Translate this page into:
Pectin mediated green synthesis of Fe3O4/Pectin nanoparticles under ultrasound condition as an anti-human colorectal carcinoma bionanocomposite
⁎Corresponding author. Tel.: +91-7686034517. bkarmakar@ghcollege.ac.in (Bikash Karmakar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This work described the one-pot synthesis of apple pectin encapsulated Fe3O4 nanoparticles (Fe3O4/Pectin NPs) which is prepared by co-precipitation of Fe(II/(III) ions in alkaline solution mediated by pectin under ultrasound condition. This process led to formation of magnetic nanoparticles within the network of pectin. Physicochemical characterization of the as-synthesized Fe3O4/Pectin NPs was carried out through electron microscopy (SEM and TEM), energy dispersive X-ray spectroscopy (EDX), vibrating sample magnetometer (VSM) and X-ray diffraction (XRD). The in vitro cytotoxic and anti-colorectal cancer effects of biologically synthesized Fe3O4/Pectin NPs against Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29 cancer cell lines were assessed. The anti-colorectal cancer properties of the Fe3O4/Pectin NPs could significantly remove Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29 cancer cell lines in a time and concentration-dependent manner by MTT assay. The IC50 of the Fe3O4/Pectin NPs were 317, 337, 187, and 300 µg/mL against Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29 cancer cell lines. The antioxidant activity of Fe3O4/Pectin NPs was determined by DPPH method. The Fe3O4/Pectin NPs showed the high antioxidant activity according to the IC50 value. It seems that the anti-human colorectal cancer effect of recent nanoparticles is due to their antioxidant effects.
Keywords
Pectin
Magnetic
Nanocomposite
Cytotoxicity
Antioxidant
Anti-colorectal cancer
1 Introduction
The reasons for the clinical trials' failed to achieve the desired multilayer results are complex and intertwined. Unfortunately, therapeutic agents (chemotherapy, biology, and nanotechnology) are so selective and effective in targeting in vitro cancer cells, and even in proportionate animal specimens (Stewart B., Wild C.P. World Cancer Report, 2014; Rasmussen et al., 2010; Felice et al., 2014; Fernandes et al., 2015). However, failing in clinical trials is not a rule but a rule. This is because the biological distribution of therapeutic agents can be a fundamental factor in these fractures. Inadequate concentration at unwanted concentration and target sites elsewhere, leading to dose-limiting poisoning (Rasmussen et al., 2010; Felice et al., 2014). The biological distribution of drug agents is controlled largely by the drugs ability to penetrate biological barriers. Strategy for Adding Targeting Sections to Therapeutic Nanoparticles to Improve Location Specification to date, despite 30 years of effort in pharmaceutical companies and many laboratories, it has not yet been able to produce clinically approved drugs (Felice et al., 2014; Fernandes et al., 2015). This failure is because the addition of molecular agents increases the targeting of cognitive characteristics. However, it does so in the face of much greater difficulty in managing biological barriers (Caputo et al., 2014; Veisi et al., 2020; Doustkhah et al., 2018; Maier-Hauff et al., 2011; Kolosnjaj-Tabi et al., 2014).
