Translate this page into:
Phytochemistry, pharmacology and clinical applications of the traditional Chinese herb Pseudobulbus Cremastrae seu Pleiones (Shancigu): A review
⁎Corresponding author at: 300 Xueshi Rd., Hanpu Science & Technology Park, Yuelu District, Changsha 410208, PR China. wangwei402@hotmail.com (Wei Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pseudobulbus Cremastrae seu Pleiones (PCsP) is a traditional Chinese herbal medicine known as “Shancigu” in China. It has the property of relieving fever, counteracting toxicity, dissipating phlegm and resolving masses. Officially recognized species of PCsP include Pleione bulbocodioides (Franch.) Rolfe (PB), Cremastra appendiculata (D. Don) Makino (CA), and Pleione yunnanensis (Rolfe) Rolfe (PY). Approximately, 234 compounds have been isolated and identified from PCsP. The most thoroughly investigated constituents are stilbenes (bibenzyls and phenanthrenes) and glucosyloxybenzyl succinate derivatives. Other compounds include lignans, flavonoids, and simple phenolics. The extracts and purified compounds of PCsP have exhibited anti-cancer, hepatoprotective, anti-inflammatory, neuroprotective and antioxidant potentials. Furthermore, pharmacological investigations support its traditional use for treating cancer. However, there is not enough data on the toxicity and quality control of this important herb. Moreover, the mechanism of action of active compounds and extracts need to be studied, with special attention to the effectiveness of PCsP against cancer. This review article aims to provide a critical overview of the botanical description, traditional uses, chemical constituents, pharmacological effects and clinical studies to provide a solid base for further research and development.
Keywords
PCsP
Traditional uses
Phytochemistry
Pharmacological activities
Clinical applications
- A549
-
Non-small-cell lung carcinoma
- A2780
-
Ovarian cancer cell line
- AKT
-
Protein Kinase B
- Bax
-
BCL2-Associated X
- BChE
-
Butyrylcholinesterase
- Bcl-2
-
B-cell lymphoma-2
- Bel7402
-
Liver cancer cell line
- BGC-823
-
Human gastric carcinoma
- CD-4+
-
Cluster differentiation 4+
- Cyt-c
-
Cytochrome c
- D-GalN
-
D-galactosamine
- EC50
-
Half-maximal effective concentration
- HCT-8
-
Human colon cancer cell line
- HepG2
-
Human hepatocellular carcinoma
- IC50
-
Half maximal inhibitory concentration
- LA795
-
Mouse lung adenocarcinoma cells
- MCF-7
-
Human breast cancer
- MDA-MB-231
-
Human breast cell line
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PC12
-
Pheochromocytomaderived cell line
- ROS
-
Reactive oxygen species
- SH-SY5Y
-
Human myeloid neuroblastoma
- THP-1
-
Human acute monocytic leukemia cell line
- VEGF
-
Vascular endothelial growth factor
Abbreviations
1 Introduction
Pseudobulbus Cremastrae seu Pleiones (PCsP), belonging to the family Orchidaceae, has been employed in Traditional Chinese Medicine (TCM) for the treatment of cancer (Wang et al., 2013b; Hao et al., 2019). Clinical studies have shown that the combined application of TCM and chemotherapy not only reduces the toxicity of chemotherapy but also reverses the drug resistance of tumors (Zhang et al., 2020). Chinese Materia Medica (CMM) has been in use for thousands of years, with due recognition from the international community. PCsP has been used to treat cancer in both the ancient and modern era. However, current pharmacological studies have revealed that it may not be very effective against cancer. This may be attributed to the additive, synergistic, suppressive, and antagonistic interactions of PCsP’s, when it is part of a multicomponent prescription, which is common in TCM. These interactions, nevertheless are sometimes essential in improving their therapeutic potential and reducing the side effects of certain toxic ingredients (Pan et al., 2020).
The dry tubers of PB, PY, CA are considered to be the official sources of the PCsP in TCM (Committee for the Pharmacopoeia of PR China., 2020). PB and PY belong to the genus Pleione (Orchidaceae), while CA belongs to the genus Cremastra (Orchidaceae). The three precious orchid plants are perennial epiphytic or terrestrial herbs with beautiful flowers, mainly produced in East China, Central China, and Southwest China. During the last few decades, several reports have been published on the phytochemistry, bioactivities, and clinical applications of PCsP. Among the three plants, PB and CA have been the most studies ones, while PY has not been studied a lot. Furthermore, Tulipa edulis (Miq.) Baker (Guangcigu in Chinese) and Iphigenia indica Kunth et Benth. (Lijiang Shancigu in Chinese) are the counterfeit drug of PCsP in folk medicine (Liu et al., 2020). These two plants belong to Liliaceae family and contain the toxic colchicine. In modern studies, some researchers have used the two Liliaceae plants as PCsP to study their toxicities, causing confusion in future studies (Si et al., 2020). The chemical constituents of PB were first of all reported by Bai et al in 1996 (Bai et al., 1996a; Bai et al., 1996b). In the next 20 years, more than 234 compounds were isolated from PCsP by only Japanese and Chinese phytochemists, including bibenzyls, phenanthrenes, glucosyloxybenzyl succinate derivatives, lignans, flavonoids, and simple phenolics. The secondary metabolites and extracts of PCsP possess anti-cancer, hepatoprotective, anti-inflammatory, neuroprotective and antioxidant activities (Si et al., 2020).
During the past few years, several Chinese research groups have made great contributions in exploring the chemical constituents, biological effects and clinical studies of the PCsP. However, no systematic review article discussing these achievements is available in the English literature. This article is focused on presenting a comprehensive overview of the botanical description, traditional uses, phytochemistry, pharmacology, and clinical applications of PCsP to provide a basis for further research on these important medicinal herbs.
2 Botanical description, distribution, and cultivation
PB and PY are mainly distributed in West China. CA could be found in China, Japan, India and Southeast Asian countries. These plants primarily grow in humus-covered soil, on mossy rocks in evergreen broad-leaved forests and at thicket margins at an elevation of 500–3600 m (https://www.iplant.cn/foc/). The morphological of PB, CA, PY, as referenced by the Flora of China is presented in Table 1 and Fig. 1.
Species
Botanical characteristics
Tubers
Leaves
Flowers
PB
ovoid to ovoid-conic, with a conspicuous neck, 1–2.5 × 1–2 cm
1-leaved; immature at anthesis, developing after flowering, narrowly elliptic-lanceolate or suboblanceolate, 10–25 × 2–5.8 cm, papery, base attenuate into a petiole-like stalk 2–6.5 cm, apex acute or acuminate.
Inflorescence erect; peduncle 7–20 cm, covered by 3 tubular sheaths below middle; floral bracts linear-oblong, (20−)30–40 mm, apex obtuse; flower solitary or rarely 2, pink to pale purple, with dark purple marks on lip.
CA
Ovoid or subglobose, 1.5–3 × 1–3 cm
1-leaved; narrowly elliptic, subelliptic, or narrowly elliptic- oblanceolate; 18–34 × 5–8 cm, base attenuate into a petiole-like stalk 7–17 cm, apex acute or acuminate.
Inflorescence erect; peduncle 27–70 cm, covered by some epibiotic tubular sheaths below middle; floral bracts lanceolate-ovate-lanceolate, (3-)5–12 mm, apex obtuse; 5–22 flowers, lavender brown.
PY
green, ovoid, narrowly ovoid, or conic, with a conspicuous neck 1.5–3 × 1–2 cm
1-leaved; very immature or undeveloped at anthesis, lanceolate to narrowly elliptic, 6.5–25 × 1–3.5 cm, papery, base attenuate into a petiole-like stalk 1–6 cm, apex acuminate or subacute.
Inflorescence erect; peduncle 10–20 cm, with several sheaths below middle; floral bracts obovate to obovate-oblong, 20–30 × 5–8 mm, shorter than ovary, apex obtuse; flowers solitary or rarely 2, purplish, pink, or sometimes white, with purple or deep red spots on lip.
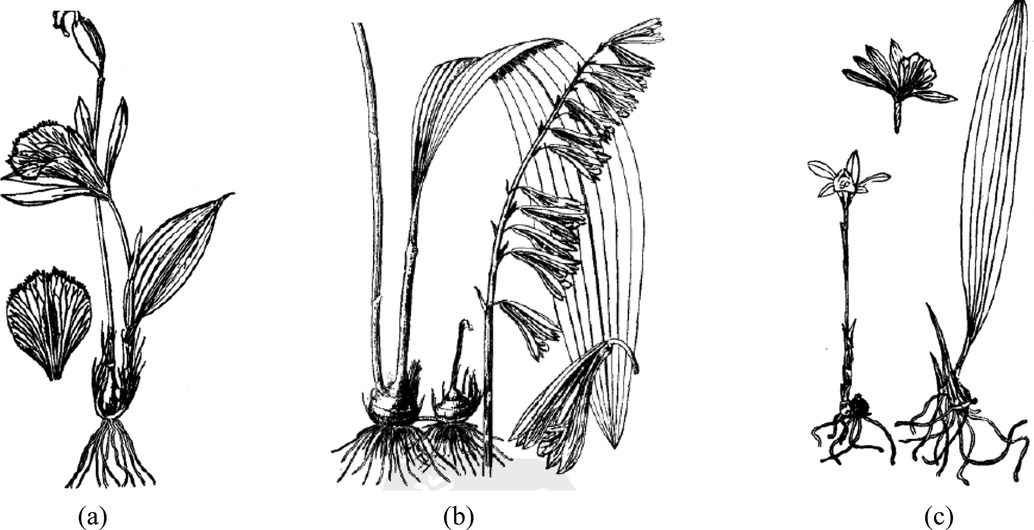
Sketch of PB (a), CA (b) and PY (c). (Cited from the Flora of China, http://www.iplant.cn/foc/).
Furthermore, over-exploitation of wild medicinal resources of PCsP, during the recent past years has caused a depletion in the natural reserves, inducing an inflation in the market price. To address the issue, cultivation on mass scale has been carried out. However, the problem associated with cultivation is a long growth period, low yields, and slow propagation. Both sexual and asexual methods of reproduction are being employed at present. Seeds are used in sexual reproduction, while the tubers are exploited for asexual reproduction method. Sexual method of reproduction does not seem very feasible or productive because of small seed size, poor maturity, technological requirements, and long seedling rate etc. The plantation period is shortened in asexual method and the method is simple and convenient, meeting the production requirements. This makes the method a better option for reproduction. It is usually cultivated in spring (May to June) and autumn (August to September) from the tubers. Tubers with plump, strong buds, no mechanical or pest/insect damage are selected for the purpose (Norimoto et al., 2021).
Under forest raising is another suitable plantation method and one for the future. Wild cultivation has the advantages of being a low cost method with high economic benefit, making full use of natural resources, realizing three-dimensional management and comprehensive utilization of forest land for improving product quality (Bing and Zhang, 2008).
3 Traditional uses
PCsP has been widely used as traditional Chinese medicine for over thousand years. The dry tubers of the plants are used for medicinal purposes. In Summer and Fall, the aerial parts of the fresh PB, CA, or PY are removed. Due to the characteristics of PCsP described by Chinese medical theories (sweet, little pungent in taste, and cool in nature), it has been used to treat conditions such as furuncles, carbuncles, scrofulous sputum, snake and insect bites, abdominal masses and lumps. The recommended dosage is 3–9 g (Wu et al., 2019) (Committee for the Pharmacopoeia of PR China, 2020).
According to Chinese medicinal books, PCsP was first recorded in “Ben Cao Shi Yi” during the Tang Dynasty (perhaps earlier). In this book, PCsP was described as a treatment for carbuncle, ulcer fistula, scrofula tuberculosis, etc. In “Ben Cao Gang Mu”, another classic book of TCM, PCsP has been reported as a cure for furuncles, carbuncles, scrofulous sputum, snake and insect bites. According to “Dian Nan Ben Cao” (Ming Dynasty), it is effective in reducing phlegm and stopping cough and sore throat. The preparations of PCsP in combination with other herbals as shown in Table 2.
Names
Prescriptions
Traditional uses
Ref.
Zijin Ding (紫金锭)
PCsP, Knoxia Root, Semen Euphorbiae Pulveratum, Galla Chinensis, Moschus, Realgar, Cinnabar
Dissipating phlegm, opening orifices, detoxification and relieving swelling and pain. It is mainly used for the treatment of abdominal distension and pain, vomiting and diarrhea, heat stroke, food and drug poisoning, headache, toothache, and traumatic injury, etc.
“Ben Cao Xin Bian” (Qing Dynasty)
Zhoushi Huisheng Wan (周氏回生丸)
PCsP, Galla Chinensis, Radix Aucklandiae, Flos Caryophylli, Semen Euphorbiae Pulveratum, Moschus, Borneol, Lignum Santali Albi, Lignum Aquilariae Resinatum, Radix Glycyrrhizae, Radix Knoxiae, Medicated Leaven, Realgar Cinnabar
Expelling summer heat and dispersing cold, detoxification, resolving dampness and relieving pain. It is used for cholera vomiting and diarrhea, acute filthy disease and abdominal pain.
Chinese Pharmacopoeia (2020 version)
Longbishu Jiaolang (癃闭舒胶囊)
PCsP, Fructus Psoraleae, Herbal Leonuri, Creeping Dichondra Herb, Spora Lygodii, Lamber
Nourishing kidney and promoting blood circulation clearing heat and freeing strangury.
Chinese Pharmacopoeia (2020 version)
Qige Tang (启膈汤)
PCsP, Eupolyphaga, Scolopendra, Herba Scutellariae Barbatae, Radix Codonopsis, Rhizoma Pinelliae
Nourishing qi, promoting blood circulation, detoxification and eliminating phlegm. It is mainly used to treat esophageal carcinoma with dysphagia.
