Translate this page into:
Oxamic acid thiohydrazides and hydrazones based on them as convenient starting compounds for the synthesis of S- and N-containing heterocyclic products. A mini-review
⁎Corresponding author. yarovladimir@yandex.ru (V.N. Yarovenko)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The chemistry of hydrazones is currently undergoing intensive development. For example, thioacylhydrazones, which are synthesized most often from thiosemicarbazides, have become very popular in recent years owing to impressive results of their extensive testing in medicinal chemistry. Their antiviral, insecticidal, antisclerotic, antioxidant, and antiparasitic activities have been demonstrated; thioacylhydrazones showed promise for the development of drugs against COVID-19 and HIV. The ligands based on these compounds with soft donor nitrogen and sulfur atoms are widely used in the creation of complexes with various bioactivities, including strong anticancer properties. Since the replacement of the oxygen atom with a sulfur atom may increase the biological activity of the compound, the desire to expand the spectrum of pharmacological action or to change the type of activity induces understandable interest in thio analogues of acylhydrazones that are known to have high bioactivity. At the same time, there are publications indicating that oxamic acid thiohydrazides containing thioamide and thiohydrazide moieties, due to their polyfunctional nature, can be effectively used in the synthesis of a wide variety of compounds, including bioactive products. This review describes the synthetic and applied potential of poorly investigated oxamic acid thiohydrazides and hydrazones based on them. A considerable benefit of these oxamic acid derivatives is the presence of an additional carboxamide group, which is itself significant for biological processes and can also be easily modified, thus providing a wealth of combinations for the synthesis of new promising products. The review describes convenient methods for the preparation of oxamic acid derivatives of this type and demonstrates their large synthetic potential, which opens up the way to new thiohydrazone analogues and to various heterocyclic compounds, including new-generation antibacterial drugs targeting the secretory system of bacteria, thus suppressing the infectious process and eliminating the pathogen from the body without affecting the proliferation of bacteria.
Keywords
Acylhydrazones
Thioacylhydrazones
Complexes
Oxamic acid thiohydrazides
Heterocycles
Bioactivity
Antivirulence drugs
1 Introduction
The interest in hydrazones as efficient broad-spectrum anti-infective drugs is permanently increasing (Sharma et al., 2020; Boulebd et al., 2022; Liu et al., 2022; Tabbiche et al., 2022; Wang et al., 2023). Acylhydrazones containing the –CO–NH–N = CH– moiety in the molecule have proved to be a versatile class of compounds applicable in various fields of organic chemistry.
A variety of drugs derived from these compounds show antimicrobial, antiviral, anti-inflammatory, antifungal, antibacterial, antitumour, anti-infective, antidepressant, anti-hemorrhagic, myorelaxant, antitrichomonal, capillary-stabilizing, antiarrhythmic, and other types of activity (Mali et al., 2021; Popiołek, 2021; Belyaeva et al., 2022; Socea et al., 2022). Acylhydrazones are investigated for the treatment of Alzheimer's disease (Bozbey et al., 2020; Yang et al., 2023); their anticancer properties have been studied by molecular docking (Çakmak et al., 2022), and promising pesticide components have been reported (Wang et al., 2024). The bioactivity of natural compounds containing acylhydrazone moieties has been studied. It was found that echinopsine has high antiviral and fungicidal activities (Cui et al., 2022), quinolines show antituberculosis and anticancer properties (Mandewale et al., 2017), curcumin (Omidi and Kakanejadifard, 2020) and isatin (de Paiva et al., 2020) have antitumor activities; and chromones possess a high algicidal activity (Tu et al., 2013) and have anticancer and antioxidant properties (Li et al., 2010). Acylhydrazones derived from vanillin were proposed as potential aldose reductase inhibitors (Demir et al., 2023). It is important that the properties of acylhydrazones are far from being limited to biological activity: they are successfully used for spectrophotometric determination of metals (Jabeen, 2022) and for the design of supramolecular complexes (Maiti et al., 2021); a review (Su and Aprahamian, 2014) describes the acylhydrazone-based molecular switches and sensors; and they have also been recommended (Meenatchi et al., 2021) for the design of high-quality nonlinear optical materials.
The thio analogues of reported acetylhydrazones, –C(=S)–NH–N = CH–, are of considerable interest since the replacement of the oxygen atom by sulfur may enhance the biological activity of the compound (for example, the piracetam thio analogues are more active than piracetam; Kadushkin et al., 1989; Soerensen and Smith, 1993), expand the range of pharmacological action (glycoluril thio analogues, Kravchenko et al., 2018), or change the type of activity, which is the case, for example, for purines and thiopurines (Bayoumy et al., 2021). Oxamic acid thiohydrazides containing proximate thioamide and thiohydrazide moieties are capable of a wide variety of transformations, owing to their polyfunctional nature and mutual influence of the moieties. Published works on oxamic acid thiohydrazides show their broad synthetic potential for creating original approaches to the synthesis of promising bioactive products such as rhodanine derivatives (Yarovenko et al., 2007), 1,3,4-thiadiazoles (Yarovenko et al., 2003), pyrazolines (Kamernitsky et al., 2007), pyridazines (Komkov et al., 2015), and thiadiazines (Komendantova et al., 2019).
The most studied derivatives are thiosemicarbazones obtained from thiosemicarbazides; they exhibit diverse bioactivity, including antiviral, insecticidal, antisclerotic, antioxidant, and antiparasitic activities, and have been used to synthesize anti-HIV agents (Acharya et al., 2021). Thiosemicarbazones were reported to exhibit anticancer activity (Shakya and Yadav, 2020; Acharya et al., 2021; Ahmed and Almalki, 2021; Dharmasivam et al., 2022) and antibacterial properties (Acharya et al., 2021; Li et al., 2021) and were studied against COVID-19 (Xu et al., 2022). A variety of biological properties were found for thiosemicarbazone metal complexes (Matesanz et al., 2021; Gupta et al., 2022; Khan et al., 2022). Their antibacterial properties have been reported (Souza et al., 2021; Scaccaglia et al., 2022); metal complexes have been tested as diagnostic radiopharmaceuticals (Parrilha et al., 2022) and anti-COVID-19 drugs (Ewert et al., 2022) and, what is especially important, they are effective against various types of tumours (He et al., 2021; Bai et al., 2022; Devi et al., 2022). The thiocarbonyl group of thioacylhydrazones is used for the design of heterocyclic compounds. It was shown that thiazolylhydrazone derivatives based on thioacylhydrazones are potent tyrosinase inhibitors (Djafarou et al., 2023). A review (Kostas and Steele, 2020) addresses the use of thiosemicarbazone complexes in catalyzed cross-coupling reactions. Oxamic acid thiohydrazides are also of undoubted interest as complex-forming structures, since they have donor atoms with both high (N,O) and low (S) electronegativity, due to which they are able to form fairly stable coordination compounds with both hard and soft Lewis acids (Yarovenko et al., 2005; Myannik et al., 2018a, 2018b).
All the foregoing attests to the relevance of investigating the synthetic and applied potential of poorly known thiohydrazides such as oxamic acid derivatives with a –NHC(O)–C(=S)–NH–N = CH– moiety. These compounds contain an additional carboxamide group, –NHC(O)–, which may be important for the formation of a particular type of bioactivity, complex formation, cyclization to form heterocycles, etc. Undoubtedly, the close proximity of the carboxamide and thiohydrazide moieties also affects the reactivity and applied properties of the compounds. Below we present the key methods for the synthesis of oxamic acid thiohydrazides (OAT) and OAT hydrazones (OATH), describe their reactivity, and give examples of synthesis of OAT- and OATH-based derivatives for biological purposes, including demonstrating the possibility of creating a new generation of drugs based on oxamic acid thiohydrazides for the treatment of chronic infections caused by pathogens.
2 Preparation and synthetic potential of oxamic acid thiohydrazides (OAT)
2.1 Synthesis of OAT
Owing to their polyfunctional nature, OAT 1 containing carboxamide and thiohydrazide moieties can undergo various transformations, some of which are depicted in Scheme 1.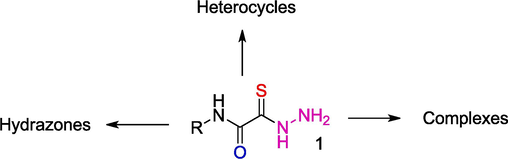
Synthetic potential of OAT.
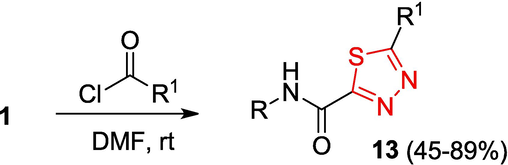
Among the reported methods for OAT synthesis (Thiel and Mayer, 1989a, 1989b), a convenient method proposed in a review (Yarovenko et al., 2003) includes treatment of monothiooxamides 4 with hydrazine (Scheme 2). It is noteworthy that this reaction smoothly proceeds only for morpholine derivatives shown in the Scheme, while the use of other amides 4 results in markedly lower yields of products 1.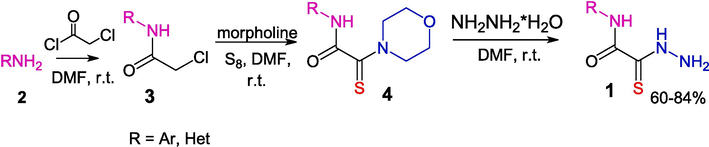
A convenient method for the preparation of monothiooxamides and OAT from amines.
In turn, these products and their various derivatives are obtained in good yields by the reactions of α-chloroacetamides with mixtures of elemental sulfur and amines prepared in advance (Yarovenko et al., 1998, 2002a, 2002b, 2007, 2009). It should be emphasized that OAT and monothiooxamides are easily obtained from amines, which makes it possible to design various heterocycles using a wide range of starting reactants, including numerous amino-containing natural compounds. In view of the simplicity of the method, it can be recommended, in particular, for the creation of extensive combinatorial libraries of potential bioactive carboxamide heterocycles based on various amines.
