Translate this page into:
Plasma protein affinity, antioxidant, and anti-lung cancer properties of O-methylated flavonol rhamnazin
⁎Corresponding author at: Huaian Hospital Affiliated to Xuzhou Medical University, 62 South Huaihai Road, Huaian 223001, China. zlq-hill@163.com (Liqing Zhou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Medicinal herbs can be used for the development of antioxidant and anticancer platforms. However, the mechanisms by which the bioactive materials show these properties remain largely unclear. Also, a detailed understanding of the interaction between small compounds and carrier proteins like albumin can provide useful information about the regulation of their stability and circulation time properties. In this paper, the interaction of rhamnazin (C17H14O7), an O-methylated flavonol exists in Rhamnus petiolaris, with human serum albumin (HSA) was investigated. Also, the antioxidant and anti-lung cancer effects of rhamnazin were assessed. The results showed that a static rhamnazin-HSA complex was formed by the contribution of hydrophobic forces, which led to an increase in the content of the α-helix structure of HSA. Cellular assays revealed that rhamnazin (10 µM) recovered cell viability in human proximal renal tubular epithelial cells (HK2) reduced by H2O2 (0.5 mM) through reduction of ROS, restoration of CAT and SOD activities and GSH content, regulation of Bax, Bcl-2, and caspase-3. Moreover, the anti-lung cancer data demonstrated that rhamnazin induced apoptosis on lung adenocarcinoma A549 cells associated with a p53-dependent manner via overexpression of apoptotic Bax and caspase-3 and down-regulation of anti-apoptotic Bcl-2. To summarize, the potential binding of rhamnazin and HSA, as well as its antioxidant/anticancer properties, are critical for understanding the solubility and stability of this bioactive compound in plasma, as well as its therapeutic implications.
Keywords
Rhamnazin
Human serum albumin
Interaction
Antioxidant
Anticancer
1 Introduction
Oxidative stress has been widely shown to be involved in the onset of several diseases including chronic diseases (Jomova, Raptova et al. 2023) and cancer (Iqbal, Kabeer et al. 2024). Oxidative stress driving from the dysregulation of endogenous oxidant signaling pathways results the in overproduction of reactive oxygen species reactive oxygen species (ROS) (Aguilar, Navarro et al. 2016). For example, it has been shown that kidney proximal tubular epithelial cells are highly sensitive to ROS-triggered adverse effects (Kishi, Nagasu et al. 2024). Chronic kidney disorder has been documented as a spreading disease worldwide, with ongoing conventional therapeutics platforms showing limited potential efficacy (Yuan, Tang et al. 2022). Some alternative approaches to promisingly inhibit the progression of kidney disorder may provide useful outcomes for patients suffering from this disease.
On the other hand, because of the wide range of signs and symptoms, non-small cell lung cancer (NSCLC) represents a heterogeneous disease that is typically detected at an advanced stage. Over the past decade, we have seen an increase in the overall number of patients suffering from NSCLC, which has driven researchers to devote themselves to the development of potential anticancer systems. In fact, NSCLC is known as the leading cause of cancer-related fatalities globally, and the frequency of newly diagnosed cases has increased in China in recent years (Chen, Liu et al. 2022).
Rhamnazin (C17H14O7) is known as an O-methylated flavonoid present in Rhamnus petiolaris, and has shown potential antioxidant activity (Patel, Kumar et al. 2018). For example, Wu et al. showed that rhamnazin provides antioxidant and anti-inflammatory impacts against lipopolysaccharide-triggered acute lung injury in vivo (Wu, Dai et al. 2017). Furthermore, Yang et al. demonstrated that rhamnazin reduces traumatic brain injury in vivo through the regulation of oxidative stress (Yang, Zhang et al. 2022). Moreover, rhamnazin mitigates lipopolysaccharide-stimulated inflammation and oxidative stress in raw macrophages (Kim 2016).
Regarding the anticancer properties of rhamnazin, Yu et al. reported that this small molecule can serve as a potential modulator of the VEGFR2 signaling pathway with promising antiangiogenic and antitumor efficiencies (Yu, Cai et al. 2015). Also, Mei et al. showed that rhamnazin inhibits hepatocellular carcinoma cell proliferation through glutathione peroxidase 4-dependent ferroptosis (Mei, Liu et al. 2022).
Small molecules are mostly transported in the body by human serum albumin (HSA) protein. HSA as a multifunctional protein has been reported to act as a versatile small molecule carrier for the regulation of several disorders (Chen and Liu 2016, Ghosh and Bhadra 2024). HSA with a flexible structure carries a number of compounds, such as bilirubin, fatty acids, amino acids, hormones, drugs, antibiotics, small compounds, and several metal cations (Quinlan, Martin et al. 2005, Kratz 2014). The interaction of small molecules with HSA not only regulates their pharmacokinetic properties but also the probable protein structural change can be one of the causes of their side effects (Yamasaki, Chuang et al. 2013, Tayyab and Feroz 2021). Therefore, the interaction of different small molecules with HSA has been widely reported in different studies (Siddiqui, Ameen et al. 2021). The secondary structure of HSA does not usually change, but its tertiary conformation, which includes the special arrangement of atoms in three-dimensional space, may undergo some significant changes upon interaction with ligands (Siddiqui, Ameen et al. 2021). In other words, HSA has a unique spatial conformation as a result of protein folding, which can play a key role in the physiological function of this protein (Ghosh and Bhadra 2024). Therefore, determining the thermodynamic and structural parameters of HSA upon interaction with small particles can provide useful information about the formation of ligand-HSA complexes and the underlying physiological properties of small molecules, which can be studied by different spectroscopic techniques (Zeinabad, Kachooei et al. 2016).
Therefore, the interaction of rhamnazin and HSA was studied by several spectroscopic techniques along with molecular docking study. Also, the protective effect of rhamnazin against cytotoxicity induced by H2O2 in HK2 cells was assessed by different cellular and molecular assays. Moreover, the anti-lung cancer properties of rhamnazin and the associated signaling pathway were assessed in vitro.
2 Materials and methods
2.1 Materials
Rhamnazin; 99 % (HPLC), HSA, Dulbecco's modified Eagle's medium (DMEM)/Ham's F12, fetal bovine serum (FBS), and tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (Saint Louis, MO, USA). Primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). FITC Annexin V Apoptosis Detection Kit I was obtained from BD Biosciences (USA).
2.2 Sample preparation
The stock solution (100 μM) of HSA was freshly prepared in phosphate buffer (100 mM, pH 7.4). UV–visible spectroscopy was used to determine the protein concentration using ε1cm% of 5.30 at 280 nm (Alam, Chaturvedi et al. 2015). Rhamnazin solution (4 mM) was prepared freshly in phosphate buffer (100 mM, pH 7.4) or cell culture medium with the addition of 1 % DMSO.
2.3 Intrinsic fluorescence quenching study
The intrinsic fluorescence quenching study was done using a fluorescence spectrophotometer (RF-5301 Shimadzu, Japan). The analysis was done at three different temperatures of 298/310/315 K with a quartz cell of 0.1 cm path length. Excitation and emission slits were set at 5 and 10 nm, respectively, where the samples were excited at 280 nm and emission signals were read in the range of 300–420 nm. The titration of rhamnazin (5–40 μM) to HSA (5 μM) solution was performed at three different temperatures. The maximum fluorescence intensity was used to calculate the quenching, binding and thermodynamic parameters. All spectra were corrected against off-signals and inner filter effect (Ali and Al-Lohedan 2018).
2.4 Circular dichroism study
The far-UV CD study (200–260 nm) was performed using a JASCO-J 813 spectropolarimeter at room temperature. The quartz cell with a path length of 0.1 cm was used to detect the ellipticity changes in the HSA solution (5 µM) upon interaction with increasing concentrations of rhamnazin (5, 10, 15, 20, 30, 40). The CD data read from 200 to 260 nm were analyzed using CDNN.
