Translate this page into:
Oxidation characteristics and hazardous of α-pinene, β-pinene and turpentine
⁎Corresponding author. laifang200609@126.com (Fang Lai), xmliu1@gxu.edu.cn (Xiongmin Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
α-Pinene, β-pinene and turpentine are renewable resources that are widely used in fine chemicals, fuels and other applications, and the safety of their production, transport and use should be considered. In this work, the characteristics and hazardous of the oxidation process of α-pinene, β-pinene and turpentine were investigated by using a novel mini closed pressure vessel testing system (MCPVT). The oxidation products were resolved by gas chromatography-mass spectrometry (GC–MS). The results showed that α-pinene, β-pinene and turpentine were stable under nitrogen atmosphere and very active chemically under oxygen atmosphere. The activation energies (Ea) of α-pinene, β-pinene and turpentine were calculated to be 78.87, 109.69 and 98.82 kJ/mol, respectively, using pressure–time (P-t) curves. So, the reaction activity sequence was α-pinene > turpentine > β-pinene. The peroxide concentrations of α-pinene (363 K), β-pinene (358 K) and turpentine (363 K) could reach 81.94, 84.70 and 75.76 mmol/kg, respectively, at relatively low temperatures. 0.6 g of α-pinene exploded at a system temperature of 369 K and an oxygen pressure of 1.23 MPa. 0.6 g of β-pinene also exploded at a system temperature of 373 K and an oxygen pressure of 1.27 MPa. 0.4 g of turpentine experienced thermal runaway at a system temperature of 384 K and an oxygen pressure of 0.81 MPa. The major oxidation products of α-pinene are α-campholenaldehyde, laevo-pinocarveol, verbenol, verbenone, bicyclo (3.1.1)heptane-2, 3-diol, 2, 6, 6-trimethyl, 2-cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, (1R,5R)-rel-, cyclobutaneacetic acid, 3-acetyl-2, 2-dimethyl, myrtenol and myrtenal. The major thermal runaway products are water, ethanol, acetone, acetic acid, 2-pentanone, propyl acetate, toluene, cymene and methylacetophenone. The major oxidation products of β-pinene are nopinone, laevo-pinocarveol, pinocarvone, myrtenol, myrtenal and perillyl alcohol. The major thermal runaway products are water, ethanol, acetone, n-ethyl propanoate, toluene, butyl acetate, cymene, apocamphor, nopinone, bornyl formate, methylacetophenone and dihydronopol. The oxidation products and thermal runaway products of pinene were significantly different. Possible reaction pathways for α-pinene and β-pinene are proposed.
Keywords
Turpentine
Thermal oxidation
Peroxide
Thermal runaway
Explosion
1 Introduction
Turpentine is an abundant and inexpensive natural product of great industrial value (García et al., 2020). It is widely used in the pharmaceutical industry (Gülçin et al., 2003), perfume industry(Bohlmann and Keeling, 2008), food additives and other chemical industries (Zhu et al., 2018). Barbara et al. used the fluoroantimonic acid-catalysed turpentine and acetic acid to synthesise terpenyl acetate, which can be used in the production of fragrances, with high conversions and selectivities of 96 % and 60 % respectively (Mendonça et al., 2022).
Turpentine is composed mainly of α-pinene and β-pinene. They are a bicyclic hydrocarbon consisting of two isoprene units resulting in the formula of C10H16 (Liu et al., 2013). Due to its C = C double bond and bicyclic structure, pinene is susceptible to oxidation during storage, use and transport when exposed to air or oxygen (Pospisilova et al., 2021). The oxidation reaction usually occurs on the α-H and C = C bond of pinene (Cherrad et al., 2022). Oxidation occurring on the α-H can give the corresponding alcohol, ketone compounds, such as verbenol/verbenone, myrtenol/myrtenal, perillyl alcohol and terpineol, etc. Myrtenal is a key precursor in the synthesis of flavours (Martins et al., 2022). Perillyl alcohol is used to treat Plasmodium falciparum infections (Sánchez-Velandia et al., 2019). For green reasons, most pinene oxidation reactions are now carried out with solid catalysts and under solvent-free conditions. Grzeszczak et al. tested the optimum conditions for catalysing the oxidation of α-pinene using ZSM-5 catalyst under solvent-free conditions. The ZSM-5 catalyst was effective in converting α-pinene to α-pinene oxide, verbenol and verbenone by reacting at 90–100 °C for 6–8 h (Grzeszczak et al., 2023). When the oxidation occurs at the C = C bond, the epoxide is formed or the bond is broken to produce the corresponding alcohols and ketones, such as α-pinene oxide, β-pinene epoxide. α-Pinene oxide and β-pinene epoxide can be used as an intermediate in drugs. To obtain highly selective α-pinene oxide, Kiełbasa et al. used coffee grounds as a carbon source and then loaded nickel onto the carbon to produce a catalyst for the catalytic synthesis of α-pinene oxide from α-pinene, which was obtained by oxidation at 100 °C for 150 min with a selectivity of 34 %(Kiełbasa et al., 2023). Therefore, the process of pinene oxidation is very complicated, which is the reason for the existence of multiple oxidation forms (Alwedian, 2017). Oxygen, as a cheap and easily available oxidant, is usually used in the oxidation reaction of pinene. However, while our attention is focused on the economic effects of the high-value oxidation products of pinene, the safety issues that these oxidation processes bring us are often overlooked. Rudz et al. determined the minimum ignition energy of the α-pinene/air mixtures using laser-induced spark ignition. This study shows the potential threat of the α-pinene/air mixture (Rudz et al., 2014). The thermal stability and oxidation characteristics of α-pinene, β-pinene and α-pinene/β-pinene mixtures have been investigated by Liu et al. using high sensitivity accelerating rate calorimeter (ARC) and C80 calorimeter. The oxidation of pinene is divided into three steps: Induction phase of the oxidation reaction, main oxidation phase with reduced pressure, thermal decomposition to gas production (Liu et al., 2021). The multiple oxidation methods described above involve continuous heating and the introduction of oxygen, which is challenging for safe production. Their thermal hazards should therefore not be underestimated and their thermal oxidation properties deserve in-depth study.
Turpentine is used as an additive in fuels (da Silva Rodrigues et al., 2011) and fuels (Dubey and Gupta, 2018; Jeevanantham et al., 2020; Karikalan and Chandrasekaran, 2017), in addition to the production of fine chemicals. Turpentine heating value, viscosity, selfignition and boiling temperatures are higher than those of gasoline (Anand et al., 2010), so it can be used in any spark ignition (SI) engines as an additive to the gasoline or gasoline-like fuel (GLF)(Anandavelu et al., 2010; Karthikeyan and Mahalakshmi, 2007; Yumrutaş et al., 2008). Studies by Kannan et al. have shown that the engine operated with turpentine performed well with little loss of brake thermal efficiency. And, proved that the turpentine is a best suited fuel for HCCI(a operating mode of internal combustion engines)operation (Kannan et al., 2014). The activation energies of α-pinene and β-pinene oxidation reactions are close to diesel and sunflower biofuel ones (Mokrani et al., 2020). Bierkandt et al. studied the combustion kinetics of α-pinene and β-pinene. The results show that α-pinene has a higher propensity to form aromatic hydrocarbons and soot (Bierkandt et al., 2021). α-Pinene and β-pinene can also be synthesized into biomass high energy density fuels via dimerization/isomerization (Liu et al., 2020; Yuan et al., 2018). Turpentine is one of the vital biodegradable fuel source which has the potential to replace partially the diesel fuel (Ballesteros et al., 2020); therefore, its oxidation characteristics and hazardous should be taken into account when used as a fuel.
To further understand the oxidation mechanism of α-pinene and β-pinene, Neuenschwander et al. studied the thermal autoxidation of α-pinene and β-pinene using both experimental and theoretical calculations. Four different types of peroxyl radicals are generated during the autoxidation of α-pinene. These radicals can generate the corresponding hydroperoxides by abstract H atoms, of which verbenyl-hydroperoxide is the most predominant product of α-pinene oxidation. Hydroperoxides are primary oxidation products and as such they are extremely unstable, decomposing when exposed to heat, light or vibration, leading to free radical chain reactions (Neuenschwander et al., 2010; Neuenschwander and Hermans, 2010). During the autoxidation of β-pinene, two main peroxyl radicals were generated: pinocarvyl peroxyl radical, and myrtenyl peroxyl radical. It was found that the relatively high peroxyl radical concentration, combined with the high rate of peroxyl cross-reaction, makes the radical–radical reaction surprisingly important for β-pinene (Neuenschwander et al., 2011). In general there are two different types of radical propagation steps for the autoxidation of α-pinene and β-pinene: The abstraction of the allyl H-atom and the addition of unsaturated C = C bonds. The efficiency for both pathways depend on the structure of the peroxyl radical.
