Translate this page into:
Potential antimicrobial compounds in flower extract of Plumeria alba
⁎Corresponding author. malikferdosi@yahoo.com (Malik F. H. Ferdosi) fiaz.iags@pu.edu.pk (Malik F. H. Ferdosi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study was carried out to investigate the antimicrobial activity of methanolic flower extract of Plumeria alba and identification of the possible antimicrobial compounds through GC–MS analysis. Antimicrobial efficacy of the methanolic extract of 5 ppm to 1000 ppm concentrations was assessed against five fungal (Trichoderma viride, T. reesei, T. harzianum, T. hamatum and T. koningii) and five bacterial species (Escherichia coli, Salmonella sp., Pseudomonas sp., Bacillus sp. and Staphylococcus sp.). In general, all the concentrations of the extract significantly reduced growth of all the fungal and bacterial species. However, there was a significant variation in susceptibility of different fungal and bacterial species to the applied concentrations of the extract. The extract was the most effective against T. reesii followed by T. viride, T. harzianum, T. hamatum and T. koningii causing 11–97%, 36–90%, 24–86%, 7–77% and 9–76% reduction in their growth. Similarly, there was 23–93%, 12–92%, 36–90%, 19–90% and 8–84% reduction in growth of Bacillus sp., Staphylococcus sp., E. coli, Pseudomonas sp., and Salmonella sp., respectively, due to different concentrations of methanolic extract. GC–MS analysis of the extract showed the presence of 21 constituents. The most abundant compound was benzofuran, 2,3-dihydro- (27.95%). Other important compounds included cyclononanone (14.59%), 9-octadecyne (14.29%), 9,12,15-octadecatrienoic acid, methyl ester, (Z,Z,Z)- (10.28%), benzyl alcohol (5.57%), 1-decanol, 2-hexyl- (5.44%), 2,6-octadien-1-ol, 3,7-dimethyl-, acetate, (Z)- (3.76%), 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (2.48%) and heptadecane (2.43%). It is concluded that methanolic extract of P. alba flowers has the potential to control both fungal as well as bacterial growth.
Keywords
Antibacterial
Antifungal
Flower extract
GC–MS analysis
Plumeria alba
1 Introduction
Plants are often affected by pathogenic fungi and bacteria that results in major constraints in agricultural productivity (Kohl et al., 2019). The estimated worldwide economic losses in crop yield due to pathogenic infections range between 30 and 50% every year. Efficient and sustainable control of phytopathogens is an exigency for all agricultural systems (Ghorbanpour et al., 2018). The pathogens not only reduce crop yield but also lowers the crop quality through release of toxins having hazardous effects on human health (Joshi et al., 2020). Nowadays, a number of management strategies including cultural, biological and chemical are in practice to control or reduce the populations of plant pathogens but each has its own limitations (Shad et al., 2020; Kong et al., 2020; Khan and Javaid, 2021). Among the various disease management practices, use of pesticides is more widespread although it has many disadvantages as it pollutes the environment and causes health hazards (Mitran et al., 2018). To ensure the global food security and environment, the management practices should be ecologically safe, more sustainable, less fungicide dependent, economically viable and socially acceptable (Chen and Wang, 2020).
Use of plant derived substances has been considered as an eco-friendly and less expensive alternative approach for disease management (Javaid et al., 2020; Banaras et al., 2020; Jabeen et al., 2021). In nature, plants are the richest bio-resources of secondary metabolites, which show a wide range of physical, biological and chemical activities for preparation of new plant-based drugs (Zulfiqar et al., 2020; Khan and Javaid, 2020a). A number of phytoconstituents such as terpenoids, tannins, diterpene, triterpenes, flavonoids, flavonols, coumarins, steroids, saponins, tannins, alkaloids, quinones, phenols and glycosides generally play a vital role related to antifungal, antibacterial, antibiotic and antinutritional properties (Isah, 2019; Erb and Kliebenstein, 2020; Banaras et al. 2021). Over the last two decades, intensive efforts have been made to discover commercially useful antimicrobial products (Khan and Javaid, 2020b; Javed et al., 2021; Javaid et al., 2022). However, so far out of several hundred thousand medicinal plant species, only a few of them have been investigated pharmacologically and phytochemically (Delgoda and Murray, 2017).
