Translate this page into:
Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater
⁎Corresponding author at: Department of Chemistry, College of Sciences, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. benharbmarwa@yahoo.fr (Marwa Ben Harb)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In reinforced concrete, a high alkaline medium, steel is commonly protected by formation a passive oxide film. Nevertheless, contamination with chlorides or carbonation causes the deterioration of protective film and the initiation of pitting corrosion. Hence, the need to enrich the fresh concrete solution with a good corrosion inhibitor. In accordance with green chemistry to evade toxicity and minimize waste, we have chosen to replace the usual inhibitors with an alternative not only ecological but also derived from biological wastes, in a perspective of circular economy emphasizing that waste can offer important compounds at an affordable price and easily available. In this context this work seeks to valorize the dried olive leaves extract as an inhibitor in alkaline chloride solution (pH 13). The targeted plant was grown in arid zones of Saudi Arabia and their leaves are usually disposed of as solid waste or animal feed. Four extraction solvents of different polarities were used; methanol, ethyl acetate, dichloromethane and hexane. The anticorrosive activity was performed using different electrochemical techniques; polarization curves, electrochemical impedance spectroscopy and Mott-Schottky analyses. The polarization studies proved that extract from olive leaves is a mixed type inhibitor in a solution of NaOH (0.1 M) + NaCl (0.5 M),with a predominant anodic effectiveness. The best inhibition of 91.9% is provided with methanol extract. GC–MS analysis showed the presence of compounds containing the heteroatoms N and O with the π electrons which are responsible for the corrosion inhibition activity. The olive leaves have been found to be high in phenol and flavonoid content. Inhibition efficiency of the olive leaves extracts increases with the polarity of extraction solvents but also it appears that it depends on another factors.
Keywords
Olive leaves
Corrosion inhibitor
Concrete
Electrochemical techniques
Phenol
Flavonoid
1 Introduction
Reinforced concrete is the judicious and intimate assembly of metal reinforcements, usually of mild steel, and of concrete which provides a physical barrier due to the thickness of the coating.
Concrete is the second material consumed next the water (International Energy Agency, 2009; Chakri, 2015). It represents 90% of construction market (Pierre Ollivier and Vichot, 2008). It is a composite material, alkaline (pH > 12.5) and fragile, consisting of various aggregates and cement (Chakri, 2015). Its basic pH allows the formation of a protective layer for the steels by constituting an electrochemical barrier. The degradation of this passive layer occurs either by carbonation of concrete, a natural phenomenon caused by the penetration of co2 through the porous network of concrete, or by penetration of chlorides (Chakri, 2015; Sassine, 2018). As soon as the depassivation has taken place, the steel will consist of an active zone (depassivated) and a passive zone. In the presence of water, the potential difference between these two zones causes galvanic corrosion (Sassine, 2018). Corrosion of steel is a major problem that has affected the world for centuries (Wang et al., 2019; Dai et al., 2019). According to a study published by NACE in 2016 (Koch.et al., 2016) the overall charge of corrosion was valued at 2,5 trillion dollars, relative to 3,4% of global gross domestic product. The same study showed that the use of available control practices results in a 15–35% decrease in overall cost (Chakri, 2015; Fedrizzi et al., 2005; Orhorhoro et al., 2017). As an economical technique for protecting metals against corrosion, the use of inhibitors remains the most effective, simplest and most applied solution. Corrosion is essentially an electrochemical process (Browne, 1973). Therefore the inhibitory action can be done only at one of the stages of the elementary reactions; transport of species in solution, adsorption of species on the surface of solid phases and electronic charge transfer (Epelboin et al., 1972).
According to NACE, “ An inhibitor is a chemical substance which, when added small concentrations to an environment, effectively checks, decreases, or prevents the reaction of the metal with the environment ” (NACE, 1965). The popularity of synthetic composites, often derived from petroleum products (Boukhedenna et al., 2005), against corrosion decreases thanks to severe ecological protocols following their poisonous impacts on ecosystems and living beings. Therefore, the discovery of natural and ecological inhibitors has recently been a persistent obsession. Indeed, plant compounds are capable of removing oxygen also the different species of reactive oxygen in a biological environment, further preventing scale formation and inhibiting microbial corrosion.
The organics extracts of medicinal plants and aromatic herbs have played a major role in this respect (Orhorhoro et al., 2017) and achieved high efficiency rates against metal protection (Khoudali et al., 2014). Its efficiency is associated to the existence of heterocyclic compounds, π electrons and especially polar functions via sulfur, oxygen or nitrogen atoms. Where the polar function presents frequently the “reaction center” that creates the adsorption process (Orhorhoro et al., 2017).
