Translate this page into:
Polymethoxyflavones transcends expectation, a prominent flavonoid subclass from Kaempferia parviflora: A critical review
⁎Corresponding author. maisarah_abdulmutalib@msu.edu.my (A.M. Maisarah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
A Thai-native herbal species, Kaempferia parviflora (K. parviflora) is a dark purple, stemless rhizome that belongs to the Zingiberaceae family with historical medicinal benefits. The rhizome has been used to treat gastrointestinal disorders, allergic, and pain relief for centuries, which gave rise to its discovery as an anti-inflammatory agent. Phytochemical investigations of K. parviflora extract (KPE) showed flavones as a major compound in the crude extract, with multiple methoxy functional groups in its chemical structure known as polymethoxyflavones (PMFs). To date, 15 PMFs with methoxy-group varied from 1 to 5 have been discovered. Furthermore, the hydroxylation of PMFs compounds further expands its promising bioactivities. The PMFs in crude extract constitute numerous biological activities, including anticancer, antidiabetic, antioxidant, antimicrobial, and more, despite its high lipophilic character and low water solubility reported that hindered its maximum potential in drug discovery. Nonetheless, the mechanism induced by the specific PMFs from crude KPE has yet to be thoroughly discussed. Thus, we aim to discuss the major biological activities of the crude KPE and its isolated PMFs, together with the mechanism of action for the respective compound. Furthermore, the phytochemical investigations and structure–activity relationship of PMFs were reviewed to provide additional evidence of the chemistry of PMFs and their reported biological activities. In addition, the toxicology reports of crude extract and PMFs were summarized to determine the optimal dosage for future studies. Therefore, this article includes advanced strategies of the crude KPE and PMFs to improve drug delivery and bioavailability through structural modification, transport vehicle, microencapsulation, nanosuspension, metal nanoparticles, and self-micro and -nano emulsifying drug delivery system (SMEDDS & SNEDDS). Overall, the reviews provide a comprehensive analysis of the current therapeutics’ potential of PMFs and crude KPE, besides its obstacles and several strategies for potential applications in future studies.
Keywords
Kaempferia parviflora
Flavonoid
Polymethoxyflavones
Biological activities
Drug delivery
Bioavailability
- Aβ
-
amyloid beta
- ABTS
-
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- Ache
-
acetylcholinesterase
- AGEs
-
advanced glycation end products
- AKT
-
protein kinase B
- ALD
-
approximate lethal dose
- AMPK
-
AMP-activated protein kinase
- AP-1
-
activator protein 1
- ATGL
-
adipose triglyceride lipase
- AuNPs
-
gold nanoparticles
- BACE1
-
Beta-secretase 1
- Bax
-
bcl-2-associated X protein
- BChe
-
butyrylcholinesterase
- BH3
-
interacting-domain death agonist
- BNDF
-
brain-derived neurotrophic factor
- β3AR
-
Beta-3 adrenergic receptor
- cAMP
-
cyclic adenosine monophosphate
- CHOP
-
C/EBP homologous protein
- CML
-
N-carboxymethylysine
- COX-2
-
cyclooxygenase-2
- CRE
-
cAMP-response element
- CUPRAC
-
CUPric reducing antioxidant capacity
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
- ER
-
endoplasmic reticulum
- ERK
-
extracellular signal-regulated kinase
- FICI
-
fractional inhibitory concentration index
- GATA
-
GATA-Binding Protein
- GLUT4
-
Glucose transporter type 4
- GO
-
glyoxal
- GSK3β
-
glycogen synthase kinase-3 beta
- HaCaT
-
human epidermal keratinocytes
- HDF
-
human dermal fibroblast
- HO-1
-
heme oxygenase-1
- HPβ-CD
-
Hydroxypropyl-β-cyclodextrin
- HPMC
-
hydroxypropyl methylcellulose
- HSL
-
hormone-sensitive lipase
- HUVEC
-
human umbilical vein endothelial cells
- IκBα
-
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- IC50
-
half-maximal inhibitory concentration
- IFN-β
-
Interferon beta-1b
- IGF1
-
insulin-like growth factor 1
- IL-1β
-
interleukin-1 beta
- iNOS
-
nitric oxide synthase
- IPM
-
isopropyl myristate
- JNK
-
jun N-terminal kinase
- KP
-
Kaempferia parviflora
- K.parviflora
-
Kae mpferia parviflora
- KPE
-
K. parviflora extract
- LA
-
lactic acid
- LPS
-
lipopolysaccharides
- MAPK
-
mitogen-activated protein kinase
- MCL-1
-
myeloid cell leukemia-1
- MCP-1
-
monocyte chemoattractant protein-1
- MCT1
-
monocarboxylate transporter 1
- MDCK
-
madin-darby canine kidney
- MGO
-
methylglyoxal
- MIC
-
minimum inhibitory concentration
- MMP
-
matrix metalloproteinase
- mTOR
-
mammalian target of rapamycin
- NF-κB
-
nuclear factor-κB
- ORAC
-
oxygen radical absorbance capacity
- PeMF
-
3,5,7,3′,4′-Pentamethoxyflavone
- PGE2
-
prostaglandin-2
- PI
-
preferential index
- PI3K
-
phosphoinositide 3-kinases
- PKA
-
protein kinase A
- PLCγ1
-
Phospholipase C gamma 1
- PMFs
-
polymethoxyflavones
- PPARγ
-
peroxisome proliferator-activated receptor gamma
- PVA-co-PEG
-
polyvinyl alcohol-polyethylene glycol grafted copolymer
- ROS
-
reactive oxygen species
- SD
-
Sprague–dawley
- SMEDDS
-
self-microemulsifying delivery system
- SNEDDS
-
self-nanoemulsifying delivery system
- Syk
-
spleen tyrosine kinase
- STAT3
-
signal transducer and activator of transcription 3
- TG
-
triglycerides
- TNF-α
-
tumor necrosis factor-alpha
- TSNO
-
tsumura suzuki non-obesity
- TSOD
-
tsumura, suzuki, and obese diabetes
- UCP-1
-
uncoupling protein 1
- VPA
-
Valproic acid
- 3DG
-
3-deoxyglucosone
- 3,5,7-TMF
-
3,5,7-Trimethoxyflavone
- 3,5,7,4′-TeMF
-
3,5,7,4′-Tetramethoxyflavone
- 5,7,4′-TMF
-
5,7,4′-Trimethoxyflavone
- 5,7,3′,4′-TeMF
-
5,7,3′,4′-Tetramethoxyflavone
- 5H-3,7,3′,4′-TeMF
-
5-Hydroxy-3,7,3′,4′-Tetramethoxyflavone
- 5H-3,7-DMF
-
5-Hydroxy-3,7-Dimethoxyflavone
- 5H-3,7,4′-TMF
-
5-Hydroxy-3,7,4′-Trimethoxyflavone
- 5H-7-MF
-
5-Hydroxy-7-Methoxyflavone
- 5H-7,4′-DMF
-
5-Hydroxy-7,4′-Dimethoxyflavone
Abbreviations
1 Introduction
The global market for herbal medicines stands at over US $165 billion in 2022 and is projected to exceed US $347 billion by 2029 (Insight, 2022). Many pharmaceutical industries have a long history of the use of herbal remedies. The use of medicinal plants is advancing worldwide, given the remarkable development of traditional medicine and a growing interest in herbal remedies. Kaempferia parviflora or kunyit hitam is an herbal plant species in the Zingiberaceae family. It’s a native species in the tropical area of Southeast Asia, particularly in Thailand, the Malay peninsula, West Java, and Borneo Island. The rhizome of K. parviflora has been traditionally utilized for centuries to treat allergic, stomach ulcers and pain relief (Saokaew et al., 2017). Pharmacological investigation of K.parviflora rhizome extract reveals its bioactivities such as anticancer (Thaklaewphan et al., 2021), antidiabetic (Yagi et al., 2019), antioxidant (Varghese et al., 2021), antiglycation (Yagi et al., 2021), antidepressant (Wattanathorn et al., 2013), antifungal (Kummee et al., 2008), antimicrobial (Sitthichai et al., 2022), antiviral (Sornpet et al., 2017), antibacterial (Jeong et al., 2016), antiparasitic (Leesombun et al., 2019) antiallergic (Tewtrakul et al., 2008), anti-inflammatory (Takuathung et al., 2021) and anticholinesterase activities (Seo et al., 2017). The efficacy of PMFs with diverse methoxy-group positions contributed to the potent bioactivities of the crude KPE. Phytochemistry of K. parviflora rhizome revealed 15 major constituents from the flavones group, dominated by 5,7-Dimethoxyflavone, 5,7,4′-Trimethoxyflavone, and 3,5,7,3′,4′-Pentamethoxyflavone (Phung et al., 2021). In this study, some PMFs exhibited hydroxyl groups in the chemical structure, further providing distinct features and interacting with the target site compared to the conventional polymethoxyflavones. As a previous study demonstrates, quercetin, with multiple hydroxy groups, exhibits numerous biological activities (Kim & Park, 2018). The synergistic effect between both functional groups in the chemical structure may provide helpful information for future studies. The current progress on the biological activity of PMFs and the crude KPE with its mechanism of action has not been fully discussed. Therefore, this critical review will cover a wide range of topics comprised of recent progress in the phytochemical investigation, structure–activity relationship, toxicity, biological activities, and future strategies of PMFs as a prominent flavonoid subclass from KPE.
1.1 Phytochemicals study of K. parviflora
Flavones, which consist of multiple methoxylated substituents, specifically PMFs, are the major components in K. parviflora, followed by acetophenone, chalcone derivatives, kaempferiaosides, and other phenolic compounds. To date, 15 major PMFs have been successfully identified, and their respective biological activities are summarized in Table 1. The methoxy group in the chemical structure varied from position C-3, C-3′, C-4′, C-5, and C-7; meanwhile for hydroxy group (C-5, C-3′ & C4′).
Chemical structure
Name
Biological activities
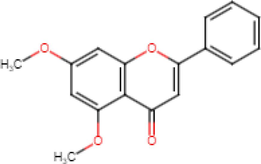
5,7-Dimethoxyflavone
Anti-inflammatory (Lee et al., 2022) Anticancer
(Kim et al., 2018) Alzheimer’s
(Youn et al., 2016) Antioxidant
(Thao et al., 2016) Antidiabetic
(Kobayashi et al., 2015) Anti-acetylcholinesterase
(Sawasdee et al., 2009)
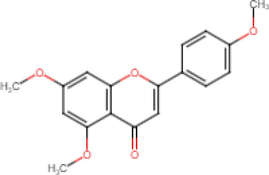
5,7,4′-Trimethoxyflavone
Antibacterial (Sookkhee et al., 2022) Anti-inflammatory
(Phung et al., 2021) Alzheimer’s
(Natsume et al., 2020) Antioxidant
(Thao et al., 2016) Anti-acetylcholinesterase (Seo et al., 2017)
Antiglycation
(Nakata et al., 2014) Anticancer
(Hossain et al., 2012) Antidiabetic
(Azuma et al. 2011) Antimycobacterial
(Yenjai et al., 2004) Antifungal
(Yenjai et al., 2004) Anti-parasites
(Yenjai et al., 2004)
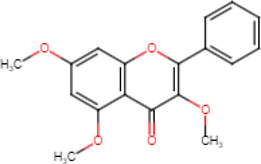
3,5,7-Trimethoxyflavone
Antibacterial (Sookkhee et al., 2022)
Antioxidant (Thao et al., 2016) Anti-inflammatory
(Sae-Wong et al., 2011) Antifungal
(kummee et al., 2008) Antiallergy
(Tewtrakul et al., 2008)
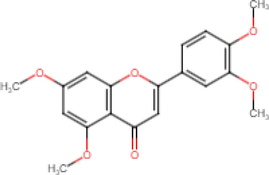
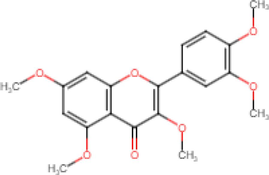
5,7,3′,4′-Tetramethoxyflavone
Anti-inflammatory (Ongchai et al., 2021) Alzheimer’s
(Natsume et al., 2020) Antidiabetic
(Azuma et al., 2011) Anti-parasites
(Yenjai et al., 2004)
3,5,7,4′-Tetramethoxyflavone
Antioxidant (Thao et al., 2016) Anti-inflammatory
(Toda et al., 2016a) Antiglycation
(Nakata et al., 2014) Antidiabetic
(Horikawa et al., 2012) Anticancer
(Hossain et al., 2012) Antiallergy
(Tewtrakul et al., 2008) Antimycobacterial
(Yenjai et al., 2004) Antifungal
(Yenjai et al., 2004)
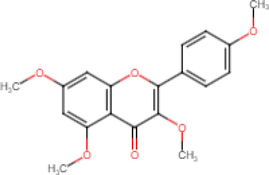
3,5,7,3′,4′-Pentamethoxyflavone
Antibacterial (Sookkhee et al., 2022) Anticancer
(Kim et al., 2018) Anti-acetylcholinesterase
(Seo et al., 2017) Alzheimer’s
(Youn et al., 2016) Anti-inflammatory
(Jakhar et al., 2014) Antiglycation
(Nakata et al., 2014)
Antidiabetic (Okabe et al., 2014) Antioxidant
(Jakhar et al., 2014)
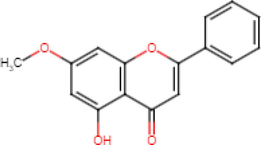
5-Hydroxy-7-Methoxyflavone
Anticancer (Sun et al., 2021) Antioxidant
(Thao et al., 2016) Antidiabetic
(Shimada et al., 2011) Anti-inflammatory
(Tewtrakul et al., 2009) Antiallergy
(Tewtrakul et al., 2008)
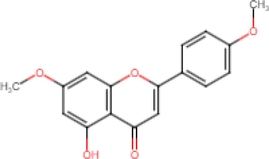
5-Hydroxy-7,4′-Dimethoxyflavone
Antioxidant (Thao et al., 2016) Anti-inflammatory
(Horigome et al., 2014) Antidiabetic
(Shimada et al., 2011) Antiallergy
(Tewtrakul et al., 2008)
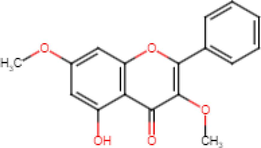
5-Hydroxy-3,7-Dimethoxyflavone
Antioxidant (Thao et al., 2016) Anti-inflammatory
(Toda et al., 2016a) Antidiabetic
(Shimada et al., 2011) Antiallergy
(Tewtrakul et al., 2008)

5-Hydroxy-7,3′,4′-Trimethoxyflavone
Anti-allergy (Kobayashi et al., 2015)
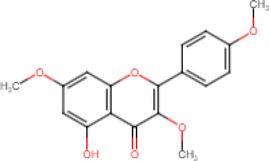
5-Hydroxy-3,7,4′-Trimethoxyflavone
Antioxidant (Thao et al., 2016) Anti-inflammatory
(Toda et al., 2016a) Antiallergy
(Kobayashi et al., 2015) Antidiabetic
(Shimada et al., 2011)
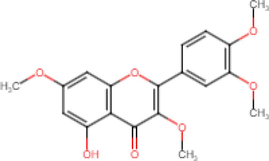
5-Hydroxy-3,7,3′,4′-Tetramethoxyflavone
Anti-inflammatory (Lee et al., 2022) Antioxidant
(Thao et al., 2016) Anticancer
(Hossain et al., 2012) Antiallergy
(Tewtrakul et al., 2008)
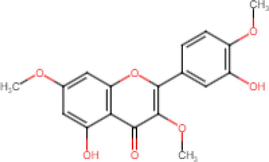
5,3′-Dihydroxy-3,7,4′-Trimethoxyflavone
Antiallergy (Kobayashi et al., 2015) Anti-inflammatory
(Sae-Wong et al., 2011)
5,4′-Dihydroxy-7-Methoxyflavone
Antioxidant (Thao et al., 2016) Antidiabetic
(Kobayashi et al., 2015)
4′-Hydroxy-5,7-Dimethoxyflavone
Antioxidant (Thao et al., 2016) Antiallergy
(Kobayashi et al., 2015)
2 Structure-activity relationship of K. parviflora and its PMFs
Polymethoxyflavones (PMFs) are a subclass of flavonoid with a skeleton structure that incorporates multiple methoxy groups bonded to the phenyl rings A & B or heterocyclic ring C with carbonyl functional groups attached at position C-4 (Fig. 1). Methoxylation of PMFs and its positions in the chemical structure play significant roles in influencing the lipophilicity of the compounds. Higher lipophilicity generally will improve the activity of PMFs through enhanced permeability in the plasma membrane, which leads to higher PMFs influx into the cytosol of the target cells (Wang et al., 2014). Nevertheless, the presence of the hydroxy group in several PMFs may also alter the bioactivities due to its hydrophilicity properties (Tung et al., 2019). Therefore, incorporating both functional groups present in the PMFs of K. parviflora and its effect on bioactivities is an interesting topic to be discussed.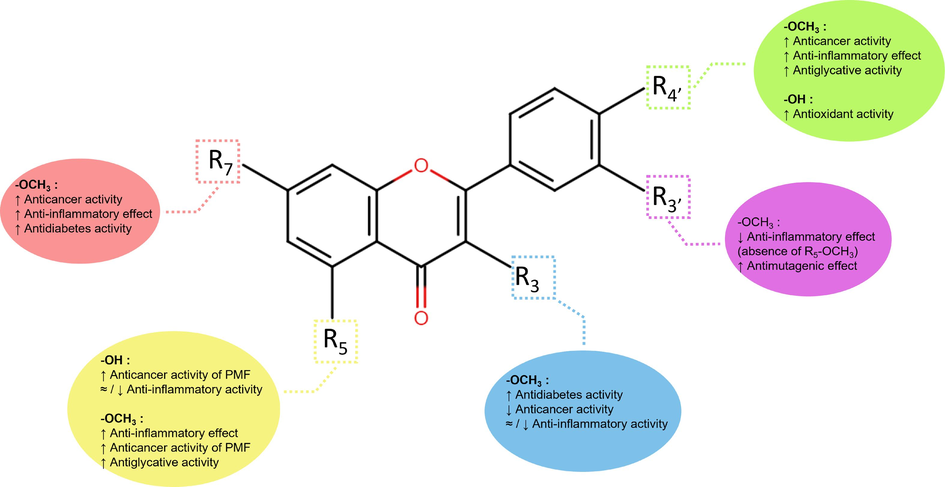
Structure-activity relationship of reported PMFs from K. parviflora.
