Translate this page into:
Phytochemical investigation and antioxidant activities of methanol extract, methanol fractions and essential oil of Dillenia suffruticosa leaves
⁎Corresponding author. miqbal@ums.edu.my (Mohammad Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oxidative stress has been known as a key factor of many disorders affecting human beings. Reactive oxygen species (ROS) attack vital biomolecules, weakening their functioning, thus exacerbating diseases. To attenuate oxidative stress-associated diseases a novel approaches of antioxidant therapies have been anticipated. Antioxidants have the potential to inhibit the propagation and formation of ROS. Dillenia suffruticosa is a medicinal plant, used by the local people for the treatment of various ailments. The study aimed to evaluate the phytochemical screening, antioxidative activity, total phenolic and flavonoid contents of methanol extract, fractions and essential oil of D. suffruticosa. Furthermore, the analysis of phytochemicals was done using gas chromatography and mass spectrometry (GCMS). The result showed the existence of alkaloids, anthraquinones, flavonoids, phytosterol, saponins, tannins, triterpenoids and steroids in the methanol extract and fractions of D. suffruticosa. The butanol fraction and methanol extract showed high phenolic (379.00 ± 9.25 and 277.00 ± 3.50 mg/g) and flavonoid values (74.44 ± 2.18 and 34.83 ± 0.71 mg/g) as compared to ethyl acetate, n-hexane and chloroform fractions. The scavenging capacity of butanol fraction and methanol extract was also higher than other fractions. GCMS analysis indicated the presences of various compounds in methanol extract, fractions and essential oil including methyl glycolate, lauryl acetate, phenol, 2,4-bis (1,1-dimethylethyl), 9,12-octadecadienoic acid, hexadecanoic acid, methyl ester, methyl stearate, phenol, benzyl alcohol, 3-hexen-1-ol, acetate and phytol. Thus, methanol extract, fractions and essential oil of D. suffruticosa leaves mainly contain vital phytochemical and shows good antioxidant activity.
Keywords
Phytochemical
Antioxidant activity
Methanol extraction
Essential oil
Dillenia suffruticosa
1 Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) such as superoxide (O∙2), hydroxyl (OH∙), peroxyl radical (ROO∙), alkoxy radical (RO∙), nitrogen dioxide (NO∙2) and nitric oxide (NO∙) play a dual role, both helpful and harmful (Halliwell, 2005; Nordberg and Arnér, 2001; Valko et al., 2007). In low quantity they are required by the body for immune system responses, however, overproduction of these molecules cause oxidative stress and nitrosative stress (Dröge, 2002). The oxidative stress causes significant damage to lipids (Ylä-Herttuala, 1999), DNA (Marnett, 2000) and protein (Stadtman and Levine, 2006) biomolecules which further lead to various chronic diseases including diabetes mellitus, cardiovascular, cancer, rheumatoid arthritis, cataracts, respiratory, ageing, hepatic damages etc (Mantena et al., 2008; Nordberg and Arnér, 2001; Pham-Huy et al., 2008; Phaniendra et al., 2015; Prakash et al., 2007; Valko et al., 2007). Hence maintenance of a balance between oxidant and antioxidant intracellular systems is vital for cell function, regulation, and adaptation to diverse growth conditions (Nordberg and Arnér, 2001). Phytochemical compounds of the medicinal plants with antioxidant activities have the potential to scavenge ROS and RNS in the human body and delay or inhibit the development of oxidative stress-associated maladies (Miller et al., 2000; Shah et al., 2017; Velmurugan et al., 2018). The antioxidant effects of natural products vary depending on their content of vitamin C, phenolic components, carotenoids, vitamin E and flavonoids (Saura-Calixto and Goñi, 2006; Zhang et al., 2015).
Dillenia suffruticosa (Griff ex Hook.f. & Thomson) Martelli (Dilleniaceae) is a medicinal plant, locally known as “Simpoh air” (Corners, 1997). The plant is mostly found on alluvial places such as swamps, mangroves, riversides or eroded soil, wasteland, forest edges, and native to Malaysia, Singapore, Brunei, Indonesia, and Sri Lanka (Corners, 1997; Kaore and Kaore, 2014; Shiew et al., 1985). The local people in Sabah Malaysia use the plant for various purposes. The Ibans use the plant to relieve stomach pain, the Dusun use for the treatment of fever and headache while the Kedayans use it for a post-childbirth tonic drink (Chiam, 2011). The plant has been reported with antimicrobial and anticancer properties. The antimicrobial properties of the methanol extracts of the plant have been reported against Bacillius subtilis, Bacillus cereus, Candida albicans, and Pseudomonas aeruginosa (Wiart et al., 2004). The chromatographic fractions of D. suffruticosa extract have been reported with the potential to prevent the proliferation of cancer cell through the induction of apoptosis (Armania et al., 2013; Saiful Yazan and Armania, 2014).
Regardless of the availability of several synthetic drugs applied to counter oxidative stress, the adverse side effects and high costs related to them limit their usefulness (Lourenço et al., 2019). Thus alternative nontoxic, natural and affordable antioxidants are required to manage oxidative stress, thereby thwarting the associated diseases (Liu et al., 2018). Hence, this study was designed to investigate the in vitro antioxidant activities of methanol extract, fractions and essential oil of D. suffruticosa in the quest for cheap and safer antioxidant sources.
2 Materials and methods
2.1 Sample collection
Both mature and young leaves of the plant were collected on a sunny day (9 am., 1 pm., and 5 pm) in September 2019 with maximum temperature 33 °C and min temperature 24 °C from the lower land of Papar, Sabah. Malaysia. The young leaves were 5 to 10 cm in length with reddish colour while the mature leaves were 12 to 35 cm in length with dark green colour. The plant was shrubby and 5 to 6 m tall. The identification of the leaves was done by Mr. Johnny Gisil, a botanist at the Institute of Tropical Biology and Conservation (ITBC) Herbarium, Universiti Malaysia Sabah and voucher of the specimen (MDS 002) was deposited (Fig. 1).
Dillenia suffruticosa.
