Translate this page into:
One-dimensional copper oxide nanorods with potential anticancer effects against melanoma cells
⁎Corresponding author at: No 87, Xiangya Road, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China. hucjxy@csu.edu.cn (Chenchen Zuo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, one-dimensional copper oxide nanorods (CuO NRs) as new type anticancer particles were synthesized by hydrothermal method along with sonication. Afterward, the biological properties of synthesized CuO NRs against murine B16F10 melanoma with high metastatic potential and normal fibroblast cell line (3 T3) were evaluated. XRD pattern of synthesized CuO NRs revealed a crystalline structure and FT-IR study disclosed that bands at 438–607 cm−1 and 1010 cm−1 are typically associated with the stretching and bending vibrations of the Cu–O bond. DLS study indicated that prepared NRs have a size distribution in the range of 37–99 nm in solution, with a zeta potential value of −37.00 mV. Finally, TEM analysis demonstrated that CuO NRs have a nanorod-like morphology with an average particle size of 5 nm in width and 25–100 nm in length. Furthermore, we discovered that CuO NRs provided an apparent and selective antiproliferative effect on B16F10 melanoma cells at a minimum concentration of 10 µg/ml, associated with induction of membrane leakage and elevation of oxidative stress. Also, induction of apoptotic process was observed in melanoma cells following exposure to CuO NRs mediated by elevation of cytochrome c, AIF, caspase-3, caspase-9, Bax, and downregulation of Bcl-2 genes and proteins. Therefore, it was deduced that both caspase-dependent and caspase-independent pathways were associated with anticancer mechanisms of CuO NRs against B16F10 melanoma. In conclusion, CuO NRs could be appointed as potential nanostructures in the modulation or prevention of skin cancer and may be an indication of a new potential anticancer compound in the treatment of melanoma, which warrants further investigations in future studies.
Keywords
Copper oxide nanorods
Melanoma
Signaling pathway
1 Introduction
Nanotechnology has revolutionized the fields of biology and medicine (Vo-Dinh, 2007). Today, new products based on nanotechnology have entered the market more than ever, and the results of research in this field are rapidly being commercialized. This technology improves production processes with potential specifications and functions. In the coming years, nanotechnology-related applications are expected to cover almost all industrial sectors and enter the consumer market widely. Therefore, due to the bright future of nanotechnology, different countries are making large investments in this field. Increased cancer cell resistance to anticancer drugs causes serious health problems. Therefore, there is a strong incentive to improve alternative therapeutic modalities. The size of nanomaterials is similar to that of many biomolecules, which enables them as useful compounds for biomedical research and applications (Goodsell, 2000).
The synthesis of nanostructure-based particles with specific physicochemical properties has recently received much attention in medical science. In comparison with the size/dimension control, morphology/shape regulation of nanostructures is also demanded by biomedical researchers. Recently, several reports have depicted that the anticancer activity of nano-based particles relies on not only their sizes but also their shapes (Kessentini and Barchiesi, 2012; Mao et al., 2020). Hence, for biomedical application, especially cancer therapy the regulation of shape in addition to the size of nanostructure-based particles is crucial.
As the population ages, skin tumor morbidity in China is becoming a major concern, where age, gender and occupation are known as the main factors significantly influencing skin tumors (Huang et al., 2023). A malignant alteration of melanocytes found in the epidermis is referred to as melanoma (Shain and Bastian, 2016). Although the early stage of melanoma can be almost curable using appropriate treatments, the metastatic melanoma could bring unfavorable consequences for prognosis and therapy. Also, due to the occurrence of drug resistance in malignant melanoma, possibly hinders its curation with the currently used chemotherapeutics, which results in minimizing the survival rate of melanoma patients (Grossman and Altieri, 2001; Kong et al., 2022). Additionally, the currently used chemotherapeutics trigger several side effects (Bhatia et al., 2009). Based on these facts, it is crucial to explore the new potential chemotherapeutics which are able to selectively inhibit the proliferation of malignant melanoma cells.
Due to a wide range of anticancer applications of CuO nanostructure-based particles based on serving as responsive platforms against photothermal tumor ablation (Gao et al., 2021) or apoptosis induction in several kinds of cancers such as breast (Mahmood et al., 2022), cervical (Chen et al., 2021), prostate (Wang et al., 2017), and other cancer cells (Wang et al., 2017), the production of nanostructured CuO has attracted considerable attention in this field. The unique characteristics of one-dimensional (1D) nanostructured particles have made nanorods (NRs) very interesting in biomedical applications. Even though different synthesis routes have been reported in the literature to manipulate the morphologies and nanostructures, the hydrothermal method is known as one of the most feasible approaches to produce ID nanoscale CuO particles (Vargeese and Muralidharan, 2012). Due to the anisotropic nature of the crystal formation, it is more likely to grow slowly in the direction of the ID under hydrothermal conditions (Vargeese and Muralidharan, 2012).
Although, the induction of apoptosis have been reported to be exclusively associated with the caspase proteases (Donovan and Cotter, 2004), it is becoming increasingly known that apoptosis can also occur through a caspase-independent signaling pathway (Donovan and Cotter, 2004, Bhadra, 2022, Hussar, 2022) which can be regulated through overexpression or leakage of apoptosis-inducing factor (AIF) from mitochondria. It should be noted that mitochondrial function plays a key role in both caspase-dependent and-independent apoptosis (Bhadra, 2022). However, the associated mechanisms induced by nanostructure materials to govern the efflux of pro-apoptotic or anti-apoptotic molecules are largely unknown. Therefore, in the present study, we aim to report a bottom-up strategy for preparing CuO NRs via the hydrothermal/ultrasonication method followed by exploring their anticancer effects against melanoma cells through evaluation of apoptosis markers.
2 Materials and methods
2.1 Materials
Copper acetate monohydrate, Cu (CH3COO)2 H2O, urea, and other materials used in this study were of analytical grade (Merck China Ltd.) and used without further purification.
