Translate this page into:
Performance evaluation of functionalized ordered mesoporous silica for VOCs adsorption by molecular dynamics simulation
⁎Corresponding author. jma3@gzu.edu.cn (Jun Ma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Volatile organic compounds (VOCs) are growing pollutants now that cause the serious environmental pollution and threaten human health. The functionalized ordered mesoporous silica (FOMS) has attracted considerable attention in adsorbing VOCs. In this paper, the molecular dynamics simulation was used to simulate the adsorption performance of FOMS on VOCs (acetone, ethyl acetate and toluene). After simulating different pore sizes (2 nm, 3 nm and 4 nm) adsorption performances of ordered mesoporous silica (OMS) on VOCs, OMS with a pore size of 4 nm was selected to further study the influence of functional groups (vinyl, methyl, and phenyl). The following law was obtained: the saturated adsorption capacities of vinyl-functionalized OMS (V-FOMS) to acetone, ethyl acetate and toluene were 3.045 mmol.g−1, 2.568 mmol.g−1 and 1.976 mmol.g−1 respectively; the saturated adsorption capacities of methyl-functionalized OMS (M-FOMS) to acetone, ethyl acetate and toluene were 2.798 mmol.g−1, 2.312 mmol.g−1 and 1.698 mmol.g−1 respectively; the saturated adsorption capacities of phenyl-functionalized OMS (P-FOMS) to acetone, ethyl acetate and toluene were 2.124 mmol.g−1, 1.941 mmol.g−1 and 1.539 mmol.g−1 respectively. These results show that the adsorption ability of FOMS for different adsorbates follows the sequence of acetone > ethyl acetate > toluene. Furthermore, the interaction between functional groups (vinyl, methyl and phenyl) in FOMS and VOCs was explored. It is found that the interaction between different functional groups and adsorbates is different (interaction energy effect). This interaction energy effect promotes FOMS to better adsorb VOCs. This work would provide fundamental understanding and guidance for the development of novel adsorption materials for the adsorption of VOCs.
Keywords
Functionalized mesoporous silica
VOCs
Adsorption
Molecular dynamics simulation
1 Introduction
Volatile organic compounds (VOCs) refer to organic compounds with saturated vapor pressure greater than 70 Pa at 25 °C and boiling point less than 260 °C at 101.325 kPa, or volatile organic compounds with saturated vapor pressure greater than or equal to 10 Pa at 20 °C (Dai et al., 2021; Gao et al., 2021). VOCs originating from solvent production, transportation, applications and incomplete combustion of fossil fuels, etc. (Li et al., 2020; Sha et al., 2021; Zhang et al., 2018). It could cause serious environmental problems, including formation of secondary organic aerosol (SOA) and photochemical pollution (Baysal et al., 2021; Hu et al., 2021; Arneth et al., 2011). Those compounds would also lead to health issues, such as respiratory diseases or even cancer (Zhu et al., 2021; Srikrishnarka et al., 2020). Many technologies have been proposed to eliminate the emission of VOCs, for example, adsorption (Liu et al., 2016; Kim et al., 2018), catalytic combustion (Wang et al., 2006; Li et al., 2009), condensation (Belaissaoui et al., 2016), photocatalytic degradation (Liu et al., 2019; Peng et al., 2019), etc. At present, adsorption has been recognized as an effective technology for its high purification efficiency and low operating cost. In the adsorption process of VOCs, the adsorbent’s performance plays the key role in overall capital and operating costs, and therefore the choice of suitable adsorbent is crucial for adsorption of VOCs. Among the nanoporous materials, activated carbons (Kante et al., 2019; Laskar et al., 2019), silica gels (Sui et al., 2018; Sui et al., 2019), zeolites (Li et al., 2021; Wu et al., 2021), metal organic frameworks (MOFs) (Zhu et al., 2017; Li et al., 2021), and mesoporous silica have been developed as adsorbents (Liu et al., 2018; Ziarani et al., 2020). The activated carbons are excellent adsorbents for their large surface area and high porosity. However, due to the sensibility to high temperature and difficulty in regeneration, the activated carbons are limited for some applications. Different from the activated carbons, silica gel and zeolites are non-combustible materials. However, the hydrophilicity of these kind of materials makes the adsorption capacity to be highly dependent on the humidity conditions of the VOCs gases. In addition, due to the high cost for preparation of MOFs, which limits its large-scale application in the adsorption of VOCs (Lai et al., 2021; Virdis et al., 2021).
As is known to all, the mesoporous silica possess excellent properties, such as high surface area, large pore volume, good thermal stability, and safety. Therefore, it has attracted considerable attention in catalysis and gas adsorption. Especially, the mesopores in silica allow desorption of VOCs to be conducted in a relatively easy and energy-saving way (Zhang et al., 2017; Sui et al., 2017). For example, Xingang Li et al synthesized an organic–inorganic hydrophobic mesoporous silica. This functionalized ordered mesoporous silica (FOMS) performes well in adsorbing p-xylene (Li et al., 2020). Relevant studies showed the pore sizes and functional groups in mesoporous silica also have a significant effect on its adsorption performance of VOCs (Muir et al., 2021; Costa et al., 2021; Li et al., 2021). However, limited to the detection method, It is extremely difficult to examine the effects of specific pore sizes and functional groups by experimental methods. It is also exceedingly difficult to explain adsorption mechanism on the FOMS adsorb VOCs from the molecular point of view by experimental methods. Molecular dynamics simulation can solve all these problems well and can be used to investigate the adsorption data of VOCs in mesoporous silica with arbitrary pore size and functional groups at any pressure and temperature.
