Translate this page into:
Phenolics of selected species of Persicaria and Polygonum (Polygonaceae) in Egypt
⁎Corresponding author. salwasharkawy@windowslive.com (Salwa Kawashty)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Four selected species of family Polygonaceae Juss. viz. Persicaria salicifolia (Brouss. ex Willd.) Assenov, Persicaria senegalensis (Meisn.) Soják, Polygonum bellardii All. and Polygonum equisetiforme Sm. were subjected to botanical, chemical and numerical studies. The botanical part deals with macro- and micromorphological characters of the whole plant. The chemical part deals with extraction and identification of 17 compounds including flavones, flavonols, flavone C-glycosides and phenolic acids. The botanical and chemical results of the four selected species were subjected to a numerical analysis.
Keywords
Persicaria
Polygonum
Macromorphology
Micromorphology
Flavonoids
Chemosystematics
1 Introduction
Polygonaceae Juss. is a family comprising about 50 genera and 1120 species of monoecious and dioecious herbs, shrubs and small trees (Li et al., 2003). In Egypt, Polygonaceae includes about 22 wild species under six genera; Atraphaxis, Calligonum, Emex, Oxygonum, Polygonum and Rumex (Montasir and Hassib, 1956). Täckholm (1974) had recognized 28 species and added one more genus viz. Bilderdykia to the genera cited by Montasir and Hassib (1956) while Boulos (1999) treated Polygonum and Persicaria as two different genera.
Persicaria (L.) Mill. is distributed mainly in the Northern hemisphere and seven species are recorded in Egypt (Boulos, 1999). On the other hand Polygonum L. is the largest genus of Polygonaceae, which comprises about 30 species of much-branched annual herbs, and distributed in the temperate regions of the northern hemisphere (Hong et al., 2005). According to Boulos (1999) only six species are found in Egypt.
Several studies dealt with the macro- and micromorphological characters of Persicaria and Polygonum (Simmonds, 1945; Haraldson, 1978; Ronse Decraene and Akeroyd, 1988; Hamed and Tantawy, 1990, 1991; Leresten and Curtis, 1992; Brandbyge, 1993; Boulos, 1999; Partridge, 2001; Li et al., 2003; Tantawy et al., 2005). The taxonomy of Polygonum has been unsettled since Linnaeus established it and has been subdivided into various sections and subsections, split into segregate genera, and some elements have even been treated as different tribes (Leresten and Curtis, 1992).
Quercetin glycosides were commonly reported in the Polygonaceae while myricetin glycosides and acylated flavonoids were rare and methylated flavonols were detected in some species of sections Echinocaulon and Persicaria (Hegnauer, 1969; Kuroyanagi and Fukushima, 1982; Kawasaki et al., 1986; Park, 1987).
The survey involving Persicaria salicifolia indicated that flavonol glycosides are predominant (Calis et al., 1999). Chalcone, dihydrochalcone and quercetin glycosides were isolated and identified from Persicaria senegalensis (Maradufu and Ouma, 1978; Dossaji and Kubo, 1980; Midiwo and Owuor, 1992; Midiwo et al., 1994; Midiwo et al., 2002, 2007). Quercetin and three quercetin glycosides in addition to isorhamnetin were isolated and identified from Polygonum equisetiforme by Ghazal et al. (1992).
The specific objectives of this study are to: investigate the macro- and micromorphological characters of both vegetative and reproductive organs of the investigated taxa in comparison with those of Hamed & Tantawy (1990 and 1991) and Tantawy et al. (2005); study the flavonoid chemistry of the four investigated taxa in comparison with the other investigated taxa in the literature and clarify the relationship between Persicaria and Polygonum and should they be treated as two separate entities or as one taxonomic unit.
2 Material and methods
2.1 General
UV spectra were recorded on Shimadzu, model 2401. EIMS spectra were carried out on Finnigan-Mat SSQ 7000 spectrometer. ESIMS spectra on Micromass Quattro-LC triple quadruple mass spectrometer equipped with a Z-Spray electro-spray ion source while NMR measurements were carried out using Jeol EX-500 spectroscopy; 500 MHz (1H NMR) and 125 MHz (13C NMR) and Joel JNM-EX 270 spectroscopy; 270 MHz (1H NMR) and 67.5 MHz (13C NMR). Sugars of O-glycosides were identified by enzymatic hydrolysis (β-glucosidase and β-galactosidase) or acid hydrolysis followed by co-chromatography with reference standards. C-glycoside flavonoids were determined using ferric chloride degradation (Mabry et al., 1970). Authentic samples were obtained from the department of phytochemistry and plant systematics, NRC.
