Translate this page into:
Polyvinyl alcohol–sulphanilic acid water soluble composite as corrosion inhibitor for mild steel in hydrochloric acid medium
*Corresponding author. Tel.: +91 9942460747 srimathichem@gmail.com (M. Srimathi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 2 December 2010

Peer review under responsibility of King Saud University.
Abstract
The inhibitive action of synthesised polyvinyl alcohol–sulphanilic acid (PVASA) composite on the corrosion of commercial mild steel in 1 M HCl medium has been investigated by weight loss, potentiodynamic polarization, and electrochemical impedance spectroscopic (EIS) methods. Characterization of PVASA composite has been carried out using Fourier transform infrared spectroscopy (FTIR). Experimental results reveal that PVASA composite acts as an inhibitor in the acid environment. The inhibition efficiency increases with an increase in the concentration of the inhibitor. Maximum inhibition efficiency of PVASA composite was found to be 84% at 6000 ppm. Thermodynamic and kinetic parameters have been obtained from temperature studies. Electrochemical measurement reveals that PVASA composite acts as a mixed inhibitor and the adsorption follows Langmuir adsorption isotherm.
Keywords
Corrosion
Polymer composite
Weight loss method
Electrochemical measurements
FTIR spectroscopy

1 Introduction
Corrosion is a serious environmental problem in the oil, fertilizer, metallurgical and other industries (Eddy and Mamza, 2009; Eddy, 2009, 2010; Aytac et al., 2005). Valuable metals, such as mild steel, aluminum, copper and zinc are prone to corrosion when they are exposed to aggressive media (such as, acids, bases and salts) (Ebenso et al., 2009; Odoemelam et al., 2009; Odiongenyi et al., 2009). Therefore, there is a need to protect these metals against corrosion. The use of inhibitors has been found to be one of the best options available for the protection of metals against corrosion (Eddy et al., 2009a). The most efficient corrosion inhibitors are organic compounds containing electronegative functional groups and π electrons in their triple or conjugated double bonds (Emregu et al., 2006). The initial mechanism in any corrosion inhibition process is the adsorption of the inhibitor on the metal surface (El Ashry et al., 2006; Eddy et al., 2009b; Khaled, 2008; Xia et al., 2008). The adsorption of the inhibitor on the metal surface can be facilitated by the presence of hetero atoms (such as N, O, P and S) as well as an aromatic ring. The inhibition of the corrosion of metals can also be viewed as a process that involves the formation of a chelate on the metal surface, which involves the transfer of electrons from the organic compounds to the surface of the metal and the formation of a coordinate covalent bond. In this case, the metal acts as an electrophile while the nucleophilic centre is in the inhibitor.
Polymers find applications as effective corrosion inhibitors for steel (Olivares et al., 2006). The use of polymers as corrosion inhibitors have drawn considerable attention recently due to their inherent stability and cost effectiveness. Owing to the multiple adsorption sites, polymeric compounds adsorb more strongly on the metal surface compared with their monomer analogues (Ali and Saeed, 2001). Therefore, it is expected that the polymers will be better corrosion inhibitors.
The Literature reveals that a wide range of polymeric compounds have been successfully investigated as potential inhibitors for the corrosion of metals in aggressive media. Polymers such as polyethylenimine and polyvinylpyrrolidone, poly(o-phenylenediamine), polyanthranilic acid, polyacrylic acid, polyvinyl pyridine and polyvinylpyrrolidone, maleic anhydride and N-vinyl-2-pyrrolidone, polyamino-benzoquinone, polyvinyl alcohol, and polyethylene glycol have been reported (Schweinsberg et al., 1996; Abd El Rehim et al., 2010; Shukla et al., 2008; Amin et al., 2009; Mostafa Abo El-Khair, 1986; Achary et al., 2008; Muralidharan et al., 1995; Umoren et al., 2006a,b).
In continuation of our quest for developing corrosion inhibitors with high effectiveness and efficiency, the present paper aims at the utilization of oxidatively polymerized PVASA composite as corrosion inhibitor by weight loss, potentiodynamic polarization and electrochemical impedance spectroscopy. The effect of temperature on corrosion and inhibition processes are thoroughly assessed and discussed. Thermodynamic parameters governing the adsorption process were also calculated and discussed. FTIR spectroscopic technique was used to reveal the formation of PVASA composite.
2 Experimental method
2.1 Chemicals and reagents
Ammonium per sulphate (APS), p-sulphanilic acid (p-aminobenzene sulphonic acid), polyvinyl alcohol (Mw = 14,000) from Merck chemicals.
