Translate this page into:
Polarity guided extraction, HPLC based phytochemical quantification, and multimode biological evaluation of Otostegia limbata (Benth.) Boiss
⁎Corresponding author. Iffatkhattak@yahoo.com (Iffat Naz) I.Majid@qu.edu.sa (Iffat Naz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Purpose of study
Otostegia limbata (Benth.) Boiss. (Family: Lamiacae) is an important underexplored ethnomedicinal plant that has been used as antinflammatory, anticancer and antibacterial herbal remedy previously. The present work was aimed to evaluate the antioxidant, antimicrobial, antileishmanial, and anticancer prospective of O. limbata stem and leaf extracts.
Results
The highest amount of phenolic and flavonoid content was obtained in the methanol-acetone and methanol stem extracts i.e., 53.29 ± 1.33 and 28.64 ± 1.16, respectively with highest DPPH scavenging in MeH stem extract (IC50 = 34.5 ± 1.34 μg/ml). Significant amount of catechin, gallic acid, apigenin and rutin was quantified. A moderate antibacterial and substantial antifungal activity was observed. Cytotoxicity against brine shrimps categorized 21% of stem (3 out of 14 extracts) and 57% (8 out of 14 extracts) of leaf extracts as potent. Substantial cytotoxicity against THP-1 cell line (IC50 = 3.46 ± 0.25 μg/ml) and Leishmania (IC50 = 1.50 ± 0.23 μg/ml) was exhibited by methanol-distilled water leaf extract while noteworthy antiproliferative activity against Hep-G2 (IC50 = 0.44 ± 0.45 μg/ml) was manifested by n-hexane stem extract. Absence of hemolysis in normal RBCs signified plant’s selective cytotoxicity. Methanol-distilled water and chloroform stem extracts displayed prominent protein kinase inhibition and antidiabetic potential of plant.
Conclusion
The results of present study recommend O. limbata as a potential source of antifungal, antileishmanial, anticancer, and α-amylase inhibitory agents.
Keywords
Otostegia limbata
THP-1 human leukemia cell line
Hep-G2 hepatoma cell line
1 Introduction
Nature has bestowed us with medicinal plants as a valued gift. An indefinite place has been secured by medicinal plants since ancient civilizations for curing various ailments (Anand et al., 2019). Natural products and their preparations offer a great contribution to humans, livestock, agriculture, veterinary, food items, cosmetics, and other fields to resolve practical problems (Drasar and Khripach, 2020).
One of the well-acknowledged Genus Otostegia belongs to the family Lamiaceae and is geographically distributed throughout the world. This flowering plant family has about 4000 species and 220 genera. Otostegia limbata (Benth.) Boiss. is an important medicinal plant of this genus and is recognized by the synonyms; Rydingia limbata (Benth.) Scheen & V. A. Albert and Ballota limbata. Benth. (Scheen and Albert, 2007). Plant species is identified by the common names “Spin aghzai”, ‘’Chiti booti’’, ‘’Chitti jharri”, ‘’Spin azghay ‘’ and “Bui”. The plant is characterized by a pale yellow bunch of flowers and oblong leaves with thick headed apex, sharp bract and small petiole. Plant species contain various chemical constituents including limbatolide A, B, C, D, ballotenic and ballodiolic acids (Sadaf et al., 2016). In Pakistan, O. limbata is widely associated with several ethnomedicinal uses including jaundice, cancer, scabies, boils, goiter, ulcer, cuts, wounds, dental problems, and animal infections (Rosselli et al., 2019). In Azad Jammu and Kashmir, KPK, Punjab, and Himalayan regions of Pakistan, infusion of fresh leaves is consumed by traditional healers in acidity, hypertension, depression, ulcer, jaundice, gum, and ophthalmic infection (Ahmad et al., 2015; Rashid et al., 2015). Dried powder mixed with butter is applied topically for wound healing in humans and cattle(Bibi et al., 2017). Plant powder is also consumed in jaundice (Hussain et al., 2017). Previous studies have reported the use of methanol extract and fraction of whole plant as antibacterial, antioxidant, anticancer, and haem agglutinant (Maqsood et al., 2015; Shakoor and Zaib, 2014; Waheed et al., 2013). Butanol fraction of whole plant has been associated with antitussive action in mice (Haq et al., 2011).
A comprehensive review of literature shows that despite being a well-acknowledged ethnomedicinal plant, O. limbata has not been scientifically validated thoroughly. Phytochemical contents are distinct in different parts of the same plant (Sadaf et al., 2016), therefore bioactivity profiling of individual plant organ is important. Previous studies on O. limbata included whole plant only proposing a research gap for appraisal of various parts individually (Haq et al., 2011). Furthermore, earlier reports included methanol and butanol extracts , thus, polarity-guided extraction for retrieval of diverse phytochemicals proposed us another significant investigation prospect (Sadaf et al., 2016). Keeping in view the identified research gaps, the present work aims to evaluate the extract library of O. limbata phytochemically and biologically. In line with this aim, the following objectives were acheived; Preparation of extract library to peruse polarity guided extraction, phytochemical quantification of polyphenols, and in vitro biological evaluation of antioxidant, antimicrobial, α-amylase inhibitory, and cytotoxic potential. The present study describes the impact of solvents of wide polarity range to demonstrate the correlation of solvent polarity and bioactivity. To the best of our knowledge, the cytotoxic potential of subject plant against Artemia salina and human monocytic leukemia THP-1 cell line, antiproliferative activity against Leishmania tropica, antifungal activity (against A. flavus and Mucor species), α-amylase inhibition as well as the selective cytotoxicity and biocompatibility (Hemolytic assay) studies have not been reported hitherto.
2 Materials and methods
2.1 Plant material collection and identification
The plant material was collected in September 2019 and was confirmed as O. limbata by Prof. Dr. Rizwana Aleem Qureshi, Department of Plant Sciences, Faculty of Biological Sciences, Quaid-i-Azam University (QAU) Islamabad, Pakistan. The voucher specimen of dry plant was preserved in the Herbarium of medicinal plants, Quaid-i-Azam University Islamabad under herbarium number PHM-487-A.
2.2 Solvents and reagents
The analytical grade reagents and solvents were used in the current study. Solvents needed for the extraction: Ethanol, Methanol, Ethyl acetate, Chloroform, Acetone, and n-Hexane were procured from Merck (Darmstadt, Germany). Folin–Ciocalteu reagent, 2, 2-diphenyl, 1-picrylhydrazine (DPPH), Trichloroacetic acid (TCA), Doxorubicin, Vincristine, 5 Flourouracil, Triton X 100 and standard antibiotics (Cefixime, Roxithromycin, Clotrimazole) were bought from Sigma–Aldrich (Steinheim, Germany). All additional chemicals (analytical grade), and reagents: Sodium hydroxide, Ferrous chloride, Aluminum chloride, Potassium dihydrogen phosphate, Dipotassium hydrogen phosphate, Quercetin, Gallic acid, Ascorbic acid, Caffeic acid, Rutin, Kaempferol, (+)-Catechin and Myricetin used in this study were obtained from Merck (Darmstadt, Germany). Medium ISP4 was designed in the lab whereas Sabouraud dextrose agar (SDA) was procured from Oxoid, England, and Tween-20 was acquired from Merck-Schuchardt, USA.
2.3 Preparation of crude extract
After complete washing with running water to eliminate the contamination, plant material was shade dried at ambient conditions with active ventilation for about three weeks. Dried stem and leaves were milled individually to a fine powder by using an electric knife miller and kept in airtight jars. The powdered material was extracted by ultra-sonication supported maceration using analytical grade solvents i.e., n-hexane (Hex), Ethyl Acetate-n-hexane (EtOAc-Hex), Ethanol-n-hexane (EtOH-Hex), Chloroform (CHCl3), Ethyl Acetate (EtOAc), Methanol-Chloroform (MeOH-CHCl3), Methanol-Ethyl Acetate (MeOH-EtOAc), Acetone (ACET), Methanol (MeOH), Methanol-Acetone (MeOH-ACET), Ethanol (EtOH), Distilled Water-Acetone (D.W.-ACET), Methanol-Distilled water (MeOH-D.W.) and Distilled Water (D.W.). The extraction solvents were employed either alone or in combination (1:1) as described earlier. After careful measurement, plant material (40 g) was macerated in one liter Erlenmeyer flask with 200 ml solvent for 24 h at room temperature through intermittent ultra-sonication (room temperature, 30 min). Same procedure was repeated two times on the marc. Filtration was carried out using muslin cloth and Whattman No. 1 filter paper. Afterward, the filtrates were concentrated through vacuum evaporation (Rotary evaporator R-210 Buchi, Switzerland) at 60 °C and were dried in a vacuum oven (Yamato, Japan) at 45 °C to obtain crude extracts which were then stored at −20 °C till further use.
2.4 Phytochemical analysis
2.4.1 Determination of total phenolic content
Folin–Ciocalteu reagent-based colorimetric assay was employed for phenolic content determination as described earlier (Zahra et al., 2017). From the stock solution of each test sample (4 mg/ml DMSO), an aliquot of 20 μl was transferred in the specific well of 96 well plate. Then 90 μl of Folin–Ciocalteu reagent was added. After incubation (5 min), 90 μl of sodium carbonate was added to the reaction mixture. Gallic acid with concentrations ranging from 0 to 25 μg/ml worked as positive control while DMSO served as a negative control. The absorbance was measured at 630 nm via a microplate reader (Biotech USA, microplate reader Elx 800). A calibration curve (y = 0.1198x + 0.1663, R2 = 0.9982) was developed in parallel under the same working circumstances using gallic acid as a standard. The analysis was performed in triplicate. Results are expressed as μg gallic acid equivalent per milligram of extract (μg GAE/mg extract).
2.4.2 Determination of total flavonoid content
The total flavonoid content was assessed by the aluminum chloride colorimetric method (Zahra et al., 2017). From the stock solution (4.0 mg/ml DMSO) of each extract, an amount of 20 μl was added to the respective well of 96 well plate. Then, 10% aluminum chloride and 1.0 M potassium Acetate (10 μl each) were added to a specific well with the subsequent addition of distilled water (160 μl) and set aside at room temperature (30 min). The absorbance was assessed at 415 nm via a microplate reader. The calibration curve was developed by using quercetin as a positive control with two-fold serial dilutions (0, 2.5, 5, 10, 20, 40 μg/ml). The flavonoid content was expressed in μg quercetin equivalents per milligram of extract (μg QE/mg extract). The equation used for the calibration curve was y = 0.0727x + 0.0246 (R2 = 0.9974). The assay was performed in triplicate.
2.4.3 HPLC-DAD quantitative analysis
Reverse-phase high-performance liquid chromatography (RP-HPLC) was performed by previously documented procedure (Ahmed et al., 2019). The components of the device consisted of column oven (CTO-20A), auto-sampler (SIL-20A), and diode array detector (SPD-M20A) along with HPLC system (Shimadzu LC-20AT). The analytical column Nucleosil C18, 5 µm (particle size, 4.6 × 250 mm. Phenomenex) coupled with a guard column (KJO-4282, Phenomenex) was used. The polyphenols detection was accomplished by binary gradient system with mobile phase A (methanol: water: acetic acid: acetonitrile in 10:85:1:5 ratio) and mobile phase B (acetonitrile: methanol: acetic acid in 40:60:1 ratio). The flow rate was adjusted at 1 ml/min and the column temperature was maintained at 25 °C. The sample solution (20 µl in methanol) was injected into the column. Column was reconditioned for 10 min before injecting new sample. The gradient volume of (mobile phase B) was 0–50% in (0–20 min), 50–100% in (20–25 min) and 100% in last 25 to 30 min. Standards were prepared and further diluted to get the stock solution with two-fold serial dilutions (10, 20, 50, 100, 200 μg/ml of methanol). Qualitative analysis of monomers has been conducted through UV absorption spectra and retention time of corresponding standards. The absorbance of various extracts was noted at the λmax of standard polyphenols employed i.e., 257 nm for rutin, 368 nm for myricetin, kaempferol and quercetin, 325 nm for apigenin and caffeic acid, 279 nm for gallic acid and catechin. The whole analysis was performed in triplicate at ambient conditions.
