Translate this page into:
Piperonal protects neuron-like and retinalpigment epithelial (RPE) cells from oxidative stress and apoptosis through inhibition of α-synuclein aggregation
⁎Corresponding author. peizengyangcmu@126.com (Peizeng Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Protein fibrillation is a crucial process in the onset of several neurodegenerative and retinal disorders due to the formation of cytotoxic species. Because of their capacity to prevent protein aggregation, small molecules have the potential to be appointed as therapeutic agents. Here, we examined the inhibitory impacts of the piperonal as a carbaldehyde-derived compound on the fibrillation process of α-synuclein and underlying cytotoxicity against neuron-like (PC12) and human retinal pigment epithelial (RPE) (ARPE-19) cells. The results showed that the values of kapp and lag time of α-synuclein were modulated with piperonal. Moreover, ANS fluorescence intensity analysis indicated that piperonal can inhibit the formation of a molten global (misfolded) state of α-synuclein, which is a necessary step in the formation of protein amyloid fibrils. Congo red absorption and circular dichroism spectroscopy also verified the inhibition of β-sheet structure formation after treatment of α-synuclein with piperonal. Furthermore, theoretical studies displayed that piperonal interacts with VAL40:HN, GLU35:O, VAL40, and LYS43 amino acid residues and forms a complex. In addition, cytotoxicity assays demonstrated that piperonal as a safe small molecule could mitigate the induced cytotoxicity by α-synuclein amyloids in PC12 and ARPE-19 cells through reduction of ROS and Bax/Bcl2 mRNA overexpression. Taken together, these outcomes showed that piperonal as a natural aldehyde compound can inhibit α-synuclein fibrillation and underlying cytotoxicity which may be developed for potential therapeutic applications in vivo.
Keywords
α-Synuclein
Protein amyloid fibrils
Piperonal
Toxicity
Parkinson
Retinal
Data availability
Data will be made available on request.
1 Introduction
Protein aggregation has been shown to be associated with the progression of neurodegenerative disorders of the brain and retina (Gupta, Gupta et al. 2016). Indeed, ocular neurodegenerative disorders have been reported as an interconnection between the retina and cortical areas (Marchesi, Fahmideh et al. 2021). α-Synuclein is known as a protein with 140-amino acid residues and minor ordered conformer that show the ability to bind to lipid biomembranes to adopt an α-helical structure (Beyer 2007, Musteikytė, Jayaram et al. 2021). This protein has seven defined repeats, in the NH3-terminal region (positive charge) and the middle region (hydrophobic), with the COOH-terminal region (negative charge) (Doherty, Ulamec et al. 2020). Recent reports of the biochemical functions of α-synuclein showed that this protein is involved in synaptic vesicle transport, and overexpression of α-synuclein because of genetic mutations or low clearance may participate in the onset of Parkinson's disease (PD) and associated disorders (Bellucci, Zaltieri et al. 2012). Multiple structural species of α-synuclein, such as oligomers, protofibrils and fibrils, have a key role in α-synuclein-related cytotoxicity (Bennett 2005). In fact, it has been indicated that the propagation and transmission of α-synuclein play the main role in the pathogenesis of neurodegenerative disorders, especially PD (Hansen and Li 2012, Karpowicz Jr, Trojanowski et al. 2019). Also, retinal α-synuclein accumulation in PD patients and animal models has been reported as a non-motor symptom that lead to visual disturbances and retinal abnormalities (Veys, Vandenabeele et al. 2019). A growing body of clinical research has explored the application of in vivo evaluations of retinal structure and vision-driven tasks as novel approaches for recognizing disease progression in PD patients (Veys, Vandenabeele et al. 2019, Di Pippo, Fragiotta et al. 2023, Camacho-Ordonez, Cervantes-Arriaga et al. 2024). Indeed, oxidative stress derived from α-synuclein deposits may play a key role in the pathogenesis of human retinal diseases. Therefore, any agent that inhibit the induction of α-synuclein aggregation can be nominated as a potent compound against the onset of PD and retinal abnormalities.
Small molecules derived from a variety of biological sources have been introduced as promising compounds against the formation of protein amyloid fibrils (Doig and Derreumaux 2015, Peña-Díaz, García-Pardo et al. 2023, Siwecka, Saramowicz et al. 2023). It has been shown that natural aldehydes can be used as potential small molecules against α‐synuclein aggregation and cytotoxicity (Aldini, Orioli et al. 2011, Morshedi, Aliakbari et al. 2015, Kumar, Hsu et al. 2019). It was reported that aldehyde-based small molecule can inhibit protein aggregation into β-structural aggregates, which could be attained by the Schiff base reaction with amine moieties of protein (Morshedi, Aliakbari et al. 2015). Piperonal (C8H6O3), 2H-1,3-Benzodioxole-5-carbaldehyde, as an organic small molecule is commonly found in Piper nigrum which belongs to the plant family Piperaceae (Jin, Ro et al. 2022). Piperonal-based derivatives have shown promising biological activities (Akash, Dushyant et al. 2020, Haq, Imran et al. 2021). Although, de Oliveira et al. showed that piperonal-based hybrids have potential anti-neurodegenerative properties (de Oliveira C. Brum, Neto et al. 2019). the protective effects of this aromatic aldehyde-based small molecule against neurodegenerative diseases and retinal abnormalities remains largely unknown. Therefore, the present paper tried to evaluate the potential protective effects of piperonal against α-synuclein aggregation and its underlying cytotoxicity in neuron-like cells and human retinal pigment epithelial (RPE) cells. To inhibit visual defects that appeared in PD patients, a comprehensive study was done to provide evidence for the protective effects of piperonal on the formation of α-synuclein aggregates and associated cytotoxicity.