2.2 Plant material
Four selected plant species were collected from different localities representing Egyptian taxa of the family Polygonaceae viz Persicaria salicifolia (Brouss. ex Willd.) Assenov, P. senegalensis (Meisn.) Soják, Polygonum bellardii All. and P. equisetiforme Sm. The investigated taxa were identified according to Boulos (1999), and voucher specimens were deposited in the herbarium of the National Research Centre (CAIRC). Details of the collected samples are given below, with an asterisk (*) indicating herbarium samples: P. salicifolia (Brouss. ex Willd.) Assenov, Ismailia Canal and Nile-Delta region 15 Nov. 2008, leg. S.R. Hussein and U.I.A. EL-Magly, s.n.623. P. senegalensis (Meisn.) Soják El-Kanater, Nile-Delta region 2 Oct. 2007, leg. S.R. Hussein and U.I.A. EL-Magly, s.n.532. P. bellardii∗ All. Shakshouk near lake Qarun along a canal 24 Mar. 1981, leg. L. Boulos s.n.1725 and P. equisetiforme Sm. El-Arish & Rafah Desert Road. 25 Apr. 2007 leg. S.R. Hussein and U.I.A. EL-Magly, s.n.513.
2.3 Extraction and isolation
The aerial parts of P. senegalensis and P. equisetiforme were dried in the shade and ground. Each powder was extracted twice with 70% methanol. The extract was filtered and concentrated then partitioned with solvents of increasing polarity (petroleum ether, diethyl ether, chloroform, acetone, ethyl acetate, methanol and water). Isolation of flavonoids was carried out using column chromatography (MN-polyamide 6S) and paper chromatography (Whatman No. 1 and 3MM) using butanol–acetic acid–water 4:1:5 (BAW upper phase), water and 15% AcOH (water–acetic acid 17–3), (Harborne, 1967; Mabry et al., 1970). The compounds were further purified on a Sephadex LH-20 column with standard solvent systems (Markham, 1982). Trace compounds were identified by co-chromatography with authentic samples. Details of the isolated compound are outlined below.
2.3.1 Apigenin (5,7,3′-trihodroxy-flavone) (1)
Rf: 0.85 (BAW), 0.03 (H2O), 0.15 (15% AcOH). UV/Vis λmax (MeOH): 272, 332; (+NaOMe): 283, 333, 400; (+AlCl3): 280, 305, 350, 386sh; (+AlCl3 + HCl): 279, 305, 347, 385sh; (+NaOAc): 281, 306sh, 387; (+NaOAc + H3BO3): 276, 321, 347sh. EI-MS: m/z 270 equivalent to molecular formula of C15H10O5.
2.3.2 3,6-Dimethoxy-kaempferol (2)
Rf: 0.81 (BAW), 0.04 (H2O), 0.16 (15% AcOH). UV/Vis λmax (MeOH): 271, 342; (+NaOMe): 276, 327, 399; (+AlCl3): 274, 306sh, 349; (+AlCl3 + HCl): 280, 303sh, 352, 406sh; (+NaOAc): 276, 300sh, 351; (+NaOAc + H3BO3): 276, 346. 1H NMR in DMSO-d6: δ 7.92 (2H, d, J = 8.7 Hz, H-2′, H-6′), δ 6.93 (2H, d, J = 8.7 Hz, H-3′, H-5′), δ 6.52 (1H, s, H-8), δ 3.77 (3H, s, OCH3), δ 3.74 (3H, s, OCH3). EI-MS: m/z 330 equivalent to molecular formula of C17H14O7.
2.3.3 3,7,4′-Trimethoxy-kaempferol (3)
Rf: 0.93 (BAW), 0.01 (H2O), 0.18 (15% AcOH). UV/Vis λmax (MeOH): 276, 333; (+NaOMe): 278, 291sh, 390; (+AlCl3): 263sh, 279, 303, 349; (+AlCl3 + HCl): 260, 283, 300, 350; (+NaOAc): 276, 298sh, 350; (+NaOAc + H3BO3): 276, 336. 1H NMR in DMSO-d6: δ 8.04 (2H, d, J = 8.7 Hz, H-2′, H-6′), δ 7.12 (2H, d, J = 8.7 Hz, H-3′, H-5′), δ 6.87 (1H, d, J = 2 Hz, H-8), δ 6.61 (1H, d, J = 2 Hz, H-6), δ 3.86 (6H, s, OCH3), δ 3.75 (3H, s, OCH3).
