Translate this page into:
Polypyrrole and rice husk composite potential for the adsorptive removal of 2,4,6-trichloro phenol from aqueous medium
⁎Corresponding author. munawar.iqbal@ue.edu.pk (Munawar Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The rice husk (RH) composite with polypyrrole (PPY-RH) was fabricated and investigated for the abatement of TCP (2,4,6-trichlorophenol) in column adsorption mode. The flow rate, bed height and TCP inlet concentration are the influential parameters, which were optimized for the enhanced removal of TPC. The breakthrough time declined with bed depth decrease, while it was reverse for flow rate and TPC inlet concentration. It was also depicted that with a higher in flow rate, less bed height and inlet TCP concentration, the biosorption capacity decreased. Column models viz. Thomas and Bed Depth Service fitted better with the TCP adsorption data of TPC. Scanning electron microscope (SEM) analysis was also performed -pre and post adsorption and it was observed that the surface characteristics of the composite was changed significantly after the adsorption of TCP and similar observation was observed in FTIR analysis. The thermogravimetry analysis (TGA) was accomplished in order the check the stability, which revealed promising stability of the prepared composite. The rice husk composite with PPY has potential for the removal of TCP, which has promising capability for the adsorptive removal of TCP from the effluents.
Keywords
Tricholorophenol
Column mode
Sorption
Kinetics
Composite
Wastewater
1 Introduction
Since technological revolution natural assets of the universe specifically air, soil and water have been spoiled with different types of chemicals in various ways. Among them, water pollution is playing an adverse role in taking organisms on the verge of extinction. The concentration of several pollutants in wastewater is observed beyond a certain permissible level. They can be characterized as inorganic and organic or their particular compound depending on their source of origin. According to the United Nations Report (Connor, 2015), the population will rise to 9.3 billion by 2050, the world may face severe water scarcity (Khalid et al., 2021, Nazir et al., 2021).
Phenolic compounds are one of POPs (persistent organic pollutants), which frequently mixed into the water resources causing an unpleasant odor and taste of the water. It is toxic and can be absorbed through the skin and is also mutagenic beyond the permissible concentration limit (Ghezali et al., 2018, Iqbal et al., 2019). The waterborne diseases of chlorophenols on aquatic species have been reported. The bacteria and fish toxicity of some phenols results showed that phenols were toxic to the aquatic species. Phenolic compounds frequently occur where industries use petroleum products and commonly used in petroleum refining industries along with various other industries. Phenol is readily soluble in water and its wet chemical analysis contributes to many other phenolic derivatives. Mono- to penta-substituted chlorophenols are broadly used as synthesis intermediates in dyestuffs. Phenolic compounds can also be formed by the degradation of complex molecules such as phenoxy acetic acid and chlorobenzenes (Iqbal and Bhatti 2015, Ashar et al., 2016, Ahamd et al., 2017, Shafique et al., 2020).
At current, there is a great concern for the purification of water by employinmg several techniques viz, filtration, sedimentation, precipitation, chlorination, adsorption etc (Benabdallah et al., 2017, Daij et al., 2017, Djehaf et al., 2017, Legrouri et al., 2017, Minas et al., 2017, Ghezali et al., 2018, Ibrahim et al., 2020, Awwad et al., 2021, Elsherif et al., 2021). Each technique has its own merits and demerits. Various studies in past focused on adsorption for water purification because of simple design, cost-effective, wide pH range, and high performance and the use of agro-waste which is termed as biosorption is found one of promising techniques in this regard. Biosorption investigation is performed in batch and column mode. Batch mode is employed for optimizing the input (reaction time, pH, concentration of the adsorbate, the mass of adsorbent, etc) variables, which are then applied in column mode for scale-up of adsorption process for practical use (Kausar et al., 2017, Kausar et al., 2021, Khan et al., 2021), i.e., Amiri et al. (Bahrami et al., 2018) studied the removal of 2,4-dichloro acetic acid using rice husk as a sorbent in column mode and the efficiency was highly promising. Similarly, Liu et al. (Liu et al., 2020) investigated the removal of different types of pollutants using functionalized straw as a sorbent with efficiency adsorption efficiency. Also, the performance of the activated carbon in column study for phenolic compounds from refinery wastewater has been studied (El-Naas et al., 2017). Fitting of different kinetic models revealed that activated carbon the best choice for phenolic compounds adsorption.
Several methods have been used for phenolic compounds removal from (Iqbal and Bhatti 2015, Ashar et al., 2016, Ahamd et al., 2017, Ghezali et al., 2018, Bhatti et al., 2020, Shafique et al., 2020), which are regarded as costly and non-ecofriendly. In this investigation, a cost-effective technique was adopted for TCP adsorptive removal. The potential of rice husk (RH) composite with polypyrrole (PPY) was prepared and applied for TCP removal in a column mode. Different kinetic models like Thomas and BDST models were employed for the interpretation of adsorption data. The TGA and SEM techniques were used for the characterization of the prepared composite.
2 Material and methods
2.1 Reagents
The chemicals, i.e., hydrochloric acid (HCl), pyrrole (C4H4NH), ferric chloride (FeCl3), sodium hydroxide (NaOH), sulfuric acid (H2SO4), HCHO and methanol (CH3OH) were precured from Merck. The RH biomasses were collected from the farmer fields.
2.2 Sorbent preparation
Biomass has a size between 300 and 500 nm was chemically reacted with formaldehyde for their color removal. In this practice, solution of 100 mL (0.1 M) sulphuric acid (H2SO4) and 5 mL of formaldehyde (HCHO) was taken in a flask containing 10 g biomass and agitated at 50 0C for 36 h in an orbital shaker at 120 rpm. The same procedure was done with other sorbents used for screening. After agitation, sorbents were remised with water and dried at 60 0C (Yadamari et al., 2011). The screening was performed with eight sorbents (barley Husk (BH), rice husk (RH), mustard (MD), peanut husk (PH), sugarcane bagasse (SB), citrus (CT), sawdust (SD) and eucalyptus bark (EB)) in batch mode.
2.3 Biocomposite preparation (PPY-RH)
To enhance the biosorption characteristics of RH, a composite with polypyrrole (PPY-RH) was prepared. The biomass was mixed in pyrrole solution (0.2 M) and kept 12 h at 25 0C. After 24 h, the polymerization process was initiated by introducing a FeCl3 (0.5 M) slowly in the above contents of RH (Ishtiaq et al., 2020). In the end, polymer-coated RH was filtered, washed, and dried at 60 0C for 4 h.