(Wu and Chen, 1993)
Xiaoai Jiedufang消癌解毒方
PCsP, Radix Ophiopogonis, Radix Pseudostellariae, Fruit of austral Akenia, Bombyx Batryticatus, Scolopendra
Eliminating cancer, detoxification, strengthening body and eliminating pathogen
(Guo et al., 2017)
4 Phytochemisrty
The PCsP is rich in stilbenes and glucosyloxybenzyl succinate derivatives. Till date, a total of 234 compounds, including 35 bibenzyls, 92 phenanthrenes, 36 glucosyloxybenzyl succinate derivatives, 7 lignans, 14 flavonoids, 22 simple phenolics, and 28 other compounds (Table 2) have been isolated and identified from PB, CA, and PY. Among the three orchid plants, 127, 104, and 61 compounds were isolated from PB, CA, and PY, respectively. Most of these compounds were isolated from the tubers of these plants. The name and chemical structures of these compounds are shown in Table 3 and Figs. 2–6. Plausible biogenetic pathway of some different types of stilbenes is given in Fig. 7 (Shao et al., 2019; Sun et al., 2021).
NO.
Compounds
Species
Ref.
Bibenzyls
1
3,5-dimethoxy-3′-hydroxybibenzyl
PB, PY
(Liu et al., 2011a; Xu et al., 2020)
2
3′-O-methylbatatasin III
PB, PY
(Bai et al., 1997a; Dong et al., 2013)
3
gigantol
PB, CA
(Li et al., 2015b; Li et al., 2020)
4
batatasin III
PB, PY, CA
(Bai et al., 1997a; Dong et al., 2013; Wang et al., 2013b)
5
bauhinol C
PB
(Li et al., 2015b)
6
2,5,2′,5′-tetrahydroxy-3-methoxybibenzyl
PB
(Li et al., 2015b)
7
3,5,3′-trihydroxybibenzyl
CA
(Li et al., 2015a)
8
batatsin III-3-O-glucoside
PB, PY
(Bai et al., 1997a; Dong et al., 2013)
9
3′,5-dimethoxybibenzyl-3-O-β-D-glucopyranoside
PB, PY
(Bai et al., 1997a; Dong et al., 2013)
10
shancigusin F
PY
(Dong et al., 2013)
11
5,4′-dihydroxy-bibenzyl-3-O-β-D-glucoside
CA
(Li et al., 2015a)
12
5-methoxyl-bibenzyl-3,3′-di-O-β-D-glucopyranoside
PB, CA
(Liu et al., 2008a; Han et al., 2019)
13
shancigusin E
PY
(Dong et al., 2013)
14
gymconopin D
PB
(Liu et al., 2007a)
15
bulbocol
PB
(Bai et al., 1998b)
16
shancigusin D
PY
(Dong et al., 2010)
17
3,3′-dihydroxy-2-(p-hydroxybenzyl)-5-methoxybibenzyl
PB, PY, CA
(Dong et al., 2010; Qin and Shen, 2011; Zhang et al., 2013)
18
3′,5-dihydroxy-2-(p-hydroxybenzyl)-3-methoxybibenzyl
PB, PY, CA
(Dong et al., 2010; Qin and Shen, 2011; Zhang et al., 2013)
19
shancigusin C
PY
(Dong et al., 2010)
20
5-O-methylshanciguol
PB, PY, CA
(Dong et al., 2010; Li et al., 2015b; Lin et al., 2016)
21
blestritin B
PB
(Li et al., 2015b)
22
shanciguol
PB, PY
(Bai et al., 1996a; Dong et al., 2010)
23
shancigusin A
PY
(Dong et al., 2010)
24
shancigusin B
PY
(Dong et al., 2010)
25
bulbocodin
PB
(Li et al., 2015b)
26
bulbocodin C
PB
(Bai et al., 1998a)
27
bulbocodin D
PB, CA
(Bai et al., 1998a; Liu et al., 2016c)
28
arundinin
PB, PY, CA
(Bai et al., 1998b; Dong et al., 2010; Wang et al., 2013b)
29
shanciol A
PB
(Bai et al., 1998c)
30
shanciol B
PB
(Bai et al., 1998c)
31
2-(4′'-hydroxybenzyl)-3-(3′-hydroxy-phenethyl)-5-methoxy-cyclohexa-2,
5-diene-1,4-dionePB
(Liu et al., 2008b)
32
dusuanlansin A
PB
(Li et al., 2015b)
33
dusuanlansin B
PB
(Li et al., 2015b)
34
dusuanlansin C
PB
(Li et al., 2015b)
35
dusuanlansin D
PB
(Li et al., 2015b)
Phenanthrenes
Dihydrophenanthrenes
36
coelonin
PB, PY, CA
(Xuan et al., 2005; Dong et al., 2013; Li et al., 2017)
37
lusianthridin
PB, PY, CA
(Dong et al., 2013; Li et al., 2017; Li et al., 2020)
38
hircinol
PB
(Wang et al., 2014a)
39
isohircinol
CA
(Xuan et al., 2005)
40
7-hydroxy-2,4-dimethoxy-9,
10-dihydrophenanthreneCA
(Wang et al., 2013b)
41
1,2,7-trihydroxy-4-methoxy-9,10-dihydroxyphenanthrene
PY
(Xu et al., 2020)
42
1,4,7-trihydroxy-2-methoxy-9,10-dihydroxyphenanthrene
PY
(Xu et al., 2020)
43
2,5,7-trihydroxy-4-methoxy-9,10-dihydroxyphenanthrene
PY
(Xu et al., 2020)
44
4,7-dihydroxy-1-(p-hydroxybenzyl)-2-methoxy-9,10-dihydrophenanthrene
PB, PY
(Bai et al., 1996a; Dong et al., 2013)
45
2,7-dihydroxy-4-methoxy-1-(p-hydroxy-benzyl)-9,10-dihydrophenanthrene
PB, PY
(Dong et al., 2013; Li et al., 2015b)
46
1-(4-hydroxybenzyl)-4,7-dimethoxy-9,
10-dihydrophenanthrene-2-olPB, PY
(Li et al., 2015b; Xu et al., 2020)
47
1-(3′-methoxy-4′-hydroxybenzyl)-7-methoxy-9,10-dihydrophenanthrene-2,4-diol
CA
(Liu et al., 2013)
48
shancigusin G
PY, CA
(Dong et al., 2013; Wang et al., 2013b)
49
7-hydroxy-4-methoxy-9,10-dihydro-phenanthrene-2-O-β-D-glucopyranoside
CA
(Wang et al., 2013b)
50
4-methoxy-9,10-dihydrophenanthrene-2,7-di-O-β-D-glucopyranoside
CA
(Wang et al., 2013b)
Phenanthrenes
51
7-hydroxy-2,4-dimethoxy-phenanthrene
CA
(Qin and Shen, 2011)
52
2-hydroxy-4,7-dimethoxy-phenanthrene
CA
(Xue et al., 2006)
53
2,7-dihydroxy-4-methoxy-phenanthrene
PY, CA
(Wang et al., 2013b; Li et al., 2021)
54
3,5-dihydroxy-2,4-dimethoxy-phenanthrene
CA
(Liu et al., 2014)
55
7-hydroxy-4-methoxyphenanthrene-2-O-β-D-glucoside
CA
(Xia et al., 2005)
56
7-hydroxy-4-methoxyphenanthrene-2,8-di-O-β-D-glucoside
CA
(Liu et al., 2016c)
57
8-hydroxy-4-methoxyphenanthrene-2,7-di-O-β-D-glucoside
CA
(Liu et al., 2016c)
58
1-(p-hydroxybenzyl)-2,7-dihydroxy-4-methoxy-phenanthrene
PB, PY, CA
(Qin and Shen, 2011; Dong et al., 2013; Wang et al., 2014a)
59
1-(3′-methoxy-4′-hydroxybenzyl)-4-methoxyphenanthrene-2,7-diol
CA
(Liu et al., 2013)
60
1-(3′-methoxy-4′-hydroxybenzyl)-4-methoxyphenanthrene-2,6,7-triol
CA
(Liu et al., 2013)
61
cremaphenanthrene L
CA
(Liu et al., 2015)
62
cremaphenanthrene M
CA
(Liu et al., 2015)
63
cremaphenanthrene N
CA
(Liu et al., 2015)
64
cremaphenanthrene O
CA
(Liu et al., 2015)
65
cremaphenanthrene P
CA
(Liu et al., 2015)
Biphenanthrenes
66
cremaphenanthrene A
CA
(Liu et al., 2016b)
67
cremaphenanthrene B
CA
(Liu et al., 2016b)
68
blestriarene C
PY, CA
(Dong et al., 2013; Wang et al., 2013b)
69
monbarbatain A
PB, PY, CA
(Liu et al., 2014; Wang et al., 2014a)
70
2,7,2′-didroxy-4,4′,7′-trimethoxy-1,
10-biphenanthrenePB, CA
(Wang et al., 2014a; Liu et al., 2015)
71
2,2′-dihydroxy-4,7,4′,7′-tetramethoxy-1,1′-biphenanthrene
CA
(Xue et al., 2006)
72
blestriarene B
CA
(Wang et al., 2013b)
73
4,7,4′-trimethoxy-9′,10′-dihydro
(1,1′-biphenanthrene)-2,2′,7′-triolCA
(Liu et al., 2015)
74
blestriarene A
PB, PY, CA
(Wang et al., 2013b; Wang et al., 2014a; Wang et al., 2014b)
75
isoarundinin I
PB
(Li et al., 2015b)
76
blestrianol A
PB, CA
(Wang et al., 2013b; Wang et al., 2019)
77
cremaphenanthrene C
CA
(Liu et al., 2016b)
78
gymconopin C
CA
(Wang et al., 2013b)
79
9′,10′-dihydro-4,5′-dimethoxy-(1,3′-biphenanthrene)-2,2′,7,7′-tetrol
CA
(Wang et al., 2013b)
80
cremaphenanthrene F
CA
(Liu et al., 2021)
81
cremaphenanthrene G
CA
(Liu et al., 2021)
82
phochinenin B
CA
(Liu et al., 2016b)
83
cremaphenanthrene D
CA
(Liu et al., 2016b)
84
cremaphenanthrene E
CA
(Liu et al., 2016b)
85
blestrin D
CA
(Liu et al., 2016b)
86
blestrin C
CA
(Liu et al., 2016b)
87
M-bulbocodioidin E
PB
(Wang et al., 2019)
88
P-bulbocodioidin E
PB
(Wang et al., 2019)
89
M-bulbocodioidin F
PB
(Wang et al., 2019)
90
P-bulbocodioidin F
PB
(Wang et al., 2019)
91
M-bulbocodioidin G
PB
(Wang et al., 2019)
92
P-bulbocodioidin G
PB
(Wang et al., 2019)
Dihydrophenanthrene/phenanthrene and bibenzyl polymers
93
M-bulbocodioidin H
PB
(Wang et al., 2019)
94
P-bulbocodioidin H
PB
(Wang et al., 2019)
95
phoyunnanin A
PB
(Li et al., 2015b)
96
shancilin
PB
(Bai et al., 1996a)
Triphenanthrene
97
2,7,2′,7′,2′'-pentahydroxy-4,4′,4′',7′'-tetramethoxy-1,8,1′,1′'-triphenanthrene
CA
(Xue et al., 2006)
Dihydrophenanthrene and phenylpropanoid polymers
98
pleionesin B
PB, PY
(Dong et al., 2011; Wang et al., 2014a)
99
pleionesin C
PY
(Dong et al., 2011)
100
(2,3-trans)-2-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-10-methoxy-2,3,4,5-tetrahydro-phenanthro[2,1-b]furan-7-ol
CA
(Wang et al., 2013b)
101
pleionesin A
PY
(Dong et al., 2011)
102
shanciol H
PB, PY, CA
(Dong et al., 2011; Wang et al., 2013b; Wang et al., 2014a)
103
9-(4′-hydroxy-3′-methoxyphenyl)-10-(hydroxymethyl)-11-methoxy-5,6,9,10-tetrahydrophenanthro[2,3-b]furan-3-ol
PB
(Liu et al., 2009)
104
