Translate this page into:
Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens
⁎Corresponding authors at: Department of Plant Protection, Faculty of Agriculture, Universiti Putra Malaysia Serdang, Selangor, Malaysia (A. Khairulmazmi). khairulmazmi@upm.edu.my (A. Khairulmazmi), yasmeen@upm.edu.my (S. Yasmeen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The objectives of this study were to profile ginger essential oils (EOs) phytochemical constituents and antimicrobial activity against important phytopathogens. Ginger EOs was extracted using a modified Clevenger-type apparatus by hydro-distillation then followed by GCMS and headspace analysis of its phytochemical constituents. The phytoconstituents identified were monoterpenes and sesquiterpene hydrocarbons. Food poisoned and disc diffusion techniques were applied to determine the percentage inhibition of fungal mycelial and bacterial growth respectively. The EOs produced mycelial growth inhibition in all the test fungal pathogens after five days of incubation. The MIC and MFC of the EOs on the tested fungi were in the range of 1 μl/ml and 5–6 μl/ml, respectively. The bacterial growth of all the tested isolates was also affected by EOs at 100–500 µl/ml, from weak to strong antibacterial activity. The EOs affected the Xanthomonas oryzae pv. oryzae-strain A isolate most at a higher concentration of 400–500 μl/ml with mean inhibition of 20.66 mm and 22.66 mm respectively, which are found to be effective. The MIC values on the bacterial pathogens were at100 μl/ml. The inhibition zone of positive control (streptomycin) at 15 µg/disc was 25.00 mm and appeared to be efficient. Metabolomics analysis to concurrently quantify variability among multiple compounds in the data sets and identify such compounds responsible for the X. oryzae pv. oryzae-strain-A inhibition were determined. The cross-validated PLS model has shown a strong correlation between ginger EOs and bioactivity. The action of ginger EOs on the cell structure was fully identified using SEM by observing the changes in morphology and integrity of X. oryzae pv. oryzae-strain-A cells. The DMSO treatment (control) showed a normal rod shape cell, while treatment with the ginger EOs showed irregular shape with sunken surfaces, and treatment with antibiotics display abnormal growth of the cells. These findings can, therefore, propose that the ginger EOs could be used as a new antimicrobial agent in suppressing the growth of phytopathogens and as possible new alternatives to synthetic fungicides and bactericides.
Keywords
Phytochemical profiling
Antifungal activity
Antibacterial activity
Essential oils
Zingiber officinale
Metabolomics
1 Introduction
Phytopathogens are the main cause of plant diseases that result in significant crop losses, especially in tropics. Prevention or reduction of plant disease infestation is a major concern of farmers. In spite of the advances in production agriculture, effective management of plant disease remains a challenge because of the side effects of existing pesticides as most of them chemically synthetic (bactericides, fungicides, among others) (Shaheen and Issa, 2020). Excessive used of synthetic pesticides has been implicated in their negative effects on the environment such as soil and water pollutions, long periods of degradation, residual accumulation in the food chain, and less control efficacy against pathogenic microbes with long-term usage (Ghormade et al., 2011; Nega, 2014; Bhavaniramya et al., 2019). Globally, the use of some of the existing antibiotics in agriculture is being banned because of the adverse effects of the antibiotics such as severe or extreme pathogens resistance, higher cost of production, prolonged cycles of chemicals degradation, and environmental pollutions (Hajano et al., 2012; Sundin et al., 2016; Buttimer et al., 2017). In terms disease resistance, streptomycin which is the main antibiotic that is used to control bacterial diseases in plants is no longer effective because of the resistant of bacterial to streptomycin (Xu et al., 2010). The limitations aforementioned limitations are posing a serious challenge for achieving sustainable management of plant diseases in many farming systems (Juroszek and Von Tiedemann, 2011; Dara, 2019). In particular, disease management systems are of the major concern because there is a strong need for natural antimicrobial agents or biopesticides that are effective, non-toxic, and ecologically safe to control plant diseases. Research on the alternative pesticides and antimicrobials, including natural plant products such as ginger essential oils is essential. The introduction of new generation/innovation of biopesticides could provide solutions for the pathogenic microbes that developed resistance to synthetic chemicals (Saha et al., 2016).
Ginger essential oils (EOs) are one of the natural products that could be an alternative class of natural antimicrobials with a wide range of metabolite spectrum to pave the way for new and more effective compounds in controlling plant pathogens. It is becoming clear that these natural products have the ability to influence the modern agrochemical solutions for their biological and antimicrobial activities (Abdel-Kader et al., 2015) as well as economic viability and low toxicity (Brusotti et al., 2014). They are also well known for their antibacterial, antiviral, antifungal activities, insect repellents (Venkateshwarlu, 2014; Sendanayake et al., 2017; Azhari et al., 2017), biodegradable, and generally embraced by many societies. Thus, it is a new solution for protecting plants from being attacked by pathogens (Lim et al., 2012; Lanzotti et al., 2013).
Considering the economic importance of the plant diseases caused by the bacterial and fungal pathogens besides the toxic effects of synthetic chemicals, it is important to explore an alternative way that is eco-friendly for the management of plant diseases. To date, the knowledge on the use of ginger EOs constituents in plant disease control is limited. The current research efforts are directed towards to profile ginger EOs phytochemical constituents so as to determine antimicrobial activity against selected major phytopathogens. The outcomes of this study may serve as one of the new control alternatives for controlling plant diseases in the tropics. This is considered as effective and sustainable solution to synthetic chemicals by exploring the untapped potentials of ginger EOs (natural products) for its antimicrobial activity against phytopathogenic bacteria and fungi.
2 Materials and methods
2.1 Collection and preservation of plant sample
The plant materials used were the rhizomes of wild and domestic gingers. To reduce variations between samples, fresh ginger rhizomes raised in the same environmental and growth conditions were obtained in February 2019 from local producers in Bentong, Pahang (GPS coordinates: 3.8126°N, 103.3256°E). The matured rhizomes were divided into 3 kg of six experimental groups after which the ginger rhizomes were kept in an ice chest and transported to the laboratory for extracting EOs.
2.2 Extraction of the essential oils
The EOs were extracted from the ginger rhizomes using standard procedures. The rhizomes were washed with tap water to remove dirt followed by washing them with distilled water after which they were cut into pieces and ground using a blender. The extractions were performed using hydro-distillation (Clevenger-type apparatus). The device is a custom-sized machine coupled with an electric boiler as well as condenser and glass decanter to separate oil from water condensate. The ratio of 3:5 of pretreated ginger rhizomes with solvent (distilled water) was used. The process was maintained for five hours. The ground ginger rhizomes were put directly inside the 10 L flask and boiled. The hot steam enables the aromatic compounds (essential oils) in the plant parts to be released. Thereafter, the molecules of these volatile oils escaped from the plant material and evaporated into the vapor within the system. The steam temperature was carefully regulated. The sample was boiled at 100 °C for 20 min after which the temperature of the sample was reduced to 45 °C for 5 h. This was to ensure that the EOs were extracted. Afterwards, the samples with the EOs were condensed and separated (Mesomo et al., 2013). The yield OEs was computed using the below formula after which the samples were stored at − 20 °C in Bacteriology Laboratory, Faculty of Agriculture, Universiti Putra Malaysia, until when it was required for the antimicrobial bioassays.