2.4 Characterizations
For pHpzc determination, adsorbent was soaked in 0.1 M solution of KNO3 and pH was set in 2 to 10 range. The contents left for two days at room temperature and measure the pH change. pH effect was also investigated using a batch approach in 2–8 range, while keeping all other factors constant. Results were predicted in the form of biosorption capacity. Prepared sample was subjected to different characterization techniques viz, SEM, BET, FTIR and TGA.
2.5 Adsorption studies
For adsorption study, a column system was used having a glass column with dimensions 20 mm diameter (internal) and 42 cm height, filled with a measured quantity of adsorbent (RH and PPY-RH). A TCP solution was pumped using a peristaltic pump and effluent was collected after regular intervals followed by the measurement of absorbance at 296 nm (CE Cecil 7200 UK). Biosorption capacity was calculated by employing Eq. (1).
2.6 Kinetics study
For this study, regression analysis was used and the adsorption data was statistically analyzed. The adsorption capacity was the dependent variable in this study, while bed height, TCP inlet concentration and flow rate were independent variables. To check the adsorption kinetics, Thomas (Thomas 1944) and BDST (Bohart and Adams 1920) models were applied (Table 1).
Model
Equation
Plot
Thomas
ln [(Co/Ct)-1] vs t
BDST
Z vs t
3 Results and discussion
3.1 Biomasses efficiency comparison
The experiment was executed to evaluate the effective adsorption potential of various biomasses such as barley husk (BH), mustard (MD), sugarcane bagasse (SB), peanut husk (PH), rice husk (RH), sawdust (SD), citrus (CT) and eucalyptus bark (EB) to remove TCP from aqueous solution. The rice husk shows the promising effect for TCP adsorption (Fig. 1). This might be due to the difference in properties, i.e., binding sites available for the attachment of TCP ions. The rice husk (RH) was further used for the preparation of composite with PPY and employed for TCP sequestration in a column mode and various input variables were optimized for the same.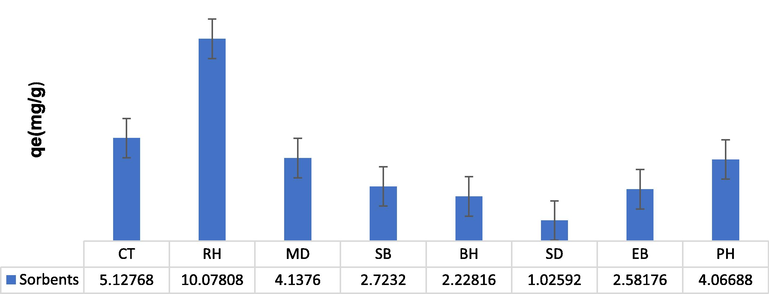
The adsorption capacity of different agro-wastes for TCP removal (adsorbent dose 0.05 g; temperature, 30 °C; contact time 90 min; particle size ≤ 300 µm).
3.2 Properties of the adsorbent
The pHpzc (point of zero charge) analysis was employed to determine the surface charge of the adsorbent since pHpzc impact for the adsorption of adsorbate on to the adsorbent is highly crucial sue to change in the surface charges of the adsorbent. For this, the adsorbent bears a positive charge if < pHpzc and this is reversed for > pHpzc and vice versa. It is reported that the pH is changed and surface activity of the adsorbent also also changed, which may favor the adsorption process or the adsorption process may decline with change in pH of the medium. As function of pHpzc, the cationic or anionic species are adsorbed or repelled by the adsorbent (Shoukat et al., 2017) (Fig. 2). The pHpzc in case of RH and PPY-RH were 7.2 and 4.8, respectively (Fig. 3). The pH influence was considered in 2–8 range and the optimum pH for RH and PPY-RH were 4 and 2, respectively (Fig. 4). Also, previous studies revealed that the pHpzc has substantial impact on the sequestration of adsorbate, i.e., 2-chlorophenol and 2, 4, 6-trichlorophenol removal was investigated using APS MCM 41 in a 1–12 pH range. The comparative uptake was significantly affected by changing the solution pH. The removal efficiency was higher in 7–11 pH range, which was reduced significantly in the acid pH range (Anbia and Lashgari 2009).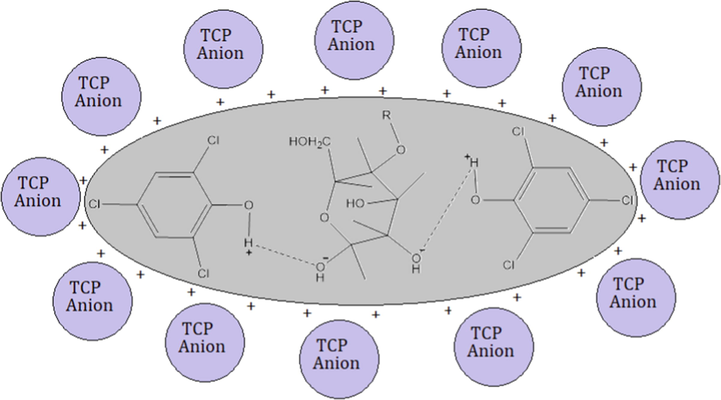
Proposed mechanism for the adsorption of TCP on the composite adsorbent.
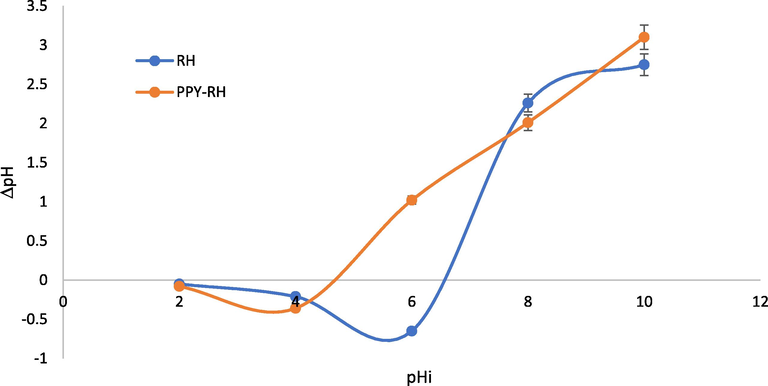
Point of zero-charges analysis of the prepared adsorbents.
