Translate this page into:
One-spot synthesis of a benzene-rich triazine-based hyperbranched charring agent and its efficient intumescent flame retardant performance for thermoplastic polyester elastomer
⁎Corresponding author. wuwei@ecust.edu.cn (Wei Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A highly effective hyperbranched charring agent (CDS) based on triazine and rich in benzene was synthesized. The intumscent flame retardant composed of CDS and AlPi can effectively flame retard TPEE and inhibit its droplets. Deep research has been done on the TPEE/AlPi/CDS condensed phase flame retardant mechanism.
Abstract
An intumescent flame retardant (IFR) system was created using a benzene-rich triazine-based hyperbranched charring agent (CDS) and aluminum diethlyphosphinate (AlPi) to enhance the flame resistance of thermoplastic polyester elastomers (TPEE). Thermogravimetric analysis (TGA) results revealed that CDS had substantial char residue (50.8 wt%) and good thermal stability at 700 °C. The limiting oxygen index (LOI) value achieved 30.2% and it passed the V-0 rating in vertical combustion (UL-94) with the integration of 20 wt% (wt%) IFR (AlPi:CDS = 3:1) into TPEE matrix. Scanning electron microscope (SEM), laser Raman spectroscopy (LRS), and heating infrared spectroscopy were used to examine the morphology and chemical makeup of char residues, as well as the evolution of the structure during the heating process. The outcomes demonstrated the compatibility of CDS and AlPi in both the gas phase and the condensed phase. After burning, the high-efficiency flame-retardant TPEE composite created a dense, continuous char layer that contained triazine rings and aromatic ring structures.
Keywords
Intumescent flame retardant
Triazine charring agent
Thermoplastic polyester elastomer
Aluminium diethlyphosphinate
1 Introduction
Thermoplastic polyester elastomer (TPEE), a polymer with the high elasticity of conventional vulcanized rubber at room temperature and the ability to be plasticized and molded at high temperatures, has recently gained popularity due to its many advantages in use and processing performance(Fang et al., 2019). Because of this, TPEE can be widely used in various fields such as automotive parts, wire and cable cladding, household appliances and industrial manufacturing, etc. In addition, with the renewal of electronic devices, flame retardant performance has become a common requirement for various standards. However, because to limitations imposed by their molecular structure, TPEE are combustible, melt and drip during combustion, and they are unable to self-extinguish(Huang et al., 2016; Wang et al., 2019). The study of flame retardant TPEE has become a prominent topic in recent years due to the constant increase of the requirements for material fire resistance and environmental protection performance.(Zhou et al., 2007; Kim et al., 2017; Zou et al., 2018; Bhatnagar and Mahanwar, 2020).
A fast evolving halogen-free flame retardant known as a “intumescent flame retardant” (IFR) typically consists of three components: acid source, gas source, and carbon source(Bourbigot and Duquesne, 2007). The dehydration of the carbon source into char is catalyzed by the acid source. The char layer is expansion and the concentration of combustibles in the combustion environment is reduced thanks to the incombustible gas the gas source produces(Chen et al., 2022; Chen et al., 2022; Shi et al., 2020). Halogen-free intumescent flame retardant systems are thought to benefit greatly from the acidity provided by phosphorus-based flame-retardants.(Hu et al., 2009; Li et al., 2016; Lu and Hamerton, 2002). It is known from Braun and Schartel(Braun et al., 2007; Gallo et al., 2009; Gallo et al., 2011); AlPi, an aluminum diethlyphosphinate, has demonstrated success in improving polyester's fire resistance. Our earlier research revealed that the intumescent flame retardant made of aluminum diethlyphosphinate and melamine phosphate may be employed to enhance the combustion performance of thermoplastic polyester elastomer in order to address the issue of melt dripping in the combustion process.(Zhong et al., 2014; Zhong et al., 2016; Zhang et al., 2018; Zhong et al., 2015; Liu et al., 2019). The system still requires the addition of some synergists, such as 0D(MoS2), 1D(CNTs(Zhong et al., 2015), 2D(montmorillonite(Zhong et al., 2016) and LDHs(Yue, 2019) and 3D(graphite(Zhang et al., 2018) fillers and biomaterials (lignin-derived bio-based flame retardant synergists(Yang et al., 2020), in order to enhance the charring performance of TPEE and meet the goal of avoiding combustion. However, if inorganic components are added in excess, TPEE's mechanical characteristics may deteriorate. Therefore, it's crucial for creating a new class of charring agent that is very effective and stable.
Triazine flame retardants, which have the advantages of low toxicity, low smoke, recyclability, and environmental protection, are triazine derivatives in molecular structure and nitrogen flame retardants in flame retardant elements(Mishra and Vasava, 2020). By copolymerizing cyanuric chloride and various organic diamines, Junfeng Zhou(Zhou et al., 2016), Caimin Feng(Feng et al., 2016; Feng et al., 2015), and others were able to create macromolecules containing various triazines that exhibit outstanding flame retardant qualities in polymer composites, particularly the capacity to produce char. In our previous research, we successfully synthesized a liner triazine-based char agent and compounded with AlPi to flame retard TPEE composites, with the char residue of only 11.31 wt%(Liu et al., 2019). However, hyperbranched polymers, due to their special structure, can be cross-linked to form a better graphitized structure and improve the charring performance(Tang et al., 2019; Wen et al., 2014).Furthermore, the benzene ring structure was a key component of the residual char, and benzene-rich char agent can effective improve the charring performance of composites, thereby improving flame retardancy(Zhou et al., 2016; Feng et al., 2012; Feng et al., 2016).
In this work, a benzene-rich triazine-based hyperbranched char agent (abbreviated as CDS in this article) was successfully synthesized. Flame-retardant TPEE composites were prepared using CDS and AlPi as intumescent flame retardants. Compared with the analogous triazine charring agents reported by other researchers, the incorporation of benzene-rich diamine as a chain extender enhance the charring ability of CDS in the intumescent flame retardant TPEE systems, which can degrade to produce aromatized structural units to be spliced into graphitized char layers. The further research was conducted on the mechanism of char forming process. The fire behavior, the thermal decomposition and the char formation mechanism of the flame retardant composites were studied by LOI, UL-94, CCT, TGA, SEM and LRS tests.