α-Pinene, β-pinene and turpentine may have the risk of thermal runaway and explosion in use. Therefore, the purpose of this study is to investigate their thermal oxidation characteristics and the hazardous of the thermal runaway in order to provide theoretical support for safe application in different fields. Mini closed pressure vessel test (MCPVT) was used to investigate the characteristics and thermal hazards of α-pinene, β-pinene and turpentine oxidation. The MCPVT has the following advantages (Huang et al., 2013; Schreck et al., 2004; Wang et al., 2018): (1) It has a relatively short test time and can be used for rapid testing of the oxidation characteristics and hazards of organic compounds. (2) As a pressurised test device it is closer to the actual intermittent production equipment. It can also simulate the high pressure environment of an internal combustion engine. (3) The MCPVT is less expensive than the ARC, C80 and VSP2, making it a cost-effective and safe device for evaluating chemical thermal hazards. The thermal safety parameters of α-pinene, β-pinene and turpentine were tested using MCPVT including: the initial oxygen absorption temperature (Tab), the runaway onset temperature (Tc), maximum temperature (Tmax), the maximum rate of temperature rising (dT/dt) max and the maximum rate of pressure rising (dP/dt) max. The kinetics of the oxidation of α-pinene, β-pinene and turpentine were calculated. The oxidation products were examined using GC–MS and possible oxidation pathways for α-pinene and β-pinene were postulated. The data obtained may be quite instructive for avoiding fire and explosion accidents in production, transportation and application of α-pinene, β-pinene and turpentine. The results can be applied to the design of fire rescue systems.
2 Experimental
2.1 Materials
α-Pinene (purity ≥ 95.0 %) and β-pinene (purity ≥ 95.0 %) was obtained from Shanghai Macklin Biochemical Co., Ltd., China. Turpentine (AR) was purchased from Guangdong Guanghua Sci-Tech Co., Ltd., China. Table 1 shows the composition of turpentine. KI (purity 99.50 %) and Na2S2O3 (purity 99.50 %) were obtained from Aladdin Industrial Corporation, China. The O2 (purity 99.99 %) and N2 (purity 99.99 %) gases were obtained from Nanning Zhong Yi Chuang Gas Co., Ltd., China.
Compounds
Content (%)
α-Pinene
57.6
Camphene
7.4
β-Pinene
6.4
α-Phellandrene
0.8
3-Carene
5.9
1,4-Cineole
1.7
4-Carene
0.9
p-Cymene
4.4
Limonene
7.1
β-Phellandrene
1.9
1,8-Cineole
1.6
γ-Terpinene
0.5
Terpinolene
3.8
2.2 Thermal oxidation reaction of α-pinene, β-pinene and turpentine by MCPVT
A custom-designed MCPVT was used to trace the thermal oxidation behavior of α-pinene, β-pinene and turpentine (Liang et al., 2022). MCPVT consists mainly of a gas cylinder, stainless steel vessel, heating and stirring system, pressure sensor, temperature sensor, safety relief device and signal logger, as shown in Fig. 1.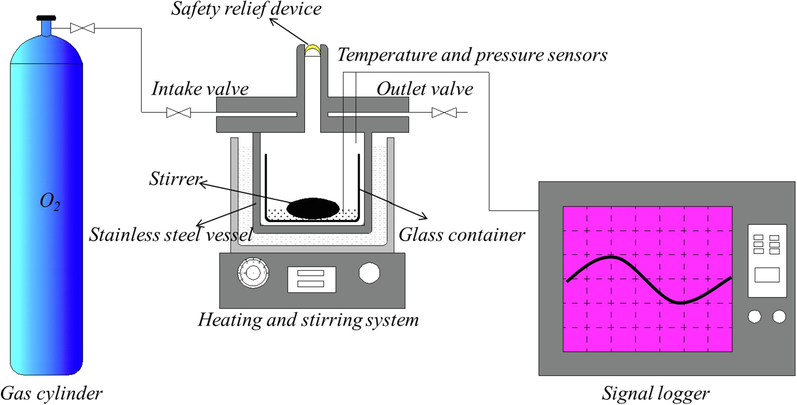
Experimental installation of α-pinene, β-pinene and turpentine oxidation.
The experimental process is as follows: To avoid possible contamination, approximately 0.6 g sample was loaded in a small glass container (10 mL), and then introduced into the stainless steel vessel. Oxygen is delivered from the cylinder into a stainless steel vessel until a predetermined pressure is reached. Heating process: The stainless steel vessel was heated from 303 to 433 K at a rate of approximately 1.5 K/min with constant stirring, and the temperature and pressure inside the stainless steel vessel were recorded throughout this period. Isothermal process: The stainless steel vessel is heated continuously for 7 h at a fixed temperature (330–371 K). The temperature and pressure data inside the stainless steel vessel during the reaction were recorded. At the end of the reaction the reactor is cooled down quickly for subsequent experiments. We filled the stainless steel container with nitrogen for comparative experiments.
2.3 The thermal oxidation of α-pinene and β-pinene and turpentine by DSC under air atmosphere
Thermal stability of α-pinene and β-pinene and turpentine was evaluated by differential scanning calorimeter (DSC). Dynamic temperature-programmed screening experiments were analyzed on a TA Q2000 coupled with a stainless steel crucible (maximum withstood pressure of 15.0 MPa). The sample was placed into the test cell, and then sealed manually. The heat effects were investigated in the nitrogen atmosphere and the detection sensitivity is 0.2 μW. The DSC test was performed at temperatures from 310 to 470 K with heating rate of 5 K/min.
2.4 Analysis of α-pinene, β-pinene and turpentine products by GC–MS
Oxidation products were analyzed by gas chromatography-mass spectrometry (GCMS-QP2010 Ultra, Shimadzu Corp) coupled with an electron impact ionization detector (EID: 70 eV) equipped with a DB-WAX fused silica capillary column (30 m × 0.25 mm i.d., film thickness 0.25 mm). Thermal runaway reactions occurring in stainless steel vessels produce both gaseous and liquid products, both of which are analysed by GC–MS. At the end of the reaction, the stainless steel vessel is placed in ice water to rapidly reduce its internal temperature to room temperature. Finally, the gas is collected in a special gas collection bag. The liquid product remains in the vessel. The test conditions are as follows:
Gas: Injection volume was 2 µL. Column temperature was held at 305 k for 5 min. The EI detector was operated in full scan mode from m/z 11 to 400, and detector temperatures was 493 k.
Liquid: Injection volume was 0.8 µL. The inlet temperature was set at 443 K, and carrier gas was helium gas at a flow rate of 1.5 mL/min. The split ratio was 30:1 and the injection volume was 0.8 µL. The following column temperature program was used: maintained 343 K for 3 min, and then increased to 363 K (1 K/min), and finally increased to 473 K (10 K/min). The mass spectrometer used an EI ion source with anelectron energy of 0.8 kV, and detector temperature was 503 K. The EI detector was operated in full scan mode from m/z 14 to 500. The product peaks detected were identified by the National Institute of Standards and Technology 2011 library of mass spectra.
2.5 Peroxide analysis by iodimetry
Peroxide concentration was determined by the iodometry (Fábos et al., 2009; Yu et al., 2020). The detailed steps are as follows: Approximately 0.1 g of the oxidation product is dissolved in chloroform, followed by the addition of an aqueous solution of starch potassium iodide and stored away from light for 1 h. The solution will undergo the reaction shown in equation (1) to produce iodine as a monomer. An equivalent amount of iodine was liberated with starch, and the solution tured blue. The solution was then titrated with standardized sodium thiosulfate solution until the blue color disappeared (equation (2). The results were quantified as millimoles per kilogram (mmol/kg) of peroxide.
The generated peroxides were analyzed by thin-layer chromatography (TLC). A mixture of petroleum ether and ethyl acetate (V/V, 6:1) was used as the unfolding solvent, and starch potassium iodide solution was used as color developing reagent. If a blue spot was shown on the TLC plate, it was considered as peroxide (Wang et al., 2021).
3 Results and discussion
3.1 Isothermal oxidation of α-pinene, β-pinene and turpentine
The isothermal oxidation reaction can simulate the conditions of α-pinene, β-pinene and turpentine during use, which is an important means to understand their oxidation properties. Fig. 2 shows the pressure behaviour of the α-pinene, β-pinene and turpentine oxidation under isothermal temperature conditions. The pressure behaviour of α-pinene, β-pinene and turpentine was first tested under nitrogen conditions. The pressure in the reactor remained constant even at temperatures up to 373 K. This indicates that the volatilization of α-pinene, β-pinene and turpentine was negligible and no decomposition reaction occurred. Their oxidation behaviour was then tested at a constant temperature in the range 330–371 K. The oxidation of α-pinene and β-pinene with turpentine is a gas–liquid reaction, and if the pressure inside the MCPVT is reduced then an oxidation reaction has occurred. It can be observed from the P-t curves that α-pinene, β-pinene and turpentine have been oxidized at 331 K, 339 K and 330 K, respectively. Therefore, α-pinene, β-pinene and turpentine are very active under oxygen atmosphere. After neglecting the vapour pressure of α-pinene, β-pinene and turpentine during the constant temperature, the oxygen consumption can be obtained by calculating the amount of oxygen at the beginning and end of the reaction using the ideal equation of state (PV = nRT), where R = 8.314 J·mol−1·K−1. As the isothermal oxidation temperature increases the rate of oxygen consumption is accelerating, and its oxygen consumption is also increasing. Because the initial reaction stage produces peroxides, with the increase of reaction temperature, the peroxides gradually decompose and accumulate free radicals in the reactants, and then the free radicals trigger a rapid oxygen uptake reaction, which makes the oxygen pressure drop rapidly.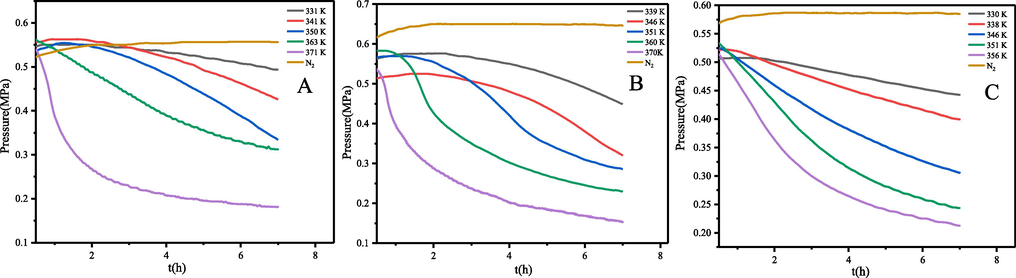
Pressure versus time for α-pinene (A), β-pinene (B) and turpentine (C).
