Translate this page into:
Phytochemical prospection, evaluation of antibacterial activity and toxicity of extracts of Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz
⁎Corresponding authors. hdmcoutinho@gmail.com (Henrique Douglas Melo Coutinho), hdmcoutinho@urca.br (Henrique Douglas Melo Coutinho), asiyadatpanah@yahoo.com (Abolghasem Siyadatpanah), polrat.wil@mahidol.ac.th (Polrat Wilairatana),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz is a arboreal species found in the Caatinga from Northeast of Brazil that has been used in popular medicine as an anti-inflammatory, healing, analgesic and for the treatment of respiratory system disorders. Therefore, the objective of this work was to evaluate the composition of ethanol extracts from the leaves and inner bark of Libidibia ferrea, as well as to verify its antibacterial activity and as a potential inhibitor of the TetK efflux pump in Staphylococcus aureus strains, in addition to investigating the toxicity of the extracts in a Drosophila melanogaster model. The analysis and quantification of the extracts markers was performed by High Performance Liquid Chromatography (HPLC). To determine the Minimum Inhibitory Concentration (MIC) broth microdilution tests were carried out. The evaluation of efflux pump inhibition was performed by modifying the MIC of antibiotics and ethidium bromide. Mortality and negative geotaxis tests were used to verify the toxicity of extracts on D. melanogaster. Hydrolysable tannins (gallic acid and ellagic acid) and flavonoids were found in HPLC analysis. The extracts did not show antibacterial activity, demonstrating a MIC ≥ 1024 µg/mL, however the ethanolic extract of the leaves decreased the MIC of the antibiotic from 64 µg/mL to 16 µg/mL, but this effect is not associated with the inhibition of the efflux pump. The extracts did not show toxicity in a D. melanogaster model. This is the first study to evaluate the antibacterial activity of L. ferrea extracts on the IS-58 strain of S. aureus, as well as the first to investigate its toxicity using D. melanogaster. From the results, further studies are needed to determine the mechanisms of action of the extract with other antibiotics.
Keywords
Libidibia ferrea
Microorganisms
Phenolic acids
Antibacterial activity
Toxicity
HPLC
- BHI
-
Brain Heart Infusion
- BHIA
-
Brain Heart Infusion Agar
- BOD
-
Biochemical Oxygen Demand
- CCCP
-
Carbonylcyanide m-chlorophenylhydrazone
- CPMZ
-
Chlorpromazine
- DMSO
-
Dimethylsulfoxide
- EEELF
-
Ethanol Extract of L. ferrea Inner Bark
- EEFLF
-
Ethanol Extract of L. ferrea Leaves
- EtBr
-
Ethidium Bromide
- HCDAL
-
Caririense Dárdano de Andrade Lima Herbarium
- HPLC
-
High Performance Liquid Chromatography
- MDR
-
Multidrug-resistant Microorganisms
- \MIC
-
Minimum Inhibitory Concentration
- URCA
-
Regional University of Cariri
Abbreviations
1 Introduction
There are a variety of Caatinga species that have been reported in the literature due to their use in popular medicine and in the implementation for formulation of herbal medicines, however, studies that prove the pharmacological properties of these species are still scarce (Luna et al., 2020). Among these species Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz, popularly known as Jucá or Pau-ferro, belonging to the Fabaceae family, is an endemic species from Brazil (Flora do Brasil, 2020).
The use of L. ferrea in popular medicine has been documented in the literature, with important biological properties reported as activities: anti-inflammatory, antioxidant, antiproliferative, healing, antifungal and antibacterial (Falcão et al., 2019a; Guerra et al., 2017; Jozala et al., 2020; Kobayashi et al., 2015; Nascimento et al., 2017; Soares et al., 2018). However, there are no studies evaluating the effects of L. ferrea extracts on strains that carry specific antibiotic resistance mechanisms, such as efflux pumps.
Efflux pumps are proteins that are inserted in the cytoplasmic membrane of cells and may be present in Gram-positive and Gram-negative bacteria, being able to actively extrude antibiotics and toxic substances out of the cell (Bambeke et al., 2003). In adittion, it is known that drug resistance mediated by efflux pumps can occur through intrinsic, acquired or phenotypic resistance pathways (Hernando-Amado et al., 2016), being frequently expressed by Staphylococcus aureus strains.
S. aureus is a Gram-positive bacterium, present in the natural microbiota of human beings. However, this pathogen can act opportunistically and affect immunocompromised people, especially in nosocomial environments, triggering severe inflammatory processes such as endocarditis, sepsis and necrotizing pneumonia (Haaber et al., 2017; Spaulding et al., 2013). In addition, S. aureus has become resistant to a wide range of antibiotics used in the clinic, due to practices such as incorrect and negligent use of these drugs, leading to the emergence of Multidrug-resistant Microorganisms (MDR) (Davies and Behroozian, 2020; Wang et al., 2021).
Therefore, natural products are an important source for the discovery and development of new products or active substances, which in association with synthetic and/or biological substances, as well as, in isolation, present themselves as an alternative for the formulation of efficient drugs on various human diseases (Newman and Cragg, 2020).