2 Materials and methods
2.1 Materials
Thioflavin T (ThT), 8-Anilino-1-naphthalenesulfonic acid (ANS), Congo red, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), and piperonal [Synonym(e): 3,4-Methylendioxy-benzaldehyd, Heliotropin, Empirische Formel (Hill-System): C8H6O3] were purchased from Sigma-Aldrich (Shanghai, China). Fetal bovine serum (FBS), RPMI-1640 medium, trypsin, and penicillin/streptomycin were obtained from Gibco Co. (Gibco™, Thermo Fisher Scientific). All other materials were of analytical grades and used without further purification.
2.2 Aggregation assay
Expression and purification of human α-synuclein was done based on the previous study (Ji, Zhao et al. 2016). The induction of α-synuclein amyloid fibrils was then performed based on the previous study (Semenyuk, Kurochkina et al. 2020). Briefly, the α-synuclein samples incubated with different concentrations (0.5 and 50 µM) of piperonal were exposed to intense shaking in aggregation buffer “(10 mM potassium phosphate buffer, pH 4.0, having 137 mM sodium chloride and 2.7 mM potassium chloride) at 37 °C”.
2.3 ThT assay
5 μl of protein samples was mixed with 495 μl of ThT solution (20 μm). Fluorescence intensity was then read using a Hitachi FL-2700 fluorometer (Tokyo, Japan) with 448-nm excitation wavelength and 484-nm emission wavelength. Slit widths were set at 5 nm and the experiment was done at room temperature. The ThT fluorescence intensity of α-synuclein samples was corrected against buffer and piperonal emission intensity.
2.4 ANS binding assay
The α-synuclein samples co-incubated with or without different concentrations (0.5 and 50 µM) of piperonal were added by ANS (30 µM) and incubated for 30 min. Afterwards, the fluorescence intensity of the sample was recorded in the emission spectra of 400–600 nm upon excitation at 380 nm. The experiment was done at room temperature and the ANS fluorescence intensity of protein samples were corrected against control samples.
2.5 Congo red absorption assay
The Congo red absorption assay was carried out using UV–vis spectrophotometer (Shimadzu, Japan) with a scanning range of 440–650 nm. The concentration of protein and Congo red were set at 3 and 6 μM, respectively. All samples were incubated for 30 min at room temperature in the dark before the UV–vis experiment.
2.6 Far-UV circular dichroism spectroscopy
Structural changes in protein samples (3 µM) were assessed with a circular dichroism spectrometer (Jasco J-810) over a spectral range of 260–190 nm at room temperature, with a 1 nm bandwidth, and a scanning speed of 50 nm/min. The ellipticity of the both buffer and small molecule alone was also measured and corrected against the protein spectrum.
2.7 Molecular docking
Molecular docking analysis was carried out using the AutoDock Vina software package. The RCSB PDB code 1XQ8 was used for α-synuclein structure and the 3D structure of the piperonal compound was downloaded directly from the PubChem database. The docking box was centered on the α-synuclein structure to cover the entire α-synuclein. The grid box size was set to X=66, Y=66, Z=66 and center set to X=237.746, Y=63.01, Z= − 15.164.The preparation and analysis of the molecular docking study was performed based on the previous report (Jayaraj and Elangovan 2014).
2.8 Cell culture
The neuron-like cells (PC12) and human RPE cells (ARPE-19) obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in RPMI-1640 medium supplemented with 10 % FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated in a humidified incubator at 37 °C and 5 % CO2.
2.9 MTT assay
First, the cells were seeded into 96-well plates (5000 cells/ well) with fresh medium and incubated for 24 h. The cell culture media were then replaced with fresh media containing protein samples incubated without or with piperonal for 10 h. The plates were then incubated for another 24 h followed by determining the cell viability using the MTT assay based on a previous study (Jia, Wang et al. 2019). The absorbance of samples was read using a microplate reader (Biotek, VT, USA) at 570 nm.
2.10 ROS determination
The cells seeded in 96-well black-wall plates (2 × 104 cells per well) for 24 h were treated for a further 24 h with protein samples incubated without or with piperonal for 10 h. Then, ROS levels in the cells were measured using H2DCFDA (5 μM, 40 min, 37 ˚C). The fluorescence of cells was read using a Multimode plate reader at Ex/Em wavelengths of 485 nm/535 nm.
2.11 Real-time PCR assay
Following incubation, the cells were harvested and total RNA was extracted employing the TRizol reagent (Invitrogen, US). cDNA was then synthesized based on the manufacturer's protocols of the PrimeScript® RT reagent kit (Takara Bio Inc.), which was used as a template for the expression assay with SYBR®-Green PCR Master mix (Takara Bio Inc.). The real-time PCR procedure and primers were done based on the previous studies (Liu, Lao et al. 2015, Chen, Wang et al. 2017). The relative gene expression fold was expressed according to the 2−ΔΔCT formula in which data was normalized to β-actin expression in cell samples.
2.12 Statistical analysis
Some experiments were done in triplicate and the statistical analysis was carried out to determine standard deviations using SPSS software and the P-value was determined using student t-test.
