Translate this page into:
Oxone activated TiO2 in presence of UV-LED light for the degradation of moxifloxacin: A mechanistic study
⁎Corresponding authors. majid.chemist@yahoo.com (Majid Muneer), bosalvee@yahoo.com (Munawar Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This work provides new insight into the development of the TiO2/Oxone/UV-LED process for organic contaminant degradation as well as hospital waste management. The Moxifloxacin (MOX) degradation using Oxone activated TiO2 under UV-LED was studied. The TiO2/Oxone/UV LED process was carried out by the addition of Oxone (0.025, 0.05, 0.1 and 0.2 mM) activated by TiO2 different concentrations 0.0125, 0.025, 0.05, 0.1 and 0.5 g/L. The degradation efficiency was studied by HPLC having UV/Vis detector, C18 column (5µ, 4.6 × 250 mm2). The complete removal of 10 ppm of MOX occurred at 0.1 g/L TiO2 and 0.1 mM Oxone with UV-LED exposure time of 12 min. The TOC analysis was performed and 55% TOC reduction was observed at described procedure. The parameters such as drug initial concentration, Oxone, TiO2 dosages and pH were optimized and their effects on degradation were noted. The pseudo-first order reaction kinetics was observed for MOX degradation. It was revealed from the mechanism of activation that and have played a key role in the degradation. The effect of pH (3.6–11) was observed to evaluate the degradation rate of Moxifloxacin. The pH 9.4 achieved the maximum degradation. The UPLC-ESI-MS analysis was performed to identify the intermediates and degraded end-products.
Keywords
Moxifloxacin
Photocatalytic degradation
UV-LED
Advanced Oxidation Process
1 Introduction
The Moxifloxacin is a Fluoroquinolone (FQ) and is a broad-spectrum antibiotic and has been frequently utilized to cure infection (Sayed et al., 2015a; Sayed et al., 2015b; Kümmerer, 2009; Speltini et al., 2010). The minute amount of this antibiotic affects the aquatic bodies badly when get mixed in any water body (Robinson et al., 2005; Celiz et al., 2009; Jjemba, 2006; Petrovic et al., 2003). The pharmaceutical based wastewater requires treatment prior to its discharge. The routine treatment methods have been applied to treat, but these exhibited a less performance for the removal of the pollutants (Adu et al., 2020; Jamil et al., 2020; Sohail et al., 2020). The methods such as filtration, coagulation, chlorination, etc are technologically deficit to meet the complete degradation and transfer pollutants from one phase to other rather elimination (Nebot et al., 2015; Muneer, 2020; Kanjal et al., 2020; Alsager et al., 2018; Saeed et al., 2018; Basfer et al., 2018). The advanced oxidation processes (AOPs) are efficient and innovative for the treatment of antibiotics as well as other toxic compounds (Liu et al., 2014; Iqbal and Bhatti, 2015; Kanjal et al., 2020). These include O3/UV/H2O2, gamma/H2O2, UV/H2O2, UV/Fe2+/H2O2, and UV/TiO2 etc. are effective methods to degrade the organic matter present in the aquatic environment (Van Doorslaer et al., 2015, 2012, 2011; Paul et al., 2007; Muneer et al., 2020; Saeed et al., 2015; Muneer et al., 2012). Among AOPs, the TiO2 assisted photo-catalysis is an efficient option due to production of h+, OH•, e- and •O2– when exposed to UV light as shown in equation (1) (Yahya et al., 2017; Marugan et al., 2010; Izumi et al., 1981).
While, e- and h+ represent the electron and hole generated during photo-catalysis respectively (Jaeger and Bard, 1979; Kessler, 2013). The UV-LED lamps are eco-friendly than conventional UV lamps (Yoshihiko et al., 2014; Ibrahim et al., 2014; Crawford et al., 2005). However, the e- and h+ recombine and decrease the photo-catalysis efficiency. To fill this gap, the electron acceptors species such as hydrogen peroxide, persulfate, and Oxone were used during photocatalytic process (Liu et al., 2014). The use of Oxone as an electron acceptor is a better option in order to degrade the organic compounds via reactive radicals generation as shown in equation (2) (Moradi et al., 2014; Oh et al., 2016).
The Oxone activation by UV-LED can be achieved by transition metals or photo-catalysts such as TiO2 (Chen et al., 2012). In the established literature, a few studies were reported about the TiO2/Oxone/UV-LED process for the drug degradation. In the present study, the TiO2/Oxone/UV-LED was used to degrade the Moxifloxacin antibiotic. The degradation study and intermediate/end-product analysis was carried out by using the UPLC-ESI-MS.