2.5 Molecular docking study
Vina Autodock was used to perform the docking study. The 3D structures of HSA (PDB ID: 1AO6) and rhamnazin (PubChem CID: 5320945) were obtained from Protein Data Bank and PubChem, respectively. Water molecules as well as ions were removed, and hydrogen atoms and partial Kollman charges were added/assigned to rigid HSA. The grid size was set at 51 Å, 108 Å, 90 Å along X, Y, and Z axes, respectively. Spacing, mode number, energy range and exhaustiveness were fixed at 1, 10, 5 and 8, respectively.
2.6 Cell culture
Human proximal renal tubular epithelial cells (HK2, purchased from Procell Life Science and Technology Co., Wuhan, China) and lung adenocarcinoma A549 cells (purchased from ATCC) were cultured in DMEM/Ham's F12 and DMEM, respectively, supplemented with 10 % FBS, penicillin (1000 U/ml) and streptomycin (1000 U/ml) in 5 % CO2 at 37 °C.
2.7 Treatments
A fixed concentration of H2O2 (0.5 mM) for 24 h was used to trigger apoptosis in HK2 cells based on the previous studies (Small, Morais et al. 2014, Shahzad, Small et al. 2016). Also, concentration-response assays (range 1–50 µM) were done to determine the maximum rhamnazin concentration that did not stimulate cytotoxicity in HK2 cells. Based on this pilot assay, a maximum safe concentration of 10 µM rhamnazin for HK2 cells at an incubation time of 24 h was determined. HK2 cells were then incubated for 24 h with H2O2 (0.5 mM) alone or co-incubated with H2O2 (0.5 mM) and rhamnazin (10 µM). For lung adenocarcinoma A549 cells, the cells were incubated with different concentrations of rhamnazin (1–50 µM) for 24 h.
2.8 MTT assay
MTT assay was done to assess the cell viability based on the previous study (Shahzad, Small et al. 2016). Briefly, the culture medium was replaced with 100 μL of fresh medium supplemented with MTT (0.5 mg/ml), incubated for 60 min, and followed by removal of the medium and replacement with 100 μL of DMSO. The absorbance was then determined at 570 nm using a microplate reader (RT-2100C Microplate Reader, China).
2.9 Determination of oxidative stress
To analyze the formation of intracellular ROS, the HK2 cells incubated with different samples were added by 2,7-dichlorodihydrofluorescein diacetate acetyl ester (H2DCFDA) (10 mM) for 60 min at 37 °C. Then, the cells were washed with PBS and the fluorescence intensity of samples was detected using a fluorescence microplate reader (Bio-Tek Instruments, Winooski, USA) by excitation and emission wavelengths of 485 and 528 nm, respectively. CAT activity (Abcam, ab83464), SOD activity (Abcam, ab65354), and GSH content (Abcam, ab239709) in HK2 cells were examined following the product manual.
2.10 Quantitative real-time PCR
The relative expression of Bax, Bcl-2, and caspase-3 mRNA in HK2 cells and relative expression of Bax, Bcl-2, caspase-3, and p53 mRNA in A549 cell was assessed by quantitative real-time PCR following standard methods (Kong, Zhang et al. 2017, Yuan, Yang et al. 2021) Briefly, RNA from cells were extracted using Trizol kit (Invitrogen, CA, USA) and was reverse-transcribed using a Revert Aid First Strand cDNA Synthesis kit (Bio-Rad, CA, USA) following the product manual. The relative expression of mRNA was assessed through real-time PCR using SYBR green master mix. The primer sequences were used as reported in the previous studies (Kong, Zhang et al. 2017, Yuan, Yang et al. 2021). The data was analyzed through the 2−ΔΔCt method and GAPDH was used as the target gene to normalize data.
2.11 Western blot analysis
Western blot analysis was done based on the previous study (Shahzad, Small et al. 2016). Briefly, the HK2 cells were washed, scraped with RIPA cell lysis buffer supplemented with phosphatase and protease inhibitors, sonicated, and centrifuged (3,000g, 20 min, 4 °C). Protein concentration was calculated based on the Bradford assay. Then protein samples were run on an SDS-polyacrylamide gel (12 %), transferred onto PVDF membrane, soaked in a blocking buffer for 60 min for 1 h, and incubated with primary antibodies (1:1000) 24 h at 4 °C. Afterwards, the membrane was washed and added by anti-rabbit HRP-conjugated secondary antibody (1:2000). Finally, protein bands were detected using enhanced chemiluminescence.
2.12 Apoptosis induction
The apoptotic A549 cells after exposure to rhamnazin (IC50 concentration) for 24 h was assessed using FITC Annexin V Apoptosis Detection Kit I according to the manufacturer’s protocol and instructions reported previously (Akram, Al-Saffar et al. 2022). Briefly, after exposure, the A549 cells were collected, resuspended in the binding buffer, stained, and incubated in the dark place for 15 min. at room temperature. Cells were screened using flow cytometry (FACS ARIA III, BD Biosciences).
2.13 Determination of the level of expression of p53, Bcl-2, and Bax
Rhamnazin (IC50 concentration)-treated and −untreated A549 cells were lysed. The level of expression of caspase-3, p53, Bax, and Bcl-2 was analyzed through colorimetric assays using human p53 ELISA kit (ab171571), human cleaved caspase-3 (Asp175) ELISA Kit (ab220655), human Bcl-2 ELISA kit (ab119506) and human Bax ELISA kit (ab199080), according to the protocols provided by the manufacturer. The expression of proteins was determined by detecting the absorbance at 450 nm using a microplate reader (RT-2100C Microplate Reader, China).
2.14 Statistical analysis
Data were presented as mean ± standard deviation of triplicate experiments for cellular assays (n = 3). Statistical analyses were analyzed with one-way ANOVA, followed by Tukey’s test. Statistical significance was set at P < 0.05.
3 Results and discussion
The investigation of interactions between rhamnazin with HSA has not been fully investigated in the literature. Therefore, we tried to analyze the interactions of rhamnazin with HSA using different spectroscopic techniques. The analysis was done to explore the quenching mechanism, thermodynamic and binding parameters, and structural changes of HSA. Collectively, we tried to analyze the biochemical interactions existing between HSA and rhamnazin and furnish a detailed characterization of the biological properties of rhamnazin.