2.3 Analysis of the chemical contents of domestic and wild ginger essential oils using GCMS
The GCMS analysis was carried out at the Institute of Bioscience (IBS), Universiti Putra Malaysia. The analysis was conducted to determine the volatile compounds and their amounts in the ginger EOs. The approach used by Bhattarai et al. (2018) to analyze the volatile compounds was adopted using Shimadzu QP-2010 GCMS system that consists of gas chromatograph interfaced with a mass spectrometer and equipped with a Zebron ZB5-MS capillary fused silica column (30 m × 0.25 nm I.D. × 0.25 m film thickness). The initial temperature of the oven was set at 70 °C (isothermal for 3 min) with a 10 °C/min after which it was increased to 240 °C followed by increasing it to 300 °C for 10 min isothermal. A scan interval of 0.5 s and fragments of 45 to 480 Da were taken at 70 ev. Total GC running time was 45 mins for the domestic ginger and 51 mins for the wild ginger. Carrier gas, helium (99.999%) with a flow rate of 1 ml/min; injection volume 1 μl with a split ratio of 10:1 and injector temperature 250 °C were used in this present study. The mass spectrum of the obscured constituents of both gingers was identified by comparing their retention times (RT), similarity index (SI), and mass spectral data with those from FFNSC1.3.lib, NIST11.lib, and WILEY229. Lib mass spectral databases, as well as with related literature (Wei et al., 2010; Nagappan and Palaniveloo, 2012; Trimanto et al., 2018). The composition of EOs was expressed as a percentage of total peak area. The chemical compound’s names, percentages, retention time, and molecular formula were determined.
2.4 Analysis of the chemical contents of domestic and wild ginger by headspace
The analysis was carried out using the procedure of Yang et al., (2009). A 0.5 g amount of ginger rhizome particle was hermetically sealed in a 4 ml screw-top amber vial. The sealed vial was exposed to the extraction of the ginger component by immediately inserting it into the GC injector and the fiber thermally desorbed. A desorption time of 2 min at 250 °C was used in splitless mode with a valve oven temperature at 110 °C and transfer line temperature of 120 °C. Thereafter, the above stated GCMS condition was established using a Shimadzu QP-2010 GCMS system. The chemical compounds names, percentages, retention time, and molecular formula were recorded.
2.5 Description and retrieval of fungal and bacterial pathogens
The phytopathogenic bacteria and fungi were obtained from the Culture Collections Unit, Department of Plant Protection, Faculty of Agriculture, Universiti Putra Malaysia. Isolates of five plant pathogenic fungi and bacteria were Fusarium oxysporum, Pyricularia oryzae, Colletotrichum falcatum, Ganoderma boninense, and Rigidoporus microporus as well as Xanthomonas oryzae pv. oryzae- strain A, X. oryzae pv. oryzae- strain B, Ralstonia solanacearum, Bacillus sp. and Klebsiella sp. Confirmation of the identity of the resultant cultures was based on their morphological and molecular characteristics (Table 1).
Fungal and Bacterial Pathogens
Disease
Host
Reference
Fusarium oxysporum (Foc-TR4)
Fusarium wilt of banana
Isolated from infected roots of banana
(Ahmad et al., 2020; Wong et al., 2019)
Pyricularia oryzae
Rice blast
Isolated from infected rice leaves
(Awla et al., 2016)
Colletotrichum falcatum
Red rot of sugarcane
Isolated from infected stalks of sugarcane
(Hossain et al., 2020)
Ganoderma boninense
Basal stem rot (BSR) of oil palm
Isolated from infected root of oil palm
(Rakib et al., 2017)
Rigidoporus microporus
White root rot of rubber
Isolated from infected root of rubber
Un-published
Xanthomonas oryzae pv. oryzae- strain A and B
Bacterial leaf blight (BLB) of rice
Isolated from infected rice leaves
(Azman et al., 2017; Chibuike et al., 2019)
Ralstonia solanacearum
Bacterial wilt of eggplant
Isolated from infected root of eggplant
Un-published
Bacillus sp.
–
Isolated from agriculture soil
–
Klebsiella sp.
–
Isolated from agriculture soil
–
2.6 Determination of antifungal activity of ginger essential oils
The ginger EOs were screened against the fungal pathogens using the food poison technique (Talibi et al., 2012). The test concentrations 1, 2, 3, 4, 5, and 6 µl/ml were prepared by adding an appropriate amount of ginger EOs containing 0.5% (v/v) of Tween 80 to the cooled molten PDA (45 °C) followed by manual rotation in a sterile Erlenmeyer flask to disperse the oil in the medium and thoroughly mixed before solidification. After the samples had solidified, 6 mm bits of fungus culture was cut from a seven day old culture with sterilized cork borer after which it then placed at the center of Petri dish plates with a sterilized inoculation needle in three replications of each treatment. All Petri dishes were sealed with sterile laboratory parafilm to prevent the EOs from evaporating. The Petri dish plates were incubated at 25 ± 2 °C. Negative control was maintained in the medium that was not mixed with anything but inoculated with the pathogen whereas the positive control was mixed with 60 µl/ml of Azoxystrobin/Difenoconazole. Data were collected when the control petri plates were fully grown with mycelium. The data of the radial growth of the fungal colony was measured in millimeters. The percentage inhibition over control was calculated by the below formula (Aman and Rai, 2015):
Where C = Growth of fungus in control T = Growth of fungus in treatment.
To determine whether the EOs have fungicidal effect on the test pathogens, a plug of 6 mm PDA from the plate with no growth or suppressed growth was transferred to un-amended PDA medium. The treatment in which the mycelial did not growth after additional seven days of incubation was considered fungicidal to the test pathogens (Talibi et al., 2012).
2.7 Determination of antibacterial activity of ginger essential oils
The analysis was done using the method of Rajip et al., (2016) but with modifications. A standard disc diffusion method was used to determine the antibacterial activity of the EOs against the bacterial pathogens in triplicate using 24–48 h grown bacterial species reseeded on nutrient media. The cultures were adjusted with saline water to obtain a suspension at concentration of 1 × 106 CFU/ml using spectrophotometer, then 100 µl of the suspension was spread on Muller Hinton (MH) agar media plates to obtain uniform microbial growth using a sterile glass rod. Sterile filter paper discs (Whatman’s No. 6 mm in diameter) was impregnated with 10 µl of oils diluted in dimethyl sulfoxide (DMSO) and obtained different concentrations range from 50, 100, 200, 300, 400 and 500 µl/ml and finally placed on the surface of the agar test plate at intervals. The positive control discs were saturated with 10 µl of streptomycin (15 µg/ml/disc) and negative control was DMSO buffer. All Petri dishes were sealed with sterile laboratory parafilm to prevent the EOs from evaporating. The dishes were left for 30 min at room temperature to enable the oil to diffuse. Afterwards, the plates were incubated at 37 °C for 24 h. The appearance or absence of a zone of inhibition has been used as a means of identifying active or inactive concentrations of the EOs, they were determined by measuring the growth inhibition diameter in millimeter (mm).
2.8 Metabolomics’ Profiling of chemical constituents of ginger essential oils
After GCMS analysis of domestic (DMT) and wild (WLD) ginger EOs, the chromatographic and spectral data were properly processed and analyzed by multivariate data analysis (MDA). The metabolomic analysis started by obtaining the comma-separated values (CSV) file, which enables data to be saved in a tabular format. The deconvolution was done to convert these data into a data matrix suitable for multivariate data analysis (MDA) using Microsoft Excel 2010. After these procedures, a multivariate data matrix containing information about sample identities (sample code), ion identities (RT and m/z values), and normalized peak intensities were introduced into the analytical software called SIMCA (MKS Umetrics, version 14.1.0.2047 (32-bit) whereby the generated normalized metabolite peaks were transformed into variables. The data were mean centered and Pareto scaled. The PCA was then used to see the discrimination among the different observations from different ginger samples, (DMT and WLD). A model containing PCs (PC1 and PC2) were established representing a portion of examined data set. Results were visualized in the score plot of the two main components (DMT and WLD), in which each point was a representative of an individual sample spectrum. The analysis was checked and validated by default seven-fold internal cross validation based on the values R2X and Q2 which are the fitness and goodness of prediction respectively. The PCA, which is an unsupervised analysis that was performed to evaluate summary of the possible differences or similarities between sample groups based on the score plots observation. The corresponding loading plot were created to show the metabolites leading to group separation.
The supervised partial least square (PLS) was subsequently conducted to classify the metabolites associated with bioactivity addition via the score plot, that is, PLS model was used to find the correlation between the antimicrobial activities to the metabolites identified in each of the two different ginger EOs. There was validation by determining R2Y and Q2 through permutation test. Thereafter, the most significant metabolites (VIP > 1.0) were then identified with the bioactivity and subsequently, the bi-plot gave the correlation of bioactivity in a single plot.