2.3.4 Calycopterin (5,4′-dihydroxy-3,6,7,8-tetramethoxy-flavone) (4)
Rf: 0.88 (BAW), 0.05 (H2O), 0.27 (15% AcOH). UV/Vis λmax (MeOH): 273, 341; (+NaOMe): 278, 302sh, 398; (+AlCl3): 274, 306sh, 350; (+AlCl3 + HCl): 280, 303sh, 352; (+NaOAc): 273, 300sh, 351; (+NaOAc + H3BO3): 276, 346. 1H NMR in DMSO-d6: δ 7.91 (2H, d, J = 8.5 Hz, H-2′, H-6′), δ 6.92 (2H, d, J = 8.5 Hz, H-3′, H-5′), δ 3.9 (3H, s, OCH3), δ 3.88 (3H, s, OCH3), δ 3.78 (3H, s, OCH3), δ 3.69 (3H, s, OCH3). ESI-MS: m/z [M−H]− 373 corresponding to a molecular weight of 374 and a molecular formula of C19H18O8.
2.3.5 Quercetin (3,5,7,3′,4′-pentahydroxy-flavone) (5)
Rf: 0.65 (BAW), 0.09 (H2O), 0.05 (15% AcOH). UV/Vis λmax (MeOH): 258, 267sh, 298sh, 360; (+NaOMe): 274, 324, 409; (+AlCl3): 275, 304sh, 333sh, 429; (+AlCl3 + HCl): 269, 300sh, 358sh, 403; (+NaOAc): 269, 323,373; (+NaOAc + H3BO3): 261, 301sh, 379. ESI-MS: m/z [M−H]− 301 corresponding to a molecular weight of 302 and a molecular formula of C15H10O7.
2.3.6 Quercetin-3-O-glucopyranoside (6)
Rf: 0.57 (BAW), 0.08 (H2O), 0.38 (15% AcOH). UV/Vis λmax (MeOH): 256, 267sh, 298sh, 359; (+NaOMe): 272, 326, 408; (AlCl3): 275, 304sh, 333sh, 429; (+AlCl3 + HCl): 269, 300sh, 358sh, 403; (+NaOAc): 271, 323, 373; (+NaOAc + H3BO3): 262, 301sh, 379. 1H NMR in DMSO-d6: δ 7.66 (1H, d, J = 8.5 Hz, H-6′), δ 7.55 (1H, d, J = 2 Hz, H-2′), δ 6.80 (1H, d, J = 8.5 Hz, H-5′), δ 6.37 (1H, d, J = 2 Hz, H-8), δ 6.17 (1H, d, J = 2 Hz, H-6), δ 5.37 (1H, d, J = 7.5 Hz, H-1″), δ 3–4 (5H, m).
2.3.7 Quercetin-3-methoxy-3′-O-glucopyranoside (7)
Rf: 0.52 (BAW), 0.13 (H2O), 0.39 (15% AcOH). UV/Vis λmax (MeOH): 258, 264sh, 359; (+NaOMe): 272, 327, 402; (AlCl3): 263, 300sh, 366, 405sh; (+AlCl3 + HCl): 264, 300sh, 360, 402sh; (+NaOAc): 269, 321, 369; (+NaOAc + H3BO3): 262, 370. 1H NMR in DMSO-d6: δ 8.37 (1H, d, J = 2 Hz, H-2′), δ 7.26 (1H, d, J = 7.7 Hz, H-6′), δ 6.77 (1H, d, J = 8.4 Hz, H-5′), δ 6.33 (1H, s, H-8), δ 6.13 (1H, s, H-6), δ 5.11 (1H, d, J = 7.0 Hz, H-1″), δ 3.60 (3H, s, OCH3), δ 3–4 (5H, m). ESI-MS: m/z [M−H]− 477 corresponding to a molecular weight of 478 and a molecular formula of C22H22O12.
2.3.8 5-Hydroxy-7-methoxy-isoflavone (8)
Rf: 0.91 (BAW), 0.33 (H2O), 0.41 (15% AcOH). UV/Vis λmax (MeOH): 279; 320sh (+NaOMe): 275, 361, 413sh; (+AlCl3): 271; (+AlCl3 + HCl): 272; (+NaOAc): 278; (+NaOAc + H3BO3): 279. 1H NMR in DMSO-d6: δ 8.45 (1H, s, H-2), δ 7.07 (5H, m, phenyl ring), δ 6.64 (1H, d, J = 2 Hz, H-8), δ 6.58 (1H, d, J = 2 Hz, H-6), δ 3.67 (3H, s, OCH3). 13C NMR in DMSO-d6: δ 175.8 (C-4), 167.3 (C-7), 158.0 (C-9), 156.0 (C-5), 151.9 (C-2), 136.4 (C-4′), 132.0 (C-1′), 124.0 (C-2′), 124.0 (C-6′), 122.0 (C-3′), 122.0 (C-5′), 121.0 (C-3), 106.5 (C-10), 100.1 (C-6), 97.0 (C-8), 56.3 (OCH3). EI-MS: m/z [M+H] 269 corresponding to a molecular weight of 268 and a molecular formula of C16H12O4.