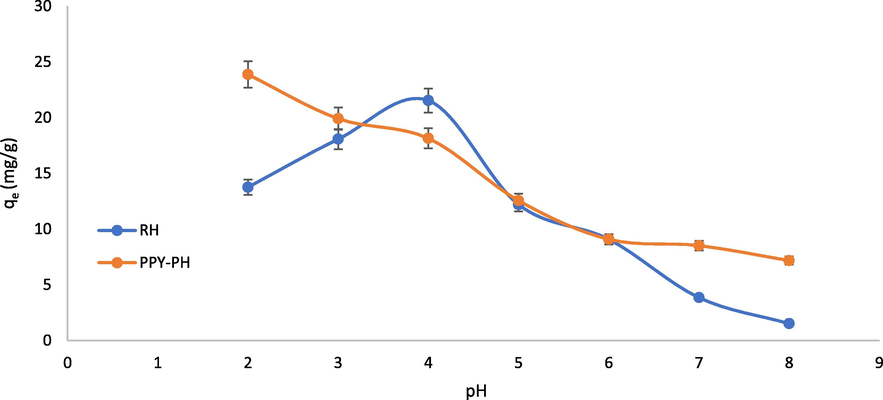
Effect of pH on the removal of TCP using RH and PPY-RH (adsorbent dose, 0.05 g; 30 °C; contact time 90 min; particle size ≤ 300 µm).
The surface properties of the prepared was studied by SEM analysis. As sorption is a surface dependent phenomenon, hence, this study is helpful to observe the change in surface morphology after adsorption. The surface of the adsorbents found to be highly heterogeneous and in comparison, the composite surface found to be rougher and have more pores, which might be more helpful in accommodating the adsorbate ions of the surface and intraparticle diffusion (Fig. 5). The adsorption studies also revealed that the PPY-RH furnished higher adsorption efficiency versus RH.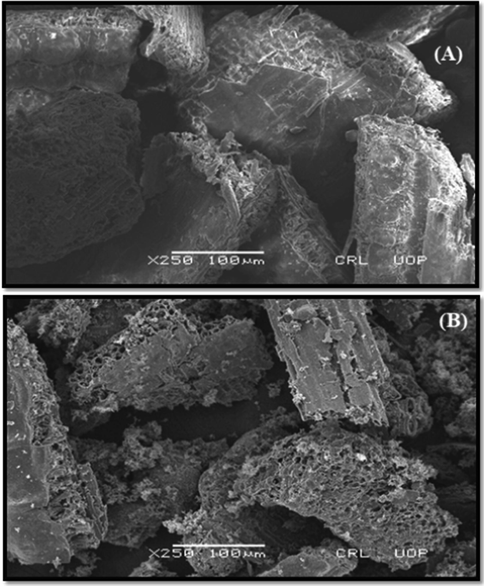
Surface morphological analysis of the prepared adsorbents, (A) RH and (B) RH-PPY.
The TGA was undertaken to estimate the stability of the adsorbent. The TGA curve shows three different weight loss regions, at 100 OC is due to dehydration of water molecule adsorbed on the adsorbent surface (Fig. 6). The second weight loss is due to degradation of cellulosic components in the adsorbent, i.e., lignin and hemicellulose degradation in 350 0C range (Chong et al., 2019).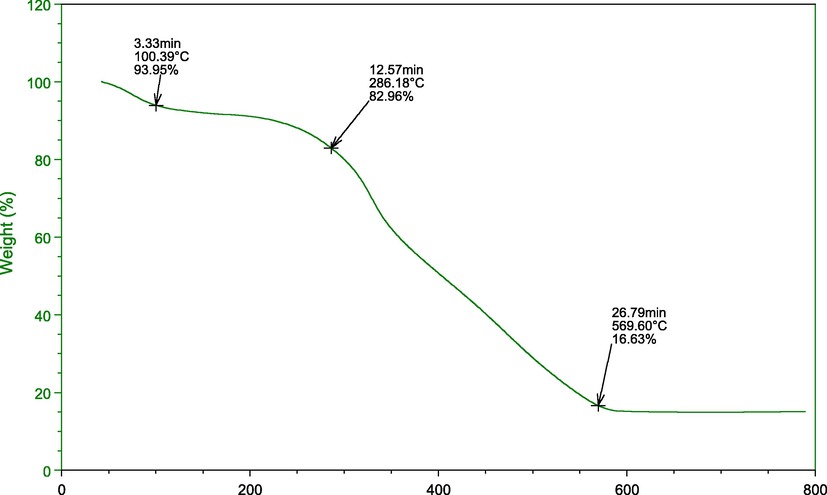
The thermogravimetric analysis (TGA) of the adsorbent.
The reactive moieties in the adsorbent were identified by FTIR analysis. The RH and PPY-RH FTIR spectra (in 650–4000 cm−1 range) and responses thus estimated are presented in Figs. 7-8. The RH spectra showed a broader band at 3334 cm−1 before adsorption, which confirmed the presence of –OH group. While, after adsorption, the RH biomass did not show the band at 3334 cm−1, which designates the involvement of –OH group in bonding with TCP. In PPY-RH spectra, an absorption band for NH– group was observed, which were vanished due the adsorption of TCP. The shifting of absorption band after adsorption of TCP indicated that the involvement of the respective functional group in the binding of TCP. BET and BJH techniques were used for surface area and surface pore analysis. Through investigation, 24.8 m2/g surface area and 23.21 μm pore diameter was observed. The graph between relative pressure and biosorption capacity confirmed Type III material according to IUPAC (Fig. 9).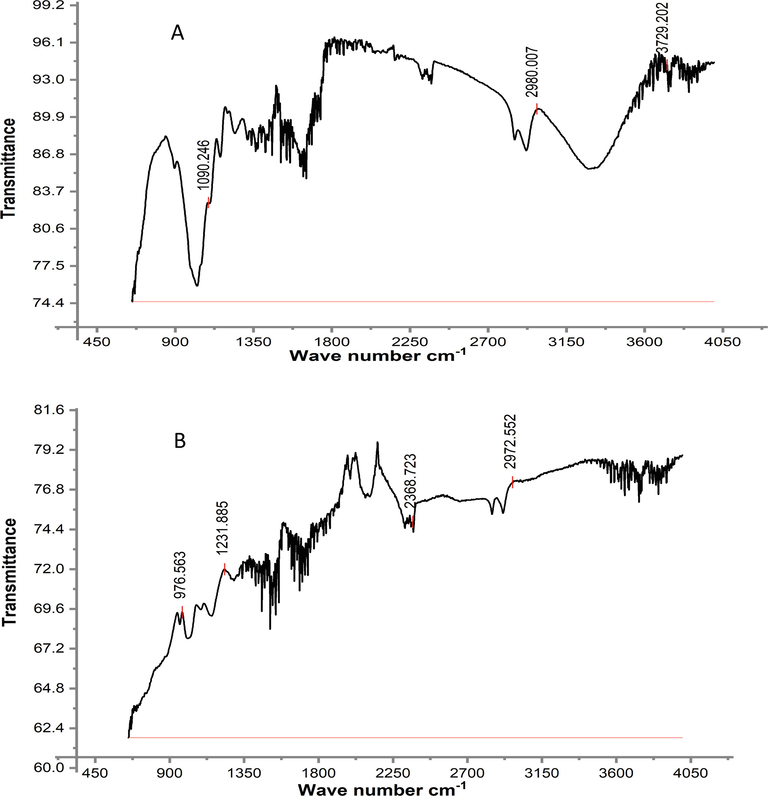
FTIR analysis of RH adsorbent, (A) before adsorption (B) after adsorption.