In anticancer activities, the absence of methoxy group (–OCH3) in position C-3 and C-3′ with an additional hydroxy group positioned at C-5 of PMFs shows enhancement in cell cytotoxicity. The statement was supported by the significant cytotoxicity effect of PMFs from K. parviflora on human pancreatic cancer cells, PANC-1 (Sun et al., 2021), colorectal cancer cells, HCT-15 (Sun et al., 2021) and lens epithelial cell lines, SRA01/04 (Miyata et al., 2019). Nonetheless, although methoxy substituents at C-5 reduced anticancer activity, 5,7-DMF significantly alleviates inflammation (Lee et al., 2022; Fuchino et al., 2018). Compound 5,7-DMF potently suppressed matrix metalloproteinase (MMP) MMP-1 and MMP-3 action (Kobayashi et al., 2018), which is responsible for the regulation of inflammatory cytokines and chemokines (Nissinen and Kähäri, 2014). In ring B, the substitution of methoxy group in C-4′ forming trimethoxy compound, 5,7,4′-Trimethoxyflavone (5,7,4′-TMF) significantly ameliorates the suppression of nitric oxide (NO), Interleukin-6 (IL-6), Interleukin-1 beta (IL-1β), and cyclooxygenase-2 (COX-2); a key inflammatory mediator compared with the same compound without methoxy group (Phung et al., 2021; Tewtrakul & Subhadhirasakul, 2008).
High glucose level increases glucose autoxidation and generates excessive ROS, leading to oxidative stress induction and the formation of advanced glycation end products, AGEs (Ha and Lee, 2000). The hyperglycemic complication and oxidative stress further escalate AGEs released that are associated with diabetes pathogenesis and aging (Suji and Sivakami, 2004). Many herbal products have potent antiglycation activities. The glycation inhibition by PMFs in K. parviflora, such as 5,7,4′-TMF, substantially enhanced the anti-glycation activity with the presence of methoxy in C-4′ (Nakata et al., 2014). However, substituting another methoxy group at C-3′; 5,7,3′,4′-Tetramethoxyflavone (5,7,3′,4′-TeMF) results in lower anti-mutagenic activity (Azuma et al., 2011). Nevertheless, PMFs bearing similar methoxy positions (C-3′ and C-4′), such as TeMF, demonstrated potent antidiabetic activity by inhibiting the α-glucosidase enzyme (Azuma et al., 2011). Another study showed that PMFs' antidiabetic activity diminished with a single methoxy constituent at C-3′ or C-4′ (Toda et al., 2016b). In addition, 5,7,4′-TMF also demonstrates potent acetylcholinesterase inhibition with the presence of methoxy group at C-4′ and C-5; however, at positions 3 and 3′, the inhibitory effect declined (Sawasdee et al., 2009). Concurrently, all studies agree that methoxylation at the C-5 position significantly influences the bioactivities of PMFs and mediate diabetes and mutagenicity (Horikawa et al., 2012), cognitive deterioration (Natsume et al., 2020) and Alzheimer’s (Youn et al., 2016).
3 Toxicology study of K. parviflora extract (KPE)
3.1 In-vivo toxicity study
Although KPE has been used in traditional remedies for centuries, no toxicity guidelines have been utilized throughout its consumption. The safety profiles of K. parviflora in various doses were discussed in toxicology in vivo studies on rats from 2006 to 2019. While no optimal amounts have been confirmed, intake of 2.0 g/kg body weight of K. parviflora was categorized as safe based on a 7-day acute single-oral toxicity study, with low toxicity levels recorded (Sae-wong et al., 2009). The results were within the satisfactory range given that in another 14-day toxicity test, the approximate lethal dose (ALD) recorded was more than 5.0 g/kg, with no apparent difference in body weight changes for both female and male Sprague-Dawley rats (SD rats) (Han & Park, 2018). However, contradicting the earlier report, a 6-month chronic toxicity test of high intake of KPE at 500 mg/kg body weight /day revealed a significant decline in male rats’ body weight starting from week 8 of treatment, possibly due to lower food consumption. Meanwhile, for female rats, a notable surge was visible in glucose and cholesterol levels at a higher dose (174.96 & 116.18 mg/dL) compared to the control, 141.59 and 68.93 mg/dL, respectively, which may be attributed to overdose with a high level of ALT and BUN level detected, a sign of kidney and liver disease (Chivapat et al., 2010). Besides, the platelet count of female rats in low doses of K. parviflora (5 and 50 mg/kg /day) was decreased (880.5 × 103/mm3) compared to the control study (932.0 × 103/mm3). In contrast, increases in platelet counts were recorded with consumption of 25 mg/kg /day doses in 90-day hematological analysis (100.4 × 1012/L) against the control groups (82.8 × 1012/L); however, no apparent difference in body weight changes, glucose, and cholesterol level for female and male rats recorded in this study (Yoshino et al., 2019). Meanwhile, in other hematological parameters analysis, K. parviflora remained non-toxic to both female and male rats in various doses, and no adverse effect was reported on red blood cell count, white blood cell count, Hemoglobin count, and hematocrit count, in agreement with the initial findings (Sudwan et al., 2006). Meanwhile, consumption of 100 mg/kg body weight of K. parviflora twice a day does not exert any changes on the level of kidney and liver enzymes; creatinine, blood urea nitrogen, alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), which supported by other toxicology studies (Yorsin et al., 2014). However, at high doses (249 mg/kg/day), the creatinine level was lowered to 24.75 mmol/L, while ALP observed a higher count (291.8 U/L) than the control. Nevertheless, the changes were noticeable yet within the optimum range in regulating kidney and liver functions (Yoshino et al., 2019).
3.2 In vitro toxicity study
On another note, the toxicity effect of crude extract and its PMFs were investigated on normal cell lines to determine its selectivity activity and optimum concentration level. Aqueous extract of crude K. parviflora showed low toxicity on normal Madin-Darby canine kidney (MDCK) cells with an IC50 value of 468.2 μg/ml. However, ethanolic extract's toxicity was unexpectedly higher, with an IC50 value of 2.2 μg/ml (Sornpet et al., 2017). In another study, at the highest concentration of KPE (200 μg/ml), the normal human epidermal keratinocytes (HaCaT) cell growth was maintained at 90 % (Wang et al., 2022). In another study with similar types of cells, no toxicity was observed in the cell when administered with crude extract at a concentration of 400 μg/ml (Lee et al., 2018). All 6 PMFs compounds isolated from the crude extract exhibit no toxicity up to 100 µM concentration (Lee et al., 2022). Meanwhile, the crude extract demonstrated high selectivity against normal human umbilical vein endothelial cells (HUVEC) cells, with low toxicity recorded after 24 h (IC50: 88.85 μg/ml) (Tangjitjaroenkun et al., 2021). Treatment of up to 100 μg/ml of crude extract does not exhibit significant toxicity against normal human dermal fibroblast cells (HDF), with cell growth maintained above 90 % (Sitthichai et al., 2022). In another toxicity study of the isolated compound from KPE, the HDF cell viability retained above 90 % when treated up to 100 µM of compound 3,5,7,3′,4′-Pentamethoxyflavone (PeMF), followed by 5,7-DMF (25 µM) and 5,7,4′-TMF (25 µM). In another study, the reduction of normal fibroblast HS68 cells from cellular senescence was suppressed with increased cell growth by the crude extract up to 10 µM concentration (Park et al., 2017). The in vitro study of crude KPE and its isolated compounds demonstrate potent inhibition of normal cell death.
4 Anti-inflammatory activity
Inflammation is a complex immune response due to damage in living tissues that causes soreness, redness, and swelling. Suppression of excessive inflammatory response by KPE and its isolated PMFs is crucial to alleviate chronic inflammatory diseases such as psoriasis (Takuathung et al., 2021), skin aging (Phung et al., 2021), acne (Sitthichai et al., 2022) and arthritis (Ongchai et al., 2021). Anti-inflammation study shows that K. parviflora and its PMFs are involved in mediating mRNA expression and released of matrix metalloproteinases (MMPs), reactive oxygen species (ROS), nitric oxide synthase (iNOS), nitric oxide (NO), proteins kinases (MAPK, IκBα, AKT), interleukins (IL-4, IL-6, IL-8 & IL-1β), tumor necrosis factor-alpha (TNF-α), protein transcription factors (NF-κB, AP-1), prostaglandin-2 (PGE2) and cyclooxygenase-2 enzymes (COX-2), as summarized in Table 2.
Sample
Experimental model
Treatment
Molecular target
References
Crude extract
In vitro: H2O2-induced senescent HS68 cells
1–10 µg/ml
↓ IL-6, IL-8, NFκB, COX-2
(Park et al., 2017)
In vitro: Anti-acne on RAW 264.7 cell
0.05 mg/ml
↓ NO
(Sitthichai et al., 2022)
In vitro: LPS-induced inflammation in HUVEC
1000 μg/ml
↓ NO, IL-1β, TNF-α, IL-6, COX-2, MCP-1, ROS
(Horigome et al., 2014)
In vitro: LPS-induced inflammatory marker release in RAW264.7 cell
100 μg/ml
↓ NO
(Tewtrakul and Subhadhirasakul, 2008)
20 μg/ml
↓ iNOS, NO, COX-2, IκBα, NFκB, pNFκB
(Jin and Lee, 2018)
100 μM
↓ PGE2, iNOS, COX-2
(Sae-wong et al., 2009)
H. pylori-induced inflammation in AGS cell
16 μg/ml
↓ IL-8
(Nemidkanam et al., 2020)
In vivo: UVB-induced photoaging in hairless mice
200 mg/kg/day
↓ MMP-2, MMP-9, AP-1, NFκB, IL-1β, COX-2
(Park et al., 2014)
In vitro: sUV-induced oxidative damage in JB6 P+ & HaCaT cells
100 μg/ml
↓ COX-2, PGE2, NFκB, JNK, p38, ERK, MKK, MEK,
(Lee et al., 2018)
In vivo: sUV-induced oxidative damage in mouse skin
100 mg/kg
↓ COX-2, JNK, p38, ERK
(Lee et al., 2018)
In vitro: IL-1β -induced inflamed Human knee-derived chondrocytes
10 – 20 μg/ml
↓ MMP-1, MMP-3, MMP-13
(Kobayashi et al., 2018)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μg/ml
↓ IL-6, TNF-α
(Toda et al., 2016a)
LPS-induced inflammation on RAW 264.7 and HaCaT cells
3.75, 7.5 & 15 μg/ml
↓ NO, IL-1β, IL-6, iNOS, COX-2, and TNF-α, NFκB, IκBα, ERK, JNK, p38
(Takuathung et al., 2021)
In vitro: Antigen-stimulated inflammation in RBL-2H3 cells
250 μg/ml
↓ TNF-α, IL-4, MCP-1
(Horigome et al., 2014)
In vitro: SW1353-stimulated inflammation
10 μg/ml
↓ IL-1β, IL-6, TNF-α, COX-2, NFκB, JNK, ERK, p38
(Ongchai et al., 2021)
In vitro: cytokines-induced inflammation on SW982 cell
3–30 μg/ml
↓ IL-1β, TNF-α, IL-6, NO, PGE2, p38, STAT1, STAT3
(Kongdang et al., 2019)
5,7-Dimethoxyflavone
In vivo: LPS-induced inflammation in HDF
3 μg/ml
↓ ROS, TNF-α, MMP-1
(Klinngam et al., 2022)
In vitro: P. acnes-induced inflammation in HaCaT cells
3 & 4 μg/ml
↓ iNOS, IκB-α, NFκB
(Jin and Lee, 2018)
In vitro: LPS-Induced inflammation in RAW 264.7 cell
1–100 μg/ml
↓ NO, TNF-α, iNOS, ERK, JNK
(Sae-Wong et al., 2011)
In vitro: LPS-induced inflammation in HUVEC
1–100 μM
↓ NO, IL-1β, TNF-α, IL-6, COX-2, MCP-1, ROS
(Horigome et al., 2014)
In vitro: IL-1β -induced inflamed Human knee-derived chondrocytes
5 μM
↓ MMP-1
(Kobayashi et al., 2018)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
In vivo: anti-sarcopenic effect
25–––50 mg/kg/day
↑ PI3K & Akt
↓ IL-6, TNF-α, NFκB(Kim and Hwang, 2020)
In vitro: antigen-stimulated inflammation in RBL-2H3 cells
50 μM
↓ TNF-α, IL-4, MCP-1
(Horigome et al., 2014)
In vitro: SW1353-stimulated inflammation
3.3 μg/ml
↓ IL-1β, IL-6, TNF-α, COX-2
(Ongchai et al., 2021)
In vitro: cytokines-induced inflammation on SW982 cell
10 μg/ml
↓ TNF-α, IL-6, MMP-13, ZIP8, IL-1β, COX-2
(Kongdang et al., 2019)
5,7,4′-Trimethoxyflavone
In vitro: TNF-α-induced inflammation in NHDF
6.25 & 12.5 µM
↓ COX-2, IL-1β, IL-6, JNK, ERK, p38, ROS,
(Phung et al., 2021)
In vivo: LPS-induced inflammation in NHDF
3 μg/ml
↓ ROS, IL-6, MMP-1
(Klinngam et al., 2022)
In vitro: LPS-Induced inflammation in RAW 264.7 cell
1–100 μg/ml
↓ NO, TNF-α, iNOS, ERK, SYK
(Sae-Wong et al., 2011)
In vitro: IL-1β -induced inflamed Human knee-derived chondrocytes
5 μM
↓ MMP-1, MMP-3
(Kobayashi et al., 2018)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
In vitro: SW1353-stimulated inflammation
2.6 μg/ml
↓ IL-1β, IL-6, TNF-α, COX-2
(Ongchai et al., 2021)
In vitro: cytokines-induced inflammation on SW982 cell
8 μg/ml
↓ TNF-α, IL-6, MMP-13, ZIP8, IL-1β, COX-2
(Kongdang et al., 2019)
3,5,7-Trimethoxyflavone
In vitro: TNF-α-induced inflammation in NHDF
50 & 100 µM
↓ MMP-1, ROS, TNF-α, AKT, COX-2, HO-1, IL-1β, IL-6, IL-8
(Lee et al., 2022)
In vitro: LPS-induced inflammation in RAW264.7 cell
–
↓ NO
(Tewtrakul et al., 2009)
5,7,3′,4′-Tetramethoxyflavone
In vitro: LPS-Induced inflammation in RAW 264.7 cell
1–100 μg/ml
↓ NO, TNF-α, iNOS, ERK, JNK
(Sae-Wong et al., 2011)
3,5,7,4′-Tetramethoxyflavone
In vitro: IL-1β -induced inflamed Human knee-derived chondrocytes
5 μM
↓ MMP-1
(Kobayashi et al., 2018)
In vitro: LPS-induced inflammation in RAW264.7 cell
–
↓ NO
(Tewtrakul et al., 2009)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
3,5,7,3′,4′-Pentamethoxyflavone
In vivo: LPS-induced inflammation in NHDF
3 μg/ml
↓ ROS, MMP-1
(Klinngam et al., 2022)
In vitro: Protective effect of PMFs against DNA damage
100 μg/ml
↓ NO
(Jakhar et al., 2014)
In vitro: IL-1β -induced inflamed Human knee-derived chondrocytes
5 μM
↓ MMP-1, MMP-3
(Kobayashi et al., 2018)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
In vitro: SW1353-stimulated inflammation
2.2 μg/ml
↓ TNF-α
(Ongchai et al., 2021)
In vitro: Cytokines-induced inflammation on SW982 cell
7 μg/ml
↓ TNF-α, IL-6, MMP-13, ZIP8
(Kongdang et al., 2019)
5-Hydroxy-7-Methoxyflavone
In vitro: LPS-induced inflammation in RAW264.7 cell
–
↓ NO
(Tewtrakul et al., 2009)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
5-Hydroxy-7,4′-Dimethoxyflavone
In vitro: LPS-induced inflammatory marker release in RAW 264.7 cell
1 – 100 μg/ml
↓ NO
(Tewtrakul et al., 2009; Tewtrakul and Subhadhirasakul, 2008)
5-Hydroxy-3,7-Dimethoxyflavone
In vitro: LPS-induced inflammation in RAW 264.7 cell
–
↓ NO
(Tewtrakul et al., 2009)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
5-Hydroxy-7,3′,4′-Trimethoxyflavone
In vitro: LPS-induced inflammation in RAW 264.7 cell
–
↓ NO
(Tewtrakul et al., 2009)
5-Hydroxy-3,7,4′-Trimethoxyflavone
In vitro: LPS-Induced inflammation in RAW 264.7 cell
1–100 μg/ml
↓ NO
(Sae-Wong et al., 2011; Tewtrakul and Subhadhirasakul, 2008
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
5-Hydroxy-3,7,3′,4′-Tetramethoxyflavone
In vitro: LPS-induced inflammation in HUVEC
1–100 μM
↓ NO, IL-1β, TNF-α, IL-6, COX-2, ROS
(Horigome et al., 2014)
In vitro: LPS-induced inflammation production in RAW 264.7 cell
100 μg/ml
↓ NO, PGE2
(Tewtrakul and Subhadhirasakul, 2008)
100 μM
↓ iNOS, COX-2
(Sae-wong et al., 2009)
–
↓ NO, PGE2, TNF-α
(Tewtrakul et al., 2009)
In vitro: LPS and LA-induced muscular inflammation C2C12 myoblasts
10 μM
↓ IL-6, TNF-α
(Toda et al., 2016a)
In vitro: Antigen-stimulated inflammation in RBL-2H3 cells
50 μM
↓ TNF-α, IL-4, MCP-1
(Horigome et al., 2014)
5,3′-Dihydroxy-3,7,4′-Trimethoxyflavone
In vitro: LPS-induced inflammation in RAW 264.7 cell
1–100 μg/ml
↓ NO
(Sae-Wong et al., 2011)
4.1 KPE inhibits inflammatory markers
Inhibition of cytokines is a primary pathway to suppress inflammatory release. In the study by Nemidkanam et al. (2020), the mRNA expression of IL-8 was significantly suppressed at 16 μg/ml crude extract by 2.3-fold and 2.07-fold after 6 and 12 h, respectively. Remarkably, the secretion was also reduced to 57.68 pg/ml and 72.15 pg/ml in the same period, respectively, compared to control. Besides, crude extract with a concentration range of 10 ng/ml significantly inhibits the expression and release of IL-6, IL-1β, and TNF-α in a dose-controlled manner (Table 2) (Ongchai et al., 2021). The crude extract at 1000 μg/ml effectively decreased the expression of these cytokines by 7 %, 39 %, and 57 %, respectively (Horigome et al., 2017). Mitogen-activated protein kinase (MAPK) family comprises proteins ERK, p38, JNK, and protein kinase B (PKB/Akt), which are predominantly associated with the activation of many inflammatory mediators. The crude extract potently suppressed MAPK proteins (Table 2) (Lee et al., 2018), with phosphorylation of ERK, p38, and JNK reduced by 29.8 %, 40.6 %, and 39.0 %, respectively (Ongchai et al., 2021). Furthermore, 15 μg/ml of KPE also downregulated the activity of Nuclear factor-κB (NF-κB) and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) by 3 and 0.97-fold, respectively (Takuathung et al., 2021). Meanwhile, 3 h of treatment significantly suppressed activator protein 1 (AP-1) activity, which is responsible for elevated COX-2 expression (Lee et al., 2018).
