Translate this page into:
Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes
⁎Corresponding author. Tel.: +964 7901782816. emad_yousif@hotmail.com (Emad Yousif)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The photostabilization of poly(methyl methacrylate) (PMMA) films by new types of 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole with Sn(II), Ni(II), Zn(II), and Cu(II) complexes was investigated. The PMMA films containing concentration of complexes 0.5% by weight were produced by the casting method from chloroform solvent. The photostabilization activities of these compounds were determined by monitoring the hydroxyl index with irradiation time. The changes in viscosity average molecular weight of PMMA with irradiation time were also tracked (using benzene as a solvent). The quantum yield of the chain scission (Φcs) of these complexes in PMMA films was evaluated and found to range between 5.22 × 10−5 and 7.75 × 10−5. Results obtained showed that the rate of photostabilization of PMMA in the presence of the additive followed the trend: According to the experimental results obtained, several mechanisms were suggested depending on the structure of the additive. Among them, UV absorption, peroxide decomposer and radical scavenger for photostabilizer mechanisms were suggested.
Keywords
Photochemistry
PMMA
UV–vis spectroscopy
Photostabilizer
UV absorber
1,3,4-Oxadiazole
Carboxylate

1 Introduction
Ultraviolet light stabilizers are used widely in plastics, cosmetics, and films. In outdoor applications where the materials are exposed to UV solar radiation, the energy of this radiation is sufficient to initiate photochemical reaction leading to degradation. Plastics are commonly protected against such deterioration by the addition of antioxidants, light and heat stabilizers (Chmela et al., 2001). The main purpose of the UV-stabilizer is to prevent polymers from photodegradation or photocrosslinking caused by ultraviolet light presented in sunlight and an artificial light source. Ultraviolet light stabilizers are divided into inorganic UV-stabilizers, organic UV-stabilizers, and other kinds. Inorganic UV-stabilizers, such as iron oxide, titanium oxide, chromic oxide, and carbon black, usually cannot evenly distribute in the plastic substrate so much as to be incompatible with the polymer matrix. The final effects depend on the particle size and concentration (Zhao and Dan, 2006). These drawbacks limit the application of inorganic UV-stabilizers.
Organic UV-stabilizers, generally with small molecular weight, include fluorescent compounds, phenyl-ester of benzoic acid, hydroxylbenzophenone, benzotriazoles, etc. By the addition of these stabilizers to plastic materials, problems such as migration, incompatibility, volatility, and solvent extraction will inevitably occur. It leads to a strong diminution of the materials’ utilization. To resolve such problems, many approaches have been developed, such as preparing a reactive UV-stabilizer; (Grassie and Scott, 1985) introducing compatible side chains, or chemical anchoring of the additive to the polymer backbone, etc. (Andrady et al., 1988). Among these methods, preparing the high molecular weight UV stabilizer is a highlight because, for most of the polymer materials, blending is the first choice to enhance their UV-resistance. Meanwhile, different high molecular weight UV-stabilizers can be prepared by the copolymerization of a reactive UV-stabilizer with other monomers. At this point, it is very convenient to ameliorate the compatibility between the UV-stabilizer and plastic matrix by preparing suitable high molecular weight UV-stabilizer (Harper et al., 1974). As part of our on-going research on the photostabilization of PMMA, the photostabilization of PMMA was studied using 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole with Sn(II), Ni(II), Zn(II), and Cu(II) complexes. To our knowledge there is no attempt to investigate the photostabilization of PMMA films by complexes containing 1,3,4-oxadiazole rings, therefore, in this article we report the designing of some oxadiazole complexes and studied their use as a photostabilizing reagent.
2 Experimental
2.1 Materials
2-Thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes were all prepared by the method previously described by Ibraheem et al. (2010a,b).
2-Thioacetic acid-5-phenyl-1,3,4-oxadiazole
(L)
Bis[2-thioacetic acid-5-phenyl-1,3,4-oxadiazole]tin(II)
Sn(L)2
Bis[2-thioacetic acid-5-phenyl-1,3,4-oxadiazole]nickel(II)
Ni(L)2
Bis[2-thioacetic acid-5-phenyl-1,3,4-oxadiazole]zinc(II)
Zn(L)2
Bis[2-thioacetic acid-5-phenyl-1,3,4-oxadiazole]copper(II)
Cu(L)2
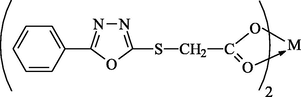 where
.
where
.
