Translate this page into:
Phytochemicals from Zingiber capitatum rhizome as potential α-glucosidase, α-amylase, and glycogen phosphorylase inhibitors for the management of Type-II diabetes mellitus: Inferences from in vitro, in vivo and in-silico investigations
⁎Corresponding authors at: Department of Pharmacy, Noakhali Science and Technology University, Sonapur, Noakhali 3814, Bangladesh (A.F.M. Shahid Ud Daula) and Pharmacy Discipline, Khulna University, Khulna 9208, Bangladesh (Md. Amirul Islam). ma.islam@pharm.ku.ac.bd (Md. Amirul Islam), shahid@nstu.edu.bd (A.F.M. Shahid Ud Daula)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Diabetes mellitus is known to be one of the most challenging public health issues of the 21st century. The unwanted side effects associated with conventional synthetic antidiabetic medications have compelled the quest for natural antidiabetic drugs. Therefore, in this study, in vitro, in vivo, and in silico antidiabetic effects of Zingiber capitatum methanol extract (ZcME) were evaluated. ZcME exhibited potent inhibitory activity against α-glucosidase as evidenced by an IC50 value of 0.45 mg/ml compared to standard voglibose (IC50 = 0.31 mg/ml). The administration of ZcME at 400 mg/kg significantly (p < 0.05) reduced the fasting blood glucose level compared to the diabetic control group after 20 days of treatment. ZcME decreased blood glucose levels 25.80% on day 1, 45.58% on day 10, and up to 59.17% on day 20 when given at 400 mg/kg BW. Furthermore, GC–MS examination of the plant extracts revealed the presence of sterols, aliphatic acids, aromatics, and esters. In silico study affirm the experimental findings, where the identified compounds showed strong binding affinities against α-glucosidase (7.9 kcal/mol), α-amylase (9.7 kcal/mol), and glycogen phosphorylase enzymes (7.7 kcal/mol), highlighting the antidiabetic potential of ZcME.
Keywords
Zingiber capitatum
GCMS analysis
α-Glucosidase
α-Amylase
Glycogen phosphorylase
Molecular docking
1 Introduction
Diabetes mellitus (DM) has been an extensively discussed metabolic disorder in the scientific literature over centuries (Gilinsky et al., 2015; Liu et al. 2022). It is a clinically and genetically diverse metabolic condition defined by hyperglycemia due to inadequate insulin secretion or impaired insulin sensitivity, resulting in changes in the metabolism of proteins, carbohydrates, and lipids (Shobana et al., 2009). Statistics showed that this disease affects about 1.3% of the world's population (Mukundi et al., 2015). Some common symptoms of DM are polydipsia, polyuria, polyphagia, and weight loss (Kipasika et al., 2020). Numerous investigations have revealed that people with diabetes are more prone to people with glomerular lesions, urinary tract infections, papillary necrosis, and renal atherosclerosis (Turrent-Carriles et al., 2018). If the disease is ignored, it steadily advances to serious side effects such as diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, and foot ulcer. About 50% of people with diabetes complain about one or more of the abovementioned issues (Zheng et al., 2018).
As a result of modernisation, diabetes now poses a serious concern to the entire world. Worldwide, the number of diabetic patients is rising quickly, particularly in developing nations like Bangladesh, Srilanka, Pakistan, India, and China. According to recent studies, there are currently 463 million type 2 diabetics (T2D) worldwide. By 2030, that number will rise to 578 million (10.2 % of the population), and 700 million (10.9% of the population) by 2045. Due to dense populations, Bangladesh, Pakistan, India, and China would make up a significant portion of these (Wang et al., 2021). DM is a treatable condition in which the blood glucose level is examined routinely. The traditional treatment for DM entails dietary and activity changes, as well as the administration of insulin or oral hypoglycemic medications (Tanko et al., 2008). Due to the complexity, side effects, and expensive maintenance involved with allopathic drugs, there has been a recent increase in interest in herbal formulations and nutraceuticals made from medicinal plants. Since alternative therapies are thought to be cheap and without side effects, this has motivated medical professionals and most of the world's population to seek them out (Mukundi et al., 2015).
Higher plants, animals, and microbes have been found to create a wide variety of protein inhibitors of alpha amylases and alpha glucosidases (Choudhury et al. 1996). Some of these enzyme inhibitors work by physically obstructing the enzyme's active center at different local sites (Sama et al. 2012). By decreasing the rate at which alpha amylase converts starch to simple sugars in animals, alpha amylase inhibitors reduce the high glucose levels that can develop after a meal (Boivin et al. 1987). This is significant for diabetics since low insulin levels limit the blood's ability to remove extracellular glucose. As a result, diabetics frequently have low levels of alpha amylase in an effort to control their blood glucose levels. Alpha amylase inhibitors are also used by plants as an insect defense strategy. These inhibitors prevent insects from feeding normally by changing how alpha amylases and proteinases in their guts break down food. As a result, alpha amylase inhibitors may play a role in regulating blood sugar levels and crop protection (Gong et al. 2020).