The practice of biological products in inhibiting corrosion of steel in acidic medium (HCl, H2SO4) have been established by many researchers (Uwah et al., 2013; Singh et al., 2019; M’hiri et al., 2016) but their application in reinforced concrete remains very limited. Loto et al. (2013) investigated the inhibitory effect of Vernonia amygdalina by electrochemical and gravimetric measurements and concluded the inhibitory effect at low concentrations even in the presence of a significant amount of chloride in concrete. Still according to Loto et al. (2011) kola nut extracts, its leaves and its bark also has a corrosion inhibiting effect; their adsorption prevents the chloride ions from accessing the surface of the armature. Asipita et al. (2014) used the extract from Bambusa arundinacea as a corrosion inhibitor. It has a good adsorption and it stabilizes calcium silicates (CSH), which prevents the transformation of hydroxide from calcium to calcite. Also, its pore blocking effect, prevents the differential aeration of concrete which favors the corrosion of steel. Okeniyi et al. (2016) used electrochemical techniques to study the inhibition effect of extract from Phyllanthus muellerianus leaves against corrosion of immersed metal reinforcement in an aggressive environment, 3.5% NaCl, to simulate the sea environment. They proved the inhibitory power of this plant and concluded that its mechanism follows the Langmuir adsorption model. El-Sayed et al. (2001) showed an augmentation in mass of concrete in the presence of 0.5 M NaCl relative to the immersion time, which is due to corrosion. By the addition of liquor extracted from banana, this augmentation decreases with the increase in concentration. For their part, Tantawi and Selim (1996) showed that the addition of 0.2% of banana extract reduced the potential and the corrosion current of steel immersed in a solution representative of the interstitial solution of concrete and containing chlorides. Chakri (2015); have studied the methanol extracts of dwarf palm leaves, Chamaerops humilis L. as an antioxidant and corrosion inhibitor. Their results revealed that this extract has an important antioxidant activity with 81% of inhibition. Concerning the corrosion of steel, tests highlighted 45% of inhibition with chloride ions at 0.5 g/L of the extract. And its phytochemical screening proven the existence of Polyphenols, Catechol, Gallic Tannins, Flavonoids, Saponins, Terpenoids, Anthracenosides and Cardiac glycosides (Chakri, 2015).
Most of the previous studies in this field studies have used valuable sources, but we believe that there is a need to take advantage of bio-wastes and organic residues, frequently released into the environment, in a context of circular economy. So we have chosen to benefit from olive leaves thanks to antioxidants, anti-inflammatory and anti-microbial activities as a cheap source of various biologically active compounds. The significance is not only for the start of bio-sources, but also of taking advantage of by-products and wastes (Paula Pereira et al., 2007; Salah et al., 2012).
Olea europaea L., the botanical classification of olive tree, is among of the most famous plants in Mediterranean Sea. Mediterranean Population have used the olive tree (bark, roots and leaves) in home-remedies for over two centuries. Egyptians too utilized olive leaf in their formula to preserve their mummies. Olden civilizations have utilized olive leaves to brew teas to treat colds besides else anxieties. Many olive extracted products known by their antioxidant activity, protect the corps against oxidation harm induced by damaging molecular called free radicals that abet ageing and maladies (Issaoui et al., 2012). Olea europaea L. extract presents a great tenor in polyphenols (oleuropein, hydroxytyrosol and their derivatives) related to its antioxidant effects. Hence Olive leaves polyphenols have been intended to perform a significant role in inhibition against corrosion (Paula Pereira et al., 2007; Salah et al., 2012).
Recently some researchers have proposed olive leaves as a corrosion inhibitor (Pustaj et al., 2016), but according to our knowledge none have tried in the concrete.
An important step in the use of bioactive compounds from plant resources is the extraction process. It is somewhat complex owing to the variety of structures as well polarities of the chemical compounds. Several parameters can affect the extraction yield and therefore the biological activity of plant extracts, the most important of which is the nature of extraction solvent. Its choice depends on the targeted bioactive compound. In 2012, Quezada and Cherian (2012) showed that the amount of phenolic content extracted from flax seeds depends on the polarity of the solvent. Indeed, the polarity of a molecule is a characteristic value known as the electric moment. Thermodynamically, thanks to the solvent-solute forces, the polar substances dissolve in the polar substances. So in order to properly study the antioxidant activity of olive leaves, we will use four organic solvents with different polarity indices (Abarca-Vargas et al., 2016).
In this context, our project seeks to assess the inhibitory effect of Olea europaea L. extracts against corrosion of mild steel in synthetic solution which simulates the concrete polluted with chlorides, exploiting four organic solvents of different degrees of polarity. The anticorrosive activity was carried out by using polarization curves, Mott-Schottky analyses and electrochemical impedance spectroscopy (EIS). And to get a clearer idea about these leaves, we looked for the chemical structure of its compounds by Gas chromatography-mass spectrometry (GC–MS) analysis and the entire amount of flavonoid and phenolic by respectively the aluminum chloride colorimetric assay and Folin-Ciocalteu phenol reagent method.
2 Experimental conditions
2.1 Preparation of the plant extract
The Olea europaea L. was grown in arid zones of Dammam city in Saudi Arabia. After collection and washing, the fresh leaves were weighed and dried in the umber until a stable mass was obtained. Next they were led to a soxhlet extraction for 6 h using four organic solvents: methanol, dichloromethane, ethyl acetate and hexane separately. The solution obtained was concentrated in the rotavapor, until a solid was obtained. Then it was used for tests of anti-corrosion activity.