We calculated the oxidation kinetics via the constant temperature oxidation experiments of α-pinene, β-pinene and turpentine, and the calculation procedure was similar to that of Yu et al (Yu et al., 2020). The kinetic calculations start from the stabilisation temperature. It was assumed that α-pinene, β-pinene and turpentine oxidation occurred as follows:
Compound (l) + O2 (g) → product
Kinetic equation (eqn (3)):
Where t is the time, CA0 is the initial concentration of compound, CB0 is the initial concentration of oxygen, x is the concentration of the reaction products at t, k is the rate constant of oxidation, and α and β are the reaction order of compound and oxygen, respectively. The kinetic calculations start after temperature stabilisation. Approximate kinetics can be derived from the reaction rate data by assuming the chemical reaction order. If this assumption holds true, a plot of the natural logarithm of the reaction rate as a function of inverse temperature will yield a straight line.
We have made four assumptions:
(1) The oxidation kinetics was assumed to be zero, first and second order reactions and it was found that the second order reaction gave the best fit. (2) Compound vapor pressure is P = 0. (3) Oxygen is an ideal gas, the remaining molar number of oxygen can be calculated by the ideal gas equation: nt = (B0 - x) = PV/RT, (P is the pressure, R is the gas constant, and T is the temperature). (4) All oxidation products are liquid. So, eqn (3) is simplified by eqn (4):
For the oxidation experiment of α-pinene, β-pinene and turpentine, nA0 = nB0.
Then, eqn (5) can be obtained by the integral of eqn (4):
Where q is a constant.
Eqn (6) is obtained by doing indefinite integration of the Areneus equation in differential form.
Where Z is a constant.
Fig. 3 (D1, E1 and F1) showed the plots of 1/CA versus t at different temperatures, which indicated that lnk had a good linear relationship with T. The initial oxidation reaction of α-pinene, β-pinene and turpentine is a secondorder reaction. The test results are shown in Table 2, the reaction rate increased with increasing temperature. Fig. 3 (D2, E2 and F2) represents the ln k versus (1/T) curve. The parameters Z and Ea for the well-known Arrhenius equation, can be derived from the intercept and the slope of the line. The results of fitting the linear equation are shown in Table 3. The correlation coefficients (R2) for α-pinene, β-pinene and turpentine were 0.9848, 0.9533, 0.9906, respectively, indicating significant linearity. Substituting the slopes of the lines into eqn (5) gave activation energies of 78.87, 109.69 and 98.82 kJ/mol for α-pinene, β-pinene and turpentine, respectively.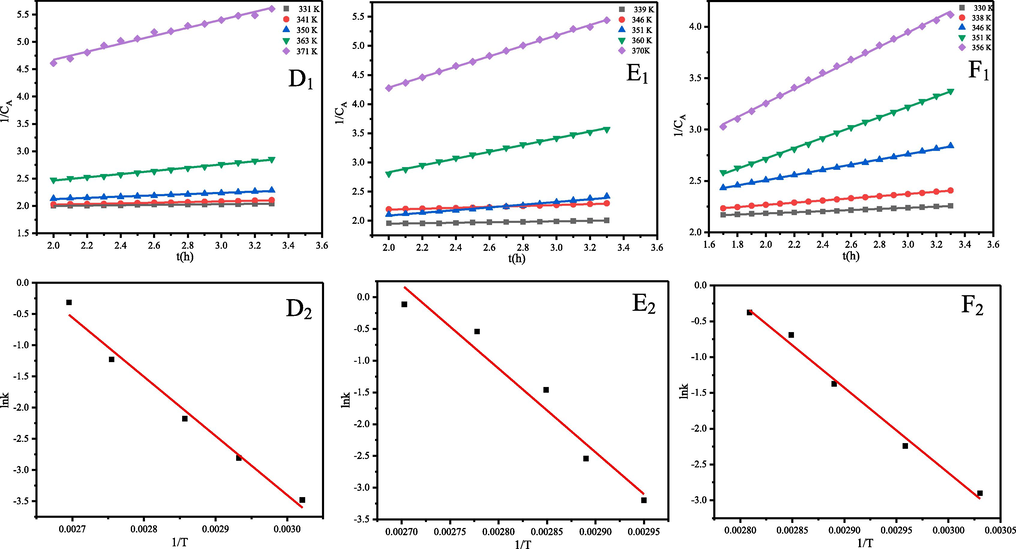
Plots of 1/CA versus time and lnk versus 1/T at different temperatures, α-pinene (D1, D2), β-pinene (E1, E2) and turpentine (F1, F2).
Compound
T(K)
k × 3600(L/(mol·s))
R2
α-Pinene
331
0.0308
0.9495
341
0.0604
0.9817
350
0.1135
0.9861
363
0.2930
0.9955
371
0.7307
0.9822
β-Pinene
339
0.0408
0.9637
346
0.0788
0.9842
351
0.2326
0.9822
360
0.5828
0.9975
370
0.8932
0.9985
Turpentine
330
0.0550
0.9946
338
0.1064
0.9997
346
0.2529
0.9995
351
0.5013
0.9993
356
0.6852
0.9978
Compound
Equation
R2
Ea/(kJ/mol)
α-Pinene
Lnk = -9486.07×(1/T) + 25.06
0.9848
78.87
β-Pinene
Lnk = -13193.18×(1/T) + 35.82
0.9533
109.69
Turpentine
Lnk = -11886.27×(1/T) + 33.04
0.9906
98.82
From the experimental results, it can be seen that β-pinene has the highest activation energy, α-pinene has the lowest activation energy and turpentine is in between them. So, the reaction activity sequence was α-pinene > turpentine > β-pinene. The activation energies of α-pinene, β-pinene and turpentine are all relatively low compared to the activation energies of the oxidation reactions of ethyl tert-butyl ether, methyl tert-butyl ether, dimethyl ether, diethyl ether and diisopropyl ether measured by Liu et al (Liu et al., 2016). They are susceptible to oxidation with oxygen or air, which poses a significant safety hazard for transport, storage and production.
3.2 The formation of α-pinene, β-pinene and turpentine peroxides
Since TLC can be operated at room temperature without breaking the -O-O- bond. Therefore, TLC was used to analyze the peroxides in α-pinene, β-pinene and turpentine oxides. Fig. 4 shows the results of TLC analysis of α-pinene (353 K, 7 h), β-pinene (363 K, 7 h) and turpentine (353 K, 7 h). Multiple blue spots appeared in the TLC plots of α-pinene, β-pinene and turpentine, indicating that they produced multiple peroxide species during the oxidation process. So, the oxidation process of α-pinene, β-pinene and turpentine is very complicated.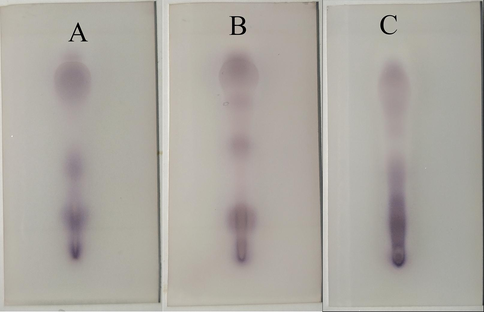
TLC analysis for α-pinene(A), β-pinene(B) and turpentine(C) peroxides.
