Translate this page into:
Polymeric ionic liquid as novel catalyst for the Prins reaction
⁎Corresponding authors. shy@licp.cas.cn (Heyuan Song), chenj@licp.cas.cn (Jing Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of micro-mesoporous polymeric ionic liquids (PILs) have been successfully synthesized by the method of anion and cation copolymerization. Then use FT-IR, N2 adsorption–desorption isotherms, SEM, and TG to characterize them. Furthermore, the catalytic performance of the synthesized PILs was investigated for the Prins reaction of propylene with 1, 3, 5-trioxane. Among the four PILs synthesized, VIMBs-DVB-SSA has better catalytic activities for the Prins reaction. Under the optimal conditions, 100 °C, 8 wt%, 4 h and n (propylene)/n (1, 3, 5-trioxane) = 4:1, the conversion of formaldehyde (99.8%) and selectivity (81.7%) for 4-methyl-1, 3-dioxane. In addition, the effects of reaction time, catalyst dosage, and reaction temperature and mole ratio of reaction substrates on the reaction were also investigated. The prepared catalyst had good thermal stability and can be reused easily.
Keywords
Polymeric ionic liquids
Prins reaction
4-methyl-1, 3-dioxane
1 Introduction
The Prins reaction, the condensation of olefins with aldehydes catalyzed by acid (Pricecc et al., 1949), is an effective way to form cyclic carbon and epoxy compounds (Vardhan et al., 2018; Sidorenko et al., 2019), and also one of the important ways to form C-O and C-C bonds (Wang et al., 2019; Sekerová et al., 2019). Its main products, 1, 3-dioxane and its derivatives, are important intermediates or solvents for organic synthesis, and also exist in many structural units of natural macromolecules (Harnying et al., 2017; Wang and Iglesia, 2017; Dubbu and Vankar, 2017). Other compounds, such as alcohols, diols and conjugated dienes, are also widely used in the chemical industry, medicine and daily chemical industry (Yu et al., 2018; Almohseni et al., 2018; Vyskočilová et al., 2018) materials used for the preparation of 4-alkyl-1, 3-dioxane from Prins reaction are cheap and easy to obtain, with high yield. The products can be further hydrolyzed to 1, 3-diols, which have attracted wide attention (Rambabu et al., 2015; Liu et al., 2014). The reaction is usually catalyzed by strong protonic acid, but it has some disadvantages such as strong corrosiveness, difficult separation of catalyst and low yield. Therefore, to overcome these shortcomings and improve the yield, many other catalysts have been developed, such as heteropoly acid, molecular sieve, metal oxide, ionic liquid, etc. (Carmeladerisi et al., 2008; Yadav et al., 2003; Li Guixian et al., 2004; Marakatti et al., 2015).
Ionic liquids are “room temperature liquid salts” composed of cations and anions (Larsen et al., 2000). Since the first case of ionic liquid was reported in 1914 (Walden, 1914), ionic liquid has been applied from the initial electrochemical field to many fields such as catalysis, separation, extraction, lubrication, etc (Yang et al., 2015; Yong et al., 2016; Zhou, et al., 2020; Chen et al., 2018; Reyna-González et al., 2020). It is the forefront of green chemistry, because of its characteristics, such as good solubility, low vapor pressure, designability, good electrochemical performance (Oliver, et al., 2008; Shiguo et al., 2017). However, some defects of ionic liquids, such as synthesis process is complicated, and purification difficulties lead to higher production costs, and easy dissolution in water and so on, limit their large-scale industrial application (Wytze Meindersma and De Haan, 2013; Lopes and Najwa, 2017). To overcome these shortcomings, immobilized ionic liquid technology came into being (Itoh et al., 1990; Ning, et al., 2020).
Polymerization is a good immobilization method because it does not need expensive coupling agents, and the monomers are linked by chemical bonds and are very stable (Formosa Plastics Corporation, 2017). Polymeric ionic liquids (PILs) prepared by polymerization not only have the mechanical properties of polymers, but also retain the excellent physical and chemical properties of ionic liquids, so they are widely studied (Bian et al., 2019; Kamaz et al., 2019). As a new polymer, Polymeric ionic liquids has been applied in many fields, such as chemical adsorption, fuel desulfurization, membrane separation, biodiesel production, electrolyte, catalysis, etc. (Shen et al., 2020; Al-kharabsheh and Bernstein, 2018; Zhou et al., 2018; Yuan et al., 2017). In the field of catalysis, Polymeric ionic liquids modified by acid groups are mainly used as acid catalysts for various organic reactions, such as polymerization (Koyilapu et al., 2020), epoxidation (Hua et al., 2014) and oxidation (Dandan et al., 2016). However, the Prins reaction of olefins and aldehydes catalyzed by Polymeric ionic liquids has not been reported.
Therefore, we have prepared some series of Polymeric ionic liquids containing sulfonic groups, which are copolymerized by cations and anions. The catalyst was characterized by FT-IR, N2 adsorption–desorption, SEM and simultaneous thermal analysis. These catalysts were used to catalyze the preparation of 4-methyl-1, 3-dioxane from propylene and 1, 3, 5-trioxane. At the same time, various factors affecting the reaction, such as reaction time, amount of catalyst, reaction temperature and molar ratio of reaction substrate, were also investigated.