Nanotechnology is defined in different ways in several countries, which affects the nanodrugs clinical validation. However, what these different definitions have in common is the use of nanoscale structures (Caputo et al., 2014; Veisi et al., 2020; Doustkhah et al., 2018). There are several distinct benefits to using nanotechnology in the diseases treatment. Nanoparticles, especially metal nanoparticles and metal oxides, have been widely used by medical consumers and manufacturers. The mechanism of nanoparticle-induced toxicity against cancer cells is the production of reactive oxygen species (ROS) (Maier-Hauff et al., 2011; Kolosnjaj-Tabi et al., 2014; Bhattacharyya et al., 2011; Hilger and Kaiser, 2012). Excessive production of reactive oxygen species can lead to oxidative stress, disruption of normal physiological maintenance, and oxidation regulation. These effects in turn lead to DNA damage, unregulated cell signaling pathways, changes in cell evolution, cytotoxicity, apoptotic death, and the onset of cell death (Orel et al., 2015; Van Landeghem et al., 2009; Silva et al., 2011; Johannsen et al., 2010). Critical-deterministic factors can affect the production of reactive oxygen species. These critical-deterministic factors include shape, size, nanoparticle surface area, particle surface baroelectricity, surface-forming groups, Particle solubility, metal ion emission from nanomaterials and nanoparticles, optical activation, model of cell reactions, inflammatory effects and ambient pH (Johannsen et al., 2010; Bañobre-López et al., 2013; Klein et al., 2014; Chatterjee et al., 2008; Zhang and Sun, 2004). Metal nanoparticles and oxides of metal nanoparticles due to their optical properties due to the large active area and high atomic number, amplify the photoelectric and Compton effects of both X-ray and gamma-ray interactions with the adsorbent in the diagnostic and therapeutic range (Thevenot et al., 2008; Colon et al., 2010; Wason et al., 2013; Tarnuzzer et al., 2005). Finally, they can lead to the development of methods for the destruction of tumor cells and reduce their survival with minimal side effects in radiation therapy. As a result, increasing industrial knowledge in the field of scalable nanoparticle synthesis, along with the design of multifunctional nanoparticles, will dramatically change the strategies of microenvironmental preparation and therapeutic-diagnostic nanoparticles for cancer treatment (Ali et al., 2014; Neri and Supuran, 2011; Seo et al., 2007; Hou et al., 2015; Cui et al., 2013; Lucky et al., 2015; Idris et al., 2014).
Pectin is a natural biopolymer, which is usually obtained from fruit. Therefore, this polymer, polygalacturonic acid, is a polysaccharide that contains carboxylate groups in each monomer. This fact can cause pectin to have some additional advantages rather to other analogues. Carboxylate groups can act as a covalently chelating agent to Fe3O4 nanoparticles and surrounds it as shell. A general and schematic pathway for one-pot preparation of Fe3O4/Pectin NPs that presented in Scheme 1. In the current research, the properties of Fe3O4/Pectin NPs against common colorectal cancer cell lines i.e. Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29 were evaluated.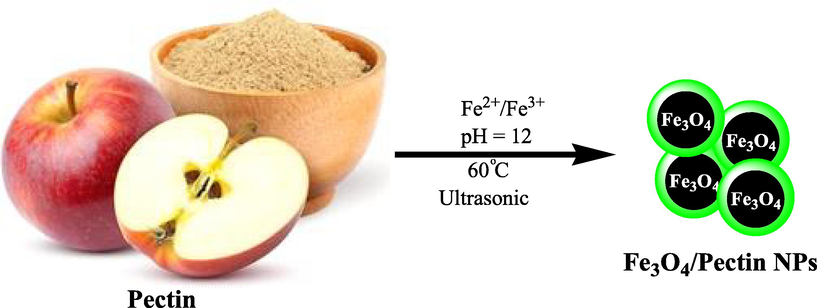
Schematic one-pot preparation of Fe3O4/Pectin NPs under ultrasound condition.
2 Experimental
2.1 Preparation of orange pectin coated Fe3O4 nanoparticles (Fe3O4/Pectin NPs)
In a typical procedure, 0.2 g of apple pectin was dissolved in deionized water (50 ml) in a 100-mL three-necked flask equipped with a stirrer and dropping funnel. Then, FeCl3·6H2O (0.84 g) and FeCl2·4H2O (0.34 g) were slowly added into the mixture. The mixture was sonicated for 15 min at 60 °C, and then 1 mol/L NaOH aqueous solution was added under ultrasonic conditionby dropping into the mixture until the pH increased to 12. The products from this system were magnetized for 30 min by a magnet. The precipitates were cooled, washed and diluted to neutrality by deionized water. The powder of Fe3O4/Pectin NPs was obtained by air drying.