It is known (Daly and Brown, 1973) that the reaction of amines with elemental sulfur occurring in the solution as an eight-membered cyclic molecule is accompanied by accumulation of considerable amounts of polysulfide anions (Scheme 3). It was assumed (Yarovenko et al., 2003) that the polysulfide anions 6, when present in a sufficiently high concentrations, react with chloroacetamides 4 via nucleophilic substitution of chlorine to give polysulfides 7, in which amine molecules induce proton elimination simultaneously with sulfide bond cleavage, thus forming thioaldehyde moiety 8. Thioaldehyde 8 reacts with amine to be converted to imine 9, which is then oxidized with sulfur to give the corresponding thioamide moiety 10.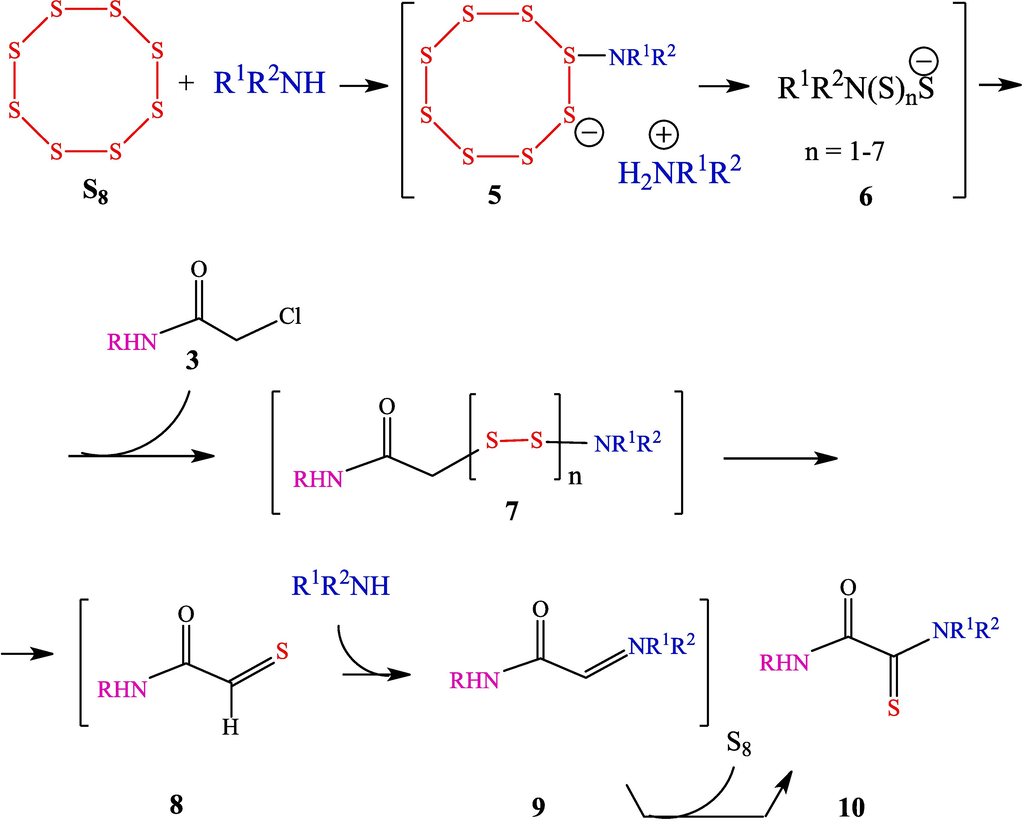
Scheme of the reaction of α-chloroacetamides with a mixture of amines and elemental sulfur prepared in advance.
Presumably the yield of monothiooxamides 10 considerably decreases due to the side reaction of amines with chloroacetamides 3, which affords relatively unreactive α-aminoacetamides 11 (Shirokov, 2005) (Scheme 4).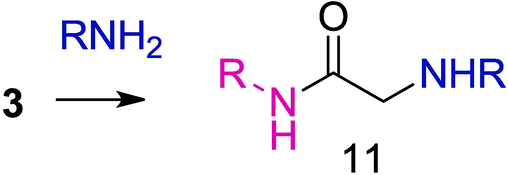
Side reaction in the synthesis of OAT.
This reaction can be prevented by keeping a mixture of elemental sulfur and amine for some time before the reaction.
The structure of OAT was confirmed using X-ray diffraction in relation to compound 12 (Shirokov, 2005) (Fig. 1).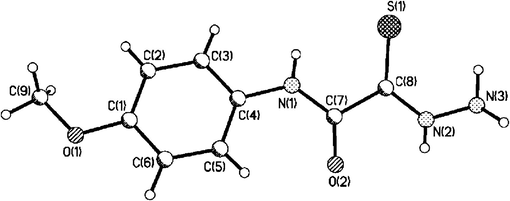
Molecular structure of OAT 12 (Shirokov, 2005).
The crystals of compound 12 refer to the monoclinic system with the unit cell parameters: a = 24.910(6) Å, b = 3.9567(11) Å, c = 10.141(3) Å, β = 91.589(11)°, V = 999.1(5) Å3, Z = 4, space group P21/c. The amide group in 12 is rotated relative to the benzene ring plane by 9.3° (C(5)-C(4)-N(1)-C(7). There is no conjugation in the O = C–C = S system, and the distance between the thiocarbonyl and carbonyl groups along the C(7)–C(8) bond is 1.532(3).
2.2 Synthetic potential of OAT
The following Sections present data demonstrating the extensive synthetic potential of OAT for the design of heterocyclic compounds.
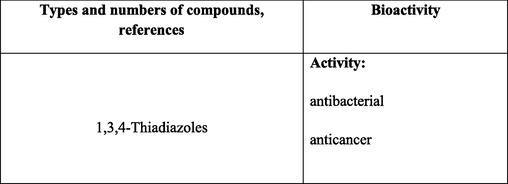
2.2.1 Synthesis of 1,3,4-thiadiazoles
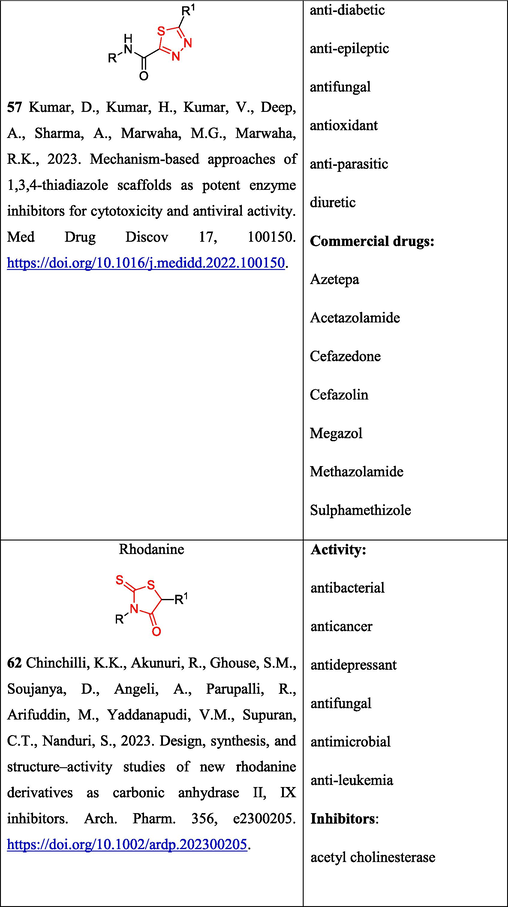
The synthesis of 1,3,4-thiadiazole derivatives is obviously a promising trend in the development of biologially active compounds (Kumar et al., 2023) (Table 2 Biological activity of the compounds). The preparation of 5-carbamoyl-1,3,4-thiadiazoles 13 under mild conditions by the reaction of OAT with acid chlorides has been reported (Yarovenko et al., 2003) (Scheme 5). * The 1H NMR spectra were recorded in DMSO‑d6 at T = 24℃.
Compounds*
R
R1
41(%)
40(%)
39a
H
Ph
90
10
39b
3,4-Cl2
Ph
0
100
39c
H
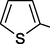
70
30
39d
H
4-NO2-Ph
100
0
39e
3,4-Cl2
4-NO2-Ph
80
20
39f
3,4-Cl2
2-NO2-Ph
100
0
39 g
2,3-Me2
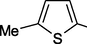
60
40
39 h
3-Me
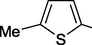
50
50
Synthesis of 5-carbamoyl-1,3,4-thiadiazoles.
The resulting thiadiazoles contain reactive substituents capable of being further modified, in particular converted to heterocycles. For example, it was shown that the chloromethyl group of 5-chloromethyl-1,3,4-thiadiazole-2-carboxamides, synthesized by the reaction of OAT with chloroacetyl chloride (Yarovenko et al., 2003), can be converted to a dithiocarboxylic acid salt by treatment with sulfur and triethylamine. The subsequent treatment with methyl iodide gives dithiocarboxylic acid ester 14, which smoothly reacts with ethylenediamine and 1,3-diaminopropane to give dihydroimidazoles 15a and tetrahydropyrimidines 15b, respectively (Shirokov, 2005). The reaction of OAT with carbon disulfide in the presence of bases furnishes N-aryl-5-thioxo-4,5-dihydro-1,3,4-thiadiazole-2-carboxamides 16, which are converted to sulfides 17 upon alkylation. The oxidation of sulfides 17 with m-chloroperbenzoic acid yields products 18 with sulfoxide moieties, which are effective leaving groups in nucleophilic reactions. The oxidation of the thioamide group in thiadiazoles with C(NO2)4 leads to disulfides 19 (Yarovenko et al., 2003). Unsubstituted 5-carbamoyl-1,3,4-thiadiazoles 20 are formed in good yields by reactions of OAT with a new cyclization agent, a solution of diethyl chlorophosphate in DMF (Yarovenko et al., 2004). An OAT-involving synthesis of 3-(1,3,4-thiadiazol-2-yl) propanoic acids 21, new agonists of free fatty acid receptors (GPR40), has been reported (Krasavin et al., 2017)(Fig. 2).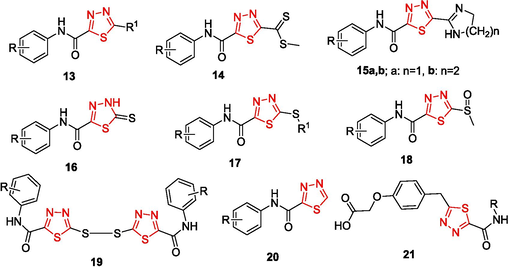
1,3,4-Thiadiazoles obtained by cyclization of OAT.
The reaction of OAT with chlorocarbonylsulfenyl chloride at room temperature affords 6-phenylcarbamoyl-5,6-dihydro (Sharma et al., 2020; Boulebd et al., 2022; Tabbiche et al., 2022; Wang et al., 2023)dithiazin-3-one 22, which eliminates sulfur on heating and is thus converted to 5-phenylcarbamoyl-2-oxy-1,3,4-thiadiazole 23 (Yarovenko et al., 2003) (Scheme 6). The OAT cyclization on treatment with 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethylammonium tetrafluoroborate (TBTU) results in the formation of 2-dimethylamino-1.3.4-thiadiazoles 24 (Lukin et al., 2018).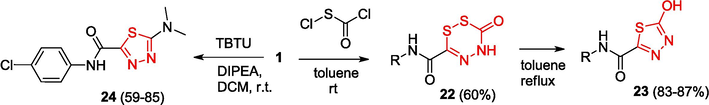
Reaction of OAT with chlorocarbonylsulfenyl chloride and TBTU.
2.2.2 Synthesis of rhodanines
Rhodanines are important heterocycles possessing a wide range of biological activity, including anticancer, antibacterial and anti-mycobacterial properties (Chinchilli et al., 2023) (Table 2. Biological activity of compounds); they are utilized for the treatment of diabetes mellitus (Epalrestat drug).