2 Experimental
2.1 Chemicals and materials
All of the chemicals, 1,4-butylene sulfone (>98 wt%, Energy Chemical), N-vinyl imidazole (98 wt%, Energy Chemical), 4-vinyl pyridine (>95 wt%, TCI (Shanghai)), Divinylbenzene (80 wt%, Energy Chemical), Sodium p-styrenesulfonate (90%, Innochem), Azobisisobutyronitrile (AIBN), ethyl acetate, ethanol 1, 3, 5-trioxane (TOX), propylene, toluene, silver nitrate (>99.8%), hydrochloric acid (36%) and anhydrous methanol are analytical reagents. All reagents are used directly without further purification.
2.2 Catalysts preparation
2.2.1 Synthesis of vinyl -SO3H functionalized imidazolium or pyridine zwitterions
According to the method reported in the previous literature (Heyuan Song, 2019). In a 250 mL round-bottom flask, 1, 4- butylene sulfone (0.3 mol, 40.85 g) was added drop wise into a solution of N-vinyl imidazole (0.2 mol, 18.83 g) or 4-vinyl pyridine (0.2 mol, 21.03 g) in ethyl acetate (100 mL) at room temperature under stirring, and then heated at 60 °C for 24 h. After cooling to room temperature, the 1-Vinyl-3-sulfonic butyl imidazolium zwitterions (VIMBs) or 4-vinyl-N-sulfonic butyl pyridine zwitterions (VPyBs) was filtered, washed thoroughly with ethyl acetate (50 mL × 3) and dried in vacuum for further characterized.
2.2.2 Synthesis of p-styrenesulfonic acid (SSA)
The ethanol solution of p-styrenesulfonic acid was prepared according to the literature (Frenzel et al., 2019) but slightly modified. First, equimolar quantities of sodium p-styrenesulfonate (20.619 g, 0.1 mol) mix with silver nitrate (16.988 g, 0.1 mol) that were separately dissolved in distilled water (150 mL and 20 mL). The reaction was carried out during stirring and in the absence of light for 2 h at room temperature. The precipitated monomer was filtrated, washed with water (3 × 50 mL), and dried under vacuum at 35 °C for 48 h resulting in silver-p-styrene sulfonate as a white solid. Then, silver-p-styrenesulfonate (5.8164 g, 0.02 mol) was suspended in ethanol (50 mL) followed by adding equal moles of concentrated hydrochloric acid (2.0256 g, 0.02 mol). The solution was stirred for 1 h at room temperature resulting in precipitation of silver chloride. The precipitate was filtered off and washed with ethanol (3x20mL).
2.2.3 Synthetic method for PILs
PILs were prepared according to the literature reports (Heyuan Song, 2019). As shown in Scheme 1, VIMBs or VPyBs (0.05 mol), SSA (0.05 mol), azobisisobutyronitrile (3.2 wt%), or divinylbenzene (0.05 mol), were mixed in 250 mL ethanol in a round-bottom flask under stirring and refluxing. Then heated gradually to 75 °C for an additional 24 h. After cooling to room temperature, filtering and vacuum drying, VPyBs-SSA, VIMBs-SSA, VPyBs-DVB-SSA, VIMBs-DVB-SSA can be obtained.
2.3 Catalysts characterization
FT-IR spectra were acquired on a Nicolet NEXUS 870 system (USA) with a scan range of 400–4000 cm−1 using anhydrous KBr as standard. The BET isotherms were recorded on Micromeritics ASAP2010 instrument (USA). The pore size and volumes distributions were calculated by the Barrett-Joyner-Halenda (BJH) technique and the surface areas were estimated using the BET method. SEM images were recorded on a Hitachi SU8020 microscope (Tokyo, Japan) to assess the particle dimensions and morphology. TG analysis was conducted on the Netzsch STA 449 F3 simultaneous thermal analysis system (Selb, Germany) between 20 and 600 °C in an N2 atmosphere. The acid capacity of PILs (50 mg) was measured through acid-base titration with NaCl solution (20 mL; 2 mol/L) as an ion-exchange agent at room temperature for more than 24 h, and then the filtrate was titrated by NaOH solution (0.01 mol/L).
2.4 Catalysts activity tests
Adding catalyst, solvent toluene, and TOX into the stainless steel autoclave and then seal it. Purging nitrogen into the reactor to 2.0 MPa, and then release the gas. Repeating the above operation three times to replace the air in the stainless steel autoclave. After that, propylene is charged, and the mass of propylene is weighed out with an electronic scale. Putting the stainless steel autoclave into an oil bath and heat it under magnetic stirring. After the reaction, the reaction solution was taken for GC analysis, tetrahydrofuran was used as an internal standard for quantitative analysis, and the amount of residual formaldehyde was analyzed by titration.
3 Results and discussion
3.1 Catalysts characterization
3.1.1 FT-IR analysis
The Fourier transform infrared spectrum of four PILs as shown in Fig. 1. In Fig. 1(a), the peaks at 1034.9 and 1167.3 cm−1 were characteristic of -SO3H (Jinmei Miao et al., 2011). The peaks at 3140.0, 1561.1 and 1455.0 cm−1 were stretching vibrations of pyridine C—H, C⚌C and C⚌N (Li et al., 2014; Zhao and Yua, 2010), whereas a broadband around 3439.1 cm−1 was ascribed to the stretching vibration of hydrogen bonds of ILs with physisorbed water which overlap with the characteristic peak of N-H (Li et al., 2014). The results showed that vinyl monomers were successfully polymerized together. Similarly, in Fig. 1(b), the peaks by 3056.0, 1640.5 and 1469.4 cm−1 were attributed to imidazole C—H, C⚌C and C⚌N stretching vibrations. The peaks at 1175.5 and 1035.2 cm−1 belong to —SO3H.