2 Experimental section
2.1 Materials
TPEE resin (H605, Tm = 193℃, Shore Hardness = 55D) was offered by Sunplas co., ltd (China). Cyanuric chloride (CNC, purity 99%) was purchased from Macklin Biochemical Co., Ltd. (Shanghai, China) and Diethyl aluminum hypophosphite(AlPi, OP935) was supplied by Clariant (Germany). 4,4-Diaminodiphenyl sulfone (DDS) was obtained from Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China). Sodium carbonate (NaCO3), 1, 4-dioxane was supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Synthesis of CDS
As showed in Scheme1, the specific preparation route of CDS are as follows. 4,4-Diaminodiphenyl sulfone (0.09 mol) and 400 ml 1, 4-dioxane were added to a 1000 ml four-necked flask with a thermometer and mechanical stirring. And then the solution of cyanuric chloride (0.06 mol) dissolved in 200 ml 1, 4-dioxane was dropwise added into the flask in 2 h at 25℃. After all the ingredients were added in the reaction system, the reacting mixture was stirred at 95℃ for 10 h. The by-products HCl generated during the reaction were neutralized by the reaction with Na2CO3. Finally, the reaction mixture was filtered and washed several times to remove the solvent and the excessive NaCl. After drying in a vacuum oven at 120℃ for 4 h, light yellow product was obtained and the yield is 92.2%.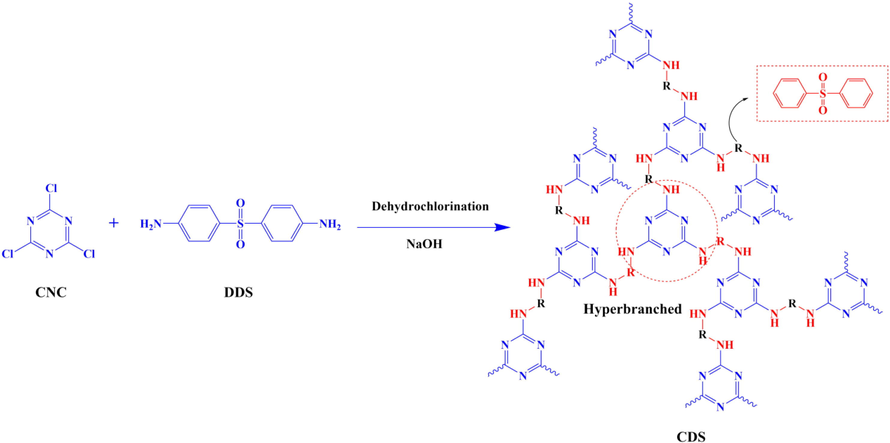
Synthesis of CDS.
2.3 Preparation of flame retardant TPEE samples
TPEE, AlPi, CDS were dried in a 90℃ constant temperature drying oven (DHG-9055A, Shanghai Hecheng Instrument Manufacturing Co., Ltd.) for 8 h, and weighed according to the research formula. All the components were melt-mixed in a torque rheometer (160 Nm/Polab QC, Germany Haake Company) at 205℃ and 60 r/min rotor speed. Specifically, the premix of TPEE and CDS was firstly added into torque rheometer and mixed for 3.5 min, and then AlPi were added and melt-mixed for another 2.5 min. After that, all the components were press molding on a flat vulcanizer (QLB-50, Shanghai Rubber Machinery First Factory) at 205℃. After hot-pressed for 5 min and holding pressure for 4 min, all samples were cold-pressed to room temperature to obtain suitable test specimens.
2.4 Characterization
The Fourier transform infrared was obtained with a Nicolet FTIR 6700 infrared spectrophotometer where the samples were prepared with KBr pellets.
The 13C solid-state NMR spectra were measured on a Thermo Varian INOVA500NB spectrometer at 500 MHz.
The vertical burning test (UL-94) was performed with a CFZ-3 type instrument (Jiangning Analysis Instrument Company, China) according to ASTM D3801-10 standard with specimen size of 130.0 mm × 13. 0 mm × 1.6 mm.
The Limiting oxygen index (LOI) was performed by an oxygen index instrument (JF-3, Jiangning Analysis Instrument Factory, China) with specimen size of 130.0 mm × 6.0 mm × 3.0 mm according to ASTM D2863-17.
Cone calorimeter test (CCT) was performed by a FTT2000 cone calorimeter at a heat flux of 50 kW∙m−2 with specimen size of 100.0 mm × 100.0 mm × 4.0 mm according to ISO 5660–1 standard.
The thermogravimetric analysis (TGA) was performed using a STA 409 PC/PG thermogravimetric analyzer (Netzsch, Germany) at a heating rate of 10 ℃/min, heating from room temperature to 700 ℃ under N2 atmosphere (40 ml/min).
The microstructure of char residues was examined with field emission scanning electron microcopy (SEM S-3400, Japan) with accelerating voltage was 15.0 kV. The sample were sputter-coated with a conductive layer of gold before analysis.
The graphitic degree of char residues was characterized by Laser Raman spectroscopy (SPEX, USA) with a 532 nm argon laser line at room temperature, the scanning range was 400 ∼ 2000 cm−1 region.
3 Results and discussion
3.1 Characterization of CDS
The structural characterization of CDS was performed by FTIR and 13C NMR. The FTIR spectra of DDS, CDS and CNC were presented in Fig. 1a. Clearly, several functional groups from DDS and CNC, such as N–H(3320–3530 cm−1), C = C (1620–1450 cm−1), C = N(1530 cm−1), S = O = S(1140 cm−1 and 1105 cm−1) can be observed in the FTIR spectra of CDS. The absorption peak of C-N (1265 cm−1) appeared, and the absorption peak of C-Cl (850 cm−1) disappeared, which indicated the completion of polymerization. The structures of CDS was further confirmed by 13C solid-state NMR and the result was shown in Fig. 1b. The signals at 165.75 ppm, 142.49 ppm, 136.08 ppm, 128.85 ppm, 121.30 ppm were attributed to C(a, δtr-C), C(b,δ-C=C-in para-point of benzene ring), C(e, δ-C=C-in para-point of benzene ring), C(d, δ-C=C-in meta-point of benzene ring), C(c, δ-C=C-in ortho-point of benzene ring), respectively.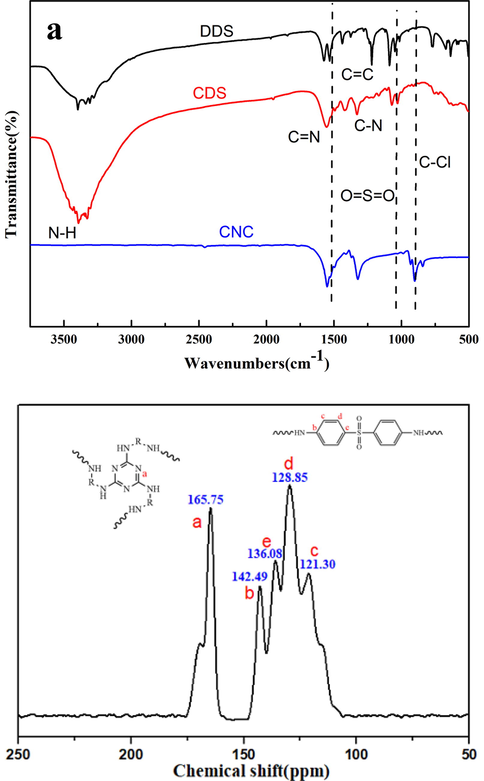
a FTIR spectrum of DDS, CDS and CNC and Fig. 1b 13C solid-state NMR spectrum of CDS.
3.2 Flame retardancy of TPEE composites
The formula and the results of LOI values and UL-94 ratings of neat TPEE and TPEE composites are shown in Table 1. Neat TPEE was very flammable and burned to the clamp after the first ignition, and was accompanied by severe droplets with the LOI value of 17.9. CDS was acted as both charring and blowing agents in the intumescent flame retardant system. There was hardly any improvement for the LOI values and UL-94 ratings by the addition of 20% CDS separately. It revealed that the lack of acid source for the intumescent flame retardant system is not efficient enough. When 15 wt% or 20 wt% of AlPi was added, the TPEE sample were extinguished in about 5 s after the Bunsen burner is ignited for 10 s, and then during the secondary combustion, droplets dripped into the fire Bunsen burner constantly. Therefore, it reached the UL-94 V-2 grade. In addition, compared with neat TPEE, the LOI values of TPEE/15AlPi and TPEE/20AlPi increased to 26.5 and 29.8, which showed that aluminum hypophosphite was able to play a flame retardant effect in both condensed phase and gas phase. In the gas phase, free radical capture leaded to flame suppression, and in the condensed phase, it initiated the formation of char or inorganic residues(Tang et al., 2012). LOI: limiting oxygen index; t1: average combustion time after the first application of flame; t2, average combustion time after the second application of flame; BC: burns to clamp; NR: no rating.