Alpha glucosidase inhibitors which are utilized as oral anti-diabetic medications, work by stopping the breakdown of carbohydrates like starch. Normally, carbohydrates are broken down into simple sugars that can pass through the colon and be absorbed (Bischoff, 1994). The alpha glucosidase enzyme required for carbohydrate digestion is competitively inhibited by alpha glucosidase inhibitors. In the small intestine, intestinal alpha glucosidases hydrolyze complex carbohydrates into glucose and other monosaccharides. The pace at which carbohydrates are digested can be slowed down by inhibiting these enzyme systems (Mechchate, et al. 2021). Because the carbohydrates are not converted into glucose molecules, less glucose is absorbed. These pharmacological properties that inhibit enzymes have the short-term impact of lowering high blood glucose levels in diabetics. Synthetic enzyme inhibitors, which are now in use, have gastrointestinal adverse effects such diarrhea, flatulence, abdominal bloating, etc. (Bray and Greenway, 1999). As a result, dietary plants' natural alpha amylase and glucosidase inhibitors can be utilized as an efficient medication with few adverse effects for treating postprandial hyperglycemia.
Zingiber capitatum Roxb (common name: wild ginger) is a member of the family Zingibaraceae. Its roots and rhizome are traditionally used in skincare and have antiseptic properties. The plants are indigenous to Bangladesh, Assam, Madhya Pradesh, Orissa, Himalayan region (Kumaun to Sikkim), and many other parts of India and are typically found in evergreen rainforests (Joshi 2011). Many species of this family have been identified for their biological activities, such as antifungal, anti-oxidants, insecticidal, and anti-inflammatory activities (Gupta 1994). However, there is no evidence of the Z. capitatum extract's antidiabetic effectiveness in both in-vivo and in-vitro models, which are the main focus of our investigation. Additionally, GC–MS analysis was conducted to determine the extract’s phytochemicals and explore the possible anti-diabetic mechanism by targeting α-glucosidase, α-amylase, and glycogen phosphorylase enzymes through in silico study.
2 Materials and methods
2.1 Plant materials and extraction
The rhizome of Z. capitatum was collected from Fatehabad, Chittagong, Bangladesh and identification and authentication were carried out by a senior taxonomist at Chittagong University Herbarium, Chittagong, Bangladesh. (Accession number: DACB-44932). The powder (about 600 g) was soaked in methanol (about 2500 mL) for about 15 days while being jerked and stirred intermittently. Following filtration with the filter cloth and Whatman paper, the solution was evaporated using a rotary evaporator. The reddish-cloggy concentrate was stored as a crude ZcME for further experiments and kept at 4 °C in a refrigerator in a sealed glass vial.
2.2 α-glucosidase inhibition assay
α-Glucosidase inhibition experiment was conducted following Oboh et al. (2004) method with minor modification (Oboh et al., 2014). Initially, 50 μL potassium phosphate buffer (PBS, pH 6.8) and 10 μL enzyme solution (1 unit/mL) were added to 20 μL of varying concentrations of extracts(ZcME) or standard (voglibose) dissolved in methanol were incubated in a 96-well plate at 37 °C for 15 min. p-Nitrophenyl-α-D-glucopyranoside solution (5 mM) in a 0.1 M phosphate buffer (pH 6.9) was then added to 50 μL of the mixture. After letting the solutions sit at 25 °C for 5 min, the spectrophotometer was used to measure the absorbance at 405 nm.
2.3 Ethical statement and use of experimental animals
The International Center for Diarrheal Disease Research in Bangladesh supplied thirty (30) adult Swiss albino mice weighing 25–35 g each. The mice were housed in a plastic cage and accustomed to a half-dark, half-light habitat in a standard laboratory setting. During the experiment, food and drink were freely provided. The Animal Ethics Committee of Noakhali Science and Technology University (No. 59/2021) approved and permitted all animal procedures and protocols for the experiments.
2.4 Induction of diabetes
Alloxan monohydrate, dissolved in a cold, isotonic solution of 0.9 percent sodium chloride, was administered intraperitoneally (i.p.) to mice at a dose of 150 mg/kg body weight (BW) to induce hyperglycemia (Reza et al., 2020). To prevent deadly alloxan-induced hypoglycemia, a 0.2 mL dose of a 5 percent glucose solution was given to every mouse right after the alloxan injection. Following 72 h, blood glucose levels were assessed. Fasting blood glucose levels exceeding 10 mmol/L accompanied by demonstrable hyperglycemia symptoms such as polyuria, polydipsia, and hyperphagia were considered a successful induction of diabetes. The mice that met these criteria were chosen for experimental tests (Harley et al., 2022). To demonstrate stable diabetes induction, the fasting glucose level was reassessed after 96 h.
2.5 Experimental procedure
The chosen mice were separated into five groups (n = 5) in order to assess ZcME's antidiabetic effects. The standard group received glibenclamide (10 mg/kg BW), while the diabetic control group received normal saline (10 mL/kg BW). ZcME was given to two groups in doses of 200 mg/kg and 400 mg/kg. The mice in the normal group did not have diabetes (not treated with alloxan). The diabetic control group, the standard group, and the extract group received saline, glibenclamide, and ZcME orally once daily for twenty days. Tail vein blood samples were collected from all five groups on days 0, 1, 10, and 20, and glucose levels were measured.
2.6 GC–MS analysis of ZcME
The methanol extract of rhizomes of Z. capitatum was analysed by GC (Agilent, Santa Clara, USA) coupled with MS (GC MS-QP Plus). Helium was delivered as carrier gas (flow rate 1.0 mL/min) in Rxi-5Sil MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness). The injector temperature was adjusted to 250 °C. Initially, the oven temperature was maintained at 100 °C for 2 min and then increased by 4 °C/min to 230 °C with a hold of 5 min. The temperature was then again raised by 5 °C/min to 280 °C. Mass spectra of the chromatographic peaks were recorded at 70 eV electron energy in the range of 40 to 700 Da. The identification of different phytochemicals was carried out by comparing their retention time and mass spectral data with the corresponding retention time and mass spectral database of NIST and Wiley 7 library.