2.2 Polyvinyl alcohol–sulphanilic acid composite preparation (PVASA composite)
A standard procedure was adopted to prepare polyvinyl alcohol–sulphanilic acid polymer composite (Trivedi, 1997; Gangopadhyay et al., 2001; Mirmohseni and Wallace, 2003). 1% p-Sulphanilic acid was well mixed with 10% polyvinyl alcohol solution. The system was cooled at 0–5 °C followed by the addition of 20 ml of aqueous oxalic acid solution of ammonium persulphate. Sulphanilic acid to ammonium persulphate mole ratio was maintained 1:1. Polymerization was allowed to proceed for 3 h. The polymer composite was formed. This was treated with ammonium hydroxide for deprotonation. The pH of this solution was kept around 9.0 by adding drops of 1 M NH4OH and kept for 5 h. It was isolated from the medium by precipitation technique using a non-polar solvent and dried under vacuum.
2.3 Weight loss methods
Weight loss measurements were carried out using a Denvar balance. The mild steel samples were obtained from a locally available industrial Fe–C steel with very low concentration of carbon. A large sheet of cold rolled mild steel coupons with a chemical composition of carbon 0.106%, manganese 0.196%, silicon 0.006%, phosphorus 0.027%, sulphur 0.016%, chromium 0.022%, molybdenum 0.003%, nickel 0.012% and iron 99.612% were utilized for the present study. The mild steel samples, with an active surface of 1 × 5 cm2 were mechanically abraded, degreased, washed in double distilled water and dried in warm air.
The experiments were performed in 1 M HCl solution without inhibitor and in the presence of PVASA composite at different concentrations: 600, 1200, 1800, 2400, 3000, 3600, 4200, 4800, 5400 and 6000 ppm at various immersion times: 0.5, 1, 3, 6, 12, 24 and 48 h and at various temperatures 303, 313, 323, 333 and 343 K.
2.4 Electrochemical measurements
2.4.1 Polarization and impedance studies
The experiments were performed in a classical three-electrode electrochemical cell. Mild steel specimen of 1 cm2 area was used as the working electrode and platinum electrode as a counter electrode and saturated calomel electrode as a reference electrode. Prior to each experiment the working electrode surface was polished with emery paper. Solartron Electrochemical analyzer (model 1280B) interface with an IBM computer and corrware and z-plot corrosion software were used for data acquisition and analysis. For polarization and impedance studies the period of immersion was for 30 min. Polarization technique was carried out using corrware software from a cathodic potential of −0.1 V to an anodic potential of −1 V with respect to corrosion potential at a sweep rate 2 mV/s. AC signals of 10 mV amplitude and a frequency spectrum from 20 to 0.1 Hz were impressed and the Nyquist representation of the impedance data were analysed with Z view software.
2.5 FTIR spectra
To characterize the synthesized PVASA composite, the FTIR analysis of PVA, SA and PVASA were carried out (Fig. 1). The important peaks are assigned and discussed with references.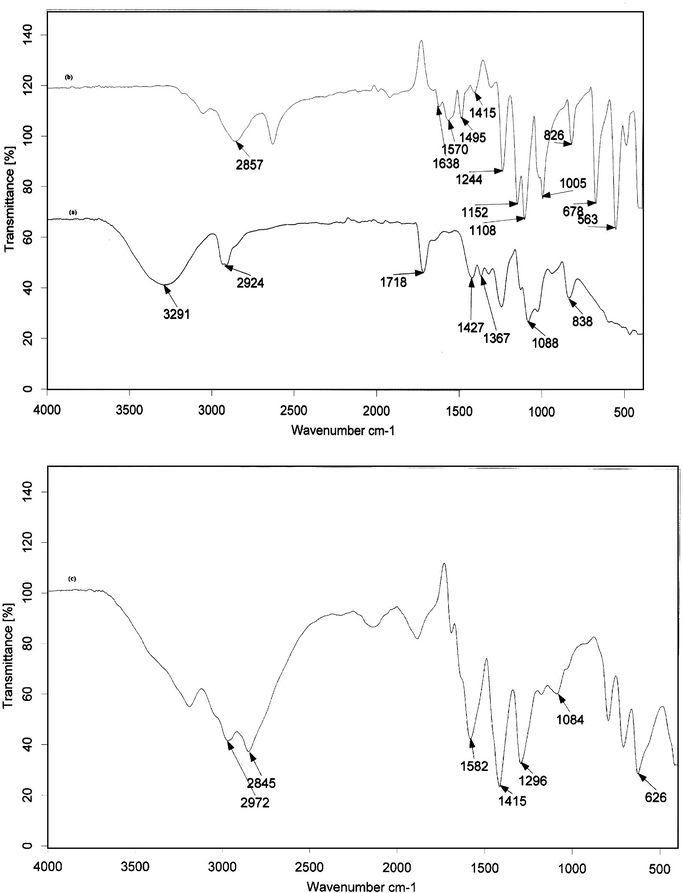
IR spectrum of (a) polyvinyl alcohol (PVA); (b) sulphanilic acid (SA); (c) polyvinyl alcohol–sulphanilic acid composite (PVASA).