Sample
LOI (%)
UL-94,1.6 mm Bar
t1/t2
Dripping
Rating
Neat TPEE
17.9 ± 0.5
BC
Yes/-
NR
TPEE/15AlPi
26.5 ± 0.5
6.2/14.2
No/Yesa
V-2
TPEE/20AlPi
29.8 ± 0.5
4.5/10.5
No/Yes
V-2
TPEE/20CDS
22.5 ± 0.5
BC
Yes/-
NR
TPEE/18AlPi/2CDS
28.2 ± 0.5
4.5/8.9
No/Yes
V-2
TPEE/16AlPi/4CDS
29.1 ± 0.5
2.5/6.7
No/No
V-0
TPEE/15AlPi/5CDS
30.2 ± 0.5
2.2/5.9
No/No
V-0
TPEE/14AlPi/6CDS
29.3 ± 0.5
3.7/7.2
No/Yes
V-2
TPEE/12AlPi/8CDS
27.8 ± 0.5
4.6/8.2
No/Yes
V-2
TPEE/10AlPi/10CDS
26.2 ± 0.5
4.5/8.9
No/Yes
V-2
The formulation of the intumescent flame retardant played a vital role in the flame retardancy of the flame retardant polymer composites. As the mass ratio of the acid source and the char source increased, it can be seen that when 2 wt% CDS was added into the intumescent flame retardant system, the LOI of the TPEE/18AlPi/2CDS was increased to 28.2. However, droplets were still produced during the secondary combustion and V-2 rating was reached, which meant less char agent was unable to form a thick and dense char layer to prevent melt dripping. With the increase in the proportion of CDS, TPEE/15AlPi/5CDS can stop burning during the first combustion in 2.2 s and the UL-94 rating was enhanced to V-0 from V to 2 with inhibited dripping during the secondary combustion. However, with the further increase of the charring agent, it can be found that the LOI value of TPEE/10AlPi/10CDS was decreased to 26.2 and dripped again within 10 s in the ignition process of secondary flame. The reason was that the char layer formed after expansion and combustion was too much to cover the surrounding of the sample, making the heating area larger. It can be concluded that an appropriate ratio of AlPi and CDS in the intumescent flame retardant system was particularly important for the flame retardant performance of TPEE composites.
3.3 Charring behavior and thermal degradation of CDS and AlPi
The charring behavior and thermal degradation of CDS, AlPi and IFR in nitrogen atmosphere was investigated by TGA. IFR is AlPi/CDS mixture whose mass fraction was 15:5 and calculation IFR is the result calculated from the experimental results of AlPi and CDS based on their percentage in the IFR system in accordance with the follow formula:
WAlPi /WCDS: The mass of AlPi/CDS in the TGA tests.
As can be seen from Table.2, AlPi showed excellent thermal stability with a high char residue (20.98%) at 700℃ under N2 atmosphere. In addition, it decomposed into one-steps, starting from 436.6℃and the maximum mass rate at 482.8℃. AlPi can catalyze dehydration and cross-linking reaction of charring agents in IFR, and served as an acid source and CDS can be used as a char-forming agent. The thermal degradation process of CDS was divided into two steps: the first step roughly occurred at 300 to 400℃(corresponding to the chemical reaction of dehydration and release of SO2)(Tang et al., 2019), and the second step occurred at 400 to 550 °C, which may be allocated to the decomposition of macromolecular framework. Finally, CDS degraded into expanded char during the pyrolysis process with the increase of temperature, and the char residue is about 50.8% at 700 °C. These results showed that CDS had good thermal stability, which can be attributed to the presence of triazine and benzene ring.
Sample
T5%
(℃)T10%
(℃)Tmax
(℃)dw/dt(max)
(wt%/min−1)Residue (%)
700(℃), N2
CDS
336.1
361.3
403.2
−0.30
50.8
AlPi
436.6
451.4
482.8
−2.28
20.98
IFR
394.3
423.8
391.0
−1.22
32.24
Calculation IFR
409.2
445.6
396.0
−1.31
28.44
The initial decomposition temperature (T 5%) of IFR was 394.3℃ and the char residue was 32.24% at 700℃, which proved that IFR had excellent thermal stability and charring performance. Compared with Calculation IFR, the calculated value of initial decomposition temperature was 409.2 ℃, which was higher than the actual measured temperature value. This result revealed that with the incorporation of CDS into AlPi, the overall thermal degradation process changed. Meanwhile, the char residue (28.44%) of calculation IFR was lower than the IFR, which indicated that AlPi can improved the charring performance of CDS and accelerated the formation of the char layer.
3.4 Thermal degradation of TPEE and TPEE composites
Fig. 3a and Fig. 3b showed the TGA and DTG curves of TPEE and flame-retardant TPEE composites under N2 atmosphere. Table 3 summarized detailed data such as 5% mass loss, 10% mass loss, maximum mass loss rate, and the experimental and calculation value of char residue at 700℃. Through these data, we tried to explore the mechanism of the condensed phase and clarify the thermal stability of different formulations. The decomposition of neat TPEE started at 370.2℃, and the highest loss rate at 403.2℃ was −2.53 wt%/min−1. After the TGA test, TPEE was completely degraded into gaseous molecules, so it can be decomposed in one-step with almost no residue. After AlPi was added to the TPEE matrix, the initial thermal decomposition temperature (T5%) and the maximum thermal decomposition rate temperature (Tmax) of the TPEE composite did not change much, which indicated that AlPi can cooperate with the TPEE matrix well, and the experimental value of char residue of the composite was much higher than the calculated value, which indicated that the phosphorus in AlPi can catalyze TPEE to form a char layer during the combustion process. When the intumescent flame retardant composed of CDS and AlPi was added to TPEE, the initial thermal decomposition temperature of the composite material was slightly reduced, which was due to the lower initial thermal decomposition temperature of CDS. The proportion increased gradually, and it was found that when the addition amount of the charring agent CDS was 5%, the synergistic efficiency of AlPi and CDS was the highest at this time, and the char residue of the TPEE composite material was increased to 23%, which meant the surface can form a thick and dense char layer to prevent the TPEE composite from dripping so that the flame retardancy would be improved, which was consistent with the UL-94 measurement result. Comparing all formulas from TPEE/18AlPi/2CDS to TPEE/10AlPi/10CDS, the experimental values of the char residue rate were always much higher than the calculated values, which showed that the role of the intumescent flame retardant was not a simple linear addition, but was caused by the good synergy between the acid source, the charring agent and the matrix. T5%: temperature of 5 wt% mass loss; Tmax: temperature of the maximum mass loss rate; dw/dt (max): maximum mass loss rate; Residue: weight of the residue at 700℃ after TGA test; Cal: calculation value of char residue; Exp: experimental Value of char residue.