2.2 Extraction and fractionation
The leaves of the plant were washed with tap water, clean from contamination and oven-dried at 37 °C for 3–4 days. Dried leaves were ground to a fine powder using a heavy-duty grinder. Plant powder about 60 g was extracted with pure methanol (300 ml) using the Soxhlet method at 50–60 °C and 72 h. The methanol residues were removed from the extract using a vacuum rotary evaporator. The dried methanol crude extract was further fractionated with solvents according to increasing order of polarity starting with n-hexane, ethyl acetate, chloroform and butanol in a separatory funnel. The solvents were removed from the fractions under vacuum. The samples were kept at minus 80 °C for 24 h and then lyophilized using a freeze drier. The freeze-dried samples were then stored in the freezer for further studies (Osadebe et al., 2012).
2.3 Extraction of essential oil by hydrodistillation
For the extraction of essential oil from D. suffruticosa, 100 g of leaves were subjected to hydro distillation for 3 h by a Clevenger apparatus. At the end of the distillation, an aqueous phase (aromatic water) and an organic phase (essential oil) were noticed. The essential oil was collected, dried under anhydrous sodium sulphate and stored in sealed vials at 4 °C until further use (Atti-Santos et al., 2005).
2.4 Radical scavenging activity using the DPPH method
Different concentrations ranging from 0.012 to 0.50 mg/g of methanol extract, fractions and essential oil the plant was diluted with distilled water at various ratios. Further 0.3 ml of each dilution was mixed with 2.7 ml of DPPH (150 μM) prepared in methanol and left in the dark for 1 h. The absorbance was recorded at 512. Ascorbic acid was employed as a positive control (Brand-Williams et al., 1995).
2.5 Determination of total phenolic content
Briefly, the test sample (0.2 ml) was treated with 1.5 ml of Folin-Ciocalteu reagent in a tube and mixed thoroughly. After 5 min, 1.5 ml sodium carbonate (60 g/l) was added to the mixture and mixed thoroughly. Finally, the samples were kept for 90 min at room temperature in the darkness. The absorbance was recorded at 725 nm using a spectrophotometer. Gallic acid was used as a positive control (Velioglu et al., 1998).
2.6 Determination of total flavonoid content
Briefly, the test samples (0.25 ml) were mixed with 1.25 ml of distilled water and 0.075 ml of sodium nitrate (5%). The mixture was well shaken and kept for 6 min in a dark place. Further, the mixture was vortexed after the addition of 0.15 ml of aluminium chloride (10%) and kept for 5 min at room temperature. Finally, 0.5 ml of sodium hydroxide (4%) was added to the mixture, followed by the addition of distilled water to obtain a final volume of 2.5 ml. The mixture was shaken strongly and absorbance was recorded at 510 nm against the blank. Catechin was used as a positive control (Zou et al., 2004).
2.7 Preliminary phytochemical screening
The preliminary phytochemical screening of the methanol extract and methanol fractions (1 mg/ml) of D. suffruticosa was carried out to access the presence or the absence of different phytochemical components such as alkaloids, steroids, flavonoids, triterpenoids, saponin, tannins, anthraquinones and phytosterol (Harborne, 1998).
2.8 Gas chromatography-mass spectrometry (GC–MS) analysis of D. Suffruticosa
GCMS analysis for the methanol extract, fractions and essential oil of D. suffruticosa were performed using GCMS system consisting of an Agilent 7890A gas chromatograph system coupled with an Agilent 5975C mass spectrometry detector as described by Shah et al., (2014). Briefly, 1 µL of the reconstituted sample was injected into an Agilent J&W HP-5MS capillary column (30 m × 0.25 mm × 0.25 µm) with purified helium gas used as a carrier gas. The split less mode was selected for all analysis and identification of compounds was carried out by matching the MS spectral against the built-in National Institute of Standards and Technology (NIST) library (version 2011).
2.9 Statistical analysis
All the experiments were conducted in triplicates. The values are expressed as the mean ± standard deviation (SD). The statistical analyses were done using SPSS 25.0 windows statistical package software (SPSS Inc., Chicago, IL). Significant differences between extract, fractions and essential oil were analysed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test.
3 Results
3.1 The percentage (%) yield, calculated effective concentration (EC50), total phenolic and flavonoid contents of methanol extract, fractions and essential oil of D. Suffruticosa
The % yields, EC50, total phenolic and flavonoid contents of methanol extract, fractions and essential oil of D. suffruticosa are revealed as shown in Table 1. The methanol extract showed a high % yield followed by n-hexane, butanol, ethyl acetate and chloroform fractions. The essential oil of the plant indicated high EC50 value while butanol fraction showed low EC50 value as compare to hexane, ethyl acetated and chloroform fractions and methanol extract. The butanol fraction indicated high phenolic and flavonoid contents followed by methanol extract and other fractions of the plant sample (Table 1). Each value represents the mean ± SD of 3 replicates. Different letters with each mean a statistical difference.
Extract/fractions
Percentage (%) yield
EC50 (mg/g)
Total phenolic content (mg/g)
Total flavonoid content (mg/g)
Mean ± SD
Mean ± SD
Mean ± SD
Mean ± SD
Methanol extract
9.27 ± 0.75
0.216 ± 0.001
277.00 ± 3.50
34.83 ± 0.71
Hexane fraction
5.77 ± 0.83a
0.230 ± 0.002a
206.50 ± 3.34a
26.56 ± 1.45a
Ethyl acetate fraction
0.91 ± 0.18b
0.231 ± 0.000b
234.33 ± 3.46b
32.49 ± 1.92
Chloroform fraction
0.58 ± 0.06c
0.271 ± 0.000c
129.33 ± 4.28c
20.88 ± 1.66c
Butanol fraction
1.05 ± 0.10d
0.074 ± 0.000d
379.00 ± 9.25d
74.44 ± 2.18d
Essential oil
0.50 ± 0.08e
2.27 ± 0.001e
ND
9.78 ± 1.13 ,e
3.2 DPPH free radical scavenging activity of methanol extract, fractions and essential oil of D. Suffruticosa
The DPPH free radical scavenging activity of the D. suffruticosa methanol extract, fractions and essential oil was determined at different concentrations ranging from 0.012 to 0.5 mg/g. The methanol extract and butanol fraction showed high % DPPH scavenging activity followed by ethyl acetate, hexane and chloroform fraction and essential oil (Fig. 2).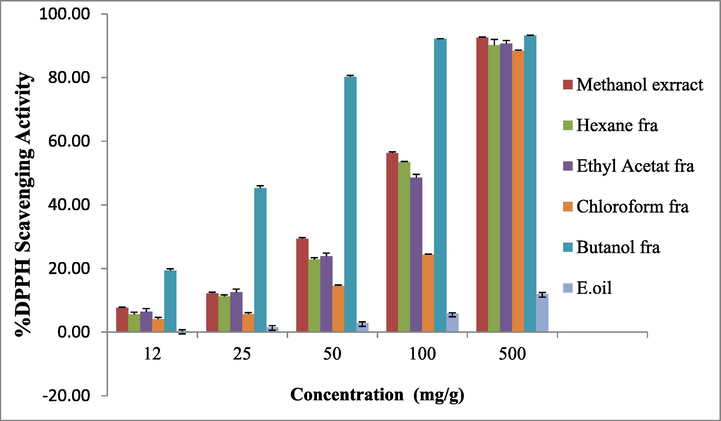
Radical scavenging activity of D. suffruticosa methanol extract, methanol fractions and essential oil by DPPH method.