2.2 DPPH assay protocol
DPPH is a free radical that changes color in the presence of substances with antioxidant properties and captures electrons. Yellow to purple color change is the basis of antioxidant properties. Solutions with different concentrations (10 to 1000 μg/ml) were prepared from phenolic powder and BHT synthetic antioxidant in methanol solvent. 1 ml of DPPH methanol solution was added to 1 ml of concentrate to 3 ml of nanoparticles and the resulting mixture was stirred vigorously. The test tubes were placed in a dark place for 30 min. After this period, the absorbance at the wavelength of 517 nm was read. It should be noted that in the control sample, the nanoparticles was replaced with 3 ml of methanol. Finally, the DPPH radical's inhibition percentage was calculated with this formula (Chen et al., 2006; Das et al., 2007; Korsvik et al., 2007; Hong et al., 2006; Zangeneh et al., 2019):
2.3 MTT assay protocol
Cell culture is the process of culturing eukaryotes in a culture medium where the culture conditions are different for each cell type and these conditions must be provided for its better growth; But in general, artificial environments should contain nutrients such as vitamins, minerals, sugars, amino acids, hormones and gases. In this environment, physical and chemical conditions must also be adjusted for the cells to grow better. Due to the differences in cell types, some of these cells need a surface to attach to grow, and others can grow floating in the culture medium. In the laboratory, there is a specific cell culture medium for specific cells. The culture medium suitable for hepatocytes is different from the culture medium for neurons. For the better proliferation of cells in the culture medium, the cell density in the culture medium should not be high. The cells should be transferred to a new medium (passage) every few days. If the density and volume of the cells increase, the proliferation decreases due to lack of contact and the cells differentiate. Evaluation of cells in terms of growth, nutritional requirements and growth arrest, changes in cell morphology under a microscope are the goals of cell culture. To study the cell growth cycle, control the growth of cancer cells and study the expression of genes, cell culture must be performed outside the body to better study the type of growth and growth rate of cells. The study of animal evolution is one of the applications of cell culture. How can a fertilized cell become a percellular organism, and how does each cell differ in morphology under a microscope? To answer this question, it is better to culture the fertilized a cell in the laboratory and evaluate the stages of cell culture based on evolution. In cell culture medium, cells are prepared that are in the process of differentiation and differentiate into new cells with hormones and growth factors. By cell culture, identical cells are produced and intracellular functions such as DNA replication, DNA transcription, RNA and protein synthesis, and cell metabolism are examined. After the molecule binds to its membrane receptor, intracellular reactions such as complexes, intracellular messages, and message transmission are evaluated. The cultured cells can be stored at a very low temperature. The low temperature maintained the growth rate or genetic composition of the cells. Cells can be used when needed, for example, a month later. This method prevents cell aging. In studies with animals such as rats and rabbits, animal homeostasis and experimental stress should be considered. However, this is not the case in cell culture. Standardizing laboratory tests is easier than studying animals; in the laboratory, it is easier to control physicochemical factors, cell environments such as acidity, heat, osmotic pressure, and oxygen and carbon dioxide pressures (Jalalvand et al., 2019).
In this study, the anticancer effects of Fe3O4/Pectin NPs samples against the colorectal cancer cells (Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29) were investigated.
These cells in DMEM culture medium (Gibco, USA) with 10% FBS (Gibco, USA) and penicillin/streptomycin (100 μl/100 μg/ml) in an incubator containing 5 % Carbon dioxide with 90% humidity was stored at 37 °C. Then, when about 80% of the flask was filled, cell passage was performed and about 5 × 104 cells (per square centimeter) were placed in 24 house bacterial petri dishes in the usual environment. The cells were treated with different concentrations of nanoparticles 24 h later and kept in this condition for 3 days. The survival rate of cultured cells was prepared with different concentrations. In this experiment, cells were cultured at 3 × 104 cells/well in 24-well plates and kept in an incubator at 37 °C for 24 h. Then the old culture medium was taken out of the wells and the cells were treated with different concentrations of nanoxidro. This test was performed on the first, second and third days after exposing the cells to the compounds; thus, at the appropriate time after culturing the cells in plates of 24 cells, the culture medium was removed and about 300 μl of fresh medium containing 30 μl of MTT solution was added to each cell. After 3–4 h of incubation at 37 °C, MTT solution is removed and 200 μl (Dimethyl Sulfoxide, Merck, USA, 100%) DMSO is added to each house. Then the sample absorption was read at 570 wavelengths using ELISA rider (Expert 96, Asys Hitch, Ec Austria). This experiment was repeated 3 times and each time, four wells were considered for each nano oxide concentration. Cell survival percentage was evaluated by the following formula (Jalalvand et al., 2019):
The results were evaluated as Mean ± SE using SPSS software version 12 and statistical tests of variance of completely randomized block design. Drawing graphs in Excel software was performed and the significance level of the differences was considered p < 0.01.