Sample
T5%
Tmax
dw/dt(max)
Residue(700℃, N2)
(℃)
(℃)
(wt%/min−1)
Cal
Exp
Neat TPEE
370.3
403.2
−2.53
–
1.68
TPEE/20AlPi
368.3
397.0
−2.17
5.54
16.50
TPEE/18AlPi/2CDS
360.5
402.1
−1.71
6.14
19.12
TPEE/15AlPi/5CDS
352.0
391.0
−1.82
7.03
23.52
TPEE/10AlPi/10CDS
341.4
396.0
−1.50
8.52
17.72
TPEE/15AlPi
368.7
396.0
−2.14
4.58
16.08
3.5 Fire properties of TPEE composites
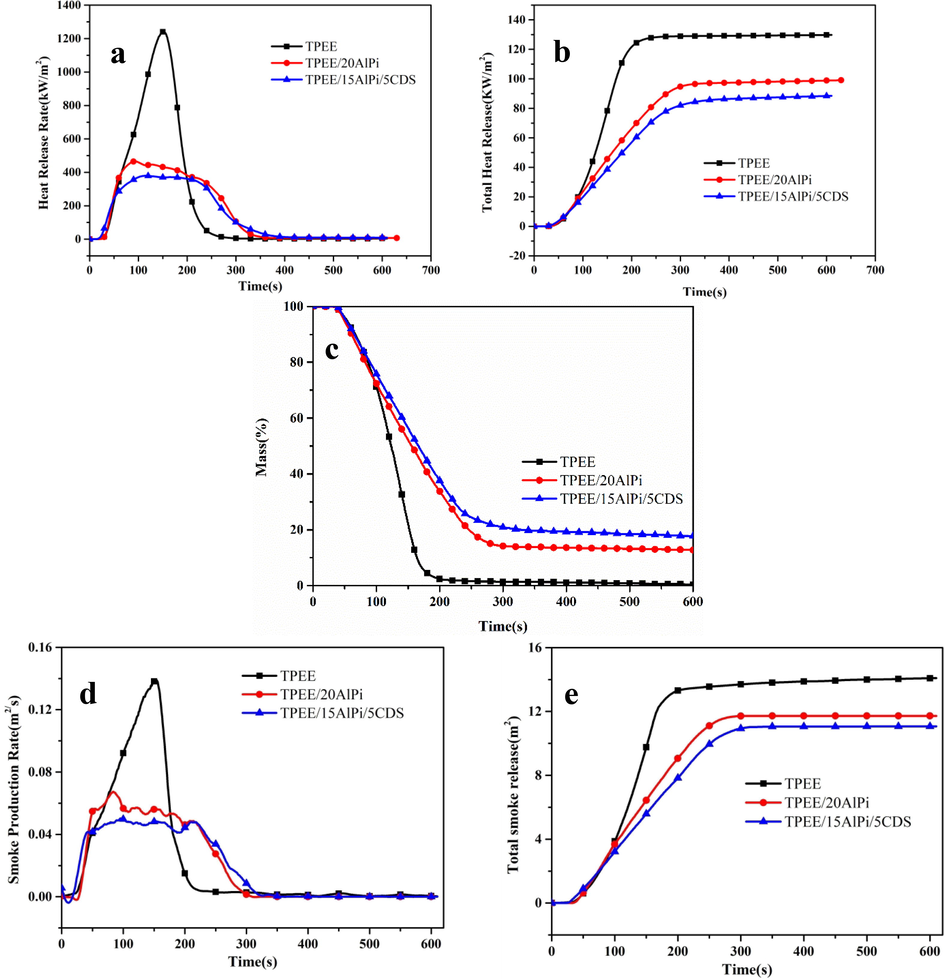
The cone calorimeter test was considered to be an effective method for assessing the combustion performance of polymeric materials in real fires. This method allowed the burning of a material to be represented by curves of heat release rate (HRR), total heat release rate (THR), ignition time (TTI) and mass loss curves. Fig. 4 (a,b,c) show the HRR, THR and Mass Loss curves for samples TPEE, TPEE/20AlPi and TPEE/15AlPi/5CDS, and the data can be seen from the Table.4, Pure TPEE ignited rapidly after 29 s with a peak heat release rate (PHRR) of 1234.1 kW/m2 and a total heat release (THR) of 129.3 MJ/m2. After the addition of 20 wt% AlPi, TPEE/20AlPi ignited after 32 s with a significantly lower HRR and PHRR of 468.9 kW/m2and 99.3 MJ/m2, which indicated that the addition of AlPi can significantly reduce the heat release rate and the amount of heat release during the combustion of the composites, which was because AlPi can generate diethyl phosphate at high temperatures, it can catalyze the dehydration of the matrix into char, thus reducing the amount of combustible material and playing a role in reducing heat release.(Lau et al., 2022) Subsequently, TPEE/15AlPi/5CDS, an intumescent flame retardant system with the addition of 5 wt% charring agent CDS, showed a better effect in reducing heat release. The sample ignited after 45 s and the PHRR and THR value were further reduced to 374.1 kW/m2 and 88.6 MJ/m2, which was due to the presence of the triazine-charring agent that can generate a char layer with a graphitic structure during combustion, which played a role in heat insulation and oxygen barrier. And, moreover, AlPi can have a P-N synergistic flame retardant effect with CDS, thus further reducing the heat release of the composite(Liu et al., 2019). Furthermore, as can be seen from the graph, the residual char rate of the TPEE composites increases from 0.1 wt% to 12.6 wt% and 17.7 wt% with the addition of AlPi and CDS, which indicated an increase in the charring ability, thus improving the flame retardant properties of the TPEE composites. In addition, smoke release was also one of the serious hazards in case of fire, which can easily lead to the death of evacuated people during the fire due to inhalation of released toxic gases or asphyxiation. The smoke release rate (SPR) and total smoke release (TSP) data of TPEE composites were shown in Fig. 4(d, e). Compared to pure TPEE, the smoke release rate (SPR) and total smoke release (THR) of TPEE/20AlPi and TPEE/15AlPi/5CDS were reduced. The maximum smoke production rate (pSPR) and smoke release production (TSP) of TPEE/20AlPi were 0.067 m2/s and 11.74 m2, respectively, which were 51.7% and 17.1% lower relative to 0.139 m2/s and 14.16 m2 of pure TPEE. In addition, the pHRR and TSP of TPEE/15AlPi/5CDS were 0.048 m2/s and 11.05 m2, which were reduced by 65.5% and 22%, respectively. This phenomenon was due to the fact that more residual char fragments can be stitched together into a dense and thick char layer due to the presence of the charring agent CDS, thus blocking the release of smoke.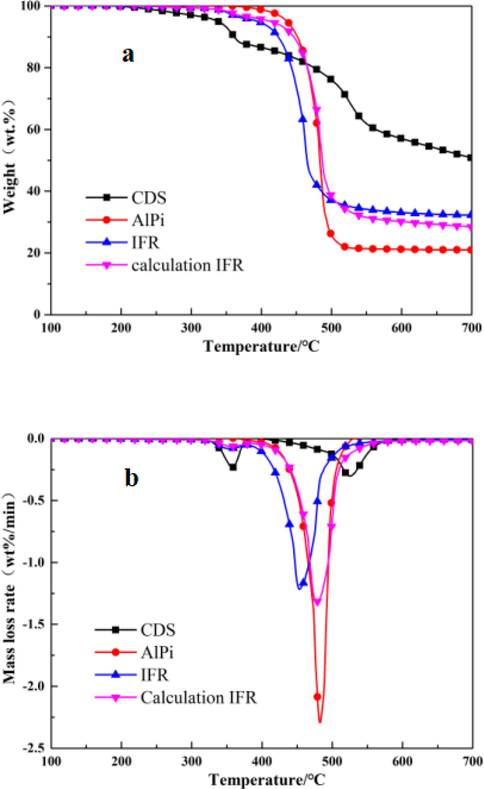
A Mass loss curves and Fig. 2b Mass loss rate curves CDS, AlPi, IFR and calculation IFR of CDS, AlPi, IFR and calculation IFR (under nitrogen atmosphere, heating rate of 10℃/ min−1).
