Translate this page into:
Palmatine ameliorates cisplatin-induced acute kidney injury through regulating Akt and NF-κB/MAPK pathways
⁎Corresponding authors at: College of Biotechnology, Tianjin University of Science & Technology, Tianjin, 300457, China. liuzhen5957@tust.edu.cn (Zhen Liu), yupeng@tust.edu.cn (Peng Yu), tyo201485@tust.edu.cn (Yuou Teng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cisplatin-induced acute kidney injury (CIAKI) is a major dose-limiting toxicity of cisplatin treatment. The mechanisms of CIAKI involve the production of inflammation, apoptosis and oxidative stress. Palmatine, an isoquinoline alkaloid has anti-inflammatory activity. Here, we investigated the protective effect and underlying mechanism of palmatine on CIAKI by in vitro and in vivo experiments based on network pharmacology. Network pharmacology was used to analyze the relationship and potential mechanisms of palmatine and CIAKI. The protective effect of palmatine was validated in cisplatin induced 293 T cell injury model and mice model. Furthermore, the mechanism of palmatine on CIAKI was determined by detecting inflammatory factors and related signaling pathways. A total of 61 targets of palmatine against CIAKI and the closely related signaling pathways including oxidative stress, MAPK and Akt were found. Palmatine effectively protected 293 T cells and mouse kidney against CIAKI. Mechanistically, palmatine reduced CIAKI inflammation by suppressing the NF-κB/MAPK pathway. Meanwhile, palmatine inhibited apoptosis by activating the Akt pathway and reduced oxidative stress. The results of in vitro and in vivo experiments were consistent with those of network pharmacology. Furthermore, the cytotoxicity of cisplatin to H460 and HCT116 cells was slightly improved by palmatine. In conclusion, palmatine protects against CIAKI by inhibiting inflammation and apoptosis through regulation of NF-κB/MAPK and Akt pathways. Palmatine is a potential adjunctive treatment during the use of cisplatin.
Keywords
Palmatine
Cisplatin
Acute kidney injury
Inflammation
Oxidative stress
Apoptosis
- CIAKI
-
Cisplatin-induced acute kidney injury
- GO
-
Gene Ontology
- KEGG
-
Kyoto Encyclopedia of Genes and Genomes
- MTT
-
Methyl thiazolyl tetrazolium
- CRE
-
Serum creatinine
- BUN
-
Blood urea nitrogen
- SOD
-
Superoxide dismutase
- MDA
-
Malondialdehyde
- GSH
-
Glutathione
Abbreviations
Data availability
All data generated or analyzed during this study are included in this published article.
1 Introduction
Cisplatin is a frontline chemotherapy drug and is part of the treatment regimen for patients with solid tumors including head and neck, testicular, lung, ovarian and breast cancer (Zhang et al., 2021). Unfortunately, the severe side effects caused by cisplatin limit its clinical use. During metabolism, most of the cisplatin is trapped in the kidney, and the concentration of cisplatin in proximal renal tubular cells is five times higher than that in serum, leading to severe nephrotoxicity (Volarevic et al., 2019). The most several and common form of nephrotoxicity is acute kidney injury (AKI), which occurs in 20–30 % of patients treated with cisplatin. Cisplatin-induced AKI (CIAKI) increases the risk of death by 10 to 15 times and results in a mortality rate of 50 % (Ozkok and Edelstein, 2014), especially in elderly patients (Latcha et al., 2016). The production of inflammation, apoptosis and oxidative stress underlie the molecular mechanisms of CIAKI (Holditch et al., 2019). The main therapeutic intervention for CIAKI in clinical practice is to increase urinary excretion.
We have previously found that Zhibai Dihuang Granule, a traditional Chinese medicine, prevents CIAKI by inhibiting cell apoptosis and inflammation (Liu et al., 2022a). Based on the results, we found that one of the CIAK-related active ingredients in Zhibai Dihuang Granule is palmatine. Palmatine is a natural isoquinoline alkaloid and has a variety of biological activities, including antioxidant, anti-inflammatory, antibacterial and anticancer activities (Tarabasz and Kukula-Koch, 2020). Palmatine has been reported to exert a protective effect on SIN-1 induced renal cell damage (Yokozawa et al., 2005). Therefore, we speculated that palmatine plays a crucial role in preventing CIAKI in combination with our previous experimental results. In this study, we first investigated the relationship between palmatine and CIAKI by network pharmacology. Then, we evaluated the protective effect of palmatine on cisplatin induced 293 T cell damage and CIAKI mouse model. Finally, the mechanism of palmatine in 293 T cells and CIAK animal model was analyzed based on the results of network pharmacology.
2 Methods and materials
2.1 Target prediction and protein–protein interaction (PPI) network construction
The targets of palmatine were predicted by TCM systematic pharmacology database (TCMSP, https://tcmspw.com/tcmsp.php), Pharmmapper database (http://www.lilab-ecust.cn/pharmmapper/) and Swiss target prediction database (http://swisstargetprediction.ch/). All gene targets related to CIAKI were obtained from Genecards database (https://www.genecards.org/), OMIM database (https://www.omim.org/) and Disgenet database (https://www.disgenet.org/). Standardized targets were obtained from the UniProt database(https://www.uniprot.org/). The overlapping genes and Venn diagram of palmatine and CIAKI targets were obtained from the online Venn map platform (https://bioinfogp.cnb.csic.es/tools/venny/https://bioinfogp.cnb.csic.es/tools/venny/). The gene targets were further imported into STRING database (https://string-db.org/) to explore the interactions between the known and predicted proteins. The topological parameters in the PPI network were analyzed using Cytoscape 3.9.0 software.
2.2 Enrichment of gene ontology (GO) terms and kyoto encyclopedia of genes and genomes (KEGG) pathways
We imported the obtained potential targets of palmatine for CIAKI treatment into the target gene name list through the DAVID database (https://david.ncifcrf.gov/home.jsp), restricting the species to human. GO enrichment analysis and KEGG pathway annotation analysis were performed on the potential targets to screen the important signaling pathways of palmatine in CIAKI.