3 Experimental techniques
3.1 Films preparation
Commercial PMMA was re-precipitated from chloroform solution by alcohol several times and finally dried under vacuum at room temperature for 24 h. Fixed concentrations of PMMA solution (5 g/100 ml) in chloroform were used to prepare polymer films with 40 μm thickness (measured by a micrometer type 2610 A, Germany). The prepared complexes (0.5% concentrations) were added to the films starting at 0 concentration (blank). It was necessary to control the hygrometry and the rate of evaporation of the solvent during casting to maintain good optical quality and very limited turbidity. The film transmission should be greater than 80% in the near-UV range. The films were prepared by evaporation technique at room temperature for 24 h. To remove the possible residual chloroform solvent, film samples were further dried at room temperature for 3 h under reduced pressure. The films were fixed on stands especially used for irradiation. The stand is provided with an aluminum plate (0.6 mm in thickness) supplied by Q-panel company.
3.2 Irradiation experiments
3.2.1 Accelerated testing technique
Accelerated weather-meter Q UV tester (Q panel, company, USA), was used for irradiation of the polymer films. The accelerated weathering tester contains a stainless steel plate, which has two holes in the front side and a third one behind. Each side contains a lamp (type fluorescent ultraviolet lights) 40 W each. These lamps are of the type UV-B 313 giving a spectral range between 290 and 360 nm with a maximum wavelength of 313 nm. The polymer film samples were vertically fixed parallel to the lamps to make sure that the UV incident radiation is perpendicular to the samples. The irradiated samples were rotated from time to time to ensure that the intensity of light incident on all the samples is the same.
3.3 Photodegradation measuring methods
3.3.1 Measuring the photodegradation rate of polymer films using infrared spectrophotometry
The degree of photodegradation of the polymer film samples was followed by monitoring the FTIR spectra in the range of 4000–400 cm−1 using FTIR 8300 Shimadzu Spectrophotometer. The position of hydroxyl absorption is specified at 3430 cm −1 (Rabek and Ranby, 1975).
The progress of photo-degradation during different irradiation times was followed by observing the changes in the hydroxyl peak. Then the hydroxyl index (IOH) was calculated by comparison of the FTIR absorption peak at 3430 cm−1 with a reference peak at 1450 cm−1. This method is called band index method which includes (Rabek and Ranby, 1975):
3.3.2 Determination of average molecular weight using viscometry method
The viscosity property was used to determine the average molecular weight of the polymer, using the Mark–Houwink relation (Mark, 2007).
4 Results and discussion
2-Thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes were used as additives for the photostabilization of PMMA films. In order to study the photochemical activity of these additives for the photostabilization of PMMA films, the hydroxyl index was monitored with irradiation time using IR spectrophotometry. The irradiation of PMMA films with UV light of wavelength, λ = 313 nm led to a clear change in the FTIR spectrum. Appearance of bands in 3430 cm−1 was attributed to the formation of the hydroxyl group (Andrady and Searle, 1989). The absorption of the hydroxyl group was used to follow the extent of polymer degradation during irradiation. This absorption was calculated as the hydroxyl index. It is reasonable to assume that the growth of the hydroxyl index is a measure of the extent of degradation. However, in Fig. 1, the IOH of Sn(L), Zn(L)2, Cu(L)2 and Ni(L)2 showed a lower growth rate with irradiation time with respect to the PMMA control film without additives. Since the growth of the hydroxyl index with irradiation time is lower than the PMMA blank, as seen in Fig. 1, it is suitable to conclude that these additives might be considered as photostabilizers of the PMMA polymer. An efficient photostabilizer shows a longer induction period. Therefore, [1] is considered as the most active photostabilizer, followed by Ni(L)2, Cu(L)2, Zn(L)2 and Sn(L)2 which is the least active.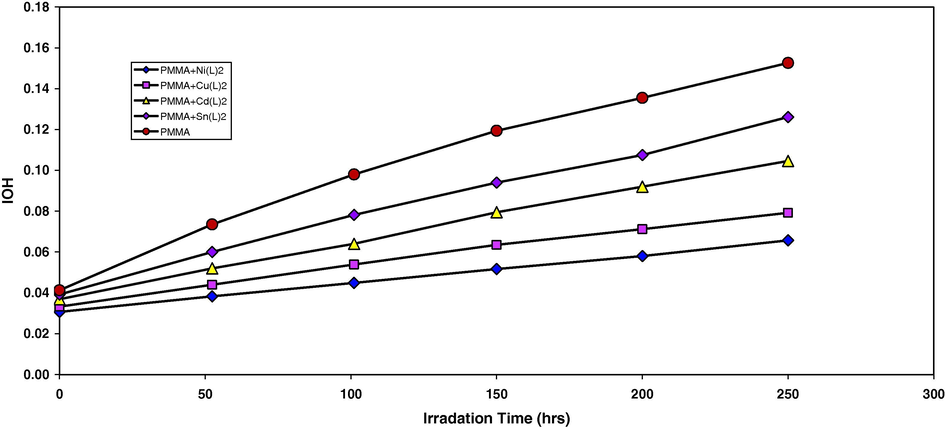
The relationship between the hydroxyl index and irradiation time for PMMA films (40 μm thickness). Containing different additives, concentration of additives are fixed at 0.5% by weight.