(3-hydroxy-9-(4′-hydroxy-3′-methoxyphenyl)-11-methoxy-5,6,9,10-tetrahydrophenanthro [2,3-b] furan-10-yl) methyl acetate
PB
(Liu et al., 2007a)
105
bletilol A
PB
(Bai et al., 1998c)
106
bletilol B
PB
(Bai et al., 1996b)
107
shanciol F
PB, PY
(Bai et al., 1998a; Dong et al., 2011)
108
shanciol C
PB
(Bai et al., 1998c)
109
shanciol
PB
(Bai et al., 1998c)
110
shanciol E
PB
(Bai et al., 1998a)
111
shanciol D
PB
(Bai et al., 1998c)
112
bletilol C
PB
(Bai et al., 1998c)
113
(2,3-trans)-3-[(2,7-dihydroxy-4-methoxy-phenanthren-1-yl)methyl]-2-(4-hydroxy-3-methoxyphenyl)-10-methoxy-
2,3,4,5-tetrahydro-phenanthro[2,1-b]furan-7-olCA
(Wang et al., 2013b)
114
(2,3-trans)-3-[2-hydroxy-6-(3-hydroxy-phenethyl)-4-methoxybenzyl]-2-(4-hydroxy-3-methoxyphenyl)-10-methoxy-2,3,4,5-tetrahydro-phenanthro[2,1b]furan-7-ol
CA
(Wang et al., 2013b)
Phenanthraquinones
115
(R)-bulbocodioidin A
PB
(Shao et al., 2019)
116
(S)-bulbocodioidin A
PB
(Shao et al., 2019)
117
(R)-bulbocodioidin B
PB
(Shao et al., 2019)
118
(S)-bulbocodioidin B
PB
(Shao et al., 2019)
119
(R)-bulbocodioidin C
PB
(Shao et al., 2019)
120
(S)-bulbocodioidin C
PB
(Shao et al., 2019)
121
(R)-bulbocodioidin D
PB
(Shao et al., 2019)
122
(S)-bulbocodioidin D
PB
(Shao et al., 2019)
123
2,6-dihydroxy-8-methoxy-1,4-phenanthraquinone
CA
(Li et al., 2020)
124
1-hydroxy-4,7-dimethoxy-1-(2-oxopropyl)-1H-phenanthren-2-one
CA
(Xue et al., 2006)
125
1,7-dihydroxy-4-methoxy-1-(2-oxopropyl)-1H-phenanthren-2-one
CA
(Xue et al., 2006)
126
bulbocodioidin I
PB
(Shao et al., 2020)
127
bulbocodioidin J
PB
(Shao et al., 2020)
Glucosyloxybenzyl succinate derivatives
128
pleionoside G
PB
(Han et al., 2019)
129
pleionoside H
PB
(Han et al., 2019)
130
pleionoside I
PB
(Han et al., 2019)
131
gymnoside I
PB, PY
(Dong et al., 2013; Han et al., 2019)
132
militarine
PB, PY, CA
(Dong et al., 2013; Wang et al., 2013b; Han et al., 2019)
133
(−)-(2R,3S)-1-[(4-O-β-D-glucopy-ranosyloxy)benzyl]-4-methyl-2-isobutyltartrate
PB, CA
(Wang et al., 2013b; Han et al., 2019)
134
loroglossin
PB, PY, CA
(Wang et al., 2013b; Han et al., 2019; Han et al., 2021)
135
1-[4-(β-D-glucopyranosyloxy)benzyl]-4-methyl-(R)-2-hydroxy-2-isobutylsuccinate
PY, CA
(Wang et al., 2013b; Han et al., 2021)
136
1-(4-β-D-Glucopyranosyloxybenzyl) 4-ethyl (2R)-2-isobutylmalate
CA
(Wang et al., 2013b)
137
pleionoside R
PY
(Han et al., 2021)
138
pleionoside S
PY
(Han et al., 2021)
139
pleionoside U
PY
(Han et al., 2021)
140
pleionoside Q
PY
(Han et al., 2021)
141
pleionoside T
PY
(Han et al., 2021)
142
pleionoside P
PY
(Han et al., 2021)
143
pleionoside M
PY
(Han et al., 2021)
144
pleionoside N
PY
(Han et al., 2021)
145
pleionoside O
PY
(Han et al., 2021)
146
shancigusin H
PY
(Han et al., 2021)
147
dactylorhin A
PB, PY
(Han et al., 2019; Han et al., 2021)
148
gymnoside III
PY
(Han et al., 2021)
149
dactylorhin E
PY
(Han et al., 2021)
150
pleionoside A
PB
(Han et al., 2019)
151
pleionoside B
PB
(Han et al., 2019)
152
pleionoside C
PB
(Han et al., 2019)
153
pleionoside D
PB
(Han et al., 2019)
154
vandateroside II
PB
(Han et al., 2019)
155
pleionoside E
PB
(Han et al., 2019)
156
pleionoside F
PB
(Han et al., 2019)
157
grammatophylloside B
PB
(Han et al., 2019)
158
grammatophylloside A
PB
(Han et al., 2019)
159
cronupapine
PB
(Han et al., 2019)
160
1-(4-β-D-glucopyranosyloxybenzyl) 4-methyl (2R)-2-benzylmalate
CA
(Wang et al., 2013b)
161
(−)-(2S)-1-[(4-O-β-D-glucopyranosyloxy) benzyl]-2-isopropyl-4-[(4-O-β-D-glucopyranosyloxy)benzyl] malate
PB
(Han et al., 2019)
162
bletillin A
PB
(Li et al., 2015b)
163
(Z)-2-(2-methylpropyl)butenedioic acid bis(4-β-D-glucopyranosyloxybenzyl) ester
PB
(Wang et al., 2013a)
Lignans
164
sanjidin A
PB
(Bai et al., 1997b)
165
sanjidin B
PB
(Bai et al., 1997b)
166
pleionin A
PB
(Bai et al., 1997b)
167
(−)-syringaresinol
PY
(Dong et al., 2013)
168
syringaresinol mono-O-β-Dglucoside
PB
(Han et al., 2019)
169
phillygenin
PB
(Zhang et al., 2013)
170
(7S,8R)-dehydrodiconiferyl alcohol-
9′-O-β-D-glucopyranosidePB
(Han et al., 2019)
Flavonoids
171
quercetin
CA
(Liu et al., 2014)
172
quercetin 3′-O-β-D-glucopyranoside
CA
(Liu et al., 2014)
173
genkwanin
CA
(Liu et al., 2014)
174
isorhamnetin-3,7-di-O-β-D-glucopyranoside
PB
(Li et al., 2017)
175
3′-O-methylquercetin-3-O-β-D-glucopyranoside
PB
(Li et al., 2017)
176
5,7-dihydroxy-8-methoxyflavone
PB
(Zhang et al., 2013)
177
3,5,3′-trihydroxy-8,4′-dimethoxy-7-(3-methylbut-2-enyloxy)flavone
PB
(Li et al., 2017)
178
3,5,7,3′-tetrahydroxy-8,4′-dimethoxy-6-(3-methylbut-2-enyl)flavone
PB
(Li et al., 2017)
179
kayaflavone
PB
(Yuan and Liu, 2012)
180
amentoflavone
PB
(Yuan and Liu, 2012)
181
corylin
CA
(Lin et al., 2016)
182
neobavaisoflavone
CA
(Lin et al., 2016)
183
isobavachalcone
CA
(Lin et al., 2016)
184
5,7-dihydroxy-3-(3-hydroxy-4-methoxy-benzyl)-6-methoxychroman-4-one
CA
(Shim et al., 2004)
Simple phenolics
185
p-hydroxybenzcic acid
PB
(Yuan and Liu, 2012)
186
p-hydroxy benzaldehyde
PB
(Yuan and Liu, 2012)
187
4-(methoxymethyl) phenol
PB
(Liu et al., 2011a)
188
4-(ethoxymethyl) phenol
PB
(Liu et al., 2011a)
189
p-dihydroxy benzene
PB
(Yuan and Liu, 2012)
190
methyl(4-OH) phenylacetate
PB
(Yuan and Liu, 2012)
191
p-hydroxyphenylethyl alcohol
CA
(Xuan et al., 2005)
192
ethyl p-hydroxyhydrocinnamate
PB
(Liu et al., 2011a)
193
syringic acid
CA
(Liu et al., 2014)
194
vanillin
CA
(Liu et al., 2014)
195
3-hydroxybenzcic acid
PB, CA
(Liu et al., 2011b; Yuan et al., 2017)
196
protocatechuic acid
CA
(Liu et al., 2008a)
197
p-coumaric acid
CA
(Zhang et al., 2011)
198
3-methoxy-4-hydroxy phenylethanol
CA
(Liu et al., 2014)
199
ethyl 3-hydroxyhydrocinnamate
PB
(Liu et al., 2011a)
200
vanillic acid
CA
(Zhang et al., 2011)
201
trans-cinnamic acid
PB
(Liu et al., 2011a)
202
3,4-dihydroxyphenylethyl alcohol
CA
(Xuan et al., 2005)
203
4,4′-dihydroxy-bisphenxyl
PB
(Zhang et al., 2013)
204
(E)-p-hydroxycinnamic acid
PY
(Wang et al., 2014b)
205
(E)-ferulic acid
PY
(Wang et al., 2014b)
206
(E)-ferulic acid hexacosyl ester
PY
(Wang et al., 2014b)
Others
207
β-sitosterol
PB, PY, CA
(Dong et al., 2013; Zhang et al., 2013; Lin et al., 2016)
208
daucosterol
PY, CA
(Xuan et al., 2005; Liu et al., 2011b; Dong et al., 2013)
209
ergosta-4,6,8(14),22-tetraen-3-one
PB
(Wang et al., 2014a)
210
cyclolaudanol
CA
(Qin and Shen, 2011)
211
(−)-cadin-4,10(15)-dien-11-oic acid
CA
(Li et al., 2008)
212
(+)-24,24-dimethyl-25,32-cyclo-5α-lanosta-9(11)-en-3β-ol
CA
(Li et al., 2008)
213
(−)-ent-12b-hydroxykaur-16-
en-19-oic acid 19-β-D-xylopyranosyl-
(1 → 6)-O-β-D-glucopyranosideCA
(Li et al., 2008)
214
pleionol
PB
(Bai et al., 1998b)
215
pleionoside K
PB
(Han et al., 2020)
216
pleionoside L
PB
(Han et al., 2020)
217
emodin
CA
(Liu et al., 2014)
218
chrysophanol
PB, CA
(Zhang et al., 2013; Liu et al., 2014)
219
physcion
PB, CA
(Zhang et al., 2013; Liu et al., 2014)
220
adenosine
PY, CA
(Xia et al., 2005; Dong et al., 2013)
221
aurantiamide acetate
CA
(Qin and Shen, 2011)
222
cremastrine
CA
(Ikeda et al., 2005)
223
gastrodin
PB, CA
(Wang et al., 2013b; Han et al., 2019)
224
tyrosol 8-O-β-D-glucopyranoside
CA
(Xia et al., 2005)
225
4-(2-hydroxyethyl)-2-methoxypheny l-O-β-D-glucopyranoside
CA
(Xia et al., 2005)
226
shancigusin I
PY, CA
(Dong et al., 2013; Yuan and Liu, 2015)
227
pleionoside K
CA
(Han et al., 2019)
228
2,6,2′,6′-tetramethoxy-4,4′-bis(2,3-epoxy-1-hydroxypropyl)biphenyl
CA
(Lin et al., 2016)
229
2-furoic acid
CA
(Zhang et al., 2011)
230
5-hydroxymethyl furfural
PB, CA
(Liu et al., 2011b; Qin and Shen, 2011)
231
4-(4′'-hydroxybenzyl)-3-(3′-hydroxy-phenethyl)furan-2(5H)-one
PB
(Liu et al., 2007b)
232
3-(3′-hydroxyphenethyl)furan-2(5H)-one
PB
(Liu et al., 2007b)
233
tephrosin
CA
(Tu et al., 2018)
234
succinic acid
PY
(Dong et al., 2013)
Structures of bibenzyls isolated from PCsP.
Structures of phenanthrenes isolated from PCsP.
Structures of glucosyloxybenzyl succinate derivatives isolated from PCsP.
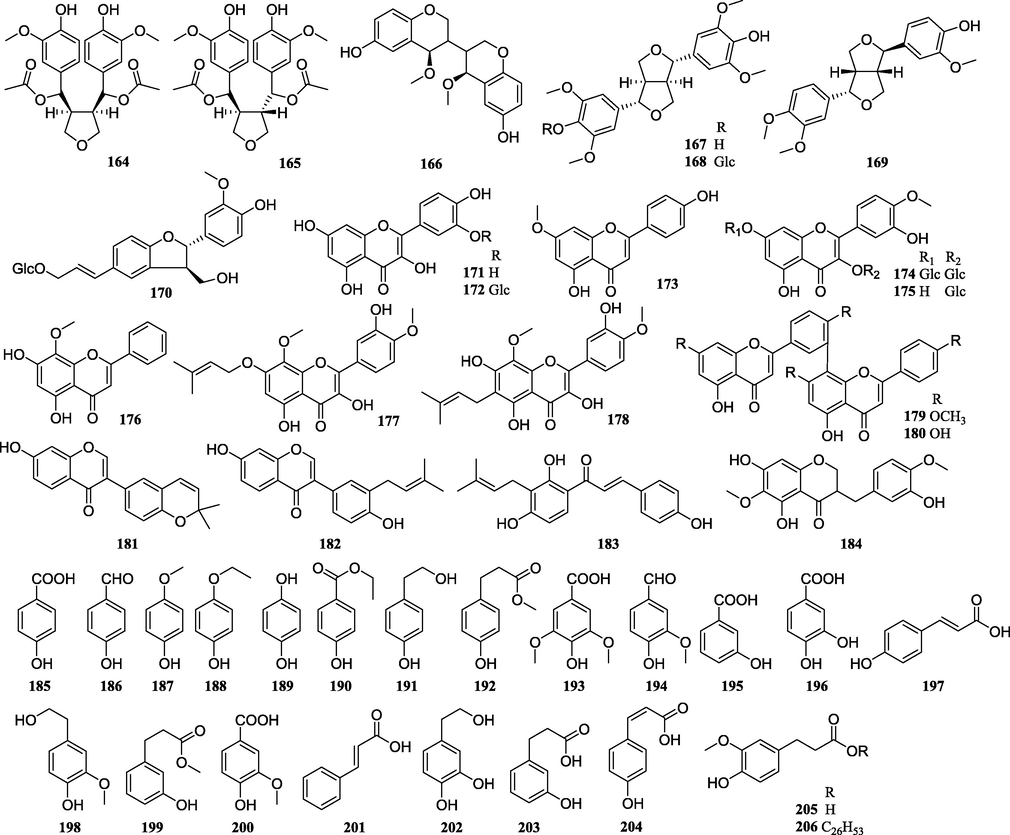
Structures of lignans, flavonoids, and simple phenolics isolated from PCsP.