Molecular dynamics (MD) simulation method is a computational method emerging in past decades (Rapaport, 1999). It is gradually applied in various fields. With the help of high speed computer, we can not only solve the above problems by establishing an idealized model, but also get the adsorption data that is difficult to obtain by experiments, and further explain the adsorption mechanism at the molecular level. At present, some researchers applied the molecular dynamics simulation to study the adsorption on mesoporous silica. For example, Yan Cao et al (Cao et al., 2021) researched the adsorption of cationic dyes in amino-functionalized mesoporous silica adsorbents by MD simulation method. The results of this research provided deeper atomic/molecular understanding into the adsorption mechanism of dye molecules on adsorbent surface, which could help to get new insights about interactions of molecules. Basaran et al (Basaran et al., 2021) investigated the CO2 adsorption mechanism over amine-functionalized mesoporous silica. The results demonstrated that carbamate and carbamic acid were the two main adsorption products. Hydrogen bonding interactions with the surface silanols as well as the neighboring amines were critical for the formation and stabilization of the products. Besides, surface silanols not only played a significant role on the thermodynamics and kinetics of the reaction mechanism, but also could directly participate in the reaction. However, there is no systematic study on the effects of ordered mesoporous silica (OMS) pore size and different functional groups on the adsorption performance of many kinds of VOCs with different structure and properties. In addition, the adsorption mechanism of VOCs by functionalized mesoporous silica is still not clear.
Accordingly, herein, MD simulation method was used to evaluate the adsorption performance of ordered mesoporous silica (OMS) with different pore sizes (2 nm, 3 nm and 4 nm) and functionalized ordered mesoporous silica (FOMS) for typical VOCs (acetone, ethyl acetate and toluene). Furthermore, in order to reveal the adsorption mechanism of FOMS to VOCs, the interaction effects of different functional groups in FOMS with VOCs were also analyzed in a systematic way. In short, we hope that this study will help designing new functional materials with desired adsorption properties towards VOCs.
2 Simulation methodolgy
2.1 Selection and construction of calculation model
The selected VOCs are acetone, ethyl acetate and toluene. The original silica model is disordered silica crystal. These structures of geometry optimization by Materials Studio Software is shown in Fig. 1. As shown in Fig. 2, the FOMS structure models with different pore sizes (2 nm, 3 nm and 4 nm) and functional groups (vinyl, methyl and phenyl) were obtained by editing disordered silica crystal in Materials Studio Software. The energy change curves of the above structural models in the process of geometric structure optimization were shown in Figure S1.
Molecular structure model of adsorbates (acetone, ethyl acetate and toluene) and disordered silica crystal.
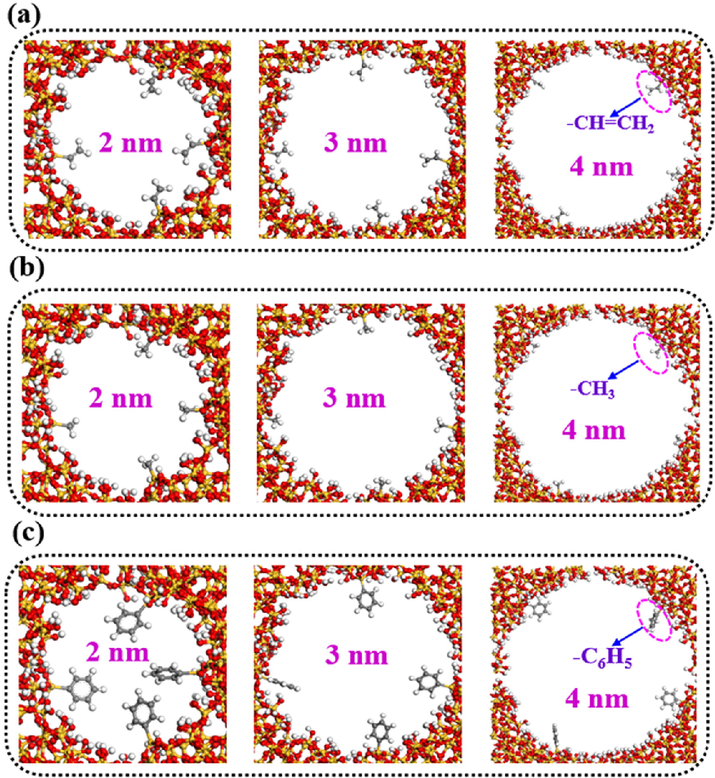
Schematic diagram of local structure model of ordered mesoporous silica with different pore sizes and functional groups (a: Vinyl-functionalized OMS, b: Methyl-functionalized OMS, c: Phenyl-functionalized OMS).
2.2 Sorption simulation details
In order to evaluate the adsorption performance of OMS and FOMS for selected VOCs. The adsorption of VOCs in OMS and FOMS were carried out by Sorption module of the Materials Studio Software. The calculation parameters in the Sorption module were set as follows: the calculation method was set to metropolis and the calculation accuracy was chosen ultra-fine. The equilibration and production steps were set to 1 × 105 and 1 × 106, respectively. The adsorbates fugacity was set according to saturated vapor pressure (Table 1) of adsorbates at 298.15 K. The COMPASS II was selected as the calculated force field. Van der Waals and electrostatic interaction were based on atom based and ewald & group, cut off distance was 1.85 nm. Simulation box size and total number of atoms were shown in Figure S2-Figure S5.