4.1 Variation of PMMA molecular weight during photolysis in the presence of by 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes
Analysis of the relative changes in viscosity average molecular weight
has been shown to provide a versatile test for random chain scission. Fig. 2 shows the plot of
versus irradiation time for the PMMA film with and without 0.5% (wt/wt) of the selected additives, with absorbed light intensity of 1.052 × 10−8 ein dm−3 s−1.
is measured using Eq. (3) with benzene as the solvent at 25 °C. It is worth mentioning that traces of the films with additives are not soluble in chloroform indicating that cross-linking or branching in the PMMA chain does occur during the course of photolysis (Mori et al., 1997). For better support of this view, the number of average chain scissions (average number cut per single chain) (S) (Shyichuk and White, 2000) was calculated using the relation:
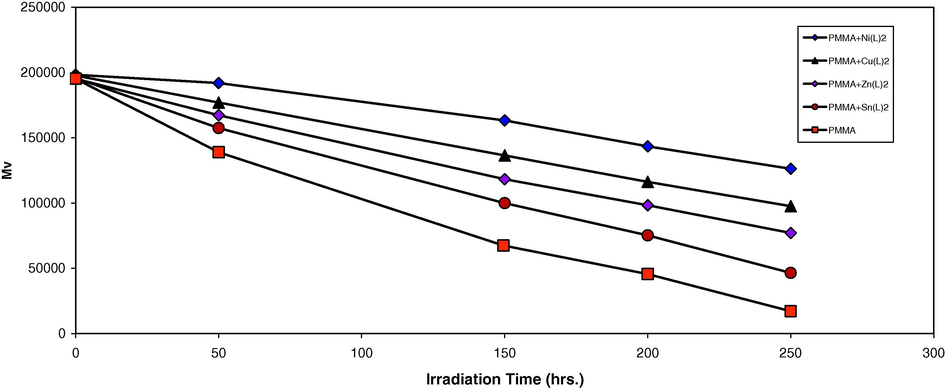
Changes in the viscosity average molecular weight
during irradiation of PMMA films (40 μm) (control) and with 0.5 wt% of additives.
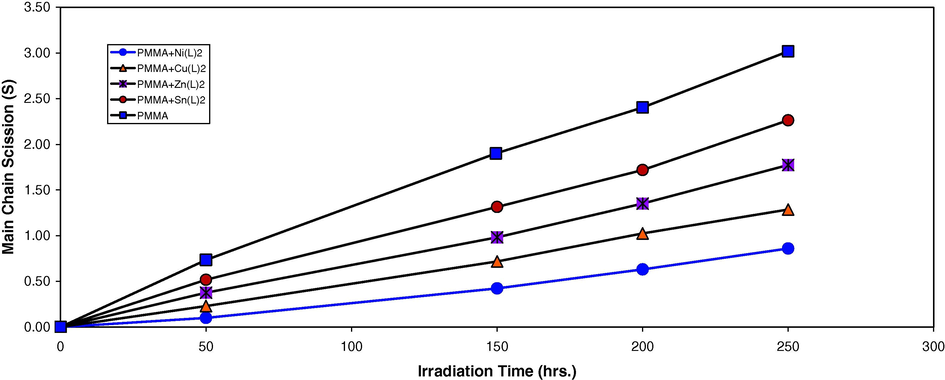
Changes in the main chain scission (S) during irradiation of PMMA films (40 μm) (control) and with 0.5 wt% of additives.