2.7 Molecular docking
The crystal structure of protein molecules of α –glucosidase (PDB ID: 5NN8), α-amylase (PDB ID: 3BAJ), and glycogen phosphorylase (PDB ID: 1NOI) were downloaded from the PDB library to carry out molecular docking. Using PyMol, the water molecules and other interfering hetero atoms were eliminated from the crystal structures. Using the Swiss PDB viewing tool, the protein structure's energy was minimised (version 4.1.0). The protein's pdb files were modified using the autodock program to combine non-polar hydrogens and add polar hydrogens (Ravi and Kannabiran 2016). The active sites of the proteins were used to build the grid box. Following that, pdbqt representations of the protein structure were saved for the docking research. The 3D structures of GC–MS ligands were retrieved in sdf format from the PubChem database and converted to pdb format using PyMOL. Using Auto Dock tools, the pdb formats of the ligands were further transformed to pdbqt formats. Autodock Vina was used to accomplish docking at the end (v.1.2.0). With the aid of BIOVIA Discovery studio, the docking expressions were visualised.
2.8 Statistical analysis
Every piece of information is presented as mean ± SEM. GraphPad Prism was used to conduct the statistical analysis (version 9.4.1). A two-way analysis of variance (ANOVA) was performed for alloxan-induced antidiabetic tests against each time, followed by Dunnett's test. P < 0.05 was regarded as statistically significant.
3 Results
3.1 In vitro α -glucosidase inhibitory activity of ZcME
The α-glucosidase inhibitory activity and IC50 value of ZcME are summarised in Fig. 1(A) and 1(B). The inhibitory effect of the plant extract was contrasted with the standard α-glucosidase inhibitor voglibose. Both doses of the plant extract and standard demonstrated a concentration-dependent effect; the higher the concentration, the greater the effect rule. However, the plant extract showed a remarkable enzyme inhibitory effect compared to the standard. The IC50 values of the plant extract and voglibose were 0.45 mg/ml and 0.31 mg/ml, respectively.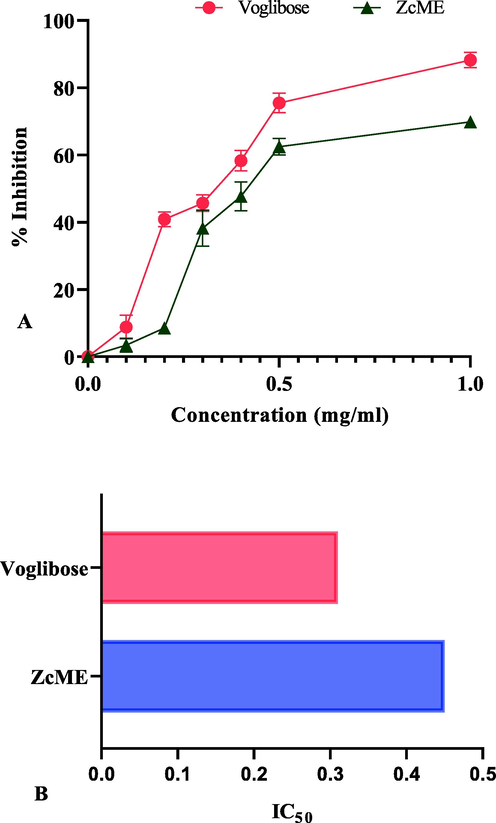
Effect of ZcME on 1(A) % inhibition of α-glucosidase and 1(B) IC50 value. Here, ‘ZcME’ stands for Z. capitatum methanol extract. Values are mean ± SD (n = 5).
3.2 Antidiabetic activity of ZcME in alloxan-induced diabetic mice
Fig. 2 shows how ZcME affected the blood glucose levels of diabetic mice. After 20 days of treatment, the diabetic control group still had the same fasting blood glucose level as on day 0. Blood glucose levels in the glibenclamide-treated group dropped from 21.5 2.94 mmol/L on day 0 to 6.64 1.38 mmol/L on day 20, reflecting a 69.12% decrease in glucose levels. Alloxan-induced diabetic mice treated with ZcME at doses of 200 and 400 mg/kg BW for 20 days exhibited strong, concentration-dependent antihyperglycemic effects. Fasting blood glucose levels were significantly altered when 200 mg/kg BW ZcME was delivered, decreasing by 22.05% on day 1, 37.42% on day 10, and 50.96% on day 20 relative to the level on day 0 of the matching group. When administered at 400 mg/kg BW, ZcME reduced blood glucose levels from day 0 by 25.80% on day 1, 45.58% on day 10, and up to 59.17% on day 20. (Table 1). Zc 200, Zingiber capitatum 200 mg/kg; Zc 400, Zingiber capitatum 400 mg/kg. ‘↓’ indicates a decrease, and ‘↑’ indicates an increase in blood glucose concentration compared to day 0.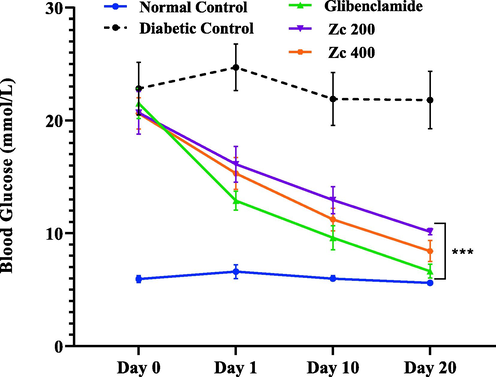
Effect of rhizome extract of ZcME on blood glucose level in diabetic mice. ‘Zc’ stands for Z. capitatum, and 200 and 400 indicate extract doses in mg/kg B.W. Value repesented mean ± SD, n = 5 (P < 0.001 (***), P < 0.01 (**), and P < 0.05 (*) vs diabetic control).
