Translate this page into:
Physicochemical features and antioxidant activity of polysaccharides from Herba Patriniae by gradient ethanol precipitation
⁎Corresponding author at: Gansu Vocational College of Agriculture, No. 425, Duanjiatan Rd, Lanzhou 730020, PR China. lzdxhui@163.com (Heping Hui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Four polysaccharides BJP50, BJP60, BJP70 and BJP80 (total named BJPs) were separated from Herba Patriniae via gradient ethanol precipitation with 50%, 60%, 70% and 80%, respectively. After decolorization and deproteinization, their physicochemical features and antioxidant activities were investigated. The results showed that the total sugar content of BJPs accounted above 50% but no protein contained, while BJP50 and BJP60 contained a small amount of uronic acid. GC analysis indicated that BJPs were mainly composed of galactose, arabinose, glucose, mannose, xylose and rhamnose. From BJP50 to BJP80, the types of monosaccharides and the content of arabinose, glucose and mannose increased but that of galactose and rhamnose decreased, and their molecular weights gradually reduced from 2.3 × 106 to 4.5 × 103. BJPs had a good thermal stability with the order of BJP80 > BJP70 > BJP60 > BJP50. The vitro bioactivity assay showed that BJP80 and BJP70 exhibited stronger scavenging capacities for DPPH, ABTS and hydroxyl free radicals than that of BJP60 and BJP50. As the concentration reached 4 mg/mL, the scavenging capacities of BJP80 and BJP70 on DPPH and hydroxyl free radical were up to 92% and 95%, respectively, and their antioxidant activities gradually approached to the positive control.
Keywords
Herba Patriniae polysaccharides
Gradient ethanol precipitation
Physicochemical features
Antioxidant activity
1 Introduction
Herba Patriniae is a perennial herb belonging to the family of Patriniae. The medicinal plants are Patrinia scabiosaefolia Fisch. Ex Trev, Patrinia villosa Juss or the whole plants with roots of their relatives (Li et al., 2021). They are distributed in most areas of China and commonly used as edible wild vegetables in the folk. The Herba Patriniae has been used for thousands of years in China as a traditional Chinese medicine with dispelling blood stasis, relieving pain, heat-clearing, detoxification, eliminating carbuncle, purging pus, calming the mind and nerves, protecting the liver and promoting choleretic effects (Gong et al., 2021; Cheng et al., 2007; Xie et al., 2015; Zhang et al., 2006). It is applied widely for the treatment of rheumatoid arthritis, diarrhea, acute hepatitis, pelvic inflammatory disease and ulcerative colitis in clinic (Li et al., 2016). Studies have shown that Herba Patriniae is rich in polysaccharides, which have obvious anti-fatigue, anti-diarrhea, anti-constipation, anti-viral, anti-oxidant, immune regulation, and anti-tumor growth and other pharmacological activities (Li et al., 2016; Chi et al., 2014; Lu et al., 2017; Zhang et al., 2008; Wang et al., 2019; Wei et al., 2020; Lu et al., 2009; Hui et al., 2019a, 2019b). Herba Patriniae polysaccharides have extremely high medical value and widespread application prospects such as cosmetics, food, health cares, beverage and feed additives. Nevertheless, few in-depth comparative studies on the physicochemical features and antioxidant activities of Herba Patriniae polysaccharides have been reported in recent years.
Polysaccharides have attracted more and more attention for their naturally non-toxic, biodegradable and biocompatible properties, as well as their diverse biological activities (Hui et al., 2019a; Barbosa et al., 2021; Xie et al., 2021). However, the bioactivities of polysaccharide could be strongly influenced by its physicochemical features (Qu et al., 2020; Chen et al., 2016), which are greatly affected by the methods of purification. Generally, fractional precipitation with ethanol is the most convenient and effective method for initial purification of aqueous extracts to obtain polysaccharides due to its simple, rapid and easy concentration (Chen et al., 2016; Xing et al., 2018; Xu et al., 2018). What’s more, the physicochemical features and biological activities of polysaccharides are closely associated with the concentration of ethanol precipitation (Chen et al., 2016; Xing et al., 2018; Xu et al., 2018; Xu et al., 2014; Li et al., 2015; Zhang et al., 2018). As far as we know, the Herba Patriniae polysaccharides reported previously were only precipitated by the concentration of ethanol around 80%, but lack of the lower concentrations of ethanol-precipitated polysaccharides and bioassays for their antioxidant activities. The systematic researches of the Herba Patriniae polysaccharides by graded alcohol precipitation are insufficient, and it is not conducive to elucidate the structure–activity relationship of the polysaccharides from Herba Patriniae. Therefore, the current study uses graded alcohol precipitation to obtain the crude polysaccharides from the Herba Patriniae, and further decolorized and deproteinized to gain the four fractions of preliminarily purified Herba Patriniae polysaccharides, then their physicochemical features and antioxidant activities in vitro have been identified correspondingly. The findings will lay a foundation for researching on the structure–activity relationship of polysaccharides and seeking new natural antioxidant compounds from Herba Patriniae.
2 Material and methods
2.1 Material
Herba Patriniae was purchased from the Yellow River medicine market in Lanzhou, Gansu province, China, which was identified as Patrinia villosa by Prof. Ma Zhigang from the School of Pharmacy of Lanzhou University. The raw material of Herba Patriniae was powdered with a food grinder (FW100, Tianjin Taisite Instrument Co. Ltd., China), and the powders were passed through a 60 mesh sieve and stored at vacuum desiccator until being extracted.
2.2 Chemicals
Bovine serum albumin and standard monosaccharides including D-mannose (Man), D-xylose (Xyl), D-glucose (Glc), D-galactose (Gal), L-arabinose (Ara), L-rhamnose (Rha), D-glucuronic acid (GlcU) and D-galactosal acid (GalU) were purchased from the Chinese Institute for the Control of Pharmaceutical and Biological Products. Vitamin C, 2, 2-Azinobis-3-ethylbenzthiazoline-6-sulfonate acid (ABTS) radical, 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radical, were purchased from Sigma Aldrich (St. Louis, MO, USA). All other reagents used were of analytical grade.
2.3 Preparation of the Herba Patriniae polysaccharides
Taken 200 g of Herba Patriniae powder, added 80 % ethanol to reflux degreasing in a water bath three times, and the residues were placed in the ventilation cabinet dry standby. 20 times of distilled water was added to the dried dregs for boiling extraction for 3 times (2 h/time), then filtered by multi-layer gauze, and the filtrate was collected and centrifuged at 3000 rmp for 5 min. The supernatant was concentrated under reduced pressure to 1/10 of the original volume, then precipitated by ethanol with the final concentration of 50% at 4℃ for 24 h. After centrifugation, the precipitation was collected and freeze-dried with a freeze drier (Labconco, FreeZone®, American) to obtain Herba Patriniae polysaccharide BJP50. The supernatant was then added with ethanol to make its final concentration 60%, placed overnight at 4 °C, centrifuged, and the precipitate was collected and freeze-dried to obtain the polysaccharide BJP60. According to this method, the ethanol concentration was successively increased to obtain Herba Patriniae polysaccharides BJP70 and BJP80.