2.2 Anti-corrosive activity
For the anti-corrosive activity part, we used a standard three-electrode electrochemical cell. The counter electrode was graphite sheet, the reference was saturated calomel electrode and for that the working electrode, it was a cylindrical rod made of mild steel, C ≈ 0.22%, with a diameter of 5 mm. A cataphoretic epoxy amine paint (PPG; WT724 P962) covered the side part, so that the active surface, 0.29 cm2, would be restricted to the cross part of the steel rod implanted in the epoxy resin. To simulate the interstitial electrolyte of concrete polluted with salt-water, the solution of corrosion test was NaOH (0.1 M) + NaCl (0.5 M) with pH around 13. The electrochemical tests were carried out at ambient temperature without stirring or purging the dissolved oxygen, and to reduce the carbonation of solution the cell was sealed.
2.3 Electrochemical techniques
2.3.1 Polarisation measurements
The Autolab instrument was used to record the potentiodynamic polarisation curves. To allow sufficient potential stability, the electrode was maintained at an open circuit during 1 h before recording. A freshly abraded electrode was used in each experiment. We applied a scan rate of 1 mV/s and a potential array of ± 500 mV. All tests were performed three times.
2.3.2 Mott-Schottky analysis
The electrical properties of protective film were investigated by exploiting the M-S plots.The M-S measurements were carried out at a frequency of 1 kHz AC signal with a polarization potential ranging from −0.8 V to 0.8 V (relative to the SCE) at a scan rate of 50 mV/s and a scan potential of 10 mV.
The space charge capacity (Csc) in the passive film related to the applied potential (Em) was calculated using the equation of Csc = −1/(ωz″), where ω is the angular frequency and Z '' is the imaginary part of the alternative impedance.
The electrical properties of the semiconductors were gotten from the M-S curves on the basis of M-S eq (Hakiki et al., 2000):
Efb is the flat band potential and it is acquired from linear extrapolation to
2.3.3 Impedance measurements (EIS)
The Autolab equipment was used to determine the EIS. A sinusoidal voltage of 10 mV has been applied in a frequency range starting from 100 kHz to 10 mHz, with 7 points per decade. We carried out experiments in potentiostatic mode EOC. Prior to any impedance measurement, the electrode was maintained under open circuit conditions for 1 h for system stabilization.
2.4 Phytochemical screening
2.4.1 GC–MS analysis
We used Hewlett Packard HP 5890 series II Gas Chromatograph, associate with a fused HP 5MS capillary column, and coupled with HP 5972A mass selective detector (Palo Alto, CA, USA). The injector temperature was set at 250 °C and the transfer line was set at 280 °C (split ratio:1/100). We used helium (99.995% purity) for carrier gas and 1.2 ml/min for flow rate. We injected 2 µl of the sample. About the oven temperature program we followed the next steps; beginning with 50 °C for 1 min, increased to 250 °C by rate of 7 °C/min and finishing by maintained 5 min at 250 °C. And when as the mass spectrometer detection, an ionization voltage of 70 eV, 150 °C for the ion source temperature and a mass range of 50–550 m/z was adopted to acquire the electron ionization mass spectra.
2.4.2 Determination of total phenolic compound
The entire amount of phenolic existent in olive leaf extracts was measured according to Singleton et al. where they use the Folin-Ciocalteau phenol reagent for the oxidation of all phenols (Singelton et al., 1999). Which is a combination of phosphomolybdic acid and phosphotungstic acid. The oxidation of phenolic compounds induces the reduction of this reagent to a complex of tungsten oxides and blue molybdenum. So the amount of phenol oxides is relative to this blue color which absorbs at 760 nm (Salah et al., 2012). In the experiment, 20% of Na2CO3 (1 ml) and distilled water (5 ml) were added to an aliquot of diluted methanolic extract (1 ml). Once shaking, we incubated the blend during 3 min at room temperature. And lastly, we added 1 ml of the diluted reagent solution (F-C) to the solution before it was maintained during 30 min at 40 °C. The reading wavelength was 760 nm. Based on the standard gallic acid curve, we have expressed the entire amount of phenolic as mg of gallic acid equivalent per gram dry weight (mg GAE/g). Our calibration curve range was 200–800 mg/l (R2 = 0.998). We were analyzed each sample three times.
2.4.3 Determination of total flavonoid
The entire amount of Flavonoids was determined as described by Dewanto et al.
We added 5%NaNO2 (75 μl) to methanolic extracts diluted (250 μl). After shaking for 6 min, 150 μl of 10% AlCl3then500 μl of NaOH (1 M) were added to the previous preparation. And to finish, we was adjusted our preparation by adding deionized water to reach 2.5 ml. The reading wavelength was 420 nm. Based on the standard catechin curve, we have expressed the entire amount of flavonoid as mg catechin equivalents per gram of dry weight (mg EC/g). We adopted the range of 100–600 mg/l (R2 = 0.997) in calibration curve. (Salah et al., 2012) We analyzed each sample three times.
3 Results and discussions
3.1 Polarisation curves
The evaluation of inhibitory effect of Olea europaea L. extract was effected using four solvents of different polarities; methanol (MeOH), ethyl acetate (EtOAc), dichloromethane (DCH) and hexane (hex). Their polarity index are depicted in Table 1.