2.2 Synthesis of CuO NRs
The hydrothermal method along with sonication was used to synthesize CuO NR based on a previous study with some modifications (Vargeese and Muralidharan, 2012). Cu(CH3COO)2·H2O and urea dissolved in double deionized water (DDW) with a stock concentration of 0.025 M were mixed (10 ml each), heated in a Teflon-lined stainless steel oven at 150 °C for 45 min followed by cooling down to room temperature, washed with ethanol, collected, dried at room temperature for 50 min, redispersed in ethanol, and exposed to ultrasonication (150 T, 40 kHz, 320 W) for 2 h. Finally, the colloidal solution was dried under reduced pressure at room temperature for 2 h and utilized for further analyses.
2.3 Characterization of CuO NRs
X-ray diffraction (XRD) pattern of synthesized CuO NRs was detected using a Shimadzu XRD-6000 X-ray diffractometer (Shimadzu, Japan) with Kα radiation (λ = 1.54059 nm). Fourier transform infrared spectroscopy (FT-IR) analysis was performed to reveal probable vibrational bonds presented in synthesized CuO NRs using an 8400S FT-IR (Shimadzu, Japan), with a standard potassium bromide pellet technique. The mean hydrodynamic size and zeta potential of synthesized CuO NRs were done on a Zetasizer analyzer (HPPS-5001; MI, Worcestershire, UK) at room temperature. Transmission electron microscopy (TEM) analysis was conducted on a Zeiss Libra 120 model (Carl Zeiss, Oberkochen, Germany).
2.4 Cell culture
Murine B16F10 melanoma with a high metastatic potential and normal fibroblast cell line (3 T3) was purchased from the American Type Culture Collection (ATCC). The cells were cultured in DMEM (Sigma-Aldrich, Shanghai, China) medium supplemented with fetal calf serum (FCS) (10 %) (GIBCO, Shanghai, China) and antibiotics (1 %).
2.5 Cell viability and lactate dehydrogenase (LDH) assays
Cell viability of B16F10 melanoma and 3T3 normal fibroblast cells was done with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma Aldrich, Shanghai, China) assay. The cells (3 × 105 cells/well) were cultured in 96-well microplates for 24 h followed by the addition of various concentrations (0.1–100 μg/ml) of CuO NRs prepared in the medium. After 24 h of incubation, 20 μL of MTT solution (5 mg/ml) was added, incubated for 4 h at 37 °C, and finally cell viability was assessed spectrophotometrically at 570 nm using a microplate reader (Biotech Inc., USA). The maximum concentration of CuO NR which has a cytotoxic effect against B16F10 melanoma while is nontoxic against 3T3 normal fibroblast cells was considered for further cellular assays. To detect the LDH release, after incubation of B16F10 melanoma cells with a single concentration of CuO NRs for 24 h, LDH activity was assessed using a LDH Cytotoxicity Detection Kit (Cat. #MK401 v201906, Takara Bio, Japan) according to the protocol which was also reported previously (Filipova et al., 2018).
2.6 Intracellular glutathione (GSH) and reactive oxygen species (ROS) contents
Intracellular GSH and ROS generation were determined as per the methods reported by Mukhopadhyay et al. (Mukhopadhyay et al., 2018). Briefly, for determination of GSH content, B16F10 cells treated with a single concentration of CuO NRs for 24 h were lysed and centrifuged (3000 rpm for 15 min). Afterward, aliquots of supernatants were collected, diluted, and incubated for 10 min at ambient temperature. The absorbance of samples was read at 412 nm using a microplate reader (Biotech Inc., USA). For ROS assay B16F10 cells were added with a single concentration of CuO NRs for 24 h. After incubation, the media was replaced with 10 μM diacetyldichlorofluorescein (DCFDA-DA) and incubated in the dark for 30 min. After washing twice, the cells with PBS, the fluorescence intensity was read on a microplate reader in the excitation wavelength of 485 nm and emission wavelength of 538 nm.
2.7 Caspase activity assay using a microplate reader
To further explore the induction of apoptosis, the activity of caspase-3 and caspase-9 in B16F10 melanoma cells treated with a single concentration of CuO NRs was determined using Caspase Assay kits (Geno Technology) as reported previously (Shiraga and Adamus, 2002). Briefly, CuO NR-treated cells were lysed and centrifuged (14,000g, 4 °C, 30 min). Then, 50 μL of the supernatant with the similar volume of caspase assay buffer was mixed. The used caspase substrates were LEHD-AFC (Caspase 9) and DEVD-AFC (Caspase 3) and the assay was done at 37 °C for 2 h. The absorbances of samples was then read after 2 h using a microplate reader (Biotech Inc., USA).
2.8 Quantitative real-time PCR analysis
Quantitative real-time PCR analysis was done based on the previous study with minor modifications (Mukhopadhyay et al., 2018) on an ABI PRISM 7000 Real-Time system (Applied Biosystems). Briefly, B16F10 cells seeded, exposed to 10 μg/ml of CuO NRs for 24 h, were exposed to total RNA extraction using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) based on the standard protocols. Then, the RNA pellets were quantified using a NanoDrop ND-3000 spectrophotometer (Thermo-Fisher Scientific, Waltham, MA, USA). Reverse transcription was carried out by using a cDNA synthesis kit (Ribobio, Guangzhou, China) following the manufacturer’s protocol using a PrimeScript™ RT reagent kit (Thermo Scientific, Shanghai, China). The apoptotic genes such as Bax, caspase9, caspase3, AIF, and Bcl-2 were used for analysis, while β-actin was taken as the reference gene. The primer sequences were used as reported previously (Mukhopadhyay et al., 2018). The expression of genes was calculated based on the threshold (Ct) method, and the fold changes of mRNA were compared with the control samples.