2 Material and methods
2.1 Chemicals
The physicochemical properties of Moxifloxacin are shown in Table 1. The titanium dioxide (TiO2) was used as a catalyst and purchased from Degussa Company, Germany. The Acetonitrile and formic acid were used as mobile phases. While methanol (CH3OH), sodium thiosulfate (Na2S2O3), hydrochloric acid (HCl), sodium hydroxide (NaOH) and Oxone (2KHSO5.KHSO4.K2SO4) were procured from Sigma-Aldrich, USA.
Name of drug
Molecular formula
Molecular weight
λmax
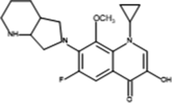
Moxifloxacin
C21H24 FN3O4
401.438 g/mol
2.2 Experimental setup
The MOX solutions having concentrations 10 to 40 mg/L were prepared using ultra-pure water; photodegradation was performed in photo-reactor as described in Fig. 1. The UV-LED lamp having 100 W intensity, emitting 365 nm light with self-cooled chamber with agitation. A catalyst (TiO2) was mixed with 100 mL of drug (10 mg/L) solution and was placed in dark. The Oxone (oxidant) was then added and after predetermines time interval solution was utilized for further analysis. The UV-LED lamp was fixed vertically 8 cm above the solution.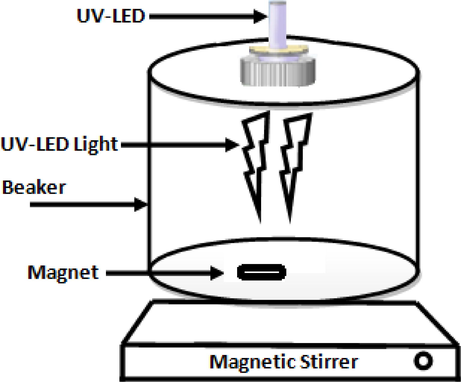
Experimental setup of UV chamber.
2.3 Analytical procedures
The decrease in MOX concentrations was monitored by HPLC, C18 column with UV/Vis detector. A mixture of Acetonitrile, water and phosphoric acid (20%, 80% and 0.1% respectively) was used as a mobile phase with flow rate of 1 mL/min while degassing was done prior to run. The Shimadzu TOC-L analyzer was used for TOC analysis. The intermediate were identified using UPLC-ESI-MS detector. The Tribrid Mass Spectrometer Orbitrap Fusion Lumos (Thermo Fisher Scientific, USA) and the UPLC Waters Acquity (Waters, USA) comprised with Acquity BEH C18 column (1.7 µm, 2.1 mm × 50 mm) was employed. The mobile phase used for intermediates detection was a mixture of acetonitrile and formic acid (100% and 0.1% respectively) with the flow rate of 0.3 mL/min.
3 Results and discussion
3.1 Degradation of MOX using UV-LED/TiO2/PMS
The performance of different processes such as TiO2/dark, Oxone/dark, TiO2/Oxone/dark, UV-LED, Oxone/UV-LED, TiO2/UV-LED and TiO2/Oxone/UV-LED was examined and then the MOX degradation efficiency was compared (Fig. 2). The obtained data showed 4% and 10% degradation for 10 ppm solution using the adsorption (TiO2/dark) and photolysis (UV-LED) respectively. The degradation was noted as 20% and 56% after Oxone/UV-LED and TiO2/UV-LED treatments respectively. The complete degradation was achieved after TiO2/Oxone along with UV-LED for 12 min exposure time. The degradation of MOX suggests the seminal role of Oxone (HSO5-) in capturing the generated h+ and may cause to produce excess sulfate and hydroxyl radicals as shown in Eqs. 3–6 (Zhang et al., 2015; Chen et al., 2007).
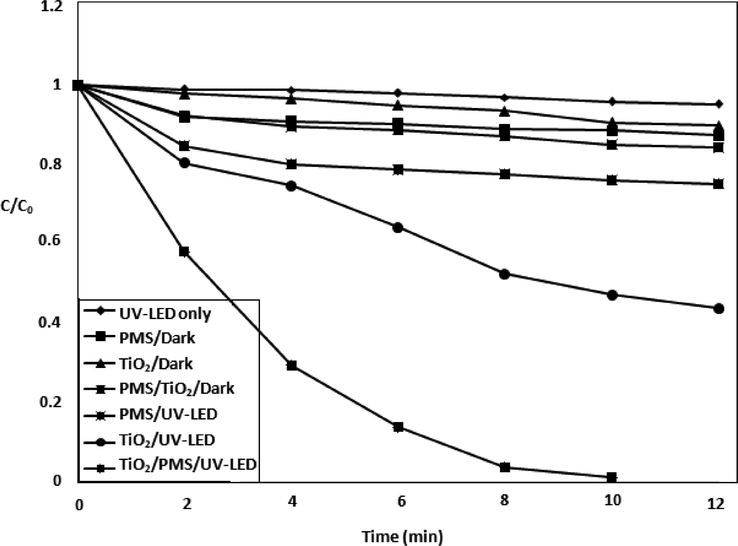
Degradation kinetics of MOX by using the oxidant along with catalyst.
