0.13a,b
2,000
10.08 ± 0.14a,b
9.25 ± 0.22a,b
p-value
0.000
0.048
Ethyl acetate fraction
0
9.22 ± 0.20c
8.88 ± 0.41c
250
10.65 ± 0.79a
10.98 ± 0.55a,b
500
9.70 ± 0.30b,c
10.18 ± 0.65b
1,000
10.25 ± 0.44a,b
11.80 ± 1.06a
2,000
10.50 ± 0.23a,b
10.22 ± 0.61b
p-value
0.014
0.005
The antibacterial activity of the methanol extract against S. mutans at a concentration of 1,000 ppm showed a larger inhibition zone than 500 ppm, 13.78 ± 1.81 mm. However, based on Fisher's One-way ANOVA analysis, the zone of inhibition at 1,000 ppm did not significantly differ from the inhibition zone obtained at a concentration of 500 ppm, so that a concentration of 500 ppm could provide strong antibacterial activity. Meanwhile, the antibacterial activity of methanol extract against E. coli which was not significantly different (p > 0.05), had an inhibition zone of 13.03 ± 2.92 mm at a concentration of 500 ppm. Based on the One-way ANOVA analysis by Fisher's method, the inhibition zone shows a significant difference to the negative control, namely 0 ppm (DMSO without extract) with an inhibition zone of 8.63 ± 1.12 mm, so that at a concentration of 500 ppm it can be stated to have antibacterial activity against E. coli (Fig. 2).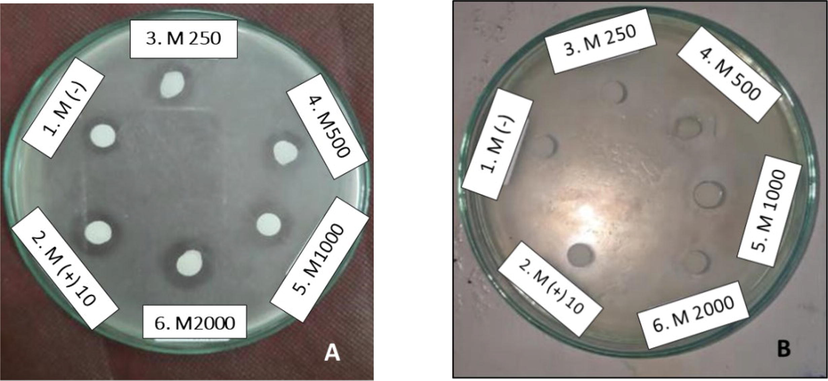
Determination of the inhibition zone of the methanol extract of the Central Java M. pruriens seeds against S. mutans ATCC 35,668 (A) and E. coli ATCC 25,922 (B). 1. DMSO solvent (negative control); 2. Chloramphenicol 10 ppm (positive control); 3. 250 ppm methanol extract; 4. 500 ppm methanol extract; 5. methanol extract 1,000 ppm; 6. 2,000 ppm methanol extract.
The water fraction had significant antibacterial activity against S. mutans (p < 0.001) and E. coli (p < 0.05). The strongest antibacterial activity against S. mutans was shown at a concentration of 250 ppm with an inhibition zone of 10.08 ± 0.14 mm and against E. coli at the same concentration as an inhibition zone of 10.13 ± 1.59 mm. The antibacterial activity of the water fraction against S. mutans at a concentration of 500 ppm showed a larger inhibition zone (10.45 ± 0.18 mm) than that obtained at a concentration of 250 ppm but did not differ significantly so that a concentration of 250 ppm was sufficient provides strong antibacterial activity against S. mutans. Also, the antibacterial activity of the water fraction against E. coli at a concentration of 500 ppm showed a larger zone of inhibition (10.85 ± 1.03 mm) than the inhibition zone obtained at a concentration of 250 ppm but did not differ significantly so that it was sufficient a concentration of 250 ppm can provide strong antibacterial activity against E. coli.
Ethyl acetate fraction also had significant antibacterial activity against S. mutans (p < 0.05) and E. coli (p < 0.001). The strongest antibacterial activity against S. mutans was shown at a concentration of 250 ppm with an inhibition zone of 10.65 ± 0.79 mm and against E. coli at the same concentration as an inhibition zone of 10.98 ± 0.55 mm. Antibacterial activity against E. coli at a concentration of 1,000 ppm showed a larger inhibition zone (11.80 ± 1.06 mm) than the inhibition zone obtained at a concentration of 250 ppm, but not significantly different so that a concentration of 250 ppm could provide strong antibacterial activity against E. coli. The negative control (concentrated DMSO solvent) was also gave antibacterial activity against S. mutans and E. coli with a zone of about 8 mm (including the diameter of disc paper). This was also obtained by Rajeshwar et al. (2005), in which 10% DMSO solvent provides antibacterial activity against several Gram-positive and Gram-negative bacteria. Every organic solvent commonly used as a diluting agent in the pharmacological test (for example methanol, ethanol, Tween 20, and DMSO) has its toxicity characteristics (Geetha et al., 2013). Therefore, the DMSO solvent provided antibacterial activity in this study. According to Kirkwood et al. (2018), the use of high concentrations of DMSO solvents needs to be avoided and it is recommended to use appropriate experimental controls, that is, it is necessary to observe the antibacterial activity response after DMSO is diluted several times, to avoid reporting false positive antimicrobial effects. Rajeshwar et al. (2005) succeeded in showing that the methanol extract of the Indian M. pruriens seeds had antibacterial activity against E. coli with an inhibition zone up to 18 mm. The method used to determine the antibacterial activity is disc diffusion. Then, Venkatkumar & Rajeshkumar (2017) determined the antibacterial activity against pathogenic bacteria Streptococcus sp from the ethanol extract of Indian M. pruriens seeds using the good diffusion method. The results showed that there was an antibacterial activity with the inhibition zone reaching ± 18 mm. In this study, antimicrobial activity tests were carried out on S. mutans ATCC 35,668 and E. coli ATCC 25922. The reason of using S mutans rather than S.aureus because the bacteria S. mutans is also as the gram-positive bacteria, and plays a role in the formation of biofilms on the teeth surface and gums, known as dental caries, where this condition can lead to infection of the oral cavity (Krzyściak et al., 2014).
