Pectin based nanocomposite membranes as green electrolytes for direct methanol fuel cells
⁎Corresponding authors at: Department of Chemistry, Periyar University, Salem, Tamilnadu, India (V. Raj). suganthichempower91@gmail.com (V. Raj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Facile proton transport and restricted methanol passage through PC-PVA/s-TiO2 hybrid nanocomposite membranes.
Abstract
Biocomposite materials are highly attractive for research and industrial application due to biodegradability, nontoxicity and sustainability. Solid electrolyte membrane derived from biodegradable materials broadens scope of using sustainable polymers and is easily disposable at the end of life cycle. In this study, Pectin (PC) is blended with polyvinyl alcohol (PVA) in order to fabricate new class of hybrid nanocomposite followed by addition of sulfonated titanium dioxide (s-TiO2) nanoparticles as inorganic proton conducting material. PVA and PC is in situ cross-linked using dual cross-linker comprising a mixture of sulfosuccinic acid and glutaraldehyde followed by solvent casting. Rheological studies with polymer solutions are conducted to study alignment and disentanglement of polymer chains at molecular level. It is shown that rational design of membrane microstructure with proper arrangement of hydrophobic/hydrophilic domains has been formulated by blending PVA with PC. PC-PVA blends with a flexible polymeric network which is appropriate to disperse rigid s-TiO2 nanoparticles resulting in improved proton conductivity and restricted methanol permeability. With further enriched proton conductivity by the presence of s-TiO2 nanoparticles, fabricated PC-PVA/s-TiO2 hybrid nanocomposite membrane exhibit a peakpower density of 27 mW/cm2 at 70 °C in DMFCs.
Keywords
Pectin
Polyelectrolytes
Hydrophobic/hydrophilic domains
Methanol permeation
1 Introduction
Owing to the growing demand for clean and sustainable energy, fuel cells have been identified as eco-friendly and efficient alternate energy conversion systems. Direct methanol fuel cells (DMFCs) being environmentally gracious are considered to be one of the greatest promising candidates for moveable and stationary applications in the view of their benefits such as high energy density of methanol, high competence as well as they operate with liquid fuel which could be used without needful any fuel-processing units (Kreuer, 1996; Deluca and Elabd, 2006; Nerburchilov et al., 2007). Polymer Electrolyte Membrane (PEM) stand for an integral component of DMFC and an ideal DMFC membrane should facilitate transfer of protons from anode to cathode at the same time should effectively prevent passage of fuel and electrons. Widely used perfluorosulfonic acid ionomer (Nafion®) membrane is advantageous for its high proton conductivity, excellent thermo-mechanical stabilities and good oxidative stability, but high methanol permeation rate, higher cost and environmental inadaptability associated with Nafion® severely prohibit its extensive application as well stimulated continuing interest in the direction of developing alternate low-cost, bio-degradable, methanol impermeable polymer elelctrolytes. (Smitha et al., 2004; Shirdast et al., 2016). Accordingly, the various alternative methods for resolving PEMs based on sulfonated aromatic polymers, irradiation graft polymers, cross-linked polymers, and blend polymers were the various alternative methods for resolving successfully demonstrated as DMFC electrolytes as a substitute for Nafion® (Hu et al., 2017). In line with recent literatures, effective application of DMFC devices with enhanced efficiency is feasible only through developing alternate PEMs based on non-fluorinated polymers (Lufranco et al., 2013). For instance, modified polysulfone composite membranes have been demonstrated as a composite electrolyte for DMFC that delivered a maximum peak power of about 140–180 mW/cm2 during high temperature operation (Lufranco et al., 2008; Lufranco et al., 2006). Also, stable prolonged operation of DMFC using low-cost ethylene-tetrafluoroethylene based grafted membranes with −0.13 mA cm−2 linear decay of current has been demonstrated by Arico et al. (2003) Several hybrid organic–inorganic composite membranes are fabricated and evaluated for DMFC application (Kim et al., 2014). Organic-inorganic non-fluorinated membranes, acidic-basic membranes and natural polymer precursor derived membranes are continuously investigated as alternate membrane materials (Munjewar et al., 2017).
There exists a demand for environmentally-benign raw materials particularly in the areas in which an increasing industrial requirement is expected that include energy storing/converting devices, solar cells and supercapacitors, etc.,. Biopolymers such as starch, carboxy methyl cellulose, cellulose, chitosan, carrageenan, pectin, chitin, lignocellulosic materials, hyaluronic acid, agarose, polylactides, polyhydroxyalkanoates (bacterial polyesters), sago and soy-based plastics provide the impetus for new electrolyte host materials for rechargeable batteries (Zolin et al., 2016; Avetta et al., 2013; Samsudin et al., 2014; Ma et al., 2007; Harun et al., 2012; Buraidah et al., 2009; Samsudin et al., 2012). Recently, banana peel pseudo graphite offers superb dual functionality for sodium ion battery and lithium ion battery anodes, while silk worm cocoon derived biopolymer has been proposed as host matrix for lithium-sulphur battery cathode materials (Zhang et al., 2014). Besides, dye-sensitised solar cells has been developed with bio source-derived polymers such as carboxy methyl cellulose, microfibrillated cellulose, carrageenan, chitosan, etc., that contributed significantly to the step forwards of the systems into sustainability by developing devices (Barolo et al., 2017; Bella et al., 2014; Ma et al., 2007; Harun et al., 2012; Admassie et al., 2014). Bio-cathodes in microbial fuel cells with an emphasis on the classification according to the final electron acceptors and the comparison with the traditional abiotic cathode of microbial fuel cells has been reviewed by Song et al. (2019). With the goal of utilising environmentally-being materials, biopolymers such as lignin, sodium alginate are demonstrated as super capacitor materials for efficient energy storing medium (Borchardt et al., 2014; Raymundo et al., 2006). This emphasizes the prominent of developing cost-effective, eco-friendly materials derived from natural sources such as polysaccharides, proteins, lipids, etc.
Hence, following a line of investigation towards fabricating environmentally safe materials for industrial application has become a booming field of research. Biopolymer and biodegradable polymers such as polysaccharides are chitosan (CS), alginate, carrageenan and pectin these are all of meticulous interest, owing to their good film-forming capability along with inherent methanol-impermeable characteristics. Many of CS based PEMs have been successfully demonstrated for DMFC application (Mohanapriya et al., 2009; Mohanapriya et al., 2011; Osifo and Masala, 2010; Wu et al., 2016; Tripathi and Shahi, 2008; Bai et al., 2014; Xiang et al., 2009; Vijayalekshmi and Khastgir, 2017) and a few reports studied behaviour of sodium alginate based PEMs (Mohanapriya et al., 2010; Smitha et al., 2005). Pectin (PC) is a least explored class of polysaccharide with respect to DMFC application and investigations on PC based is hardly available in literature except preliminary evaluation on the utility of amidated PC as a DMFC electrolyte proposed by Mishra et al. (2009). Recently, biocomposite membranes of highly methylated PC/Diethanolamine composite membrane could be tailored for DMFC applications (Mishra et al., 2012).
PC is a natural anionic polysaccharide which plays an important role of regulating mechanical behaviour of plant cell wall and it is extracted from outer cells of most of the plants. From the structural point of view, PC fit into a family of pectic polysaccharides containing 1,4-linked α-D-galacturonic residues with partial methyl ester groups of carboxylic acid residues and is recognized for its widespread applications in the field of food processing, cosmetics, medical and pharmaceutical industries (Espitia et al., 2014; Fidalgo et al., 2014; Thakur et al., 1997; Cabello et al., 2017). In the present study, PC has been blended with Poly (vinyl alcohol) (PVA) in order to fabricate polymeric membranes with adequate mechanical stability, ionic conductivity and well-known material for separation of alcohol mixtures with good selectivity (Murthy and Shah, 2017). It is quite interesting to combine natural polymer with a synthetic polymer; accordingly, PC is blended with PVA. In order to further promote ionic conductivity, sulfonated titanium dioxide nanoparticles (s-TiO2) are incorporated into the blends to formulate nanocomposite membranes (Wang et al., 2012). It is illustrious that s-TiO2 acts as a filler and leads to an increase in net acid content by increasing the number ion-conducting groups per unit volume (Wang et al., 2008).
Eventually, the purpose of this investigation is to develop PC based nanocomposite membranes, evaluating their functional properties such as proton conductivity and methanol permeability and also substantiating their suitability as DMFC electrolyte. These membranes are wisely designed to possess higher selectivity for conducting protons rather than methanol. PC inserts an incredible impact on hydrophilic/hydrophobic microstructure of polymer network, consequently helps to improve ionic conductivity and mitigate methanol permeation simultaneously. Cross-sectional area of hydrophilic domains through which proton and methanol transport occurs has been condensed due to the presence of pectin and resulting polymeric blends, preferentially transport protons (or hydronium ions) and greatly reduces methanol transport. s-TiO2 nanoparticles introduced in the flexible polymer blend further ameliorates this selective sorption feature and improves mechanical stability which is seminal for desirable DMFC performance.
2 Experimental
2.1 Materials
PVA, (99.7% hydrolyzed, M.W. 115,000), PC (M. Wt 3.0 × 104−1.0 × 105) was obtained from LobaChemie, India. Hydrophilic fumed TiO2 with an average particle size of 21 nm was procured from Aeroxide. Methanol and Sulfosuccinic acid (SSA) were obtained from SRL chemicals. Sulfuric acid (98%) and Glutaraldehyde (25% aqueous) were obtained from S.D. Fine Chemicals, India. All the chemicals were used as received. De-ionized (DI) water (Resistivity 18.4 MΩ cm) from Millipore was used throughout the experiments.
2.2 Membrane preparation
2.2.1 Preparation of s-TiO2
Sulfonation of TiO2 was carried out as mentioned in the earlier literature (Tsai et al., 2010). In brief, 1 g of TiO2 nanoparticles was added to 15 mL of 0.5 M sulfuric acid. These mixed solutions kept under ultrasonication for 1 h. Solid mass was filtered and washed thoroughly with deionised water untilfiltrate become neutral to remove residual acid. s-TiO2 was obtained by drying the sample at 80° C for 24 h.