2.9 Whole cell extract and western blotting
SDS-PAGE and western blots for proteins (whole or nuclear) were done based on the standard protocols. Detection analysis was carried out with HRP-conjugated secondary antibodies and visually assessed using the chemiluminescence detection approach. Primary antibodies used in this study were as following: anti-caspase-3 (1:2000, #9662, Cell Signaling), anti-caspase-8 (1:2000, #1C12, Cell Signaling), anti-Bcl-2 (1:1000, #2772, Cell Signaling), anti-Bax (1:1000, #2872, Cell Signaling), anti-AIF (1:5000, # 4642, Cell Signaling), and anti-β-actin (1:1000, #4970, Cell Signaling). The secondary antibody was anti-rabbit IgG-HRP (1:5000, #7074, Cell Signaling).
2.10 Statistical analysis
Data were analyzed using one-way ANOVA test and presented as mean ± SD. P < 0.05 was reported significant.
3 Result and discussion
3.1 Characterization of CuO NRs
Fabricating particles with dimensions < 100 nm has been known as an interesting area for researchers in the nanomedicine field. As the size of particles is reduced to the nanoscale dimension, nanomaterials tend to show unique and novel features that do not mimic those of their bulk counterparts (Auffan et al., 2009). These new properties make NRs, especially inorganic ones, to be used potentially in the biomedical field, especially cancer therapy (Pugazhendhi et al., 2018). Although these characteristics make nanostructures attractive for medical applications, complicated synthesis and characterization processes hamper their wide utilization. Therefore, the concern lies in determining the precise characterization methods/techniques with optimum capability for exploring the unique nanostructure's characteristics. As a result of this, in addition to the synthesis and production procedures, NRs must be characterized accurately in order to evaluate their properties. Several kinds of techniques can be utilized for nanostructure-based particle characterization, such as FT-IR, XRD, DLS, and TEM (Teimouri et al., 2018). Therefore, in this paper, after the synthesis of CuO NRs mediated by the hydrothermal method along with sonication, they were characterized by the above-mentioned techniques.
The crystalline structure of the hydrothermal method along with sonication-mediated prepared CuO NRs was analyzed through the XRD technique. Fig. 1A depicts the XRD pattern of synthesized CuO NRs, which depicts that the NRs provide a crystalline structure. The sharpness and narrowness of diffraction peaks reflect the high crystalline nature and tiny crystallite size of prepared NRs (Rao et al., 2020). According to the JCPDS card Nr. [00–048-1548], the detected diffraction patterns are associated with the appearance of the monoclinic CuO (space group C2/c) (Rao et al., 2020). The diffraction patterns were indexed as 32.2, 35.6, 38.6, 48.4, 53.2, 58.2, 62.0, 66.6, 68.6, 73.0, and 75.1 and could be assigned to the 1 1 0, 1 1 −1, 1 1 1, 2 0 −2, 0 2 0, 2 0 2, 1 1 −3, 3 1 −1, 2 2 0, 3 1 1, and 0 0 4 planes, respectively (Rao et al., 2020). A imilar XRD pattern has been also reported upon synthesizing CuO NRs assisted by electrochemical (Mousali and Zanjanchi, 2019) and surfactants (Rao et al., 2020)-based approaches.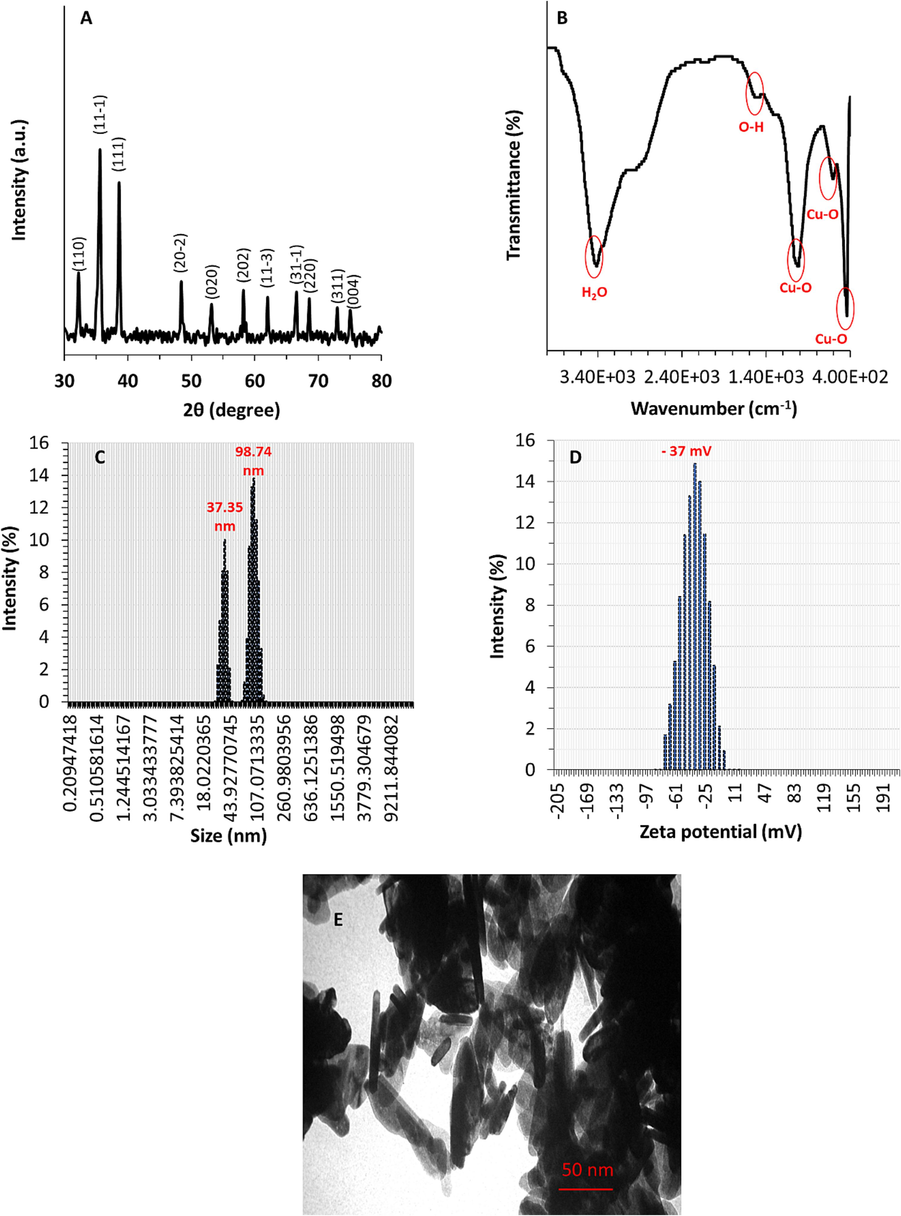
Characterization of CuO NRs synthesized through hydrothermal method along with sonication. (A) XRD, (B) FT-IR, (C) DLS, (D) Zeta potential, (E) TEM analyses.