2.9 Determination of antibacterial activity against Xanthomonas oryzae pv. oryzae- strainA
The potential antibacterial effects of EOs were evaluated using the electron microscopy (EM) methods. Scanning electron microscopy (SEM) for visual observation of the pathogen response to the in-situ antibacterial activity of the EOs. The test pathogen was treated with the MIC concentration (100 µl/ml), streptomycin (15 µg/ml/disc) and control with DMSO. The method as described by de Oliveira et al., (2011) was used for this analysis. The samples were then fixed with modified Karnovsky’s fixative (Karnivsky, 1965) containing 2% (v/v) glutaraldehyde and 2% (v/v) paraformaldehyde in 0.05 M sodium cacodylate buffer solution (pH 7.2) and left at 4 °C overnight. The samples were washed with 0.1 M sodium cacodylate buffer in three changes after 30 mins each, followed by post-fixation in 1% osmium tetroxide in 0.2 M PBS for 2 h, dehydrated through series of graded acetone (35, 50, 75, 95%) for 10 min each and 100% for 15 min. After completing the dehydration process, the samples were transferred into specimen basket and put into critical dryer for 30 min and observed with a scanning electron microscope (SEM: JSM − 5610LV, JOEL, Japan) at the IBS, Universiti Putra Malaysia.
2.10 Data analysis
The data in this present study were analyzed using PROC ANOVA by SAS 9.4 version and significant differences among the means were determined using least significant difference (LSD) at probability level of 0.05. The EPA probit analysis program version 1.5 was used to determine LC values among different concentrations of the essential oil and their overall antimicrobial activity.
3 Results
3.1 The extracted oil and yield (%)
The volume of EOs for the domestic ginger was 8 ml per 3 kg fresh rhizome. The highest yield obtained was 2.46% and the lowest yield obtained was 0.41% per 3 kg whereas for that of wild ginger, the oil yield was poor, the volume of EOs was 0.3 ml per 3 kg. The highest yield obtained was 0.28% and the lowest yield was 0.046% per 3 kg fresh rhizome.
3.2 Chemical constituents of the domestic and wild ginger essential oils by GCMS
The essential oil's chemical constituents of the domestic and wild ginger were identified using GCMS analysis. In total, 82 (42 from domestic and 51 from wild) chemical components were identified, representing 99.0% and 98.9% of the total constituents detected in the EOs from both domestic and wild ginger, respectively. The volatile phytochemical composition of domestic ginger EOs with the most abundant compounds were α-zingiberene (18.56%), geranial (13.88%), neral (10.75%), Trans-caryophyllene (9.64%), Eucalyptol (5.05%), β-phellandrene (5.51%), camphene (5.34%), α-pinene (2.05%) and heptan-2-ol (1.05%). The detailed identification and concentrations of the compounds found in both domestic and wild ginger EOs are presented in Table 2. The chemical compounds in the wild ginger EOs were monoterpenes such as isoeugenol, camphene, geranial, geranyl acetate, anethole, fenchyl acetate and neral as well as sesquiterpene hydrocarbons mainly α-humulene, α-urcumene, β-bisabolene, β-sesquiphellandrene, thus the most abundant compounds were isoeugenol (42.17%), caryophyllene (6.72%), β-bisabolene (5.10%), anethole (4.60%), Eucalyptol (1.41%), β-isabolol (1.98%), (-)-globulol (1.90%), α-curcumene (1.54%), α-humulene (1.74%), fenchyl acetate (1.49%), β-pinene (1.85%), alloaromadendrene (1.49%) and geranial (1.15%). DMT = Domestic ginger EOs, WLD = Wild ginger EOs, - (Absent).
S/No
Chemical component
R. Time (mins)
Concentrations (%)
Formula
DMT
WLD
1
Heptan-2-ol
6.874
1.04812
0.33076
C7H16O
2
α-Pinene
8.084
2.04791
0.75519
C10H16
3
Camphene
8.635
5.33798
0.48012
C10H16
4
β-Pinene
9.677
0.4184
1.85
C10H16
5
6-Methyl-5-hepten-2-one
10.023
0.34674
–
C8H14O
6
Myrcene
10.228
1.81418
–
C10 H16
7
α-Phellandrene
10.771
0.2967
–
C10 H16
8
β-Phellandrene
11.861
5.51193
0.54087
C10 H16
9
Eucalyptol
11.941
5.04681
1.40716
C10H18O
10
Terpinolene
14.474
0.27852
–
C10H16
11
1,6-Octadien-3-ol,3,7-dimethyl
15.011
1.07906
–
C10H18O
12
Citronellal
17.474
0.57812
–
C10H18O
13
Isogeranial
18.024
0.40472
–
C10H16O
14
Borneol
18.111
0.85195
0.67653
C10H18O
15
4-Terpineol
18.651
–
0.70908
C10H18O
16
cis-Verbenol
18.88
0.65463
–
C10H16O
17
α-Terpineol
19.284
–
0.9668
C10H18O
18
Linalyl propionate
19.298
1.04619
–
C13H22O2
19
Fenchyl acetate
20.683
–
1.69652
C12H20O2
20
Citronellol
21.128
0.97955
–
C10H20O
21
Neral
21.761
10.75466
0.85664
C10H16O
22
Geraniol
22.328
0.43753
–
C10H18O
23
Geranial
23.183
13.87864
1.14875
C10H16O
24
Anethole
23.755
–
4.60316
C10H12O
25
2-Undecanone
24.112
0.69251
–
C11H22O
26
α-Copaene
27.944
0.32066
–
C15H24
27
Geranyl Acetate
28.196
0.21411
–
C12H20O2
28
β-Elemene
28.665
0.79624
0.66772
C15H24
29
Cyperene
29.033
–
0.90321
C15H24
30
Methyleugenol
29.14
–
1.00219
C11H14O2
31
α-Bergamotene
29.679
–
0.41094
C15H24
32
Caryophyllene
29.913
–
6.72124
C15H24
33
Elemene
30.477
0.27864
–
C15H24
34
α-Humulene
31.39
–
1.73865
C15H24
35
Trans-β-Farnesene
31.452
0.42654
–
C15H24
36
Aromadendrene
31.701
0.29104
–
C15H24
37
Alloaromadendrene
31.71
–
1.49034
C15H24
38
Selina-4(14),11-diene
32.305
–
0.64802
C15H24
39
α-Selinene
32.323
0.21036
–
C15H24
40
α-Curcumene
32.613
4.42463
1.54084
C15H22
41
β-Chamigrene
32.713
–
0.73115
C15H24
42
Eremophilene
32.739
0.20921
–
C15H24
43
β-Humulene
32.802
–
0.85724
C15H24
44
α-Zingiberene
33.264
18.56259
–
C15H24
45
Isoeugenol
33.286
–
42.16819
C11H14O2
46
α-Bisabolene
33.456
–
0.63301
C15H24
47
β-Bisabolene
33.684
–
5.19785
C15H24
48
Trans-Caryophyllene
33.728
9.64171
–
C15H24
49
γ- Amorphene
33.927
0.36408
–
C15H24
50
(Z)-γ Bisabolene
34.014
–
0.59183
C15H24
51
3, 7(11)-Eudesmadiene
34.148
–
0.40932
C15H24
52
α-Panasinsene
34.183
0.14748
–
C15H24
53
β-Sesquiphellandrene
34.42
6.46214
2.46761
C15H24
55
(E)-γ -Bisabolene
34.681
–
0.37421
C15H24
55
Elemol
35.414
0.5595
–
C15H26O
56
Germacrene B
35.788
0.34994
–
C15H24
57
(E, E)-Farnesol
35.933
0.57294
C15H26O
58
Trans-Nerolidol
35.935
–
0.52933
C15H26O
59
Spathulenol
36.613
–
0.65573
C15H24O
60
Globulol
36.86
–
1.90228
C15H26O
61
Viridiflorol
37.21
–
0.76439
C15H26O
62
Guaiol
37.415
–
1.26692
C15H26O
63
Ledol
37.652
–
0.46106
C15H26O
64
Humulene epoxide
37.897
–
0.26243
C15H24O
65
Levomenol
38.005
0.54691
–
C15H26O
66
γ-Eudesmol
38.323
0.14552
–
C15H26O
67
Zingiberenol
38.669
0.79597
–
C15H26O
68
Spathulenol
39.025
–
0.57582
C15H24O
69
Isoelemicin
39.444
–
0.39723
C12H16O3
70
Rosifolio
39.514
0.36652
–
C15H24O
71
Cadin-4-en-10-ol
39.653
0.30705
–
C15H26O
72
Intermedeol
39.683
–
0.90742
C15H26O
73
Juniper Camphor
39.856
–
0.77779
C15H26O
74
β-isabolol
40.198
–
1.97755
C15H24O
75
Carotol
40.922
–
–
C15H26O
76
1-Chlorooctadecane
41.279
–
0.26508
C18H37 cl
77
Farnesal
42.909
–
0.43101
C15H24O
78
Squalene
45.164
–
0.49606
C30H50
79
Farnesyl Acetate
46.5
–
0.6923
C17H28O2
80
Kauran-18-al
51.637
–
1.0494
C21H34O
81
β-copaen-4-α-ol
56.145
–
0.37397
C15H24O
82
Trispiro [4.2.4.2.4.2.] heneicosane
60.969
–
0.83048
C21H36
Total
–
–
99.0
98.9
–
3.3 Chemical constituents of domestic and wild ginger rhizomes by headspace
Using headspace analysis of the domestic and wild ginger rhizomes revealed 27 (24 from domestic and 10 from wild) constituents, representing 99.4% and 99.9%, respectively of the total constituents detected. The dominant constituents of the domestic ginger rhizome were camphene (16.93%), Bisacurone epoxide (16.35%), Eucalyptol (14.90%), β-phellandrene (11.60%), α-zingiberene (7.17%), α-pinene (5.18%), geranial (4.14%), myrcene (4.08%), neral (2.87%), α-farnesene (2.81%), heptan-2-ol (2.31%), β-sesquiphellandrene (2.26%) and β-bisabolene (1.09%). For the wild ginger were β-phellandrene (72.73%), isoeugenol (3.98%), β-pinene (17.21%), and α-pinene (4.21%) (Table 3). DMT = Domestic ginger rhizome, WLD = Wild ginger rhizome, - (Absent).