Solvent Polarity
Index (PI)
Hexane
0
Dichloromethane
3,7
Ethyl acetate
4,4
Methanol
6,6
The cathodic and anodic polarization curves of mild steel after immersion in NaOH (0.1 M) + NaCl (0.5 M) in the absence and presence of different organic extracts from Olea europaea L. are illustrated in Fig. 1.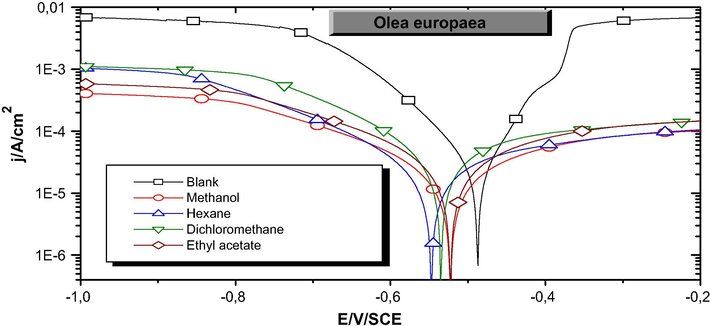
Polarization curves of mild steel after 1 h of immersion in NaOH (0.1 M) + NaCl (0.5 M) in the absence and presence of different organic extracts of Oleaeuropaea L.
A first analysis of these curves shows that the presence of all organic extracts from Olea europaea L. induces the decrease of anodic and cathodic current density translating the inhibition of both anodic metal dissolution and cathodic reduction reactions.
The values of the corrosion current density (jcorr), the corrosion potential (Ecorr), the cathodic and anodic Tafel slopes (βc and βa) and the inhibition efficiency (IEj%) for different polarities solvents are reported in Table 2. The inhibitory efficacy is defined as follows:
where
and
are the values of corrosion current density of steel, by extrapolation of cathodic or anodic straight lines of Tafel, after immersion in an alkaline medium respectively without and with addition of organic extracts.
Solvent
Ecorr/V/ECS
jcorr/µA/cm2
βa/mVdec−1
βc/mVdec−1
EIj (%)
Blank
−0.48
28.4
95.3
−160.5
–
Oleaeuropaea
Methanol
−0.52
2.3
64.2
−153.4
91.9
Hexane
−0.55
5.2
68.7
−158.6
81.7
Dichloromethane
−0.53
7.6
71.3
−154.5
73.2
Ethyl acetate
−0.52
4.2
65.4
−155.2
85.2
In reinforced concrete, Ecorr represents the potential value of reinforcement implanted in concrete resulting from the equilibrium between the anodic oxidation of iron and the cathodic reduction of dissolved oxygen (aerated medium) or water (des-aerated medium) (Constantin, 2011). The result shows that the Ecor values are practically unaffected by the adding of all the organic extracts, which indicates the mixed nature of these extracts. The corrosion current densities (jcorr) decrease translating the decrease of corrosion rates. The slope of anode Tafel line (βa) relative to the polarization of steel in NaOH (0.1 M) + NaCl (0.5 M) is 95.3 mV, by the addition of Olea europaea L. extracts it reflects a decrease in the oxidation current densities. However, the value of cathodic Tafel slopes (βc) has not almost changed. Thence we suppose that the impact of olive leaf extracts is more significantly on anodic reaction than cathodic reaction (Pustaj et al., 2016).
The inhibitory effect (EIj%) acquired is in the following order: MeOH, EtOAc, Hex then DCM.
So, it increases by increasing the polarity of the extraction solvent. The higher inhibition efficiency was obtained with the methanol extract, it reached 91.9%. This propose that the main phytochemicals of the olive leaf are very polar.
But there is a contradiction in hexane and dichloromethane, and knowing that:
-
The anti-corrosive effect and subsequently the antioxidant effect of the Olea europaea L. extract is attributed mainly to the phenolic compounds (Paula Pereira et al., 2007; Salah et al., 2012; Issaoui et al., 2012).
-
The extraction of compounds having the highest antioxidant potential, is efficient with methanol seen its index of high polarity (100%).
-
Semi-polar solvents, Ethyl acetate and Dichloromethane, allow the extraction of proportionally polar compounds.
-
Only the lipophilic compounds can be extracted with hexane following its zero as a polarity index (Abarca-Vargas et al., 2016).
We can say that with this study it hasn’t been possible to specify the chemical compound responsible for anticorrosive activity, but it has been confirmed that the entire plant is recommended for a noble source of inhibitor. And we think that other factors may influence the inhibition efficiency other than the polarity of the extraction solvent; such as the molecular size, the carbon chain length, the conjugated bonding and aromaticity, the aptitude of film to be dense or reticulated, the resistance of the bond to the metal substrate, the number and nature of bonding groups and atoms within molecule and an appropriate solubility of phenolic compounds in solvent extraction.