Additionally, Fig. 1B displays the FT-IR spectrum of CuO NRs recorded in the range of 4000–400 wave number (cm−1) range using the FT-IR spectrophotometer assisted with the KBr pellet technique. The broad and sharp absorption bands were detected upon FT-IR analysis of synthesized CuO NRs. The bands at 438–607 cm−1 are typically associated with the stretching vibrations of Cu–O. The appearance of 1010 cm−1 band is attributed to the bending vibration of the Cu–O bond. A band at 1501 cm−1 corresponds to surface-bound OH moieties, and the broadband centered 3410 cm−1 is derived from stretching vibrations of water molecules in the CuO powder. FT-IR findings indicated that the as-prepared CuO NRs are of high purity. This data is in good line with previous studies, which implied that CuO NRs were synthesized through different methods with negligible contamination (Chen et al., 2008; Sonia et al., 2014; Rao et al., 2020).
Furthermore, DLS analysis was performed to characterize the size and zeta potential of as-synthesized CuO NRs. Fig. 1 C depicts that prepared CuO NRs possess two distinct peaks with the hydrodynamic size of around 37.35 nm and 98.75 nm, revealing that these NRs show a size distribution in the range of 37–99 nm in solution. Fig. 1D exhibits the zeta potential of prepared CuO NRs, revealing a charge distribution of about −37.00 mV. This data signified that the produced CuO NRs possess a high negative surface charge, which induces electrostatic resolution among NRs and prevents them from aggregating into big pieces (Pochard et al., 2003; Hu et al., 2023).
Fig. 1E depicts a high magnification TEM photograph of CuO NRs prepared via hydrothermal method along with sonication. From the TEM image of prepared CuO NRs, it was revealed that particles have a nanorod-like morphology with an average particle size of 5 nm in width and 25–100 nm in length. Furthermore, the CuO NRs were observed to be aggregated, however the sizes were almost regular. The sonication used in the synthesis approach plays a key role in dictating the colloidal stability and disaggregation of CuO NRs. However, it should be indicated that most inorganic nanostructure-based particles tend to aggregate upon TEM analysis.
3.2 Cytotoxicity assays
MTT as a tetrazolium dye with a yellow color can transform into formazan crystals with purple color catalyzed by mitochondrial enzymes. This transformation occurs in viable cells with functional mitochondria and cell damage can be reflected by assessing the optical density of formazan. Inorganic NRs can be used as efficient nanostructures in cancer therapy (Zhou et al., 2014; Wang et al., 2019; Hu et al., 2021). In this study, both B16F10 melanoma cells and 3T3 normal fibroblasts were incubated with various concentrations of CuO NRs (ranging between 0.1 and 100 μg/ml) for 24 h and cell viability percentage was determined based on MTT assay. CuO NRs showed concentration-dependent cytotoxicity, mainly in cancer cells, while in normal cells they do not display a significant (*P < 0.05) cytotoxicity up to 100 μg/ml (Fig. 2A). The 50 % reduction in cell viability value of the B16F10 cell line was observed following exposure to 100 μg/ml whereas around 80.37 % of 3T3 fibroblast cells were detected to be viable when incubated with the same concentration of CuO NRs (Fig. 2A). This data was in line with the previous study displayed that 102 μg/ml CuO nanostructure-based particles (around 40 nm) stabilized with Quisqualis indica extract could inhibit the proliferation of B16F10 and 3T3 fibroblast cells up to 50 % and 20 % compared to control cells, respectively (Mukhopadhyay et al., 2018).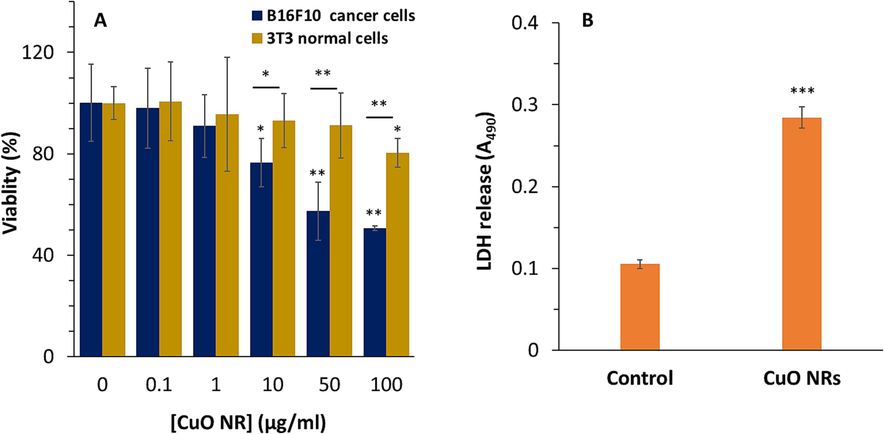
(A) The effect of increasing concentrations of CuO NRs after 24 h on cell viability of B16F10 melanoma cancer and 3 T3 fibroblast cells evaluated by MTT assay. (B) Cytotoxic influence of 10 µg/ml CuO NRs after 24 h on B16F10 cells determined by lactate dehydrogenase (LDH) assay. Data presented are mean ± SD of three experiments, (n = 3). Statistically significant at *P < 0.05, **P < 0.01, ***P < 0.001.
