Polyphenolic characterization and evaluation of multimode antioxidant, cytotoxic, biocompatibility and antimicrobial potential of selected ethno-medicinal plant extracts
⁎Corresponding authors at: College of Pharmacy, Gachon University, No. 191, Hambakmoero, Yeonsu-gu, Incheon 21936, Republic of Korea. amnaparvin@gachon.ac.kr (Parveen Amna), hfchughtai@qau.edu.pk (Humaira Fatima)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Introduction
Scientific evidence about biological profile of natural products can support their traditional uses. The current work was aimed to assess phytochemical and biological profile of nine medicinal plants collected from Herbalists.
Methods
Extracts prepared in different solvents were subjected to phytochemical, antioxidant, enzyme inhibitory, cytotoxic, and antimicrobial activities. Reverse phase-high performance liquid chromatography (RP-HPLC) analysis was performed for the quantification of polyphenols.
Results
Results showed methanol extract (M) being potent as compared to others. Gentian lutea M showed maximum extract recovery (15.00 ± 0.11 % w/w) and TFC (30.82 ± 0.21 μg QE/mg extract). Nigella sativa M displayed highest TPC (44.99 ± 0.43 μg GAE/mg extract) and TAC (334.72 ± 0.35 μg AAE/ mg extract). Results showed noteworthy quantities of vanillic acid, rutin, kaempferol, emodin in ethyl acetate (EA) and methanol (M) extracts of plants assessed by RP-HPLC. Gentisic acid was highest (11.75 µg/mg extract) in T. arjuna M extract. Similarly, maximum %FRSA (82.28 ± 0.03 %) and TRP (160.40 ± 0.38 μg AAE/ mg extract) were depicted by Terminalia chebula and Chamomilla recutita, respectively. Moreover, Mentha longifolia and G. lutea M demonstrated noteworthy (p < 0.05) antibacterial activity against Staphylococcus aureus (14 ± 0.7 mm) and Klebsiella pneumoniae (12 ± 0.3 mm), respectively. Curcuma amada, C. recutita, Murraya koenigii and G. lutea M had significant α-glucosidase activity. Another good solvent for extraction was ethyl acetate (EA), whose extracts were secondary to methanol in producing significant biological profile. For example, EA of N. sativa (TPC: 1.46 ± 0.45 µg GAE/ mg extract), G. lutea (TRP: 160.33 ± 0.52 μg AAE/mg extract: ZOI of 12 ± 0.5 mm in K. pneumoniae) and Mormodica charantia (α-amylase inhibition: 39.5 ± 0.10 %) showed significant bioactivities. All extracts displayed mild antifungal protein kinase inhibition activities and were significantly (greater than80 %: p < 0.05) cytotoxic to brine shrimps with negligible hemolytic activity.
Conclusion
Briefly, variable polarity solvent extracts of studied plants will be processed for isolation of antioxidant, cytotoxic, carbohydrate enzyme inhibitory and antibacterial compounds.
Keywords
Natural products
Phytoconstituents
Antioxidant
Antibacterial
Antidiabetic potential
Cytotoxic activity
- EA
-
Ethyl acetate
- M
-
Methanol
- A
-
Aqueous
- TPC
-
Total phenolic contents
- TFC
-
Total flavonoid contents
- GAE
-
Gallic acid equivalent
- QE
-
Quercetin equivalent
- %FRSA
-
Free radical scavenging activity
- DPPH
-
2 2-Diphenyl-1-picrylhydrazyl
- TAC
-
Total antioxidant capacity
- AAE
-
Ascorbic acid equivalent
- TRP
-
Total reducing power
- DMSO
-
Dimethyl sulfoxide
- MIC
-
Minimum inhibitory concentration
- IC50
-
50% Inhibitory concentration
- LC50
-
Lethal concentration causing 50% mortality
- ZOI
-
Zone of inhibition
- BZ
-
Bald zone
- CZ
-
Clear zone
Abbreviations
1 Introduction
The current world is plagued with plethora of diseases. These range from minor ailments like cough and flu to major diseases like life threatening infections and cancer. Although, multiple medicines are available to cater the needs of people; however, there are issues of efficacy, side effects and compliance. To furnish the medical needs, there is continuous research on new medicines either from synthetic or natural sources. The most replenishing source is the medicinal plants containing multitude of phytochemicals, which can be used as supplements, supportive remedies or as treatment of particular diseases (Wadood et al. 2013, Dias et al. 2012). Historically medicinal plants serve as the foundation of traditional herbal medicine. There are about 422,000 flowering plant species world-wide, out of which approximately 50,000 are considered as an integral part of traditional herbal medicine (Rashid et al. 2021). In developed countries, about 80 % of the world’s people are totally dependent on herbal drugs for primary healthcare needs, and more than 25 % of the prescribed medications are derived from wild plant species (Schultes and Hofmann 1992). A recent study showed that consumption of cruciferous vegetable can prevent colorectal cancer formation and reduce its incidence (Ağagündüz et al. 2022). Moreover, medicinal plants including Abelmoschus esculentus (Haque et al. 2022), Linum usitatissimum and Coriandrum sativum (Mechchate et al. 2021) are reported for their antihyperglycemic activities. Researchers have also demonstrated the role of Spirulina platensis and Ophiorrhiza rugose in the management of pain (Chy et al. 2021, Freitas et al. 2021). All these studies indicate the clinical importance of medicinal plants and natural products obtained from those. Therapeutic applications of natural products have always spiked the interest of researchers that facilitated the discovery process of innovative and effective compounds (Bibi et al. 2017).
Natural products are basically the phytochemicals that have variable physical, chemical and biological properties (Khan et al. 2019, Goyal et al. 2011). Chemically, phytochemicals include mixture of primary and secondary metabolites such as proteins, carbohydrates, alkaloids, glycosides, terpenoid, phenolics and flavonoids (Wadood et al. 2013). These have different physical natures when extracted, i.e., as gums, semisolids, crystals of alkaloids etc. Furthermore, these phytochemicals have reported significant biological properties including immunomodulatory, antioxidant, anti-inflammatory, enzyme stimulation/inhibition, antimicrobial, antiplatelet and hormonal regulating activities (Zohra et al. 2019). For instance, Küpeli Akkol et al., have reported the significance of coumarin polyphenols in cancer (Küpeli Akkol et al. 2020). polyphenols found in natural products exhibit free radical scavenging ability with inhibition of enzymes accountable for production of reactive oxygen species (ROS). Phenolic compounds have gained an attention as potent agents in controlling and treating oxidative stress related diseases including cardiovascular problems, inflammation, diabetes mellitus, cancer, and microbial diseases (Li et al. 2014). In vivo studies on polyphenols demonstrated that these exert their function by controlling plasma membranes permeability/integrity, acting as transcription factors and regulating enzymatic activities (Selby-Pham et al. 2017). Despite the significance of phytochemicals, it is always mandatory to evaluate or repurpose the properties of phytochemicals since these are obtained from various medicinal plants or from the same plant collected during different seasons. Thus, a battery of up-to-date isolation and phytochemical evaluation methods are utilized to provide a validated scientific data on bioactive natural products (Omotayo and Borokini 2012, Goyal et al. 2011).
Some of the important natural products have been obtained from Terminalia (T) arjuna, Momordica (M) charantia and Curcuma (C) amada. The M. charantia, commonly called bitter melon, has been reported with antioxidant, anti-inflammatory, hepatoprotective, antihyperglycemic, antibacterial, immunomodulatory, antiulcer, and antitumor activities that can be attributed to the presences of various phytochemicals like flavonoids, phenols, and terpenoids (Naqvi et al. 2020). T. arjuna (Combretaceae) is used as aphrodisiac, diuretic, expectorant, cardioprotective, tonic and as supplement in cancer patients (Javed et al. 2016). C. amada, commonly known as Mango ginger, possess curcuminoids, amadaldehyde, phenolic acids and amadannulen exhibiting anti-inflammatory, brine-shrimp lethality, analgesic and antiplatelet activities (Policegoudra et al. 2011).
Following these lines, this work was designed to assess the phytochemical parameters and biological potential of Mentha (M) longifolia, Gentian (G) lutea, Nigella (N) sativa, T. arjuna, Chamomilla (C) recutita, M. charantia, Murraya (M) koenigii, C. amada and Terminalia (T) chebula. We assessed the phytochemicals in different extracts prepared using solvents of variable polarities and evaluated the antimicrobial, cytotoxic, enzyme inhibition, blood biocompatibility and antioxidant activities of selected plants. This study is a good contribution to research in natural products and provides scientific evidence of some of the traditional uses of selected plants.
2 Methods
2.1 Plant collection and verification
Native plants M. longifolia, G. lutea, N. sativa, T. arjuna, C. recutita, M. charantia, M. koenigii, C. amada and T. chebula (Table 1) were collected from local herbalists of Islamabad, Pakistan. All plants were verified by Prof. Dr. Rizwana Aleem Qureshi, Department of Plant Sciences, Faculty of Biological Sciences, Quaid-i-Azam University Islamabad, Pakistan. Specified herbarium number was assigned to all plants including M. longifolia (PHM-527), G. lutea (PHM-528), N. sativa (PHM-529), T. arjuna (PHM-530), C. recutita (PHM-531), M. charantia (PHM-532), M. koenigii (PHM-533), C. amada (PHM-534) and T. chebula (PHM-535). Shade dried specimen of subject plants were archived within Herbarium of Medicinal Plants, Quaid-i-Azam University, Islamabad.
| Botanical name | Local name | Part used | Family | Ethno medicinal uses | References |
|---|---|---|---|---|---|
| Mentha longifolia | Mint, podina | Aerial parts | Lamiaceae | Carminative, indigestion, wound healing, anti-microbial, anti-inflammatory, stomachache, antimutagenic, anti-arthritis, carminative and colitis. | (Farzaei et al., 2017) |
| Gentian lutea | Pakhan peid | Roots | Gentianaceae | Effective for stomach, anti-inflammatory, wound healing, gastritis and antioxidant. | (Niiho et al., 2006, Mathew et al., 2004) |
| Nigella sativa | Kalonji | Seeds | Ranunculaceae | Inflammation, gastro disorders, cancers, antioxidant properties, analgesic, anti-diarrheal, antimicrobial, cardiovascular disorders, anticarcinogenic and antidiabetic properties. | (Ahmad et al., 2013, Majeed et al., 2021) |
| Terminalia arjuna | Arjun | Bark | Combretaceae | Anti-ulcerative and anti H.pylori effect, antioxidant, anti-inflammatory, immunomodulatory, Anticarcinogenic, antimutagenic potential, antiatherogenic and cardiovascular activities. | (Javed et al. 2016) |
| Chamomilla recutita | Gul-e- baboon | Flower | Asteraceae | Active against H. pylori, analgesic, hepato protective, GIT disorders, anti-inflammatory, carminative, antispasmodic properties, wound-healing, and antimicrobial effects. | (Singh et al., 2011, Zadeh et al., 2014) |
| Mormodica charantia | Kareela |
Fruit | Cucurbitaceae | Antimalarial, antidiabetic, pneumonia, psoriasis, cancer, infectious diseases, asthma, ulcers, bronchitis, anti-neoplastic properties, cholera, anemia and gout. | (Naqvi et al. 2020) |
| Murraya koenigii | Curry patta, Mitha Neem | Leaves | Rutaceae | Antidiabetic, anti-cancer, antioxidant, stomachic, carminative, antihelminthic, analgesic, antimicrobial activity, blood purifier, antidepressant, antidysenteric and antidiarrheal |
(Handral et al., 2012, Saini and Reddy, 2015) |
| Curcuma amada | Amba haldi, mango ginger | Rhizome | Zingiberaceae | Inhibition of H.pylori growth, antioxidant, antimicrobial, hypoglycemic and cyto-toxic activity | (Policegoudra et al. 2011) |
| Terminalia chebula | Hareer | Fruit | Combretaceae | Antimicrobial (Anti H. pylori), antidiabetic, antiretroviral, antioxidant, antiparasitic, antitumour, diuretics, antitussive and wound healer | (Das et al., 2020, Nigam et al., 2020) |
2.2 Chemicals and solvents
Ethyl acetate, Methanol and Distilled water (analyrical grade) were purchased from Merck (Darmstadt, Germany). Folin–Ciocalteu reagent, Alpha amylase, Alpha gucosidase, and 2, 2-diphenyl, 1-picrylhydrazine (DPPH) were obtained from Sigma–Aldrich (Steinheim, Germany). All other chemicals were were procured from Merck (Darmstadt, Germany), unless the source is specified.
Ethical statement
The experiments where human blood was required, was performed after approval from Ethical Committee of Quaid-i-Azam University (No. BEC-FBS-QAU2021-259) and in consensus with the WHO guidelines for drawing blood (2010). Informed consent was taken from all individuals who volunteered for blood sampling.
2.4 Crude extract preparation
Selected plants were rinsed, dried in shade (37 °C; 3 weeks), powdered with commercial miller and stored in a sealed container until use. Accurately weighed plant powder (750 g) was soaked in 1500 ml analytical grade solvent using Erlenmeyer flask at room temperature (20–25 °C) for 72 h and extracted by maceration with frequent sonication at the temperature 25 °C (frequency 25 kHz; two cycles; 30 min) for 24 h. The Marc was filtered, filtrates concentrated (rotary evaporator; Buchi, Switzerland), vacuum dried (Yamato, Japan) at 45 °C and stored at − 20 °C for use.
2.5 Estimation of extraction yield
The percent extract recovery of dried extracts was estimated using the following formula.
Where, X is the total weight of dried extract and Y is total weight of plant powder used for extraction.