2.3 Cell culture and evaluation of cell viability (MTT assay)
Plamatine and cisplatin were purchased from MACKLIN and MCE (Shanghai, China), respectively. Human embryonic kidney 293 T and human colon cancer HCT116 were provided by the Peking Union Cell Center. Human non-small cell lung cancer H460 was provided by Professor Zhi Yao (Tianjin Medical University, Tianjin, China). 293 T, H460 and HCT116 cells were cultured in DMEM, 1640 and IMDM media supplemented with 10 % (v/v) fetal bovine serum (FBS), 100 IU/mL penicillin and 100 IU/mL streptomycin, respectively. All cells were maintained in a humidified air/CO2 incubator (5 % v/v) at 37 °C.
The MTT assay was used to determine the cell viability. Cells were inoculated into 96-well plates at a density of 5 × 10-4 for 24 h and treated with cisplatin (12.5 μM) and/or palmatine (0.0001, 0.001, 0.01, 0.1, 1, 10, 100, 1000, 10000, 100000 nM) for a further 48 h. Then, DMSO was added for 10 min, after which MTT (5 mg/mL) was added for 4 h. Absorbance was measured using a microplate reader (Tecan, Austria) at 492 nm/630 nm.
2.4 Cell apoptosis assay and intracellular reactive oxygen species (ROS) assay
The FITC-Annexin V/PI double staining kit (Sanjian Biotechnology Co., LTD., Tianjin) was used to examine cell apoptosis. After palmatine and cisplatin treatment, 293 T cells were collected after trypsinization and centrifugation. Before adding PI solution (5 μL), the cells were incubated with Annexin V-FITC (5 μL) for 10 min at room temperature in the dark. After co-incubation for 10 min, apoptosis was detected by flow cytometry (Accuri, USA).
The peroxide-sensitive fluorescent probe 2, 7-dichlorofluorescein diacetate (DCFH-DA) (Solebo Technology Co., LTD., Beijing) was used to measure the level of intracellular ROS. After exposure to palmatine and cisplatin, cells were harvested and cultured in serum-free medium containing DCFH-DA (10 μM) for 30 min, followed by detection using flow cytometry (Accuri, USA).
2.5 CIAKI animal experiments
Male C57BL/6J mice (6–8 weeks) were purchased from Sikebeisi Biotechnology Co. Ltd (Henan, China; permit number SYXK (Tianjin), 2020–0005). All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Tianjin University of Science and Technology and approved by the Animal Ethics Committee of Tianjin University of Science and Technology. All mice were randomly divided into four groups (n = 8 ∼ 10) including normal, model, low dose of palmatine (PAL-L), high dose of palmatine (PAL-H). Normal and model mice received 5 % glucose (10 mL/kg) by intraperitoneal injection for 7 days. PAL-L and PAL-H mice were injected intraperitoneally with palmatine at 5 mg/kg and 10 mg/kg, respectively, for 7 days. Except for the normal group, all other groups were injected with a single dose of cisplatin (20 mg/kg) on day 4. Normal mice received 0.9 % NaCl (10 mL/kg) on day 4.
On day 7, all mice were sacrificed. Blood samples were collected and centrifuged at 3000 rpm for 10 min to obtain serum, which was stored at −80 °C. Kidneys were weighed and pathological examination was performed by HE staining. The renal tubular injury score was calculated based on the degree of lesion: no obvious lesion, 0 points; slight or very little, 0.5 points; mild or small, 1 point; moderate or more, 2 points; severe or more quantity, 3 points; extremely severe or a lot, 4 points (Li et al., 2019). The concentrations of creatinine (CRE), blood urea nitrogen (BUN), superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione (GSH) were determined using commercial assay kits purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). The levels of TNF-α, IL-6 and IL-1β were determined by enzyme-linked immunoassay (ELISA) purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China).
2.6 Western blot
Cell or kidney samples were rapidly lysed with protein lyase. Protein samples were obtained after centrifugation and determined by the BSA method. Protein was separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was incubated with Bax (41162S, 1:1000), Bcl-2 (15071S, 1:1000), p65 (8242S, 1:1000); p-p65 (3033S, 1:1000); p-ERK (4370S, 1:2000), ERK (4695S, 1:2000), p-p38 (4511S, 1:1000), p38 (8690S, 1:1000), p-Akt (4060S, 1:2000), Akt (7292S, 1:2000), p-mTOR (5536S, 1:1000), mTOR (2893S, 1:1000) or α-Tubulin (2148S, 1:1000) overnight. Subsequently, the membranes were incubated with the appropriate secondary antibodies (Invitrogen-Thermo Fisher Scientific, USA) for 1 h. α-Tubulin was used as an internal reference protein. All antibodies were purchased from Cell Signaling Technology (USA). The grey intensity of the bands was analyzed and quantified using ImageJ software.
2.7 Statistical analysis
All data were represented as mean ± SD. One-way analysis of variance (ANOVA) was used for statistical analysis. p < 0.05 was considered statistically significant.