2.5 Biological evaluation
2.5.1 Free radical scavenging assay
The capability to reduce (quench) the stable free radical 2, 2-diphenyl 1-picrylhydrazyl (DPPH) was examined to estimate the antioxidant proficiency of extracts (Zahra et al., 2017). An aliquot of 20 µl from sample stock solution (4 mg/ml DMSO) was transferred in the respective well of 96 well plate having 180 µl of DPPH (9.2 mg/100 ml methanol) solution. The reaction mixture was allowed to incubate at 37 °C for 30 min. Percentage of free radical scavenging activity (%FRSA) was determined and extracts showing>50% scavenging were evaluated at lower concentrations (200, 66.66, 22.22, and 7.406 μg/ml) to determine 50% inhibitory concentration (IC50). Absorbance was noted via a microplate reader at 517 nm. Percent free radical scavenging activity (%FRSA) was estimated by the following formula:
Abs represents the absorbance of the sample, whereas Abc shows the absorbance of negative control (containing DMSO instead of the sample). Ascorbic acid was employed as a standard and analysis was carried out three times.
2.5.2 Antimicrobial assays
2.5.2.1 Antileishmanial assay
The antileishmanial potential of test samples (extract) was assessed by MTT colorimetric assay according to previously documented procedure with minor changes (Ahmed et al., 2019). The Medium-199 (supplemented with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin sulphate, 100 IU/ml penicillin G) was used to culture Leishmania tropica kwh23 followed by incubation for 6–7 days at 24 °C. The promastigote culture (180 µl, seeding density of 1 × 106 promastigotes/ml) was transferred to the respective well of 96 well plate already provided with test samples (20 µl). The test sample had not more than or equal to 1% DMSO in PBS with an absolute concentration of 100 µg/ml. Amphotericin B (0.33–0.004 µg/ml) was used as a standard while 1% DMSO in PBS served as a negative control. After incubation for 72 h at 24 °C, a stock solution of 4 mg MTT reagent per ml in distilled H2O (20 µl, pre-filter, sterilized) was added to plate followed by another period of incubation (24 °C, 4 h). Next, the supernatant was isolated cautiously avoiding the disturbance of coloured formazan sediments. DMSO (100 µl) was mixed in well and allowed to set aside (1 h) to confirm the complete dissolution of formazan sediments. The absorbance was calculated via a microplate reader at 540 nm. The extracts with>50% cell mortality at 100 µg/ml were further studied at lower concentrations i.e., 33.3, 11.1, 3.7, and 1.23 µg/ml. IC50 was evaluated using Graph pad prism 2D version 5.01 software.
2.5.2.2 Antibacterial assay
The agar disc diffusion method was employed to evaluate the antibacterial potential (In vitro) of test samples with minor amendments as described earlier (Khan et al., 2015). The refreshed bacterial cultures lawn: Staphylococcus aureus (ATCC-6538), Bacillus subtilis (ATCC-6633), Escherichia coli (ATCC-25922), Klebsiella pneumoniae (ATCC-1705), and Pseudomonas aeruginosa (ATCC- 15442) with pre-adjusted turbidity was developed on Petri plates imbedded with nutrient agar. The sterile filter paper discs infused with test extract (5 μl, 20 mg/ml DMSO) were directly placed on the surface of seeded plates. The cefixime and roxithromycin-infused disc (4 mg/ml in DMSO) worked as positive control whereas DMSO infused disc served as a negative control. After incubation (37 °C, 24 h), the average diameter of the inhibition zone adjacent to the discs was noted. The samples (extracts) showing an inhibition zone greater than or equal to 10 mm in diameter were considered as active followed by further screening to determine minimum inhibitory concentrations (MIC: lowest concentration of the extract inhibiting the growth of bacterial strain) by standard three-fold micro broth dilution method. The serial dilution method was employed on the stock solution of active samples in 96 well plate using Mueller Hinton broth to attain a range of concentrations (100, 33.33, 11.11, and 3.70 μg/ml). A standardized inoculum of every single bacterial strain (5 × 104 CFU/ml) was transferred to the respective well. Following incubation (37 °C, overnight), MIC was assessed by calculating OD at 600 nm and the assay was completed as triplicate analysis.
2.5.2.3 Antifungal assay
The agar disc diffusion method was applied to assess the antifungal potential of test samples (extracts) in triplicate (Khan et al., 2015). The spores of given fungal strains [Aspergillus fumigatus (FCBP- 66), Mucor species (FCBP-0300), Aspergillus niger (FCBP-0198), and Aspergillus flavus (FCBP-0064)] were gathered in Tween 20 solution (0.02%). The turbidity of spores was adjusted conferring to McFarland 0.5 turbidity standard. Then harvested fungal strains (100 μl) were streaked on the plate’s surface having Sabouraud Dextrose agar. Sterile filter paper discs infused with test extract (5 μl, 20 mg/ml DMSO) were placed on the seeded plates. DMSO soaked disc acted as a negative control whereas standard drug clotrimazole served as a positive control. Following incubation (28 °C for 2448 h), the average diameter (mm) of the zone of inhibition around the disc was measured. Samples (extracts) showing inhibition zone ≥ 10 mm were further examined for MIC (minimum inhibitory concentration) estimation at lower concentrations (50, 25, 12.5, 6.25, and 3.12 μg/disc) by standard disc diffusion method.
2.5.3 α-amylase inhibition
The α-amylase inhibition assay was performed to assess the α-amylase inhibitory potential of test extracts by previously documented procedure with minor changes (Ahmed et al., 2019). The reaction mixture containing 15 μl phosphate buffer (pH 6.8), 25 μl α-amylase enzyme (0.14 U/ml), 10 μl sample (4 mg/ml DMSO), and 40 μl of starch solution (2 mg/ml in potassium phosphate buffer) was allowed to incubate at 50 °C for 30 min in 96 well plate. Subsequently, an aliquot of 20 μl of HCl (1 M) solution was added to stop the reaction. Next an amount of 90 μl of iodine reagent (5 mM of each potassium iodide and iodine in phosphate buffer) was mixed into each well. Blank was formulated by utilizing DMSO and phosphate buffer as a substitute of plant extract and enzyme solution respectively while negative control was prepared by using DMSO instead of test extract. 250 μM of acarbose acted as standard (positive control). Absorbance was noted at 540 nm. The percent α-amylase inhibition was calculated by the subsequent equation:
Whereas, On = Absorbance of negative control, Os = Absorbance of the sample, and Ob = Absorbance of the blank well.
2.5.4 Cytotoxicity assays:
2.5.4.1 Brine shrimp lethality assay
Brine shrimp lethality assay was carried out against Artemia salina (Brine shrimp) in 96 well plates for 24 h by the formerly adopted procedure with minor changes (Zahra et al., 2017). Eggs of Artemia salina (Ocean 90, USA) were allowed to hatch at 30–32 °C under direct light and constant provision of oxygen in a specially designed vessel with two compartments separated by a porous partition in sea water (38 g/l supplemented with 6 mg/l dried yeast) for 24–48 h. The mature nauplii were harvested with help of a Pasteur pipette and assembled in a small container. Next, the nauplii were transferred into 96 well plates containing test extracts (with serial dilutions), and volume was adjusted with sea water so that the concentration of DMSO was lower than 1%. The lethality of extracts was tested at four different concentrations i.e. 200, 100, 50, and 25 μg/ml. Doxorubicin (1.25–10 μg/ml) worked as standard, while DMSO was employed as a negative control. The percent lethality shown by each sample was measured by considering the number of alive nauplii after 24 h period. The test samples showing greater than or equal to 50% mortality were measured for median lethal concentration (LC50) via Graph pad prism 2D version 5.01 software. The whole procedure was carried out three times.
2.5.4.2 Hemolytic assay
The hemolytic assay was carried out to assess the effect of various extracts from stem and leaf parts of O. limbata by previously documented procedure (Nasar et al., 2018). One ml of fresh human blood was taken in Eppendorf (1.5 ml) followed by centrifugation (14,000 rpm) to get RBCs (erythrocytes). After discarding the supernatant, an aliquot of 200 µl from blood (pellet) was kept in a falcon tube with the subsequent addition of phosphate buffer saline (PBS, 9.8 ml) and another cycle of centrifugation (10 min, 2000 rpm). The supernatant was thrown out and the washing process was repeated three times. A portion of 100 µl of red blood cell suspension (suspended in PBS) was transferred to a 96 well plate followed by the addition of stem and leaf extracts of O. limbata (dissolved in PBS). The plates were allowed to incubate for one hour (35 °C) followed by centrifugation at 1000 rpm for 10 min. The supernatant was collected and dispensed into 96 well plates. The release of hemoglobin was monitored by a microplate reader at 540 nm. Triton X-100 (0.5%) worked as a positive control, whereas PBS served as a negative control. Percent hemolysis was assessed using the following formula;
Whereas, Abs = Absorbance of the sample, Abn = Absorbance of negative control, and Abp = absorbance of positive control.
2.5.4.3 Cytotoxicity against THP-1 human leukemia cell line
Human leukemia (THP-1) cell line (ATCC # TIB-202) was used to assess the in vitro cytotoxic potential of test samples (crude extract) according to previously documented procedure with minor changes (Ahmed et al., 2019). Concisely, the complete growth medium comprising of RPMI-1640 buffered with 2.2 g/l NaHCO3 and supplemented with 10% HIFBS (heat-inactivated fetal bovine serum) was used to culture leukemia cells at 37 °C (7.4 pH) in a 5% humidified CO2 incubator (Panasonic, Japan MCO-18 AC-PE) for 72 h. A portion of 190 μl of THP-1 cells was transferred to the respective well of 96 well plate with a defined seeding density (5 × 105 cells per ml). Afterward, the sample (10 μl) having not>1% DMSO in phosphate buffer saline was added. Samples were evaluated at a concentration of 20 μg/ml three times. The reaction mixture was allowed to incubate in a humidified CO2 incubator with 5% CO2 at 37 °C for 72 h. The standard drugs used were vincristine and 5-florouracil prepared at a final concentration of 20 μg/ml. 1% DMSO solution in PBS served as a negative control. An amount of 20 µl of MTT solution (4 mg/ml in distilled water, sterile filtered) was infused in plate followed by incubation in a CO2 incubator (5%) at 37 °C for 4 h. Afterward, the formazan sediments were carefully removed. DMSO (100 µl) was mixed in the respective well and set aside for 1 h to make sure the complete dissolution of formazan sediments. Eventually, the absorbance was noted via a microplate reader at 540 nm. Samples presenting ≥ 50% inhibition were further examined at lesser concentrations of 6.66, 2.22, 0.74, and 0.24 μg/ml. The analysis was carried out three times. The inhibitory concentration (IC50) was measured through Graph pad prism 2D version 5.01 software.