3 Results and discussion
3.1 Catalyst characterization data analysis
We have used co-precipitation of Fe(II) and Fe(III) in the presence of orange pectin leading to formation of Fe3O4/Pectin NPs in one-pot (Scheme 1). The structural, morphological and physicochemical properties of the Fe3O4/Pectin NPs were determined by various analytical techniques including FE- SEM, EDX, TEM, VSM and XRD study.
In order to have an idea of structural morphology, shape and size of Fe3O4/Pectin NPs nanocomposite, the electronic microscopic analysis (TEM and SEM) were performed (Figs. 1 and 2).The particles are of globular shape. The grey and black particles represent the Fe3O4 NPs that encapsulated in the pectin matrix. The homogeneous growth in the form of a thin layer of pectin polymer can be detected by close observation (Fig. 1 and Fig. 2). The pectin also stabilizes the corresponding NPs not to agglomerate together.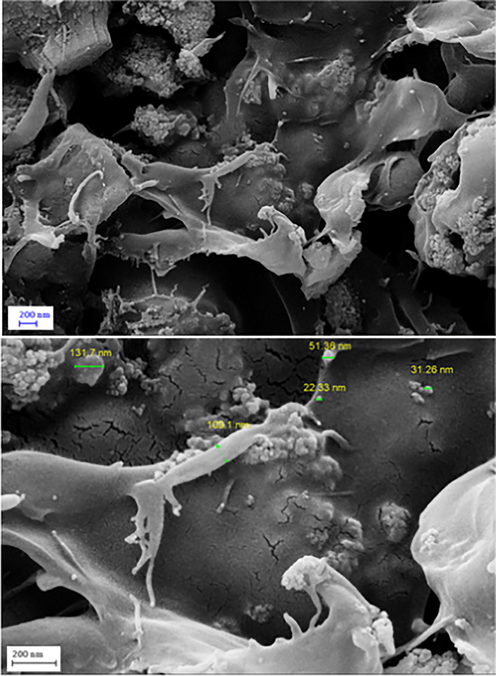
FE-SEM images of Fe3O4/Pectin NPs.
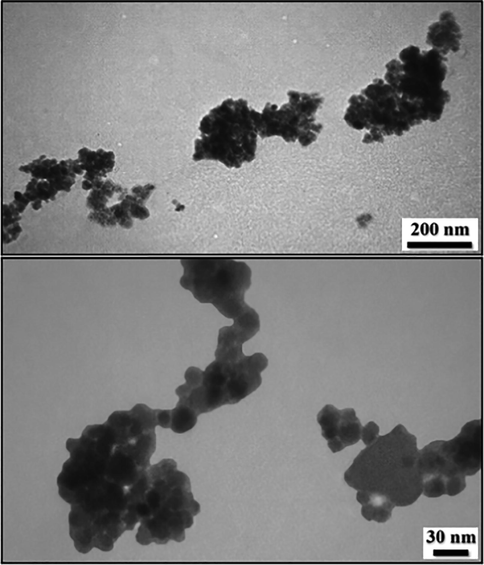
TEM images of Fe3O4/Pectin NPs.
Chemical composition of the nanocomposite was assessed from EDX analysis and the profile is shown in Fig. 3. It represents Fe, O, and C as elemental components. The occurrence of carbon confirmed the presence of pectin and successful fabrication of Fe3O4/Pectin NPs.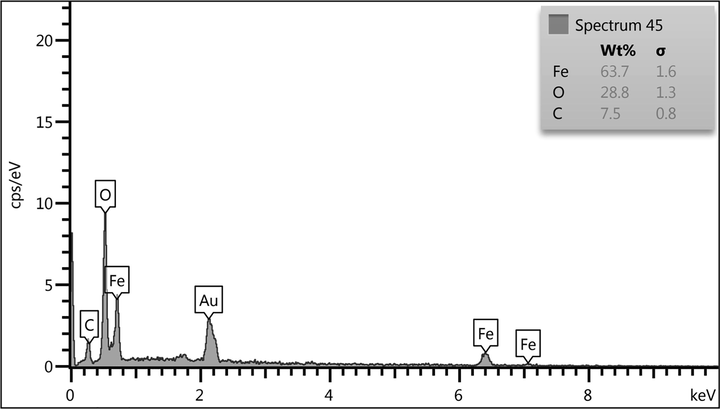
EDX spectrum of Fe3O4/Pectin NPs.