As mentioned in Section 2., MMPs are an inflammatory modulator regulating various inflammatory markers. The activity of MMPs is essential in various cell biological and physiological processes, including tissue remodeling, such as wound healing, angiogenesis, and bone development. Nonetheless, it’s associated with multiple inflammatory-mediated diseases such as arthritis, sclerosis, diabetes, and cancer progression (Chen et al., 2017). In a study by Kobayashi et al. (2018), low concentration of KPE (20 µg/ml) potently suppressed expression of IL-1β-induced inflammation enzyme MMP-1 (0.74-fold), MMP-3 (0.82-fold) and MMP-13 (0.95-fold) activity, compared to control (1.00-fold). In another study in vivo, 100 and 200 mg/kg/day of K. parviflora potently suppressed the expression of MMPs (MMP-2, MMP-3, MMP-9 & MMP-13) in a concentration-controlled behaviour, compared to the control (Park et al., 2014).
ROS regulates inflammatory markers in various stages of inflammatory responses (Chelombitko, 2018). Numerous studies discovered flavonoids with potent antioxidants capable of scavenging ROS free radicals from oxidative damage and reducing oxidative stress (Liu et al., 2018). KPEs were reported to reduce the ROS level by 13 % between 1 and 10 μg/ml and 67.6 % at 1000 μg/ml (Park et al., 2017; Horigome et al., 2017). Additionally, KPE at 20 μg/ml (Jin and Lee, 2018) and up to 1000 μg/ml show potent suppression of COX-2 expression. However, at low concentrations (5 & 10 μg/ml), COX-2 suppression was ineffective (Horigome et al., 2014). Astonishingly, 15 μg/ml of the crude extract can reduce COX-2 expression and secretion by 18-fold and 30 %, respectively (Table 2) (Takuathung et al., 2021). In an in vivo study, consuming 50 and 100 mg/kg body weight significantly suppressed COX-2 expression (Lee et al., 2018). Meanwhile, the crude extract inhibited PGE2 released by COX-2 in JB6 P + cells in a concentration-dependent manner (Lee et al., 2018). The extract suppressed the PGE2 level in macrophage cells with an IC50 value of 9.2 µg/ml (Sae-wong et al., 2009). Additionally, treatment with KPE reduced the expression of iNOS and NO in a concentration-controlled manner (Jin and Lee, 2018; Kongdang et al., 2019). The K. parviflora extract at concentrations of 3.75 and 15 μg/mL increased the iNOS expression by 6- and 18-fold, while NO levels decreased to 45 and 30 μM, respectively (Takuathung et al., 2021)., in another study, iNOS expression was unchanged. Nonetheless, NO production was significantly suppressed (IC50: 482.6 μg/ml) (Horigome et al., 2017).
4.2 Isolated compound of KPE inhibits MAPK and AKT proteins
Treatment of 50 & 100 µM 3,5,7-Trimethoxyflavone (3,5,7-TMF) significantly suppressed MAPK proteins with reduction of ERK (1.30 & 0.77-fold), JNK (4.88 & 4.86-fold), p38 (5.58 & 5.20-fold) and AKT protein phosphorylation by 4.88 & 4.86-fold, respectively (Table 2) (Lee et al., 2022). Similarly, 5,7,4′-TMF possesses a comparable effect at lower concentrations (6.25 & 12.5 µM) (Phung et al., 2021). However, the inconsistent result was observed in RAW 264.7 cells with 5,7,4′-TMF, and 5,7-DMF only demonstrated moderate suppression on phosphorylation of JNK and ERK (Sae-Wong et al., 2011); nonetheless, 5,7-DMF was capable of alleviating AKT phosphorylation (Kim & Hwang, 2020).
4.3 Isolated compound of KPE inhibits MMPs activities
At 50 µM, 3,5,7-TMF attenuated MMP-1 mRNA expression and secretion in HDF cells by 2.53- and 1.99-fold, respectively (Lee et al., 2022). Compound 5,7-DMF, 5,7,4′-TMF, and PeMF show potent activity in suppressing the MMP-1 gene, ranging from 34 % to 47 %. Long-term treatment of these PMFs contributes to the reduction of MMP-1 activity by 68.57 %, 71.43 %, and 28.57 %, respectively (Table 2) (Klinngam et al., 2022).
4.4 Isolated compound of KPE as oxidative stress suppressor
Other than that, ROS, COX-2, and HO-1 expression were significantly suppressed by 3,5,7-TMF in a dose-dependent behaviour, as shown in Table 2. At 50 and 100 µM, ROS expression was suppressed by 1.88-fold and 1.33-fold, COX-2 (4.78 and 2.36-fold), and HO-1 (1.68 and 3.03-fold) (Lee et al., 2022). Likewise, 5,7,4′-TMF concomitantly suppressed COX-2 and ROS expression at lower concentrations (Phung et al., 2021), with ROS level reduced by 40 % at the lowest concentration (3 μg/ml) (Klinngam et al., 2022). In addition, a strong inhibitory effect was demonstrated by 5-hydroxy-3,7,3′,4′-Tetramethoxyflavone (5H-3,7,3′,4′-TeMF) with 102 % suppression on COX-2 level (Horigome et al., 2017) and significant inhibition on PGE2 level with IC50 of 16.3 µM (Tewtrakul et al., 2009). Inhibition of protein transcription factors like NF-κB and AP-1 complexes capable of reducing oxidative stress. Isolated K. parviflora, 5,7,4′-TMF significantly reduced the expression of these complexes in a dose-controlled manner (Park et al., 2014). Meanwhile, 5,7-DMF also exerts similar suppression activity towards the phosphorylation of IκB-α and NF-κB (Table 2) (Jin and Lee, 2018).
4.5 Isolated compound of KPE suppressed inflammatory cytokines mediators
Pro-inflammatory cytokines are involved in elevating inflammation processes. Compound 5,7,4′-TMF, 3,5,7-TMF, 5,7-DMF, and PeMF show potent suppression of IL-1β and IL-6 mRNA expression and released in a dose-controlled manner (Phung et al., 2021), with 5,7-DMF, demonstrated the most potent effect (Ongchai et al., 2021). Compound 5,7,4′-TMF alleviate secretion of IL-1β and IL-6 at low concentration (12.5 µM) by 1.03 and 1.12-folds, respectively (Lee et al., 2022). Meanwhile, in another study, 5,7-DMF (50 µM) only reduced it by 2 % and 4 %, respectively (Horigome et al., 2017), while no change was observed in IL-1β when treated with PeMF (Kongdang et al., 2019). In comparison, 50 µM of 5H-TeMF significantly suppressed the expression of IL-6, IL-1β, and TNF-α with reductions of 73 %, 64 %, and 36 %, respectively (Horigome et al., 2017). Compound 5,7-DMF and 5H-3,7,3′,4′-TeMF demonstrate potent suppression of TNF- α expression with 100 % inhibition in antigen-induced RBL-2H3 cells (Horigome et al., 2014). In human THP-1 monocytes, 5,7-DMF reduced TNF-α released by 56.89 % (Klinngam et al., 2022). Meanwhile, in RAW 264.7 cells, 5,7,4′-TMF, 5,7-DMF, and 5,7,3′,4′-TeMF moderately suppressed TNF- α secretion with an IC50 value of 30 to 100 µg/ml (Sae-Wong et al., 2011). In contrast, consumption of 25 and 50 mg/kg/day of 5,7-DMF potently suppressed the release of IL-6 and TNF-α levels and downregulated NF-κB expression (Kim & Hwang, 2020).
4.6 Isolated compound of KPE inhibits iNOS and NO activities
Nitric oxide (NO) involves inflammation's pathogenesis with three major isotypes: iNOS, eNOS, and iNOS (Sierra et al., 2014). iNOS is notably expressed in macrophages and microglia during cell damage induced by inflammatory markers (Brown & Neher, 2010). Meanwhile, overexpression of NO in cytokines-mediated macrophages is a primary factor contributing to inflammatory progression with the presence of NOSs (Sharma et al., 2007). In a study of Propionibacterium acnes-induced iNOs, it was reported that 5,7-DMF and 5H-3,7,3′,4′-TeMF reduced the expression of iNOS level significantly in a concentration-controlled manner (Jin and Lee, 2018; Sae-wong et al., 2009). In contrast, lipopolysaccharides (LPS)-induced iNOS mRNA expression was weakly suppressed by 5,7-DMF and 5H-TeMF in RAW264.7 cells (Horigome et al., 2017). Although weak LPS-induced iNOS expression was reported by 5H-TeMF, the compound shows the highest NO inhibitory effect (IC50 = 16.1 µM) (Tewtrakul and Subhadhirasakul, 2008; Tewtrakul et al., 2009). Besides, 5,7-DMF remarkably suppressed LPS-induced NO level with the lowest IC50 of 9.03 µM (Fuchino et al., 2018), followed with 5,7,4′-TMF and 5,7,3′,4′-TeMF, both recorded IC50 values of 14.2 µM and 16.6 µM, respectively (Sae-Wong et al., 2011). A similar effect was observed in human umbilical vein endothelial cells (HUVEC) (Horigome et al., 2017). Nevertheless, 3,5,7-TMF shows weak NO inhibitory activity with IC50 of 44 to 60 µg/ml (Sae-Wong et al., 2011). Meanwhile, PeMF at 25, 50, and 100 µg/ml concentration inhibit NO activity by 17.57 %, 21.72 %, and 31.67 %, respectively. However, the result is lower than quercetin, which shows 53.2 % inhibition at 100 µg/ml concentration (Jakhar et al., 2014).
5 Anticancer activity
Flavonoid roles in anticancer activity encompass its effects on the early stages of cancer cell proliferation and apoptosis, which include cell invasion, migration, and metastasis progression (Khan et al., 2021). PMFs isolated from various plants have demonstrated potent activity in inhibiting cell growth and induced cell death by apoptosis, such as tangeritin (Surichan et al., 2018), nobiletin (Liu et al., 2018) and quercetin (Mohammed et al., 2021). Thus, the anticancer properties of KPE and its major compound, PMFs, were discussed in this review to understand its efficacy and their mechanism of cell death.
5.1 Anticancer activity of PMFs isolated from KPE
Isolated PMFs from KPE potently induced apoptotic cell death in a dose-dependent behaviour. Kim et al. (2018) reported that compound 5,7,4′-TMF (50 µM) demonstrated the most potent inhibitory effect on gastric cancer cells, SNU-16, with almost 50 % cell growth reduction. In the same study, other PMFs, like 5,7-DMF and PeMF, required at least 100 µM to obtain similar results (Kim et al., 2018). Compound 5,7,4′-TMF induced SNU-16 cell death in the apoptotic pathway, with the percentage of cell accumulation in the sub-G-1 phase increased in a dose-controlled manner, from 3.9 % to 35.1 % at 50 µM. Meanwhile, 5-hydroxy-7-methoxyflavone (5H-7-MF) induced apoptosis in pancreatic cancer cells, PANC-1, through cleavage in caspase-3 (Sun et al., 2021). On another note, 5,7,4′-TMF, 5H-3,7,3′,4′-TeMF, and 3,5,7,4′-TeMF induced cytotoxicity on human colorectal carcinoma cells (HCT15) in the concentration range from 25 to 500 µM. Among these PMFs, 5,7,4′-TMF significantly reduced cell growth by almost 80 % at an optimal concentration of 100 µM. Meanwhile, the HCT15 cell growth was weakly suppressed by 5H-3,7,3′,4′-TeMF and 3,5,7,4′-TeMF with 29.2 % and 16.1 % inhibition, respectively, which may be due to the position of methoxy groups (Hossain et al., 2012). The result suggests that 5,7,4′-TMF shows the most potent anticancer activity by inducing apoptotic cell death. The compound stimulates cell death via four major apoptotic pathways, as confirmed via reverse-transcription polymerase chain reaction, RT-PCR (Kim et al., 2018). Firstly, the apoptotic effect of the PMFs was activated via suppression of the AKT/Mammalian target of rapamycin (mTOR)/Glycogen synthase kinase-3 beta (GSK3β) pathway. Suppressing AKT's phosphorylation reduced cancer cells' survival and mediated apoptotic protein activity. Secondly, a reduction in AKT-Phosphoinositide 3-kinases (PI3K) activity elevates the endoplasmic reticulum (ER) stress. These combinations overstimulate the C/EBP homologous protein (CHOP) gene in the ER-stress-stimulated apoptotic mechanism, resulting in higher activity on caspase-8 and −4, thus inducing apoptotic cell death (Siu et al., 2002). In addition, compound 5,7,4′-TMF was also reported to induce apoptotic cell death via extrinsic and intrinsic pathways. The extrinsic mechanism was stimulated by caspase-8 activation and cleavage on pro-apoptotic BH3 interacting-domain death agonist (Bid), thus activating the intrinsic pathway via oligomerization of Bcl-2-associated X protein (Bax), another pro-apoptotic protein. The PMFs stimulate increased Bax/BCl-2 and Bax/Bcl-xL ratio in a concentration-controlled behaviour, activating caspase-9 and −3 that initiate apoptosis cell death (Kim et al., 2018).
5.2 Anticancer activity of KPE
KPE within 1.00 mg/ml demonstrates potent cytotoxicity against ovarian cancer SKOV3 cells with a reduction of more than 70 % (IC50: 0.53 mg/ml) (Table 3) (Paramee et al., 2018). The cancer cell doubling time improved to 32.6 h compared to untreated cancer cells (24 h) with 0.025 mg/ml extract before decreasing to 31.5 h at a higher concentration. Treatment with 0.05 mg/ml crude extract reduced the cell migration and invasion by almost 50 % and 70 %, respectively. In cancer treatment, MMP-2 and MMP-9 activity inhibition could minimize tumor invasion and metastasis. KPE reduced the activity of the MMPs in a concentration-controlled manner. At 0.01 mg/ml, the activity of MMP-9 and MMP-2 were mildly suppressed by 11.34 % and 7.48 % and improved to 31.17 % and 18.08 %, respectively, at higher concentrations. A similar result was observed in Hela 229 cells with suppression of MMP-2 activity by 30 % (IC50: 0.22 mg/ml) (Potikanond et al., 2017). ERK and AKT activities are significantly involved in stimulating the cell death pathway. KPE at 0.01 mg/ml potently inhibits the phosphorylation of these proteins by 0.85 and 0.87-fold and 0.64 and 0.58-fold at 0.05 mg/ml, respectively. In similar concentrations, KPEs also demonstrated potent suppression of these proteins on Hela 229 cells (Potikanond et al., 2017) and HL-60 cells (Banjerdpongchai et al., 2008). The study shows that KPEs induced an apoptotic effect, with approximately 15.67 % and 26.33 % cell death at concentrations of 0.1 and 0.25 mg/ml, respectively. At higher concentrations (0.30 & 0.50 mg/ml), the percentage of a cell undergoing apoptosis significantly increased to 22.13 % and 41.13 %, respectively.