2.5.4.4 Cytotoxicity against Hep-G2 cell line
Cytotoxic potential of the samples was assessed via SRB colorimetric assay against Hep-G2 cell line (RBRC-RCB1648) according to a previously documented process with minor changes (Ahmed et al., 2019). DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 100 μg/ml streptomycin sulphate, 10% FBS, 100 IU/ml penicillin G sodium and 0.25 μg/ml amphotericin B was employed for the proliferation of Hep-G2 cells. The plate was allowed to incubate in CO2 humidified incubator (5% CO2) at 37 °C for 72 h to achieve 70–80% confluence of cells. The fresh medium was used and another 24 h incubation period was subjected to cells followed by trypsonisation and dilution to achieve the required seeding density (1 × 105 cells/ml). The test sample (20 μl) containing 1% DMSO in PBS was added to a specific well of 96 well plate. Afterward, an aliquot of 180 μl is added to each well from Hep-G2 culture to achieve a final concentration of 20 μg/ml. Doxorubicin with concentrations 20, 6.66, 2.22, 0.74, 0.24, and 0.08 μg/ml in Phosphate Buffer Saline served as positive control whereas 1% dimethyl sulfoxide in PBS worked as a negative control. After another period of humidified incubation (37 °C for 72 h in a CO2 incubator), the culture plate was supplied with 50 μl of cold TCA solution for cell fixation (20% w/v) followed by washing with tap water (4 times). Subsequently, the plate was dried and stained with SRB reagent (50 μl of 0.057% w/v SRB in 1% w/v acetic acid) for 30 min at room temperature. After a series of repeated washing with acetic acid (1% v/v), plates were allowed to dry overnight. An amount of 200 μl of Tris base (10 mM) at pH 10 was used to dissolve the bound dye and maintained at room temperature for 1 h. A microplate reader (Biotech USA, microplate reader Elx 800) was used to measure the optical density at 515 nm and percent survival of cells was noted. Zero-day control was run by the addition of cells in 16 wells in equal amount for 1 h at 37 °C and the same procedure was followed as described earlier. Percent inhibition was measured by the following formula:
2.5.4.5 Protein kinase inhibition assay
The protein kinase inhibition assay was accomplished by using a purified strain of Streptomyces 85E (Zahra et al., 2017). The tryptone soya broth medium was utilized to refresh the strain of Streptomyces 85E for 96 h at 37 °C. The mycelia fragments (spores) of Streptomyces were dispersed in minimal ISP4 medium on sterilized plates to grow the bacterial lawn. An amount of 5 μl from each sample stock solution (20 mg extract /ml of DMSO) was loaded onto a sterile filter paper disc (6 mm). The saturated paper discs having an absolute concentration (100 μg/disc) were placed on the surface of plates imbedded with Streptomyces 85E. Surfactin acted as positive control while DMSO served as a negative control. The plates were incubated approximately for 72 h (time necessary for hyphae formation) at 30 °C. The results were inferred as a bald zone of inhibition around the discs.
2.6 Statistical analysis
The results obtained for cytotoxic, antimicrobial, alpha-amylase inhibitory, and phytochemical assays were analyzed statistically by one-way analysis of variance (ANOVA) followed by Tukey and Duncan’s test using the statistical package PASW Statistics 18 and P < 0.05 was considered as significant when appropriate. Data were expressed as mean ± SD. Data were expressed as mean ± standard deviation (SD). Correlation of the phytochemical activities and %FRSA was performed via correlation and regression by Microsoft Excel program.
3 Results
3.1 Phytochemical analysis
3.1.1 Total phenolic content (TPC)
The total phenolic content of stem and leaf extracts of O. limbata prepared in different solvents is shown in Fig. 1. Among the stem extracts, the highest phenolic content i.e., 53.29 ± 1.33 µg GAE/mg extract was found in the MeOH stem extract. In the case of leaf extracts, maximum phenolic content i.e. 21.14 ± 1.41 µg GAE/mg extract was recorded in D.W.-ACET and MeOH-ACET extracts. The total phenolic content was maximum (MeOH-ACET = 53.29 ± 1.33 µg GAE/mg extract) in moderately polar extract while minimal content was reported in fairly non-polar extract (Hex = 0.59 ± 1.22 µg GAE/mg extract) of stem and leaf respectively.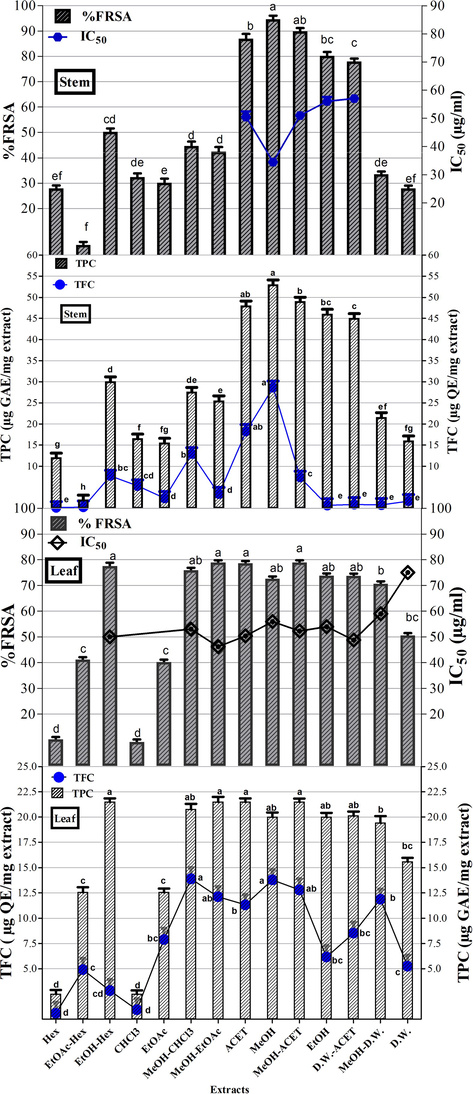
TPC (Total phenolic content μg GAE/mg extract), TFC (Total flavonoid content μg QE/mg extract), and %FRSA (%Free radical scavenging activity) of O. limbata in different solvents. Values are presented as mean ± standard deviation from the triplicate investigation. The columns with different superscript (a-h) letters show significantly (P < 0.05) different means. Note:- Hex: n-hexane, EtOAc-n-Hex: Ethyl acetate-n-hexane, EtOH-Hex: Ethanol-n-hexane, CHCl3: Chloroform, EtOAc: Ethyl acetate, MeOH-CHCl3: Methanol-Chloroform, MeOH-EtOAc: Methanol-Ethyl acetate, ACET: Acetone, MeOH: Methanol, MeOH-ACET: Methanol-Acetone, EtOH: Ethanol, D.W.-ACET: Distilled Water-Acetone, MeOH-D.W.: Methanol-Distilled Water and D.W.: Distilled Water extracts.
3.1.2 Total flavonoid content (TFC)
The total flavonoid content in terms of µg quercetin equivalent/mg extract is presented in Fig. 1. In stem extracts, the maximum flavonoid content i.e., 28.64 ± 1.16 µg quercetin equivalent/mg extract (μg QE/mg extract) has been documented in the MeOH extract Among the leaf extracts, the highest flavonoid content was quantified in MeOH-CHCl3 extract (13.89 ± 1.22 µg QE/mg extract).
3.1.3 HPLC-DAD analysis
Quantification of specific polyphenols via reverse-phase HPLC-DAD technique by comparing the UV spectra and retention time of the standard with those of test extracts has been presented in Table 1 and Figs. 2a-d. A substantial amount of rutin, gallic acid, apigenin, and caffeic acid was observed in several extracts. Both stem and leaf extracts exhibited a noteworthy quantity of caffeic acid in the MeOH-EtOAc (12.10 ± 0.8 and 3.09 ± 0.7 μg/mg extract respectively) and EtOH (7.65 ± 0.4 and 1.31 ± 0.6 μg/mg extract respectively) extracts. A significant amount of gallic acid and apigenin was also measured in MeOH-EtOAc (2.20 ± 0.23 and 1.89 ± 0.31 μg/mg extract respectively) and EtOH (0.74 ± 0.43 and 0.81 ± 0.46 μg/mg extract respectively) extract of the stem. A significant quantity of rutin, gallic acid, and apigenin was observed in the MeOH extract of the stem.
-- not detected.
Extracts
Polyphenols (μg/mg)
Rutin
Gallic acid
Catechin
Caffeic acid
Apigenin
Stem
MeOH
3.79 ± 0.03
0.58 ± 0.001
–
0.51 ± 0.02
0.65 ± 0.001
EtOH
–
0.74 ± 0.003
–
7.65 ± 0.41
0.81 ± 0.002
MeOH-EtOAc
0.31 ± 0.05
2.20 ± 0.004
–
12.10 ± 0.81
1.89 ± 0.01
Leaf
MeOH
–
–
–
–
–
EtOH
–
–
–
1.31 ± 0.04
0.41 ± 0.004
EtOAc
–
–
–
0.47 ± 0.002
–
MeOH-EtOAc
0.62 ± 0.01
–
–
3.09 ± 0.7
0.31 ± 0.001
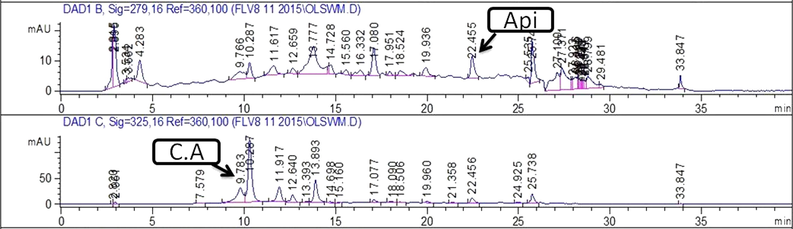
Chromatograms showing peaks of phenolics detected in EtOH extract of leaf. Note:- C.A: Caffeic acid; Api: Apigenin.
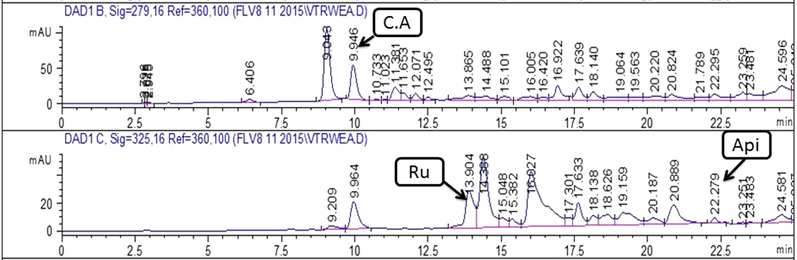
Chromatograms showing peaks of phenolics detected in MeOH-EtOAc extract of leaf . Note:- C.A: Caffeic acid; Api: Apigenin; Ru: Rutin.
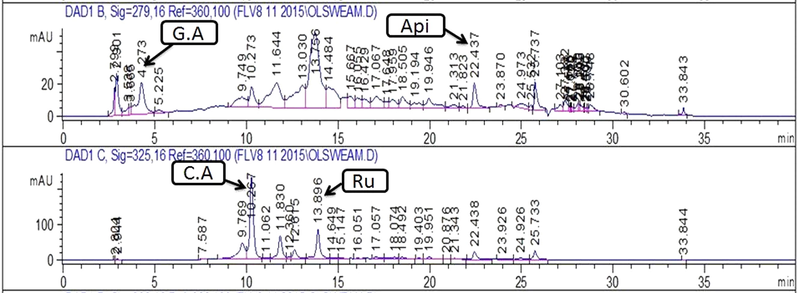
Chromatograms showing peaks of phenolics detected in MeOH-EtOAc extract of stem. Note:- C.A: Caffeic acid; Api: Apigenin; Ru: Rutin; G.A: Gallic acid.