3 Results
3.1 ThT analysis
Inhibition of α-synuclein aggregation by piperonal was assessed using a well-known ThT fluorescence assay. It is well-documented that although ThT has a negligible fluorescence intensity in the unbounded state, its fluorescence intensity increases remarkably after interaction with the β-sheet structure of amyloid fibrils (Minakawa and Nagai 2021). Therefore, fluorescence intensity of ThT remained almost unchanged when interacted with native α-synuclein, however, an apparent enhancement in the fluorescence intensity of ThT was detected following its interaction with α-synuclein in aggregation buffer, confirming the induction of amyloid fibrils of α-synuclein (Wördehoff and Hoyer 2018, Galkin, Priss et al. 2024). α-Synuclein was also treated with different concentrations (0.5 and 50 µM) of piperonal in aggregation buffer. The ThT emission intensity was decreased upon increasing the concentration of piperonal (Fig. 1a). The percentage prevention of α-synuclein aggregation by different concentrations of piperonal is tabulated in Table 1, which reveals the inhibitory effect of this small molecule against the protein misfolding.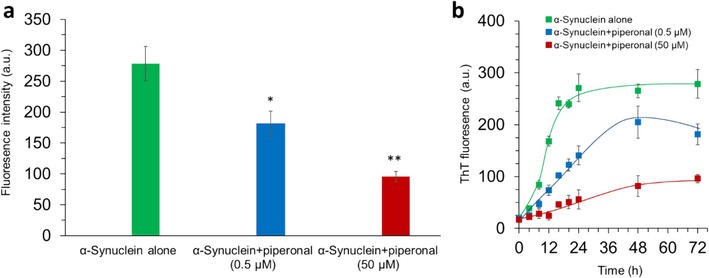
(a) ThT fluorescence assay of α-synuclein samples incubated with different concentrations (0.5 and 50 µM) of piperonal for 72 h. (b) Kinetic study of α-synuclein aggregation incubated with different concentrations (0.5 and 50 µM) of piperonal assessed by ThT fluorescence study. *P<0.05 and **P<0.01 relative to control (α-synuclein samples incubated alone).
[Piperonal] (µM)
Inhibition (%)
kapp (h−1)
Lag time (h)
0
−
0.29 ± 0.05
2.93 ± 0.07
0.5
65.07
0.18 ± 0.03
3.19 ± 0.13
50
34.40
0.04 ± 0.01
12.47 ± 0.33
Furthermore, the kinetics of protein fibrillation was analyzed with and without piperonal derived from ThT emission intensity over a time period (Fig. 1b), where the obtained data were fitted to the equation reported by Lee, Nayak et al. (Lee, Nayak et al. 2007).
Therefore, the apparent rate constant (kapp) and the lag time of fibril formation were determined.
Then, the values of kapp determined based on Fig. 1b for the amyloid fibril formation of α-synuclein with different concentrations (0, 0.5 and 50 µM) of piperonal were 0.29 ± 0.05, 0.18 ± 0.03, and 0.04 ± 0.01 h−1, respectively (Table 1). Moreover, the lag time of protein fibrillation for α-synuclein, α-synuclein-piperonal (0.5 µM) and α-synuclein-piperonal (50 µM) were 2.93 ± 0.07, 3.19 ± 0.13 and 12.47 ± 0.33 h respectively (Table 1). The kinetic parameters of α-synuclein fibrillation calculated from this study were not comparable to the reported value of other studies (Zand, Khaki et al. 2019, Atarod, Mamashli et al. 2022). This difference might be derived from the variation in the experimental setup used for protein fibrillation. In general, from the calculated kinetic parameters, it can be inferred that piperonal not only potentially delayed the lag period, but also regulated the ‘kapp’ of α-synuclein aggregation process, suggesting that this small molecule can prevent fibrillation of the protein (Save, Rachineni et al. 2019, Mehrabi, Bijari et al. 2023).
3.2 Congo red adsorption assay
It is important to study the inhibitory effects of small molecules against protein aggregation by some other probes besides ThT fluorescence intensity. Indeed, inhibition outcomes derived from ThT emission assay may be biased upon exploring the anti-aggregation role of small molecules (Sharma, Kesamsetty et al. 2023). In this case, Congo red absorption assay has been widely employed to analyze the anti-amyloidogenic properties of some small molecules (Marchiani, Mammi et al. 2013, Zhang, Zhang et al. 2024). Therefore, in addition to ThT fluorescence study, we used Congo red absorption assay to investigate the inhibition efficacy of piperonal against α-synuclein fibrillation. We tried to verify the inhibition of α-synuclein misfolding at two different concentrations of piperonal. It was found that Congo red with an absorption maximum around 490 nm showed a significant increase in the absorbance and a red-shift to 524 nm following the addition of α-synuclein fibrils (Fig. 2a). However, when the α-synuclein samples co-incubated with piperonal, the Congo red absorbance and underlying red-shift were reduced.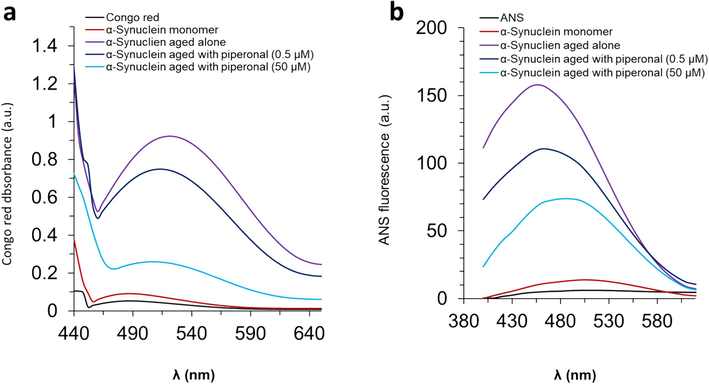
(a) Congo red absorption assay of α-synuclein samples incubated with different concentrations (0.5 and 50 µM) of piperonal for 72 h. (b) ANS emission intensity assay of α-synuclein samples incubated with different concentrations (0.5 and 50 µM) of piperonal for 72 h.