In an effort to expand the spectrum of antimicrobial agents from natural resources, Plumeria alba belonging to Apocynaceae family was selected. It is native to tropical America and is found in northern South America, Southern Mexico and South Asian subcontinent (Kakishima et al., 2017). Various Plumeria species are now being cultivated extensively in warmer regions of the world (Semenya and Maroyi, 2020). It is a small deciduous tree with fleshy branches that produce milky juice when the branches or leaves are cut down (Bihani, 2020). It is commonly known as champa grown mainly for its fragrant and spiral-shaped ornamental flowers (Idrees et al., 2020). It is a medicinal plant which contains a wide range of bioactive constituents with therapeutic properties that can be used for the treatment of malaria, diabetes, inflammation, hypertension, abdominal tumors, rheumatism, leprosy, skin cancer, fungal, viral and bacterial infections (Bihani, 2020; Imrana and Asif, 2020). However, information regarding antimicrobial activity of its flower extract are rare. Therefore, this study was carried out to evaluate the antimicrobial potential of methanolic flower extract of Plumeria alba and the identification of possible phytoconstituents through GC–MS analysis.
2 Materials and methods
2.1 Preparation of extract
Healthy flowers of P. alba were collected from Lahore, Pakistan during September 2019 and stored in paper bags to carry to the laboratory. After washing, petals were separated from the flowers and kept in trays to evaporate water from their surface. Flowers were dried under shade and thoroughly crushed in a pestle and mortar. Flower extract was prepared in 95% methanol by gentle heating at 40 °C for 60 min in a closed container. The solvent was filtered and then evaporated on a rotary evaporator at 40 °C under reduced pressure and the remaining gummy material (methanolic extract) was used for antimicrobial bioassays.
2.2 Evaluation of antibacterial activity
Five bacterial species namely Bacillus sp., Salmonella sp., Pseudomonas sp., Escherichia coli and Staphyllococcus sp. were procured from First Culture Bank of Pakistan (FCBP). LBA growth medium (beef extract 10 g + typton 10 g + NaCl 5 g + agar 20 g + water 1000 mL) was autoclaved at 121 °C for 30 min. For preparation of different concentrations of the methanolic extract, procedure of Kanwal et al. (2009) was followed with little modifications. To 75 mL of LBA media, appropriate quantity of the methanolic extract was added and mixed well to prepare 5, 10, 20, 50, 100, 500 and 1000 ppm concentrations. This volume was divided into three equal portions and poured in sterilized Petri plates aseptically to serve as replicates. Agar discs (5 mm) containing bacterial inocula were placed in the centers of the plates and incubated at 37 °C for 24 h, and radial growth of bacteria in each plate was recorded thereafter (Balouiri et al., 2016).
2.3 Evaluation of antifungal activity
Five fungal species namely Trichoderma koningii, T. viride, T. hamatum, T. harzianum and T. reesei were obtained from FCBP. A 2% malt extract agar (20 g malt extract + 20 g agar + 1000 mL water) was autoclaved at 121 °C for 30 min. To avoid bacterial growth, streptomycene capsule was added after autoclaving during the cooling stage. A volume of 75 mL of the autoclaved growth media was poured in 250-mL flasks and appropriate quantities of the flower extract were added to prepare 5, 10, 20, 50, 100, 500 and 1000 ppm concentrations. Control was without the flower extract. Media was poured in autoclaved Petri plates, each containing 25 mL of the media. Media was allowed to solidify and thereafter the plates were inoculated in the center (5 mm disc) with the test fungal species. Experiment was carried out in a completely randomized design using three replications. After 7-day incubation at 27 °C, radial growth of the fungi was measured (Kanwal et al., 2010).