Study
Cancer cell
IC50
Treatment period (Hour)
Crude extract/ PMF
References
Human ovarian cancer
SKOV3
0.53 mg/ml
24
Crude extract
(Paramee et al., 2018)
Human ovarian cancer
TOV-21G
30.00 µg/ml
48
Crude extract
(Thaklaewphan et al., 2021)
Human cervical cancer
Hela
0.22 mg/ml
24
Crude extract
(Potikanond et al., 2017)
Human bile duct cancer
HuCCA-1
46.13 µg/ml
48
Crude extract
(Leardkamolkarn et al., 2009)
RMCCA-1
61.97 µg/ml
48
Crude extract
HuCCA-1 & RMCCA-1
No data
48
5,7,4′-Trimethoxyflavone
Human myeloid leukemia
U937 cell
70 µg/ml
48
Crude extract
(Banjerdpongchai et al., 2009)
Human myeloid leukemia
HL-60
18.5 mg/ml
48
Crude extract
(Banjerdpongchai et al., 2008)
Human urinary bladder cancer
T24
29.62 µg/ml
24
Crude extract
(Tangjitjaroenkun et al., 2021)
Human Prostate cancer
DU145
∼ 40 µg/ml
24
Crude extract(Tangjitjaroenkun et al., 2021)
PC3
∼ 80 µg/ml
LNCaP
∼ 60 µg/ml
Human gastric cancer
SNU-16
NIL
24
5,7-Dimethoxyflavone
(Kim et al., 2018)
5,7,4′-Trimethoxyflavone
3,5,7,3′,4′-Trimethoxyflavone
Human colorectal cancer
HCT15
48
3,5,7,4′- Tetramethoxyflavone
(Hossain et al., 2012)
5,7,4′-Trimethoxyflavone
5-Hydroxy-3,7,3′,4′-Tetramethoxyflavone
In cervical cancer, the crude extract significantly inhibits the proliferation of Hela 229 cells within the concentration range of 0.01 to 1 mg/ml. Maximum cytotoxicity effect was observed at 0.5 mg/ml with 90 % cell reduction (IC50: 0.22 mg/ml) (Table 3). Cell treated with 0.3 and 0.5 mg/ml crude extract induced a higher percentage of apoptosis cell death with 39.8 % and 69.85 %, respectively, through cleavage on caspase-7 and caspase-9. In addition, 0.01, 0.05, and 0.1 mg/ml of KPE suppressed cell migration by 52.21 %, 63.23 %, and 84.54 %, respectively, and cell invasion (52.21, 42.17 and 78.12 %) (Potikanond et al., 2017). In the human urinary bladder cancer cell line (T24), treatment of ethanolic KPE for 1, 4, and 7 days induced potent cytotoxicity (IC50: 29.62, 16.91, and 7.56 μg/ml) (Table 3). Meanwhile, in human prostate cancer cell lines (DU145, LNCaP & PC3), the crude extract significantly suppressed cell growth in a concentration-dependent manner. The mechanism of apoptotic cell death in prostate cancer was induced through a potent expression of the tumor protein p53 gene up to 4-fold via the intrinsic-mitochondria pathway (Tangjitjaroenkun et al., 2021).
In another study, KPE induced bile duct apoptotic cancer cell death (HuCCA-1 & RMCCA-1) with an IC50 value of 46.13 and 61.97 µg/ml, respectively (Table 3) (Leardkamolkarn et al., 2009). A study by Banjerdpongchai et al. (2009 & 2008) demonstrates the potent anticancer activity of KPE on leukemic and HL-60 cancer cells, respectively. Treatment of KPE (24, 48, & 72 h) stimulates potent cytotoxicity on HL-60 cancer cells at IC50 values of 25.5, 18.5, and 14.5 mg/ml, respectively (Table 3). Meanwhile, significant leukemic cancer cell (U937) reduction was also observed (IC50: 92, 70, and 61 µg/ml). Both HL-60 and U937 cancer cells induced apoptotic cell death through cleavage on caspase-3 in similar crude extract concentration ranges. However, the crude extract triggered HL-60 cell death in the apoptotic pathway up to 80 mg/ml, followed by necrosis when treated at 100 mg/ml. Meanwhile, the crude extract demonstrates antiapoptotic roles on U937 cancer cells at low concentrations (10 and 20 µg/ml) based on the low mitochondrial transmembrane potential (MTP) recorded. Nonetheless, the antiapoptotic role of KPE was not thoroughly investigated as a study by Erster et al. (2004) and Padanilam (2003) demonstrated that the reduction of MTP may be attributed to changes in mitochondria membrane permeability by the release of other pro-apoptotic genes such as cytochrome c during the apoptotic process that modulated by Bcl-2 proteins. Furthermore, drug combinations between the crude extract and commercialized drug, paclitaxel, camptothecin, and doxorubicin significantly ameliorate the cell proliferation and cytotoxicity in a dose-controlled manner through an apoptotic effect on both leukemic and HL-60 cancer cells.
5.3 Anticancer activities of KPE via suppression of inflammatory mediators
Cancer cell proliferation and the progression of tumors occur through complex mechanisms and pathways, which include inflammatory mediators. Interestingly, expression of cytokines TNF-α and IL-6 activate signaling cascades and upregulation of protein kinases (MAPK, Akt, PI3k, ERK) and protein transcription (NF-kB & STAT3) eventually contribute to elevated levels of antiapoptotic protein (MCL-1), cancer cell proliferation, migration, and invasion in various organ system (Wang & Lin, 2008). Therefore, this review highlights the effective inhibition of pro-inflammatory mediators via modulating various signaling by KPE and isolated PMFs compound.
In this review, the anticancer activity of KPE was explored through the suppression of inflammatory mediators in Hela cervical cancer cells (Suradej et al., 2019) and ovarian carcinoma cancer cells (TOV-21G) (Thaklaewphan et al., 2021). In Hela cells, 7.5 and 15 µg/ml of KPE significantly inhibited the expression of IL-6 by 326.8 and 242.6-fold, respectively. A similar result was observed in TOV-21G, with the secretion reduced to 9077.78 and 6151.85 pg/ml when treated with 5 and 10 µg/ml crude extract, respectively. In addition, KPE also suppressed the phosphorylation of the transcription factors (NF-κB & STAT3). Nonetheless, no significant change was recorded on the NF-κB level in Hela cells. Treatment of 7.5 and 10 µg/ml of the crude extract significantly suppressed phosphorylation of protein kinase AKT in both cells, respectively. In TOV-21G cells, 10 µg/ml of KPE significantly suppressed phosphorylation of ERK and inhibited MCP-1 protein to 2.41-fold. In both cancer cells, deactivation of the inflammatory mediators significantly suppressed phosphorylation of antiapoptotic protein MCL-1 in a concentration-controlled manner, which induced apoptotic cell death. As a result, TOV-21G and Hela cell proliferation were potently reduced in a dose-controlled manner, with a 30 µg/ml IC50 value recorded in TOV-21G; meanwhile, a significant reduction in cell growth was observed in Hela cells.
Previous studies reported that KPEs and PMFs potently reduced cancer cell proliferation and induced apoptosis cancer cell death through various pathways, including extrinsic pathway via caspase-8 and intrinsic pathway with activation on caspase-9. Both pathways resulted in cleavage on caspase-3 and 7. Among the PMFs studied, 5,7,4′-TMF was the most potent anticancer compound. In addition, crude extract and PMFs also strongly inhibit cell migration and invasion in the 50 % to 80 % range. Meanwhile, the inhibition of MMP-2 and MMP-9 was also significant, with a suppression range of 10 to 30 %. However, most current studies focus on crude extract only, and the potential use of PMFs as a chemopreventive agent was not thoroughly studied. Nonetheless, the review suggests that both crude extract and PMFs metabolites demonstrate anticancer properties and may reduce cell progression and metastasis.
6 Antidiabetes activity
Obesity is associated with an excessive intake of calories that leads to an elevated risk of metabolic diseases, which include insulin resistance, hypertension, and diabetes (Ye & Gimble, 2011). The elevation of metabolic disorders can be lessened through various pathways that suppress body weight gain, abdominal fats, and postprandial blood glucose levels. Several anti-obesity and antidiabetic pathways have been discovered through treatment with KPE and its PMFs to alleviate the effect, which includes inhibition of α-glucosidase and α -amylase (Azuma et al., 2011), improve adipokines secretion (Okabe et al., 2014), induction of adipogenesis and thermogenesis on adipocytes ((Horikawa et al., 2012).
6.1 In vitro anti-obesity activity of K. parviflora and its PMFs
Inhibition of α-amylase and α-glucosidase enzymes delays the breakdown of polysaccharides through competitive binding at the enzymatic site, which helps to alleviate postprandial blood glucose levels (Lebovitz, 1997). Treatment of PMFs isolated from ethanolic KPE demonstrates potent inhibitions on the α-glucosidase enzyme (Azuma et al., 2011). Compound 3,7,3′4′-TeMF and 5,7,4′-TMF significantly inhibit the enzyme (IC50 = 20.4 and 54.3 µM), higher than PMF's quercetin (59.5 µM). The position of the methoxy group greatly influenced the binding affinity of the PMFs, as discussed in the previous sections (2.). In another study, the crude extract significantly inhibits α-amylase and α-glucosidase enzymes with IC50 values of 433.3 μg/ml and 3.722 μg/ml. However, the inhibitory effect was weak compared to the well-known α-glucosidase inhibitors, acarbose, with IC50 values of 5.10 μg/ml and 0.06 μg/ml, respectively (Yagi et al., 2019).
6.1.1 Reduction of triglycerides (TG) by lipolysis
Obesity is linked to increased hypertrophy of adipocytes due to the stimulation of inflammatory mediators by macrophage infiltration that mediates adipokines secretion and insulin-sensitizing signaling in adipocytes, elevating insulin resistance (Okabe et al., 2014); meanwhile, high-calorie intake suppressed lipolysis, which reduced the hydrolysis of triglycerides. The combined effect of these factors results in lower adipocyte differentiation and reduced storage of synthesized triglycerides. Excessive triglycerides accumulated in adipocyte tissue cause adipocyte hypertrophy, which leads to obesity (Schweiger et al., 2006; Ye and Gimble, 2011). KPE and its PMFs significantly upregulate genes and protein expression of mature adipocytes and reduce TG accumulation via lipolysis (Fig. 2) in 3 T3-L1 cells (Okabe et al., 2014). Treatment of 3 and 10 µg/ml crude extract significantly stimulates adiponectin mRNA expression and enzyme lipases (ATGL & HSL) in a concentration-dependent manner. At higher concentrations (10 to 30 µg/ml), isolated PMFs significantly reduced the accumulation of TG with 5,7,4′-TMF, demonstrating the most potent effect (0.3 mg/mg) compared to the vehicle control (0.7 mg/mg). High glycerol levels proved that hydrolysis of TG takes place.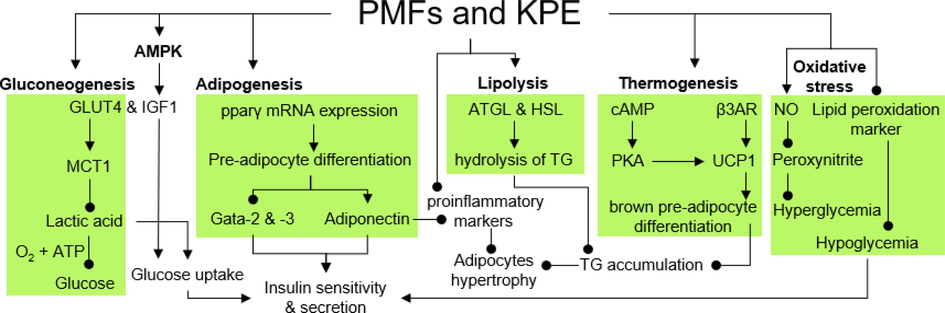
The mechanism of anti-obesity and antidiabetes effect of reported PMFs and KPE.
6.1.2 Adiponectin improves insulin resistance
Meanwhile, another study focused on releasing adiponectin through adipogenesis by stimulating pre-adipocyte differentiation. Adipogenesis is a complex process controlled by various adipogenesis markers through cell differentiation to mature adipocytes, forming adipose tissue that is stored in the form of subcutaneous fat (Zhao et al., 2022). Nonetheless, regulating the PPARγ, an adipogenesis marker, is one of the unique pathways to regulate insulin resistance through the induction of adiponectin expression (Moseti et al., 2016). In a study by Horikawa et al. (2012), KPE, 3,5,7,4′-TeMF, and PeMF potently induced differentiation of the pre-adipocytes, confirmed by increased accumulation of TG and lipids droplets. The induction of adipogenesis was contributed by the upregulation of PPARγ and adiponectin by the crude extract and PMFs, subsequently deactivation of adipogenesis inhibitor, GATA-Binding Protein (Gata-2 & −3) mRNA expression in a dose-controlled manner (Fig. 2). A high differentiation rate enhanced the adiponectin secretion by mature adipocytes, contributing to lower cytokines-induced insulin resistance (Horikawa et al., 2012).
6.1.3 GLUT4 induces glucose uptake and improves energy metabolism
It has been widely accepted that insulin accelerates the movement rates of GLUT4 and stimulates glucose uptake into adipocytes and skeletal muscle (Blot & McGraw, 2006). A similar mechanism was studied on muscular tissue by investigating the energy metabolism of pre-differentiate (pC2C12) and differentiated (dC2C12) myocytes to improve glucose uptake and reduced insulin secretion. The crude extract at the concentration of 0.1 to 1.0 µg/ml potently induced the uptake of glucose molecules into both myocytes’ cells (Toda et al., 2016b). Interestingly, the mRNA expression of GLUT4 and IGF1 was significantly elevated by 5H-7-MF and 5,7-DMF, while higher expression was observed on 5-Hydroxy-3,7,4′-Trimethoxyflavone (5H-3,7,4′-TMF), and 3,5,7,4′-TeMF, compared to crude extract. Moreover, KPE (10 µg/ml) significantly suppressed lactic acid secretion in both cells with the enhancement of its transporter, monocarboxylate transporter 1 (MCT1) expression up to 2-fold (Fig. 2), leading to higher lactic acid utilization and enhancement of glucose metabolism (Juel & Halestrap, 1999; Halestrap & Prince, 1999). On another note, upregulation of AMPK phosphorylation was recorded in both cells by isolated PMFs and the crude extract in a dose-controlled behavior. Phosphorylation of AMPK alleviates diabetes complications through a reduction in insulin resistance, thus improving glucose uptake and energy metabolism (Entezari et al., 2022).
6.1.4 Elevation of brown adipocytes delays metabolic syndrome
Brown adipose tissue is involved in energy uptake that elevates insulin sensitivity, loss of weight, and lower risk of atherosclerotic disease. An in-vitro study by Kobayashi et al. (2016) demonstrated KPE mechanisms of action in improving the thermogenesis process of brown adipocytes and enhancing the cell differentiation of brown adipocytes. As a result, the brown pre-adipocyte differentiation to mature adipocytes was increased in a concentration-controlled manner. The result was confirmed with a high accumulation of TG in the brown adipocytes treated with 10 μg/ml crude extract. Nevertheless, the TG growth was low compared with positive control Troglitazone, a well-known antidiabetic drug. Interestingly, the TG accumulation was also investigated among 12 isolated PMFs from KPE, with 5,7-DMF, 5-Hydroxy-7,4′-Dimethoxyflavone (5H-7,4′-DMF), 5H-7-MF, and 5,7,4′-TMF demonstrating potent increment in TG accumulation from adipocyte differentiation process. In addition, the mRNA expression of pparγ was the highest when the brown adipocytes were treated with 30 μg/ml, further elevating the cell differentiation. Meanwhile, the thermogenesis of brown adipocytes was also upregulated due to enhancement in mRNA expression of UCP-1 and β3AR gene. Thermogenesis occurred through activating UCP-1 and its marker, the β3AR gene (Fig. 2). Expression of these genes could induce thermogenesis, thus delaying metabolic syndrome (Zhang et al., 2016).
6.2 In-vivo anti-obesity activity of KPE and its PMFs
Antidiabetic studies on TSOD mice were conducted by Kobayashi et al. (2016) on brown adipose tissues. At both concentrations (0.3 % & 1 %), KPE significantly reduced the brown adipose tissue weight dose-dependently. The mRNA expression of UCP-1 and its marker, β3AR, was upregulated at a higher concentration than the control. The result was congruent with the previous study, with the treatment of 1 % crude extract significantly elevated UCP-1 expression to more than 200 % compared to the control (Yoshino et al., 2014). Both concentrations of KPE (0.5 & 1.0 %) potently reduced abdominal fat weight and TG buildup. At 0.5 % crude extract, the neurotransmitters (noradrenaline and adrenaline) concentration was significantly elevated compared to the control. Neurotransmitters are responsible for increased cyclic adenosine monophosphate (cAMP) levels that regulate UCP-1 mRNA expression in brown adipocytes through the cAMP/PKA pathway (Chen et al., 2013; Koh et al., 2007). In another study, KPE reduced the adipose tissue weight and plasma glucose level and elevated the release of adiponectin and stronger pparγ activity, contributing to lower insulin resistance, adipocyte differentiation, and fat accumulation (Ochiai et al., 2019). In an 8-week study on similar mice models, the growth of visceral and subcutaneous fats was suppressed moderately (Akase et al., 2011). Crude extract at both concentrations (1 % & 3 % KPE) significantly reduced the risk of dyslipidemia in TSOD mice, with potent inhibition was observed when treated with 3 % crude extract. Among the parameters, insulin and TG levels were the most suppressed by crude extract to 2.9 and 166.0 mg/dL, compared to the vehicle control 10.3 and 234 mg/dL, respectively. On another note, the crude extract also reduced the blood pressure and glucose levels of TSOD mice. At the same time, no significant change was recorded in Tsumura Suzuki Non-Obesity (TSNO) mice, proving that the crude extract did not affect the healthy or non-diseased group.
In another study, a low dosage of KPEs reduced the metabolic risk in TSOD and TSNO mice (Shimada et al., 2011). On week 8, 1 % crude extract potently reduced abdominal fat in the TSOD group. In addition, 1 % crude extract concentration decreased the blood glucose level to less than 400 mg/dL compared to the control and 0.3 % crude extract in the TSOD group. At low concentrations (0.3 %), the blood glucose level decreased after 1-hour treatment. One of the possible pathways to reduce the risk of obesity is inhibiting the lipase enzyme's activity (Heck et al., 2000). The crude extract, 5-Hydroxy-3,7-Dimethoxyflavone (5H-3,7-DMF), 5H-7,4′-DMF, and 5H-7-MF potently inhibit lipase enzyme activity compared to other PMFs with IC50 values of 487, 291, 220, and 291 µg/ml, respectively. In a study by Hidaka et al. (2017), three different extracts of K. parviflora; crude KPE (KPE), PMFs-rich extract (PMF), and PMFs-poor extract (KPX), were investigated for anti-obesity effect. The result shows that the PMFs group improved the expression of the pparγ gene, which is involved in adipocyte differentiation. Meanwhile, a similar result was obtained from the previous study, with a reduction in visceral and subcutaneous fat for all three KPEs. The result was contributed by the downregulation of subcutaneous fat thickness, with the size of adipocytes decreasing to smaller cells.