Therefore, the extent of cytotoxicity induced by CuO NRs was not very apparent against normal fibroblasts. Also, it was found that 10 μg/ml of CuO NRs triggered a significant cytotoxicity (*P < 0.05) against B16F10 cell line, while more than 90 % of 3T3 fibroblast cells were viable when incubated with this concentration. Therefore, 10 μg/ml of CuO NRs was chosen for further cellular and molecular assays. Sahoo et al. indicated that multifunctional CuO nanostructure-based particles (with a diameter of ∼13 nm) synthesized through green chemistry could be used for photothermal therapy, as 30 µg/ml of these particles exhibit the ability to damage ∼50 % of B16F10 melanoma cells assisted by the photothermal effect (Sahoo et al., 2023).
Lactate dehydrogenase (LDH) is an enzyme located in the cell cytoplasm that can be leaked to the cell culture medium after damaging the cell membrane induced by nanostructure-based particles. In the present study, a significant enhancement in LDH leakage from B16F10 melanoma cells incubated with 10 μg/ml of CuO NRs (for 24 h) was detected (Fig. 2B). Also, Mukhopadhyay et al. reported that CuO nanostructure-based particles modified with plant extract can induce antiproliferative activity against melanoma cancer cells mediated by cell membrane damage analyzed by LDH assay (Mukhopadhyay et al., 2018). It seems that cell membrane damage is one of the main mechanisms by that inorganic nanostructures with rod-like morphology induce their anticancer effects (Ahamed et al., 2017; Pan et al., 2017; Ali et al., 2022).
3.3 Intracellular GSH and ROS estimation
Reduced glutathione as a non-enzymatic antioxidant molecule demonstrating free radical scavenging capability. In the present study, B16F10 melanoma cells incubated with 10 µg/ml Cuo NRs expressed a significant (***P < 0.001) reduction in the content of cellular GSH (Fig. 3A).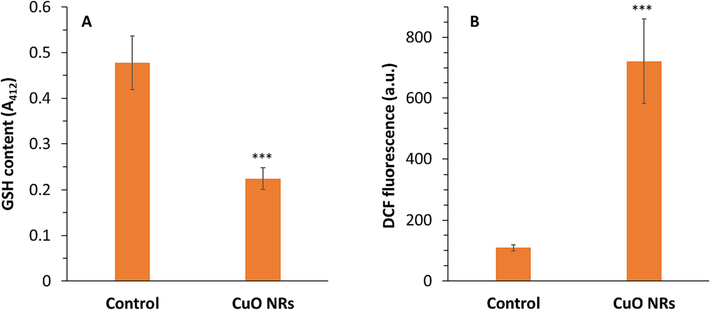
(A) The effect of 10 µg/ml CuO NRs after 24 h on reduced glutathione (GSH) content of the B16F10 cells determined by GSH assay. (B) The effect of 10 µg/ml CuO NRs after 24 h on intracellular reactive oxygen species (ROS) level of the B16F10 cells determined by DCF fluorescence intensity. Data presented are mean ± SD of three experiments, (n = 3). Statistically significant at ***P < 0.001.
Furthermore, it was determined that B16F10 cells incubated with 10 µg/ml CuO NRs experienced intracellular oxidative stress and this, in turn, led to the generation of ROS (Fig. 3B). In fact, the excessive free radical due to oxidative stress caused the intracellular generation of fluorescent 2′,7′-dichlorofluorescein (DCF). The level of ROS in cells treated with CuO NRs was found to be significantly (***P < 0.001) elevated relative to control cells. The above study indicated the generation of excessive ROS triggered-oxidative stress in cancer cells after CuO NR exposure. Indeed, the low content of GSH in CuO NRs-incubated cancer cells can be attributed to the elevated generation of ROS which is in good agreement with the findings reported by several studies (Ahamed et al., 2017; Mukhopadhyay et al., 2018). As a matter of fact, inorganic NRs can decrease the intrinsic antioxidant defense system of cells, which can be exploited to boost their therapeutic benefits (Cheng et al., 2019).
3.4 Apoptotic study
Overactivation of oxidative stress signaling pathways leads to DNA damage and cell proliferation reduction, finally resulting in the induction of apoptotic cell death (Obeng, 2020). Apoptosis as a process of programmed cell death can be induced by the activation of caspase (Brentnall et al., 2013). Caspase-9 and caspase-3 play a key role in the activation and execution of intrinsic apoptosis (Brentnall et al., 2013). In the present study, the activation of the caspase-mediated apoptosis pathway upon exposure of melanoma cells to CuO NR was qualitatively determined by caspase-9 and caspase-3 activity assay. In the present study treatment of the B16F10 cells with 10 μg/ml CuO NRs for 24 h was seen to result in a significant (***P < 0.001) increase in caspase-9 activity and caspase-3 activity (Fig. 4).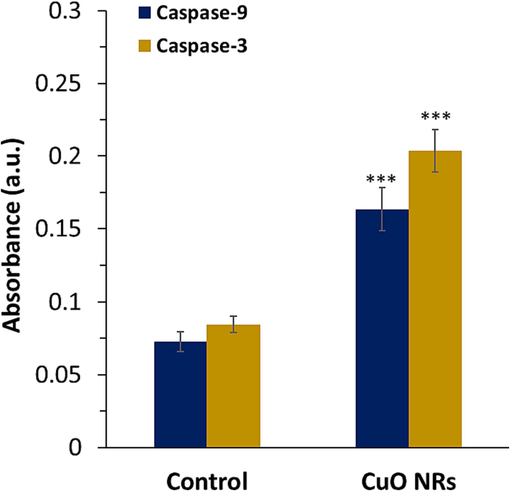
The effect of 10 µg/ml CuO NRs after 24 h on caspase-9 and −3 activity in the B16F10 cells determined by caspase activity assay. Data presented are mean ± SD of three experiments, (n = 3). Statistically significant at ***P < 0.001.