Percentage decreases in the fungal growths due to different concentrations were calculated using the following equation (Khan et al., 2021):
2.4 GC–MS analysis
The gas chromatograph (GC) machine model was 7890B and that of mass spectroscopy (MS) was 5977A, used for the identification of different compounds from the sample while both were branded by Agilent Technologies. The column used was DB 5 MS (30 m × 0.25 µm × 0.25 μm). Injection volume was 1 µL and the carrier gas was helium. Oven ramping; initial temperature was 80 °C and then raised 10 °C per minute up to 300 °C. Inlet temperature was 280 °C. MS conditions were as mode: scan 50–500 m/z, the source temperature was 230 °C and quadrupole temperature was 150 °C. Chemical compounds were identified by comparison of their spectra with library and arranged in the ascending order of their retention times and retention indices. The relative abundance was reported by using their peak areas (Ferdosi et al., 2021).
2.5 Statistical analysis
All the experimental data regarding the effect of flower extract on growth of different bacterial and fungal species were subjected to two-way ANOVA using the software Statistix 8.1. The significant differences among the treatment means were determined by LSD test at P ≤ 0.05.
3 Results and discussion
3.1 Antibacterial activity
The effect of bacterial species (B), extract concentration (C) and B × C was significant for bacterial growth (Table 1). The highest growth was recorded in negative control treatments of all the five bacterial species. Flower extract of P. alba significantly reduced growth of all the bacterial species over control. However, the effect was variable against different bacterial species (Fig. 1A). There was 36–90%, 8–84%, 19–90%, 23–93% and 12–92% reduction in growth of E. coli, Salmonella sp., Pseudomonas sp., Bacillus sp., and Staphylococcus sp., respectively, over corresponding control treatments (Fig. 1B).
Sources of variation
df
SS
MS
F values
Bacterial species (B)
4
4.60
1.15
226*
Concentration (C)
7
179.1
25.58
5032*
B × C
28
3.32
0.12
23.32*
Error
80
0.41
0.0051
Total
119
187.4

Antibacterial activity of methanolic flower extract of Plumeria alba. Different letters indicate significant differences (P ≤ 0.05) among the treatments as determined by LSD Test.
In general, the extract was effective against all the five bacterial species, however, different bacterial species showed variable growth responses to the extract. Similar variable antibacterial effects of different products such as extracts and essential oil of this plant have been reported earlier in other parts of the world. Zaheer et al. (2010) studied the antibacterial activity of essential oils of P. alba flower and reported that the oil was more toxic to Gram positive than Gram-negative bacteria. The oil was more effective against Staphylococcus aureus and Bacillus subtilis than against Escherichia coli and Salmonella typhi. Radha et al. (2008) reported that methanolic extract (MIC 200 μg/mL) of P. alba flowers and its triterpene rich fraction (MIC 200 μg/mL) showed pronounced antibacterial activity against S. aureus, E. coli, S. typhi and Pseudomonas aeuroginosa. Gold nanoparticles of P. alba flower extract showed more antibacterial activity than the standard antibiotics. Moreover, the nanoparticles were more effective against E. coli than against Streptobacillus sp. (Nagaraj et al., 2012).
3.2 Antifungal activity
The effect of fungal species (F), extract concentration (C) and their interaction (F × C) was significant (P ≤ 0.001) for fungal growth (Table 2). All the concentrations of the applied extract significantly suppressed growth of all the five test fungal species. The effectiveness of the extract was concentration dependant as there was a gradual decrease in fungal growth with increase in extract concentration. Growth response to the applied extract also varied with the change in fungal species (Fig. 2A). Different concentrations of the extract reduced growth of T. viride, T. harzianum, T. hamatum, T. reesei and T. koningii by 31–90%, 24–86%, 7–77%, 11–97% and 9–76%, respectively, over the respective control treatments (Fig. 2B).