In the process of discovering new antibacterial agents, the assessment of the toxicity of the natural product is a crucial step to determine the toxicological profile and safety on eukaryotic cells (Li et al., 2021). A widely used model to trace the toxicological profile of substances is the fruit fly, Drosophila melanogaster, due to its similarity in physiology to mammals, as it is also an organism with a short life cycle, the creation and maintenance processes are simple, in addition to the need for low maintenance costs (Ng et al., 2019; Ong et al., 2017).
In this sense, the present study aimed to evaluate the phytochemical composition of ethanol extracts from the leaves and inner bark of L. ferrea, as well as to verify their antibacterial potential and efflux pump inhibitor potential on the IS-58 strain of S. aureus carrier of the TetK efflux pump, in addition to investigating the toxicity of extracts in a D. melanogaster model.
2 Materials and methods
2.1 Collection and preparation of extracts
The plant material of the leaves was collected in the region of Cariri, in the city of Crato, Ceará, Brazil with geographic coordinates 7° 14′ 19.36″ South latitude and 39° 24′ 53.71″ West longitude of Greenwich. The inner bark were collected at the Cantinho small farm, located in the city of Exu, Pernambuco, Brazil with the following geographic coordinates: 7° 30′ 50.2″ South latitude and 39° 50′ 42.7″ West longitude of Greenwich. The leaves were collected on November 12, 2021, while the bark was collected on February 12, 2021. The plant species was identified by Prof. Dr. Maria Arlene Pessoa da Silva and a voucher specimen was deposited in the Caririense Dárdano de Andrade Lima Herbarium - HCDAL of the Regional University of Cariri - URCA, cataloged under the registration number #13905.
The collected parts were crushed and submerged in ethanol PA solvent, separately at room temperature for 72 h. After this period, the solution obtained was filtered and subjected to solvent distillation in a vacuum rotary evaporator apparatus (Fisatom Scientific Equipment Ltd., Brazil), where the product obtained was taken to the water bath (Quimis Scientific Equipment Ltd., Brazil) for evaporation of the ethanol excess. The yield of the obtained extract was 13.69% for the leaves, 5.33% for the inner bark. The Ethanol Extract of L. ferrea Leaves was called EEFLF and the Ethanol Extract of L. ferrea Inner Bark was called EEELF.
2.2 Sample preparation and High Performance Liquid Chromatography
It was weighed 20 mg of the extracts (EEFLF and EEELF) and later this volume was transferred to a 10 mL volumetric flask and solubilized in 10 mL of ethanol. 5 mL aliquots of the obtained solutions were transferred to 10 mL volumetric flasks and the volume was measured with ultrapure water (Purelab Classic UV, Elga®).
Subsequently they were filtered into vials with the help of a PVDF filter (0.45 µm; Chromafil®). Chromatographic conditions: HPLC analysis was performed on an Ultimate 3000 HPLC system (Thermo Fisher Scientific, USA), coupled to a photodiode array detector (DAD; Thermo Fisher Scientific) and equipped with a binary pump (HPG-3x00RS, Thermo Fisher Scientific), degasser and autosampler equipped with a 20 µL loop (ACC-3000, Thermo Fisher Scientific). The wavelength was set at 270 nm. Chromatographic separations were obtained with a C18 column (250 mm × 4.6 mm i.d., 5 µm) Supelco® equipped with guard column (4 mm × 3.9 µm C18; Phenomenex®). The separations were carried out at a temperature of 25 ± 1 °C. The mobile phase consisted of ultrapure water (A) and methanol (B), both acidified with 0.05% trifluoroacetic acid, and flow adjusted to 0.9 mL/min. A gradient program was applied as follows: 0–10 min, 12.5–25% B; 10–15 min, 25–40% B; 15–25 min, 40–75% B; 25–30 min, 75–75% B; 30–33 min, 75–12.5% B (Ferreira et al., 2016a, 2016b). Data were analyzed, after injection in triplicate, and processed using Chromeleon 6.8 software (Dionex/Thermo Fisher Scientific). The standards ellagic acid (95% of purity) and gallic acid (analytical purity) were used to confirme the presence of compounds in the extracts by UV spectra and retention time, and to calculate the content of each compound in the extracts. Both were purchased from Sigma-Aldrich®.
2.3 Microbiological tests
2.3.1 Bacterial strains
The strain of Staphylococcus aureus used was IS-58 encoded by plasmid pT181, which carries the gene that expresses the Tetracycline efflux protein - TetK. The strain was provided by Prof. S. Gibbons (University of London), being maintained in blood agar (Laboratórios Difco Ltda., Brazil) and, before the experiments, it was cultivated for 24 h at 37 °C in a solid Brain Heart Infusion (BHI)-Agar (BHI, Acumedia Manufacturers Inc.).
2.3.2 Culture media
To performe the microbiological tests, the following culture media were used: Brain Heart Infusion Agar (BHIA) (BHI, Acumedia Manufacturers Inc.), prepared according to the manufacturer, and Brain Heart Infusion (BHI, Acumedia Manufacturers Inc.) prepared at the same concentration of 10%.