3 Results
3.1 Network pharmacological analysis of the relationship between palmatine and CIAKI
A total of 245 palmatine-related target genes were obtained by searching the TCMSP, Swiss target prediction and Pharmmapper database. In addition, a total of 570 CIAKI targets were obtained from Genecards, OMIM and the Disgenet database. After discarding duplicate data, 61 potential palmatine targets against CIAKI were obtained (Fig. 1A). The overlapping targets were imported into the String database to achieve PPI network with a confidence score > 0.4, and the network of core proteins were constructed by Cytoscape software (Fig. 1B). Among the core proteins, the top ten proteins of palmatine against CIAKI were acquired based on the degree values and other topological properties (Table 1).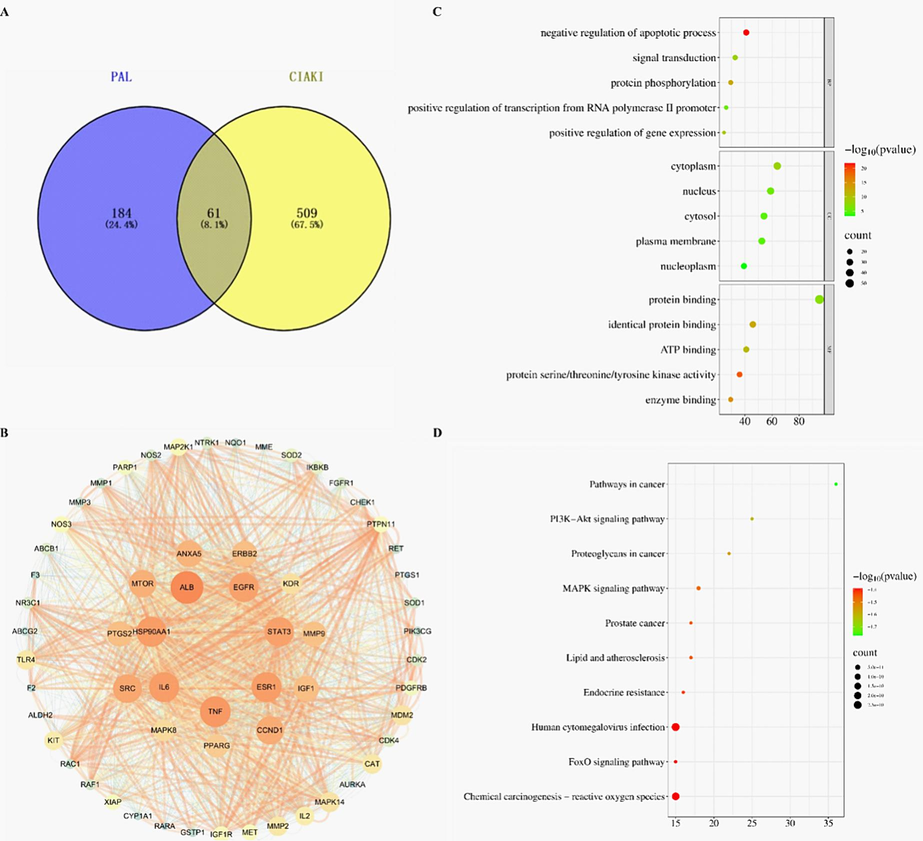
Prediction of potential core targets, construction of PPI networks and enrichment analysis of palmatine against CIAKI. (A) Venn diagram of potential anti-CIAKI targets. (B) PPI network of proteins. (C) Top 5 Gene Ontology biological processes, cellular components and molecular functions. (D) Top 10 KEGG pathways. The abscissa represents the gene proportion, the ordinate represents pathway name, the bubble size represents the number of targets in the pathway and the color represents the p value.
name
Degree
Average Shortest Path Length
Betweenness
CentralityCloseness
CentralityNeighborhood
Connectivity
ALB
53
1.12
1.15
0.90
29.83
TNF
50
1.17
1.15
0.86
30.54
HSP90AA1
49
1.18
0.78
0.85
31.22
IL6
49
1.18
1.01
0.85
30.86
ESR1
49
1.18
1.13
0.85
29.63
STAT3
48
1.20
0.62
0.83
31.71
EGFR
48
1.20
0.83
0.83
30.81
SRC
47
1.22
0.66
0.82
31.55
CCND1
46
1.23
0.71
0.81
31.22
MTOR
45
1.25
0.47
0.80
32.64
To comprehensively describe the mechanism of palmatine against CIAKI, we used the DAVID database to perform GO and KEGG enrichment analysis. A total of 767 GO enrichment items related to CIAKI were obtained, of which 89 were related to cellular component, 412 were related to biological process, and 266 were related to molecular function. KEGG pathway enrichment analysis showed that the potential targets were mapped to 140 pathways. As shown in Fig. 1C-D, only the top 5 related functions were shown in the bubble diagrams. The key targets of the interaction between palmatine and CIAKI were mainly involved in the negative regulation of apoptotic process, positive regulation of kinase activity, PI3K signaling and other biological processes. The signaling pathways revealed by palmatine against CIAKI were mainly involved in oxidative stress, FoxO, PI3K-Akt and MAPK.
3.2 Palmatine inhibited cisplatin-induced renal cell injury and improved cisplatin cytotoxicity to cancer cells
The concentrations of palmatine within 1 μM were safe for 293 T cells (Fig. 2A). As shown in Fig. 2B, cell viability was decreased when exposed to cisplatin (12.5 μM) alone, while it was significantly increased in the presence of palmatine. The data suggested palmatine attenuated cisplatin-induced toxicity in 293 T cells.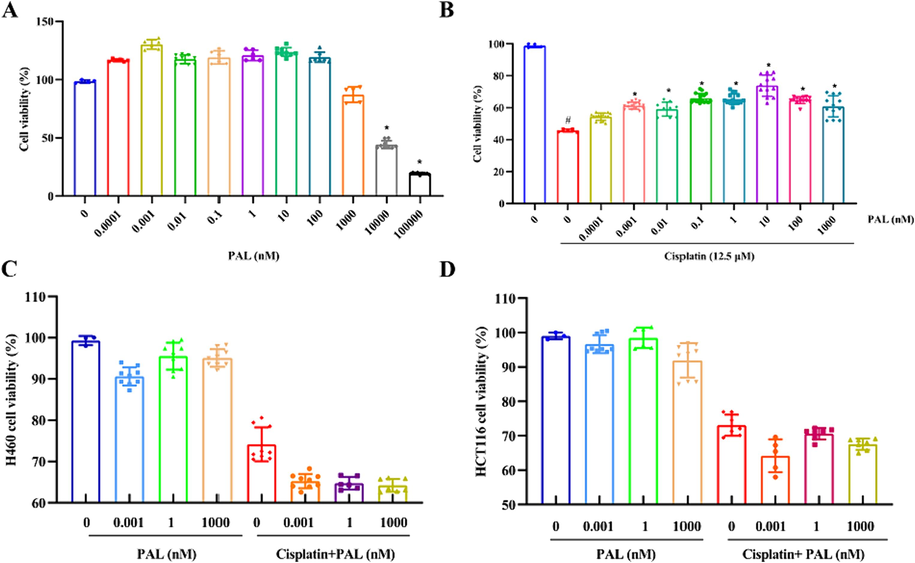
The effect of palmatine (PAL) on cisplatin induced damage to 293 T cells. Palmatine could increase the cell viability within the concentration of 100 nM, but significantly decreased the cell viability at 10000–100000 nM (A). Cisplatin (12.5 μM) significantly decreased cell viability at 12.5 μM, while palmatine significantly improved cell viability (B). Palmatine had little effect on H460 (C) and HCT116 cells (D), while cisplatin was cytotoxic to both cancer cells. Palmatine showed a slight ameliorating effect on the cytotoxicity of cisplatin. #p < 0.05 vs control and * p < 0.05 vs cisplatin.