3.1 Intrinsic fluorescence spectroscopy
Fluorescence spectroscopy is widely used to characterize ligand-macromolecule (protein/DNA) interaction mechanisms to provide details on the quenching mechanisms, binding parameters, as well as thermodynamic changes (Van de Weert and Stella 2011, dos Santos Rodrigues, Delgado et al. 2023). Principally, alterations in the intrinsic emission intensity of proteins are determined at the λmax. The addition of rhamnazin to HSA results in the fluorescence quenching of HSA (excitation at 280 nm) at three different temperatures of 298/310/315 K with different quenching rates (Fig. 1).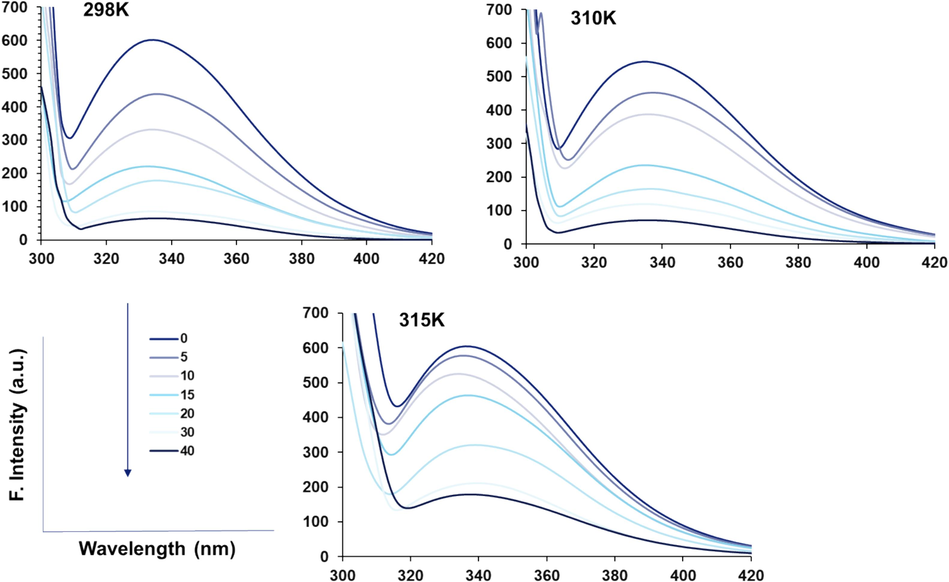
Fluorescence quenching study of HSA (5 µM) upon interaction with different concentrations of rhamnazin ranging from 5 to 40 µM at three different temperatures of 298/310/315 K.
The observed changes in fluorescence intensity patterns at different temperatures could be attributed to the reorganization of intra and intermolecular forces that contribute to protein stability and folding.
3.1.1 Quenching mechanism
Titration of rhamnazin to the HSA solution can lead to the potential interaction of this ligand with HSA and may form a complex (Kenoth and Kamlekar 2022). HSA fluorescence intensity was found to be quenched upon the addition of rhamnazin, concentration-dependently (Fig. 1). Quenching of HSA fluorescence emission with the addition of rhamnazin was assessed by the well-known Stern–Volmer Eq. (1) (Leilabadi-Asl, Divsalar et al. 2024):
The Eq. (1) in Fig. 2a2a was plotted to determine the KSV at three different temperatures of 298/310/315 K.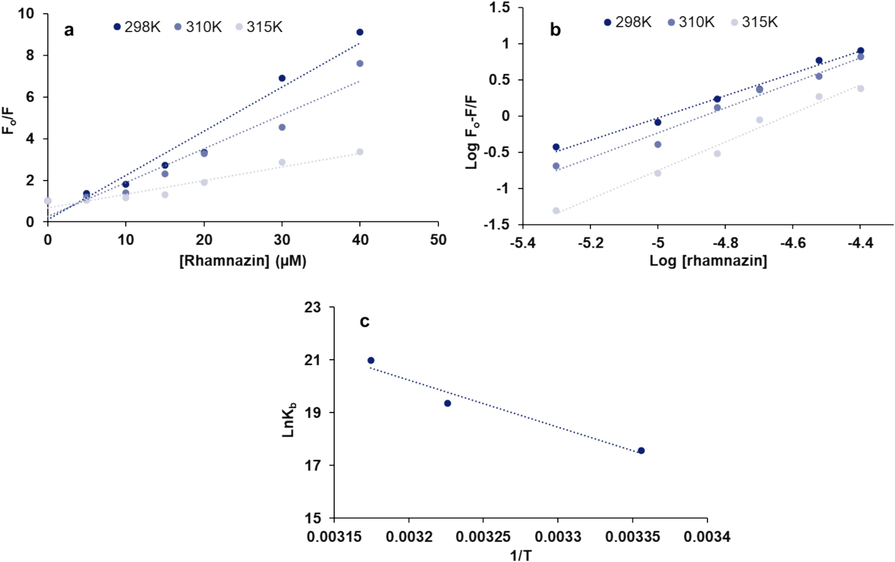
(a) Stern-Volmer plot, (b) modified Stern-Volmer plot, (c) Van’t Hoff plot for the interaction of HSA (5 µM) with different concentrations of rhamnazin ranging from 5 to 40 µM at three different temperatures of 298/310/315 K.
Fluorescence quenching can be categorized into three different classes: static, dynamic, or combined mechanisms, where they show different behaviors against temperature (Fossum, Johnson et al. 2023). The quenching constants under a dynamic quenching mechanism increase upon elevating temperatures, leading to large KSV values. Nevertheless, higher temperatures can lead to a reduction in the stability of the formed complex, which can result in a decline in the KSV values for static quenching. The formation of the static or dynamic complex is also can be verified using the calculation of quenching rate constant (kq) values (Eq. (2)) (Roy, Tapan Kumar Das et al. 2016):
Fig. 2a displays the Stern–Volmer plot at three different temperatures of 298/310/315 K for the interaction of rhamnazin and HSA, and the resultant calculated KSV and kq values based on Eq. (1) and Eq. (2) are summarized in Table 1. Decreasing the KSV values after increasing the system temperature revealed that the interaction of rhamnazin and HSA results in a static quenching mechanism. Furthermore, the order of magnitude of the kq for rhamnazin-complex was found to be 1013, which was greatly higher than the bimolecular dynamic quenching rate constant (2 × 1010 L mol−1 s−1) (Patar, Jalan et al. 2024), indicating a static quenching of the binding of rhamnazin to HSA.
T (K)
Ksv
(105 L/mol)
kq
(1013 L/mol s−1)
logKb
(L/mol)
n
ΔH°
(kJ/mol)
ΔS°
(J/mol K−1)
ΔG°
(kJ/mol)
298
2.12
2.12
7.64
1.53
147.922
641.258
−43.172
310
1.63
1.63
8.42
1.73
147.922
641.258
−50.867
315
0.65
0.65
9.12
1.97
147.922
641.258
−54.073
3.1.2 Determination of the binding parameters
The binding parameters were calculated using modified Stern–Volmer Eq. (3) (Wang, Wang et al. 2023):
3.1.3 Determination of thermodynamic parameters
The ligand-macromolecule interaction occurs through four main driving forces, including hydrophobic, hydrogen bonding, van der Waals, and electrostatic forces (Siddiqui, Ameen et al. 2021). The thermodynamic parameters [enthalpy change (ΔHο), entropy change (ΔSο), and Gibbs free energy (ΔGο)] determine the type of binding interactions. The association between thermodynamic changes and binding interaction has been well documented. It has been manifested that when both ΔHο and ΔSο values are positive, hydrophobic forces are involved in the interaction of proteins with ligands, while van der Waals forces and hydrogen bonding mainly contribute to the interaction mechanism when both these values are negative. Electrostatic forces are mainly involved in the formation of ligand–protein complexes when ΔHο and ΔSο show different negative and positive values. The Van’t Hoff Eq. (4) could be used for determining ΔHο and ΔSο values if temperature changes is minimum (Liu, Wang et al. 2022):
The straight line upon plotting ln Kb versus 1/ T can be used to determine ΔHο and ΔSο values (Fig. 2c). Then, the Gibbs-Helmholtz Eq. (4) can be used to determine ΔGο values (Mariño-Ocampo, Rodríguez et al. 2023):
3.2 Circular dichroism study
Circular dichroism (CD) has been utilized as a sensitive spectroscopic technique to analyze the secondary structural change of protein in the presence of ligands (Tongkanarak, Loupiac et al. 2024). In order to explore the secondary structural alterations of HSA triggered by rhamnazin, a far-UV CD (200–260 nm) study was performed. In fact, upon interaction of rhamnazin, intermolecular forces involved in the stability of HSA structure may undergo some partial or substantial rearrangement, leading to conformational changes in the protein which is detected by monitoring the chirality changes (Tongkanarak, Loupiac et al. 2024). Fig. 3 depicts the far-UV CD signals of HSA without and with increasing concentrations of rhamnazin. It is evident that ellipticity changes of native HSA show two minima centered at 208 and 222 nm, representing the characteristic minima of typical α-helix structure (Tongkanarak, Loupiac et al. 2024).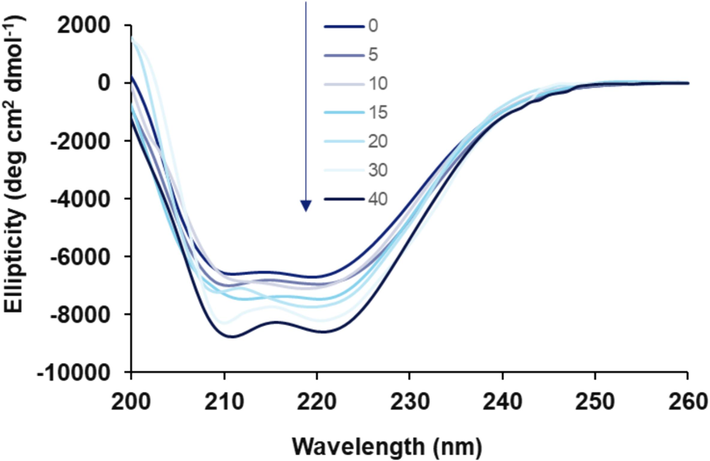
Far-UV CD (200–260 nm) for the interaction of HSA (5 µM) with different concentrations of rhamnazin ranging from 5 to 40 µM at room temperature.