3.2 Electrochemical impedance spectroscopy (EIS)
In reinforced concrete, seen its alkaline medium, carbon steel is spontaneously protected by forming a passive oxide film. However, contamination by chlorides or carbonation causes a deterioration of the passive layer and subsequently the initiation of pitting corrosion (Chakri, 2015; Constantin, 2011; Kumar and Ramesh Singh, 2013). This phenomena, characterized by a low polarization resistance indicating the rapid dissolution of passive film, is represented by a constant platform as it is clear in the curve of blank solution in Fig. 2. After adding the Olea europaea L. extracts, intended to act as an inhibitor, we noticed a disappearance of the platform with all different organic extracts used with an increase in the polarization resistance, proving the effectiveness of the extract in the protection of the oxide film already formed.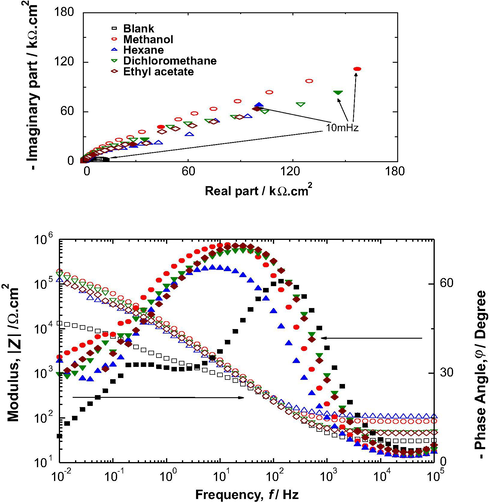
Impedance spectra in Nyquist and Bode plots of system: mild steel in NaOH(0.1 M) + NaCl(0.5 M)in the absence and presence of organic extracts fromOleaeuropaea leaves.
The Nyquist diagrams plotted with the different organic extracts have a capacitive arc whose diameter probably depends on the polarity of the extraction solvents used, reflecting the protective nature of the oxide layer thus formed. In practice, the higher the low-frequency limit of the impedance or polarization resistance Rp, the more protective the oxide layer is and therefore the more the solution is passive. As of Fig. 3, it appears that the highest impedance modulus was obtained with the addition of the methanolic extract of Olea europaea L. in the aggressive solution, which is in agreement with the polarization curves.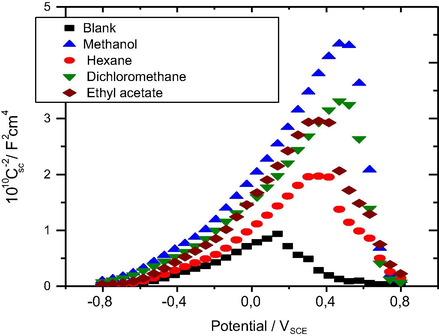
Mott-Schottky plots of the capacitance behavior of system: mild steel/NaOH (0.1 M) + NaCl (0.5 M) without and with Oleaeuropaea L.extracts.
It has been reported in previous studies that organic compounds acting as a corrosion inhibitor contain an active center (such as nitrogen) which by adsorbing onto the steel surface reacts chemically with the oxide layer (Deposition of rust); changing thus its structure to generate a barrier surface network against the diffusion of oxygen improving the passivity of the oxide layer (Chakri, 2015; Constantin, 2011; Faustin, 2013).
The adsorption of organic compounds is a direct result of the electrostatic interactions between the comparatively weakly bound electrons, such as the anionic and organic molecules and the heterocyclic compound having single-pair electrons, or the p-electrons with the vacant d-orbitals of metallic iron atoms (Tezeghdenti et al., 2015).
The difference in the inhibitory effect obtained between the various organic extracts can be explained by the fact that the adsorption mechanism, or the inhibition action, could be affected mainly by the polarity which contributes to the formation of strong bonds but also by other parameters such as the size of organic molecules, the number of functional groups and the adsorption rate of inhibitory compounds (Tezeghdenti et al., 2015).
3.3 Mott-Schottky analysis
Fig. 3 depicts the Mott-Schottky curves for the protective layer formed around mild steel immersed for 1 h in alkaline solution before and after the addition of organic extracts from Olea europaea L.
With all the solvents M-S plots are characterized by two linear zones. In the potential zone the positive slope proves the n-type semi-conductivity of protective layer on carbon steel surface, indifferent to the presence of Cl− in the solution. On the other hand the negative slope indicates the p-type semi-conductivity. We therefore believe that this transformation is due to the deterioration of the protective film, which can be attributed to the predominance of iron ion deficiency on oxygen deficiency in the protective film (Azumi et al., 1987).
3.4 Chemical composition
As the efficacy of organic inhibitor is related to its structure, the chemical identification of our plant is a necessity. We present in Table 3 the compounds identified by GC–MS analysis from the methanol Olea europaea L. extract.