The cytotoxicity activity of cisplatin with or without palmatine was performed on H460 and HCT116 cells. As shown in Fig. 2C-D, palmatine had little cytotoxicity to the cancer cell lines. Cisplatin (12.5 μM) could reduce the cell viability of H460 and HCT116 cells. Meanwhile, palmatine could improve the cytotoxicity of cisplatin to H460 and HCT116 cells. This means that when cisplatin exerted anti-tumor effects, it was toxic to normal kidney cells, and palmatine could alleviate this toxicity while improving the anti-tumor effect of cisplatin.
3.3 Palmatine inhibited cisplatin-induced renal cell apoptosis and intracellular ROS accumulation
We further investigated the effect of palmatine on cisplatin-induced apoptosis and ROS accumulation in 293 T cells. Cisplatin-induced apoptosis of 293 T cells was significantly inhibited by pretreatment with palmatine in a dose-dependent manner (Fig. 3A and B). Cisplatin caused the accumulation of endogenous ROS, which contributed to cell death, while palmatine alone had almost no effect on ROS. However, the cellular ROS accumulation induced by cisplatin was reduced after palmatine treatment (Fig. 3C and D). The results revealed that palmatine attenuated the ability of cisplatin to induce apoptosis and ROS production in kidney cells.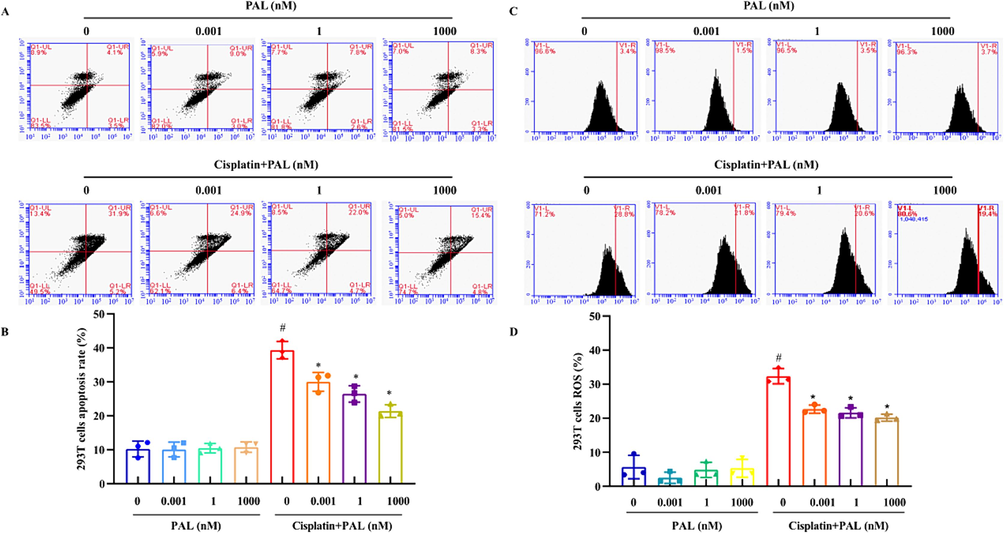
Effects of palmatine (PAL) on cisplatin-induced apoptosis and ROS in 293 T cells. Cells were treated with palmatine (0.001, 1, 1000 nM) and cisplatin (12.5 μM) for 48 h. The cells were stained with Annexin V-FITC/PI fluorescence and detected by flow cytometry to test the apoptosis (A). Palmatine alone had little effect on cell apoptosis, while it could significantly decrease cell apoptosis induced by cisplatin (B). The cells were stained with DCFH-DA fluorescence and detected by flow cytometry to evaluate ROS (C). Cisplatin caused the accumulation of ROS in 293 T cells, which was significantly reduced by palmatine (D). #p < 0.05 vs control and *p < 0.05 vs cisplatin-treated group.
3.4 Palmatine attenuated cisplatin-induced renal cell injury by suppressing NF-κB/MAPK and activating PI3K/Akt pathways
Apoptosis related proteins were detected. In 293 T cells exposed to cisplatin, palmatine markedly blocked increased Bax and significantly increased decreased Bcl-2 (Fig. 4A). The results suggested that palmatine could inhibit cisplatin-induced apoptosis in 293 T cells.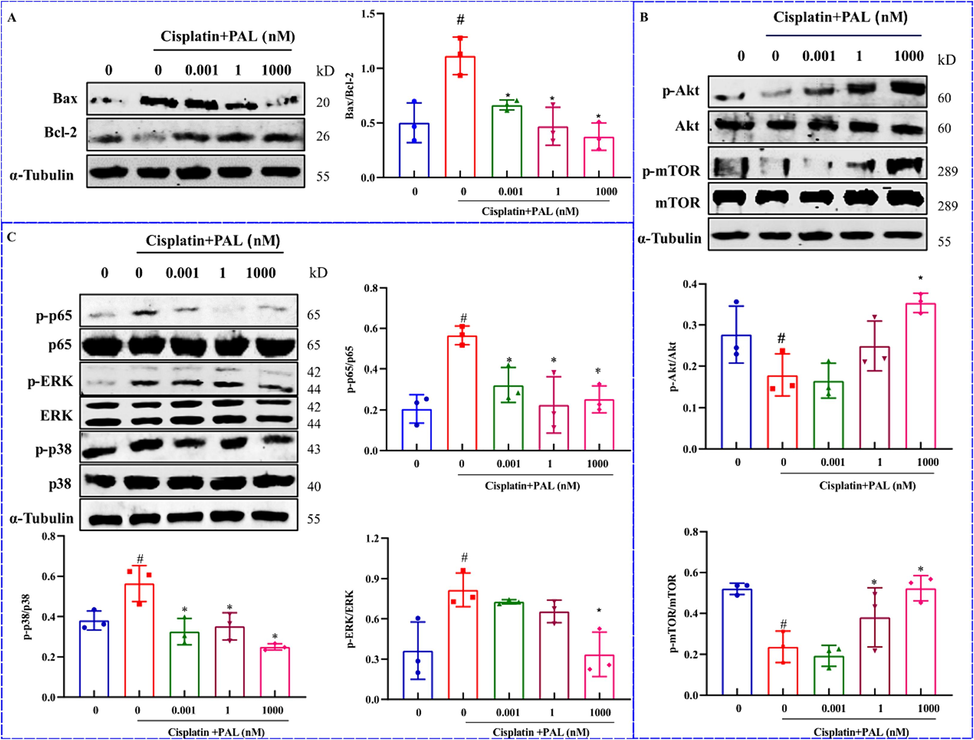
Proteins regulation by palmatine (PAL) on cisplatin-induced injury of 293 T cells. The ratio of Bax/Bcl-2 was significantly increased after cisplatin, while palmatine reversed the increase (A). Cisplatin significantly decreased the levels of Akt and mTOR, which were restored to normal levels by palmatine (B). In the MAPK pathways, the levels of p65, ERK and p38 were significantly increased by cisplatin and decreased after treatment with palmatine (C). #p < 0.05 vs control, *p < 0.05 vs cisplatin group.