The pseudo-first order kinetics was observed for data (Eq. (7)).
Where k and C indicate the reaction rate constant and concentrations of MOX at time t respectively. The slope of ln (C/C0) versus t gives the value of k and C0 represents the initial concentration of the drug.
3.2 Initial concentration influence on MOX degradation
The TiO2/Oxone/UV-LED process was used to check the effect of the initial MOX concentration on the photodegradation efficiency (Fig. 3) having concentrations 10–40 mg/L of drug. The degradation efficiency was reduced as the MOX concentration increased. This can be rationalized by the increase the number of targeted molecules, compete with a limited number of reactive species (•OH and
) and may decrease the rate of drug degradation due to competition among the molecules. Furthermore, the active sites of the catalyst become over occupied can render the production of •OH and O2•- (Tokode et al., 2012). The results are in accordance to the.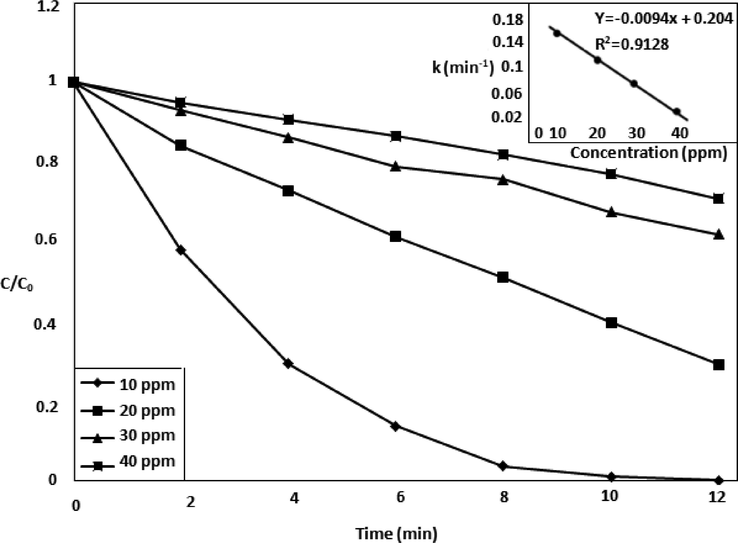
Effect of drug concentration in presence of oxidant and catalyst.
findings of Van Doorslaer and his co-workers performed in the degradation of MOX by UV/TiO2 process (Van Doorslaer et al., 2011).
3.3 pH influence on MOX degradation
The pH of the media affects the photocatalytic activity (Hermann, 1999) and may alter the catalyst’s surface property and may influence the adsorption nature of the pollutant (Abdelhaleem and Chu, 2017; Hsiung et al., 2016; Natarajan et al., 2011; Ahn et al., 2016). The effect of change in pH from 3.6 to 11 using 0.1 mM Oxone and 0.1 g/L TiO2 using UV-LED light was examined (Fig. 4). The complete degradation at pH 9.4 while 80% and 90% in case of acidic and neutral media was observed respectively. In acidic and alkaline media, the TiO2 is positively and negatively charged respectively as shown in eqs. (9) & (10) (Langlois et al., 2005).
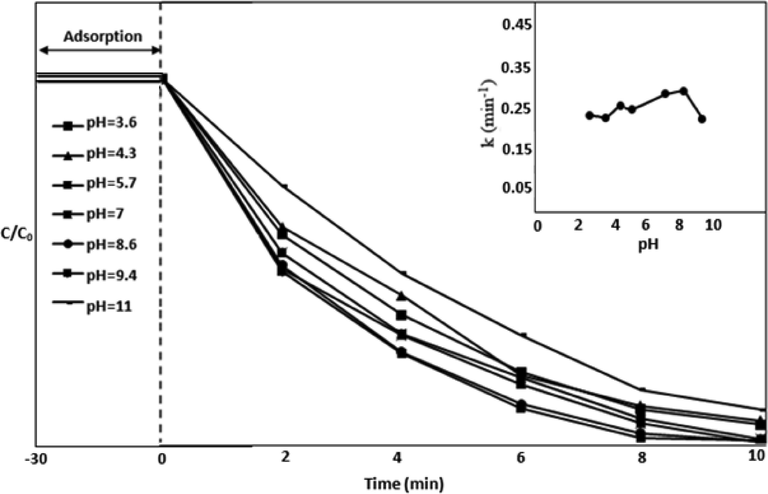
The effect of pH on MOX along with oxidant and catalyst.