2.2.2 Preparation of Pectin-PVA/s-TiO2 hybrid nanocomposites
PC-PVA/s-TiO2 hybrid nanocomposite membranes were prepared by using solution-casting technique. Briefly, 30 mL of 4 wt% PVA solution was prepared by dissolving the considered essential amount of PVA in water at 60° C followed by stirring until a clear solution was obtained. Similarly, 20 mL of 25 wt% Pectin in respect to PVA was dissolved in de-ionised water followed by stirring until a homogeneous solution was obtained. Both the solutions were mixed together and stirred for 4 h to form a compatible blend. Required quantity of sulfonated TiO2 was dispersed in de-ionised water under ultra sonication for 2 h. The resultant s-TiO2 solution was added drop-wise into the above Pectin-PVA blend solution under continuous stirring for 24 h. Sequential binary cross-linking with a mixture consisting of 10 wt% sulfosuccinic acid (SSA) and 5 wt% glutaraldehyde (GA) optimisation (with respect to PVA polymer) was done by following the procedure stated elsewhere (Wang et al., 2012). The plexiglass plate was fixed on the flat surface and the resulting solution was cast on a flat Plexiglass plate then the solution dried at room atmospheric temperature and the membranes were peeled off from the glass plate and washed thoroughly before subjecting to further studies. PC-PVA blend membranes were prepared in the similar manner without addition of s-TiO2. The composition of s-TiO2 was varied from 0.75 wt% to 9.75 wt% in relation to PC-PVA blend. PC-PVA/s-TiO2 hybrid nanocomposite membranes comprising 0.75 wt%, 4.75 wt% and 9.75 wt% of s-TiO2 are designated as PC-PVA/s-TiO2-I, PC-PVA/s-TiO2-II and PC-PVA/s-TiO2-III respectively. It is to be noted that when level of s-TiO2 is beyond 10 wt%, membrane develops fragility and brittleness. The thickness of all the membranes was maintained at ∼160 µm. Scheme 1 provides pictorial representation of structural modification created due to the presence of PC when combined with PVA.
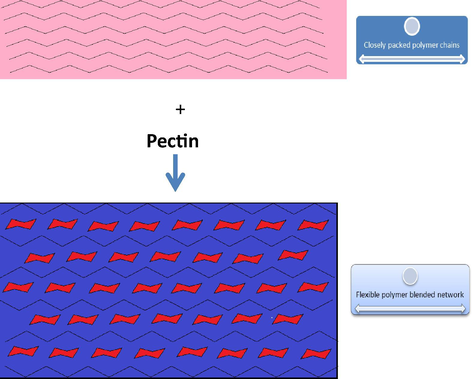
- Pictorial representation of structural modification created due to the presence of PC when combined either with PVA.
2.3 Rheological measurements
Rheological characterization is useful for understanding interaction between polymeric chains. Rheological properties of PC and PC-PVA polymer precursor solutions were measured in dynamic flow mode isothermally at 30 °C using Physica MCR 51 rheometer (Antonpar, Germany). Samples are first subjected to a strain sweep test to define linear viscoelastic region. Experiments at imposed shear rate were performed on DG26 (Double Gap), fitted with parallel plate geometry. The quantity of the fluid on the plate was 4 mL and temperature was controlled by Viscotherm VT2 system. Relative viscosities of the above solutions were determined in relation to shear rate.
2.4 Ion-exchange capacity and oxidative stability measurements
A measure of the number of mille-equivalent of ions in 1 g of the membrane is indicated by Ion-exchange capacity (IC). IC for the membranes was determined by following the procedure reported in the literature and the IC is calculated using following relation (Vijayalekshmi and Khastgir, 2017)
The oxidation stability of each membrane was estimated by the weight changes of the membranes in Fenton’s reagent (Ke et al., 2011). Small pieces of membrane samples (2 cm × 2 cm) were located into Fenton’s reagent (4 ppm FeSO4 in 3% H2O2) at 80 °C under stirring. The samples were taken out of the solution at regular intervals and quickly weighed after removing the surface liquid with filter paper.
2.5 Sorption and proton conductivity measurements
The circularly-cut membrane sample was immersing into the de-ionized water for 24 h to obtain equilibrium and weighed (diameter = 2.5 cm) before dipping into sorption chamber after sorption measurements were conducted by the membrane. The weight measurements were made on wet membranes after blotting their surface dry. Sorption values for aforesaid membranes were calculated using Eq. (2) given below.
In Eq. (2), W∞ and Wo denote to the weights of sorbed and dry membranes, respectively. The experiments were repeated for at least three different membrane samples, and average value was considered.
Proton conductivity measurements were carried out with PC-PVA blend, PC-PVA/s-TiO2 hybrid nanocomposite membranes in a two-probe conductivity cell by AC impedance technique. The conductivity cell comprised two electrodes, each of 20 mm diameters. The membrane was placed between these two electrodes to have a through plane contact and then mounted in a Teflon block and kept in a closed glass-container filled with water to maintain 100% humidity which also has the provision to heat. The membranes were equilibrated for 24 h in this condition before measuring ionic conductivity. Conductivity measurements were done by varying the temperature from 30 °C to 100 °C. AC impedance measurements were carried out using Autolab PGSTAT-30 equipment. An AC frequency range between 1 MHz and 10 Hz with an amplitude of 10 mV was recorded. The intercept with the real axis of the impedance at high-frequency corresponds to the membrane resistance (R) the membrane conductivity was calculated from the membrane resistance, R, using Eq. (3) as follows:
In Eq. (3), σ is the proton conductivity of the membrane in S/cm, l is the membrane thickness in cm and A is the membrane cross sectional area in cm2.
2.6 Physicochemical characterization
Mechanical properties of the membranes were studied by the equipment used for carrying out the test was a Universal testing machine (UTM) (Model AGS-J, Shimadzu) with instrument operating head-load of 10 kN.
ASTM D-882 standards were followed to cut the test samples in dumb-bell shape. The membranes were then placed in the sample gauze of the machine. The film was stretched using a strain rate (the cross-head speed) of 1 mm/min (10% min−1) and its tensile strength was estimated using Eq. (4).
Surface morphologies of PC-PVA blend and PC-PVA/TiO2 hybrid nanocomposite membranes were studied using JEOL JSM 35CF Scanning Electron Microscope (SEM). Gold film of thickness <100 nm was sputtered on the membrane surfaces using a JEOL Fine Coat Ion Sputter-JFC-1100 Unit prior to their examination under SEM, before subjecting it to actual measurements. Thermogravimetric analysis for all the membranes were studied using a SDT Q600 V8.2 TGA/DTA instrument in the temperature range between 30 °C and 1000 °C at a heating rate of 5 °C/min with nitrogen flushed at 60 mL/min.
The FTIR spectra for TiO2 powder, sulfonated TiO2, PC-PVA blend and PC-PVA/TiO2 hybrid nanocomposite membranes were recorded using a Nicolet IR 860 spectrometer (Thermo Nicolet Nexus-670) in the frequency range between 4000 and 400 cm−1. The band gaps for TiO2 and polymers were investigated by optical absorption studies carried out using a UV–Vis spectrophotometer (T80- PG instruments Ltd.,) in the range 200–700 nm.
2.7 Methanol permeability
In our earlier studies, we have developed a method to determine methanol crossover in situ, which relies on a principle of mass balance between supplied fuel and methanol utilized for electrochemical reaction. However, this method is applicable only during the operation of fuel cell and it needs DMFC operation for longer time period. Present study involves a simple but efficient way of determining methanol permeability.
A diffusion cell consists of two 50 mL chambers in which 2 M methanol solution was filled with one compartment and the other compartment filled with deionised water. The membrane (effective area 4 cm2) was placed between the two compartments which were kept under stirring for 12 h during experiment. The obvious methanol permeability of the membranes was measured at 70 °C by conducting density measurements of liquids. The disparity of methanol concentration with time in the receiver reservoir (CB) was determined by means of density measurements. Amount of permeated methanol from donor reservoir (CA) to receiver reservoir (CB) was calculated by determining the difference in the density of methanol before and after conducting the experiment. The densities of methanol were measured using a density meter (Mettler Toledo, DB51) by taking 20 mL of the collected methanol sample. The molarity of the methanol was calculated from the measured density values using Eq. (5) given below (Perry et al., 1997).
2.8 Membrane performance evaluation in DMFC
The DMFC performance of the membranes was evaluated by preparing its membrane electrode assemblies. Commercial DMFC electrodes (supplied by Alfa Aesar Johnson Matthey US) comprising Pt-Ru/C (1:1 atomic ratio, 2 mg/cm2 metal loading) as anode and Pt/C (2 mg/cm2 metal loading) as cathode were used for the experiments. Before sandwiching the membrane between these two electrodes, a binding layer of Nafion-IPA (1:1 vol%) mixture is coated on the surface of the electrode. These were pressed at a compaction pressure of 20 kg/cm2 in a hot press (Flow-Mech) at 60 °C for 1 min to form membrane electrode assembly (MEA). MEAs performance was evaluated using a conventional fuel cell fixture with parallel serpentine flow-field machined on graphite plates. The cells were tested at 70 °C with 2 M aqueous CH3OH at a flow rate of 2 mL/min at the anode side and oxygen at the cathode side at a flow rate of 300 mL/min at atmospheric pressure. Steady state cell polarization is done in galvanostatic mode and is noted in terms of cell voltage in relation to current using electronic load Model-LCN4-25-24/LCN 50–24 from Bitrode Instruments (US).