Through network pharmacological enrichment analysis, the mechanism of palmatine against CIAKI was related to PI3K/Akt and MAPK pathways. We detected the protein expressions in the Akt and MAPK pathways. As shown in Fig. 4B, the levels of p-Akt and p-mTOR were decreased after cisplatin treatment, while they were significantly increased by the addition of palmatine. The MAPK pathway was significantly associated with the inflammatory response and played an important role in the regulation of apoptosis. The expressions of p-p65, p-p38 and p-ERK were activated after cisplatin treatment in 293 T cells, but they were obviously suppressed by palmatine (Fig. 4C).
3.5 Palmatine prevented CIAKI in animal model
The CIAKI animal model was established by a single injection of cisplatin (20 mg/kg) on the fourth day (Fig. 5A). The body weight of mice in the model group was obviously decreased, which was significantly increased by palmatine (Fig. 5B). The kidney index was elevated in the model, while it was reduced in palmatine (Fig. 5C). The high serum BUN and CRE levels indicated impaired renal function. We found that palmatine decreased the levels of BUN and CRE, especially in the high dose group (Fig. 5D and E).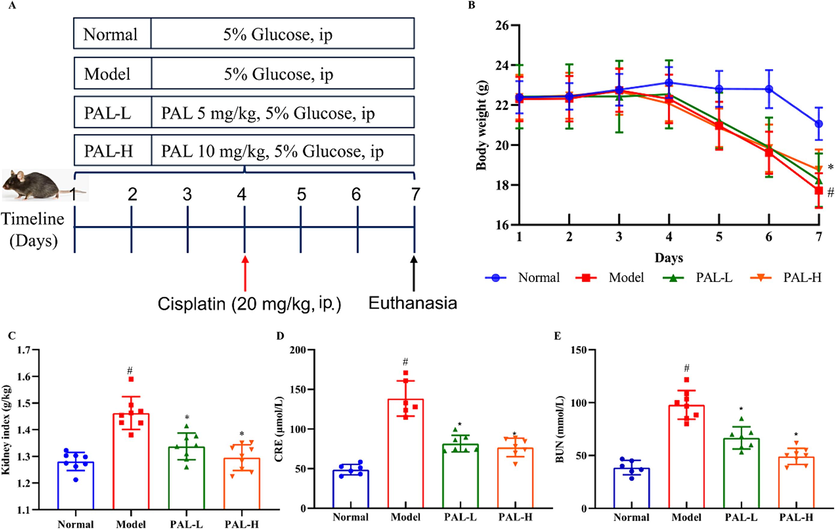
Effect of palmatine (PAL) on the CIAKI mice model. Flow chart of drug administration (A) and body weight of mice (B) during the experiment. Kidney index (C), serum levels of CRE (D) and BUN (E) were significantly increased in the CIAKI model, while they were restored to normal after palmatine treatment. #p<0.05 vs normal group, *p<0.05 vs model group.
3.6 Palmatine inhibited cisplatin-induced oxidative damage and inflammation
HE staining showed that CIAKI mice exhibited pathological changes, including proximal tubular cell necrosis, inflammatory cell infiltration, tissue vacuolation and luminal enlargement. In contrast, palmatine significantly alleviated the above symptoms especially in the high dose group (Fig. 6A-B). The tubular injury score of mice was significantly increased after cisplatin treatment, while it was reversed after palmatine treatment (Fig. 6C). To evaluate the oxidative stress in the mice, the levels of MDA, SOD and GSH in kidney tissue were determined. As shown in Fig. 6D-F, cisplatin induced an increase in MDA and a decrease in SOD and GSH, which was reversed by palmatine. Interestingly, palmatine restored MDA, SOD and GSH to normal expression levels. Moreover, we measured the concentrations of pro-inflammatory factors in the mice. The levels of TNF-α, IL-6 and IL-1β were significantly increased in the cisplatin group, while palmatine significantly decreased the expressions of these cytokines (Fig. 6G-I). The results indicated that palmatine prevented CIAKI by inhibiting oxidative stress and inflammation.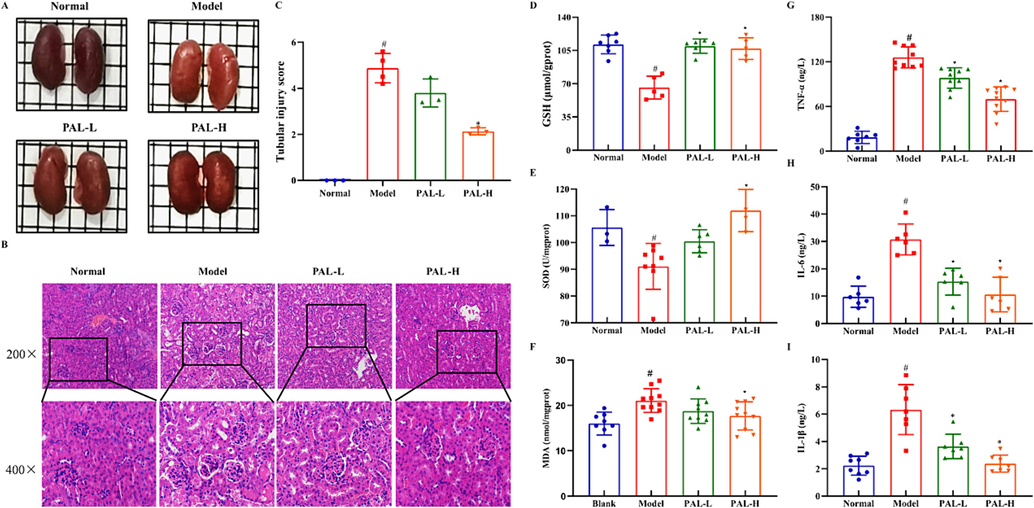
Effect of palmatine (PAL) on renal tissue pathology, oxidative stress indicators and inflammatory factors in the CIAKI mice model. The renal appearance of palmatine was similar to that of the normal group (A). HE staining showed significant changes in the renal tissue structure of the model group, while palmatine alleviated these changes (scale bar, 20 μm, B). The renal tubular injury score in HE was quantitatively analyzed according to the lesion (C). Indicators of oxidative stress including GSH (D), SOD (E) and MDA (F) in the kidney were apparently regulated in the model group, but were restored to normal levels by palmatine treatment. The concentrations of TNF-α (G), IL-6 (H) and IL-1β (I) in kidney were significantly increased in the model group, while palmatine restored them to normal levels. #p < 0.05 vs normal group, *p < 0.05 vs model group.