Adsorbates
Saturated vapor pressure (kPa)
Acetone
Ethyl acetate30.788
12.617
Toluene
3.792
2.3 Forcite simulation details
The interaction between adsorbate molecules (acetone, ethyl acetate and toluene) and FOMS were calculated by full atom molecular dynamics simulation. It was calculated by Forcite module in the Materials Studio Software. Calculation accuracy was chosen ultra-fine. Molecular dynamic (MD) simulation was carried out at NVT-ensemble (constant number of atoms (N), volume (V) and temperature (T) for the established sorption system). Temperature and pressure were set at 298 K and 1 atm, respectively, using the algorithm described by Berendsen et al. (1984). The COMPASS II was selected as the force field. Van der Waals and electrostatic interaction were all based on group based, cut off distance was 1.85 nm. The simulation time and time step were 500 ps and 1 fs, respectively. Full trajectory was saved and frames were output every 5 ps. The system was equilibrated when the energy and temperature fluctuated around a constant value.
2.4 Analysis of adsorption mechanism
In order to understand the interaction between functional groups in FOMS and VOCs, the adsorption mechanism of FOMS is described from three aspects (isosteric heats, adsorption energy and interaction energy).
The isosteric heats (E) was displayed by the equation (1). The first section is the Coulomb equation, which calculates the electrostatic interaction force between the intermolecular i and j charge groups. The second section is the 9–6 Lennard-Jones (LJ) potential model that represents the intermolecular non-bonding effect and the van der Waals interaction force. q, rij represents the charge of the adsorption site and the distance between the two adsorption sites, respectively. ε is the potential well depth and r0 is the hard sphere radius of the atom. Moreover, the 9–6 LJ parameters of different atoms are determined by using the equations (2) and (3) (Waldman and Hagler, 1993).
The adsorption energy between adsorbate molecules and differently FOMS were calculated by formula (4). The interaction energy between adsorbate molecules and variously functional groups in the FOMS can be described by radial distribution function (RDF). The RDF can be calculated using formula (5) (Duan et al., 2017; Song et al., 2018; Xiang et al., 2016).
Where, represents adsorption energy; represents energy of FOMS adsorbing adsorbate molecules to form stable structure; represents energy of FOMS after MD simulation; represents energy of adsorbates after MD simulation. V is the system volume, Ni and Nj are number of i and j, respectively, and ΔNij(r → r + Δr) is the ensemble averaged number of i around j within a shell from r to r + Δr.
3 Results and discussions
3.1 Adsorptions in OMS with different pore sizes
Fig. 3 shows the adsorption isotherms of the VOCs in OMS with various pore sizes (2 nm, 3 nm and 4 nm) at the temperature of 293.15 K and the respective threshold pressure in the range of 0–1.0 P0 of the adsorbate. These adsorption data were all modeled independently through sorption module in Materials Studio Software. The adsorption capacity represents amount of gas adsorbed per gram of adsorbent. Fig. 3a-c shows the adsorption isotherms of acetone, ethyl acetate and toluene in OMS respectively. Obviously, the pore size has a significant effect on the adsorption isotherms of VOCs, and the effects of different VOCs are diverse from each other.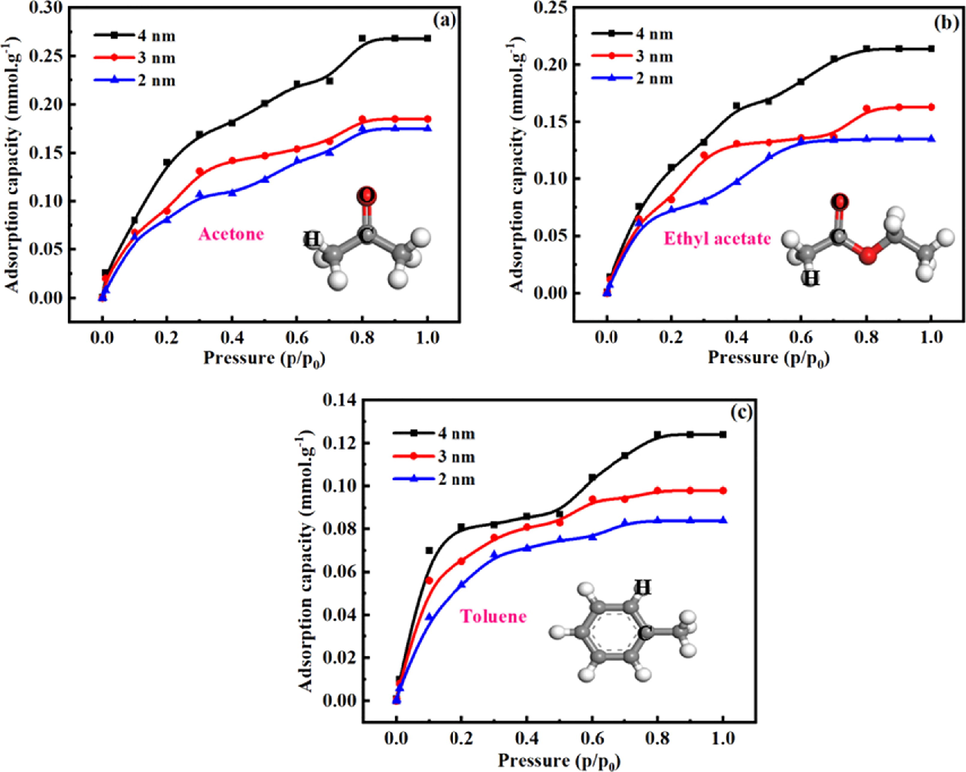
Adsorption isotherms of VOCs (acetone, ethyl acetate and toluene) in OMS with different pore sizes.