It was shown that OAT react with trithiocarbonyl diglycolic acid in the presence of dicyclohexylcarbodiimide or carbonyldiimidazole to give 2-[(4-oxo-2-thioxo-1,3-thiazolidin-3-yl)amino]-N-(hetero)aryl-2-thioxamides 25 (Yarovenko et al., 2006) (Scheme 7).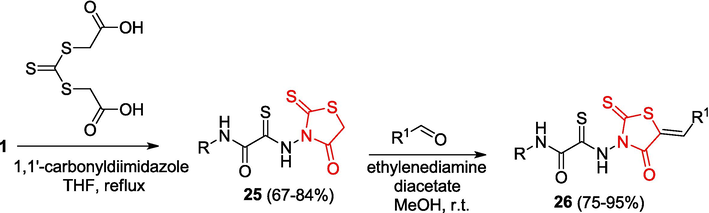
Synthesis of rhodanines and arylidenerhodanines.
In view of the fact that functional rhodanine derivatives are widely used for the synthesis of various biologically active compounds, considerable attention is paid to the synthetic potential of rhodanine-containing OAT, modification of which can substantially extend the range of derivatives of this heterocycle. The reactions of 2-thioxo-1,3-thiazolidin-4-one 25 with aromatic aldehydes resulted in the formation of 5-arylidenerhodanines 26 (Yarovenko et al., 2007);some of these products inhibit endonuclease APE1 and have an antiproliferative effect against a cancer cell line; this makes them appropriate objects for the development of antiviral agents and anticancer drugs (Ramkumar et al., 2010). The reaction of rhodanines 25 with triethyl orthoformate affords ethoxy methylenerhodanines 27, which are converted to enamines 28 on treatment with amines; the reactions of 25 with diazonium salts furnish 5-arylhydrazonorhodanines 29; the acylation of rhodanines with benzoic acid chlorides produces 5-acylrhodanines 30, while the bromination of rhodanines followed by amination gives arylaminorhodanines 31 (Yarovenko et al., 2007). The readily produced rhodanines 26–31 with OAT moieties were used in the synthesis of various fused heterocycles (Fig. 3).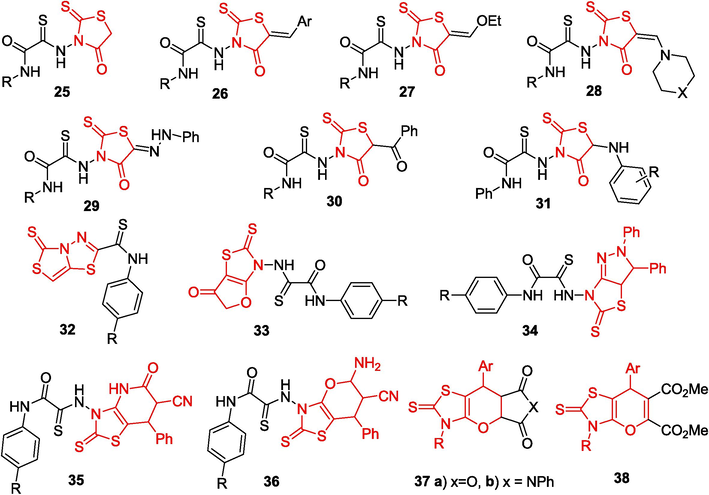
Rhodanine derivatives based on OAT.
Refluxing of rhodanine 25 with the Lawesson's reagent or with phosphorus pentasulfide in toluene gives N-aryl-5-thioxo[1.3]thiazolo[4,3-b] (Sharma et al., 2020; Liu et al., 2022; Tabbiche et al., 2022)thiadiazole-2-carbothioamides 32; the condensation of rhodanines 26 with chloroacetic acid affords 2-thioxo-2,3-dihydrofuro[2,3-d] (Sharma et al., 2020; Liu et al., 2022)thiazol-6-ones 33; when 5-benzylidenerhodanines 26 react with phenylhydrazine, tetrahydro-5H-pyrazolo[3,4-d] (Sharma et al., 2020; Liu et al., 2022)thiazole-5-thiones 34 are formed; refluxing of benzylidene rhodanine derivatives 26 with ethyl cyanoacetate in an acetic acid solution of ammonium acetate gives substituted 2-thioxothiazolopyridine-6-carbonitriles 35; and the condensation of rhodanines 26 with malononitrile or the condensation of rhodanines 25 with benzylidenemalononitrile results in the formation of 3,7-dihydro-2H-pyrano[2,3-d] (Sharma et al., 2020; Liu et al., 2022)thiazole-6-carbonitriles 36. It is important to note that microwave radiation has a pronounced effect on the formation of fused heterocycles (Yarovenko et al., 2007). For instance, the cycloaddition of arylidene rhodanines 26 to dienophiles can be successfully performed only under microwave irradiation. When these rhodanines react with maleic anhydride and with dimethyl acetylenedicarboxylate, pyrano[2,3-d]thiazoles 37a,b and 38 are formed in 78–––90 % yields, whereas this reaction without irradiation gives products in low yields, if at all (Yarovenko et al., 2008).
Oxamic acid thiohydrazide-hydrazones are successfully used in the synthesis of various heterocycles and complex structures. Although these compounds are products of OAT transformation, they are set out in a separate Section due to their exceptional importance.
3 Oxamic acid thiohydrazide-hydrazones (OATH)
3.1 Synthesis and reactions of OATH
In the vast majority of cases, the synthesis of oxamic acid thiohydrazide-hydrazones (OATH) does not present any difficulty and is traditionally carried out by the reaction of OAT with compounds containing aldehyde groups. For example, this approach was used to prepare the hydrazones of gossypol 47 (Stepanov et al., 2024), chromones 48–50, which are of interest as antituberculosis agents (Myannik, 2018), coumarins 51 with high fungal toxicity (Milevsky et al., 2012), and formyl-nitrofuran 52 with antibacterial activity (Lukin et al., 2020). Analogues of the drugs thioacetazone 53 and metisazone 54 (Shirokov, 2005) exhibiting bactericidal activity have been obtained (Fig. 4).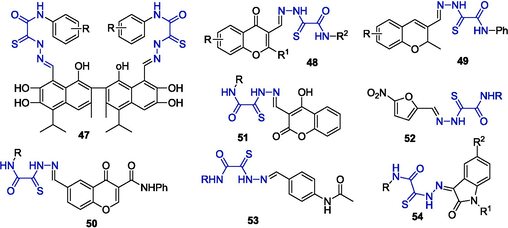
Hydrazones resulting from reactions of OAT with various aldehydes.
OATH are convenient starting compounds for the synthesis of dihydro-1,3,4-thiadiazoles and 1,3,4-thiadiazoles. However, researchers may face a number of problems related to the identification of hydrazones. This is caused by both product tautomerism and the possibility of spontaneous conversion to heterocycles. For example, the reaction of aryl OAT derivatives 39a-h with aldehydes yields hydrazones, which can exist in solutions as an equilibrium mixture of open- 40a-h and closed-ring 41a-h forms, which are converted to 2-carbamoyl-1,3,4-thiadiazoles 42a-h on treatment with oxidants (Yarovenko et al., 2003; Shirokov, 2005) (Scheme 8).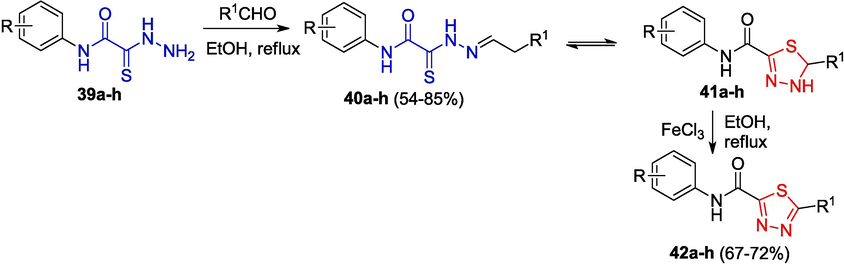
Synthesis of N-aryl-1,3,4-thiadiazole-2-carboxamides.
The ratio of the tautomers depends on the substituents in both the aldehyde and thiohydrazide moieties of the molecule (Table 1) (Shirokov, 2005).
It can be seen from the Table that the presence of an electron-withdrawing group in the aldehyde shifts the equilibrium towards the cyclic product (compounds 41a and 41d), whereas electron-withdrawing substituents in OAT may have the opposite effect (compounds 40b and 40e). The position of substituents in the benzene rings of the reactants also plays a certain role (cf. data for derivatives of 39e and 39f).
1,3,4-Thiadiazoles are synthesized using heterocyclic derivatives of OAT. For example, the oxidation of hydrazones 55 and 57 furnished quinolines 56 (Aksenov et al., 2021) and steroids 58 (Zavarzin et al., 2013) containing 1,3,4-thiadiazole rings (Scheme 9).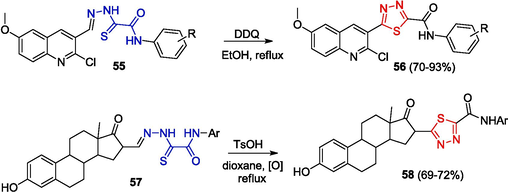
Synthesis of quinolines and steroids containing 1,3,4-thiadiazole rings.
In some cases, it is impossible to isolate hydrazones, as their closed-ring form is immediately converted to 1.3.4-thiadiazoles upon auto-oxidation with air oxygen: for example, compounds 44 are formed in the reaction of OAT with formylpyridine isomers 43 (Myannik et al., 2017) (Scheme 10).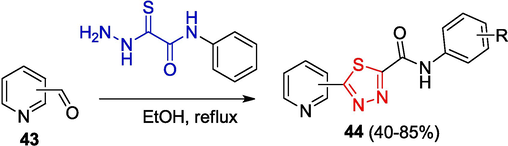
Synthesis of pyridines containing 1.3.4-thiadiazole rings.
The treatment of hydrazones with acyl chloride may serve as a simple preparative method for the synthesis of dihydro-1,3,4-thiadiazoles and their derivatives. For example, the acylation of a mixture of hydrazones 40 and 41 affords 4-acetyl-2-carbamoyl-4,5-dihydro-1,3,4-thiadiazoles 45, which are oxidized with hydrogen peroxide in acetic acid to give 4-acetyl- 2-carbamoyl-4,5-dihydro-1,3,4-thiadiazole 1-oxides 46 (Shirokov, 2005) (Scheme 11).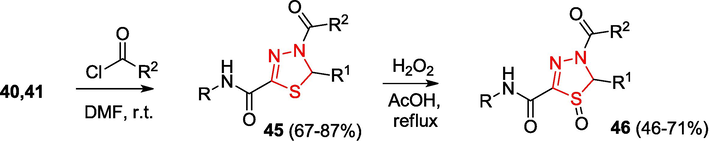
Synthesis of dihydro-1,3,4-thiadiazoles.