Tr (min)
Compounds
Structure
5.816
Benzene, 1-ethyl-3-methyl-
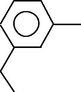
6.319
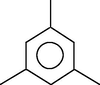
Mesitylene
6.966
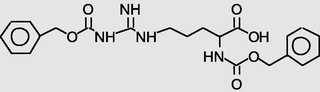
N-α,N-ω-Di-cbz-L-arginine
8.213
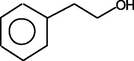
PhenylethylAlcohol
8.992
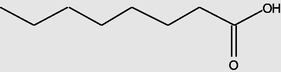
Octanoicacid
9.404
L-α-Terpineol
9.758
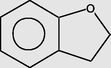
Benzofuran, 2,3-dihydro-
10.416
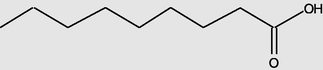
Nonanoicacid
10.783
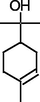
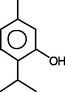
Thymol
10.937
Phenol, 2-methyl-5-(1-methylethyl)-

11.063
Benzaldehyde, 2-hydroxy-3-methoxy-
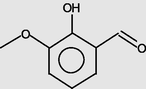
11.149
2-Methoxy-4-vinylphenol
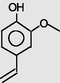
11.584
Geranicacid
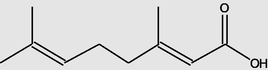
11.658
3-Pyridinecarboxylic acid, 5-ethenyl-, methyl ester
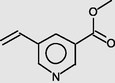
11.727
Eugenol
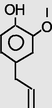
11.853
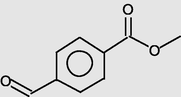
Benzoic acid,4-formyl-, methyl ester
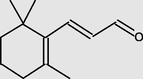
12.31
2-Propenal,3-(2,6,6-trimethyl-1-cyclohexen-1-yl)-
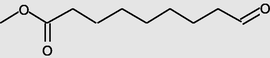
12.659
Nonanoicacid, 9-oxo-, methyl ester
13.678
Phenol,2,4-bis(1,1-dimethylethyl)-
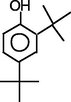
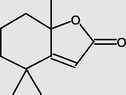
14.078
2(4H)-Benzofuranone,5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)-
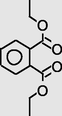
14.702
DiethylPhthalate
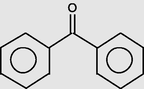
15.183
Benzophenone
15.372
Cyclopentaneacetic acid,3-oxo-2-pentyl-, methyl ester
16.27
1,7-di-iso-propylnaphthalene
16.464
Tetradecanoicacid
16.676
Phenol,2,6-bis(1,1-dimethylethyl)-4-ethyl-

16.808
1-Octadecanol
17.792
Phenethylamine, 3-benzyloxy-2-fluoro-β-hydroxy-
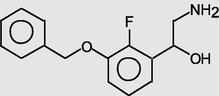
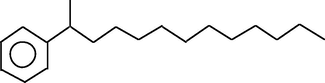
18.078
Benzene, (1-methyldodecyl)-
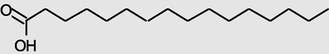
18.542
n-Hexadecanoicacid
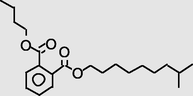
18.616
1,2-Benzenedicarboxylica cid,butyl 8-methylnonyl ester
18.833
1-Eicosene
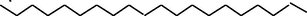
20.229
OleicAcid
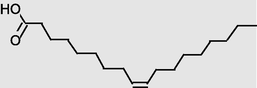
20.424
Octadecanoicacid
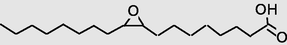
21.637
Oxiraneoctanoicacid, 3-octyl-, methyl ester, cis
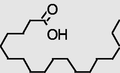
22.341
9-Octadecenamide, (Z)-
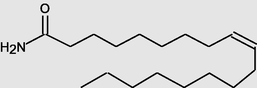
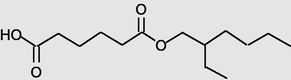
22.650
Hexanedioicacid, mono(2-ethylhexyl)ester
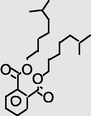
24.103
Diisooctylphthalate
The GC–MS analysis shows that the Olea europaea L. extract is rich in molecules containing the N and O heteroatoms in the aromatic ring and in the side chain in addition to double bonds and polar functional groups (—OH, —NH2). We therefore believe, based on previous studies that the corrosion inhibition of steel by this plant is due to the adsorption of phytochemicals that contain π-electrons besides the oxygen and azote atoms in their molecules. It is known that they represent the adsorption centers onto the metal surface.
And this confirms that most ecological inhibitors consist of organic compounds containing polar groups that enhance the adsorption on the metal surface. Indeed, several studies have shown that organic inhibitors containing heteroatoms particularly N, O and S either in the ring or in the side chain in the addition to multiple bonds, aromatic rings and polar functional; such as —NH2, —OH, NO2, —CN or —SH, act as effective corrosion inhibitors. Also many N-heterocyclic compounds have been proved to be effective inhibitors (Sebhaoui et al., 2017; Marzorati et al., 2019).
3.5 Total phenols and flavonoids
The total phenols content in the extract from Olea europaea L. was 201.07 mg GAE per gram of dry extract. And the entire amount of flavonoids was 46.08 mg catechin per gram of dry extract. So, it yielded a high phenol and flavonoid content comparing with other plants (Dieng and Fall Dior, 2015). Certainly, Olea europaea L. is abundant in polyphenols, where the Oleuropein present the main phenolic compound and the rutin, one of the most bioactive flavonoids, is the second abundant component (Dekanski et al., 2009). In addition to verbacoside, luteolin-7-glucoside and apigenin-7-glucoside (Paula Pereira et al., 2007; Salah et al., 2012; Issaoui et al., 2012; Silva et al., 2006).
Recent studies evidenced the antioxidant and antimicrobial activity of Olea europaea L. extract (Silva et al., 2006). In fact, the aromatic rings (C6) accompanied by hydroxyl groups that characterize the structure of phenolic compounds are responsible for this antioxidant activity (Michalak, 2006), by offering the aptitude of trapping free radicals, donating electrons and hydrogen and chelating metals, what is called the structure activity relationships “SAR” (Michalak, 2006; Balasundram et al., 2006).