According to Stockbroekx et al. (2022), S. mutans is more susceptible to antibiotics than S. aureus. So we choose S. mutans to make sure that the bacteria used in the assay has less possibility of resistance (Stockbroekx et al., 2022).
Overall, the antibacterial activity with a relatively larger range of inhibition zones was shown by the methanol extracts of the seeds of Central Java M. pruriens. In a previous study conducted by Venkatkumar & Rajeshkumar (2017), methanol extract of Indian M. pruriens accession seeds also showed greater antibacterial activity than water extract, hexane extract, petroleum ether extract, and benzene extract. In the methanol extract of the seeds of Indian M. pruriens, it is known that they contain compounds such as alkaloids, flavonoids, tannins, and terpenoids (Murugan & Mohan, 2011). These compounds have been shown to play a role in antibacterial activity through different mechanisms (Rahman et al., 2017). Then, this study showed that the extract from the seeds of Central Java M. pruriens was relatively more effective against Gram-negative bacteria compared to Gram-positive bacteria. Theoretically, Gram-negative bacteria have a cell wall arrangement that makes them more resistant to the presence of antibacterial agents, because they have an additional layer, namely the outer membrane (Kapoor et al., 2017). However, this study is inversely related to the theory. Rajeshwar et al. (2005) also showed that methanol extract from seeds of Indian M. pruriens had an antibacterial activity that was more effective against E. coli (Gram-negative bacteria) than Staphylococcus aureus is included in Gram-positive bacteria.
When viewed from the size of the bacteria, both S. mutans and the genus Staphylococcus in the form of coccus have a diameter of 0.5–1.0 μm ( Krzyściak et al., 2014; Foster, 1996). Meanwhile, the size of the E. coli bacteria has a length of 2.0–6.0 μm and the wide bacilli reach 1.1–1.5 μm (Percival & Williams, 2014). This shows that at the time of the preparation of bacterial colony suspensions by comparing the turbidity to the standard McFarland No. 1 (3 × 108 CFU / mL), the actual number of colonies obtained in the S. mutans and E. coli suspension was not the same so that it affected the inhibition zone obtained. Although the McFarland turbidity standard is used universally in testing for antibacterial activity, it is not accurate with other organisms because bacterial species vary in size and shape. To obtain a more accurate number of colonies or cell concentrations, it can be determined using a Densimat photometer (Zapata & Ramirez-Arcos, 2015). We used NA media in the disc diffusion test were according to the several articles, like the article of Banjara et al. (2012), and Bakht et al. (2011). And according to the article of Festus & Emmanuella (2020), they compared of MHA media with NA media on the diffusion test, showed that the Standard discs zones of inhibition on MHA and NA media were not significant (Festus & Emmanuella, 2020).
Neuroprotective effects and possible mechanisms of anti-infective agents in Parkinson’s disease have already showed by researchers: Ceftriaxone, as the antimicrobial agent can also attenuating oxidative stress and neuroinflammation that correspond with Parkinson disease (Bisht et al., 2014; Kaur & Prakash, 2017). Doxycycline as the antimicrobial agent have also activity on protection against nigral dopaminergic degeneration, correspond with Parkinson disease (Cho et al, 2009).
3.4 Analytical thin layer chromatography
This analytical TLC was performed to determine the Rf value of the standard and sample. The solvent system will then be used for adsorption column chromatography and preparative thin-layer chromatography. The isocratic mobile phase consisted of butanol: acetic acid: water (6:1:2). This solvent ratio can separate the target spots from those that fluoresce under UV light at λ = 365 and other stains. The Rf value from this analytical TLC is 0.37, and the Rf value is similar to the Rf value obtained by Sampath et al. (2014), 0.36. However, the mobile phase ratio is different. In the area of methanol extract and water fraction, there are stains parallel to the standard, this indicates that both contain l-Dopa which can be further isolated. However, the stains on both of them are not a single spot, so further purification by adsorption column chromatography is needed.
3.5 Adsorption column chromatography
Fractions of 1 mL were collected and analyzed for their content using TLC. The result of TLC indicates that the target compound is within the fraction range and these fractions still have some impurities. Therefore, further purification need to be performed in order to get more pure GA3. Preparative TLC was chosen for the purification method.