A broad and strong peak centred at 3291 cm−1 is due to the stretching vibrations of –OH group with strong hydrogen bonding of intra and inter types in PVA. This peak is found to be shifted to 3188 cm−1 in the PVASA composite (Chen, 2002). The adsorption peak at 2924 cm−1 assigned to –CH and –CH2 asymmetric stretching vibrations in PVA has been shifted to 2972 and 2845 cm−1 in PVASA composite (Rajendran et al., 2004a). A peak at 1718 cm−1 associated with C⚌O stretching vibrations of PVA backbone is found to be shifted to a lower wave number in (1696 cm−1) in the PVASA composite (Gandhi et al., 2011). The absorption peak due to –CH2 bending observed in PVA at 1427 cm−1 is found to be shifted to a lower wave number (1415 cm−1) in PVASA composite (Yu et al., 2003). A band at 1367 cm−1 associated with C–H/OH bending is found in PVA (Rajendran et al., 2004b). A sharp band at 1088 cm−1 due to C–O–C stretching of acetyl group present in PVA backbone is found to be shifted to 1084 cm−1 in the PVASA composite (Yurudu et al., 2006). The absorption band at 838 cm−1 corresponds to CH rocking vibrations of PVA (Rajendran et al., 2004a,b).
A band at 2857 cm−1 corresponds to CH symmetric and anti-symmetric vibrations of SA (Saravanan et al., 2006). A peak at 1495 cm−1 in SA indicates the presence of benzene ring in aromatic compounds (ring stretching). Bands at 1570 and 1638 cm−1 of SA are due to charged amine derivatives in sulphanilic acid and –NH2 group (NH2 deformation in primary amines). This band was shifted to 1582 cm−1 in the PVASA composite. A peak at 1244 cm−1 is due to C–N stretching of aromatic amine of SA. This band was shifted to 1296 cm−1 in the PVASA composite (Bhandari et al., 2010). The band at 1152 cm−1 is due to asymmetrical stretching of SO3 group in SA (Bhandari et al., 2010). The peak at 1005 and 1033 cm−1 are related to symmetric stretching of S⚌O bond in SA. Another peak at 1415 cm−1 is also related to stretching of SO3 group in SA. This was also present in the PVASA composite. A peak at 1108 cm−1 due to the stretching vibrations of Ar–S bond in SA is shifted to a lower wave number 1084 cm−1 in PVASA composite (Xu et al., 2007). A peak at 678 cm−1 is related to special stretching of C–S bond in SA. This was shifted to 626 cm−1 in PVASA composite. A band at 826 cm−1 is due to the aromatic C–H out of plane bending vibrations of disubstituted benzene ring of SA (Bhandari et al., 2010).
The changes in the vibrational frequencies of the above mentioned peaks in the FTIR spectrum of PVA, SA and PVASA confirm the formation of PVASA composite.
3 Results and discussion
3.1 Weight loss method
3.1.1 Effect of concentration
Effect of PVASA composite on the corrosion of mild steel in 1 M HCl was studied by weight loss measurements at room temperature (303 K) and the results are tabulated in Table 1. From the weight loss values, the percentages of inhibition efficiencies (IE) were calculated using the following equation (Al Shamma et al., 1987):
S. No.
Concentration of inhibitor (ppm)
Immersion time (h)
0.5
1
3
6
12
24
48
1.
600
74.2
75.1
70.0
67.8
50.4
58.8
32.3
2.
1200
75.9
79.2
73.9
73.0
60.5
64.4
35.5
3.
1800
78.0
82.8
79.6
73.7
63.9
67.8
44.1
4.
2400
78.7
84.3
79.6
75.3
67.1
69.5
45.8
5.
3000
78.9
84.3
80.9
76.5
68.0
72.6
55.3
6.
3600
79.7
85.2
82.4
76.9
69.9
73.7
56.0
7.
4200
79.8
86.1
83.2
80.5
71.5
77.9
56.8
8.
4800
79.8
86.5
84.3
80.6
71.8
78.1
57.4
9.
5400
80.9
86.8
85.2
82.8
73.4
79.3
59.7
10.
6000
80.8
90.5
85.7
83.9
74.1
79.9
63.0
3.1.2 Effect of immersion time
Table 1 illustrates the variation of inhibition efficiency with time for the corrosion of mild steel in 1 M HCl containing various concentrations of PVASA composite. From Table 1, it can be seen that the inhibition efficiency varies from 84% (at 1 h) to 80% (at 24 h) and then reached 63% (at 48 h). This behaviour can be explained on the basis that prolonged immersion of steel in acidic solutions, (a) allows the cathodic or hydrogen evolution kinetics to increase presumably as more cathodic or carbon containing sites are exposed by the corrosion process (Zakvi and Mehta, 1987) and (b) increase the concentration of ferrous ion which is known for its stimulation of corrosion attack of the acid on the base metal. PVASA composite is found to be a good inhibitor from immersion studies results.