Sources of variation
df
SS
MS
F values
Fungal species (F)
4
8.76
2.19
365*
Concentration (C)
7
176
25.1
4183*
F × C
28
7.36
0.263
44*
Error
80
0.48
0.006
Total
119
192
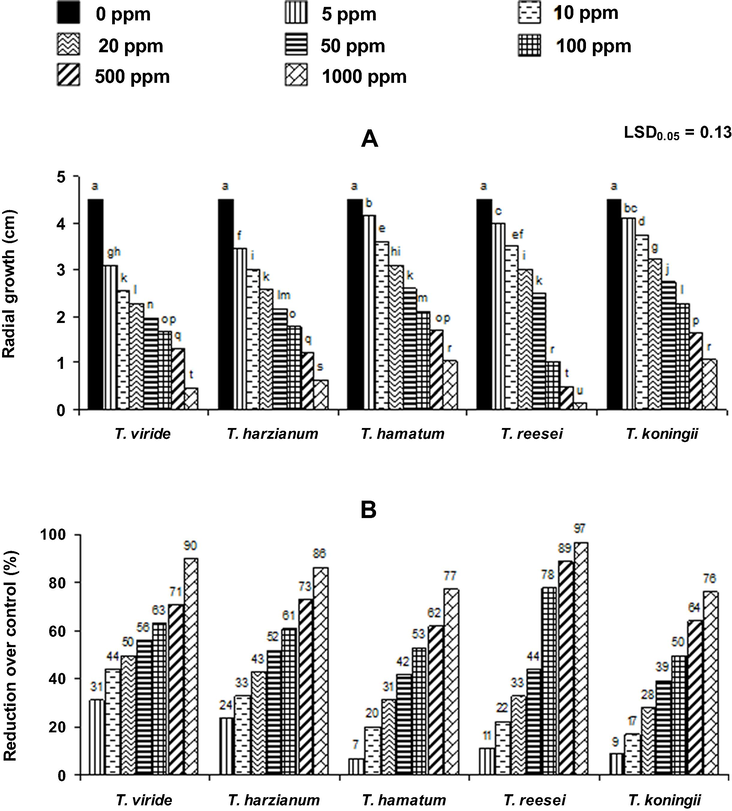
Antifungal activity of methanolic flower extract of Plumeria alba against Trichoderma species. Different letters indicate significant differences (P ≤ 0.05) among the treatments as determined by LSD Test.
Like bacteria, the methanolic flower extract also significantly reduced growth of all the fungal species and the susceptibility of the different fungi was variable to the applied extract. Earlier, petroleum ether extract of P. alba showed pronounced fungistatic activity against Penicillium digitatum, Aspergillus flavus and Rhizopus arrhizus but not against Aspergillus fumigatus and A. niger (Sibi et al., 2012). Similarly, Kumari et al. (2012) reported that essential oil of P. alba flowers was highly toxic to the growth of Candida albicans, Aspergillus niger, and Penicillium chrysogenum while Ralstonia entropha and Phaenorochaete chrysporium showed less susceptibility to the applied oil. Alkaloids are the major compounds in flowers of P. alba followed by flavonoids and glycosides in addition to phenols, tannins and terpenoids (Sibi et al., 2012), which could be responsible for antifungal activity (Yadava and Tiwari, 2007; Kanwal et al., 2010; Javed et al., 2021). Radha et al. (2008) reported antifungal activity of methanolic flower extract of P. alba against A. niger, P. chrysogenum and Microsporum gypseum, and correlated the activity with triterpenes present in the extract. Extracts of other parts of this plant also known to exhibit antifungal activity against various fungal species, such as ethyl acetate bark extract showed marked activity against C. albicans (Murniana et al., 2011).