Group
Blood glucose (mmol/L)
Day 1
Day 10
Day 20
Normal control
11.11 ↑
0.67 ↑
5.72 ↓
Diabetic control
8.23 ↑
4.03 ↓
4.47 ↓
Glibenclamide
40.09 ↓
55.35 ↓
69.12 ↓
Zc 200
22.05 ↓
37.42 ↓
50.96 ↓
Zc 400
25.80 ↓
45.58 ↓
59.17 ↓
The impact of ZcME on the body weight of mice with alloxan-induced diabetes is shown in Table 2. As the intervention proceeded, the body weight of the diabetic control mice were reduced by 21.15 % after 20 days of treatment, compared to the BW for this group on day 0. On the other hand, the body weights were increased by 11.68 % and 21.17 %, respectively, following the administration of ZcME at 200 and 400 mg/kg BW compared with the body weights for each group on day 0. Treatment with glibenclamide also increased body weight (15.67%) compared to day 0. Value represented mean ± SD, n = 5. Note: Zc 200, Zingiber capitatum 200 mg/kg; Zc 400, Zingiber capitatum 400 mg/kg. ‘↓’ indicates a decrease, and ‘↑’ indicates an increase in body weight compared to day 0.
Group
Body weight (g)
Day 0
Day 1
Day 10
Day 20
% of change
Normal control
27 ± 1.66
28.2 ± 3.11
29.4 ± 4.28
32 ± 2.35
+ 18.51
Diabetic control
31.2 ± 3.90
29.6 ± 3.56
26.4 ± 3.91
24.6 ± 3.51
− 21.15
Glibenclamide
26.8 ± 2.59
29 ± 3.46
30.2 ± 2.17
31 ± 1.87
+ 15.67
Zc 200
27.4 ± 1.82
26.2 ± 1.30
28.6 ± 4.16
30.6 ± 3.97
+ 11.68
Zc 400
27.4 ± 3.21
25.4 ± 2.19
29.2 ± 4.21
33.2 ± 2.68
+ 21.17
3.3 Compound fingerprinting using GC–MS
Multiple bioactive chemicals were found in ZcME after GC–MS analysis (Table 3 and Fig. 3). Those compounds are caryophyllene oxide, isolongifolol, γ-muurolol, τ-muurolol, Acetic acid, 4a-methyldecahydronaphthalen-1-, α-vetivol, pentadecanoic acid, 14-methyl-, methyl ester, geranyl-α-terpinene, cis-9-hexadecenal, heptadecanoic acid, 15-methyl-, methyl ester, cis-Z-α-bisabolene epoxide, pseduosarsasapogenin-5,20-dien, pseduosarsasapogenin-5,20-dien methyl ether, retinal, cholestane, 4,5-epoxy-, (4.alpha.,5.alpha.)-, 6-epi-shyobunol, stigmastane-3,6-dione, 4,8,13-cyclotetradecatriene-1,3-diol, 1,5,9-tri, cis-11-eicosenamide, 1-naphthalenepropanol,.alpha.-ethenyldecahy, β-sitosterol and γ-sitosterol. The aforesaid compounds were reported for many important biological properties, including anticancer, analgesic, anti-inflammatory, antifungal, antimicrobial, antioxidant, antidiabetic, larvicidal, insecticidal, antiulcer, anticonvulsant, hepatoprotective, and antipyretic activities (Fidyt et al., 2016).
Compound name
Retention Time
Area
m/z
γ.-Muurolene
8.859
407,005
161.00
Caryophyllene oxide
9.747
721,768
79.00
Isolongifolol
10.080
407,692
67.00
τ-Muurolol
10.577
555,165
95.00
Acetic acid, 4a-methyldecahydronaphthalen-1-
11.509
1,113,916
43.00
α.-Vetivol
12.563
256,964
43.00
Pentadecanoic acid, 14-methyl-, methyl ester
13.440
3,352,164
74.00
geranyl-α.-terpinene
14.595
611,856
69.00
cis-9-Hexadecenal
15.226
327,376
55.00
Heptadecanoic acid, 15-methyl-, methyl ester
15.473
611,694
74.00
cis-Z-α-Bisabolene epoxide
15.955
9,708,302
43.00
Pseduosarsasapogenin-5,20-dien methyl ether
16.191
90,485
73.00
Cholestane, 4,5-epoxy-, (4.alpha.,5.alpha.)-
16.803
158,865
69.00
Retinal
16.804
74,707
41.00
Pseduosarsasapogenin-5,20-dien
17.072
237,875
43.00
6-epi-shyobunol
17.870
201,208
69.00
Cholestane, 4,5-epoxy-, (4.alpha.,5.alpha.)-
18.144
127,656
43.00
Stigmastane-3,6-dione, (5.alpha.)-
22.522
104,508
98.00
4,8,13-Cyclotetradecatriene-1,3-diol, 1,5,9-tri
22.858
117,551
81.00
cis-11-Eicosenamide
24.505
1,659,908
59.00
1-Naphthalenepropanol,.alpha.-ethenyldecahy
25.396
73,414
207.00
β-Sitosterol
30.127
58,925
207.00
γ-Sitosterol
31.564
21,206
207.00
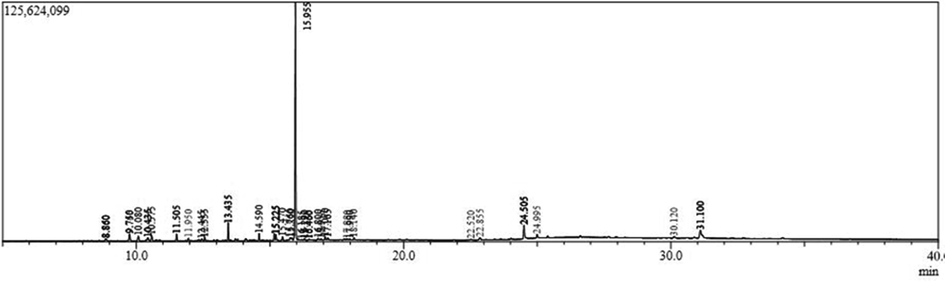
Phytochemicals identified in ZcME using GC–MS.