For acetone, ethyl acetate and toluene, the adsorption isotherms in the OMS were quite similar. The amount of VOCs adsorbed by OMS increases with the increase of pore size. However, the OMS possess different adsorption capacity for various VOCs. Because there are hydrophilic silanol groups on the surface of silica, and in the selected VOCs, the order of hydrophilicity was acetone > ethyl acetate > toluene. Therefore, its’ adsorption capacity for selected VOCs follows the sequence of acetone > ethyl acetate > toluene. The adsorption of VOCs in OMS reaches saturation state when the pressure was 0.8 P0. When the pore sizes of OMS were 2 nm, 3 nm and 4 nm respectively, the correspondingly saturated adsorption capacity of acetone in the OMS were 0.175 mmol.g−1, 0.185 mmol.g−1 and 0.268 mmol.g−1. For ethyl acetate, the correspondingly saturated adsorption capacity in the OMS were 0.135 mmol.g−1, 0.163 mmol.g−1 and 0.214 mmol.g−1. For toluene, the correspondingly saturated adsorption capacity in the OMS were 0.084 mmol.g−1, 0.098 mmol.g−1 and 0.124 mmol.g−1. These results show that increasing the pore size of OMS is beneficial to enhance its adsorption capacity for VOCs. Therefore, the OMS model with pore size of 4 nm was further used to investigate the effects of various functional groups (methyl, phenyl and vinyl) functionalized OMS on the adsorption of VOCs.
3.2 Adsorptions in OMS with functional groups
Fig. 4 shows the adsorption isotherms of VOCs (acetone, ethyl acetate and toluene) in different functional groups-functionalized OMS with 4 nm pore size. Obviously, when OMS was functionalized with different functional groups (vinyl, methyl and phenyl), the adsorption capacity of OMS for VOCs can be significantly increased. Besides, the adsorption capacity of FOMS for VOCs increases with the increase of pressure, and when the pressure reaches 0.8 P0, the adsorption of FOMS for VOCs reaches the saturated state, which is consistent with the results in Fig. 3. However, for vinyl-functionalized OMS (V-FOMS), the saturated adsorption capacities of acetone, ethyl acetate and toluene were 3.045 mmol.g−1, 2.568 mmol.g−1 and 1.976 mmol.g−1 in V-FOMS. For methyl-functionalized OMS (M−FOMS), the saturated adsorption capacities of acetone, ethyl acetate and toluene were 2.798 mmol.g−1, 2.312 mmol.g−1 and 1.698 mmol.g−1 in M−FOMS. For phenyl-functionalized OMS (P-FOMS), the saturated adsorption capacities of acetone, ethyl acetate and toluene were 2.124 mmol.g−1, 1.941 mmol.g−1 and 1.539 mmol.g−1 in M−FOMS. These results show that the adsorption ability of FOMS for different adsorbates follows the sequence of acetone > ethyl acetate > toluene. It may be due to the different interaction of selected VOCs and FOMS, resulting the affinity and interaction of adsorbates in FOMS is various, further affect the adsorption capacity of FOMS for acetone, ethyl acetate and toluene. Hence, the interaction between functional groups of FOMS and VOCs needs to be further studied to reveal the adsorption capacity of FOMS from the perspective of adsorption mechanism.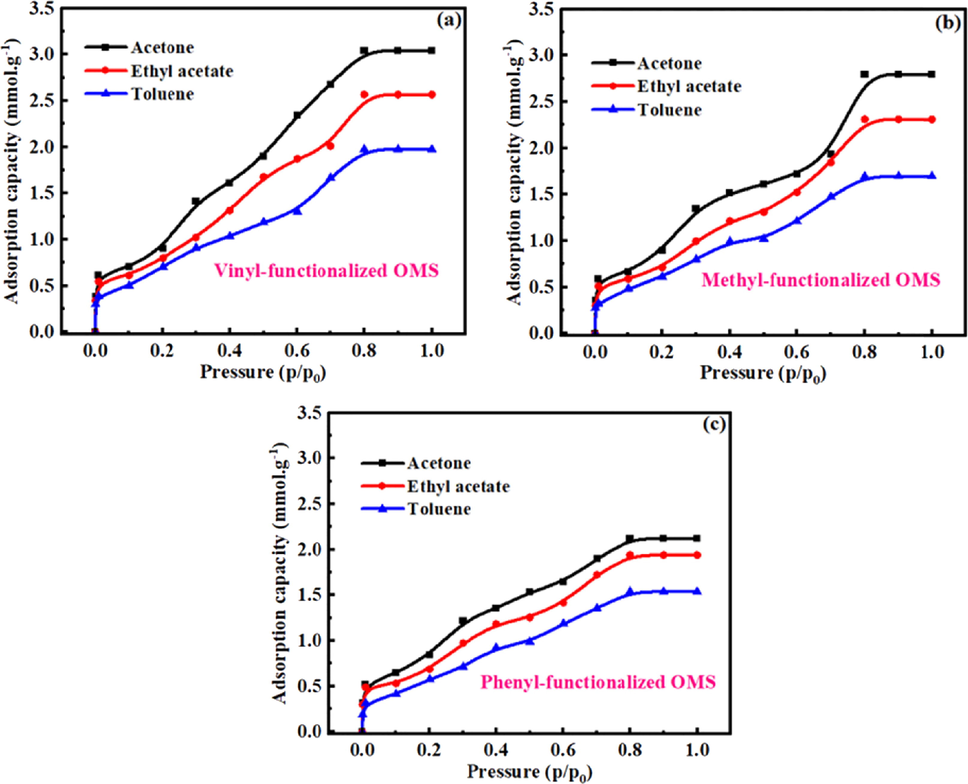
Adsorption isotherms of VOCs (acetone, ethyl acetate and toluene) in differently functional groups-functionalized OMS with 4 nm pore size.