S/No
Chemical component
R. Time (mins)
Concentrations (%)
Formula
DMT
WLD
1
Bisacurone epoxide
1.773
16.35215
–
C15H24O4
2
2-Heptanone
5.809
0.36243
–
C7H14O
3
Heptan-2-ol
6.015
2.31573
–
C7H16O
4
α--pinene
6.928
5.47721
4.20737
C10H16
5
Camphene
7.321
16.93417
0.08067
C10H16
6
β-Pinene
8.055
0.99324
17.20679
C10H16
7
Myrcene
8.359
4.07577
0.49867
C10 H16
8
Octanal
8.663
0.42944
–
C8H16O
9
α-Phellandrene
8.781
0.79561
–
C10H16
10
β-Phellandrene
9.504
11.60409
72.72907
C10H16
11
Eucalyptol
9.561
14.99832
0.40929
C10H18O
12
Butyl 2-methylvalerate
9.76
0.74342
C10H18O
13
Caryophyllene
10.937
–
0.49867
C15H24
14
Isoeugenol
11.276
–
3.98642
C11H14O2
15
Linalool
11.503
0.7712
–
C10H18O
16
Citronellal
13.084
0.79028
–
C10H18O
17
Neral
15.717
2.87096
–
C10H16O
18
Geranial
16.564
4.13926
–
C10H16O
19
2-Undecanone
17.198
0.3768
–
C11H22O
20
Copaene
19.716
0.43628
–
C15H24
21
α-Curcumene
22.495
0.50593
–
C15H22
22
Germacrene D
22.591
0.59153
–
C15H24
23
α-Zingiberene
22.83
7.16701
–
C15H24
24
Fenchyl acetate
22.624
–
0.61332
C12H20O2
25
α-Farnesene
23.077
2.81488
–
C15H24
26
β-Bisabolene
23.175
2.09026
0.07273
C15H24
27
β-Sesquiphellandrene
23.583
2.26332
–
C15H24
Total
–
–
99.4
99.9
–
However, monoterpenes were present in the constituents of the two ginger rhizomes. The constituents were monoterpenes alcohols (heptan-2-ol, 4-terpineol); bicyclic monoterpenes (phellandrenes, camphene, borneol, pinenes); acyclic monoterpenoids like myrcene, geranyl acetate, geranial, neral and citronellol. Even though present were several sesquiterpenes such as farnesenes, β-bisabolene, α-zingiberene, curcumenes, caryophyllene, globulol. Some compounds were present in the wild ginger but absent in the domestic ginger rhizomes Tables 2 and 3. Furthermore, the wild ginger oils have isoeugenol (42.17%) as the major component identified, whereas α-zingiberene (18.56%) is the major constituent in the domestic ginger oils.
3.4 Determination of antifungal activity of essential oils (in-vitro)
Findings of this study showed that the assayed EOs had different degrees of growth inhibition against the five fungal species. The antifungal activity of the EOs (Table 4) suggests that the ginger EOs has antifungal effect. Considerable variation in the concentrations of the EOs ranging from 1 to 6 µl/ml was observed to have effect on the fungal pathogens. The fungal pathogens exhibited considerable inhibition even at a lower concentration (1 µl/ml). Fusarium oxysporum exhibited the highest inhibition (50.38 ± 0.5) and the lowest was G. boninense (27.46 ± 0.5) at a concentration of 1 µl/ml. The order of the sensitivity (descending order) was F. oxysporum > C. falcatum > P. oryzae > R. microporus > G. boninense. Mycelial inhibition was dependent on the index of concentrations because the diameter of inhibition increased with the increasing concentration of the EOs. Mycelial growth inhibition was observed in F. oxysporum and C. falcatum to be the same at 2 µl/ml (57.19 ± 0.3). Means in a row with different superscripts are significantly different (P < 0.05). PC-positive control and NC– negative control.
Phytopathogen
Inhibition of Radial Growth (%)
1 µl/ml
2 µl/ml
3 µl/ml
4 µl/ml
5 µl/ml
6 µl/ml
PC
Fusarium oxysporum
50.38e ± 0.5
57.85d ± 0.1
67.43c ± 0.6
78.73b ± 0.7
100.00a ± 0.0
100.00a ± 0.0
100.00a ± 0.0
Pyricularia oryzae
42.62e ± 0.8
46.90d ± 0.2
55.42c ± 0.5
71.32b ± 0.9
100.00a ± 0.0
100.00a ± 0.0
100.00a ± 0.0
Colletotrichum falcatum
45.02e ± 0.6
57.19d ± 0.3
64.05c ± 0.4
76.82b ± 0.8
100.00a ± 0.0
100.00a ± 0.0
100.00a ± 0.0
Ganoderma boninense
27.46f ± 0.5
32.75e ± 0.2
42.41d ± 0.4
59.82c ± 0.9
71.33b ± 0.7
100.00a ± 0.0
100.00a ± 0.0
Rigidoporus microporus
33.46f ± 0.8
41.65e ± 0.4
49.78d ± 0.3
63.48c ± 0.8
76.67b ± 0.6
100.00a ± 0.0
100.00a ± 0.0
Diameter of Inhibition Zone (mm)
50 µl/ml
100 µl/ml
200 µl/ml
300 µl/ml
400 µl/ml
500 µl/ml
PC
NC
Xanthomonas oryzae pv. oryzae- strain A
0.00g
15.67f
17.33e
19.33d
20.67c
22.67b
25.00a
0.00g
Ralstonia solanacearum
0.00g
13.67f
15.33e
16.67d
17.67c
18.67b
21.33a
0.00g
Xanthomonas oryzae pv. oryzae- strain B
0.00f
15.67e
16.67d
16.33d
17.33c
18.67b
20.67a
0.00f
Bacillus sp.