Therefore, the aptitude of acting like a reactive oxygen scavengers gives polyphenols class, especially flavonoids as well as phenylcarboxylic acid derivatives, the highest priority to be the most recommended substitute to biological inhibitors (Pirvu, 2014). Hence, olive leaf extract seems to be one of the best inhibiting products combining both anticorrosion and anti-biodegradation properties.
4 Conclusion
In conclusion, we can say that the choice of the Olea europaea leavesis successful for effective protection against corrosion of steel in reinforced concrete made forsea environment. It provides a highly effective protection of mild steel against deterioration in basicchloride solution, especially with methanol extract which reaches a maximum of inhibition of 91.9%. This inhibition activity against corrosion could be due to the presence of N, O and π-electrons, shown by GC–MS analysis. The Olea europaea leaves have been found to be high inphenol and flavonoid content.
Polarisation studies showed that extract from olive leaves is a mixed type inhibitor in NaOH (0.1 M) + NaCl (0.5 M), with a prevailing influence on the anode process. Electrochemical impedance spectroscopy, polarization curves and Mott-Schottky analyses give coherent results. The polarity of the extraction solvents influences the effectiveness of inhibition, which increases with polarity. The inhibition rate of hexane and dichloromethane extract shows that other factors besides the polarity affect the adsorption mechanism.
Acknowledgements
This work received support from Grant N°2015319 awarded by the Research Center, Scientific Research Deanship, Imam Abdulrahman Bin Faisal University, KSA.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Characterization of chemical compounds with antioxidant and cytotoxic activities in bougainvillea x buttiana Holttum and Standl, (var. Rose) extracts. Antioxidants. 2016;5(4):45.
- [CrossRef] [Google Scholar]
- Green BambusaArundinacea leaves extract as a sustainable corrosion inhibitor in steel reinforced concrete. J Clean Prod.. 2014;67:139-146.
- [CrossRef] [Google Scholar]
- Mott-Schottky plot of the passive film form don iron in neutral borate and phosphate solutions. J. Electrochem. Soc.. 1987;134:11352-11357.
- [Google Scholar]
- Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem.. 2006;99:191-203.
- [CrossRef] [Google Scholar]
- Boukhedenna, W., Fiala, A., Daghboudj, S., 2005. Inhibition de la corrosion de l’acier au carbone en milieu HCl 1M par un composé organique. https://www.researchgate.net/publication/307967520.
- In The performance of concrete structures in the marine environment. In: Symposium on Corrosion in the marine environment, International Corrosion Conference. London: Institute of Marine Engineers; 1973. p. :50-57.
- [Google Scholar]
- Compréhension des mécanismes d’inhibition de la corrosion des armatures métalliques des bétons par des molécules d’origine biologique. Génie chimique, Université Pierre et Marie Curie - Paris VI; 2015.
- [Google Scholar]
- Etude de l'efficacité d'inhibiteurs de corrosion utilisés dans les liquides de refroidissement. INSA de Lyon, Universitée de Pitesti (Roumanie); 2011.
- [Google Scholar]
- Prestress loss diagnostics in pre-tensioned concrete structures with corrosive cracking. J. Struct. Eng. 2019
- [CrossRef] [Google Scholar]
- Phytochemical analysis and gastroprotective activityof an olive leafextract. J. Serb. Chem. Soc.. 2009;74(4):367-377.
- [CrossRef] [Google Scholar]
- Dosing of polyphenols and antioxidant activity of leaves and inflorescences males of Borassusaethiopum, Mart. (Arecaceae) Int. J. Biol. Chem. Sci.. 2015;9(1):1067-1071.
- [CrossRef] [Google Scholar]
- Identification and utilization of banana plant juice and its pulping liquor as anti-corrosive materials. J. Sci. Ind. Res.. 2001;60(9):738-747.
- [Google Scholar]
- Use of impedance measurements for determination the instant rate of metal corrosion. J. Appl. Electrochem.. 1972;2:71-79.
- [CrossRef] [Google Scholar]
- The use of migrating corrosion inhibitors to repair motorways' concrete structures contaminated by chlorides. Cement Concr. Res.. 2005;35(3):551-561.
- [CrossRef] [Google Scholar]
- Semiconducting properties of thermally grown oxide films on AISI 304 stainless steel. Corros. Sci.. 2000;42(4):687-702.
- [CrossRef] [Google Scholar]
- International Energy Agency, 2009. World Business Council for Sustainable Development, Cement Technology roadmap- Carbon emissions reductions up to 2050. https://www.iea.org/newsroom/news/2018/april/cement-technology-roadmap-plots-path-to-cutting-co2-emissions-24-by-2050.html.
- Composition of the olive tree bark: richness in Oleuropein. Trends Chem. Eng.. 2012;14
- [Google Scholar]
- Study of antioxidant activity and anticorrosion action of the methanol extract of dwarf palm leaves (Chamaeropshumilis L.) from Morocco. J. Mater Environ. Sci.. 2014;5(3):887-898.
- [Google Scholar]
- Koch, G., Varney, J., Thompson, N., Moghissi, O., Gould, M., Payer, J., 2016. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study, Gretchen Jacobson, NACE International ed., Houston, Texas. http://impact.nace.org/documents/Nace-International-Report.pdf.