3.6 High-performance liquid chromatography of l-Dopa isolate
As shown in Fig. 3 the l-Dopa standard was detected at retention time 5.573 min with an area of 2,966,054. The number of the area is according to the concentration of l-Dopa standard, 230 ppm (2.3 mg of l-Dopa standard in 10 mL of methanol: water (1:1 v/v). In Fig. 4, the l-Dopa isolate chromatogram, there is a dominant peak detected at retention time 5.345 with an area of 668,921. The retention time of the sample and standard are relatively the same. The purity of l-Dopa isolate is 91.70%. The concentration of l-Dopa isolate from M. pruriens seeds Central Java accession can be calculated by comparing the peak area of the l-Dopa isolate and the l-Dopa standard. The isolated l-Dopa from M. pruriens Central Java accession is 0.49 mg/g, dry weight.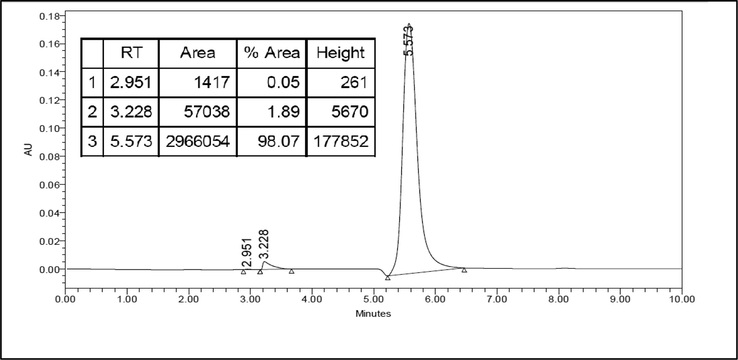
HPLC chromatogram for l-Dopa standard. HPLC was carried out using a reverse-phase C-18 nucleosil ODS column and a UV detector with λ = 230 nm. The mobile phase was 20 mM potassium dihydrogen phosphate (pH 2.5) and methanol HPLC grades (97:3 v/v). With a flow rate of 1 mL/min. The retention time of the l-Dopa standard was 5.573 min.
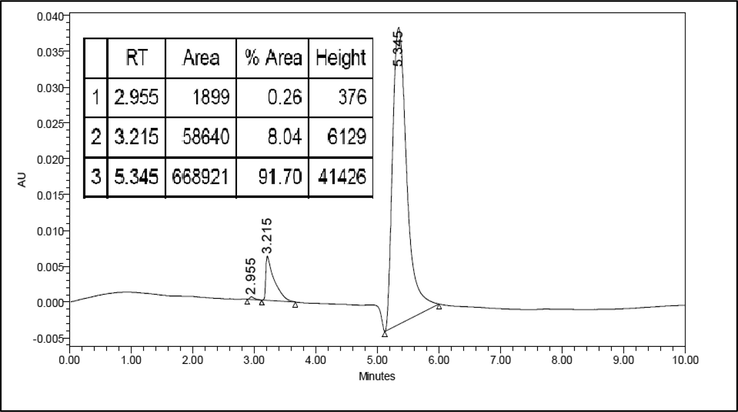
HPLC chromatogram for l-Dopa isolate from M. pruriens seeds Central Java accession. HPLC was carried out using a reverse-phase C-18 nucleosil ODS column and a UV detector with λ = 230 nm. The mobile phase was 20 mM potassium dihydrogen phosphate (pH 2.5) and methanol HPLC grades (97:3 v/v). With a flow rate of 1 mL/min. The retention time of the l-Dopa standard was 5.345 min.
The l-Dopa content in the seeds of M. pruriens, especially from India accession is known to be around 0.89–6.42% (Pulikkalpura et al., 2015). Meanwhile, the l-Dopa content in M. pruriens seeds from Indonesia was around 7.56–13.9% (Sardjono et al., 2012; Sardjono et al., 2016).
3.7 Fourier-transform infrared spectrometer of l-Dopa isolate
Fourier-transform infrared spectroscopy (FTIR) analysis was carried out to classify functional groups in the l-Dopa isolate. The FTIR spectrum in the range 4000–400 cm−1 of l-Dopa standard and l-Dopa isolate is shown in (Fig. 5) The wide absorption peak found in wave number 3308.27 cm−1 (standard) and 3308.57 cm−1 (isolate) is the stretching of the O—H bond, the peak in wave number 2942.26 cm−1 (standard) and 2941.62 cm−1 (isolate) is the symmetrical stretching of the N—H bond and the peak that presence at 2830.37 cm−1 (standard) and 2830.42 cm−1 (isolate) is aromatic stretching of C—H bond (Sukker et al., 2015). The absorption peak at a wave number of 1448.13 cm−1 (standard) and 1447.97 cm−1 (isolate) is aliphatic bending of C—H bond, FTIR peaks at 1416.94 cm−1 (standard) and 1417.42 cm−1 (isolate) could be ascribed to the C⚌O bond of the carboxylate group, and the peak that presence at 630.34 cm−1 (standard) and 610.47 cm−1 (isolate) is the bending of the N—H bond (Kumari et al., 2019).![FTIR spectrum of l-Dopa standard [red line] (A) and l-Dopa isolate [green line] (B). The FTIR spectrum of l-Dopa standard and l-Dopa isolates showed the presence of O—H(stretching); N—H(stretching) symmetrical; C—H(stretching) aromatic; C—H(bending) aliphatic; C⚌O(stretching); and N—H(bending) bonds at wave numbers 3308.27 and 3308.57 cm−1; 2942.26 and 2941.62 cm−1; 2830.37 and 2830.42 cm−1; 1448.13 and 1447.97 cm−1; 1416.19 and 1417.42 cm−1; 630.34 and 610.47 cm−1, respectively.](/content/184/2023/16/1/img/10.1016_j.arabjc.2022.104390-fig5.png)
FTIR spectrum of l-Dopa standard [red line] (A) and l-Dopa isolate [green line] (B). The FTIR spectrum of l-Dopa standard and l-Dopa isolates showed the presence of O—H(stretching); N—H(stretching) symmetrical; C—H(stretching) aromatic; C—H(bending) aliphatic; C⚌O(stretching); and N—H(bending) bonds at wave numbers 3308.27 and 3308.57 cm−1; 2942.26 and 2941.62 cm−1; 2830.37 and 2830.42 cm−1; 1448.13 and 1447.97 cm−1; 1416.19 and 1417.42 cm−1; 630.34 and 610.47 cm−1, respectively.