In acidic condition, the H-bonding between hydrogen ion and O-O group in
reduces the positive charge on
and decreases the photodegradation by hindering the interaction of
with surface of catalyst (Liu et al., 2015; Guan et al., 2011). Furthermore, H+ can scavenge sulfate and hydroxyl radicals at low pH (pH 3 or below) based on equations 11–13 (Sun et al., 2012; Jaafarzadeh et al., 2017). The complete degradation was achieved in alkaline condition (at pH 9.4) which is due to increase in
formed due to adsorption of OH− on the surface of catalyst (Guan et al., 2011). This is also the optimum pH. Further increase in pH may reduce the process efficiency due to less adhesion of drug molecules on the surface of catalyst as a result of repulsion between TiO2 and MOX (Muruganandham and Swaminathan, 2004).
3.4 Catalyst dose influence on MOX degradation
The Fig. 5 shows the effect of catalyst dosage (0.0125–0.5 g/L) on the degradation efficiency of MOX. The complete degradation was achieved for 10 ppm drug aqueous solution by using UV-LED light in combination of oxidant and catalyst at UV-LED exposure time of 12 min. The availability of more active sites due to increased amount of catalyst enhanced the drug degradation. The degradation reduced suddenly when TiO2 was overdosed (0.5 g/L) due to the reduction in the penetration of light at higher catalyst dosages (Chu and Wong, 2004).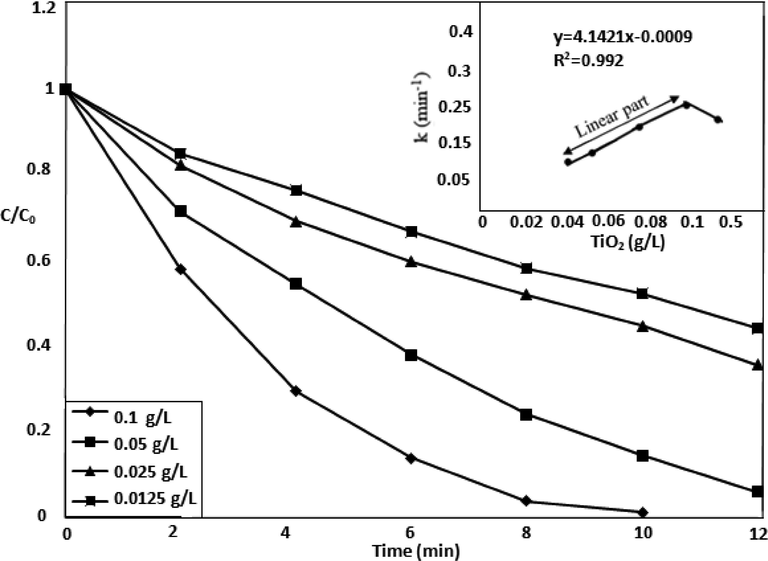
Effect of TiO2 dosage on the degradation of MOX.
3.5 Oxidant influence on MOX degradation
During the photocatalysis, electrons and the holes may recombine, which result limited degradation. Electron acceptor such as oxone is added to the reaction system in order to overcome the recombination. The TiO2/UV-LED process have shown 55% degradation, which enhanced to 100% along with Oxone (0.2 mM) as shown in Fig. 6. The increase in MOX degradation efficiency can be justified by the production of reactive radicals through the Oxone decomposition by the photo-catalyst (Madhavan et al., 2006) as shown by the equations 14–16 (Dhanalakshmi et al., 2008).