2.3.3 Chemicals and reagents
The antibiotic (Tetracycline), ethidium bromide (EtBr), carbonylcyanide m-chlorophenylhydrazone (CCCP) were obtained from Sigma Aldrich Co. Ltd. (St. Louis, U.S.A.), while Chlorpromazine (CPMZ) was obtained from Aché Pharmaceuticals Laboratories (Pernambuco, Brazil). The antibiotic Norfloxacin, as well as and EEFLF and EEELF, were diluted in Dimethylsulfoxide (DMSO) and in sterile water. The proportion of DMSO used was less than 5%. CPMZ and EtBr were dissolved in sterile distilled water, while CCCP was dissolved in methanol/water (1:3, v/v). All substances were diluted to a standard concentration of 1024 µg/mL.
2.3.4 Determination of Minimum Inhibitory concentration (MIC)
MIC was determined for compounds EEFLF and EEELF according to the Broth Microdilution Method proposed by (Javadpour et al., 1996) with adaptations. The strains used in the tests were sown 24 h before the experiments. After this period, the bacterial inoculum was suspended in saline, corresponding to 0.5 of the McFarland scale, approximately 1.5 × 108 (CFU)/mL. Eppendorfs® were then filled with 900 µL of BHI and 100 µL of the inoculum and the plates were filled with 100 µL of the final solution. Microdilution was performed with 100 µL in serial dilutions up to the penultimate well of the plate (1:1), the latter being used as a growth control. The concentrations of the compounds ranged from 512 µg/mL to 8.0 µg/mL. After 24 h of incubation, readings were performed by adding 20 µL of Resazurin (7-hydroxy-10-oxidophenoxazin-10-ium-3-one) (Exodo, Brazil). Resazurin was oxidized in the presence of the acid medium caused by bacterial growth, promoting the color change of the blue to pink (Elshikh et al., 2016). The MIC was defined as the lowest concentration in which no growth can be observed (Andrews, 2001). The tests were performed in triplicate.
2.3.5 Evaluation of efflux pump inhibition by modification of MIC of antibiotics and ethidium bromide
To observe whether EEFLF and EEELF act as potential inhibitors of the TetK Efflux Pump, a comparative study between the effects of the standard inhibitors of the Efflux Pump was used, evaluating the ability of both to decrease the MIC of EtBr and the antibiotic Tetracycline. The standard CCCP inhibitors and Chlorpromazine were used to provide the expression of the TetK pump by the strains tested. The inhibition of the Efflux Pump was tested using a Sub-inhibitory Concentration (MIC/8) of inhibitors and EEFLF and EEELF. In the tests, 170 µL of each bacterial inoculum suspended in saline, corresponding to 0.5 of the McFarland scale, approximately 1.5 × 108 (CFU)/mL, was added together with the inhibitors and EEFLF and EEELF (MIC/8) and completed with BHI. These were then transferred to 96-well microdilution plates, to which 100 µL of antibiotic or EtBr were added in serial dilutions (1:1) ranging from 512 to 0.25 µg/mL. The plates were incubated at 37 °C for 24 h and bacterial growth was evaluated with resazurin (7-hydroxy-10-oxidophenoxazin-10-ium-3-one). Resazurin was oxidized in the presence of acid medium caused by bacterial growth, causing the color change from blue to pink (Elshikh et al., 2016). The MIC was defined as the lowest concentration in which no growth can be observed (Andrews, 2001). The MIC of the controls was evaluated using only plates with antibiotic Tetracycline and with EtBr and the tests were performed in triplicate.
2.3.6 Negative control
For negative control, the last rows of microdilution plate cavities were used, where only the culture medium and bacterial inoculum were added without the addition of the tested substances, in view of the maximum formation of the number of bacterial colonies, in addition to proving that the inoculum was properly added to the eppendorfs®. However, these numbers were not added to the results graphs due to the impossibility of measuring the number of colonies formed in a period of 24 h after the microbiological tests. The methodology used does not allow the counting of bacterial colonies, however we used Resazurin as a dye to identify bacterial growth through oxidation-reduction.
2.4 Toxicity tests
2.4.1 Drosophila melanogaster stock
Drosophila melanogaster (Harwich strain) was obtained from the National Species Stock Center, Bowling Green, OH. Flies were raised in 340 mL glass jars (15 cm high and 6.5 cm in diameter) grown with the medium containing: (83% corn mass, 4% sugar, 4% lyophilized milk, 4% soybean meal, 4% wheat or oat bran and 1% salt). When the mixture was cooked, 1 g of Nipagin (Methylparaben) was added. After cooling in the growth flasks, 1 mL of solution containing Saccharomyces cerevisiae was added. The flies were raised in BOD photoperiod greenhouses at a temperature of 25 °C ± 1 °C and a relative humidity of the air at 60%.
2.4.2 Survival tests
The ingestion bioassay method proposed (Da Cunha et al., 2015) was used to assess the toxicity of EEFLF and EEELF. Where adult flies (male and female aged approximately 4 days), in number of 20, were placed in 130 mL flasks, previously prepared with 1 mL of sucrose solution in distilled water, at a concentration of 20%, allowing the flies fed ad libitum. The other treatments received volumes of 25, 50 and 100 mg/mL of ethanol extract from the leaves and inner bark of L. ferrea, and these volumes were diluted in a 20% sucrose solution. All bioassays were conducted in a BOD type greenhouse with a light and dark cycle every 12 h, temperature controlled at 26 °C ± 1 °C and 60% relative humidity of the air. The tests were performed in triplicate and the readings of mortality rates were taken at 3,6, 9, 12, 24 and 48 h (Da Cunha et al., 2015).