3.7 Palmatine prevented CIAKI by regulating Akt and NF-κB/MAPK pathways
Consistent with the results in 293 T cells, the ratio of Bax to Bcl-2 was remarkably increased in the CIAKI model, which was significantly reversed by palmatine (Fig. 7A). Cisplatin decreased the phosphorylation of Akt and increased the phosphorylation of p65, p38 and ERK. After palmatine treatment, the levels of Akt, p65, p38 and ERK returned to normal expression (Fig. 7B and C). The results demonstrated that palmatine prevented CIAKI by activating the Akt pathway and suppressing the NF-κB/MAPK pathway.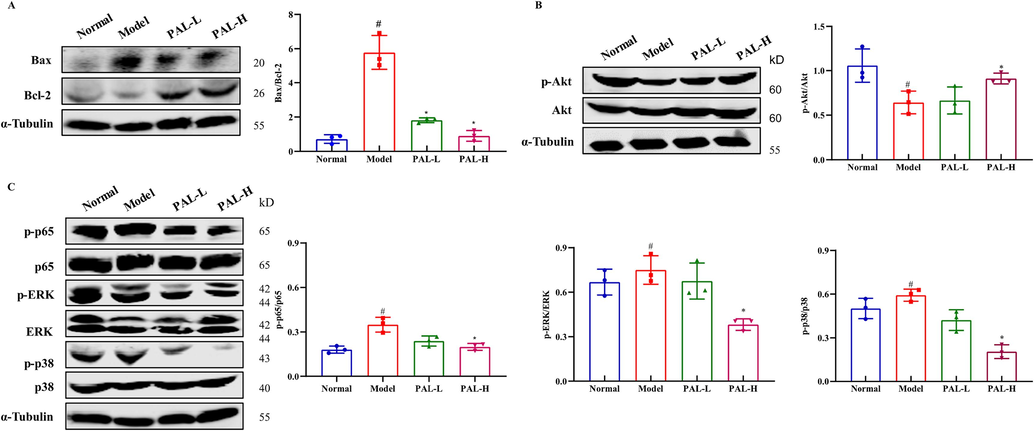
Effects of palmatine (PAL) on kidney related protein levels in the CIAKI mice model. The ratio of Bax to Bcl-2 was significantly increased in the CIAKI model, but returned to normal after palmatine treatment (A). Akt (B) and MAPK (C) pathway proteins were affected by cisplatin and restored by palmatine. #p < 0.05 vs normal group, *p < 0.05 vs model group.
4 Discussion
Cisplatin has been widely used as first-line chemotherapy in combination with other anti-tumor drugs (Rottenberg et al., 2021). However, due to the non-specific anti-tumor activity of cisplatin, it is often accompanied by toxic and side effects, in particular CIAKI. Inflammation, oxidative stress and apoptosis have been reported to contribute to CIAKI. Therefore, some anti-inflammatory natural products show great potential in alleviating CIAKI, such as quercetin (Tan et al., 2020), kaempferol (Wang et al., 2020) and 6-Shogaol (Gwon et al., 2021). Palmatine is a potent component of traditional Chinese medicine and has antioxidant and anti-inflammatory activities. We investigated the protective effect of palmatine on CIAKI using network pharmacology and preclinical experiments. Network pharmacology showed the possibility of palmatine against CIAKI and its related molecular signaling pathways were related to inflammation and oxidative stress. Palmatine was able to alleviate the cisplatin induced toxicity, apoptosis and ROS accumulation in 293 T cells. Similarly, palmatine was shown to protect against CIAKI in an animal model. The mechanism of palmatine against CIAKI was to inhibit inflammation, oxidative stress and apoptosis through regulation of Akt and NF-κB/MAPK pathways.
Network pharmacology can rapidly screen and integrate drug and disease targets, predict signaling pathways, and systematically analyze drug-disease interactions (Nogales et al., 2022). Through the analysis of the integration of palmatine and CIAKI targets, TNF-α, IL-6 and mTOR were involved in the process of palmatine against CIAKI. TNF-α is a key element in a network of proinflammatory chemokines and cytokines that are activated in cisplatin induced nephrotoxicity (Ramesh and Reeves, 2002). IL-6 is a pleiotropic cytokine with pro-inflammatory functions and anti-inflammatory properties (Kaur et al., 2020). It has been reported that mTOR plays a complex role in various pathophysiological aspects of CIAKI, such as oxidative stress (Sakul et al., 2019), proximal tubular injury and renal dysfunction (Xing et al., 2019). KEGG enrichment analysis showed that palmatine against CIAKI had a strong relationship with PI3K/Akt and MAPK pathways, which tipically target apoptosis and inflammation.
According to the reported method (Tanimura et al., 2019), we established the CIAKI mice model by injecting 20 mg/kg of cisplatin, which caused a sharp increase in CRE and BUN, as well as damage with obvious pathological changes such as renal tubular cell necrosis or apoptosis, edema and inflammation. In our study, palmatine reversed cisplatin induced renal damage and reduced the levels of CRE and BUN. Cisplatin caused toxicity in 293 T cells, which was consistent with previous report (He et al., 2019), while palmatine effectively increased cell viability. Our results indicated that palmatine could confer a protective effect against CIAKI in vitro and in vivo.