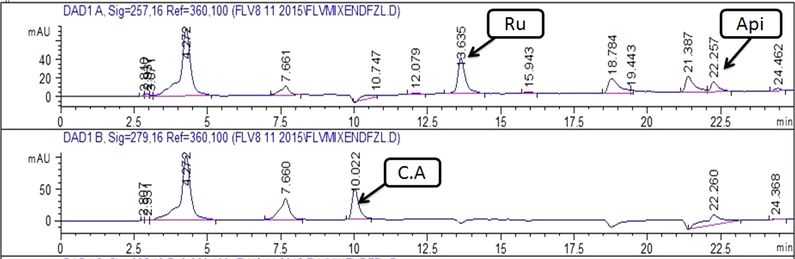
Chromatograms showing peaks of phenolics detected in EtOAc extract of stem.Note:- C.A: Caffeisc acid; Api: Apigenin; Ru: Rutin; G.A: Gallic acid.
3.2 Biological evaluation
3.2.1 Antioxidant activity
-
Free radical scavenging activity
The results of percent free radical scavenging activity (%FRSA) of stem and leaf extracts have been presented in Fig. 1. Most proficient antioxidant activity was displayed by MeOH (34.5 ± 1.34 μg/ml) stem extract followed by ACET (IC50 = 50.52 ± 1.14 μg/ml), MeOH-ACET (IC50 = 51.04 ± 1.04 μg/ml), EtOH (56.00 ± 1.30 μg/ml) and D.W.-ACET (57.04 ± 1.24 μg/ml) extracts. In the case of leaf extracts, 64% of extracts exhibited significant free radical quenching capability whereas the greatest antioxidant prospective was confirmed by the MeOH-EtOAc extract (IC50 = 46.05 ± 1.39 μg/ml). D.W. extract (IC50 = 75.02 ± 1.30 μg/ml) has shown the lowest antioxidant activity among leaf extracts. Polyphenols are less in MeOH extract in comparison to EtOH and MeOH-EtOAc but exhibited highest antioxidant potenial because pytochemicals other than polyphenols act as antioxidants such as diterpenoids as some previoius studies have reported the presense of clerodane type diterpenoids (Limbatolide, Limbatenolide) in of O. limbata.
3.2.2 Antimicrobial activity
3.2.2.1 Antileishmanial activity
Antiproliferative activity against Leishmanial promastigotes based on MTT assay is summarised in Fig. 3. Among the stem extracts, noteworthy activity was observed in Hex, CHCl3, EtOAc-Hex, and MeOH-EtOAc extracts (IC50 values ranging from 4.45 ± 0.45 to 11.67 ± 0.45 μg/ml) while MeOH-CHCl3, ACET, and D.W.-ACET extracts showed moderate activity (IC50 = 21.75 ± 0.45 μg/ml). In the case of leaf extracts, remarkable inhibition was recorded in MeOH-D.W., MeOH, EtOH, and MeOH-CHCl3 extracts with IC50 of 1.50 ± 0.23, 4.66 ± 0.23, 5.78 ± 0.20, and 11.11 ± 0.01 μg/ml whereas EtOAc-Hex, EtOH-Hex, ACET, MeOH-ACET, D.W.-ACET and D.W. samples showed moderate activities (IC50 = 100 ± 0.45 μg/ml). The positive control (amphotericin B) showed an IC50 value of 0.01 μg/ml while DMSO (negative control) did not show any activity.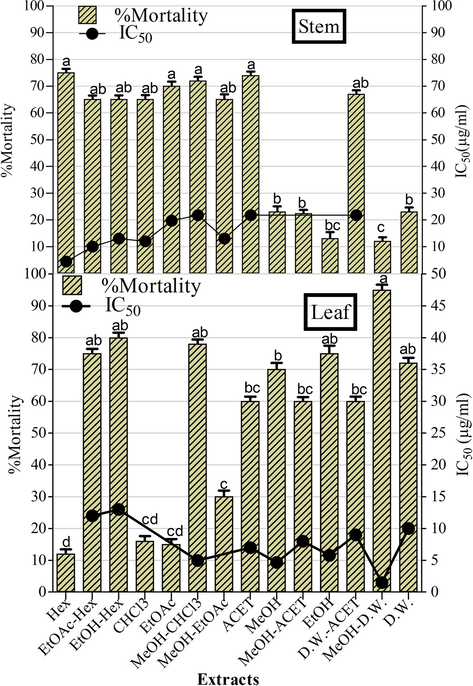
The antileishmanial potential of O. limbata extracts. Amphotericin B (positive control) showed IC50 value of 0.01 μg/ml. Values are presented as mean ± standard deviation from the triplicate investigation. The columns with different superscript (a-d) letters show significantly (P < 0.05) different means.
3.2.2.2 Antibacterial activity
The antibacterial potential of O. limbata extracts against various bacterial strains is documented in Table 2. Most of the extracts were found to be active with a minimum zone of inhibition ≥ 10 mm. A prominent zone was observed against P. aeruginosa by EtOH extract (13 ± 0.56 mm, MIC = 33.3 ± 0.52 μg/ml) followed by 71% stem extracts with zone ranging from 10 to 12 mm at a concentration of 100 μg/disc. The Hex extract of stem displayed the best inhibitory potential against B. subtilis with 16 ± 0.44 mm diameter (MIC = 33.3 ± 0.53 μg/ml) followed by 35% extracts (ZOI = 10–12 mm). A noticeable zone of inhibition was detected against S. aureus by various stem extracts including D.W.-ACET and MeOH-D.W. (MIC = 33.33 ± 0.37 μg/ml) extracts. Several leaf extracts have shown noteworthy antibacterial affect against E. coli, P. aeruginosa, S. aureus, B. subtilis, and K. proteus. In the case of leaf extracts, the most significant activity was documented against P. aeruginosa by EtOH extract (MIC = 33.3 ± 0.55 μg/ml) followed by EtOH-Hex, MeOH-CHCl3, CHCl3, and MeOH-D.W. extracts with MIC value of 33.3 ± 0.46 μg/ml. Noticeable activity against B. subtilis was recorded by MeOH-ACET and ACET extracts (MIC = 33.3 ± 0.56 μg/ml) whereas CHCl3 extract showed potent activity against S. aureus (MIC = 33.3 ± 0.65 μg/ml) in leaf extracts. The zones produced by the extracts were comparable to the standard cefixime which produced 18 ± 0.45 mm zone of inhibition at 10 μg/disc concentration. The activity was more intensified in non-polar and moderately polar extracts. The negative control (DMSO) showed no inhibition. Zone of bacterial growth inhibition was measured including 5 mm of the disc. The concentration of the sample on each disc was 100 μg/ml. Triplicate analysis (n = 1 × 3) was performed to express the statistical data as mean ± standard deviation. The columns with different superscript (a-d) letters show significantly (P < 0.05) different means, – shows no activity.
Extracts
Diameter of growth inhibition zone (mm ± SD) at 100 μg/disc : MIC; μg/ml
E. coli
MIC
K. proteus
MIC
P. aeruginosa
MIC
B. subtilis
MIC
S. aureus
MIC
Stem
Hex
10 ± 0.56d
100
–
–
–
–
16 ± 0.44bc
33.3
–
–
EtOAc-Hex
–
–
–
–
10 ± 0.44d
100
11 ± 0.54 cd
100
–
–
EtOH-Hex
–
–
–
–
–
–
–
–
11 ± 0.66 cd
100
CHCl3
–
–
–
–
–
–
10 ± 0.45d
100
–
–
EtOAc
–
–
–
–
11 ± 0.44 cd
100
10 ± 0.74d
100
–
–
MeOH-CHCl3
–
–
–
–
11 ± 0.44 cd
100
–
–
11 ± 0.55 cd
100
MeOH-EtOAc
–
–
–
–
10 ± 0.44d
100
–
–
–
–
ACET
–
–
–
–
11 ± 0.44 cd
100
–
–
–
–
MeOH
–
–
–
12 ± 0.55 cd
33.3
10 ± 0.55d
100
10 ± 0.55d
100
MeOH-ACET
9 ± 0.45
–
–
–
12 ± 0.55 cd
33.3
–
–
–
–
EtOH
9 ± 0.45
–
–
–
13 ± 0.56c
33.3
–
–
–
–
D.W.-ACET
–
–
–
–
11 ± 0.44 cd
100
–
–
13 ± 0.55c
33.3
MeOH-D.W.
–
–
–
–
11 ± 0.33 cd
100
10 ± 0.46d
100
12 ± 0.44 cd
33.3
D.W.
10 ± 0.44d
100
–
–
–
–
Leaf
Hex
–
–
–
–
–
–
–
–
11 ± 0.44 cd
100
EtOAc-Hex
12 ± 0.76 cd
33.3
–
–
–
–
–
–
12 ± 0.44 cd
33.3
EtOH-Hex
–
–
–
–
13 ± 0.44c
33.3
–
–
–
–
CHCl3
–
–
–
–
12 ± 0.55 cd
33.3
–
–
14 ± 0.45bc
33.3
EtOAc
–
–
9 ± 0.34
–
–
–
–
–
12 ± 0.45 cd
33.3
MeOH-CHCl3
–
–
–
–
13 ± 0.89c
33.3
–
–
11 ± 0.45 cd
100
MeOH-EtOAc
11 ± 0.76 cd
100
–
–
–
–
10 ± 0.45d
100
–
–
ACET
11 ± 0.43 cd
100
–
–
11 ± 0.56 cd
100
13 ± 0.45c
33.3
11 ± 0.34 cd
100
MeOH
–
–
10 ± 0.34d
100
12 ± 0.56 cd
100
12 ± 0.45 cd
33.3
10 ± 0.44d
100
MeOH-ACET
10 ± 0.43d
100
10 ± 0.34d
100
–
–
13 ± 0.45c
33.3
–
–
EtOH
11 ± 0.54 cd
100
–
–
14 ± 0.56bc
33.3
11 ± 0.45 cd
100
–
–
D.W.-ACET
12 ± 0.76 cd
33.3
–
–
–
–
11 ± 0.45 cd
100
–
–
MeOH-D.W.
–
–
–
–
13 ± 0.56c
33.3
–
–
–
–
D.W.
12 ± 0.34 cd
33.3
–
–
–
–
–
–
–
–
Roxithromycin
25. ± 0.45a
0.33
24 ± 0.55a
0.33
23 ± 0.56a
0.33
24 ± 0.33a
0.33
25 ± 0.45a
0.33
Cefixime
19 ± 0.55b
1.11
20 ± 0.55a
0.33
22 ± 0.56a
0.33
18 ± 0.45b
1.11
24 ± 0.44a
0.33
DMSO
–
–
–
–
–
3.2.2.3 Antifungal assay
The results of antifungal propensity against four kinds of fungal strains are depicted in Table 3. In the case of stem extracts, CHCl3 and ACET exhibited most prominent antifungal activities against A. fumigatus, Mucor sp, A. flavus, and A. niger with MIC values ranging from 6.25 to 50 µg/disc respectively. The most significant activity against A. flavus waspresented by 85% stem extracts wherein EtOAc-Hex extract showed the best activity with a MIC value of 6.25 µg/disc. Hex, CHCl3, EtOAc, ACET, and EtOH extracts also presented good antifungal potential against the Mucor species with MIC = 6.25 µg/disc. In the case of leaf extracts, the most prominent antifungal potential was presented against A. niger by Hex, CHCl3 and EtOAc extract with the MIC value of 12.5 µg/disc. The noticeable activity against A. fumigatus was revealed by 42% leaf extracts whereas the most potent activities were documented by EtOH extract (MIC = 6.25 µg/disc). Substantial antifungal action was also exhibited against Mucor species by several leaf extracts. Clotrimazole as a positive control revealed the maximum antifungal propensity however DMSO did not show any antifungal tendency. Zone of growth inhibition was measured including 5 mm of the disc. The concentration of the sample on each disc was 100 μg. Values are represented as mean ± standard deviation by triplicate analysis. The columns with different superscript (a-d) letters show significantly (P < 0.05) different means, –: shows no activity.