3.4 Molecular docking
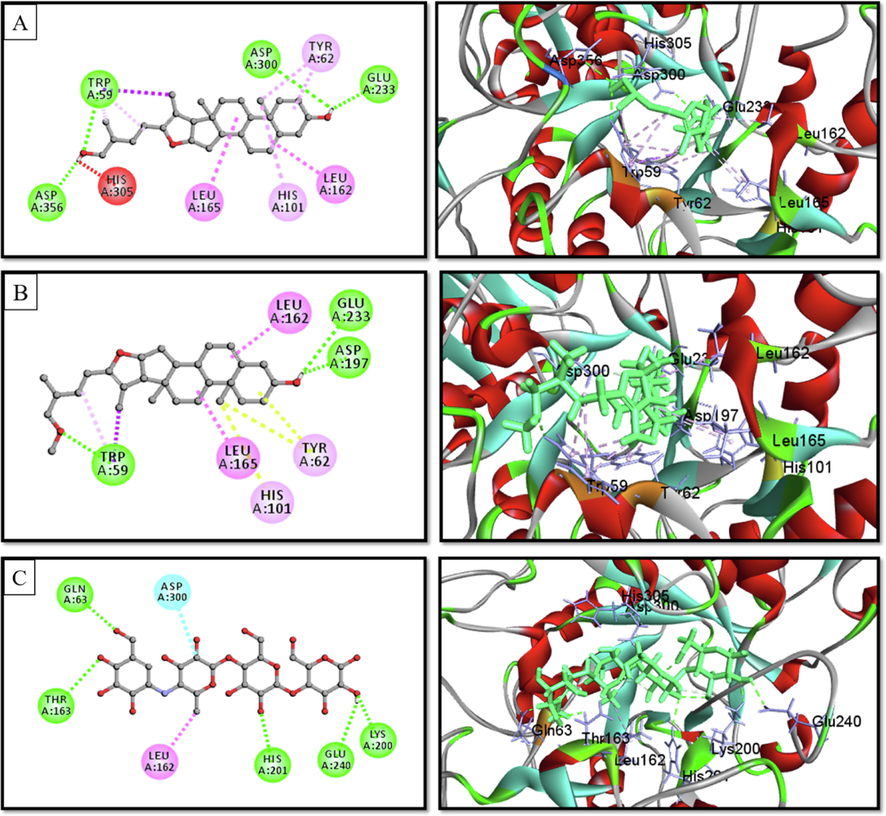
Docking experiments were done to forecast the type and strength of interaction between the target (α-glucosidase, α-amylase, and glycogen phosphorylase) and the phytochemicals discovered by GC–MS analysis (Table 4). Each substance manifested a binding propensity to α-glucosidase, α-amylase, and glycogen phosphorylase. Pseduosarsasapogenin-5,20-dien revealed the highest binding affinity towards α-glucosidase (7.9 kcal/mol) and glycogen phosphorylase (7.7 kcal/mol), whereas Pseduosarsasapogenin-5,20-dien methyl ether exhibited a high binding score of 9.7 kcal/mol towards α-amylase. β-Sitosterol, and γ-Sitosterol, two members of the phytosterol family, also showed favourable binding affinity with these three enzymes. According to an examination of the compounds' interactions with the enzyme, hydrophobic elements were primarily responsible for the interaction along with a minuscule amount of hydrogen bonds (Figs. 4-6).