3.1.3 Effect of temperature
The influence of temperature on the corrosion behaviour of mild steel in acid medium in the presence of various concentrations of PVASA composite is investigated by weight loss trends in the temperature range of 303–343 K. The variation of inhibition efficiency with increasing temperature is shown in Fig. 2. The behaviour of PVASA composite at 303–343 K may be attributed to the adsorption PVASA composite up to 6000 ppm and a further increase in temperature brings about desorption of the PVASA composite under study.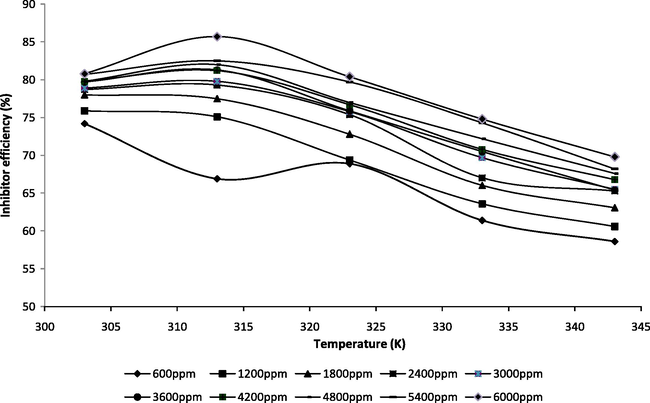
Variation of inhibition efficiency with temperature at various inhibitor concentrations.
This may be explained as follows, adsorption and desorption of inhibitor molecules continuously occur at the metal surface and the equilibrium exists between these two processes at particular temperature, with the increase of temperature the equilibrium between adsorption and desorption process is shifted leading to a higher desorption rate than adsorption until equilibrium is again established at a different value at equilibrium constant. It explains the lower inhibition efficiency at higher temperature. Fig. 2 clearly infers that the inhibition efficiency of PVASA composite for mild steel corrosion decreases with increase in temperature, supporting the mechanism of physical adsorption. For a physical adsorption mechanism, the inhibition efficiency of the inhibitor is expected to decrease with the increase in temperature as observed in this work (Ebenso and Oguzie, 2005).
3.2 Adsorption isotherm
Adsorption isotherms are very important in determining the mechanism of organo electrochemical reaction (Damaskin et al., 1971). The most frequently used isotherms are Langmuir, Frumkin, Temkin, Flory–Huggin, Freundlich, Dhar–Flory–Huggin, kinetic/thermodynamic model of El-Awady et al. and Bockris-Swinkels (Ikedia et al., 1982; Dhar et al., 1973; Umoren et al., 2008). All these isotherms are of the general form:
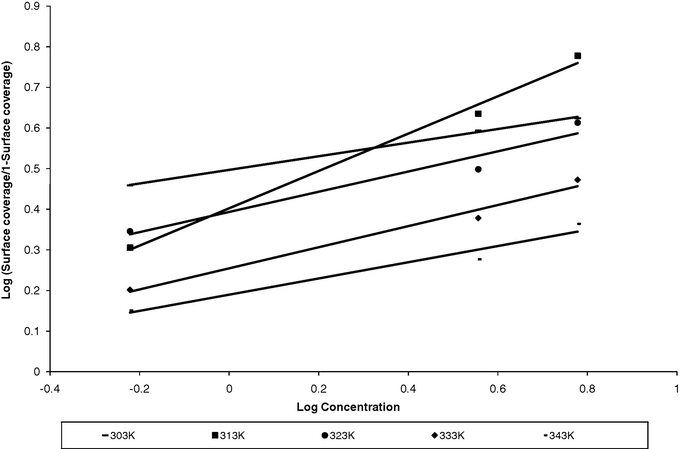
Plot of Langmuir isotherm.
Temperature
R2
303
0.9983
313
0.9926
323
0.9484
333
0.9815
343
0.9577
3.3 Energy of activation and thermodynamic parameters
3.3.1 Activation energy
The kinetic parameter of the system under consideration was evaluated using the data obtained from weight loss method for various concentration of the PVASA composite at different temperature. Activation energy (Ea) of corrosion reaction was calculated from Arrhenius equation (Taha et al., 1995):
It is clear that the addition of PVASA composite to the acid solution increases the value of Ea. The increase in Ea is proportional to the inhibitor concentration. Such a trend suggests that the corrosion reaction will be further pushed to the surface sites that are characterized by progressively higher values of Ea as the concentration of the inhibitor becomes larger. This means that the adsorption of PVASA composite on mild steel surface leads to the formation of a barrier layer that retards the metal activity in the electrochemical reactions of corrosion. Szauer and Brand (1981) explained that the increase in activation energy can be attributed to an appreciable decrease in the adsorption of the inhibitor on the mild steel surface with increase in temperature and a corresponding increase in corrosion rates due to the fact that a greater area of metal is exposed to the acid environment.