2.3.9 Gentisic acid 5-O-(6′-O-galloyl)-glucopyranoside (9)
Rf: 0.29 (BAW), 0.48 (H2O), 0.43 (15% AcOH). UV/Vis λmax (MeOH): 279, 361sh; (+NaOMe): 275, 361sh; (+AlCl3): 277, 322sh; (+AlCl3 + HCl): 276, 331sh; (+NaOAc): 278; (+NaOAc + H3BO3): 279. 1H NMR in DMSO-d6: δ 7.63 (1H, d, J = 2 Hz, H-6), δ 7.19 (2H, s, H-2″, H-6″), δ 6.85 (1H, d, J = 8.6 Hz, H-4), δ 6.57 (1H, d, J = 8.6 Hz, H-3), δ 4.69 (1H, d, J = 7.5 Hz, H-1′), δ 3–4 (5H, m). 13C NMR in DMSO-d6: δ 172.9 (COOH), 166.7 (CO), 157.7 (C-2), 149.5 (C-5), 146.4 (C-3″),146.4 (C-5″), 139.0 (C-4″), 122.6 (C-1″), 119.9 (C-4), 119.2 (C-3), 116.8 (C-6), 115.9 (C-1), 109.0 (C-2″), 109.0 (C-6″), 102.4 (C-1′), 76.5 (C-3′), 75.2 (C-2′), 73.8 (C-5′), 70.7 (C-4′), 65.3 (C-6′). ESI-MS: m/z [M−H]− 467 corresponding to a molecular weight of 468 and a molecular formula of C20H20O13.
2.3.10 Gentisic acid 5-O-(2′-O-glucopyranosyl)-rhamnoside (10)
Rf: 0.39 (BAW), 0.65 (H2O), 0.71 (15% AcOH). UV/Vis λmax (MeOH): 266sh, 309; (+NaOMe): 272sh, 308. 1H NMR in DMSO-d6: δ 7.57 (1H, d, J = 2 Hz, H-6), δ 6.87 (1H, dd, J = 3.5, 8.6 Hz, H-4), δ 6.52 (1H, d, J = 8.6 Hz, H-3), δ 5.41 (1H, br. s, Δν1/2 = 2.5 Hz, H-1′), δ 4.59 (1H, d, J = 8.6 Hz, H-1″), δ 3–4 (9H, m), δ 1.12 (3H, d, J = 6.5 Hz , CH3-rhamnose). 13C NMR in DMSO-d6: δ 174.2 (COOH), 157.8 (C-2), 148.5 (C-5), 122.1 (C-4), 121.3 (C-3), 118.6 (C-6), 116.8 (C-1), 104.5 (C-1″), 102.5 (C-1′), 82.1 (C-2′), 77.3 (C-3″), 77.0 (C-5″), 75.6 (C-2″), 73.6 (C-4′), 72.8 (C-3′), 70.2 (C-5′), 69.5 (C-4″), 61.1 (C-6″), 21.0 (C-6′). ESI-MS: m/z [M+H]+ 463 corresponding to a molecular weight of 462 and a molecular formula of C19H26O13.
2.3.11 Apigenin-6-C-arabinopyranosyl-8-C-glucopyranoside (11)
Rf: 0.13 (BAW), 0.36 (H2O), 0.48 (15% AcOH). UV/Vis λmax (MeOH): 273, 333, (+NaOMe): 284, 332, 399; (+AlCl3): 279, 305, 344, 387sh; (+AlCl3 + HCl): 280, 304, 343, 387sh; (+NaOAc): 282, 308sh, 392; (+NaOAc + H3BO3): 275, 320, 347sh. 1H NMR in DMSO-d6: δ 7.9 (2H, d, J = 7.6 Hz, H-2′, H-6′), δ 6.85 (2H, d, J = 7.9 Hz, H-3′,H-5′), δ 6.5 (1H, s, H-3), δ 4.8 (1H, d, J = 8 Hz, H-1″), δ 4.6 (1H, d, J = 8 Hz, H-1‴), δ 3.2–4 (9H, m). 13C NMR in DMSO-d6: δ 182.6 (C-4), 164.5 (C-2), 161.6 (C-5), 161.6 (C-7), 158.7 (C-4′), 155.6 (C-9), 129.4 (C-2′), 129.4 (C-6′), 121.9 (C-1′), 116.4 (C-3′), 116.4 (C-5′), 108.4 (C-6), 105.3 (C-8), 103.9 (C-10), 103 (C-3), 82.2 (C-5‴), 79 (C-3″), 74.6 (C-1″), 74 (C-2″), 73.7 (C-1‴), 71.5 (C-2‴), 71 (C-3‴), 70.6 (C-4‴), 69.6 (C-4″), 69 (C-5″), 61.4 (C-6‴). ESI-MS: m/z [M−H]− 563 corresponding to a molecular weight of 564 and a molecular formula of C26H28O14.