3.1.2 SEM studies
The SEM images of PILs are illustrated in Fig. 2. It is found that the polymer synthesized by adding divinylbenzene as cross-linker shows regular small spheres, and insoluble in water (Fig. 2(c) and (d)). Therefore, they can be used not only in catalytic non-aqueous system, but also in the catalytic aqueous system. On the contrary, the polymer without divinylbenzene showed irregular shape and soluble in water (Fig. 2(a) and (b)). Divinylbenzene has two vinyl groups in the para position, so it can act as a “bridge” between linear molecules. In the presence of an initiator, many linear molecules can be bonded and cross-linked to form porous materials.
3.1.3 TG analysis
The thermal stability of PILs was measured via TG, and the weight losses are illustrated in Fig. 3. The small weight loss at the first stage occurred before 300 °C due to the release of adsorption water, solvent, and then monomer of polymer that confined inside some pore channels. However, the weight loss of PILs drops from 300 °C to 600 °C, which attributed to the polymer decomposition. This fact revealed that the PILs demonstrated good thermal stability, and suitable as catalysts.
3.1.4 BET isotherms
The BET isotherms results of PILs as follows (Fig. 4). The four PILs are typically type IV with hysteresis loops at the pressures P/Po = 0.2–1.0, distinctive of micro-mesoporous materials. From Table 1, we can see that the PILs prepared by adding divinylbenzene as a crosslinking agent have a larger BET surface area (66.36 m2/g), total pore volume (0.28 cm3/g) and average pore width (16.84 nm) than that without (Table 1).
3.2 Catalytic performance of the synthesized PILs
The Prins reaction of 1, 3, 5-trioxane with propylene as the model reaction to evaluate and compare the catalytic performance of PILs (Scheme 2).
The effects of various catalysts on the Prins reaction were studied under the following conditions: 8.0 wt% catalyst, propylene: HCHO (TOX) = 4:1 mol ratio, 100 °C, 4 h; the results were summarized in Table 2. When VPyBs-SSA and VIMBs-SSA were used as catalysts, the conversion of TOX was 54.7% and 77.4%, the selectivity of Product was 31.6% and 46.8% respectively (entries 1 and 2, Table 2). In contrast, VPyBs-DVB-SSA and VIMBs-DVB-SSA which co-polymerized by DVB with cations and anions (entries 3 and 4, Table 2) exhibited moderate catalytic activities, the conversion of TOX was 98.2% and 99.3% respectively. But when VPyBs-DVB-SSA as a catalyst, the selectivity of the product was only 74.5%, VIMBs-DVB-SSA up to 81.4%. What’s more, under the condition of keeping the same acidity of the catalyst, the catalytic activity of the porous PILs prepared by us is better than that of the traditional proton acid (entries 4–6, Table 2). When concentrated sulfuric acid as a catalyst, the conversion of TOX is 86.4% and the selectivity of the product is 71.7% (entry 5, Table 2). The catalytic activity of phosphoric acid is worse, the conversion of TOX is 79.6% and the selectivity of the product is 64.3% (entry 6, Table 2). Therefore, based on the above results and bet isotherms (Table 1), we believe that the pore structure of the catalyst may also an important factor affecting the catalytic activity. The results of Table 2 showed that the catalytic activity was good with large specific surface area and pore volume.
VIMBs-DVB-SSA was selected as the catalyst to investigate the influence of various reaction parameters, and the results were summarized in Table 3. The amount of catalyst was confirmed as one of the major parameters; the conversion of TOX steadily increased from 80.8% to 99.4% as the catalyst loading was increased from 2 to 10 wt% (entries 1–5, Table 3). However, the Selectivity of product increases then decreases with the increase of catalyst loading. The selectivity reached the maximum (79.2%) when the loading was 8 wt% (entry 4, Table 3). When the amount of catalyst reached 10%, selectivity down to 72.5% (entry 5, Table 3). These results indicated that excess acid may promote the generation of other byproducts. Temperature is another important factor affecting the catalytic activity of the catalyst; while the reaction temperature was increased from 80 °C to 100 °C, the peak value for the conversion (99.8%) and selectivity (81.7%) was observed (entry 7, Table 3). However as the temperature continues to rise to 140 °C, the conversion was constant and selectivity down to 72.5%from 81.7% (entries 8, 7, Table 3). The influence of reaction time, ranging from 1 to 6 h, was also investigated (entries 7 and 9–11, Table 3). Moreover, the TOX conversion enhanced gradually by prolonging the reaction time reached 99.1% at 2 h and then stay the same. At 4 h, the most favorable reaction time, which the selectivity for 4-methyl-1, 3-dioxane was 81.7%. In the end, the mole ratio of Propylene: HCHO (TOX) was a key factor for achieving the higher catalytic performance; the conversion of TOX remained basically unchanged. The selectivity for 4-methyl-1, 3-dioxane increased from 78.5% to 81.7% when the ratio of Propylene: HCHO was enhanced from 1:1 to 4: 1 (entries 7 and 12, 13, Table 3) and then dropped to 77.2% at Propylene: HCHO = 6: 1 (entry 14, Table 3). Therefore, the optimal condition can be obtained. Under the optimized conditions, we evaluated the reproducibility of the catalyst. From Table 3, we can see that the experimental results of the three repeated experiments (entries 15–17, Table 3) can better repeat the first experimental results (entry 7, Table 3).