3.3 GC–MS analysis
GC–MS chromatogram presented in Fig. 3 demonstrated the presence of 21 natural compounds in methanolic flower extract of P. alba whose details are given in Table 3. Principal components of this extract included benzofuran, 2,3-dihydro- (27.95%), 9-octadecyne (14.29%), and cyclononanone (14.59%). Moderately abundant compounds included 9,12,15-octadecatrienoic acid, methyl ester, (Z,Z,Z)- (10.28%), benzyl alcohol (5.57%), 1-decanol, 2-hexyl- (5.44%), and 2,6-octadien-1-ol, 3,7-dimethyl-, acetate, (Z)- (3.76%). Compounds such as 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (2.48%), heptadecane (2.43%), 1,3-propanediamine, N-methyl- (1.86%), 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl-, [S-(Z)]- (1.35%), hexadecanoic acid, 15-methyl-, methyl ester (1.19%), 4-methyl-1,5-heptadiene (1.07%), and phenol (1.04%) were identified as less abundant ones. Four compounds namely hydroquinone (1.01%), catechol (0.99%), 3,3′-dimethoxybenzil (0.99%), 3,5-dimethylanisole (0.95%), 3,7,11-tridecatrienenitrile, 4,8,12-trimethyl- (0.94%), 2-cyclopenten-1-one, 2-hydroxy- (087%), and 1,4-cyclohexanedimethanamine (0.86%), with peak area less than 1% were ranked as the least abundant. The most abundant compound in this study was benzofuran, 2,3-dihydro-. Earlier, Idan et al. (2015) also reported this compound from methanolic fruit extract of Citrullus colocynthis. Compounds possessing benzofuran nucleus (bicyclic ring system) are known for their broad-spectrum bioactivities including antimicrobial activities (Kamal et al., 2011). Recently, Zaher et al. (2022) identified a benzofuran glucoside in ethyl acetate extract as a new and the potent antimicrobial agent inhibiting growth of two Gram-positive and one Gram-negative bacteria, and a fungal species. Some of the uother identified compounds are also known to possess antimicrobial potential. 2-Cyclopenten-1-one, 2-hydroxy- is a diterpene with good antimicrobial activity (Soonthornchareonnon et al., 2012; Revathi et al., 2015). Phenol is known to have antibacterial activity (Bennett, 1959). Phenolic compounds inhibit bacterial growth by damaging the bacterial membrane, inhibiting the virulence factors like toxins and enzymes, and by suppressing the biofilm formation (Miklasińska-Majdanik et al., 2018). Benzyl alcohol, an aromatic alcohol, is a naturally occurring substance present in many fruits and in many essential oils, and is known for its antimicrobial activity (Lucchini et al., 1990). Catechol is a phenolic allelochemical produced in plants. It showed antibacterial activity against Corynebacterium xerosis, Pseudomonas putida, P. pyocyanea, and antifungal