3 Results and discussion
3.1 Characterization of polymer solutions
3.1.1 Rheological behavior of polymer solutions
Studies on rheological behaviour of polymer precursor solutions are important to evaluate processing conditions and provide insight into the interfacial interaction between polymers. Rheological properties of PC polymer solution and PC-PVA blend solution are performed by evaluating variation of viscosity (μ) in relation to shear rate (δu/δy). Variation of viscosity upon changing shear rate is shown in Fig. 1. PC exhibits a typical shear-thinning behaviour because viscosity is higher at lower shear rate and vice versa under the adapted experimental conditions as shown in Fig. 1a. The drop of viscosity is due to the molecular alignments and disentanglements of long polymer chains under the influence of shear stress. Materials possessing shear-thinning behaviour are pseudo plastics (Peres et al., 2016). When PVA is blended with PC, apparent viscosity has been changed and there is a decrease in viscosity with shear rate. It is associated with the internal friction, is mainly related to the orientation of the macromolecules with increasing shear force. Attractive forces between PC and PVA polymeric components in the blend solutions may cause decrease of in apparent viscosity with increase of shear rate. Variation of shear rate with shear stress of different polymer solutions is shown in Fig. 1b. Nature of flow characteristics of polymers is closely related to their mechanical properties and specific interaction between polymers which is seminal to enhance tensile strength of polymer blend.

- (a) Variation of viscosity with shear rate for PC and PC-PVA polymer solutions. (b) Variation of shear rate with shear stress PC and PC-PVA polymer solutions. (c) Power law plots for PC and PC-PVA viscous polymer solutions.
Typical flow behaviour of PC and PC-PVA viscous solutions are described by Non-Newtonian power law given by Eq. (7).
In Eq. (7), K is flow consistency index (pa sn), δu/δy is the shear rate or velocity gradient (1/s), and n is the flow behaviour index. The power-law plots for PC and PC-PVA are shown in Fig. 2c. Typical pseudo plastic characteristics of these viscous solutions are evidenced by calculated value and since n < 1, it is to be noted that all polymer solutions under the study are pseudoplastics (Esfe and Rostamian, 2017). Power law parameters are tabulated in Table 1. Potential influence of inter and intra-molecular hydrogen bonds upon rheological behaviour of polymer solutions are evident from the results.
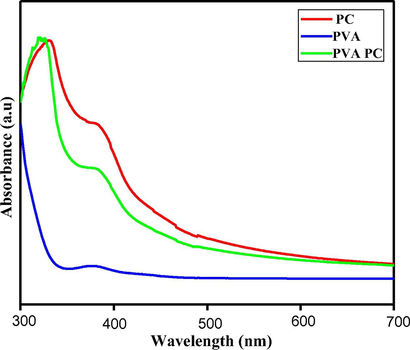
- UV-Visible electronic absorption spectra of PVA, PC and PC-PVA polymer solutions.
| PC | PC-PVA | |
|---|---|---|
| n (Dimensionless) | 0.29 | 0.33 |
| K (Pa sn) | 16.98 | 27.8 |
Analysis of UV spectra of PVA, PC and PC-PVA blend provides evidence for successful blending of polymers and results are shown in Fig. 2. Pectin exhibits a distinctive band with maximum at 352 nm related to chromospheres groups. In case of PVA-PC blend, it is observed that absorption peak of PC shifted towards a lower wavelength and the blend spectra are superimposed to constituent a spectrum which shows a characteristic absorption at 345 nm. From these results it is clear that, functional groups of PC interact strongly with hydroxyl groups of polymers through hydrogen bonding.
3.2 Micro-structural characterization
3.2.1 Characterization of TiO2 nanoparticles
Sulfonation of TiO2 nanoparticles is verified by FT-IR. Fig. 3 shows FT-IR spectra of pure TiO2 with that of s-TiO2. Characteristic wide peaks related to —OH groups at 1662 and 3355 cm−1, and broad peak below 1200 cm−1 attributed due to Ti-O-Ti vibration are prominently observed for unmodified TiO2 spectrum. Sulfonation of TiO2 is evidenced by the emergence of a pair of important peaks at 1037 cm−1 and 1220 cm−1 corresponding to the symmetric and asymmetric stretching vibrations of S⚌O, respectively in the s-TiO2 spectrum. Interestingly, broad band noticed from 2400 to 3400 cm−1 is ascribed to stretching vibrations of OH groups that is widened and deepened owing to the formation of hydrogen bonds caused by sulfonation. FTIR spectral studies prove successful functionalisation of TiO2 with —SO3H groups.
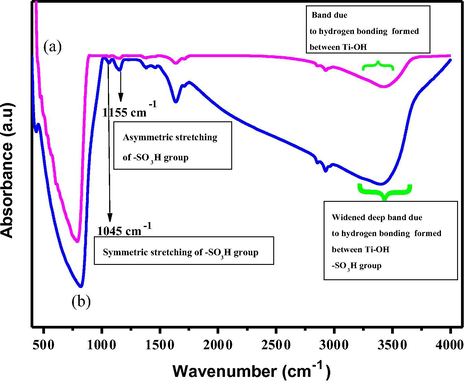
- FTIR spectra for (a) prisitne TiO2 (b) s-TiO2 nanoparticles.
To further establish the surface functionalisation of TiO2 particles, thermal studies of pristine and s-TiO2 are performed and results are as shown in Fig. 4 that presents markedly higher weight-loss in case of s-TiO2 which is attributed due to the strongly absorbed water molecules over sulphonic acid attached TiO2. The difference in the thermal behaviour clearly provide evidence for surface functionalisation of TiO2.
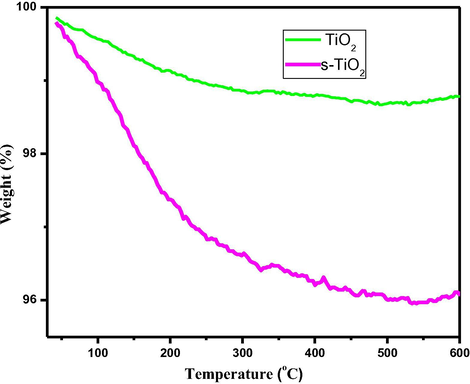
- TGA plots for (a) prisitne TiO2 (b) s-TiO2 nanoparticles.
In order to further confirm the surface attachment of –SO3H groups, XPS spectra associated with unmodified pristine TiO2 as well as s-TiO2 nanoparticles are recorded and presented as shown in Fig. 5 and Fig. 6. XPS spectra of s-TiO2 presents additional peaks at binding energy levels of 166.5 eV and 170 eV that are assigned to the oxidized sulfur groups, such as sulfones, sulfate or sulfonate and attributed due to S2s level. This observation confirmed the attachment of —SO3H groups to TiO2. It can be seen that Ti2p3/2 core level for TiO2 and s-TiO2 appear at 458.22 eV and 458.62 eV respectively with a noticeable shift of 0.4 eV. Variation of electronic states of Ti 2p core level in pristine TiO2 and s-TiO2 clearly manifested by the shift in the binding energy level. There arises a possible modification of TiO2 matrix due to —SO3H attachment probably due to surface induced strain that contributes to the difference in the binding energy level. These results are in agreement with earlier studies (Ayyaru et al., 2016); XPS results provide evidence for successful surface attachment of —SO3H group.
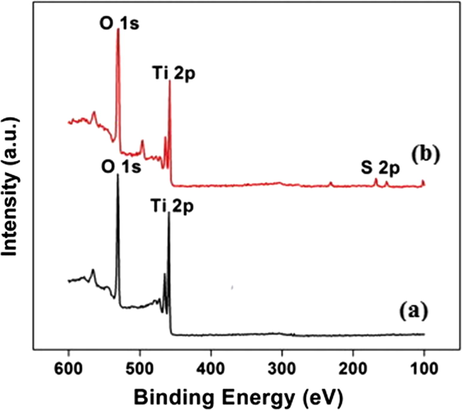
- XPS Survey spectra of (a) prisitne TiO2 (b) s-TiO2 nanoparticles.
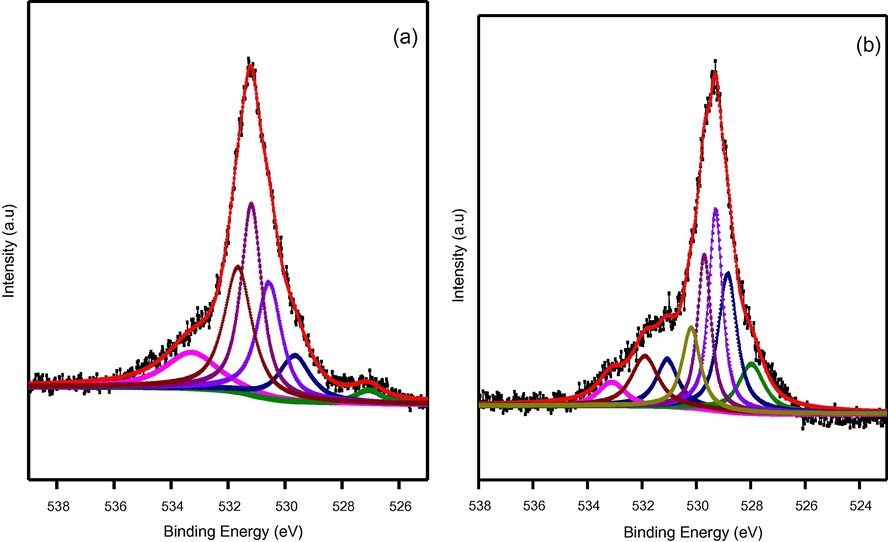
- XPS spectra of Ti 2p core level for (a) pristine TiO2 (b) s-TiO2 nanoparticles.
Fig. 7 consists of SEM image and related EDAX data of s-TiO2 nanoparticles. Clear spherical nanostructure with a grain size of approximately 30 nm is visible from SEM image. EDAX data with sulphur peaks prove that TiO2 nanoparticles are surface modified with —SO3H groups.