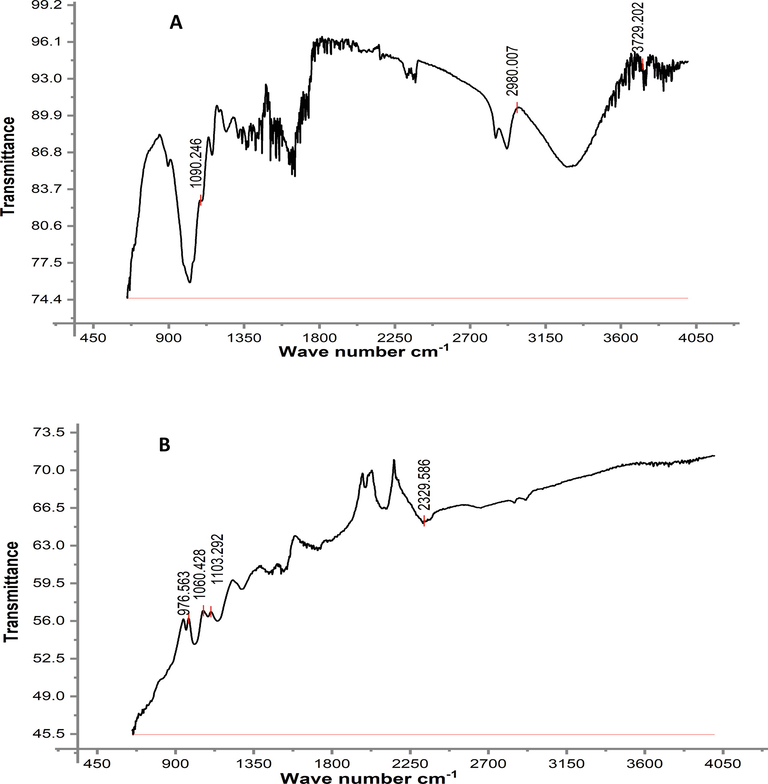
FTIR analysis of PPY-RH, (A) before adsorption (B) after adsorption.
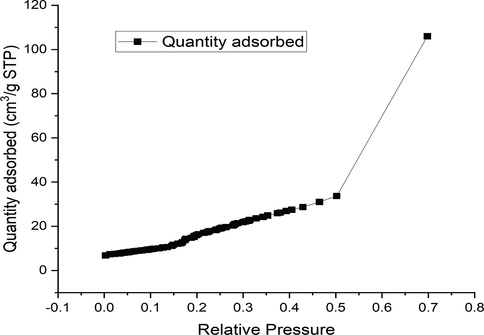
Brunauer-Emmett-Teller (BET) analysis of the PPY-RH adsorbent.
3.3 Adsorption studies
3.3.1 Bed height influence
For this study, the adsorption analysis was conducted at 2, 3 and 4 cm a bed height by keeping other factors constant, i.e., 1.8 mL/min and 50 ppm of flow rate and TCP inlet concentration and the adsorption responses thus observed are depicted in Fig. 10. The breakthrough time (increased from 90 to 185 min for RH) was enhanced with bed height increase from 2 to 4 cm, while 85 to 190 min for PPY-RH. This increase in the breakthrough time indicated an excellent intraparticle diffusion process, which enabled the higher removal capacity for TCP. Table 2 shows that the efficient TCP removal was achieved at a bed height of 4 cm, which was 6.9 and 7.72 (mg/g) for RH and PPY-RH, respectively. With bed height increase, the number of adsorbent sites increases, which enhance the removal capacity of the TCP (Noreen et al., 2016). Similarly, the bed height impact on the adsorption of adsorbate was explored and observations were in accordance with the present investigation. The saturation and breakthrough time value found to be stalwartly depended on the bed height, which is due the reason that more binding sites were available for binding the adsorbate ions at higher bed height. The removal efficiencies for different adsorbate were found to 85.53 to 93.05 (%) for the bed height in 5 to 15 cm range (Charumathi and Das 2012).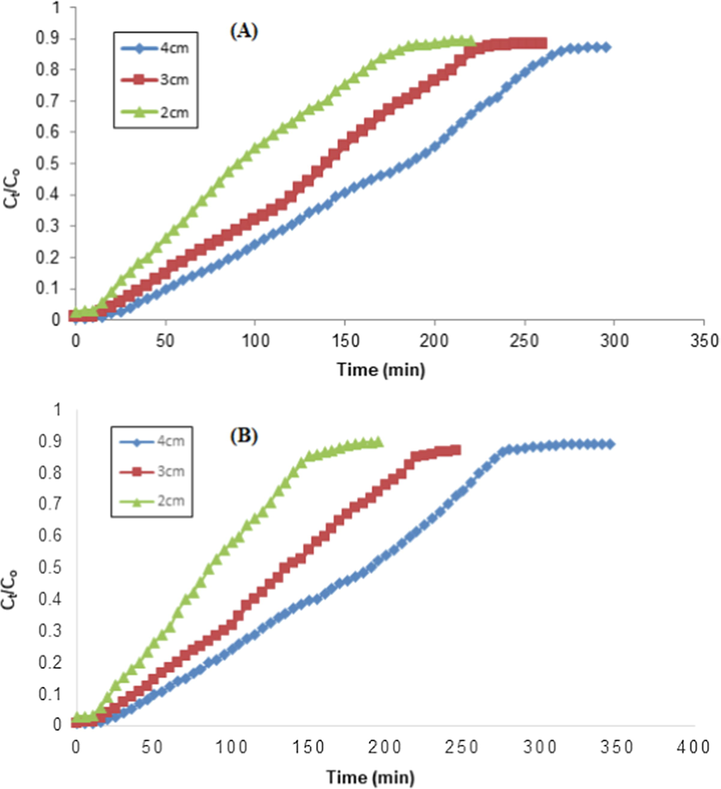
(A) Effect of bed height on the removal of TCP using RH and (B) Effect of bed height on the removal of TCP using PPY-RH (particle size 300–500 µm; pH 2, flow rate 1.8 mL/min, TCP concentration, 50 ppm).