Extracts
Diameter of growth inhibition zone at 100 µg/disc (mm ± SD): MIC; μg/disc
A. fumigatus
MIC
Mucor sp
MIC
A. niger
MIC
A. flavus
MIC
Stem
Hex
–
–
16 ± 0.65bc
6.25
14 ± 0.66bc
25
14 ± 0.22bc
12.5
EtOAc-Hex
–
–
14 ± 0.65bc
12.5
–
–
15 ± 0.33bc
12.5
EtOH-Hex
12 ± 0.65d
100
–
–
–
–
14 ± 0.33bc
12.5
CHCl3
13 ± 0.65c
50
15 ± 0.65bc
6.25
13 ± 0.66c
25
15 ± 0.33bc
12.5
EtOAc
–
–
17 ± 9.65b
6.25
12 ± 0.67d
25
13 ± 0.66c
50
MeOH-CHCl3
–
–
12 ± 0.65d
100
–
–
14 ± 0.3bc
12.5
MeOH-EtOAc
–
–
13 ± 0.66c
50
–
–
13 ± 0.33c
25
ACET
14 ± 0.65bc
12.5
18 ± 0.56b
6.25
13 ± 0,56c
25
13 ± 0.33c
50
MeOH
–
–
–
–
–
–
13 ± 0.43c
50
MeOH-ACET
–
–
–
–
–
–
12 ± 0.43d
50
EtOH
–
–
18 ± 0.45b
6.25
–
–
13 ± 0.33c
50
D.W.-ACET
–
–
–
–
–
–
–
–
MeOH-D.W.
–
–
–
–
–
–
–
–
D.W.
–
–
–
–
–
–
13 ± 0.43c
25
Leaf
Hex
–
–
–
–
14 ± 0.56bc
12.5
–
–
EtOAc-Hex
13 ± 0.66c
50
–
–
–
–
–
EtOH-Hex
14 ± 0.67bc
12.5
–
–
13 ± 0.56c
50
–
–
CHCl3
–
–
–
–
15 ± 0.56bc
12.5
–
–
EtOAc
13 ± 0.45c
50
–
–
13 ± 0.56c
12.5
–
–
MeOH-CHCl3
–
–
–
–
13 ± 0.56c
25
–
–
MeOH-EtOAc
–
–
12 ± 0.43d
100
–
–
–
–
ACET
13 ± 0.45c
50
12 ± 0.44d
100
14 ± 0.67bc
25
–
–
MeOH
14 ± 0.45bc
12.5
–
–
13 ± 0.66c
25
–
–
MeOH-ACET
–
–
12 ± 0.44d
100
15 ± 0.56bc
25
–
–
EtOH
17 ± 0.45b
6.25
–
–
–
–
–
–
D.W.-ACET
–
–
–
–
–
–
–
–
MeOH-D.W.
–
–
–
–
–
–
–
–
D.W.
–
–
–
–
–
–
–
–
Clotrimazole
30a
5
30a
25
30a
5
31a
10
DMSO
–
–
–
–
–
–
–
–
3.2.3 α-amylase inhibition
The recent study investigated the antidiabetic propensity of O. limbata extracts for the first time via alpha-amylase inhibition assay with results described in Fig. 4. A remarkable α-amylase inhibitory was exhibited by non-polar leaf extracts including Hex and CHCl3 with percent inhibition of 90% and 95% with IC50 of 36.2 ± 0.49 and 33.1 ± 0.54 μg/ml respectively. The positive control used was acarbose with α-amylase inhibition of 87% (IC50 = 33.73 ± 0.12 μg/ml). The most significant α-amylase inhibitory potential was exhibited by CHCl3 (IC50 = 33.1 ± 0.54 μg/ml) and Hex (IC50 = 36.2 ± 0.49 μg/ml) leaf extracts was comparable to acarbose (IC50 of 33.73 ± 0.12 μg/ml) employed as a positive control in this study.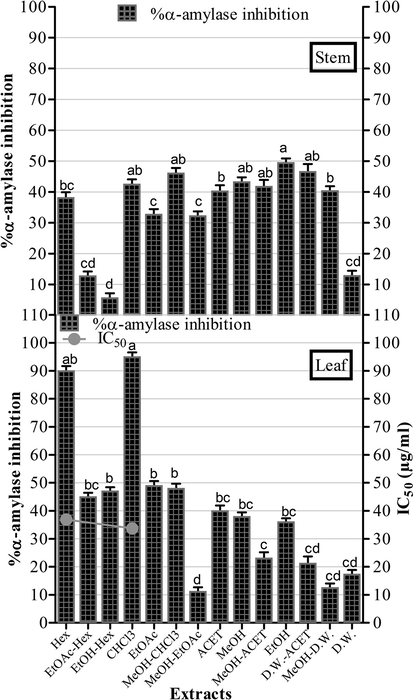
α amylase inhibition by O. limbata extracts. The IC50 of acarbose (positive control) = 33.73 ± 0.12 μg/ml. The experiment was performed in triplicate and values are presented as mean ± standard deviation. The columns with different superscript (a-d) letters show significantly (P < 0.05) different means.
3.2.4 Cytotoxicity appraisal
3.2.4.1 Brine shrimp lethality test
Preliminary cytotoxicity was assessed by brine shrimp lethality assay and results have been presented in Table 4. Among the leaf extracts, 57% of the extracts (8 out of 14 extracts) showed moderate toxicity with LC50 values of 50–200 μg/ml while Hex, MeOH-CHCl3, MeOH, CHCl3 and ACET leaf extracts were most potent (LC50 = 50–59 μg/ml). In the stem extracts, 21% extracts (3 out of 14 extracts) were found to be moderately cytotoxic (LC50 = 70–83 μg/ml) while the remaining extracts were weakly cytotoxic. The positive control, doxorubicin revealed LC50 value of 5.63 ± 0.21 µg/ml while the negative control (≤1% DMSO in sea water) did not show any toxicity.
Extracts
Brine shrimp lethality
Hemolytic activity
THP-1 cytotoxicity
Hep-G2 cytotoxicity
Protein kinase inhibition
%Mortality
200
LC50
%Hemolysis
200
%Inhibition
20
IC50
%Inhibition
20
IC50
Clear
Bald
MIC
(μg/ml)
(μg/ml)
(μg/ml)
(μg/ml)
1ZOI (mm)
μg/disc
Stem
Hex
92 ± 0.34a
70 ± 0.54
37 ± 0.31 cd
79 ± 0.43bc
6.6 ± 0.43
98 ± 0.21a
0.44 ± 0.45
–
20 ± 0.21b
25
EtOAc-Hex
23 ± 0.21
>200
7 ± 0.43
15 ± 0.33
>20
26 ± 0.25
> 20
–
–
–
EtOH-Hex
20 ± 0.02
>200
32 ± 0.33 cd
17 ± 0.54
>20
31 ± 0.55d
> 20
–
–
–
CHCl3
90 ± 0.12a
77 ± 0.44
37 ± 0.34 cd
51 ± 0.57 cd
19.3 ± 0.4
77 ± 0.24ab
1.1 ± 0.56
–
–
–
EtOAc
20 ± 0.22
>200
14 ± 0.34
22 ± 0.22
> 20
9 ± 0.34
> 20
–
–
–
MeOH-CHCl3
33 ± 0.34
>200
30 ± 0.41 cd
40 ± 0.45d
> 20
62 ± 0.03bc
3.24 ± 0.33
–
16 ± 0.13bc
50
MeOH-EtOAc
33 ± 0.54
>200
40 ± 0.23c
80 ± 0.54b
5.9 ± 0.43
65 ± 0.23bc
2.02 ± 0.45
–
–
–
ACET
56 ± 0.23c
83 ± 0.43
35 ± 0.45 cd
50 ± 0.65 cd
20 ± 0.45
73 ± 0.42ab
1.86 ± 0.36
–
–
–
MeOH
11 ± 0.24
>200
20 ± 0.44
35 ± 0.34
> 20
10 ± 0.46
> 20
–
20 ± 0.32b
25
MeOH-ACET
20 ± 0.44
>200
25 ± 0.21
77 ± 0.44bc
7.01 ± 0.4
69 ± 0.27b
1.98 ± 0.52
–
22 ± 0.32ab
25
EtOH
30 ± 0.33
>200
18 ± 0.48
33 ± 0.53
> 20
19 ± 0.28
> 20
–
18 ± 0.2b
50
D.W.-ACET
20 ± 0.22
>200
12 ± 0.24
41 ± 0.23 cd
> 20
5 ± 0.44
> 20
–
–
–
MeOH-D.W.
40 ± 0.02d
>200
7 ± 0.25
43 ± 0.13 cd
> 20
3 ± 0.65
> 20
–
26 ± 0.52a
12.5
D.W.
13 ± 0.24
>200
8 ± 0.54
40 ± 0.75d
> 20
0.1 ± 0.66
> 20
–
24 ± 0.42a
12.5
Leaf
Hex
90 ± 0.23a
50.38 ± 0.43
15 ± 0.23
50 ± 0.22 cd
20 ± 0.51
94 ± 0.4a
1.44 ± 0.65
–
24 ± 0.31a
12.5
EtOAc-Hex
36 ± 0.4
>200
6 ± 0.43
13 ± 0.35
> 20
13 ± 0.2
> 20
–
26 ± 0.11a
12.5
EtOH-Hex
40 ± 0.5d
>200
6 ± 0.44
18 ± 0.45
> 20
39 ± 0.3c
> 20
–
18 ± 0.41bc
50
CHCl3
79 ± 0.3bc
56.57 ± 0.55
16 ± 0.54
35 ± 0.33
> 20
3 ± 0.22
> 20
–
24 ± 0.31a
12.5
EtOAc
73 ± 0.4bc
70.76 ± 0.58
17 ± 0.55
48 ± 0.45 cd
> 20
91 ± 0.03a
2.19 ± 0.54
–
–
–
MeOH-CHCl3
93 ± 0.5a
50.65 ± 0.45
29 ± 0.23
82 ± 0.56ab
4.58 ± 0.43
90 ± 0.3a
2.56 ± 0.66
–
14 ± 0.44c
50
MeOH-EtOAc
36 ± 0.63
>200
24 ± 0.44
39 ± 0.65
> 20
5 ± 0.4
> 20
–
22 ± 0.41ab
25
ACET
86 ± 0.27b
59.45 ± 0.67
40 ± 0.24c
70 ± 0.44c
9.01 ± 0.43
7 ± 0.33
> 20
–
20 ± 0.31b
25
MeOH
92 ± 0.26a
52.36 ± 0.23
26 ± 0.42
84 ± 0.32ab
3.98 ± 0.42
88 ± 0.12ab
3.55 ± 0.67
–
26 ± 0.13a
12.5
MeOH-ACET
40 ± 0.27d
>200
14 ± 0.24
78 ± 0.13bc
8.43 ± 0.32
89 ± 0.11ab
3.26 ± 0.33
–
–
–
EtOH
73 ± 0.25bc
69.97 ± 0.31
62 ± 0.34b
80 ± 0.76b
5.67 ± 0.36
88 ± 0.31ab
3.46 ± 0.37
–
–
–
D.W.-ACET
76 ± 0.26bc
62.43 ± 0.14
16 ± 0.45
44 ± 0.45 cd
> 20
35 ± 0.4d
> 20
–
–
–
MeOH-D.W.