3.3 Catalytic reaction mechanism
The mechanism of the Prins reaction was first studied by Ross et al. with isobutylene and formaldehyde as raw materials. Later, Lloyd J. Dolby, Smissman E. et al. and other scientist also studied it (Yang et al., 1959; Dolby and Schwarz, 1965; Smissman et al., 1965; Dolby et al., 1966, 1968a, 1968b; Kovacs and Kovari, 1966; Watanabe, 1966; Schowen et al., 1968; Wilkins et al., 1968; Wilkins and Marianelli, 1970; Dai et al., 1989; Dumitriu et al., 1997, 1999; Kocovsky et al., 1999). Based on the above results (Table 2), the catalytic activity was good with large specific surface area and pore volume compared with microporous PILs and homogeneous catalysts. We believe that the pore structure of the catalyst may also an important factor affecting the catalytic activity. So, we propose the possible reaction mechanism as follows (Scheme 3). Firstly, 1, 3, 5-trioxane is dissociated into formaldehyde monomer under acidic conditions. Then reaction substrates diffuse into the pores of PILs and interact with the acidic active sites. Formaldehyde generates carbon positive ion under the action of protonic acid of catalyst, and then carbon positive ion and olefin have conjugate addition, After that, the carbonyl oxygen in formaldehyde attacks the positive carbon ion, and C⚌O double bond opens to form a new positive carbon ion. Finally, the hydroxyl attacks the positive carbon ion to form a ring, that is, to generate 4-substituted 1, 3-dioxane compounds.
3.4 Recyclability of the catalysts
The recyclability of VIMBs-DVB-SSA in the Prins reaction was also tested under the optimized reaction conditions i.e., 8.0 wt%, propylene: HCHO(TOX) = 4:1 mol ratio, 100 °C, 4 h, and the results were showed in Fig. 5.
The catalyst could be recovered from the reaction system by a simple centrifugation and used again after drying. After reused four times, the conversion of TOX was decline from 97.8% to 74.6%, and the selectivity of 4-methyl-1, 3-dioxane was decreased from 79.7% to 70.3%. The acid strength of the catalyst before and after use was determined by acid-base titration. Compared with before use, the acid strength of the catalyst after use decreased from 2.00 to 1.41. In addition, the structure of the catalyst after reused four times as compared with that of fresh VIMBs-DVB-SSA using FT-IR analysis (Fig. 6) and SEM (Fig. 7). It is obvious that the morphology of the catalyst changes slightly after 4 times of repeated use. In a word, the reuse of the catalyst is a little poor, which needs further study.
4 Conclusions
In conclusion, we have synthesized four kinds of micro-mesoporous PILs for the Prins reaction of propylene with TOX to synthesize 4-methyl-1, 3-dioxane. The PILs copolymerized by DVB with cations and anions displayed superior catalytic activity and selectivity, together with the advantages of easy separation and recovery. In particular, VIMBs-DVB-SSA exhibited the greatest catalytic activity, giving a high formaldehyde (99.8%) and selectivity (81.7%) for 4-methyl-1, 3-dioxane at 100 °C , 4 h and n(propylene)/n(1,3,5-trioxane) = 4:1. Moreover, the properties of catalysts were characterized in detail using FT-IR spectroscopy, BET isotherms, SEM and TG. In a word, it was demonstrated that PILs might be significant and promising catalysts for the Prins reaction.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Thin-Film Composite Polymeric Liquid Gel Membranes and Their Potential for Nanofiltration in Organic Solvents. Adv. Mater. Interfaces. 2018;5(21)
- [Google Scholar]
- On the Prins reaction of terminal olefins and formaldehyde in trifluoroacetic acid. Chem. Heterocycl. Com.. 2018;54:474-477.
- [Google Scholar]
- Synthesis of Polymeric Liquid by Phenolic Condensation and Its Application in Esterification. ACS Sustain. Chem. Eng.. 2019;7(20):17220-17226.
- [Google Scholar]
- Carmeladerisi, Giuliafanton, GianpollinI, et al., 2008. Recent advances in the stereo selective synthesis of trans-3,4-disubstituted-piperi-dines:applications to(-)-paroxetine. Tetrahedron:Asymmetry 19(1), 131–155.
- Improvement in lubricating properties of TritonX-100/n-C10H21OH/H2O lamellar liquid crystals with the amphiphilic ionic liquid 1-alkyl-3-rnethylirnidazolium hexafluorophosphate. J. Colloid Interface Sci.. 2018;522:200-207.
- [Google Scholar]
- Research of stereochemistry on the Prins reaction of cyclohexene. Chin. Sci. Bull.. 1989;34:2045-2049.
- [Google Scholar]
- polymer ionic liquid, by Manyu, Song Hua. Synthesis of Polymeric liquid phosphotungstate and catalytic oxidation desulfurization of simulated oil. J. Fuel Chem.. 2016;44(07):876-881.
- [Google Scholar]
- The Mechanism of the Prins Reaction. IV. Evidence against Acetoxonium Ion Intermediates1. J. Organ. Chem.. 1965;30(11):3581-3586.
- [Google Scholar]
- The mechanism of the Prins reaction. V. The Prins reaction of styrenes. J. Org. Chem.. 1966;31:1110-1116.
- [Google Scholar]
- The mechanism of the Prins reaction. VI. The solvolysis of optically active trans-2-hydroxymethylcyclohexyl brosylate and related arenesulfonates. J. Org. Chem.. 1968;33:3060-3066.
- [Google Scholar]
- Mechanism of the Prins reaction. VII. Kinetic studies of the Prins reaction of styrenes. J. Org. Chem.. 1968;33:4155-4158.