Upon the addition of rhamnazin, a notable increase in the ellipticity changes at minima was detected without any significant shift, indicating an increase in the helical content and stability of HSA. In fact, it was deduced that the α-helix content of HSA was increased from 51.35 % to 59.77 % upon increasing the concentration of rhamnazin from 0 to 40 µM, respectively, determined by CDNN software.
3.3 Molecular docking study
A theoretical simulation tool, molecular docking study, can be used to evaluate the nature of the ligand-receptor interaction (Fan, Wang et al. 2024). A molecular docking study was run to support experimental data about binding affinity and forces as well as amino acid residues at the binding pocket (Fig. 4a). Outcomes are detected from this assay, indicating that the rhamnazin shows a strong interaction and binding affinity (ΔG°= − 33.89 kJ/mol) with HSA at the domain B (Fig. 4b). The rhamnazin conformer was revealed to interact with the active site residues R186, R145, R114, L112, E425, P110, L463, D108, V426, Y148, P147, H146, K190, S193, and F148 at domain B (Fig. 4c). Moreover, it was displayed that rhamnazin provided hydrophobic interaction with HSA through residue R145 (CB), whereas H146 is involved in the cation-pi interaction, D108, R145 (NH1), and R197 are involved in the formation of hydrogen bonding. Data obtained from the molecular modeling study is in good agreement with the experimental outcomes, indicating the formation of rhamnazin-HSA binding mostly through hydrophobic forces.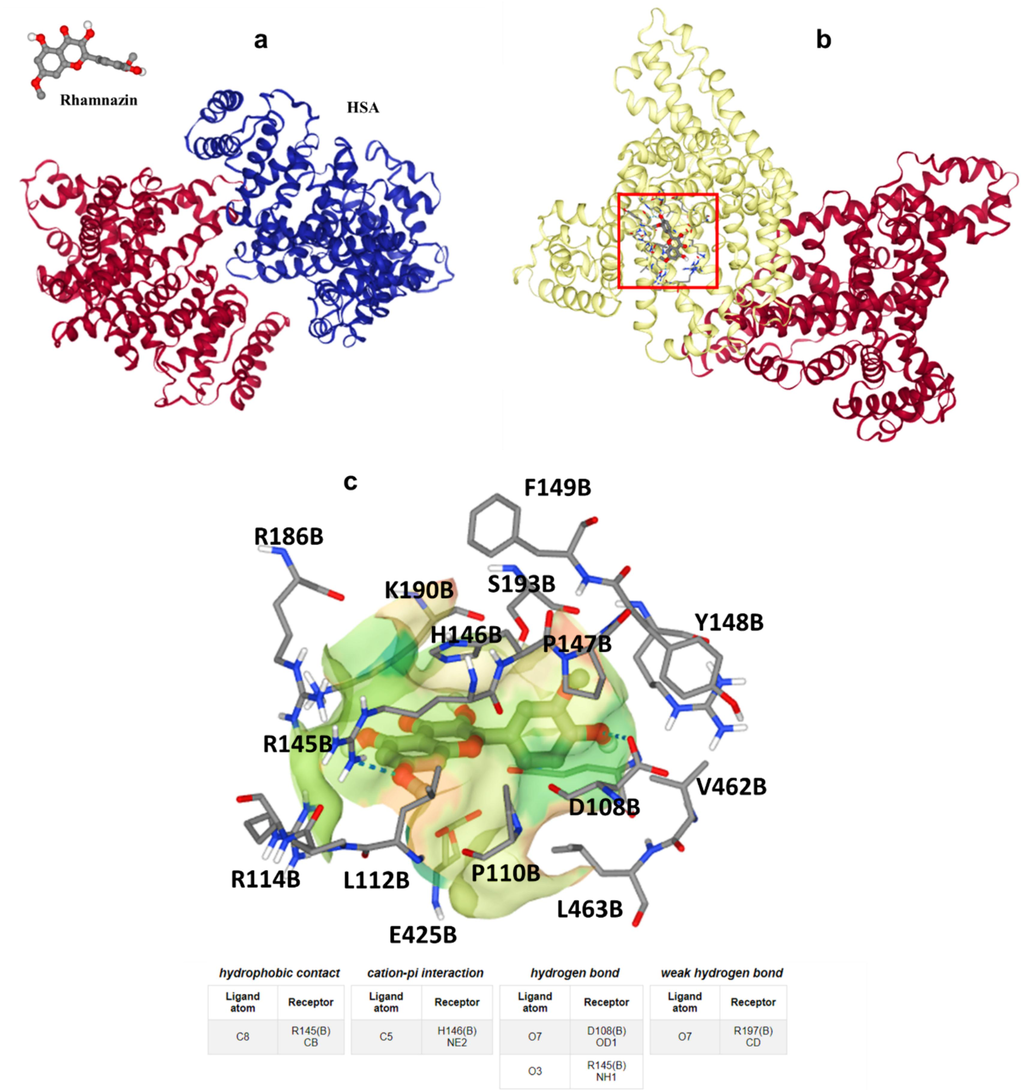
(a) Rhamnazin and HSA structures, (b) binding site of HSA upon interaction with rhamnazin, (c) amino acid residues in the binding pocket.