It was detected that the optical density was increased from 0.072 unit to 0.163 unit for caspase-9 and from 0.084 to 0.203 upon incubation of the B16F10 cell line with 10 μg/ml CuO NRs for 24 h. This study indicated that CuO NRs triggered cell death in the B16F10 cell line mediated by stimulating apoptosis, which is in good accordance with the previous study reported by Murugadas et al. (Murugadas et al., 2016).
In general, induction of apoptosis pathways would result in the overactivation of pro-caspase-3 into caspase-3, leading to cleavage of DNA and formation of apoptotic bodies (Obeng, 2020).
3.5 Gene expression assay of apoptotic mRNA
Further studies were conducted to elucidate the initiation and progression of apoptosis in B16F10 melanoma cell line following exposure to 10 μg/ml CuO NRs for 24 h. Therefore, the expression levels of several apoptotic marker mRNAs such as Bax, caspase9, caspase3, AIF, and Bcl-2 in the B16F10 cells were assessed upon exposure to CuO NRs.
Our results displayed that the mRNA levels of the apoptotic markers such as Bax, caspase-9, caspase-3, and AIF were significantly elevated in melanoma cells due to exposure to CuO NRs for 24 h (Fig. 5). Furthermore, it was detected that the expression of antiapoptotic mRNA, Bcl-2, was reduced after incubation of B16F10 melanoma cell line with 10 μg/ml CuO NRs for 24 h, which resulted in a significant increase in Bax/bcl-2 ratio. The mRNA level of AIF as an important marker of apoptosis which stimulates chromatin condensation/DNA fragmentation was 1.61-fold (**P < 0.01) higher in treated cells in comparison with the untreated cells. Furthermore, the effect of CuO NRs on the mRNA expression of Bax, caspase-9 and caspase-3 were evaluated. It was then determined that the expression levels of Bax, caspase-9 and caspase-3 were 1.61- (**P < 0.01), 1.34- (*P < 0.05) and 2.53-fold (***P < 0.001) higher in tread cells in comparison with the control cells. Finally, it was manifested that expression of Bcl-2 mRNA was 0.77-fold (*P < 0.05) less in tread cells in comparison with the control cells, which elevated the Bax/Bcl-2 ratio significantly (***P < 0.001) (Fig. 5).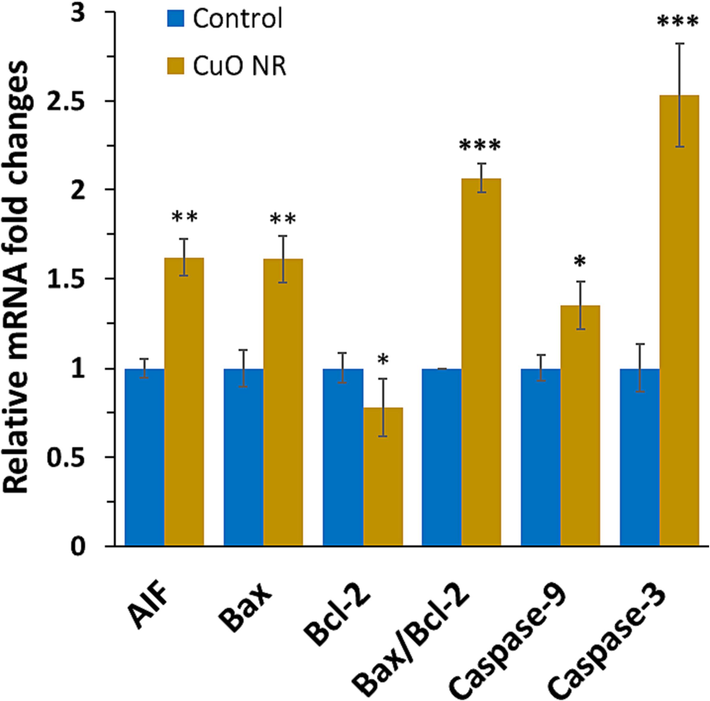
The effect of 10 µg/ml CuO NRs after 24 h on gene expression of the B16F10 cells determined by quantitative real-time PCR. Data presented are mean ± SD of three experiments, (n = 3). Statistically significant at *P < 0.0.05, **P < 0.01, ***P < 0.001.
Therefore, it can be suggested that in this study CuO NR incubation reduced the expression of anti-apoptotic mRNA, Bcl-2, while enhancing the levels of apoptotic AIF, Bax, caspase-9, and caspase-3. These data substantiate the induction of apoptosis in melanoma cells after incubation with CuO NRs maybe through a mitochondria-assisted intrinsic pathway (Murugadas et al., 2016), which needs further investigations in future studies. In general, this study revealed that CuO NRs promoted the expression of Bax and downregulation of Bcl-2, leading to the activation of caspase-9 and caspase-3, then apoptosis in B16F10 melanoma cells. Also, AIF has been reported to directly associate mitochondrial membrane permeabilization with apoptosis through caspase-independent pathways (Cande et al., 2004).