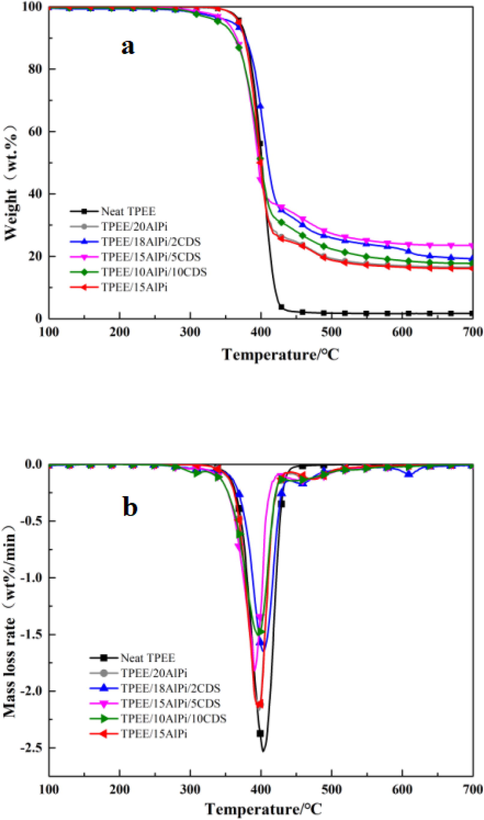
A Mass loss curves and Fig. 3b mass loss rate curves of all the investigated samples (under nitrogen atmosphere, heating rate of 10℃/ min−1).
Sample
TPEE
TPEE/20AlPi
TPEE/15AlPi/5CDS
TTI(s)
29
32
45
pHRR(kW/m2)
1234
468.9
374.1
tPHRR(s)
150
89.3
121
THR(MJ/m2)
129
99.3
88.6
Residual mass (wt%)
0.10
12.6
17.7
pSRP(m2/s)
0.139
0.067
0.048
TSP(m2)
14.16
11.74
11.05
FPI(m2·s/kW)
0.02
0.07
0.12
FIGRA(kW/m2·s)
8.22
5.25
3.09
Av-EHC(MJ/kg)
21.25
16.57
12.18
Fire Performance Index (FPI) and Fire Growth Rate (FIGRA) are useful indicators for assessing the fire risk of composites. B. Schartel and his team (Schartel and Hull, 2007)found that the FPI value correlates with the flashover time when the material burns, with lower FPI values resulting in earlier flashover time and higher fire risk. FIGRA is defined as the ratio of PHRR to tPHRR, therefore a smaller FIGRA value indicates a greater time to reach PHRR and a lower fire risk(Breulet and Steenhuizen, 2005). The formulas for both are:
As can be seen from the Table. 4, the FPI value for pure TPEE is 0.02 m2·s/kW, while TPEE/20AlPi and TPEE/15AlPi/5CDS correspondingly increased to 0.07 m2·s/kW and 0.12 m2·s/kW respectively, in addition, the FIGRA value for the composite decreased from 8.22 kW/m2·s to 5.25 kW/m2·s and 3.09 kW/m2·s, indicating that with the addition of AlPi and CDS, the flame growth trend would be suppressed, which would facilitate evacuation and firefighting during a fire.
The effective average heat of combustion (Av-EHC) reflected the heat released from the combustion of the pyrolytic volatiles. The results showed that the Av-EHC of combustion of the TPEE composites decreased from 21.25 MJ/kg to 16.57 MJ/kg and 12.18 MJ/kg, indicating that the addition of AlPi and CDS can exert some flame retardant effect through the gas phase flame retardant, which was due to the fact that the PO· generated by the cleavage of AlPi during combustion can reduce the concentration of hydrogen radicals in the flame area and extinguish the flame, as well as the non-flammable gases such as N2 and NH3 generated by CDS during combustion, thus diluting the concentration of combustible materials in the gas phase and thus reducing the heat generated by the continued combustion of volatiles.
3.6 Morphology and chemical structure of char residues
The residual char morphology obtained after the TPEE/20AlPi and TPEE/15AlPi/5CDS cone calorimetric tests was shown in the Fig. 5(a1, a2 and b1, b2). The pure TPEE sample had almost no residual char remaining after CCT test. As can be seen from the Fig. 5(a1, a2), TPEE/20AlPi produced a thick char layer after combustion, which helped to prevent the entry of combustible substance and the transfer of heat. However, there were many obvious defects on its surface and the surface is irregular, such a structure was not effective in preventing the drops produced after combustion and thus only achieves UL-94 V-2 grade. As can be seen from the Fig. 5(b1, b2), the addition of CDS accelerated the charring rate of the composite, thus producing a dense and thick char layer on the surface of the sample. Such a residual char structure can play an effective role in heat insulation and oxygen barrier, thus acting as a melt drip suppressor and flame retardant, and these reasons also contributed to the composite passing the V-0 UL-94 grade. The above results showed that the intumescent flame retardant system 15CDS/5AlPi had a good flame retardant effect and can play an excellent role in char formation.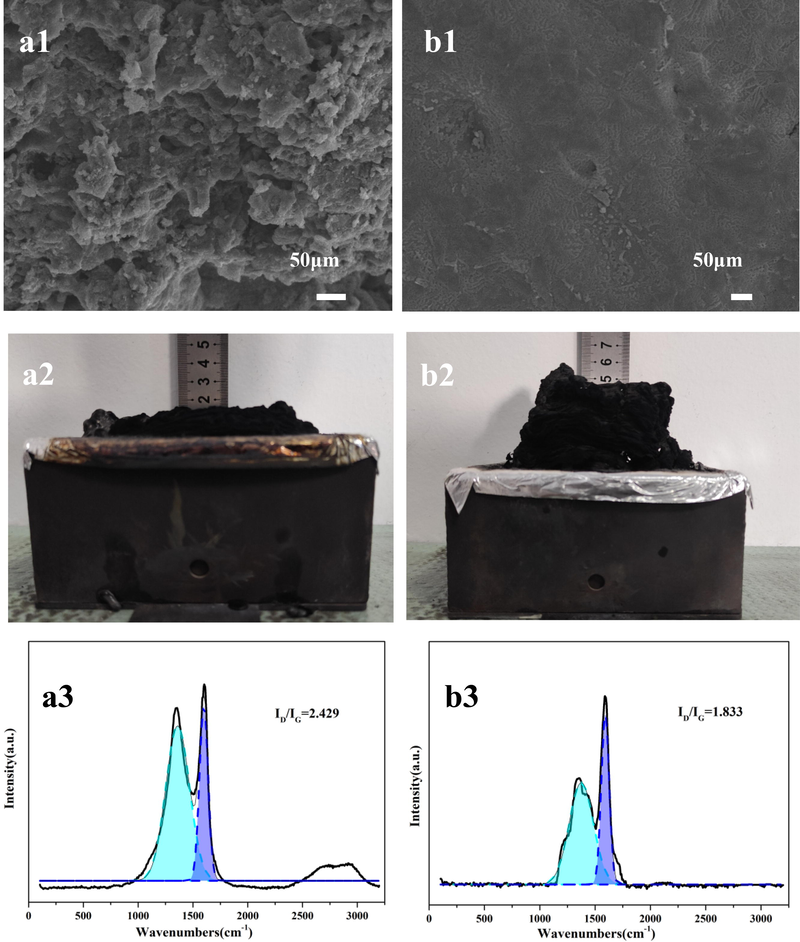
Characterization of residual char by CCT of TPEE/20AlPi (a1, a2, a3) and TPEE/15AlPi/5CDS (b1, b2, b3).