0.00e
10.67d
10.67d
11.67c
12.33bc
13.00b
17.67a
0.00e
Klebsiella sp.
0.00e
14.67c
10.67d
14.33c
14.33c
16.33b
20.33a
0.00e
However, mycelial growth inhibition was also observed in all the sampled fungi. The percentage range of the suppression was from 50% to 100%. The positive control (Azoxystrobin/Difenoconazole) was effective at lower concentration because it demonstrated higher inhibition zones against all the fungal strains. Inhibition zones were not observed with the negative control. The minimum fungicidal concentrations of the ginger EOs on the tested isolates were in the range of 5–6 µl/ml, suggesting the fungicidal effects on the five fungi at > 5 µl/ml (Table 5). LC50 = lethal concentration of the EOs that kills 50% of the cell, LC90 = lethal concentration of the EOs that kills 90% of the cell, MIC = minimum inhibitory concentration and MFC/MBC = minimum fungicidal concentration/ minimum bactericidal concentration.
No
Pnytopathogens
LC50 (µl/ml)
LC90 (µl/ml)
MIC (µl/ml)
MFC (µl/ml)
1.
Fusarium oxysporum
1.3
5
1
5
2.
Pyricularia oryzae
2.8
5
1
5
3.
Colletotrichum falcatum
1.5
5
1
5
4.
Ganoderma boninense
2.5
6
1
6
5.
Rigidoporus microporus
3.5
6
1
6
6.
Xanthomonas oryzae pv. oryzae- strain A
300
400
100
7.
Ralstonia solanacearum
400
500
100
8.
Xanthomonas oryzae pv. oryzae- strain B
400
500
100
9.
Bacillus sp.
500
>500
100
10.
Klebsiella sp.
500
>500
100
3.5 Determination of in-vitro antibacterial activity of the essential oils
The ginger EOs showed different degrees of growth inhibition of the assayed EOs against the five bacterial species. The growth of the tested pathogens was affected by the concentration EOs which ranged from 100 to 500 µl/ml. In this present study, X. oryzae pv. oryzae-strain-A was affectively controlled by the EOs at 400 µl/ml and 500 µl/ml. The mean diameters of inhibition zone for 400 µl/ml and 500 µl/ml were 20.66 and 22.66 mm, respectively. The inhibition areas of X. oryzae pv. oryzae-strain-B at similar concentration were 17.33 and 18.66 mm, respectively (Table 4). Bacillus sp. recorded the lowest effect in which the mean diameter of the inhibition zones at 400 µl/ml and 500 µl/ml were 13.00 and 17.66 mm, respectively. These findings suggest significant antibacterial activity against X. oryzae pv. oryzae-strain-A, X. oryzae pv, oryzae-strain-B, R. solanacearum, Klebseilla sp. and least effective against Bacillus sp. The EOs' minimum inhibitory concentration (MIC) was determined and reported in Table 5. The MIC value of ginger EOs was 100 µl/ml. The effectiveness of antibacterial activity varied depending on the species of pathogens.
3.6 Metabolomic analysis of chemical constituents of ginger essential oils
The results obtained from the multivariate analysis of GC-mass spectral data of EOs for the domestic (DMT) and wild (WLD) gingers are shown in the PCA model against X. oryzae pv. oryzae-strain-A. The validity of PCA model was measured by the relative values of R2X and Q2 respectively, which are the goodness of a model's fit and predictive performance. Model fitness and predictive capacity are considered good when the values Q2 and R2 are>0.5. R2X (cum) = 0.87 and Q2 = 0.69, indicating how well the model fit and good predictively, each point represented one sample in the score plot.
The PC1 explained 56.5% of the variation between the two different observations whereas PC2 explained 30.5% of the variability. Hence, the model explained a total of 87.0% of the data set variation. Fig. 3 shows the score plot of all the variables from the two EOs group (DMT and WLD) with good separation by PC1. Significant outliers were not observed. The respective loading plot shows a concentrated distribution of metabolites in the middle, suggesting that the two groups might share high similarity in metabolites (Fig. 1).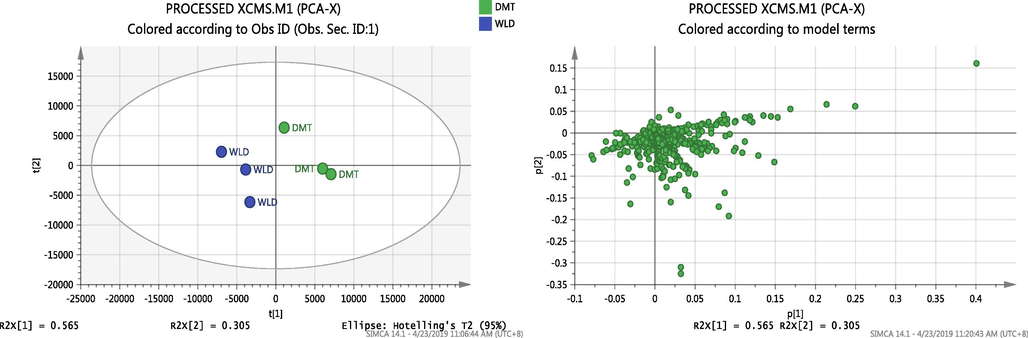
Score and scattered plot of the observation (PCA) showing all the variables from the two EOs groups (DMT and WLD) have good separation by PC1 suggesting that the two groups might share high similarity in metabolites from the scattered plot.
In the loading column plot (Fig. 2), the elements of the PCA loading vector represent the weights that combine the X-variables (domestic (DMT) and wild (WLD) ginger) to form the score vector. They are also proportional to the correlations between the scores and each X-variable.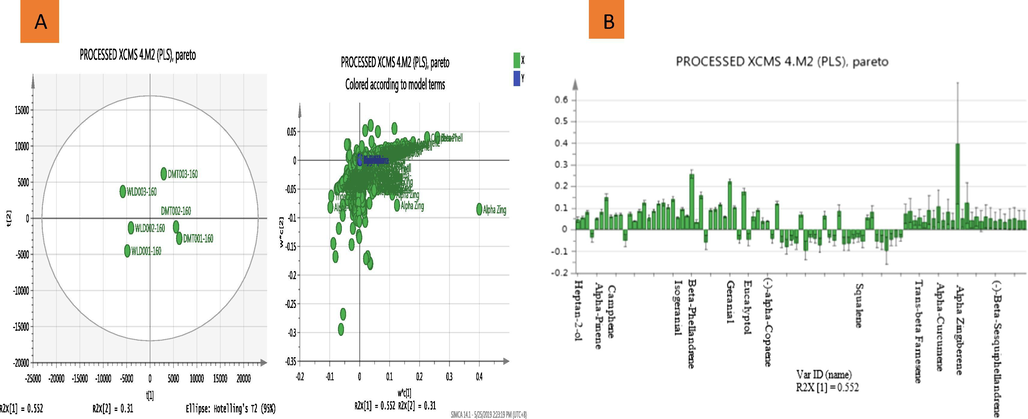
Metabolomic analysis of domestic and wild EOs. (A) Score and scattered plot of the observation (PLS) showing all the variables from the two EOs group (DMT and WLD) have good separation by PC1. Suggesting that the two groups might share high similarity in metabolites from the scattered plot and (B) Loading column plot (PLS model) of simplified relevant metabolites in a threshold of VIP > 1 representing a total of 13 discriminating metabolites.
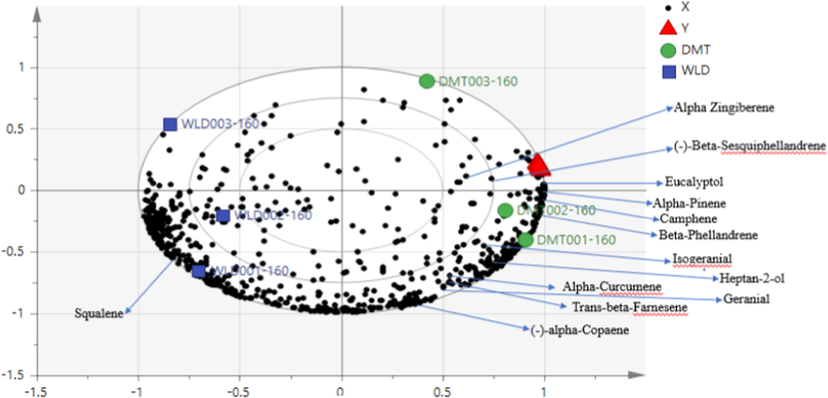
The bi-plot of the simplified relevant metabolites in a threshold of VIP > 1 showing 13 metabolites with clear contribution from α-Zingiberene, (-)-β-Sesquiphellandrene and Eucalyptol in the bioassay activity.