2.6 Phytochemical investigation
2.6.1 Folin–Ciocalteu method for total phenolic content estimation (TPC)
Folin–Ciocalteu reagent was used for estimating the total phenolic content following reported protocols (Bray et al. 1952). Concisely, individual extracts (20 μl; 4 mg/ml DMSO) and Folin–Ciocalteu reagent (90 μl) were incubated for 5 min in a 96 well microtiter plate and subsequently mixed with sodium carbonate (90 μl; 6 % w/v). Absorbance values were measured at 630 nm with Elx 800 microplate reader (Biotech USA). Calibration curve was prepared using gallic acid (3.125–50 μg/ml) as internal control (y = 0.0282x + 0.0189, R2 = 0.9972) (Ul-Haq et al. 2012). The assay was done in triplicate, and outcomes are stated as μg gallic acid equivalent per mg of dry weight extract (µg GAE/mg extract).
2.6.2 Total flavonoid content (TFC) measurement via aluminium chloride
Colorimetric analysis by means of aluminum chloride was conducted to determine total flavonoid content according to previously described protocols (Phull et al. 2021). Briefly, crude extract (20 μl; 4 mg/ml DMSO), potassium acetate (1.0 M; 10 μl), aluminum chloride (10 %; 10 μl) and distilled water (160 μl) were combined for 30 min at 37 °C in 96 well plate. Using microplate reader (Elx 800; Biotech USA), the reading was taken at 415 nm and calibration curve (y = 0.0329x + 0.0369; R2 = 0.9954; p < 0.05) was drawn using twofold dilutions of quercetin (2.5–40 μg/ml). After testing the extracts in triplicate, the results of flavonoid content are reported in µg quercetin equivalent per mg of dry weight extract (µg QE/mg extract).
2.7 Reverse-phase high performance liquid chromatography (RP-HPLC) of polyphenols
Polyphenol constituents in the crude extracts were quantified by RP-HPLC as reported previously (Ullah et al. 2014a, Nasir et al. 2017) using Agilent Chem Station and Zorbex C8 analytical column (4.6 × 250 nm and 5 µm particle size) equipped with DAD detector (Agilent technologies, Germany). Mobile phases A & B contained acetonitrile: methanol: water: acetic acid in ratios of 5:10:85:1 and 40:60:0:1, respectively. Mobile phases were eluted through the column at the flow rate of 1 ml/min. The mobiles phase was eluted at different gradients. The gradient of mobile phase changed over a period ot 30 min where conentration of mobile phase A declined from 100 to 0 % while mobile phase B was increased from 0 to 100 %. The gradient was run as follows in terms of mobile phase B: Initially for 0–20 min at 0–50 % B, then for 20–25 min at 50–100 % B and lastly for 25–30 min at isocratic 100 % B. Extracts (20 μl; 10 mg/ml) were filtered using 0.45-µm membrane filters and injected into the column with 10 min reconditioning phase between two samples. Methanol was used to recondition the columns. Phenolic standards including emodin, vanillic acid, myricetin, syringic acid, rutin, catechin, coumaric acid, kaempferol and gentisic acid were prepared in concentrations of 10, 20, 50, 100, 200 μg/ml in methanol. The UV absorption spectra of samples was recorded at 368 nm (myricetin and kaempferol), 325 nm (gentisic acid), 279 nm (syringic acid, coumaric acid, emodin and catechin) and 257 nm (rutin and vanillic acid). Polyphenols were quantified as μg/mg of sample from the calibration curve. The limits of detection and quantification were calculated by formula 3.3 × (σ/b) and 10 × (σ/b), respectively. Here, σ and b stand for the standard deviation of response and slope of the calibration curve, respectively.
2.8 Biological assessment
2.8.1 Free radical scavenging activity (%FRSA)
Scavenging action of the extracts was evaluated by measuring its ability to quench the stable DPPH radical (Fatima et al. 2015). The capacity of extracts (4 mg/ml DMSO; 7.406–200 μg/ml) to discolor (37 °C for 30 min) purple colored DPPH (0.092 mg/ml methanol) was measured at 517 nm using microplate reader (Elx 800; Biotech USA) to quantify the percent free radical scavenging activity (%FRSA) by following formula.
Where, Xs is the sample absorbance and Xc is the absorbance of the negative control.
Ascorbic acid was utilized as a positive control and IC50 values were calculated for each extract by GraphPad Prism 9 software.
2.8.2 Ammonium molybdate reduction capacity of extracts
Total antioxidant capacity (TAC) of all extracts was evaluated by measuring the reducing capacity of ammonium molybedate. The absorbance of a mixture of extract or ascorbic acid (100 µg/ml) or DMSO (0.1 ml; 4 mg/ml of DMSO) and reagent (900 µl, H2SO4 0.6 M, sodium phosphate 28 mM, ammonium molybdate 4 mM) stabilized at 95 °C for 90 min. (Ul-Haq et al. 2012) was measured at 695 nm. The mixture was stabilized at 95 °C for 90 min. After cooling, absorbance was measured at 695 nm using Agilent Technologies 8354 spectrophotometer (Germany) was used for triplicate analysis and all the results were calculated as μg of ascorbic acid equivalent per mg of dry weight extract (μg AAE/mg extract) (Phull et al. 2021).
2.8.3 Determination of total reducing power by potassium ferricyanide colorimetric analysis (TRP)
TRP of extracts was estimated following reported method (Zahra et al. 2017). Briefly, the extract (200 μl; 4 mg/ml DMSO; n = 3) was incubated for 20 min with phosphate buffer (400 μl; 0.2 M; pH 6.6) and potassium ferricyanide (500 µl; 1 % w/v) at 50 °C. After centrifuging (3000 rpm for 10 min) with 10 % trichloroacetic acid (400 μl), the supernatant (500 μl) was combined with o ferric chloride (100 μl; 0.1 %) and distilled water (500 μl). The absorbance of all samples was recorded at 700 nm. DMSO and ascorbic acid replaced extracts as negative and positive controls, respectively. TRP of the extracts was presented as mg of ascorbic acid equivalent per mg of dry weight extract (mg AAE/mg extract).
2.8.4 Inhibition of carbohydrate metabolizing enzymes
2.8.4.1 α-Amylase blocking activity
The extracts analyzed for α-amylase blocking activity followed previously reported procedure (Fatima et al. 2022b). In each well of 96 well plate, a mixture of enzyme α-amylase (0.14 U/ml; 25 μl), extract (10 μl; 4 mg/ml DMSO), phosphate buffer (15 μl; pH 6.8) and freshly prepared starch solution (40 μl; 2 mg/ml) was stabilized at 50 °C for 30 min. To stop the further reaction, 1 M HCl (20 μl) and iodine reagent (5 mM) were added. Positive and negative control wells had acarbose and DMSO replacing the extract, respectively, while in the blank well both enzyme and extract were replaced with an equal volume of phosphate buffer. After incubation, the absorbance was read at 540 nm ((Elx 800 microplate reader; Biotech USA) and the activity was stated as percent inhibition of α-amylase per mg of dry weight of extract.
Enzyme inhibitory potential was estimated by the following equation:
Where, Xs is the absorbance of test extracts, Xb is absorbance of blank well and Xn is absorbance of negative control.
2.8.4.2 α-Glucosidase blocking activity
Procedure describe by Kim et al. (2000) was used to assess the α-glucosidase inhibition potential of the extracts (Kim et al. 2000). Substrate solution of p-nitrophenyl-d-glucopyranose (PNG; 25 µl; 20 mM) was added in 96 well plate already filled with phosphate buffer (69 µl; 50 mM). Afterwards, 1 µl of enzyme (3 units/ml) and 5 µl of extract (4 mg/ml DMSO) were mixed and an initial reading was obtained at 405 nm with microplate reader ((Elx 800; Biotech USA). After incubating samples at 37 °C for 30 min, the reaction was stopped using 100 µl of sodium bicarbonate (0.2 mM) and absorbance was again measured at 405 nm. Acarbose worked as a positive control and in blank both enzyme and extract were replaced with phosphate buffer. Percent of inhibition for α-glucosidase action was analyzed using the formula given below:
Xs = sample absorbance, Xb = blank absorbance and Xn = negative control absorbance. The test was carried out in triplicate.
2.8.5 Cytotoxicity assays
2.8.5.1 Brine shrimp lethality assay
Brine shrimps (Artemia salina) analysis is an easy tool to appraise the cytotoxic effect of crude extracts (Ahmed et al. 2017). Briefly, eggs of A. salina (Ocean star, USA) were bred in artificial sea water (38 g/l plus yeast 6 mg/l) over a period of 2448 h in bi-compartment plastic plate ensuring required illumination, oxygen supply and a temperature of 30–32 °C. The hatched nauplii were collected by a Pasteur pipette in a beaker having sea salt water. In a 96 well plate, 10 nauplii along with 150 µl of sea salt water were added. Sequential dilution (200, 100, 50 and 25 µg/ml; n = 3) of extracts (DMSO < 1 %) were added in nauplii containing well and filled to maximum of 300 µl with ocean water. Doxorubicin (4 mg/ml stock; final concentrations: 10, 5, 2.5 and 1.25 μg/ml; n = 3) and DMSO (1 %) worked as positive and negative controls, respectively. Then, after incubating for 24 h at 37 °C, the extent of nauplii death was assessed by counting the number of surviving nauplii under an inverted microscope. Median lethal concentration (LC50; n = 3) of studied extracts was evaluated using GraphPad Prism 9 software.
2.8.5.2 Protein kinase inhibition assay
The protein kinase inhibition test was conducted in triplicate by discerning the effect of extracts on hyphae growth of Streptomyces 85E strain (Yao et al. 2011). Fresh Streptomyces 85E spores were dispered on sterile petri plates contaning ISP4 medium. Sterile filter paper discs (6 mm) were soaked with (5 μl; 20 mg/ml of DMSO; 100 μg/disc) individual extracts and positioned on the plates implanted with Streptomyces 85E. An aliquote of 5 μl of DMSO and Surfactin (4 mg/ml of DMSO; 20 µg/disc) permeated discs worked as negative and positive controls, respectively. Later, sample plates were keep warm at 30 °C for 3 days and the results were recorded as bald (BZ) and clear zones (CZ) of inhibition surrounding the test sample and control discs.
2.8.6 Biocompatibility evaluation
2.8.6.1 Hemolytic assay
Hemolytic assay assessed the biocompatibility of selected plants extract with physiological system by following the procedure described by Nasar et al (2019) (Qasim Nasar et al. 2019). An aliquot of 1 ml human blood was centrifuged (14,000 rpm) to isolate RBCs. After discarding plasma, 200 µl of pellet was re-suspended in phosphate buffer saline (PBS; 9.8 ml), centrifuged (2000 rpm; 10 min) and washed thrice (PBS). Later, erythrocyte suspension (100 µl) was mixed with test extracts (200 µl; 200, 100, 50, 25 μg/ml) in PBS and incubated for 1 h at 35 °C in 96 well plate followed by centrifugation (1000 rpm) for 10 min. Absorbance of the supernatant (200 µl; 96 well plate) was recorded at 450 nm by microplate reader. Triton X-100 (200 µl; 0.5 %) and PBS (200 µl; 1X) were the internal controls. Following formula was applied to calculate percent hemolysis.
2.8.7 Antimicrobial screening
2.8.7.1 Antibacterial assay
Antibacterial activity of selected plant extracts was tested against non-resistent bactrail strains by agar disc diffusion method (Madiha et al. 2016). Gram positive bactria including Staphylococcus (S) aureus ATCC-6538 and Bacillus (B) subtilis ATCC-6633 and gram negative bacteria ncluding Escherichia (E) coli ATCC-25922, Klebsiella (K) pneumoniae ATCC-1705 and Pseudomonas (P) aeruginosa ATCC- 15,442 were used in this assay. Filter discs permeated with extract (sterile; 5 μl; 20 mg/ml DMSO), Ciprofloxacin, Cefixime (positive control; 4 mg/ml DMSO; 5 μl) or DMSO (negative control; 1 %; 5 μl) were positioned on fresh bacterial lawn (seeding density 1 × 106 CFU/ml) on nutrient agar. After the incubation for 24 h at 37 °C, the average diameter of zone of inhibition (ZOI) around each sample disc was recorded. Extracts creating ZOI ≥ 12 mm were taken as active and subjected to minimum inhibitory concetration (MIC) determination using twofold microbroth dilution method (Kaushik and Goyal 2008). Serial dilutions of extracts (12.5–100 μg /ml) in Mueller Hinton broth (Thermo Fischer Scientific, US) were incubated with individual bacterial strains (5 × 104 CFU/ml) in 96 well plate. After overnight incubation (37 °C), the MIC was calculated by determining the optical desnsity (OD) at 600 nm. The experiment was executed thrice.
2.8.7.2 Antifungal assay
Antifungal effect of the extracts was assessed in triplicate by agar disc diffusion method (Nasir et al. 2017). Fungal spores of the selected strains of Aspergillus (A) niger (FCBP-0198), Aspergillus (A) fumigatus (FCBP-66), Aspergillus (A) flavus (FCBP-0064), Fusarium (F) solani (FCBP-0253) and Mucor (M) species (FCBP-0300) were dispersed in Tween-20 solution (0.02 %) and concentration was matched with McFarland 0.5 turbidity. After swabing 100 μl of individual strains on Sabouraud Dextrose Agar plates, sterie filter paper discs (60 mm) soaked with extracts (5 μl; 20 mg/ml DMSO) were positioned on the petri plates. DMSO and Terbinafine permeated discs served as internal controls. Subsequently incubating for 24–48 h at 28 °C, ZOI around the discs was measurd. MIC was determind for extracts with ZOI ≥ 10 mm in diameter using 3.12–50 μg/disc cocentraions (Nasir et al. 2017).
2.9 Statistical analysis
Results are presented as mean ± SD of respective values in triplicate. The resuls are analyzed by one way ANOVA using PASW Statistics 18 and GraphPad Prism 9 at significane values of p < 0.05, p < 0.01, or p < 0.001.