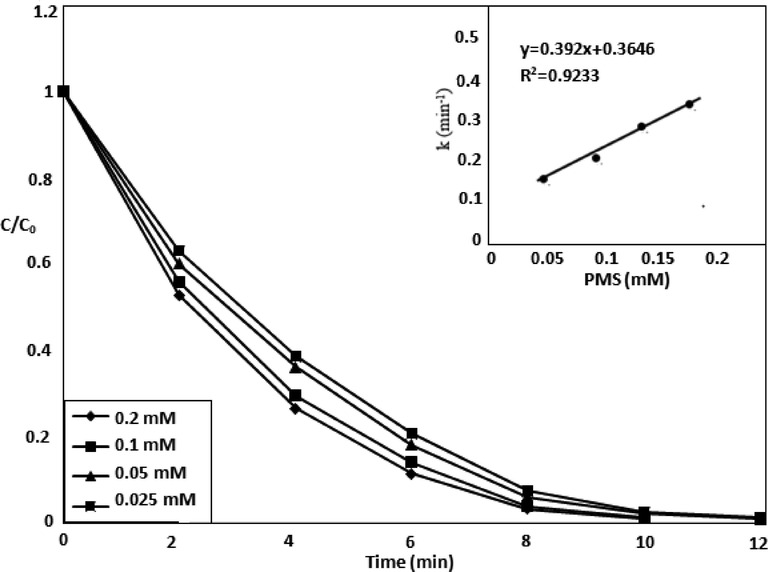
Effect of Oxone on degradation efficiency along with catalyst.
3.6 Mineralization efficiency
The organic compounds could be more harmful to the environment as compared to parent compounds when degraded to smaller molecules via oxidation process. The mineralization was carried out by using total organic carbon (TOC) analysis (Zazouli et al., 2007). Fig. 7 shows the mineralization results of Moxifloxacin at different experimental conditions. During TiO2/Oxone/UV-LED process, about 55% of TOC reduction was observed while only 18% TOC removal was observed in case of Oxone/UV-LED process. The mineralization took place in the following order TiO2/Oxone/UV-LED > UV-LED/TiO2 > UV-LED/Oxone. The TOC reduction in the TiO2/Oxone/UV-LED process reveals that the process could be effective technique for the removal of the intermediates resulting from the photodegradation of Moxifloxacin.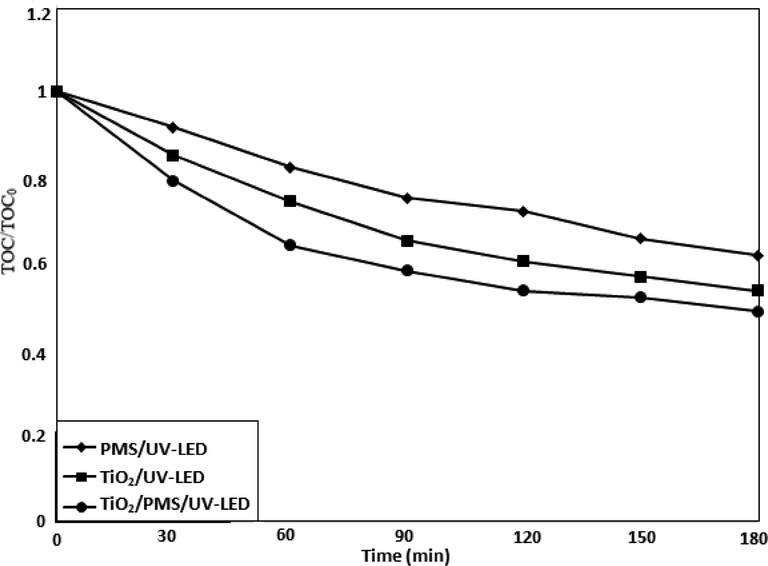
TOC measurements in UV-LED/TiO2/Oxone process.
3.7 Byproducts distribution
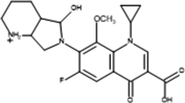
The proposed mechanism of MOX photocatalytic degradation by TiO2/Oxone/UV-LED process is shown in scheme 1. The ion [M + H+] where M is the molecular mass of the respective analyte generated because of a proton in order to get positively charged molecular ion in all analytes while Table 2 summarizes the intermediates’ information with structure. The MS spectra of MOX (m/z 402) yields ions at m/z 374 and 358 as a result of CO and CO2 loss, respectively. Whereas, the intermediate specie at m/z 321 identified with the loss of HF at m/z 341 (Chai et al., 2011). The proton-bound complex resulted complementary products at m/z 293 and 110 (Zhang et al., 2012) formed by the rearrangement of hydrogen (Hudson and McAdoo, 2007). Due to loss of CH3 and H2O, the ion with m/z 293 produced an ion at m/z 279 (Kingston et al., 1975). The fragment pathways for protonated moxifloxacin have shown conflict with the fragment pathways reported by (Raju et al., 2012). However, the elimination of neutral species such as CO, H2O and CO2 took place via same fragment pattern in both studies, while, a significant variation in relative abundance of the ions was observed.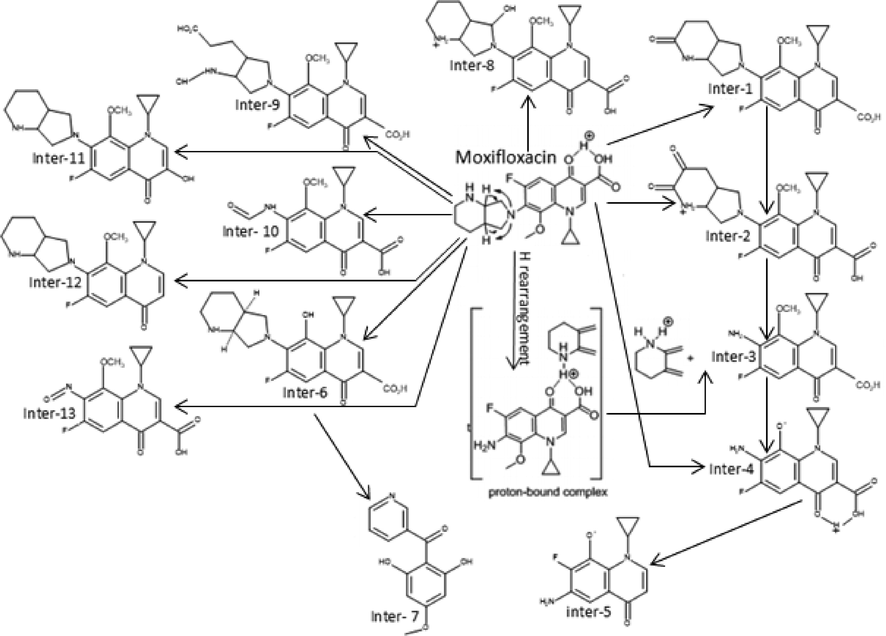
Proposed pathways of Moxifloxacin using UV-LED/TiO2/Oxone process.
Product ID
Molecular Ion [M + H+]
Proposed Structure
Inter-1
293