The peroxide generated by the oxidation reaction is a key factor in the occurrence of thermal runaway. In order to observe the process of peroxide generation and decomposition in MCPVT and to obtain the variation of peroxide (ROOH) concentration, we have used the iodometric method to analyse the products after pressurised oxidation. The iodometric method is an effective means of detecting peroxides, both to prove their presence and to obtain information on their concentration (Fábos et al., 2009). The peroxide values of α-pinene, β-pinene and turpentine as a function of temperature and time are shown in Fig. 5. The peroxide concentration is the average of three experiments.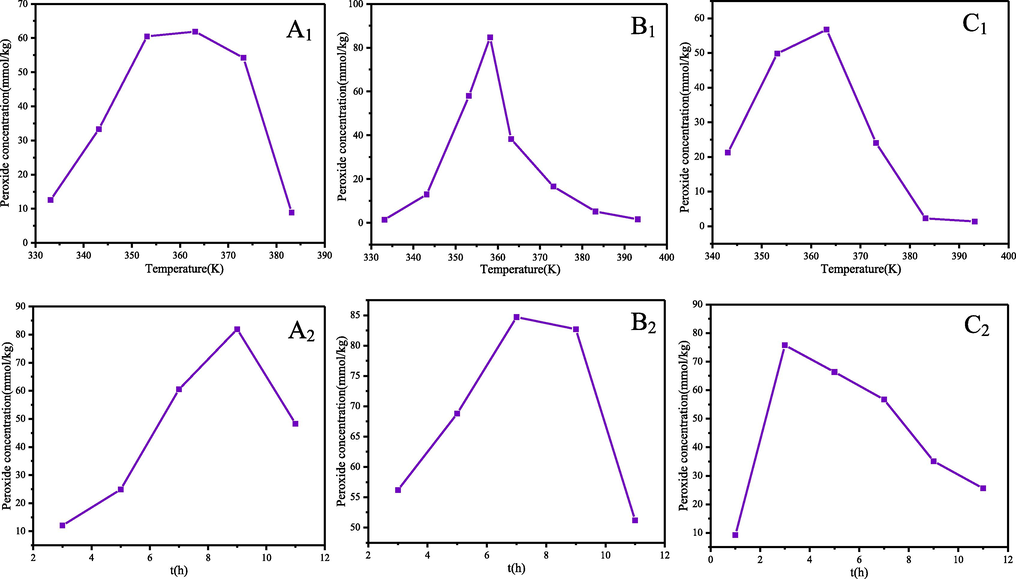
Peroxide concentration versus α-pinene(A1: T-333, 343, 353, 363, 373 and 383 K, t-7 h, A2:t-3,5,7,9 and 11 h, T-363 K), β-pinene (B1: T-333, 343, 353, 358, 363, 373, 383 and 393 K, t-7 h, B2: t-3,5,7,9 and 11 h, T-358 K) and turpentine (C1: T- 343, 353, 363, 373, 383 and 393 K, t-7 h, C2: t-1, 3,5,7,9 and 11 h, T-363 K) oxidation reaction temperature and time.
Temperature is an important factor that affects the formation and decomposition of peroxides (Liu et al., 2019; Yu et al., 2020). Therefore, the effect of the reaction temperature on peroxide concentration was investigated by varying the temperature from 333 K to 393 K, while other factors were kept constant, and the results are shown in Fig. 5 (A1, B1 and C1), when the reaction time was 7 h. The peroxide values of α-pinene, β-pinene and turpentine all increase and then decrease with increasing oxidation temperature. The maximum peroxide concentration for α-pinene was 61.90 mmol/kg at 363 K, for β-pinene 84.70 mmol/kg at 358 K, and for turpentine 56.74 mmol/kg at 363 K. Before the peak temperature is reached the rate of peroxide production is greater than the rate of decomposition, peroxide is accumulating, after reaching the peak due to the increase in temperature the rate of decomposition of peroxide accelerates, exceeds the rate of peroxide production, making the peroxide value decreases. When the temperature exceeded 380 K, the peroxide value dropped sharply and the final product turned dark brown, indicating that a large number of free radicals were released initiating the deep oxidation of α-pinene, β-pinene and turpentine and oxygen.
The α-pinene, β-pinene and turpentine were oxidised at the temperature corresponding to their respective highest peroxide values and the effect of different oxidation times on the peroxide values was tested in the range of 1 h to 11 h (Fig. 5 (A2, B2 and C2)). The oxidation times required for α-pinene, β-pinene and turpentine to reach maximum peroxide concentration were 9, 7 and 3 h, corresponding to values of 81.94, 84.70 and 75.76 mmol/kg, respectively. As the reaction time increases the peroxide concentration in the system rises and then falls, similar to the experiments with temperature. This is due to the low bond energy of the O-O bond of the peroxide (43 kcal/mol), which is susceptible to decomposition reactions, and this contributes to its extreme instability. The maximum peroxide values for α-pinene and β-pinene are similar and both are capable of accumulating large amounts of peroxide. The maximum peroxide value for turpentine is relatively low, but it only needs to be oxidised for 3 h to reach a peroxide concentration of 75.76 mmol/kg. These are all red flags. α-Pinene, β-pinene and turpentine are already capable of producing peroxides at temperatures as low as 333 K. If these peroxides are not eliminated in time, they can lead to deterioration in product quality and even to explosions when produced, transported, stored and used. Turpentine, as a potential biomass fuel, should be taken seriously for its potential explosion risk. Antioxidant measures should be taken to inhibit the formation of peroxides, which is an important way to prevent explosive accidents. And during the production, storage, transport and use of turpentine, the concentration of peroxide should be checked regularly and antioxidants added in a timely manner.
3.3 The hazardous of α-pinene, β-pinene and turpentine
The thermal hazardous of α-pinene, β-pinene and turpentine were investigated using MCPVT from 303 to 433 K at a slow temperature rise (1.5 K/min approx). Experimental results of T-t (temperature vs. time) and P-t (pressure vs. time) are shown in Fig. 6. The black and red curves in the graph show the results of the experiments in oxygen and nitrogen atmospheres respectively. The T-t (pressure vs. time, 1) and P-t (pressure vs. time, 2) red curve of Fig. 6 have all no obvious change point, indicating that no significant chemical reaction occurred for α-pinene, β-pinene and turpentine in nitrogen atmosphere. Black curve of Fig. 6 (A1) exhibits a sharp exothermic peak, oxidation temperature was rapidly elevated to about 406 K by self-heating when reaction heated to about 369 K. Black curve of Fig. 6 (B1) exhibits a sharp exothermic peak, oxidation temperature was rapidly elevated to about 408 K by self-heating when reaction heated to about 373 K. This indicates that both α-pinene and β-pinene underwent a vigorous exothermic oxidation reaction. For safety reasons, the amount of turpentine added in the heating experiment was changed from 0.6 g to 0.40 g, and the amount of oxygen charged was changed from 1.0 MPa to 0.646 MPa. Black curve of Fig. 6 (C1) exhibits a sharp exothermic peak, oxidation temperature was rapidly elevated to about 396 K by self-heating when reaction heated to about 384 K. In the MCPVT pressure changes was more sensitive relative to temperature. In the P-t curves (Fig. 6 (A2, B2, C2) black curves), the pressure in the reactor containing pinene instantaneously increased and exploded. The pressure of the α-pinene and β-pinene reaction systems instantaneously reached 12.82 MPa and 3.13 MPa from 1.23 MPa and 1.27 MPa, respectively. Due to the presence of a safety relief device, the pressure relief was triggered when the pressure inside stainless steel vessel was too high, thus releasing the gas from the reactor. The actual temperature and pressure in the reactor were higher than the recorded temperature and pressure. The P-t plots for turpentine (Fig. 6C2) show that a period of constant oxygen pressure occurs as the temperature rises. Subsequently, when the exotherm started (Fig. 6C1) the oxygen pressure dropped again from 0.81 MPa, indicating that a thermal runaway of turpentine has occurred.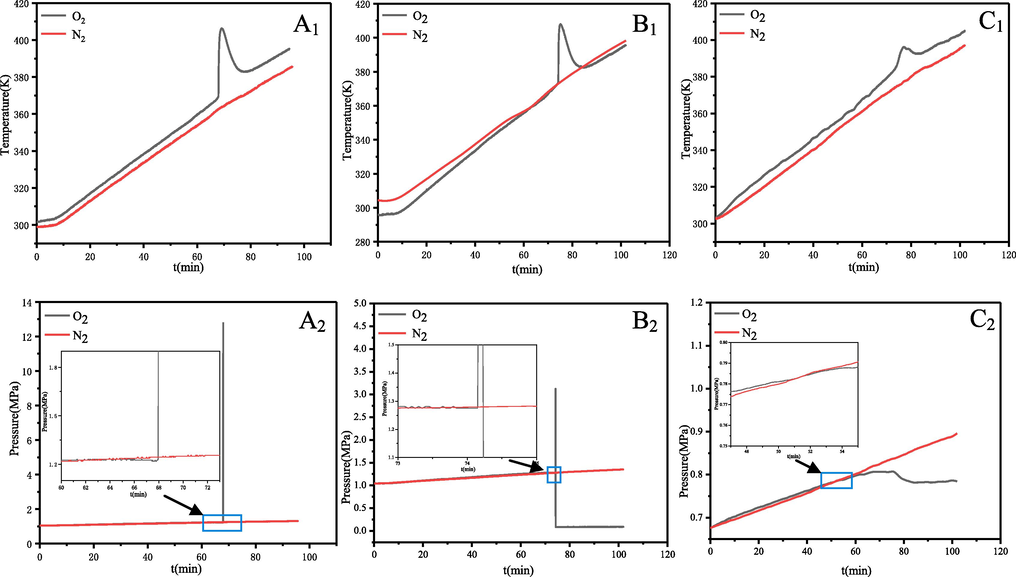
Temperature vs. time and pressure vs. time plots for the process of α-pinene (A1: mass-0.6 g, A2: pressure-1.0 MPa), β-pinene (B1: mass-0.6 g, B2: pressure-1.0 MPa) and turpentine (C1: mass-0.4 g, C2: pressure-0.646 MPa) oxidation.