Bed height (cm)
Flow rate (mL/min)
TCP conc. (mg/L)
Breakthrough point (min)
Adsorption capacity (mg/g)
2
1.8
50
90
5.7
3
1.8
50
140
6.6
4
1.8
50
185
6.9
4
3.6
50
85
6.3
4
5.4
50
50
5.65
4
1.8
60
160
7.2
4
1.8
70
145
7.6
3.3.2 Flow rate influence
The flow rate effect was studied in 1.8 to 5.4 mL/min while other all factors were constant. By increasing the flow rate, the resident time was decreased from 185 to 50 for RH, while 190 to 45 min for PPY-RH (Fig. 11). The earlier achievement of breakthrough time might be due to less contact time between pollutants and sorbent. It was also investigated that biosorption capacity decreases from 6.9 to 5.65 mg/g for RH, it might be due to the less resident time of TCP in the column (Noreen et al., 2016). Different column parameters are listed in Tables 2 and 3. Noreen et al. (Noreen et al., 2016) also reported similar observations, i.e., the effect of flow rate on the adsorption using sugarcane bagasse and the removal capacity was decreased from 20.2 to 13.5 mg/g by augmenting the flow rate from 7.2 to 3.6 mL/min.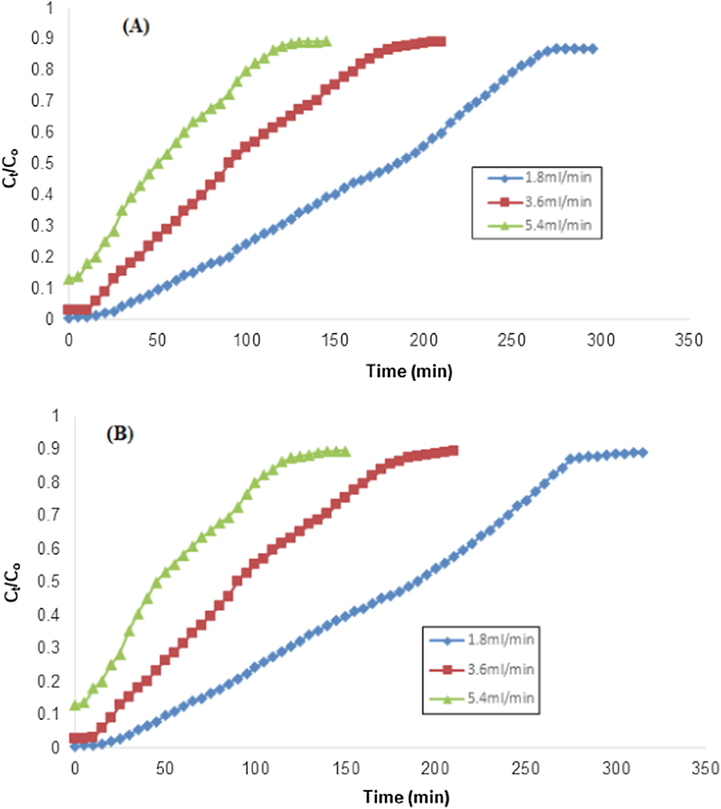
Effect of flow rate on the removal of TCP using RH and (B) Flow rate effect on the removal of TCP using PPY-RH (particle size 500–700 µm; pH 6, bed height 4 cm, TCP concentration 50 ppm).
Bed height (cm)
Flow rate (mL/min)
TCP concentration (mg/L)
Breakthrough point (min)
Adsorption capacity (mg/g)
2
1.8
50
85
6.4
3
1.8
50
135
7.15
4
1.8
50
190
7.72
4
3.6
50
85
6.9
4
5.4
50
45
5.52
4
1.8
60
170
8.35
4
1.8
70
150
8.6
3.3.3 Inlet TCP concentration influence
The TCP inlet concentration is one of imperative factors that may affect the adsorption potential of an adsorbent. The initial TCP concentration effect was studied in from 50 to 70 mg/L range and other parameters were kept constant, i.e., bed height and flow rate and responses are depicted in Fig. 12. By increasing the TCP inlet concentration from 50 to 70 mg/L, the breakthrough time was declined from 185 to 145 min for RH and in case of PPY-RH, it was reduced from 190 to 150 mg/L (Tables 2 and 3). This rapid saturation reason may be the more binding sites available at higher concentration of TCP (Noreen et al., 2016). In accordance with this study, Afroze et al. (Afroze et al., 2016) also investigated the initial concentration impact in column mode study using eucalyptus bark as an adsorbent and adsorption capacity was increased from 160 to 228 mg/g as the inlet concentration of adsorbate was enhanced from 50 to 100 mg/L.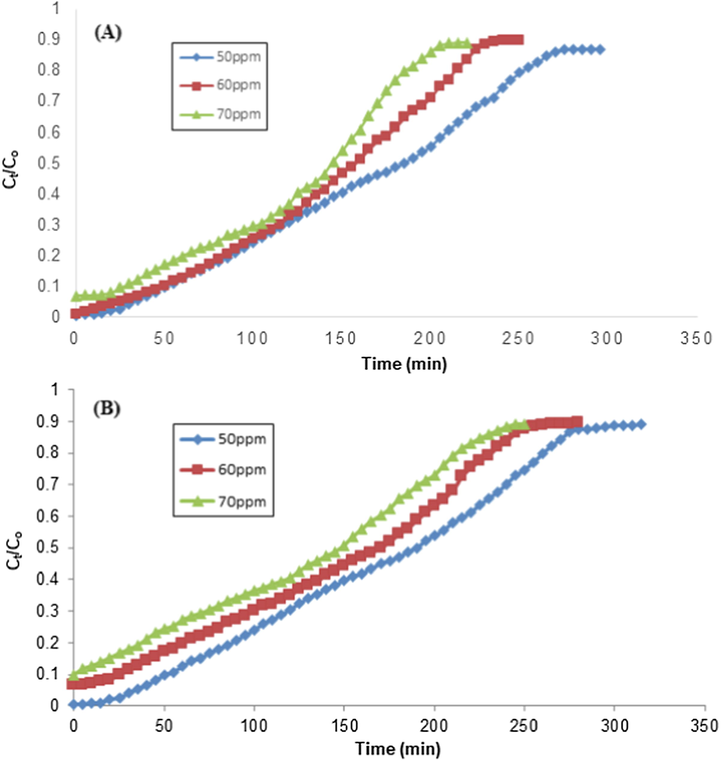
Effect of TCP initial concentration on the removal of TCP using RH and (B) Effect of TCP initial concentration on the removal of TCP using PPY-RH (particle size 300–500 µm; pH 2; bed height 4 cm; flow rate 1.8 mL/min).