The four polysaccharides were dissolved in distilled water, respectively, decolored with 6% hydrogen peroxide, deproteinized by the Sevage method (Staob, 1965), and dialyzed against deionized water for 72 h with a dialysis bag (MW3500). The dialysis liquid was collected and precipitated again by 50%, 60%, 70% and 80% ethanol in sequence, placed overnight at 4 °C and centrifuged. The precipitate was collected and freeze-dried. Finally, the preliminarily purified polysaccharides BJP50, BJP60, BJP70 and BJP80 can be obtained and kept in the desiccator for later use.
2.4 Determination of total carbohydrate, uronic acid and protein contents
The total carbohydrate contents were determined by the phenol–sulfuric acid method (Li et al., 2020), using D-glucose as the standard. The protein contents were measured according to Bradford’s method (Wang et al., 2021a), using bovine serum albumin as the standard. The uronic acid contents were estimated by the carbazole-sulfuric acid method (He et al., 2021a), using D-glucuronic acid as standard. In addition, prepared 1 mg/mL polysaccharide solution, and FeCl3 reaction was carried out to detect whether the polysaccharide contained the phenolic substances.
2.5 Monosaccharide components analysis
Monosaccharide composition was analyzed by aldoononitrile acetate derivative method referred to our previous literature (Hui et al., 2019b). Briefly, 5 mg of polysaccharide was hydrolyzed by the TFA (2 mL, 4 M) at 110 °C for 6 h, and then derivatized with nitrile acetate. The standard monosaccharides were derivatized according to the same method, and then entered the GC (Agilent 6890 N, santa Clara, CA, USA) fitted with a flame ionization detector (FID) and a quartz capillary column DB-1 (30 m × 0.32 mm × 0.3 μm) to determine the monosaccharide composition. The injection port and detector temperature was 250℃ and 260℃, respectively. The column temperature programmed warming up from 100 °C (2 min) to 220 °C (5 min) at a rate of 3℃ /min. The diffuse proportion was 50:1. The carrier gas was N2 and its flow rate was 2 mL/min.
2.6 Molecular weight determination
The molecular weights of BJPs were determined by the GPC method. The measurement was perfumed on the Shimadzu LC-10A (Shimadzu Co. Japan) equipped with differential refractive index detector (DRI, Optilab Rex, Wyatt), and the chromatographic column was combined with Shodex OH pak SB-804 and 803 (8.0 mm × 300 mm, Showa Denko k.k., Japan). The dextran standards were series of different molecular masses of dextran with 1152, 11600, 23800, 48600, 80900, 148000, 273000, 409800, 535600, 1050000 Da. The mobile phase was 0.05 mol/L NaCl solutions with the flow rate at 0.6 mL/min, and the temperature of the column oven was 40 °C. The sample and standard was prepared to a 5 mg/mL solution, centrifuged at 12000 rpm for 10 min, filtered the supernatant with a 0.22 μm microporous membrane, then transferred the samples to a 1.8 mL injection vial and injected 20 μL. The standard curve was drawn with different molecular masses of standard dextran, and the molecular weight of each polysaccharide was determined according to the above standard curve.
2.7 UV–vis spectroscopic analysis
The BJPs were dissolved in distilled water to prepare 1 mg/mL polysaccharide solution, scanned in the range of 190–900 nm with an ultraviolet spectrophotometer (UV-1700, Shimadzu Co. Japan), and detected whether the polysaccharides contained protein (280 nm) and nucleic acid (260 nm) conjugates.
2.8 FT-IR spectroscopic analysis
2 mg of the dried samples were mixed with an appropriate amount of dried potassium bromide powder, grinded uniformly and pressed the tablet, and then recorded the FT-IR spectra by using a Thermo Nicolet 5700 infrared spectrograph (Thermo-Electron, Madison, WI, US) from 4000 to 400 cm−1.
2.9 Thermogravimetric (TG) analysis
TG analysis was performed in the temperature range of 30–800 °C, with a heating rate of 10 °C /min using a NETZSCH STA 449C instrument, air environment, scanning speed as 1 °C/min.
2.10 Determination of antioxidant activities in vitro
2.10.1 ABTS free radical scavenging activity assay
The effect of scavenging ABTS radical was measured according to our reports with minor modification (Hui et al., 2019a). ABTS radical cations were prepared by adding 140 mM Sodium persulfate to 7 mM ABTS solution. The mixture solution reacted in dark at room temperature for 16 h, subsequently the ABTS solution was diluted using PBS (pH 7.0) to an absorbance of 0.70 ± 0.05 at 734 nm. 0.1 mL of polysaccharide aqueous solution (0.125–4.0 mg/mL) was mixed evenly with 3.9 mL of the ABTS solution, allowing the mixed solution to react at room temperature for 6 min, and immediately recorded the absorbance of mixed solution at 734 nm. The same concentration of Vc was used as reference antioxidant. The activity of scavenging ABTS radical was calculated as follows:
Where Ai is the absorbance of the test sample mixed with the ABTS solution; A is the absorbance of the sample without the ABTS solution; and A0 is the absorbance of the distilled water replaced the sample solution.
2.10.2 DPPH free radical scavenging activity assay
The DPPH radical scavenging activity was carried out by Chen’s method (Chen et al., 2021a) with slight modification. 2 mL of polysaccharide aqueous solution with various concentrations (0.125–4.0 mg/mL) was mixed with 1 mL of freshly prepared DPPH in ethanol (0.1 mM). The mixture solution was shaken vigorously and incubated in the dark at room temperature for 60 min, immediately the absorbance of the reaction solution was measured at 517 nm against a blank. Vc was taken as the positive control. The DPPH radical scavenging activity was calculated as follows:
Where Ai is the absorbance of the test sample mixed with the DPPH solution, A is the absorbance of methanol replaced the DPPH solution, and A0 is the absorbance of the distilled water replaced the sample solution.
2.10.3 Hydroxyl radical scavenging activity assay
The effect of scavenging hydroxyl radical was performed in the light of the method we reported recently (Hui et al., 2019a). Briefly, 2.0 mL of polysaccharide aqueous solution (0.125–4.0 mg/mL) was mixed with 2.0 mL FeSO4 (6 mM) and 2.0 mL H2O2 (2.4 mM), blended well and hold at room temperature for 10 min. The mixture was added to 2.0 mL salicylic acid (6 mM), shaken well and kept in 37 °C water bath for 30 min, centrifuged. The absorbance of supernatant was measured at 500 nm, taking Vc as reference antioxidant. The effect of scavenging hydroxyl radicals were calculated as follows:
Where Ai is the absorbance of the test sample mixed with the reaction solution; A is the absorbance of water instead of the salicylic acid solution; and A0 is the absorbance of the control reaction.