The PLS model carried out the detection and comparison of metabolites present in ginger EOs with respect to the bioactivity. The developed PLS model was efficient as shown by parameters R2Y = 0.986 and Q2 = 0.96, respectively. These values indicate the fit and predictability of goodness. The scores plot shows PCs and sample classification. Hence, the model explained 86.2% of the data set variation (Fig. 2A). Bioassay of related metabolites are conveniently defined in the loading plot by their correlation with the PCs that distinguish sample groups. Differential metabolites contributing to the correlation were identified using variable importance for the projection (VIP) value. In general, a threshold of VIP > 1 was considered as the relevant metabolites for interpreting the discrimination. Subsequently, accurate mass determination, elemental composition analysis, GCMS fragmentation and search of metabolite spectral databases establish the chemical identities of bioactive phytochemicals/reactive metabolites (putative compounds). The robustness of the model was also verified through further testing by a 100-permutation test which signifies a very good prediction. A total of 13 discriminating metabolites (Table 6) resulting from model group were obtained when compared with reported metabolites presented in Table 2. RT- retension time, VIP -variable importance for the projection. Source: https://pubchem.ncbi.nlm.nih.gov.
S/No
Putative compounds
VIP number
RT
Conc.
Formula
Biological activities
1
Heptan-2-ol
1.38276
6.874
1.04812
C7H16O
Disruption of the mitochondrial membrane - cell viability test thus, antimicrobial
2
α-Pinene
2.60823
8.084
2.04791
C10H16
Antifungal and antibacterial -Cellular toxicity
3
Camphene
6.40926
8.635
5.33798
C10H16
Insecticidal against cotton leafworm and Cytotoxicity against Homo sapiens (human) HeLa cells.
4
β-Phellandrene
7.3729
11.861
5.51193
C10 H16
Antifungal against Colletotrichum gloeosporioides, C. fragariae and C. acutatum
5
Eucalyptol
4.29913
11.941
5.04681
C10H18O
Insecticidal against cotton leafworm.
6
Isogeranial
1.04368
18.024
0.40472
C10H16O
No information found
7
Geranial
2.18994
23.183
13.87864
C10H16O
Antimicrobial via cytotoxicity Assay
8
α-Copaene
1.40911
27.944
0.320066
C15H24
No information found
9
Trans-β-Farnesene
2.03628
31.452
0.42654
C15H24
Antimicrobial via cytotoxicity Assay
10
α-Curcumene
2.75977
32.613
4.42463
C15H22
Insecticidal, repellents and insect feeding deterrents.
11
α-Zingiberene
11.3492
33.264
18.5629
C15H24
Antifungal and Antibacterial against Gram-positive bacteria
12
β-Sesquiphellandrene
1.46082
34.42
6.46214
C15H24
Antimicrobial and antioxidant
13
Squalene
1.49348
45.164
0.6923
C30H50
Antibacterial through membrane permeation.
The cross-validated PLS model showed a strong correlation between ginger EOs and Xoo-A bio-efficacy. The results indicate that 13 metabolites in the bioassay (Fig. 2B). This suggest clear activity by α-zingiberene, β-sesquiphellandrene and Eucalyptol (Fig. 3). Their functions in inhibiting X. oryzae pv. oryzae-strain-A growth were thoroughly ascertained. However, the PLS is a supervised calibration approach which enables the details of ginger components to be compared with the response data from the bioactivity instead of describing the variance of both gingers as PCA does. Conclusively, the results for multivariate analysis of GC-mass spectral data of both ginger EOs were considered with a good fitness and predictability of the constructed PCA and PLS models accordingly. It also showed that the metabolomics approach is an effective way of gaining insights into the metabolite responses to the X. oryzae pv. oryzae-strain-A bio-efficacy.
3.7 Scanning electron microscopy (SEM) observation on the mode of action of ginger EOs against Xanthomonas oryzae pv. oryzae-strain A
The examination of the changes on morphology and integrity of X. oryzae pv. oryzae-strain-A cells in response to ginger EOs treatment at MIC concentration (100 µl/ml), streptomycin (15 µg/ml/disc) and control with DMSO was carried out using scanning electron microscopy. The scanning electron micrograph revealed that normal untreated cells of X. oryzae pv. oryzae-strain-A was typically rod shaped with a normal, smooth and bright surface without any apparent cellular debris (Fig. 4A). However, the Xoo cells with ginger EOs demonstrated irregular shape with sunken surfaces and severely disruption of cells (Fig. 4B). Similarly, effects of the antibiotics on X. oryzae pv. oryzae-strain-A cells caused abnormal growth, lysis, shrinkage, disruption, and aggregation of cells (Fig. 4C). Thus, by electron microscopy (SEM), it can be inferred that the EOs interrupts the cell's structure with sunken surfaces and produces substantial damage that ultimately leads to X. oryzae pv. oryzae-strain-A growth inhibition.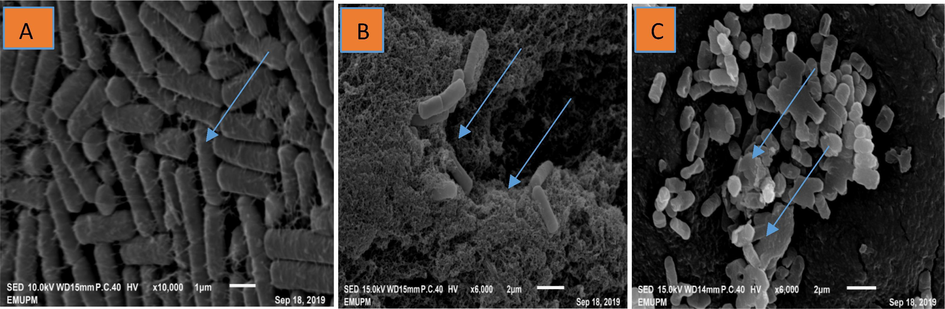
Scanning electron micrograph of ginger EOs causes ultrastructural modifications in Xanthomonas oryzae pv. oryzae-strain A cells. (A) The Xoo cells treated with DMSO (control) showing Xoo cells with a normal rod shape, smooth and bright surface, (B) The Xoo cells treated with the ginger EOs showed irregular shape with sunken surfaces, severely disruption of the cells and (C) The Xoo cells treated with (Streptomycin (15 µg/ml) display abnormal growth, shrinkage, disruption and aggregation of the cells.
4 Discussion
Considerable amount of EOs were extracted the domestic and wild gingers. The EOs extracted from domestic ginger yielded the highest amount (2.46%) than wild ginger (0.28%). The differences in the yields was due to differences of rhizomes texture and odor. Moreover, the amount of the yield is influenced by geographical origin, period of harvest, and condition of the ginger varieties. Similarly, the yield percentage of EOs depends upon on the amount of time spent during extraction, as prolonged extraction time would have a high chance of interaction between the solvent and the sample materials (Hoferl et al., 2015; Lopez et al., 2017). In this present study, the extraction time and geographic region were similar but the oil contents and yield differed because of the difference in the ginger species which might have resulted from variability in genetic composition of the species, plant maturity, and climatic and seasonal conditions in the regions where the crop is cultivated.