activity against Penicillium italicum, Colletotrichum circinans and Fusarium oxysporum (Farkas and Kiraly, 1962; Kocaçalişkan et al., 2006). This compound is also effective against harmful intestinal bacterial species namely Escherichia coli, Clostridium perfringes, C. difficile (Jeong et al., 2009). Hydroquinone showed strong antibacterial activity against Staphylococcus aureus by destroying cell wall and membrane, and increasing permeability leading to leakage of intracellular substances, and by influencing gene expression (Ma et al., 2019).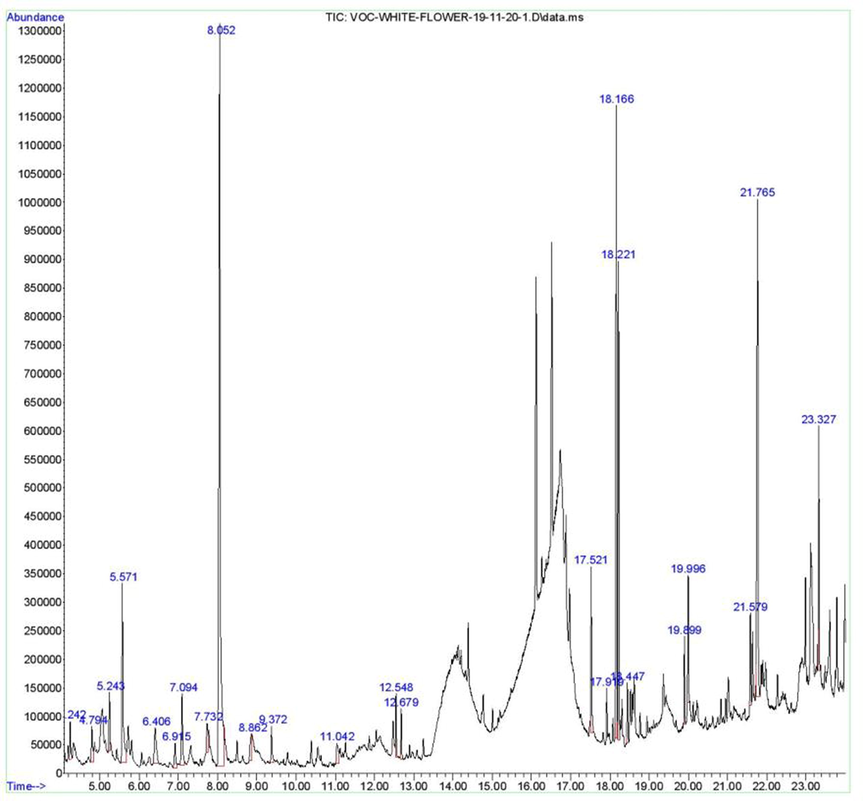
GC–MS chromatogram of methanolic flower extract of Plumeria alba.
Sr. No.
Names of compounds
Molecular formula
Molecular weight
Retention time (min)
Peak area (%)
1
2-Cyclopenten-1-one, 2-hydroxy-
C5H6O2
98.09
1.242
0.87
2
Phenol
C6H6O
94.11
4.794
1.04
3
Benzyl alcohol
C7H8O
108.14
5.571
5.57
4
1,3-Propanediamine, N-methyl-
C4H12N2
88.15
6.406
1.86
5
1,4-Cyclohexanedimethanamine
C8H18N2
142.24
6.915
0.86
6
4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-
C6H8O4
144.12
7.094
2.48
7
Catechol
C6H6O2
110.11
7.732
0.99
8
Benzofuran, 2,3-dihydro-
C8H8O
120.15
8.052
27.95
9
Hydroquinone
C6H6O2
110.11
8.862
1.01
10
3,3′-Dimethoxybenzil
C16H14O4
270.28
9.372
0.99
11
3,5-Dimethylanisole
C9H12O
136.19
11.042
0.95
12
1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, [S-(Z)]-
C15H26O
222.36
12.548
1.35
13
4-Methyl-1,5-heptadiene
C8H14
110.2
12.679
1.07
14
2,6-Octadien-1-ol, 3,7-dimethyl-, acetate, (Z)-
C12H20O2
196.28
17.521
3.76
15
3,7,11-Tridecatrienenitrile, 4,8,12-trimethyl-
C16H25N
231.38
17.919
0.94
16
9-Octadecyne
C18H34
250.46
18.166
14.29
17
9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)-
C19H32O2
292.45
18.221
10.28
18
Hexadecanoic acid, 15-methyl-, methyl ester
C18H36O2
284.47
18.447
1.19
19
Heptadecane
C17H36
240.5
19.996
2.43
20
Cyclononanone
C9H16O
140.22
21.765
14.59
21
1-Decanol, 2-hexyl-
C16H34O
242.44
23.327
5.44
4 Conclusion
This study concludes that methanolic flower extract of P. alba has antifungal and antibacterial activity against a variety of fungal and bacterial species. Compounds such as benzofuran, 2,3-dihydro-; 2-cyclopenten-1-one, 2-hydroxy-; phenol; benzyl alcohol; catechol; and hydroquinone could be responsible for antimicrobial activity of the extract.
Ethical approval
This article does not contain any studies with human participants or animals performed by either of the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal.. 2016;6(2):71-79.
- [Google Scholar]
- Potential antifungal constituents of Sonchus oleraceous against Macrophomina phaseolina. Int. J. Agric. Biol.. 2020;24:13760-111382.