- [Google Scholar]
- Diversity-Oriented Synthesis of Carbohydrate Scaffolds through the Prins Cyclization of Differently Protectedd-Mannitol-Derived Homoallylic Alcohols. Eur. J. Org. Chem.. 2017;2017:5986-6002.
- [Google Scholar]
- Isoprene by Prins condensation over acidic molecular sieves. J. Catal.. 1997;170:150-160.
- [Google Scholar]
- Prins condensation of isobutylene and formaldehyde over Fe-silicates of MFI structure. Appl. Cat. A. 1999;181:15-28.
- [Google Scholar]
- Formosa Plastics Corporation, 2017. Livingsto, Catalyst system for olefin polymerization and method for producing olefin polymer. Focus on Catal. 2017(11).
- Charge transport and glassy dynamics in polymeric ionic liquids as reflected by their inter and intra-molecular interactions. Soft Matter. 2019;15(7):1605-1618.
- [Google Scholar]
- Wells-Dawson type molybdovanadophoric heteropolyacids catalyzed Prins cyclization of alkene with paraformaldehyde under mild conditions a facile and efficient method to 1,3-dioxane derivatives. J. Mol. Catal. A Chem.. 2004;218:147-152.
- [Google Scholar]
- Catalytic Prins Reaction Effected by Molecular Iodine in the Presence of Bis(trifluoromethanesulfonyl)imide Salts. Synthesis. 2017;49:269-274.
- [Google Scholar]
- Novel polymeric acidic ionic liquids as green catalysts for the preparation of polyoxymethylene dimethyl ethers from the acetalation of methylal with trioxane. RSC Adv.. 2019;9:40662-40669.
- [Google Scholar]
- Immobilization of polyoxometalate-based ionic liquid on carboxymethyl cellulose for epoxidation of olefins. New J. Chem. 2014:3953-3959.
- [Google Scholar]
- Solid state ionic behavior of liquid electrolytes immobilized in a microporous membrane. Elsevier; 1990. p. :40-41.
- Poly (ionic liquid) augmented membranes for π electron induced separation/fractionation of aromatics. J. Membr. Sci.. 2019;579:102-110.
- [Google Scholar]
- New Lewis-Acidic Molybdenum (II) and Tungsten (II) Catalysts for Intramolecular Carbonyl Ene and Prins Reactions. Reversal of the Stereoselectivity of Cyclization of Citronellal. J. Org. Chem.. 1999;64:2765-2775.
- [Google Scholar]
- Chemistry of 1,3-diols. IV. Stereochemistry of the Prins reaction of 4-tert-butylcyclohexene. Acta Chim. Acad. Sci. Hung.. 1966;48:147-160.
- [Google Scholar]
- Grafting of vinylimidazolium-type poly (ionic liquid) on silica nanoparticle through RAFT polymerization for constructing nanocomposite based PEM. Polymer. 2020;195:122458
- [Google Scholar]
- Am. Chem. Soc.. 2000;122(30):7264-12083.
- MOR zeolite supported Bronsted acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for ketalization. RSC Adv.. 2014;4:12160-12167.
- [Google Scholar]
- An Efficient Prins Cyclization for Stereoselective Synthesis of Tetrahydropyran from Imines and Homoallyl Alcohols. Chinese J. Chem.. 2014;32:1095-1098.
- [Google Scholar]
- Molybdenum oxide/γ-alumina: an efficient solid acid catalyst for the synthesis of nopol by Prins reaction. RSC Adv.. 2015;5(113):93452-93462.
- [Google Scholar]
- Preparation of cellulose aerogels from ionic liquid solutions for supercritical impregnation of phytol. J. Supercrit. Fluids. 2017;130
- [Google Scholar]
- Synthesis of immobilized Brønsted acidic ionic liquid on silica gel as heterogeneous catalyst for esterification. Catal. Commun.. 2011;12:353-356.
- [Google Scholar]
- Catalytic Synthesis of Bisphenol F over Short-ChanneledMesoporous Molecular Sieve Zr-Ce-SBA-15 Supported Acidic Ionic Liquids. Chin. J. Appl. Chem.. 2020;37(9):1038-1047.
- [Google Scholar]
- Microemulsions with an ionic liquid surfactant and room temperature ionic liquids as polar pseudo-phase. J. Phys. Chem. Part B: Condens. Matter, Mater. Surf. Interfaces Biophys.. 2008;113(2)
- [Google Scholar]
- The preparation of some 2-ar-yl-1, 3-butadienes. J. Am. Chem. Soc.. 1949;71(8):2860-2862.
- [Google Scholar]
- Simple and efficient synthesis of 4-arylamino-1, 3-dioxanes in aqueous medium: a new route for the Prins reaction. Res. Chem. Intermediat.. 2015;41:8441-8450.
- [Google Scholar]
- Effect of SCN- and NO3- ions on the extraction of heavy metals from aqueous solutions with the ionic liquid trihexylammonium octanoate. Sep. Purif. Technol.. 2020;247
- [Google Scholar]
- Mechanism of the Prins reaction. Kinetics and product composition in acetic acid. J. Org. Chem.. 1968;33:1873-1876.
- [Google Scholar]
- Methyltrioxorhenium as a Lewis Acid in the Prins Cyclization of Benzaldehyde and Isoprenol. React. Kinet. Mech. Catal.. 2019;126:869-878.