3 Results
3.1 Extraction yield versus extraction solvents
It is imperative to analyze the extractive yields of medicinal plants to ensure the efficacy of extraction process and the solvent system used (Celeghini et al. 2001). The results showed variable exaction yields with different solvents as well as with the same solvent system (Fig. 1). The maximum extraction yield was exhibited by methanol (M) extract of G. lutea (15.03 ± 0.42 % w/w). It was followed by aqueous (A) extracts of C. recutita and M. charantia, and ethyle acetate (EA) extract of G. lutea with percentage recovery of 9.68 ± 0.52, 8.06 ± 0.30 and 7.266 ± 0.38 % w/w, respectively. Conversely, the M. koenigii EA extract was recovered in least amount with 1.94 ± 0.22 % w/w of extraction yield.
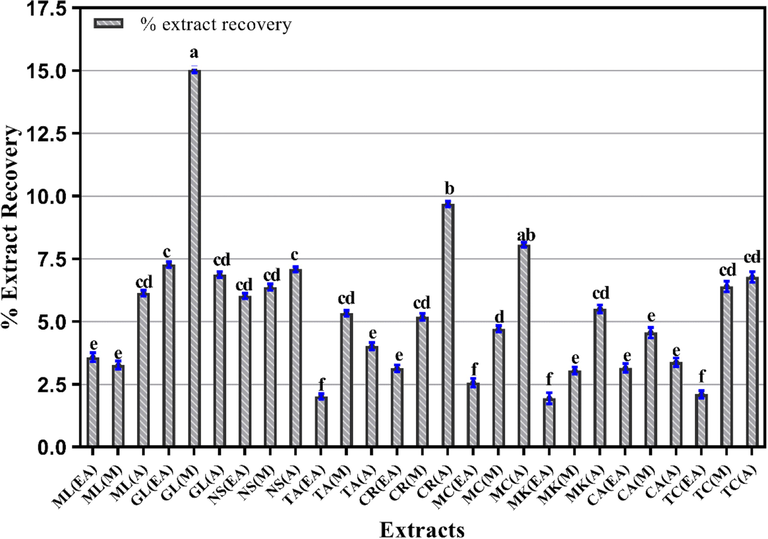
- Percent extract recovery of plant extracts using various solvent for extraction. Columns with different superscript (a-f) letters show significantly (P < 0.05) different means. EA. Ethyl acetate; M. Methanol and A. Aqueous extracts.
3.2 Phytochemical investigation
Extracts prepared in different solvent may yield variable types or amounts of phytochemical. In general, polyphenolic compounds are considered essential for the biological profile of any medicinal plant. Phenolic compounds are the major class of secondary metabolites that have reported to possess strong correlation with antioxidant and anti-inflammatory activities of medicinal plants (Sarawong et al. 2014). Hence, phenolic compounds in all plant extracts were estimated in vitro in triplicate and calculated from the calibration curve of the respective standards.
3.2.1 Folin–Ciocalteu method for TPC
TPC analysis of all extracts showed that the phenolic compounds ranged from 44.99 ± 0.43 to 17.63 ± 0.32 μg GAE/mg extract (Fig. 2). The highest TPC was shown by N. sativa M (extract with value of 44.99 ± 0.43 μg GAE/mg extract (p < 0.05). Likewise, M extracts of T. chebula, C. amada and G. lutea demonstrated 42.20 ± 0.23, 41.42 ± 0.12 and 40.68 ± 0.45 μg GAE/mg extract, respectively of TPC. The EA extracts of N. sativa and M. longifolia showed 41.46 ± 0.45 and 28.23 ± 0.53 µg GAE/mg extract of TPC, respectively. The value gradually declined in A extracts of N. sativa (33.80 ± 0.12 µg GAE/mg extract) and T. chebula (29.37 ± 0.17 µg GAE/mg extract), whereas A extract of M. longifolia exhibited the least quantity of TPC (17.63 ± 0.32 µg GAE/mg extract. Overall, the comparative decline in TPC of plant extracts was in the following order: M > EA > A (Fig. 2).
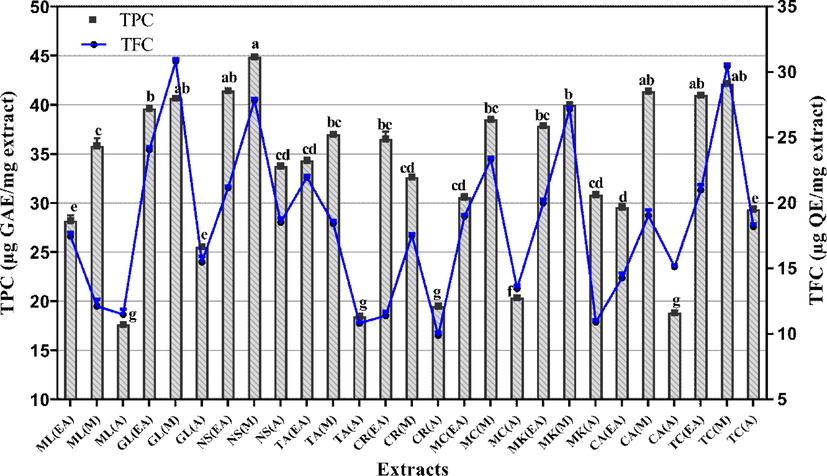
- Graphical presentation of total phenolic and total flavonoid content of all tested extracts. Values are accessible as mean ± standard deviation from the triplicate analysis. Columns with different superscript (a-g) letters show significantly (P < 0.05) different means. TPC. total phenolic content (μg GAE/mg extract); TFC. total flavonoid content (μg QE/mg extract); EA. Ethyl acetate; M. Methanol and A. Aqueous extracts.
3.2.2 TFC measurement via aluminium chloride
Next, the assessment of TFC assay in all extracts demonstrated that M extracts of G. lutea (30.82 ± 0.21 μg QE/mg extract) and T. chebula (30.46 ± 0.17 μg QE/mg extract) showed the maximum value of TFC (p < 0.05). Among the EA extracts, G. lutea depicted significant TFC with value of 24.08 ± 0.23 μg QE/mg extract. On the contrary, the A extracts of N. sativa and C. recutita showed 18.54 ± 0.33 and 9.91 ± 0.23 μg QE/mg extract of TFC, respectively where C. recutita had the least value. Overall, the A extracts showed less amount of TFC as compared with EA and M extracts.
3.3 Quantification of polyphenols
It is imperative to know about the concentrations of particular phytochemical in the extracts in order to relate the bioactivity profile. Moreover, the amount of phytochemical may directly relate with the extent of bioactivity and may represent the potency of that particular extract. Thus, polyphenols in test extracts were quantified by RP-HPLC procedure by comparing UV and retention time of 09 reference standards with extracts (Table 2). Results of HPLC-DAD analysis (Table 3) showed significant quantities of emodin, gentisic acid, kaempferol, myricetin and rutin within analyzed extracts. Maximum amount of emodin was expressed in A and M extracts of G. lutea (74.43 and 29.29 μg/mg, respectively), whereas T. arjuna (M) exhibited maximum gentisic acid (11.75 μg/mg). A substantial quantity of kaempferol was also seen in EA and M extracts of T. arjuna with values of 8.39 and 4.94 μg/mg, respectively. M. koenigii A and T. arjuna M extracts have 4.57 and 4.23 μg/mg, respectively of myricetin. Chromatograms of detected phenols and standards are presented in Fig. 3 (a-f). This indicates that variable polarity solvents affect the extracted quantities of polyphenols from each plant.
| Sr. No. | Polyphenols | Wavelength (nm) | Retention time | Limit of detection (µg/ml) | Limit of quantification (µg/ml) |
|---|---|---|---|---|---|
| 1 | Vanillic acid | 257 | 9.714 | 0.0091 | 0.0277 |
| 2 | Rutin | 257 | 13.233 | 0.048 | 0.145 |
| 3 | Catechin | 279 | 7.463 | 0.066 | 0.200 |
| 4 | Syringic acid | 279 | 10.1 | 0.008 | 0.024 |
| 5 | Coumaric acid | 279 | 14.907 | 0.0025 | 0.0075 |
| 6 | Emodin | 279 | 29.125 | 0.080 | 0.242 |
| 7 | Gentisic acid | 325 | 8.233 | 0.0973 | 0.294 |
| 8 | Myricetin | 368 | 15.639 | 0.015 | 0.045 |
| 9 | Kaempferol | 368 | 21.387 | 0.038 | 0.115 |
| Plant name | Extract code | Vanillic acid | Rutin | Catechin | Syringic acid | Coumaric acid | Emodin | Gentisic acid | Myricetin | Kaempferol |
|---|---|---|---|---|---|---|---|---|---|---|
|
M. longifolia |
ML(EA) | 1.89 | Nd | 0.23 | 0.38 | 0.10 | Nd | Nd | 0.31 | 2.66 |
| ML(M) | 0.10 | 1.30 | Nd | 0.03 | Nd | 7.31 | Nd | 1.32 | 0.42 | |
| ML(A) | 0.10 | 0.40 | Nd | Nd | Nd | Nd | Nd | Nd | 1.28 | |
|
G. lutea |
GL(EA) | 1.43 | 1.09 | Nd | Nd | Nd | 4.69 | Nd | Nd | Nd |
| GL(M) | Nd | 1.54 | Nd | Nd | Nd | 29.29 | Nd | Nd | Nd | |
| GL(A) | Nd | 0.53 | Nd | 0.11 | Nd | 74.43 | Nd | Nd | 0.17 | |
|
N. sativa |
NS(EA) | Nd | 1.82 | 0.32 | 0.46 | 0.01 | Nd | Nd | Nd | 2.55 |
| NS(M) | 0.46 | Nd | Nd | 0.15 | 0.01 | 1.77 | Nd | 0.06 | 0.83 | |
| NS(A) | 0.45 | Nd | Nd | 0.13 | Nd | Nd | Nd | Nd | Nd | |
|
T. arjuna |
TA(EA) | 0.24 | 1.66 | Nd | 0.02 | 0.23 | 0.83 | Nd | Nd | 8.39 |
| TA(M) | 0.74 | Nd | 1.11 | 0.70 | 0.07 | Nd | 11.75 | 4.23 | 4.94 | |
| TA(A) | 0.10 | 1.30 | Nd | 0.03 | Nd | 7.31 | Nd | 1.32 | 0.42 | |
| C. recutita |
CR(EA) | 0.57 | 0.61 | Nd | Nd | Nd | 5.97 | Nd | 0.00 | 0.76 |
| CR(M) | Nd | 2.57 | Nd | 0.04 | Nd | 12.96 | Nd | Nd | Nd | |
| CR(A) | Nd | 2.74 | Nd | Nd | Nd | 12.80 | Nd | Nd | Nd | |
|
M. charantia |
MC(EA) | 0.90 | 0.24 | 2.24 | 0.04 | 0.02 | Nd | 3.52 | 0.63 | Nd |
| MC(M) | 0.23 | 4.63 | 1.29 | Nd | 0.11 | Nd | Nd | 2.86 | Nd | |
| MC(A) | 0.87 | 2.30 | 0.30 | 0.07 | 0.01 | Nd | 4.89 | Nd | Nd | |
|
M. koenigii |
MK(EA) | Nd | 1.46 | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| MK(M) | 0.45 | Nd | Nd | 0.13 | Nd | Nd | Nd | Nd | Nd | |
| MK(A) | 1.23 | 0.69 | 1.21 | 0.08 | 0.61 | Nd | Nd | 4.57 | Nd | |
|
C. amada |
CA(EA) | 1.49 | 1.35 | Nd | 0.52 | 0.06 | 1.72 | Nd | Nd | Nd |
| CA(M) | Nd | 2.69 | 0.87 | 0.07 | Nd | Nd | Nd | 0.34 | Nd | |
| CA(A) | 1.396 | 6.25 | 13.47 | 1.12 | Nd | 0.28 | 3.88 | 0.30 | 0.99 | |
|
T. chebula |
TC(EA) | 0.771 | Nd | 0.23 | Nd | Nd | Nd | Nd | 0.31 | 2.66 |
| TC(M) | 0.105 | 1.30 | Nd | 0.36 | Nd | 7.31 | Nd | Nd | Nd | |
| TC(A) | 0.469 | Nd | Nd | 0.15 | Nd | 1.77 | Nd | 0.06 | 0.83 |
Mentha longifolia (ML), Gentian lutea (GL), Nigella sativa (NS), Terminalia arjuna (TA), Chamomilla recutita (CR), Momordica charantia (MC), Murraya koenigii (MK), Curcuma amada (CA), Terminalia chebula (TC). Nd means not detected. M = methanol; EA = ethyl acetate; A = aqueous.
![Chromatograms of polyphenols. The figure represents chromatograms obtained from reverse-phase high performance liquid chromatography analysis for [a] polyphenolic standards, [b] Curcuma amada aqueous extract, [c] Nigella sativa ethyl acetate extract, [d] Terminalia arjuna methanol extract, [e] Murraya koenigii aqueous extract and [f] Mentha longifolia methanol extract.](/content/184/2023/16/2/img/10.1016_j.arabjc.2022.104474-fig3.png)
- Chromatograms of polyphenols. The figure represents chromatograms obtained from reverse-phase high performance liquid chromatography analysis for [a] polyphenolic standards, [b] Curcuma amada aqueous extract, [c] Nigella sativa ethyl acetate extract, [d] Terminalia arjuna methanol extract, [e] Murraya koenigii aqueous extract and [f] Mentha longifolia methanol extract.
3.4 Biological activities
Extracts prepared in different extraction solvents may present variable bioactivity profile owning to the presence of particular phytochemicals. Since, the extracts showed significant presence of polyphenols and flavonoids; therefore, their bioactivities were assessed to identify the best extract and extraction solvent for further isolation of active compounds.