6.2.1 Reduction of oxidative stress
Sripanidkulchai et al. (2020) conducted an anti-obesity study on the streptozotocin-stimulated diabetic rats’ model. Crude extract at 300 mg/kg dose significantly lowered blood glucose level on week 8 (234 mg%) compared to the control and vehicle group (394 & 327 mg%); however, a lower dose of crude extract reversed the effect. Inhibition of lipid peroxidation markers ameliorated the hypoglycemic effect through reduced oxidative stress in chronic diabetes (Hassan et al., 2015). Treatment of 1 % and 3 % KPE reduced the peroxidation marker level in a concentration-dependent manner (16.2 & 11.6 nmol/mg protein) compared to control (18.6 nmol/mg protein), as summarized in Fig. 2. In addition, histological analysis demonstrates potent cell recovery and improvement in islet count, contributing to the upregulation of insulin secretion similar to the commercial drug, glibenclamide. In another study, peroxynitrite is formed due to the upregulation of superoxide in neutralizing nitric oxide levels, escalating free radicals and leading to endothelial damage (Malakul et al., 2011). Treatment of 0.1 to 100 µg/ml crude extract in the aorta of the rats decreased the superoxide’s expression and reversed the effect. The upregulation of nitric oxide levels and reduction of superoxide production contribute to the protective effect of K. parviflora from metabolic disease (Sokolovska et al., 2020).
6.2.2 Human in vivo anti-obesity activity of KPE and its PMFs
An in vivo antidiabetic study by Yoshino et al. (2014) demonstrated a potent increase in energy expenditure through the upregulation of UCP-1 and thermogenesis in brown adipose tissue. The energy expenditure of 20 healthy male subjects was the highest after 60 min (2969 kJ/d) when administered 100 mg KPE (Matsushita et al., 2015). In another study by Yoshino et al. (2018), 74 subjects were chosen with the consumption of 150 mg KPE for 12 weeks. The crude extract reduced the visceral and subcutaneous fat area to 90.23 and 257.3 cm2 compared to the placebo subjects, 90.30 and 258.13 cm2, respectively. High TG level correlates with the inability of lipase to break down triglycerides, resulting in the accumulation of TG in adipose tissue (Daud et al., 2018). At week 12, TG growth was significantly reduced in the active subjects (-10.3 mg/dL) compared to the control (+5.2 mg/dL). Another anti-obesity study was conducted on 77 overweight Japanese adults for 12 weeks with 6 mg KPE (Yoshino et al., 2021). Among the female subjects, the significant difference in calorie reduction was only observed on week 4 (1693.5 kcal) compared to the control (1709.0 kcal); however, it elevated on week 12 compared to the placebo female subjects. For male subjects, calorie reduction was observed on the final week (1816.5 kcal), compared to the control, 1880.9 kcal. Meanwhile, the abdominal fat growth area decreased time-dependent on 12-week treatment with the crude extract. On week 12, visceral and subcutaneous fat areas decreased to 81.62 and 227.55 cm2, respectively, compared to placebo subjects. Low dose consumption may be attributed to the insignificant effect of the crude extract. Thus, previous studies suggest that optimum doses of KPE (100–150 mg) may reduce accumulated human abdominal fat area.
This study shows that KPE and its PMFs can induce potent antidiabetic effects by targeting adipocyte-mediated pathways. Inhibition of enzyme lipase by crude extract reduces the absorption of fats in the gastrointestinal consequently reducing the abdominal fats; however, it may elevate TG accumulation and increase the size of adipocytes. Therefore, optimization on the crude extract concentration may provide a synergistic effect between adipocyte differentiation and lipolysis by lipases to regulate TG levels. Nevertheless, the crude extract can induce adipokines in adipocytes, reducing insulin resistance and thus reducing blood glucose levels. Adipocytes are known to exist in 2 types, white and brown adipocytes, with both cells significantly involved in reduced metabolic disorder through adipogenesis and thermogenesis via their respective markers. On another note, blood glucose levels could be reduced by inhibiting α-glucosidase and α-amylase. However, the results were weak and insignificant compared to the common antidiabetic drugs.
7 Antiglycation activity
The AGEs of fluorescent, pentosidine, CML, and intermediates glycation product (3DG, GO & MGO) were elevated due to non-enzymatic activity of protein and lipid with sugars. Excessive AGEs levels are associated with chronic metabolic and vascular diseases (Goldin et al., 2006). In the antiglycation study by Yagi et al. (2021), the KPE demonstrated potent inhibition of AGEs and their intermediates in a dose-dependent manner. The crude extract significantly suppressed the formation of fluorescent AGEs, pentosidine, and CML with IC50 values of 0.078 mg/ml, 0.292 mg/ml, and 0.031 mg/ml, respectively. In addition, a potent effect was also observed on intermediates 3DG, GO, and MGO with IC50 values of 0.028, 0.038, and less than 0.010 mg/ml, respectively, higher than positive control aminoguanidine and epigallocatechin gallate. The AGEs' potent degradation by the crude extract was confirmed with the activation of AGEs crosslink cleavage action and suppression of oxidized protein hydrolase enzyme in a dose-controlled manner, as demonstrated by another antiglycation study by Perera and Handuwalage (2014).
In another study, the antiglycation effect of KPE was significantly strong, with an IC50 value of 25.1 μg/ml (Nakata et al., 2014). Interestingly, the isolated PMFs, PeMF exhibit the highest activity (IC50: 5.87 μg/ml), followed by 5,7,4′-TMF (8.69 μg/ml) and 3,5,7,4′-TeMF (9.57 μg/ml). The result suggests that another active compound may disrupt the unexpected moderate activity of the crude extract in the rhizome. However, the antiglycation activity of KPE was contradicted by the previous study that demonstrated potent inhibition with a low IC50 value of 2.8 μg/ml (Kusirisin et al., 2009). The surprising result may be attributed to the ethanolic solvent used to extract the rhizome, contrary to the hot aqueous extract used in the previous study. Antiglycation activity was significantly dependent and strongly correlated with the antioxidant activity of the extracted compounds (Safari et al., 2018). The ethanolic extract was known to mediate and improve the antioxidant activity of the extracted compound compared to hot and cold aqueous extract (Ramdan et al., 2017). Thus, we suggest that KPE demonstrates potent antiglycation activity, nonetheless dependending on the extraction technique and solvent used.
8 Neuroprotection and anti-neurodegenerative effect
8.1 Inhibition on Ache
Inhibition of acetylcholinesterase (AChe) and butyrylcholinesterase (BChe) enzymes has been a therapeutic target to alleviate Alzheimer’s disease through inhibition of hydrolysis of AChe (Nordberg et al., 2013; Rees & Brimijoin, 2003). Among the isolated PMFs, treatment of 0.1 mg/ml of compound 5,7,4′-TMF and 5,7-DMF was the most potent in suppressing both AChe and BChe enzymes, respectively (Sawasdee et al., 2009). The percentages of inhibition by 5,7,4′-TMF on each respective enzyme were 47.1 % and 46.2 %; meanwhile, 5,7-DMF were 42.6 & 84.6 %, respectively. Significant inhibition of both enzymes by PMFs contributes to another in-depth study by Seo et al. (2017) to investigate the anti-acetylcholinesterase activity of 5,7-DMF, 5,7,4′-TMF, and PeMF. Interestingly, PeMF demonstrates the most potent inhibitory effect at a concentration of 40 µM with a reduction of up to 80 % activity in contrast to 5,7,4′-TMF and 5,7-DMF (20 %) when treated at high concentration (100 µM). However, the potent activity of PMFs was insignificant due to the high neurotoxicity recorded against PC12 cells.
Therefore, PC12 cell lines were extensively used in an in vitro Alzheimer’s disease study to investigate the target compounds' neurotoxicity and neuron damage effect (Tong et al., 2018). Meanwhile, no significant cell damage and neurite outgrowth were observed for 5,7,4′-TMF and 5,7-DMF, which makes it the potent Ache inhibitor.
8.2 Increase neurite outgrowth
Interference of CRE-dependent transcription has been conjectured to promote neuronal dysfunction and induced death. Furthermore, the cAMP-stimulated signaling pathway inactivation mediates the neurite outgrowth through protein kinases (ERK/MAP) associated with cognitive impairment (Natsume et al., 2020). Isolated PMFs from KPE show intense activity to facilitate activation of the CRE-mediated transcription to ameliorate memory loss (Natsume et al., 2020). KPE and 4 PMFs compounds (30 μg/ml) demonstrated upregulation of CRE-mediated transcription by 3 to 9-fold through firefly luciferase assay. On another note, all PMFs and the crude extract do not exhibit significant toxicity against PC12D cells, a new subline from normal cells PC12D, including neurite outgrowth response from cAMP (Katoh-Semba et al., 1989), compared to the previous study.
8.3 Inhibition of beta-secretase 1 (BACE1) activity
The progression of the neurodegenerative disorder is accelerated by the growth of amyloid plaque, neurotoxic β-amyloid (Aβ) (Hardy & Allsop, 1991). Formation of Aβ peptides as a result of enzymatic cleavage of amino acid position 1 on the Aβ precursor protein by BACE1 (Vassar & Kandalepas, 2011). Inhibition of BACE1 reduced the generation of Aβ peptides responsible for elevated levels of cognitive impairment, neuronal damage, oxidative stress, and inflammatory activity (Fukumoto et al., 2010). Aβ peptides, together with the formation of neurofibrillary thread with neurons by Tau proteins, are responsible for alleviating cognitive dysfunction (Tong et al., 2005). PMFs isolated from KPE significantly inhibit the activity of the BACE1 enzyme in a dose-controlled manner. Compounds 5,7,4′-TMF demonstrate the most potent inhibitory activity with 80 % secretion reduction of BACE1 enzyme and IC50 value of 36.9 µM. Meanwhile, 5,7-DMF and PeMF display significant suppression on BACE1 (49.5 µM & 59.8 µM), respectively (Youn et al., 2016). Nevertheless, all compounds exhibit higher activities than polyphenol resveratrol, a notable natural compound with therapeutic potential in Alzheimer's disease (Jabir et al., 2018). Furthermore, the molecular docking simulation portrayed the binding orientation of the PMFs on the allosteric site of the BACE1 enzyme, demonstrating the non-competitive binding inhibition by the PMFs.
8.4 Inhibition of neurotransmitter glutamate toxicity
Oxidative stress injury is a leading pathway of glutamate neurotoxicity (Lau & Tymianski, 2010). Excessive levels of glutamate neurotransmitters are correlated with overexpression of oxidative stress and damage by ROS and RNS that upregulate protein kinases and transcription factors, thus inducing apoptotic HT-22 cell death (Xin et al., 2019). The antioxidant properties of K. parviflora rhizome had a therapeutic potential to reduce toxicity induced by neurotransmitter glutamate in neuronal HT-22 cells. KPE significantly reduced glutamate-induced toxicity in HT-22 cells in a dose-controlled manner (Tonsomboon et al., 2021). Although 5 μg/ml of the crude extract could not reverse the toxicity effect, higher concentrations of crude extract (50 & 75 μg/ml) significantly ameliorated the glutamate-induced toxicity, with cell viability increasing to 115.97 % and 102.11 %, respectively. The elevated level of glutamate-induced toxicity of more than 100 % due to the upregulation of ROS expression was significantly inhibited by KPE at 50 and 75 μg/ml concentrations. Both concentrations of crude extract reduced the ROS level by 26.44 % and 46.37 %, respectively. The crude extract demonstrates antiapoptotic roles on HT22 cells by reducing cell death to 22.49 % and 28.00 %, respectively. The antiapoptotic pathway shown by KPE was revealed with suppression of MAPK pathway and ERK phosphorylation, along with upregulation of brain-derived neurotrophic factor, BNDF expression, indicating the KPE displays neuroprotective effect against glutamate-induced toxicity in neuronal HT022 cells.
8.5 Ameliorates VPA-induced cognitive impairments
Meanwhile, an in vivo study was conducted on Male Sprague Dawley rats by Welbat et al. (2016) to investigate the protective effect of KPE against drug-stimulated spatial memory impairment, valproic acid (VPA). VPA is an anti-epileptic drug for the treatment of bipolar and schizophrenia disorder, which is categorized with a high safety profile. However, several studies have reported its side effect, which includes mild memory impairment (Hommet et al., 2007) and lower hippocampal neurogenesis (Umka et al., 2010). Synergistically, combine treatment of KPEs and VPA significantly reversed the spatial cognitive impairment with higher mean preferential index (PI) in object exploratory tests on the rats. In addition, the crude extract did not show any toxicity indicator against Ki-67 cells; a higher cell number was recorded when treated with KPE compared to VPA treatment alone. Furthermore, administering VPA significantly suppressed the expression of BNDF and doublecortin proteins, a neurogenesis marker. Co-treatments of the crude extract with VPA potently reversed the effect and upregulated the expression of BNDF and doublecortin proteins up to 100 % activity.
As discussed in the previous section (8.4.), elevated free radicals are associated with oxidative damage to the cells, affecting cognitive and psychological function. Therefore, inhibiting oxidative stress by introducing antioxidant agents could provide an alternative mechanism to prevent neurodegenerative effects (Gilgun-Sherki et al., 2001). Consumption of 150 to 250 mg/kg of KPE shows neuropharmacological activities in rats’ model in 2 2-week study with antidepressant behavior and moderate enhancement of cognitive function (Hawiset et al., 2011). Meanwhile, introducing 200 mg/kg KPE significantly ameliorated the duration of escape latency of rats’ models in 7,14, and 21 days studied, compared to the vehicle-stress models (Wattanathorn et al., 2013). Nonetheless, 100 mg/kg and 300 mg/kg crude extract did not influence changes compared to the vehicle-stress models. On another note, in similar doses, the treated rats demonstrated the development of cholinergic neurons excitatory; however, the effect was only observed on neurons C3. Nonetheless, the results have shown that the properties of K. parviflora improved cognitive deterioration, as supported by the previous study at 200 mg/kg crude extract doses (Phachonpai et al., 2012).
9 Antioxidant activity
High levels of ROS free radicals promote the development of chronic diseases, including diabetes, cancer, arthritis, and neurodegenerative disorders due to DNA, lipid, and protein damage (Sharifi-Rad et al., 2020); (Collin, 2019). The antioxidant activity of KPE is crucial to suppress free radicals’ levels through several chemical mechanisms: hydrogen atom transfer, single electron transfer, and transition metals chelating effect (Kumar et al., 2016). Several studies have highlighted the potent antioxidant properties of the crude extract and its isolated PMFs through DPPH and ABTS radical scavenging assay. DPPH assay of KPE shows moderate inhibition of free radical activity with an IC50 value of 438.3 µg/ml (Table 4). Nonetheless, the incorporation of gold nanoparticles with KPE (KP-AuNPs) significantly suppressed the free radical activity with a lower IC50 value (94.5 µg/ml) (Table 4). The enhanced antioxidant effect may be due to the synergistic effect between the crude extract and gold particles, as previous studies had demonstrated the ability of metal nanoparticles to mediate anti-inflammatory, anticancer, antimicrobial, and antioxidant effects of the plant crude extract (Varghese et al., 2021). In another study by Malakul et al. (2011), KPE macerated with 95 % ethanol showed significant improvement in antioxidant activity with an IC50 value of 161.9 µg/ml (Table 4), suggesting the improvement in antioxidant activity was contributed by the solvent used (Do et al., 2014). Nevertheless, the antioxidant activity was lower than ascorbic acid (IC50: 50 μg/ml). Nonetheless, treatment of 200 μg/ml crude extract significantly inhibits free radicals by approximately 90 % and 30 % in ABTS and DPPH assay, respectively (Table 4) (Lee et al., 2018). The difference in antioxidant activity displayed was confirmed with ORAC activity determination. At similar concentrations, the crude extract depicts ORAC activity in the range of 50 to 60 µM in TROLOX equivalence and improved to 100 µM when treated at a higher concentration (400 μg/ml). Meanwhile, a low concentration of KPE dichloromethane extract (10 µg/ml) demonstrates poor antioxidant activity, with ORAC and Cupric Reducing Antioxidant Capacity (CUPRAC) values recorded were 7.85 µM and 3.35 µM, respectively, lower than the methanol extract (Thao et al., 2016).
Solvent
Sample
% Inhibition
IC50 (µg/ml)
Trolox Equivalent, µM
Reference
DPPH
ABTS
ORAC
CUPRAC
Ethanol (95 %)
Crude
35.34
54.89
–
–
–
(Tonsomboon et al., 2021)
Hot water
Crude
36.00
–
438.3
–
–
(Varghese et al., 2021)
CO2
3,5,7,3′,4′-Pentamethoxyflavone
17.31
2.07
234.74
–
–
(Jakhar et al., 2014)
Ethanol (95 %)
Crude
–
–
161.9
–
–
(Malakul et al., 2011)
Ethanol (60 %)
Crude
30.00
90.00
–
60.00
–
(Lee et al., 2018)
CH2Cl2
Crude
–
–
–
7.85
3.35
(Thao et al., 2016)
Methanol
Crude
–
–
–
9.11
4.53
5-Hydroxy-7-Methoxyflavone
–
–
–
0.94
0.84
5,7-Dimethoxyflavone
–
–
–
1.26
0.08
5-Hydroxy-3,7-Dimethoxyflavone
–
–
–
3.59
0.80
3,5,7-Trimethoxyflavone
–
–
–
1.11
0.00
4′-hydroxy-5,7-Dimethoxyflavone
–
–
–
8.47
0.17
5,7,4′-Trimethoxyflavone
–
–
–
1.51
0.17
5-Hydroxy-7,4′-Dimethoxyflavone
–
–
–
1.01
0.17
3,5,7,4′-Tetramethoxyflavone
–
–
–
1.01
0.34
5-Hydroxy-3,7,4′-Trimethoxyflavone
–
–
–
0.95
0.04
3,5,7,3′,4′-Pentamethoxyflavone
–
–
–
0.79
0.00
5-Hydroxy-3,7,3′,4′-Tetramethoxyflavone
–
–
–
2.66
1.05
4′,5-dihydroxy-3,7,4′-Trimethoxyflavone
–
–
–
1.70
0.00
In another antioxidant study, the crude extract shows the potent activity of 21.21 mg Trolox/g crude extract (Kusirisin et al., 2009). The inhibition activity was average compared to other medicinal plants. The highest and lowest antioxidant activity was recorded by Phyllanthus emblica (226 mg Trolox/g crude extract) and Lycopersicon esculentum (0.43 mg Trolox/g crude extract), respectively. As the previous study depicts the low antioxidant activity of crude KPE, PMFs were isolated to investigate its antioxidant effect. Compound 4′-Hydroxy-DMF from the crude extract exhibited the highest ORAC activity at 1 µM and 10 µM concentration with the radical-scavenging activity of 2.23 µM and 8.47 µM, respectively. Meanwhile, other PMFs demonstrated moderate activity with a range of 0.01 µM and 7.04 µM (Table 4). The finding concurrent with a study by Jakhar et al. (2014), which compound PeMF isolated from KPE shows insignificant antioxidant activity in DPPH assay with only 17.31 % inhibition when treated at the highest concentration (100 µg/ml). Meanwhile, in the ABTS assay, PeMF only exhibits 2.07 % inhibition of free radicals. The results suggest that KPE and its isolated PMFs demonstrate moderate antioxidant activity.