40 ± 0.36d
>200
19 ± 0.46
86 ± 0.12ab
3.46 ± 0.25
100 ± 0.2a
1.33 ± 0.43
–
–
–
D.W.
3 ± 0.46
>200
6 ± 0.35
40 ± 0.13 cd
> 20
22 ± 0.2
> 20
–
14 ± 0.34c
50
Doxorubicin
96 ± 0.21a
5.63 ± 0.21
98.37 ± 0.44a
5.30 ± 0.67
*5 Flouro
99.2 ± 0.23a
5.13 ± 0.21
Vincristine
99.33 ± 0.22a
8.24 ± 0.22
Triton X 100
90 ± 0.35a
Surfactin
28 ± 0.13a
DMSO
–
–
1% DMSO in sea water
–
1% DMSO in PBS
–
–
–
–
–
3.2.4.2 Hemolytic assay
The results of hemolytic activity of extracts estimated against freshly isolated erythrocytes (human RBCs) are presented in the Table 4. Both stem and leaf extracts presented very low percent hemolysis at concentrations of 200 μg/ ml. This model was used to evaluate the hemolytic activity of extracts to normal body cells (RBCs).
3.2.4.3 Cytotoxicity against THP-I cell line
All extracts were tested at 20 μg/ml against leukemia (THP-1) and samples presenting inhibition>50% were further evaluated at lower concentrations (6.66, 2.22, 0.74 μg/ml) to determine the IC50 as given in Table 4. The leading cytotoxic activity was displayed by MeOH-EtOAc, Hex, MeOH-ACET, CHCl3, and ACET with IC50 of 5.9 ± 0.43, 6.6 ± 0.43, 7.01 ± 0.4, 19.3 ± 0.4, and 20 ± 0.45 μg/ml regarding stem extracts. A remarkable cytotoxic potential was displayed by leaf extracts including MeOH-D.W., MeOH, MeOH-CHCl3, EtOH, MeOH-ACET, ACET, and Hex with IC50 values of 3.46 ± 0.25, 3.98 ± 0.42, 4.58 ± 0.43, 5.67 ± 0.36, 8.43 ± 0.32, 9.01 ± 0.43, and 20 ± 0.51 μg/ml respectively greatly comparable to standard drugs. The standard drugs employed were 5-florouracil and vincristine having IC50 of 5.13 ± 0.21 and 8.24 ± 0.22 μg/ml respectively.
3.2.4.4 Cytotoxic potential against Hep-G2 cell line
The results of cytotoxic potential of O. limbata extracts against Hep-G2 cell line have been mentioned in Table 4. Among stem extracts, the most promising activity was found in Hex, CHCl3, ACET, MeOH-ACET, MeOH-EtOAc, and MeOH-CHCl3 with IC50 values of 0.44 ± 0.45, 1.1 ± 0.56, 1.86 ± 0.36, 1.98 ± 0.52, 2.02 ± 0.45, and 3.24 ± 0.33 μg/ml respectively even superior to standard doxorubicin (IC50 = 5.30 ± 0.67 μg/ml) while other extracts (57.14%) showed moderate activity. In the case of leaf extracts, most powerful activities were shown by; MeOH-D.W., Hex, EtOAc, MeOH-CHCl3, MeOH-ACET, EtOH, and MeOH extracts with IC50 values of 1.33 ± 0.43, 1.44 ± 0.65, 2.19 ± 0.54, 2.56 ± 0.66, 3.26 ± 0.33, 3.46 ± 0.37, and 3.55 ± 0.67 μg/ml respectively whereas remaining 50% of extracts exhibited a moderate Hep-G2 inhibition with IC50 values>20 μg/ml. Standard drug (doxorubicin) unveiled an IC50 of 5.30 ± 0.67 μg/ml
3.2.4.5 Protein kinase inhibition potential
After a sound in vitro anticancer study, the extracts were further screened for protein kinase inhibitory potential with results arranged in Table 4. In the case of stem extracts, a noteworthy bald zone of growth inhibition was observed around MeOH-D.W. (26 mm) and D.W. (24 mm) samples with IC50 = 12.5 μg/disc followed by descending order of MeOH-ACET > Hex = MeOH > EtOH > MeOH-CHCl3 extracts. In the case of leaf extracts, noticeable hyphae formation inhibition (bald) zones were shown by MeOH, EtOAc-Hex, CHCl3, and Hex extracts impregnated discs with an IC50 value of 12.5 μg/disc. The rest of the active samples showed prominent bald zones with the following decreasing order; MeOH-EtOAc > ACET > EtOH-Hex > D.W. = MeOH-CHCl3. As a negative control (DMSO) did not exhibit a bald zone whereas surfactin (positive control) showed a prominent bald zone (28 mm).
4 Discussion
4.1 Phytochemical analysis
Several methods have been used in the extraction of medicinal plants such as maceration, infusion, decoction, percolation, digestion and Soxhlet extraction, superficial extraction, ultrasound-assisted, and microwave-assisted extraction (Shams et al., 2015). The choice of an appropriate extraction method depends on the nature of the plant material, solvent used, pH of the solvent, temperature, and solvent to sample ratio. Heat-stable plant material is extracted using Soxhlet extraction or microwave-assisted extraction, whereas plant materials that are not heat stable are extracted using maceration or percolation (Azwanida, 2015). This study adopted maceration as an extraction method .
As antioxidants help to defend the body against oxidative stress by maintaining the homeostatic balance, disparity among antioxidant and prooxidants leads to certain chronic conditions for example diabetes, melanoma, heart diseases, and atherosclerosis (Engwa, 2018). The prominence of flavonoids among polyphenols against these diseases may be attributed to the presence of aromatic ring using OH substitution as well as their functional groups that aids in metal ion chelation of reactive oxygen species thus inhibiting the lipid peroxidation (Treml and Šmejkal, 2016). The highest percentage of phenolic and flavonoid components observed in MeOH stem extract of O. limbata directs its treatment prospects of diseases linked with oxidative stress. Identification of polyphenolics including flavonoids in methanolic extracts of O. persica was reported previously(Sadeghi et al., 2015).
Manifestation of secondary metabolites (apigenin, gallic acid, rutin, and caffeic acid) by HPLC analysis shows a positive correlation of O. limbata with its therapeutic potentials observed in the current study. MeOH, EtOH, and MeOH-EtOAc stem and leaf extracts exhibited prominent antifungal, anticancerous, leishmanicidal, and α-amylase inhibitory bioactivities. Based on these results, we propose that some polyphenols may produce stronger activity than the others and potent extracts may have a combination of those polyphenols. Moreover, these bioactivities may be attributed to the presence of compounds such as cis-clerodane type ditepenoids including limbatolide A, B, C, beta-setosteryl, and oleanic acid (Ahmad et al., 2004). Polyphenols have documented potential to inhibit cancer cell proliferation, and downregulation of drug efflux transporters (Hussain et al., 2016). Also, a study showed significant relation of polyphenolic content and inhibition of in vivo tumor growth by controlling MAPK/ERK, STAT3, and PI3K/AKT pathways in breast cancer stem cells and reduced lung metastasis (Vuong et al., 2016).
Caffeic acid as hydroxycinnamic acid derivative prevents LDL oxidation and can prevent coronary heart disease and atherosclerosis (Kabała-Dzik et al., 2017). Rutin is a flavonol glycoside showing various bioactivities such as antiinflammatory, antitumor, antidiabetic, antimicrobial, and antiasthmatic. Anticancer proficiency of rutin (flavonol) was observed by both in vitro and in vivo studies. Rutin also exhibited activities against rheumatoid arthritis (Saleh et al., 2019). Apigenin is a 5, 7,4-trihydroxy flavone that belongs to the subclass of flavones and acts as a free radical scavenger presenting antibacterial, anti-proliferative, antispasmodic, antiphlogistic, and anti-inflammatory effects (Sen et al., 2016). Gallic acid or 3,4,5-trihydroxybenzoic acid is one of the most abundant phenolic acids in the plant kingdom. Several in vitro and in vivo studies illustrate treatment prospects of gallic acid regarding ulcer, nephrotoxicity, pulmonary fibrosis, myocardial injury, colorectal cancer, and diabetes (Kahkeshani et al., 2019). Therefore, the plant may be sought as a potential source of bioactive polyphenolic compounds and cis-clerodane type diterpenoids which would serve as cheap, safe, and effective sources of anticancer, antibacterial, and leishmanicidal drugs (Cory et al., 2018).
4.2 Biological evaluation
4.2.1 Antioxidant activity
Both stem and leaf extracts of O. limbata exhibited a positive association between TPC, TFC, and %FRSA that infer the role of polyphenols in antioxidant potential. Phenolic compounds play a part in antioxidant potential via the formation of peroxyl radical (ROO•) (Mileo and Miccadei, 2016). To find the main components responsible for the free radical scavenging activity of the extracts, we developed a correlation of %FRSA with total phenolic content and total flavonoid content of leaf (R2 = 0.9950 and 0.7457 respectively) and stem (R2 = 0.9935 and 0.5934 respectively) extracts. Both stem and leaf extracts exhibited a positive association that infer the role of polyphenols in antioxidant potential. The presence of apigenin imparts free radical scavenging potential to plants that is confirmed via RP-HPLC analysis in the current study (Sen et al., 2016). Several in vitro and in vivo studies showed antioxidant propensity of caffeic acid, gallic acid, and rutin (Kabała-Dzik et al., 2017; Kahkeshani et al., 2019; Saleh et al., 2019). These phytochemicals have been confirmed via chromatographic fingerprinting (RP-HPLC) in the present study. Moreover, bioactivities may be ascribed to the presence of certain cis and trans-clerodane type diterpenoids including limbatolide A-C, limbatenolide A-D, oleanic, ballotenic and ballodiolic acid illustrated in previous studies (Ahmad et al., 2007, 2005). Based on the present results, it is concluded that the plant has produced good scavenging activity more concentrated in polar extracts than non-polar ones. Antioxidant activity of methanol extract of O. limbata leaves was reported in earlier studies that are in agreement with the current study (Shakoor and Zaib, 2014).
4.2.2 Antimicrobial activity
4.2.2.1 Antileishmanial potential
Leishmaniasis is a widespread disease universally affecting 12 million people in 88 countries with almost 350 million individuals threatened. There are various species of Leishmania responsible for visceral, cutaneous, and mucocutaneous leishmaniasis (Bhunia and Shit, 2020). Leishmania tropica is a flagellated parasite causing cutaneous leishmaniasis (Masoudzadeh et al., 2020). Pentavalent antimonials are standard drugs for the treatment of leishmaniasis nevertheless leading to renal toxicity (Marques et al., 2019). The emergence of resistance to antimonials, some serious side effects, and incidence of disease worldwide emphasizes the discovery of leishmanicidal agents (Mambro et al., 2019).