2.4.3 Negative geotaxy test
The damage to the locomotor system was determined by the negative geotaxy test, as described by (Coulom and Birman, 2004). Briefly, shortly after counting the mortality of the flies every 3, 6, 9, 12, 24 and 48 h. The negative geotaxy test was carried out concomitantly with the surviving flies, which consists of counting the number of flies that ascend in the column of the experiment's own glass, above 5 cm, in a time interval of 5 s. Assays were repeated twice at 1 min intervals. Results were presented as the mean time(s) ± SE obtained in two independent experiments.
2.5 Statistical analysis
The central data and standard deviations of microbiological assays were obtained according to the methodology of (Freitas et al., 2021), on microbiological analysis in microdilution plates. The data were analyzed using the statistical program GraphPad Prisma 6.01 through a one-way ANOVA test. Afterwards, a post hoc Bonferroni test was performed (where p < 0.05 was considered significant and p > 0.05 non-significant). To analyze the toxicity data, a two-way ANOVA test was performed, followed by a Tukey multiple comparison test. There is no statistical difference with the same concentration versus time.
3 Results
3.1 High-Performance Liquid Chromatography (HPLC)
In this study, the HPLC-DAD method based on Ferreira et al. (2016a, 2016b) was evaluated to obtain a chromatographic system capable of eluting and providing resolution in the separation of compounds in the samples of Libidibia ferrea. By analyzing the chromatographic profile of the EEELF sample (Fig. 1), it is possible to observe the presence of 7 main compounds, not being possible to identify all substances. However, the peaks observed at retention times of 6.27 min (peak 1) and 23.68 min (peak 7) indicate the presence of hydrolyzable tannin monomers, gallic acid and ellagic acid, respectively. Peaks 4, 5 and 6 correspond to the presence of hydrolyzable tannins (derived from ellagic acid) according to the scanning spectra. Peaks 2 and 3 indicate the presence of condensed tannins (derived from catechin) according to the scanning spectra with maxima at 200 and 279 nm approximately.
EEELF sample chromatogram and scan spectrum of peaks observed at 270 nm.1 – Gallic acid; 2 and 3 – Catechin derivatives; 4, 5 and 6 – Ellagic derivatives; 7 –Ellagic acid.
As for the chromatographic profile of the EEFLF sample (Fig. 2), the presence of 6 main compounds can be observed. The peaks observed at retention times 6.10 min (peak 1) and 23.89 min (peak 3) indicate the presence of hydrolyzable tannin monomers, gallic acid and ellagic acid, respectively. Peaks 2, 4, 5 and 6 correspond to the presence of flavonoids, and although it is not possible to identify which they are, according to the scanning spectra it is possible to infer that they belong to the class (with maxima of absorption at 268 and 350 nm approximately).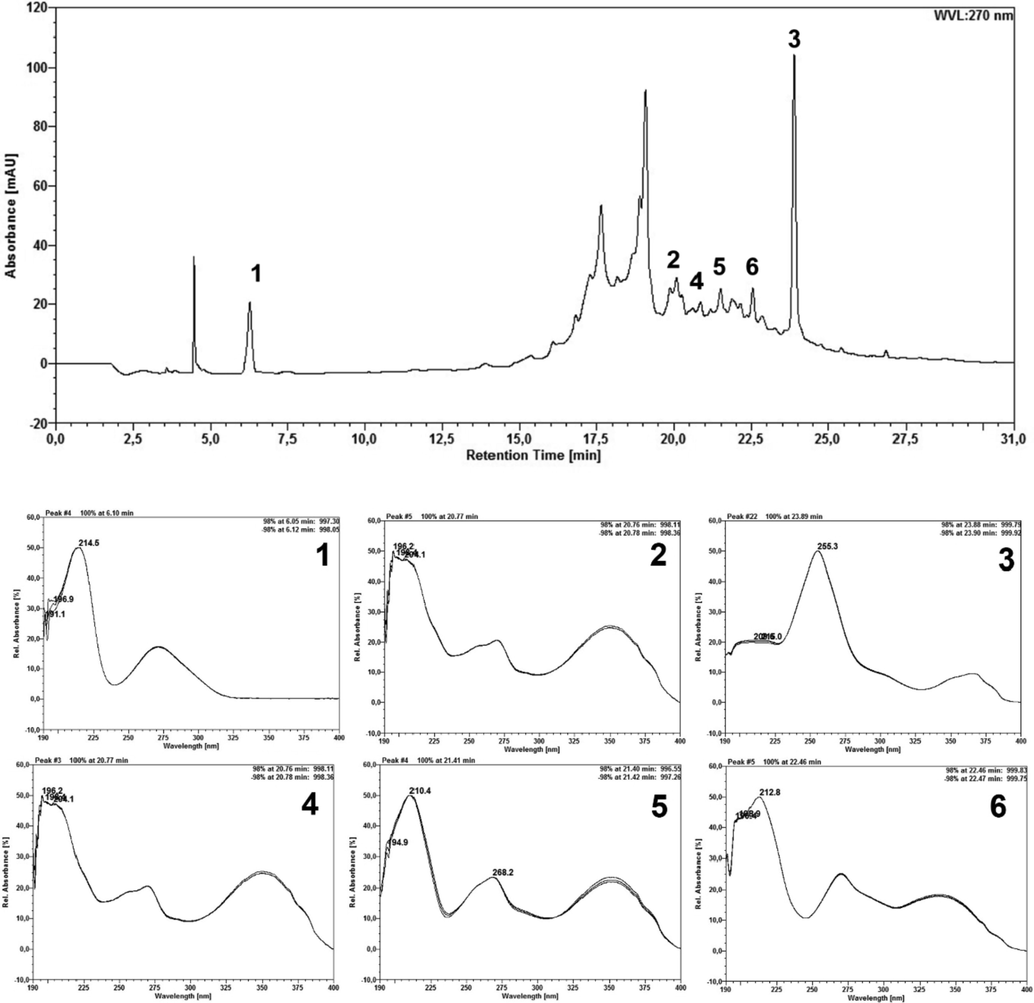
EEFLF sample chromatogram and scan spectrum of peaks observed at 270 nm. 1– Gallic acid; 3 – Ellagic acid; 4, 5 and 6 – Flavonoid derivative.