- [Google Scholar]
- Fabrication of antifouling membranes by blending poly (vinylidene fluoride) with cationic Polymeric liquid. J. Appl. Polym. Sci.. 2020;137(29)
- [Google Scholar]
- Highly selective Prins reaction over acid-modified halloysite nanotubes for synthesis of isopulegol-derived 2H-chromene compounds. J. Catal.. 2019;374:360-377.
- [Google Scholar]
- Smissman, E., RA, S., PS, P., 1965. Mechanism of the Prins Reaction. Stereoaspects of the Formation of 1, 3-Dioxanes. J. Organ. Chem. 30(3), 797–801.
- Covalent Organic Framework Decorated with Vanadium as a New Platform for Prins Reaction and Sulfide Oxidation. ACS Appl. Mater. Inter.. 2018;11:3070-3079.
- [Google Scholar]
- Prins cyclization in 4-methyl-2-phenyl-tetrahydro-2H-pyran-4-ol preparation using smectite clay as catalyst Reaction Kinetics. Mech. Catal.. 2018;124:711-725.
- [Google Scholar]
- Bull. Acad. Imper. Sci. 1914:1800-1803.
- Catalytic diversity conferred by confinement of protons within porous aluminosilicates in Prins condensation reactions. J. Catal.. 2017;352:415-435.
- [Google Scholar]
- Synthesis of Oxa-Bridged Medium-Sized Carbocyclic Rings via Prins Cyclization. Org. Lett.. 2019;21:1881-1884.
- [Google Scholar]
- Thermal Prins reaction. II. The mechanism of the thermal Prins reaction. Kogakubu Kenkyu Hokoku (Chiba Daigaku) 1966:17-21.
- [Google Scholar]
- Mechanism of the Prins reaction of styrenes Prins reaction of trans- -deuterostyrene. Tetrahedron. 1970;26:4131-4138.
- [Google Scholar]
- Stereochemical applications of deuterium magnetic resonance. I. Prins reaction of transstyrene- -d. Tetrahedron Lett. 1968:5109-5112.
- [Google Scholar]
- ChemInform Abstract: Cyano-Containing Ionic Liquids for the Extraction of Aromatic Hydrocarbons from an Aromatic/Aliphatic Mixture. ChemInform. 2013;44(52)
- [Google Scholar]
- Lewis acidic chloroaluminate ionic liquids: novel reaction media for the synthesis of 4-chloropyrans. Eur. J Organ. Chem.. 2003;2003(9):1779-1783.
- [Google Scholar]
- Long-chain fatty acid-based phosphonium ionic liquids with strong hydrogen-bondbasicity and good lipophilicity:synthesis, characterization, andapplication in extraction. ACS Sustain. Chem. Eng.. 2015;3(2):309-316.
- [Google Scholar]
- Novel choline-based ionic liquid as safe electrolytes for high-voltage lithium-ion batteries. J. Power Sources. 2016;328(2):397-404.
- [Google Scholar]
- Effect of Phosphoric Acid on HZSM-5 Catalysts for Prins Condensation to Isoprene from Isobutylene and Formaldehyde. Chem. Res. Chinese U.. 2018;34:485-489.
- [Google Scholar]
- Heterogeneous oxidative desulfurization for model fuels using novel PW-coupled Polymeric liquids with carbon chains of different lengths. J. Taiwan Instit. Chem. Eng.. 2017;76
- [Google Scholar]
- Task-specific basic ionic liquid immobilized on mesoporous silicas: Efficient and reusable catalysts for Knoevenagel condensation in aqueous media. Micropor. Mesopor. Mater.. 2010;136:10-17.
- [Google Scholar]
- Oxidative NHC catalysis for base-free synthesis of benzoxazinones and benzoazoles by thermal activated NHCs precursor ionic liquid catalyst using air as oxidant. Mol. Catal.. 2020;492
- [Google Scholar]
- Preparation of carboxylatocalixarene functionalized magnetic Polymeric liquid hybrid material for the pre-concentration of phthalate esters. J. Chromatogr. A. 2018;1565
- [Google Scholar]
Appendix A
See Figs. 1–7, Schemes 1–3, and Tables 1–4. Reaction conditions a: catalyst: 8.0 wt% (1.23 g), Propylene: HCHO (TOX) = 4:1 mol ratio, 100 °C, 4 h. b: catalyst: 0.1231 g, Propylene: HCHO (TOX) = 4:1 mol ratio, 100 °C, 4 h. c: catalyst: 0.0945 g, Propylene: HCHO (TOX) = 4:1 mol ratio, 100 °C, 4 h.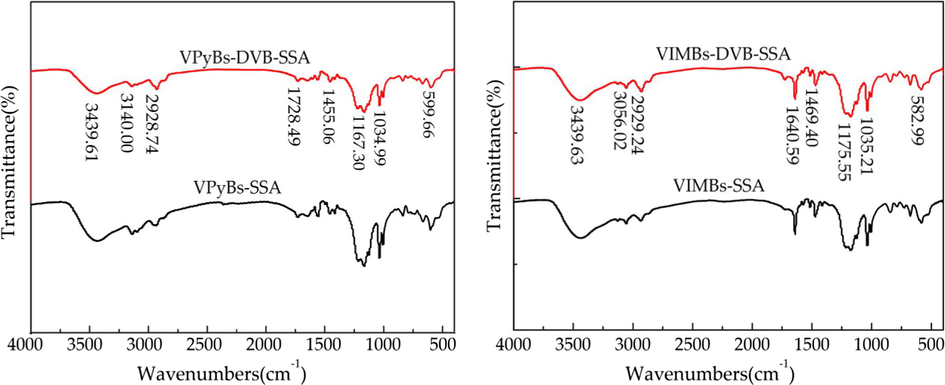
FT-IR spectra of PILs. (a) Pyridinium-based PILs, (b) Imidazolium-based PILs.