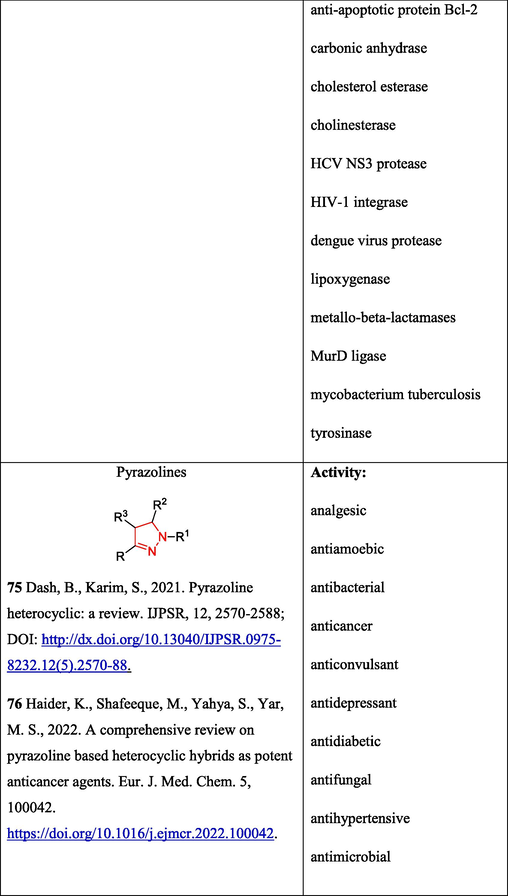
The diverse biological activity of pyrazolines (Table 2. Biological activity of compounds), formed upon the reaction of unsubstituted ketones with OAT thiohydrazides, is summarized in reviews (Dash and Karim, 2021; Haider et al., 2022). According to the authors (Kamernitsky et al., 2007) (Scheme 12), the reactions starts with the formation of hydrazones 59; this is followed by NH-nucleophilic addition to the activated double bond to give pyrazolines 60. The subsequent 1,3[H] rearrangement and deprotonation of intermediates 61 furnishes reaction products 62. It was found that heterocyclization is promoted in the case of thiohydrazides with electron-donating substituents in the aryl moiety.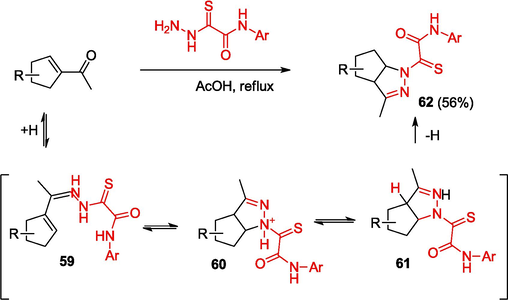
Synthesis of pyrazolines.
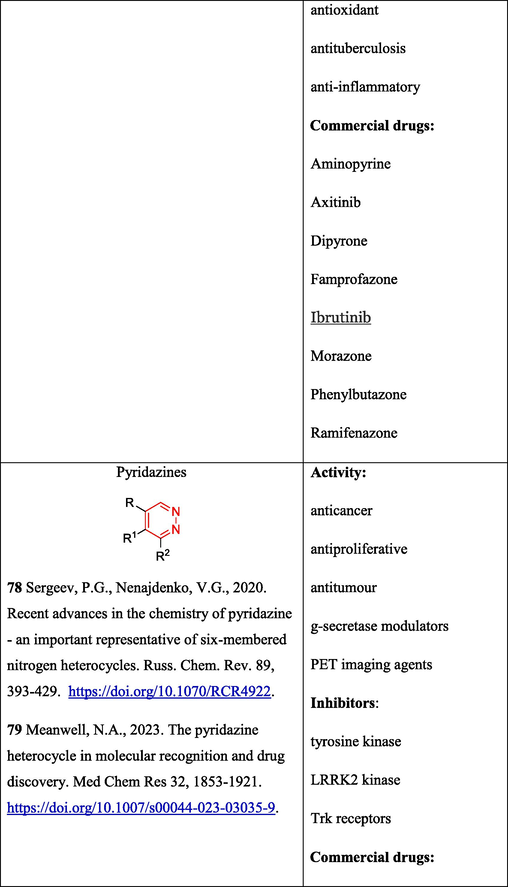
It is noteworthy that not only 5-membered but also 6-membered heterocycles, which are used in the synthesis of various biologically active compounds, can be obtained using OAT hydrazones. In particular, pyridazines were prepared as biologically active compounds (Sergeev and Nenajdenko, 2020; Meanwell, 2023) (Table 2. Biological activity of compounds) from ketones and OAT according to Scheme 13, which includes Vilsmeier–Haack chloroformylation of enolyzable ketones 63 giving chlorides 64 followed by imination to give OAT hydrazones 65 and cascade electrocyclization/aromatization resulting in the formation of pyridazines 66 (Komkov et al., 2015).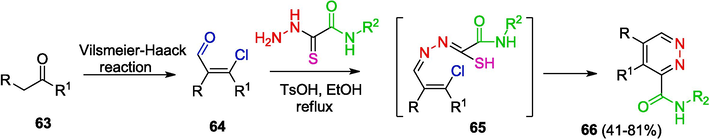
Synthesis of pyridazines.
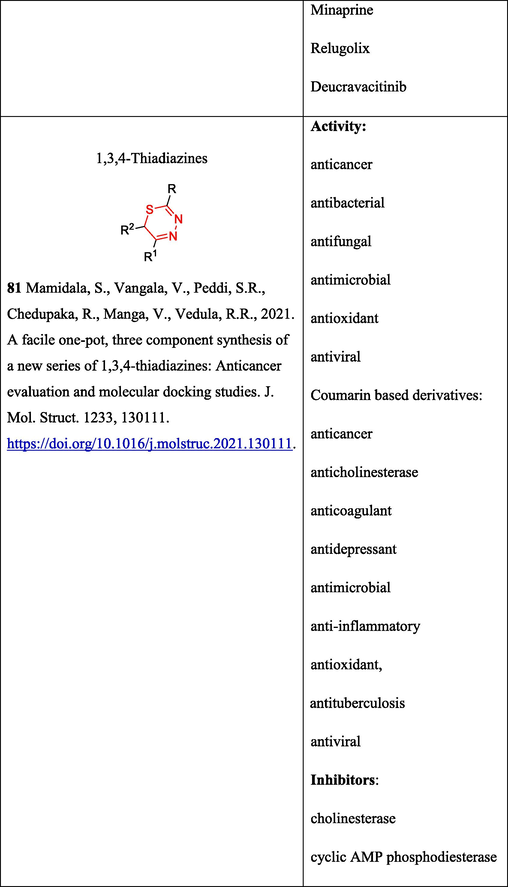
The reactions of OAT with phenacyl bromides 67 (Scheme 14), which affect the thione moiety and lead to 1,3,4-thiadiazines 68 and 5,6-dihydro-4H-1,3,4-thiadiazin-5-ols 69, possessing a broad range of biological activity (Mamidala et al., 2021), have been reported (Komendantova et al., 2019) (Table 2. Bioloigal activity of compounds). The reaction was carried out using an equimolar mixture of OAT and α-bromoacetophenones under basic conditions. It was noted that the ratio of cyclocondensation products substantially depends on the substituents and conditions of the reaction.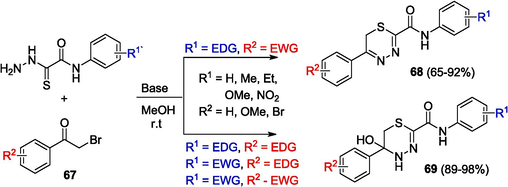
Synthesis of thiadiazines.
The above transformations have been widely used in the chemistry of steroids possessing diverse pharmaceutical and bioactive properties (Schiffer et al., 2019; Birukova et al., 2023; Sharma et al., 2023). OAT hydrazones have been successfully used for the design of molecules containing pyridazine 70 (Volkova et al., 2016), pyrazoline 71 (Kamernitskii et al., 2006), and 1,3,4-thiadiazine moieties 72 (Chernoburova et al., 2019) (Fig. 5).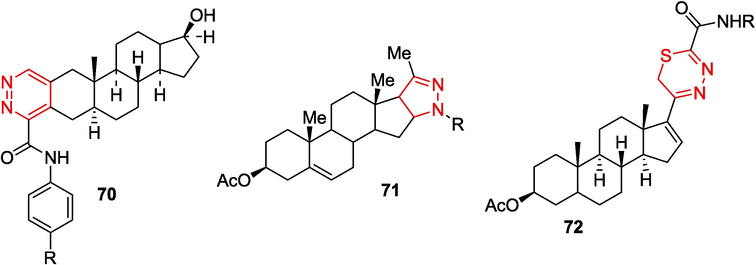
Synthesis of steroids containing various heterocycles on the basis of OAT.
OAT are of obvious interest as complex forming agents, as they contain donor atoms both with high (N,O) and low (S) electronegativity; therefore, they can form fairly stable coordination bonds with both hard and soft Lewis acids.
3.2 Complexes of OAT hydrazones
It is known that metal complexes with thiosemicarbazones, resulting from reactions of salicylaldehyde with thiosemicarbazide and its derivatives, inhibit the growth of human leukemia cells and possess antibacterial and antifungal properties (Pahontu et al., 2013). Tridentate ligands based on OATH 75 were obtained. In particular, the complex [Ni(C15H11N3O2S)(C5H5N)] (76) was synthesized by the reaction of OAT 73 (R = H) with salicylaldehyde and NiCl2 in the presence of pyridine (Yarovenko et al., 2005) (Scheme 15).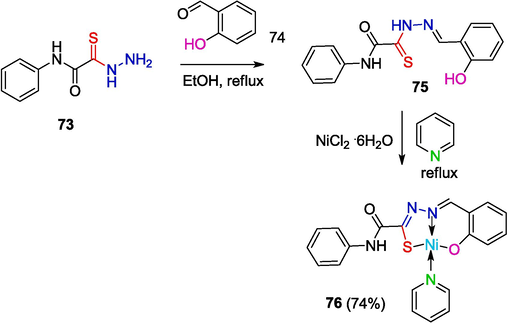
Synthesis of complex 76 in the presence of pyridine.