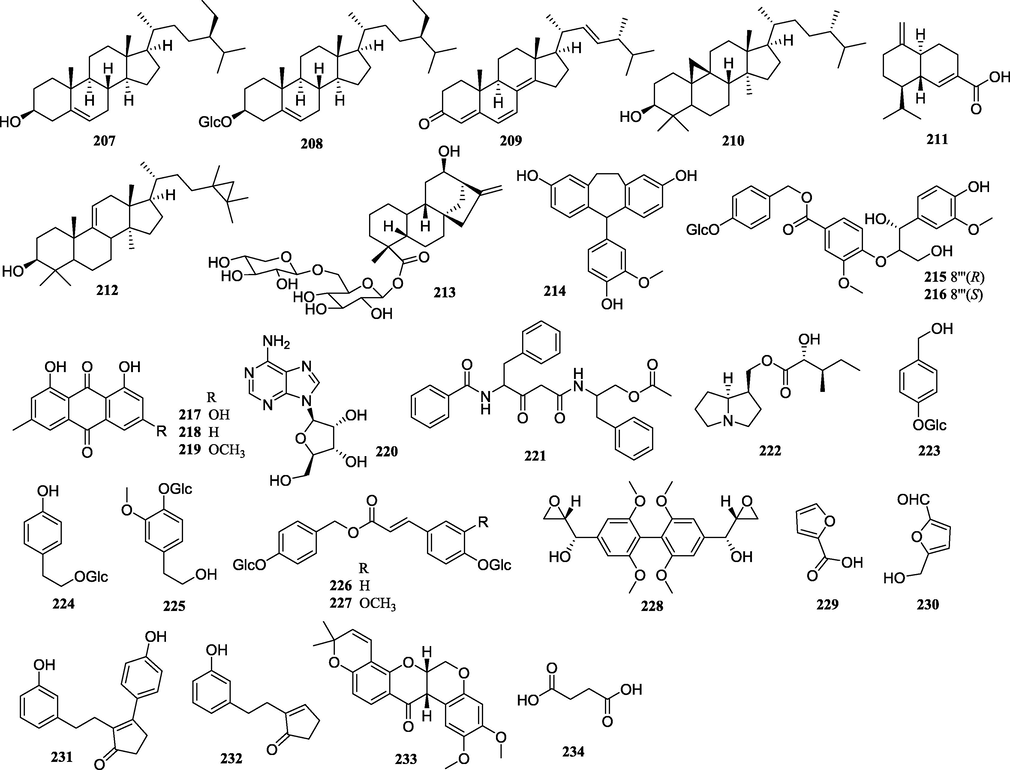
Structures of other compounds isolated from PCsP.
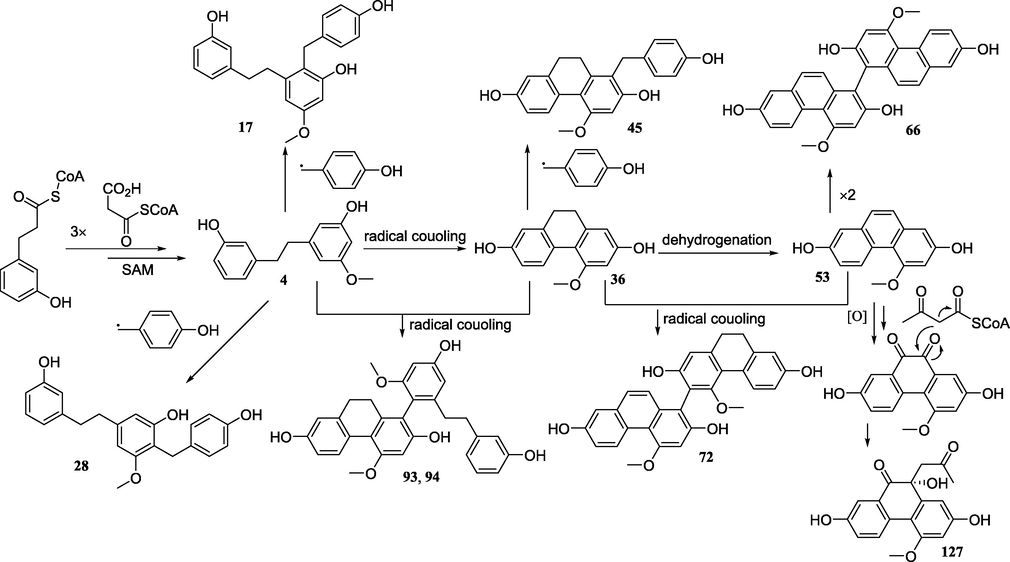
Plausible biogenetic pathway of some different types of stilbenes.
4.1 Bibenzyls
Bibenzyls contains a simple skeleton, just like one hydrogen on each of the two carbons in ethane being replaced by a phenyl group. These are usually found in bryophytes and Orchidaceae plants (Asakawa et al., 2013). Even so, their structural diversity stems from the number and positions of the substituents. Compounds 1–13 are simple bibenzyls, substituted with methoxy, hydroxy, and glucosyl groups. In 1997, 3′-O-methylbatatasin III (2), batatsin III-3-O-glucoside (8), and 3′,5-dimethoxybibenzyl-3-O-β-D-glucopyranoside (9) were isolated from the tubers of PB for the first time (Bai et al., 1997a). Compounds 14–28 are complex bibenzyls which have a 4-hydroxybenzyl group at C-2 or C-4, at least. Shanciguol (22) was isolated from the tubers of PB in 1996 (Bai et al., 1996a). Four compounds identified as shancigusins A-D (16, 19, 23 and 24) were isolated from the tubers of PY (Dong et al., 2010). Furthermore, some other interesting bibenzyls (29–35) found in PB are also given below. Shanciol A (29) and shanciol B (30) are flavan-3-ols which are simple bibenzyls combined with a phenylpropanoid moiety (Bai et al., 1998c); Compound 31 has a p-benzoquinone skeleton (Liu et al., 2008b), while compounds 32–35 are pyrrolidone substituted bibenzyls (Li et al., 2015b).
4.2 Phenanthrenes
The phenanthrenes are a rather uncommon class of aromatic metabolites which are presumably formed by oxidative coupling of the aromatic rings of stilbene precursors (Kovács et al., 2008). Ninety-two phenanthrenes, classified into 7 types have been isolated from the tubers of PB, CA, and PY. They can be classified as dihydrophenanthrenes (36–50), phenanthrenes (51–65), biphenanthrenes (66–92), dihydrophenanthrene/phenanthrene and bibenzyl polymers (93–96), triphenanthrene (97), dihydrophenanthrene and phenylpropanoid polymers (98–114), and phenanthraquinones (115–127).
The synthetic precursors of dihydrophenanthrenes are bibenzyls (Reinecke and Kindl, 1993). Coelonin (36) and lusianthridin (37) have been reported from all the plants of PCsP (Xuan et al., 2005; Dong et al., 2013; Li et al., 2017; Li et al., 2020). 4,7-dihydroxy-1-(p-hydroxybenzyl)-2-methoxy-9,10-dihydrophenanthrene (44), 2,7-dihydroxy-4-methoxy-1-(p-hydroxy-benzyl)-9,10-dihydrophenanthrene (45), and 1-(4-hydroxybenzyl)-4,7-dimethoxy-9,10-dihydrophenanthrene-2-ol (46) were isolated from the tubers of PB and PY (Bai et al., 1996a; Dong et al., 2013; Li et al., 2015b; Xu et al., 2020). Isohircinol (39), 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene (40), 1-(3′-methoxy-4′-hydroxybenzyl)-7-methoxy-9,10-dihydrophenanthrene-2,4-diol (47), 7-hydroxy-4-methoxy-9,10-dihydrophenanthrene-2-O-β-D-glucopyranoside (49) and 4-methoxy-9,10-dihydrophenanthrene-2,7-di-O-β-D-glucopyranoside have only been reported from were found in the tubers of CA (Xuan et al., 2005; Wang et al., 2013b).
All the phenanthrenes (51–65) can be found in CA. 2,7-Dihydroxy-4-methoxy-phenanthrene (53) and 1-(p-hydroxybenzyl)-2,7-dihydroxy-4-methoxy-phenanthrene (58) also were isolated from PY and PB/PY, respectively (Qin and Shen, 2011; Dong et al., 2013; Wang et al., 2013b; Wang et al., 2014a; Li et al., 2021). More than 20 dimeric phenanthrene include 9,10-dihydro- and dehydro derivatives were obtained from the PCsP. The monomers are mostly 1–1′-linked, but 1–3′ and 2-O-1′ linkages also occur in these plants. The dimeric phenanthrenes derivatives have been regarded as optically pure compounds for a long time except one study which mentioned that the isolated biphenanthrene derivatives existed as racemates (Yao et al., 2008; Wang et al., 2019). Biphenanthrene atropisomers, cremaphenanthrene F (80) and cremaphenanthrene F (81) were obtained from the tubers of CA. Three pairs of racemic bi(9,10-dihydro)phenanthrene atropisomers, bulbocodioidins E-G (87–92) were isolated from the pseudobulbs of PB (Wang et al., 2019).
Four dihydrophenanthrene/phenanthrene and bibenzyl polymers, M-bulbocodioidin H (93), P-bulbocodioidin H (94), phoyunnanin A (94) and shancilin (95) were isolated from the tubers of PB (Bai et al., 1996b; Li et al., 2015b; Wang et al., 2019). 2,7,2′,7′,2′'-pentahydroxy-4,4′,4′',7′'-tetramethoxy-1,8,1′,1′'-triphenanthrene (96) was the only triphenanthrene which was isolated from the CA (Xue et al., 2006). In 2011, 5 dihydrophenanthrenes, pleionesin B (98), pleionesin C (99), pleionesin A (1 0 1), shanciol H (1 0 2) and shanciol F (1 0 7) were isolated from the tubers of PY (Dong et al., 2011). Eight dihydrophenanthrenes, bletilol A (1 0 5), bletilol B (1 0 6), shanciol F (1 0 7), shanciol C (1 0 8), shanciol (1 0 9), shanciol E (1 1 0), shanciol D (1 1 1) and bletilol C (1 1 2) were obtained from the tubers of PB by Japanese phytochemists (Bai et al., 1996b; Bai et al., 1998a; Bai et al., 1998b; Bai et al., 1998c). Bulbocodioidins A-D (115–122, 126 and 127) were the 4 pairs of phenanthrenequinone enantiomers found only in the tubers of PB (Shao et al., 2019). 2,6-Dihydroxy-8-methoxy-1,4-phenanthraquinone (1 2 3), 1-hydroxy-4,7-dimethoxy-1-(2-oxopropyl)-1H-phenanthren-2-one (1 2 4) and 1,7-dihydroxy-4-methoxy-1-(2-oxopropyl)-1H-phenanthren-2-one (1 2 5) were only isolated from the tubers of CA (Xue et al., 2006; Li et al., 2020).
4.3 Glucosyloxybenzyl succinate derivatives
A total of 36 glucosyloxybenzyl succinate derivatives have been isolated from the PCsP. These compounds can be divided into three groups, i.e., glucosyloxybenzyl 2-isobutylsuccinates (128–149 and 161–163), glucosyloxybenzyl 2-p-hydroxybenzylsuccinates (150–154), and III-glucosyloxybenzyl 2-benzylsuccinates (155–160). Militarine (1 3 2) and loroglossin (1 3 4) were isolated from all the three plants of PCsP (Dong et al., 2013; Wang et al., 2013b; Han et al., 2019; Han et al., 2021). Gymnoside I (1 3 1), (−)-(2R,3S)-1-[(4-O-β-D-glucopy-ranosyloxy)benzyl]-4-methyl-2-isobutyltartrate (1 3 3), 1-[4-(β-D-glucopyranosyloxy)benzyl]-4-methyl-(R)-2-hydroxy-2-isobutylsuccinate (1 3 4) and dactylorhin A (1 4 7) were isolated from the two plants of PCsP (Dong et al., 2013; Wang et al., 2013b; Han et al., 2019; Han et al., 2021). The other compounds were just found in one of plants of PCsP. Furthermore, this type of compounds, including 31 compounds all can be found in the tubers of PB/PY by one research group, Dr Shuai Li’s group (Han et al., 2019; Han et al., 2021). In fact, this research group has been working on the phytochemistry of PCsP for more than 10 years (Li et al., 2008; Han et al., 2021).
4.4 Lignans
Till date, only 7 lignans have been found in PCsP. Most of them are tetrahytrofunan lignans, except pleionin A (1 6 6), which is different (Bai et al., 1997b).
4.5 Flavonoids
Flavonoids are important natural products but only 14 compounds were isolated from the PCsP. Even though the amount of the compounds is limited, but the type is diverse. Quercetin (1 7 1), quercetin 3′-O-β-D-glucopyranoside (1 7 2), genkwanin (1 7 3), isorhamnetin-3,7-di-O-β-D-glucopyranoside (1 7 4), 3′-O-methylquercetin-3-O-β-D-glucopyranoside (1 7 5), and 5,7-dihydroxy-8-methoxyflavone (1 7 6) are simple flavonoids; 3,5,3′-trihydroxy-8,4′-dimethoxy-7-(3-methylbut-2-enyloxy)flavone (1 7 7), 3,5,7,3′-tetrahydroxy-8,4′-dimethoxy-6-(3-methylbut-2-enyl)flavone (1 7 8), corylin (1 8 1), and neobavaisoflavone (1 8 2) are prenylated flavonoids; kayaflavone (1 7 9) and amentoflavone (1 8 0) are bioflavonoids; isobavachalcone (1 8 3) is a chalcone and 5,7-dihydroxy-3-(3-hydroxy-4-methoxy-benzyl)-6-methoxychroman-4-one (1 8 4) is a homo-isoflavanone.
4.6 Simple phenolics
Twenty-six simple phenolics have been obtained from the PCsP. They are important natural products because they can protect plants by offering against insects, viruses, and bacteria (Heleno et al., 2015; Xu et al., 2019).