There is an oxidation induction period for α-pinene and β-pinene and turpentine during the oxidation process (enlargement of Fig. 6). As the temperature increases the P-t curve in the oxygen atmosphere gradually approaches that of nitrogen, i.e. the oxygen uptake process, with initial oxygen uptake temperatures of α-pinene = 351 K, β-pinene = 362 K, and turpentine = 347 K, respectively. In this induction period they will form peroxides, and after the accumulation of peroxides to a certain amount, it will lead to rapid oxidation reaction consumes a lot of oxygen and release heat. When the rate of heat dissipation of the system is less than the rate of heat release, the system temperature rises, peroxides decomposition is accelerated, and the concentration of free radicals also rose. Finally, a violent thermal decomposition reaction of the raw material or oxide is triggered, resulting in a large number of gaseous small molecule compounds in a short period of time and thus an explosion.
In summary, all the liquid samples reacted with oxygen, and the reactions of α-pinene and β-pinene were particularly violent and even exploded and released much gas and heat. Fig. 7 shows the destructive force of their explosion, where it can be observed that the blast sheet was broken through and the glass container inside was blown to pieces. The inside of the reactor is covered with a layer of black material, which would be carbon formed from the incomplete combustion of the organic material. The temperature rise rate (dT/dt) and pressure rise rate (dP/dt) correlate with reaction rates and are often used to evaluate thermally runaway reactions (Li et al., 2021; Yao et al., 2021). The first derivatives of P–t and T–t are calculated to obtain the dP/dt-t and dT/dt-t curves, as shown in Fig. 8 (E, F and G). The maximum rate of temperature rising (dT/dt) max or the maximum rate of pressure rising (dP/dt) max is positively associated with the thermal hazards. The dT/dt and dP/dt of α-pinene were reached the maximum value of 159.8 K/min and 232.7 MPa/min respectively, when the system temperature reached 406 K. The dT/dt and dP/dt of β-pinene were reached the maximum value of 195.8 K/min and 18.4 MPa/min respectively, when the system temperature reached 408 K. It should be noted that these are only the values recorded by the instrument and that the actual values of (dT/dt) max and (dP/dt) max are greater than the values recorded by the instrument due to the explosion which caused the safety relief device to be opened. The (dT/dt)max for turpentine is 5.6 K/min, when the system temperature reaches 396 K. Thus, in the MCPVT experiments the oxidation reaction of α-pinene and β-pinene was the most violent and destructive. The experimental parameters of MCPVT including Tab, Tc, Tmax, (dT/dt)max, (dP/dt)max are compared and summarised in Table 4. Tab is the starting temperature of pressure reduction, Tc is the runaway onset temperature, Tmax is maximum temperature.
The aftermath of α-pinene and β-pinene oxidation (a) rupture disk (b) glass container.
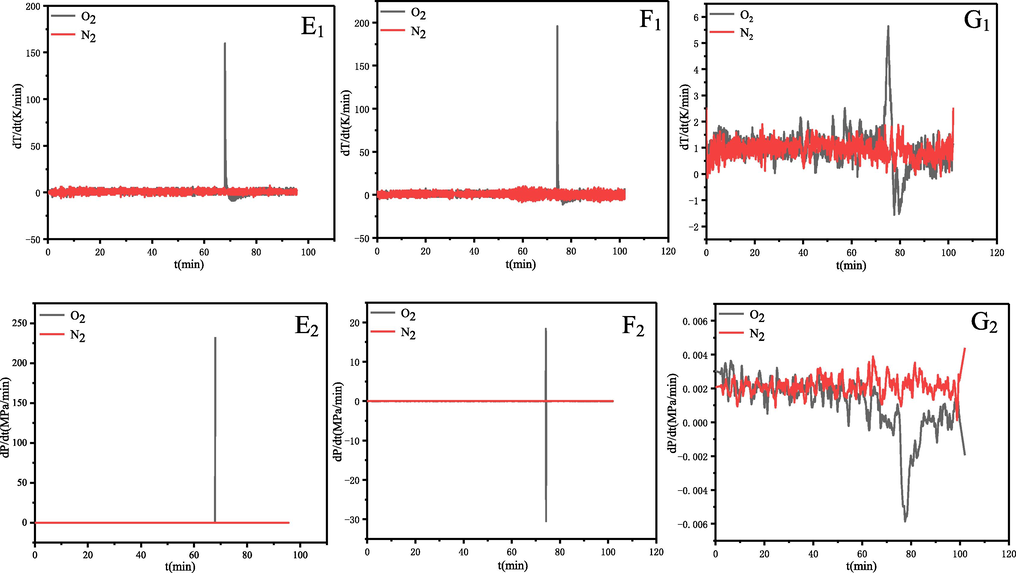
The dT/dt and dP/dt plots for the explosion of α-pinene (E1, E2), β-pinene (F1, F2) and turpentine (G1, G2) oxidation.
Sample
Mass/(g)
Tab/(K)
Tc/(K)
Tmax/(K)
(dT/dt)max (K/min)
(dP/dt)max (MPa/min)
α-Pinene
0.62
351
369
406
159.8
232.7
β-Pinene
0.62
362
373
408
195.8
18.4
Turpentine
0.40
347
384
396
5.6
–
Exothermic reactions are the cause of runaway reactions, which are usually described in terms of kinetic and thermodynamic controls in organic chemistry. In general, the activation energy of the reaction is smaller at kinetically controlled reactions than at thermodynamically controlled reactions. So that at lower temperatures, mainly kinetically controlled reactions are carried out, at higher temperatures, mainly thermodynamically controlled reactions are carried out. For the oxidation of α-pinene, β-pinene and turpentine, before the runaway reaction, the reaction is still mainly kinetically controlled. As the temperature in the reactor rises, the exothermic rate of the sample also rises, and when the temperature is higher than the thermal decomposition temperature of the product the reaction changes to a thermodynamic control and the reaction releases a large amount of heat and pressure, which eventually leads to a runaway reaction. Hence, to prevent explosions the temperature in the kinetic control phase needs to be reduced and heat dissipated in time.
The advantage of DSC is that the sample amount is small (typically mg level), and the high level of safety. Both α-pinene and β-pinene exploded in the MCPVT experiment, so their thermal characteristics were investigated using DSC. The sample of 2–4 mg pinene and turpentine was injected into the test cell by a pipette, and then sealed manually in air. The DSC test was performed at temperatures from 310 K to 470 K with heating rate of 5 K/min, as illustrated in Fig. 9.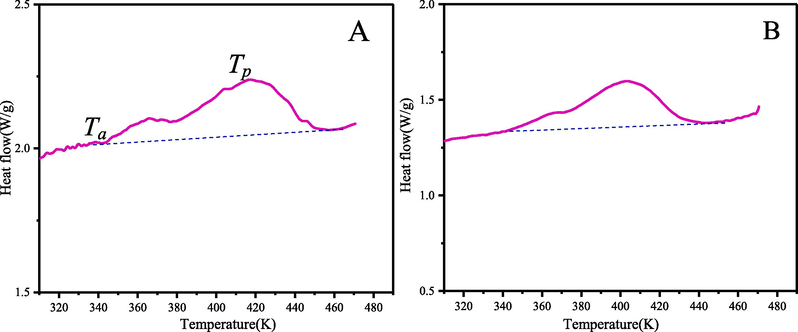
The DSC curve of α-pinene (A) and β-pinene (B).
The starting reaction temperature is a very important indicator of the ease with which a chemical reaction can occur and the thermal hazardous of the substance. The starting reaction temperature (Ta) of α-pinene and β-pinene in the air atmosphere are 342.4 K and 343.4 K respectively, which were similar to the results of MCPVT. The relatively low starting reaction temperature of α-pinene and β-pinene, making the oxidation of α-pinene and β-pinened relatively easy and less thermally stable. Thermal characteristics data obtained by DSC are shown in Table 5. The DSC results indicate that the presence of small amounts of oxygen in the air still triggers the exothermic reaction of α-pinene, β-pinene. Hence, attention should be paid to the exothermic reaction of pinene when it is stored in large quantities, as well as the possible thermal runaway.
Sample
Mass/(mg)
Heating rate /(K/min)
Ta/(K)
Tp/(K)
α-Pinene
2.79
5
342.4
417.0
β-Pinene
3.53
5
343.4
403.1
3.4 Products of α-pinene, β-pinene and turpentine oxidation
The analysis of oxidation products is essential to study the oxidation properties and oxidation pathway of α-pinene, β-pinene and turpentine. The gas and liquid products of α-pinene, β-pinene and turpentine oxidation were detected by GC–MS, and the relative content of each identified component were estimated based on the peak areas. The distribution of oxidation products is shown in Table 6-8.
