Potentiometric and surface topography studies of new carbon-paste sensors for determination of thiamine in Egyptian multivitamin ampoules
⁎Corresponding author. yousrymi@yahoo.com (Yousry M. Issa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
We report here for the first time two potentiometric carbon-paste sensors for determination of thiamine chloride hydrochloride in aqueous solutions. The proposed sensors use the ion-pair of thiamine with tetraphenylborate as an electro-active species and dibutyl phthalate (sensor I) or o-nitrophenyloctyl ether (sensor II) as solvent mediators. The effect of solvent mediator was studied using dibutyl phthalate, o-nitrophenyloctyl ether, ethylhexyl adipate, dioctyl phthalate, tricresyl phosphate and paraffin oil. The slopes of the calibration graphs are 29.49 ± 0.24 and 29.60 ± 0.15 mV/decade for sensors (I) and (II), respectively. The sensors are able to detect down to 5.25 × 10−6 and 3.57 × 10−6 for (I) and (II), respectively. Both sensors show reasonable thermal stability and fast response time. The selectivity coefficients obtained from the matched potential method indicate high selectivity of the proposed sensors toward thiamine over commonly interfering cations. Sensor (I) has a lifetime of only 1–2 days; however, sensor (II) remains usable for up to one month. Analytical applications to pure solutions and Egyptian multivitamin ampoules show excellent recovery values ranging from 97.92 to 103.72% and 97.21 to 102.19% for sensors (I) and (II), respectively. Moreover, the precision and reproducibility of the sensors are indicated from the low values of %RSD of five replicate measurements. In addition, the surface topography of the sensors was studied using scanning electron microscopy to investigate the effect of chemical modification on the surface structure.
Keywords
Carbon-paste
Sensor
Thiamine
Potentiometry
Microscopy
Topography
1 Introduction
Vitamins are important biological molecules which play the role of cofactors in enzyme catalyzed reactions inside the bodies of the living organisms. They cannot be synthesized inside the human body and must be taken from an external source (Li, 2014). Thiamine hydrochloride (TH) is the first identified vitamin (Brumback, 2012). It belongs to a group of vitamins called the B-complex family. It is also known as vitamin B1 and its IUPAC name is 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methylthiazolium chloride hydrochloride (Abiola et al., 2011). TH acts as a co-enzyme in many enzymatic processes inside the human body such as the biosynthesis of neurotransmitters, production of pentoses for nucleic acid synthesis and the production of the reducing agents which is used in the defense against oxidative stress (Guilland, 2013).
Sodium tetraphenylborate (STPB) is the sodium salt of the tetraphenylborate (TPB) anion which has a molar mass of 342.2 g/mol. This organic compound is usually used as a precipitating agent due to its high molecular weight. It also plays an important role in chemical synthesis (Ciattini et al., 1992; King and Bryant, 1992). Moreover, STPB is used as a precipitating agent for preparing the electrode materials of electrochemical potentiometric sensors (Abu Shawish et al., 2016b; Issa et al., 2017; Shao et al., 2016). Scheme 1 shows the chemical structures of TH and STPB.
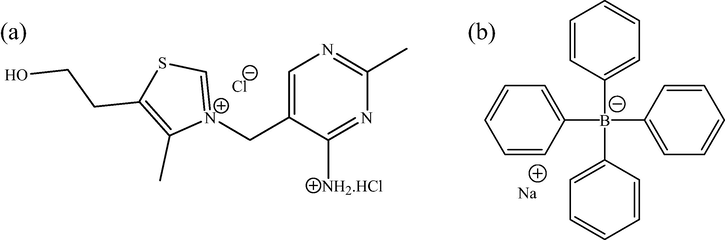
- Chemical structures of TH (a) and STPB (b).
Many researchers proposed analytical methods for determination of TH such as spectrophotometry and spectrofluorimetry based on thiochrome-formation reaction (Fujiwara and Matsui, 1953; Karlberg and Thelander, 1980; Weber and Kewitz, 1985). In addition, high performance liquid chromatography (HPLC) methods were developed to determine TH (Dinç et al., 2000; Marszałł et al., 2005; Tang et al., 2006; Weber and Kewitz, 1985; Yantih et al., 2011). Hassan et al. proposed a membrane sensor based on thiamine-picrolonate ion-pair complex for the determination of thiamine (Hassan et al., 1985). Moreover, Hassan and Elnemma used thiamine-reineckate in nitrobenzene to prepare a membrane sensor selective for thiamine (Hassan and Elnemma, 1989). Another membrane sensor was recently developed by Hosseinzadeh and Khorsandi. The sensor is based on the ion-pair of thiamine and the anti-inflammatory drug, diclofenac sodium (Hosseinzadeh and Khorsandi, 2015). From the abovementioned methods, it is clear that there are few electrochemical methods developed for determination of TH and, to the best of our knowledge, there are no carbon-paste sensors (CPSs) used for determination of TH. The spectrophotometric methods have the disadvantages of relatively high detection limit compared with the other methods and the consumption of a massive amount of chemicals. On the other hand, HPLC usually requires the use of toxic organic solvents and expensive columns. Moreover, the previously proposed potentiometric membrane sensors have the disadvantages of the use of organic solvents, tedious preparation procedures and the use of a filling solution for electrical conduction. Consequently, it is necessary to prepare carbon-paste sensors (Wei et al., 2013; Yan et al., 2013a, 2013b) for estimating TH potentiometrically.