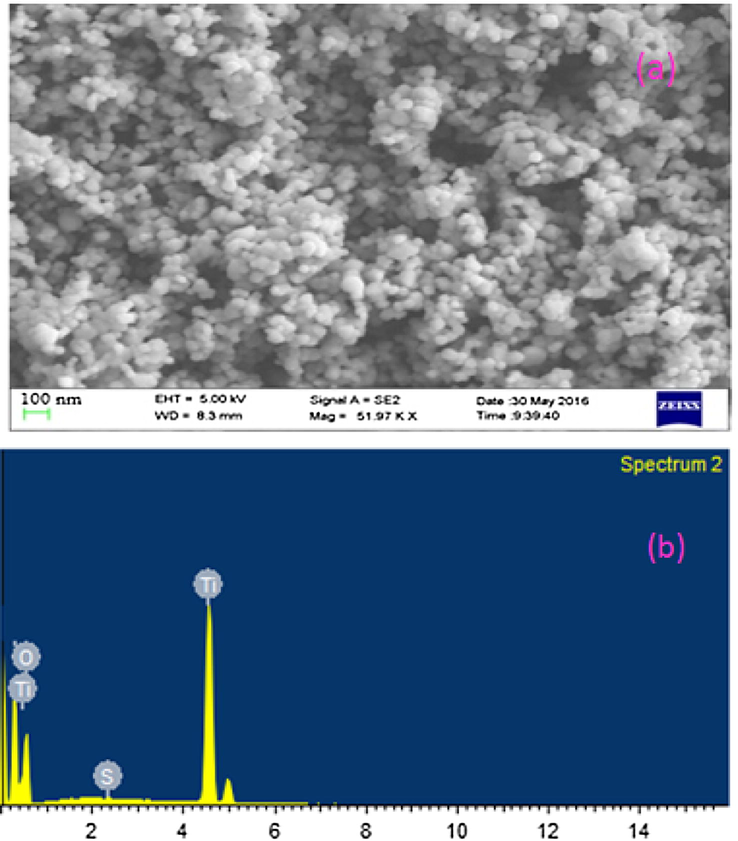
- (a) SEM morphology of s-TiO2 nanoparticles (b) corresponding EDAX data.
3.2.2 Characterization of polymer membranes
Fig. 8 represents FT-IR spectra of PC, PC-PVA blend and PC-PVA/s-TiO2 hybrid nanocomposite membranes with essential peaks being indicated by an arrow mark. Blending of PC with PVA and interaction of polymer blends with s-TiO2 are elucidated by evaluating respective FT-IR spectra. It is illustrious that both inter and intramolecular interaction between polar hydroxyl groups hydrogen bonding are expected to occur among PVA chains due to high hydrophilic forces. Spectrum of pure PC exhibits a broad band at 3800–2300 cm−1 related to stretching vibrations of O—H and C—H groups. Distinct band at 3200–3300 cm−1 is related to hydroxyl groups of pyranose rings (Gnanasambandam and Proctor, 2000). Vibration modes due to C—H and CH3 of PC backbone emerged at 2930–2940 cm−1. Band at 1370 cm−1 is attributed to symmetric methyl deformation mode of methyl ester present in galacturonic rings and rhimarose rings of PC backbone. In spectrum of PC, it is possible to detect characteristic peaks of free carboxyl group and the esterified group, typically they appear at 1650 and 1750 cm−1 respectively (Almohammed et al., 2017), but in PC-PVA spectra these peaks are shifted into a lower wavelength (1642 and 1705 cm−1) symptomatic of an interaction between both polymers.
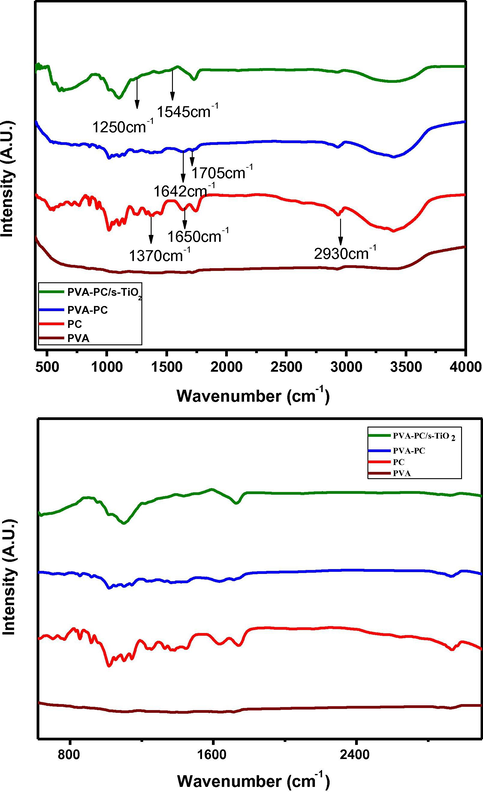
- FTIR spectra and expanded form for (a) PVA (b) PC-PVA and (c) PC-PVA/s-TiO2 hybrid nanocomposite membranes.
Spectra of PC-PVA blend presents combined characteristics of both PC and PVA with some of the bands get overlapped and a few bands being only just formed. The characteristic peak around ∼1032 cm−1 is due to the stretching vibration attributed to cross-linking of hydroxyl groups of PVA with aldehyde groups of GA. The absorption bands at 1250 cm−1 and 1545 cm−1 are assigned to —SO3 group of SSA which is added as a cross-linker. This is in agreement with the bands observed for similar kinds of membranes examined earlier (Mohanapriya et al., 2016). The absorption peaks of pure PVA at around 1022 cm−1 due to carboxyl stretching mode (C—O) gets shifted to 1044 cm−1 in the spectra of PC-PVA blend membranes. Further, intensity of band at 1430 cm−1 in PVA spectrum decreases and shifts to 1450 cm−1 in PC-PVA blend. Interaction of s-TiO2 nanoparticles with PC-PVA polymer blend is identified by the presence of characteristics peaks of s-TiO2 nanoparticles in PC-PVA/s-TiO2 nanocomposite. FTIR spectroscopy supplies additional information about intermolecular interactions between polymers and successful interaction of polymer blends with s-TiO2 nanoparticles.
3.2.3 XRD studies
Fig. 9 represents XRD patterns of PVA, PC-PVA blend and PC-PVA/s-TiO2 hybrid nanocomposites. Pristine PVA is associated with a large crystalline peak at 2θ∼20° corresponding to (1 0 1) plane and is known to be semi-crystalline in nature. Long-range order of PVA is disrupted after blending with PC, as a result PC-PVA blend membrane exhibits characteristic XRD peaks at 2θ ∼22° and peak width being broadened. Hence, in PC-PVA blend, polymer chain motion creates a dynamic disordered membrane framework that plays a critical role in facilitating proton transport (Baskaran et al., 2006). XRD studies elucidate that combination of PC results in flexible polymeric framework, which is appropriate for incorporation of inorganic fillers. Incorporation of s-TiO2 into PC-PVA blend leads to partial overlap of peaks indicating that compatibility exists between the constituents. XRD peaks for typical TiO2 particles occur at 25.2, 37.3, 37.8, 38.7, 48.2, 54, 55.1, 62.8, 68.9, 70.4, 75.1 and 83.1° corresponding to (1 0 1), (1 0 3), (0 0 4), (1 1 2), (2 0 0), (1 0 5), (2 1 1), (2 0 4), (1 1 6), (2 2 0), (2 1 5) and (2 2 4) planes in accordance with JCPDS card number 89-4921 for anatase crystallite phase of TiO2. Degree of relative crystallinity as calculated from ratio of peak are to total area under the peaks associated with PVA, PC-PVA blend and PC-PVA/s-TiO2 hybrid nanocomposite is respectively 35%, 26.5% and 14% . XRD peaks for PC-PVA/s-TiO2 hybrid nanocomposites show higher degree of amorphous state illustrating ameliorated ion-conducting nature of nanocomposites (Kim et al., 2004). These studies are corroborated with proton conductivity measurements.
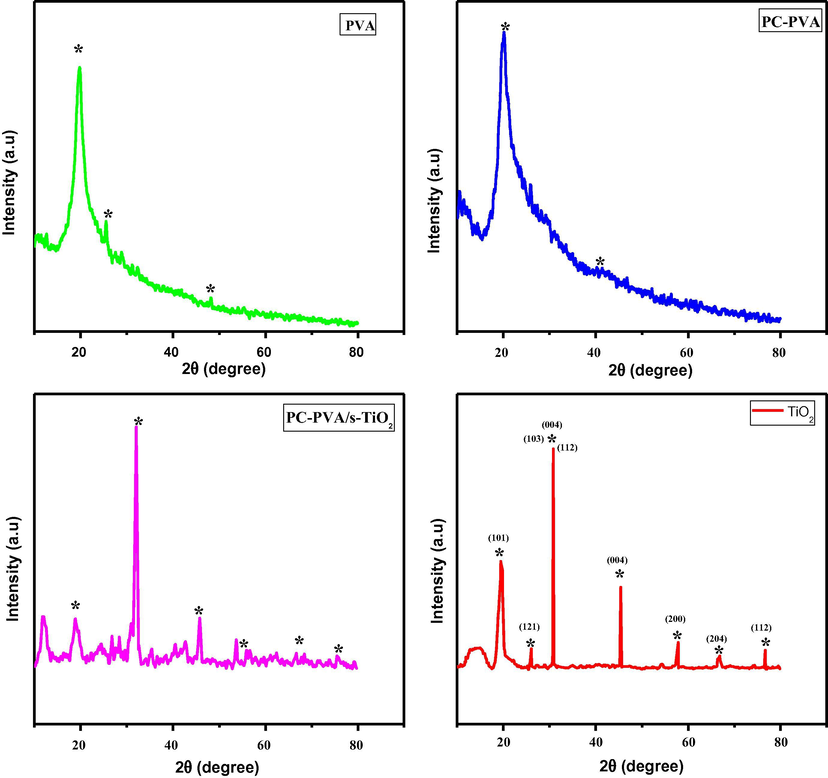
- XRD patterns for (i) PVA, (ii) PC-PVA blend and (iii) PC-PVA/s-TiO2 polymer nanocomposite membranes.