Most studies tend to suggest that cisplatin induced nephrotoxicity is due to oxidative stress induced by ROS production, lipid peroxide accumulation, and inhibition of the antioxidant system (Tang et al., 2023). Cisplatin was hydrolyzed to positively charged molecules via receptor-mediated endocytosis, which directly disrupted mitochondrial complexes, leading to mitochondrial uncoupling and ROS production (Zhu et al., 2020). Cisplatin was combined with mercaptan-containing antioxidant molecules such as GSH, leading to GSH depletion and subsequent increase in oxidative stress (Ozkok and Edelstein, 2014). The antioxidant agent berberine has been demonstrated to be effective aganist cisplatin nephrotoxicity (Shen et al., 2020). Palmatine could inhibit cisplatin induced ROS production in 293 T cells, upregulate GSH and SOD, and reduce MDA production in the CIAKI mice model. It was suggested that the protective mechanism of palmatine against CIAKI might be the prevention of oxidative stress. Cisplatin induced apoptosis of human proximal tubular epithelial cells (HK-2 cells) was significantly reduced after celastrol treatment (Yu et al., 2018). Consistently, palmatine inhibited cisplatin induced renal cell apoptosis. In addition, the expression of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 was unbalanced after treatment with cisplatin in 293 T cells and the CIAKI mice model, while palmatine significantly alleviated the unbalanced state, suggesting that anti-apoptosis was another mechanism by which palmatine prevented CIAKI.
Inflammation is an important feature of CIAKI (Tan et al., 2020). HE staining showed that inflammatory cells infiltrated the kidney tissue and caused damage. Inflammatory factors may exacerbate nephrotoxicity during cisplatin accumulation in the kidney (Vasaikar et al., 2018). Inhibition or knockout of TNF-α could alleviate CIAKI, suggesting that TNF-α plays an important role in cisplatin induced nephrotoxicity (Yu et al., 2021). Meanwhile, TNF-α can induce ROS production, further exacerbating oxidative stress (Korbecki and Bajdak-Rusinek, 2019). Palmatine has potent anti-inflammatory activity in a variety of diseases, including Alzheimer's disease (Liu et al., 2022b), sepsis (Chen et al., 2022), peptic ulcer disease (Wang et al., 2017) and colitis (Zhang et al., 2018). In this study, TNF-α, IL-6 and IL-1β in kidney tissue were reduced after palmatine treatment, demonstrating the anti-inflammatory ability of palmatine. The production of inflammatory cytokines after cisplatin administration was highly dependent on the activation of NF-κB and MAPK (Kim et al., 2022). Hydroxyl radicals generated by cisplatin were involved in the phosphorylation of p38 and regulation of TNF-α synthesis, ultimately inducing the activation of NF-κB (Achkar et al., 2018). Similarly, we found that cisplatin increased the phosphorylation levels of ERK, p38 and p65, while palmatine reversed the increase. These results indicated that palmatine attenuated CIAKI by inhibiting inflammation through the NF-κB/MAPK signaling pathway.
The PI3K/Akt/mTOR pathway has recieved more attention in relation to cisplatin induced nephrotoxic syndrome. Gagliflozin inhibits renal cell apoptosis and alleviates normal kidney function by activating the PI3K/Akt pathway in the treatment of CIAKI (Song et al., 2020). The key participant in the PI3K/Akt/mTOR pathway is Akt, which is closely associated with cell growth and survival (Kubli and Gustafsson, 2014). Our study showed that palmatine reversed the cisplatin induced decrease in Akt and mTOR. Moreover, Akt phosphorylation directly inhibits the expression of the pro-apoptotic protein FOXO3 and indirectly negatively regulates cell apoptosis (Link, 2019), which was consistent with the results of our enrichment analysis. Furthermore, we found that palmatine could improve the cytotoxicity of cisplatin to H460 and HCT116 cells, which was consistent with the reported anticancer activity of palmatine when combined with anticancer drugs (Grabarska et al., 2021). This mean that the administration of palmatine together with cisplatin may not only improve the anti-tumor effect, but also prevent CIAKI. The key signaling pathways and possible underlying molecular mechanisms associated with the therapeutic effect of palmatine against CIAKI were proposed in Fig. 8.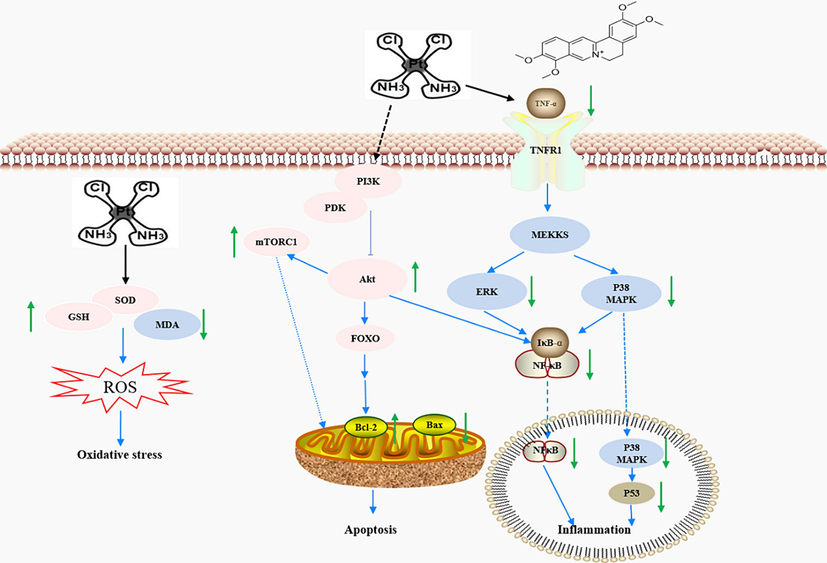
A proposed schematic model of the possible underlying molecular mechanisms associated with the therapeutic effect of palmatine against CIAKI. Cisplatin produces nephrotoxicity by inducing oxidative stress, apoptosis and inflammation. Palmatine inhibits ROS production during oxidative stress, balances Bax/Bcl-2 in the mitochondrial apoptotic pathway through the PI3K/Akt pathway, and reduces inflammatory factors via the NF-κB/MAPK pathway.