Compound name
PubChem CID
Binding score ( kcal/mol)
5NN8
3BAJ
1NOI
Pseduosarsasapogenin-5,20-dien
261,799
−7.9
−9.5
−7.7
Pseduosarsasapogenin-5,20-dien methyl ether
552,194
−7.8
−9.7
−7.2
β-Sitosterol
86,821
−7.7
−9.4
−7.2
γ-Sitosterol
457,801
−7.4
−9.0
−7.2
Retinal
638,015
−6.8
−7.5
−6.8
Cis-Z-alpha-Bisabolene epoxide
91,753,574
−6.5
−7.1
−7.2
γ-Muurolene
15,094
−6.5
−6.7
−7.5
τ-Muurolol
6,432,221
−6.4
−7.0
−6.9
α-Vetivol
5,743,467
−6.2
−7.3
−7.0
Guaia-3,9-diene
585,005
−6.0
−6.6
−6.0
Caryophyllene oxide
14,350
−5.8
−7.3
−6.5
Cis-11-Eicosenamide
5,365,374
−5.6
−5.3
−5.8
Acetic acid-4a-methyldecahydronaphthalen-1-
584,552
−5.5
−7.1
−7.0
Heptadecanoic acid-15-methyl-methyl ester
554,152
−5.5
−5.4
−5.3
Isolongifolol
12,311,096
−5.5
−7.0
−5.8
6-epi-shyobunol
520,758
−5.1
−6.3
−6.2
Cis-9-Hexadecenal
5,364,643
−5.1
−5.4
−4.5
Pentadecanoic acid-14-methyl-methyl ester
21,205
−4.8
−5.4
−5.5
Voglibose
444,020
−5.2
–
–
Acarbose
444,254
–
−7.6
–
Metformin
4091
–
–
−5.4
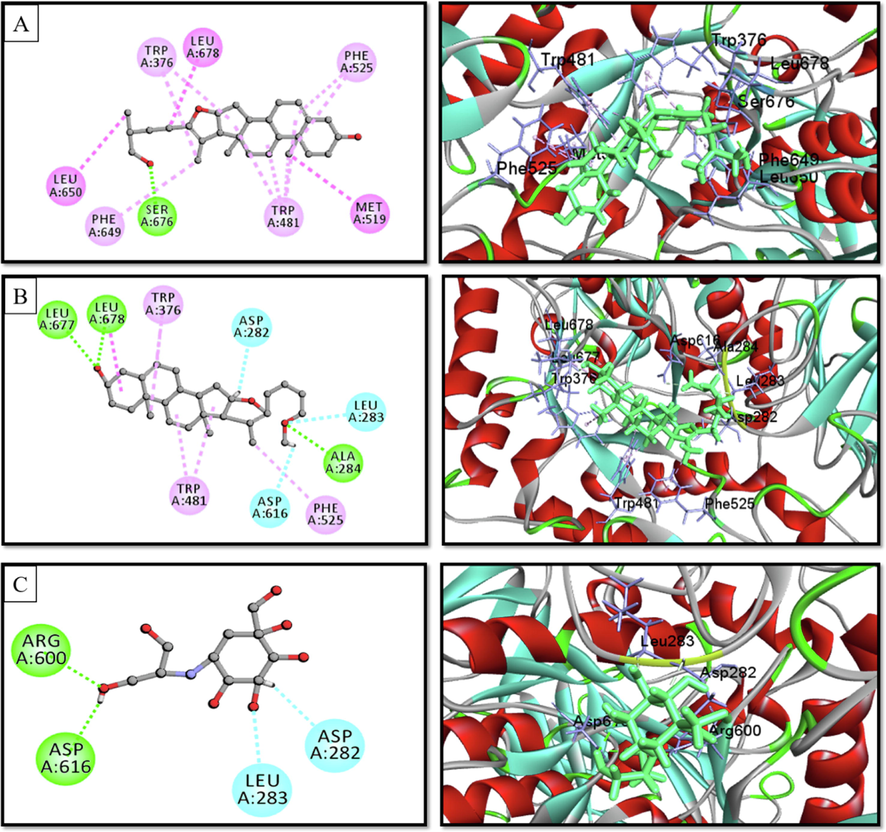
2D & 3D interactions of (A) Pseduosarsasapogenin-5,20-dien, (B) pseduosarsasapogenin-5,20-dien methyl ether, and (C) voglibose with human lysosomal α-glucosidase (PDB ID. 5NN8) (Pose predicted by AutoDock Vina).
2D & 3D interactions of (A) Pseduosarsasapogenin-5,20-dien, (B) pseduosarsasapogenin-5,20-dien methyl ether, and (C) acarbose with α-amylase (PDB ID. 3BAJ) (Pose predicted by AutoDock Vina).
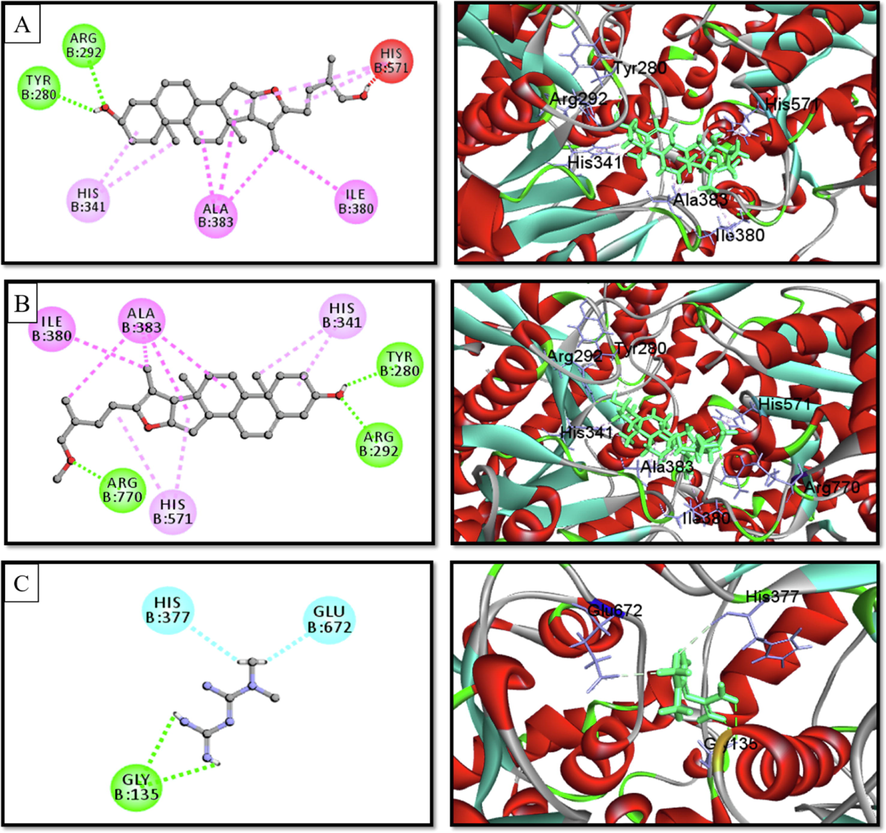
2D & 3D interactions of (A) Pseduosarsasapogenin-5,20-dien, (B) pseduosarsasapogenin-5,20-dien methyl ether, and (C) metformin with glycogen phosphorylase (PDB ID. 1NOI) (Pose predicted by AutoDock Vina).