3.3 Sorption and IC studies on membranes
Sorption data associated with PC-PVA blend and PC-PVA/s-TiO2 hybrid nanocomposites measured at 70 °C with water and aqueous methanol is shown in Fig. 10. Carboxyl group of PC molecule ionically interact with functional groups of PVA creating large inter-connected structure which provide more space for water binding to the material itself, thereby increasing water uptake. Besides, carboxylic group itself is a hydrophilic functional group therefore contributes to water-binding capability of membrane. Also, it is noteworthy that degree of water uptake has been considerably increased after incorporating s-TiO2 nanoparticles, which demonstrates tendency of TiO2 to absorb water molecules. Also % water sorption rapidly increases on increasing s-TiO2 from 1 wt% to 5 wt% but tend to saturate when increased from 5 wt% to 10 wt%. By contrast, methanol sorption follows a reverse trend in behaviour when s-TiO2 is incorporated in the polymeric matrix revealing the fact that nanocomposite membranes exhibit a selective affinity towards water rather than methanol. Lower methanol sorption with increased s-TiO2 filler content could be explained on the basis of formation of closely packed network due to inter-locking created by s-TiO2. Hence, it can be inferred that PC-PVA based polymeric membranes are suitable for DMFC applications.
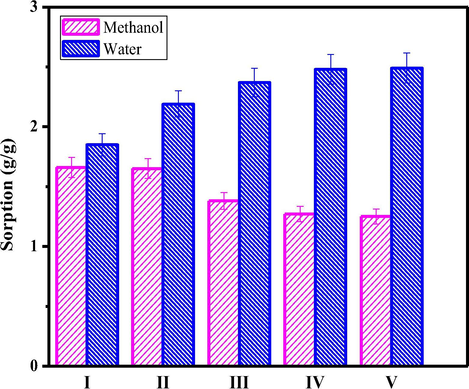
- Sorption properties of PC-PVA blend, PC-PVA/s-TiO2-I, PC-PVA/s-TiO2-II and PC-PVA/s-TiO2-III hybrid nanocomposite membranes measured with water and methanol.
IC is an important property of membranes which is determined by appropriate segregation of hydrophilic and hydrophobic nanodomains to form per location paths for proton transport. PC-PVA polymeric blend offer a composite structure comprising flexible polymeric network, which is appropriate for accommodation of brittle inorganic s-TiO2 nanoparticles. These s-TiO2 nanoparticles greatly contribute to IC through increasing number of charged ionic groups per unit area. Interestingly, IC shows a formal linear increase with an increase in s-TiO2 content in polymer blends hence it is assumed that ionic inorganic fillers trapped in separation between the hydrophobic and hydrophilic domains might provide additional pathways for proton transport. Concentration of ion-exchangeable groups has been increased upon addition of s-TiO2 into polymeric blends. It is to be noted that IC of PC-PVA/s-TiO2 hybrid nanocomposites are distinctly higher as shown in Table 1. PC-PVA/s-TiO2-II hybrid nanocomposite membranes with optimal content of s-TiO2 display higher IC compared to other membranes. However, excessive addition of s-TiO2 leads to decrement in IC, possibly due to the disruption of proton conducting paths that arises due to membrane inhomogenity. The results suggest that quantity and density of s-TiO2 nanoparticles on the membrane surface directly influence the efficiency of surface modification and ionic conductivity through reducing water permeation resistance.
3.4 SEM studies on membranes
Fig. 11 shows SEM images of PC-PVA/s-TiO2 hybrid nanocomposite membranes along with EDS data. s-TiO2 nanoparticles are well incorporated into the matrix and average size of it is ∼40 nm. Presence of s-TiO2 is confirmed using energy-dispersive X-ray analysis (EDS). s-TiO2 embedded within the matrix is beneficial to suppress methanol passage and facilitate ionic transport. Hydrogen bonding between sulfonic acid groups (—SO3H) of s-TiO2 and functional groups (amino and carboxylic groups) of polymers lead to favourable interaction along with homogenous dispersion. It is inferred from EDS that carbon and oxygen are main constituents of the membrane. Titanium and sulphur are present in low quantities and almost evenly distributed.
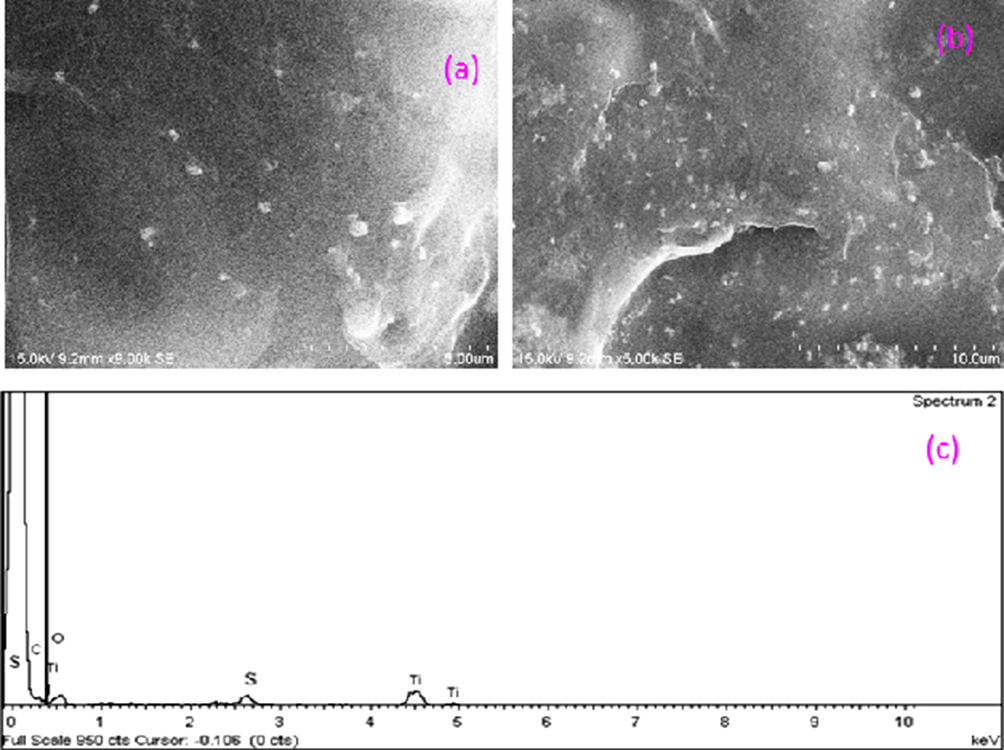
- (a,b) SEM images of PC-PVA/s-TiO2 hybrid nanocomposite membrane under different magnification and (c) corresponding EDAX data.
3.5 Thermo-mechanical stabilities
Fig. 12a represents TG profiles of PVA, PC-PVA blend and PC-PVA/s-TiO2 hybrid nanocomposite membranes. PVA show two-stage weight loss, by contrast all PC-PVA/s-TiO2 are accompanying with three-stage weight loss. Closer assessment reveals the fact that weight-loss associated with PC-PVA/s-TiO2 nanocomposites around 100 °C due to dehydration process are higher than that of PC-PVA blend illustrating the fact that PC-PVA/s-TiO2 hybrid nanocomposites are more hydrophilic compared to blend membranes. First stage weight loss from 30 to 200 °C is attributed to the removal of adsorbed water from the membranes and the quick weight-loss between 200 °C and 450 °C is due to the thermal degradation of polymeric blend membranes (Ke et al., 2011). This second-stage weight-loss region is also associated with thermal breakdown of PC-PVA/s-TiO2 matrix. Decomposition of the main polymer chains, acetal linkage created during cross-linking with GA, de-sulfonation of SSA as well as s-TiO2 nanoparticles together contributes to this observed weight loss. Interfacial interactions between functional groups of PVA and s-TiO2 nanoparticles require higher energy to breakdown the polymer framework thereby enhancing thermal stabilities of PC-PVA/s-TiO2 hybrid nanocomposites. The third stage weight-loss between 450 °C and 800 °C is due to decomposition of main polymeric chains namely PC, and PVA. PC-PVA/s-TiO2 hybrid nanocomposite membranes are characterized by lower weight-loss at second and third stage of thermal decomposition in relation to PC-PVA blends which is a clear manifestation of higher stability due to the embedded s-TiO2 nanoparticles (Kumar et al., 2017). However, it is clear from thermal studies that both PC-PVA/s-TiO2 hybrid nanocomposites are thermally stable under DMFC operating conditions because thermal degradation onset of these membranes begins after 200 °C.
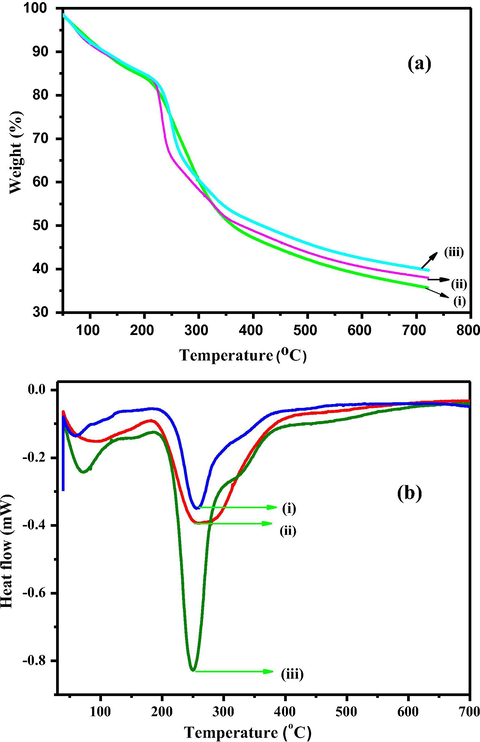
- (a) TGA plots for (i) PVA (ii) PC-PVA blend and (iii) PC-PVA/s-TiO2-III hybrid nanocomposite. (b) DSC plots for (i) PVA (ii) PC-PVA blend and (iii) PC-PVA/s-TiO2-III hybrid nanocomposite.