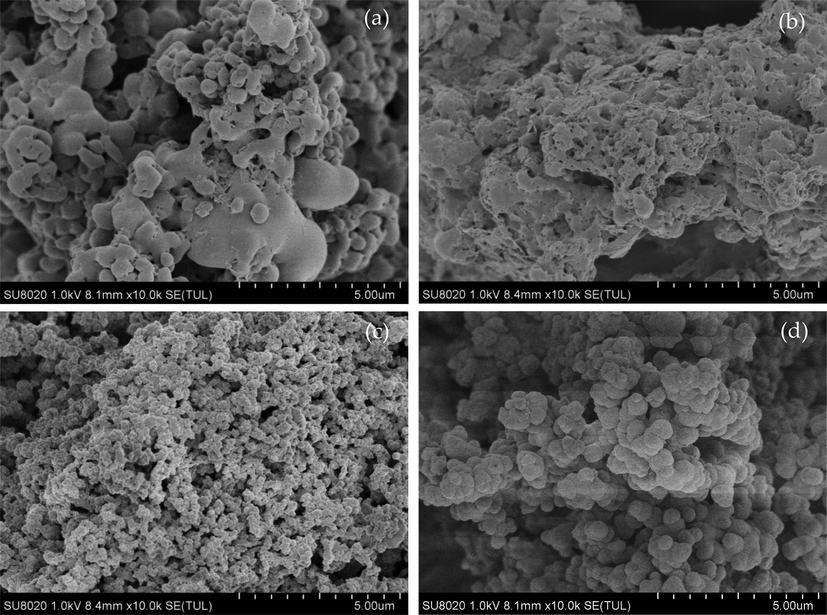
The SEM of PILs. (a) VPyBs-SSA, (b) VIMBs-SSA, (c) VPyBs-DVB-SSA, (d) VIMBs-DVB-SSA.
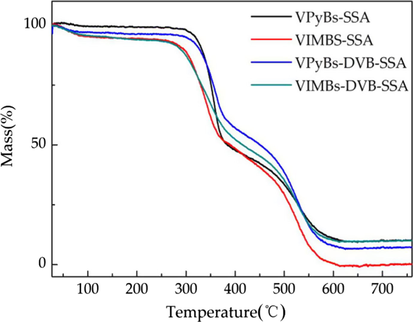
The TG of PILs.
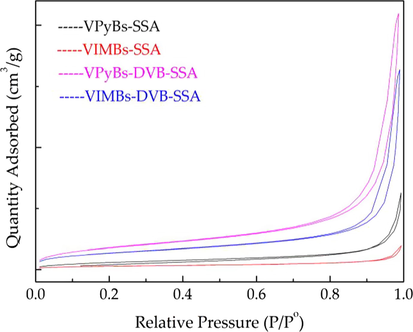
N2 adsorption–desorption isotherms of PILs.
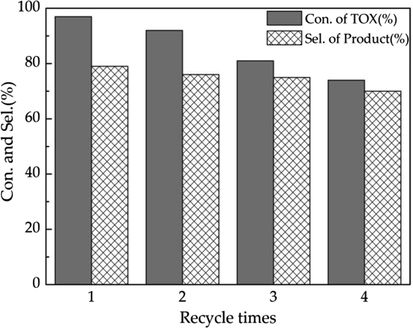
The recycle test of VIMBs-DVB-SSA in the Prins reaction of propylene with TOX. Reaction conditions: 8.0 wt%, propylene: HCHO (TOX) = 4: 1 mol ratio, 100 °C, 4 h.
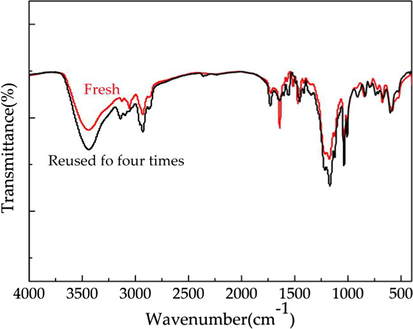
FT-IR spectra of the VIMBs-DVB-SSA fresh and reused four times.
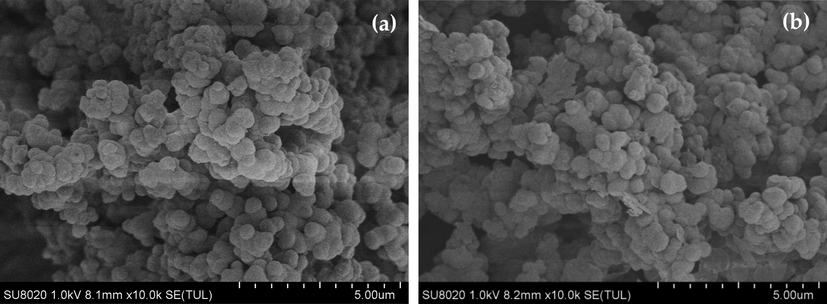
The SEM images of PILs. (a) Fresh VIMBs-DVB-SSA, (b) Reused for 4 times.