3.3 Phytochemical analysis methanol extract and methanol fractions of D. Suffruticosa
The presence of various phytochemicals such as alkaloids, anthraquinones, flavonoids, phytosterol, saponins, tannins, triterpenoids and steroids in the methanol extract and fractions of D. suffruticosa is revealed in Table 2. Alkaloids were detected strongly in methanol extract and all the fractions. Anthraquinones were noticed only in the chloroform and butanol fractions and methanol extract while absent in hexane and ethyl acetate fractions. Flavonoids were noticed strongly in butanol fractions and methanol extract as compared to other fractions. Phytosterols were detected in methanol extract and fractions except for butanol. Saponins were strongly detected in methanol extract and fractions except for butanol. Triterpenoids were absent in butanol fraction while strongly present in the hexane fraction and methanol extract as compared to ethyl acetate and chloroform fractions. Steroids were also absent in butanol fractions while strongly present in the methanol extract and other fractions. + = present; ++ = Strong present; – = absent.
Phytochemicals
Extract/fractions
Hexane fraction
Ethyl acetate fraction
Chloroform fraction
Butanol fraction
Methanol extract
Alkaloids
++
++
++
++
++
Anthraquinones
–
–
+
+
+
Flavonoids
+
+
+
++
++
Phytosterols
+
+
+
–
+
Saponins
++
++
++
–
++
Tannins
–
+
+
+
++
Triterpenoids
++
+
+
–
++
Steroids
++
++
++
–
++
3.4 GCMS analysis of methanol extract, fractions and essential oil of D. Suffruticosa
3.4.1 GC–MS chromatogram of methanol extract, fractions and essential oil of D. Suffruticosa
Figs. 3a-3f indicates the GC–MS chromatogram of various bioactive compounds detected in methanol extract, fractions and essential oil of D. suffruticosa.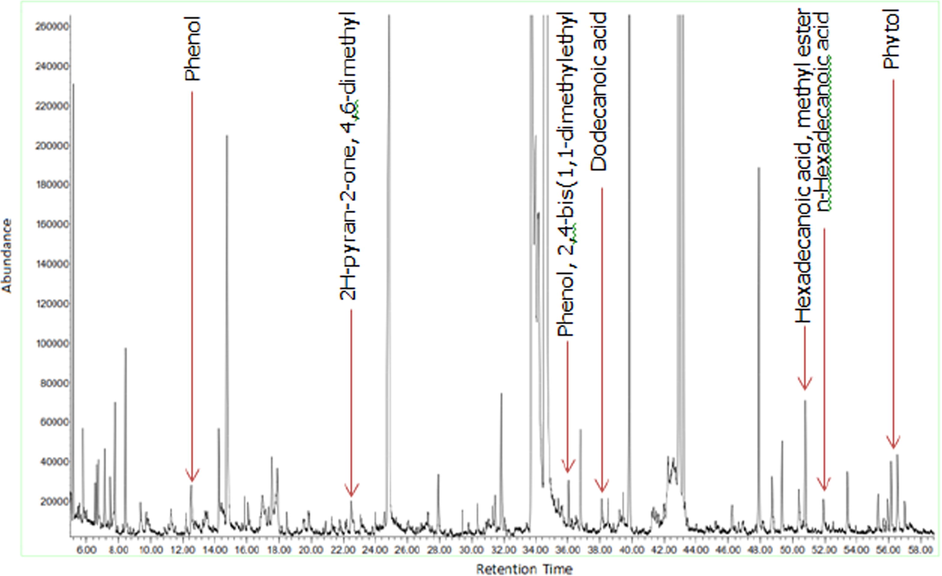
GC–MS chromatogram of the methanol extract of D. suffruticosa.
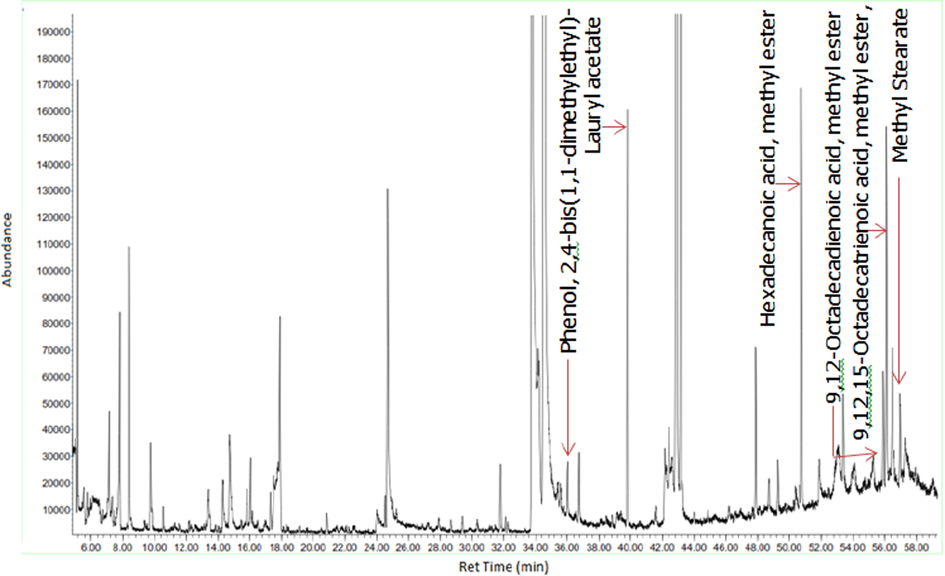
GC–MS chromatogram of the hexane fraction of D. suffruticosa methanol extract.