10 Anti-gastric activity
The K. parviflora crude extract demonstrated anti-gastric activity in the in vivo study of Male Sprague–Dawley rats through inhibition of gastric ulcer formation and elevated gastric mucus secretion in a dose-controlled manner (Rujjanawate et al., 2005). Consumption of 30, 60, and 120 mg/kg crude extract significantly inhibits indomethacin-stimulated gastric ulcer formation with an ulcer index value of 1.1, 0.3, and 0.5 mm, respectively. A similar effect was observed on HCl/EtOH, and the water mixture restrained stress-induced gastric ulcers when treated with the crude extract. In addition, the gastric mucus content induced by HCl/EtOH in the male rats was significantly upregulated by a similar concentration of crude extract (438, 588, and 663 µg Alcian blue/g wet stomach) compared to the control (331 µg Alcian blue/g wet stomach). Increased gastric wall mucus content protects against gastrointestinal impairment in rats model study (Davenport, 1968). A similar outcome was also observed on other plant extracts, with A. marmelos extract from fruits and roots demonstrating gastroprotective properties through its related antioxidant mechanism. The crude extract shows potent ulcer-protecting activity in a dose-controlled manner in both acute and chronic gastric ulcer rat models (Das & Roy, 2012; Verma et al. 2010).
11 Antimicrobial activity
11.1 Antifungal
The crude KPE demonstrates antifungal activity by inhibiting dermatophyte fungi activity. Moderate antifungal effect of crude extract (200 µg/ml) was recorded on T. rubrum, T. mentagrophytes, and M. gypseum with minimum inhibitory concentration (MIC) values of 62.5, 125, and 250 µg/ml, respectively. The result demonstrates that all PMFs had a weaker antifungal activity, with the highest recorded by 3,5,7-TMF (MIC value: 250 μg/ml) (Kummee et al., 2008). The low antifungal activity of PMFs contradicted the previous study by Yenjai et al. (2004), which reported PMFs 3,5,7,4′-TeMF and 5,7,4′-TMF exhibit potent antifungal activity on Candida albicans with IC50 value of 39.71 and 17.63 μg/ml. In addition, both compounds possessed moderate antimycobacterial activity (MIC: 200 & 50 μg/ml) compared to the antifungal drug. The moderate antifungal activity of PMFs was further supported by the recent antifungal activity of methoxyflavones derivatives isolated from the roots of Deguelia duckeana. The isolated 7-Methoxyflavones derivatives demonstrate weak antifungal activity on C. albicans, C. gattii, and C. neoformans, with all MIC50 recorded exceeding 320 µg/ml. However, isolated chalcone derivatives, 4-Hydroxylonchocarpine, from the same crude extract demonstrated potent MIC50 with the lowest value recorded on C. neoformans (20 µg/ml). The result suggests that different bioactive PMFs compounds may result in distinct antifungal activities that require further studies.
11.2 Antibacterial activity
One of the earliest antibacterial studies of K. parviflora extract by Kummee et al. (2008) demonstrated negligible activity against gram-positive bacteria (S. aureus, S. epidermidis & E. faecalis) and gram-negative bacteria (E. coli). Among the genus Kaempferia studied, K. parviflora shows the most promising antibacterial activity against bacteria K. pneumoniae, P. aeruginosa, and A. baumannii, with MIC values of 64, 64, and 32 μg/ml, respectively (Sookkhee et al., 2022). Meanwhile, the crude extract also inhibits bacterial growth of Cronobacter spp and Enterohemorrhagic E. coli in a concentration-controlled manner (Jeong et al., 2016). In another study, the crude extract demonstrated potent antibacterial on P. acnes and S. Aureus with 100 % suppression on both bacteria growth when treated at a concentration of 250 and 500 μg/ml, respectively (Jin and Lee, 2018).
The influence of solvent extraction on the antibacterial activity of K. parviflora was elucidated by Sitthichai et al. (2022). Ethyl acetate extract demonstrates the most potent activity on bacteria S. epidermis with an MIC value of 3.84 mg/ml. Meanwhile, acetone, ethyl acetate, and dichloromethane-crude extract show potent inhibition on bacteria growth of C. acnes with an MIC value of 0.03 mg/ml. However, no significant difference in antibacterial activity was reported for S. aureus when treated with all crude extract. In another study, a similar result was obtained with ethyl acetate crude extract exhibiting the highest inhibition growth against H. pylori (MIC = 32 μg/ml), followed by hexane (64 μg/ml), methanol (64 μg/ml) and volatile oil (1024 μg/ml), respectively (Chaichanawongsaroj et al., 2010). Extraction of plant extract with ethyl acetate solvent to achieve maximum antimicrobial activity was also supported by another study on seeds of Peganum harmala L by (Arif et al., 2022). The ethyl acetate extract demonstrates the highest activity against S. aureus (81.10 %) and E. coli (61.29 %). Nevertheless, in a similar study on stem extract, n-butanol is the best solvent against K. pneumonia (32.44 %) and E. coli (37.61 %).
The crude extract was purified and isolated to obtain PMFs. Compounds 3,5,7-TMF, 5,7,4′-TMF, and PeMF demonstrate the most potent antibacterial activity with MIC value range between 50 and 100 μg/ml. However, the activity of the PMFs was unsatisfactory compared to the positive control, Gentamicin. Nevertheless, the combination of antibiotic Gentamicin and the PMFs produced a synergistic effect, with compound 5,7,4′-TMF recording the highest median fractional inhibitory concentration index (FICI) value (FICI value range: 0.4–0.5), compared to 3,5,7-TMF and PeMF alone. Interestingly, the combination therapies result in more potent inhibition with lower IC50 than single Gentamicin treatment alone. The mechanisms responsible for the effect were not elucidated. However, it may be attributed to the anti-efflux activity, which had been reported by various metabolites from plant extracts against gram-negative bacteria (Soto, 2013). The positive results indicate potential combined uses of isolated herbal KPE against emerging Carbapenem-resistant bacteria (Iovleva & Doi, 2017).
11.3 Antiviral activity
The antiviral activity of KPE from water and ethanol was investigated against the H5N1 virus (Sornpet et al., 2017). The solvent crude extract significantly inhibits the virus replication in the first 24 h post-infection and 100 % inhibition upon 72 h infection. The potent antiviral effect of the crude extract contributed by the upregulation of cytokines TNF-α and Interferon beta-1b (IFN-β) expression in the MDCK cells on 24 and 48-hour post infections. Both cytokines are well-known for their roles as a defense mechanism in reducing disease risks by the hepatitis C virus (Mochizuki et al., 2004) and the human influenza virus (Seo & Webster, 2002). In addition, the antiviral activity of K. parviflora methanolic extract against HIV-1, Hepatitis C, and human cytomegalovirus shows promising activity (Sookkongwaree et al., 2006). K. parviflora methanolic extract shows higher activity and moderately inhibits the respective virus’s replication by 37.1 %, 17.7 %, and 29.6 %, respectively. In addition, higher concentration crude extract (200 μg/ml) significantly elevated the virus protease inhibition to 81.5 %, 71.9 %, and 74.7 %, respectively. Due to the remarkable activity of the KPE, PMFs were isolated from the crude extract to investigate the potent compounds responsible for the antiviral effect. Compound 5H-7-MF and 5,7-DMF show potent antiviral activity against the HIV-1 protease virus with IC50 values of 19.04 and 19.54 µM. Nonetheless, PMFs show weak inhibition with negligible IC50 value against hepatitis C and human cytomegalovirus. Nevertheless, flavone groups are well-known for their antiviral activity through multiple mechanisms of action. Compound quercetin demonstrates potent activity by mediating the Internal Ribosome Entry Sites (IRES) translation and reducing HSP40 and HSP70 interaction with NS5A (Gonzalez et al., 2009). Meanwhile, a similar compound approached a different mechanism by suppressing the activity and replication of HCV NS3 protease (Bachmetov et al., 2012). Apart from that, inhibition of both viruses' reverse transcriptase and DNA polymerase enzyme and suppressing nucleoprotein production are other possible pathways to achieve antiviral activity of flavones, a flavonoid subclass group (Ben-Shabat et al., 2020).
11.4 Antiparasitic activity
In addition, inhibition of parasitic growth is crucial to preserving human health. Parasites Plasmodium spp. was responsible for the widespread malaria disease, anemia, and acidosis (Phillips et al., 2017). A study by Yenjai et al. (2004) showed that the isolated PMFs, 5,7,4′-TMF and 5,7,3′,4′-TeMF demonstrate potent suppression on Plasmodium falciparum (IC50: 3.70 and 4.06 μg/ml). On another note, the infectious parasite Toxoplasma gondii may cause miscarriages and neurological issues (Montoya & Liesenfeld, 2004). A recent study depicts that KPEs can suppress parasite growth of Plasmodium and T. gondii with IC50 values of 28.7 μg/ml and 53.5 μg/ml, respectively (Leesombun et al., 2019). The crude extract's inhibitory effect on T. gondii was significantly more potent than the commercialized sulfadiazine, indicating that an in-depth study on the antiparasitic effect of KPE may provide an alternative treatment for toxoplasmosis.
12 Antiallergic activity
The KPE potently suppressed allergic reactions compared to other medicinal Thai herbs in a study by Tewtrakul and Subhadhirasakul (2007). Treatment of 100 μg/ml crude extract significantly inhibits the release of β-hexosaminidase in RBL-2H3 cell lines by 101.3 % with an IC50 value of 10.9 μg/ml. The allergic effects are associated with mast cell degranulation, which releases β-hexosaminidase to mediate the allergic reaction (Shahari et al., 2017). Inhibition of cell degranulation by the crude extract was also contributed by calcium ionophore, which activates Ca2+ influx through the Ca2+ release-activated Ca2+ (CRAC) channel, an inducer responsible for releasing β-hexosaminidase. Thus, inhibition of β-hexosaminidase released may control the degranulation level of the mast cell. Moreover, the same author further elucidates the potent PMFs compound responsible for antiallergic activity (Tewtrakul et al., 2008). Compound 5-hydroxy-3,7,3′,4′-Tetramethoxyflavone demonstrated the most potent inhibition on β-hexosaminidase released with IC50 value of 8.0 µM, compared to other PMFs (IC50: 20.6 µM − 66.5 µM). Another approach was investigated by Kobayashi et al. (2015), which focused on the inhibition of calcium ionophores in RBL-2H3 cell lines by the PMFs isolated from KPE to alleviate the allergic effect. Compound 5,3′-Dihydroxy-3,7,4′-Trimethoxyflavone and 5,7,4′-TMF demonstrate the strongest influence in reducing the cell degranulation with IC50 values of 3.5 and 7.2 µM, respectively. Meanwhile, the secretion of calcium ionophore (A23187 & DTBHQ + PMA) was significantly suppressed by 5,3′-Dihydroxy-3,7,4′-Trimethoxyflavone with IC50 values of 25.7 and 68.82 µM, respectively. However, 5,7,4′-TMF is unable to demonstrate a similar effect on both calcium ionophores. The antidegranulation mechanism of the PMFs was exhibited through inhibition of antigen-induced phosphorylation of Phospholipase C, gamma 1, (PLCγ1), and spleen tyrosine kinase (Syk), which may provide an insight into treatment against allergic responses (Kobayashi et al., 2015).
13 Advance strategy of KPE and its PMFs
The crude extract of K. parviflora rhizome and its PMFs constitutes various biological activities, as discussed in the previous sections. However, the presence of multiple methoxy groups in the PMFs structure enhanced its lipophilicity characteristics, thus reducing the compound's solubility and bioavailability (Kim et al., 2014; Tsuji et al., 2006). Therefore, several strategies have been investigated to improve the bioavailability and drug delivery of the PMFs and crude extract.
13.1 Structural modification of PMFs isolated from KPE
A study by Yenjai et al. (2009) hypothesized that structural modification of PMFs isolated from the crude extract might improve the polarity and solubility. Compound 5,7-DMF was modified through a series of reactions to produce flavanones, dihydroxyflavone, chalcone, and oximes derivatives. In anticancer analysis, derived compound 5,7-Dihydroxyflavone shows potent cytotoxicity against human liver cancer cell (HepG2) and breast cancer cell lines (T47D), with IC50 value of 21.36 and 25.00 μg/ml, respectively. However, in antifungal activity, only 5,7-Dihydroxyflavanone oxime demonstrates potent activity (IC50: 48.98 μg/ml); meanwhile, for antimalarial activity, compound 5,7-DMF exhibits potent inhibitory (IC50: 5.73 μg/ml), comparable with the standard drug, Dihydroartemisinin (IC50: 5.73 μg/ml). In another study, compound 5,7-DMF was modified to incorporate the amino and nitro derivatives for anticancer activity against the human epithelial carcinoma cancer KB cell line (Wanich & Yenjai, 2009). The modified compounds, chalcone and amino flavones, induced potent cytotoxicity against the cancer cell with IC50 values of 6.80 and 5.84 μg/ml, respectively. Likewise, in another study, oxime derivatives from 5,7-DMF show potent inhibition against KB and NCI-H187 cancer cell lines with IC50 values of 0.26 and 0.014 μg/ml, respectively, more potent than the Ellipticine control agent (IC50: 1.79 & 2.77 μg/ml) (Yenjai & Wanich, 2010). In another study, a combination of selenium nanoparticles (SeNP) and KPE shows potent cytotoxicity toward gastric cancer cells (AGSr) (Wang et al., 2022). The new approach was introduced as a previous study demonstrated the SeNP potent’s bioactivities in medical applications due to its low toxicity, biocompatibility, and high bioavailability properties (Bisht et al., 2022). Based on the study, treatment of 200 μg/ml complexes significantly induced cytotoxicity in a dose-dependent manner against AGS gastric cancer cell line with more than 70 % reduction in cancer cell growth. Interestingly, the KP-SeNP complex exhibits high selectivity in normal cells. The normal cell growth remained above 80 %, compared to 50 µM cisplatin, which inhibits cell viability by 70 %. The complex induced apoptotic cell death via inhibition of PI3K/AKT/mTOR phosphorylation, which activates the BCL-2 pro-apoptotic protein and caspase 3, resulting in cell death. The result suggests modification of PMFs from KPE capable of elevating cytotoxicity against cancer cells and reducing its lipophilicity character.
13.2 Transdermal delivery mechanism of KPE
In addition, the bioavailability of KPE was potently enhanced through the combination of transdermal vehicles isopropyl myristate (IPM), ethanol, and propylene glycol, with the addition of permeation enhancers and fatty acids. The solubility of K. parviflora in the IPM vehicle was recorded as 15.6 mg/ml and significantly improved when combined with a higher ethanol-to-propylene glycol ratio. Interestingly, the combination of ethanol and IPM vehicle in all proportions (1:9, 2:8 & 3:7) significantly improved the solubility to 94.1, 182.4, and 204.4 mg/ml, respectively. A higher ethanol ratio shows significant enhancement in crude extract solubility. Nonetheless, it reduced the flux of the crude extract. Ethanol with a low IPM ratio (1:9) demonstrates the highest flux in the crude section (314.7 μg/cm2/h). Higher flux value indicates improvement in solubility properties of KPE in ethanol: IPM vehicles (Tuntiyasawasdikul et al., 2014). On another note, adding fatty acid and oleic acid with ethanol: IPM vehicle significantly improved the solubility and flux of KPE to 109.8 mg/ml and 358.5 μg/cm2/h, respectively. The study suggests that adding fatty acid could reduce the resistance of the outer layer of skin during transdermal transport to improve its effectiveness. A similar study was designed by Tuntiyasawasdikul et al. (2015) to investigate the permeability of a monolithic drug-in-adhesive type patch of isolated PMFs from crude KPE with a combination of the delivery vehicle and permeation enhancer, oleic acid, and methanol. Combining oleic acid and methanol produced maximum flux values for all compounds, indicating a synergistic effect between K. parviflora, oleic acid, and methanol content. Thus, an in vivo study on rats with patch combinations significantly released the PMFs into the systemic circulation through transdermal delivery with plasma drug concentration and time maximum was recorded at 218.08 ng/ml and 8 h, respectively, with less than 1 % retained in the skin (1.43 μg/cm2). Based on these results, microemulsion and microgels of KPE were introduced through combination with oleic acid and surfactants, which has been shown to improve the crude extract solubility and dissolution rate in transdermal delivery mechanism (Rangsimawong et al., 2018). The microemulsion of KPE improved the skin permeation in a time and dose-dependent manner.
Interestingly, the addition of Limonene at a higher ratio (10 %) improved the permeation capacity and recorded the highest flux value (0.132 μg/cm2/h) compared to the control (0.013 (μg/cm2/h). The result was consistent with the study by (Lu et al., 2014). Limonene is a nonpolar compound with a lipophilic character that facilitates drug diffusion into the skin. Meanwhile, microgels of KPE produced similar results with higher permeability than the control. However, the permeation flux was lower than the microemulsion state, with 0.044 μg/cm2/h recorded. Microgels are associated with high viscosity, leading to lower drug diffusion to penetrate the skin. Thus, the permeation and flux rates were drastically reduced (Gannu et al., 2010).