- [Google Scholar]
- Bioassays guided fractionation of Ageratum conyzoides extract for the identification of natural antifungal compounds against Macrophomina phaseolina. Int. J. Agric. Biol.. 2021;25:761-767.
- [Google Scholar]
- Factors affecting the antimicrobial activity of phenols. Adv. Appl. Microbiol.. 1959;1:123-140.
- [Google Scholar]
- Plumeria rubra L. – a review on its ethnopharmacological, morphological, phytochemical, pharmacological and toxicological studies. J. Ethnopharmacol.. 2020;264:113291
- [Google Scholar]
- Electroanalytical methods for detecting pesticides in agricultural products: a review and recent developments. Int. J. Electrochem. Sci.. 2020;15:2700-2712.
- [Google Scholar]
- Evolutionary perspectives on the role of plant secondary metabolites. In: In. Pharmacognosy. Academic Press; 2017. p. :93-100.
- [Google Scholar]
- Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol.. 2020;184:39-52.
- [Google Scholar]
- Role of phenolic com-pounds in the physiology of plant diseases and diseaseresistance. Phytopathology. 1962;44:105-150.
- [Google Scholar]
- Analysis of n-butanol flower extract of Cassia fistula through GC-MS and identification of antimicrobial compounds. Pak. J. Phytopathol.. 2021;33:103-107.
- [Google Scholar]
- Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control. 2018;117:147-157.
- [Google Scholar]
- Spectral analysis and anti-bacterial activity of methanolic fruit extract of Citrullus colocynthis using gas chromatography-mass spectrometry. Afr. J. Biotechnol.. 2015;14:3131-3158.
- [Google Scholar]
- Idrees, S., Hanif, M.S., Ayub, M.A., Jilani, M.I., Memon, N., 2020. Frangipani. In: Medicinal. Plants. of South. Asia. Elsevier. pp. 287–300
- Morphological, ethnobotanical, pharmacognostical and pharmacological studies on the medicinal plant Plumeria alba Linn. (apocynaceae) Arab. J. Med. Arom. Plants. 2020;6:54-84.
- [Google Scholar]
- Stress and defense responses in plant secondary metabolites production. Biol. Res.. 2019;52:39.
- [Google Scholar]
- Management of southern blight of bell pepper by soil amendment with dry biomass of Datura metel. J. Plant Pathol.. 2021;103(3):901-913.
- [Google Scholar]
- Control of the chickpea blight, Ascochyta rabiei, with the weed plant, Withania somnifera. Egypt. J. Biol. Pest Control. 2020;30:114.
- [Google Scholar]
- Potential health-related phytoconstituents in leaves of Chenopodium quinoa. Adv. Life Sci.. 2022;9(4):574-578.
- [Google Scholar]
- Lupeol acetate as a potent antifungal compound against opportunistic human and phytopathogenic mold Macrophomina phaseolina. Sci. Rep.. 2021;11:8417.
- [Google Scholar]
- Antimicrobial activity of catechol isolated from Diospyros kaki Thunb. roots and its derivatives toward intestinal bacteria. Food. Chem.. 2009;115:1006-1010.
- [Google Scholar]
- A spotlight on the recent advances in bacterial plant diseases and their footprint on crop production. In: Recent Advancements in Microbial Diversity. Academic. Press; 2020. p. :37-69.
- [Google Scholar]
- Geographic expansion of a rust fungus on Plumeria in Pacific and Asian countries. N. Z. J. Bot.. 2017;55:178-186.
- [Google Scholar]
- Benzofurans: a new profile of biological activities. Int. J. Med. Pharmaceut. Sci.. 2011;1:1-15.
- [Google Scholar]
- Flavonoids from mango leaves with antibacterial activity. J. Serb. Chem. Soc.. 2009;74(12):1389-1399.
- [Google Scholar]
- Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat. Prod. Res.. 2010;24:1907-1914.
- [Google Scholar]
- Anticancer, antimicrobial and antioxidant compounds of quinoa inflorescence. Adv. Life. Sci.. 2020;8:68-72.