2.10.4 Superoxide radical scavenging activity assay
The superoxide radical scavenging was assessed by our previous reports (Hui et al., 2019b). Firstly, the rate of auto-oxidation of pyrogallol was determined. Tris-HCl buffer (50 mmol/L, pH 8.2, 4.5 mL) was mixed with distilled water (4.2 mL), incubated at 25 °C for 20 min, and quickly added 0.3 mL of pyrogallol solution (3 mmol/L, prepared with 10 mmol/L HCl and preheated at 25 °C). After blended, the change of absorbance was recorded at 320 nm within 5 min. And the change of absorbance per unit time of the reactants was calculated in the linear range to obtain pyrogallol’ self-oxidation rate by plotting the time (t)-absorbance (A) curve. The auto-oxidation rate equation for pyrogallol is y = 0.035x + 0.641 (R2 = 0.9993).
The following steps were used to determine the auto-oxidation rate of pyrogallol under the test samples. Tris-HCl buffer (50 mmol/L, pH 8.2, 4.5 mL) was mixed with pure water (3.3 mL), incubated at 25 °C for 20 min, then quickly added 0.9 mL of the test sample and 0.3 mL of pyrogallol solution (3 mmol/L, prepared with 10 mmol/L hydrochloric acid, preheated at 25 °C). The mixture was blended well and recorded the absorbance change at 320 nm within 5 min. Draw the time (t)-absorbance (A) curve and calculate the change of absorbance per unit time in the linear range, when the auto-oxidation rate of pyrogallol under the test sample was obtained. Vc was taken as the positive control. The superoxide radical scavenging activity was calculated by the following formula:
Where V is auto-oxidation rate of pyrogallol under the test sample, V0 is auto-oxidation rate of pyrogallol.
2.11 Statistical analysis
All the experiments were carried out in triplicate. The data were analyzed with SPSS software (version 18.0 for Windows, SPSS Inc., Chicago, IL, USA) and recorded as means ± standard deviations (SD).
3 Results and discussion
3.1 Yields and physicochemical properties
The yields and physicochemical properties of four BJPs polysaccharides from Herba Patriniae were showed in Table 1. It can be seen that the highest yield of BJPs is BJP60 and BJP80, followed by BJP50 and BJP70. This indicated that BJP60 and BJP80 were the main components of Herba Patriniae polysaccharides. After decolorization and deproteinization, the four polysaccharides contained no protein, and the total sugar contents exceeded 50%. Additionally, a small amount of uronic acid was found in the BJP60 and BJP50, and the ferric chloride reaction was negative, indicating that the four BJPs polysaccharides contained no phenolic compounds.
Sample
BJP50
BJP60
BJP70
BJP80
Yield (w%)a
4.1 ± 0.12
5.7 ± 0.23
3.9 ± 0.26
5.4 ± 0.08
Total sugar content (w%)a
53.8 ± 0.22
62.8 ± 0.28
56.6 ± 0.36
63.6 ± 0.29
Protein content (w%)a
–c
–c
–c
–c
Uronic acid content (w%)a
1.1 ± 0.28
5.5 ± 0.23
–c
–c
Monosaccharide composition (mol%)b
Man
–c
0.3 ± 0.05
0.2 ± 0.08
0.9 ± 0.12
Glc
–c
–c
–c
1.3 ± 0.26
Ara
0.7 ± 0.02
0.9 ± 0.13
1.1 ± 0.15
1.5 ± 0.18
Gal
1.1 ± 0.08
1.1 ± 0.32
0.8 ± 0.09
1.3 ± 0.22
Rha
0.7 ± 0.12
0.5 ± 0.09
0.4 ± 0.12
0.5 ± 0.16
Xyl
–c
–c
–c
0.4 ± 0.22
Molecular Weight
2.4 × 106 (95)d
2.1 × 106 (97)d
1.3 × 105 (46)d
9.1 × 104 (93)d
4.4 × 104 (1)d
8.4 × 103 (3)d
2.4 × 104 (51)d
8.3 × 103 (7)d
8.5 × 103 (4)d
8.8 × 103 (3)d
GC was employed to determine the monosaccharide composition. The results revealed that the four Herba Patriniae polysaccharides were composed of different monosaccharides (Fig. 1) with different molar ratios (Table 1). Obviously, the four BJPs polysaccharides were all heteropolysaccharides. BJP50 was composed of Ara, Gal and Rha with the molar ratio of 0.7: 1.1: 0.7. BJP60 and BJP70 were composed of Ara, Gal, Rha and Man with the molar ratios of 0.9: 1.1: 0.5: 0.3 and 1.1: 0.8: 0.4: 0.2, respectively. BJP80 was composed of Ara, Gal, Rha, Man, Glc and Xyl with the molar ratio of 1.5: 1.3: 0.5: 0.9: 1.3: 0.4. These were different from the Herba Patriniae polysaccharides reported previously in the composition and proportion (Chi et al., 2014; Zhang et al., 2008; Tang et al., 2016), indicating that the four polysaccharides were new polysaccharides obtained from Herba Patriniae. In addition, the composition analysis showed that the number of monosaccharides in the corresponding polysaccharides increased gradually with the increase of ethanol concentration, and the contents of Gal and Rha decreased relatively, while the content of Ara increased comparatively. This might be one of the main reasons for the different antioxidant activities of polysaccharides from the Herba Patriniae.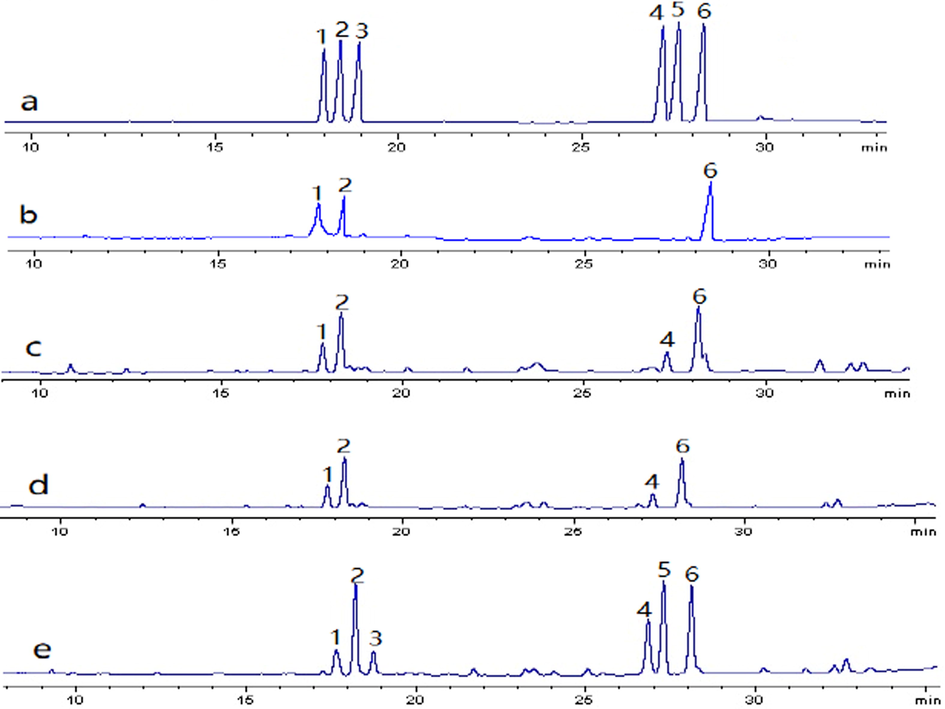
(a) GC profile of monosaccharide standards (1. Rha, 2. Ara, 3. Xyl, 4. Man, 5. Glc, 6. Gal). (b–e) GC profiles of the polysaccharides of BJP50, BJP60, BJP70 and BJP80 obtained from Herba Patriniae.