13.3 Self-emulsifying delivery system of KPE
The self-microemulsifying delivery system (SMEDDS) was introduced on KPE by Mekjaruskul et al. (2013). Previous study has demonstrated that combining the crude extract with oil and surfactants improved the solubility and permeability of drug delivery. SMEDDS is a microemulsifying process through chemical interaction contrary to mechanical methods prepared by the previous study to form a homogenous mixture of crude KPE (Kim et al., 2019). In an in vitro study, the combination of KPE with polyethylene castor oil and propylene glycol in a ratio of 2:1 (80 & 85 %) with triglycerides of coconut oil (20 & 15 %) improved the dissolution rate compared to the crude extract with 100 % unloaded within 20 min. In addition, the crude extract combined with the cyclodextrin complex demonstrates a higher dissolution rate of 100 % within 10 min. Meanwhile, for the in vivo study, a similar result was obtained, with all complexes showing enhancement in oral bioavailability compared to crude extract. In a separate study, a self-nanoemulsifying delivery system (SNEDDS) significantly improved the dissolution rate of crude extract. However, it recorded lower capacity than SMEDDS, with 92 % unloading after 60 min. The significant differences may be attributed to the type and ratio of oil, surfactant, and co-surfactant used (Chairuk et al., 2020). However, the formation of nanosuspension crude extract in liquid form improved the dissolution rate to over 80 % in both 3 % SLS and PBS stabilizer at pH 6.8, comparable with the SMEDDS. Meanwhile, in solid form, both stabilizers slow down the dissolution rate in the first 30 min before achieving a similar rate as a liquid form within 1 h. The result indicates that micro and nano-drug delivery may improve the lipophilicity character and bioavailability of the PMFs from KPE (Mekjaruskul and Sripanidkulchai, 2020).
13.4 Improvement of solubility and dissolution rate of KPE and its PMFs
In another study, introducing complex Hydroxypropyl-β-cyclodextrin (HPβ-CD) and 5,7-DMF from KPE significantly improved the solubility compared to free 5,7-DMF from 0.00304 to 1.10 mg/ml (Songngam et al., 2014). The complex was investigated on BChE inhibitory, as a previous study reported 85 % inhibition on the enzyme by 5,7-DMF (Sawasdee et al., 2009). The HPβ-CD/5,7-DMF complex significantly inhibits the enzyme activity in a concentration-dependent manner with an IC50 value of 23.5 µM, 2.7-fold stronger than free 5,7-DMF (IC50: 62.4 µM). HPβ-CD has been widely used to improve the solubility of flavonoid metabolites due to its low toxicity and high compatibility (Loftsson and Duchêne, 2007). The solid dispersion method was introduced to enhance the dissolution rate of PMFs with a combination of hydroxypropyl methylcellulose (HPMC) and polyvinyl alcohol-polyethylene glycol grafted copolymer (PVA-co-PEG) polymer (Weerapol et al., 2017). In the solid dispersion technique, the hydrophobic drug (5,7,4′-TMF) was mixed with a hydrophilic matrix (polymer) to form a homogenous mixture of amorphous or crystalline particles (Paudel et al., 2013). In the absence of a polymer matrix, there was no significant difference in the dissolution rate of 5,7,4′-TMF dissolved within 120 min of exposure. Interestingly, the formation of 5,7,4′-TMF and HPMC with a ratio of 1:2 demonstrate the dissolution rate of 43.71 % and 62.11 %, respectively. Interestingly, the mixture with polymer PVA-co-PEG shows the highest dissolution rate compared to the HPMC polymer combination. PVA-co-PEG ratio (1:1) enhanced the solubility rate to 75.74 % and 84.83 %, respectively. However, further increases in polymer ratios with the compound 5,7,4′-TMF (4:1) significantly reduced the dissolution rate. Compared to the isolated compound from KPE, the excess polymer ratio causes increased mixtures' viscosity, thus reducing the dissolution rate (Weerapol et al., 2017).
14 Conclusion
This review has recapped astonishing biological activity findings from the rhizome of K. parviflora, contributed by the PMFs as a major compound in the crude extract. The structure–activity relationship of the PMFs revealed that methoxylation on different carbon positions significantly influenced the biological properties of each PMFs compound and was independent of the number of methoxy-groups bonded to the structure. Interestingly, some functional group positions (5-methoxy & 5-Hydroxy) were selectively favoured in several biological activities reported. In fact, the addition of the hydroxy group within the flavones scaffold presents numerous advantages and provides an appealing scope of studies due to the electron-withdrawing and donating effects differences between both hydroxy and methoxy functional groups. Nevertheless, the outcome of this review elucidates the research gap in the biological properties of PMFs, given the remarkable pharmacological effects of KPE, especially in anticancer and antidiabetes, that need to be extensively explored, which may provide an exciting outcome for future studies. In addition, the toxicology reports summarized the superior safety of the crude KPE with high dosage in vitro and in vivo studies. However, no toxicology investigations have been reported on PMFs from KPE. Due to the non-polarity and high lipophilicity of the PMFs mentioned by multiple studies that may impede their potential in drug discovery, several strategies have been outlined to enhance their bioavailability. This review concluded multiple approaches and techniques, which include emulsification, encapsulation, suspension, dispersion, nanoparticles, and transport vehicles by utilizing vast co-aided agents to produce highly stabilized, excellent loading and released capacity and significant improvement in water solubility and bioavailability of PMFs and the crude KPE.
Acknowledgement
This article review was supported by the Ministry of Higher Education, Fundamental Research Grant Scheme, FRGS/1/2021/SKK0/MSU/02/1 and the MSU Publication & Conference Grant (MPCG) (MPCG-001-012023-SGS) from the Management & Science University, Shah Alam, Malaysia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med.. 2011;65(1):73-80.
- [CrossRef] [Google Scholar]
- Antimicrobial activities of different solvent extracts from stem and seeds of Peganum Harmala L. PLoS One. 2022;17(4):e0265206.
- [Google Scholar]
- Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem.. 2011;125(2):471-475.
- [CrossRef] [Google Scholar]
- Bachmetov, L., Gal-Tanamy, M., Shapira, A., Vorobeychik, M., Giterman-Galam, T., Sathiyamoorthy, P., Golan-Goldhirsh, A., Benhar, I., Tur-Kaspa, R., & Zemel, R. 2012. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. Journal of Viral Hepatitis, 19(2), e81–e88. https://doi.org/10.1111/j.1365-2893.2011.01507.x.
- Ethanolic rhizome extract from Kaempferia parviflora Wall. ex. Baker induces apoptosis in HL-60 cells. Asian Pac. J. Cancer Prev.: APJCP. 2008;9(4):595-600.
- [Google Scholar]
- Induction of apoptosis in the human Leukemic U937 cell line by Kaempferia parviflora Wall.ex.Baker extract and effects of paclitaxel and camptothecin. Asian Pac. J. Cancer Prev.: APJCP. 2009;10(6):1137-1140.
- [Google Scholar]
- Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res.. 2020;10(2):354-367.
- [CrossRef] [Google Scholar]
- Selenium nanoparticles: a review on synthesis and biomedical applications. Mater. Adv.. 2022;2022(3):1415-1431.
- [CrossRef] [Google Scholar]
- GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. EMBO J.. 2006;25(24):5648-5658.
- [CrossRef] [Google Scholar]
- Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol.. 2010;41(2–3):242-247.
- [CrossRef] [Google Scholar]
- The effects of Kaempferia parviflora on anti-internalization activity of Helicobacter pylori to HEp-2 cells. Afr. J. Biotechnol.. 2010;9(30):4796-4801.
- [Google Scholar]
- Enhancing oral absorption of poorly water-soluble herb (Kaempferia parviflora) extract using self-nanoemulsifying formulation. Pharm. Dev. Technol.. 2020;25(3):340-350.
- [CrossRef] [Google Scholar]
- Role of Reactive Oxygen Species in Inflammation: A Minireview. Moscow Univ. Biol. Sci. Bull.. 2018;73:199-202.
- [CrossRef] [Google Scholar]
- Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204-7218.
- [CrossRef] [Google Scholar]
- Synergism between cAMP and PPARγ Signalling in the Initiation of UCP1 Gene Expression in HIB1B Brown Adipocytes. PPAR Res.. 2013;2013:476049
- [CrossRef] [Google Scholar]
- Chronic Toxicity Study of Kaempferia parviflora Wall ex. Extract. Thai J. Vet. Med.. 2010;40:377-383.
- [Google Scholar]
- Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci.. 2019;20(10):2407.
- [CrossRef] [Google Scholar]
- The protective role of Aegle marmelos on aspirin-induced gastro-duodenal ulceration in albino rat model: a possible involvement of antioxidants. Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association. 2012;18(3):188-194.
- [CrossRef] [Google Scholar]
- Prevalence and association between triglyceride level and lifestyle factors among Malay obese class I and II adults. Enferm. Clin.. 2018;28:310-315.
- [CrossRef] [Google Scholar]
- Destruction of the gastric mucosal barrier by detergents and urea. Gastroenterology. 1968;54(2):175-181.
- [Google Scholar]
- Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal.. 2014;22(3):296-302.
- [CrossRef] [Google Scholar]
- AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother.. 2022;146:112563
- [CrossRef] [Google Scholar]
- In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol. Cell. Biol.. 2004;24(15):6728-6741.
- [CrossRef] [Google Scholar]
- Fortune Business Insight, 2022. Herbal Medicine Market Size, Share & COVID-19 Impact Analysis, By Application (Pharmaceutical & Nutraceutical, Food & Beverages, and Personal Care & Beauty Products), By Form (Powder, Liquid & Gel, and Tablets & Capsules), and Regional Forecast, 2022-2029. Fortune Business Insight, https://www.fortunebusinessinsights.com/herbal-medicine-market-106320.
- Inhibitory effect of black ginger (Kaempferia parviflora) constituents on nitric oxide production. Japanese Journal of Food Chemistry and Safety. 2018;25(3):152-159.
- [CrossRef] [Google Scholar]
- A noncompetitive BACE1 inhibitor TAK-070 ameliorates Aβ pathology and behavioral deficits in a mouse model of Alzheimer's disease. J. Neurosci.. 2010;30(33):11157-11166.
- [CrossRef] [Google Scholar]
- Enhanced bioavailability of lacidipine via microemulsion based transdermal gels: formulation optimization, ex vivo and in vivo characterization. Int. J. Pharm.. 2010;388(1–2):231-241.
- [CrossRef] [Google Scholar]
- Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959-975.
- [CrossRef] [Google Scholar]
- The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology (Baltimore, MD). 2009;50(6):1756-1764.
- [CrossRef] [Google Scholar]
- Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int. Suppl.. 2000;77:S19-S25.
- [CrossRef] [Google Scholar]
- The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J.. 1999;343 Pt 2(Pt 2):281-299.
- [Google Scholar]
- Single oral toxicity test and safety classification for Kaempferia parviflora. Kor. J. Herbol. 2018;33(4):53-58.
- [CrossRef] [Google Scholar]
- Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci.. 1991;12(10):383-388.
- [CrossRef] [Google Scholar]
- Hassan, S. K., El-Sammad, N. M., Mousa, A. M., Mohammed, M. H., Farrag, A. el R. H., Hashim, A. N. E., Werner, V., Lindequist, U., & Nawwar, M. A. E.-M. 2015. Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine, 5(6), 462–471. https://doi.org/10.1016/j.apjtb.2015.03.004.
- Screening Neuropharmacological Activities of Kaempferia parviflora (Krachai Dam) in Healthy Adult Male Rats. Am. J. Appl. Sci.. 2011;8(7):695-702.
- [CrossRef] [Google Scholar]
- Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20(3):270-279.
- [CrossRef] [Google Scholar]
- Efficacy of Kaempferia parviflora in a mouse model of obesity-induced dermatopathy. J. Nat. Med.. 2017;71(1):59-67.
- [CrossRef] [Google Scholar]
- Reversible cognitive and neurological symptoms during valproic acid therapy. Journal of the American Geriatrics Society. 2007;55(4):628.
- [CrossRef] [Google Scholar]
- Identification and evaluation of anti-inflammatory compounds from Kaempferia parviflora. Biosci. Biotech. Bioch.. 2014;78(5):851-860.
- [CrossRef] [Google Scholar]
- Inhibitory effects of Kaempferia parviflora extract on monocyte adhesion and cellular reactive oxygen species production in human umbilical vein endothelial cells. Eur. J. Nutr.. 2017;56(3):949-964.
- [CrossRef] [Google Scholar]
- Polymethoxyflavonoids from Kaempferia parviflora induce adipogenesis on 3T3-L1 preadipocytes by regulating transcription factors at an early stage of differentiation. Biol. Pharm. Bull.. 2012;35(5):686-692.
- [CrossRef] [Google Scholar]
- Cytotoxic effects of polymethoxyflavones isolated from Kaempferia parviflora. J. Korean Soc. Appl. Biol. Chem.. 2012;55:471-476.
- [CrossRef] [Google Scholar]
- Carbapenem-Resistant Enterobacteriaceae. Clin. Lab. Med.. 2017;37(2):303-315.
- [CrossRef] [Google Scholar]
- Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther.. 2018;24(9):753-762.
- [CrossRef] [Google Scholar]
- 3,5,7,3′,4′-Pentamethoxyflavone, a quercetin derivative protects DNA from oxidative challenges: Potential mechanism of action. J. Photochem. Photobiol. B Biol.. 2014;131:96-103.
- [CrossRef] [Google Scholar]
- Antibacterial Effect of Crude Extracts of Kaempferia parviflora (Krachaidam) against Cronobacter spp. and Enterohemorrhagic Escherichia coli (EHEC) in Various Dairy Foods: A Preliminary Study. J. Milk Sci Biotechnol.. 2016;34(2):63-68.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity. Int. J. Mol. Sci.. 2018;19(11):3457.
- [CrossRef] [Google Scholar]
- Lactate transport in skeletal muscle - role and regulation of the monocarboxylate transporter. J. Physiol.. 1999;517(Pt 3) (Pt 3):633-642.
- [CrossRef] [Google Scholar]
- Glycosaminoglycan composition of PC12 pheochromocytoma cells: a comparison with PC12D cells, a new subline of PC12 cells. J. Neurochem.. 1989;52(3):889-895.
- [CrossRef] [Google Scholar]
- Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. European Journal of Medicinal Chemistry Reports. 2021;3:100010
- [CrossRef] [Google Scholar]
- Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. Int. J. Nanomed.. 2019;14:4949-4960.
- [CrossRef] [Google Scholar]
- The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients. 2020;12(4):1079.
- [CrossRef] [Google Scholar]
- Metabolism of Kaempferia parviflora polymethoxyflavones by human intestinal bacterium Bautia sp. MRG-PMF1. J. Agric. Food Chem.. 2014;62(51):12377-12383.
- [CrossRef] [Google Scholar]
- Induction of ER Stress-Mediated Apoptosis by the Major Component 5,7,4′-Trimethoxyflavone Isolated from Kaempferia parviflora Tea Infusion. Nutr. Cancer. 2018;70(6):984-996.
- [CrossRef] [Google Scholar]
- Quercetin and its role in biological functions: an updated review. EXCLI J.. 2018;17:856-863.
- [CrossRef] [Google Scholar]
- Polymethoxyflavones from Kaempferia parviflora ameliorate skin aging in primary human dermal fibroblasts and ex vivo human skin. Biomed. Pharmacother.. 2022;145:112461
- [CrossRef] [Google Scholar]
- Effects of ethyl acetate extract of Kaempferia parviflora on brown adipose tissue. J. Nat. Med.. 2016;70(1):54-61.
- [CrossRef] [Google Scholar]
- Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods. 2015;13:100-107.
- [CrossRef] [Google Scholar]
- Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med.. 2018;72(1):136-144.
- [CrossRef] [Google Scholar]
- Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem. J.. 2007;403(3):473-481.
- [CrossRef] [Google Scholar]
- Ethanolic extract of Kaempferia parviflora interrupts the mechanisms-associated rheumatoid arthritis in SW982 culture model via p38/STAT1 and STAT3 pathways. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2019;59:152755
- [CrossRef] [Google Scholar]
- Kumar, B., Smita, K., Cumbal, L., & Debut, A. 2016. Ficus carica (Fig) Fruit Mediated Green Synthesis of Silver Nanoparticles and its Antioxidant Activity: a Comparison of Thermal and Ultrasonication Approach. BioNanoSci, 6, 15–2. https://doi.org/10.1007/s12668-016-0193-1.
- Antimicrobial activity of the ethanol extract and compounds from the rhizomes of Kaempferia parviflora. Songklanakarin J. Sci. Technol.. 2008;30(4):463-466.
- [Google Scholar]
- Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Medicinal Chemistry (shariqah (united Arab Emirates)). 2009;5(2):139-147.
- [CrossRef] [Google Scholar]
- Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Archiv : European Journal of Physiology. 2010;460(2):525-542.
- [CrossRef] [Google Scholar]
- Pharmacological activity of Kaempferia parviflora extract against human bile duct cancer cell lines. Asian Pac. J. Cancer Prev.: APJCP. 2009;10(4):695-698.
- [Google Scholar]
- alpha-Glucosidase inhibitors. Endocrinol. Metab. Clin. North Am.. 1997;26(3):539-551.
- [CrossRef] [Google Scholar]
- Antiskin Inflammatory Activity of Black Ginger (Kaempferia parviflora) through Antioxidative Activity. Oxid. Med. Cell. Longev.. 2018;2018:5967150.
- [CrossRef] [Google Scholar]
- Improvement of Damage in Human Dermal Fibroblasts by 3,5,7-Trimethoxyflavone from Black Ginger (Kaempferia parviflora) Antioxidants. 2022;11(2):425.
- [CrossRef] [Google Scholar]
- Ethanol Extracts from Thai Plants have Anti-Plasmodium and Anti-Toxoplasma Activities In Vitro. Acta Parasitol.. 2019;64(2):257-261.