3.6 Protein expression assay for determination of caspase-dependent/independent pathways
Mitochondria can be influenced by different signaling pathways in several ways, including translocation of the mitochondrial matrix, modification of proteins, and formation of channels (Green, 2022). Nanostructure-based particles can trigger the permeability of the mitochondrial membranes and the resultant leakage of proteins into the cytosol and nucleus (Asweto et al., 2017). The pro-apoptotic proteins, Bak, Bax, and Bok, with oligomerization properties can result in the macropore formation in the mitochondrial membrane which enables the release of some mitochondrial proteins, while anti-apoptotic Bcl-2 can negatively regulate this process (Yang et al., 1997, Green, 2022). For example, cytochrome c following cytosolic release can mediate the formation of apoptosomes which activate caspase-9 and caspase-3. The caspase-independent pathway is associated with the release of AIF from mitochondria (Harmand et al., 2005). A drop in mitochondrial membrane potential is typically associated with AIF release from mitochondria, which can translocate to the nucleus to trigger DNA condensation and fragmentation (Harmand et al., 2005). Therefore, we investigated the release of cytochrome c, AIF and overexpression of Bax, Bcl-2, caspase-9, and caspase-3 protein in B16F10 melanoma cells following exposure to 10 µg/ml CuO NRs for 24 h. As revealed in Fig. 6, CuO NRs induce cytochrome c and AIF release from mitochondria in B16F10 melanoma cells. Also, the protein expressions of Bax, caspase-9 and caspase-3 were increased upon exposure of B16F10 melanoma cells to CuO NRs.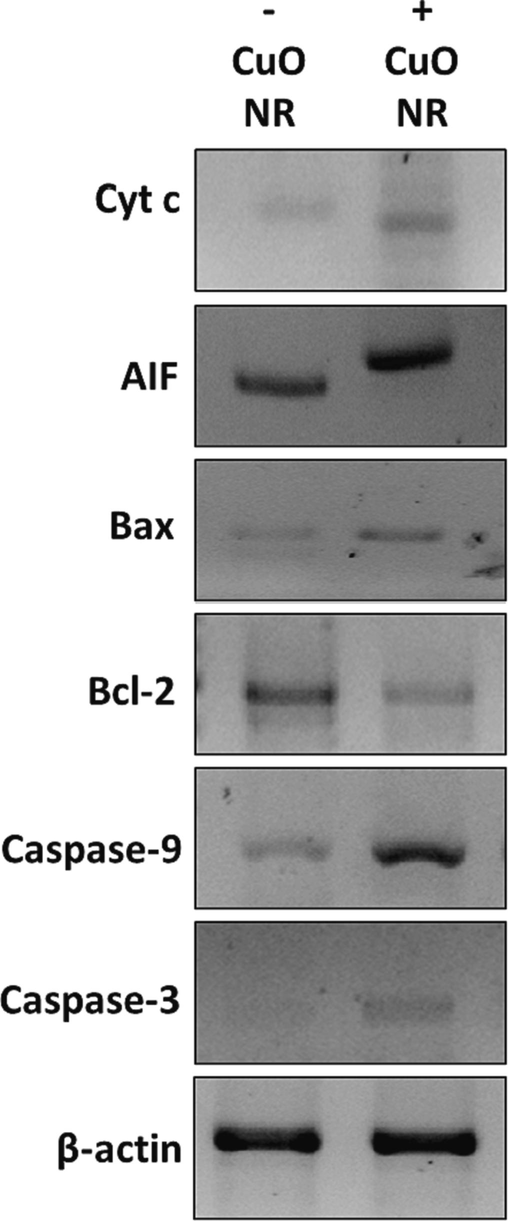
The effect of 10 µg/ml CuO NRs after 24 h on protein expression of the B16F10 cells determined by western blot.
This data clearly indicated that CuO NRs stimulated apoptosis in B16F10 melanoma cells through both caspase-dependent and caspase-independent pathways.
4 Conclusion
In conclusion, one-dimensional CuO NRs were synthesized through the hydrothermal method along with sonication and characterized by several techniques. Then, the anticancer mechanisms of CuO NRs against murine B16F10 melanoma were assessed by different techniques. It was observed that the size distribution of CuO nanostructures in solution was in the range of 37–99 nm with nanorod-like morphology and an average particle size of 5 nm in width and 25–100 nm in length in the powder form. Then, CuO NRs depicted a selective and significant anticancer effect in B16F10 melanoma cells. To our knowledge, these findings explicitly revealed for the first time that CuO NRs stimulate apoptosis in melanoma malignant cells. The induction of apoptosis contributed in CuO NR incubation was explicitly associated with the stimulation of the mitochondrial intrinsic pathway. Finally, we divulged that CuO NR incubation induced caspase overexpression and relevant activation as well as cytochrome c and AIF leakage, indicating that CuO NRs stimulated the induction of both caspase-dependent and caspase-independent signaling pathways. Therefore, CuO NRs can be used as encouraging nanostructures in the treatment or prevention of skin cancer, although other in vivo and clinical studies should be done in future studies to address the main limitation of this work.
Acknowledgements
The authors are grateful to AJC editor and reviewers for constructive comments on this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. Nanomedicine in Cancer, Jenny Stanford Publishing 2017:347-368.
- [Google Scholar]
- Anticancer and photocatalytic activities of zinc oxide nanorods synthesized from Manilkara littoralis leaf extract. Mater. Chem. Phys.. 2022;277:125541
- [Google Scholar]
- Cellular pathways involved in silica nanoparticles induced apoptosis: a systematic review of in vitro studies. Environ. Toxicol. Pharmacol.. 2017;56:191-197.
- [Google Scholar]
- Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol.. 2009;4(10):634-641.
- [Google Scholar]
- A mini review on molecules inducing caspase-independent cell death: a new route to cancer therapy. Molecules. 2022;27(19):6401.
- [Google Scholar]
- Treatment of metastatic melanoma: an overview. Oncology (Williston Park). 2009;23(6):488.
- [Google Scholar]
- Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol.. 2013;14:1-9.
- [Google Scholar]
- Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ.. 2004;11(6):591.