After GPC determination, the standard curve was obtained by plotting the logarithm of the retention time and molecular weight of the series of dextran standards measured on the gel column (log MW=﹣0.1973 RT + 12.456, R2 = 0.9957, where MW is the average molecular weight, RT is retention time). Bring the RT value of the retention time of polysaccharides measured by passing the sample through the high-efficiency gel column into the standard curve, the relative molecular weights of polysaccharides were calculated out. As shown in Fig. 2, the four polysaccharides were not homogeneous polysaccharide. BJP50 showed three peaks meant it contained three types of polysaccharides (Fig. 2a), and the dominant was the polysaccharide with the highest molecular weight of 2.4 × 106, which accounted for 95% of the total sugars according to peak area (Table. 1). BJP60 exhibited two peaks implied it contained two types of polysaccharides (Fig. 2b), and the predominant was the polysaccharide with the higher molecular weight of 2.1 × 106, which accounted for 97% of the total sugars according to peak area (Table 1). BJP70 displayed three consecutive and baseline no separation peaks (Fig. 2c), and the middle peak with the molecular weight of 2.4 × 104 was slightly dominant (Table 1). BJP80 showed two peaks but the predominant peak was the lower molecular weight of 8.3 × 103 (Fig. 2d), which accounted for 93% of the total sugars according to peak area (Table 1). Obviously, the order of the molecular weight of four polysaccharides were BJP50 > BJP60 > BJP70 > BJP80. As the concentration of ethanol precipitation increased, the molecular weight of the polysaccharide decreased. This revealed that the molecular weight of the polysaccharide depended on the concentration of ethanol precipitation. In addition, GPC determination demonstrated that the purity of four BJPs polysaccharides with different molecular weight were not enough to further analyze their chemical structure. Therefore, the current studies pay more attention to their physicochemical features.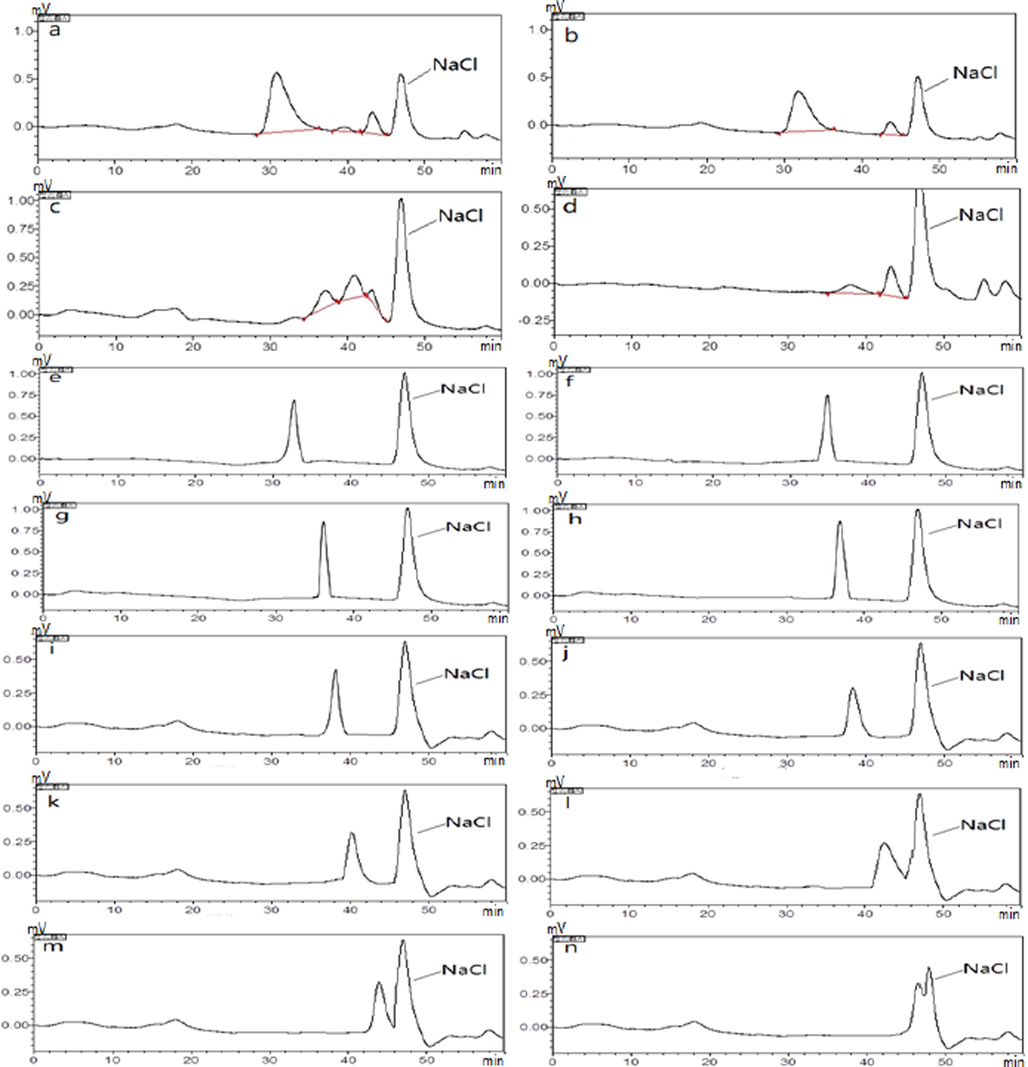
GPC spectra of BJP50 (a), BJP60 (b), BJP70 (c), BJP80 (d), dextran standards of 1152 (e), 11,600 (f), 23,800 (g) , 48,600 (h), 80,900 (i), 148,000 (j), 273,000 (k), 409,800 (l), 535,600 (m) and 1,050,000 (n).