Additionally, Table 1 showed the content of gallic acid and ellagic acid that were calculated according to the calibration curves of the standards and the results were obtained in triplicate and expressed as mean ± standard deviation (relative standard deviation).
Sample
Gallic acid
Ellagic acid
EEFLF
0,224 ± 0,0021 g% (0,94%)
0,339 ± 0,0014 g% (0,42%)
EEELF
0,097 ± 0,0017 g% (1,75%)
0,162 ± 0,0020 g% (1,26%)
3.2 Microbiological tests
The assays to obtain the MIC of the EEFLF and the EEELF against the strain of S. aureus carrying the TetK efflux pump demonstrated an irrelevant antibacterial activity, with a MIC value ≥ 1024 µg/mL for both extracts. The MIC of the antibiotic Tetracycline was 64 µg/mL.
Compared to the assays of association between Tetracycline and EEFLF, there was a reduction in the antibiotic MIC from 64 µg/mL to 16 µg/mL, characterized as a potentiating effect on the antibiotics efficacy (Fig. 3). As for the association of Tetracycline with EEELF, there was no statistical difference.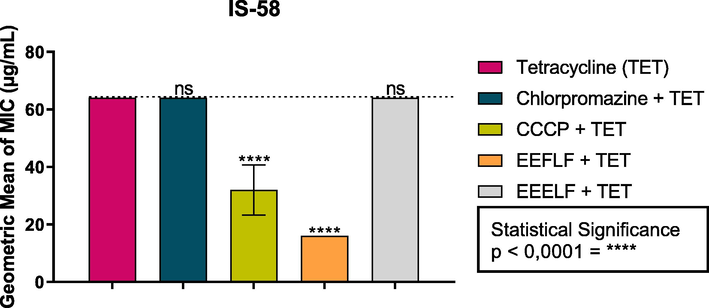
Effect of the combination of EEFLF and EEELF with the antibiotic tetracycline on the IS-58 strain of S. aureus carrying the TetK efflux pump. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) and Chlorpromazine are standard inhibitors.
In the evaluation of the inhibition of the efflux pump by decreasing the MIC of ethidium bromide, it was verified that the association of both extracts had no effect on the functioning of the efflux pump, since the values did not differ statistically from the control (Fig. 4).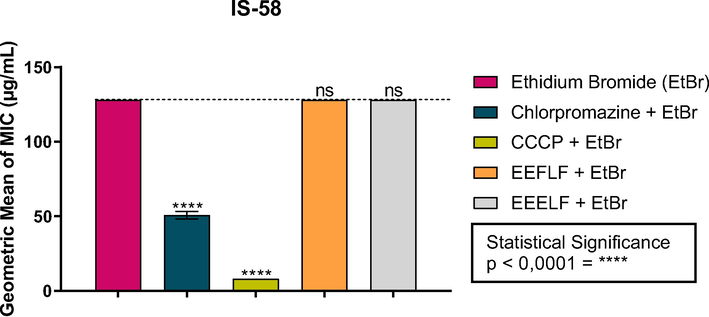
Effect of the combination of EEFLF and EEELF with ethidium bromide on the IS-58 strain of S. aureus carrying the TetK efflux pump. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) is a standard inhibitor.
3.3 Toxicological tests
The toxicological tests using the arthropod-model D. melanogaster demonstrated that both extracts did not show toxicity at all tested concentrations of 25, 50 and 100 mg/mL, since there was no mortality in the treatment groups, which is why the EC50 was not calculated.