3.3 ANS fluorescence intensity assay
ANS binding assay is widely used to analyze the exposure of hydrophobic portions on the surface of the protein derived from protein misfolding and fibrillation (Ikenoue, Oono et al. 2023, Zhang, Zhang et al. 2024). Upon binding of ANS with hydrophobic surfaces, the emission intensity of this probe is usually enhanced accompanied by a blue-shift (Zhang, Zhang et al. 2024). In Fig. 2b, the emission intensity of ANS was realized to be unchanged upon the interaction of α-synuclein monomer with ANS. However, a remarkable enhancement in the emission intensity along with a blue-shift was detected when α-synuclein amyloid fibrils interacted with ANS. This data confirms the formation/exposure of hydrophobic portions on the α-synuclein surface during misfolding, which are a deriving factor for the induction of protein fibrils (Ikenoue, Oono et al. 2023). When α-synuclein samples co-incubated with piperonal, the emission intensity was detected to be much less than that measured for α-synuclein fibrils incubated alone. This data supports that piperonal could minimize the formation/exposure of the hydrophobic surface of the α-synuclein, causing inhibition in protein aggregation.
3.4 Secondary structure study
Changes in the secondary structure of α-synuclein derived from protein fibrillation with and without piperonal were measured using far-UV circular dichroism spectroscopy. Fig. 3 displayed the spectrum of native α-synuclein with a minimum of 195 nm, indicating the predominance of random coil structure in the α-synuclein (Zhang, Zhang et al. 2024). It was shown that the direct interaction of piperonal with α-synuclein resulted in the formation of a more unfolded structure of protein. Furthermore, it was found that the incubation of α-synuclein under aggregation conditions resulted in a notable reduction in random coil signal and appearance of a minimum around 217 nm, corresponding to the formation of β-sheet structure in protein. However, when α-synuclein was co-incubated with piperonal, secondary structural perturbation of protein was reduced. Based on the previous studies (Srivastava, Tyagi et al. 2024, Zhang, Zhang et al. 2024), this outcome may indicate that piperonal can prevent fibrillation of α-synuclein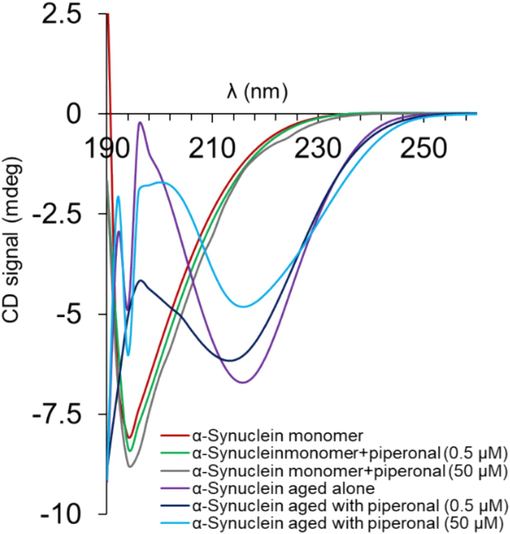
Circular dichroism spectroscopy study of α-synuclein monomers and α-synuclein samples incubated alone or with different concentrations (0.5 and 50 µM) of piperonal for 72 h.
3.5 Molecular docking results
The molecular docking simulations could be used in the initial processes of bioactive molecular development through the prediction and analysis of biomolecular complex formation.
According to molecular docking results, the spontaneous interaction between protein and piperonal was assessed by the negative binding energy value of −4.0 kcal/mol. Fig. 4 and Table 2 presents the best conformation of the piperonal in the binding site of protein. According to the docking results, piperonal formed two hydrogen bonds with the VAL40:HN and GLU35:O amino acids. Furthermore, two hydrophobic interactions with VAL40 and LYS43 amino acid residues are responsible for the formation of the piperonal- α-synuclein complex. It can be concluded that based on the docking data, both hydrogen bonds and hydrophobic interactions contribute to the binding of piperonal with protein.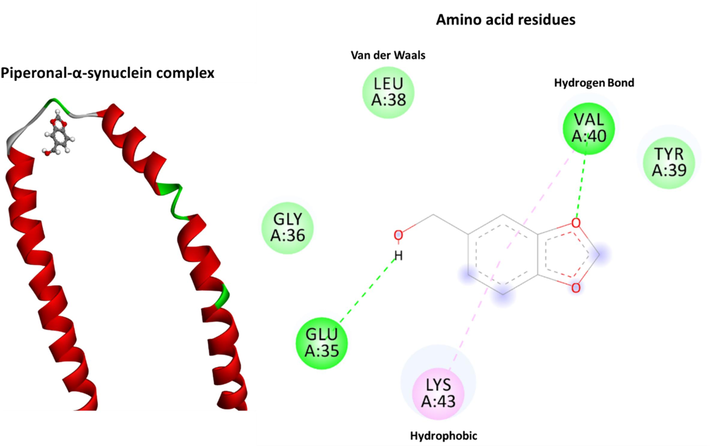
The docking conformation of the piperonal-α-synuclein system analyzed by AutoDock. Molecular interactions between the piperonal and residues of the protein are provided with the Discovery Studio.
Interactions
Distance
Category
Type
Piperonal:O1- A:VAL40:HN
2.07
Hydrogen Bond
Conventional Hydrogen Bond
Piperonal:H6 − A:GLU35:O
2.91
Hydrogen Bond
Conventional Hydrogen Bond
Piperonal − A:VAL40
5.29
Hydrophobic
Pi-Alkyl
Piperonal − A:LYS43
4.26
Hydrophobic
Pi-Alkyl
This predicted data advances our understanding about the structural basis of inhibitory effects of piperonal on α-synuclein aggregation.