3.4 Rhamnazin protects HK2 cells against H2O2 –induced cytotoxicity
Fig. 5a displays the data obtained from the MTT assay, expressed as a percentage of cell viability in HK2 cells. For rhamnazin, it was shown that the maximum safe concentration at 10 µM was achieved, while further increasing the rhamnazin concentration up to 50 µM induced a significant reduction in cell viability (Fig. 5a). Therefore, rhamnazin with a concentration of 10 µM was used for further studies. It was also shown that the HK2 cell viability was significantly (***P < 0.001) decreased after incubation of cells with 0.5 mM H2O2 for 24 h competed to control cell culture, as it was in good agreement with the previous study (Shahzad, Small et al. 2016). However, co-incubation of HK2 cells with rhamnazin with a concentration of 10 µM for 24 h markedly ($$P < 0.01) restored the cell viability compared with the H2O2-treated sample (Fig. 5b). Based on these data, it can be deduced that the rhamnazin (10 µM) can protect cells against cytotoxicity induced by H2O2 (0.5 mM).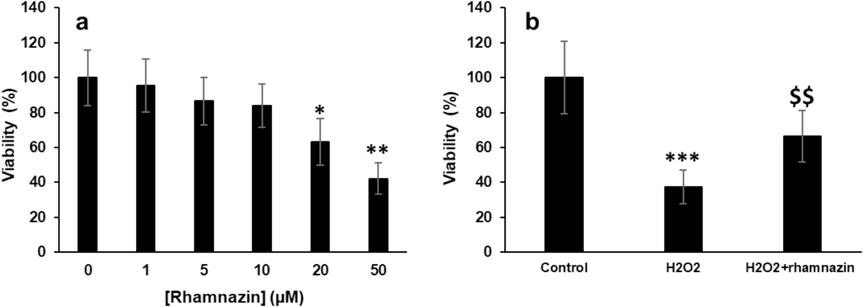
Cell viability was assessed by the MTT assay. (a) Incubation of HK2 cells with increasing concentrations of rhamnazin for 24 h. (b) Incubation of cells with H2O2 (0.5 mM), or co-incubation of HK2 cells with H2O2 (0.5 mM) and rhamnazin (10 µM) for 24 h. All data are expressed as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, relative to untreated negative control cells; $$P < 0.01 relative to H2O2-treated cells.
3.5 Rhamnazin protects HK2 cells against H2O2–induced oxidative stress
The potential of rhamnazin to inhibit the generation of ROS was assessed using the DCFH-DA assay. As illustrated in Fig. 6a the significant intracellular ROS generation in the cells exposed to H2O2 (0.5 mM) was potentially inhibited upon co-incubation of cells with H2O2 (0.5 mM) and rhamnazin (10 µM) compared to the H2O2-treated group. Furthermore, the activities of the antioxidant enzymes, including CAT and SOD in HK2 cells were analyzed after 24 h of incubation with H2O2 (0.5 mM) and co-incubation with H2O2 (0.5 mM) and rhamnazin (10 µM). As expected, H2O2 decreased SOD (Fig. 6b) and CAT (Fig. 6c) activities in HK2 cells and co-incubation of cells with H2O2 (0.5 mM) and rhamnazin (10 µM) restored significantly the enzyme activities. Also, the analysis of GSH content (Fig. 6d) verified the antioxidant capacity of rhamnazin.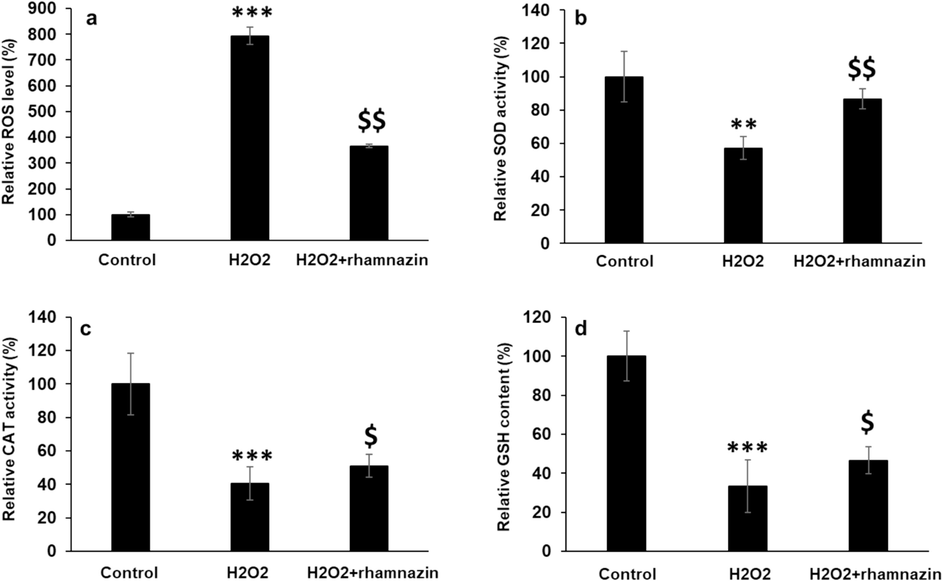
Antioxidant properties of rhamnazin after incubation of HK2 cells with H2O2 (0.5 mM) or co-incubation with H2O2 (0.5 mM) and rhamnazin (10 µM) for 24 h. (a) ROS activity, (b) SOD activity, (c) CAT activity, (d) GSH content. All data are expressed as means ± SD. **P < 0.01, ***P < 0.001, relative to untreated negative control cells; $P < 0.05, $$P < 0.01 relative to H2O2-treated cells.
ROS production can be triggered by some mechanisms, including disruption of the mitochondrial membrane and activation of NADPH oxidase (Magnani and Mattevi 2019). ROS overproduction can result in some serious side effects against biomacromolecules, eventually triggering apoptosis (Juan, Pérez de la Lastra et al. 2021). As a result, any agent that reduces ROS production can be used as an antioxidant to counteract the cytotoxic effects of oxidative stress. In this study, we showed that rhamnazin can reduce ROS production by restoring the activity of antioxidant enzymes and biomolecules maintaining a redox state. It has been also shown that quercetin and rhamnazin can mitigate lipopolysaccharide-induced oxidative stress and inflammation in porcine intestinal cells (Karancsi, Kovács et al. 2022). Moreover, rhamnazin was shown to reduce traumatic brain injury through the inhibition of apoptosis and oxidative stress (Yang, Zhang et al. 2022).
3.6 Rhamnazin protects HK2 cells against H2O2–induced apoptosis
It was shown that incubation of HK2 cells with H2O2 (0.5 mM) induced apoptosis by upregulating caspase-3 and Bax mRNA and protein expression and downregulating Bcl-2 mRNA and protein expression (Fig. 7). However, rhamnazin (10 µM) co-incubation decreased Bax mRNA expression (Fig. 7a) and increased Bcl-2 mRNA expression (Fig. 7b). Also, it was shown that rhamnazin (10 µM) co-incubation mitigated the overexpression of caspase-3 mRNA induced by H2O2 (0.5 mM) in HK2 cells (Fig. 7c). Western blot analysis also verified that rhamnazin (10 µM) co-incubation resulted in the downregulation of apoptotic proteins (Bax and cleaved caspase-3) and upregulation of anti-apoptotic protein (Bcl-2) (Fig. 7d).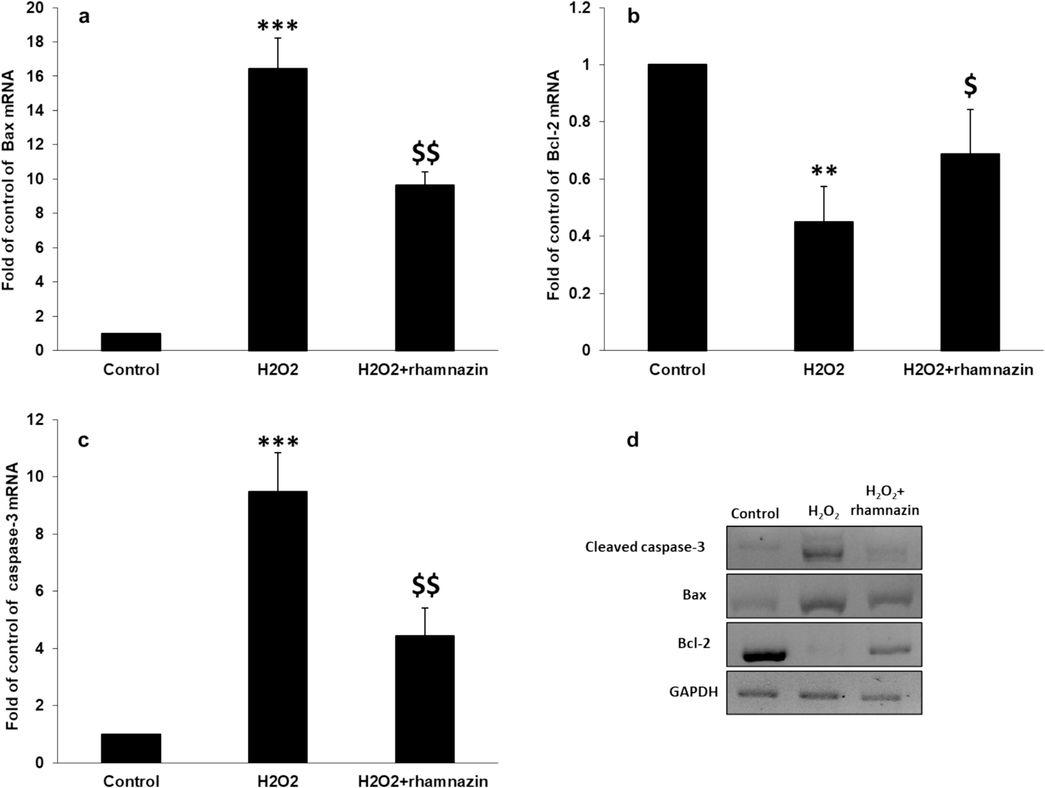
Antiapoptotic properties of rhamnazin after incubation of HK2 cells with H2O2 (0.5 mM) or co-incubation with H2O2 (0.5 mM) and rhamnazin (10 µM) for 24 h. (a) Bax mRNA expression assay, (b) Bcl-2 mRNA expression assay, (c) caspase-3 mRNA expression assay, (d) western blot analysis. All data are expressed as means ± SD. **P < 0.01, ***P < 0.001, relative to untreated negative control cells; $P < 0.05, $$P < 0.01 relative to H2O2-treated cells.