3.3 Adsorption mechanism
To provide unique insights into the intrinsic nature of the FOMS for the adsorption of VOCs, the adsorption mechanism of VOCs in FOMS are discussed in this section.
Fig. 5 shows the isosteric heats of different VOCs in FOMS (V-FOMS, M−FOMS and P-FOMS) and OMS with 4 nm pore size. Obviously, one the one hand, with the increase of adsorption pressure, the isosteric heats of VOCs in FOMS and OMS gradually increase. When the adsorption pressure reaches 0.8p0, the isosteric heats tend to be stable. On the other hand, for V-FOMS, M−FOMS, P-FOMS and OMS. The isosteric heats of different VOCs in FOMS and OMS follows the sequence of acetone > ethyl acetate > toluene. However, the isosteric heats of VOCs in FOMS is larger than that of VOCs in OMS. It shows the groups (vinyl, methyl, phenyl) functionalized OMS is beneficial to improve the isosteric heats of VOCs in OMS. In addition, as illustrated in Fig. 6. For FOMS and OMS, the adsorption energies of different VOCs in FOMS and OMS also follows the sequence of acetone > ethyl acetate > toluene, and the adsorption energies of VOCs in FOMS is larger than that of VOCs in OMS. Besides, the isosteric heats and adsorption energies between V-FOMS and VOCs are greater than that between the other two FOMS (M−FOMS and P-FOMS) and VOCs, it indicates that vinyl possess stronger affinity interaction with VOCs. The above results show that the adsorption capacity of FOMS for VOCs is stronger than that of OMS. It further indicates that the interaction between VOCs and FOMS is greater than that between VOCs and OMS, and the groups (vinyl, methyl, phenyl) functionalized OMS is beneficial to improve the interaction between OMS and VOCs.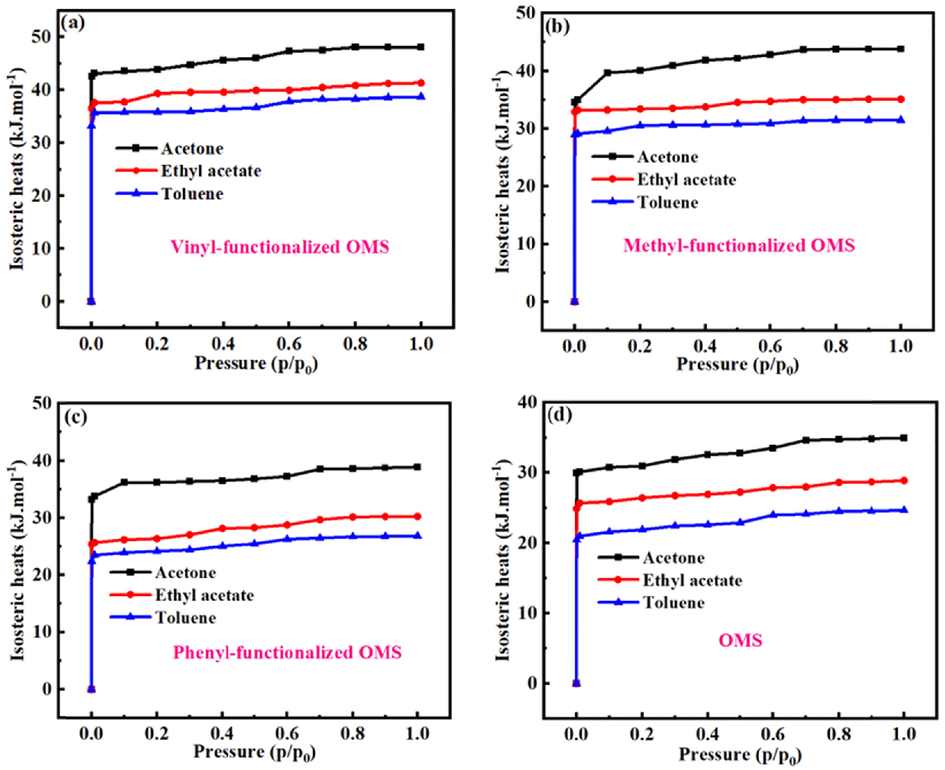
Isosteric heats of VOCs (acetone, ethyl acetate and toluene) in different FOMS (V-FOMS, M−FOMS and P-FOMS) and OMS with 4 nm pore size.
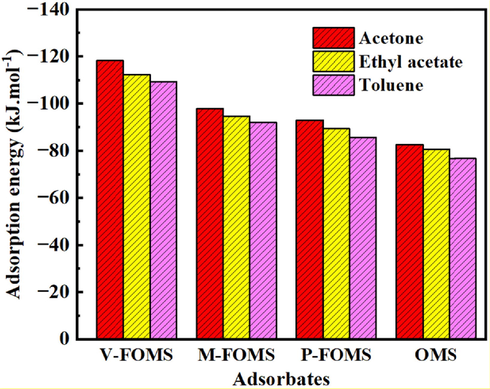
Adsorption energies of different VOCs in different FOMS (V-FOMS, M−FOMS and P-FOMS) and OMS with 4 nm pore size.