3.2 UV–vis spectra properties
The results were shown in Fig. 3, four BJPs polysaccharides presented a maximum absorption peak around 200 nm, which attributed to the polysaccharides. On the UV scanning spectrum at 260 nm and 280 nm were not found the obvious absorption peaks, suggesting that the BJPs were basically free of nucleic acids and proteins (Abuduwaili et al., 2022; Wang et al., 2021b).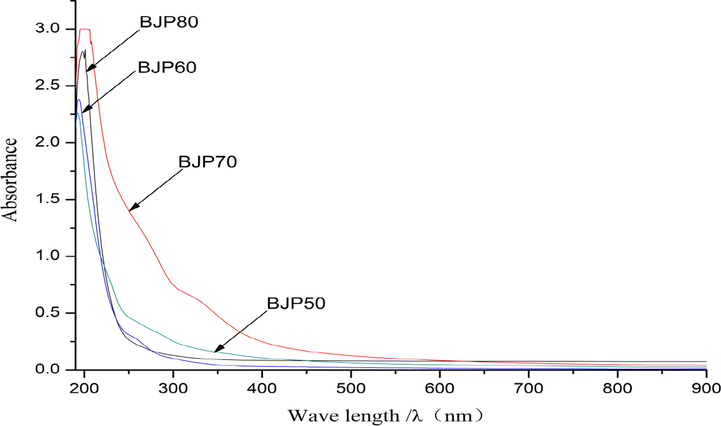
The UV–Vis spectra of polysaccharides from Herba Patriniae.
3.3 FT-IR spectra properties
The FT-IR spectra of four BJPs polysaccharides (Fig. 4a–d) were similar, and they showed the characteristic absorption of typical polysaccharides. The broad peak at 3397–3426 cm−1 was —OH stretching vibration (Wang et al., 2021b; He et al. 2021b; Khemakhem et al., 2018). The absorption peak at 2919–2931 cm−1 was the C–H stretching vibration. BJP60 and BJP50 had a weak absorption peak at 1735 cm−1, which indicated that both polysaccharides were a weak acidic polysaccharide, the findings were consistent with the results of uronic acid content determination. 1617–1654 cm−1 was the absorption peak of the amorphous region of bound water, while 1441–1460 cm−1 was the C—H curve-vibration absorption peak (Thimmaraju et al., 2022; Eljoudi et al., 2022; Chen et al., 2021b). Three absorption peaks appearing at 1250–900 cm−1 were the C—O—C or C—O stretching vibration of pyranose ring (Chen et al., 2021b; Kim et al., 2022), and the peaks at 759–772 cm−1 indicated the presence of α-pyranose (Khemakhem et al., 2018; Thimmaraju et al., 2022; Eljoudi et al., 2022; Chen et al., 2021b).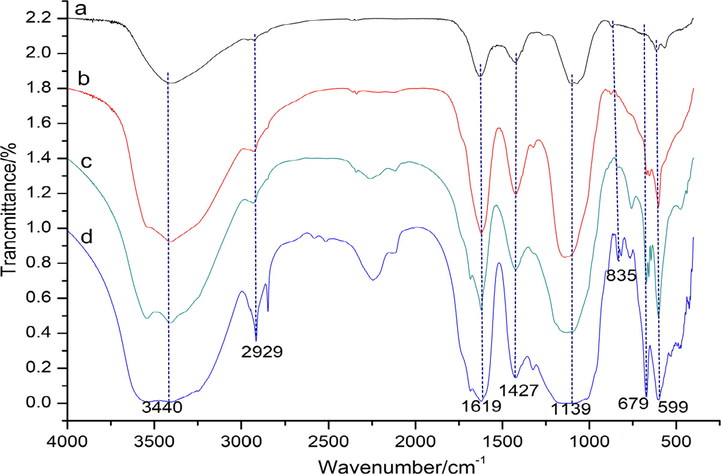
The FT-IR spectra of BJP80 (a), BJP60 (b), BJP70 (c) and BJP50 (d).
3.4 TG properties
The TG can investigate the thermal weight loss of materials, which were closely related to their properties of molecular structure and reflected their thermal stability. To a certain extent, the applicability of polysaccharides depended on their thermal properties. The thermal characteristics of four BJPs polysaccharides within 30–800 °C were shown in Fig. 5. TG curves indicated that the weight loss of BJPs mainly experienced three stages except BJP80. The first stage of BJPs started differently, a slight weight loss of 8%, 6%, 5% and 4% of BJP50, BJP60, BJP70 and BJP80 happened before 280.2 °C, 263.1 °C, 142.3 °C and 116.5 °C, respectively, which might be caused by undried crystal water or other volatile substances (Hui et al., 2019b; Ding et al., 2021). The second stage of BJP50, BJP60 and BJP70 appeared at range of 280.2–323.5 °C, 263.1–353.3 °C and 142.3–483.5 °C, and their weight loss rate was about 55%, 50% and 32%, respectively, while the weight loss