The negative geotaxy data demonstrate the ability of EEFLF and EEELF to cause damage to the flies' locomotor system. In this sense, it was possible to observe that EEFLF caused damage to the locomotor system of flies from 12 h of exposure at concentrations of 50 and 100 mg/mL, with a statistical difference in relation to the control, Fig. 5A. After a 24-hour period of exposure to EEFLF, the concentration of 25 mg/mL also caused damage to the locomotor system of the flies.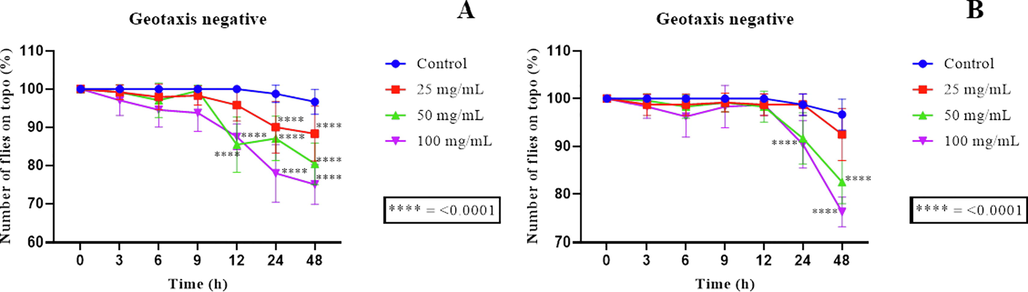
Negative geotaxy assays using the D. melanogaster model. (A) and (B) Negative geotaxy data using the D. melanogaster model against the EEFLF and EEELF, respectively; **** represents significance in relation to control.
As for the EEELF, it was shown that only the concentration of 100 mg/mL differed statistically from the control after 24 h of exposure, while the concentration of 50 mg/mL only differed from the positive control in the last reading of 48 h of exposure to the extract (Fig. 5B).
4 Discussion
Several studies in the literature about the phytochemical composition of extracts from L. ferrea bark and leaves demonstrate the presence of ellagic, gallic and caffeic acid, in addition to catechin, epicatechin, quercertin and kaempferol (Andrade et al., 2019; Da Silva et al., 2018; Indriani et al., 2018; Pedrosa et al., 2016). Chemical classes such as tannins, flavonoids, coumarins, saponins, steroids, terpenoids were also identified in phytochemical screening of L. ferrea bark and leaf extracts (Falcão et al., 2019b; Leandro et al., 2019; Luna et al., 2020). These studies corroborate our results, since the phytochemical analysis of ethanol extracts from the leaves and innner bark of pau-ferro through the HPLC showed the presence of hydrolysable tannins, flavonoids, gallic acid and ellagic acid.
Bioactive compounds such as those included in chemical classes such as flavonoids, alkaloids, terpenoids and steroids, as well as other phenolic compounds are used for the formulation and development of new drugs for the treatment of respiratory disorders, diseases of the urinary tract and skin infections caused by microorganisms (Mayekar et al., 2021).
In the literature there are reports on the antibacterial activity of L. ferrea leaves against Bacillus subtilis, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa and S. aureus with MIC ranging from 0.039 to 25 mg/mL (Luna et al., 2020). There are also studies evaluating the antibacterial activity of the inner bark against standard and multiresistant strains of E. coli, P. aeruginosa and S. aureus, which verified that there was no inhibition of bacterial growth by determining the MIC ≥ 1024 µg/mL (Ferreira et al., 2016a, 2016b). The MIC is defined as the lowest concentration of the substance capable of inhibiting bacterial growth, and a three-point reduction in MIC is required to indicate inhibition of bacterial growth (Davies and Wright, 1997; Davies and Behroozian, 2020).
The results of the present study demonstrated that EEFLF and EEELF did not show antibacterial activity against the IS-58 strain of S. aureus, which carries the TetK efflux pump and did not inhibit the functioning of the efflux pump when evaluated using ethidium bromide. However, the pau-ferro leaf extract, when associated with the antibiotic, reduces its MIC twice, being characterized as a potentiative action of effectiveness of the antibiotic, which may be acting on another resistance mechanism. There are no studies evaluating the antibacterial activity of L. ferrea extracts on the IS-58 strain of S. aureus, and this study is the first report.
The TetK Efflux Pump present in S. aureus is inserted in the Major Facilitator Superfamily – MFS that use the driving force of the proton gradient to actively expel the Tetracycline to the outside of the bacterial cell, reducing its concentration in the intracellular environment and making it difficult its action to inhibit the functioning of ribosomes (Chopra and Roberts, 2001; Hobson et al., 2021; Kumar et al., 2020).
In addition to investigating the antibacterial potential of natural substances, it is essential to analyze their toxic profile, since the therapeutic activity of a natural substance is considered safe when there is no toxicity on animal cells (Li et al., 2021). The present study demonstrated that both extracts did not show toxicity over the alternative D. melanogaster model.
D. melanogaster is considered an important alternative model to evaluate the toxicity of natural products in vivo due to its speed in obtaining results compared to traditional models, such as rodents (Pandey and Nichols, 2011). In addition, D. melanogaster has more than 75% of disease genes homologous to those of humans, as well as being highly sensitive to low concentrations of compounds (Chifiriuc et al., 2016). Here is the first report on the toxicity of L. ferrea extracts on the alternative D. melanogaster model.