In order to reveal the interaction between functional groups (vinyl, methyl and phenyl) in FOMS and VOCs molecules. The radial distribution function (RDF) was also introduced to describe the interaction between functional groups and adsorbate molecules, and it represent the molecular configuration and orderly molecular combination. The RDF between adsorbate molecules and different functional groups in FOMS was computed with a distance less than the cutoff value. The peak of RDF is larger, the more ordered arrangement between the adsorbate molecules and functional groups, the stronger the interaction between the adsorbate molecules and functional groups (Ma et al., 2021). As shown in Fig. 7a, 7c and 7e, the RDF of VOCs and functional groups (vinyl, methyl and phenyl) in FOMS showed strong peaks in the 1.5 Å. In addition, the RDF between hydroxyl and VOCs in OMS was used as a blank control (Fig. 7g). Obviously, the peaks between VOCs and functional groups (vinyl, methyl and phenyl) in FOMS were sharper than that between VOCs and hydroxyl in OMS. It indicated that the groups (vinyl, methyl and phenyl) functionalized OMS can significantly improve the interaction between OMS and VOCs. However, the peaks between acetone molecule and functional groups (vinyl, methyl and phenyl) in FOMS were sharper than that between the other two adsorbate molecules (ethyl acetate and toluene) and functional groups in FOMS. Besides, the peak intensity of RDF between VOCs and functional groups (vinyl, methyl, phenyl and hydroxyl) follows the sequence of acetone > ethyl acetate > toluene. The results suggested that FOMS possess better affinity interaction with adsorbate molecules, and the affinity interaction is the strongest between V-FOMS and acetone.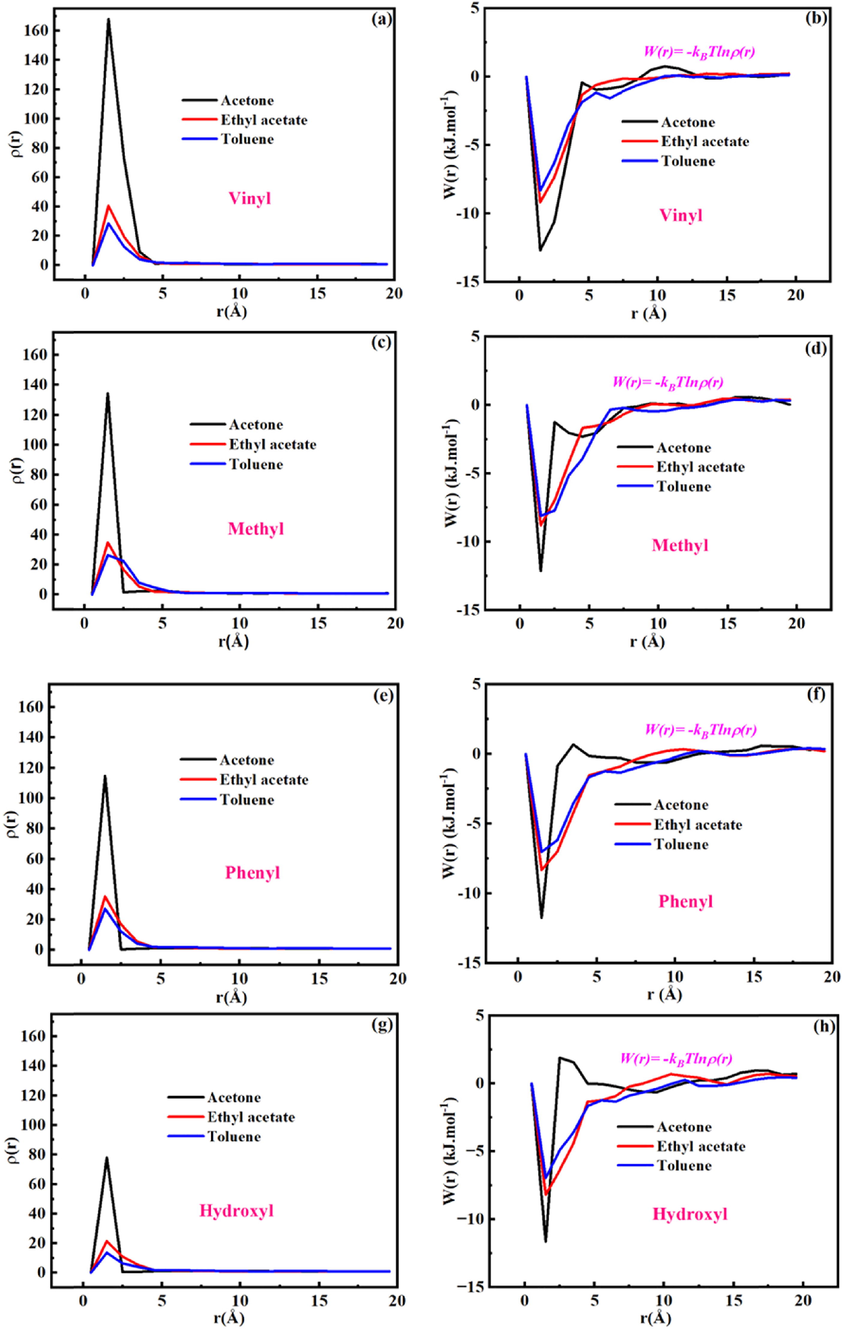
Radial distribution function (a, c, e and g) and potential of mean force (b, d, f and h) between functional groups in FOMS (Vinyl, Methyl, Phenyl) and OMS (Hydroxyl) and adsorbate molecules.