2.3.12 Apigenin-6,8-di-C-glucopyranoside (12)
Rf: 0.13 (BAW), 0.34 (H2O), 0.48 (15% AcOH). UV/Vis λmax (MeOH): 273, 333, (+NaOMe): 283, 332, 400; (+AlCl3): 280, 305, 346, 388; (+AlCl3 + HCl): 279, 305, 345, 387; (+NaOAc): 282, 309sh, 3902; (+NaOAc + H3BO3): 274, 323, 349sh. 1H NMR in DMSO-d6: δ 7.9 (2H, d, J = 8.5 Hz, H-2′, H-6′), δ 6.85 (2H, d, J = 8.5 Hz, H-3′,H-5′), δ 6.5 (1H, s, H-3), δ 4.8 (1H, d, J = 8.6 Hz, H-1″), δ 4.6 (1H, d, J = 8.6 Hz, H-1‴), δ 3–4 (10H, m). ESI-MS: m/z [M−H]− 593 corresponding to a molecular weight of 594 and a molecular formula of C27H30O15.
2.3.13 Quercetin-3, 7-di-O-glucopyranoside (13)
Rf: 0.18 (BAW), 0.33 (H2O), 0.63 (15% AcOH). UV/Vis λmax (MeOH): 258, 267sh, 355; (+NaOMe): 270, 308sh, 405; (AlCl3): 270, 309sh, 436; (+AlCl3 + HCl): 266, 303sh, 357sh, 406; (+NaOAc): 261, 310sh, 387; (+NaOAc + H3BO3): 263, 306sh, 377. 1H NMR in DMSO-d6: 7.55 (2H, dd, J = 2.5 Hz, H-2′; J = 7.5 Hz, H-6′), δ 7.15 (1H, d, J = 8.5 Hz, H-5′), δ 6.80 (1H, d, J = 2 Hz, H-8), δ 6.45 (1H, d, J = 2 Hz, H-6), δ 5.35 (1H, d, J = 8 Hz, H-1″), δ 4.85 (1H, d, J = 8 Hz, H-1‴), δ 3–3.8 (10H, m).
2.3.14 Rhamnetin-3-O-rhamnopyranoside (14)
Rf: 0.71 (BAW), 0.25 (H2O), 0.54 (15% AcOH). UV/Vis λmax (MeOH): 257, 264 sh., 350; (+NaOMe): 267, 303sh, 398; (+AlCl3): 274, 304sh, 427; (+AlCl3 + HCl): 271, 301sh, 352, 399; (+NaOAc): 260, 302sh, 366; (+NaOAc + H3BO3): 262, 290sh, 372. 1H NMR in DMSO-d6: δ 7.33 (1H, d, J = 2 Hz, H-2′), δ 7.27 (1H, dd, J = 2, 8.2 Hz, H-6′), δ 6.85 (1H, d, J = 8.1 Hz, H-5′), δ 6.69 (1H, d, J = 2 Hz, H-8), δ 6.39 (1H, d, J = 2 Hz, H-6), δ 5.28 (1H, d, J = 2.5 Hz, H-1″), δ 3.86 (3H, s, OCH3), δ 0.81 (3H, d, J = 5.5 Hz , CH3-rhamnose).
2.3.15 Myricetin (3,5,7,3′,4′,5′-heahyroxy-flavone) (15)
Rf: 0.43 (BAW), 0.05 (H2O), 0.26 (15% AcOH). UV/Vis λmax (MeOH): 265, 300 sh, 361; (+NaOMe): 269, 328sh, 408; (+AlCl3): 272, 310, 427; (+AlCl3 + HCl): 271, 306, 362, 411; (+NaOAc): 270, 401; (+NaOAc + H3BO3): 266, 391. 1H NMR in DMSO-d6: δ 7.15 (2H, d, J = 2 Hz, H-2′, H-6′), δ 6.33 (1H, d, J = 2 Hz, H-8), δ 6.16 (1H, d, J = 2 Hz, H-6).
2.3.16 Myricetin-3-O-glucopyranoside (16)
Rf: 0.48 (BAW), 0.07 (H2O), 0.27 (15% AcOH). UV/Vis λmax (MeOH): 264, 285 sh, 357; (+NaOMe): 272, 397; (+AlCl3): 272, 361sh, 411; (+AlCl3 + HCl): 270, 361, 402; (+NaOAc): 269, 362; (+NaOAc + H3BO3): 265, 384. 1H NMR in DMSO-d6: δ 7.20 (2H, d, J = 2 Hz, H-2′, H-6′), δ 6.35(1H, d, J = 2 Hz, H-8), δ 6.20 (1H, d, J = 2 Hz, H-6), δ 5.42 (1H, d, J = 8.5 Hz, H-1″), δ 3–3.8 (5H, m).