According to the Fig. 5 showed that the FTIR spectrum of the isolated sample was similar with the FTIR spectrum of l-Dopa standard, it is also show indication that the isolated sample was in high purity.
3.8 Antioxidant activity of l-Dopa isolate with radical DPPH method
The antioxidant activity of l-Dopa isolate and concentrated methanol extract is determined using the radical method of DPPH. Measurements are made of DPPH solutions using a UV–vis spectrophotometer at (=517 nm. The presence of antioxidant activity is indicated by the reduction of purple to yellow. The principle of this DPPH method is the interaction of DPPH antioxidants as free radicals either through the transfer of electrons and hydrogen radicals in DPPH and will neutralize free radicals from DPPH (Marjoni & Zulfisa, 2017). The average value of IC-50 obtained from M. pruriens extract in Central Java accession is 23.75 ± 0.44 ppm, ascorbic acid is 15.84 ± 0.16 ppm, and l-Dopa isolate is 8.92 (0.03). IC-50 (inhibitory concentration) values indicate the concentration of an antioxidant substance that can inhibit free radicals by 50 %. The smaller IC50 value indicates an increasingly active antioxidant activity (Marjoni & Zulfisa, 2017). Based on one-way-ANOVA analysis of Tukey methods. IC-50 values obtained from all three test samples differed significantly (p < 0.001). Nonetheless, all three have antioxidant activity with a very active intensity (Table 5). Description: The data presented is the result of three repetitions that was analyzed using the One Way-ANOVA Tukey method. Data that does not share same letter (a,b,c) mean significantly different.
Test Sample
Average IC50 (ppm)
Intensity
Methanol extract
23,75 ± 0,44a
Very active
Ascorbic acid
15,84 ± 0,16b
Very active
Isolate of l-Dopa
8,92 ± 0,03c
Very active
IC-50 value obtained from l-Dopa isolate M. pruriens accession of Central Java has a value relatively similar to the l-Dopa isolate obtained by Biswas et al. (2010) with an IC-50 value of 9.22 ppm. The l-Dopa constituent contains a catechol structure with two hydroxyls bonded to adjacent carbon (dihydroxyfenol) (Norris & Carr, 2013). The structure of catechol is widely distributed in many natural antioxidants and plays a role in warding off free radicals. The catechetical structure of l-Dopa in methanol solvents has better DPPH radical counteracting activity than ascorbic acid (Shimizu et al., 2010). This was also evident in this study where the IC-50 value of l-Dopa isolates was smaller than IC-50 values of ascorbic acid, and that mean the l-Dopa isolates showed stronger DPPH radical counteracting activity than the ascorbic acid (Table 5).