4 Discussion
Diabetes is a metabolic condition brought on by impaired insulin production or the emergence of insulin resistance. Nowadays, it is considered one of the leading causes of death worldwide. Diabetes that is not treated or controlled can lead to long-term health problems, including cardiopathy, blindness, and hepatic and renal abnormalities (Association 2009). Many hypoglycemic medications have been used to control it, but they are associated with severe adverse effects (Ogbonnia et al., 2010). As a result, the demand for natural products increases day by day. Free radical production from oxidative stress is a harmful process that speeds up the development of diseases like diabetes (Francisqueti et al., 2017). The reducing capacity, free radical scavenging, hydrogen ion donation, and singlet oxygen quenching abilities of medicinal plants can all be used to gauge their anti-oxidant qualities (Seifu et al., 2012, Kasote et al., 2015). Studies have revealed the existence of strong anti-oxidants in foods and medicinal plants, which may be able to mitigate the consequences of oxidative stress in conditions like diabetes (Luisi et al., 2019). A previous study has shown that Z. capitatum rhizome extract has potent anti-oxidant activity (Jena et al., 2011). Therefore, the objective of this study was to assess the hypoglycemic effects of ZcME.
One of the core attributes of DM is hyperglycemia, which leads to several physical complications in the body (Altaş et al., 2011). The considerable increase in fasting plasma glucose levels shown in diabetic control mice may be explained by decreased glucose entry to muscle, peripheral tissues, adipose tissue, increased gluconeogenesis, increased glycogen breakdown, and increased hepatic glucose synthesis (Ahmed et al., 2012). The hyperglycemia seen in this experiment could be related to the glucose-fatty acid cycle, in which high levels of free fatty acids (FFAs) inhibit glucose uptake and consumption by increasing endogenous glucose synthesis (Kasper et al., 2015).
When creating new antidiabetic medications, inhibition of α -glucosidase and α -amylase is still a powerful method (Khan et al., 2016). Long-chain carbohydrates are broken down into smaller carbohydrate moieties by the intestinal enzyme α -glucosidase, which also hydrolyses starch and disaccharides to glucose (Kawamura-Konishi et al., 2012). Glycogen Phosphorylase (GP) is another rate-limiting enzyme that catalyses the initial stage of the glycogen breakdown process. Therefore, if they are also inhibited, it is possible to manage T2DM in a novel way. Thus, if this catalysing enzyme can be inhibited, it is anticipated that hepatic glucose synthesis and excessive blood sugar levels can be controlled along with stopping glycogenolysis (Treadway et al., 2001). Therefore, for a better understanding of the ani-diabetic action of ZCME, molecular docking (MD) was performed between the phytochemicals and α-amylase, α-glucosidase and glycogen phosphorylase receptors. The in silico results from this study revealed that phytochemicals from ZcME efficiently bound to the active site of the target enzymes. The molecular docking of secondary plant metabolites with α-glucosidase, α-amylase, and GP demonstrated that all of the examined ligand molecules exhibited high binding energy. More than 30 compounds showed comparable binding affinity with their respective standard drug. The methoxy and hydroxyl groups in these ligands’ structures may cause their high binding energies. Especially, pseduosarsasapogenin-5, 20-dien, pseduosarsasapogenin-5, 20-dien methyl ether, β-sitosterol and γ-sitosterol revealed the strongest binding affinity with, α-glucosidase, α-amylase and GP. The aforementioned chemicals, therefore, possess strong hypoglycemic drug characteristics. These results further imply that plant extracts may decrease the breakdown of disaccharides and polysaccharides, leading to reduced glucose absorption from the small intestine and, ultimately, reduced blood glucose levels.
5 Conclusion
Based on the results of the current study, ZcME showed remarkable antidiabetic activities in both in vitro α-glucosidase inhibition assay and in vivo mouse model of diabetes induced by alloxan. Docking study suggests that pseduosarsasapogenin-5, 20-dien, pseduosarsasapogenin-5, 20-dien methyl ether, β-sitosterol and γ-sitosterol were found to be the most attractive molecules for hypoglycemic effects in anti-diabetic targets such as α-glucosidase, α-amylase, and GP. However, in order to develop the extract as an effective anti-diabetic medicine, long term investigations on chronic animals are required to clarify the precise mode of action of the extract and its constituents.
Acknowledgement
The authors are grateful to the Research Cell, Noakhali Science and Technology University, for partial financial assistance to conduct this research. We are also thankful for the Research and Innovation Center, Khulna University, Khulna for financial support to Azmira Islam and Professor Dr. Md. Amirul Islam for conducting the research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat.. 2012;41:53-67.
- [Google Scholar]
- Protective effect of Diyarbakır watermelon juice on carbon tetrachloride-induced toxicity in rats. Food Chem Toxicol.. 2011;49(9):2433-2438.
- [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes care.. 2009;32:S62-S67.