According to the curve of RDF changing with distance, the change of mean force potential (PMF) with distance between functional groups and different VOCs were calculated based on formula (6) (Roux, 1995), as shown in Fig. 7b, 7d, 7f and 7 h.
Where, W(r) is potential of mean force, kB is the Boltzmann constant (1.38 × 10-23 J.K−1), T is temperature (K), is probability density (obtained by RDF).
According to formula (6). The interaction energies between functional groups (vinyl, methyl, phenyl and hydroxyl) and VOCs were shown in Table 2. Obviously, the interaction energies between functional groups (vinyl, methyl and phenyl) in FOMS and VOCs were higher than between hydroxyl in OMS and VOCs. It also showed groups (vinyl, methyl and phenyl) functionalized OMS can significantly improve the interaction between OMS and VOCs. In addition, this result also indicated that the interaction between functional groups in FOMS and acetone is strongest. Besides, it is found that the interaction energies between different functional groups and adsorbate molecules are different (interaction energy effect). Under the interaction energy effect, FOMS possess better ability to adsorb VOCs.
AdsorbatesW(r) (kJ.mol−1)
Adsorbates-VinylW(r) (kJ.mol−1)
Adsorbates-MethylW(r) (kJ.mol−1)
Adsorbates-PhenylW(r) (kJ.mol−1)
Adsorbates-Hydroxyl
Acetone
−12.687
−12.134
−11.740
−11.643
Ethyl acetate
−9.173
−8.785
−8.312
−8.178
Toluene
−8.309
−8.097
−7.013
−6.954
4 Conclusions
In this work, effects of mesoporous silica pore sizes (2 nm, 3 nm and 4 nm) and functional groups (vinyl, methyl, and phenyl) on adsorption capacity of VOCs (acetone, ethyl acetate and toluene) were studied by molecular dynamics simulation, and the pore sizes, functional groups and adsorption mechanism were discussed in detail. With the increase of pore sizes, the adsorption capacity of mesoporous silica for VOCs is enhanced. When ordered mesoporous silica was functionalized with variously functional gropus (vinyl, methyl, and phenyl), the adsorption capacity of FOMS for VOCs was significantly enhanced. And different FOMS’s adsorption capacity for selected VOCs follows the sequence of V-FOMS > M−FOMS > P-FOMS. In addition, the affinity between V-FOMS and VOCs was the best. It is also found that the interaction energies between different functional groups and adsorbate molecules are different (interaction energy effect). In this interaction energy effect, FOMS possess better ability to adsorb VOCs. These findings not only evaluated the adsorption performance of FOMS for selected VOCs, but also reveal the adsorption mechanism of FOMS. In addition, the research findings of this study also provide potential guidance for the development of novel adsorption materials for the adsorption of VOCs in the future.
Acknowledgements
This research were funded by Guizhou Provincial Science and Technology Projects (Grant NO. ZK[2022]088), Natural Science Special Foundation of Guizhou University (Grant NO. X2021073 Special Post D), The cultivation project of Guizhou University (Grant NO. [2020]38), Innovation Group Project of Education Department in Guizhou Province (Grant NO. 2021010), One Hundred Person Project of Guizhou Province (Grant NO. 20165655), Excellent Young Scientific and Technological Talent Program of Guizhou Province (Grant NO. 20195645) and Zunyi City Innovative Talent Team Project (Grant NO. 20195645).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Global terrestrial isoprene emission models: Sensitivity to variability in climate and vegetation. Atmos. Chem. Phys.. 2011;11:8037-8052.
- [Google Scholar]
- Theoretical investigation of CO2 adsorption mechanism over amine-functionalized mesoporous silica. J. CO2 Util.. 2021;47:101492
- [Google Scholar]
- Development of a new needle trap-based method for the determination of some volatile organic compounds in the indoor environment. Chemosphere. 2021;277:130251
- [Google Scholar]
- Energy efficiency of a hybrid membrane/condensation process for VOC (Volatile Organic Compounds) recovery from air: A generic approach. Energy. 2016;95:291-302.
- [Google Scholar]
- Molecular dynamic simulations and quantum chemical calculations of adsorption process using amino-functionalized silica. J. Mol. Liq.. 2021;330:115544
- [Google Scholar]
- Adsorption of benzene and toluene from aqueous solution using a composite hydrogel of alginate-grafted with mesoporous silica. J. Hazard. Mater.. 2021;418:126405
- [Google Scholar]
- Theoretical exploration of VOCs removal mechanism by carbon nanotubes through persulfate-based advanced oxidation processes: Adsorption and catalytic oxidation. J Hazardpus Mater.. 2021;405:124684
- [Google Scholar]
- Layer-by-layer assembled film of asphaltenes/polyacrylamide and its stability of water-in-oil emulsions: A combined experimental and simulation study. J. Phys. Chem. C. 2017;121(8):4332-4342.
- [Google Scholar]
- Source profiles and emission factors of VOCs from solvent-based architectural coatings and their contributions to ozone and secondary organic aerosol formation in China. Chemosphere. 2021;275:129815
- [Google Scholar]
- Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal.. 2021;4(3):242-250.
- [Google Scholar]
- Exploring resistance changes of porous carbon upon physical adsorption of VOCs. Carbon. 2019;146:568-571.