of BJP80 was about 40% from 116.5 °C to 600 °C. It could be seen that the weight loss of BJPs increased abruptly at this stage except for BJP80, suggesting that BJP80 was the most stable one. This is because the polysaccharide at this stage underwent severe degradation and depolymerization reactions, and the long chains fractured and decomposited (Ding et al., 2021). In the third stage, there was a slow weight loss process in BJP50, BJP60 and BJP70 within 401.3–500 °C, 422.8–500 °C and 543.8–700 °C. At this time, the decomposition process of the polysaccharide was completed, and the residual weight of BJP50, BJP60, BJP70 and BJP80 was about 2%, 5%, 40% and 45% at 800 °C. Obviously, the order of thermal stability was BJP80 > BJP70 > BJP60 > BJP50, this might be related to the types and numbers of monosaccharides in BJPs, and the specific reasons needed to be further studied. In all, BJPs had a good thermal stability. Especially for BJP80 and BJP70, they were expected to be used as additives in food industries.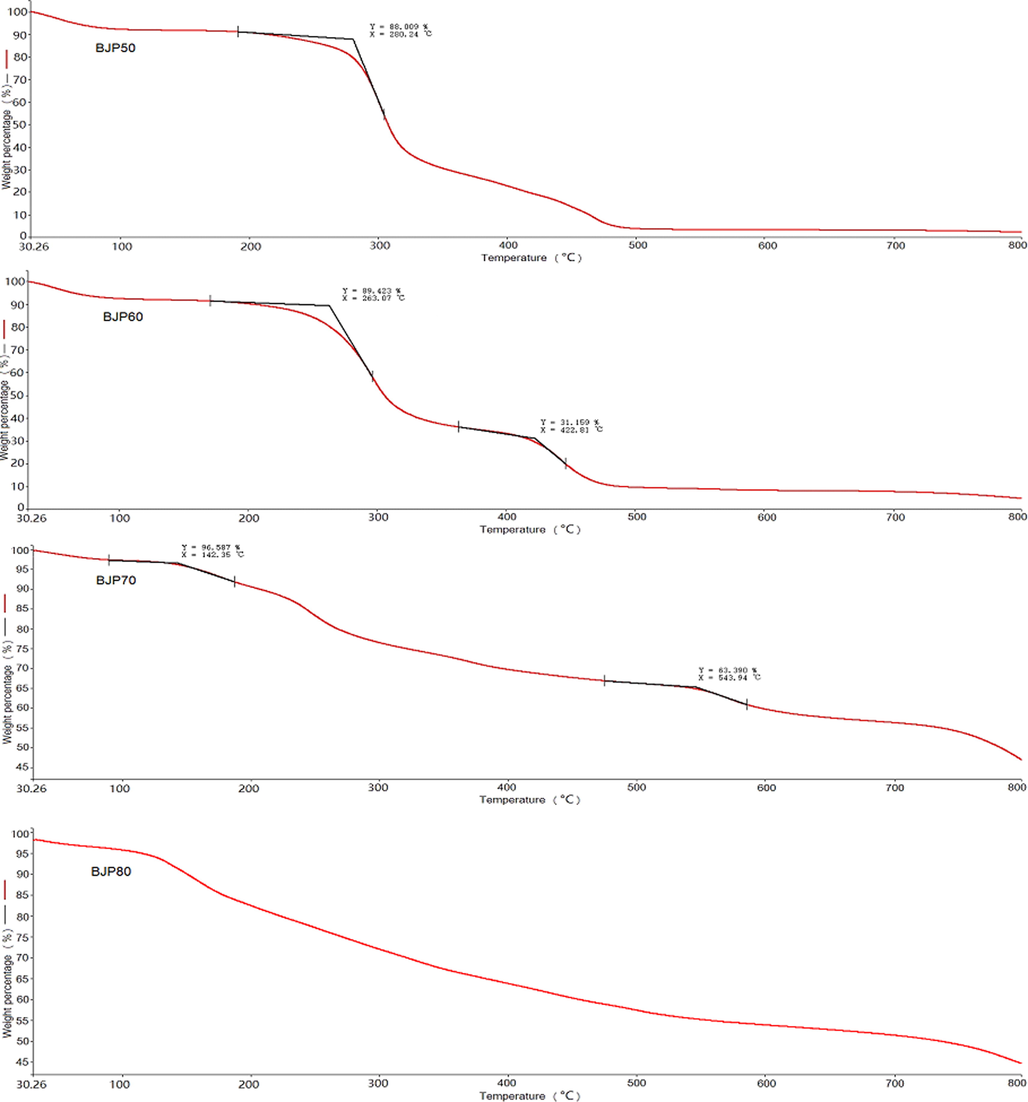
Thermogravimetric analysis curves of BJP50, BJP60, BJP70 and BJP80.
3.5 Antioxidant activities in vitro
3.5.1 ABTS radical scavenging activity
ABTS+ method is widely used to evaluate the antioxidant capacity of natural products in vitro due to its simple operation and good repeatability (Wang et al., 2021c). ABTS reacts with potassium persulfate to form blue-green cation ABTS free radical, and the antioxidant component reacts with ABTS free radical to fade the system, thus reflecting the antioxidant capacity of the substance (Eljoudi et al., 2022). The four BJPs polysaccharides had certain scavenging ability on ABTS+ free radicals, and showed a certain dose-dependent relationship, the results as shown in Fig. 6a. When the polysaccharide concentration reached 4.0 mg/mL, the ability of BJP50, BJP60, BJP70 and BJP80 to scavenge ABTS+ were 43.5 ± 1.6%, 49.7 ± 2.6%, 56.2 ± 2.5% and 75.8 ± 2.4%, respectively. BJP80 exhibited the highest scavenging ability (p < 0.05) in the range of 0–4.0 mg/mL, with BJP70, BJP60, and BJP50 having lower but similar abilities. And their half inhibition concentration (IC50) decreased in the order of BJP80 (154.8 ± 0.2 μg/mL) < BJP70 (2313. ± 0.2 μg/mL) < BJP60 (3771.1 ± 0.1 μg/mL) < BJP50 (5112.8 ± 0.2 μg/mL), compared to the IC50 of Vc (6.8 ± 0.1 μg/mL), the value of BJPs was much weak.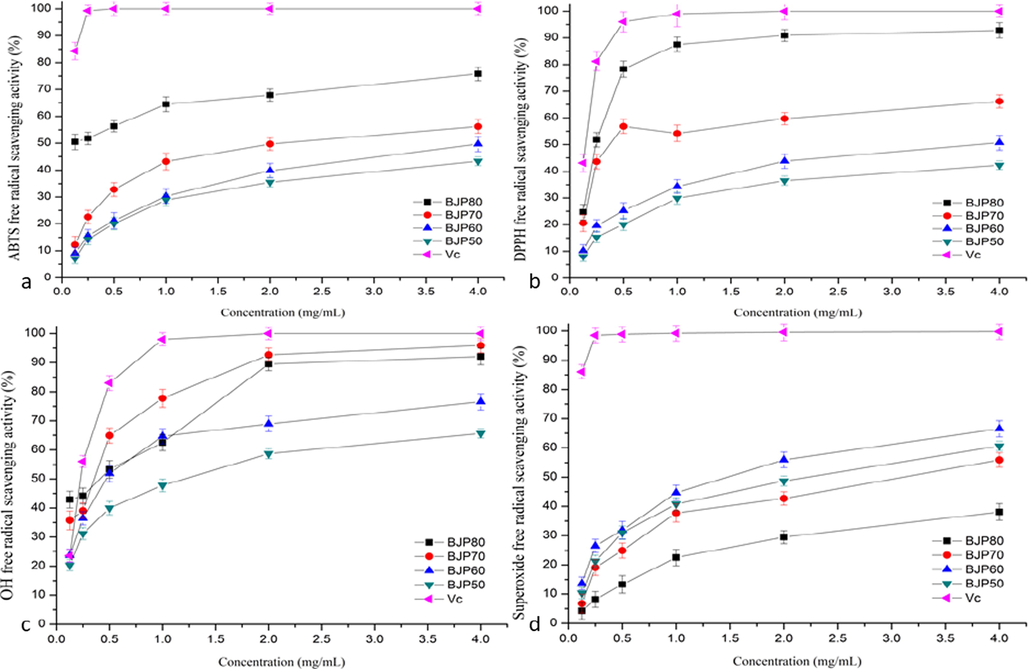
The antioxidant activities of polysaccharides from Herba Patriniae at different concentrations. (a) ABTS free radical scavenging activity; (b) DPPH free radical scavenging activity; (c) OH free radical scavenging activity; (d) superoxide free radical scavenging activity.