3.4.1 FRSA evaluation
The capability of crude extracts to neutralize DPPH radical is representative of their antioxidant capacity (Fig. 4). The maximum %FRSA was exhibited by M extracts of T. chebula (82.28 ± 0.03 %), N. sativa (81.65 ± 0.09 %), M. charantia (80.83 ± 0.02 %), C. amada (78.65 ± 0.23 %), M. koenigii (76.38 ± 0.02 %) and C. recutita (75.02 ± 0.05 %) where M. koenigii M extract had the least IC50 value (20.87 ± 0.12 µg/ml; p < 0.05). Among other extracts, G. lutea EA extract had maximum %FRSA (66.39 ± 0.11 %), whereas, M. charantia A extract showed least %FRSA (24.61 ± 0.15 %).
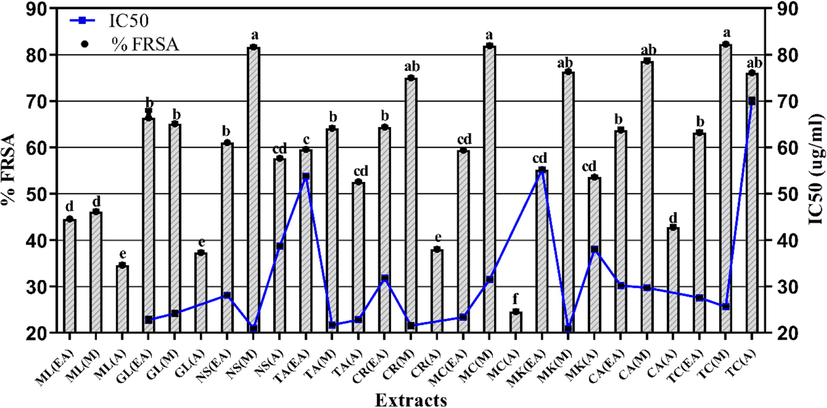
- Graphical presentation of free radical scavenging activity of tested extracts. Values are presented as mean ± standard deviation from triplicate investigation. The columns with superscript (a-f) letters displayed significantly (P < 0.05) different means. %FRSA. percent free radical scavenging activity; EA. Ethyl acetate; M. Methanol and A. Aqueous extracts.
3.4.2 TAC determination by ammonium molybdate reduction capacity
Subsequent evaluation of antioxidant activity by TAC test demonstrated that N. sativa M extract has the highest TAC with a value of 334.72 ± 0.35 μg AAE/mg extract (Fig. 5). Significant (p < 0.05) antioxidant capacities were shown by M. koenigii M and C. amada M extracts with values of 307.17 ± 0.75 and 256.48 ± 0.44 μg AAE/mg extract, respectively. The A extracts of M. charantia (71.52 ± 0.28 μg AAE/mg extract) and C. recutita (73.81 ± 0.77 μg AAE/mg extract) exhibited minimum TAC. Overall, the trend in TAC values declined in following way: M > EA > A.
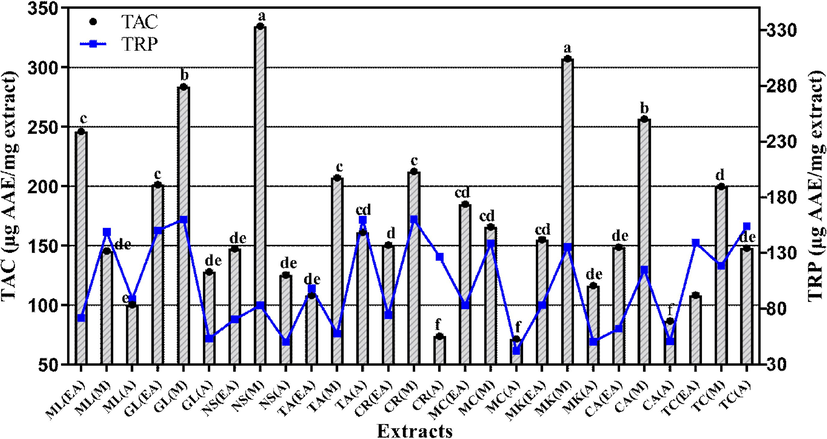
- Total antioxidant capacity and total reducing power of the selected plant extract. Values are presented as mean ± Standard deviation (n = 3) and columns with different superscript (a-f) letters show significantly (P < 0.05) different means. TAC. total antioxidant capacity; TRP. total reducing power; EA. Ethyl acetate; M. Methanol and A. Aqueous extracts.
3.4.3 Evaluation of TRP by potassium ferricyanide colorimetric analysis
Assessment of TRP as another method for antioxidant activity evaluation confirmed the results obtained by previous two assays. TRP analysis showed that maximum reducing power was exhibited by C. recutita M and G. lutea EA extracts with the values of 160.40 ± 0.38 and 160.33 ± 0.52 μg AAE/mg extract, respectively (p < 0.05; Fig. 5). Similarly, T. arjuna A extract (159.94 ± 0.53 μg AAE/mg extract) and G. lutea M extract (159.36 ± 0.12 µg AAE/mg extract) demonstrated significant antioxidant activity. Least TRP value of 42.36 ± 0.29 μg AAE/mg extract was expressed by A extract of M. charantia.
3.4.4 Inhibition of carbohydrate metabolizing enzymes
Carbohydrate metabolizing enzymes like α-amylase and α-glucosidase break complex carbohydrates to simple sugars and aid in the intestinal absorption of dietary carbohydrates. Studies have shown that antioxidant polyphenols in plant extracts can inhibit carbohydrate metabolizing enzymes and have beneficial effects in reducing blood glucose levels (Jemaa et al. 2017, Kalita et al. 2018). Thus, we next assessed the inhibitory effect of our extracts on α-amylase and α-glucosidase enzymes to open a prospect for their potential antidiabetic profile.
3.4.4.1 α-Amylase blocking activity
Enzyme inhibition activity of all extract and acarbose showed significant variance from negative control (p < 0.05; Fig. 6). Acarbose, the positive control, inhibited α-amylase enzyme activity by 59.14 ± 1.7 %. Comparatively, most significant (p < 0.05) inhibition was displayed by EA and M extracts of M. charantia (39.5 ± 0.10 % and 29.6 ± 0.3 %). Rest of the sample showed moderate or negligible activity. The EA extract of N. sativa and C. amada exhibited the percent inhibition of 18.4 ± 0.1 % and 27.3 ± 0.5 %, respectively. Likewise, M extracts of M. koenigii and C. recutita showed mild to moderate α-amylase inhibition activity (25.4 ± 0.06 % and 24.9 ± 0.3 %, respectively). On the contrary, EA extracts of M. longifolia and G. lutea indicated least percent inhibition in this assay.
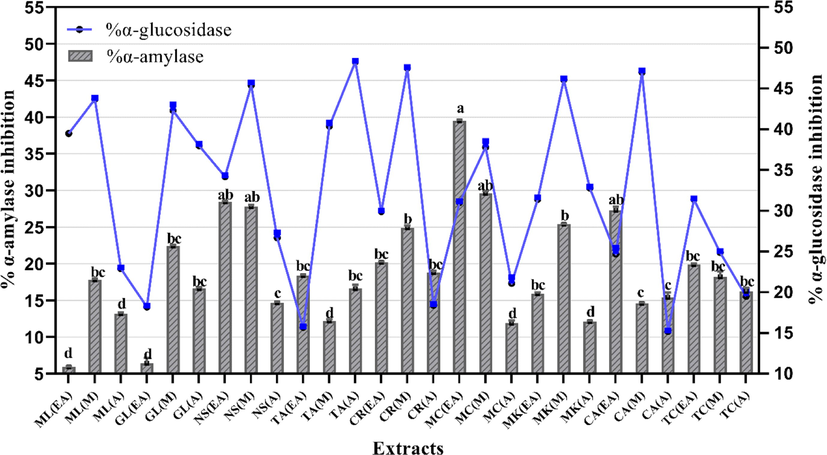
- Inhibition of carbohydrate metabolizing enzymes by selected plant extracts. Values are presented as mean ± Standard deviation (n = 3). The columns with different superscript (a-d) letters show significantly (P < 0.05) different means. EA. Ethyl acetate; M. Methanol and A. Aqueous extracts.
3.4.4.2 α-Glucosidase blocking activity
Next, another test was executed to calculate the α-glucosidase enzyme blocking potential of selected extract. Among all the samples, significant (p < 0.05) α-glucosidase inhibition was shown by M extracts of C. amada, C. recutita, M. koenigii and G. lutea with values of 47.0 ± 0.22 %, 47.5 ± 0.1 %, 46.1 ± 0.15 % and 42.3 ± 0.76 % inhibition, respectively (Fig. 6). Maximum inhibition was exhibited by A extract of T. arjuna (48.3 ± 0.12 % inhibition). A substantial activity was exhibited by EA extracts of T. chebula (31.4 ± 0.12 %) and G. lutea (38.0 ± 0.22 %). On the other hand, T. arjuna EA and C. amada A extracts showed least inhibition of α-glucosidase enzyme (Fig. 6).
3.4.5 Cytotoxicity assays
Assessment of in vitro toxicity of extract provides information about their safety as well as possibility of use as targeted therapy in cancer. Since, the extracts exhibited significant antioxidant activity; hence, their capacity to impede oxidative stress may aid in combating various diseases. Consequently, we used brine shrimp and protein kinase inhibition assays to depict the likelihood of extracts for further investigation in cancer models.
3.4.5.1 Brine shrimp lethality assay
Cytotoxic potential assessment in brine shrimps showed significant (p < 0.05) results for all extracts where most of the extract had greater than 80 % cytotoxicity at 200 µg/ml (Table 4). M. longifolia A extract had least LC50 value of 48.15 ± 0.13 µg/ml. This indicates that it is most potent among all other extracts. Furthermore, C. recutita EA, G. lutea A, and M. koenigii EA extracts also displayed potent cytotoxic action with LC50 values of 50.58 ± 0.12, 48.47 ± 0.15 and 49.00 ± 0.19 µg/ml, respectively. Doxorubicin (6.25 ± 1.25 µg/ml) was still most potent among all tested samples while the negative control DMSO showed no toxicity. Overall, the extracts LC50 values ranging between 48 and 96 μg/ml.