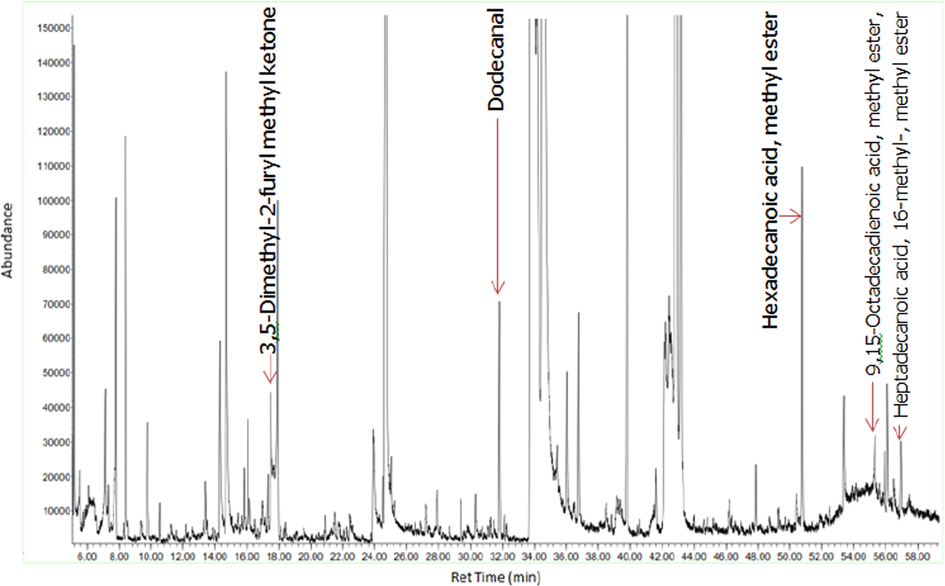
GC–MS chromatogram of the ethyl acetate fraction of D. suffruticosa methanol extract.
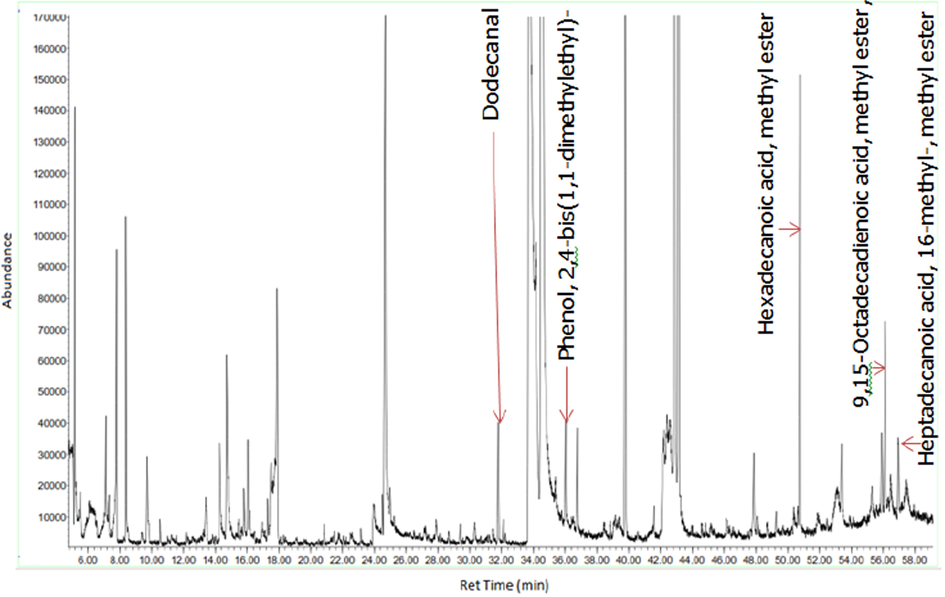
GC–MS chromatogram of the chloroform fraction of D. suffruticosa methanol extract.
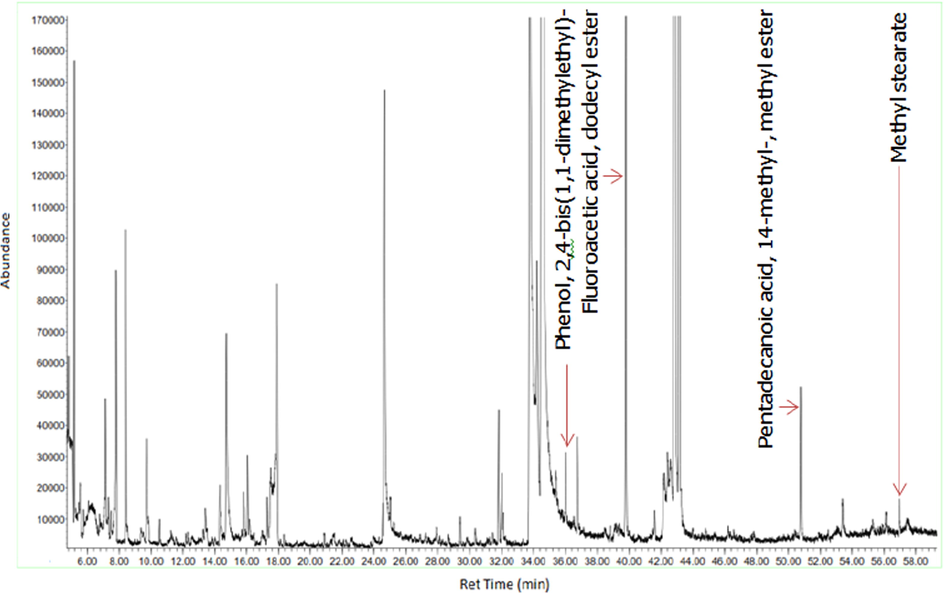
GC–MS chromatogram of the butanol fraction of D. suffruticosa methanol extract.