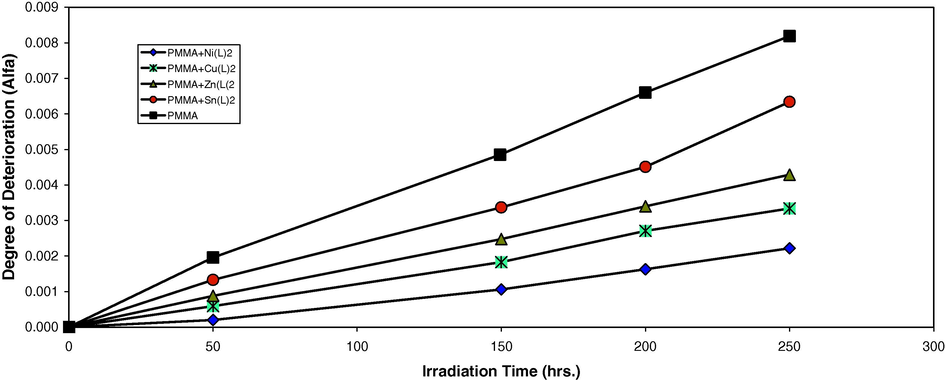
Changes in the degree of deterioration during irradiation of PMMA films (40 μm) (control) and with 0.5 wt% of additives.
The Φcs values for complexes are tabulated in Table 1.
Additive (0.5%wt)
Quantum yield of main chain scission (Φcs)
PMMA + Ni(L)2
5.22 × 10−5
PMMA + Cu(L)2
4.45 × 10−5
PMMA + Zn(L)2
7.56 × 10−5
PMMA + Sn(L)2
7.75 × 10−5
PMMA (blank)
4.55 × 10−5
The Φcs values for PMMA films in the presence of additive are less than that of additive free PMMA (blank), which increase in the order:
4.2 Suggested mechanisms of photostabilization of PMMA by 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes
Depending on the overall results obtained, the efficiency of 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes as stabilizer for PMMA films can be arranged according to the change in the hydroxyl concentration as a reference for comparison as shown in Figs. 1–4, as follows:
Metal chelate complexes generally known as photostabilizers for PMMA through both peroxide decomposer and excited state quencher. Therefore, it is expected that these complexes act as a peroxide decomposer through the following proposed mechanism Scheme 1. This mechanism is adopted by Yousif et al. (2011).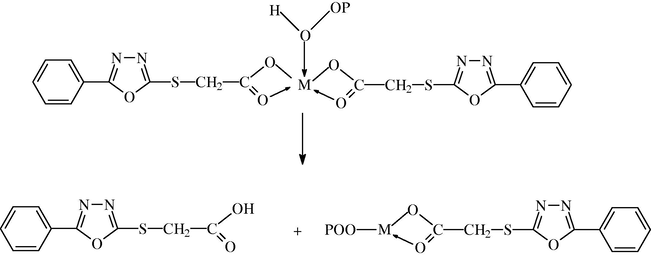
Suggested mechanism of photostabilization of complexes as peroxide decomposer.
These metal chelate complexes also function as radical scavengers through energy transfer and by forming un-reactive charge transfer complexes between the metal chelate and excited state of the chromophore (POO•) and stabilize through resonating structures as shown in Scheme 2. This mechanism is in agreement with that reported by Adil et al. (2011).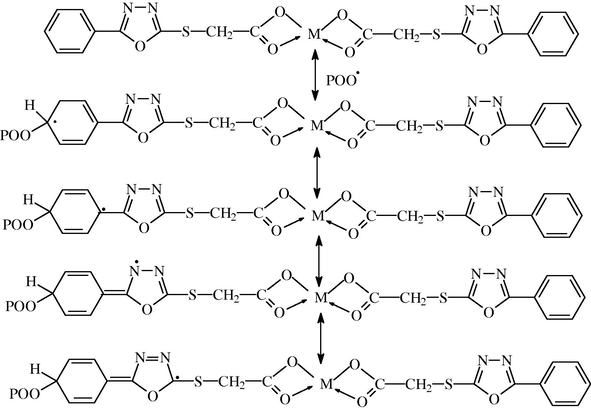
Suggested mechanism of photostabilization of carboxylates complexes as radical scavengers through energy transfer and forming unreactive charge transfer and stabilize through resonating structure.