Fig. 12b represents DSC of PVA, PC-PVA blend and PC-PVA/s-TiO2-III hybrid nanocomposite membranes. Tg of a polymer corresponds to amorphous regions of polymer and is therefore useful to reflect changes that arise in these regions. Miscibility of blend polymers over entire composition is indicated by a single Tg value. It is evident from DSC plots that Tg of pure PVA is 246 °C which has been shifted to 242 °C for blend membranes. However, addition of s-TiO2 enhances thermal stability and PC-PVA/s-TiO2-III hybrid nanocomposite membrane possesses Tg value of 249.3 °C (Wu et al., 2017; Aminabhavi and Patil, 2010).
Mechanical properties of the membranes were also monitored using tensile strength and elongation at break. Tensile strength reflects mechanical resistance of membrane while elongation at break is related to capability of film to extend before breaking. Mechanical parameters of PC-PVA/s-TiO2 nanocomposite membranes are shown in Table 2. It is evident from the data that blending PC causes only marginal improvement in tensile strength of PC-PVA polymeric blend but its elongation properties are enhanced considerably. Generally, strong interaction between constituent polymers generate highly stable polymeric network. Blending PC constitutes additional hydrogen bonds in the membrane framework, which perform as reinforcing bridge elements. Hence, hydrogen bonding and electrostatic interactions existing between polymeric chains provide good mechanical stability to the membrane structure. Nanocomposite membranes comprising s-TiO2 are found to be more stable compared to the blend membrane.
| Membrane type | IEC (meq./g) | Tensile strength (MPa) | Elongation-at-break (%) | Arrhenius activation energy (kJ/mol) | Proton diffusion coefficient ×107 (cm2/s) |
|---|---|---|---|---|---|
| PC-PVA Blend | 0.48 | 20.5 ± 0.5 | 34 ± 0.07 | 29.7 | 0.2 |
| PC-PVA/s-TiO2-I | 0.59 | 31.8 ± 0.7 | 27 ± 0.05 | 26.5 | 1.8 |
| PC-PVA/s-TiO2-II | 0.73 | 32.9 ± 0.83 | 28 ± 0.08 | 21.9 | 3.3 |
| PC-PVA/s-TiO2-III | 0.68 | 34.6 ± 0.78 | 27 ± 0.06 | 22.32 | 2.7 |
Elongation of polymers has been facilitated upon blending with PC. PC-PVA polymer blend displayed better elongating properties than their respective constituents. It is attributed to greater chain movement which implies that material changes partially from hard and brittle glassy state to more flexible amorphous state. Apparently, elongation-at-break is fairly increased due to facile segmental mobility of individual polymer chains. Nevertheless, when s-TiO2 nanoparticle is incorporated, elongation properties of nanocomposite membranestend to decrease because it hinders the free movement of polymer chains. PC-PVA/s-TiO2-II hybrid nanocomposite membrane displays maximum tensile strength along with good elongation characteristics, which is essentially ascribed to organic and inorganic phases exhibit the strong interaction between two phases. Dependence of mechanical properties on s-TiO2 content is ascribed to molecular-level dispersion of s-TiO2 and strong hydrogen bonding between polymer blend and s-TiO2. The results are concurrent with rheological behavior of polymers. Generally, prepared membranes are strong and flexible enough for DMFC application.
3.6 Proton conductivity measurements
Fig. 13a shows the proton conductivities of PC-PVA based membranes measured at different temperatures from 30 °C to 100 °C, the conductivity of all the membranes increased with temperature. Apparently, proton conductivity of PC has been enhanced by blending with PVA polymer, incorporation of s-TiO2 into polymer blend further promotes proton conductivity. Degree of hydration of a PEM produces a profound effect on proton conductivity, being hydrophilic in nature, PC attracts more number of water molecules, and because of these water molecules which are confined within hydrophilic domain which enhanced proton mobility in the polymer framework takes place (Rambabu and Bhat, 2014). Combining PC with PVA generates a flexible polymer framework, resulting PC-PVA blend with stretchable polymeric chains offer more space for water molecules to reside and these water molecules facilitate ionic mobility. In other words, appropriate balance between hydrophobic and hydrophilic domains is established in PC-PVA polymer matrix, which allows a fast exchange between mobile ions and fixed charged sites through hydrated hydrophilic network. Besides, flexible nature of PC-PVA blend offer suitable matrix for incorporating rigid s-TiO2 nanoparticles, which helps to further enhance ionic conductivity while maintaining good mechanical properties. Proton conductivities of PC-PVA-s-TiO2 nanocomposite membranes are obviously higher compared to their blend membrane. s-TiO2 nanoparticle promotes proton conduction both through hopping and vehicular mechanism. They rapidly transmit protons through inter-linking hydrophilic domains. As well, hydrogen bonds formed between functional groups of polymers and ionic groups of s-TiO2 nanoparticles offer a path for proton transfer which is explained as a elementary feature of hydrogen bonds (Rambabu et al., 2016). It is noteworthy that -SO3H functional groups present in SSA (cross-linking agent) also involved in hydrogen bonding, thereby increasing the density of hydrogen bonding netwrok. Vehicular transfer of protons is also facilitated as bridged water complexes are formed in the nancomposites. Water molecules entrapped by dynamic functional groups through the hydrogen bonds transfer the hydrated protons along these bridges. The proton diffuses through the medium together with a vehicle (e.g., as H3O+) where the counter diffusion of unprotonated vehicles (H2O) allows the net transport of protons. Accordingly, both Grotthuss and vehicular mechanisms are being operated in the PC-PVA/s-TiO2 hybrid polymeric nanocomposites. Hence proton conductivity of membranes followed the sequence: PC < PC-PVA < PC-PVA/s-TiO2-I < PC-PVA/s-TiO2-II > PC-PVA/s-TiO2-III. When s-TiO2 is added in excess, proton conductivity decreases due to aggregation of additive in the polymer matrix. Hence proton conductivity of PC-PVA/s-TiO2-III is decreased in comparison to PC-PVA/s-TiO2-II.
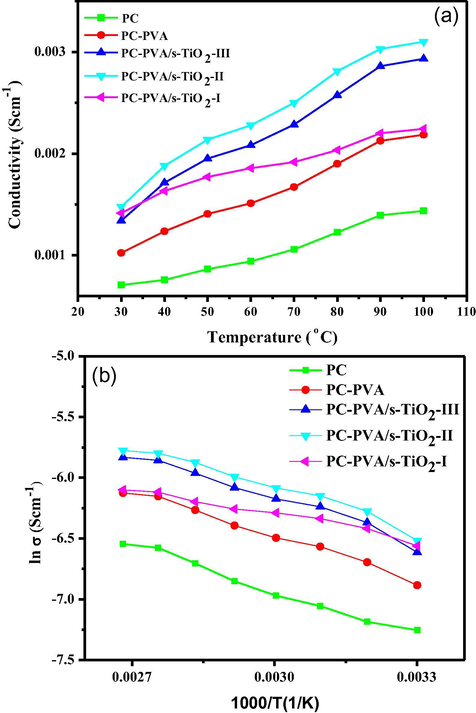
- (a) Variation of proton conductivity with temperature and (b) ln vs. 1000/T plot for PC,PC-PVA blend, PC-PVA/s-TiO2-I, and PC-PVA/s-TiO2-II and PC-PVA/s-TiO2-III hybrid nanocomposite membranes.
All the membranes indicate an Arrhenius-type temperature dependence of proton conductivity indicating thermally-activated process. The activation energy (Ea), which is the lowest energy, required for proton transport, is obtained from the slope of Arrhenius plots by plotting ln σ vs. 1/T according to Eq. (8) given below
In Eq. (6), σ is the proton conductivity, σo is the pre-exponential factor, Ea is the activation energy in (kJ/mol), R is the universal gas constant (8.314 J/mol K), and T is the absolute temperature (K). As represented in Fig. 10b, conductivity increases with increasing temperature pointing out that the proton conductivity is a thermally activated process. Ea values for all the membranes are given in Table 2. From the data it is obvious that Ea associated with proton conduction decreases after incorporation of s-TiO2 nanoparticles into polymeric blend.
3.7 Proton diffusion coefficient and oxidative stability of membranes
A Comprehensive transport mechanism in PEMs has not yet been advanced due to their complex nanostructure and inhomogeneous nature when hydrated. Mobility associated with total charge carrier concentration is the most significant parameter to characterise and estimate ionic transport through PEM. The mobility of the proton is abnormally high as compared with other ions of a size similar to hydronium ion, and is explained in terms of contribution by the so-called Grotthuss mechanism, or the “relay” mechanism, in which the transport of protons is determined by the rate at which the hydrogen bond between a hydronium ion and a water molecule forms rather than by the slower rate at which hydronium ions may migrate. Based on assumption that all ionic capacity into PEM is involved in the distribution of ions that can move freely through the membrane, proton diffusivity can be expressed according to Einstein relation as:
PEMs should have a good oxidative stability in the energy storage and production in space requires rugged operating environment of fuel cells. During a typical fuel cell operating cycle, degradation of membrane is likely due to HO and HO2 radicals formed by diffusion of oxygen molecules through the membrane and incomplete reduction of it. Fenton’s reagent was adopted to pretend the practical operation environment of fuel cells as indicate earlier (Salleh et al., 2017) and to evaluate oxidative durability time dependant weight changes associated with membranes is evaluated. Fig. 14 illustrates weight losses of the membranes as a function of time. A primary sharp decrease in weight percentage was observed for initial period. Weight of membranes tends to maintain as constant after certain time except for PC membrane. Stability of membranes is in the order of PC < PC-PVA < PC-PVA/s-TiO2-I < PC-PVA/s-TiO2-II ∼ PC-PVA/s-TiO2-III. Results suggest that PC-PVA/s-TiO2 nanocomposite membranes show promising oxidation stability for application in DMFC.