3.5.2 DPPH radical scavenging activity
DPPH radical is a free radical with a single electron but relatively stable structure, the color of the solution will be changed once it interacts with polysaccharide. It was one of the commonly used methods for evaluating the antioxidant activity of natural products in laboratories (Li et al., 2021; Wu et al., 2015). The DPPH radical scavenging activity of polysaccharide may be due to the reaction of the electrons or hydrogen atoms provided by the hydroxyl group of polysaccharide with DPPH radical, which reduces the DPPH radical (Huang et al., 2020; Eljoudi et al., 2022). The results of BJPs on DPPH free radicals scavenging were shown in Fig. 6b. Four BJPs polysaccharides had a certain scavenging effect on DPPH free radicals, in the experimental concentration range, the elimination ability of BJPs on DPPH free radicals gradually enhanced with the continuous increase of the concentration of polysaccharides except BJP70, showing certain dose–effect dependence. While the BJP70 for DPPH radical scavenging ability increased first, followed by decreased and then increased gradually. As the concentration of polysaccharides climbed up to 4 mg/mL, the scavenging rates of BJP80, BJP70, BJP60 and BJP50 on DPPH free radicals were 92.8 ± 2.8%, 66.2 ± 2.5%, 50.7 ± 2.8% and 42.3 ± 1.6%, respectively. The IC50 was 187.8 ± 0.1 μg/mL for BJP80, 790.3 ± 0.2 μg/mL for BJP70, 3366.9 ± 0.2 μg/mL for BJP60 and 5340.3 ± 0.2 μg/mL for BJP50. Obviously, BJP80 showed the highest DPPH radical scavenging activity (p < 0.01) in the test concentration range, and its scavenging ability for DPPH radical was almost close to the positive control Vc at 4 mg/mL, which might be the concentration of Ara and Gal in BJP80 greatest than that of other Herba Patriniae polysaccharide (Yarley et al., 2021).
3.5.3 Hydroxyl radical scavenging activity
Hydroxyl radicals, as the most active free radicals, which reacted with biomolecules in living cells, such as lipids, peptides, proteins, nucleic acids, etc. The hydroxyl radicals would produce the strongest oxidative damage effect on biological macromolecules, and cause oxidative damage to cells and tissues, leading to organ disease and body aging (Abuduwaili et al., 2022; Zhang et al., 2010; Khemakhem et al., 2018; Yang et al., 2016; Xu et al., 2018). As shown in Fig. 6c, when the polysaccharide concentration increased from 0 to 2.0 mg/mL, the scavenging capacity of BJPs on hydroxyl radicals significantly trended upward. As the concentration exceeded 2 mg/mL, the growth trend was relatively slow. At the concentration of 4.0 mg/mL, the scavenging capacity of BJP80, BJP70, BJP60 and BJP50 on hydroxyl radicals was 92.1 ± 2.8 %, 95.9 ± 2.5%, 76.5 ± 2.8 % and 65.6 ± 1.6 %, respectively. The IC50 of BJP80, BJP70, BJP60, BJP50 and Vc were 324.5 ± 0.2 μg/mL, 280.6 ± 0.2 μg/mL, 642.9 ± 0.3 μg/mL, 1337.5 ± 0.1 μg/mL and 143.9 ± 0.2 μg/mL, respectively. Obviously, BJP70 had the strongest scavenging ability on hydroxyl radicals and its scavenging ability was almost approached to Vc, followed by BJP80, BJP60 and BJP50. Moreover, the change curve of the scavenging ability of BJP70 was similar to Vc since the concentration varied in range 0–4.0 mg/mL. Hydroxyl radicals, as one of the most reactive radicals in ROS, might directly or indirectly cause tissue damage, induce multiple human diseases including cancer and aging (Hui et al., 2019b; Wang et al., 2021a, 2021b, 2021c). As the strong scavenging activity of Herba Patriniae polysaccharide on hydroxyl radical, especially BJP70 and BJP80, they will have a certain potential to be developed as antioxidant to prevent or treat diseases caused by excessive hydroxyl radicals.
3.5.4 Superoxide free radical scavenging activity
The ability to scavenge superoxide anion radicals is one of the critical indicators of anti-oxidation activities. It was due to not only the superoxide anion was a highly toxic radical species that caused the damage of the cells and organs, but also it related to the occurrence of some major diseases such as aging and cancer (Hui et al., 2019b; Gong et al., 2018; Zhang et al., 2019). The Fig. 6d displayed the scavenging ability of BJPs and Vc closely depended on concentration. BJPs had certain scavenging ability on superoxide free radicals, and presented a good dose-dependent manner, at concentration of 4 mg/mL, the scavenging rate of BJP80 on superoxide free radicals was 38.1 ± 2.6%, 55.9 ± 2.5% for BJP70, 66.5 ± 2.8% for BJP60, 60.6 ± 1.6% for BJP50 and 99.7 ± 2.6% for Vc. The IC50 of BJP80, BJP70, BJP60, BJP50 and Vc were 6274.1 ± 0.3 μg/mL, 2845.6 ± 0.2 μg/mL, 1661.0 ± 0.4 μg/mL, 2208.9 ± 0.3 μg/mL and 1100.1 μg/mL, respectively. Compared with other free radicals, BJP60 showed the highest scavenging capacity on superoxide radicals, followed by BJP50 and BJP70, while BJP80 exhibited the lowest scavenging activity, this might be possibly due to the relatively high uronic acid concentration in BJP60 (Yarley et al., 2021).
4 Conclusions
Four polysaccharides (BJP50, BJP60, BJP70 and BJP80) were successfully obtained from Herba Patriniae combined ethanol fractional precipitation method with decolorization and deproteinization, their physiochemical features and antioxidant activities were preliminary determined. The findings demonstrated that the four BJPs polysaccharides were all heteropolysaccharides that mainly composed of Ara, Gal, Rha, Man, Glc and Xyl with different types and ratios, and the types of monosaccharides and the content of Ara, Glc and Man increased from BJP50 to BJP80, while the content of Gal and Rha decreased inversely. The UV–vis spectra presented a strong characteristic absorption peak of carbohydrates around 200 nm, which was proved by the typical characteristic absorption of polysaccharides at 3440, 2929, 1427 cm−1 in FT-IR. The four BJPs polysaccharides contained no nucleic acids, proteins and phenolic compounds. Their molecular weights increased in the order of BJP50 > BJP60 > BJP70 > BJP80, while the order of thermal stability was BJP80 > BJP70 > BJP60 > BJP50. BJP80 and BJP70 were remarkably resistant to the high temperatures at 600 °C, which was expected to be used as food additives for high-temperature applied in the cooking, refining and other industries.
Antioxidant activity assays showed that the four BJPs polysaccharides exhibited strong scavenging capacities on ABTS, DPPH, superoxide radicals and hydroxyl radicals in a concentration-dependent manner. Especially for DPPH and hydroxyl radicals, BJP70 and BJP80 displayed highest scavenging ability and their scavenging ability on DPPH and hydroxyl radicals almost closed to Vc as the concentration reached 4 mg/mL. However, BJP80 and BJP70 expressed weaker scavenging activities for superoxide radicals than that of BJP60 and BJP50. Regrettably, no direct correlation could be found between the antioxidant activity and their physicochemical features. Thus, the upcoming research will be carried out to further purify the four BJPs polysaccharides and reveal the relationship of their structure features and antioxidant activities.
Author contributions
H.P.Hui. designed the research and performed the experiments, and wrote the manuscript. W.J.Gao. revised the manuscript..
Acknowledgments
The Gansu Province Higher Education Innovation Fund of China (2021A-260) supported this work.
References
- Isolation, structural modification, characterization, and bioactivity of polysaccharides from Folium Isatidis. Ind. Crops Prod.. 2022;176:114119
- [Google Scholar]
- Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol.. 2021;108:223-235.