As for the findings of GCMS, the wild ginger chemical components of the EOs demonstrated higher number of volatile compounds compared with the domestic ginger. The domestic ginger also showed significantly higher volatile compound content as well as high volume oil and yield. Thus, both ginger EOs concentrations are diverse and present in different proportions. The major contents and compositions of the two EOs were slightly different. The wild ginger EOs had isoeugenol (42.17%) as the major chemical constituent, whereas, the α-zingiberene (18.56%) was the major constituent in the domestic ginger EOs. These results are comparable to those reported in the literature (Hoferl et al., 2015; Sharma et al., 2016.; Khayyat and Roselin, 2018). For some ginger EOs, geranial is the major component while others α--zingiberene and β-sesquiterpene were the main components in 10 to 60% range (Sharma et al., 2016.;Sharifi-Rad et al., 2017).
However, different analyses of natural products yield different efficiencies (Sharifi-Rad et al., 2017), the contents of both methods of analysis and the types of ginger which are defined by many compounds in the GCMS analysis of EOs but low compounds in the Headspace approach. This could be due to many factors such as the time taken for the analysis was short and the rhizomes were not efficiently utilized by headspace. In other words, some of the phytoconstituents were hidden in the rhizomes compared to the extracted EOs. The established Headspace analysis adopted in this present study proved to be simple, rapid, and convenient method for fingerprinting the volatile organic compositions characteristic. However, for better qualitative and quantitative scrutiny, extracted EOs should be harness.
The results of the in-vitro antifungal screening showed that ginger EOs are highly antifungal and potent against all the tested fungal pathogens. The LC50 value resulting from exposure to the EOs varied among fungal pathogens because of the ability of the pathogens to resist against the active volatile organic components. As the EOs concentration increased, the activity against the fungi increased. The findings of the present study are consistent with the reported in the literature. The in-vitro tests indicated that the ginger EOs exhibited effective antimicrobial activity against major phytopathogenic fungi. A study by El-Baroty et al. (2010) revealed that ginger EOs inhibited the growth of the common spoilage fungus Aspergillus niger at dilutions of 75 µg/ml and 100 µg/ml. Moreover, the EOs showed similar inhibitory effect against P. notatum, M. heimalis and F. oxysporum. Similarly, the antimicrobial activity of EOs of Z. officinale showed that all of the fungal strains were sensitive (Lopez et al., 2017). Furthermore, Kumar et al. (2016) reported that EOs exhibited significant antimicrobial activity against B. subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans and A. niger compared with standards. The EOs are made up of many sesquiterpenes and monoterpenes and exhibited significant antimicrobial activity against pathogenic microorganisms. The MIC and MFC of the ginger EOs on the tested isolates showed fungicidal effect on F. oxysporum, P. oryzae, C. falcatum, G. boninense and R. microporus at 5–6 μl/ml. Gakuubi et al. (2017) studied EOs of Eucalyptus camaldulensis Dehnh on the antifungal activity against Fusarium spp and found that MIC and MFC values of the EOs on the test pathogens were at 7–8 μl/ml and 8–10 μl/ml, respectively.
However, the results obtained for antibacterial activity against bacterial pathogens including X. oryzae pv. oryzae-strain A, R. solanacearum, Klebseilla sp., X. oryzae pv. oryzae-strain B and Bacillus sp. showed different degrees in growth inhibition of the assayed EOs. The bacterial growth of the tested pathogens was affected by the EOs concentration (100–500 µl/ml). In this present, the inhibition increased with the increasing concentration of the EOs used. The results obtained is in conformity Wonni et al., (2016) who also reported the antibacterial effects of EOs. Rajip et al., (2016) revealed that EOs of palmarosa (Cymbopogon martinii) at 5% and 1% concentrations demosntrated the highest pathogen inhibition followed by lemongrass oil (Cymbopogon flexuous), cinnamon oil (Cinnamomum zeylanicum) and vetiver oil (Chrysopogon zizanioides).
In the present study, the Intensity of the antibacterial activity varied depending on the species of bacteria. These findings are consistent with those reported in the literature. The study by Debbarma et al., (2013) on the antibacterial activity of ginger EOs (Z. officinale), eucalyptus (E. camaldulensis), and sweet orange (Citrus sinensis) were assessed against fish spoilage caused by pathogenic bacteria. Among the three EOs analyzed, ginger EOs demonstrated the best antibacterial activity against all the examined bacteria. In some studies, ginger EOs and extracts demonstrated strong antimicrobial activity and inhibitory to selected food-spoilage microorganisms (Nikolic et al., 2014; Bohme et al., 2014).
The metabolomics of GC-mass spectral data of the wild and domestic ginger EOs provides a systematic solution in addition to addressing the challenges of recognizing novel phytoconstituents in a complex interaction among phytochemicals and the microbial organisms using analytical software called SIMCA. Metabolomics ability to distinguish antimicrobials from different sources and expose the correlation with the bioassay arises from the advanced scientific base of data acquisition of GCMS. Data collection and preparation methods adopted in metabolomics-based research are used to promote and recognize novel phytoconstituents variability in the complex interaction of metabolites. The identification of the principal components (PC1 and PC2) in a complex dataset are meant to get clear sample clustering in the scores plot as well as the contribution of individual ions to PCs and group separation in the loading plot, which portrays the relationships between ions and PCs using the unsupervised method. The supervised multivariate data analysis (MDA) is used for model construction purposes by PLS whereby data properties and intent of the MDA analysis are usually determined by their correlation with antimicrobial activity against the tested microbes.
The action of EOs on the cell structure was fully identified by observing the changes on morphology and integrity of the Xoo cells with the MIC concentration of ginger EOs (100 µl/ml) and streptomycin (15 µg/ml). The integrity of the cell is very important for the survival of organisms as it is the key factor for critical biological activities taking place in the cells (Chouhan et al., 2017). The cell or cell membrane establishes an effective barrier between internal and external structures; important substances and chemicals are exchanged through the cell membrane. Thus, the effects of antimicrobial activity is achieved when cell morphology is disturbed (Wu et al., 2019). The work of de Oliveira et al. (2011) on assessment of the antibiotic efficacy of extracellular compounds generated by Pseudomonas strain against X. citri pv, citri 306 strain revealed that the cell integrity was completely disrupted by the action of the said compounds. Similarly, Sahu et al., (2018) found that niclosamide inhibited Xoo growth by impeding the formation of biofilms and disrupting Xoo cells. Based on the findings of this present study, ginger EOs have the potential application for controlling plant diseases caused by various phytopathogens. However, there are some limitations in our understanding on the regulating synergism and/or antagonism of the individual bioactive compounds in the EOs. Consequently, research in the future should explore the mechanism of action of the individual EO components, along with an initiation in systematically investigating on the mechanisms of synergistic interaction between components. New techniques for synergistic studies could provide an important platform for this field of research.
5 Conclusion
The chemical constituents in the two ginger EOs are diverse and present in different proportions. The overall finding of this study suggested that ginger EOs could be used as a new antimicrobial agent in suppressing the growth of phytopathogens and as a potential alternative for synthetic fungicides and bactericides for sustainable production agriculture.
6 Compliance with ethical principles
This research conducted by the authors includes no experiments with human and animal participant, rather than phytopathogens.
Acknowledgement
The authors thank the staffs of Department of Plant Protection and Institute of Bioscience for their technical assistance in this study. Special appreciation to the Ministry of Higher Education Malaysia for proving a research funding under Long-term Research Grant Scheme (LRGS/1/2019/UPM/2).
Declaration of Competing Interest
The authors have declared no conflict of interest.
References
- Biological and Chemical Resistance Inducers as Seed priming for Controlling Faba Bean Root rot Disease under Field Conditions. Int. J. Eng. Innov. Technol.. 2015;4:300-305.
- [Google Scholar]
- Molecular characterization of Fusarium oxysporum f. sp. cubense Tropical Race 4 (Foc-TR4) isolates from Malaysian banana using secreted in Xylem (SIX) effector genes. Arch. Phytopathol. Plant Prot.. 2020;1–16
- [Google Scholar]
- Antifungal activity of fungicides and plant extracts against yellow sigatoka disease causing Mycosphaerella musicola. Curr. Res. Environ. Appl. Mycol.. 2015;5:277-284.
- [Google Scholar]
- Bioactive Compounds Produced by Streptomyces sp. Isolate UPMRS4 and Antifungal Activity against Pyricularia oryzae. Am. J. Plant Sci.. 2016;7:1077-1085.