3.6 Cytotoxicity assays
To assess the cytotoxicity of α-synuclein aggregated species and the formation of less toxic protein aggregates in the presence of piperonal, cellular and molecular assays were performed. The cells incubated with different α-synuclein species for 24 h were assessed by MTT and real-time PCR assays (Figs. 5, 6). It was found that the presence of α-synuclein aggregates incubated alone for 10 h (corresponding to the formation of neurotoxic oligomers) induced a significant cytotoxic effect on PC12 cells (Fig. 5a) mediated by high generation of ROS (Fig. 5b) overexpression of Bax mRNA (Fig. 5c), and downregulation of Bcl-2 mRNA (Fig. 5d). However, it was found that the cytotoxicity and induction of apoptosis were mitigated when the PC12 cells were exposed to α-synuclein aggregates incubated with piperonal, concentration-dependently, indicating the formation of less toxic α-synuclein aggregated species in the presence of piperonal.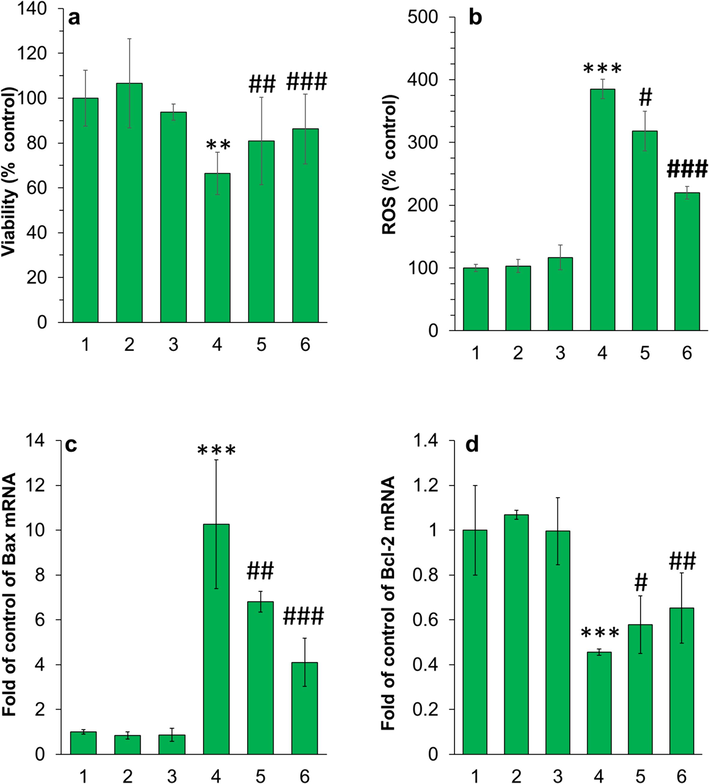
(a) MTT assay, (b) ROS assay, (c, d) real-time PCR for evaluation of the cytotoxic effect of different species on PC12 cells. The cells were incubated with different samples for 24 h. For both protein and piperonal samples, the final concentration was diluted at 5 µM. The protein samples were withdrawn after 10 h (corresponding to the formation of neurotoxic oligomers) and used for cellular assays. The data were reported as mean ± SD of three experiments. *P<0.05, **P<0.01, ***P<0.001 relative to control (negative treated cells). #P<0.05, ##P<0.0, and ###P<0.001 relative to control (α-synuclein samples incubated alone). 1: control cells, 2: piperonal, 3: α-synuclein monomer, 4: α-synuclein samples incubated alone in aggregation condition, 5: α-synuclein samples incubated with piperonal (0.5 µM) in aggregation condition, 6: α-synuclein samples incubated with piperonal (50 µM) in aggregation condition.
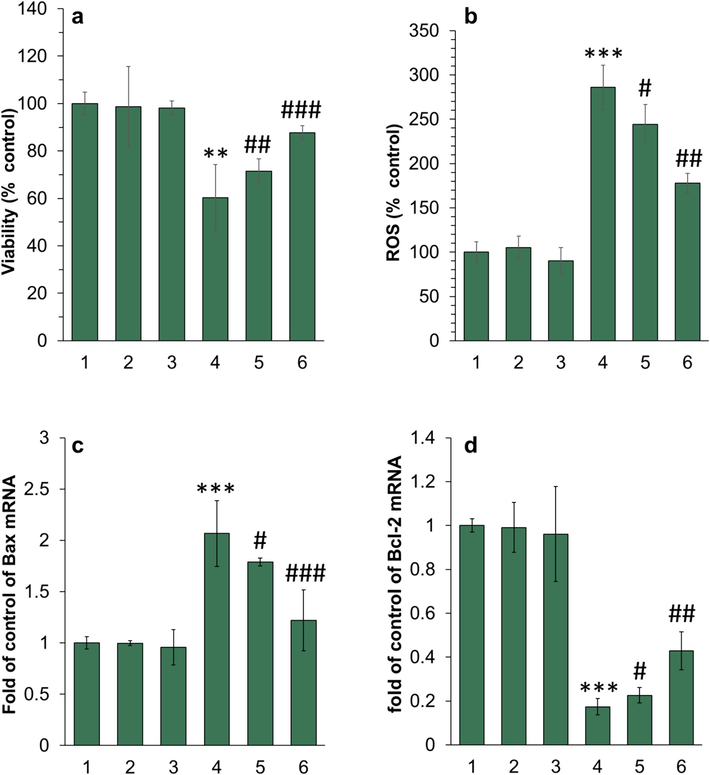
(a) MTT assay, (b) ROS assay, (c, d) real-time PCR for evaluation of the cytotoxic effect of different species on ARPE-19 cells. The cells were incubated with different samples for 24 h. For both protein and piperonal samples, the final concentration was diluted at 5 µM. The protein samples were withdrawn after 10 h (corresponding to the formation of neurotoxic oligomers) and used for cellular assays. The data were reported as mean ± SD of three experiments. **P<0.01, ***P<0.001 relative to control (negative treated cells). #P<0.05, ##P<0.0, and ###P<0.001 relative to control (α-synuclein samples incubated alone). 1: control cells, 2: piperonal, 3: α-synuclein monomer, 4: α-synuclein samples incubated alone in aggregation condition, 5: α-synuclein samples incubated with piperonal (0.5 µM) in aggregation condition, 6: α-synuclein samples incubated with piperonal (50 µM) in aggregation condition.