No
Product
Molecular formula
Relative content (%)
Similarity (%)
α-Pinene
β-Pinene
Turpentine
1
3-Carene
C10H16
8.46
92
2
α-Pinene
C10H16
29.30
1.03
26.00
97
3
β-Pinene
C10H16
42.56
2.02
96
4
Nopinone
C9H14O
8.64
97
5
Camphene
C10H16
1.72
5.70
96
6
o-Cymene
C10H14
3.19
95
7
D-Limonene
C10H16
1.92
94
8
α-Pinene oxide
C10H16O
4.13
93
9
α-Campholenaldehyde
C10H16O
3.10
5.74
94
10
Laevo-pinocarveol
C10H16O
1.64
8.70
1.73
93
11
Pinocarvone
C10H14O
0.68
3.07
1.88
92
12
Verbenol
C10H16O
5.59
8.50
95
13
Myrtenal
C10H14O
2.03
6.87
2.60
97
14
Verbenone
C10H14O
29.37
10.83
97
15
2-Cyclohexen-1-ol,2-methyl-5-(1-methylethenyl)-,(1R,5R)-rel-
C10H16O
2.89
1.80
97
16
6,6-Dimethyl-2-(3-oxobutyl)bicyclo[3.1.1]heptan-3-one
C13H20O2
1.64
84
17
Bicyclo(3.1.1)heptane-2,3-diol,2,6,6-trimethyl-
C10H18O2
5.25
1.49
86
18
Myrtenol
C10H16O
8.16
13.79
8.7
96
19
Perillyl alcohol
C10H16O
2.09
92
20
2-Ethenyl-1,1-dimethyl-3-methylenecyclohexane
C11H18
4.15
84
21
4,4-Propane-2,2-diyldicyclohexanone
C15H24O2
3.47
81
22
Trans-p-mentha-2,8-dienol
C10H16O
1.24
82
23
p-menth-1-ene-3β,7-diol
C10H18O2
5.43
3.66
81
24
3-Methyl-1-pentanol
C10H16O2
2.13
93
25
Cyclobutaneacetic acid,3-acetyl-2,2-dimethyl-
C10H16O3
1.66
90
26
Unknown components
1.05
4.39
0.01
No
No
Product
Molecular formula
Relative content (%)
Similarity (%)
α-Pinene
β-Pinene
Turpentine
1
Oxygen
O2
79.56
82.03
94.12
92
2
Water
H2O
5.5
5.54
1.84
100
3
Acetaldehyde
C2H4O
1.47
2.31
0.77
87
4
Methyl formate
C2H4O2
5.90
3.71
1.74
99
5
Methyl-1-butene
C5H10
0.42
0.04
0.08
89
6
Acetone
C3H6O
5.82
5.26
1.37
98
7
Methyl acetate
C3H6O2
0.38
0.19
–
96
8
Isopropyl formate
C4H8O2
0.23
0.29
0.06
93
No
Product
Molecular formula
Relative content (%)
Similarity (%)
α-Pinene
β-Pinene
Turpentine
1
Water
H2O
15.34
25.48
10.66
97
2
Ethanol
C2H6O
18.56
6.56
6.21
98
3
Acetone
C3H6O
8.79
11.16
11.04
96
4
Isopropyl formate
C4H8O2
0.74
89
5
Acetic acid
C2H4O2
17.86
10.07
94
6
2-Pentanone
C5H10O
2.57
2.83
93
7
2-Butanone,4-hydroxy-3-methyl-
C5H10O2
1.80
82
8
Propanoic acid
C3H6O2
0.52
92
9
n-Ethyl propanoate
C5H10O2
4.45
91
10
Propyl acetate
C5H10O2
1.96
91
11
Butyl formate
C5H10O2
0.39
82
12
3-Pentanol
C5H12O
1.02
0.34
0.87
92
13
Isobutyric acid
C4H8O2
0.59
96
14
Toluene
C7H8
1.69
0.80
2.24
96
15
3-Methyl-2-butenal
C5H8O
0.15
82
16
Mesityl oxide
C6H10O
0.23
92
17
Butyl acetate
C6H12O2
0.80
4.45
1.30
96
18
5-Butyl-1, 3-cyclohexadiene
C10H16
1.21
86
19
Cyclene
C10H16
0.67
1.82
2.01
94
20
α-Pinene
C10H16
0.47
1.12
97
21
Camphene
C10H16
2.77
4.48
7.85
97
22
Cymene
C10H14
14.81
15.43
21.18
97
23
Limonene
C10H16
0.47
1.25
96
24
Apocamphor
C9H14O
0.84
5.38
1.54
96
25
Fenchone
C10H16O
0.20
0.65
97
26
Fenchol
C10H18O
0.39
0.96
0.63
96
27
Nopinone
C9H14O
1.73
97
28
Camphor
C10H16O
0.54
1.05
0.49
97
29
Isopinocamphone
C10H16O
0.29
95
30
Bornyl formate
C11H18O2
1.58
4.61
2.05
96
31
Methylacetophenone
C9H10O
5.31
5.01
11.62
97
32
7-Octen-3-ol, 2, 3, 6-trimethyl-
C11H22O
0.41
80
33
2(3H)-Furanone,dihydro-5,5-dimethyl-4-(3-oxobutyl)-
C10H16O3
0.87
88
34
Dihydronopol
C11H20O
1.39
82
35
Ethyl 6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-carboxylate
C12H18O
0.59
79
36
Verbenone
C10H14O
1.11
96
37
Unknown components
0.7
1.11
1.9
No
The oxidation products of α-pinene, β-pinene and turpentine before thermal runaway reactions are important to elucidate the pathway and stability of the chemical reaction. Table 6 shows the liquid products of the isothermal oxidation of α-pinene, β-pinene and turpentine obtained at the temperature conditions with the highest peroxide values. According to the results of GC–MS, the similarity of each component was retrieved from the mass spectrometry library. The results showed that α-pinene, β-pinene and turpentine were all easily oxidized in oxygen. The main oxidation products of α-pinene were α-campholenaldehyde, laevo-pinocarveol, verbenol, verbenone, bicyclo (3.1.1)heptane-2, 3-diol, 2, 6, 6-trimethyl, 2-cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, (1R,5R)-rel-, cyclobutaneacetic acid, 3-acetyl-2, 2-dimethyl, myrtenol and myrtenal, with the verbenyl compounds (34.96 %) being the most abundant one.
The main oxidation products of β-pinene were nopinone, laevo-pinocarveol, pinocarvone, myrtenol, myrtenal and perillyl alcohol, with the highest content of myrtenyl compounds (20.66 %). The high amount of nopinone in the product mixture, which proves the generation of singlet oxygen (1O2), indicates that the importance of radical–radical reactions in the oxidation reaction of β-pinene cannot be ignored. The main oxidation products of turpentine are α-pinene oxide, α-campholenaldehyde, laevo-pinocarveol, pinocarvone, verbenol, verbenone, myrtenal, myrtenol. The most abundant of them is verbenyl compounds (19.33 %). The main oxidation products of turpentine basically contained the oxidation products of α-pinene and β-pinene.
α-Pinene and β-pinene generated peroxyl radicals in the presence of oxygen, and these peroxyl radicals can abstract allylic H atoms, yielding hydroperoxide, or they can add to the C = C double bond, yielding the corresponding epoxide and alkoxyl radicals. The differences in the oxidation products of α-pinene and β-pinene are mainly due to the different positions of the C = C bonds, one is an intra-ring double bond and the other is an extra-ring double bond, which leads to the differences in their chemical activities. They are isomers of each other and are the main components of turpentine. The study of their thermal oxidation reactions can help to understand the oxidation characteristics of turpentine and provide theoretical basis for the safe production and transportation of turpentine. The results in Table 6 show that almost all of the pressurized isothermal oxidation reactions produced alcohols and aldehydes compounds at a relatively high α-pinene, β-pinene and turpentine conversion rate. The above is the state before thermal runaway, which is still in the kinetic control stage.
In order to obtain the gas as well as liquid products of the thermal runaway reaction we did the heating experiments again. For safety reasons, the amount of α-pinene, β-pinene and turpentine added and the amount of oxygenation were decreased in equal amounts when the heating experiment was repeated. The reaction conditions were as follows: 0.4 g of sample, 0.646 MPa oxygen, heating range 303–433 K(1.5 K/min approx). Liquid as well as gaseous products were obtained after the thermal runaway reaction as shown in Tables 7 and 8. α-Pinene, β-pinene had the same composition of gaseous products including acetaldehyde, methyl formate, methyl-1-butene, acetone, methyl acetate isopropyl formate. The above substances have lower boiling points and volatilize easily as gases at room temperature and pressure. Methyl formate and acetone are the main gaseous products, among which acetone is the decomposition product of α-pinene and β-pinene initiated by O or H radicals (Van den Bergh et al., 2002). This indicates that the explosion occurred when the peroxides generated by the oxidation of α-pinene and β-pinene underwent a decomposition reaction to generate a large number of OH radicals, which further triggered the thermal degradation of pinene or pinene oxide at high temperatures to form small molecule compounds, which are characterized by exothermic reactions and significant pressure increase and belong to free radical oxidation. So, the small molecule gas produced by thermal decomposition is the culprit of the explosion.
The main liquid products after the thermal runaway reaction of α-pinene were water, ethanol, acetone, acetic acid, 2-pentanone, propyl acetate, toluene, cymene and methylacetophenone. The conversion of α-pinene is over 99 % and the thermal runaway products contain more complex ketones, alcohols, esters and cyclic isomerisation products (e.g., cyclenes and camphene, etc.) in addition to the common compounds such as water, ethanol, acetone and acetic acid. The main liquid products of β-pinene oxidation were water, ethanol, acetone, n-ethyl propanoate, toluene, butyl acetate, cymene, apocamphor, nopinone, bornyl formate, methylacetophenone and dihydronopol. Since β-pinene was not detected in the products indicating that β-pinene was fully converted in the heating oxidation reaction. Compared with α-pinene, β-pinene produced a certain amount of nopinone, indicating that the reaction also released a large amount of singlet oxygen (1O2)(Neuenschwander et al., 2011). This is sufficient to show that the reactions under thermal runaway conditions are very complex and diverse. The main liquid products of turpentine oxidation are similar to products of α-pinene and β-pinene oxidation.