4.7 Other compounds
Other compounds including 3 steroids (207–209), 4 terpenoids (210–213), 1 polyphenol (2 1 4), 2 phenylpropanoid glycosidic (215–216), 3 anthraquinones (217–219), 3 nitrogen compounds (220–222), 5 simple glycosidics (223–227), and 7 others (228–234) have been reported from the PCsP. The terpenoids including 2 triterpenoids (210 and 212), 1 sesquiterpenoid (2 1 1), and 1 diterpenoid (2 1 3) were only found in the tubers of CA (Li et al., 2008; Qin and Shen, 2011; Liu et al., 2013).
5 Pharmacology
5.1 Anti-cancer activity
5.1.1 The cytotoxicity effect
The cytotoxic effects of batatasin III (4), P-bulbocodioidin H (94), shanciol F (1 0 7), (R)-bulbocodioidin A (1 1 5), (S)-bulbocodioidin D (1 2 2) and bulbocodioidin J (1 2 7) isolated from the ethanolic extract of tubers of PB were evaluated against several human cancer cell lines. Compounds 4 and 107 showed weak cytotoxic activity against the growth of LA795 tumor cell line with IC50 value of 76 and 21 μM (Liu et al., 2007a). Compound 115 showed moderate inhibition activities against HepG2, BGC-823, MCF-7 cell lines with respective IC50 values of 8.1, 8.4 and 3.9 μM. Compounds 94 and 122 exhibited marked cytotoxic activities against HCT-116, HepG2, MCF-7 cell lines with IC50 values of 7.6, 3.8, 3.4 μM and 8.3, 2.3, and 2.5 μM, respectively (Taxol as the positive control with IC50 values of 0.001–0.03 μM) (Shao et al., 2019; Wang et al., 2019). Compound 127 exhibited cytotoxic activity against MCF-7 with the IC50 value of 2.1 μM (Taxol was used as the positive control) (Shao et al., 2020).
According to the cytotoxicity assay, 14 compounds, 1-(3′-methoxy-4′-hydroxybenzyl)-7-methoxy-9,10-dihydrophenanthrene-2,4-diol (47), 1-(3′-methoxy-4′-hydroxybenzyl)-4-methoxyphenanthrene-2,7-diol (59), 1-(3′-methoxy-4′-hydroxybenzyl)-4-methoxyphenanthrene-2,6,7-triol (60), cremaphenanthrene L (61), Blestriarene C (68), 2,2′-dihydroxy-4,7,4′,7′-tetramethoxy-1,1′-biphenanthrene (71), blestriarene B (72), blestriarene A (74), 2,7,2′,7′,2′′-pentahydroxy-4,4′,4′′,7′′-tetramethoxy-1,8,1′,1′′-triphenanthrene (97), pleionesin C (99), shanciol H (1 0 2), (2,3-trans)-3-[(2,7-dihydroxy-4-methoxy-phenan-thren-1-yl)methyl]-2-(4-hydroxy-3 meth-oxyphenyl)-10-methoxy-2,3,4,5 tetra-hydrophenanthro[2,1-b]furan-7-ol (1 1 3), (2,3-trans)-3-[2-hydroxy-6-(3-hydroxyphenethyl)-4-methoxybenzyl]-2-(4-hydroxy-3 met-hoxyphenyl)-10-methoxy-2,3,4,5-tetrahydro-phenanthro[2,1-b]furan-7-ol (1 1 4), and (+)-24,24-dimethyl-25,32-cyclo-5α-lanosta-9(11)-en-3β-ol (2 1 2) isolated from the tubers of CA showed different activities against several human cancer cell lines. Compounds 72, 74, 99, 102, 113, and 114 showed weak or moderate cytotoxic activity against A549 cells with IC50 values of 48.2, 47.5, 33.6, 42.8, 38.0, and 16.0 μM, respectively, with bufalin as the positive control (IC50 = 0.05 μM) (Wang et al., 2013b). Compounds 47 and 59 exhibited moderate cytotoxicity against MDA-MB-231 cell line with IC50 values of 10.42 and 11.92 μM, respectively; while compound 60 was found moderately active against HCT-116 cell line with IC50 value of 14.22 μM, while the IC50 values of paclitaxel against HCT-116 and MDA-MB-231 cell lines were 2.33 and 0.002 μM, respectively (Liu et al., 2013). Compounds 68, 71 and 97 exhibited moderate cytotoxicity against A549, A2780, Bel7402, BGC-823, HCT-8, and MCF-7 cell lines with IC50 values ranging from 8.4 to 17.8 μM, 9.5–11.9 μM, and 8.0–11.6 μM, respectively. Topotecan was used as positive control with IC50 values ranging from 1.1 to 4.4 μM (Xia et al., 2005; Xue et al., 2006). Compound 61 showed moderate cytotoxic activities against HCT-116, MCF-7, and MDA-MB-231 cancer cell lines with IC50 values of 19.01, 24.18, and 15.84 μM, respectively, (paclitaxel, IC50 values of 2.33, 0.08, and 0.002 μM, respectively) (Liu et al., 2015). Compound 212 showed in vitro-selective cytotoxicity against MCF-7 cell line with an IC50 of 3.18 μM (Li et al., 2008). It also was found that the ethyl acetate fraction of the pseudobulb of PB and CA had significant anti-tumor activity by MTT method (Liu et al., 2011b; Li et al., 2015a).
5.1.2 Inhibit the proliferation of tumor cells
Liu et al and Wu et al found that the water decoction of CA could significantly inhibit the proliferation of 4 T1 and SW579 cells in dose-dependent manner (Wu, 2014; Liu et al., 2016d). Xing et al studied the inhibitory effect of different concentrations of CA water extracts on MDA-MB-231 cell proliferation. They concluded that the mechanism may be related to the inhibition of PI3K/Akt signaling pathway (Xing et al., 2020). Yu et al reported the anti-proliferative and apoptotic effects of CA on thyroid cancer SW579 cells. The effect was attributed to the down-regulation of Bcl-2 protein (Yu et al., 2018).
5.1.3 Induce the apoptosis of tumor cells
Xu et al studied the anti-tumor effect and mechanism of PY polysaccharides on mice with H22 hepatocellular carcinoma and found that the probable route of inhibitory effect of PYRP on mice with H22 solid tumor is immune enhancement and acceleration of cell apoptosis (Xu et al., 2016). The water extracts of CA have shown significant effects on inducing the apoptosis of 4T1 cells in a dose-dependent manner (Liu et al., 2016d). The CA extract can also induce the apoptosis in HT29 cells by enhancing the expression of Cyt-C, Bax and Caspase-3 protein and at the same time suppressing Bcl-2 protein (Yu and Zhai, 2016). The polysaccharides from PY can promote mice H22 solid tumor cell fragmentation observed by HE staining and inhibit the expression of antiapoptotic factor Bcl-2 in different degrees. The expression of p53 has been found to increase with a decrease in differentiation of tumor cells (Xu, 2015). Hao et al studied the effects of an ethyl acetate extract of PB on the cell viability and apoptosis of THP-1 (human acute monocytic leukemia cell line) and its interaction with possible apoptotic pathways. The results showed that the EtOAc extract of PB significantly inhibits cell viability and induces cell apoptosis in THP-1 through a mitochondria-regulated intrinsic apoptotic pathway (Hao et al., 2019). It has been studied that the 55% ethanol extract of PCsP can inhibit the proliferation of human breast cancer MDA-MB-231 cells in vitro. The activity may be related to the down-regulation of Bcl-2 expression and up-regulation of Bax and Caspase-3 protein, inducing apoptosis (Xing et al., 2020). Similarly, the ethyl acetate extract of CA has also been found to induce apoptosis in lung cancer A549 cells (Zhang et al., 2020).
5.1.4 Inhibition of tumor angiogenesis
The aqueous extracts of PCsP has been found effective against breast cancer in vivo in a dose dependent manner by inhibiting the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9) (Yang et al., 2018). The homoisoflavanone (1 8 4), obtained from the tubers of CA, inducing angiogenesis of the chorioallantoic membrane (CAM) of chick embryo both in vitro and in vivo. The compound was found to be non-toxic and at the same time effectively inhibited the bFGF-induced invasion of human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner in vitro with IC50 value of 0.5 μM using retinoic acid as positive control (IC50 = 0.3 μM) (Shim et al., 2004).
5.1.5 Improving human immunity
Jiang et al found that PCsP polysaccharide exhibited potent anti-tumor activity by enhancing lymphocyte proliferation capacity and macrophage phagocytic activity, increasing the levels of CD4 + and CD8 + T cells in mice and elevating the CD4+/CD8 + ratio, interleukin-2, tumor necrosis factor-α and interferon-γ levels, that may be related to the improvement of immune function (Jiang et al., 2018).
5.2 Hepatoprotective activity
Five compounds, pleionoside C (1 5 2), pleionoside D (1 5 3), pleionoside F (1 5 6), pleionoside K (2 1 5) and pleionoside L (2 1 6), isolated from the pseudobulbs of PB exhibited moderate hepatoprotective activity against the N-acetyl-p-aminophenol (APAP)-induced HepG2 cell damage in in vitro assays, with cell survival rates of 31.89%, 31.52%, 31.97%, 25.83% and 28.82% at 10 μM, respectively (Han et al., 2019; Han et al., 2020).
The chemical constituents of the pseudobulbs of PY showed interesting hepatoprotective activities, tested through the MTT method. pleionoside Q (1 4 0), pleionoside R (1 3 7), shancigusin H (1 4 6), and 1-[4-(β-D-glucopyranosyloxy)benzyl]-4-methyl-(R)-2-hydroxy-2-isobutylsuccinate (1 3 5) showed significant in vitro hepatoprotective activity against (D-galactosamine) D-GalN-induced toxicity in HL-7702 cells with increasing cell viability by 27%, 22%, 19%, and 31% compared to the positive group (cf. bicyclol, 14%) at 10 μM, respectively. On the other hand, Pleionoside P (1 4 2), pleionoside U (1 3 9), and dactylochin A (1 4 7) exhibited moderate activity by enhancing the cell viability by 9%, 16%, and 12% compared to the positive group (cf. bicyclol, 9%) at 10 μM, respectively (Han et al., 2021). However, the pathophysiological mechanisms of D-GalN and APAP-induced liver injury are different and complex. Therefore, it is necessary to further study the mechanisms by which these compounds play a protective role in these two different liver injury models in the future.
5.3 Anti-inflammatory activity
2,5,2′,5′-Tetrahydroxy-3-methoxybibenzyl (6), and 4,7-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (37), obtained from the pseudobulbs of PB, have shown potent anti-inflammatory activity against LPS-stimulated NO production in BV-2 microglial cells, with IC50 values of 2.46 and 5.44 μM, respectively. Quercetin was used as a positive control with IC50 value of 3.8 μM (Li et al., 2015b; Li et al., 2017). 3′-Hydroxy-3,5-dimethoxybibenzyl (2), 1,2,7-trihydroxy-4-methoxy-9,10-dihydroxyphenanthrene (41), and 2,5,7-trihydroxy-4-methoxy-9,10-dihydroxyphenanthrene (43), isolated from the tubers of PY, have exhibited strong inhibitory effects on NO production in LPS-activited RAW264.7 cells without showing any obvious cytotoxicity toward RAW264.7 cells at the highest concentration, and their IC50 values ranged from 6.02 to 12.25 μM (Xu et al., 2020). 2,5,2′,5′-Tetrahydroxy-3-methoxybibenzyl (6) and 4,7-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (37) possessed strong anti-inflammatory effects on LPS-stimulated NO production in BV-2 microglial cells with IC50 values of 2.46 and 3.14 μM, respectively (Li et al., 2015b). The anti-inflammatory mechanism of PCsP needs further study.
5.4 Neuroprotective activity
The 55% ethanol extract of the tubers of CA has been found to significantly reduce excessive ROS production due to oxidative stress in PC12 cells, exerting thus exert its neuroprotective effect by inhibiting mitochondrial apoptosis pathway (Huo et al., 2018). Moreover, the 95% ethanol extract of the tubers of CA was found to significantly inhibit the butyrylcholinesterase (BChE) enzyme (IC50 = 23.66 µg/mL) and β-amyloid peptide aggregation (74.09% at 100 μg/mL). Coelonin (36) and orchinol (40), 7-hydroxy-2,4-dimethoxy-phenanthrene (51), obtained from this plant exhibited potent BChE inhibitory effects with IC50 values of 19.66, 32.80, and 37.79 µM, respectively. Kinetic studies indicated that both compounds 40 and 51 were mixed-type BChE inhibitors. Meanwhile, compounds 40 and 51 also inhibited the β-amyloid peptide aggregation (64.49% and 29.50% at 20 μM, respectively), indicating that they could serve as multifunctional potential agents against Alzheimer′s disease (AD) (Tu et al., 2018).
Keeping in view the inhibitory potential of the crude extract of CA tubers against apoptosis of human myeloid neuroblastoma (SH-SY5Y), the isolates of the same were probed for the same activity, revealing that compounds 132 and 228 performed well in neuroprotection assay in a hydrogen peroxide model, through weakening of the oxidative damage (Lin et al., 2016).