3.9 Determination of anti-Parkinson activity of isolated l-Dopa using classical labirin test
The determination of anti-Parkinson activity was carried out using the classic maze test. This method is used to evaluate the behavior of rodents such as rats and mice. The behavior of the animal in question is such as learning and memory skills (Gasmi, 2018). In this study the mice were divided into six groups: vehicle group, negative control group, positive control 1 (l-Dopa standard), positive control 2 (Levodopa: Benserazid HCl), methanol extract of M. pruriens seeds, and l-Dopa isolate. The mice used were mice that met the inclusion criteria, namely-seven-week-old male white rats weighing 200–250 g, meeting test criteria and healthy conditions, able to pass through the maze from the starting point to the end point with a time of ≤ 10 min and/or 2 (10 min). This anti-Parkinson test consists of training, induction (days 1 to 7) and recovery (days 8 to 17). During the training period, each mouse was given the opportunity to explore the maze for 10 min. Then, it is tested by measuring the time it takes to travel through the maze from the starting point to the stopping point. Rats were given a smelly stimulant in the form of feed. On the last day of the training period, the travel time of mice from the control group was negative; positive control 1; positive control 2; methanol extract; isolated l-Dopa; and vehicles respectively are 0.97 ± 0.59; 0.98 ± 0.65; 0.98 ± 0.69; 0.98 ± 0.73; 0.98 ± 0.32; and 0.98 ± 0.07 min (Table 6). Based on Tukey's One Way-ANOVA analysis, mice from each group had a significant travel time with a p value of 1.00 (p > 0.05). Next, the mice entered the induction period. Each mouse from the negative control group; positive control 1; positive control 2; methanol extract; And l-Dopa isolates were induced with rotenone (2.5 mg/kg) intraperitoneal (IP) for seven consecutive days. Meanwhile, the vehicle group was only induced with a solvent without rotenone which is 0.1% DMSO in PBS. Rotenone is a hydrophobic compound that plays a role in strongly inhibiting mitochondrial complex I and can induce dopamine neurodegeneration (Bose & Beal, 2016). Rotenone is able to cross the blood–brain barrier and cause disturbances in the central nervous system (Bisbal & Sanchez, 2019). Chronic IP rotenone administration at toxic doses of 2.3–2.5 mg/kg is commonly used to model parkinsonism. After 5–7 days, this substance can induce typical symptoms of parkinsonism in mice (catalepsy and oligo kinesis), along with non-specific neurotoxic effects (weight loss to death) (Gmiro et al., 2019). In this study, administration of rotenone led to significantly increased travel time of mice from negative control group, positive control 1, positive control 2, methanol extract, and l-Dopa isolate significantly to the training period (p < 0.001). The mice's travel time on the last day of the induction period of the negative control group, positive control 1, positive control 2, methanol extract, and l-Dopa isolates were consecutively 5.42 ± 0.39; 5.19 ± 0.51; 5.47 ± 0.51; 6.82 ± (0.02; and 5.92 ± 0.90 min (Table 6). Meanwhile, mice in the vehicle group had a travel time that was not significantly different from the training period with a p value of 0.141 (p > 0.05) which is 1.27 (0.25 min. This maze test is a very challenging task because it requires test animals to remember the fastest path between the starting point and the stopping point (Gasmi, 2018). Later on, at the recovery period, each mouse from the control group was negative; positive control 1; positive control 2; methanol extract; and l-Dopa isolates are treated according to those shown in Table 6. The vehicle group was given a 0.2% ascorbic acid in a PBS. On the last day of the recovery period, the negative control group experienced a very significant increase in time (p < 0.001) to the training period and the induction period to 7.92 ± 0.13 min. Meanwhile, the positive control-1, positive control-2, methanol extract, and l-Dopa isolate experienced a significant decrease in time (p < 0.001) against the induced period successively to 1.98 ± 0.22; 1.78 ± 0.70; 1.72 ± 0.23; and 2.16 ± 0.17 min. Meanwhile, mice in the vehicle group had an in no significant difference from the previous 1.27 ± 0.15 min (p > 0.05) as shown in the vehicle group data in Table 6. Description: The data presented is the result of three repetitions. The data presented was analyzed using the One Way-ANOVA Tukey method. Data that doesn't share the same letter means significantly different. Capital letters indicate grouping in a single column. Lowercase indicates grouping in a single line.
Group (Test sample)
Mouse travel time on classic maze test (minutes)
Training (Day 0)
After rotenone induction (Day 7)
After feeding the test sample (Day 17)
Negative control
0,97 ± 0,59A;c
5,42 ± 0,39AB;b
7,92 ± 0,13A;a
Positive control-1 (l-Dopa standard; 10 mg/kg)
0,98 ± 0,65A;b
5,19 ± 0,51B;a
1,98 ± 0,22B;b
Positive control-2 (Levodopa:Benserazid-HCl;10 mg/kg)
0,98 ± 0,69A;b
5,47 ± 0,51AB;a
1,78 ± 0,70B;b
Methanol extract (200 mg/kg)
0,98 ± 0,73A;b
6,82 ± 0,02A;a
1,72 ± 0,23B;b
Isolate of l-Dopa (10 mg/kg)
0,98 ± 0,32A;b
5,92 ± 0,90AB,a
2,16 ± 0,17B;b
Group
Training (Day 0)
After induction with 0,1% DMSO in PBS (Day7)
After feeding 2 % of ascorbic acid in PBS (Day 17)
Vehicle
0,98 ± 0,07A;a
1,27 ± 0,25C; a
1,27 ± 0,15B; a
The methanol extract of M. pruriens seeds (200 mg/kg) and l-Dopa isolate (10 mg/kg) had the ability to significantly decrease symptoms of non-motor Parkinsonism such as memory ability (p < 0.001). And both the ability of l-Dopa isolate and l-Dopa standard (positive control-1) to lower parkinsonism symptoms was no better than positive control-2. This is because the oral administration of l-Dopa was treated without decarboxylase inhibitors (such as carbidopa or benserazid). That means, l-Dopa can be decarboxylated into dopamine in extra-cerebral tissue, especially in the digestive tract, so that only a few l-Dopa make it to the central nervous system. Oral administration of l-Dopa without decarboxylase inhibitors is almost entirely absorbed and only 2% is excreted in feces. However, only 30% of l-Dopa concentrations can enter the circulatory system without being metabolized in the digestive tract (Khor & Hsu, 2007).