The geometric parameters of the complex were studied by X-ray diffraction (Fig. 6), (Yarovenko et al., 2005). The needle-shaped dark red crystals of 76 crystallize in the monoclinic system with the unit cell parameters: a = 41.91(2), b = 3.952(3), c = 28.840(13)Å, β = 126.87(4)°, V = 3821(4)Å3, Z = 8, space group C2/c. The structure has an intramolecular hydrogen bond with N(12)–H(12) = 0.81(3)Å, H(12)…N(9) = 2.25(3)Å, N(12)…N(9) = 2.662(4)Å, and N(12)–H(12)…N(9) = 112(3)°. The deviation of atoms from the planes does not exceed 0.02 Å in any of the benzene rings. The square planar coordination of the central atom is typical of nickel. The deviation of the N(8), O(1), N(21), and S atoms from the plane does not exceed 0.1 Å. The six-membered N(21)/C(26) heterocycle is rotated relative to the plane of the above square by 58.5(1).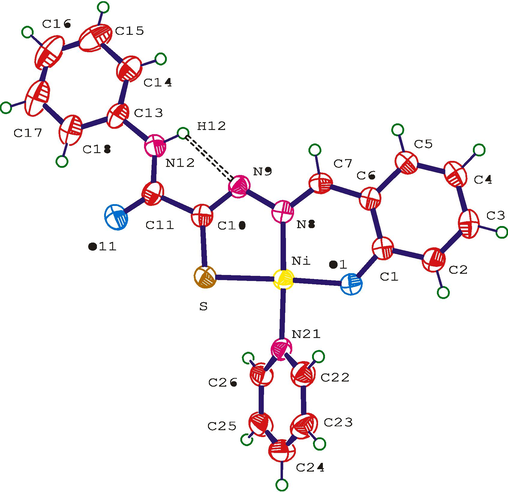
Molecular structure of complex 76 (Yarovenko et al., 2005).
The square planar nickel coordination is completed by two additional atoms, Si- (3.407(2)Å distance) and Oii (3.451(4)Å distance; symmetry code i: x, y + 1, z and i: x, y-1, z) to give a distorted octahedron around the nickel atom. This gives rise to an infinite chain (Yarovenko et al., 2005) (Fig. 7).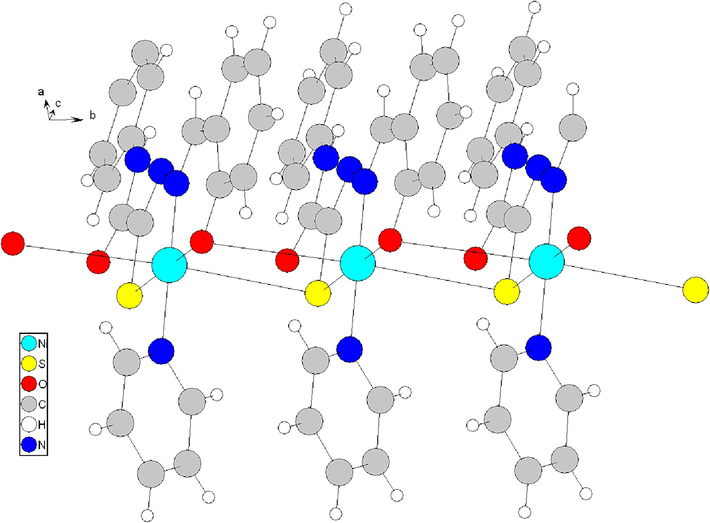
Formation of polymer chains of complex 76 (Yarovenko et al., 2005).
Complex 77 containing the aminoadamantane drug as a ligand was prepared by the reaction of the drug with hydrazone 75 and NiCl2 in the presence of triethylamine (Shirokov, 2005)(Scheme 16).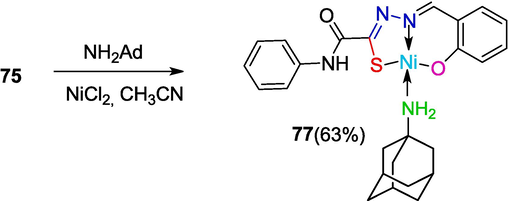
Synthesis of complex 77 containing aminoadamantane.
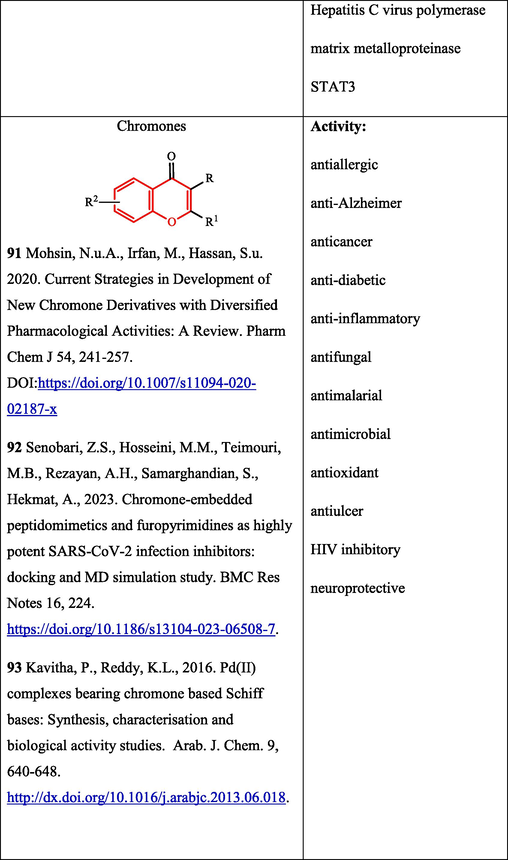
Chromones are known to possess a broad range of biological activity, including anti-HIV (Mohsin et al., 2020) and anti-SARS-CoV-2 (Senobari et al., 2023) activities (Table 2. Biological activity of compounds); the unique ability of chromones to be compatible with various types of receptors was noted (Mohsin et al., 2020). It was shown that Pd complexes with Schiff bases based on formylchromones have antimicrobial properties (Kavitha and Reddy, 2016).
When metal chlorides (CuII, CoII, NiII) react with hydrazones 78–102, formed by the reaction of formylchromones with OAT, binuclear complexes (L-H)2M2Cl2 103–126 are produced (Scheme 17). The electrochemical behaviour of the ligands and the complexes was investigated by cyclic voltammetry (Myannik et al., 2018a, 2018b).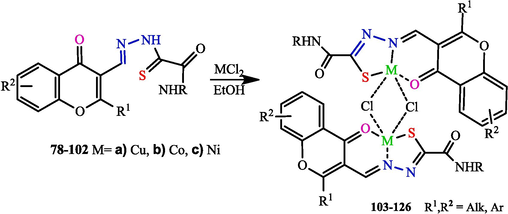
Synthesis of binuclear complexes 103–126.
The structure of the Cu, Co, and Ni coordination compounds is almost the same for all of the ligands. The IR spectra of the complexes show significant shifts of the C = O, N-N, and C = N absorption bands to the 1620–1600 range, which confirms the coordination of the metal ion to the nitrogen atom. The C = S vibration band is missing, indicating that the ligand exists in the complex as the thioenol tautomer. One N–H band also disappears, which confirms the presented structure.
The absolute configuration of compound 103a was established by X-ray diffraction analysis (Fig. 8). According to the results, complex 103a consists of two tridentate moieties linked by two bridging chlorine atoms. The copper atom is coordinated to the carbonyl oxygen atom of the pyranone ring, aldimine nitrogen atom, and the sulfur atom of the thiolate group.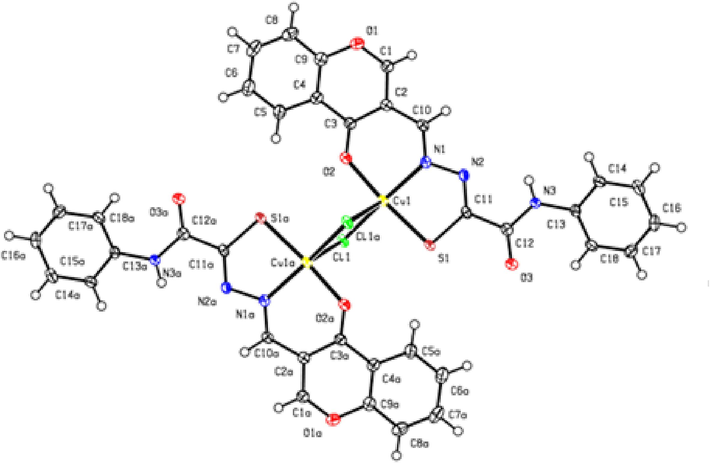
Molecular structure of complex 103a (Myannik et al., 2018a, 2018b).
Note that the reactions of hydrazones 78–102 with copper chloride in ethanol give binuclear complexes, unlike their reported analogues containing carbonyl group in place of the thiocarbonyl group, which form mononuclear complexes. It is known that binuclear copper complexes have a higher catalytic activity in the azide–alkyne cycloaddition reaction giving triazoles than the corresponding mononuclear complex (Ye et al., 2017).
To summarize, we would like to conclude that the synthesis of complexes based on OAT hydrazones is not complicated, but the main challenges are related to determination of fine structure of the compounds.
4 Design of bioactive compounds based on OATH
Considerable progress was made in the development of promising bioactive compounds based on OATH. Below we present examples of synthesis of compounds of this type according to Scheme 18, which either proved to be efficient against model infections in animals, or were approved for clinical trials, or have already been registered. The purpose of these data is to extend information on the reactivity of OATH.
Syntheses and transformations of OATH.
The key conclusion drawn from the years of experience in combating antibiotic resistance can apparently be formulated in the following way: it is necessary to reduce the selective pressure of drugs on pathogens, i.e., the paradigm of treatment of infections should be changed: a drug should inhibit the bacterial virulence rather than kill the bacteria (Clatworthy et al., 2007; Czaplewski et al., 2016).We will consider this statement in relation to the effect of OATH on the type III secretion system (T3SS), a fairly attractive therapeutic target (Sheremet et al., 2020; Chen and Goldberg, 2023) for determining the bacterial virulence. Among known T3SS inhibitors (Zigangirova et al., 2012), the best studied compounds are hydrazones based on aromatic hydrazides of carboxylic acids and salicyladehydes 127–130, which are depicted in Fig. 9.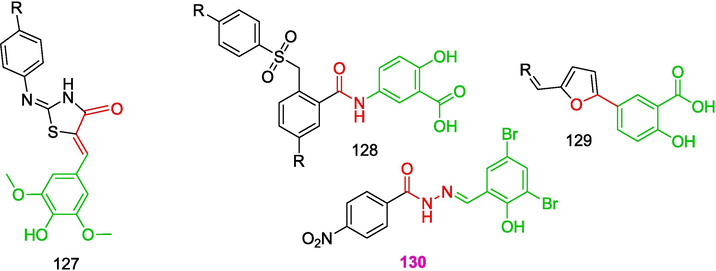
Structures of T3SS inhibitors.