A similar trend was also observed upon incubation of ARPE-19 cells with α-synuclein aggregates incubated alone or with piperonal (Fig. 6 a-d).
4 Discussion
In recent years, the main mechanism of protein amyloid fibrils has remained unknown. It has been revealed that misfolding of proteins induces transmissible neural- and retinal-related disorders derived from the aggregated species which are insoluble in physiological media (Veys, Vandenabeele et al. 2019). Retina abnormalities could serve as a marker of the protein deposits-mediated pathological processes that are underway in the brain. Based on this fact, further investigations into protein aggregation inhibition are necessary in order to alleviate the PD pathophysiology in the retina. The inhibition of protein misfolding mediated by small molecules has been widely used to mitigate protein aggregation-linked cytotoxicity (Doig and Derreumaux 2015).
However, the specific stages of protein misfolding and aggregated species that are influenced by natural small molecule inhibitors, as well as the associated biochemical processes that mediate the amyloid-triggered cytotoxicity and retinal abnormalities are not yet fully explored. Therefore, several studies have aimed to disclose a detailed investigation of the related mechanisms that regulate these processes (Pena-Diaz, Pujols et al. 2020, Louros, Schymkowitz et al. 2023). In general, anti-aggregation small molecules can possibly exert their actions by inducing the formation of covalent bonds and/or a wider range of non-covalent forces, including π-π interactions, H-bonding, or electrostatic interactions with different amino acid residues of the protein that may play a key role in the regulation of one or all phases of the amyloid fibril processes (Velander, Wu et al. 2017, Pena-Diaz, Pujols et al. 2020). However, non-covalent interactions between inhibitors and proteins could not fully inhibit the formation of protein aggregation in all amyloid systems (Popovych, Brender et al. 2012, Velander, Wu et al. 2017). For that reason, several studies have reported that covalent binding might also occur between the thiols/amines as nucleophilic centers and the carbonyls in o-quinones and aldehydes as electrophilic moieties (Sato, Murakami et al. 2013, Velander, Wu et al. 2017, Cawood, Guthertz et al. 2020, Grcic, Leech et al. 2024). Covalent adduct formation remarkably influences the anti-aggregation impacts of baicalein (Zhu, Rajamani et al. 2004) and dopamine derivatives (Sivakumar, Nagashanmugam et al. 2023) on α-synuclein. Such a mechanism seems to be crucial to inhibit amyloid fibril formation of α‐synuclein by catechol derivatives (Inciardi, Rizzotto et al. 2024) and aldehydes (Ma, Yang et al. 2020), although it needs further investigation in the future studies.
The piperonal due to its unique structure probably binds with amine groups and hydrophobic regions of the partially misfolded α-synuclein through benzodioxole and carbaldehyde moieties, respectively and thereby inhibits the exposure of hydrophobic patches and corresponding formation of protein amyloid fibrils to some extent. A similar outcome was reported earlier as cuminaldehyde as a natural aldehyde of Cuminum cyminum can significantly inhibit α‐synuclein aggregation and associated cytotoxicity (Morshedi, Aliakbari et al. 2015).
In general, the molten globule of α-synuclein was analyzed through interaction with ANS. It was found that the emission intensity of ANS was enhanced apparently following the addition of this probe to α-synuclein amyloid fibrils, suggesting that the fibrillation process and induced cytotoxicity was mediated by the formation of protein molten globules (Fazili and Naeem 2013, Gadhe, Sakunthala et al. 2022, Mondal, Dolui et al. 2023). Upon the interaction of ANS with α-synuclein samples incubated in the presence of piperonal, the ANS emission intensity was lower than that read for α-synuclein incubated alone. This data suggested the inhibiting the formation of α-synuclein molten globule structure in the presence of piperonal and underlying cytotoxicity. Indeed, the hydrophobic patches formed during the protein fibrillation can induce cytotoxicity through overexpression of ROS-mediated apoptosis signaling pathways (Liu, Yu et al. 2011, Teng, Zhao et al. 2019, Khan and Khan 2022, He, Zhang et al. 2024). In this study, it was reported that although α-synuclein species incubated alone induced significant cytotoxicity against neuron-like and RPE cells through excessive generation of ROS, overexpression of apoptotic marker (Bax mRNA), and downregulation of antiapoptotic marker (Bax mRNA), the treatment of α-synuclein with piperonal could control these adverse effects which were in agreement with previous reports (Morshedi and Aliakbari 2012, Zohoorian-Abootorabi, Meratan et al. 2023, Cui, Guo et al. 2024).
5 Conclusion
In conclusion, we assessed the inhibitory effects of the piperonal as a benzodioxole-carbaldehyde compound on α-synuclein fibrillation and cytotoxicity against neuron-like and RPE cells by different spectroscopic, theoretical, cellular, and molecular assays. The outcomes displayed that the piperonal could interact with α-synuclein and affect the kinetic parameters of protein fibrillation, reduce the formation of molten globule and β-sheet structures. Also, it was found that piperonal mitigates the cytotoxicity of α-synuclein fibrils against neuron-like and RPE cells through regulation of ROS generation as well as Bax and Bcl-2 expression. In conclusion, this study revealed that piperonal as a natural small molecule with a unique aldehyde-based structure could show efficacy in inhibiting α-synuclein fibrillation which could be a benefit for the modulation of retinal α-synuclein deposits in PD patients. Therefore, further studies should be developed to examine the potential therapeutic application of piperonal in vivo.