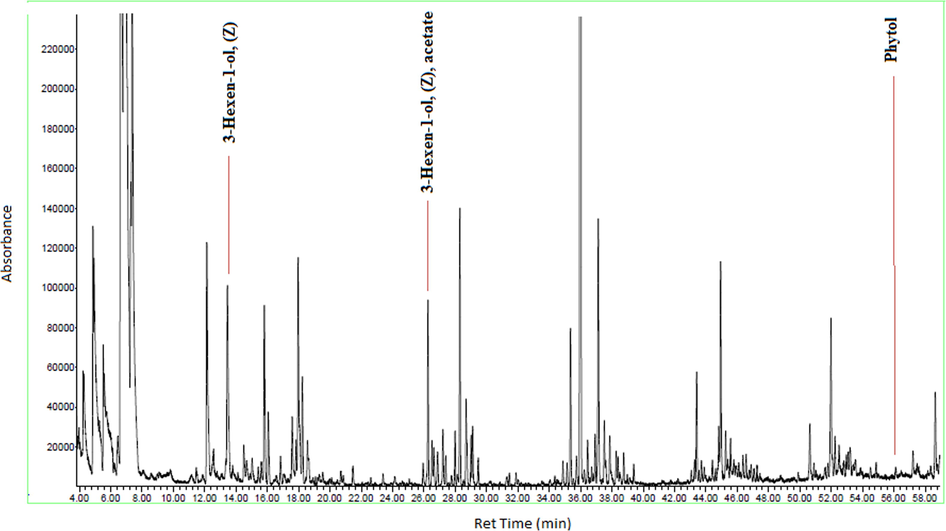
GC–MS chromatogram of the chemical constituents of essential oil of D. suffruticosa.
3.4.2 List of phytochemical compounds detected in the methanol extract, fractions and essential oil of D. Suffruticosa
The retention time, compound name and area percentage (%) of the phytochemicals compound detected in the methanol extract, fractions and essential oil of D. suffruticosa by GCMS are shown in Table 3. In the methanol extract total 19 compounds were detected while in hexane 8, ethyl acetate 11, chloroform 10 and butanol fraction 6 and essential oil 6 compounds were noticed. The structures of the identified bioactive compounds are shown in Fig. 4.
Extract/Fractions/ Essential oil
No
Retention Time
Compound name
Area (%)
Methanol
1.
4.25
Methyl Glycolate
2.95
2.
7.44
2-Furanmethanol
0.77
3.
12.52
Phenol
1.39
4.
14.26
Benzyl Alcohol
2.04
5.
17.09
Undecane
11.97
6.
22.5
2 h-Pyran-2-One, 4,6-Dimethyl-
1.19
7.
30.35
1-Undecanol
0.78
8.
31.48
Tetradecane
0.62
9.
31.83
Tridecanal
0.03
10.
33.71
Cyclododecane
35.78
11.
34.19
1-Dodecene
19.46
12.
36.06
Phenol, 2,4-Bis(1,1-Dimethylethyl)
0.83
13.
36.8
n-Tridecan-1-ol
1.98
14.
38.01
Dodecanoic acid
0.84
15.
39.83
Lauryl acetate
12.26
16.
42.57
3-Chloropropionic acid, heptadecyl
1.28
17.
50.79
Hexadecanoic acid, methyl ester.
2.66
18.
51.9
n-Hexadecanoic acid
0.96
19.
56.51
Phytol
2.13
Hexane
1.
17.78
Undecane
7.81
2.
33.76
Cyclodecane
21.76
3.
36.03
Phenol, 2,4-bis(1,1-dimethylethyl)-
3.62
4.
39.79
Lauryl acetate
18.04
5.
50.75
Hexadecanoic acid, methyl ester
19.05
6.
55.91
9,12-Octadecadienoic acid, methyl ester
5.95
7.
56.11
9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)-
18.17
8.
56.96
Methyl stearate
5.59
Ethyl acetate
1.
17.5
3,5-Dimethyl-2-furyl methyl ketone
17.68
2.
17.69
Undecane
11.61
3.
31.8
Dodecanal
17.05
4.
35.45
1,4-Benzenedicarboxylic acid, dimethyl ester
11.29
5.
38.46
Chloroacetic acid, tridecyl ester
3.55
6.
50.75
Hexadecanoic acid, methyl ester
26.39
7.
55.92
9,15-Octadecadienoic acid, methyl ester, (Z,Z)-
6.17
8.
56.96
Heptadecanoic acid, 16-methyl-, methyl ester
6.26
11.
56.96
Methyl stearate
6.20
Chloroform
1.
4.73
2,2-Dimethoxybutane
0.81
2.
17.78
Undecane
3.54
3.
31.8
Dodecanal
1.87
4.
33.7
Cyclododecane
76.86
5.
36.03
Phenol, 2,4-bis(1,1-dimethylethyl)-
1.69
6.
39.79
Fluoroacetic acid, dodecyl ester
0.31
7.
50.75
Hexadecanoic acid, methyl ester
7.50
8.
55.916
E,Z-1,3,12-Nonadecatriene
1.75
9.
56.11
9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)-
4.09
10.
56.97
Heptadecanoic acid, 16-methyl-, methyl ester
1.58
Butanol
1.
17.89
Undecane
35.53
2.
31.8
Dodecanal
12.95
3.
36.03
Phenol, 2,4-bis(1,1-dimethylethyl)-
10.35
4.
39.79
Fluoroacetic acid, dodecyl ester
56.66
5.
50.75
Pentadecanoic acid, 14-methyl-, methyl ester
15.50
6.
56.97
Methyl stearate
4.53
Essential oil
1.
6.85
3-Hexen-1-ol, (Z)
19.95
2.
13.44
3-Hexen-1-ol, acetate, (Z)
10.39
3.
18.24
Decane, 3,7-dimethyl-
1.24
4.
26.02
Hexadecane
1.21
5.
56.46
Phytol
1.74
6.