Crystalline phases and the diffraction planes of the Fe3O4/Pectin NPs was ascertained by XRD study, that shown in Fig. 4. It represents a single phase profile indicating a united entity of the assembled counterparts. The typical diffraction peaks due to Fe3O4 are observed at 2θ = 30.1, 35.4, 43.3, 53.6, 57.1, 62.7° corresponding to (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) Bragg reflection planes respectively (JCPDS file, PDF No. 65–3107). Three additional broad diffraction peaks observed at 2θ = 10-20° are contributed from pectin polymers.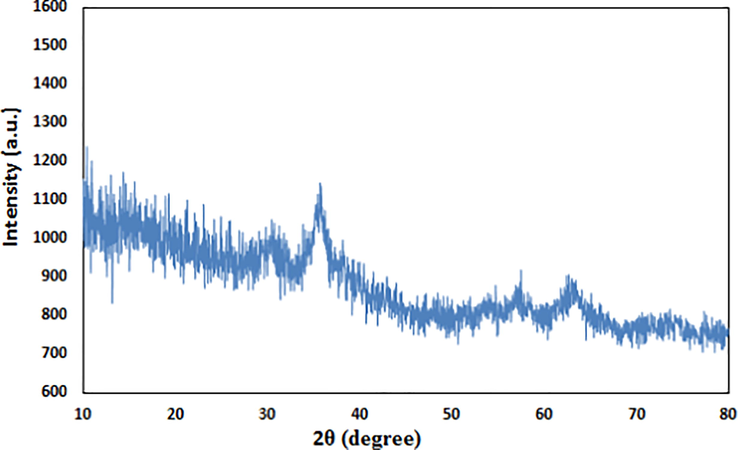
XRD pattern of Fe3O4/Pectin NPs.
VSM study was carried out to determine the magnetic properties of the Fe3O4/Pectin NPs. On passing an external magnetic field of −20kOe to + 20kOe, a magnetic hysteresis curve is obtained and the corresponding saturation magnetization (Ms) value of Fe3O4/Pectin NPs was 52.5 emu/g (Fig. 5). It clearly reveals the material to be superparamagnetic in nature.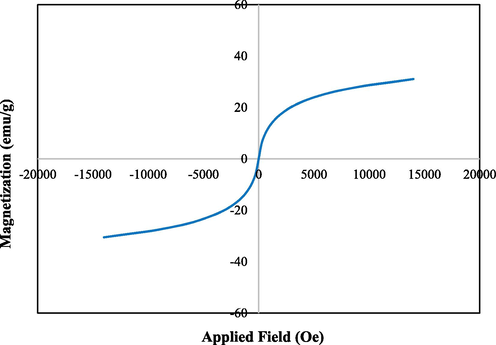
VSM analysis of Fe3O4/Pectin NPs.
3.2 The bio application of Fe3O4/Pectin NPs
Regarding cancer, efforts have been made to use smart nanomaterials (nanoparticles, nanostructures), which have a greater ability to target cancer cells, to treat such patients. That is, they kill malignant cells by irradiating them, providing a microscopic therapeutic effect within electrons (Hilger and Kaiser, 2012; Orel et al., 2015; Van Landeghem et al., 2009; Silva et al., 2011; Johannsen et al., 2010). Nanoparticles are programmed to achieve optimal therapeutic efficacy, delivering therapeutic loads to target cells. Studies have also been performed on several nanocarriers based on lipids, polymers, and peptides for delivery to the respiratory system (Bañobre-López et al., 2013; Klein et al., 2014; Chatterjee et al., 2008; Zhang and Sun, 2004). Properties of nanoparticles for targeted delivery of nanoparticles to tumors is the motivation for targeted drug delivery in cancer treatment to kill cancer cells. In a way, that has the least damage to healthy cells (Thevenot et al., 2008; Colon et al., 2010; Wason et al., 2013; Tarnuzzer et al., 2005; Ali et al., 2014). One of the nanotechnology goals is to mount drugs on carriers, send them and release them into the target cell, which is called targeted drug delivery. Using nanoparticles, the drug can be intelligently delivered to the desired tissue, and improve the tissue without damaging other tissues (Neri and Supuran, 2011; Seo et al., 2007; Hou et al., 2015; Cui et al., 2013; Lucky et al., 2015; Idris et al., 2014).