In addition to the analysis of the morphology of the residual char, the study of the structure of the residual char was also of great importance for the study of the flame retardant mechanism. Raman spectroscopy was widely used to study the crystal structure and molecular structure of carbon materials. The Fig. 5(a3, b3) showed the Raman spectra of TPEE/20AlPi, and TPEE/15AlPi/5CDS after CCT testing. It can be found that there are broad peaks of 1332 cm−1 and 1590 cm−1 in the Raman spectra. The former (D-band) was caused by vibrations of carbon atoms oscillating on disordered graphite sp3 hybridized atoms or amorphous carbon layers, while the latter (G-band) corresponded to vibrations of sp2 hybridized atoms of graphite flakes. The degree of graphitization of the system can be expressed as the ratio of the peak areas in the D and G bands (AD/AG)(Liu et al., 2020). In general, the lower the AD/AG, the more graphitic carbon there was in the char layer and the higher the quality of the layer. The higher the degree of graphitization, the denser and more stable the structure of the char layer. The graphitized carbon formed during combustion was important in controlling the release of heat and volatiles from the carbon structure which was stable at high temperatures, the AD/AG ratio of TPEE/20AlPi (2.429) was higher than that of TPEE/15AlPi/5CDS (1.833), which indicated that the degree of graphitization and thermal stability of TPEE/15AlPi/5CDS increases with the addition of CDS.
3.7 The flame retardant mechanism of TPEE composites
Through the characterization of the infrared spectrum of the TPEE composites during the heating process, the synergistic effect between flame-retardants and the mechanism and form of action in the matrix can be studied. Fig. 6 (a, b, c) showed the infrared spectra of Neat TPEE, TPEE/20AlPi and TPEE/15AlPi/5CDS at a heating rate of 10 °C/min−1. 2962 cm−1 and 2878 cm−1 (–CH2, stretching vibration), 1712 cm−1 (-C = O, stretching vibration), 1458 cm−1 (–CH2, fracture vibration), 1410 cm−1 (aromatic) ring), 1274 cm−1 (–CO-O-ester), 1106 cm−1 (–CH2-O-CH2-ether) and 727 cm-1 (–CH, bending vibration of aromatic ring) were the characteristic peaks of TPEE. With the increase of heating temperature, the band intensity at 2956 and 2861 cm−1 (–CH2) dropped rapidly, which was caused by the scission of the α-methylene group in the soft segment, which was consistent with our previous research results(Zhong et al., 2014).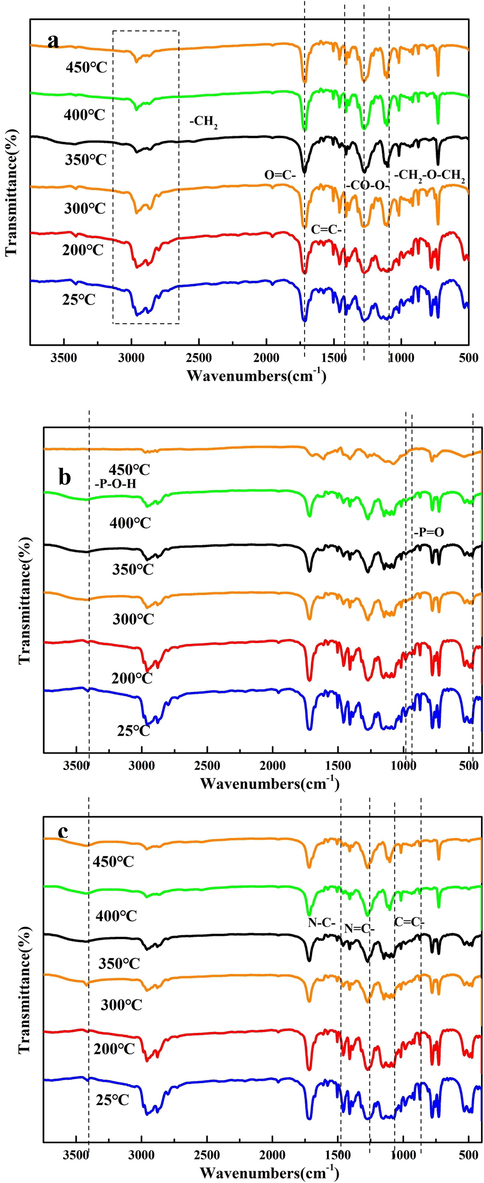
FTIR spectra of the solid phase in TPEE (a), TPEE/20AlPi (b) and TPEE/15AlPi/5CDS (c) at different temperatures.
For the formula TPEE/20AlPi, due to the addition of AlPi, the typical absorption bands of AlPi appeared in the spectrum, such as 1151, 1079 and 780 cm-1 (-P = O); 1018 cm-1 (PO43-); and 475 cm-1 (O = PO, AlPi). Same as Neat TPEE, TPEE/20AlPi in Fig. 6 (b) was also decomposed on the soft segment due to the active a-methylene group of the ether bond. In the hard segment (C = O), due to the presence of carboxyl groups, there was a shoulder gap at 1680 cm−1. As the temperature rose, due to the strong interaction between Al3+ and carboxyl groups, electron migration occurred, which also improved the thermal stability of the composite. There was no P-O–H unit (3400–3800 cm−1) from the curve at 450℃, which indicated that the AlPi may played a flame retardant effect in the gas phase after being released. The solid phase spectrum of TPEE/AlPi showed that the interaction between TPEE and AlPi improved thermal stability and increased the residue rate, and may formed intermediate products in the gas phase to suppress flames.
The infrared spectrum of TPEE/15AlPi/5CDS during the heating process was shown in Fig. 6(c). It can be seen that the N–H peak at 3400 cm−1 gradually weakened with the increase of temperature, which indicated that the triazine charring agent CDS decomposed non-combustible gases such as ammonia and nitrogen, thereby exerting a flame retardant effect in the gas phase. In addition, C-N (1265 cm−1), C = N (1500 cm−1) and characteristic bands of aromatic structure 737 cm−1 and 1411 cm−1 appeared in the residue at 450℃, which indicated that the addition of triazine charring agent CDS can make the TPEE composites form the char layer spliced by benzene ring and triazine ring after combustion, thereby increasing the strength of char.