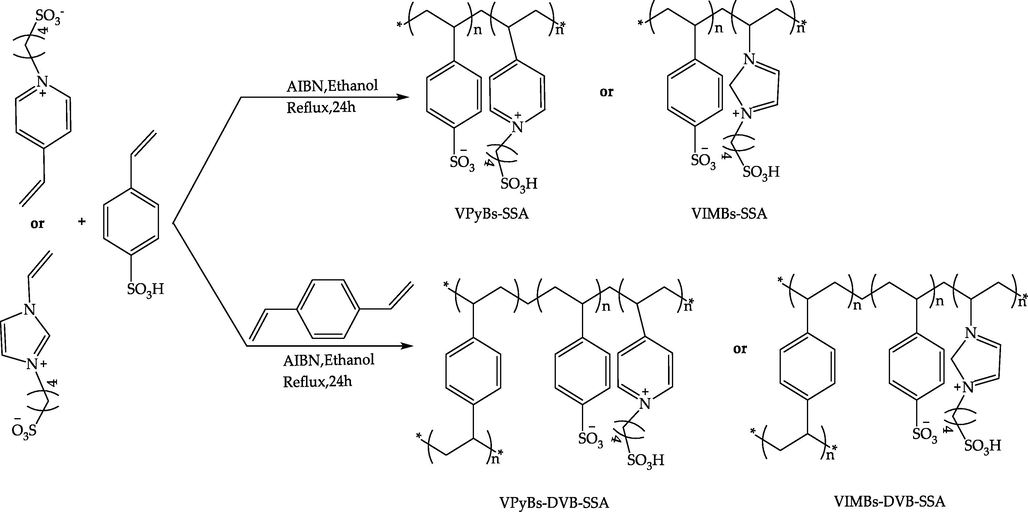
The preparation method of PILs.
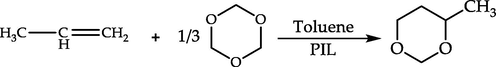
The Prins reaction of 1, 3, 5-trioxane with propylene catalyzed by PILs.
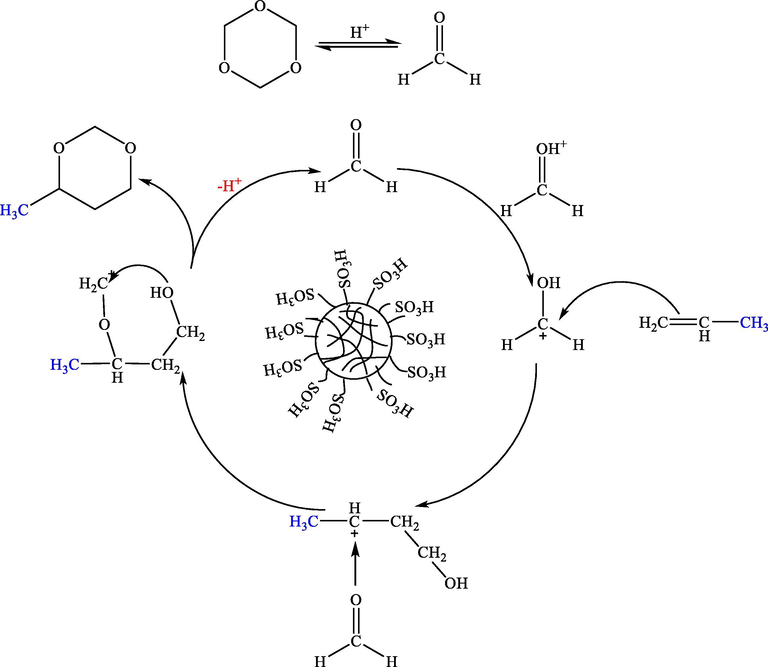
The possible reaction mechanism of propylene with 1, 3, 5-trioxane catalyzed by PILs.
Catalysts
BET surface area (m2/g)
Total pore volume (cm3/g)
Average pore width (nm)
VPyBs-SSA
10.24
0.024
9.48
VIMBs-SSA
23.13
0.074
12.82
VPyBs-DVB-SSA
66.36
0.28
16.84
VIMBs-DVB-SSA
48.90
0.20
16.04
Entry
Catalysts
Conversion of TOX (%)
Selectivity of 4-methyl-1, 3-dioxane (%)
1
VPyBs-SSA
54.7
31.6
2
VIMBs-SSA
77.4
46.8
3
VPyBs-DVB-SSA
98.2
74.5
4
VIMBs-DVB-SSA
99.3
81.4
5
H2SO4b
86.4
71.7
6
H3PO4c
79.6
64.3
Entry
Catalyst (wt %)
Propylene: TOX (molar ratio)
Temperature (°C)
Time (h)
Conversion of TOX (%)
Selectivity of 4-methyl-1, 3-dioxane (%)
1
2
4:1
120
4
80.8
76.5
2
4
4:1
120
4
88.6
73.7
3
6
4:1
120
4
93.5
75.8
4
8
4:1
120
4
99.2
79.4
5
10
4:1
120
4
99.4
72.5
6
8
4:1
80
4
96.8
79.2
7
8
4:1
100
4
99.8
81.7
8
8
4:1
140
4
99.3
72.5
9
8
4:1
100
1
93.6
79.4
10
8
4:1
100
2
99.1
78.3
11
8
4:1
100
6
99.2
75.6
12
8
1:1
100
4
98.6
75.7
13
8
2:1
100
4
99.2
78.5
14
8
6:1
100
4
99.4
77.2
15
8
4:1
100
4
99.5
81.6
16
8
4:1
100
4
99.7
81.5
17
8
4:1
100
4
99.8
82.1
Catalysts
Acid capacity (mmol/g)
1
2
Average
VIMBs-DVB-SSA
1.99
2.01
2.00
VIMBs-DVB-SSA(4)
1.40
1.41
1.41