- [Google Scholar]
- Structural characterization and in vitro fermentation by rat intestinal microbiota of a polysaccharide from Porphyra haitanensis. Food Res. Int.. 2021;147:110546
- [Google Scholar]
- Polysaccharides from ginger stems and leaves: Effects of dual and triple frequency ultrasound assisted extraction on structural characteristics and biological activities. Food Biosci.. 2021;42:101166
- [Google Scholar]
- Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol.. 2016;91:505-509.
- [Google Scholar]
- Studies on polysaccharides from Patrinia villosa Juss. Chongqing: Southwest University; 2007.
- Composition of Polysaccharides from Herba Patriniae and Their Anti-fatigue and Anti-hypoxia Activities. Food Sci.. 2014;35(21):212-215.
- [Google Scholar]
- Polysaccharides from the lignified okra: Physicochemical properties and rheological properties. Bioact. Carbohydr. Dietary Fibre. 2021;26:100274
- [Google Scholar]
- New polysaccharides extracted from Malcolmia triloba: Structure characterization, biological properties and application to beef meat preservation. J. Food Compos. Anal.. 2022;107:104380
- [Google Scholar]
- Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol.. 2018;109:611-618.
- [Google Scholar]
- The Herba Patriniae (Caprifoliaceae): A review on traditional uses, phytochemistry, pharmacology and quality control. J. Ethnopharmacol.. 2021;265:113264
- [Google Scholar]
- The structure elucidation of novel arabinogalactan LRP1-S2 against pancreatic cancer cells growth in vitro and in vivo. Carbohydr. Polym.. 2021;267:118172
- [Google Scholar]
- Isolation, purification, structure characterization and antioxidant activity of cornus officinalis seeds polysaccharides. Food Sci. 2021
- [Google Scholar]
- Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol.. 2020;155:1262-1269.
- [Google Scholar]
- Purification, characterization and antioxidant activities of a polysaccharide from the roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol.. 2019;135:1208-1216.
- [Google Scholar]
- Structural characterization, antioxidant and antibacterial activities of two heteropolysaccharides purified from the bulbs of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol.. 2019;133:306-315.
- [Google Scholar]
- Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol.. 2018;106:425-432.
- [Google Scholar]
- Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol.. 2018;106:425-432.
- [Google Scholar]
- Structure and antiviral activity of a pectic polysaccharide from the root of Sanguisorba officinalis against enterovirus 71 in vitro/vivo. Carbohydr. Polym.. 2022;281:119057
- [Google Scholar]
- The protective effect of Herba Patriniae polysaccharide on fatigue rats liver tissue. Food Res. Dev.. 2016;37(12):174-177.
- [Google Scholar]
- Graded ethanol precipitation method on physicochemical properties and antioxidant activities of polysaccharides extracted from Astragalus Radix. China J. Chinese Materia Medica.. 2015;40(11):2112-2116.
- [Google Scholar]
- Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym.. 2020;250:116958
- [Google Scholar]
- Physicochemical properties and antioxidant activity of Maillard reaction products derived from Dioscorea opposita polysaccharides. LWT-Food Sci. Technol.. 2021;149:111833
- [Google Scholar]
- Study on immunomodulatory effect of polysaccharides from Herba Patriniae on S180 tumor-bearing mice. J. Chinese Medicinal Mater.. 2017;40(1):212-215.
- [Google Scholar]
- Study on anti-cervical cancer mechanism of polysaccharides of Pateinia Heterophylla Bugge. Yangling: Northwest A & F University; 2009.
- Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol.. 2020;161:24-34.
- [Google Scholar]
- Physicochemical characteristics and improvement of exercise fatigue and chronic fatigue syndrome of bitter vegetable polysaccharides. Xian: Shaanxi normal university; 2016.
- Novel studies of characterization, antioxidant, anticoagulant and anticancer activity of purified polysaccharide from Hypsizygus ulmarius mushroom. Bioact. Carbohydr. Dietary Fibre. 2022;27:100308
- [Google Scholar]
- Structural elucidation and anti-diabetic osteoporotic activity of an arabinogalactan from Phellodendron chinense Schneid. Carbohydr. Polym.. 2021;271:118438
- [Google Scholar]
- Structural characterization of a novel polysaccharide from Pleurotus citrinopileatus and its antitumor activity on H22 tumor-bearing mice. Int. J. Biol. Macromol.. 2021;168:251-260.
- [Google Scholar]
- Study on Extraction and identification of polysaccharide from Patrinia. Food Saf. Rep.. 2019;2(6):130.
- [Google Scholar]
- Anti-oxidant Activity of Different Polysaccharide Components of Inonotus obliquus in Vitro and in Vivo. J. Chinese Institute Food Sci. Technol. 2021
- [Google Scholar]
- Extraction optimization and antioxidant activity estimation for polysaccharides from Patrinia. World Chinese Med.. 2020;15(14):2026-2030.
- [Google Scholar]
- Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydr. Polym.. 2015;132:31-40.
- [Google Scholar]
- Anti-hypertensive and cardioprotective activities of traditional Chinese medicine-derived polysaccharides: A review. Int. J. Biol. Macromol.. 2021;185:917-934.
- [Google Scholar]
- Preparation and antioxidant activity of crud polysaccharide from Bitter herbs. Farm Products Process.. 2015;385(6):15-22.
- [Google Scholar]
- Physicochemical properties of polysaccharides from Dendrobium ofcinale by fractional precipitation and their preliminary antioxidant and anti-HepG2 cells activities in vitro. Chem. Cent. J.. 2018;12(1):1-10.
- [Google Scholar]
- Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. Int. J. Biol. Macromol.. 2014;67:205-209.
- [Google Scholar]
- Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem.. 2018;243:26-35.
- [Google Scholar]
- Characterization and antioxidant activity of a novel polysaccharide from Pholidota chinensis Lindl. Carbohydr. Polym.. 2016;138:327-334.
- [Google Scholar]
- Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol.. 2021;183:2262-2273.
- [Google Scholar]
- In vitro anti-respiratory syncytial virus effect of an active compound (AP4) from Herba patriniae. Heilongjiang Med. Pharm.. 2006;29(1):48-50.
- [Google Scholar]
- Extraction, purification, identification and in vitro anti-RSV effect of polysaccharide from Patrinia. J. Chinese Med. Mater.. 2008;31(12):1879-1881.
- [Google Scholar]
- Polysaccharides obtained from bamboo shoots (Chimonobambusa quadrangularis) processing by-products: New insight into ethanol precipitation and characterization. Int. J. Biol. Macromol.. 2018;112:951-960.
- [Google Scholar]
- Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int. J. Biol. Macromol.. 2010;47(4):546-550.
- [Google Scholar]
- Characterization and biological activities of polysaccharides from artificially cultivated Phellinus baumii. Int. J. Biol. Macromol.. 2019;129:861-868.
- [Google Scholar]