4 Conclusions
M. pruriens seeds from Central Java accession have antibacterial activity against S. mutans ATCC 35,668 and E. coli ATCC 25922, as the Gram-positive and Gram-negative bacteria. M. pruriens seeds extract has a moderate to a high level of toxicity. The l-Dopa isolates showed stronger DPPH radical counteracting activity than the ascorbic acid. The l-Dopa isolate had anti-Parkinson activity in rotenone-induced mice by significantly lowering symptoms of non-motoric Parkinsonism (p < 0.001). For the future work, proliferation assay should be done to demonstrate the efficacy of the l-Dopa isolate in in-vivo studies.
Acknowledgements
The authors are grateful to the Directorate of Research and Community Service and Innovative Universitas Padjadjaran; Dean of the Faculty of Mathematics and Natural Sciences, Head of the Department of Chemistry, and Head and the entire staff of Biochemistry Laboratory. We are also grateful to the laboratory facilities provided by Department of Chemistry, Faculty of Mathematics and Natural Sciences Universitas Padjadjaran and to thank to all those who have given advice, motivation, and material support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors are grateful to the Directorate of Research and Community Service Universitas Padjadjaran.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative phytochemical profiling of different soybean (Glycine max (L.) Merr) genotypes using GC–MS. Saudi J. Biol. Sci.. 2018;25(1):15-21.
- [CrossRef] [Google Scholar]
- Official methods of analysis. 16 th. Virginia: Association of official analytical chemist Inc.; 1990.
- Antimicrobial potentials of Eclipta alba by disc diffusion method. Afr. J. Biotechnol.. 2011;10(39):7658-7667.
- [CrossRef] [Google Scholar]
- Antibacterial activity of di-2-ethylaniline phosphate screened by paper disc diffusion method. J. Appl. Pharma. Sci.. 2012;02(07):230-233.
- [CrossRef] [Google Scholar]
- Nutritional quality evaluation of velvet bean seeds (Mucuna pruriens) exposed to gamma irradiation. Int. J. Food Sci. Nutr.. 2008;59(4):261-278.
- [CrossRef] [Google Scholar]
- Neurotoxicity of the pesticide rotenone on neuronal polarization: a mechanistic approach. Neural Regener. Res.. 2019;14(5):762-766.
- [CrossRef] [Google Scholar]
- Ceftriaxone mediated rescue of nigral oxidative damage and motor deficits in MPTP model of Parkinson’s disease in rats. Neurotoxicology. 2014;44:71-79.
- [Google Scholar]
- Antioxidant activity of Mucuna pruriens seeds, cultured callus and L-Dopa. Indian Drugs. 2010;47(12):22-25.
- [Google Scholar]
- Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem.. 2016;139(Suppl. 1):216-231.
- [CrossRef] [Google Scholar]
- Potentiation of Mucuna pruriens (L.) DC. for insecticidal activity, insect repellency and brine shrimp lethality tests under the laboratory conditions. J. Entomol. Zool. Stud.. 2017;5(56):692-696.
- [Google Scholar]
- Doxycycline is neuroprotective against nigral dopaminergic degeneration by a dual mechanism involving MMP-3. Neurotox. Res.. 2009;16:361-371.
- [Google Scholar]
- Daffodil, E.D., Tresina, P.S., Mohan, V.R. 2016. Nutritional and antinutritional assessment of Mucuna pruriens (L.) DC var. utilis (Wall ex. Wight) Bak. Ex Burck and Mucuna deeringiana (Bort) Merril: An underutilized tribal pulse. International Food Research Journal. 23(4), 1501–1513. DOI: 10.1016/B978-0-12-384947-2.00036-2.
- Comparative genetic analysis of trichome-less and normal pod genotypes of Mucuna pruriens (Fabaceae) Genet. Mol. Res.. 2011;10(3):2049-2056.
- [CrossRef] [Google Scholar]
- A new validated HPLC method for the determination of levodopa: application to study the impact of a ketogenic diet on the pharmacokinetics of levodopa in Parkinson's participants. Biomed. Chromatogr.. 2019;33(1):1-26.
- [CrossRef] [Google Scholar]
- Effect of methanolic extract of Mucuna pruriens seed on the immune response of mice. Comp. Clin. Pathol.. 2012;21(6):1343-1347.
- [CrossRef] [Google Scholar]
- Festus, O.D. Emmanuella, O.O. 2020. Testing the efficacy of Mueller Hinton agar over Nutrient agar for optimal antibiotic sensitivity testing response by selected clinical bacterial pathogens. GSC Advanced Research and Reviews. 05(02), 061–07. DOI: 10.30574/gscarr.2020.5.2.0037.
- Staphylococcus. Chapter 12. In: Baron S., ed. Medical Microbiology ((4 edition).). Galveston: University of Texas Medical Branch at Galveston; 1996.
- [Google Scholar]
- Gandjar, I., Slamet, D.S., Moeljono. 1973. Kadar zat gizi dalam tempe benguk. Penelitian Gizi dan Makanan. 3, 65–71
- Classic labyrinth test for neurobehavioral evaluation in Wistar rats. Bio-protocol. 2018;8(18):e3007
- [Google Scholar]
- Effects of Deltamethrin on striatum and hippocampus mitochondrial integrity and the protective role of Quercetin in rats. Environ. Sci. Pollut. Res. Int.. 2017;24(19):16440-16457.