3.3.2 Thermodynamic parameters
With the help of the temperature study results, thermodynamic parameters such as ΔG, ΔH and ΔS were calculated. Standard free energy values of adsorption at different temperatures were derived from Langmuir plot using the relation (Vashi and Champaneri, 1997):
S. No.
Concentration of inhibitor (ppm)
Activation energy, Ea (kJ/mol)
Free energy of adsorption, −ΔG (kJ/mol)
Change in enthalpy, ΔH (kJ/mol)
Change in entropy, ΔS (kJ/mol)
303 K
313 K
323 K
333 K
343 K
1.
Blank
51.8
–
–
–
–
–
–
–
2.
600
61.4
14.0
13.6
14.3
13.8
13.9
−14.3
1.3
3.
1200
63.6
12.5
12.8
12.5
12.1
12.1
−17.5
16.0
4.
1800
64.8
11.8
12.1
11.4
11.3
12.1
−12.3
1.7
5.
2400
64.9
11.2
11.6
11.4
10.6
11.3
−13.8
8.1
6.
3000
64.5
10.6
11.1
10.9
10.4
10.1
−16.3
17.8
7.
3600
64.8
10.3
10.9
10.4
10.0
9.6
−17.9
23.9
8.
4200
64.2
9.9
10.5
10.1
9.6
9.3
−16.8
21.7
9.
4800
64.1
9.6
10.3
9.8
9.4
9.3
−14.3
14.4
10.
5400
63.6
9.5
10.1
9.9
9.4
8.8
−16.0
20.2
11.
6000
62.8
9.2
10.4
9.7
8.8
8.7
−17.6
25.9
3.4 Electrochemical measurements
3.4.1 Potentiodynamic polarization studies
The potentiodynamic polarization curves of mild steel in 1 M HCl with the addition of various concentrations of PVASA composite is shown in Fig. 4. The corrosion kinetic parameters such as corrosion potential (Ecorr), corrosion current density (Icorr), anodic Tafel slope (ba) and cathodic Tafel slope (bc) deduced from the curves are given in Table 4. The corrosion current density values decrease from 130.6 mA/s of the blank acid to 24.6 mA/s for the addition of 6000 ppm of PVASA composite resulting in 81.1% of inhibition efficiency. The increase in concentration of the inhibitor decreases the Icorr values. Ecorr, ba and bc values do not change appreciably with the addition of the inhibitor indicating that the inhibitor is not interfering the anodic dissolution or cathodic hydrogen evolution reactions independently but acts as a mixed type of inhibitor. Polarization resistance values (Rp) obtained from the LPR method showed an increase in value from 1.9 ohm/cm2 for that of blank to 10.6 ohm/cm2 for the addition of the highest concentrations of PVASA composite.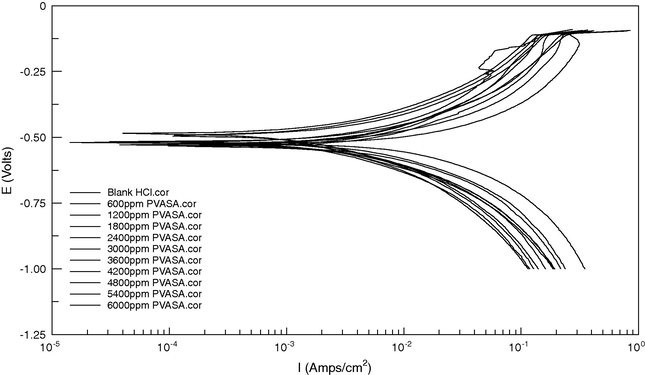
Potentiodynamic polarization curves for mild steel in 1 M HCl in absence and presence of different concentration of PVASA composite.
S. No.
Conc. of inhibitor (ppm)
−Ecorr (mV/s)
Icorr (×10−4 mA/s)
ba (mV/dec)
bc (mV/dec)
IE (%)
Rp (ohm/cm2)
IE (%)
Rct (ohm cm2)
IE (%)
Cdl (×10−4 μF/cm2)
θ
1.
Blank
535
130.6
199
153
–
1.9
–
6.46
–
3.24
–
2.
600
535
75.6
177
141
42.1
3.4
42.1
26.6
75.7
2.44
0.2
3.
1200
531
61.0
143
147
53.3
4.2
53.3
33.6
80.8
2.05
0.4
4.
1800
531
46.6
165
132
64.3
5.5
64.3
41.2
84.3
1.77
0.5
5.
2400
532
42.1
184
172
67.7
6.2
67.7
54.2
88.1
1.76
0.5
6.
3000
537
41.8
139
114
68.0
6.1
67.3
55.0
88.3
1.55
0.5
7.
3600
536
42.7
188
177
67.3
6.2
68.0
58.3
88.9
1.38
0.6
8.