3.8 Proposed flame-retardant mechanism
According to the described structure characterization and char formation behavior analysis, the possible flame retardant mechanism of TPEE/15AlPi/5CDS composite was shown in Scheme 2 and Fig. 7. Firstly, the charring agent CDS decomposed at about 336 °C to release incombustible gas, and then cracked to generate triazine ring structures and aromatic structures, which were easy to assemble and splice into a char layer. Subsequently, the thermal oxidative degradation of the TPEE matrix occurred selectively in the soft segment of the polyether, and at the same time, the ester bond was formed. When the temperature continued to be oxidized, the polyester segment will also be broken. The chain scission reaction passed through a six-membered ring intermediate product, –COO abstracted the H atom from the methylene group at the β position to form an oligomer with a carboxyl group and an unsaturated double bond at the end, which continued to oxidize to produce tetrahydrofuran, butadiene, benzoic acid, water, CO2, etc.(Mi et al., 2017; Ma et al., 2019). Subsequently, the Al3+ provided by AlPi after the temperature rose and the carboxyl-containing oligomers produced by the degradation of TPEE formed a compound, and finally a variety of inorganic aluminum phosphate salts such as pyrophosphate were formed(Lau et al., 2022). This phosphate had a certain strength and can help to isolate the heat and oxygen exchange. In addition, the phosphate closely covered the surface of the substrate to further catalyze the carbonization of the TPEE substrate and the triazine charring agent CDS. Finally, the decomposition of CDS produced an aromatic structure that was assembled with the pyrolysis product of TPEE, and the graphitized char layer was formed on the surface of the composites to isolate heat and played a flame retardant effect.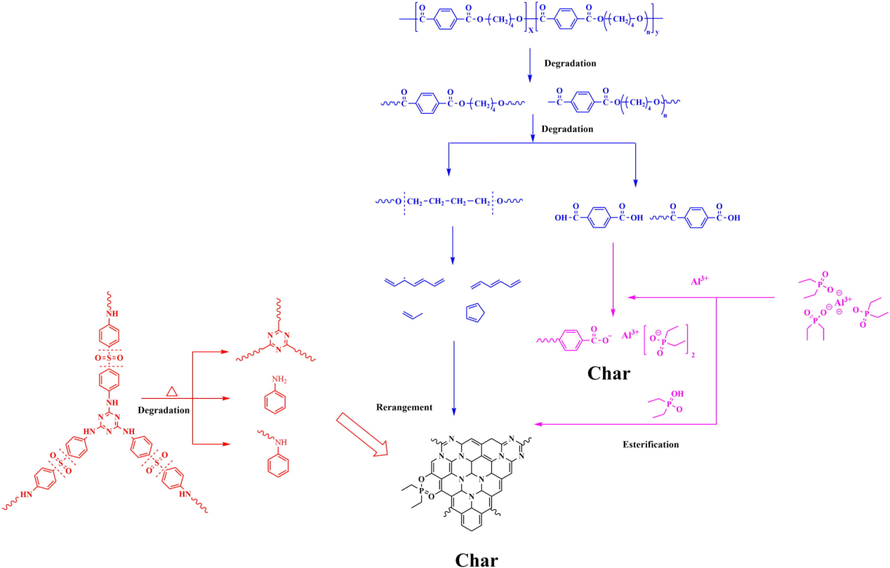
Main decomposition mode of TPEE/15AlPi/5CDS composite.
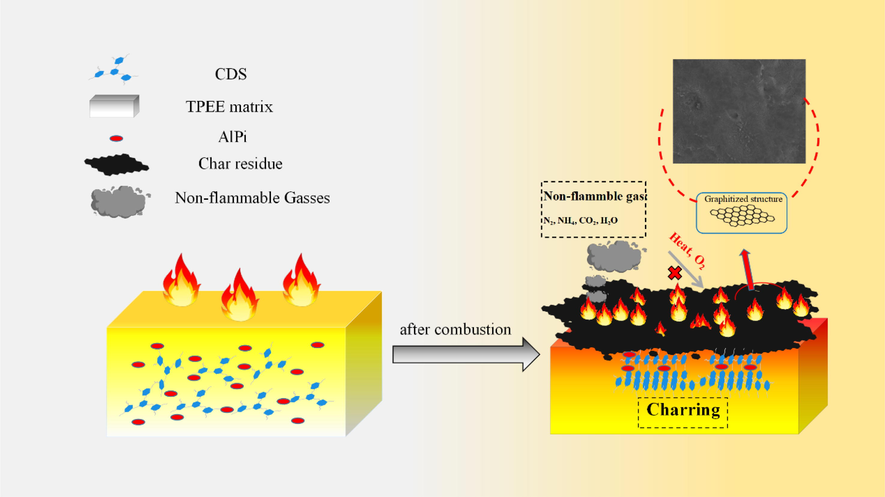
Schematic illustration of flame-retardant mechanism for CDS and AlPi in flaming of TPEE composites.
3.9 Mechanical properties of TPEE composites
As a high-performance polymer, TPEE has excellent mechanical properties. However, in general, the mechanical properties of flame retardant composites were reduced with the addition of flame-retardants. In order to evaluate the effect of the intumescent flame retardant system AlPi/CDS on the mechanical properties of TPEE composites, the tensile strength and elongation at break data of TPEE composites were shown in Fig. 8.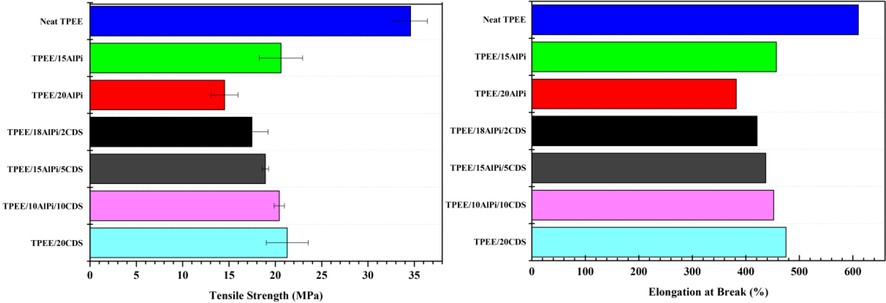
Tensile strength (left) and elongation (right) at break curves of TPEE composites.
From the Fig. 8, it can be seen that the tensile strength and elongation at break of TPEE composites had different degrees of reduction with the addition of flame retardants AlPi and CDS. When AlPi is added alone, the tensile strength and elongation at break of TPEE composites decreased from 34 MPa to 14 MPa and 600% to 385%, respectively, which indicated that the poor compatibility between the inorganic flame retardant AlPi and TPEE. Furthermore, with the increase of organic macromolecule flame retardant CDS addition, the tensile strength and elongation at break of TPEE composites gradually increased, which indicated that CDS and TPEE showed good compatibility with each other.
4 Conclusions
In this paper, a new type of charring agent CDS was synthesized by CNC, DDS through condensation reaction, which can form an IFR system together with AlPi, and the IFR significantly improved the flame retardancy of TPEE. When only 20% IFR was loaded, the TPEE composite can obtain a high LOI value of 30.2% and passed UL-94 V-0 rating. Through the analysis of TGA, it can be seen that CDS has an extremely high char residue rate and had a certain synergistic flame retardant effect with AlPi. Combining the structure analysis of heating infrared and the charring behavior analysis of SEM and LRS, it can be concluded that CDS was an agent with excellent thermal stability and charring performance. It can be flame retardant when combined with AlPi to form intumescent flame retardant TPEE materials, and can form a high-quality graphitized carbon layer on the surface of the substrate.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Investigating the compatibility of thermoplastic polyester elastomer/high-density polyethylene blends and its effect on the horizontal flame propagation[J] Plast., Rubber Compos.. 2020;49(2):66-78.
- [Google Scholar]
- Fire retardant polymers: recent developments and opportunities[J] J. Mater. Chem.. 2007;17(22):2283-2300.
- [Google Scholar]
- Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6[J] Polym. Degrad. Stab.. 2007;92(8):1528-1545.