Funding
This study received no external funding.
CRediT authorship contribution statement
Meiqi Wang: Study designing, Data curation, Routine analysis, Experiments execution, Writing. Tao Yang: Experiments execution. Weiying Chen: Experiments execution. Jian Bai: Investigation. Peizeng Yang: Conceptualization, Data curation, Formal analysis, Investigation.
Acknowledgments
We appreciate all lab members helped in the conceptualization and discussion of this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protein modification by acrolein: relevance to pathological conditions and inhibition by aldehyde sequestering agents. Mol. Nutr. Food Res.. 2011;55(9):1301-1319.
- [Google Scholar]
- Bivalent metal ions induce formation of α-synuclein fibril polymorphs with different cytotoxicities. Sci. Rep.. 2022;12(1):11898.
- [Google Scholar]
- From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson's disease. Brain Res.. 2012;1476:183-202.
- [Google Scholar]
- The role of α-synuclein in neurodegenerative diseases. Pharmacol. Ther.. 2005;105(3):311-331.
- [Google Scholar]
- Mechanistic aspects of Parkinson’s disease: α-synuclein and the biomembrane. Cell Biochem. Biophys.. 2007;47:285-299.
- [Google Scholar]
- Is there any correlation between alpha-synuclein levels in tears and retinal layer thickness in Parkinson's disease? Eur. J. Ophthalmol.. 2024;34(1):252-259.
- [Google Scholar]
- Modulation of amyloidogenic protein self-assembly using tethered small molecules. J. Am. Chem. Soc.. 2020;142(49):20845-20854.
- [Google Scholar]
- Paeoniflorin exerts neuroprotective effects against glutamate-induced PC12 cellular cytotoxicity by inhibiting apoptosis. Int. J. Mol. Med.. 2017;40(3):825-833.
- [Google Scholar]
- Brazilin-7-acetate, a novel potential drug of Parkinson's disease, hinders the formation of α-synuclein fibril, mitigates cytotoxicity, and decreases oxidative stress. Eur. J. Med. Chem.. 2024;264:115965
- [Google Scholar]
- Synthesis of new quinoline-piperonal hybrids as potential drugs against Alzheimer’s disease. Int. J. Mol. Sci.. 2019;20(16):3944.
- [Google Scholar]
- The role of alpha-synuclein deposits in Parkinson’s disease: a focus on the human retina. Int. J. Mol. Sci.. 2023;24(5):4391.
- [Google Scholar]
- A short motif in the N-terminal region of α-synuclein is critical for both aggregation and function. Nat. Struct. Mol. Biol.. 2020;27(3):249-259.
- [Google Scholar]
- Inhibition of protein aggregation and amyloid formation by small molecules. Curr. Opin. Struct. Biol.. 2015;30:50-56.
- [Google Scholar]
- In vitro hyperglycemic condition facilitated the aggregation of lysozyme via the passage through a molten globule state. Cell Biochem. Biophys.. 2013;66:265-275.
- [Google Scholar]
- Intermediates of α-synuclein aggregation: Implications in Parkinson's disease pathogenesis. Biophys. Chem.. 2022;281:106736
- [Google Scholar]
- Navigating α-Synuclein Aggregation Inhibition: Methods, Mechanisms, and Molecular Targets. Chem. Rec.. 2024;24(2):e202300282.
- [Google Scholar]
- Targeting misfolding and aggregation of the amyloid-β peptide and mutant p53 protein using multifunctional molecules. Chem. Commun.. 2024;60(11):1372-1388.
- [Google Scholar]
- One protein, multiple pathologies: multifaceted involvement of amyloid β in neurodegenerative disorders of the brain and retina. Cell. Mol. Life Sci.. 2016;73:4279-4297.
- [Google Scholar]
- Beyond α-synuclein transfer: pathology propagation in Parkinson's disease. Trends Mol. Med.. 2012;18(5):248-255.
- [Google Scholar]
- He, M., X. Zhang, X. Ran, Y. Zhang, X. Nie, B. Xiao, L. Lei, S. Zhai, J. Zhu and J. Zhang (2024). “Black phosphorus nanosheets protect neurons by degrading aggregative α‐syn and clearing ROS in parkinson's disease.” Adv. Mater. 2404576.
- A RaPID Macrocyclic Peptide That Inhibits the Formation of α-Synuclein Amyloid Fibrils. Chembiochem. 2023;24(12):e202300320.
- [Google Scholar]
- Catechol-induced covalent modifications modulate the aggregation tendency of α-synuclein: An in-solution and in-silico study. Biofactors 2024
- [Google Scholar]
- In silico identification of potent inhibitors of alpha-synuclein aggregation and its in vivo evaluation using MPTP induced Parkinson mice model. Biomedicine & Aging Pathology. 2014;4(2):147-152.
- [Google Scholar]
- Inhibition effects of tanshinone on the aggregation of α-synuclein. Food Funct.. 2016;7(1):409-416.
- [Google Scholar]
- Dihydromyricetin inhibits α-synuclein aggregation, disrupts preformed fibrils, and protects neuronal cells in culture against amyloid-induced cytotoxicity. J. Agric. Food Chem.. 2019;67(14):3946-3955.
- [Google Scholar]
- Piperonal synthase from black pepper (Piper nigrum) synthesizes a phenolic aroma compound, piperonal, as a CoA-independent catalysis. Applied Biological Chemistry. 2022;65(1):20.