| Plant name | Extract code | Brine shrimp cytotoxicity | Protein kinase inhibition | %Hemolytic analysis | %Hemolysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| % Mortality | LC-50 |
Diameter (mm) 100 μg/disc |
%Hemolysis |
%Hemolysis | %Hemolysis | ||||
| (200 µg/ml) | Bald zone | Clear zone | (200 μg/ml) | (100 μg/ml) | (50 μg/ml) | (25 μg/ml) | |||
|
M. longifolia |
ML(EA) | 90 ± 5.77a | 70.71 ± 0.21 | 8 mm ± 0.30 cd | 9.13 ± 0.58ab | 7.43 ± 0.34 cd | 3.86 ± 1.42e | 2.74 ± 0.21f | |
| ML(M) | 90 ± 0.11a | 59.62 ± 0.19 | – | 7 mm ± 0.53d | 9.56 ± 0.54b | 6.55 ± 0.30 | 4.35 ± 0.70de | 2.31 ± 0.15 fg | |
| ML(A) | 80 ± 0.51b | 48.15 ± 0.13 | – | 8 mm ± 0.21 cd | 10.42 ± 0.36a | 8.49 ± 0.36 cd | 4.24 ± 0.82de | 3.17 ± 0.17ef | |
|
G. lutea |
GL(EA) | 90 ± 5.72a | 51.00 ± 0.61 | 7 mm ± 0.4d | – | 9.72 ± 0.94b | 8.11 ± 0.34 cd | 3.97 ± 0.89e | 2.74 ± 0.06f |
| GL(M) | 70 ± 1.02c | 56.19 ± 0.89 | 8 mm ± 0.18 cd | – | 9.08 ± 0.82ab | 7.79 ± 0.33d | 4.56 ± 1.12de | 3.17 ± 0.15ef | |
| GL(A) | 90 ± 0.00a | 48.47 ± 0.19 | 7 mm ± 0.22d | – | 9.40 ± 0.56ab | 8.33 ± 0.36 cd | 4.29 ± 0.39de | 3.38 ± 0.03ef | |
|
N. sativa |
NS(EA) | 90 ± 0.69a | 50.00 ± 0.74 | – | 7 mm ± 0.11d | 8.38 ± 0.74 cd | 7.52 ± 0.52d | 3.97 ± 0.82e | 2.79 ± 0.09f |
| NS(M) | 80 ± 0.58b | 48.24 ± 0.26 | 8 mm ± 0.17 cd | – | 8.91 ± 0.68c | 6.34 ± 0.36de | 2.20 ± 0.61 fg | 1.77 ± 0.05 g | |
| NS(A) | 80 ± 0.00b | 59.62 ± 0.39 | – | 8 mm ± 0.42 cd | 9.56 ± 0.82b | 8.17 ± 0.34 cd | 3.54 ± 0.32e | 2.36 ± 0.12 fg | |
|
T. arjuna |
TA(EA) | 90 ± 0.10a | 83.86 ± 0.19 | 7 mm ± 0.19d | – | 9.88 ± 1.22b | 7.52 ± 0.56d | 4.08 ± 0.59de | 3.06 ± 0.03ef |
| TA(M) | 60 ± 1.77d | 88.99 ± 0.97 | 9 mm ± 0.30c | – | 9.61 ± 0.94b | 6.82 ± 0.39de | 3.92 ± 0.41e | 2.84 ± 0.18f | |
| TA(A) | 90 ± 0.00a | 49.02 ± 0.32 | – | 8 mm ± 0.28 cd | 10.69 ± 0.82a | 7.47 ± 0.46 cd | 2.68 ± 0.77f | 2.04 ± 0.16 fg | |
|
C. recutita |
CR(EA) | 90 ± 0.13a | 50.58 ± 0.12 | 9 mm ± 0.61c | – | 10.58 ± 0.82a | 8.76 ± 0.36c | 4.29 ± 0.82de | 2.31 ± 0.15 fg |
| CR(M) | 80 ± 0.00b | 49.02 ± 0.31 | – | 8 mm ± 0.9 cd | 10.80 ± 0.94a | 7.74 ± 0.36d | 3.86 ± 0.58e | 3.33 ± 0.09ef | |
| CR(A) | 90 ± 0.31a | 70.71 ± 0.15 | 10 mm ± 0.77ab | 7 mm ± 0.36d | 10.37 ± 0.86a | 8.60 ± 0.31c | 3.54 ± 0.82e | 2.90 ± 0.07f | |
|
M. charantia |
MC(EA) | 70 ± 5.77c | 50.00 ± 0.23 | 11 mm ± 0.10ab | 7 mm ± 0.14d | 10.26 ± 0.82a | 8.49 ± 0.16 cd | 4.51 ± 1.82de | 3.33 ± 0.10ef |
| MC(M) | 90 ± 0.27a | 50.00 ± 0.82 | – | 7 mm ± 0.7d | 9.77 ± 0.57b | 8.01 ± 0.36 cd | 4.24 ± 0.82de | 3.65 ± 0.9e | |
| MC(A) | 60 ± 1.75d | 88.99 ± 0.19 | – | 7 mm ± 0.24d | 10.42 ± 1.42a | 6.66 ± 0.36de | 2.68 ± 0.76f | 2.20 ± 0.05 fg | |
|
M. koenigii |
MK(EA) | 80 ± 0.64b | 49.00 ± 0.19 | 13 mm ± 0.38a | 9.99 ± 0.82b | 7.25 ± 0.32 cd | 4.83 ± 0.43de | 2.68 ± 0.06f | |
| MK(M) | 90 ± 0.00a | 59.62 ± 0.27 | 11 mm ± 0.13ab | – | 9.67 ± 0.69b | 8.92 ± 0.36c | 3.38 ± 0.85ef | 1.82 ± 0.14 g | |
| MK(A) | 90 ± 0.23a | 70.57 ± 0.91 | 9 mm ± 0.52c | 7 mm ± 0.19d | 10.20 ± 0.80a | 7.04 ± 0.38 cd | 3.81 ± 0.62e | 2.74 ± 0.7f | |
|
C. amada |
CA(EA) | 90 ± 0.00a | 70.71 ± 0.26 | – | 12 mm ± 0.10ab | 9.08 ± 0.72b | 8.87 ± 0.36c | 4.19 ± 0.49de | 3.27 ± 0.04ef |
| CA(M) | 90 ± 0.44a | 96.94 ± 0.15 | 7 mm ± 0.15d | 10 mm ± 0.41b | 9.45 ± 1.15ab | 7.47 ± 0.32 cd | 3.92 ± 0.82e | 2.95 ± 0.09f | |
| CA(A) | 60 ± 1.73 | 96.71 ± 0.89 | – | 7 mm ± 0.73c | 10.53 ± 1.05a | 8.65 ± 0.31c | 4.35 ± 0.88de | 2.63 ± 0.5f | |
|
T. chebula |
TC(EA) | 90 ± 0.77a | 48.24 ± 0.53 | 8 mm ± 0.22 cd | – | 8.97 ± 1.17c | 8.11 ± 0.36 cd | 3.81 ± 0.56e | 2.84 ± 0.6f |
| TC(M) | 90 ± 5.61a | 49.02 ± 0.91 | 7 mm ± 0.36d | – | 8.59 ± 1.01c | 6.45 ± 0.37de | 3.27 ± 0.42de | 1.55 ± 0.9 g | |
| TC(A) | 80 ± 0.58b | 56.19 ± 0.34 | 8 mm ± 0.11 cd | – | 9.94 ± 1.13b | 7.95 ± 0.31d | 4.62 ± 0.61de | 2.09 ± 0.11 fg | |
| Surfactin | 27 ± 0.06 a | ||||||||
| Doxorubicin | 96 ± 0.21a | 6.25 ± 1.25 | |||||||
| Triton-X | 100 ± 0.63a | ||||||||
| DMSO | 0.5 % | ||||||||
ZOI (Zone of inhibition) represented as diameter (mm) of clear zone and bald zone. Values are presented as mean from triplicate investigation ± standard deviation by triplicate investigation. The columns with different superscript (a-g) letters show significantly (P < 0.05) different means, –: shows no activity. EA: Ethyl acetate, M: Methanol and A: aqueous extract.
3.4.5.2 Protein kinase inhibition assay
Another test to estimate the cytotoxic potential was inhibition of protein kinases that are responsible for hyphae formation in Streptomyces 85E strain. Results (Table 4) showed moderate to low protein kinase inhibition with maximum bald zone of 13 ± 0.38 mm shown by M. koenigii EA extract. It was followed by M. charantia EA (11 ± 0.10 mm) and M. koenigii M (11 ± 0.13 mm) extracts. Rest of the extracts had mild protein kinase inhibition activity with bald zones between 7 and 9 mm. It appears that non-polar solvent extracts have some phytochemicals that are more effective in inhibiting hyphae formation than polar solvent extracts.
3.4.6 Biocompatibility evaluation
3.4.6.1 Hemolytic assay
Hemolytic assay was executed to check the toxic effects of extracts on erythrocytes to ensure their safety profile. It was observed that there was 10–8 %, 8–6 %, 4–2 % and 3–1.5 % hemolysis at 200, 100, 50 and 25 µg/ml concentrations (Table 4). The maximum hemolysis at 200 µg/ml was shown by T. arjuna A extract with the value of 10.69 ± 0.82 % as compared to Triton-X 100 (positive control; 100 ± 0.63). There was dose dependent decline in the hemolytic effects of the extracts. This indicates that all the extracts are safe at ≥ 200 µg/ml where majority of the biological activities are observed.
3.4.7 Antimicrobial activities
Prevention and management of infections standalone or associated with other diseases like diabetes, cancer and injuries require the use of multiple antibiotics. Plant extracts, which are safe and have good antioxidant and antimicrobial profile, can be alternative to allopathic medicines (Manso et al. 2021). Hence, afterwards we investigated the extracts for their potential antibacterial and antifungal activities.
3.4.7.1 Antibacterial assay
The antibacterial assay of plant extracts in different non-resistant bacterial strains showed mild to moderate activity (Table 5). The extracts with zone of inhibition (ZOI) ≥ 8 mm and ≤ 14 mm is considered moderately active, extracts with ZOI ≥ 15 mm and ≤ 21 is considered active, while the highly active extracts exhibited ZOI ≥ 22 mm and ≤ 29 mm. M. longifolia EA and M extracts exhibited significant (p < 0.05) activity against S. aureus with ZOI of 12 ± 0.4 mm and 14 ± 0.7 mm at 100 μg/disc. EA and M extracts of G. lutea also revealed significant (p < 0.05) effect against K. pneumoniae with ZOI of 12 ± 0.5 mm and 12 ± 0.3 mm, respectively. Likewise, A and M extracts of T. chebula presented a potent antibacterial activity against K. pneumoniae (13 ± 0.1 mm) and P. aeruginosa (14 ± 0.2 mm), respectively. This means that though moderate, the extracts showed variable antibacterial profile against different strains that possibly will be accredited to the existence of diverse phytochemicals. No ZOI was present in negative control that established that DMSO did not affect the results whereas Ciprofloxacin (14 ± 0.10 mm to 18 ± 0.24 mm) and Cefixime (23 ± 0.1 mm to 26 ± 0.6 mm) showed highly significant (p < 0.05) antibacterial activities. The MIC value of 50 μg/disc was observed for M extract of M. charantia and M and EA extracts of M. longifolia against S. aureus. Results indicated that M extracts of most plants presented better antibacterial potential than others.
| Diameter (mm) of growth inhibition zone at 100 μg/disc and MIC (μg/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extract code | E. coli | MIC | K. pneumoniae | MIC | B. Subtilis | MIC | P. aeruginosa | MIC | S. aureus | MIC |
| M. longifolia | ||||||||||
| ML(EA) | – | – | – | – | – | – | 9 ± 0.5de | – | 12 ± 0.4d | 50 |
| ML(M) | – | – | 11 ± 0.3de | – | – | – | – | – | 14 ± 0.7c | 50 |
| ML(A) | – | – | 10 ± 0.8de | – | – | – | – | – | – | – |
| G. lutea | ||||||||||
| GL(EA) | – | – | 12 ± 0.5d | 50 | – | – | – | – | – | |
| GL(M) | – | – | 12 ± 0.3d | 100 | 9 ± 0.5de | – | – | 10 ± 0.1de | – | |
| GL(A) | – | – | 9 ± 0.5de | – | – | – | – | 9 ± 0.6de | – | |
| N. sativa | ||||||||||
| NS(EA) | 9 ± 0.4de | – | – | – | – | – | – | 7 ± 0.8e | – | – |
| NS(M) | – | – | – | – | 8 ± 0.4de | – | 10 ± 0.5de | – | 12 ± 0.3d | – |
| NS(A) | – | – | – | – | 9 ± 0.1de | – | – | – | – | |
| T. arjuna | ||||||||||
| TA(EA) | 8 ± 0.5de | – | 10 ± 0.3de | – | – | – | – | – | 10 ± 0.5de | – |
| TA(M) | – | – | 12 ± 0.6d | 100 | – | – | – | – | – | – |
| TA(A) | – | – | 9 ± 0.8de | – | – | – | 11 ± 0.5d | – | – | – |
| C. recutita | ||||||||||
| CR(EA) | 8 ± 0.5de | – | – | – | 10 ± 0.3de | – | – | – | – | – |
| CR(M) | – | – | – | – | 8 ± 0.5de | – | – | – | – | – |
| CR(A) | 7 ± 0.5e | – | – | – | – | – | 9 ± 0.5de | – | – | – |
| M. charantia | ||||||||||
| MC(EA) | 8 ± 0.5de | – | – | – | – | – | 11 ± 0.2de | – | – | – |
| MC(M) | – | – | 8 ± 0.5de | – | 10 ± 0.4de | – | 11 ± 0.3de | – | 14 ± 0.2c | 50 |
| MC(A) | – | – | – | – | 9 ± 0.5de | – | 11 ± 0.5de | – | – | – |
| M. koenigii | ||||||||||
| MK(EA) | – | – | 11 ± 0.5de | – | 10 ± 0.5de | – | 12 ± 0.5d | 25 | 10 ± 0.5de | – |
| MK(M) | 8 ± 0.5de | – | 9 ± 0.5de | – | 9 ± 0.5de | – | – | – | – | |
| MK(A) | 9 ± 0.5de | – | 10 ± 0.3de | 9 ± 0.5de | – | – | 9 ± 0.7de | – | – | – |
| C. amada | ||||||||||
| CA(EA) | 8 ± 0.5de | – | 10 ± 0.5de | – | – | – | 7 ± 0.5e | – | 9 ± 0.6de | – |
| CA(M) | – | – | 9 ± 0.5de | – | – | – | 11 ± 0.1de | – | – | – |
| CA(A) | 10 ± 0.5de | – | – | – | 9 ± 0.4de | – | 9 ± 0.6e | – | – | – |
| T. chebula | ||||||||||
| TC(EA) | – | – | 10 ± 0.5de | – | – | – | – | – | 7 ± 0.5e | – |
| TC(M) | 9 ± 0.5de | – | – | – | – | 7 ± 0.5e | 14 ± 0.2c | 50 | 9 ± 0.3e | – |
| TC(A) | – | – | 13 ± 0.1 cd | 50 | 11 ± 0.5de | – | – | – | – | – |
| Controls | ||||||||||
| Ciprofloxacin | 14 ± 0.4b | – | 16 ± 0.6b | 1.25 | 16 ± 0.6b | 1.25 | 18 ± 0.9b | 1.25 | 14 ± 0.6b | 1.25 |
| Cefixime | 26 ± 0.6a | – | 23 ± 0.2a | 1.25 | 26 ± 0.2a | 2.50 | 24 ± 0.7a | 2.50 | 23 ± 0.2a | 1.25 |
| DMSO | – | – | – | – | – | – | – | – | – | |
Results are mean ± standard deviation of bacterial growth inhibition zone that was measured including 6 mm of disc. Sample concentration on disc was 100 μg/disc. n = 3; Columns with superscript (a-e) letters displayed significant P value < 0.05, different means, – – means no activity.