5.5 Antioxidant activity
Six compounds, pleionoside E (1 5 5), syringaresinol mono-O-β-D-glucoside (1 6 8), (7S,8R)-dehydrodiconiferyl alcohol-9′‐O-β-D-glucopyranoside (1 7 0), 3′-hydroxyl-5-methoxyl bibenzyl-3-O-β-D-glucopyranoside (8), pleionoside K (2 1 5) and pleionoside L (2 1 6), isolated from the pseudobulbs of PB were tested for antioxidant activity against H2O2-induced toxicity in SK-N-SH cells by means of MTT method, exhibiting moderate antioxidant activity with the cell viability increasing by 36.1%, 45.0%, 25.5%, 20.7%, 24.9% and 34.6%, respectively at 10 μM in comparision with the positive group (Han et al., 2019; Han et al., 2020). Meanwhile, coelonin (36) and orchinol (40) isolated from the tubers of CA exhibited the effective DPPH and ABTS radical scavenging activities (EC50 < 11 μM) (Tu et al., 2018). Furthermore, the polysaccharides from PCsP could have antioxidative effects by scavenging superoxide anion radicals, DPPH free radicals and hydroxyl free radicals (Fang et al., 2017). Furthermore, the antioxidant mechanism of PCsP needs further research.
6 Clinical applications
Clinical studies have shown that the combined application of traditional Chinese medicine (TCM) and chemotherapy not only reduces the toxicity of chemotherapy but also reverse the resistance of tumors against drugs (Zhang et al., 2020). There is limited data on the clinical studies of PCsP. Most of the formulations involve the crude extracts along with other herbs. Statistics suggest that the prescriptions with PCsP as an ingredient, are effective in lowering body temperature and detoxification, which could be helpful for treatment of liver and lung cancer (Shen, 2008). Upon analysis of the clinical symptoms, tumor markers, and tumor foci of 20 patients with liver cancer, surviving without surgical treatment, it was revealed that “Xiaoaijiedu” prescription could improve the clinical symptoms of patients with advanced liver cancer and stabilize the disease (Zhu et al., 2019). Another group of 198 patients with advanced tumors showed marked improvement in health when treated with “Xiaoaijiedu”, avoiding the side effects of chemotherapy. Thus PCsP shows good prospects of development as an anti-cancer drug (Zhu et al., 2019). Furthermore, a group of 60 patients diagnosed with malignant tumors were treated with “Ketong San”, applied externally. The product showed good analgesic effects on mild pain (83% relief), however it was not much effective against sever pain. More importantly, no side effects were reported (Jiang et al., 2010). In another study, carried out on a group of 90 advanced lung cancer patients, PCsP formulation was found very effective as compared to chemotherapy (Gu and Wu, 2016). PCsP also exhibits good analgesic effects in metastatic bone pain, revealed through a study conducted on 41 patients with this disorder (Gao and Feng, 2011). A total of 74 patients with advanced non-small cell lung cancer were selected to study the clinical effect of the adjuvant treatment of PCsP and the results showed that PCsP had a high clinical remission rate and promoted the patients′ physical function status that good for improving the prognosis (Xiao, 2021).
7 Conclusions
Significant breakthrough has been made during the last two decades regarding the phytochemical and biological studies on PCsP. Almost all of the contribution have been made by Chinese research groups, i.e., Dr. Shui Li, Xinqiao Liu, Pengfei Tu, et al, in recent years. The larger contribution ratio by Chinese research groups may be attributed to the distribution of these plants in China. Stilbenes and glucosyloxybenzyl succinate derivatives are regarded as the major chemical constituents (Fig. 8a) of PCsP. The crude extracts and compounds from the PCsP have been shown to exhibit anti-cancer, anti-inflammatory, anti-microbial, hepatoprotective, and neuroprotective actions (Table 4 and Fig. 8c).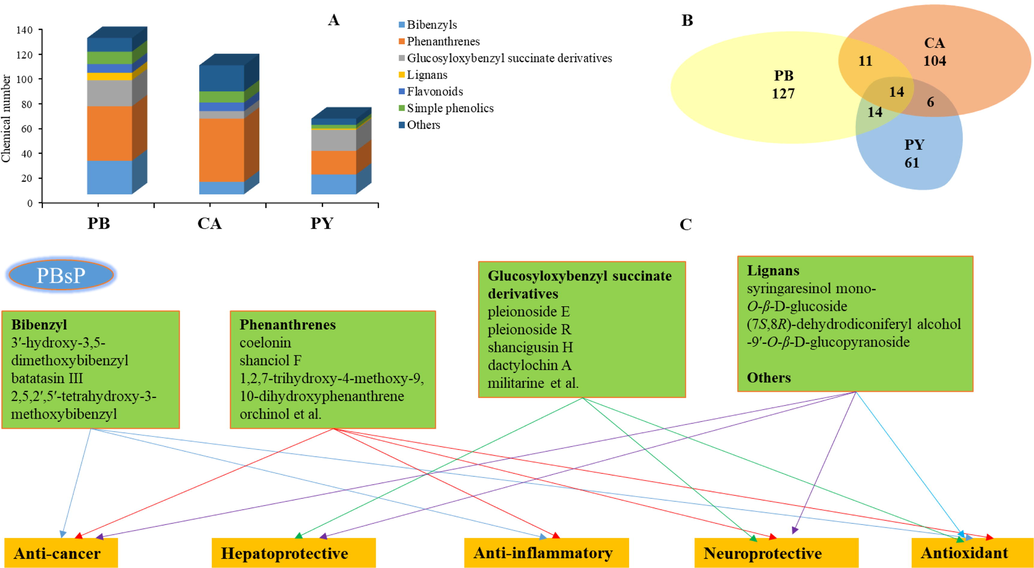
Comparative understanding the phytochemistry and pharmacology of PCsP.
Effects
Species
Extracts or compounds
Results
Reference
Anti-cancer
PB
Ethyl acetate extract
It significantly inhibits cell viability and induces cell apoptosis in THP-1 through a mitochondria-regulated intrinsic apoptotic pathway.
(Hao et al., 2019)
batatasin III (4)
Showed weak cytotoxic activity against the growth of LA795 tumor cell line with the IC50 value of 76 μM.
(Liu et al., 2007)
P-bulbocodioidin H (94)
Exhibited marked cytotoxic activities against HCT-116, HepG2, MCF-7 cell lines with IC50 values of 7.6, 3.8, 3.4 μM (Taxol as the positive control with IC50 values of 0.001–0.03 μM).
(Shao et al., 2019; Wang et al., 2019)
shanciol F (1 0 7)
Showed weak cytotoxic activity against the growth of LA795 tumor cell line with the IC50 value of 21 μM.
(Liu et al., 2007)
(R)-bulbocodioidin A (1 1 5)
Exhibited moderate inhibition activities in HepG2, BGC-823, MCF-7 cell lines with respective IC50 values of 8.1, 8.4 and 3.9 μM.
(Shao et al., 2019; Wang et al. 2019)
(S)-bulbocodioidin D (1 2 2)
Exhibited marked cytotoxic activities against HCT-116, HepG2, MCF-7 cell lines with IC50 values of 8.3, 2.3, 2.5 μM, respectively (Taxol as the positive control with IC50 values of 0.001–0.03 μM).
(Shao et al., 2019; Wang et al., 2019)
bulbocodioidin J (1 2 7)
Showed cytotoxic activity against MCF-7 with the IC50 value of 2.1 μM (Taxol was used as the positive control).
(Shao et al., 2020)
Hepatoprotection
PB
pleionoside C (1 5 2)
Exhibited moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced HepG2 cell damage in in vitro assays, with cell survival rates of 31.89% at 10 μM.
(Han et al., 2020; Han et al., 2019)
pleionoside D (1 5 3)
Exhibited moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced HepG2 cell damage in in vitro assays, with cell survival rates of 31.52% at 10 μM.
(Han et al., 2020; Han et al., 2019)
pleionoside F (1 5 6)
Exhibited moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced HepG2 cell damage in in vitro assays, with cell survival rates of 31.97% at 10 μM.
(Han et al., 2020; Han et al. 2019)
pleionoside K (2 1 5)
Exhibited moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced HepG2 cell damage in in vitro assays, with cell survival rates of 25.83% at 10 μM.
(Han et al., 2020; Han et al. 2019)
pleionoside L (2 1 6)
Exhibited moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced HepG2 cell damage in in vitro assays, with cell survival rates of 28.82% at 10 μM.
(Han et al., 2020; Han et al., 2019)
Anti-inflammation
PB
2,5,2′,5′-tetrahydroxy-3-methoxybibenzyl (6)
Exhibited potent anti-inflammatory activities on LPS-stimulated NO production in BV-2 microglial cells, with IC50 values of 2.46 μM. Quercetin was used as a positive control with IC50 value of 3.8 μM.
(Li et al., 2017; Li et al., 2015b)
4,7-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (37)
Exhibited potent anti-inflammatory activities on LPS-stimulated NO production in BV-2 microglial cells, with IC50 values of 5.44 μM. Quercetin was used as a positive control with IC50 value of 3.8 μM.
(Li et al., 2017; Li et al., 2015b)
Anti-inflammation
PY
3′-hydroxy-3,5-dimethoxybibenzyl (2)
Showed strong inhibitory effects on NO production in LPS-activited RAW264.7 cells without showing any obvious cytotoxicity toward RAW264.7 cells at the highest concentration, and their IC50 values of 11.20 μM.
(Xu et al., 2020)
1,2,7-trihydroxy-4-methoxy-9,10-dihydroxyphenanthrene (41)
Showed strong inhibitory effects on NO production in LPS-activited RAW264.7 cells without showing any obvious cytotoxicity toward RAW264.7 cells at the highest concentration, and their IC50 values of 6.02 μM.
(Xu et al., 2020)
2,5,7-trihydroxy-4-methoxy-9,10-dihydroxyphenanthrene (43)
Showed strong inhibitory effects on NO production in LPS-activited RAW264.7 cells without showing any obvious cytotoxicity toward RAW264.7 cells at the highest concentration, and their IC50 values of 12.25 μM.
(Xu et al., 2020)
Hepatoprotection
PY
1-[4-(β-D-glucopyranosyloxy)benzyl]-4-methyl-(R)-2-hydroxy-2-isobutylsuccinate (1 3 5)
Showed significant in vitro hepatoprotective activity against D-GalN-induced toxicity in HL-7702 cells with increasing cell viability by 31% compared to the positive group (cf. bicyclol, 14%) at 10 μM.
(Han et al., 2021)
pleionoside R (1 3 7)
Showed significant in vitro hepatoprotective activity against D-GalN-induced toxicity in HL-7702 cells with increasing cell viability by 22% compared to the positive group (cf. bicyclol, 14%) at 10 μM.
(Han et al., 2021)
pleionoside U (1 3 9)
Exhibited moderate hepatoprotective activity against APAP-induced toxicity in HepG2 cells with increasing cell viability by 16% compared to the positive group (cf. bicyclol, 9%) at 10 μM.
(Han et al., 2021)
pleionoside Q (1 4 0)
Showed significant in vitro hepatoprotective activity against D-GalN-induced toxicity in HL-7702 cells with increasing cell viability by 27% compared to the positive group (cf. bicyclol, 14%) at 10 μM.
(Han et al., 2021)
pleionoside P (1 4 2)
Exhibited moderate hepatoprotective activity against APAP-induced toxicity in HepG2 cells with increasing cell viability by 9% compared to the positive group (cf. bicyclol, 9%) at 10 μM.
(Han et al., 2021)
shancigusin H (1 4 6)
Showed significant in vitro hepatoprotective activity against D-GalN-induced toxicity in HL-7702 cells with increasing cell viability by 19% compared to the positive group (cf. bicyclol, 14%) at 10 μM.
(Han et al., 2021)
dactylochin A (1 4 7)
Exhibited moderate hepatoprotective activity against APAP-induced toxicity in HepG2 cells with increasing cell viability by 12% compared to the positive group (cf. bicyclol, 9%) at 10 μM.
(Han et al., 2021)
Anti-cancer
CA
Ethyl acetate extract
Had effect of inducing apoptosis in lung cancer A549 cells.
(Zhang et al., 2020)
1-(3′-methoxy-4′-hydroxybenzyl)-7-methoxy-9,10-dihydrophenanthrene-2,4-diol (47)
Had moderate cytotoxicity against MDA-MB-231 cell line with IC50 values of 10.42 μM.
(Liu et al., 2013)
1-(3′-methoxy-4′-hydroxybenzyl)-4-methoxyphenanthrene-2,7-diol (59)
Had moderate cytotoxicity against MDA-MB-231 cell line with IC50 values of 11.92 μM.
(Liu et al., 2013)
1-(3′-methoxy-4′-hydroxybenzyl)-4-methoxyphenanthrene-2,6,7-triol (60)
Showed moderate cytotoxicity against to HCT-116 cell line with IC50 value of 14.22 μM, while the IC50 values of paclitaxel against HCT-116 and MDA-MB-231 cell lines were 2.33 and 0.002 μM, respectively
(Liu et al., 2013)
cremaphenanthrene L (61)
Showed moderate cytotoxic activities against HCT-116, MCF-7, and MDA-MB-231 cancer cell lines with IC50 values of 19.01, 24.18, and 15.84 μM, respectively, paclitaxel as a positive control with IC50 value of 2.33, 0.08, and 0.002 μM, respectively.
(Liu et al., 2015)
Blestriarene C (68)
Had moderate cytotoxicity against A549, A2780, Bel7402, BGC-823, HCT-8, and MCF-7 cell lines with IC50 values ranging from 8.4 to 17.8 μM. Topotecan was used as positive control with IC50 values ranging from 1.1 to 4.4 μM.
(Xia et al., 2005; Xue et al., 2006)
2,2′-dihydroxy-4,7,4′,7′-tetramethoxy-1,1′-biphenanthrene (71)
Had moderate cytotoxicity against A549, A2780, Bel7402, BGC-823, HCT-8, and MCF-7 cell lines with IC50 values ranging from 9.5 to 11.9 μM. Topotecan was used as positive control with IC50 values ranging from 1.1 to 4.4 μM.