4200
536
32.5
182
135
75.1
7.8
74.3
77.2
91.6
1.03
0.7
9.
4800
530
28.4
172
133
78.3
8.0
75.1
95.2
93.2
1.02
0.7
10.
5400
530
33.5
183
130
74.3
9.1
78.3
104.6
93.8
1.01
0.7
11.
6000
530
24.6
143
101
81.1
10.6
81.1
135.1
95.2
0.98
0.7
3.4.2 Electrochemical impedance measurements (EIS)
The Nyquist representation of electrochemical impedance spectroscopic values for mild steel in 1 M HCl containing different concentrations of PVASA composite is presented in Fig. 5. These plots, having the shape of semicircle, indicated the activation controlled nature of the reactions with single charge transfer process. The existence of the depressed nature of the semicircle with its centre below the x axis is the characteristic of solid electrodes and is attributed to the increased micro-roughness of the surface and other inhomogeneties of solid electrodes during corrosion (Juttner, 1990; Pajkossy, 1994). The diameter of the semicircle gives the charge transfer resistance Rct equivalent to the polarization resistance Rp which is inversely proportional to corrosion rate. With the increase in concentration of PVASA composite the charge transfer resistance increases. The charge transfer resistance Rct and the interfacial double layer capacitance Cdl derived from these curves are given in Table 4. The Rct value increased from 6.46 ohm cm2 for the blank acid to 135.1 ohm cm2 for the highest concentration of PVASA composite. The interfacial double layer capacitance Cdl decreased from 3.24 μF/cm2 for the blank to 0.98 μF/cm2 for PVASA composite. The surface coverage θ was estimated from the measured double layer capacitance Cdl values using the relationship:
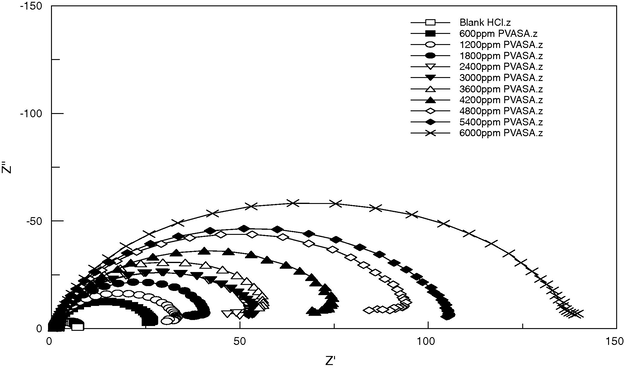
Nyquist plots for mild steel in 1 M HCl in the absence and presence of different concentration of PVASA composite.
4 Mechanism of inhibition
The mechanism of corrosion inhibition of mild steel in acidic solution by the PVASA composite molecules can be explained on the basis of adsorption of PVASA composite on the metal surface. The adsorption on the inhibitor molecules on the mild steel surface is due to the donor atoms N, O and aromatic rings of inhibitors and vacant d orbitals of the iron surface PVASA composite molecules are macromolecules and it can be adsorbed on a mild steel surface and it may lie flat on a mild steel surface, hence block more surface area of the mild steel surface. The PVASA composite molecule may also coordinately bond with the metal.
5 Comparison of inhibition efficiency obtained by weight loss method and electrochemical measurements
Bar graph obtained using inhibition efficiency obtained by various techniques reveal that the results are quite comparable are shown in Fig. 6.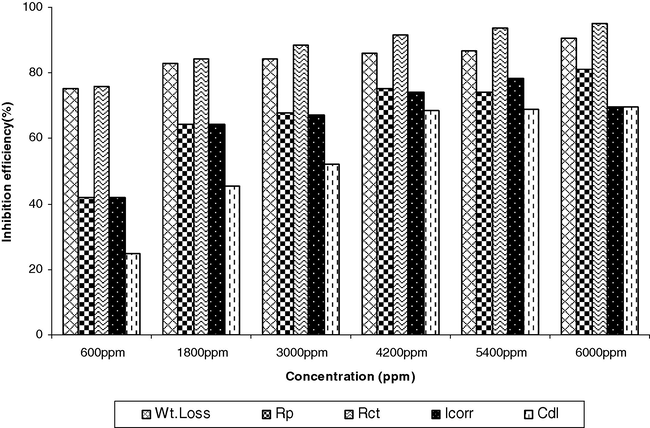
Comparative graphs for electrochemical methods and weight loss method.
6 Conclusion
-
Polyvinyl alcohol–sulphanilic acid (PVASA) composite acted as an effective corrosion inhibitor for mild steel in 1 M HCl medium.
-
PVASA composite formation was confirmed by FTIR technique.
-
The inhibition efficiency of PVASA composite increases with increase in concentration, but decreases with increase in temperature.
-
Maximum inhibition efficiency was 84% at 6000 ppm concentration at 24 h.