Analogues of the well-known hydrazone 130 will be considered below in more detail. Thiohydrazones 131a-j were synthesized (Zayakin, 2009; RU 2 402 531 C2, 2010; Zigangirova et al., 2012), and the imine bond in thiohydrazones 131a-j was regioselectively reduced with sodium borohydride, with the carbonyl and thiocarbonyl groups remaining intact. This gave rise to N-(hetero)aryl-2-(2-arylhydrazino)-2-thioxoacetamides 132a-j (Zayakin, 2009; RU 2 400 471 C1, 2010; Zigangirova et al., 2012)1(Scheme 19).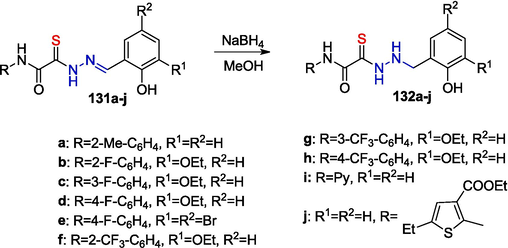
Reduction of OATH in methanol.
The reaction is sensitive to the structure of OAT hydrazones. The reduction of compounds 131a-j was carried out in methanol; however, an attempted reduction of thiohydrazones 133a-d in the same solvent resulted in the formation of complex mixtures. Instead, compounds 133a-d were smoothly reduced in THF (Zayakin, 2009) (Scheme 20).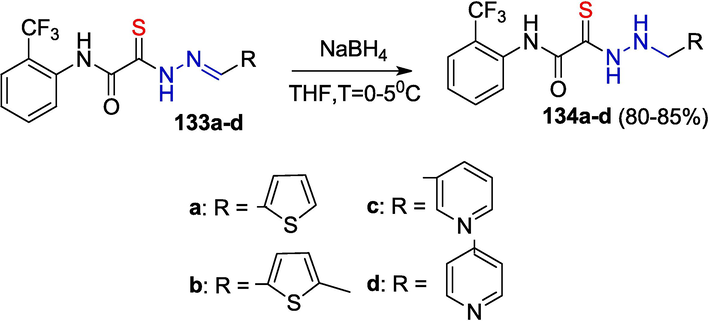
Reduction of OATH in THF.
A variety of heterocyclizations involving the OATH reduction products and giving five-, six-, and seven-membered heterocycles have been reported. It was shown that 4-arylmethyl-5-oxo-N-aryl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamides 135a-c can be obtained by treatment with 1,1′-carbonyldiimidazole (Zayakin, 2009; Zigangirova et al., 2012)(Scheme 21).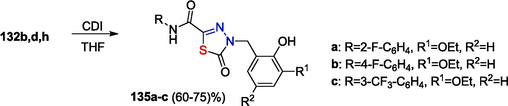
Reaction of OATH reduction products with carbonyldiimidazole.
4-Arylmethyl-5-thioxo-N-aryl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamides 136a-c, thio analogues of compounds 135a-c, were obtained by the reaction of thiohydrazides 132c,d,g with carbon disulfide and an alkali in methanol. The reactions of thiohydrazide with 3-ethoxy-2-hydroxybenzaldehyde afford the corresponding 4,5-dihydro-1,3,4-thiadiazole-2-carboxamides 137a-c. The reactions of thiohydrazide with diethyl chlorophosphate yield phosphorus-containing heterocycles, dihydrothiadiazaphospholane oxides 138a-c (Zayakin, 2009) (Scheme 22).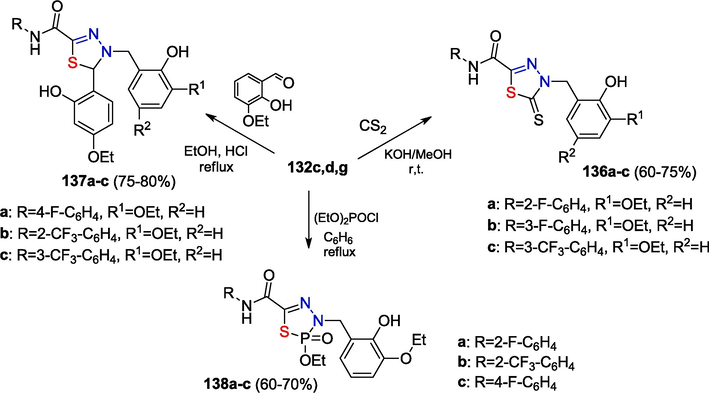
Synthesis of five-membered heterocycles.
Six-membered thiadiazinones 139 are formed in the reactions of thiohydrazides with chloroacetic acid in the presence of ammonium acetate. Traditionally, thiadiazinone modification involving the methylene group is performed by refluxing thiadiazinones with aldehydes in acetic acid and results in the formation of condensation products 141. The interaction of thiohydrazides with oxalyl chloride in DMFA in the presence of triethylamine produces sulfoxide 140; the reaction with aromatic alpha-bromoketones leads to thiadiazines 142. Seven-membered tetrahydrothiadiazepines 143 are the products of reaction of thiohydrazides 132 with bromopropionic acid (Scheme 23) (Zayakin, 2009; RU 2 447 066 C2, 2012; Zigangirova et al., 2012).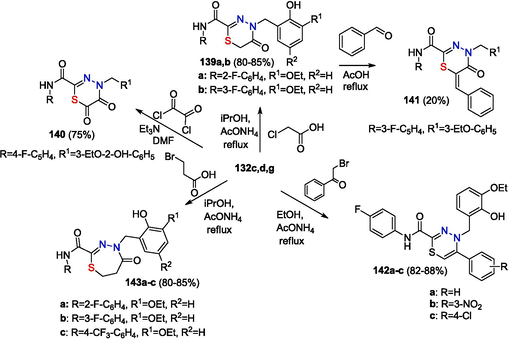
Synthesis of six-membered heterocycles.
The synthesis of indole thiadiazinone derivatives 148 by the reaction of reduced hydrazone 147 with chloroacetic acid has been reported (Scheme 24) (RU 2 495 036 C1, 2013).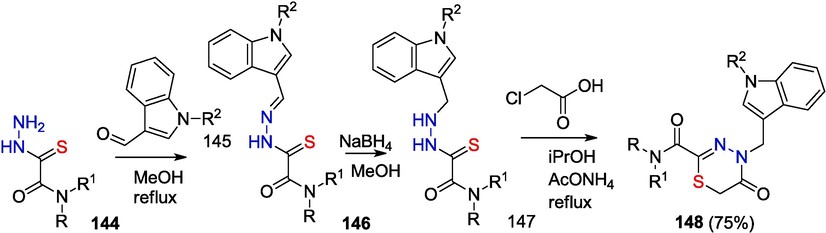
Synthesis of indole derivative of thiadiazinone 148.
The biological activity assays (Zigangirova et al., 2012)of heterocyclic compounds based on the reduction products of OAT hydrazones and specifically targeting the Chlamydial type III secretion system revealed the most effective compound, thiadiazinone 152, the synthesis of which is depicted in Scheme 25 (RU 2 447 066 C2, 2012).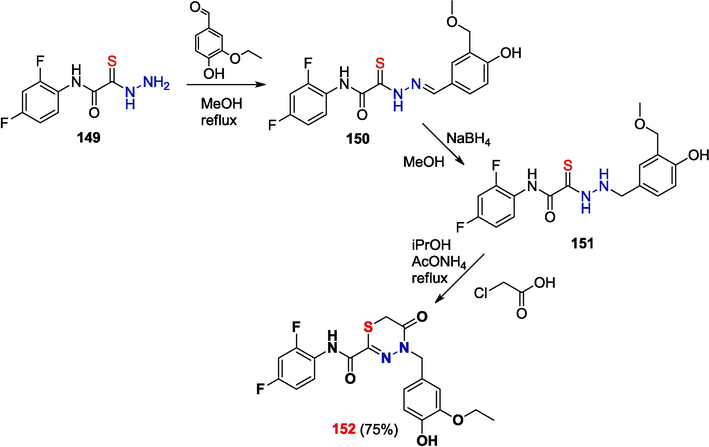
Synthesis of fluorothiazinone 152.
Compound 152 was used for the subsequent, more advanced testing of biological properties, which showed that this compound is a low-molecular-weight inhibitor of type III secretion system and many gram-negative pathogenic bacteria. Product 152 exhibited in vivo and in vitro activity against Chlamydia trachomatis, Salmonella enterica serovar Typhimurium, and multidrug-resistant Pseudomonas eruginosa and Acinetobacter baumannii. It was found that this agent easily penetrates into peripheral tissues, is retained in the body for a long time, and is mainly metabolized to fluorothiazinone glucuronide (Savitskii et al., 2023; Zigangirova et al., 2021; Tsarenko et al., 2023).
5 Conclusions
The interest in the synthesis of thiohydrazone-based compounds possessing a broad spectrum of bioactivity such as antiviral, insecticidal, antisclerotic, antioxidant, antiparasitic, anti-COVID-19, and anti-HIV activities has considerably increased in recent years. The ligands based on these compounds with soft donor nitrogen and sulfur atoms are widely used to obtain metal complexes with various bioactivities, including strong anticancer properties. There is a growing interest in oxamic acid thiohydrazides containing proximate thioamide and thiohydrazide moieties. Owing to their polyfunctional nature and mutual influence of the moieties, these compounds are capable of a wide variety of transformations. The review describes publications in this area, indicating good prospects of using poorly studied derivatives of oxamic acid thiohydrazides for the synthesis of new types of thioacylhydrazones as bioactive products. Convenient methods for the synthesis of oxamic acid thiohydrazides and related hydrazones are considered. Their high synthetic potential is demonstrated in relation to the design of diverse structures, including complexes and a plethora of heterocyclic compounds: pyridazines, pyrazolines, 1,3,4-thiadiazines, 1,3,4-thiadiazoles, thiadiazinones, dihydrothiadiazaphospholanes, thiadiazines, and tetrahydrothiadiazepines, in particular those prepared from natural compounds. Oxamic acid thiohydrazide-hydrazones were shown to function as innovative antivirulence drugs targeting the bacterial secretory system, thus suppressing the infectious process and eliminating the pathogen from the body without affecting the reproduction of bacteria. There are broad prospects for further research in this area owing to the sharp increase in the combinatorial opportunities via structural changes of the initial components and modification of carboxamide groups of oxamic acid derivatives.
CRediT authorship contribution statement
M.M. Krayushkin: Writing – original draft. V.N. Yarovenko: Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review on development of bio-active thiosemicarbazide derivatives: recent advances. J. Mol. Struct.. 2021;1226:129268.
- [CrossRef] [Google Scholar]
- Design, synthesis, antiproliferative activity, and cell cycle analysis of new thiosemicarbazone derivatives targeting ribonucleotide reductase. Arab. J. Chem.. 2021;14:102989.
- [CrossRef] [Google Scholar]
- Synthesis of (2-chloroquinolin-3-yl)-1,3,4-thiadiazole-2-carboxamides. Russ. Chem. Bull.. 2021;70:1131-1134.
- [CrossRef] [Google Scholar]
- Advances in thiosemicarbazone metal complexes as anti-lung cancer agents. Front. Pharmacol. 2022
- [Google Scholar]
- A.B. Bayoumy F. Crouwel N. Chanda T.H.J. Florin Buiter,·H.J.C., Mulder, C.J.J., Boer, N.K.H., Advances in Thiopurine Drug Delivery: The Current State-of-the-Art Eur J Drug Metab Pharmacokinet 46 2021 743 758 10.1007/s13318-021-00716-x.