In this study, the treated cells with different concentrations of the present Fe3O4/Pectin NPs were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) and colorectal malignancy cell lines i.e. Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29. The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for Fe3O4/Pectin NPs (Table 1 and Fig. 6,7). The viability of malignant colorectal cell line reduced dose-dependently in the presence of Fe3O4/Pectin NPs. The IC50 of Fe3O4/Pectin NPs were 317, 337, 187, and 300 µg/mL against Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29 cell lines, respectively (Table 1 and Figs. 6 and 7).
Fe3O4/Pectin NPs (µg/mL)
IC50 against Ramos.2G6.4C10
317 ± 0b
IC50 against HCT-8 [HRT-18]
337 ± 0b
IC50 against HCT 116
187 ± 0a
IC50 against HT-29
300 ± 0b
IC50 against HUVEC
–
![The anti-colorectal carcinoma properties of Fe3O4/Pectin NPs against Ramos.2G6.4C10 (I), HCT-8 [HRT-18] (II), HCT 116 (III), and HT-29 (IV) cell lines.](/content/184/2022/15/6/img/10.1016_j.arabjc.2022.103867-fig7.png)
The anti-colorectal carcinoma properties of Fe3O4/Pectin NPs against Ramos.2G6.4C10 (I), HCT-8 [HRT-18] (II), HCT 116 (III), and HT-29 (IV) cell lines.
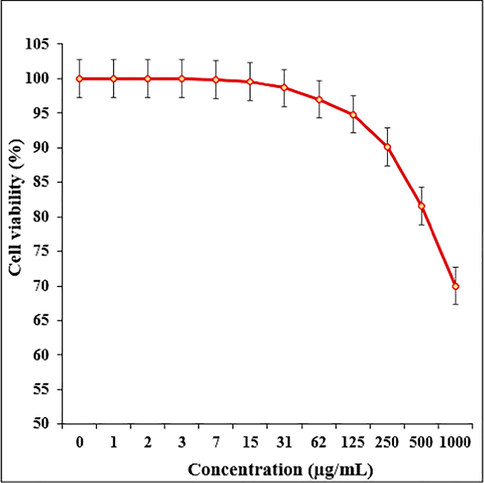
The cytotoxicity effects of Fe3O4/Pectin NPs against normal (HUVEC) cell line.
Nanoparticles have several compounds, each with a different structure. The extraction of these compounds depends on several factors, the most important of which are the type of solvent and the extraction method. It will be very difficult to choose a solvent for any group of nanoparticles compounds, because with these compounds, there are other substances that affect the degree of solubility of these substances (Thevenot et al., 2008; Colon et al., 2010; Wason et al., 2013; Tarnuzzer et al., 2005; Ali et al., 2014). When extracting nanoparticles, it should be noted that always use a method that has the best performance in the survival of antioxidant compounds. Usually nanoparticles have unique antioxidant effects (Seo et al., 2007). Recent studies have shown that when the plant extracts are placed in metal nanoparticles as stabilizing and reducing compounds, they form nanocomposites with extraordinary antioxidant effects. Antioxidants are generally referred to as substances that can delay, slow down, and even stop oxidation processes (Zangeneh et al., 2019; Jalalvand et al., 2019; Barclay et al., 1990; Hamelian et al., 2018; Esumi et al., 2003). These compounds can optimally prevent changes in the color and taste of food because of oxidation reactions. The antidote to the mechanism of oxidants is that they prevent the spread of oxidation chain reactions by giving hydrogen atoms to free radicals. In recent years, the synthetic antioxidants use such as BHT, BHA, TBHQ as well as other chemical additives has been limited due to their potential toxicity and carcinogenicity. Today, most research in this area focuses on the use of new and safe antioxidants from plant, animal, microbial and food sources (Chen et al., 2006; Das et al., 2007; Korsvik et al., 2007; Hong et al., 2006).