To the best of our knowledge, this study discloses the potential against L. tropica for the first time. Plants constitute a different kind of phytoconstituents such as alkaloids, terpenoids, flavonoids, and tannins as a source of antioxidants in many studies. The lack of certain enzymes such as GSH peroxidases and catalases render the parasite more vulnerable to reactive oxygen species (ROS) mediated programmed cell death (apoptosis). Flavonoids such as quercetin show antileishmanial action by inhibition of topoisomerase II causing cell cycle arrest (Ahmad et al., 2020). Some former studies reported that clerodane diterpenoids revealed 86.9%, 83.7%, and 84.2% inhibition of L. donovani amastigotes in spleen, liver, and bone marrow respectively at 100 mg/kg dose in hamsters (Duarte et al., 2019). Farooq et al (2004) revealed the presence of some clerodane type diterpenoids such as limbatolide and limbatenolide in methanol extract of O. limbata providing evidence of leishmanicidal action in the current study. Therefore, the incidence of flavonoids confirmed by HPLC in the current study along with diterpenoids reported in earlier studies may be attributed to the antileishmanial potential of O. limbata. Overview of whole data led us to the conclusion that stem contains most active compounds in non-polar region and leaves have bioactive compounds concentrated in the polar region. Therefore, this information could be further employed to isolate and characterize pure compounds with the potential to develop antileishmanial drugs in the future.
4.2.2.2 Antibacterial potential
The manifestation of antibacterial action highly depends upon the plant material utilized, methods applied for testing, growth medium composition, and the strains used (Vieitez et al., 2018). The extracts are specifically active against B. subtilis strain thus qualifying as a valuable source of narrow-spectrum antibiotics. Monotherapy with narrow-spectrum drugs provides the advantage of a targeted approach which is considered valuable to avoid the emergence of resistance widely seen in the case of broad-spectrum antibiotics (Khameneh et al., 2016). Moreover, phytochemical analysis confirmed the presence of rutin, caffeic acid, apigenin, and gallic acid in the current study. A significant antibacterial potential is reported by rutin and apigenin (Saleh et al., 2019; Sen et al., 2016). Inhibition of certain bacteria including P.aurogenosa, S.aureus, and Streptococcus mutants by gallic acid has also been reported. Mechanism of inhibition involves hindrance in motility, adherence as well as biofilm formation. Gallic acid may also affect the permeability and charge of the membrane. Some studies have reported the effect of gallic acid on some metabolic enzymes including dihydrofolate reductase and topoisomerase IV leading to DNA cleavage. Chelation via divalent cations may also contribute to membrane disruption of gram-negative bacteria (Kahkeshani et al., 2019).
Previous studies have reported the role of methanol extract and the fraction of the whole O. limbata plant in antibacterial proficiency (Shakoor and Zaib, 2014). Other studies also showed the presence of diterpenoids (Ahmad et al., 2005). It can be deduced that this plant is rich in the aforementioned chemicals as confirmed by the phytochemical analysis and it could be a source of natural compounds to develop new antiinfective agents.
4.2.2.3 Antifungal potential
The current data suggests that the most substantial antifungal action (MIC = 6.25–50 μg/ml) is prominent in the stem part compared to the leaf part. Flavonoids (Caffeic acid, apigenin, rutin, and gallic acid) occurrence in several extracts of both stem and leaf parts of O. limbata has been confirmed by chromatographic fingerprinting (RP-HPLC) in the current study. Flavonoids are widely used to treat several human ailments. The antifungal mechanism of flavonoid involves several fundamental principles such as inhibition of cell membrane and cell wall formation, inhibition of RNA and protein synthesis, dysfunction of the mitochondrial system, and efflux pumps (Aboody and Mickymaray, 2020). To the best of our knowledge, the current study reveals the antifungal activity against A. flavus and Mucor species for the first time. The potent antifungal action of the subject plant needs to be further investigated for isolation of bioactive to treat fungal infections.
4.2.3 α-amylase inhibition activity
4.2.3.1 Several methods
Clerodane type diterpenoids have been documented in O. limbata extracts in some earlier reports (Ahmad et al., 2007). Previous studies have described the role of clerodane diterpenoids in the inhibition of α-amylase (Huang et al., 2019; Luyen et al., 2019). This enzyme catalyzes the hydrolysis of glycosidic linkages (α 1–4) from the non-reducing region of polysaccharides like amylopectin, starch, and glycogen to produce maltose leading to increased glucose absorption. The α-amylase inhibition decreases glucose absorption. Hence postprandial glucose upsurge is reduced (Bashary et al., 2020). Polyphenols and flavonoids act as an antioxidant, decrease oxidative stress, obstruct the free radicals production, and inhibit digestive enzymes hence reducing postprandial glucose rise (Gutiérrez-Grijalva et al., 2019). The current study reveals the diabetic-friendly potential of O. limbata for the first time that should be further investigated via purification and characterization to develop more safe targets for this chronic disease.
4.2.4 Cytotoxic activity
4.2.4.1 Brine shrimp lethality
These results suggest that a non-polar and moderately polar binary solvent system is the best option for cytotoxic compound isolation as compared to a most polar solvent systems in O. limbata stem and leaf extracts. Previous studies reported that the whole plant of O. limbata was evaluated for cytotoxic potential on rhabdomyosarcoma (RB) cell line using methanolic extract and it exhibited anticancer activity emphasizing the cytotoxic potential of the plant (Maqsood et al., 2015). The brine shrimp lethality assay is a well-planned method for primary assessment of toxicity (Hamidi et al., 2014). The current study revealed the brine shrimp lethality potential by various parts of O. limbata for the first time. The LC50 value below 1000 μg/ml is considered cytotoxic during bioactivity evaluation via brine shrimp assay in plant extracts. Therefore, the cytotoxic potential was further assessed by hemolytic assay, cancer cell lines (THP-1 and Hep-G2), and protein kinase inhibition assays.
4.2.4.2 Hemolytic activity
Hemolytic potential illustrated the mechanism of cytotoxicity to cell membrane since RBCs membrane resembles other cell membranes (Alonso and Alonso, 2016). Both stem and leaf extracts displayed very low percent hemolysis indicating the biocompatibility with normal body cells. Negligible hemolysis at a concentration of 200 μg/ ml represents the selective cytotoxicity of extracts towards the THP-1 and Hep-G2 cancer cell line in comparison to normal body cells (RBCs). The entire data of hemolytic assay led us to the conclusion that stem and leaf extracts of O. limbata have notable biocompatibility and do not distress the normal body cells. To the best of our knowledge, this is the first-ever report regarding the hemolytic potential of O. limbata. The hemolytic activity should be further investigated to use these extracts for food and drug products.
4.2.4.3 Cytotoxicity against THP-1 cell line
The stem extracts of O. limbata showed more cytotoxic potential in non-polar and moderately polar binary solvents. The leaf extracts showed more percent inhibition against THP-1 cell line in moderately polar binary solvents to polar solvents which can be correlated to a previous study on methanol extract of O. limbata that proved effective against rhabdomyosarcoma cell line (Maqsood et al., 2015). Anti-cancer properties of rutin (flavonol) were observed by in vitro and in vivo studies (Saleh et al., 2019). Apigenin as a free radical scavenger possesses antibacterial, anti-proliferative, and anti-inflammatory effects (Sen et al., 2016). Several in vitro and in vivo studies illustrated treatment prospects of gallic acid regarding ulcer, colorectal cancer, and diabetes (Kahkeshani et al., 2019). Quantification of these phytochemicals (Rutin, apigenin, and gallic acid) based on RP-HPLC analysis along with clerodane type diterpenoids such as limbatolide and limbatenolide reported by Farooq et al. (2007) may be attributed to the antileukemic potential of tested samples. Vincristine employed as a positive control in the current study has been widely used in several categories of cancer including acute lymphoblastic leukemia, Hodgkin lymphoma, Wilms’ tumor, sarcomas, and neuroblastoma. Vincristine-induced hemolytic anemia is extensively reported in clinical settings (Michlitsch et al., 2019). The tested extracts of O. limbata revealed negligible percent hemolysis at a concentration of 200 μg/ml. Accordingly, the results validate that samples from both stem and leaf extracts have greater selectivity for THP-1 cell line compared to normal body cells (RBCs).
This is the first report describing the anticancer potential against monocytic leukemia. This suggests that a high degree of selective cytotoxicity of stem and leaf extracts of O. limbata against human monocytic leukemia should be employed further for purification and characterization of the cytotoxic compounds from potent extracts use candidates for chemotherapeutic drug development.
4.2.4.4 Cytotoxicity against Hep G2 cell line
The greater cytotoxic potential was documented by several extracts in comparison to standard drugs employed in the current study. The data shows that the cytotoxic profile of the stem extracts mainly resides in the non-polar region which convinces the non-polar nature of active moieties. The presence of clerodane type diterpenoids in previous reports may be correlated to the anticancer effect of Hex stem extract against cancer cell lines in the current study. Similarly, clerodane type diterpenoids isolated from Scutellaria barbata imparts anticancer effects as observed by in vitro studies against various human carcinoma cells, including, breast cancer, colon cancer, and hepatocellular carcinoma by cell cycle arrest, apoptosis, and autophagic pathways (Wang et al., 2019). Therefore, literature also supports the anticancer potential of non-polar extracts clerodane diterpenoids. Among leaf extracts, polar and moderately polar extracts reported greater cytotoxic potential. Anticancer properties of rutin, gallic acid, and apigenin regarding ulcer, colorectal cancer, and diabetes were described in earlier studies (Kahkeshani et al., 2019; Saleh et al., 2019; Sen et al., 2016). Significant quantification of these phytochemicals based on HPLC in the present study accentuates the cytotoxic potential of polar and moderately polar extracts of O. limbata.
The results also validate that the samples from both stem and leaf extracts have greater selectivity for Hep-G2 cell line compared to normal body cells (RBCs). This suggests that a high degree of selective cytotoxicity of stem and leaf extracts against Hep-G2 hepatoma cell line should be employed further for purification and characterization of cytotoxic compounds from potent extracts for chemotherapeutic appraisal.
4.2.4.5 Protein kinase inhibition potential
Several extracts of both stem and leaf parts exhibit notable inhibitory activities. The data exhibited that polar solvents would be a better choice for the extraction of active constituents from the stem part while non-polar and moderately polar solvents would efficiently extract the phytochemicals from the leaf part of O. limbata with kinase inhibitory activity.
Cancer pathogenesis involves the accumulation of mutations in the genes crucial for the cellular differentiation, growth, and apoptosis. The mutations constitutively upraise the activity of kinase at serine/threonine residues, frequently observed in human cancers. The serine/threonine kinases are an important factor in carcinogenesis (Roskoski, 2019). Similarly a subfamily of serine/threonine kinases was mutated in ovarian carcinomas (Bhullar et al., 2018). The tyrosine kinases control the normal cell functioning and also play a main role in oncogenesis (Qu et al., 2020). All these kinases are reflected as exceptional targets for cell signaling involved in the cancer pathogenesis as well as to limitize the toxicities exhibited by conventional therapy (Montagnani and Stecca, 2019). The protein kinase inhibitory potential could be determined by use of Streptomyces 85E strain. This strain closely looks like the eukaryotic counterpart. The inhibition this strain by several extracts helps to recognize the anti-infective, antitumor agents and antimycobacterial potential of plant (Belknap et al., 2020; Zahra et al., 2017). Further investigations are required to exploit these bioactivities of the plant.