5 Conclusion
In this study, the phytochemical analysis of L. ferrea leaf and inner bark extracts by HPLC demonstrated the presence of hydrolyzable tannins, flavonoids, gallic acid and ellagic acid. However, both extracts did not show direct antibacterial activity against the IS-58 strain of S. aureus, nor did they act as efflux pump inhibitors. However, the association of the ethanol extract of the L. ferrea leaf with Tetracycline resulted in a synergistic association. Furthermore, the extracts did not show toxicity on the D. melanogaster model. It is concluded that the ethanol extract of L. ferrea leaf potentiated the effect of the antibiotic tetracycline, but this effect is not due to the reversal of the efflux pump. New studies must be carried out to identify this mechanism of action.
Acknowledgements
The authors are thankful to the Federal University of Pernambuco – UFPE; Regional University of Cariri – URCA; Geopark Araripe.
Formatting of funding sources
Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico - FUNCAP (BPI 02/2020 NÚMERO: BP4-0172-00168.01.00/20 SPU N°: 09673071/2020); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES; Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bark of the Stem of Libidibia Ferrea Associated with Mycorrhizal Fungi: An Alternative to Produce High Levels of Phenolic Acids. Open Microbiol. J.. 2018;12:412-418.
- [CrossRef] [Google Scholar]
- Andrade, B. de A., Corrêa, A.J.C., Gomes, A.K.S., Neri, P.M. da S., Sobrinho, T.J. da S.P., Araújo, T.A. de S., Castro, V.T.N. de A. e, Amorim, E.L.C. de, 2019. Photoprotective Activity of Medicinal Plants From the Caatinga Used as Anti-inflammatories. Pharmacogn. Mag. 15, 356–361. 10.4103/pm.pm_482_18.
- Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother.. 2001;48:5-16.
- [CrossRef] [Google Scholar]
- Antibiotic efflux pumps in prokaryotic cells: Occurence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antimicrob. Chemother.. 2003;51:1055-1065.
- [CrossRef] [Google Scholar]
- Drosophotoxicology: An emerging research area for assessing nanoparticles interaction with living organisms. Int. J. Mol. Sci.. 2016;17:1-14.
- [CrossRef] [Google Scholar]
- Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev.. 2001;65:232-260.
- [CrossRef] [Google Scholar]
- Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci.. 2004;24:10993-10998.
- [CrossRef] [Google Scholar]
- Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: Involvement of oxidative stress mechanisms. Toxicol. Res. (Camb). 2015;4:634-644.
- [CrossRef] [Google Scholar]
- Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol.. 1997;5:234-240.
- [CrossRef] [Google Scholar]
- An ancient solution to a modern problem. Mol. Microbiol.. 2020;113:546-549.
- [CrossRef] [Google Scholar]
- Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett.. 2016;38:1015-1019.
- [CrossRef] [Google Scholar]
- Libidibia ferrea Fruit Crude Extract and Fractions Show Anti-Inflammatory, Antioxidant, and Antinociceptive Effect in Vivo and Increase Cell Viability in Vitro. Evidence-based Complement. Altern. Med.. 2019;2019
- [CrossRef] [Google Scholar]
- Crude extract from Libidibia ferrea (Mart. ex. Tul.) L.P. Queiroz leaves decreased intra articular inflammation induced by zymosan in rats. BMC Complement. Altern. Med.. 2019;19:1-10.
- [CrossRef] [Google Scholar]
- Ferreira, J.V. de A., Ferreira, L. de L., Gomes, F.F., Matias, E.F.F., Sobral, E. de S., Cosmo, J.A., Tintino, S.R., Leite, N.F., Albuquerque, R.S., Morais Braga, M.F.B., Cunha, F.A.B. da, Costa, J.G.M. da, Coutinho, H.D.M., 2016. Evaluation of antimicrobial and modulatory activity of the ethanol extract of Libidibia ferrea (Mart. ex tul.) l.p. queiroz. Rev. Cuba. Plantas Med. 21, 71–82.
- Chromatographic and spectrophotometric analysis of phenolic compounds from fruits of Libidibia ferrea Martius. Pharmacogn. Mag.. 2016;12:285.
- [CrossRef] [Google Scholar]
- Flora do Brasil, 2020. Jardim Botânico do Rio de Janeiro [WWW Document]. URL http://floradobrasil.jbrj.gov.br (accessed 7.20.21).
- Freitas, T.S., Campina, F.F., Costa, M.S., Rocha, J.E., Cruz, R.P., Pinheiro, Ja.C.A., Pereira-Júnior, F.N., Lima, M.A., Sá, M.F.Ca.P. de, Teixeira, A.M.R., Coutinho, H.D.M., 2021. UPLC-QTOF-MS/MS analysis and antibacterial activity of the Manilkara zapota (L.) P. Royen against Escherichia coli and other MDR bacteria. Cell. Mol. Biol. 67, 116–124. 10.14715/cmb/2021.67.1.18.
- Guerra, A.C.V. de A., Soares, L.A.L., Ferreira, M.R.A., Araújo, A.A. de, Rocha, H.A. de O., Medeiros, J.S. de, Cavalcante, R. dos S., Júnior, R.F. de A., 2017. Libidibia ferrea presents antiproliferative, apoptotic and antioxidant effects in a colorectal cancer cell line. Biomed. Pharmacother. 92, 696–706. 10.1016/j.biopha.2017.05.123.