17.78
Undecane
7.81
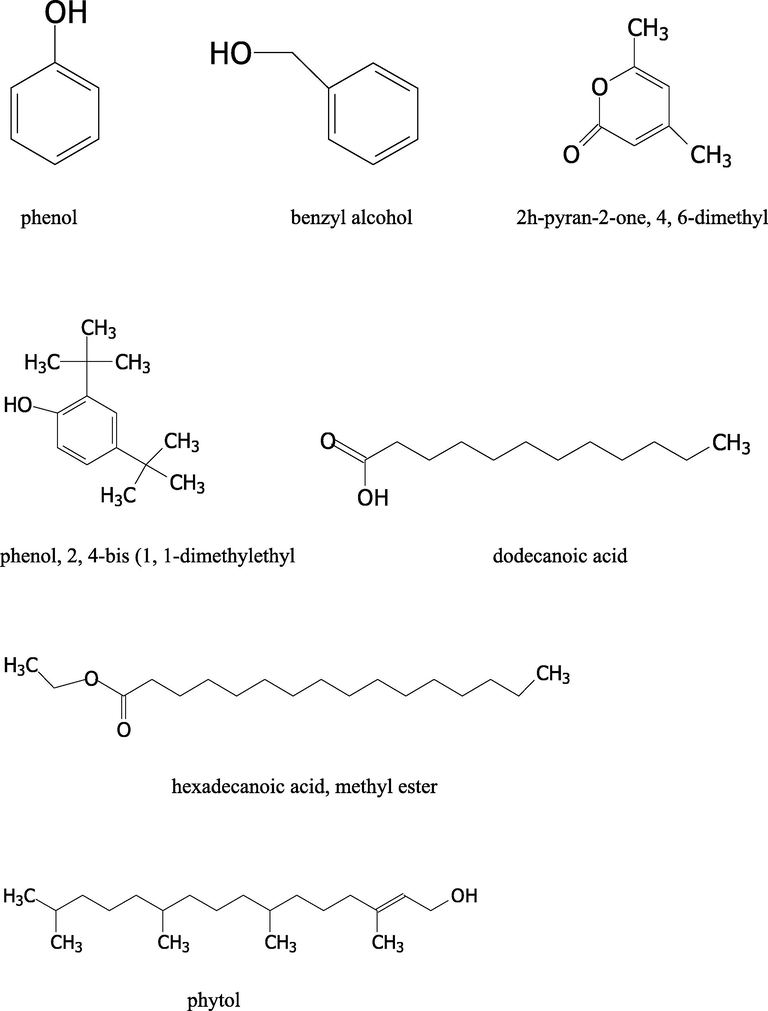
Structure of the identified bioactive compounds in the methanol extract, fractions and essential oil of D suffruticosa.
4 Discussion
Antioxidants have the potential to neutralize the actions of free radicals (Anju and Sarita, 2010). In our sample, the butanol fraction and methanol extract showed the highest antioxidant activity with EC50 values of 0.07 and 0.21 mg/g. The highest activity of butanol fraction and methanol extract could be due to the presence of the high amount of phenolic and flavonoid compounds. Phenolics and flavonoids are secondary plant metabolites and are widely distributed in various plants (Bonoli et al., 2004; Kaisoon et al., 2011; Tungmunnithum et al., 2018). The compounds have attracted great attention recently due to their potential to neutralize reactive oxygen species and other free radicals. Phenolic and flavonoid compounds display anti-tumour, anti-adhesive, antimicrobial and anti-inflammatory properties and protection against chronic diseases by the reduction of oxidative stress solely or synergistically with other phenolic-containing amalgams. (Brighente et al., 2007; Kylli et al., 2011; Nemudzivhadi and Masoko, 2014; Rasmussen et al., 2005; Tungmunnithum et al., 2018). Therefore, the amount of total phenolics and flavonoids present in the methanol extract, fractions and essential oil of D. suffruticosa were investigated and significantly highest results were recorded in the order butanol > methanol > ethyl acetate > hexane > chloroform for total phenolics and total flavonoids. Similarly, the antioxidant activity, phenolic and flavonoid content of extract, fractions and essential oil of Commelina nudiflora (Commelinaceae), Dillenia indica (Dilleniaceae), Ballota limbata (Lamiaceae) plant have also been reported (Abdille et al., 2005; Shah and Iqbal, 2018; Waheed et al., 2014). Further, the preliminary phytochemical analysis revealed that D. suffruticosa methanol extract and its fraction are very rich in phytochemical compounds (alkaloids, anthraquinones, flavonoids, phytosterol, saponins, tannins, triterpenoids and steroids). These phytochemical compounds have potential health-promoting effects including antioxidant, anti-inflammatory, antitumor, anti-HIV, anti-cholinesterase, antibacterial etc. (Dillard and German, 2000; Hussain et al., 2019, 2018; Kuo et al., 2009; Othman et al., 2019; Panche et al., 2016; Patel and Savjani, 2015). Anthraquinones possess antioxidant, antiparasitic and anticancer properties (Dave and Ledwani, 2012; Yadav et al., 2019). The current results demonstrated that anthraquinones were noticed only in the butanol of D. suffruticosa. Alkaloids have been reported to possess antimicrobial, anti-inflammatory, neuroprotective effects (Hussain et al., 2018; Peng et al., 2019). Tannins have been reported with neuroprotection (Hussain et al., 2019). Plant-derived steroids have been reported with anti-inflammatory properties (Patel and Savjani, 2015). Phytosterol and saponins have been reported to have hypocholesterolemic and anti-diabetic effects (Edeoga et al., 2005; Moreau et al., 2002). While triterpenoids have been reported with antitumor and anti-HIV properties (Kuo et al., 2009). The strong presence of phytosterol, saponins and triterpenoids were noticed in methanol extract and its fractions excluding butanol fraction. The above mentioned bioactive compounds have been also detected in the solvent extract and fractions of C. nudiflora and B. limbata (Shah and Iqbal, 2018; Waheed et al., 2014).