3.4.7.2 Antifungal assay
Subsequently, plant extracts were tested against five fungal strains and the results showed mild antifungal activity. ZOI for A. niger ranges between 7.00 ± 0.34 mm to 8.23 ± 0.55 mm. In case of the A extracts, T. chebula (9.00 ± 0.45 mm), G. lutea (9.00 ± 0.50 mm) and C. recutita (7.00 ± 0.42 mm) exhibited the slightly significant (Table 6; p < 0.05) activity against Mucor species. Moreover, A extracts of T. arjuna (9.00 ± 0.30 mm) and C. amada (9.00 ± 0.65 mm) displayed noteworthy antifungal potential against F. solani. Likewise, M extracts of N. sativa, C. amada and T. chebula showed mild (p < 0.05) activity against A. niger with ZOI of 8.00 ± 0.16 mm, 8.00 ± 0.58 mm, and 8.23 ± 0.55 mm, respectively. Against A. fumigatus, EA extract of T. chebula (9.00 ± 0.31 mm) and M. koenigii (8.00 ± 0.13 mm) were slightly significant (p < 0.05). Terbinafine, positive control showed highly significant antifungal activity (ZOI: 15–18 mm; p < 0.05) against all test strains, whereas DMSO did not produce any effect.
| Diameter (mm) of growth inhibition zone (100 µg/disc) | |||||
|---|---|---|---|---|---|
| Extract code | F. solanni | A. niger | A. fumigatus | A. flavus | Mucor sp. |
| M. longifolia | |||||
| ML(EA) | 7.00 ± 0.33c | – | – | – | 9.00 ± 0.21ab |
| ML(M) | – | – | 7.00 ± 0.41 cd | – | – |
| ML(A) | – | – | – | 8.00 ± 0.35b | – |
| G. lutea | |||||
| GL(EA) | – | 8.00 ± 0.43 cd | |||
| GL(M) | 7.00 ± 0.11d | – | – | – | – |
| GL(A) | – | – | 7.00 ± 0.45c | – | 9.00 ± 0.50ab |
| N. sativa | |||||
| NS(EA) | 8.00 ± 0.35b | – | 8.00 ± 0.22d | ||
| NS(M) | – | 8.0 ± 0.16b | – | – | – |
| NS(A) | – | 7.00 ± 0.65 cd | – | – | 7.00 ± 0.31c |
| T. arjuna | |||||
| TA(EA) | 7.00 ± 0.51d | – | – | 7.00 ± 0.30 cd | 8.00 ± 0.27b |
| TA(M) | – | – | – | – | |
| TA(A) | 9.00 ± 0.30ab | – | – | – | – |
| C. recutita | |||||
| CR(EA) | – | 7.00 ± 0.43c | – | – | |
| CR(M) | – | – | – | – | – |
| CR(A) | – | – | – | – | 7.00 ± 0.42c |
| M. charantia | |||||
| MC(EA) | – | 7.00 ± 0.36c | – | 7.00 ± 0.51c | |
| MC(M) | – | – | 8.00 ± 0.21d | – | – |
| MC(A) | 7.00 ± 0.44c | – | – | – | – |
| M. koenigii | |||||
| MK(EA) | – | – | 8.00 ± 0.31b | – | – |
| MK(M) | – | 7.00 ± 0.34 cd | – | – | – |
| MK(A) | – | – | – | 7.00 ± 0.47c | – |
| C. amada | |||||
| CA(EA) | – | – | – | – | 8.00 ± 0.51 cd |
| CA(M) | – | 8.00 ± 0.58b | – | – | |
| CA(A) | 9.00 ± 0.65ab | – | – | – | 8.00 ± 0.52b |
| T. chebula | |||||
| TC(EA) | 7.00 ± 0.41c | 9.00 ± 0.13ab | – | – | |
| TC(M) | – | 8.23 ± 0.55b | – | – | |
| TC(A) | 7.00 ± 0.53 cd | – | – | 9.00 ± 0.45ab | |
| Controls | |||||
| Terbinafine | 15.0 ± 0.1 | 14 ± 0.5 | 18 ± 0.14a | 17 ± 0.2a | 17.7 ± 0.1a |
| DMSO | – | – | – | – | – |
Zone of growth inhibition was measured with 6 mm of disc. The sample concentration was 100 µg/disc. Values are presented as mean ± SD that was calculated in triplicate analysis. Superscript (a-d) letters on columns means significantly (p < 0.05) different from negative control, –: shows no activity. A. flavus = Aspergillus flavus, A. fumigatus = Aspergillus fumigatus, A. niger = Aspergillus. Niger and F. solanni = Fusarium solani.
4 Discussion
Treatment of diseases using herbal remedies has been in practice since ages. According to WHO the characteristics of safety, efficacy, cost effectiveness and easy accessibility have made natural products a major source in resolving primary health problems world-wide (Hussain et al. 2011). Pakistan has plenty of herbal resources where more than 1000 species with documented medicinal value are used by herbalists to cure different diseases. Natural products obtained from biologically active plants are either used as crude drugs or as isolated phytochemicals from the subject plant. It is very challenging to ensure the quality of natural products owing to the complex nature of phytochemicals (Chothani and Patel 2012). Researchers and pharmaceutical industries has made substantial contribution to investigate the biologically active phytochemicals and standardize the natural products with approved efficacy and safety (Khan and Rauf 2014). Since, the characteristics of natural products vary depending upon extracts solvents and composition of extracts ultimately affecting their biological profile (Ullah et al. 2014b); therefore, it is imperative to evaluate the biological profile of natural products to validate their efficacy and provide scientific evidence of their use in traditional remedies by herbalists (Sasidharan et al. 2011). Following these lines, we attempted to investigate the phytochemical and biological profile of nine medicinal plants collected from Herbalists in Islamabad, Pakistan. Here, we report medicinal value of selected crude extracts prepared in solvents of varying polarity. We performed a range of assays to prepare an initial profile for all plants that will direct towards bioactivity mediated isolation of efficacious compounds.
4.1 Extraction yield versus extraction solvents
A total of 27 crude extracts of nine plants were prepared by sonication assisted maceration technique. It is the simple, reproducible and an exhaustive process where phytochemicals can be extracted using solvents of varying polarity (Wojdyło et al. 2021). Although, different conventional techniques are employed for extraction process because of their efficiency, wide-range application and ease of use (Stalikas 2007); however, risk–benefit ratio of each technique has to considered to achieve the better quality of extract. For instance, exposure to high temperatures during extraction can destroy bioactive glycosides and polyphenols (Mahmoudi et al. 2013). In the current study, sonication assisted maceration provided optimal conditions for penetration of solvent in particles of dry plant powder and extracting significant quantities of phytochemicals (Wojdyło et al. 2021). Using distilled water, methanol and ethyl acetate ensured the extraction of polar and non-polar phytochemicals in different extracts. It was observed that extraction efficiency of methanol was higher as compared to non-polar solvent, which was also reflected in overall biological profile of the extracts. However, greater extract yield may not always direct towards absolute biological action (Fatima et al. 2015) and more pronounced activity may be observed in lower yield extracts depending on the nature of extracted phytochemicals.
4.2 Phytochemical investigation
As the first step in our investigation, polyphenols were quantified in all extracts. Recognition of phytochemicals within selected plants can be correlated with respective biological activities. Flavonoids and phenols are widely accepted as therapeutic agents due to their antioxidant, and immuno-protective properties that can protect the body from environmental and endogenous toxins. Distinct classes of flavonoids are isolated with significant biological interests such as antidiabetic, anticancer, antimicrobial, antimalarial, neuro-protective and anti-inflammatory. Involvement of flavonoids in routine diet is well recommended to reduce the risk of numerous life-threatening diseases for instance heart attack, diabetes mellitus and cancer (Ullah et al. 2020).
The current study revealed significant polyphenols in all extracts, with the highest content in methanol extracts of G. lutea, T. chebula, N. sativa and M. koenigii. Literature shows that phenolic acids can alter gene expression of certain proteins to induce cytotoxic, anti-mutagenic and anticancer effects (Ribarova and Atanassova 2005). Flavonoids can block the free radical generation pathway by inhibiting lipid peroxidation and also possess well established anti-inflammatory activity (Inomata et al. 2013). Most of the effects are attributed to the aromatic ring and hydroxyl groups in polyphenolic compounds (Treml and Šmejkal 2016). Previous studies have reported a significant correlation between polyphenols and in vivo antitumor effect by regulating PI3K/AKT and MAPK/ERK pathways (Vuong et al. 2016).
4.3 RP-HPLC quantification
Preliminary screening of phytoconstituents give the clue for natural aptitude of different plant extracts. Through extraction process, various secondary products are obtained, responsible for therapeutic capacities of traditional plants in various diseases (Gurjar et al. 2012). The presence of polyphenols in EA, M and A extracts confirmed by RP-HPLC demonstrated significant quantities of coumaric acid, gentisic acid, kaempferol, emodin, catechin, rutin and synergic acid. These polyphenols have established pharmacological profile. For example, rutin, emodin, myricetin and coumaric acid exhibit antioxidant, anti-inflammatory and antibacterial activities (Dai and Mumper 2010). Gentisic acid has effective biological activities such as neuroprotective, anti-inflammatory, antimicrobial, and antioxidant interests that make it a good remedy for prospective treatment of different ailments (Choubey et al. 2015). Epidemiological findings also revealed a substantial relationship between the consumption of phenolic acids enriched diet and the prevention of diseases (Akilandeswari and Ruckmani 2016). Furthermore, Kaempferol, a dietary flavonoid, acts as potent chemo-preventive substance for lung, breast and gastric cancer by restricting multiple signaling pathways, boosting the apoptosis mechanism via the suppression of cell cycle genes and activating p53 function (Khan et al. 2021). Consequently, in recent study, differentiated fingerprinting using 9 polyphenolic standards was performed for selected plant extracts confirmed substantial quantities of polyphenols that may be responsible for the biological activities of selected plants.
4.4 Biological activities
4.4.1 Antioxidant activity
Oxidative stress is the root of majority of diseases. Oxidative stress was found related to cardiovascular diseases, diabetes, cancer, alzheimer’s disease, kidney disorders and chronic obstructive pulmonary disease (Liguori et al. 2018). Plant extracts containing polyphenols have been known to combat oxidative stress by scavenging or blocking the free radical formation (Dahlia et al. 2017, Saha and Verma 2016, Mammad et al. 2017). In line with this, antioxidant activity of the extracts was determined by measuring the DPPH free radical scavenging activity (FRSA), ammonium molybdate reduction capacity (TAC) and potassium ferricyanide colorimetric analysis (TRP). Previous studies have shown antioxidant (Sheng et al. 2018) anti-inflammatory (Bordoni et al. 2019) and antimicrobial activities (Ali et al. 2021) of the selected plants. Here, we re-evaluated antioxidant activity to compare the effects of different extraction solvents.
DPPH free radical scavenging capacity is detected by change in the color of DPPH from violet to yellow (Yazdanparast et al. 2008). This happens because it can accept electrons from electron donors such as phenolics and flavonoids (Ahmed et al. 2017). Out of 27 extracts, M and EA extracts exhibited well defined DPPH scavenging activity, while A extracts showed the least potential. This was significantly (p < 0.05) correlated with phenolic (R2 = 0.656) and flavonoid (R2 = 0.447) content estimated in this study. Literature also supports the current findings (Dahlia et al. 2017, Saha and Verma 2016, Mammad et al. 2017) of free radical scavenging by phenolic and flavonoid content of the plant extracts.
Subsequent analysis of TAC also provided evidence of better results in methanol extracts as compared to other solvent extracts. Maximal TAC values were in the range of 145.45 ± 0.76 to 334.41 ± 0.35 µg QE/mg extract. TAC assay measures the electron and hydrogen donating capacity of test samples (Kasote 2013). Electron or hydrogen donating molecules like carotenoids, alpha-tocopherol, cysteine, ascorbic acid, terpenoids, polyphenols and aromatic amines (with OH aromatic rings) possess the potential to conduct a series of redox reactions and are considered as good antioxidants. Correlation (R2 = 0.4431) was found between TFC and TAC values and it can be concluded that the antioxidant capacity of extracts can be attributed to the significant presence of polyphenols and other phytochemicals in the M and EA extracts. Polyphenols have been previously reported to combat oxidative stress by reducing ROS activity (Stagos 2019). The mechanism of polyphenols involves the formation of peroxyl radical by the exchange of hydrogen atom from polyphenol structure with oxygen or electron of the free radical, thus quenching their oxidation potential. Phenols have ability to obstruct oxidation of LDL that is associated with reduction in critical diseases e.g., leukemia, Alzheimer’s disease, breast, gastrointestinal, and ovarian cancers (Mileo et al. 2016).
The antioxidant activity was further proved by measuring the reduction process of ferric (Fe+3) to ferrous (Fe+2) under the action of extracts that transformed the yellow color of test solution to green (Wang et al. 2016). All extracts possess proton donating phytochemicals that provided reducing power to the samples. It has been reported that flavonoids (naringenin and catechin), volatile oils (carvacrol, thymol and menthol), phenolic acid (caffeic acid and gallic acid), phenolics diterpenes (carnosic acid, carnosol and rosmanol) show good reducing power (Brewer 2011). Furthermore, it has been documented that natural products with high phenolic and flavonoid content show good reducing potential with beneficial effects on health (Akbari et al. 2022). Since, polyphenols are natural antioxidant compounds; these can protect cells from ROS induced damage by quenching mechanism. Consequently, these help to inhibit the process of lipid oxidation and protect human body cells against the oxidative damage that leads to decreased risk of some oxidative-stress associated diseases, such as cardiovascular, cancer and neurodegenerative dysfunctions (Afshari and Sayyed-Alangi 2017). A recent study showed that rutin in combination with vitamin C reduced TNFα levels, inflammation and oxidative stress in hemodialysis patients (Omar et al. 2022). Experimental evidence supports that coumaric acid inhibits melanin synthesis in murine melanoma cells and human epidermal cells and has implications in reducing pigmentation in human study (Boo 2019). Presence of these polyphenols in our extracts indicate that the selected extracts have cane be likely used to manage inflammation and pigmentation and become the source for isolation of antioxidant, anti-inflammatory and antipigmentation compounds. Concisely, it can be concluded from the results that polar (methanol) plant extracts have notable antioxidant proficiency as compared to the other solvent extracts.
4.4.2 Catalytic inhibition of carbohydrate metabolizing enzymes
Carbohydrates are the primary source of energy for metabolic reactions in the body. Carbohydrates are metabolized by α-amylase and α-glucosidase enzymes to simple sugars. α-Amylase hydrolyzes α bonds of α-linked polysaccharides like starch and glycogen and converts those into glucose and maltose. α-glucosidase primarily catalyzes the hydrolysis of dietary starch into glucose for intestinal absorption. These provide sufficient glucose in blood that can cause post-prandial hyperglycemia in patients of diabetes mellitus (Zinjarde et al. 2011) and can be considered promoters of diabetes complications (Sangeetha and Vedasree 2012). Hence, herbal remedies inhibiting α-amylase and α-glucosidase enzymes are effective strategy to minimize exaggerated spikes of post-prandial hyperglycemia. The present study showed that methanol and aqueous extracts have inhibited α-amylase and α-glucosidase enzyme activity in vitro. Methanol extracts of N. sativa, C. amada, C. recutita, and M. koenigii and aqueous extract of T. arjuna have shown significant enzyme inhibition. These results are supported by reports of antidiabetic activity of quercetin, rutin, and vanillic acid in N. sativa (Dalli et al. 2021), myricetin and kaempferol in M. koenigii (Ashokkumar et al. 2013) and luteolin and quercetin in C. recutita (Kato et al. 2008). Thus, in our study, potential antihyperglycemic activity of the polar solvent extracts can be linked to the diverse polyphenols in sample. Literature also demonstrates that effects linked with diabetes could be attributed to existence of phenolic content and also relate with antioxidant capability of commonly used herbs and medicinal plants (Ranilla et al. 2010). Furthermore, C. amada, N. Sativa and M. charantia have been traditionally used for the treatment of diabetes (Habicht et al. 2014, Hamdan et al. 2019, Mitra et al. 2019). Evidence of their inhibitory effects on carbohydrate metabolizing enzymes supports the traditional use of these plants and provides basis to isolated active phytoconstituents that can be used for clinical management of diabetes after thorough investigations.