- [CrossRef] [Google Scholar]
- Interference from ordinarily used solvents in the outcomes of Artemia salina lethality test. J. Adv. Pharm. Technol. Res.. 2013;4(4):179-182.
- [CrossRef] [Google Scholar]
- Comparison of the antiparkinson activity of levodopa, memantine, and guanidine-containing analogs of amantadine and memantine (IEM-2151 and IEM-2163) in rats with rotenone-induced parkinsonism. Neurosci. Behav. Physiol.. 2019;49(4):502-507.
- [CrossRef] [Google Scholar]
- New Antimicrobials of Plant Origin. In: Janick J., ed. Perspectives on New Crops and New Uses. Alexandria, VA: ASHS Press; 1999. p. :457-462.
- [CrossRef] [Google Scholar]
- Analysis of bioactive components from ethyl acetate and ethanol extract of Mucuna pruriens linn seeds by GC-MS technique. J. Chem. Pharm. Res.. 2016;8(8):403-409.
- [Google Scholar]
- Levodopa-reduced Mucuna pruriens seed extract shows neuroprotective effects against Parkinson’s disease in murine microglia and human neuroblastoma cells, Caenorhabditis elegans, and Drosophila melanogaster. Nutrients. 2018;10(9):1-14.
- [CrossRef] [Google Scholar]
- Action and resistance mechanisms of antibiotics: a guide for clinicians. J. Anaesthesiol. Clin. Pharmacol.. 2017;33(3):300-305.
- [CrossRef] [Google Scholar]
- Phytolacca octandra (L.), Phytolacca dodecandra (L’Herit) and Balanites aegypiaca (L.) extracts as potential molluscicides of schistosomiasis transmitting snails. J. Med. Plants Res.. 2016;10(44):823-828.
- [CrossRef] [Google Scholar]
- Ceftriaxone attenuates glutamate-mediated neuro-inflammation and restores BDNF in MPTP model of Parkinson’s disease in rats. Pathophysiology. 2017;24:71-79.
- [CrossRef] [Google Scholar]
- Registration of trichomesless, early maturing, high seed and l-Dopa yielding variety “CIM Ajar” of Kewanch (Mucuna pruriens L.) J. Med. Aromat. Plant Sci.. 2007;29:40-41.
- [Google Scholar]
- The pharmacokinetics and pharmacodynamics of levodopa in the treatment of Parkinsons disease. Curr. Clin. Pharmacol.. 2007;2(3):234-243.
- [CrossRef] [Google Scholar]
- Antimicrobial effect of dimethyl sulfoxide and N, N-Dimethylformamide on Mycobacterium abscessus: implications for antimicrobial susceptibility testing. Int. J. Mycobacteriol.. 2018;7(2):134-136.
- [CrossRef] [Google Scholar]
- The elevated plus maize test for measuring anxiety-like behavior in rodents. Methods Mol. Biol.. 2019;1916:69-74.
- [CrossRef] [Google Scholar]
- The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Diseases : Off. Publication Eur. Soc. Clin. Microbiol.. 2014;33(4):499-515.
- [CrossRef] [Google Scholar]
- Synthesis of Fe3O4-DOPA-Cu magnetically separable nanocatalyst: a versatile and robust catalyst for an array of sustainable multicomponent reactions under microwave irradiation. Catal. Lett.. 2019;149(8):2180-2194.
- [CrossRef] [Google Scholar]
- Genotype selection for agronomical trait-seed yield in kewachh {Mucuna pruriens (L.)} Industrial Crop Prod.. 2015;65:62-70.
- [CrossRef] [Google Scholar]
- The magic velvet bean of Mucuna pruriens. J. Traditional Complement. Med.. 2012;2(4):331-339.
- [CrossRef] [Google Scholar]
- Brine shrimp lethality test (BSLT) dari berbagai fraksi ekstrak daging buah dan kulit biji mahkota dewa (Phaleria macrocarpa) Buletin Penelitian Kesehatan.. 2006;34(3):111-118.
- [Google Scholar]
- Maldonado, R.G. 2018. Mucuna and Parkinson's Disease: Treatment with Natural Levodopa. Book Chapter. In Parkinson's Disease-Understanding Pathophysiology and Developing Therapeutic Strategies Interchopen. Chapter 6; 96-116. DOI:10.5772/intechopen.74062.
- Antioxidant activity of methanol extract/fractions of Senggani Leaves (Melastoma candidum D Don) Pharma. Anal. Acta. 2017;08(08):1-6.
- [CrossRef] [Google Scholar]
- Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med.. 1982;45(1):31-34.
- [CrossRef] [Google Scholar]
- Mohan, V.R., Tresina, P.S., Daffodil, E.D. 2016. Antinutritional factors in legume seeds: Characteristics and determination. In B. Caballero, P. M. Finglas, & F. B. T.-E. of F. and H. Toldrá, eds. Encyclopedia of Food and Health. Oxford: Academic Press, pp. 211–220. DOI: 10.1016/B978-0-12-384947-2.00036-2.
- Murugan, M. Mohan, V.R. 2011. Antibacterial activity of M. pruriens (L.) Dc. Var. pruriens - An ethnomedicinal plant. Science Research Reporter. 1(2), 69–72.
- How to read and interpret ftir spectroscope of organic material. Indonesian J. Sci. Technol.. 2019;4(1):97-118.