3.4 Kinetics analysis
Kinetics study was performed on the TCP adsorption data onto the RH and PPY-RH using the Thomas model and observations are presented in Figs. 13-15. The TCP adsorption data was fitted well on the Thomas model. This model revealed that, no axial dispersion happened in the column, which also follows Langmuir isotherm. The Thomas model parameters, i.e., rate constant (KTh), uptake capacity (qe) and correlation coefficient (R2) were estimated regression technique. The rate of solute transfer to the adsorbent from the liquid phase is referred as rate constant (Thomas), while uptake capacity represents the efficiency of the adsorbent. By augmenting the flow rate, the Thomas rate constant was also enhanced (Tables 4).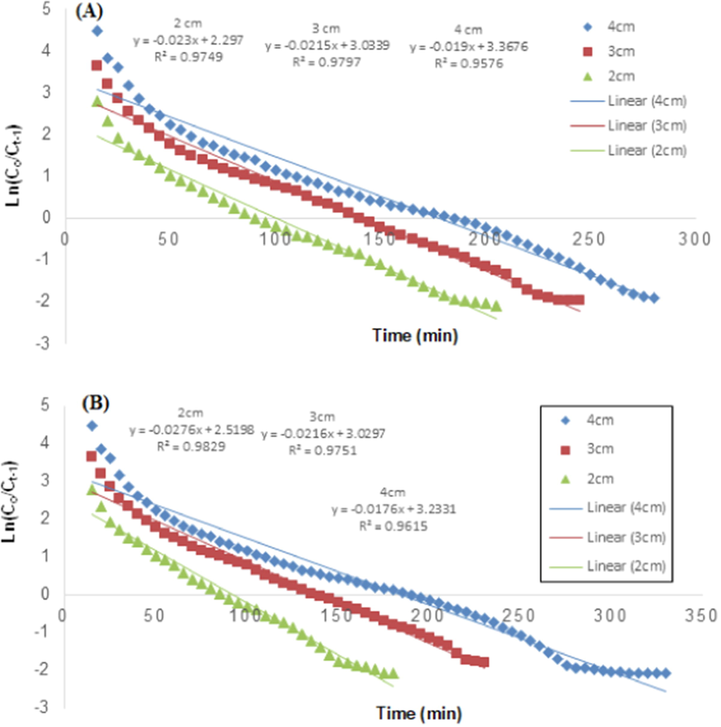
Thomas model representing the effect of bed height on TCP removal using (A) RH and (B) PPY-RH.
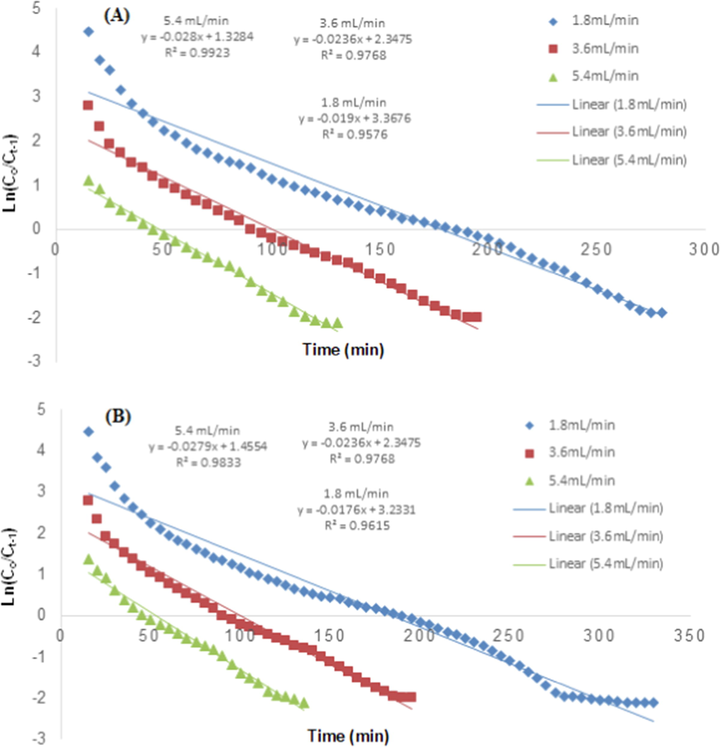
Thomas model representing the effect of flow rate for TCP removal (A) RH and (B) PPY-RH.
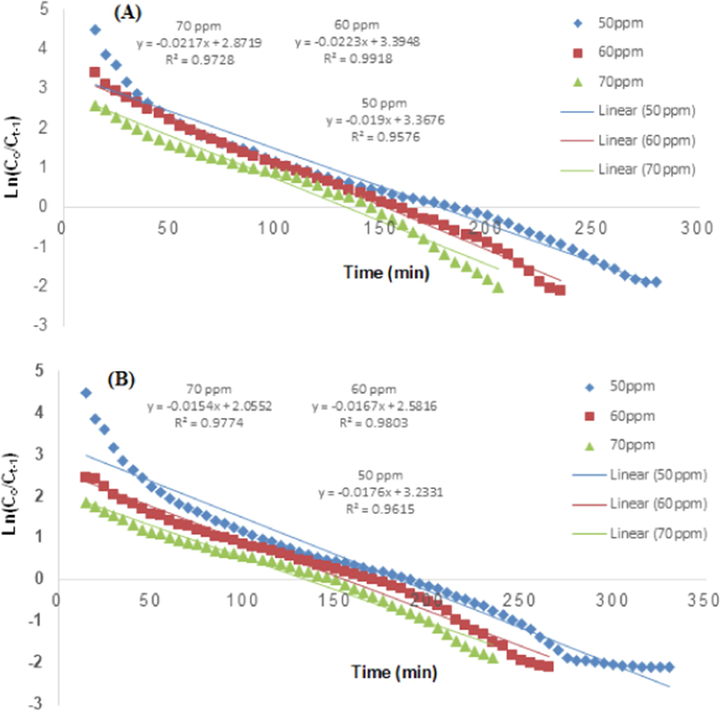
Thomas model representing the effect of initial TCP concentration (A) RH and (B) PPY-RH.