4.4.3 Cytotoxic assays
Brine shrimp lethality assay is a well-established tool for cytotoxicity evaluation (Hamidi et al. 2014). It can be used to determine the toxicity of plant extracts as a primary indicator for their potential to be tested in cancer cell models and to illustrate the tendency of their biological action using a zoological organism (Nguta et al. 2012). In the present study, all extracts showed significant cytotoxicity against brine shrimps with LC50 values between 48.2 and 96.9 μg/ml. It implies that first, these extracts can be tested and used for biological profile at concentrations lower than this to avoid toxicity of extracts and second, their cytotoxicity must be further evaluated in normal cells like lymphocytes or fibroblasts. Previous data reported that majority of the selected plant extracts (M. koenigii, N. sativa, C. amada, T. chebula and M. koenigii) displayed well defined in vitro anticancer activity highlighting the importance of cytotoxicity re-appraisal in normal versus cancer cells (Shrestha 2017, Nerdy et al. 2021, Hossain et al. 2020, Eshwarappa et al. 2016).
In line with above data, we next determined the effect of selected extracts on protein kinase activity in Streptomyces 85E. The purpose was to assess the potential inhibitory effect of extracts on protein kinase enzymes that are primary regulators of carcinogenesis. Genetic mutation in cell cycle, differentiation and cellular death processes upraise protein kinase activity at serine/threonine or tyrosine levels that are frequently detected in cancers (Fatima et al. 2022a, Montagnani and Stecca 2019). Hence, the research on drug candidates inhibiting protein kinases is ongoing for the targeted treatment of cancer (Evan and Vousden 2001). Streptomyces involves protein kinase effect of protein RamC to transform pre-Sap B to Sap B, a surfactant necessary for the formation of aerial hyphae (Waseem et al. 2017). Inhibition of RamC creates bald zones around sample discs corresponding to the inhibition of aerial hyphae formation. All extracts had mild to moderate protein kinase inhibition activity, suggesting dose adjustment and evaluation in specific kinase systems to further appraise the cytotoxic potential of tested samples. Since it will be beneficial in understanding their potential role as antitumor and anti-infective agents (Sharma et al. 2016).
4.4.4 Biocompatibility evaluation
Hemolytic analysis explores the biocompatibility of selected extracts with human normal RBCs to illustrate the cytotoxicity mechanism to cellular membrane. This was done to ensure the safety of the extracts at concentrations where significant biological activities were observed. (Alonso and Alonso 2016). Almost all the extracts displayed less than 3 % hemolysis at 25 μg/ml. Likewise, most of the extracts showed < 10 % hemolysis at 100 μg/ml. The 200 μg/ml concentration can be considered the maximum number of extracts that can be used for biological activities to prevent further damage to RBCs. Thus, further research is planned to test the biological activities of selected extracts preferably at < 100 μg/ml concentrations to ensure their safety to normal cells. Phytochemicals may influence the membrane stability of RBCs depending on their antioxidant profile as well as possible interaction of specific functional groups with cell membranes (Rubnawaz et al. 2021). Phytochemicals can have dual effect on RBC integrity based on concentration. Literature shows that higher concentration of tannic acid can cause crinkling and lysis of RBC (Deng et al. 2019). On the contrary, phytochemicals like polyphenolic compounds, being potent antioxidants have capacity to reduce oxidative stress mediated hemolysis. It has been reported that ROS particularly thiol and peroxides cause RBC membrane rigidity and fragility leading to hemolysis (Diederich et al. 2018). There is evidence of protection of RBCs from hemolysis by polyphenols due to their peroxidase and other free radical scavenging properties (Gwozdzinski et al. 2021). Although, along with the results of brine shrimp assay, the extracts showed minor toxicity in in vitro assays; however, it is necessary to evaluate their interaction with the biological system in in vivo models. Moreover, a range of sample concentrations, methanol extracts in particular, can also be assessed in cancer cell models to evaluate their prospective activity in cancer.
4.4.5 Antimicrobial potential
Treatment of infections using herbal remedies is a common practice. These contain a variety of phytochemicals that can halt the growth of bacteria or fungi depending on specific plant part and specific organism (Kaushik and Goyal 2008). Thus, traditionally used plants are being investigated progressively for the development of safe and effective antimicrobial formulations (Sukanya et al. 2009). Methanol and aqueous extracts are distinctively active against strains of S. aureus and K. pneumoniae, consequently indicating the presence of antibacterial phytochemicals. Research showed that phenolics and flavonoids such as caffeic acid, gallic acid, rutin and apigenin have well-established antibacterial activity (Sen et al. 2016). The present study reported significant amounts of TPC and TFC in plant extracts, which is supported by previous data that showed the presence of caffeic acid, gallic acid, rutin and apigenin in extracts of N. sativa, C. recutita, C. amada and T. chebula (Al-Khalifa et al. 2021, Rao et al. 2008, Mandeville and Cock 2018). Antibacterial activity of these polyphenolic compounds involves adherence, biofilm formation, hindrance in motility and influence on metabolic enzymes of bacteria (Kahkeshani et al. 2019). One study applied N. sativa seed extract topically on staphylococcal skin infection in infants and observed that the infection recovery was equivalent to standard mupirocin (Rafati et al. 2014). Additionally, in a controlled clinical study, seventy-eight patients were given mouth rinse of T. chebula extract, which significantly reduced gingival irritation and microbial plaque in comparison to 0.12 % chlorhexidine. This correlates with our results that showed significant antibacterial activity of C. amada and T. chebula laying foundation for their use in clinical practice.
Furthermore, the antifungal potential correlates with presence of tannins, flavonoid, unsaturated lactones, phenolic compounds and essential oils (Cowan 1999). Plant’s antifungal mechanism may be accredited to the flavonoid content as these can create complex with extracellular protein components present in fungi’s cell wall. Likewise, flavonoids can interrupt the fungal membrane because of high degree lipophilicity (Arif et al. 2009) and interfere with efflux pumps, protein synthesis and mitochondrial function (Al Aboody and Mickymaray 2020).The mild antifungal potential of the extracts in present study can be linked with the presence of phenolic compounds that are confirmed by phytochemical analysis.
5 Conclusions
Concisely, this study reported the effect of using solvents with different polarity for extraction on the biological profile of resultant extracts. Here, the methanol extracts contained the significant phenolic and flavonoid content. These corresponded with their significant antioxidant and antibacterial activities as well as mild to moderate protein kinase inhibition and antihyperglycemic potentials. Nigella sativa methanol extract showed maximum TPC. M. longifolia and G. lutea methanol extracts revealed notable antibacterial activity against S. aureus and K. pneumoniae. Similarly, methanol extracts of C. recutita and M. koenigii had significant α-glucosidase activity. Considering the protein kinase inhibitory and cytotoxic activities, all extracts are planned to be re-tested in normal versus cancer models at variable concentrations for their potential evaluation as anticancer agents. All these activities provide evidence for the traditional use of selected plants by herbalists. Future studies are in process for the qualitative and quantitative analysis, isolation, and characterization of individual bioactive phytochemicals.
CRediT authorship contribution statement
Sania Atta: Writing – original draft, Methodology. Durdana Waseem: Writing – review & editing. Iffat Naz: Writing – review & editing. Faisal Rasheed: Project administration. Abdul Rehman Phull: Data curation, Visualization. Tofeeq Ur-Rehman: Data curation, Visualization. Nadeem Irshad: Supervision, Investigation. Parveen Amina: Project administration. Humaira Fatima: Supervision, Investigation.
Acknowledgments
The authors are thankful to Prof. Dr. Rizwana Aleem Qureshi, Department of Plant Sciences, Faculty of Biological Sciences, Quaid-i-Azam University Islamabad, Pakistan for identifying the plants sample.
Funding source
We acknowledge the financial support by Higher Education Commission, Pakistan under the Indigenous Scholarship for PhD studies, Phase-II, Batch VI (PIN NO. 520-148048-2MD6-21 (50093084).
References
- Antioxidant effect of leaf extracts from Cressa cretica against oxidation process in soybean oil. Food Sci. Nutr.. 2017;5(2):324-333.
- [Google Scholar]
- Cruciferous vegetables and their bioactive metabolites: from prevention to novel therapies of colorectal cancer. Evid. Based Complement. Alternat Med. 2022:2022.
- [Google Scholar]
- A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed.. 2013;3(5):337-352.
- [Google Scholar]
- Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Complement. Altern. Med.. 2017;17(1):386.
- [Google Scholar]
- The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors.. 2022;48(3):611-633.
- [Google Scholar]
- Synergistic antibacterial effect of apigenin with Î2-lactam antibiotics and modulation of bacterial resistance by a possible membrane effect against methicillin resistant Staphylococcus aureus. Cell. Mol. Biol.. 2016;62(14):74-82.
- [Google Scholar]
- New approach for using of Mentha longifolia L. and Citrus reticulata L. essential oils as wood-biofungicides: GC-MS, SEM, and MNDO quantum chemical studies. Materials. 2021;14(6):1361.
- [Google Scholar]
- Evaluation of the antimicrobial effect of thymoquinone against different dental pathogens: an in vitro study. Molecules. 2021;26(21):6451.
- [Google Scholar]
- Hemolytic potential of miltefosine is dependent on cell concentration: implications for in vitro cell cytotoxicity assays and pharmacokinetic data. Biochimica et Biophysica Acta (BBA)-Biomembranes.. 2016;1858(6):1160-1164.
- [Google Scholar]
- Natural products–antifungal agents derived from plants. J. Asian Nat. Prod. Res.. 2009;11(7):621-638.
- [Google Scholar]
- Ethnomedicinal, phytochemical and antibacterial activities of medicinal flora of Pakistan used against Pseudomonas aeruginosa-A Review. Pak. J. Pharm. Sci.. 2017;30(6):2285-2300.
- [Google Scholar]
- p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants. 2019;8(8):275.
- [Google Scholar]
- Antioxidant and anti-inflammatory properties of nigella sativa oil in human pre-adipocytes. Antioxidants (Basel).. 2019;8(2):51.
- [Google Scholar]
- Kinetic studies of the metabolism of foreign organic compounds. III. The conjugation of phenols with glucuronic acid. Biochem J.. 1952;52(3):416-419.
- [Google Scholar]
- Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Comprehensive Rev. Food Sci. Food Saf.. 2011;10(4):221-247.
- [Google Scholar]
- Extraction and quantitative HPLC analysis of coumarin in hydroalcoholic extracts of Mikania glomerata Spreng:(“ guaco”) leaves. J. Braz. Chem. Soc.. 2001;12(6):706-709.
- [Google Scholar]
- Preliminary phytochemical screening, pharmacognostic and physicochemical evalution of leaf of Gmelina arborea. Asian Pacific J. Tropical Biomed.. 2012;2(3):S1333-S1337.
- [Google Scholar]
- Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharm Pat Anal.. 2015;4(4):305-315.
- [Google Scholar]
- Central and peripheral pain intervention by Ophiorrhiza rugosa leaves: potential underlying mechanisms and insight into the role of pain modulators. J. Ethnopharmacol.. 2021;276:114182
- [Google Scholar]
- In-vitro DPPH free radical scavenging activity of the plant Murraya koenigii Linn (Curry Leaf) in Rajshahi, Bangladesh. J. Complementary Alternative Med. Res.. 2017;3(4):1-9.
- [Google Scholar]
- Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. J. Mol.. 2010;15(10):7313-7352.
- [Google Scholar]
- Chemical composition analysis using HPLC-UV/GC-MS and inhibitory activity of different Nigella sativa fractions on pancreatic α-aylase and intestinal glucose absorption. BioMed. Res. Int.. 2021;2021:13.
- [Google Scholar]
- Plants of the genus Terminalia: an insight on its biological potentials, pre-clinical and clinical studies. Front Pharmacol.. 2020;11:561248.
- [Google Scholar]
- Effect of tannic acid on blood components and functions. Colloids Surf. B. Biointerfaces. 2019;184:110505
- [Google Scholar]
- A historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303-336.
- [Google Scholar]
- On the effects of reactive oxygen species and nitric oxide on red blood cell deformability. Front. Physiol.. 2018;9:332.
- [Google Scholar]
- In vivo toxicity studies on gall extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae) Pharmacognosy Res.. 2016;8:199-201.
- [Google Scholar]
- Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: an in vitro biological and phytochemical investigation. BMC Complement. Altern. Med.. 2015;15(1):376.
- [Google Scholar]
- Pharmacological activity of Mentha longifolia and its phytoconstituents. J. Trad. Chinese Med.. 2017;37(5):710-720.
- [Google Scholar]
- Polarity guided extraction, HPLC based phytochemical quantification, and multimode biological evaluation of Otostegia limbata (Benth.) Boiss. Arabia. J. Chem.. 2022;15(2):103583
- [Google Scholar]
- Polarity guided extraction, HPLC based phytochemical quantification, and multimode biological evaluation of Otostegia limbata (Benth.) Boiss. 2022;15(2):103583
- [Google Scholar]
- Involvement of opioid system and TRPM8/TRPA1 channels in the antinociceptive effect of spirulina platensis. Biomolecules.. 2021;11(4):592.