The isothermal thermal oxidation products and thermal runaway products of pinene are significantly different. The isothermal oxidation products of pinene are dominated by peroxides, alcohols, ketones and aldehydes. The thermal runaway reaction of pinene produces a large number of esters, acids and isomerization products, which are more stable at high temperatures compared to alcohols, aldehydes and ketones. It is necessary to take antioxidant measures for the sake of security.
3.5 Pathways of α-pinene, β-pinene oxidation
The study of α-pinene and β-pinene autoxidation pathway was important for understanding thermal runaway reactions and suggesting preventive measures. We deduced the possible reaction pathway of α-pinene and β-pinene by combining the results of MCPVT, kinetic calculations and GC–MS analysis mentioned above, as shown in Figs. 10-11.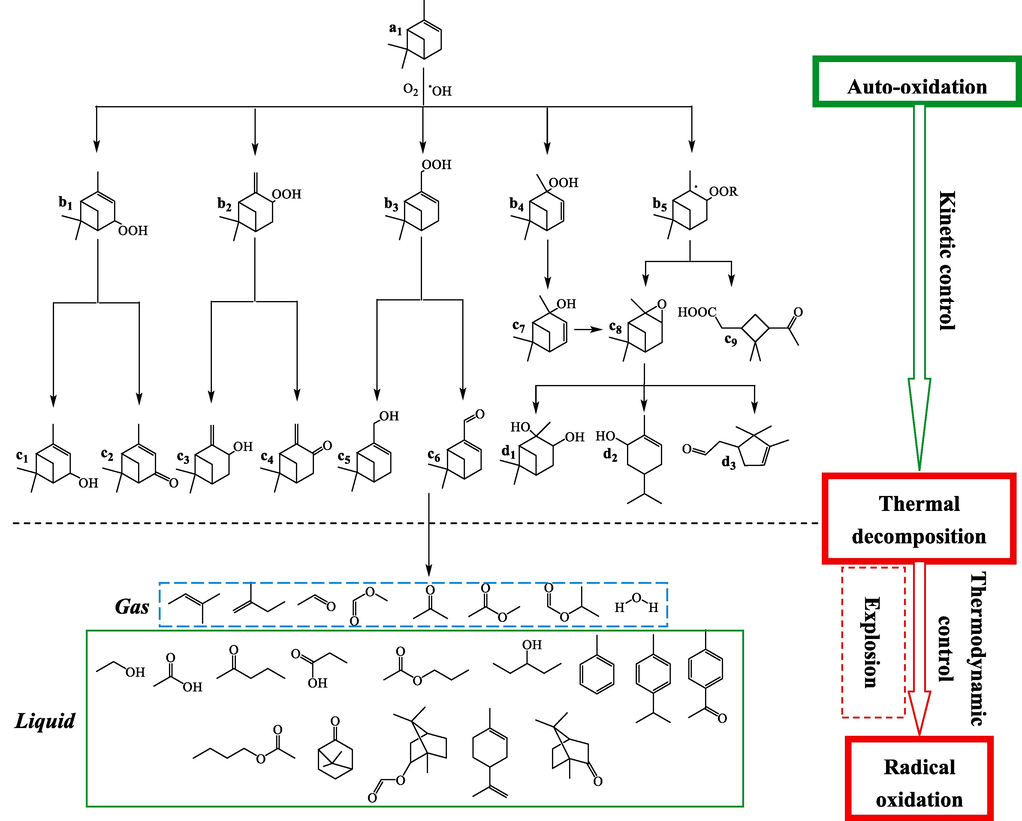
The pathway of a-pinene auto-oxidation and thermal decomposition reaction.
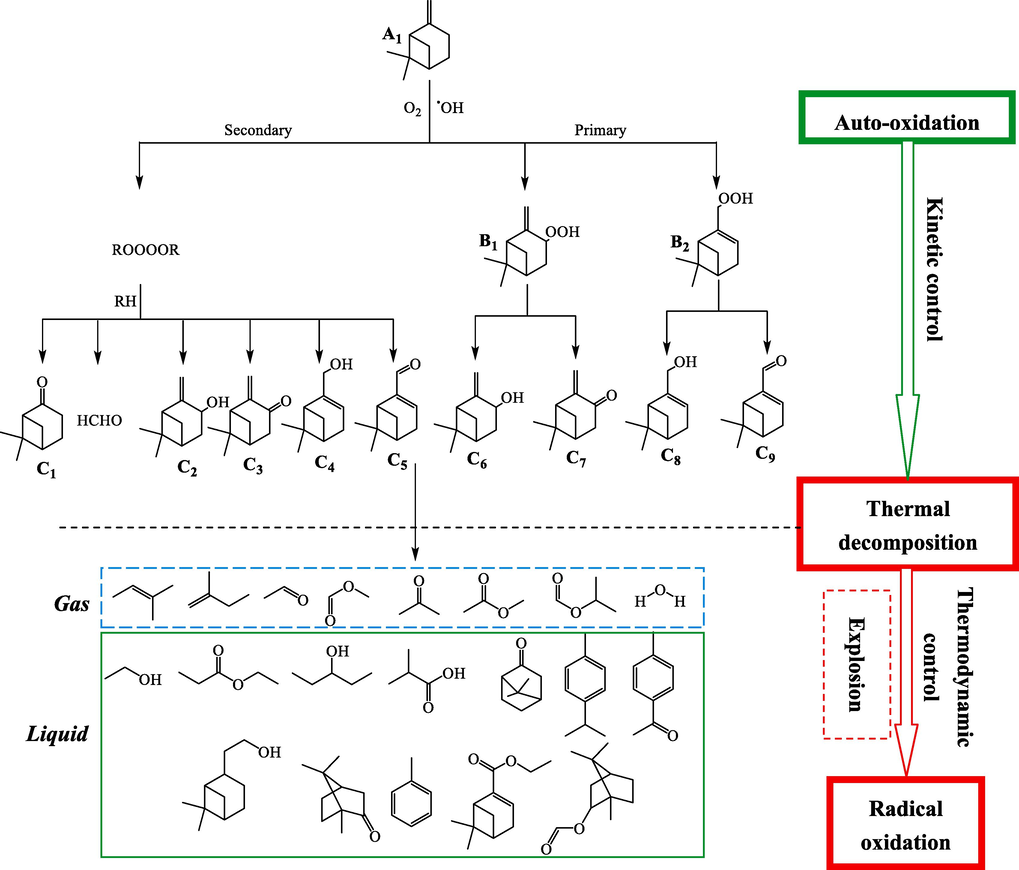
The pathway of β-pinene auto-oxidation and thermal decomposition reaction.
Fig. 10 shows the pathway of a-pinene auto-oxidation and thermal decomposition reaction. α-Pinene forms peroxides (b), which then decomposition of peroxides into alkoxy radicals and OH radicals, a large number of H atoms are abstracted by these free radicals to generate alcohol-ketone compounds (c and d). This autoxidation process from α-pinene to the generation of alcohol and ketone compounds is considered as the kinetic control stage. With the increasing temperature of the system, the heat of reaction cannot be removed in time, resulting in the rapid decomposition of peroxides generating a large number of free radicals and causing the thermal degradation of α-pinene, alcohols, ketones and ring-opening oxidation products at high temperatures, this stage will release huge amounts of heat making the whole reaction out of control. The intensity of the reaction depends mainly on the thermal stability of the substance (i.e., thermodynamic control stage), and the small molecular compounds generated can cause an instantaneous rise in pressure in the system, which can lead to an explosion. It can be seen that if the thermal runaway reaction of α-pinene is to be prevented, the number of free radicals in the reaction system must be controlled, and a good heat dissipation system and the addition of antioxidants become necessary.
Fig. 11 illustrates the pathway of the autoxidation and thermal decomposition reactions of β-pinene. Unlike a-pinene, the main products of β-pinene oxidation are myrtenyl, pinocarvyl hydroperoxide (B). Another reaction of the peroxyl radicals, which was much more important for β-pinene than for a-pinene, was the cross-reaction of two peroxyl radicals. The obtained oxidation products include alcohols corresponding to peroxides, ketones and ring-opening products (C) of β-pinene. As the temperature continues to rise, the decomposition of the peroxide accelerates and the free radical content of the system rise rapidly, deep thermal oxidation and thermal decomposition reactions occur, releasing enormous amounts of heat (thermodynamically controlled stage) and generating a large amount of gaseous small molecules in a very short time, culminating in an explosion. The liquid products are similar to α-pinene and include water and compounds containing benzene rings, as well as alcohols, ketones, acids and esters that are deeply oxidized. Therefore, in addition to timely cooling and the addition of antioxidants, to prevent α-pinene, β-pinene and turpentine and oxygen or air contact is also a good choice.
4 Conclusions
α-Pinene, β-pinene and turpentine are renewable resources with wide application prospects. This study investigated the characteristics and hazardous of α-pinene, β-pinene and turpentine oxidation by MCPVT, DSC, iodimetry and GC–MS. The conclusions are as follows.
(1) α-Pinene, β-pinene and turpentine are stable in a nitrogen environment, but become very active in the presence of oxygen and can all be oxidized at 340 K.