Inlet concentration (mg/L)
Bed height (cm)
Flow rate (mL/min)
KTH (mL/min.mg) × 103
q0cal (mg/g)
R2
50
2
1.8
0.47
6.05
0.99
50
3
1.8
0.38
6.42
0.99
50
4
1.8
0.32
6.5
0.99
50
4
3.6
0.43
5.74
0.99
50
4
5.4
0.69
4.63
0.99
60
4
1.8
0.3
6.63
0.99
70
4
1.8
0.2
8.03
0.99
Thomas model for the removal of TCP using PPY-RH
50
2
1.8
0.392
10.11
0.99
50
3
1.8
0.324
10.35
0.99
50
4
1.8
0.236
10.48
0.98
50
4
3.6
0.36
8.94
0.99
50
4
5.4
0.452
8.82
0.99
60
4
1.8
0.226
10.46
0.99
70
4
1.8
0.197
10.33
0.98
The BDST model is also commonly used for approximation of adsorption processes (Bohart and Adams 1920). The analysis was done to evaluate the adsorption behavior at various bed heights using the optimum flow rate and inlet concentration of TCP and observations are depicted in Fig. 16. The BDST parameters are presented in Tables 5. Value of regression coefficient for different cased (0.2, 0.4 and 0.6 Ct/C0 values) for both RH and PYY-RH composite are predicted, which revealed the good validation of BDST model for TCP removal using RH and PPY-RH. It was observed that N0 value increased by increasing the bed height. The BDST model plots were linear having high correlation coefficient, which revealed the validity this model for TCP adsorption using PYY-RH adsorbent and composite furnished excellent efficiency for the removal of TCP in a continuous adsorption mode.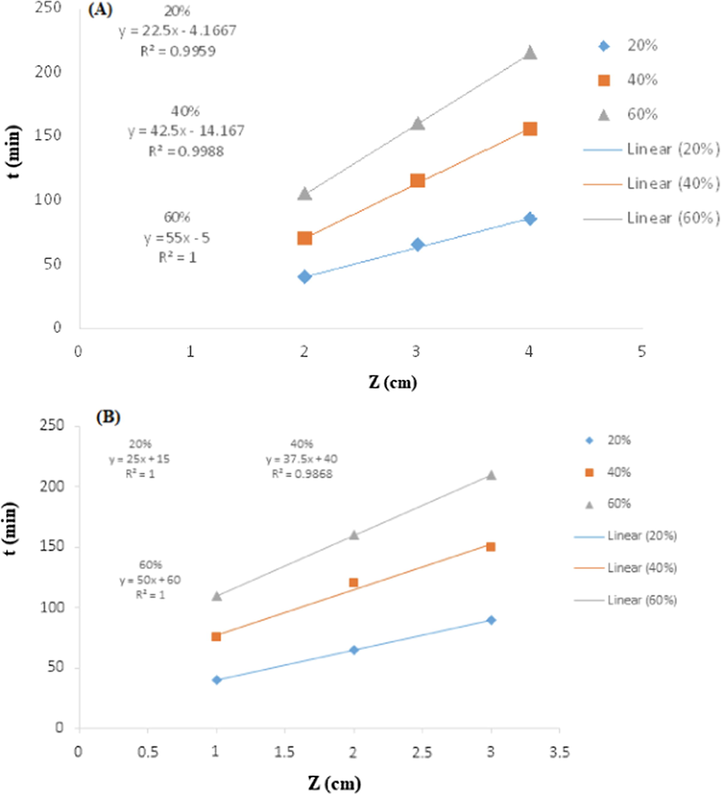
The Bed Depth Service Time (BDST) model, (A) PPY-RH (B) RH.
Ct/Co
a
b
Ka (L/min mg) × 104
No (mg/L) × 10-4
R2
0.2
0.4
0.625
37.5
50−15
−40
−60−18.5
−2.02
1.340.199
0.298
0.3981
0.99
1
Bed Depth Service Time parameters for the removal of TCP using PPY-RH
0.2
0.4
0.622.5
42.5
5.04.17
14.17
5.0−16.16
5.7
66.70.437
0.338
0.1790.996
0.998
1
3.5 Desorption and recovery study
For the application of adsorbents in repeated cycles, it is necessary to recycle and regenerate the adsorbent. The adsorbent with high adsorption potential and regeneration property makes the adsorbent cost effective. Hence, the desorption of TCP was performed for repeated cycles to recycling and regeneration of the prepared adsorbent and observation are presented in Fig. 17. The adsorption of TCP in acidic condition suggests that by providing the basic conditions, the TCP can be eluted to regenerate the adsorbent for next cycle, which was performed using NaOH solution. The TCP was eluted up to 16 % using 0.5 M NaOH, which revealed that the adsorbent can be recycled and regenerated repeated adsorption of TCP.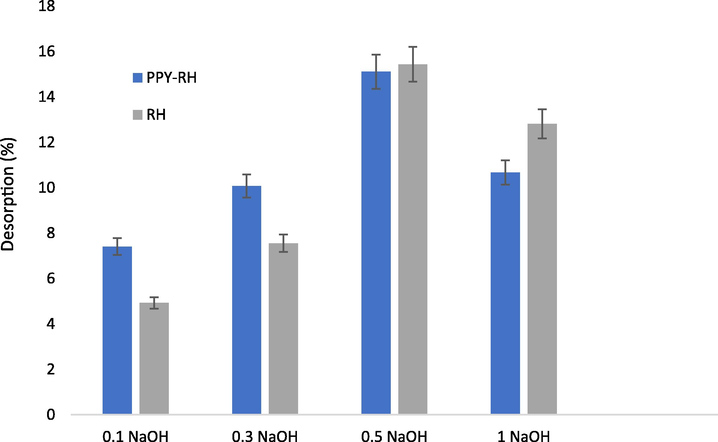
Desorption of the TCP from the RH-PPY using different concentrations of eluting agent.
4 Conclusions
The rich husk composite with polypyrrole was fabricated and utilized for the TCP removal in a column mode and flow rate, bed height and initial TCP inlet concentration impact was studied. The breakthrough time was decreased by decreasing the bed depth and reversed for flow rate and inlet concentration of TCP. At low bed height and initial TCP concentration, the sequestration efficiency was declined at higher flow rate. The Thomas and BDST models fitted well to the TCP experimental data. The composite showed promising stability, which is useful to perform the adsorption of TCP at higher temperature. The rice husk composite with PPY has potential for the sequestration of TCP, which can be utilized for the adsorptive remediation of TCP. The process variables have significant effect on the removal of TCP, which need to be optimized for effective TCP removal from the effluents in a column mode.