- [Google Scholar]
- Extraction And Chemical Compositions of Ginger (Zingiber Officinale Roscoe) Essential Oils As Cockroaches Repellent. Aust. J. Basic Appl. Sci.. 2017;11:1-8.
- [Google Scholar]
- Screening of Bacteria as Antagonist against Xanthomonas oryzae pv. oryzae, the Causal Agent of Bacterial Leaf Blight of Paddy and as Plant Growth Promoter. J. Exp. Agric. Int.. 2017;16:1-15.
- [Google Scholar]
- Chemical Constituents and Biological Activities of Ginger Rhizomes from Three Different Regions of Nepal. J. Nutr. Diet. Probiotics. 2018;1:1-12.
- [Google Scholar]
- Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol.. 2019;2:49-55.
- [Google Scholar]
- Bohme, K., Barros-velazquez, J., Calo-mata, P., Aubourg, S.P., 2014. Antibacterial, Antiviral and Antifungal Activity of Essential Oils : Mechanisms and Applications. In: Antimicrobial Compounds. pp. 51–81.
- Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. J. Pharm. Biomed. Anal.. 2014;87:218-228.
- [Google Scholar]
- Chibuike, C.S., Y, R.M., Izan, R.S., Izera, I.S., Yussuf, O., Emmanuel, O., Godwin, O., Emeka, U., Lynda, E., Senesie, S., Momodu, J., 2019. Marker-assisted selection and gene pyramiding for resistance to bacterial leaf blight disease of rice (Oryza sativa L.). Biotechnol. Biotechnol. Equip. 1–16.
- Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines. 2017;4:58.
- [Google Scholar]
- The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag.. 2019;10:1-9.
- [Google Scholar]
- de Oliveira, A.G., Murate, L.S., Spago, F.R., Lopes, L. de P., Beranger, J.P. de O., Martin, J.A.B.S., Nogueira, M.A., Mello, J.C.P. de, Andrade, C.G.T. de J., Andrade, G., 2011. Evaluation of the antibiotic activity of extracellular compounds produced by the Pseudomonas strain against the Xanthomonas citri pv. citri 306 strain. Biol. Control 56, 125–131.
- Antibacterial activity of ginger, eucalyptus and sweet orange peel essential oils on fish-borne bacteria. J. Food Process. Preserv.. 2013;37:1022-1030.
- [Google Scholar]
- Antifungal Activity of Essential Oil of Eucalyptus camaldulensis Dehnh. against Selected Fusarium spp. Int. J. Microbiol. 2017:1-8.
- [Google Scholar]
- Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv.. 2011;29:792-803.
- [Google Scholar]
- In-vitro evaluation of fungicides, plant extracts and bio-controlagents against rice blast pathogen Magnaporthe Oryzae couch. Pakistan J. Bot.. 2012;44:1775-1778.
- [Google Scholar]
- Composition and Comprehensive Antioxidant Activity of Ginger (Zingiber officinale) Essential Oil from Ecuador. Nat. Prod. Commun.. 2015;10:1085-1090.
- [Google Scholar]
- Current and Prospective Strategies on Detecting and Managing Colletotrichum falcatum Causing Red Rot of Sugarcane. Agron.. 2020;2020(10):1-19.
- [Google Scholar]
- Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol.. 2011;60:100-112.
- [Google Scholar]
- Recent progress in photochemical reaction on main components of some essential oils. J. Saudi Chem. Soc.. 2018;22:855-875.
- [Google Scholar]
- What makes Allium species effective against pathogenic microbes? Phytochem. Rev.. 2013;12:751-772.
- [Google Scholar]
- Lim, C.J., Basri, M., Omar, D., Abdul Rahman, M.B., Salleh, A.B., Raja Abdul Rahman, R.N.Z., 2012. Physicochemical characterization and formation of glyphosate-laden nano-emulsion for herbicide formulation. Ind. Crops Prod. 36, 607–613.
- Antimicrobial Activity of Essential Oil of Zingiber officinale Roscoe (Zingiberaceae) Am. J. Plant Sci.. 2017;08:1511-1524.
- [Google Scholar]
- Supercritical CO 2 extracts and essential oil of ginger (Zingiber officinale R.): Chemical composition and antibacterial activity. J. Supercrit. Fluids. 2013;80:44-49.
- [Google Scholar]
- Review on Concepts in Biological Control of Plant Pathogens. J. Biol. Agric. Healthc.. 2014;4:33-55.
- [Google Scholar]
- Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujev. J. Sci.. 2014;36:129-136.
- [Google Scholar]
- Rajip., Sumiya K. V., Dhanya S., R.K., N.M.C., 2016. Inhibitory Effect of Plant Extracts and Plant Oils on Xanthomonas Oryzae Pv Oryzae, the Bacterial Blight Pathogen of Rice. Int. J. Appl. Nat. Sci. 5, 71–76.
- Ganoderma species of basal and upper stem rots in oil palm (Elaeis Guineensis) in Sarawak, Malaysia. J. Acad. UiTM Negeri Sembilan. 2017;5:27-35.
- [Google Scholar]
- Bacterial diseases of rice : An overview Bacterial Diseases of Rice : An Overview. J. Pure Appl. Microbiol.. 2016;9:725-736.
- [Google Scholar]
- Niclosamide blocks rice leaf blight by inhibiting biofilm formation of xanthomonas oryzae. Front. Plant Sci.. 2018;9:1-16.
- [Google Scholar]
- Consumer preference, antibacterial activity and genetic diversity of ginger (Zingiber officinale Roscoe) cultivars grown in Sri Lanka. J. Agric. Sci.. 2017;12:207-221.
- [Google Scholar]
- Shaheen, H.A., Issa, M.Y., 2020. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. (Amsterdam). 264, 108940.
- Plants of the genus zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules. 2017;22:1-20.
- [Google Scholar]
- Chemical composition and antimicrobial activity of fresh rhizome essential oil of zingiber officinale roscoe. Pharmacogn. J.. 2016;8:185-190.
- [Google Scholar]
- Bacterial disease management: Challenges, experience, innovation and future prospects: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol.. 2016;17:1506-1518.
- [Google Scholar]
- Talibi, I., Askarne, L., Boubaker, H., Boudyach, E.H., Msanda, F., Saadi, B., Ait Ben Aoumar, A., 2012. Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop Prot. 35, 41–46.
- Trimanto, Hapsari, L., 2018. Short communication: A new record of Etlingera megalocheilos (Griff.) A.D. Poulsen (Zingiberaceae) in Sulawesi, Indonesia. Biodiversitas 19, 1227–1235.
- Venkateshwarlu G, J.K.A., 2014. Effect of Essential Oil and Aqueous Extract of Ginger (Zingiber Officinale) on Oxidative Stability of Fish oil-in-Water Emulsion. J. Food Process. Technol. 06, 1–5.
- Composition and Antibacterial Activity of Essential Oils from Leaves of Etlingera species (Zingiberaceae) Int. J. Adv. Sci. arts. 2010;1:1-12.
- [Google Scholar]
- Phylogenetic Analysis of Fusarium oxysporum f. sp. cubense Associated with Fusarium Wilt of Bananas from Peninsular Malaysia. Sains Malaysiana. 2019;48:1593-1600.
- [Google Scholar]
- Antibacterial activity of extracts of three aromatic plants from Burkina Faso against rice pathogen, Xanthomanas oryzae. African J. Microbiol. Res.. 2016;10:681-686.
- [Google Scholar]
- Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr.. 2019;7:2546-2555.
- [Google Scholar]
- Status of streptomycin resistance development in xanthomonas oryzae pv. oryzae and xanthomonas oryzae pv. oryzicola in China and their resistance characters. J. Phytopathol.. 2010;158:601-608.
- [Google Scholar]
- Volatile phytochemical composition of rhizome of ginger after extraction by headspace solid-phase microextraction, petrol ether extraction and steam distillation extraction. Bangladesh J. Pharmacol.. 2009;4:136-143.
- [Google Scholar]