- Transfer of Antibiotic Resistance in Staphylococcus aureus. Trends Microbiol.. 2017;25:893-905.
- [CrossRef] [Google Scholar]
- Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist. Updat.. 2016;28:13-27.
- [CrossRef] [Google Scholar]
- The Antibiotic Resistome: A Guide for the Discovery of Natural Products as Antimicrobial Agents. Chem. Rev.. 2021;121:3464-3494.
- [CrossRef] [Google Scholar]
- Arginase inhibitory activity and total flavonoid content on Caesalpinia ferrea C. Mart stem bark extracts. Pharmacogn. J.. 2018;10:1180-1183.
- [CrossRef] [Google Scholar]
- De Novo Antimicrobial Peptides with Low Mammalian Cell Toxicity. J. Med. Chem.. 1996;39:3107-3113.
- [CrossRef] [Google Scholar]
- Libidibia ferrea loaded in bacterial nanocellulose: evaluation of antimicrobial activity and wound care. J. Biomed. Biotechnol.. 2020;6:6212-6226.
- [CrossRef] [Google Scholar]
- Kobayashi, Y.T. da S., de Almeida, V.T., Bandeira, T., de Alcántara, B.N., da Silva, A.S.B., Barbosa, W.L.R., da Silva, P.B., Monteiro, M.V.B., de Almeida, M.B., 2015. Avaliaáo fotoquímica e potencial cicatrizante do extrato etanólico dos frutos de Jucá (Libidibia ferrea) em ratos Wistar. Brazilian J. Vet. Res. Anim. Sci. 52, 34–40. 10.11606/issn.1678-4456.v52i1p34-40.
- Functional and structural roles of the major facilitator superfamily bacterial multidrug efflux pumps. Microorganisms. 2020;8
- [CrossRef] [Google Scholar]
- Leandro, C. dos S., Bezerra, J.W.A., Rodrigues, M.D.P., Silva, A.K.F., Silva, D.L. da, Santos, M.A.F. dos, Linhares, K.V., Boligon, A.A., Silva, V.B. da, Rodrigues, A.S., Bezerra, J. de S., Silva, M.A.P. da, 2019. Phenolic Composition and Allelopathy of Libidibia ferrea Mart. ex Tul. in Weeds. J. Agric. Sci. 11, 109. 10.5539/jas.v11n2p109.
- Matrine: A review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches. J. Ethnopharmacol.. 2021;269:113682
- [CrossRef] [Google Scholar]
- Bioprospection of Libidibia ferrea var. ferrea: Phytochemical properties and antibacterial activity. South African J. Bot.. 2020;130:103-108.
- [CrossRef] [Google Scholar]
- Mayekar, V.M., Ali, A., Alim, H., Patel, N., 2021. A review: Antimicrobial activity of the medicinal spice plants to cure human disease. Plant Sci. Today 8, 629–646. 10.14719/PST.2021.8.3.1152.
- Avaliação do potencial antioxidante e anti-Helicobacter pylori in vitro de extratos de plantas medicinais utilizadas popularmente na região amazônica. Rev. Fitos. 2017;11:140-152.
- [CrossRef] [Google Scholar]
- Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod.. 2020;83:770-803.
- [CrossRef] [Google Scholar]
- The use of Drosophila melanogaster as a model organism to study immune-nanotoxicity. Nanotoxicology. 2019;13:429-446.
- [CrossRef] [Google Scholar]
- High prevalence of tetM as compared to tetK amongst methicillin-resistant Staphylococcus aureus (MRSA) isolates from hospitals in Perak, Malaysia. Jundishapur J. Microbiol.. 2017;10:e13935
- [CrossRef] [Google Scholar]
- Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev.. 2011;63:411-436.
- [CrossRef] [Google Scholar]
- Pedrosa, T. do N., Barros, A.O., Nogueira, J.R., Fruet, A.C., Rodrigues, I.C., Calcagno, D.Q., Smith, M. de A.C., Souza, T.P. de, Barros, S.B. de M., Vasconcellos, M.C. de, Silva, F.M.A. da, Koolen, H.H.F., Maria-Engler, S.S., Lima, E.S., 2016. Anti-wrinkle and anti-whitening effects of jucá (Libidibia ferrea Mart.) extracts. Arch. Dermatol. Res. 308, 643–654.
- Soares, M.R.P.S., Caneschi, C.A., Chaves, M. das G.A.M., Mota, M., Stroppa, P.H.F., Barbosa, W., Raposo, N.R.B., 2018. in Vitro Antifungal Activity and Cytotoxicity Screening of Dry Crude Extracts From Brazilian Amazonia Plants. African J. Tradit. Complement. Altern. Med. 15, 13. 10.21010/ajtcam.v15i4.2.
- Staphylococcal and streptococcal superantigen exotoxins. Clin. Microbiol. Rev.. 2013;26:422-447.
- [CrossRef] [Google Scholar]
- Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance. Nat. Commun.. 2021;12
- [CrossRef] [Google Scholar]