2.3.17 Gallic acid (17)
Rf: 0.81 (BAW), 0.61 (H2O), 0.60 (15% AcOH). UV/Vis λmax (MeOH): 215, 272; 1H NMR in DMSO-d6: δ 6.9 (2H, s, H-2, H-6). EI-MS: m/z 170 equivalent to molecular formula of C7H6O5.
3 Results and discussion
3.1 Botanical investigations
3.1.1 Macromorphological and micromorphological characters
The macro, and micromorphological characters of the stem, petiole and lamina were similar with those of Hamed and Tantawy (1990 and 1991) and the floral characters were given in Tantawy et al. (2005).
3.2 Chemical investigations
Seventeen compounds (14 flavonoids and 3 phenolic acids) were isolated from the investigated taxa and identified using chemical and physical investigations. Ten compounds were isolated from P. senegalensis and identified as: Apigenin (5,7,4′-trihydroxyflavone) (1), 3,6-dimethoxy-kaempferol (2), 3,7,4′-trimethoxy-kaempferol (3), calycopterin (5,4′-dihydroxy-3,6,7,8-tetramethoxy-flavone) (4), quercetin (3,5,7,3',4'-pentahydroxy-flavone) (5), quercetin-3-O-glucopyranoside (6), quercetin-3-methoxy-3′-O-glucopyranoside (7), 5-hydroxy-7-methoxy-isoflavone (8), gentisic acid-5-O-(6′-O-galloyl)-glucopyranoside (9) and gentisic acid-5-O-(2′-O-glucopyranosyl)-rhamnoside (10).
Eight compounds were isolated from P. equisetiforme and identified as: quercetin-3-O-glucopyranoside (6), Apigenin-6-C-arabinopyranosyl-8-C-glucopyranoside (11), apigenin-6,8-di-C-glucopyranosyl (12), quercetin-3,7-di-O-glucopyranoside (13), rhamnetin-3-O-rhamnopyranoside (14), myricetin (3,5,7,3',4',5'-pentahydroxy-flavone) (15), myricetin-3-O-glucopyranoside (16) and gallic acid (17).
Compound 10 (Fig. 1) which was isolated from P. senegalensis and identified as gentisic acid-5-O-(2′-O-glucopyranosyl)-rhamnoside, is a new compound identified for the first time in nature. Six compounds 2, 3, 4, 7, 8 and 9 were isolated for the first time from the plant and the remaining three compounds were previously isolated from the same plant by Midiwo and Owuor (1992), Midiwo et al. (1992, 1994, 2002). Compounds 6, 11, 12, 13, 14, 15, 16 and 17 from P. equisetiforme were isolated for the first time from the plant. The distribution of these compounds was shown in Table 1. + = Present, − = absent.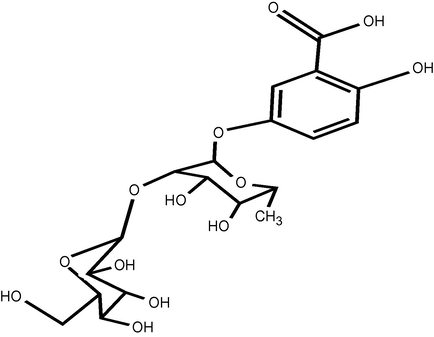
Structure of compound 10.
Compound
Persicaria salicifolia
Persicaria senegalensis
Polygonum bellardii
Polygonum equisetiforme
Apigenin (5,7,3'-trihodroxy-flavone) (1)
−
+
−
−
Apigenin-6-C-arabino-pyranosyl- 8-C-glucopyranoside (11)
+
−
−
+
Apigenin-6,8 di C-gluco-pyranoside (12)
−
−
−
+
3,6-Dimethoxy – Kaempferol (2)
−
+
−
−
3,7,4′-Trimethoxy – Kaempferol (3)
+
+
−
−
Calycopterin (5,4′-dihydroxy-3,6,7,8-tetramethoxy-flavone) (4)
−
+
−
−
Quercetin (3,5,7,3',4'-pentahydroxy-flavone) (5)
+
+
+
−
Quercetin-3-O-gluco-pyranoside (6)
+
+
−
+
Quercetin-3,7-di-O-gluco-pyranoside (13)
+
−
−
+
Quercetin-3-methoxy-3′-O-glucopyranoside (7)
−
+
−
−
Rhamnetin-3-O-rhamno-pyranoside (14)
−
−
−
+
Myricetin (3,5,7,3',4',5'-heahyroxy-flavone) (15)
−
−
−
+
Myricetin-3-O-gluco-pyranoside (16)
−
−
−
+
5-Hydroxy-7-methoxy-isoflavone (8)
+
+
−
−
Gallic acid (17)
−
−
−
+
Gentisic acid 5-O-(6′-O-galloyl)-glucopyranoside (9)
−
+
−
−
Gentisic acid 5-O-(2′-O-glucopyranosyl)-rhamnoside (10)
+
+
−
−