- Synthesis and biological activity of N-acylhydrazones. Russ. J. Bioorg. Chem.. 2022;48:1123-1150.
- [CrossRef] [Google Scholar]
- Discovery of highly potent proapoptotic antiestrogens in a series of androst-5,16-dienes D-modified with imidazole-annulated pendants. J. Steroid Biochem. Mol. Biol.. 2023;231:106309.
- [CrossRef] [Google Scholar]
- Synthesis and radical scavenging activity of new phenolic hydrazone/hydrazide derivatives: experimental and theoretical studies. J. Mol. Struct.. 2022;1249:131546.
- [CrossRef] [Google Scholar]
- A series of new hydrazone derivatives: synthesis, molecular docking and anticholinesterase activity studies. Mini-Rev. Med. Chem.. 2020;20:1042-1060.
- [CrossRef] [Google Scholar]
- Synthesis, spectral characterization, chemical reactivity and anticancer behaviors of some novel hydrazone derivatives: experimental and theoretical insights. J. Mol. Struct.. 2022;1253:132224.
- [CrossRef] [Google Scholar]
- Recent insights into type-3 secretion system injectisome structure and mechanism of human enteric pathogens. Curr. Opin. Microbiol.. 2023;71:102232.
- [CrossRef] [Google Scholar]
- Novel steroidal 1,3,4-thiadiazines: synthesis and biological evaluation in androgen receptor-positive prostate cancer 22Rv1 cells. Bioorg. Chem.. 2019;91:103142.
- [CrossRef] [Google Scholar]
- Design, synthesis, and structure–activity studies of new rhodanine derivatives as carbonic anhydrase II, IX Inhibitors. Arch. Pharm.. 2023;356:e2300205.
- [Google Scholar]
- Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol.. 2007;3:541-548.
- [CrossRef] [Google Scholar]
- Design, synthesis and biological activities of echinopsine derivatives containing acylhydrazone moiety. Sci. Rep.. 2022;12:2935.
- [CrossRef] [Google Scholar]
- Alternatives to antibiotics—a pipeline portfolio review. Alternatives Lancet Infect Dis.. 2016;16:239-251.
- [CrossRef] [Google Scholar]
- Raman spectra of sulfur dissolved in primary amines. J. Phys. Chem.. 1973;77(15):1859-1861.
- [CrossRef] [Google Scholar]
- de Paiva, R.E.Fe., Vieira, E.G., da Silva, D.R., Wegermann, C.A., Ferreira; A.M.C., 2020. Anticancer Compounds Based on Isatin-Derivatives: Strategies to Ameliorate Selectivity and Efficiency. Front. Mol. Biosci., 7, 627272. .
- Synthesis and characterization of novel acyl hydrazones derived from vanillin as potential aldose reductase inhibitors. Mol. Divers.. 2023;27:1713-1733.
- [CrossRef] [Google Scholar]
- Recent advancements of ORGANOTIN(IV) complexes derived from hydrazone and thiosemicarbazone ligands as potential anticancer agents. Inorg. Chem. Commun.. 2022;139:109208.
- [CrossRef] [Google Scholar]
- The thiosemicarbazone, DpC, broadly synergizes with multiple anti-cancer therapeutics and demonstrates temperature- and energy-dependent uptake by tumor cells. Biochim. Biophys. Acta Gen. Subj.. 2022;1866:130152.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of the antioxidant and anti-tyrosinase activities of thiazolyl hydrazone derivatives and their application in the anti-browning of fresh-cut potato. Food Chem.. 2023;414:135745.
- [CrossRef] [Google Scholar]
- Hydrazones and thiosemicarbazones targeting protein-protein-interactions of SARS-CoV-2 papain-like protease. Front. Chem.. 2022;10:832431.
- [CrossRef] [Google Scholar]
- Thiosemicarbazone derivatives of transition metals as multi-target drugs: a review. Results Chem.. 2022;4:100459.
- [CrossRef] [Google Scholar]
- A comprehensive review on pyrazoline based heterocyclic hybrids as potent anticancer agents. Eur. J. Med. Chem.. 2022;5:100042.
- [CrossRef] [Google Scholar]
- Design, synthesis and biological evaluation of novel thiosemicarbazone-indole derivatives targeting prostate cancer cells. Eur. J. Med. Chem.. 2021;210:112970.
- [CrossRef] [Google Scholar]
- A comprehensive review on analytical applications of hydrazone derivatives. JOTCSA.. 2022;9:663-698.
- [CrossRef] [Google Scholar]
- Synthesis and pharmacological study of new piracetam derivatives and their thio analogs. Pharm. Chem. J.. 1989;23:803-806.
- [CrossRef] [Google Scholar]
- Effect of ω-substituents in the hydrazones of conjugated pregnane 20-ketosteroids on their ability to cyclize to pyrazolines. Russ. Chem. Bull.. 2006;55:2117-2118.
- [CrossRef] [Google Scholar]
- Kamernitsky, A.V., Chernoburova, E.I., Chertkova, V.V., Zavarzin, I.V., Yarovenko V.N., Krayushkin, M.M., 2007. Acylhydrazones of 20-keto steroids and their transformations: I. Synthesis and properties of 1′-acyl-substituted 3′-methylandrosteno[16,17-d]pyrazolines. Russ J Bioorg Chem 33, 315-319, 33, 315-319. https://doi.org/10.1134/S1068162007030077.
- PD(II) complexes bearing chromone based schiff bases: synthesis, characterisation and biological activity studies. Arab. J. Chem.. 2016;9:640-648.
- [CrossRef] [Google Scholar]
- Medicinal utility of thiosemicarbazones with special reference to mixed ligand and mixed metal complexes: a review. Russ. J. Coord. Chem.. 2022;48:877-895.
- [CrossRef] [Google Scholar]
- Facile synthesis of carboxamide-substituted 1,3,4-thiadiazines and 5,6-dihydro-4H-1,3,4-Thiadiazin-5-ols. Chem. Heterocycl. Comp.. 2019;55:665-671.
- [CrossRef] [Google Scholar]
- A straightforward approach toward multifunctionalized pyridazines via imination/electrocyclization. Org. Lett.. 2015;17:3734-3737.
- [CrossRef] [Google Scholar]
- Thiosemicarbazone complexes of transition metals as catalysts for cross-coupling reactions. Catalysts. 2020;10:1107.
- [CrossRef] [Google Scholar]
- Continued SAR exploration of 1,2,4-thiadiazole-containing scaffolds in the design of free fatty acid receptor 1 (GPR40) agonists. Eur. J. Med. Chem.. 2017;140:229-238.
- [CrossRef] [Google Scholar]
- Synthesis of glycolurils and their analogues. Russ. Chem. Rev.. 2018;87:89-108.
- [CrossRef] [Google Scholar]
- Mechanism-based approaches of 1,3,4 thiadiazole scaffolds as potent enzyme inhibitors for cytotoxicity and antiviral activity. Med. Drug Discov.. 2023;17:100150.
- [CrossRef] [Google Scholar]
- Diaryl-substituted thiosemicarbazone: a potent scaffold for the development of New Delhi metallo-β-lactamase-1 inhibitors. Bioorg. Chem.. 2021;107:104576.
- [CrossRef] [Google Scholar]
- Synthesis, crystal structure, DNA binding properties and antioxidant activities of transition metal complexes with 3-carbaldehyde-chromone semicarbazone. Inorg. Chem. Commun.. 2010;13:1213-1216.
- [CrossRef] [Google Scholar]
- Research Progress on the biological activities of metal complexes bearing polycyclic aromatic hydrazones. Molecules. 2022;27:8393.
- [CrossRef] [Google Scholar]
- 5-Nitrofuran-2-yl thiohydrazones as double antibacterial agents synthesis and in vitro evaluation. Med. Drug Discov.. 2020;17:356-361.
- [CrossRef] [Google Scholar]
- Two new hydrogen-bonded supramolecular dioxo-MOLYBDENUM(VI) complexes based on acetyl-hydrazone ligands: synthesis, crystal structure and DFT studies. J. Mol. Struct.. 2021;1226:129346.
- [CrossRef] [Google Scholar]
- Mini-review of the importance of hydrazides and their derivatives-synthesis and biological activit. Eng. Proc.. 2021;11:21.
- [CrossRef] [Google Scholar]
- A facile one-pot, three component synthesis of a new series of 1,3,4-thiadiazines: anticancer evaluation and molecular docking studies. J. Mol. Struct.. 2021;1233:130111.
- [CrossRef] [Google Scholar]
- A review on quinoline hydrazone derivatives as a new class of potent antitubercular and anticancer agents. Univ. J. Basic Appl. Sci.. 2017;6:354-361.
- [CrossRef] [Google Scholar]
- Chemical and biological evaluation of thiosemicarbazone-bearing heterocyclic metal complexes. Curr. Top. Med. Chem.. 2021;21:59-72.
- [CrossRef] [Google Scholar]
- The pyridazine heterocycle in molecular recognition and drug discovery. Med. Chem. Res.. 2023;32:1853-1921.
- [CrossRef] [Google Scholar]
- Synthesis, crystal growth, spectroscopic characterization, hirshfeld surface analysis and DFT investigations of novel nonlinear optically active 4-benzoylpyridine-derived hydrazone. J. Mol. Struct.. 2021;1243:130858.
- [CrossRef] [Google Scholar]
- Milevsky, B.G., Solov́eva, N.P,. Chibisova, T.A., Yarovenko, V.N., Zayakin, E.S., Chernyshev, V.V., Krayushkin, M.M., Traven, V.F., 2012. Hydrazones derived from thiooxamohydrazides and 3-formyl-4-hydroxycoumarin: synthesis, structures, and fragmentation. Russ Chem Bull 61, 2311-2321. DOI https://doi.org/10.1007/s11172-012-0325-x.
- Current strategies in development of new chromone derivatives with diversified pharmacological activities: a review. Pharm. Chem. J.. 2020;54:241-257.
- [CrossRef] [Google Scholar]
- A convenient modified synthesis of 5-pyridinyl-1,3,4-thiadiazole-2-carboxamides. Arkivoc, Iii 2017:316-325.
- [CrossRef] [Google Scholar]
- Novel COPPER(II), COBALT(II) and NICKEL(II) complexes with 5-(4-oxo-4H-chromen-3-yl)-4,5-dihydro-1,3,4-thiadiazole-2-carboxamide: synthesis, structure, spectroscopic studies. Polyhedron. 2018;139:208-214.
- [CrossRef] [Google Scholar]
- Synthesis and electrochemical study of 2-carbamoyl-4,5-dihydro-1,3,4-thiadiazole-containing ligands and their complexes with cuii, coii and niii. Mendeleev Commun.. 2018;28:79-80.