In fact, this data revealed that rhamnazin can inhibit apoptosis through mitigation of ROS-associated Bax/Bcl-2 and caspase-3 signaling pathway stimulated by H2O2 in HK2 cells. Therefore, it may be discussed that rhamnazin can mitigate the induction of apoptosis in HK2 cells by regulating the mitochondrial pathway, decreasing Bax expression, and increasing Bcl-2 expression at both mRNA and protein levels as already shown by some other therapeutic compounds (Chang, Shen et al. 2014, Cao, Xu et al. 2021, Chen, Xie et al. 2023, Zhang, Tian et al. 2024). In accordance with our results, some other reports have shown that treatment with rhamnazin can control the induction of oxidative stress and apoptosis (Wu, Wang et al. 2022, Yang, Zhang et al. 2022, Ijaz, Mustafa et al. 2023).
3.7 Rhamnazin-induced cytotoxic effect in lung adenocarcinoma A549 cells
To assess the anti-lung cancer activity of rhamnazin, we assessed the MTT assay after interaction of lung adenocarcinoma A549 cells with different concentrations of rhamnazin for 24 h. The data suggested that rhamnazin induces cytotoxicity in lung adenocarcinoma A549 cells. Indeed, A549 cells were treated with different concentrations (1, 5, 10, 20, and 50 μM) of rhamnazin, and the cell viability decreased from 100 % to 98.35 %, 71.74 %, 62.27 %, 52.18 %, and 34.32 %, respectively. Then the calculated IC50 concentration was determined to be 10.03 μM (Fig. 8a).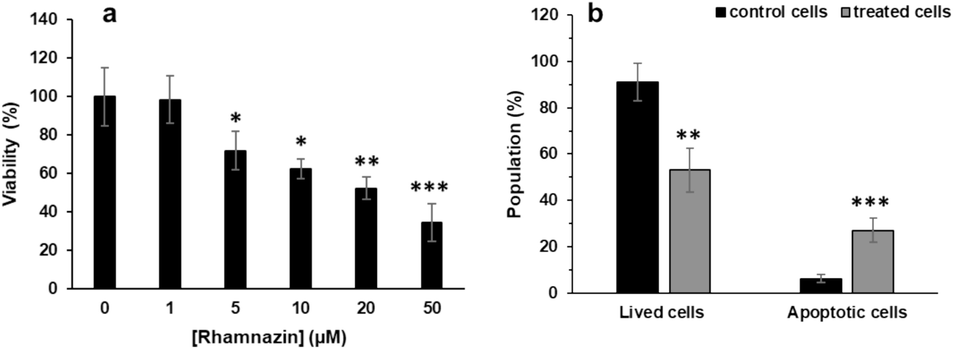
(a) Cytotoxic effects of rhamnazin on lung adenocarcinoma A549 cells after incubation for 24 h, (b) flow cytometry analysis of lung adenocarcinoma A549 cells after interaction with IC50 concentration of rhamnazin (10.00 μM) for 24 h. All data are expressed as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, relative to untreated negative control cells.
To detect whether the rhamnazin-induced cytotoxicity was mediated by apoptosis, the A549 cells were incubated with IC50 concentrations of rhamnazin for 24 h, then the cells were subjected to flow cytometry.
As shown in Fig. 8b, the control cells (cells treated without rhamnazin) had 91.17 % lived cells with 6.21 % apoptotic cells. A549 cells treated with IC50 concentration had a cell viability of 53.18 % upon treatment. The decrease in cell viability was accompanied by an elevation in the percentage of apoptotic cells to 27.15 % (Fig. 8b). Activation of the programmed cell death signaling pathway has been reported as an important mechanism for reducing tumor formation and associated progression; thus, several anticancer agents have been recognized to potentially trigger apoptosis as a primer way of effect for their cytotoxic behavior (Carneiro and El-Deiry 2020, Biswas, Roy et al. 2024). Accordingly, in the present study, we found that the pathway of rhamnazin-induced anticancer effects is mediated by the induction of apoptosis.
3.8 Rhamnazin-induced signaling pathway in lung adenocarcinoma A549 cells
Following DNA damage, the tumor suppressor gene p53 could regulate the expression of several downstream proteins such as Bax, Bcl-2, and caspases (Amaral, Xavier et al. 2010, Wei, Wang et al. 2023). A high ratio of Bax/Bcl-2 can lead to the permeabilization of the outer mitochondrial membrane, causing the release of cytochrome c and the corresponding activation of caspase-9 and -3, to trigger the process of apoptosis (Kaloni, Diepstraten et al. 2023, Saddam, Paul et al. 2024, Singh, Anand et al. 2024). Hence, it is presumed that rhamnazin triggers DNA damage in A549 cells, resulting in the overexpression of p53 which then lead to regulation of the Bax, Bcl-2, and caspases.