-
The adsorption of PVASA composite on the mild steel in 1 M HCl obeys Langmuir isotherm.
-
The values of ΔG are negative, which suggests that the inhibitors were strongly adsorbed on the mild steel surface.
-
Kinetic and thermodynamic parameters revealed that the adsorption process is spontaneous with adsorption of inhibitors on the metal surface.
-
Electrochemical studies confirmed the mixed mode of inhibition for the corrosion of mild steel in 1 M HCl without modifying the mechanism of hydrogen evolution of the inhibitor and dissolution of metal.
-
Inhibition efficiency by various techniques is quite comparable.
References
- Poly(o-phenylenediamine) as an inhibitor of mild steel corrosion in HCl solution. Mater. Chem. Phys.. 2010;123:20-27.
- [Google Scholar]
- An electroactive copolymer as corrosion inhibitor for steel in sulphuric acid medium. Appl. Surf. Sci.. 2008;254(17):5569-5573.
- [Google Scholar]
- Potentiostatic studies of the corrosion of grey castiron in sulphuric acid and sodium hydroxide solutions. Corros. Sci.. 1987;27(3):221.
- [Google Scholar]
- Synthesis and corrosion inhibition study of some 1,6-hexanediamine-based N,N-diallyl quaternary ammonium salts and their polymers. Polymer. 2001;42:2785-2794.
- [Google Scholar]
- Polyacrylic acid as a corrosion inhibitor for aluminium in weakly alkaline solutions. Part I: Weight loss, polarization, impedance EFM and EDX studies. Corros. Sci.. 2009;51:658-667.
- [Google Scholar]
- Investigation of some Schiff bases as acidic corrosion of alloy AA3102. Mater. Chem. Phys.. 2005;89(1):176-181.
- [Google Scholar]
- Enhancement of corrosion protection efficiency of iron by poly(aniline-co-amino-naphthol-sulphonic acid) nanowires coating in highly acidic medium. Thin Solid Films. 2010;519:1031-1039.
- [Google Scholar]
- Thermodynamic properties of 2,5-bis(4-methoxyphenyl)-1,3,4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. Corros. Sci.. 2006;48:2831-2842.
- [Google Scholar]
- Preparation and property of TiO2 nanoparticle dispersed polyvinyl alcohol composite materials. J. Mater. Sci. Lett.. 2002;21:1637-1639.
- [Google Scholar]
- Adsorption of Organic Compounds on Electrodes. New York: Plenum Press; 1971.
- The effect of temperature on the corrosion of mild steel in acidic media in the presence of some sulphur-containing organic compounds. Mater. Chem. Phys.. 2006;98:316-323.
- [Google Scholar]
- On the form of adsorption isotherms for substitutional adsorption of molecules of different sizes. Electrochim. Acta. 1973;18:789.
- [Google Scholar]
- Corrosion inhibition of mild steel in acidic media by some organic dyes. Mater. Lett.. 2005;59(17):2163-2165.
- [Google Scholar]
- Corrosion inhibition and adsorption properties of methocarbamol on mild steel in acidic medium. Port. Electrochim. Acta. 2009;27(1):13-22.
- [Google Scholar]
- Ethanol extract of Phyllanthus amarus as a green inhibitor for the corrosion of mild steel in H2SO4. Port. Electrochim. Acta. 2009;27(5):579-589.
- [Google Scholar]
- Part 3. Theoretical study on some amino acids and their potential activity as corrosion inhibitors for mild steel in HCl. Mol. Simul.. 2010;36(5):354-363.
- [Google Scholar]
- Inhibitive and adsorption properties of ethanol extract of seeds and leaves of Azadirachta indica on the corrosion of mild steel in H2SO4. Port. Electrochim. Acta. 2009;27(4):443-456.
- [Google Scholar]
- Ethanol extract of Terminalia catappa as a green inhibitor for the corrosion of mild steel in H2SO4. Green Chem. Lett. Rev.. 2009;2(4):223-231.
- [Google Scholar]
- Joint effect of halides and ethanol extract of Lasianthera africana on inhibition of corrosion of mild steel in H2SO4. J. Appl. Electrochem. 2009;39(6):849-857.
- [Google Scholar]
- Corrosion inhibitors. Part II: Quantum chemical studies on the corrosion inhibitions of steel in acidic medium by some triazole, oxadiazole and thiadiazole derivatives. Electrochim. Acta. 2006;51(19):3957-3968.
- [Google Scholar]
- Natural honey as corrosion inhibitor for metals and alloys. II. C-steel in high saline water. Corros. Sci.. 2000;42(4):731-738.
- [Google Scholar]
- The application of some polydentate Schiff base compounds containing aminic nitrogens as corrosion inhibitors for mild steel in acidic media. Corros. Sci.. 2006;48(10):3243-3260.
- [Google Scholar]
- Synthesis and characterizations of nano sized MgO and its nano composite with poly(vinyl alcohol) J. Non-Cryst. Solids. 2011;357:181-185.