The scavenging capacity of Fe3O4/Pectin NPs and BHT at different concentrations expressed as percentage inhibition has been indicated in Table 2 and Fig. 8. In the antioxidant test, the IC50 of Fe3O4/Pectin NPs and BHT against DPPH free radicals were 180 and 86 µg/mL, respectively (Table 2 and Fig. 8).
Fe3O4/Pectin NPs (µg/mL)
BHT (µg/mL)
IC50 against DPPH
180 ± 0b
86 ± 0a
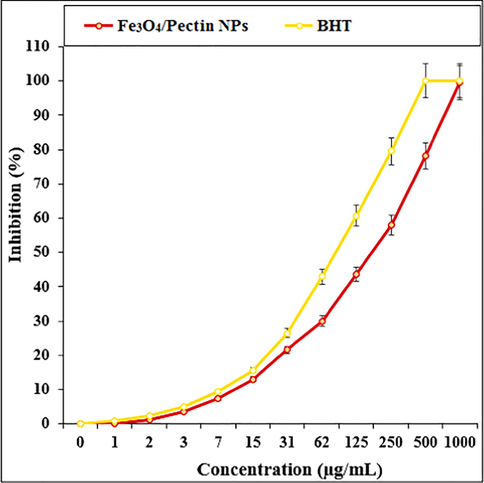
The antioxidant properties of Fe3O4/Pectin NPs and BHT against DPPH.
4 Conclusion
In summary, we demonstrate herein a unique biocompatible, natural polymer-apple pectin modified and magnetically isolable Fe3O4/Pectin NPs being successfully prepared by green method in one-pot. The prepared Fe3O4/Pectin NPs was characterized by a wide range of analytical techniques. The viability of malignant colorectal cell lines reduced dose-dependently in the presence of Fe3O4/Pectin NPs. The IC50 of Fe3O4/Pectin NPs were 317, 337, 187, and 300 µg/mL against Ramos.2G6.4C10, HCT-8 [HRT-18], HCT 116, and HT-29 cell lines, respectively. The Fe3O4/Pectin NPs showed the best antioxidant activities against DPPH. So, the findings of the recent research show that biologically synthesized Fe3O4/Pectin NPs might be used to cure colorectal cancer. In addition, the current study offer that Fe3O4/Pectin NPs could be a new potential adjuvant chemopreventive and chemotherapeutic agent against cytotoxic cells. However, it necessitates clinical trial researches to ascertain their effect as anticancer agents.
Ethics explanation
This research was approved by The People’s Hospital of Jianhu animal ethical committee, Approved No. JHQRM210412.
Acknowledgement
The authors would like to thank the Deanship of Scientific Research at King Khalid University, Abha, KSA for funding this work under Grant number (R.G.P.1/226/41). Also, the authors would like to thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R185), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
References
- Cerium oxide nanoparticles induce oxidative stress and genotoxicity in human skin melanoma cells. Cell Biochem. Biophys.. 2014;71:1643-1651.
- [Google Scholar]
- Magnetic nanoparticle-based hyper-thermia for cancer treatment. Rep. Pract. Oncol. Radiother.. 2013;18:397-400.
- [Google Scholar]
- The Antioxidant Activities of Phenolic Antioxidants in Free-Radical Peroxidation of Phospholipid-Membranes. Can. J. Chem.. 1990;68:2258-2269.
- [Google Scholar]
- Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol.. 2014;92:112-130.
- [Google Scholar]
- Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev.. 2008;60:1627-1637.
- [Google Scholar]
- Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol.. 2006;1:142-150.
- [Google Scholar]
- Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine.. 2010;6:698-705.
- [Google Scholar]
- In vivo targeted deep-tissue photodynamic therapy based on near-infrared light triggered upconversion nanoconstruct. ACS Nano.. 2013;7:676-688.