(2) The reaction activity sequence of α-pinene, β-pinene and turpentine was α-pinene > turpentine > β-pinene by comprehensive analysis.
(3) The changes of peroxide concentrations of α-pinene, β-pinene and turpentine at different oxidation temperatures and times were studied by iodometric, which is important for their stability and hazard assessment.
(4) In the heating experiment of MCPVT, α-pinene and β-pinene exploded and turpentine underwent thermal runaway reaction. The data obtained are instructive for the safe application of α-pinene, β-pinene and turpentined.
(5) The oxidation products of α-pinene, β-pinene and turpentine were analyzed by GC–MS, and significant differences were found between the products of oxidation reaction and thermal runaway reaction. The oxidation reaction pathways of α-pinene and β-pinene were proposed.
Acknowledgements
This work was supported by National Natural Science Foundation of China (21776050; 11762003), National Institute of Advanced Industrial Science and Technology Fellowship of Japan, Major Science and Technology Special Project in Guangxi (AA17204087-20), Innovation Project of Guangxi Graduate Education (YCBZ2023003) and Innovation Entrepreneurship Training Program for College Students of Guangxi University (S202210593352).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A new look at the β-pinene–ozone reaction using the atmospheric pressure reactor. Arab. J. Chem.. 2017;10:S665-S670.
- [CrossRef] [Google Scholar]
- Performance and exhaust emission of turpentine oil powered direct injection diesel engine. Renew. Energy. 2010;35:1179-1184.
- [CrossRef] [Google Scholar]
- Performance and emission evaluation of low heat rejection direct injection diesel engine fueled by diesel - Turpentine oil blends. ASME Int. Mech. Eng. Congr. Expo. Proc.. 2010;7:1581-1587.
- [CrossRef] [Google Scholar]
- Oxyfunctionalized turpentine: Evaluation of properties as automotive fuel. Renew. Energy. 2020;162:2210-2219.
- [CrossRef] [Google Scholar]
- Experimental flat flame study of monoterpenes: Insights into the combustion kinetics of α-pinene, β-pinene, and myrcene. Proc. Combust. Inst.. 2021;38:2431-2440.
- [CrossRef] [Google Scholar]
- Cherrad, S., Alrashdi, A.A., Lee, H.S., El aoufir, Y., Lgaz, H., Satrani, B., Ghanmi, M., Aouane, E.M., Chaouch, A., 2022. Cupressus arizonica fruit essential oil: A novel green inhibitor for acid corrosion of carbon steel: Cupressus arizonica fruit essential oil. Arab. J. Chem. 15, 103849. https://doi.org/10.1016/j.arabjc.2022.103849.
- Efficient oleoresin biomass production in pines using low cost metal containing stimulant paste. Biomass Bioenergy. 2011;35:4442-4448.
- [CrossRef] [Google Scholar]
- Influences of dual bio-fuel (Jatropha biodiesel and turpentine oil) on single cylinder variable compression ratio diesel engine. Renew. Energy. 2018;115:1294-1302.
- [CrossRef] [Google Scholar]
- Bio-oxygenates and the peroxide number: A safety issue alert. Energ. Environ. Sci.. 2009;2:767-769.
- [CrossRef] [Google Scholar]
- Oxyfunctionalization of turpentine for fuel applications. Energy Fuel. 2020;34:579-586.
- [CrossRef] [Google Scholar]
- The application of clinoptilolite as the green catalyst in the solvent-free oxidation of α-pinene with oxygen. Sustainability. 2023;15:10381.
- [CrossRef] [Google Scholar]
- Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol.. 2003;86:51-58.
- [CrossRef] [Google Scholar]
- Decomposition and Raman spectrum of dimethyl ether hydrate. Fuel. 2013;105:364-367.
- [CrossRef] [Google Scholar]
- Experimental study on the effect of cetane improver with turpentine oil on CI engine characteristics. Fuel. 2020;262:116551
- [CrossRef] [Google Scholar]
- Feasibility and performance study of turpentine fueled DI diesel engine operated under HCCI combustion mode. J. Mech. Sci. Technol.. 2014;28:729-737.
- [CrossRef] [Google Scholar]
- Performance and pollutants analysis on diesel engine using blends of Jatropha Biodiesel and Mineral Turpentine as fuel. Int. J. Environ. Sci. Technol.. 2017;14:323-330.
- [CrossRef] [Google Scholar]
- Performance and emission characteristics of a turpentine-diesel dual fuel engine. Energy. 2007;32:1202-1209.
- [CrossRef] [Google Scholar]
- Kiełbasa, K., Grzeszczak, J., Sre, J., 2023. Carbon-Supported Nickel Catalysts — Comparison in Alpha-Pinene Oxidation Activity 1–23.
- Effects of 1-butyl-3-metylimidazolium tetrafluoroborate on the thermal hazard of triacetone triperoxide (TATP) Process Saf. Environ. Prot.. 2021;149:518-525.
- [CrossRef] [Google Scholar]
- Oxidation reaction and thermal stability of 1,3-butadiene under oxygen and initiator. Arab. J. Chem.. 2022;15
- [CrossRef] [Google Scholar]
- Thermal oxidation process and characteristic of abietic acid and gum rosin by accelerating rate calorimeter (ARC) J. Therm. Anal. Calorim.. 2019;138:479-488.
- [CrossRef] [Google Scholar]
- Thermal stability and oxidation characteristics of α-pinene, β-pinene and α-pinene/β-pinene mixture. RSC Adv.. 2021;11:20529-20540.
- [CrossRef] [Google Scholar]
- Synthesis of strained high-energy rocket bio-kerosene via cyclopropanation of myrcene. Fuel Process. Technol.. 2020;201:106339
- [CrossRef] [Google Scholar]
- Oxidation characteristics and products of five ethers at low temperature. Fuel. 2016;165:513-525.
- [CrossRef] [Google Scholar]
- Polymerization of α-pinene using Lewis acidic ionic liquid as catalyst for production of terpene resin. Biomass Bioenergy. 2013;57:238-242.
- [CrossRef] [Google Scholar]
- Martins, G. dos S., Staudt, A., Sutili, F.K., Malafaia, C.R.A., Leal, I.C.R., 2022. Solvent screening, optimization and kinetic parameters of the biocatalytic epoxidation reaction of β-pinene mediated by Novozym®435. Biotechnol. Lett. 44, 867–878. https://doi.org/10.1007/s10529-022-03265-8.
- Superacid Catalysis: Direct Esterification of Turpentine Oil with Acetic Acid. Chem. Eng. Technol.. 2022;45:930-935.
- [CrossRef] [Google Scholar]
- Thermal degradation of α-pinene and β-pinene: An experimental study. Fuel. 2020;267:117177
- [CrossRef] [Google Scholar]
- Mechanism of the aerobic oxidation of α-Pinene. ChemSusChem. 2010;3:75-84.
- [CrossRef] [Google Scholar]
- Autoxidation of α-pinene at high oxygen pressure. PCCP. 2010;12:10542-10549.
- [CrossRef] [Google Scholar]
- Photodegradation of α-pinene secondary organic aerosol dominated by moderately oxidized molecules. Environ. Sci. Tech.. 2021;55:6936-6943.
- [CrossRef] [Google Scholar]
- Minimum ignition energy measurements for α-pinene/air mixtures. Combust. Sci. Technol.. 2014;186:1597-1605.
- [CrossRef] [Google Scholar]
- Ring-opening of Β-pinene epoxide into high-added value products over Colombian natural zeolite. Micropor. Mesopor. Mater.. 2019;287:114-123.
- [CrossRef] [Google Scholar]
- Investigation of the explosive hazard of mixtures containing hydrogen peroxide and different alcohols. J. Hazard. Mater.. 2004;108:1-7.
- [CrossRef] [Google Scholar]
- HPLC-MS determination of the oxidation products of the reaction between α- and β-pinene and OH radicals. Anal. Bioanal. Chem.. 2002;372:630-638.
- [CrossRef] [Google Scholar]
- Thermal stability and safety of dimethoxymethane oxidation at low temperature. Fuel. 2018;234:207-217.
- [CrossRef] [Google Scholar]
- Oxidation characteristics and explosion risk of 2, 5-dimethylfuran at low temperature. Fuel. 2021;302:121102
- [CrossRef] [Google Scholar]
- Thermal hazard and pyrolysis mechanism of tetrazolo[1,5-a]pyridine by TG, DSC, ARC, TG-MS and DFT methods. J. Anal. Appl. Pyrol.. 2021;159:105299
- [CrossRef] [Google Scholar]
- Characteristics and hazards of the cinnamaldehyde oxidation process. RSC Adv.. 2020;10:19124-19133.
- [CrossRef] [Google Scholar]
- Biomass high energy density fuel transformed from Α-pinene catalyzed by Br Önsted-Lewis acidic heteropoly inorganic-organic salt. Renew. Energy. 2018;123:218-226.
- [CrossRef] [Google Scholar]
- Investigation of purified sulfate turpentine on engine performance and exhaust emission. Fuel. 2008;87:252-259.
- [CrossRef] [Google Scholar]
- High value-added application of turpentine as a potential renewable source for the synthesis of heterocyclic Schiff base derivatives of cis-1,8-p-menthane-diamine serving as botanical herbicides. Ind. Crop. Prod.. 2018;115:111-116.
- [CrossRef] [Google Scholar]