- A study on corrosion of reinforcement in concrete and effect of inhibitor on service life of RCC. J. Mater. Environ. Sci.. 2013;4(5):726-731.
- [Google Scholar]
- Electrode potential monitoring of effect of plants extracts addition on the electrochemical corrosion behaviour of mild steel reinforcement in concrete. Int. J. Electrochem. Sci.. 2011;6:3452-3465.
- [Google Scholar]
- Inhibition effect of vernoniaamygdalinaextract on the corrosion of mild steel reinforcement in concrete in 3.5M NaCl environment. Int. J. Electrochem. Sci.. 2013;8:11087-11100.
- [Google Scholar]
- Faustin, M., 2013. Etude de l’effet des alcaloïdes sur la corrosion de l’acier C38 en milieu acide chlorhydrique 1M : Application à Aspidosperma album et Geissospermumleave (Apocynacées), Université des Antilles et de la Guyane, Institut del’Enseignement Supérieur de la Guyane, École doctorale pluridisciplinaire :Santé, Environnement et Sociétés dans les Amériques. https://www.theses.fr/2013AGUY0578.pdf.
- Phytochemical characteristics of Citrus peel and effect of conventional and nonconventional processing on phenolic compounds: a review. Food Rev. Int.. 2016;33(6):587-619.
- [CrossRef] [Google Scholar]
- Green corrosion inhibitors from natural sources and biomass wastes. Molecules. 2019;24(1):48.
- [CrossRef] [Google Scholar]
- Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud.. 2006;15(4):523-530.
- [Google Scholar]
- Effects of phyllanthus muellerianus leaf-extract on steel-reinforcement corrosion in 3.5% NaCl-immersed concrete. Metals. 2016;6:255.
- [CrossRef] [Google Scholar]
- Investigation and evaluation of the corrosion inhibition properties of water hyacinth extract on low carbon steel. Int. J. Emerg. Eng. Res. Technol.. 2017;5:45-50.
- [Google Scholar]
- Phenolic compounds and antimicrobial activity of olive (OleaeuropaeaL. Cv. Cobrançosa) leaves. Molecules. 2007;12:1153-1162.
- [CrossRef] [Google Scholar]
- Pierre Ollivier, J., Vichot, A, 2008. La durabilité des bétons, Association technique de l'industrie des liants hydrauliques, Paris Presses de l'École nationale des ponts et chaussées DL ed. http://bibliotheque.bordeaux.fr/in/details.xhtml?id=mgroup%3A9782859784348.
- Polyphenols and Herbal-Based Extracts at the Basis of New Antioxidant, Material Protecting Products. IntechOpen; 2014. https://doi.org/10.5772/57183
- Olive leaf extract as a corrosion inhibitor of carbon steel in CO2-saturated chloride-carbonate solution. Int. J. Electrochem. Sci.. 2016;11:7811-7829.
- [CrossRef] [Google Scholar]
- Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur. J. Lipid Sci. Technol.. 2012;114:974-982.
- [CrossRef] [Google Scholar]
- Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Med. chem.. 2012;2(5):107-111.
- [CrossRef] [Google Scholar]
- Protection cathodique du béton armé par revêtement electro-conducteur autonome. Génie civil, INSA de Toulouse; 2018.
- [Google Scholar]
- Corrosion control of mild steel in hydrochloric acid by a 1,5- benzodiazepine derivative: electrochemical and adsorption studies. JMES. 2017;8(10):3666-3675.
- [Google Scholar]
- Phenolic compounds and antioxidant activity of Oleaeuropaea L. fruits and leaves. Food. Sci. Technol. Int.. 2006;12(5):385-396.
- [Google Scholar]
- Study of passive films formed on AISI 304 stainless steel by impedance measurements and photoelectrochemistry. J. Electrochem. Soc.. 1990;137:182-187.
- [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin– Ciocalteu reagent. Meth. Enzymol.. 1999;299:152-178.
- [CrossRef] [Google Scholar]
- The litchi (Litchi Chinensis) peels extract as a potential green inhibitor in prevention of corrosion of mild steel in 0.5 M H2SO4 solution. Arab. J. Chem.. 2019;12:1035-1041.
- [CrossRef] [Google Scholar]
- Classification of the solvent properties of common liquids. J. Chromatogr. A. 1974;92(2):223-230.
- [CrossRef] [Google Scholar]
- Improvement of concrete properties and reinforcing steel inhibition using a natural product admixture. J. Mater. Sci. Technol.. 1996;12(2):95-99.
- [Google Scholar]
- Corrosion inhibition of carbon steel in 1 M sulphuric acid solution by extract of eucalyptus globulus leaves cultivated in Tunisia arid zones. J. Bio. Tribo. Corros.. 2015;1:16.
- [CrossRef] [Google Scholar]
- Inhibitive action of ethanol extracts from Nauclealatifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arab. J. Chem.. 2013;6:285-293.
- [CrossRef] [Google Scholar]
- Concrete cracking prediction under combined prestress and strand corrosion. Struct. Infrastruct. Eng.. 2019;15(3):285-295.
- [CrossRef] [Google Scholar]