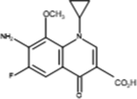
Inter-2
434
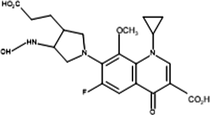
Inter-3
416
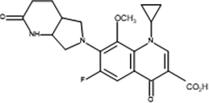
Inter-4
418
Inter-5
430
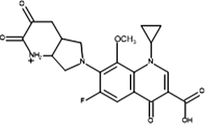
Inter-6
387
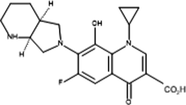
Inter-7
321

Inter-8
306
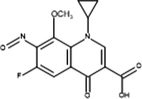
Inter-9
358
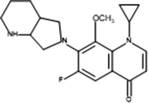
Inter-10
279
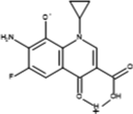
Inter-11
246
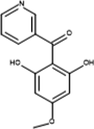
Inter-12
374
4 Conclusions
In this study, the performance of MOX degradation was assessed by using UV-LED/TiO2/Oxone process. The obtained data revealed that TiO2 had a catalytic activity for Oxone activation under the UV-LED light. A complete degradation of Moxifloxacin and 55% mineralization revealed the outstanding performance of TiO2/Oxone/UV-LED combined process for the removal of the intermediates. The TiO2/Oxone/UV-LED process could be implemented through the activation of Oxone as a sustainable and environmentally friendly approach to degrade of organic matter in aquatic environment.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2022R11), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2022R11), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photodegradation of 4-chlorophenoxyacetic acid under visible LED activated N-doped TiO2 and the mechanism of stepwise rate increment of the reused catalyst. J. Hazard. Mater.. 2017;338:491-501.
- [Google Scholar]
- Synthesis, characterization and evaluation of swelling ratio on magnetic p53-poly(MAA-co-EGDMA)@GO-Fe3O4 (MIP@GO-Fe3O4)-based p53 protein and graphene oxide from kusambi wood (Schleichera oleosa) J. Mater. Res. Technol.. 2020;9:11060-11068.
- [Google Scholar]
- Activation of peroxymonosulfate by surface-loaded noble metal nanoparticles for oxidative degradation of organic compounds. Environ. Sci. Technol.. 2016;50:10187-10197.
- [Google Scholar]
- Degradation and detoxification of 2-chlorophenol aqueous solutions using ionizing gamma radiation. Nukleonika.. 2018;62:61-68.
- [Google Scholar]
- Pharmaceutical metabolites in the environment: analytical challenges and ecological risks. Environ. Toxicol. Chem.. 2009;28:2473-2484.
- [Google Scholar]
- Gas-phase nucleophilic aromatic substitution between piperazine and halobenzyl cations: Reactivity of the methylene arenium form of benzyl cations. Chem. Eur. J.. 2011;17:10820-10824.