- [Google Scholar]
- Review on medicinal plants used by local community of Jodhpur district of thar desert. Int. J. Pharmacol.. 2011;7(3):333-339.
- [Google Scholar]
- Gurjar, M. S., Ali, S., Akhtar, M., Singh, K. S., 2012. Efficacy of plant extracts in plant disease management.
- Gwozdzinski, K., Pieniazek, A., Gwozdzinski, L. J. O. M. & Longevity, C., 2021. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease, 2021.
- Momordica charantia and type 2 diabetes: from in vitro to human studies. Curr. Diabetes Rev.. 2014;10(1):48-60.
- [Google Scholar]
- Effects of Nigella sativa on type-2 diabetes mellitus: a systematic review. Int. J. Environ. Res. Pub. Health.. 2019;16(24):4911.
- [Google Scholar]
- Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced Pharm. Bull.. 2014;60(1):9-18.
- [Google Scholar]
- A review on Murraya koenigii: multipotential medicinal plant. Asian J. Pharm. Clinical Res.. 2012;5:5-14.
- [Google Scholar]
- Abelmoschus esculentus (L.) Moench pod extract revealed antagonistic effect against the synergistic antidiabetic activity of metformin and acarbose upon concomitant administration in glucose-induced hyperglycemic mice. Biologics. 2022;2(2):128-138.
- [Google Scholar]
- Phytochemical and Biological Investigation of Curcuma amada Leaves. Dhaka Univ. J. Pharm. Sci.. 2020;19(1):9-13.
- [Google Scholar]
- Nutrient evaluation and elemental analysis of four selected medicinal plants of Khyber Pakhtoon Khwa, Pakistan. Pakakistan J. Botany. 2011;43(1):427-434.
- [Google Scholar]
- Flavones and anthocyanins from the leaves and flowers of Japanese Ajuga species (Lamiaceae) Biochem. Syst. Ecol.. 2013;51:123-129.
- [Google Scholar]
- Biological activity of Terminalia arjuna on human pathogenic microorganisms. Pakistan J. Pharm. Res.. 2016;2(1):23-27.
- [Google Scholar]
- Antioxidant activity and α-amylase inhibitory potential of Rosa canina L. Afr. J. Trad. Complement. Altern. Med.. 2017;14(2):1-8.
- [Google Scholar]
- Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci.. 2019;22(3):225-237.
- [Google Scholar]
- Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PloS One.. 2018;13(1):e0191025.
- [Google Scholar]
- Reverse phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) analysis of flavonoids profile from curry leaf (Murraya koenigii. L) J. Medicinal Plants Res.. 2013;7(47):3393-3399.
- [Google Scholar]
- Protective effects of dietary chamomile tea on diabetic complications. J. Agric. Food Chem.. 2008;56(17):8206-8211.
- [Google Scholar]
- In vitro evaluation of Datura innoxia (thorn-apple) for potential antibacterial activity. Indian J Microbiol.. 2008;48(3):353-357.
- [Google Scholar]
- Caffeic acid and its derivatives: antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem.. 2021;69(10):2979-3004.
- [Google Scholar]
- Medicinal plants: economic perspective and recent developments. World Appl. Sci. J.. 2014;31(11):1925-1929.
- [Google Scholar]
- Polyphenolic profiling of Ipomoea carnea Jacq. by HPLC-DAD and its implications in oxidative stress and cancer. Nat. Prod. Res.. 2019;33(14):2099-2104.
- [Google Scholar]
- Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem.. 2000;64(11):2458-2461.
- [Google Scholar]
- Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers. 2020;12(7):1959.
- [Google Scholar]
- Resources and biological activities of natural polyphenols. Nutrients.. 2014;6(12):6020-6047.
- [Google Scholar]
- Antioxidant, anticancer and antibacterial potential of zakhm-e-hayat rhizomes crude extract and fractions. Pak. J. Pharm. Sci.. 2016;29(3):895-902.
- [Google Scholar]
- Etude de l'extraction des composés phénoliques de différentes parties de la fleur d'artichaut (Cynara scolymus L.) Nat. Technol.. 2013;9:35.
- [Google Scholar]
- Nigella sativa L.: Uses in traditional and contemporary medicines–An overview. Acta Ecologica Sinica. 2021;41(4):253-258.
- [Google Scholar]
- Antibacterial and Antioxidant activity of Nigella sativa. Int. J. Innovation Sci. Res.. 2017;31(1):167-172.
- [Google Scholar]
- Terminalia chebula Retz. fruit extracts inhibit bacterial triggers of some autoimmune diseases and potentiate the activity of tetracycline. Indian J. Microbiol.. 2018;58(4):496-506.
- [Google Scholar]
- Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics. 2021;11(1):46.
- [Google Scholar]
- Evaluation of anti-inflammatory and wound healing activity of Gentiana lutea rhizome extracts in animals. Pharma. Biol.. 2004;42(1):8-12.
- [Google Scholar]
- Optimization of a new antihyperglycemic formulation using a mixture of Linum usitatissimum L., Coriandrum sativum L., and Olea europaea var. sylvestris flavonoids: A mixture design approach. Biologics. 2021;1(2):154-163.
- [Google Scholar]
- Mileo, A. M., Miccadei, S. J. O. m. & longevity, c., 2016. Polyphenols as modulator of oxidative stress in cancer disease: new therapeutic strategies. 2016.
- Antidiabetic and antioxidative properties of the hydro-methanolic extract (60: 40) of rhizomes of Curcuma amada roxb. (Zingiberaceae) in streptozotocin-induced diabetic male albino rat: a dose-dependent study through biochemical and genomic approaches. J. Complement. Integrat. Med.. 2019;16(4)
- [Google Scholar]
- Role of Protein Kinases in Hedgehog Pathway Control and Implications for Cancer Therapy. Cancers (Basel). 2019;11(4):449.
- [Google Scholar]
- Antioxidant, antibacterial, and anticancer activities of bitter gourd fruit extracts at three different cultivation stages. J. Chem. 2020
- [Google Scholar]
- Pharmacological evaluation of Fumaria indica (hausskn.) Pugsley; a traditionally important medicinal plant. Pak. J. Bot.. 2017;49:119-132.
- [Google Scholar]
- Brine Shrimp (Artemia salina Leach.) Lethality Test of Ethanolic Extract from Green Betel (Piper betle Linn.) and Red Betel (Piper crocatum Ruiz and Pav.) through the Soxhletation Method for Cytotoxicity Test. Open Access Macedonian J. Med. Sci.. 2021;9(A):407-412.
- [Google Scholar]
- Evaluation of acute toxicity of crude plant extracts from kenyan biodi-versity using brine shrimp, artemia salina l. (artemiidae) The Open Conference Proc. J.. 2012;3:30-34.
- [Google Scholar]
- Fruits of Terminalia chebula Retz.: A review on traditional uses, bioactive chemical constituents and pharmacological activities. Phytother Res.. 2020;34(10):2518-2533.
- [Google Scholar]
- Gastroprotective effects of bitter principles isolated from Gentian root and Swertia herb on experimentally-induced gastric lesions in rats. J. Nat. Med.. 2006;60(1):82-88.
- [Google Scholar]
- Evaluation of the combination effect of rutin and vitamin C supplementation on the oxidative stress and inflammation in hemodialysis patients. Front. Pharmacol.. 2022;13:961590
- [Google Scholar]
- Comparative phytochemical and ethnomedicinal survey of selected medicinal plants in Nigeria. Sci. Res. Essays.. 2012;7(9):989-999.
- [Google Scholar]
- Antioxidant potential, urease and acetylcholine esterase inhibitory activity and phytochemical analysis of selected medicinal plants from the Republic of Korea. Exploratory Res. Hypothesis Med.. 2021;6(2):51-59.
- [Google Scholar]
- Mango ginger (Curcuma amada Roxb.)–A promising spice for phytochemicals and biological activities. J. Biosci. (Bangalore). 2011;36(4):739-748.
- [Google Scholar]
- Seripheidium quettense mediated green synthesis of biogenic silver nanoparticles and their theranostic applications. Green Chem. Lett. Rev.. 2019;12(3):310-322.
- [Google Scholar]
- Anti-microbial effect of Nigella sativa seed extract against staphylococcal skin Infection. Med. J. Islamic Rep. of Iran.. 2014;28:42.
- [Google Scholar]
- Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol.. 2010;101(12):4676-4689.
- [Google Scholar]
- A comparative study of antimicrobial activity of Curcuma amada and Alpinia galanga of Zingiberaceae family. Asian J. Chem.. 2008;20(7):5293.
- [Google Scholar]
- Phytomedicines: Diversity, extraction, and conservation strategies. In: Phytomedicine. Elsevier; 2021. p. :1-33.
- [Google Scholar]
- Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metallurgy. 2005;40(3):255-260.
- [Google Scholar]
- Rubnawaz, S., Okla, M. K., Akhtar, N., Khan, I. U., Bhatti, M. Z., Duong, H.-Q., El-Tayeb, M. A., Elbadawi, Y. B., Almaary, K. S. & Moussa, I. M. J. P., 2021. Antibacterial, antihemolytic, cytotoxic, anticancer, and antileishmanial effects of Ajuga bracteosa transgenic plants. 10 (9), 1894.
- Antioxidant activity of polyphenolic extract of Terminalia chebula Retzius fruits. J. taibah Univ. Sci.. 2016;10(6):805-812.
- [Google Scholar]
- A review on curry leaves (Murraya koenigii): Versatile multi-potential medicinal plant. Am. J. Phytomedicine and Clinical Therapeutics. 2015;3(4):363-368.
- [Google Scholar]
- In vitro alpha-amylase inhibitory activity of the leaves of Thespesia populnea. ISRN Pharmacol.. 2012;2012:515634
- [Google Scholar]
- Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem.. 2014;143:33-39.
- [Google Scholar]
- Extraction, isolation and characterization of bioactive compounds from plants’ extracts. African J. Traditional Complementary Alternative Med.. 2011;8(1)
- [Google Scholar]
- Schultes, R. E., Hofmann, A., 1992. Plants of the gods: their sacred, healing, and hallucinogenic powers.
- Physicochemical properties of dietary phytochemicals can predict their passive absorption in the human small intestine. J. Sci. Rep.. 2017;7(1):1-15.
- [Google Scholar]
- Apigenin naturally occurring flavonoids: occurrence and bioactivity. Pharm. Biosci. J. 2016:56-68.
- [Google Scholar]
- Design strategies, structure activity relationship and mechanistic insights for purines as kinase inhibitors. Eur. J. Medicinal Chem.. 2016;112:298-346.
- [Google Scholar]
- Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PloS One. 2018;13(8):e0202368.
- [Google Scholar]
- GC-MS analysis, Antibacterial, Antioxidant and Brine Shrimp Lethality Assay of Murraya koenigii Spreng. Pharma Innovation J.. 2017;6(12):35-38.
- [Google Scholar]
- Stagos, D.J.A. 2019. Antioxidant activity of polyphenolic plant extracts. 19. MDPI.
- Chamomile (Matricaria chamomilla L.): an overview. Pharmacogn Rev.. 2011;5(9):82-95.
- [Google Scholar]
- Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci.. 2007;30(18):3268-3295.
- [Google Scholar]
- Antimicrobial activity of leaf extracts of Indian medicinal plants against clinical and phytopathogenic bacteria. African J. Biotechnol.. 2009;8(23)
- [Google Scholar]
- Flavonoids as potent scavengers of hydroxyl radicals. Comprehensive Rev. Food Sci. Food Saf.. 2016;15(4):720-738.
- [Google Scholar]
- Antioxidant and cytotoxic activities and phytochemical analysis of euphorbia wallichii root extract and its fractions. Iran J Pharm Res.. 2012;11(1):241-249.
- [Google Scholar]
- Ullah, N., Saleem, S., ul Haq, I., Mirza, B., 2014. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch.
- Ullah, A., Munir, S., Badshah, S.L., Khan, N., Ghani, L., Poulson, B.G., Emwas, A.-H., Jaremko, M.J.M., 2020. Important flavonoids and their role as a therapeutic agent. 25 (22), 5243.
- Ethnomedicinal plant use value in the Lakki Marwat District of Pakistan. J. Ethnopharmacol.. 2014;158:412-422.
- [Google Scholar]
- Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. J. Transl. Med.. 2016;14(1):1-12.
- [Google Scholar]
- Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem. Analytical Biochem.. 2013;2(4):1-4.
- [Google Scholar]
- Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev.. 2016;2016:5692852.
- [Google Scholar]
- Carboxylate derivatives of tributyltin (IV) complexes as anticancer and antileishmanial agents. DARU J. Pharm. Sci.. 2017;25(1):1-14.
- [Google Scholar]
- Effect of different pre-treatment maceration techniques on the content of phenolic compounds and color of Dornfelder wines elaborated in cold climate. Food Chem.. 2021;339:127888
- [Google Scholar]
- Citrinin derivatives from the soil filamentous fungus Penicillium sp. H9318. J. Braz. Chem. Soc.. 2011;22:1125-1129.
- [Google Scholar]
- Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem. Biol. Interact.. 2008;172(3):176-184.
- [Google Scholar]
- Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complement. Altern. Med.. 2017;17(1):443.
- [Google Scholar]
- Potent α-amylase inhibitory activity of Indian ayurvedic medicinal plants. BMC Complement. Alter. Med.?. 2011;11(1):1-10.
- [Google Scholar]
- Extraction optimization, total phenolic, flavonoid contents, HPLC-DAD analysis and diverse pharmacological evaluations of Dysphania ambrosioides (L.) Mosyakin & Clemants. Nat. Prod. Res.. 2019;33(1):136-142.
- [Google Scholar]
- Chamomile (Matricaria recutita) as a valuable medicinal plant. Int. J. Adv. Biol. Biomed. Res.. 2014;2(3):823-829.
- [Google Scholar]