5 Conclusion
Our study theoretically demonstrated the possibility of palmatine in alleviating CIAKI through network pharmacology and provided evidence for the protective effect of palmatine on CIAKI through in vitro and in vivo experiments, further supporting the potential use of palmatine as a chemoprotective agent. The preventive effect of palmatine on CIAKI was due to the suppression of oxidative stress, apoptosis and inflammation by the regulation of Akt and NF-κB/MAPK pathways. Meanwhile, palmatine enhanced the cytotoxicity of cisplatin to cancer cells. Therefore, palmatine might be a promising adjuvant therapy in cancer patients receiving cisplatin based treatment. However, further studies are needed to evaluate the potential use of palmatine as an adjuvant therapy to attenuate cisplatin-induced nephrotoxicity in a clinical context.
6 Ethics statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Tianjin University of Science and Technology and approved by the Animal Ethics Committee of Tianjin University of Science and Technology.
CRediT authorship contribution statement
Zhen Liu: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition. Lvqian Guo: Investigation, Methodology, Formal analysis, Writing – original draft. Xuan Zhu: Formal analysis, Writing – review & editing. Xinran Li: Formal analysis, Investigation. Wanshun Zhao: Writing – review & editing. Peng Yu: Software, Supervision. Yuou Teng: Conceptualization, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J. Transl. Med.. 2018;16:96.
- [CrossRef] [Google Scholar]
- The interaction of MD-2 with small molecules in huanglian jiedu decoction play a critical role in the treatment of sepsis. Front. Pharmacol.. 2022;13:947095
- [CrossRef] [Google Scholar]
- Palmatine, a bioactive protoberberine alkaloid isolated from berberis cretica, inhibits the growth of human estrogen receptor-positive breast cancer cells and acts synergistically and additively with doxorubicin. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Protective effects of 6-shogaol, an active compound of ginger, in a murine model of cisplatin-induced acute kidney injury. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Anemoside B4 attenuates nephrotoxicity of cisplatin without reducing anti-tumor activity of cisplatin. Phytomedicine. 2019;56:136-146.
- [CrossRef] [Google Scholar]
- Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem.. 2020;28:115327
- [CrossRef] [Google Scholar]
- Ojeoksan ameliorates cisplatin-induced acute kidney injury in mice by downregulating MAPK and NF-kappaB pathways. Int. J. Mol. Sci.. 2022;23
- [CrossRef] [Google Scholar]
- The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm. Res.. 2019;68:915-932.
- [CrossRef] [Google Scholar]
- Cardiomyocyte health: adapting to metabolic changes through autophagy. Trends Endocrin. Met.. 2014;25:156-164.
- [CrossRef] [Google Scholar]
- Long-term renal outcomes after cisplatin treatment. Clin. J. Am. Soc. Nephrol.. 2016;11:1173-1179.
- [CrossRef] [Google Scholar]
- Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- Active compounds and targets of yuanzhi powder in treating Alzheimer's disease and its relationship with immune infiltration based on HPLC fingerprint and network pharmacology. Evid. Based Complement. Alternat. Med.. 2022;2022:3389180.
- [CrossRef] [Google Scholar]
- Prediction of the mechanisms of action of zhibai dihaung granule in cisplatin-induced acute kidney injury: a network pharmacology study and experimental validation. J. Ethnopharmacol.. 2022;292:115241
- [CrossRef] [Google Scholar]
- Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci.. 2022;43:136-150.
- [CrossRef] [Google Scholar]
- Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int.. 2014;2014:967826
- [CrossRef] [Google Scholar]
- TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest.. 2002;110:835-842.
- [CrossRef] [Google Scholar]
- The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer.. 2021;21:37-50.
- [CrossRef] [Google Scholar]
- Squalene attenuates the oxidative stress and activates AKT/mTOR pathway against cisplatin-induced kidney damage in mice. Turk. J. Biol.. 2019;43:179-188.
- [CrossRef] [Google Scholar]
- Integrated analysis of m6A methylome in cisplatin-induced acute kidney injury and berberine alleviation in mouse. Front. Genet.. 2020;11:584460
- [CrossRef] [Google Scholar]
- Canagliflozin reduces cisplatin uptake and activates Akt to protect against cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol.. 2020;318:F1041-F1052.
- [CrossRef] [Google Scholar]
- Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-kappaB signaling maintained macrophage inflammation. Phytother. Res.. 2020;34:139-152.
- [CrossRef] [Google Scholar]
- Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat. Rev. Nephrol.. 2023;19:53-72.
- [CrossRef] [Google Scholar]
- Renal tubular injury exacerbated by vasohibin-1 deficiency in a murine cisplatin-induced acute kidney injury model. Am. J. Physiol. Renal Physiol.. 2019;317:F264-F274.
- [CrossRef] [Google Scholar]
- Palmatine: a review of pharmacological properties and pharmacokinetics. Phytother. Res.. 2020;34:33-50.
- [CrossRef] [Google Scholar]
- D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: impact on pro-inflammatory cytokines. Chem. Biol. Interact.. 2018;290:6-11.
- [CrossRef] [Google Scholar]
- Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci.. 2019;26:25.
- [CrossRef] [Google Scholar]
- Kaempferol ameliorates cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-kappaB pathways. AMB Express. 2020;10:58.
- [CrossRef] [Google Scholar]
- Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. J. Nat. Med.. 2017;71:257-264.
- [CrossRef] [Google Scholar]
- Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell. Prolif.. 2019;52:e12627.
- [Google Scholar]
- Protective role of coptidis rhizoma alkaloids against peroxynitrite-induced damage to renal tubular epithelial cells. J. Pharm. Pharmacol.. 2005;57:367-374.
- [CrossRef] [Google Scholar]
- Danshensu attenuates cisplatin-induced nephrotoxicity through activation of Nrf2 pathway and inhibition of NF-kappaB. Biomed. Pharmacother.. 2021;142:111995
- [CrossRef] [Google Scholar]
- Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-kappaB and improving mitochondrial function. EBioMedicine. 2018;36:266-280.
- [CrossRef] [Google Scholar]
- Cisplatin chemotherapy and renal function. Adv. Cancer Res.. 2021;152:305-327.
- [CrossRef] [Google Scholar]
- Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res.. 2018;137:34-46.
- [CrossRef] [Google Scholar]
- Activation of TFEB-mediated autophagy by trehalose attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Theranostics.. 2020;10:5829-5844.
- [CrossRef] [Google Scholar]