- [Google Scholar]
- Toluene and acetaldehyde removal from air on to graphene-based adsorbents with microsized pores. J. Hazard. Mater.. 2018;344:458-465.
- [Google Scholar]
- Metal-organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coord. Chem. Rev.. 2021;427:213565
- [Google Scholar]
- Competitive adsorption equilibrium modeling of volatile organic compound (VOC) and water vapor onto activated carbon. Sep. Purif. Technol.. 2019;212:632-640.
- [Google Scholar]
- Functionalized silica nanoparticles: Classification, synthetic approaches and recent advances in adsorption applications. Nanoscale. 2021;13:15998-16016.
- [Google Scholar]
- In situ synthesis of an advanced Z-scheme Bi/BiOI/black TiO2 heterojunction photocatalysts for efficient visible-light-driven no purification. Appl. Surf. Sci.. 2021;562:150250
- [Google Scholar]
- Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today. 2009;148(1):81-87.
- [Google Scholar]
- Functionalized ordered mesoporous silica by vinyltriethoxysilane for the removal of VOCs through adsorption/desorption process. Ind. Eng. Chem. Res.. 2020;59(8):3511-3520.
- [Google Scholar]
- Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol.. 2020;235:116213
- [Google Scholar]
- Adsorption and desorption characteristics of hydrophobic hierarchical zeolites for the removal of volatile organic compounds. Chem. Eng. J.. 2021;411:128558
- [Google Scholar]
- Engineering surface functional groups on mesoporous silica: Towards a humidity-resist hydrophobic adsorbent. J. Mater. Chem. A. 2018;6
- [Google Scholar]
- Enhanced adsorption of benzene vapor on granular activated carbon under humid conditions due to shifts in hydrophobicity and total micropore volume. J. Hazard. Mater.. 2016;318:425-432.
- [Google Scholar]
- Efficient photocatalytic oxidation of gaseous toluene in a bubbling reactor of water. Chemosphere. 2019;233:754-761.
- [Google Scholar]
- Mechanisms on the stability and instability of water-in-oil emulsion stabilized by interfacially active asphaltenes: Role of hydrogen bonding reconstructing. Fuel. 2021;297:120763
- [Google Scholar]
- Fundamental features of mesoporous functional materials influencing the efficiency of removal of VOCs from aqueous systems: A review. Sci Total Environ. 2021;784:147121
- [Google Scholar]
- Revisiting cocatalyst/TiO2 photocatalyst in blue light photothermalcatalysis. Catal. Today. 2019;335:286-293.
- [Google Scholar]
- The calculation of the potential of mean force using computer simulations. Comput. Phys. Commun.. 1995;91(1):275-282.
- [Google Scholar]
- A newly integrated dataset of volatile organic compounds (VOCs) source profiles and implications for the future development of VOCs profiles in China. Sci. Total Environ.. 2021;148348
- [Google Scholar]
- Encapsulation of single-walled carbon nanotubes with asymmetric pyrenyl-gemini surfactants. Chem. Eng. Sci.. 2018;187:406-414.
- [Google Scholar]
- Enhanced capture of particulate matter by molecularly charged electrospun nanofibers. ACS Sustain. Chem. Eng.. 2020;8(21):7762-7773.
- [Google Scholar]
- Removal and recovery of o-xylene by silica gel using vacuum swing adsorption. Chem. Eng. J.. 2017;316:232-242.
- [Google Scholar]
- Adsorption and desorption of binary mixture of acetone and ethyl acetate on silica gel. Chem. Eng. Sci.. 2018;197
- [Google Scholar]
- Piecewise loading bed for reversible adsorption of VOCs on silica gels. J. Taiwan Inst. Chem. Eng.. 2019;102:51-60.
- [Google Scholar]
- Multi-component ppm level adsorption of VOCs on the ZIF-8 and UiO-66 MOFs: Breakthrough analysis with selected ion flow tube mass spectrometry. J. Environ. Chem. Eng.. 2021;9(6):106568
- [Google Scholar]
- New combining rules for rare gas van der waals parameters. J. Comput. Chem.. 1993;14(9):1077-1084.
- [Google Scholar]
- Performance of the supported copper oxide catalysts for the catalytic incineration of aromatic hydrocarbons. Chemosphere. 2006;64(3):503-509.
- [Google Scholar]
- Novel preparation of binder-free Y/ZSM-5 zeolite composites for VOCs adsorption. Chem. Eng. J.. 2021;417:129172
- [Google Scholar]
- Correction: Concentration-induced structural transition of block polymer self-assemblies on a nanoparticle surface: computer simulation. RSC Adv.. 2016;6(109):107297.
- [Google Scholar]
- Current advances of VOCs degradation by bioelectrochemical systems: A review. Chem. Eng. J.. 2018;334:2625-2637.
- [Google Scholar]
- Molecular imprinting on inorganic nanozymes for hundred-fold enzyme specificity. J. Am. Chem. Soc.. 2017;139(15):5412-5419.
- [Google Scholar]
- Enhanced hydrophobic MIL(Cr) metal-organic framework with high capacity and selectivity for benzene VOCs capture from high humid air. Chem. Eng. J.. 2017;313:1122-1131.
- [Google Scholar]
- Toward healthcare diagnoses by machine-learning-enabled volatile organic compound identification. ACS Nano. 2021;15(1):894-903.
- [Google Scholar]
- The synthesis and application of functionalized mesoporous silica SBA-15 as heterogeneous catalyst in organic synthesis. Curr. Org. Chem.. 2020;24
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104192.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