- [Google Scholar]
- Comparative antifungal potential of stem extracts of four quinoa varieties against Macrophomina phaseolina. Int. J. Agric. Biol.. 2020;24:441-446.
- [Google Scholar]
- In vitro screening of Aspergillus spp. for their biocontrol potential against Macrophomina phaseolina. J. Plant Pathol.. 2021;103(4):1195-1205.
- [Google Scholar]
- Trichoderma viride controls Macrophomina phaseolina through its DNA disintegration and production of antifungal compounds. Int. J. Agric. Biol.. 2021;25(4):888-894.
- [Google Scholar]
- Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C. J. Biosci.. 2006;61:639-642.
- [Google Scholar]
- Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front. Plant. Sci.. 2019;10:845.
- [Google Scholar]
- Forest tree associated bacterial diffusible and volatile organic compounds against various phytopathogenic fungi. Microorganisms. 2020;8:590.
- [Google Scholar]
- In-vitro antifungal activity of the essential oil of flowers of Plumeria alba Linn. (Apocynaceae) Int. J. Pharm. Technol. Res.. 2012;4:208-212.
- [Google Scholar]
- Antibacterial activity of phenolic compounds and aromatic alcohols. Res. Microbiol.. 1990;141:499-510.
- [Google Scholar]
- Antimicrobial mechanism of hydroquinone. Appl. Biochem. Biotechnol.. 2019;189:1291-1303.
- [Google Scholar]
- Phenolic compounds diminish antibiotic resistance of staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health. 2018;15:2321.
- [Google Scholar]
- Advantages and disadvantages of pesticide analysis methods used in agricultural samples. Sci. Papers. Ser. B Hort.. 2018;62:709-714.
- [Google Scholar]
- Antifungal activity from ethyl acetate extract of Plumeria alba against Candida albicans. J. Nat.. 2011;11:85-88.
- [Google Scholar]
- Environmental benign synthesis of gold nanoparticles from the flower extracts of Plumeria Alba Linn. (Frangipani) and evaluation of their biological activities. Int. J. Drug Dev. Res.. 2012;4:144-150.
- [Google Scholar]
- Antibacterial and antifungal activities of methanolic extract and the isolated fraction of Plumeria alba Linn. Nat. Prod. Indian. J.. 2008;4:177-179.
- [Google Scholar]
- Revath, P., Jeyaseelansenthinath, T., Thirumalaikolundhusubramaian, P., 2015. Preliminary phytochemical screening and GC-MS analysis of ethanolic extract of mangrove plant- Bruguiera Cylindrica (Rhizho) L. Int. J. Pharmacogn. Phytochem. Res. 6, 729–740
- Assessment of useful alien plant species cultivated and managed in rural home gardens of Limpopo province. S. Afr. Sci.. 2020;2020:3561306.
- [Google Scholar]
- Evaluation of cytrol and revus fungicides against late blight of potato. Pak. J. Phytopathol.. 2020;32(2):225-229.
- [Google Scholar]
- Comparative studies of plumeria species for their phytochemical and antifungal properties against Citrus sinensis pathogens. Int. J. Agric. Res.. 2012;7:324-331.
- [Google Scholar]
- Biological activities of medicinal plants from mangrove and beach forests. J. Pharmaceut. Sci.. 2012;39:9-18.
- [Google Scholar]
- New antifungal flavone glycoside from Butea monosperma O. Kuntze. J. Enzyme. Inhib. Med. Chem.. 2007;22:497-500.
- [Google Scholar]
- Antimicrobial activity of essential oil of flowers of Plumeria alba Linn (Apocynaceae) Int. J. Pharm. Pharmaceut. Sci.. 2010;2:155-157.
- [Google Scholar]
- New antimicrobial and cytotoxic benzofuran glucoside from Senecio glaucus L. Nat. Prod. Res.. 2022;36:136-141.
- [Google Scholar]
- An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant. Sci.. 2020;295:110194
- [Google Scholar]