Compound 10 was isolated from the H2O fraction as white crystals after separation and purification on a Sephadex LH-20 column. Complete acid hydrolysis of 10 afforded rhamnose and glucose as sugar moiety, identified by co-chromatography with authentic samples. The ESI-MS of 10 showed the molecular ion peak as the base peak [M+H]+ at 463 m/z which is equal to the Mt 462 corresponding to the molecular formula C19H26O13. 1H NMR spectrum of 10 showed signals at δ 7.57, 6.87 and 6.52 assigned for H-6, H-4 and H-3, respectively, which is equivalent to gentisic acid protons (Sakushima et al., 1995). In addition, there is a broad signal at δ 5.41 for the rhamnose sugar and a doublet signal at δ 4.59 for the anomeric sugar protons with J = 8.6 Hz which is consistent for the glucose moiety. The 13C NMR spectrum confirmed that 10 is gentisic acid (Sakushima et al., 1995) with rhamnose and glucose sugar. The C-2′ of rhamnose was shifted downfield from 71 to 82.1 ppm and the upfield shift of C-1′ from 105 to 102.5 ppm, indicated that the interglycosidic linkage between the glucose and rhamnose is glucosyl (1″ → 2′) rhamnoside (Agrawal, 1989). From the above data, compound 10 was identified as gentisic acid-5-O-(2′-O-glucopyranosyl)-rhamnoside.
3.3 Chemosystematics
P. salicifolia and P. senegalensis are characterized by the presence of 3,7,4′-trimethoxy-kaempferol, 5-hydroxy-7-methoxy-isoflavone and gentisic acid glycosides. These compounds were not detected in the Polygonum species while quercetin 3,7-di-O-β-glucopyranoside and apigenin-6-C-α-arabinopyranosyl-8-C-β-glucopyranoside are present in P. salicifolia and P. equisetiforme. In addition, no methylated flavonoids were detected in P. bellardii and only one methylated flavonoid (rhamnetin-3-O-α-rhamnopyranoside) is detected in P. equisetiforme. P. equisetiforme is also characterized by the capability to form 3,4,5-trihydroxy nuclei in the form of myricetin and gallic acid.
The distribution of methylated flavonoids within the genus Persicaria in the present study agrees with the observations that methylated flavonols were detected in some species of sections Echinocaulon and Persicaria (Kuroyanagi and Fukushima, 1982; Kawasaki et al., 1986; Park, 1987). Furthermore; and according to Harborne (1977) and Kawasaki et al. (1986); methylation of flavonoids hydroxyl groups represents a more advanced step in the biosynthesis of flavonoids.
3.4 Numerical analysis
In order to determine the position of Persicaria compared to Polygonum, a dataset for numerical analysis was constructed. These data included 111 macro-, micromorphological and chemical characters. Each character used in the numerical analysis was treated as a binary character in data matrix using Minitab statistical software 13 (Minitab Inc., 1999).The dendogram formed based on the numerical analysis is illustrated in Fig. 2. At similarity level of 35.91%, the applied operational taxonomic units were separated into two main groups. Group (I) includes P. salicifolia and P. senegalensis while group (II) includes P. bellardii and P. equisetiforme. The taxa of group (I) separated at similarity level of 78.22% while the taxa of group (II) separated at similarity level of 66.76%. The grouping of Persicaria species and Polygonum species under investigation in two distinct groups is evidence for the treatment of Persicaria and Polygonum as two distinct entities.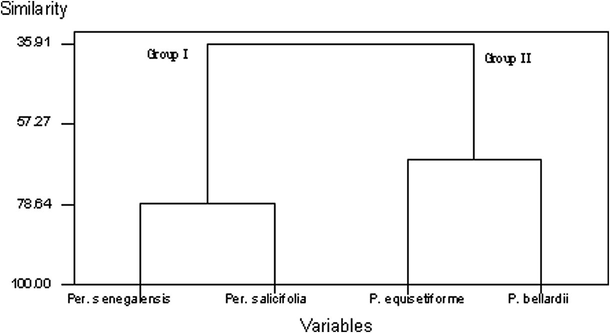
Dendrogram for the morphological, anatomical and chemical characters.
4 Conclusion
The isolation and identification of 14 flavonoids and three phenolic acids in the four investigated taxa along with their macro- and micromorphological data formed the basis for a numerical analysis of the four investigated Persicaria and Polygonum species. The data presented above supported the treatment of Persicaria and Polygonum as two distinct entities and the same result was confirmed by using the numerical analysis.
References
- Carbon-13 NMR of Flavonoids. Amsterdam, Oxford, NewYork, Tokyo: Elsevier; 1989.