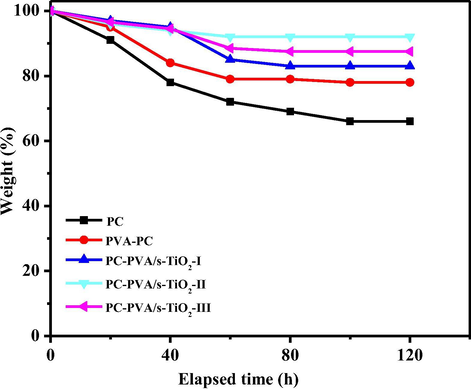
- Time course of weight loss of associated with membranes in Fenton’s reagent at 70 °C.
3.8 Methanol permeability and electrochemical selectivity
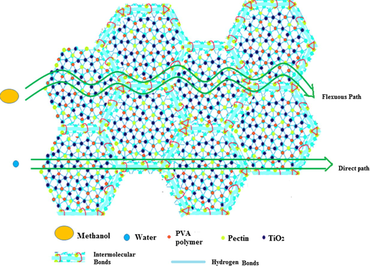
Fig. 15a shows methanol permeability data for PC-PVA based membranes. Methanol permeability values through different PC-PVA/s-TiO2 nanocomposite membranes have rapidly decreased by nearly 40% due to presence of s-TiO2. Interactions between permeate and membrane material determine selectivity and transport rate, in this respect, changes in the methanol permeability of the membranes could be attributed to many factors, including the changes in the water characteristics and cross-sectional area of ion-conducting channels (Chen et al., 2008). Hence, due to the transformation in the microstates, methanol conducting path has been turned into a tortuous one instead of direct pathway. As a result, methanol permeability was successfully decreased through the membrane. It is important to note that methyl ester groups belong to PC could exert a hydrophobic repulsion towards methanol molecules therefore helpful for selective sorption of water from aqueous methanol. In addition, dispersed s-TiO2 nanoparticles create closely packed network with lower volume of free-void space, which effectively block passage of methanol molecules (Sairam et al. (2006)). Introducing s-TiO2 nanoparticles improve affinity towards hydrophilic micropores of inorganic components by enhancing preferential water adsorption through hindering methanol diffusion through inorganic component micropores. Schematic illustration preferential of transfer of protons over methanol molecules is shown in Scheme 2.
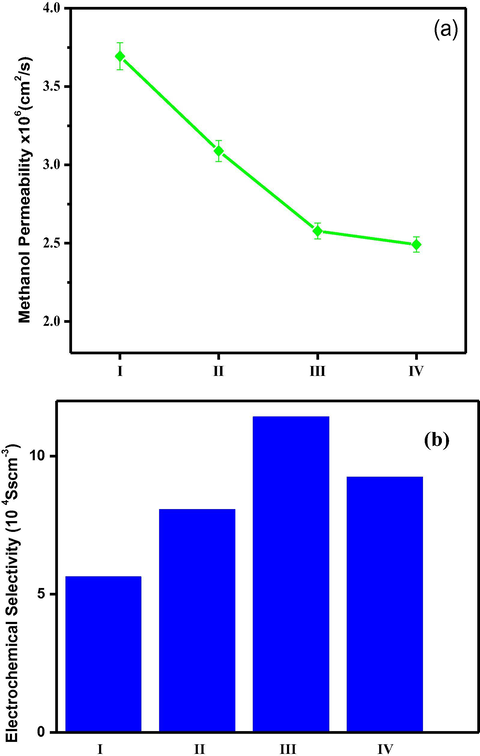
- (a) Methanol permeability associated with PC-PVA blend, PC-PVA/s-TiO2-I, PC-PVA/s-TiO2-II, PC-PVA/s-TiO2-III hybrid nanocomposite membranes measured at 70 °C. (b) Electrochemical selectivity values of PC-PVA blend, PC-PVA/s-TiO2-I, PC-PVA/s-TiO2-II, PC-PVA/s-TiO2-III hybrid nanocomposite membranes.
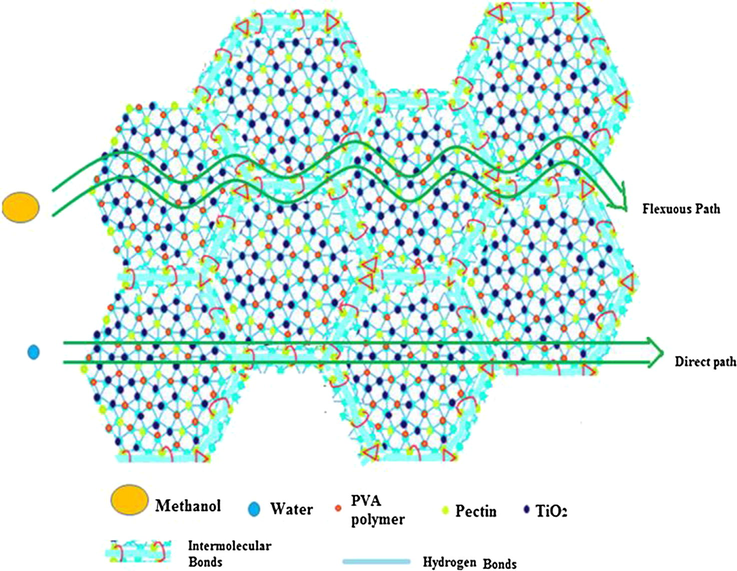
- Schematics illustrating the facile proton transport and restricted methanol passage through PC-PVA/s-TiO2 hybrid nanocomposite membranes.
Electrochemical selectivity of membranes for protons over methanol can be demarcated as the proton conductivity (σ) divided by methanol permeability (P), which is often used to evaluate the membrane-electrolyte performance in DMFC. From Fig. 15b it is apparent that due to the increase in proton conductivity and decrement in methanol cross-over flux, selectivity values of PC-PVA/s-TiO2-II hybrid nanocomposite membrane is higher. Based on these results DMFC performance of PC-PVA/sTiO2-II hybrid nanocomposite membranes is evaluated.
3.9 DMFC performance
Fig. 16 represents DMFC polarization characteristics for MEA comprising PC-PVA/sTiO2-II hybrid nanocomposite membranes at 70 °C under atmospheric pressure. The peak power-density for PVA blend and PVAs-TiO2 hybrid nanocomposite is ∼27 mW/cm2 at a load current-density of 100 mA/cm2. The observations imply that it is possible to derive new proton conducting membranes from natural sources, which are low-cost, readily available and environmentally-benign. Regardless of low power density associated with the material, the present attempt opens up utility of new biopolymer based membrane that holds good recycling potential unlike commercial membranes.

- Cell voltage and power density vs. current density for
- PC-PVA/s-TiO2-II hybrid nanocomposite membrane.
4 Conclusions
This study opens up new captivating prospects in the design of proton conducting electrolyte using recyclable, biodegradable eco-friendly precursors that are extensively beneficial for waste management as well as impact on the environment could be considerably low. PC based cost-effective natural polymeric blends tailored with s-TiO2 nanoparticles to realize a composite membrane for application in DMFCs has been explored. Combining pectin with PVA generates flexible blend polymeric network, which is appropriate for incorporation of inorganic fillers. Effect of blending pectin with polymers has been systematically followed by rheological measurements. Both pectin and Pectin-PVA blend solutions exhibit a typical shear-thinning behaviour with the exponent parameter (n) value less than 1. Pectin transforms morphological properties of PVA, results in construction of a slackly-packed network with higher affinity for water which greatly reduces methanol permeation. Fine dispersion of s-TiO2 increases the compactness of ion-conducting groups resulting in nanocomposites ultimately appropriate for DMFC application. Together with improved thermo-mechanical stabilities, low-cost eco-friendly components, ready availability and convenient method of preparation are essential highlights of these membranes that may be regarded as bio-based materials for DMFC applications.
Acknowledgements
One of the authors S. Mohanapriya is grateful to University Grants Commission (UGC), Government of India, for providing fund under the scheme of ‘UGC-Dr. D. S. Kothari Post Doctoral Fellowship’. (Ref: No. AwardLetter-No.F.4-2/2006 (BSR)/CH/14-15/0102 dated 5-5-2015). We thank Dr. V.V. Giridhar, Scientist-in-Charge, CECRI Madras Unit, and Dr. Vijaymohanan K. Pillai, Director, CECRI, Karaikudi for their constant encouragement and support.
References
- A renewable biopolymer cathode with multivalent metal ions for enhanced charge storage. J. Mater. Chem. A. 2014;2:1974-1979.
- [Google Scholar]
- Pectin recovery from sugar beet pulp enhanced by high voltage electrical discharges. Food Bioprod. Process... 2017;103:95-103.
- [Google Scholar]
- Nanocomposite membranes of poly(vinyl alcohol) loaded with polyaniline-coated TiO2 and TiO2 nanoparticles for pervaporation dehydration of aqueous mixtures of 1,4 –dioxane and tetrahydrofuran. Design. Monomers Polym.. 2010;13:497-508.
- [Google Scholar]
- Investigation of grafted ETFE-based polymer membranes as alternative electrolyte for direct methanol fuel cell. J Power Sources. 2003;123:107-115.
- [Google Scholar]
- Waste cleaning waste: photodegradation of monochlorophenols in the presence of waste-derived photosensitizer. ACS Sustain. Chem. Eng.. 2013;1:1545-1550.
- [Google Scholar]
- Ayyaru, S., Dharmalingam, S., Ahn, Y.H., 2016. Nanocomposite membranes based on sulfonated polystyrene ethylene butylene polystyrene (SSEBS) and sulfonated SiO2 for Microbial fuel cell application, Chem. Eng. J. 289, 442–451.
- Enhanced proton conduction of chitosan membrane enabled by halloy site nanotubes bearing sulfonate polyelectrolyte brushes. J. Membr. Sci.. 2014;454:220-232.