(Xia et al., 2005; Xue et al., 2006)
blestriarene B (72)
Showed weak cytotoxic activity against A549 cells with IC50 values of 48.2 μM (Bufalin was used as positive control, IC50 = 0.05 μM).
(Wang et al., 2013)
blestriarene A (74)
Showed weak cytotoxic activity against A549 cells with IC50 values of 47.5 μM (Bufalin was used as positive control, IC50 = 0.05 μM).
(Wang et al., 2013)
2,7,2′,7′,2′′-pentahydroxy-4,4′,4′′,7′′-tetramethoxy-1,8,1′,1′′-triphenanthrene (97)
Had moderate cytotoxicity against A549, A2780, Bel7402, BGC-823, HCT-8, and MCF-7 cell lines with IC50 values ranging from 8.0 to 11.6 μM. Topotecan was used as positive control with IC50 values ranging from 1.1 to 4.4 μM
(Xia et al., 2005; Xue et al., 2006).
pleionesin C (99)
Showed weak cytotoxic activity against A549 cells with IC50 values of 33.6 μM (Bufalin was used as positive control, IC50 = 0.05 μM).
(Wang et al., 2013)
shanciol H (1 0 2)
Showed weak cytotoxic activity against A549 cells with IC50 values of 42.8 μM (Bufalin was used as positive control, IC50 = 0.05 μM).
(Wang et al., 2013)
(2,3-trans)-3-[(2,7-dihydroxy-4-methoxy-phenan-thren-1-yl)methyl]-2-(4-hydroxy-3 meth-oxyphenyl)-10-methoxy-2,3,4,5 tetra-hydrophenanthro[2,1-b]furan-7-ol (1 1 3)
Showed weak cytotoxic activity against A549 cells with IC50 values of 38.0 μM (Bufalin was used as positive control, IC50 = 0.05 μM).
(Wang et al., 2013)
(2,3-trans)-3-[2-hydroxy-6-(3-hydroxyphenethyl)-4-methoxybenzyl]-2-(4-hydroxy-3 met-hoxyphenyl)-10-methoxy-2,3,4,5-tetrahydro-phenanthro[2,1-b]furan-7-ol (1 1 4)
Showed moderate cytotoxic activity against A549 cells with IC50 values of 16.0 μM (Bufalin was used as positive control, IC50 = 0.05 μM).
(Wang et al., 2013)
(+)-24,24-dimethyl-25,32-cyclo-5α-lanosta-9(11)-en-3β-ol (2 1 2)
Exhibited in vitro-selective cytotoxicity against MCF-7 cell line with an IC50 of 3.18 μM.
(Li et al., 2008)
Neuroprotection
CA
55% ethanol extract
It can significantly reduce excessive ROS production due to oxidative stress in PC12 cells, and thus exert its neuroprotective effect by inhibiting mitochondrial apoptosis pathway.
(Huo et al., 2018)
95% ethanol extract
Displayed potent inhibitory activities on butyrylcholinesterase (BChE) (IC50 = 23.66 µg/mL) and β-amyloid peptide aggregation (74.09% at 100 μg/mL).
(Tu et al., 2018)
55% ethanol extract
It can inhibit the apoptosis of human myeloid neuroblastoma (SH-SY5Y)
(Lin et al., 2016)
coelonin (36)
Exhibited potent BChE inhibitory effects with IC50 values of 19.66 µM.
(Tu et al., 2018)
orchinol (40)
Exhibited potent BChE inhibitory effects with IC50 values of 32.80 µM.
(Tu et al., 2018)
7-hydroxy-2,4-dimethoxy-phenanthrene (51)
Exhibited potent BChE inhibitory effects with IC50 values of 37.79 µM. Compounds 40 and 51 were mixed-type BChE inhibitors. Meanwhile, compounds 40 and 51 showed the inhibition effect on β-amyloid peptide aggregation (64.49% and 29.50% at 20 μM, respectively), indicating that they could serve as multifunctional potential agents for Alzheimer’s disease (AD) drugs development.
(Tu et al., 2018)
militarine (1 3 2)
Had the good performance of neuroprotection in hydrogen peroxide model. And the neuroprotective mechanism may be achieved by weakening the oxidative damage.
(Lin et al., 2016)
2,6,2′,6′-tetramethoxy-4,4-bis(2,3-epoxy-1-hydroxypropyl)biphenyl (2 2 8)
Had the good performance of neuroprotection in hydrogen peroxide model. And the neuroprotective mechanism may be achieved by weakening the oxidative damage.
(Lin et al., 2016)
Antioxidant
PB
3′-hydroxyl-5-methoxyl bibenzyl-3-O-β-D-glucopyranoside (8)
It was tested for antioxidant activity against H2O2-induced toxicity in SK-N-SH cells by means of MTT method and showed moderate antioxidant activity with the cell viability increasing by 20.7% at 10 μM compared with the positive group.
(Han et al., 2019; Han et al., 2020)
pleionoside E (1 5 5)
It was tested for antioxidant activity against H2O2-induced toxicity in SK-N-SH cells by means of MTT method and showed moderate antioxidant activity with the cell viability increasing by 36.1% at 10 μM compared with the positive group.
(Han et al., 2019; Han et al., 2020)
syringaresinol mono-O-β-D-glucoside (1 6 8)
It was tested for antioxidant activity against H2O2-induced toxicity in SK-N-SH cells by means of MTT method and showed moderate antioxidant activity with the cell viability increasing by 45.0% at 10 μM compared with the positive group.
(Han et al., 2019; Han et al., 2020)
(7S,8R)-dehydrodiconiferyl alcohol-9′‐O-β-D-glucopyranoside (1 7 0)
It was tested for antioxidant activity against H2O2-induced toxicity in SK-N-SH cells by means of MTT method and showed moderate antioxidant activity with the cell viability increasing by 25.5% at 10 μM compared with the positive group.
(Han et al., 2019; Han et al., 2020)
pleionoside K (2 1 5)
It was tested for antioxidant activity against H2O2-induced toxicity in SK-N-SH cells by means of MTT method and showed moderate antioxidant activity with the cell viability increasing by 24.9% at 10 μM compared with the positive group.
(Han et al., 2019; Han et al., 2020)
pleionoside L (2 1 6)
It was tested for antioxidant activity against H2O2-induced toxicity in SK-N-SH cells by means of MTT method and showed moderate antioxidant activity with the cell viability increasing by 34.6% at 10 μM compared with the positive group.
(Han et al., 2019; Han et al., 2020)
Antioxidant
CA
coelonin (36)
Exhibited the effective DPPH and ABTS radical scavenging activities (EC50 < 11 μM).
(Tu et al., 2018)
orchinol (40)
Exhibited the effective DPPH and ABTS radical scavenging activities (EC50 < 11 μM).
(Tu et al., 2018)
Stilbenes and glucosyloxybenzyl succinate derivatives isolated from PCsP mainly showed anti-cancer and hepatoprotective activities, respectively. Such compounds have also been reported from other genus of this family, i.e., Bletilla, Gymnadenia, Arundina, etc (Shang et al., 2017; Jiang et al., 2020; Zhang et al., 2021). However, the traditional use and pharmacology of these genus is different from PCsP, although the class of bioactive chemical constituents is the same. This is a question that needs to be answered by the researchers in future. Moreover, current studies are limited to explain the potential pharmacological mechanisms on anti-cancer and hepatoprotective activities for the PCsP. More relationships between the chemical constituents and biological activities should be established to further explain the principle of disease treatment. In addition, comparative comprehension on the chemical and pharmacological similarities and differences among these three orchid species should also be conducted to improve our understanding for the quality control and clinical study. In fact, the literature about quality control of the PCsP is rare. Cui et al found dactylorhin A (1 4 7) and militarine (1 3 2) are the high content in PCsP and want to use the two compounds as the Q-Marker to identify the PCsP and its confusions of herbs (Cui et al., 2013). However, the official Q-Marker of Bletilla striata also is militarine in Chinese Pharmacopoeia (2020 version). From the viewpoint of similarity, 14 compounds are commonly found in PB, CA and PY (Fig. 8b). These 14 common compounds might be considered as the potential quality markers (Q-Marker) for the qualitative analysis (fingerprinting) of the PCsP (Liu et al., 2016a).
As a traditional herbal medicine, the toxicity of PCsP were rarely recorded in ancient clinical applications; in modern pharmacological research, the related toxicity of this herb in animal experiments are also limited. So more toxicity studies need to be carried out on PCsP, especially in vivo. In the TCM, the PCsP was used to help cure cancers because it has strong effect of reducing phlegm and dispersing concretion and it can be used in prescriptions for the treatment of cancerous phlegm. However, in modern study, the exact mechanisms of medicinal properties of PCsP are still query; thus, more experiment researches are needed to better understand the functions and molecular targets.
Moreover, the wild resources of the three orchid plants are decreasing with the increasing use of PCsP, and the planting technology of the three plants is not mature enough. Therefore, it is necessary to use the PCsP rationally. To sum up, the herb still needs further study and the collected literature in this review can provide a reference for future researches and medicinal exploitations of PCsP.
Authors’ contribution
Sai Jiang and Mengyun Wang wrote and revised the manuscript. Lin Jiang, Jiangyi Luo, Huimin Zhao, Siying Tian, Yuqing Zhu, and Caiyun Peng collected and analyzed the references. Salman Zafar modified the language such as grammar and spelling checking. Wei Wang designed and adjusted structure of manuscript. All authors have read and approved the final manuscript.
Acknowledgements
This study was supported by (1) The National Natural Science Foundation of China (grant no. 81874369); (2) the Natural Science Foundation of Hunan Province (grant no. 2020JJ4463); (3) the Natural Science Foundation of Hunan Province (grant no. 2020JJ4064); (4) the Pharmaceutical Open Fund of Domestic First-Class Disciplines (cultivation) of Hunan Province (grant no. 2020YX10); (5) the Changsha Municipal Natural Science Foundation (grant no. kq 2014086).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical and biological studies of bryophytes. Phytochemistry. 2013;91:52-80.
- [CrossRef] [Google Scholar]
- Two bibenzyl glucosides from Pleione bulbocodioides. Phytochemistry. 1997;44:1565-1567.
- [CrossRef] [Google Scholar]
- Four stilbenoids from Pleione bulbocodioides. Phytochemistry. 1998;48:327-331.
- [CrossRef] [Google Scholar]
- A polyphenol and two bibenzyls from Pleione bulbocodioides. Phytochemistry. 1998;47:1637-1640.
- [CrossRef] [Google Scholar]
- Stilbenoids from Pleione bulbocodioides. Phytochemistry. 1996;42:853-856.
- [CrossRef] [Google Scholar]
- Lignans and a bichroman from Pleione bulbocodioides. Phytochemistry. 1997;44:341-343.
- [CrossRef] [Google Scholar]
- Flavan-3-ols and dihydrophenanthropyrans from Pleione bulbocodioides. Phytochemistry. 1998;47:1125-1129.
- [CrossRef] [Google Scholar]
- Shanciol, a dihydrophenanthropyran from Pleione bulbocodioides. Phytochemistry. 1996;41:625-628.
- [CrossRef] [Google Scholar]
- The botanical identification of Shancigu in Chinese ancient literature. J. Syst. Evol.. 2008;46:785-792.
- [CrossRef] [Google Scholar]
- Determination of dactylorhin A and militarine in three varieties of Cremastrae Pseudobulbus/Pleiones Pseudobulbus by HPLC. China J. Chin. Mater. Med.. 2013;38:4347-4350.
- [CrossRef] [Google Scholar]
- Shancigusins E-I, five new glucosides from the tubers of Pleione yunnanensis. Magn. Reson. Chem.. 2013;51:371-377.
- [CrossRef] [Google Scholar]
- New bibenzyl derivatives from the tubers of Pleione yunnanensis. Chem. Pharma. Bull.. 2010;40:513-515.
- [CrossRef] [Google Scholar]
- Complete assignments of 1H and 13C NMR data of three new dihydrophenanthrofurans from Pleione yunnanensis. Magn. Reson. Chem.. 2011;48:256-260.
- [CrossRef] [Google Scholar]
- Extraction and antioxidant activity of polysaccharides from Rhizoma Pleionis. J. Dalian Med. Univ.. 2017;039:527-531.
- [Google Scholar]
- Clinical observation on metastatic bone pain treated with application therapy with Pseudobulbus Cremastrae seu Pleiones. World J. Integr. Tradit. West. Med.. 2011;6:574-576.
- [CrossRef] [Google Scholar]
- Clinical observation on the treatment of 45 cases of lung cancer with syndrome of yin deficiency and heat-poison by “Shancigu” prescription. J. New Chin. Med.. 2016;048:205-207.
- [Google Scholar]
- ZHOU Zhongying's theory of cancer toxin treating esophageal carcinoma. Chin. Arch. Tradit. Chin. Med.. 2017;35:453-456.
- [CrossRef] [Google Scholar]
- Two new phenylpropanoid glycosidic compounds from the pseudobulbs of Pleione bulbocodioides and their hepatoprotective activity. Nat. Prod. Res.. 2020;6:1-8.
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of glucosyloxybenzyl succinate derivatives from the pseudobulbs of Pleione bulbocodioides. Phytochemistry. 2019;157
- [CrossRef] [Google Scholar]
- Hepatoprotective glucosyloxybenzyl 2-hydroxy-2-isobutylsuccinates from Pleione yunnanensis. J. Nat. Prod.. 2021;84:738-749.
- [CrossRef] [Google Scholar]
- Ethyl acetate extract of Pleione bulbocodioides (Franch.) Rolfe induces apoptosis of human leukemia K562 and HL-60 cells through intrinsic mitochondrial apoptosis pathway. Biosci. J.. 2019;35:609-619.