- [Google Scholar]
- Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: Attenuating the proliferation of cervical cancer cells. Artif. Cells Nanomed. Biotechnol.. 2021;49(1):240-249.
- [Google Scholar]
- Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J. Alloy. Compd.. 2008;464(1–2):532-536.
- [Google Scholar]
- Bismuth sulfide nanorods with retractable zinc protoporphyrin molecules for suppressing innate antioxidant defense system and strengthening phototherapeutic effects. Adv. Mater.. 2019;31(10):1806808.
- [Google Scholar]
- Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res.. 2004;1644(2–3):133-147.
- [Google Scholar]
- An effective “three-in-one” screening assay for testing drug and nanoparticle toxicity in human endothelial cells. PLoS One. 2018;13(10):e0206557.
- [Google Scholar]
- Near-infrared-light-responsive copper oxide nanoparticles as efficient theranostic nanoagents for photothermal tumor ablation. ACS Appl. Bio Mater.. 2021;4(6):5266-5275.
- [Google Scholar]
- The mitochondrial pathway of apoptosis Part II: The BCL-2 protein family. Cold Spring Harb. Perspect. Biol.. 2022;14(6):a041046
- [Google Scholar]
- Drug resistance in melanoma: mechanisms, apoptosis, and new potential therapeutic targets. Cancer Metastasis Rev.. 2001;20:3-11.
- [Google Scholar]
- Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer. 2005;114(1):1-11.
- [Google Scholar]
- Aggregation and disaggregation of Al2O3 nanoparticles: influence of solution pH, humic acid, and electrolyte cations. Colloid Polym. Sci. 2023:1-11.
- [Google Scholar]
- Se-modified gold nanorods for enhancing the efficiency of photothermal therapy: avoiding the off-target problem induced by biothiols. J. Mater. Chem. B. 2021;9(42):8832-8841.
- [Google Scholar]
- An epidemiological study on skin tumors of the elderly in a community in Shanghai, China. Sci. Rep.. 2023;13(1):4441.
- [Google Scholar]
- Quantitative comparison of optimized nanorods, nanoshells and hollow nanospheres for photothermal therapy. Biomed. Opt. Express. 2012;3(3):590-604.
- [Google Scholar]
- Endoplasmic reticulum stress in melanoma pathogenesis and resistance. Biomed. Pharmacother.. 2022;155:113741
- [Google Scholar]
- Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci. Rep.. 2022;12(1):16165.
- [Google Scholar]
- Gold nanospheres and nanorods for anti-cancer therapy: comparative studies of fabrication, surface-decoration, and anti-cancer treatments. Nanoscale. 2020;12(28):14996-15020.
- [Google Scholar]
- Electrochemical synthesis of copper (II) oxide nanorods and their application in photocatalytic reactions. J. Solid State Electrochem.. 2019;23(3):925-935.
- [Google Scholar]
- Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed. Pharmacother.. 2018;97:1373-1385.
- [Google Scholar]
- Hydra as a model organism to decipher the toxic effects of copper oxide nanorod: Eco-toxicogenomics approach. Sci. Rep.. 2016;6(1):29663.
- [Google Scholar]
- Apoptosis (programmed cell death) and its signals-A review. Braz. J. Biol.. 2020;81:1133-1143.
- [Google Scholar]
- Nuclear-targeting gold nanorods for extremely low NIR activated photothermal therapy. ACS Appl. Mater. Interfaces. 2017;9(19):15952-15961.
- [Google Scholar]
- Surface charge, effective charge and dispersion/aggregation properties of nanoparticles. Polym. Int.. 2003;52(4):619-624.
- [Google Scholar]
- Inorganic nanoparticles: a potential cancer therapy for human welfare. Int. J. Pharm.. 2018;539(1–2):104-111.
- [Google Scholar]
- Surfactant-assisted synthesis of copper oxide nanorods for the enhanced photocatalytic degradation of Reactive Black 5 dye in wastewater. Environ. Sci. Pollut. Res.. 2020;27:17438-17445.
- [Google Scholar]
- “Copper oxide nanoparticle: multiple functionalities in photothermal therapy and electrochemical energy storage.” Applied . Nanoscience 2023:1-22.
- [Google Scholar]
- Mechanism of CAR syndrome: anti-recoverin antibodies are the inducers of retinal cell apoptotic death via the caspase 9-and caspase 3-dependent pathway. J. Neuroimmunol.. 2002;132(1–2):72-82.
- [Google Scholar]
- Effect of NaOH concentration on structural, surface and antibacterial activity of CuO nanorods synthesized by direct sonochemical method. Superlattice. Microst.. 2014;66:1-9.
- [Google Scholar]
- Gold nanoparticles fabrication by plant extracts: synthesis, characterization, degradation of 4-nitrophenol from industrial wastewater, and insecticidal activity–a review. J. Clean. Prod.. 2018;184:740-753.
- [Google Scholar]
- Kinetics and mechanism of hydrothermally prepared copper oxide nanorod catalyzed decomposition of ammonium nitrate. Appl. Catal. A. 2012;447:171-177.
- [Google Scholar]
- Nanotechnology in biology and medicine: methods, devices, and applications. CRC Press; 2007.
- Dual targeted and pH-responsive gold nanorods with improved chemotherapy and photothermal ablation for synergistic cancer treatment. RSC Adv.. 2019;9(10):5270-5281.
- [Google Scholar]
- Cuprous oxide nanoparticles inhibit prostate cancer by attenuating the stemness of cancer cells via inhibition of the Wnt signaling pathway. Int. J. Nanomed. 2017:2569-2579.
- [Google Scholar]
- Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129-1132.
- [Google Scholar]
- Inhibition of cancer cell migration by gold nanorods: molecular mechanisms and implications for cancer therapy. Adv. Funct. Mater.. 2014;24(44):6922-6932.
- [Google Scholar]