- [CrossRef] [Google Scholar]
- Norris, D.O. & Carr, J.A. 2013. Synthesis, Metabolism, and Actions of Bioregulators. In D. O. Norris & J. A. B. T.-V. E. (Fifth E. Carr, eds. Vertebrate Endocrinology. San Diego: Academic Press, 41–91
- Kandungan L-3, 4-dihydroxyphenylalanine suatu bahan neuroprotektif pada biji koro benguk (Mucuna pruriens) segar, rebus, dan tempe. Jurnal Veteriner. 2017;18(1):116-120.
- [Google Scholar]
- Percival, S.L. Williams, D.W. 2014. Escherichia coli. In S. L. Percival, M. V Yates, D. W. Williams, R. M. Chalmers, & N. F. B. T.-M. of W. D. (Second E. Gray, eds. Microbiology of Waterborne Diseases: Microbiological Aspects and Risks. London: Academic Press, pp. 89–117
- Levodopa in Mucuna pruriens and its degradation. Sci. Rep.. 2015;5(11078):1-9.
- [CrossRef] [Google Scholar]
- Skrining fitokimia dan aktivitas antibakteri ekstrak etanol daun sirsak (Annona muricata L.) pada Streptococcus mutans ATCC 35668. Majalah Kedokteran Gigi Indonesia. 2017;3(1):1-7.
- [Google Scholar]
- Rajeshwar, Y., Gupta, M., Mazumder, U.K. 2005. In vitro lipid peroxidation and antimicrobial activity of Mucuna pruriens seeds. Iranian Journal of Pharmacology & Therapeutics. 4(1), 32–35. DOI: 1735-2657/05/41-32-35.
- A novel approach of the isolation of l–dopa from the methanolic extract of Mucuna pruriens seeds and its quantitative analysis by HPTLC. Int. J. Pharmacogn. Phytochem. Res.. 2014;5(4):259-262.
- [Google Scholar]
- Physicochemical composition of Indonesian velvet bean (Mucuna pruriens L) Global J. Res. Med. Plants Indigenous Med.. 2012;1(4):101-108.
- [Google Scholar]
- Sardjono, R.E., Musthapa, I., Sholihin, Subarnas, A., Herachandra, E., Ardianto, F.N. 2016. Evaluation of antiparkinson's activity of Indonesian velvet bean (Mucuna pruriens) extract. ARPN Journal of Engineering and Applied Sciences. 11(18), 10856–10861
- Antibacterial activity of medicinal plants against pathogens causing complicated urinary tract infections. Indian J. Pharma. Sci.. 2009;71(2):136-139.
- [CrossRef] [Google Scholar]
- Structure effect on antioxidant activity of catecholamines toward singlet oxygen and other reactive oxygen species in vitro. J. Clin. Biochem. Nutr.. 2010;47(3):181-190.
- [CrossRef] [Google Scholar]
- Phytochemical analysis and in vitro DPPH assay of ethanolic extract of Mucuna pruriens seed. Int. J. Curr. Pharma. Res.. 2020;12(6):93-96.
- [CrossRef] [Google Scholar]
- Stockbroekx, V., Geertsema-Doornbusch, G.I., Dijk, M., Carniello, V., Woudstra, W., Busscher, H.J. Ren, Y., 2022. A Comparison of the Adaptive Response of Staphylococcus aureus vs. Streptococcus mutans and the Development of Chlorhexidine Resistance. Frontiers in microbiology, 13, pp.861890-861890. DOI: 10.3389/fmicb.2022.861890.
- Towards understanding mode of action of L-dopa and carbidopa: DFT/TD-DFT analyses of their electronic and vibration spectra. Indian J. Chem. - Section A Inorg., Phys., Theor., Anal. Chem.. 2015;54A(11):1378-1386.
- [Google Scholar]
- The potential of velvet beans (Mucuna pruriens L.) as a source of protein in food products. Widyariset. 2014;17(3):391-397.
- [Google Scholar]
- Anti-inflammatory, antioxidant, antibacterial effect and phytochemical analysis of Mucuna pruriens seed extract. Int. J. ChemTech. Res.. 2017;10(1):273-283.
- [Google Scholar]
- Weli, A.M., AL-Hinai, J.R.K., Al-Mjrafi, J.M.A., Alnaaimi, J.R.S., Hossain, M.A., Saeed, S., Aktar, M.S. 2014. Effect of different polarities leaves crude extracts of Omani juniperus excels on antioxidant, antimicrobial and cytotoxic activities, and their biochemical screening. Asian Pacific Journal of Reproduction. 3(3), 218–223. DOI: 10.1016/S2305-0500(14)60029-4
- An important player in brine shrimp lethality bioassay: The solvent. J. Adv. Pharm. Technol. Res.. 2014;5(1):57-58.
- [Google Scholar]
- Phytochemistry and pharmacological activity of Mucuna pruriens A review. Int. J. Green Pharm.. 2017;11(2):69-73.
- [CrossRef] [Google Scholar]
- A comparative study of McFarland turbidity standards and the densimat photometer to determine bacterial cell density. Curr. Microbiol.. 2015;70(6):907-909.
- [CrossRef] [Google Scholar]