- [Google Scholar]
- Transmission of α-synuclein seeds in neurodegenerative disease: recent developments. Lab. Invest.. 2019;99(7):971-981.
- [Google Scholar]
- Protein misfolding and related human diseases: A comprehensive review of toxicity, proteins involved, and current therapeutic strategies. Int. J. Biol. Macromol.. 2022;223:143-160.
- [Google Scholar]
- Aldehyde adducts inhibit 3, 4-dihydroxyphenylacetaldehyde-induced α-synuclein aggregation and toxicity: implication for Parkinson neuroprotective therapy. Eur. J. Pharmacol.. 2019;845:65-73.
- [Google Scholar]
- A three-stage kinetic model of amyloid fibrillation. Biophys. J .. 2007;92(10):3448-3458.
- [Google Scholar]
- Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol.. 2015;8(1):11.
- [Google Scholar]
- Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol. Res.. 2011;63(5):439-444.
- [Google Scholar]
- Mechanisms and pathology of protein misfolding and aggregation. Nat. Rev. Mol. Cell Biol.. 2023;24(12):912-933.
- [Google Scholar]
- Non-polyphenolic natural inhibitors of amyloid aggregation. Eur. J. Med. Chem.. 2020;192:112197
- [Google Scholar]
- Ocular neurodegenerative diseases: interconnection between retina and cortical areas. Cells. 2021;10(9):2394.
- [Google Scholar]
- Small molecules interacting with α-synuclein: Antiaggregating and cytoprotective properties. Amino Acids. 2013;45:327-338.
- [Google Scholar]
- Effective Reduction of Tau Amyloid Aggregates in the Presence of Cyclophilin from Platanus orientalis Pollens; An Alternative Mechanism of Action of the Allergen. Curr. Protein Pept. Sci.. 2023;24(6):518-532.
- [Google Scholar]
- Protein aggregation inhibitors as disease-modifying therapies for polyglutamine diseases. Front. Neurosci.. 2021;15:621996
- [Google Scholar]
- Structure specific neuro-toxicity of α-synuclein oligomer. Int. J. Biol. Macromol.. 2023;253:126683
- [Google Scholar]
- The inhibitory effects of cuminaldehyde on amyloid fibrillation and cytotoxicity of alpha-synuclein. Pathobiology Research. 2012;15(1):45-60.
- [Google Scholar]
- Cuminaldehyde as the major component of Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicity. J. Food Sci.. 2015;80(10):H2336-H2345.
- [Google Scholar]
- Interactions of α-synuclein oligomers with lipid membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2021;1863(4):183536
- [Google Scholar]
- Development of small molecules targeting α-synuclein aggregation: a promising strategy to treat Parkinson’s disease. Pharmaceutics. 2023;15(3):839.
- [Google Scholar]
- Small molecules to prevent the neurodegeneration caused by α-synuclein aggregation. Neural Regen. Res.. 2020;15(12):2260-2261.
- [Google Scholar]
- Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP (248–286) J. Phys. Chem. B. 2012;116(11):3650-3658.
- [Google Scholar]
- Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem.. 2013;288(32):23212-23224.
- [Google Scholar]
- Natural compound safranal driven inhibition and dis-aggregation of α-synuclein fibrils. Int. J. Biol. Macromol.. 2019;141:585-595.
- [Google Scholar]
- Alpha-synuclein amyloid aggregation is inhibited by sulfated aromatic polymers and pyridinium polycation. Polymers. 2020;12(3):517.
- [Google Scholar]
- Inhibition of lysozyme amyloid fibrillation by curcumin-conjugated silver nanoparticles: A multispectroscopic molecular level study. J. Mol. Liq.. 2023;372:121156
- [Google Scholar]
- Review on the interactions between dopamine metabolites and α-Synuclein in causing Parkinson's disease. Neurochem. Int.. 2023;162:105461
- [Google Scholar]
- Inhibition of protein aggregation and endoplasmic reticulum stress as a targeted therapy for α-synucleinopathy. Pharmaceutics. 2023;15(8):2051.
- [Google Scholar]
- A natural small molecule-mediated inhibition of alpha-synuclein aggregation leads to neuroprotection in Caenorhabditis elegans. J. Neurochem.. 2024;168(8):1640-1654.
- [Google Scholar]
- Complex of EGCG with Cu (II) suppresses amyloid aggregation and Cu (II)-induced cytotoxicity of α-synuclein. Molecules. 2019;24(16):2940.
- [Google Scholar]
- Retinal α-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol.. 2019;137:379-395.
- [Google Scholar]
- α-Synuclein aggregation monitored by thioflavin T fluorescence assay. Bio-Protocol. 2018;8(14):e2941-e.
- [Google Scholar]
- Zand, Z., P. A. Khaki, A. Salihi, M. Sharifi, N. M. Qadir Nanakali, A. A. Alasady, F. M. Aziz, K. Shahpasand, A. Hasan and M. Falahati (2019). “Cerium oxide NPs mitigate the amyloid formation of α-synuclein and associated cytotoxicity.” Int. J. Nanomed., 6989-7000.
- The mechanistic interaction, aggregation and neurotoxicity of α-synuclein after interaction with glycyrrhizic acid: Modulation of synucleinopathies. Int. J. Biol. Macromol.. 2024;267:131423
- [Google Scholar]
- The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J. Biol. Chem.. 2004;279(26):26846-26857.
- [Google Scholar]
- Modulation of cytotoxic amyloid fibrillation and mitochondrial damage of α-synuclein by catechols mediated conformational changes. Sci. Rep.. 2023;13(1):5275.
- [Google Scholar]