- [Google Scholar]
- Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918-1925.
- [Google Scholar]
- (a) E. Doustkhah, M. Heidarizadeh, S. Rostamnia, A.h Hassankhani, B. Kazemi, X. Liu, Mat.Lett. 216 (2018) 139-143; (b) B. Maleki, S. Hemmati, A. Sedrpoushan, S.S. Ashrafi, H. Veisi, RSC Advances 2014, 4, 40505-40510; (c) M. Hamelian, S. Hemmati, K. Varmira, H. Veisi, Journal of the Taiwan Institute of Chemical Engineers 2018, 93, 21-30; (d) H. Veisi, S. Hemmati, H. Shirvani, H. Veisi, Applied Organometallic Chemistry 2016, 30, 387-391.
- Antioxidant-potentiality of gold-chitosan nanocomposites. Colloids Surf. B Biointerfaces. 2003;32:117-123.
- [Google Scholar]
- Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C.. 2014;41:178-195.
- [Google Scholar]
- New trends in guided nanotherapies for digestive cancers: A systemic review. J. Control Release.. 2015;209:288-307.
- [Google Scholar]
- Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B Biol.. 2018;184:71-79.
- [Google Scholar]
- Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine.. 2012;7:1443-1459.
- [Google Scholar]
- Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc.. 2006;128:1078-1079.
- [Google Scholar]
- UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: Near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano.. 2015;9:2584-2599.
- [Google Scholar]
- Photoactivation of core-shell titania coated upconversion nanoparticles and their effect on cell death. J. Mater. Chem. B.. 2014;2:7017-7026.
- [Google Scholar]
- J. Photochem. Photobiol. B.: Biol.. 2019;192:103-112.
- Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperthermia.. 2010;26:790-795.
- [Google Scholar]
- Superparamagnetic iron oxide nanoparticles as novel X-ray enhancer for low-dose radiation therapy. J. Phys. Chem. B.. 2014;118:6159-6166.
- [Google Scholar]
- Heat-generating iron oxide nanocubes: Subtle “destructurators” of the tumoral microenvironment. ACS Nano.. 2014;8:4268-4283.
- [Google Scholar]
- Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun.. 2007;1056–1058
- [Google Scholar]
- Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano.. 2015;9:191-205.
- [Google Scholar]
- Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol.. 2011;103:317-324.
- [Google Scholar]
- Interfering with pH regulation in tumors as a therapeutic strategy. Nat. Rev. Drug Discov.. 2011;10:767-777.
- [Google Scholar]
- Magnetic properties and antitumor effect of anocomplexes of iron oxide and doxorubicin. Nanomedicine.. 2015;11:47-55.
- [Google Scholar]
- Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Exp. Opin. Drug Deliv.. 2010;7:1063-1077.
- [Google Scholar]
- Development of watersoluble single-crystalline TiO2 nanoparticles for photocatalytic cancer-cell treatment. Small.. 2007;3:850-853.
- [Google Scholar]
- Silva A.C., Oliveira T.R., Mamani J.B., Malheiros S.M., Malavolta L., Pavon L.F., Sibov T.T., Amaro E., Jr., Tann_us A., Vidoto E.L. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int. J. Nanomed. 2011;6:591–603.
- Stewart B., Wild C.P. World Cancer Report 2014. International Agency for Research on Cancer World Health Organization; Lyon, France: 2014. [(accessed on 24 March 2015)]. Available online: http://www.iarc.fr/en/publications/books/wcr/wcr-order.php.
- Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett.. 2005;5:2573-2577.
- [Google Scholar]
- Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomedicine.. 2008;4:226-236.
- [Google Scholar]
- Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials.. 2009;30:52-57.
- [Google Scholar]
- Carbohyd Polymer.. 2020;235:115966
- Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine.. 2013;9:558-569.
- [Google Scholar]
- Appl. Organometal. Chem.. 2019;33:e5246
- Photocatalytic killing effect of TiO2 nanoparticles on Ls- 174-t human colon carcinoma cells. World J. Gastroenterol.. 2004;10:3191-3193.
- [Google Scholar]