A significant increase in the p53 mRNA expression was detected in the A549 cells treated with rhamnazin after 24 h of incubation (Fig. 9a). Bax (Fig. 9b) and Bcl-2 (Fig. 9c) mRNA expression in the treatment with rhamnazin after 24 h of incubation increased and reduced, respectively, in comparison with the control. Also, there was a significant change in the upregulation of apoptotic caspase-3 mRNA in the treatment with rhamnazin after 24 h of incubation (Fig. 9 d).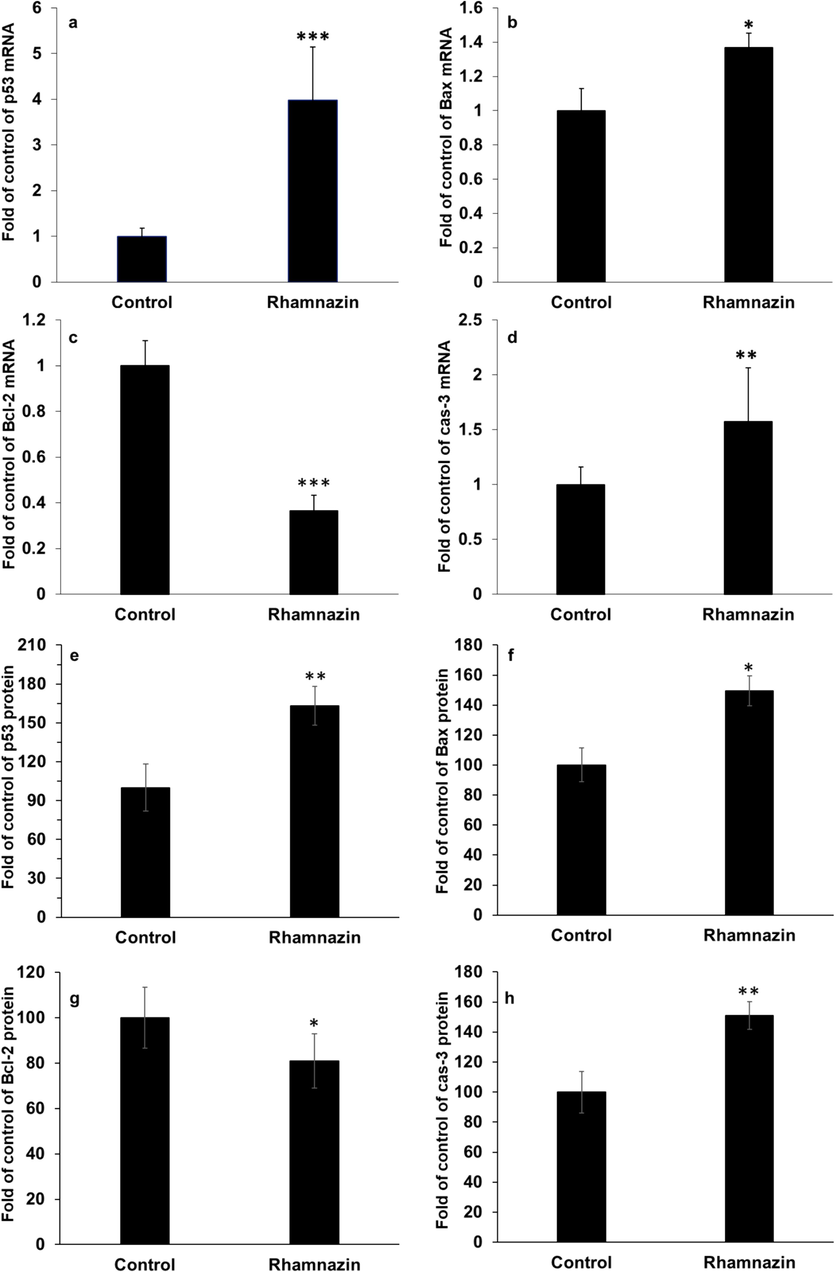
Effect of IC50 concentration of rhamnazin (10.00 μM) in lung adenocarcinoma A549 cells for 24 h on the (a) p53 mRNA expression, (b) Bax mRNA expression, (c) Bcl-2 mRNA expression, (d) caspase-3 (cas-3) mRNA expression, (e) p53 protein expression, (f) Bax protein expression, (g) Bcl-2 protein expression, (h) caspase-3 (cas-3) protein expression. All data are expressed as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, relative to untreated negative control cells.
The same data was also detected in the p53, Bax, Bcl-2, and cleaved caspase-3 protein levels in the A549 cells treated with rhamnazin after 24 h of incubation (Fig. 9 e-h).
Therefore, the data demonstrated that the anticancer effect of rhamnazin on lung adenocarcinoma A549 cells is linked with its capability to trigger apoptosis in the cells. The apoptosis induction by rhamnazin in lung adenocarcinoma A549 cells might be based on a p53-dependent manner, via upregulation of apoptotic Bax and caspase-3 and down-regulation of anti-apoptotic Bcl-2. However, the cytotoxicity of rhamnazin should be evaluated against several normal cell lines in vitro and in vivo to recognize the selectivity of this compound on cancerous cells. Therefore, we might suggest that rhamnazin could be a potential O-methylated flavonol for the treatment of lung cancer in the future.
4 Conclusion
In this paper, a characterization of the rhamnazin-HSA binding interaction was done by different spectroscopic and theoretical studies. Also, the protective effects of rhamnazin against H2O2-induced oxidative stress and apoptosis in HK2 cells were evaluated by different cellular and molecular assays. Furthermore, the anticancer effects of rhamnazin toward lung adenocarcinoma A549 cells were evaluated. It was shown that strong binding of rhamnazin to HSA was based on a static quenching, where more than one binding site was located on HSA for rhamnazin. Also, it was displayed that rhamnazin induced some structural changes in HSA conformation which resulted in an increase in the α-helix content of protein. Molecular docking analysis verified that binding interaction occurs through involvement of a hydrophobic residue, R145. Cellular and molecular assays demonstrated that rhamnazin can restore cell viability in HK2 cells treated with H2O2 (0.5 mM) for 24 h, through the reduction of oxidative stress and apoptosis. Moreover, rhamnazin showed potential cytotoxicity on lung adenocarcinoma A549 cells through a p53-dependent manner. This study may provide useful information about the valuable exploring of the pharmacokinetic characteristics along with antioxidant and anti-lung cancer activities of rhamnazin. In fact, this data may assist in revealing the molecular mechanisms behind the therapeutic effects of rhamnazin, thereby improving its pharmacological, biosafety and clinical potency, which needs further in vivo assay in future studies.
CRediT authorship contribution statement
Zheng Chen: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Yan Qiao: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization. Yu Chen: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization. Tingting Ma: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization. Wei Li: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization. Jianhong Xia: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization. Yan Yan: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization. Qian Jiang: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization. Liqing Zhou: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization, Supervision.
Acknowledgments
This work was supported by no external funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aguilar, T.A.F., Navarro, B.C.H., Pérez, J.A.M., 2016. “Endogenous antioxidants: a review of their role in oxidative stress.” A master regulator of oxidative stress-the transcription factor nrf2: 3-20.
- Utilization of novel lectin-conjugated Au nanoparticles as Thomsen-Friedenreich onco-antigen target for in vitro cytotoxicity and apoptosis induction in leukemic cell line. Life Sci.. 2022;311:121163
- [Google Scholar]
- Biophysical and molecular docking insight into the interaction of cytosine β-D arabinofuranoside with human serum albumin. J. Lumin.. 2015;164:123-130.
- [Google Scholar]
- Spectroscopic and computational evaluation on the binding of safranal with human serum albumin: role of inner filter effect in fluorescence spectral correction. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2018;203:434-442.
- [Google Scholar]
- The interplay between autophagy and apoptosis: its implication in lung cancer and therapeutics. Cancer Lett.. 2024;216662
- [Google Scholar]
- Protective effect of carnosine on hydrogen peroxide–induced oxidative stress in human kidney tubular epithelial cells. Biochem. Biophys. Res. Commun.. 2021;534:576-582.
- [Google Scholar]
- Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol. Appl. Pharmacol.. 2014;279(3):351-363.
- [Google Scholar]
- Albumin carriers for cancer theranostics: a conventional platform with new promise. Adv. Mater.. 2016;28(47):10557-10566.
- [Google Scholar]
- Protective effect of dihydromyricetin against lipopolysaccharide-induced HK2 cells by upregulating HIF-1α. Biotechnol. Genet. Eng. Rev.. 2023;1–11
- [Google Scholar]
- Applications of fluorescence spectroscopy in protein conformational changes and intermolecular contacts. BBA Advances. 2023;3:100091
- [Google Scholar]
- Research on the Interaction Mechanism and Structural Changes in Human Serum Albumin with Hispidin Using Spectroscopy and Molecular Docking. Molecules. 2024;29(3):655.