Research funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R156), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through Research Groups Program under grant number R.G.P.1:255/43.
Acknowledgments
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R156), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through Research Groups Program under grant number R.G.P.1:255/43.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using Eucalyptus sheathiana bark biomass. Res. Chem. Intermed.. 2016;42:2343-2364.
- [Google Scholar]
- Detoxification of photo-catalytically treated 2-chlorophenol: optimization through response surface methodology. Water Sci. Technol.. 2017;76:323-336.
- [Google Scholar]
- Synthesis of amino-modified ordered mesoporous silica as a new nano sorbent for the removal of chlorophenols from aqueous media. Chem. Eng. J.. 2009;150:555-560.
- [Google Scholar]
- Synthesis, characterization and photocatalytic activity of ZnO flower and pseudo-sphere: Nonylphenol ethoxylate degradation under UV and solar irradiation. J. Alloy. Compd.. 2016;678:126-136.
- [Google Scholar]
- Adsorptive removal of Pb (II) and Cd (II) ions from aqueous solution onto modified Hiswa iron-kaolin clay: equilibrium and thermodynamic aspects. Chem. Int.. 2021;7:139-144.
- [Google Scholar]
- Adsorption of 2, 4-dichlorophenoxyacetic acid using rice husk biochar, granular activated carbon, and multi-walled carbon nanotubes in a fixed bed column system. Water Sci. Technol.. 2018;78:1812-1821.
- [Google Scholar]
- Bioaccumulation of trace metals by red alga Corallina elongata in the coast of Beni Saf, west coast, Algeria. Chem. Int.. 2017;3:220-231.
- [Google Scholar]
- Biocomposites of polypyrrole, polyaniline and sodium alginate with cellulosic biomass: Adsorption-desorption, kinetics and thermodynamic studies for the removal of 2,4-dichlorophenol. Int. J. Biol. Macromol.. 2020;153:146-157.
- [Google Scholar]
- Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc.. 1920;42:523-544.
- [Google Scholar]
- Packed bed column studies for the removal of synthetic dyes from textile wastewater using immobilised dead C. tropicalis. Desalination. 2012;285:22-30.
- [Google Scholar]
- Catalytic pyrolysis of cellulose with oxides: effects on physical properties and reaction pathways. Clean Technol. Environ. Policy. 2019;21:1629-1643.
- [Google Scholar]
- The United Nations World Water Development Report 2015: Water for a Sustainable World (Vol 1). Paris: UNESCO Publishing; 2015.
- Comparative experimental study on the COD removal in aqueous solution of pesticides by the electrocoagulation process using monopolar iron electrodes. Chem. Int.. 2017;3:420-427.
- [Google Scholar]
- Textile wastewater in Tlemcen (Western Algeria): impact, treatment by combined process. Chem. Int.. 2017;3:414-419.
- [Google Scholar]
- Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. Environ. Sci. Pollut. Res.. 2017;24:7511-7520.
- [Google Scholar]
- Adsorption of crystal violet dye onto olive leaves powder: equilibrium and kinetic studies. Chem. Int.. 2021;7:79-89.
- [Google Scholar]
- Adsorption of 2, 4, 6-trichlorophenol on bentonite modified with benzyldimethyltetradecylammonium chloride. Chem. Int.. 2018;4:24-32.
- [Google Scholar]
- Polychlorinated biphenyls remediation in soil using moringa seeds and coconut shell based adsorbents. Chem. Int.. 2020;6:304-309.
- [Google Scholar]
- Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: a review. Chem. Int.. 2019;5:1-80.
- [Google Scholar]
- Gamma radiation/H2O2 treatment of a nonylphenol ethoxylates: degradation, cytotoxicity, and mutagenicity evaluation. J. Hazard. Mater.. 2015;299:351-360.
- [Google Scholar]
- Polypyrole, polyaniline and sodium alginate biocomposites and adsorption-desorption efficiency for imidacloprid insecticide. Int. J. Biol. Macromol.. 2020;147:217-232.
- [Google Scholar]
- Batch versus column modes for the adsorption of radioactive metal onto rice husk waste: conditions optimization through response surface methodology. Water Sci. Technol.. 2017;76:1035-1043.
- [Google Scholar]
- Kinetics and equilibrium of radioactive metal adsorption onto sugarcane bagasse waste: comparison of batch and column adsorption modes. Zeitschrift Phys. Chem.. 2021;235:281-294.
- [Google Scholar]
- Khalid, Q.-u.-a., A. Khan, H. Nawaz Bhatti, et al., 2021. Cellulosic biomass biocomposites with polyaniline, polypyrrole and sodium alginate: Insecticide adsorption-desorption, equilibrium and kinetics studies. Arabian Journal of Chemistry. 103227
- Composite of polypyrrole with sugarcane bagasse cellulosic biomass and adsorption efficiency for 2,4-dicholrophonxy acetic acid in column mode. J. Mater. Res. Technol.. 2021;15:2016-2025.
- [Google Scholar]
- Activated carbon from molasses efficiency for Cr (VI), Pb (II) and Cu (II) adsorption: a mechanistic study. Chem. Int.. 2017;3:301-310.
- [Google Scholar]
- Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater.. 2020;382:121040
- [Google Scholar]
- Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem. Int.. 2017;3:392-405.
- [Google Scholar]
- Zinc oxide nanoparticles fabrication using Eriobotrya japonica leaves extract: photocatalytic performance and antibacterial activity evaluation. Arabian J. Chem.. 2021;14:103251
- [Google Scholar]
- Continuous fixed bed removal of Novacron Orange P-2R using sugarcane bagasse: prediction of breakthrough curves. Desalin. Water Treat.. 2016;57:12814-12821.
- [Google Scholar]
- FeVO4 nanoparticles synthesis, characterization and photocatalytic activity evaluation for the degradation of 2-chlorophenol. Desalin. Water Treat.. 2020;187:399-409.
- [Google Scholar]
- Mango stone biocomposite preparation and application for crystal violet adsorption: a mechanistic study. Micropor. Mesopor. Mater.. 2017;239:180-189.
- [Google Scholar]
- Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc.. 1944;66:1664-1666.
- [Google Scholar]
- Biosorption of malathion from aqueous solutions using herbal leaves powder. Am. J. Anal. Chem.. 2011;2:37.
- [Google Scholar]