- [Google Scholar]
- Accelerated TiO2 Photocatalytic degradation of Acid Orange 7 under visible light mediated by peroxymonosulfate. Chem. Eng. J.. 2012;193:290-295.
- [Google Scholar]
- Photodecomposition of o-cresol by UV/TiO2 process with controlled periodic illumination. Chemosphere. 2007;69:184-190.
- [Google Scholar]
- The photocatalytic degradation of dicamba in TiO2 suspensions with the help of hydrogen peroxide by different near UV irradiations. Water Res.. 2004;38:1037-1043.
- [Google Scholar]
- Crawford, M. H., Banas, M. A., Ruby, D. S., Ross, M. P., Nelson, J. S., Allerman, A. A., Boucher, R., 2005. Sandia National Laboratories. Albuquerque, New Mexico 87185 and Livermore, California, 945-50.
- Photocatalytic degradation of phenol over TiO2 powder: The influence of peroxomonosulfate and peroxodisulphate on the reaction rate. Sol. Energy Mater. Sol. Cells. 2008;92:457-463.
- [Google Scholar]
- Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol.. 2011;45:9308-9314.
- [Google Scholar]
- Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today. 1999;53:115-129.
- [Google Scholar]
- Effects of water chemistry on the destabilization and sedimentation of commercial TiO2 nanoparticles: Role of double-layer compression and charge neutralization. Chemosphere. 2016;151:145-151.
- [Google Scholar]
- Characterization by theory of H-transfers and onium reactions of CH3CH2CH2NH=CH2. J. Am. Soc. Mass Spectrom.. 2007;18:270-278.
- [Google Scholar]
- Evaluating the impact of LED bulb development on the economic viability of ultraviolet technology for disinfection. Environ. Technol.. 2014;35:400-406.
- [Google Scholar]
- Heterogeneous photocatalytic decomposition of benzoic acid and adipic acid on platinized titanium dioxide powder. The photo-Kolbe decarboxylative route to the breakdown of the benzene ring and to the production of butane. J. Phys. Chem.. 1981;85:218-223.
- [Google Scholar]
- Gamma radiation/H2O 2 treatment of a nonylphenol ethoxylates: Degradation, cytotoxicity, and mutagenicity evaluation. J. Hazard. Mater.. 2015;299:351-360.
- [Google Scholar]
- Photocatalytic degradation of disperse dye Violet-26 using TiO2 and ZnO nanomaterials and process variable optimization. J. Mater. Res. Technol.. 2020;9:1119-1128.
- [Google Scholar]
- Efficient degradation of 2, 4- dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J.. 2017;320:436-447.
- [Google Scholar]
- Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at titanium dioxide particulate systems. J. Phys. Chem.. 1979;83:3146-3152.
- [Google Scholar]
- Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf.. 2006;63:113-130.
- [Google Scholar]
- Degradation of Methotrexate by UV/peroxymonosulfate: kinetics, effect of operational parameters and mechanism. Chinese J. Chem. Eng.. 2020;28:2658-2667.
- [Google Scholar]
- The Minamata Convention on Mercury: A First Step toward Protecting Future Generations. Environ. Health Perspect.. 2013;121(10):304-309.
- [Google Scholar]
- Intramolecular hydrogen transfer in mass spectra. III. Rearrangements involving the loss of small neutral molecules Chemical Reviews. 1975;75:693-730.
- [Google Scholar]
- Antibiotics in the aquatic environment-a review-part Ⅰ. Chemosphere. 2009;75:417-434.
- [Google Scholar]
- Protonation equilibrium and lipophilicity of moxifloxacin. J. Pharm. Biomed. Anal.. 2005;37:389-393.
- [Google Scholar]
- Activation of peroxymonosulfate with magnetic Fe3O4–MnO2 core-shell nanocomposites for 4-chlorophenol degradation. Chem. Eng. J.. 2015;262:854-861.
- [Google Scholar]
- Radiation-induced removal of sulphadiazine antibiotics from Wastewater. Environ. Technol.. 2014;35:2028-2034.
- [Google Scholar]
- Peroxomonosulphate, an efficient oxidant for the photocatalyzed degradation of a textile dye, acid red 88. Sol. Energy Mater. Sol. Cells. 2006;90:1875-1887.
- [Google Scholar]
- Analogies and differences between photocatalytic oxidation of chemicals and photocatalytic inactivation of microorganisms. Water Res.. 2010;44:789-796.
- [Google Scholar]
- Solar photocatalytic degradation of a reactive azo dye in TiO2-suspension. Sol. Energy Mater. Sol. Cells. 2004;81:439-457.