The GCMS analysis indicated the presence of various bioactive compounds in the methanol extract, fractions and essential oil of D. suffruticosa such as benzyl alcohol, 2 h-Pyran-2-one, 2,4-bis (1,1-dimethylethyl), dodecanoic acid and 4,6-dimethyl, phenol. These compounds have been reported with antioxidant, antimicrobial (Dasgupta and Humphrey, 1998; Lee and Shibamoto, 2002; Lee et al., 2005), antifungal (Mikhlin et al., 1983), anti-cancer (Ajayi et al., 2011; Rajaram et al., 2013) anti-inflammatory and anti-viral properties (Appendino et al., 2007). In addition to this, hexadecanoic acid, ethyl ester, n-hexadecanoic acid and phytol are also found in the methanol extract, fractions and essential oil of D. suffruticosa. These compounds have also been noticed in other plant extracts and reported with antioxidant, anti-cancer, anti-inflammatory, anti-microbial, hypocholesterolemic diuretic, hemolytic and hepatoprotective activities (Ajayi et al., 2011; Bülent Köse et al., 2007; Kumar et al., 2010). The above-mentioned compounds haven been also reported in the extract of Plumbago zeylanica (Plumbaginaceae.) (Ajayi et al., 2011), Peganum harmala (Peganaceae) (Moussa and Almaghrabi, 2016), Broussonetia luzonica (Moraceae) (Casuga et al., 2016), Ocimum basilicu (Lamiaceae) and Thymus vulgaris (Lamiaceae) plants (Kuete, 2017; Kumar et al., 2016; Lee et al., 2005).
These findings, therefore, imply that the studied plant extract, fractions and essential oil mainly contain vital phytochemical and shows good antioxidant activity. Thus the plant could be of considerable interest in the development of natural alternative source to reduce the damaging effects caused by oxidative stress.
5 Conclusion
The methanol extract, methanol fractions and essential oil of D. suffruticosa indicated good in vitro DPPH radical scavenging activity and high values of phenolic and flavonoid contents. The preliminary screening revealed the presence of vital phytochemicals such as alkaloids, flavonoids, anthraquinones, steroids, tannins, phytosterol, triterpenoids and saponins. Further, the GCMS analysis of methanol extract, methanol fractions and essential oil of the plant indicated various bioactive compounds including 3-hexen-1-ol, acetate, phytol, methyl glycolate, lauryl acetate, phenol, 2,4-bis (1,1-dimethylethyl), 9,12-octadecadienoic acid, hexadecanoic acid, methyl ester, methyl stearate, phenol and benzyl alcohol. These compounds have been reported with antioxidant properties. Thus, the methanol extract, methanol fractions and essential oil of the studied plant can be potential antioxidant compound sources and alternatives for the management of oxidative stress-associated disorders. The current research work endorses further studies leading to isolation and characterization of the pure antioxidant compounds, particularly those able to attenuate oxidative stress and related maladies.
Acknowledgements
This research was supported by the Research Priority Area Scheme Grant at Universiti Malaysia Sabah (SBK0027-SKK-2012). MDS is also thankful to the Ministry of Higher Education (MOHE), Malaysia for providing fellowship (Ref No: KPT.B.600-18/3/-OR212688). Authors are also thankful to Dr Zarina Amin, Director, Biotechnology Research Institute, Universiti Malaysia Sabah for support and encouragement.
Declaration of Competing Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem.. 2005;90:891-896.
- [Google Scholar]
- Gas chromatography-mass spectrometry analysis and phytochemical screening of ethanolic root extract of Plumbago zeylanica. Linn. J. Med. Plants Res.. 2011;5:1756-1761.
- [Google Scholar]
- Suitability of Foxtail millet (Setaria italica) and Barnyard millet (Echinochloa frumentacea) for development of low glycemic index biscuits. Malays. J. Nutr.. 2010;16:361-368.
- [Google Scholar]
- Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod.. 2007;70:608-612.
- [CrossRef] [Google Scholar]
- Dillenia suffruticosa extract inhibits proliferation of human breast cancer cell lines (MCF-7 and MDA-MB-231) via Induction of G2/M arrest and apoptosis. Molecules. 2013;18:13320-13339.
- [CrossRef] [Google Scholar]
- Extraction of essential oils from lime (Citrus latifolia Tanaka) by hydrodistillation and supercritical carbon dioxide. Brazilian Arch. Biol. Technol.. 2005;48:155-160.
- [CrossRef] [Google Scholar]
- Free and bound phenolic compounds in barley (Hordeum vulgare L.) flours. J. Chromatogr. A. 2004;1057:1-12.
- [CrossRef] [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25-30.
- [CrossRef] [Google Scholar]
- Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol.. 2007;45:156-161.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of the essential oil of Centaurea aladagensis. Fitoterapia. 2007;78:253-254.
- [CrossRef] [Google Scholar]
- GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac. J. Trop. Biomed.. 2016;6:957-961.
- [CrossRef] [Google Scholar]
- Chiam, A., 2011. Local plants trove of medicinal and health supplements | Borneo Post Online. URL https://www.theborneopost.com/2011/09/06/local-plants-trove-of-medicinal-and-health-supplements/ (accessed 6.5.20).
- Wayside trees of Malaya, Malayan Nature Society (4th ed.). Kuala Lumpur; 1997.
- Gas chromatographic–mass spectrometric identification and quantitation of benzyl alcohol in serum after derivatization with perfluorooctanoyl chloride: a new derivative. J. Chromatogr. B Biomed. Sci. Appl.. 1998;708:299-303.
- [CrossRef] [Google Scholar]
- A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour.. 2012;3:291-319.
- [Google Scholar]
- Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric.. 2000;80:1744-1756.
- [CrossRef] [Google Scholar]
- Free radicals in the physiological control of cell function. Physiol. Rev. 2002
- [CrossRef] [Google Scholar]
- Phytochemical constituents of some Nigerian medicinal plants. African J. Biotechnol.. 2005;4:685-688.
- [CrossRef] [Google Scholar]
- Free radicals and other reactive species in disease. In: Encyclopedia of Life Sciences. Chichester, UK: John Wiley & Sons Ltd; 2005.
- [CrossRef] [Google Scholar]
- Phytochemical methods : a guide to modern techniques of plant analysis. Chapman and Hall; 1998.
- Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: An updated review. Molecules 2019
- [CrossRef] [Google Scholar]
- Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci.. 2018;14:341-357.
- [CrossRef] [Google Scholar]
- Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods. 2011;3:88-99.
- [CrossRef] [Google Scholar]
- Citrulline: Pharmacological perspectives and role as a biomarker in diseases and toxicities. In: Biomarkers in Toxicology. Elsevier Inc.; 2014. p. :883-905.