- Flora of Egypt. Vol vol. 1. Cairo, Egypt: Al Hadara Puplishing; 1999.
- Polygonaceae. In: Kubitzki K., Rohwer J.G., Bittrich V., eds. The Families and Genera of Vascular Plants. Vol vol. 2. Berlin Heidelberg: Springer Verlag; 1993. p. :531-544.
- [Google Scholar]
- Phenylvaleric acid and flavonoid glycosides from Polygonum salicifolium. J. Nat. Prod.. 1999;62:1101-1105.
- [Google Scholar]
- Quercetin 3-(2″-O-galloylglucoside), a molluscicidal flavonoid from Polygonum senegalense. Phytochemistry. 1980;19:482.
- [Google Scholar]
- Antimicrobial activity of Polygonum equisetiforme extracts and flavonoids. Phytother. Res.. 1992;6:265-269.
- [Google Scholar]
- The stem anatomy of some polygonaceae and its diagnostic significance in identification. J. Fac. Educ.. 1990;15:249-268.
- [Google Scholar]
- The microcharacters of the petiole and blade of certain polygonaceae as an identificatory tool. Ain Shames Sci. Bull.. 1991;28B:389-406.
- [Google Scholar]
- Anatomy and taxonomy in Polygonaceae subfam. Polygonoideae Meisn. Emend. Symb. Bot. Upsal.. 1978;22:1-95.
- [Google Scholar]
- Comparative Biochemistry of Flavonoids. London: Academic; 1967.
- Flavonoids and the evolution of the angiosperms. Biochem. Syst. Ecol.. 1977;5:7-32.
- [Google Scholar]
- Polygonaceae. In: Hegnauer R., ed. Chemotaxonomie der pflanzen. Vol vol. 5. Basel: Birkhäuser Verlag; 1969. p. :361-383.
- [Google Scholar]
- Pollen morphology of the genera Polygonum s. str. and Polygonella (Polygoneae: Polygonaceae) Plant Syst. Evol.. 2005;254:13-30.
- [Google Scholar]
- Flavonoids in the leaves of twenty-eight polygonaceous plants. Bot. Mag. Tokyo. 1986;99:63-74.
- [Google Scholar]
- Highly oxygenated flavonoids from Polygonum orientale. Chem. Pharm. Bull.. 1982;30:1163-1168.
- [Google Scholar]
- Foliar anatomy of Polygonum (Polygonaceae): Survey of epidermal and selected internal structures. Plant Syst. Evol.. 1992;182:71-106.
- [Google Scholar]
- The Systematic Identification of Flavonoids. New York: Springer Verlag; 1970.
- A new chalcone as a natural molluscicide from Polygonum senegalense. Phytochemistry. 1978;17:823-824.
- [Google Scholar]
- Techniques of Flavonoid Identification. New York: Academic Press; 1982.
- Midiwo, J.O., Owuor, F.A.O., 1992. Epicuticular flavonoids of Psiadia puntulata and Polygonum senegalense. In: 5th NAPRECA Symposium on Natural Products, Antananarivo, Madagascar, p. 81.
- Flavonoids of Polygonum senegalense (Meisn) part II: more surface and internal tissue flavonoid aglycones. Bull. Chem. Soc. Ethiop.. 1992;6:119-122.
- [Google Scholar]
- Flavonoids of Polygonum senegalense Part III: Isolation of dihydrochalcone glucoside and quercetin glycosides. Bull. Chem. Soc. Ethiop.. 1994;8:79-84.
- [Google Scholar]
- Bioactive compounds from some Kenyan ethnomedicinal plants: Myrsinaceae, Polygonaceae and Psiadia punctulata. Phytochem. Rev.. 2002;1:311-323.
- [Google Scholar]
- The first 9-hydroxyhomoisoflavanone, and antiplasmodial chalcones, from the aerial exudates of Polygonum senegalense. Arkivoc (ix):21-27.
- [Google Scholar]
- Minitab, Inc., 1999. Minitab version 13 statistical software.
- Montasir, A.H., Hassib, M., 1956. Illustrated manual flora of Egypt. Part 1, Ed. 1, Bull. Fac. Sc. Ain Shams Univ., Cairo, Egypt.
- Flavonoid chemistry of Polygonum sect. Echinocaulon: a systematic survey. Syst. Bot.. 1987;12:167-179.
- [Google Scholar]
- Generic limits in Polygonum and related genera (Polygonaceae) on the basis of floral characters. Bot. J. Linn. Soc.. 1988;98:321-371.
- [Google Scholar]
- Students' Flora of Egypt (second ed.). Cairo University; 1974.
- Floral morphology of some Taxa of Polygonaceae in Egypt. J. Biotechnol.. 2005;21:308-329.
- [Google Scholar]