- [Google Scholar]
- Pharmacology of alpha-glucosidase inhibition. Eur. J. Clin. Investig.. 1994;24(Suppl 3):3-10.
- [Google Scholar]
- Effect of a purified amylase inhibitor on carbohydrate metabolism after a mixed meal in healthy humans. Mayo Clin Proc.. 1987;62(4):249-255.
- [Google Scholar]
- Current and potential drugs for treatment of obesity. Endocr. Rev.. 1999;20:805-875.
- [Google Scholar]
- Character of a wheat amylase inhibitor preparation and effects on fasting human pancreaticobiliary secretions and hormones. Gastroenterol.. 1996;111(5):1313-1320.
- [Google Scholar]
- β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med.. 2016;5(10):3007-3017.
- [Google Scholar]
- The role of oxidative stress on the pathophysiology of metabolic syndrome. Rev Assoc Med Bras (1992). 2017;63(1):85-91.
- [Google Scholar]
- Lifestyle interventions for type 2 diabetes prevention in women with prior gestational diabetes: A systematic review and meta-analysis of behavioural, anthropometric and metabolic outcomes. Prev Med Rep.. 2015;2:448-461.
- [Google Scholar]
- Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr.. 2020;8(12):6320-6337.
- [Google Scholar]
- Prospects and perspectives of natural plant products in medicine. Indian J Pharmacol.. 1994;26:1-12.
- [Google Scholar]
- Hypoglycaemic activity of Oleanonic acid, a 3-oxotriterpenoid isolated from Aidia Genipiflora (DC.) Dandy, involves inhibition of carbohydrate metabolic enzymes and promotion of glucose uptake. Biomed Pharmacother.. 2022;149(112833)
- [Google Scholar]
- Studies on antioxidant, antimicrobial and phytochemical analysis of Zingiber capitatum roxb. Rhizome extracts. Int. J. Integr. Biol.. 2011;11:127-133.
- [Google Scholar]
- Medicinal Plants of submontane forest in a part of Tarai and Bhawar of Kumaun Himalaya. Nat Sci.. 2011;9:95-99.
- [Google Scholar]
- Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci.. 2015;11(8):982-991.
- [Google Scholar]
- Harrison's principles of internal medicine, 19e. NY, USA: Mcgraw-hill New York; 2015.
- Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga Sargassum patens. J Agric Food Chem.. 2012;60(22):5565-5570.
- [Google Scholar]
- In vitro inhibitory effects on α-glucosidase and α-amylase level and antioxidant potential of seeds of Phoenix dactylifera L.Asian Pac. J Trop. Biomed.. 2016;6(4):322-329.
- [Google Scholar]
- Clinical presentation and factors associated with diabetic ketoacidosis at the onset of type-1 diabetes mellitus in children and adolescent at Muhimbili National Hospital, Tanzania: a cross section study. Int J Diabetes Clin Res.. 2020;7:126.
- [Google Scholar]
- High efficiency of Ag0 decorated Cu2MoO4 nanoparticles for heterogeneous photocatalytic activation, bactericidal system, and detection of glucose from blood sample. J. Photochem. Photobiol. B, Biol.. 2022;236:112571
- [Google Scholar]
- Anti-Oxidant and Tyrosinase Inhibitory In Vitro Activity of Amino Acids and Small Peptides: New Hints for the Multifaceted Treatment of Neurologic and Metabolic Disfunctions. Antioxidants.. 2019;8(1):7.
- [Google Scholar]
- In Vivo Anti-diabetic Effects of Aqueous Leaf Extracts of Rhoicissus tridentata in Alloxan Induced Diabetic Mice. J Develop Drugs.. 2015;4:131.
- [Google Scholar]
- In Vitro Studies on the Antioxidant Property and Inhibition of α-Amylase, α-Glucosidase, and Angiotensin I-Converting Enzyme by Polyphenol-Rich Extracts from Cocoa (Theobroma cacao) Bean. Patholog Res Int.. 2014;2014:549287
- [Google Scholar]
- Ogbonnia, S., G. Mbaka, A. Adekunle, et al., 2010. Effect of a poly-herbal formulation, Okudiabet, on alloxan-induced diabetic rats.
- A handbook on protein-ligand docking tool: AutoDock 4. Innovare. Journal of Medical Sciences.. 2016;28–33
- [Google Scholar]
- Antidiabetic and hepatoprotective potential of whole plant extract and isolated compounds of Aeginetia indica. Biomed Pharmacother.. 2020;132:110942
- [Google Scholar]
- Invitro alpha amylase and alpha glucosidase inhibition activity of crude ethanol extract of Cissus arnottiana. Asian J Plant Sci.. 2012;4:550-553.
- [Google Scholar]
- Medicinal plants as antioxidant agents: understanding their mechanism of action and therapeutic efficacy. Medicinal Plants as Antioxidant Agents: Understanding Their Mechanism of Action and Therapeutic Efficacy 2012:97-145.
- [Google Scholar]
- Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food chem.. 2009;115:1268-1273.
- [Google Scholar]
- Hypoglycemic activity of methanolic stem bark of adansonnia digitata extract on blood glucose levels of streptozocin-induced diabetic wistar rats. Int. J. Appl. Res.. 2008;1:32-36.
- [Google Scholar]
- Glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs.. 2001;10:439-454.
- [Google Scholar]
- Computationally predicting binding affinity in protein-ligand complexes: free energy-based simulations and machine learning-based scoring functions. Brief Bioinform.. 2021;22(3):bbaa107.
- [Google Scholar]
- Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol.. 2018;14:88-98.
- [Google Scholar]