- [CrossRef] [Google Scholar]
- Thymus vulgaris. In: Medicinal Spices and Vegetables from Africa. Elsevier; 2017. p. :599-609.
- [CrossRef] [Google Scholar]
- Kumar, P.P., Kumaravel, S., Lalitha, C., 2010. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo 4, 191–195.
- Herbs: composition and dietary importance. In: Encyclopedia of Food and Health. Elsevier; 2016. p. :332-337.
- [CrossRef] [Google Scholar]
- Plant-derived triterpenoids and analogues as antitumor and Anti-HIV agents. Nat. Prod. Rep. 2009
- [CrossRef] [Google Scholar]
- Lingonberry (Vaccinium vitis-idaea) and European Cranberry (Vaccinium microcarpon) Proanthocyanidins: isolation, identification, and bioactivities. J. Agric. Food Chem.. 2011;59:3373-3384.
- [CrossRef] [Google Scholar]
- Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J. Agric. Food Chem.. 2002;50:4947-4952.
- [CrossRef] [Google Scholar]
- Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem.. 2005;91(1):131-137.
- [CrossRef] [Google Scholar]
- Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. https:// 2018
- [CrossRef] [Google Scholar]
- Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019
- [CrossRef] [Google Scholar]
- Reactive oxygen species are the major antibacterials against Salmonella typhimurium purine auxotrophs in the phagosome of RAW 264.7 cells. Cell. Microbiol.. 2008;10:1058-1073.
- [CrossRef] [Google Scholar]
- Antifungal and antimicrobial activity of beta-ionone and vitamin A derivatives. Prikl. Biokhim. Mikrobiol.. 1983;19:795-803.
- [Google Scholar]
- Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J. Am. Coll. Nutr.. 2000;19:312S-319S.
- [CrossRef] [Google Scholar]
- Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res.. 2002;41:457-500.
- [CrossRef] [Google Scholar]
- Fatty acid constituents of Peganum harmala plant using Gas Chromatography-Mass Spectroscopy. Saudi J. Biol. Sci.. 2016;23:397-403.
- [CrossRef] [Google Scholar]
- In vtro assessment of cytotoxicity, antioxidant, and anti-Inflammatory activities of Ricinus communis (Euphorbiaceae) leaf extracts. Evidence-Based Complement. Altern. Med.. 2014;2014:1-8.
- [CrossRef] [Google Scholar]
- Reactive oxygen species, antioxidants, and the mammalian thioredoxin system1 1This review is based on the licentiate thesis “Thioredoxin reductase—interactions with the redox active compounds 1-chloro-2,4-dinitrobenzene and lipoic acid” by Jonas Nordberg. Free Radic. Biol. Med.. 2001;31:1287-1312.
- [CrossRef] [Google Scholar]
- Phytochemical analysis, hepatoprotective and antioxidant activity of Alchornea cordifolia methanol leaf extract on carbon tetrachloride-induced hepatic damage in rats. Asian Pac. J. Trop. Med.. 2012;5:289-293.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol 2019
- [CrossRef] [Google Scholar]
- Systematic review of plant steroids as potential anti- inflammatory agents: Current status and future perspectives. J. Phytopharm. JPHYTO. 2015;4:121-125.
- [Google Scholar]
- Plant-derived alkaloids: The promising disease-modifying agents for inflammatory bowel disease. Front. Pharmacol.. 2019;10:1-15.
- [CrossRef] [Google Scholar]
- Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem.. 2015;30:11-26.
- [CrossRef] [Google Scholar]
- Antioxidant and free radical-scavenging activities of seeds and agri-wastes of some varieties of soybean (Glycine max) Food Chem.. 2007;104:783-790.
- [CrossRef] [Google Scholar]
- Comparative bioactive studies between wild plant and callus culture of Tephrosia tinctoria Pers. Appl. Biochem. Biotechnol.. 2013;171:2105-2120.
- [CrossRef] [Google Scholar]
- Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res.. 2005;49:159-174.
- [CrossRef] [Google Scholar]
- Dillenia species: A review of the traditional uses, active constituents and pharmacological properties from pre-clinical studies. Pharm. Biol.. 2014;52:890-897.
- [CrossRef] [Google Scholar]
- Antioxidant capacity of the Spanish Mediterranean diet. Food Chem.. 2006;94:442-447.
- [CrossRef] [Google Scholar]
- The potential protective effect of Commelina nudiflora L. against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats, mediated by suppression of oxidative stress and inflammation. Environ. Health Prev. Med.. 2017;22:1-19.
- [CrossRef] [Google Scholar]
- Antioxidant activity, phytochemical analysis and total polyphenolics content of essential oil, methanol extract and methanol fractions from Commelina nudiflora. Int. J. Pharm. Pharm. Sci.. 2018;10:36.
- [CrossRef] [Google Scholar]
- Phytochemical investigation and free radical scavenging activities of essential oil, methanol extract and methanol fractions of Nephrolepis biserrata. Int. J. Pharm. Pharm. Sci.. 2014;6:269-277.
- [Google Scholar]
- A guide to the wildflowers of Singapore. Singapore Science Centre; 1985.
- Flavonoids and oher phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 2018;5:93.
- [CrossRef] [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39:44-84.
- [CrossRef] [Google Scholar]
- Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem.. 1998;46:4113-4117.
- [CrossRef] [Google Scholar]
- Investigation of phytochemical and antioxidant properties of methanol extract and fractions of Ballota limbata (Lamiaceae) Indian J. Pharm. Sci.. 2014;76:251-256.
- [Google Scholar]
- Antimicrobial screening of plants used for traditional medicine in the state of Perak, Peninsular Malaysia. Fitoterapia. 2004;75:68-73.
- [CrossRef] [Google Scholar]
- Metabolic engineering to synthetic biology of secondary metabolites production. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2019. p. :279-320.
- [CrossRef] [Google Scholar]
- Oxidized LDL and atherogenesis. In: Annals of the New York Academy of Sciences. New York Academy of Sciences; 1999. p. :134-137.
- [CrossRef] [Google Scholar]
- Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138-21156.
- [CrossRef] [Google Scholar]
- Antioxidant activity of a flavonoid-rich extract of hypericum perforatum L. in vitro. J. Agric. Food Chem.. 2004;52:5032-5039.
- [CrossRef] [Google Scholar]