- [CrossRef] [Google Scholar]
- Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol.. 2018;9:477.
- [CrossRef] [Google Scholar]
- Cyclodextrins and their pharmaceutical applications. Int. J. Pharm.. 2007;329(1–2):1-11.
- [CrossRef] [Google Scholar]
- Skin permeation of D-limonene-based nanoemulsions as a transdermal carrier prepared by ultrasonic emulsification. Ultrason. Sonochem.. 2014;21(2):826-832.
- [CrossRef] [Google Scholar]
- Effects of Kaempferia parviflora Wall. Ex Baker on endothelial dysfunction in streptozotocin-induced diabetic rats. J. Ethnopharmacol.. 2011;133(2):371-377.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. J. Nutr. Sci. Vitaminol.. 2015;61(1):79-83.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora Nanosuspension Formulation for Scalability and Improvement of Dissolution Profiles and Intestinal Absorption. AAPS PharmSciTech. 2020;21(2):52.
- [CrossRef] [Google Scholar]
- Novel formulation strategies for enhancing oral delivery of methoxyflavones in Kaempferia parviflora by SMEDDS or complexation with 2-hydroxypropyl-β-cyclodextrin. Int. J. Pharm.. 2013;445(1–2):1-11.
- [CrossRef] [Google Scholar]
- Potential Therapeutic Agents, Polymethoxylated Flavones Isolated from Kaempferia parviflora for Cataract Prevention through Inhibition of Matrix Metalloproteinase-9 in Lens Epithelial Cells. Biol. Pharm. Bull.. 2019;42(10):1658-1664.
- [CrossRef] [Google Scholar]
- Effect of in vitro interferon-beta administration on hepatitis C virus in peripheral blood mononuclear cells as a predictive marker of clinical response to interferon treatment for chronic hepatitis C. World J. Gastroenterol.. 2004;10(5):733-736.
- [CrossRef] [Google Scholar]
- Quercetin against MCF7 and CAL51 breast cancer cell lines: apoptosis, gene expression and cytotoxicity of nano-quercetin. Nanomedicine (London, England). 2021;16(22):1937-1961.
- [CrossRef] [Google Scholar]
- Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci.. 2016;17(1):124.
- [CrossRef] [Google Scholar]
- Potent SIRT1 enzyme-stimulating and anti-glycation activities of polymethoxyflavonoids from Kaempferia parviflora. Natural Product Communications. 2014;9(9):1291-1294.
- [CrossRef] [Google Scholar]
- Effect of methoxyflavones contained in Kaempferia parviflora on CRE-mediated transcription in PC12D cells. Bioorg. Med. Chem. Lett.. 2020;30(23):127606
- [CrossRef] [Google Scholar]
- Ethyl acetate extract of Kaempferia parviflora inhibits Helicobacter pylori-associated mammalian cell inflammation by regulating proinflammatory cytokine expression and leukocyte chemotaxis. BMC Complementary Medicine and Therapies. 2020;20(1):124.
- [CrossRef] [Google Scholar]
- Nordberg, A., Ballard, C., Bullock, R., Darreh-Shori, T., & Somogyi, M. 2013. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer's disease. The primary care companion for CNS disorders, 15(2), PCC.12r01412. https://doi.org/10.4088/PCC.12r01412.
- Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta. 2014;1840(8):2571-2580.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora Ethanol Extract, a Peroxisome Proliferator-Activated Receptor γ Ligand-binding Agonist, Improves Glucose Tolerance and Suppresses Fat Accumulation in Diabetic NSY Mice. J. Food Sci.. 2019;84(2):339-348.
- [CrossRef] [Google Scholar]
- Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine. 2014;21(6):800-806.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora Extract Alleviated Rat Arthritis, Exerted Chondroprotective Properties In Vitro, and Reduced Expression of Genes Associated with Inflammatory Arthritis. Molecules. 2021;26(6):1527.
- [CrossRef] [Google Scholar]
- Padanilam, B. J. 2003. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. American journal of physiology. Renal physiology, 284(4), F608–F627. https://doi.org/10.1152/ajprenal.00284.2002.
- Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement. Altern. Med.. 2018;18(1):178.
- [CrossRef] [Google Scholar]
- The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed.. 2014;30(5):237-245.
- [CrossRef] [Google Scholar]
- Standardized Kaempferia parviflora Extract Inhibits Intrinsic Aging Process in Human Dermal Fibroblasts and Hairless Mice by Inhibiting Cellular Senescence and Mitochondrial Dysfunction. Evid. Based Complement. Alternat. Med.: Ecam. 2017;2017:6861085.
- [CrossRef] [Google Scholar]
- Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm.. 2013;453(1):253-284.
- [CrossRef] [Google Scholar]
- Detection of protein glycation inhibitory potential of nine antidiabetic plants using a novel method. Asian J. Med. Sci.. 2014;6(2):1-6.
- [CrossRef] [Google Scholar]
- Effect of Dietary Kaempferia parviflora on Ischemic Brain Injury in the rat. OnLine Journal of Biological Sciences. 2012;12(1):27-33.
- [CrossRef] [Google Scholar]
- Malaria. Nat Rev Dis Primers. 2017;3:17050.
- [CrossRef]
- Protective Effect of Polymethoxyflavones Isolated from Kaempferia parviflora against TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants. 2021;10(10):1609.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora Extract Exhibits Anti-cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol.. 2017;8:630.
- [CrossRef] [Google Scholar]
- Anti-glycation and radical scavenging activities of hydro-alcohol and aqueous extracts of nine species from Lamiaceae family. Journal of Medicinal Plants Studies. 2017;5(1):331-345.
- [Google Scholar]
- Development of Microemulsions and Microemulgels for Enhancing Transdermal Delivery of Kaempferia parviflora Extract. AAPS PharmSciTech. 2018;19(5):2058-2067.
- [CrossRef] [Google Scholar]
- Rees, T. M., & Brimijoin, S. 2003. The role of acetylcholinesterase in the pathogenesis of Alzheimer's disease. Drugs of today (Barcelona, Spain: 1998), 39(1), 75–83. https://doi.org/10.1358/dot.2003.39.1.740206.
- Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol.. 2005;102(1):120-122.
- [CrossRef] [Google Scholar]
- Anti-inflammatory mechanism of Kaempferia parviflora in murine macrophage cells (RAW 264.7) and in experimental animals. J. Ethnopharmacol.. 2009;124(3):576-580.
- [CrossRef] [Google Scholar]
- Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol.. 2011;136(3):488-495.
- [CrossRef] [Google Scholar]
- Antiglycation and antioxidant activity of four Iranian medical plant extracts. Journal of Pharmacopuncture. 2018;21(2):82-89.
- [CrossRef] [Google Scholar]
- Clinical Effects of Krachaidum (Kaempferia parviflora): A Systematic Review. Journal of Evidence-Based Complementary & Alternative Medicine. 2017;22(3):413-428.
- [CrossRef] [Google Scholar]
- Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res.: PTR. 2009;23(12):1792-1794.
- [CrossRef] [Google Scholar]
- Adipose Triglyceride Lipase and Hormone-sensitive Lipase Are the Major Enzymes in Adipose Tissue Triacylglycerol Catabolism. Journalof Biological Chemistry. 2006;281(52):40236-40241.
- [CrossRef] [Google Scholar]
- Acetyl-cholinesterase Inhibitory Activity of Methoxyflavones Isolated from Kaempferia parviflora. Nat. Prod. Commun.. 2017;12(1):21-22.
- [Google Scholar]
- Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol.. 2002;76(3):1071-1076.
- [CrossRef] [Google Scholar]
- Histamine and Beta-Hexosaminidase Inhibitory Effects of Crude Alkaloid from Kopsia arborea Blume in RBL-2H3 Cell Lines. Natural Products: an Indian Journal. 2017;13(2):108.
- [Google Scholar]
- Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol.. 2020;11:694.
- [CrossRef] [Google Scholar]
- Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252-259.
- [CrossRef] [Google Scholar]
- Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia. 2011;82(8):1272-1278.
- [CrossRef] [Google Scholar]
- Expression of inducible nitric oxide synthase (iNOS) in microglia of the developing quail retina. PLoS One. 2014;9(8):e106048.
- [Google Scholar]
- Kaempferia parviflora Rhizome Extract as Potential Anti-Acne Ingredient. Molecules. 2022;27(14):4401.
- [CrossRef] [Google Scholar]
- ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. The Journal of Biological Chemistry. 2002;277(27):24120-24127.
- [CrossRef] [Google Scholar]
- Nitric oxide metabolism is impaired by type 1 diabetes and diabetic nephropathy. Biomedical Reports. 2020;12:251-258.
- [CrossRef] [Google Scholar]
- A 5,7-dimethoxyflavone/hydroxypropyl-β-cyclodextrin inclusion complex with anti-butyrylcholinesterase activity. AAPS PharmSciTech. 2014;15(5):1189-1196.
- [CrossRef] [Google Scholar]
- Synergistic Effects of Some Methoxyflavones Extracted from Rhizome of Kaempferia parviflora Combined with Gentamicin against Carbapenem-Resistant Strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Plants. 2022;11(22):3128.
- [CrossRef] [Google Scholar]
- Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharmazie. 2006;61(8):717-721.
- [Google Scholar]
- Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac. J. Trop. Med.. 2017;10(9):871-876.
- [CrossRef] [Google Scholar]
- Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013;4(3):223-229.
- [CrossRef] [Google Scholar]
- Antidiabetic activity of methoxyflavone-enriched extract of Kaempferia parviflora in streptozotocin-induced diabetic rats. Songklanakarin J. Sci. Technol.. 2020;42(6):1239-1247.
- [Google Scholar]
- Effect of Kaempferia parviflora Wall. ex. Baker on sexual activity of male rats and its toxicity. Southeast Asian J. Trop. Med. Public Health. 2006;37 Suppl 3:210-215.
- [Google Scholar]
- Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia parviflora. Plants. 2021;10(2):229.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora Extract Inhibits STAT3 Activation and Interleukin-6 Production in HeLa Cervical Cancer Cells. Int. J. Mol. Sci.. 2019;20(17):4226.
- [CrossRef] [Google Scholar]
- Anti-psoriatic and anti-inflammatory effects of Kaempferia parviflora in keratinocytes and macrophage cells. Biomed. Pharmacother.. 2021;143:112229
- [CrossRef] [Google Scholar]
- Selective Cytotoxicity of Kaempferia parviflora Extracts in Human Cell Lines. Asian Pac. J. Cancer Prev.. 2021;22(S1):73-79.
- [CrossRef] [Google Scholar]
- Anti-allergic activity of some selected plants in the Zingiberaceae family. J. Ethnopharmacol.. 2007;109(3):535-538.
- [CrossRef] [Google Scholar]
- Effects of compounds from Kaempferia parviflora on nitric oxide, prostaglandin E2 and tumor necrosis factor-alpha productions in RAW264.7 macrophage cells. J. Ethnopharmacol.. 2008;120(1):81-84.
- [CrossRef] [Google Scholar]
- Anti-allergic activity of compounds from Kaempferia parviflora. J. Ethnopharmacol.. 2008;116(1):191-193.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurate. Food Chem.. 2009;115(2):534-538.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora extract inhibits TNF-α-induced release of MCP-1 in ovarian cancer cells through the suppression of NF-κB signaling. Biomed. Pharmacother.. 2021;141:111911
- [CrossRef] [Google Scholar]
- Anti-osteoporotic and Antioxidant Activities by Rhizomes of Kaempferia parviflora Wall. ex Baker. Nat. Prod. Sci.. 2016;22(1):1060607.
- [CrossRef] [Google Scholar]
- Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon. 2016;2(5):e00115.
- [Google Scholar]
- Enhancement of energy production by black ginger extract containing polymethoxy flavonoids in myocytes through improving glucose, lactic acid and lipid metabolism. J. Nat. Med.. 2016;70(2):163-172.
- [CrossRef] [Google Scholar]
- Shikonin Protects PC12 Cells Against β-amyloid Peptide-Induced Cell Injury Through Antioxidant and Antiapoptotic Activities. Sci. Rep.. 2018;8:26.
- [CrossRef] [Google Scholar]
- Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer's disease. J. Neurosci.. 2005;25(48):11165-11174.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora Rhizome Extract Inhibits Glutamate-Induced Toxicity in HT-22 Mouse Hippocampal Neuronal Cells and Extends Longevity in Caenorhabditis elegans. Biology. 2021;10(4):264.
- [CrossRef] [Google Scholar]
- Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the Atlantic killifish. Chem. Biol. Interact.. 2006;164(1–2):85-92.
- [CrossRef] [Google Scholar]
- Polymethoxyflavones: chemistry and molecular mechanisms for cancer prevention and treatment. Curr. Pharmacol. Rep.. 2019;5:98-113.
- [CrossRef] [Google Scholar]
- Transdermal permeation of Kaempferia parviflora methoxyflavones from isopropyl myristate-based vehicles. AAPS PharmSciTech. 2014;15(4):947-955.
- [CrossRef] [Google Scholar]
- A monolithic drug-in-adhesive patch of methoxyflavones from Kaempferia parviflora: in vitro and in vivo evaluation. Int. J. Pharm.. 2015;478(2):486-495.
- [CrossRef] [Google Scholar]
- Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2010;166(1):15-22.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles using Kaempferia parviflora rhizome extract and their characterization and application as an antimicrobial, antioxidant and catalytic degradation agent. J. Taiwan Inst. Chem. Eng.. 2021;126:166-172.
- [CrossRef] [Google Scholar]
- The β-secretase enzyme BACE1 as a therapeutic target for Alzheimer's disease. Alzheimers Res. Ther.. 2011;3(3):20.
- [CrossRef] [Google Scholar]
- Investigation on Antiulcer Activity of Aegle marmelos Root as Experimental, Biochemical and Histological Study. J. Pharm. Res.. 2010;3:2523-2528.
- [Google Scholar]
- Biologically synthesized black ginger-selenium nanoparticle induces apoptosis and autophagy of AGS gastric cancer cells by suppressing the PI3K/Akt/mTOR signaling pathway. J. Nanobiotechnol.. 2022;20(1):441.
- [CrossRef] [Google Scholar]
- Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin.. 2008;29(11):1275-1288.
- [CrossRef] [Google Scholar]
- Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. Biomed. Res. Int.. 2014;2014:453972.
- [CrossRef] [Google Scholar]
- Amino and nitro derivatives of 5,7-dimethoxyflavone from Kaempferia parviflora and cytotoxicity against KB cell line. Arch. Pharm. Res.. 2009;32(9):1185-1189.
- [CrossRef] [Google Scholar]
- Anti-Stress Effects of Kaempferia parviflora in Immobilization Subjected Rats. Am. J. Pharmacol. Toxicol.. 2013;8(1):31-38.
- [CrossRef] [Google Scholar]
- Improved dissolution of Kaempferia parviflora extract for oral administration by preparing solid dispersion via solvent evaporation. Asian J. Pharm. Sci.. 2017;12(2):124-133.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Annals of Anatomy = Anatomischer Anzeiger: Official Organ of the Anatomische Gesellschaft. 2016;206:7-13.
- [CrossRef] [Google Scholar]
- Attenuated glutamate induced ROS production by antioxidative compounds in neural cell lines. RSC Adv.. 2019;9(60):34735-34743.
- [CrossRef] [Google Scholar]
- Yagi, M., Hayashi, S., Ishizaki, K., Takabe, W., Inoue, K., Sato, Y., Sakiyama, C., & Yonei, Y. (2019). Inhibitory effect of Kaempferia parviflora Wall. Ex. Baker (Zingiberaceae) rhizome on postprandial hyperglycemia. Glycative Stress Research, 6(2), 126-134. https://doi.org/10.24659/gsr.6.2_126.
- Antiglycative effect of black galangal, Kaempferia parviflora Wall. Ex. Baker (zingiberaceae). Glycative Stress Research. 2021;8(1):1-7.
- [CrossRef] [Google Scholar]
- Regulation of stem cell differentiation in adipose tissue by chronic inflammation. Clin. Exp. Pharmacol. Physiol.. 2011;38(12):872-878.
- [CrossRef] [Google Scholar]
- Cytotoxicity against KB and NCI-H187 cell lines of modified flavonoids from Kaempferia parviflora. Bioorg. Med. Chem. Lett.. 2010;20(9):2821-2823.
- [CrossRef] [Google Scholar]
- Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004;75(1):89-92.
- [CrossRef] [Google Scholar]
- Structural modification of 5,7-dimethoxyflavone from Kaempferia parviflora and biological activities. Arch. Pharm. Res.. 2009;32(9):1179-1184.
- [CrossRef] [Google Scholar]
- Effects of Kaempferia parviflora rhizomes dichloromethane extract on vascular functions in middle-aged male rat. J. Ethnopharmacol.. 2014;156:162-174.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci. Nutr.. 2014;2(6):634-637.
- [CrossRef] [Google Scholar]
- Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: a randomized, double-blind, placebo-controlled clinical study. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2018;11:447-458.
- [CrossRef] [Google Scholar]
- Toxicological evaluation of standardized Kaempferia parviflora extract: Sub-chronic and mutagenicity studies. Toxicology Reports. 2019;6:544-549. https://doi.org/110.1016/j.toxrep.2019.06.003
- [Google Scholar]
- Polymethoxyflavone purified from Kaempferia parviflora reduces visceral fat in Japanese overweight individuals: a randomised, double-blind, placebo-controlled study. Food Funct.. 2021;12(4):1603-1613.
- [CrossRef] [Google Scholar]
- Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential β-secretase (BACE1) inhibitors. J. Funct. Foods. 2016;20:567-574.
- [CrossRef] [Google Scholar]
- Influencing Factors of Thermogenic Adipose Tissue Activity. Front. Physiol.. 2016;7:29.
- [CrossRef] [Google Scholar]
- The Potential to Fight Obesity with Adipogenesis Modulating Compounds. Int. J. Mol. Sci.. 2022;23(4):2299.
- [CrossRef] [Google Scholar]