- [Google Scholar]
- Aqueous dye-sensitized solar cells: challenges in electrodes and electrolytes design. Green Chem.. 2017;19:1043-1051.
- [Google Scholar]
- Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ionics. 2006;177:2679-2682.
- [Google Scholar]
- Effect of different green cellulosic matrices on the performance of polymeric dye-sensitized solar cells. Chem. Eng. Trans.. 2014;41:211-216.
- [Google Scholar]
- Tailoring porosity in carbon materials for super capacitor applications. Mater. Horizons. 2014;1:157-168.
- [Google Scholar]
- Buraidah, M.H., Teo, L.P., Majid, S.R., Arof, A.K., 2009. Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Phys. B Condens. Matter. 404, 1373–1379.
- Influence of Pectin as a green polymer electrolyte on the transport properties of Chitosan-Pectin membranes. Carbohy. Polym.. 2017;157:1759-1768.
- [Google Scholar]
- Sodium-alginate-based proton-exchange membranes as electrolytes for DMFCs. J. Power Sources. 2008;184:44-51.
- [Google Scholar]
- Polymer electrolyte membranes for the direct methanol fuel cell. J. Polym. Sci.. 2006;44:2201-2225.
- [Google Scholar]
- Non-Newtonian power law behaviour of TiO2/SAE 50 nanolubricant; an experimental report and new correlation. J. Molecul. Liq.. 2017;232:219-225.
- [Google Scholar]
- Edible films from pectin: physical-mechanical and antimicrobial properties–a review. Food Hydrocolloids. 2014;34:287-296.
- [Google Scholar]
- Eco-friendly extraction of pectin and essential oils from orange and lemon peels. ACS Sustainable Chem Eng.. 2014;4:2243-2251.
- [Google Scholar]
- Determination of pectin degree of esterification by diffuse reflectance Fourier transform Infrared Spectroscopy. Food Chem.. 2000;68:327-332.
- [Google Scholar]
- Dielectric behaviour of cellulose acetate-based polymer electrolytes. Ionics. 2012;18:599-606.
- [Google Scholar]
- Improving the overall characteristics of proton exchange membranes via nanophase separation technologies: a progress review. Fuel cell. 2017;17:3-17.
- [Google Scholar]
- Improved performance of microbial fuel cells using sulfonated polyether ether ketone (SPEEK) TiO2–SO3H nanocomposite membrane. Int. J. Hydrogen energy.. 2011;36:3606-3613.
- [Google Scholar]
- Improved performance of microbial fuel cells using sulfonated polyether ether ketone (SPEEK) TiO2-SO3H nanocomposite membrane. Int. J. Hydrogen Energy.. 2011;36:3606-3613.
- [Google Scholar]
- A review of polymer-nanocomposite electrolyte membranes for fuel cell application. J Ind Eng Chem. 2014;21:36-52.
- [Google Scholar]
- Direct polymerization of novel functional sulfonated poly(arylene ether ketone sulfone)sulfonated poly(vinyl alcohol) with high selectivity for fuel cells. J. Membr. Sci.. 2004;240:37-48.
- [Google Scholar]
- A study on sulfonated TiO2-Poly (Vinylidene fluoride-co-hexafluropropylene) nanocomposite membranes for PEM fuel cell application. Appl. Surf. Sci.. 2017;418:64-71.
- [Google Scholar]
- Development and characterization of sulfonated polysulfone for direct methanol fuel cell. Desalination. 2006;199:283-285.
- [Google Scholar]
- Polymer electrolytes based on sulfonated polysulfone for direct methanol fuel cell. J Power Sources. 2008;243:34-41.
- [Google Scholar]
- Performance analysis of polymer electrolyte membranes for direct methanol fuel cell. J Power Sources. 2013;243:519-534.
- [Google Scholar]
- The effects of different plasticizers on the properties of thermoplastic starch as solid polymer electrolytes. Macromol. Mater. Eng.. 2007;292:503-510.
- [Google Scholar]
- Preparation and characterization of amidated Pectin based polymer electrolyte membranes, Chinese. J. Polym. Sci.. 2009;27:639-646.
- [Google Scholar]
- Development of novel pectin based membranes as proton conducting material. Int. J. Plastics Tech.. 2012;16:80-88.
- [Google Scholar]
- Bio-functionalized hybrid nanocomposite membranes for direct methanol fuel cells. J. Bionanosci.. 2009;3:131-138.
- [Google Scholar]
- Sodium-alginate-based proton-exchange membranes as electrolytes for DMFCs. Energy Environ Sci.. 2010;3:1746-1756.
- [Google Scholar]
- Bio-composite membrane-electrolytes for direct methanol fuel cells. J. Electrochem. Soc.. 2011;158:A1-A10.
- [Google Scholar]
- Bio-functionalised hybrid nanocomposite membranes for direct methanol fuel cells. RSC Adv.. 2016;6:57709-57721.
- [Google Scholar]
- A Comprehensive review on recent development of passive direct methanol fuel cell. Ionics. 2017;23:1-18.
- [Google Scholar]
- Separation of isopropyl alcohol-toluene mixtures by pervaporation using poly(vinyl alcohol) membrane. Arab. J. Chem.. 2017;10:S56-S61.
- [Google Scholar]
- Polymer electrolyte membranes for direct methanol fuel Cells. J. Power Sources. 2007;169:221-238.
- [Google Scholar]
- pH- responsive hydrogel membranes based on modified chitosan: water transport and kinetics of swelling. J. Power Sources. 2010;195:4915-4922.
- [Google Scholar]
- Evaluation of the effect of reprocessing on the structure and properties of low density polyethylene/thermoplastic starch blends. Carbohydr. Polym.. 2016;136:210-215.
- [Google Scholar]
- Perry, R.H., Green, D.W., Maloney, J.D., 1997. Perry’s Chemical Engineers Hand Book, seventh ed., McGraw-Hill.
- Simultaneous tuning of methanol crossover and ionic conductivity of sPEEK membrane electrolyte by incorporation of PSSA functionalized MWCNTs: a comparative study in DMFCs. Chem. Eng. Journal.. 2014;243:517-525.
- [Google Scholar]
- Functionalized fullerene embedded in Nafion matrix: a modified composite membrane electrolyte for direct methanol fuel cells. Chem. Eng. J.. 2016;306:43-52.
- [Google Scholar]
- A high-performance carbon for supercapacitors obtained by carbonisation of a seaweed biopolymer. Adv. Mater.. 2006;18:1877-1882.
- [Google Scholar]
- Novel dense poly (vinyl alcohol)–TiO2 mixed matrix membranes for evaporation, separation of water–isopropanol mixtures at 30 degree Celsius. J. Membr. Sci.. 2006;281:95-102.
- [Google Scholar]
- Stability of SPEEK/Cloisite®/TAP nanocomposite membrane under Fenton reagent condition for direct methanol fuel cell application. Polym. Degradat. Stab.. 2017;137:83-99.
- [Google Scholar]
- Natural polymer electrolyte system based on sago: structural and transport behaviour characteristics. Int. J. Polym. Anal. Chem.. 2012;17:600-607.
- [Google Scholar]
- Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim. Acta. 2014;129:1-13.
- [Google Scholar]
- Effect of incorporation of sulfonated chitosan/sulfonated graphene oxide on proton conductivity of chitosan membranes. J Power Sources. 2016;306:541-551.
- [Google Scholar]
- Polyelectrolyte complexes of chitosan and poly(acrylic acid) as proton exchange membranes for fuel cells. Macromolecules. 2004;37:2233-2239.
- [Google Scholar]
- Synthesis and characterization of polyelectrolyte complex N-succinyl chitosan-chitosan for proton exchange membranes. Eur. Polym. J.. 2005;41:1859-1866.
- [Google Scholar]
- Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells-A mini review. Arab. J. Chem.. 2019;12(8):2236-2243.
- [CrossRef] [Google Scholar]
- A N-o-sulphonic acid benzyl chitosan (NSBC) and N, N-dimethylene phosphonic acid propyl silane graphene oxide (NMPSGO) based multi-functional polymer electrolyte membrane with enhanced water retention and conductivity. J. Phys. Chem B.. 2008;112:15678-15690.
- [Google Scholar]
- A novel cross-linking strategy for preparing poly(vinyl alcohol)–based proton-conducting membranes with high sulfonation. J. Power. Sources.. 2010;195:2166-2173.
- [Google Scholar]
- Eco-friendly methane sulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell. J. Membr. Sci.. 2017;523:45-59.
- [Google Scholar]
- TiO2 nanoparticles with increased surface hydroxyl groups and their improved photocatalytic activity. Cataly. Commun.. 2012;19:96-99.
- [Google Scholar]
- Water-retention effect of composite membranes with different types of nanometer silicon dioxide. Electrochem. Solid-State Lett.. 2008;11:B201-B204.
- [Google Scholar]
- Doping polysulfone ultrafiltration membrane with TiO2-PDA nanohybrid for simultaneous self-cleaning and self-protection. J. Membr. Sci.. 2017;532:20-29.
- [Google Scholar]
- Novel methanol-blocking proton exchange membrane achieved via self-anchoring phosphotungstic acid into chitosan membrane with submicro-pores. J. Membr. Sci.. 2016;500:203-210.
- [Google Scholar]
- The production of sulfonated chitosan-sodium alginate found in brown algae (Sargassum sp.) composite membrane as proton exchange membrane fuel Cell (PEMFC) J. Membr. Sci.. 2009;337:318-323.
- [Google Scholar]
- Novel hierarchically porous carbon materials obtained from natural biopolymer as host matrixes for lithium−sulfur battery applications. ACS Appl. Mater. Interfaces. 2014;6:13174-13182.
- [Google Scholar]
- A simple route toward next-gen green energy storage concept by nanofibres-based self -supporting electrodes and a solid polymeric design. Carbon. 2016;107:811-822.
- [Google Scholar]