- [Google Scholar]
- Gamma and UV radiation induced treatment of anti-cancer methotrexate drug in aqueous medium: Effect of process variables on radiation efficiency evaluated using bioassays. App. Rad. Iso.. 2020;166:109371
- [Google Scholar]
- Photocatalytic degradation of azo dye using nano-ZrO2/UV/Persulfate: Response surface modeling and optimization. Kor. J. Chem. Eng.. 2014;33:539-546.
- [Google Scholar]
- Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J.. 2011;169:126-134.
- [Google Scholar]
- Introduction of human pharmaceuticals from wastewater treatment plants into the aquatic environment: a rural perspective. Environ. Sci. Pollut. Res. 2015:1-10.
- [Google Scholar]
- Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal., B. 2016;194:169-201.
- [Google Scholar]
- Visible-light-mediated TiO2 photocatalysis of fluoroquinolone antibacterial agents. Environ. Sci. Technol.. 2007;41:4720-4727.
- [Google Scholar]
- Analysis and removal of emerging contaminants in wastewater and drinking water. TrAC, Trends Anal. Chem.. 2003;22:685-696.
- [Google Scholar]
- In vivometabolic investigation of moxifloxacin using liquid chromatography-electrospray ionization tandem mass spectrometry in combination with online hydrogen-deuterium exchange experiments. Rapid Commun. Mass Spectromet.. 2012;26:1817-1831.
- [Google Scholar]
- Toxicity of fluoroquinolone antibiotics to aquatic Organisms. Environ. Toxicol. Chem.. 2005;24:423-430.
- [Google Scholar]
- VUV Photocatalytic degradation of bezafibrate by hydrothermally synthesized enhanced facets TiO2/Ti film. J. Phys. Chem. 2015
- [Google Scholar]
- Polyamidoamine (PAMAM) dendrimers synthesis, characterization and adsorptive removal of nickel ions from aqueous solution. J. Mater. Res. Technol.. 2020;9:498-506.
- [Google Scholar]
- Ag-Co3O 4: Synthesis, Characterization and evaluation of its photocatalytic activity towards degradation of rhodamine B dye in aqueous medium. Chinese J Chem. Eng.. 2018;26:1264-1269.
- [Google Scholar]
- Degradation of ciprofloxacin in water by advanced oxidation process: kinetics study, influencing parameters and degradation pathways. Environ. Technol. 2015:1-13.
- [Google Scholar]
- Fluoroquinolone antibiotics in environmental waters: sample preparation and determination. J. Sep. Sci.. 2010;33:1115-1131.
- [Google Scholar]
- Highly efficient degradation of ofloxacin by UV/Oxone/Co2+ oxidation process. Environ. Sci. Pollut. Res.. 2012;19:1536-1543.
- [Google Scholar]
- Effect of controlled periodic-b illumination on the photonic efficiency of photocatalytic decomposition of methyl orange. J. Catal.. 2012;290:138-142.
- [Google Scholar]
- Heterogeneous photocatalysis of moxifloxacin in hospital effluent: Effect of selected matrix constituents. Chem. Eng. J.. 2015;261:9-16.
- [Google Scholar]
- TiO2 mediated heterogeneous photocatalytic degradation of moxifloxacin: Operational variables and scavenger study. Appl. Catal. B: Environ.. 2012;111–112:150-156.
- [Google Scholar]
- and UV-C induced photolytic and photocatalytic degradation of aqueous ciprofloxacin and moxifloxacin: Reaction kinetics and role of adsorption. Appl. Catal. B: Environ.. 2011.UV-A;101:540-547.
- [Google Scholar]
- Degradation and mineralization of moxifloxacin antibiotic in aqueous medium by electro-Fenton process: Kinetic assessment and oxidation products. Cogent Chem.. 2017;3:1290021.
- [Google Scholar]
- Development and future of ultraviolet light-emitting diodes: UV-LED will replace the UV lamp. Semicond. Sci. Technol.. 2014;29:084004
- [Google Scholar]
- Sulfate radical and its application in Decontamination technologies. Crit. Rev. Environ. Sci. Technol.. 2015;45:1756-1800.
- [Google Scholar]
- Gas phase retro-Michael reaction resulting from dissociative protonation: Fragmentation of protonated warfarin in mass spectrometry. J. Mass Spectrom.. 2012;47:1059-1064.
- [Google Scholar]
- Photocatalytic degradation of food dye by Fe3O 4–TiO 2 nanoparticles in presence of peroxymonosulfate: The effect of UV sources. J Environ. Chem. Eng.. 2007;5:2459-2468.
- [Google Scholar]