- [CrossRef] [Google Scholar]
- Bioactivity of phenolic acids: Metabolites versus parent compounds: a review. Food Chem.. 2015;173:501-513.
- [CrossRef] [Google Scholar]
- Neuroprotection effect of the extract of Cremastra appendiculata (D. Don) Makino against glutamate-induced PC12 cells injury and its possible mechanism. Chin. J. New Drugs. 2018;027:560-565. https://doi.org/CNKI:SUN:ZXYZ.0.2018-05-014
- [Google Scholar]
- Cremastrine, a pyrrolizidine alkaloid from Cremastra appendiculata. J. Nat. Prod.. 2005;68:572-573.
- [CrossRef] [Google Scholar]
- Medicinal plant of Bletilla striata: A review of its chemical constituents, pharmacological activities, and quality control. World J. Tradit. Chin. Med.. 2020;6:393-407.
- [CrossRef] [Google Scholar]
- Effects of Pseudobulbus cremastrae seu pleiones polysaccharide on the regulation of immune function and the inhibition of tumor growth in sarcoma S180 tumor-bearing mice. Food Sci.. 2018;39:216-221.
- [CrossRef] [Google Scholar]
- Clinical study on relieving cancer pain by external application of “Ketongsan”. Guangming J. Chin. Med.. 2010;25:1163-1164.
- [Google Scholar]
- Natural phenanthrenes and their biological activity. Phytochemistry. 2008;69:1084-1110.
- [CrossRef] [Google Scholar]
- Terpenoids from the tuber of Cremastra appendiculata. J. Asian Nat. Prod. Res.. 2008;10:677-683.
- [CrossRef] [Google Scholar]
- Chemical constituents from pseudobulb of Cremastra appendiculata. Chin. Tradit. Herbal Drugs. 2015;47:41-44.
- [CrossRef] [Google Scholar]
- Phenanthranes and bibenzyls isolated from Pleione Yunnanensis. J. Kunming. Med. Univ.. 2021;42:29-32.
- [CrossRef] [Google Scholar]
- A new prenylated flavone from Pleione bulbocodioides. J. Asian Nat. Prod. Res.. 2017;19:738-743.
- [CrossRef] [Google Scholar]
- Nitrogen-containing bibenzyls from Pleione bulbocodioides: Absolute configurations and biological activities. Fitoterapia. 2015;102:120-126.
- [CrossRef] [Google Scholar]
- A new phenanthraquinone from the aerial parts of Cremastra appendiculata. Chem. Nat. Comp.. 2020;56:785-787.
- [CrossRef] [Google Scholar]
- Chemical constituents of Cremastra appendiculata and their neuroprotection. Chin. Tradit. Herbal Drugs. 2016;47:3779-3786.
- [CrossRef] [Google Scholar]
- A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin. Tradit. Herbal Drugs. 2016;47:1443-1457.
- [CrossRef] [Google Scholar]
- Chemical constituents from the tubers of Cremastra appendiculata. Acta. Pharm. Sin.. 2008;43:181-184.
- [CrossRef] [Google Scholar]
- Three new phenanthrenes from Cremastra appendiculata (D. Don) Makino. Chin. Chem. Lett.. 2013;24:737-739.
- [CrossRef] [Google Scholar]
- Five new benzylphenanthrenes from Cremastra appendiculata. Fitoterapia. 2015;103:27-32.
- [CrossRef] [Google Scholar]
- Five new biphenanthrenes from Cremastra appendiculata. Molecules. 2016;21:1089-1098.
- [CrossRef] [Google Scholar]
- Chemical constituents from tubers of Cremastra appendiculata. China J. Chin. Mater. Med.. 2014;39:250-253.
- [CrossRef] [Google Scholar]
- New biphenanthrenes with butyrylcholinesterase inhibitory activitiy from Cremastra appendiculata. Nat. Prod. Res.. 2021;35:750-756.
- [CrossRef] [Google Scholar]
- Materia medica study and modern research progress of Shancigu. China Pharmacy. 2020;31:3055-3059.
- [CrossRef] [Google Scholar]
- A new phenanthro [2,3-b] furan from Pleione bulbocodioides. Chin. Chem. Lett.. 2007;18:1089-1091.
- [CrossRef] [Google Scholar]
- Two new α, β-unsaturated butyrolactone derivatives from Pleione bulbocodioides. Chin. Chem. Lett.. 2007;18:1075-1077.
- [CrossRef] [Google Scholar]
- Two new phenanthrene glucosides from Cremastra appendiculata. Chem. Nat. Comp.. 2016;52:23-25.
- [CrossRef] [Google Scholar]
- Chemical constituents from tubers of Pleione bulbocodioides. Chin. J. Hosp. Pharm.. 2011;31:1649-1650.
- [Google Scholar]
- A new bibenzyl derivative from Pleione bulbocodioides. Chin. Chem. Lett.. 2008;19:559-561.
- [CrossRef] [Google Scholar]
- Two new stilbenoids from Pleione bulbocodioides. J. Asian Nat. Prod. Res.. 2009;11:116-121.
- [CrossRef] [Google Scholar]
- Chemical constituents of Pleione bulbocodioides. J. S. Cent. Univ. Natl. (Nat. Sci. Ed.). 2011;30:54-56.
- [Google Scholar]
- Inhibitory effect and mechanism of Shancigu water extracts on mice 4T1 cell. Hubei Agr. Sci.. 2016;55:134-137.
- [CrossRef] [Google Scholar]
- Rare and precious Chinese materia medica: Pseudobulbus Cremastrae seu Pleiones. Chin. Med. Culture. 2021;4:211-220.
- [CrossRef] [Google Scholar]
- “Shanghuo” increases disease susceptibility: modern significance of an old TCM theory. J. Ethnopharmacol.. 2020;250:112491.
- [CrossRef] [Google Scholar]
- Separation of chemical constituents from Cremastra appendiculata. J. Hebei Univ. (Nat. Sci. Ed.). 2011;31:393-396.
- [CrossRef] [Google Scholar]
- Characterization of bibenzyl synthase catalysing the biosynthesis of phytoalexins of orchids. Phytochemistry. 1993;35:63-66.
- [CrossRef] [Google Scholar]
- Gymnadenia conopsea (L.) R. Br.: A systemic review of the ethnobotany, phytochemistry, and pharmacology of an important Asian folk medicine. Front. Pharmacol.. 2017;8:24.
- [CrossRef] [Google Scholar]
- Two new phenanthrenequinones with cytotoxic activity from the tubers of Pleione bulbocodioides. Phytochem. Lett.. 2020;35:6-9.
- [CrossRef] [Google Scholar]
- Phenanthrenequinone enantiomers with cytotoxic activities from the tubers of Pleione bulbocodioides. Org. Biomol. Chem.. 2019;17:567-572.
- [CrossRef] [Google Scholar]
- Advances in clinical application research of Cremastra appendiculata. J. Practi. Tradit. Chin. Intern. Med.. 2008;022:3-4.
- [Google Scholar]
- Anti-angiogenic activity of a homoisoflavanone from Cremastra appendiculata. Planta Med.. 2004;70:171-173.
- [CrossRef] [Google Scholar]
- Research progress on chemical constituents and clinical application of Pseudobulbus Cremastrae seu Pleiones. J. Liaoning Univ. TCM.. 2020;22:157-161.
- [CrossRef] [Google Scholar]
- Phenanthrene, 9,10-dihydrophenanthrene and bibenzyl enantiomers from Bletilla striata with their antineuroinflammatory and cytotoxic activities. Phytochemistry. 2021;182:112609.
- [CrossRef] [Google Scholar]
- Anticholinesterase, antioxidant, and beta-amyloid aggregation inhibitory constituents from Cremastra appendiculata. Med. Chem. Res.. 2018;27:857-863.
- [CrossRef] [Google Scholar]
- Chemical constituents from Pleione bulbocodioides. China J. Chin. Mater. Med.. 2014;39:442-447.
- [CrossRef] [Google Scholar]
- Atropisomeric bi(9,10-dihydro)phenanthrene and phenanthrene/bibenzyl dimers with cytotoxic activity from the pseudobulbs of Pleione bulbocodioides. Fitoterapia. 2019;138:104313.
- [CrossRef] [Google Scholar]
- Chemical constituents from Pleione yunnanensis. China J. Chin. Mater. Med.. 2014;39:851-856.
- [CrossRef] [Google Scholar]
- Elution-extrusion counter-current chromatography separation of two new benzyl ester glucosides and three other high-polarity compounds from the tubers of Pleione bulbocodioides. Phytochem. Anal.. 2013;24:671-676.
- [CrossRef] [Google Scholar]
- Phenanthrenes, 9,10-dihydrophenanthrenes, bibenzyls with their derivatives, and malate or tartrate benzyl ester glucosides from tubers of Cremastra appendiculata. Phytochemistry. 2013;94:268-276.
- [CrossRef] [Google Scholar]
- Wu, J.L., 2014. The Influence of Cremastra appendiculata to the Thyroid Cancer Cell Line In Vitro and the Expression Level of the Gene NIS. Master Degree, The Master Academic Dissertation of Guangxi Medical University.
- Treatment of 36 cases of dysphagia of esophagus cancer by “Qige Tang”. Fujian J. Tradit. Chin. Med.. 1993;24 32-32
- [Google Scholar]
- Chemical constituents and biological activity profiles on Pleione (Orchidaceae) Molecules. 2019;24:3195-3220.
- [CrossRef] [Google Scholar]
- Chemical constituents from tuber of Cremastra appendiculata. China J. Chin. Mater. Med.. 2005;30:1827-1830.
- [CrossRef] [Google Scholar]
- Clinical effect of “Shancigu” of adjuvant treatment in advanced non-small cell lung cancer. Chron. Pathemat. J.. 2021;22 142–143+146
- [Google Scholar]
- Cremastra appendiculata makino affects proliferation and apoptosis of breast cancer MDA-MB-231 cells via PI3K/Akt signaling pathway. Chin. J. Immunol.. 2020;36 59–64+72. https://doi.org/CNKI:SUN:ZMXZ.0.2020-06-011
- [Google Scholar]
- Chemical constituents, pharmacologic properties, and clinical applications of Bletilla striata. Front. Pharmacol.. 2019;10:1168.
- [CrossRef] [Google Scholar]
- Bioactive chemical constituents of Pleione yunnanensis. Chem. Nat. Comp.. 2020;56:518-520.
- [CrossRef] [Google Scholar]
- Xu, X.J., 2015. Study on the Anti-tumor Effect of Pleione yunnanensis Rolfe Polysaccharides and Achyranthes Bidentata polysaccharides on the Mice Hepatocellular Carcinoma and its Mechanisms, The Master Academic Dissertation of Hunan Agricultural University.
- Study the effect of Pleione yunnanensis Rolfe polysaccharides on IL-2 and p53 protein expression. Food Res. Dev.. 2016;37:6-10.
- [Google Scholar]
- Studies on chemical constituents from the corm of Cremastra appendiculata. China J. Chin. Mater. Med.. 2005;30:511-513.
- [CrossRef] [Google Scholar]
- Mono-, bi-, and triphenanthrenes from the tubers of Cremastra appendiculata. J. Nat. Prod.. 2006;69:907-913.
- [CrossRef] [Google Scholar]
- Effect of arrowhead extract on the expressions of vascular endothelial growth factor and matrix metalloproteinase-9 in tumor tissues of breast cancer rats. Chin. J. Clin. Pharmacol.. 2018;34:838-840.
- [CrossRef] [Google Scholar]
- Stereochemistry of atropisomeric 9,10-dihydrophenanthrene dimers from Pholidota chinensis. Tetrahedron Asymmetry. 2008;19:2007-2014.
- [CrossRef] [Google Scholar]
- Effects of Cremastra appendiculata extract on the apoptosis of human colon cancer HT29 cells. Chin. J. Ethnomed. Ethnopharm.. 2016;25:17-19. https://doi.org/CNKI:SUN:MZMJ.0.2016-16-007
- [Google Scholar]
- Effect of Cremastra appendiculata makino on proliferation and apoptosis of thyroid cancer SW579 cells. Oncol. Prog.. 2018;16(1292–1294):1298.
- [CrossRef] [Google Scholar]
- Chemical constituents from Pleione bulbocodioides. J. Chin. Med. Mat.. 2012;35:1602-1604.
- [CrossRef] [Google Scholar]
- Chemical constituents from Cremastra appendiculata. J. Chin. Med. Mat.. 2015;38:298-301.
- [CrossRef] [Google Scholar]
- Chemical constituents from Cremastra appendiculata. Mod. Chin. Med.. 2017;19:639-642.
- [CrossRef] [Google Scholar]
- Chemical constituents from Pleione bulbocodioides. Chin. Tradit. Herbal Drugs. 2013;44:1529-1533.
- [CrossRef] [Google Scholar]
- Exploring the mechanism of Cremastra Appendiculata (SUANPANQI) against breast cancer by network pharmacology and molecular docking. Comput. Biol. Chem.. 2020;107396
- [CrossRef] [Google Scholar]
- Phytochemistry and pharmacological activities of Arundina graminifolia (D.Don) Hochr. and other common Orchidaceae medicinal plants. J. Ethnopharmacol.. 2021;276:114143.
- [CrossRef] [Google Scholar]
- Study on the chemical constituents from the ethyl acetate extracts of Cremastra appendiculata. J. Chin. Med. Mat.. 2011;34:1882-1883.
- [CrossRef] [Google Scholar]
- Study on the relationship between “Jun” and “Chen” of “XIAOAIJIEDU” decoction and the treatment of liver cancer based on network pharmacology. Modern Tradit. Chin. Med. Mat-World Sci. Techn.. 2019;21:2356-2366.
- [CrossRef] [Google Scholar]