- [CrossRef] [Google Scholar]
- Myannik, K. A., 2018. Synthesis of new 3-carbamoylchromones derivatives. Dis. Ph.D. chem. sciences, N. D. Zelinsky Institute of Organic Chemistry RAS, Moscow, 121. https://search.rsl.ru/ru/record/01010172227.
- A review on biological activities of schiff base, hydrazone, and oxime derivatives of curcumin. RSC Adv.. 2020;10:30186-30202.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of some new CU(II), NI(II) and ZN(II) complexes with salicylidene thiosemicarbazones: antibacterial, antifungal and in vitro antileukemia activity. Molecules. 2013;18:8812-8836.
- [CrossRef] [Google Scholar]
- Applications of radiocomplexes with thiosemicarbazones and bis(thiosemicarbazones) in diagnostic and therapeutic nuclear medicine. Coord. Chem. Rev.. 2022;458:214418.
- [CrossRef] [Google Scholar]
- Updated information on antimicrobial activity of hydrazide-hydrazones. Int. J. Mol. Sci.. 2021;22:9389.
- [CrossRef] [Google Scholar]
- Design, synthesis and structure-activity studies of rhodanine derivatives as HIV-1 integrase inhibitors. Molecules. 2010;15:3958.
- [CrossRef] [Google Scholar]
- RU 2 400 471 C1, 2010 https://patentimages.storage.googleapis.com/fd/c9/a5/341ba4c929a4b7/RU2400471C1.pdf.
- RU 2 402 531 C2, 2010 https://patentimages.storage.googleapis.com/92/98/11/c94a3533043fd1/RU2402531C2.pdf.
- RU 2 447 066 C2, 2012. https://patentimages.storage.googleapis.com/20/b2/3d/b03651dbdafb2a/RU2447066C2.pdf.
- RU 2 495 036 C1, 2013. https://patentimages.storage.googleapis.com/e3/a5/08/68e2556b244f70/RU2495036C1.pdf.
- Savitskii M.V., Moskaleva N.E., Zigangirova N.A., Soloveva A.V., Sheremet A.B., Bondareva N.E., Lubenec N.L., Pyatigorskaya N.V., Appolonova S.A., 2023. Experimental Pharmacokinetics, Metabolism and Tissue Distribution Studies Fluorothiazinon, a of Novel Antivirulence Drug. Journal Biomed. 19, 73-84. (In Russ.). https://doi.org/10.33647/2074-5982-19-1-73-84.
- M. Scaccaglia M. Rega C. Bacci D. Giovanardi S. Pinelli G. Pelosi F. Bisceglie Bismuth complex of quinoline thiosemicarbazone restores carbapenem sensitivity in NDM-1-positive Klebsiella pneumoniae J. Inorg. Biochem. 234 2022 111887 10.1016/j.jinorgbio.2022.111887.
- Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J. Steroid Biochem. Mol. Biol.. 2019;194:105439.
- [CrossRef] [Google Scholar]
- Chromone-embedded peptidomimetics and furopyrimidines as highly potent SARS-CoV-2 infection inhibitors: docking and MD simulation study. BMC. Res. Notes. 2023;16:224.
- [CrossRef] [Google Scholar]
- Recent advances in the chemistry of pyridazine - an important representative of six-membered nitrogen heterocycles. Russ. Chem. Rev.. 2020;89:393-429.
- [CrossRef] [Google Scholar]
- Thiosemicarbazones as potent anticancer agents and their modes of action. Mini-Rev. Med. Chem.. 2020;20:638-661.
- [CrossRef] [Google Scholar]
- Formation of nitrogen-containing six-membered heterocycles on steroidal ring system: a review. Steroids. 2023;191:109171.
- [CrossRef] [Google Scholar]
- Hydrazone comprising compounds as promising anti-infective agents: chemistry and structure-property relationship. Mater. Today Chem.. 2020;18:100349.
- [CrossRef] [Google Scholar]
- The type three secretion system of Pseudomonas aeruginosa as a target for development of antivirulence drugs. Mol. Genet. Microbiol. Virol.. 2020;35:1-13.
- [CrossRef] [Google Scholar]
- Shirokov, A.V. 2005. Synthesis and reactivity thiohydrazides of oxamic acids. Dis. Ph.D. chem. sciences, N.D. Zelinsky Institute of Organic Chemistry RAS, Moscow, 109. https://search.rsl.ru/ru/record/01002931047.
- Acylhydrazones and their biological activity: a review. Molecules. 2022;27(24):8719.
- [CrossRef] [Google Scholar]
- Enhancement of Y-maze learning by piracetam, 2-thio-1-pyrrolidine-acetamide and 2-thio-1-pyrrolidine-thio-acetamide in rats. J. Neural Transm. Gen. Sect.. 1993;6:139-144.
- [CrossRef] [Google Scholar]
- COPPER(II) complexes based on thiosemicarbazone ligand: preparation, crystal structure, hirshfeld surface, energy framework, antiMycobacterium activity, in silico and molecular docking studies. J. Inorg. Biochem.. 2021;223:111543.
- [CrossRef] [Google Scholar]
- Reaction of gossypol with thiohydrazides of oxamic acids. Russ. Chem. Bull.. 2024;73(2) in press
- [Google Scholar]
- Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev.. 2014;43:1963-1981.
- [CrossRef] [Google Scholar]
- New bis hydrazone: synthesis, X-ray crystal structure, DFT computations, conformational study and in silico study of the inhibition activity of SARS-CoV-2. J. Mol. Struct.. 2022;1261:132865.
- [CrossRef] [Google Scholar]
- Thiohydrazide und 1,3,4-thiadiazole durch hydrazinolyse von dithioestern. Prakt. Chem.. 1989;331:649-658.
- [CrossRef] [Google Scholar]
- Dithiocarbonsäuren, dithiocarbonsäureester oder thiocarbonsäureamide aus methylenaktiven chlormethylverbindungen und SchwefelJ. Prakt. Chem.. 1989;331:243-262.
- [CrossRef] [Google Scholar]
- A novel antivirulent compound fluorothiazinone inhibits Klebsiella pneumoniae biofilm in vitro and suppresses model pneumonia. J. Antibiot.. 2023;76:397-405.
- [CrossRef] [Google Scholar]
- Design and syntheses of novel N′-((4-oxo-4H-chromen-3-yl)methylene)benzohydrazide as inhibitors of cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase. Bioorg. Med. Chem.. 2013;21:2826-2831.
- [CrossRef] [Google Scholar]
- Access to steroidal pyridazines via modified thiohydrazides. RSC Adv.. 2016;6:42863-42868.
- [CrossRef] [Google Scholar]
- Hydrazone derivatives in agrochemical discovery and development. Chin. Chem. Lett.. 2023;35:108207.
- [CrossRef] [Google Scholar]
- Hydrazone derivatives in agrochemical discovery and development. Chin. Chem. Lett.. 2024;35:108207.
- [CrossRef] [Google Scholar]
- Hydroxamate and thiosemicarbazone: two highly promising scaffolds for the development of SARS-CoV-2 antivirals. Bioorg. Chem.. 2022;124:105799.
- [CrossRef] [Google Scholar]
- Design, synthesis, and evaluation of hydrazones as dual inhibitors of ryanodine receptors and acetylcholinesterases for Alzheimer’s disease. Bioorg. Chem.. 2023;133:106432.
- [CrossRef] [Google Scholar]
- Synthesis of carbamoylformhydroxymoyl chlorides and study of their reactivities. Russ. Chem. Bull.. 2002;51:1504.
- [CrossRef] [Google Scholar]
- New approach to the synthesis of 2-carbamoylbenzothiazoles. Russ. Chem. Bull.. 2002;51:144-147.
- [CrossRef] [Google Scholar]
- Synthesis of oxamic acids thiohydrazides and Carbamoyl-1,3,4-thiadiazoles. Russ. J. Org. Chem.. 2003;39:11331139.
- [CrossRef] [Google Scholar]
- Synthesis of carbamoyl-containing N, S-heterocyclic compounds. Phosphorus Sulfur Silicon Relat. Elem.. 2003;178:1283-1288.
- [CrossRef] [Google Scholar]
- New cyclizing reagent for the synthesis of 1,3,4-thiadiazoles. Synthesis. 2004;1:17-19.
- [CrossRef] [Google Scholar]
- {N-Anilino-2-[(2-oxidophenyl)methylenehydrazono]-2-sulfidoacetamide}pyridinenickel(II) Acta Cryst. 2005;E61:m964-m966.
- [CrossRef] [Google Scholar]
- A convenient synthesis of N-substituted 2-Thioxo-1,3-thiazolidin-4-ones. Synthesis. 2006;8:1246-1248.
- [CrossRef] [Google Scholar]
- Synthesis of 2-thioxo-1, 3-thiazolidin-4-one derivatives. Russ. Chem. Bull.. 2007;56:1624-1630.
- [CrossRef] [Google Scholar]
- Synthesis of fused heterocyclic compounds on the basis of 2-thioxo-1,3-thiazolidin-4-ones. Russ. J. Org. Chem.. 2007;43:1364-1370.
- [CrossRef] [Google Scholar]
- Synthesis of fluorine-containing analogs of ellipticine and other heterocycles from 2-nitro-and 2-amino-4,5-difluoroanilines. Russ. J. Org. Chem.. 2007;43:1387-1392.
- [CrossRef] [Google Scholar]
- Synthesis of fused heterocyclic compounds from arylidenerhodanines under microwave irradiation. ARKIVOC Iv. 2008;103–111
- [CrossRef] [Google Scholar]
- Synthesis of dihydrothiazoles and thiazoles based on monothiooxamides. J. Sulphur Chem.. 2009;327–337
- [CrossRef] [Google Scholar]
- Synthesis of mono- and binuclear CU(II) complexes bearing unsymmetrical bipyridine–pyrazole–amine ligand and their applications in azide-alkyne cycloaddition. Organometallics. 2017;36:2116-2125.
- [CrossRef] [Google Scholar]
- Interaction of 16-hydroxymethylidene derivatives of androstane and estrone with thiohydrazides of oxamic acids. Russ. Chem. Bull.. 2013;62:2603-2608.
- [CrossRef] [Google Scholar]
- Zayakin, E.S., 2009. Synthesis thiohydrazides of oxamic acids derivatives. Dis. Ph.D. chem. sciences, N. D. Zelinsky Institute of Organic Chemistry RAS, Moscow, 113. https://search.rsl.ru/ru/record/01004562945.
- Development of chlamydial type III secretion system inhibitors for suppression of acute and chronic forms of chlamydial infection. Acta Nat.. 2012;4:87-97.
- [CrossRef] [Google Scholar]
- Fluorothiazinon, a small-molecular inhibitor of T3SS, suppresses salmonella oral infection in mice. J. Antibiot.. 2021;74:244-254.
- [CrossRef] [Google Scholar]