The ring of 1,3,4-oxadiazole in this compound plays an important role in the mechanism of the stabilization process by acting as a UV absorber. The UV light absorption by these additives containing benzothiazol dissipates the UV energy to harmless heat energy (Scheme 3). Further more this ring plays a role in resonating structures conjugation of the radical in peroxide decomposer, which supports this compound as a photostabilizer (Yousif et al., 2009).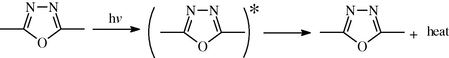
Suggested mechanism of photostabilization of 1,3,4-oxadiazole as UV absorber.
5 Conclusions
In the work described in this paper, the photostabilization of PMMA films using 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes were studied. These additives behave successfully as photostabilizer for PMMA films. The additives take the following order in photostabilization activity according to their decrease in the hydroxyl index for PMMA films. These additives stabilize the PMMA films through UV absorption or screening, peroxide decomposer and radical scavenger mechanisms. The Ni(L)2 complex was found to be the more efficient in the photostabilization process according to the photostability and mechanisms mentioned above. These mechanisms support the idea of using 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes as commercial stabilizer for PMMA.
Acknowledgments
The authors acknowledge the IIE, SRF and are grateful to the Universiti Kebangsaan Malaysia for funding (“Code UKM-GUP-NBT-08-27-113”, “UKMGGPM-NBT-164-2010” and “UKM-OUP-NBT-29-150/2011”), and the Department of Chemistry, College of Science, Al-Nahrain University.
References
- Adil, H., Emad Yousif, E., Salimon, J. 2011. New Stabilizers For PVC Based on Benzothiazole Complexes. LAMBERT Academic Publishing, Germany.
- Photodegradation of rigid PVC formulations II. Spectral sensitivity to light-induced yellowing by polychromatic light. J. Appl. Polym. Sci.. 1989;37:2789-2802.
- [Google Scholar]
- Effects of increased solar ultraviolet radiation on materials. In environmental effects of ozone depletion. J. Photochem. Photobiol. B Biol.. 1988;46:96-103.
- [Google Scholar]
- Polymer Degradation and Stabilization. London: Cambridge University Press; 1985.
- Mechanism of Polymer Degradation and Stabilization. Amsterdam: Elsevier; 1990.
- Photostabilizing effect of Ni(II) chelates in polymers II. Effect of diamagnetic chelates on polypropylene phosphorescence. J. Appl. Polym. Sci. (311):2802-2805.
- [Google Scholar]
- Synthesis, characterization and antimicrobial activity of some metal ions with 2-thioacetic-5-phenyl-1,3,4-oxadiazole. J. Al-Nahrain Univ.. 2010;13:43-47.
- [Google Scholar]
- Synthesis, characterization and antimicrobial activity of some metal ions with 2-thioacetic-5-phenyl-1,3,4-oxadiazole. J. Al-Nahrain Univ.. 2010;13:43-47.
- [Google Scholar]
- Physical Properties of Polymers Handbook. New York: Springer; 2007.
- Studies on photodegradation of poly(vinyl chloride) (part I) Die Angew. Mak-omolekulare Chemie.. 1997;64(1):89-99.
- [Google Scholar]
- Photodegradation of poly(methyl methacrylate) by monochromatic light: Quantum yield, effect of wavelengths, and light intensity. J. Appl. Polym. Sci.. 1990;41:363-889.
- [Google Scholar]
- Rabek, J., Ranby, B., 1975. Photodegradation, Photo-oxidation and Photostabilization of Polymers, John Wiley, New York.
- Analysis of chain-scission and crosslinking rates in the photo-oxidation of polystyrene. J. Appl. Polym. Sci.. 2000;77(13):3015-3023.
- [Google Scholar]
- Improved photostability of PVC films in the presence of 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes. Turk. J. Chem.. 2009;33:339-410.
- [Google Scholar]
- Synthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ring. Inter. J. Chem.. 2010;2:65-80.
- [Google Scholar]
- Improvement of the photostabilization of pvc films in the presence of thioacetic acid benzothiazole complexes. Malays. J. Anal. Sci.. 2011;15(1):81-92.
- [Google Scholar]
- Preparation and characterization of a high molecular weight UV-stabilizer based on a derivative of 2,4-dihydroxybenzophenone and Its application in polymer materials. J. Appl. Polym. Sci.. 2006;102:2203-2211.
- [Google Scholar]