- [Google Scholar]
- Polyaniline–polyvinyl alcohol conducting composite: material with easy processability and novel application potential. Synth. Met.. 2001;123(1):21-31.
- [Google Scholar]
- Adsorption of tetramethylthiourea at the mercury/water interface. J. Electroanal. Chem.. 1982;137:127.
- [Google Scholar]
- Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta. 1990;35(10):1501-1508.
- [Google Scholar]
- Molecular simulation, quantum chemical calculations and electrochemical studies for inhibition of mild steel by triazoles. Electrochim. Acta. 2008;53(9):3484-3492.
- [Google Scholar]
- Preparation and characterization of processable electro active polyaniline–polyvinyl alcohol composite. Polymer. 2003;44(12):3523-3528.
- [Google Scholar]
- The corrosion behaviour of zinc metal in acidic solutions of polyvinyl pyrrolidones and polyvinyl pyridines. Surf. Coat. Technol.. 1986;27:317-324.
- [Google Scholar]
- Polyamino-benzoquinone polymers – a new class of corrosion inhibitors for mild steel. J. Electrochem. Soc.. 1995;142(5):1478-1483.
- [Google Scholar]
- Corrosion inhibition and adsorption properties of ethanol extract of Vernonia amygdalina for the corrosion of mild steel in H2SO4. Port. Electrochim. Acta. 2009;27(1):33-45.
- [Google Scholar]
- Inhibition of the corrosion of zinc in H2SO4 by 9-deoxy-9a-aza-9a-methyl-9ahomoerythromycin A (Azithromycin) Port. Electrochim. Acta. 2009;27(1):57-68.
- [Google Scholar]
- Electrochemical and XPS studies of decylamides of α-amino acids adsorption on carbon steel in acidic environment. Appl. Surf. Sci.. 2006;252:2894-2909.
- [Google Scholar]
- Characterization of PVA–PVdF based solid polymer blend electrolytes. Physica B. 2004;348:73-78.
- [Google Scholar]
- Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes. Mater. Lett.. 2004;58:641-649.
- [Google Scholar]
- Investigations on the electrical and structural properties of polyaniline doped with camphor sulphonic acid. J. Phys. Chem. Solid. 2006;67:1496-1501.
- [Google Scholar]
- An electrochemical and SERS study of the action of polyvinylpyrrolidone and polyethylenimine as inhibitors for copper in aerated H2SO4. Corros. Sci.. 1996;38(4):587-599.
- [Google Scholar]
- A self doped conducting polymer ‘‘polyanthranilic acid”: an efficient corrosion Inhibitor for mild steel in acidic solution. Corros. Sci.. 2008;50:2867-2872.
- [Google Scholar]
- On the role of fatty acid in adsorption and corrosion inhibition of iron by amine-fatty acid salts in acidic solution. Electrochim. Acta. 1981;26:245.
- [Google Scholar]
- Evaluation of some amino acids as corrosion inhibitors for carbon steel in sulphuric acids solutions at different temperature. Egypt. J. Chem.. 1995;38(2):141-155.
- [Google Scholar]
- Nalwa H.S., ed. Handbook of Organic Conductive Molecules and Polymers. Vol vol. 2. Chichester, West Sussex, England: Wiley; 1997. p. :509-510.
- Effect of halide ions on the corrosion inhibition of mild steel in acidic medium using polyvinyl alcohol. Pigment Resin Technol.. 2006;35:284-292.
- [Google Scholar]
- Water soluble polymers as corrosion Inhibitors. Pigment Resin Technol.. 2006;35(6):346-352.
- [Google Scholar]
- Synergestic Inhibition between naturally occuring Exudate gum and Halide ions on the corrosion of mild steel in acidic medium. Int. J. Electrochem. Sci.. 2008;3:1029-1043.
- [Google Scholar]
- Chloro-substituted aniline as corrosion inhibitors for zinc in sulfamic acid. Trans. SAEST. 1997;32(1):5-14.
- [Google Scholar]
- Molecular dynamics and density functional theory study on relationship between structure of imidazoline derivatives and inhibition performance. Corros. Sci.. 2008;50(7):2021-2029.
- [Google Scholar]
- Synthesis and characterization of aniline and aniline-o-sulfonic acid copolymers. Eur. Polym. J.. 2007;43:2072-2079.
- [Google Scholar]
- Preparation and properties of poly (vinyl alcohol)–clay nanocomposite materials. Polymer. 2003;44:3553-3560.
- [Google Scholar]
- Preparation and characterization of PVA/OMMT composite. J. Appl. Polym. Sci.. 2006;102:2315-2323.
- [Google Scholar]
- Corrosion inhibition of mild steel by pyridine derivatives. J. Electrochem. Soc.. 1987;36(3):143-145.
- [Google Scholar]