- [Google Scholar]
- Insights into the mechanism of tryptophan fluorescence quenching due to synthetic crowding agents: A combined experimental and computational study. ACS Omega. 2023;8(47):44820-44830.
- [Google Scholar]
- A mini review on human serum albumin-natural alkaloids interaction and its role as drug carrier. J. Biomol. Struct. Dyn. 2024:1-18.
- [Google Scholar]
- Ijaz, M.U., Mustafa, S., Ain, Q.U., Hamza, A., Ali, S., 2023. “Rhamnazin ameliorates 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-evoked testicular toxicity by restoring biochemical, spermatogenic and histological profile in male albino rats. Human & Experimental Toxicology 42: 09603271231205859.
- Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal.. 2024;22(1):7.
- [Google Scholar]
- Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol.. 2023;97(10):2499-2574.
- [Google Scholar]
- The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci.. 2021;22(9):4642.
- [Google Scholar]
- BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis. 2023;28(1):20-38.
- [Google Scholar]
- The impact of quercetin and its methylated derivatives 3-o-methylquercetin and rhamnazin in lipopolysaccharide-induced inflammation in porcine intestinal cells. Antioxidants. 2022;11(7):1265.
- [Google Scholar]
- Kenoth, R. and R. K. Kamlekar (2022). Steady-state fluorescence spectroscopy as a tool to monitor protein/ligand interactions. Optical Spectroscopic and Microscopic Techniques: Analysis of Biological Molecules, Springer: 35-54.
- Rhamnazin inhibits LPS-induced inflammation and ROS/RNS in raw macrophages. J. Nutrit. Health. 2016;49(5):288-294.
- [Google Scholar]
- Oxidative stress and the role of redox signalling in chronic kidney disease. Nat. Rev. Nephrol.. 2024;20(2):101-119.
- [Google Scholar]
- Resveratrol raises in vitro anticancer effects of paclitaxel in NSCLC cell line A549 through COX-2 expression. Korean J. Physiol. Pharmacol.: Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol.. 2017;21(5):465.
- [Google Scholar]
- A clinical update of using albumin as a drug vehicle—a commentary. J. Control. Release. 2014;190:331-336.
- [Google Scholar]
- Unraveling the binding interactions between two Pt (II) complexes of aliphatic glycine derivatives with human serum albumin: a comprehensive computational and multi-spectral investigation. Int. J. Biol. Macromol.. 2024;266:131298
- [Google Scholar]
- Investigation of the binding properties of 3, 4-dihydroxybenzaldehyde from Salvia miltiorrhiza (Bunge) with human serum albumin via multi-spectroscopic and molecular docking techniques. BioResources. 2022;17(2):2680.
- [Google Scholar]
- Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol.. 2019;59:91-97.
- [Google Scholar]
- Direct oral FXa inhibitors binding to human serum albumin: spectroscopic, calorimetric, and computational studies. Int. J. Mol. Sci.. 2023;24(5):4900.
- [Google Scholar]
- Rhamnazin inhibits hepatocellular carcinoma cell aggressiveness in vitro via glutathione peroxidase 4-dependent ferroptosis. Tohoku J. Exp. Med.. 2022;258(2):111-120.
- [Google Scholar]
- A spectroscopic and molecular docking study on the interaction of 2ʹ-hydroxyflavanone with bovine serum albumin. Phys. Chem. Res.. 2024;12(3):709-727.
- [Google Scholar]
- Rhamnazin: A systematic review on ethnopharmacology, pharmacology and analytical aspects of an important phytomedicine. Curr. Tradit. Med.. 2018;4(2):120-127.
- [Google Scholar]
- Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41(6):1211-1219.
- [Google Scholar]
- Roy, S., Tapan Kumar Das, K., Ponra, S., Ghosh, T., 2016. Interaction of bovine serum albumin with synthetic spiropyrimidines. Adv. Mater. Lett. 7(1), 65–70.
- Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context. Egypt. J. Med. Human Genet.. 2024;25(1):12.
- [Google Scholar]
- Protection against oxidative stress-induced apoptosis in kidney epithelium by Angelica and Astragalus. J. Ethnopharmacol.. 2016;179:412-419.
- [Google Scholar]
- Siddiqui, S., Ameen, F., ur Rehman, S., Sarwar, T., Tabish, M., 2021. Studying the interaction of drug/ligand with serum albumin. J. Mol. Liquids 336, 116200.
- Targeting apoptotic cell death in cancer therapy: chemotherapeutic approaches. Int. J. Pharm. Drug Des. 2024:77-87.
- [Google Scholar]
- Oxidative stress-induced alterations in PPAR-γ and associated mitochondrial destabilization contribute to kidney cell apoptosis. Am. J. Physiol.-Renal Physiol.. 2014;307(7):F814-F822.
- [Google Scholar]
- Serum albumin: Clinical significance of drug binding and development as drug delivery vehicle. Adv. Protein Chem. Struct. Biol.. 2021;123:193-218.
- [Google Scholar]
- Tongkanarak, K., C. Loupiac, F. Neiers, O. Chambin, Srichana, T., 2024. Evaluating the biomolecular interaction between delamanid/formulations and human serum albumin by fluorescence, CD spectroscopy and SPR: effects on protein conformation, kinetic and thermodynamic parameters. Colloids Surf. B: Biointerfaces: 113964.
- Fluorescence quenching and ligand binding: a critical discussion of a popular methodology. J. Mol. Struct.. 2011;998(1–3):144-150.
- [Google Scholar]
- Exploring thyroxine binding globulin structural changes and its release from human hepatoblastoma cells upon interaction with silica particles: a prelude to unrevealing the mechanism of thyroid hormone dysregulation. Int. J. Biol. Macromol.. 2023;251:126240
- [Google Scholar]
- Structures of p53/BCL-2 complex suggest a mechanism for p53 to antagonize BCL-2 activity. Nat. Commun.. 2023;14(1):4300.
- [Google Scholar]
- Antioxidant and anti-inflammatory effects of rhamnazin on lipopolysaccharide-induced acute lung injury and inflammation in rats. Afr. J. Tradit. Complement. Altern. Med.. 2017;14(4):201-212.
- [Google Scholar]
- The alleviative effect of flavonol-type Nrf2 activator rhamnazin from Physalis alkekengi L. var. franchetii (Mast.) Makino on pulmonary disorders. Phytother. Res.. 2022;36(4):1692-1707.
- [Google Scholar]
- Albumin–drug interaction and its clinical implication. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013;1830(12):5435-5443.
- [Google Scholar]
- Rhamnazin ameliorates traumatic brain injury in mice via reduction in apoptosis, oxidative stress, and inflammation. Neuroimmunomodulation. 2022;29(1):28-35.
- [Google Scholar]
- Rhamnazin, a novel inhibitor of VEGFR2 signaling with potent antiangiogenic activity and antitumor efficacy. Biochem. Biophys. Res. Commun.. 2015;458(4):913-919.
- [Google Scholar]
- Yuan, T., N. Yang, W. Bi, J. Zhang, X. Li, L. Shi, Y. Liu and X. Gao, 2021. Protective role of sulodexide on renal injury induced by limb ischemia-reperfusion. Evidence-Based Complement. Alternat. Med. 2021.
- Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target. Ther.. 2022;7(1):182.
- [Google Scholar]
- Thermodynamic and conformational changes of protein toward interaction with nanoparticles: a spectroscopic overview. RSC Adv.. 2016;6(107):105903-105919.
- [Google Scholar]
- Aristolochic acid I aggravates oxidative stress-mediated apoptosis by inhibiting APE1/Nrf2/HO-1 signaling. Toxicol. Mech. Methods. 2024;34(1):20-31.
- [Google Scholar]