- [Google Scholar]
- Fire testing of cables: comparison of SBI with FIPEC/Europacable tests[J] Polym. Degrad. Stab.. 2005;88(1):150-158.
- [Google Scholar]
- Flexible and fire safe sandwich structured composites with superior electromagnetic interference shielding properties[J] Compos. A Appl. Sci. Manuf.. 2022;160
- [Google Scholar]
- Multi-hierarchical flexible composites towards superior fire safety and electromagnetic interference shielding[J] Nano Res.. 2022;15(10):9531-9543.
- [Google Scholar]
- Characterization and Properties of Thermoplastic Polyether Elastomer/Polyoxymethylene Blends Prepared by Melt-Mixing Method[J] Polym. Sci., Ser. A. 2019;61(6):890-896.
- [Google Scholar]
- Synthesis of novel triazine charring agent and its effect in intumescent flame-retardant polypropylene[J] J. Appl. Polym. Sci.. 2012;123(6):3208-3216.
- [Google Scholar]
- Preparation and characterization of a novel oligomeric charring agent and its application in halogen-free flame retardant polypropylene[J] J. Anal. Appl. Pyrol.. 2015;111:238-246.
- [Google Scholar]
- Synergistic effect of a novel triazine charring agent and ammonium polyphosphate on the flame retardant properties of halogen-free flame retardant polypropylene composites[J] Thermochimica Acta. 2016;627–629:83-90.
- [Google Scholar]
- Synergistic effect of ammonium polyphosphate and triazine-based charring agent on the flame retardancy and combustion behavior of ethylene-vinyl acetate copolymer[J] J. Anal. Appl. Pyrol.. 2016;119:259-269.
- [Google Scholar]
- Halogen-free flame retarded poly(butylene terephthalate) (PBT) using metal oxides/PBT nanocomposites in combination with aluminium phosphinate[J] Polym. Degrad. Stab.. 2009;94(8):1245-1253.
- [Google Scholar]
- Flame retardant biocomposites: Synergism between phosphinate and nanometric metal oxides[J] Eur. Polym. J.. 2011;47(7):1390-1401.
- [Google Scholar]
- Technology of adding phosphorus-containing flame retardant in polymers[J] J. Wuhan Univ. Natur. Sci. Ed.. 2009;55(3):283-288.
- [Google Scholar]
- Mechanical properties of thermoplastic polyester elastomer controlled by blending with poly(butylene terephthalate)[J] Polym. Test.. 2016;55:152-159.
- [Google Scholar]
- Phosphorus-containing thermoplastic poly(ether ester) elastomers showing intrinsic flame retardancy[J] J. Appl. Polym. Sci.. 2017;134(46)
- [Google Scholar]
- Aluminum Diethylphosphinate as a Flame Retardant for Polyethylene: Investigation of the Pyrolysis and Combustion Behavior of PE/AlPi-Mixtures[J] Combust. Flame. 2022;240
- [Google Scholar]
- Phosphorus-containing flame-retardant bismaleimide resin with high mechanical properties[J] Polym. Bull.. 2016;73(12):3547-3557.
- [Google Scholar]
- Synthesis of a triazine-based macromolecular hybrid charring agent containing zinc borate and its flame retardancy and thermal properties in polypropylene[J] Int. J. Polym. Anal. Charact. 2020:1-9.
- [Google Scholar]
- Synergistic effects between a triazine-based charring agent and aluminum phosphinate on the intumescent flame retardance of thermoplastic polyether ester[J] J. Macromol. Sci. A. 2019;56(7):723-732.
- [Google Scholar]
- Recent developments in the chemistry of halogen-free flame retardant polymers[J] Prog. Polym. Sci.. 2002;27(8):1661-1712.
- [Google Scholar]
- An insight into gas phase flame retardant mechanisms of AHP versus AlPi in PBT: Online pyrolysis vacuum ultraviolet photoionization time-of-flight mass spectrometry[J] Combust. Flame. 2019;209:467-477.
- [Google Scholar]
- Biocompatible, degradable thermoplastic polyurethane based on polycaprolactone-block-polytetrahydrofuran-block-polycaprolactone copolymers for soft tissue engineering[J] J. Mater. Chem. B. 2017;5(22):4137-4151.
- [Google Scholar]
- Recent developments in s-triazine holding phosphorus and nitrogen flame-retardant materials[J] J. Fire Sci.. 2020;38(6):552-573.
- [Google Scholar]
- Development of fire-retarded materials—Interpretation of cone calorimeter data[J] Fire Mater.. 2007;31(5):327-354.
- [Google Scholar]
- Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety[J] Chem. Eng. J.. 2020;399
- [Google Scholar]
- Intumescent flame retardant behavior of charring agents with different aggregation of piperazine/triazine groups in polypropylene[J] Polym. Degrad. Stab.. 2019;169
- [Google Scholar]
- Thermal Degradation and Flame Retardance of Biobased Polylactide Composites Based on Aluminum Hypophosphite[J] Ind. Eng. Chem. Res.. 2012;51(37):12009-12016.
- [Google Scholar]
- Recent advances in thermoplastic elastomers from living polymerizations: Macromolecular architectures and supramolecular chemistry[J] Prog. Polym. Sci.. 2019;95:1-31.
- [Google Scholar]
- One-pot synthesis of a novel s-triazine-based hyperbranched charring foaming agent and its enhancement on flame retardancy and water resistance of polypropylene[J] Polym. Degrad. Stab.. 2014;110:165-174.
- [Google Scholar]
- Lignin-derived bio-based flame retardants toward high-performance sustainable polymeric materials[J] Green Chem.. 2020;22(7):2129-2161.
- [Google Scholar]
- Yue, X. 2019. Flame retardant nanocomposites based on 2D layered nanomaterials: a review[J].
- Effect of graphite on the flame retardancy and thermal conductivity of P-N flame retarding PA6[J] J. Appl. Polym. Sci.. 2018;135(31)
- [Google Scholar]
- Flame-retarding mechanism of organically modified montmorillonite and phosphorous-Nitrogen flame retardants for the preparation of a halogen-free, flame-retarding thermoplastic poly(ester ether) elastomer[J] J. Appl. Polym. Sci.. 2014;131(22):n/a-n/a.
- [Google Scholar]
- The flame retarding mechanism of the novolac as char agent with the fire retardant containing phosphorous–nitrogen in thermoplastic poly(ether ester) elastomer system[J] Polym. Degrad. Stab.. 2014;105:166-177.
- [Google Scholar]
- Adding the combination of CNTs and MoS2 into halogen-free flame retarding TPEE with enhanced the anti-dripping behavior and char forming properties[J] Thermochimica Acta. 2015;613:87-93.
- [Google Scholar]
- Preparation, thermal, and flammability of halogen-free flame retarding thermoplastic poly(ether-ester) elastomer/montmorillonite nanocomposites[J] Polym. Compos.. 2016;37(3):700-708.
- [Google Scholar]
- Zhou, Y.F., Li, W.G., Chen, C.F., et al. 2007. An FT-IR study of the effect of added flame retardant on the structure of TPEE[M]. 590-592.
- s-Triazine-based functional monomers with thermocrosslinkable propargyl units: Synthesis and conversion to the heat-resistant polymers[J] Polymer. 2016;102:301-307.
- [Google Scholar]
- Flame-retardant thermoplastic polyester based on multiarm aluminum phosphinate for improving anti-dripping[J] Thermochimica Acta. 2018;664:118-127.
- [Google Scholar]