5 Conclusion
Otostegia limbata was characterized phytochemically and biologically by using polarity-guided extraction, wherein plant part and extraction solvent were found a crucial variable. The polar extracts of stem retrieved the highest amount of phenolic and flavonoid secondary metabolites and the results corroborated with their quantification via HPLC. Likewise, polar extracts of stem contained substantial antioxidant potential. The antimicrobial spectrum against fungi and bacteria was significantly recorded in diverse polarity phytochemicals of both the stem and leaf parts. Profound inhibition of proliferation of leishmanial promastigotes was manifested by both stem and leaf parts. A noteworthy antidiabetic proficiency was exhibited by nonpolar extracts of the leaf. A stupendous cytotoxic effect comparable to positive standards was recorded in wide polarity range extraction solvents of stem and leaf. Negligible hemolysis at the highest tested concentration signified the plant’s selective cytotoxicity and biocompatibility. These findings should be assessed via polarity guided isolation and detailed mechanistic studies to develop therapeutic leads for disease management 57%.
CRediT authorship contribution statement
Humaira Fatima: Supervision, Investigation. Afeefa Kainat: Software. Fazal Akbar: Methodology. Zabta Khan Shinwari: Data curation, Visualization. Iffat Naz: Visualization, Validation.
Acknowledgments
The authors would like to acknowledge the Deanship of scientific research , Qassim University for funding the publication of this project.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biogenic metal nanoparticles as a potential class of antileishmanial agents: Mechanisms and molecular targets. Nanomedicine. 2020;15:809-828.
- [CrossRef] [Google Scholar]
- Ahmad, L., Semotiuk, A., Zafar, M., Ahmad, M., Sultana, S., Liu, Q.-R., Zada, M.P., Abidin, S.Z.U., Yaseen, G., 2015. Ethnopharmacological documentation of medicinal plants used for hypertension among the local communities of DIR Lower, Pakistan. J. Ethnopharmacol. 175, 138–146. https://doi.org/https://doi.org/10.1016/j.jep.2015.09.014
- Two new diterpenoids from Ballota limbata. Chem. & Pharm. Bull.. 2004;52:441-443.
- [CrossRef] [Google Scholar]
- Three new cholinesterase-inhibiting cis-clerodane diterpenoids from Otostegia limbata. Chem. Pharm. Bull.. 2005;53:378-381.
- [CrossRef] [Google Scholar]
- Ahmad, V.U., Khan, A., Farooq, U., Kousar, F., S. Khan, S., Hussain, J., 2007. Two new trans-clerodane diterpenoids from Otostegia limbata. J. Asian Nat. Prod. Res. 9, 91–95.
- RP-HPLC-based phytochemical analysis and diverse pharmacological evaluation of Quercus floribunda Lindl. ex A. Camus nuts extracts. Nat. Prod. Res. 2019:1-6.
- [CrossRef] [Google Scholar]
- Alonso, L., Alonso, A., 2016. Hemolytic potential of miltefosine is dependent on cell concentration: implications for in vitro cell cytotoxicity assays and pharmacokinetic data. Biochim. Biophys. Acta (BBA)-Biomembranes 1858, 1160–1164.
- A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:1-13.
- [CrossRef] [Google Scholar]
- Azwanida, N.N., 2015. A review on extraction methods used in medicinal plants, principle, strength, limitation. Med. Aromat. Plants. 4(196), 2167-0412
- An insight of alpha-amylase inhibitors as a valuable tool in the management of type 2 diabetes mellitus. Curr. Diabetes Rev.. 2020;16:117-136.
- [Google Scholar]
- Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci. Rep.. 2020;10:1-9.
- [Google Scholar]
- Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer. 2018;17:1-20.
- [CrossRef] [Google Scholar]
- Bhunia, G.S., Shit, P.K., 2020. Introduction of Visceral Leishmaniasis (Kala-azar), in: Spatial Mapping and Modelling for Kala-Azar Disease. Springer, pp. 1–18.
- Ethnomedicinal, phytochemical and antibacterial activities of medicinal flora of Pakistan used against Pseudomonas aeruginosa-A review. Pak. J. Pharm. Sci.. 2017;30:2285-2300.
- [Google Scholar]
- The role of polyphenols in human health and food systems: A mini-review. Front. Nutr.. 2018;5:87.
- [Google Scholar]
- Growing importance of natural products research. Molecules. 2020;25:14-15.
- [CrossRef] [Google Scholar]
- Plant Terpenoids as Lead Compounds Against Malaria and Leishmaniasis. Studies in Natural Products Chemistry 2019
- [CrossRef] [Google Scholar]
- Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochem. Source Antioxidants Role Dis. Prev. BoD–Books Demand 2018:49-74.
- [Google Scholar]
- Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int.. 2019;116:676-686.
- [Google Scholar]
- Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced pharm bull. 2014;60:9-18.
- [Google Scholar]
- Investigation of antitussive and toxicological activity of Ballota limbata in mice. Pharm. Biol.. 2011;49:627-632.
- [CrossRef] [Google Scholar]
- Polyalthia clerodane diterpene potentiates hypoglycemia via inhibition of dipeptidyl peptidase 4. Int. J. Mol. Sci.. 2019;20:530.
- [Google Scholar]
- Conservation of indigenous knowledge of medicinal plants of Western Himalayan region Rawalakot, Azad Kashmir. Pakistan. Pak. J. Pharm. Sci.. 2017;30:773-782.
- [Google Scholar]
- Migration rate inhibition of breast cancer cells treated by caffeic acid and caffeic acid phenethyl ester: An in vitro comparison study. Nutrients. 2017;9:1144.
- [Google Scholar]
- Kahkeshani, N., Farzaei, F., Fotouhi, M., Alavi, S.S., Bahramsoltani, R., Naseri, R., Momtaz, S., Abbasabadi, Z., Rahimi, R., Farzaei, M.H., Bishayee, A., 2019. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 22, 225–237. https://doi.org/10.22038/ijbms.2019.32806.7897
- Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog.. 2016;95:32-42.
- [Google Scholar]
- Khan, K., Fatima, H., Taqi, M.M., Zia, M., ur-Rehman, T., Mirza, B., Haq, I. ul, 2015. Phytochemical and in vitro biological evaluation of Artemisia scoparia Waldst. & Kit for enhanced extraction of commercially significant bioactive compounds. J. Appl. Res. Med. Aromat. Plants 2, 77–86. https://doi.org/10.1016/j.jarmap.2015.04.002
- Wedtrilosides A and B, two new diterpenoid glycosides from the leaves of Wedelia trilobata (L.) Hitchc. with α-amylase and α-glucosidase inhibitory activities. Bioorg. Chem.. 2019;85:319-324.
- [CrossRef] [Google Scholar]
- Frontiers in Pharmacology 2019
- [CrossRef]
- Preliminary screening of methanolic plant extracts against human rhabdomyosarcoma cell line from salt range. Pakistan. Pakistan J. Bot.. 2015;47:353-357.
- [Google Scholar]
- American tegumentary leishmaniasis: severe side effects of pentavalent antimonial in a patient with chronic renal failure. An. Bras. Dermatol.. 2019;94:355-357.
- [Google Scholar]
- Molecular signatures of anthroponotic cutaneous leishmaniasis in the lesions of patients infected with Leishmania tropica. Sci. Rep.. 2020;10:1-15.
- [Google Scholar]
- Vincristine-induced anemia in hereditary spherocytosis. Exp. Biol. Med.. 2019;244:850-854.
- [Google Scholar]
- Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev.. 2016;2016
- [CrossRef] [Google Scholar]
- Role of protein kinases in hedgehog pathway control and implications for cancer therapy. Cancers (Basel).. 2019;11:449.
- [Google Scholar]
- Seripheidium quettense mediated green synthesis of biogenic silver nanoparticles and their theranostic applications. Green Chemistry Letters and Reviews 2018:310-322.
- [CrossRef] [Google Scholar]
- LINC00671 suppresses cell proliferation and metastasis in pancreatic cancer by inhibiting AKT and ERK signaling pathway. Cancer Gene Ther. 2020:1-13.
- [Google Scholar]
- Rashid, S., Ahmad, M., Zafar, M., Sultana, S., Ayub, M., Khan, M.A., Yaseen, G., 2015. Ethnobotanical survey of medicinally important shrubs and trees of Himalayan region of Azad Jammu and Kashmir, Pakistan. J. Ethnopharmacol. 166, 340–351. https://doi.org/https://doi.org/10.1016/j.jep.2015.03.042
- Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol. Res.. 2019;139:471-488.
- [CrossRef] [Google Scholar]
- A Review of the Phytochemistry, Traditional Uses, and Biological Activities of the Genus Ballota and Otostegia. Planta Med.. 2019;85:869-910.
- [CrossRef] [Google Scholar]
- Sadaf, H.M., Bibi, Y., Riaz, I., Saboon, Sultan, M.A., Bibi, F., Bibi, M., Hussain, M., Sabir, S., 2016. Pharmacological aptitude and profiling of active constituent from Otostegia limbata-Comprehensive review. Asian Pacific J. Trop. Dis. 6, 918–924. https://doi.org/10.1016/S2222-1808(16)61156-8
- Pharmacological aptitude and profiling of active constituent from Otostegia limbata-Comprehensive review. Asian Pacific Journal of Tropical Disease. 2016;6(11):918-924.
- [CrossRef] [Google Scholar]
- Antioxidant activity and total phenolic contents of some date varieties from Saravan Region, Baluchistan. Iran. J. Med. Plants Res.. 2015;9:78-83.
- [Google Scholar]
- Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn. Schmiedebergs. Arch. Pharmacol.. 2019;392:165-175.
- [Google Scholar]
- Scheen, A., Albert, V.A., 2007. Nomenclatural and Taxonomic Changes within the Leucas clade (Lamioideae ; Lamiaceae) Author (s): Anne-Cathrine Scheen and Victor A . Albert Source : Systematics and Geography of Plants , 2007 , Vol . 77 , No . 2 (2007), pp . 229-238 Published by : B 77, 229–238.
- Sen, P., Sahu, P.K., Haldar, R., Sahu, K.K., Prasad, P., Roy, A., 2016. Apigenin Naturally Occurring Flavonoids:Occurrence and Bioactivity. UK J. Pharm. Biosci. 4, 56. https://doi.org/10.20510/ukjpb/4/i6/134666
- Antioxidant and Antimicrobial Potential of Otostegia Limbata L., and Ajuga Bracteosa L. Against Pathogenic. 2014;2:179-183.
- [Google Scholar]
- Shams, A.k., Abdel-Azim, S.N., Saleh, A.I, Hegazy, F.E.M, El-Missiry, M.M. Hammouda, M.F., 2015. Green technology: Economically and environmentally innovative methods for extraction of medicinal & aromatic plants (MAP) in Egypt. J. Chem. Pharm. Res. 7(5), 1050-1074.
- Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. food Sci. food Saf.. 2016;15:720-738.
- [Google Scholar]
- Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J. Supercrit. Fluids. 2018;133:58-64.
- [Google Scholar]
- Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. J. Transl. Med.. 2016;14:1-12.
- [Google Scholar]
- Investigation of Haem Agglutination Activity in Agaricus campestris and Ballota limbata. Int. J. Adv. Pharm. Res.. 2013;5:51-56.
- [Google Scholar]
- Cytotoxic Neo-Clerodane Diterpenoids from Scutellaria barbata D. Don. Chem. Biodivers.. 2019;16:e1800499
- [Google Scholar]
- Zahra, S.S., Ahmed, M., Qasim, M., Gul, B., Zia, M., Mirza, B., Haq, I. ul, 2017. Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complement. Altern. Med. 17, 1–16. https://doi.org/10.1186/s1