The main objective of this work was to prepare and characterize ion-selective CPSs for thiamine determination in pure solution and multivitamin ampoules for the first time. The sensors are based on TH-TPB ion-pair as an electrode material. Different parameters were investigated to characterize the proposed sensors such as the type of solvent mediator, the percentage of the ion-pair, effect of interfering ions and test solution temperature. The proposed sensors were applied to the determination of TH in pure solutions and different Egyptian multivitamin ampoules.
2 Experimental
The materials used to perform this study are of chromatographic grade purity. Solutions were prepared using double distilled water from a glass distillation instrument.
2.1 Apparatus
Accurate weighing was performed using a four-digit Scientech SA 210 balance throughout the work. Potential measurements were achieved with the aid of a Jenway 3010 pH meter against an Ag/AgCl electrode as an external reference. Scanning electron microscopy images were taken by a Quanta FEG 250 electron microscope, Desert Research Center, El-Matareya, Cairo, Egypt.
2.2 Materials and reagents
Pure TH powder (337.27 g/mol) was obtained from Amriya Pharm. Ind., Egypt. STPB, carbon powder, paraffin oil, dioctyl phthalate (DOP), dibutyl phthalate (DBP), tricresyl phosphate (TCP), ethylhexyl adipate (EHA) and ortho-nitrophenyloctyl ether (NPOE) were purchased from Sigma–Aldrich, Germany. Sodium chloride, potassium chloride, magnesium sulfate heptahydrate, zinc acetate, and calcium chloride are Adwic Chemical Company products, Cairo, Egypt. Amino acids (D-alanine and DL-serine) and sugars (lactose and fructose) were obtained from Sigma–Aldrich, Germany. Neurovit® (Amriya Pharm. Ind., Egypt), Neuroton® (Amoun, Egypt), Bécozyme® (Bayer AG, Germany) and B-Com® (Amoun, Egypt) are the multivitamin ampoules used in the application of the sensors to the analysis of TH in its pharmaceutical form.
2.3 Ion-exchanger preparation
The ion-pair of TH with TPB was used as the ion-exchanger (electrode material) in the sensing carbon-paste. TH solution was prepared by dissolving 84.32 mg of TH in 25 mL of bidistilled water (solution A). A solution of STPB was prepared by dissolving 85.55 mg of STPB in 25 mL of bidistilled water (solution B). Thereafter, solution A was added to solution B and the mixture was left for 48 h for complete coagulation. The mixture was filtered and the obtained precipitate was washed several times with bidistilled water. The precipitate was covered and left to dry for one week at room temperature.
2.4 Preparation of the sensors
The sensing carbon-paste was prepared by mixing the appropriate amounts of graphite, ion-exchanger and plasticizer. The components were made homogeneous by mechanical mixing in an Agate mortar. Thereafter, the obtained paste was fixed in a hollow Teflon holder having a stainless steel rod inside its body for electrical conductance. The length of the rod is tuned (and hence ejecting the paste outside the holder body) with the aid of a rotatable screw at the upper end of the rod. The surface of the paste is smoothed against a smooth piece of paper to give a clean shiny surface. After their preparation, the sensors were stored in a dry and cold place until use.
2.5 Calculation of the selectivity coefficients
The selectivity of ion-selective sensors (ISSs) toward interfering ions can be investigated by measuring their corresponding selectivity coefficients (
2.6 Standard addition method
The standard addition method is a method applied in the analytical applications of the proposed sensors. In this method, known volumes of the TH standard solution were added to the analyte solution (containing the pure form or pharmaceutical multivitamin ampoules). The change in potential reading is recorded and the unknown concentration can be calculated by substitution in Eq. (2) (Brand and Rechnitz, 1970; Issa and Khorshid, 2011):
3 Results and discussion
3.1 Amount of the ion-exchanger
The amount of the ion-exchanger is one of the most important parameters which affect the performance of ISSs. This is because it strongly affects the sensitivity of the sensors in addition to their selectivity and limits of detection (LOD). Consequently, we have studied the effect of changing the amount of the ion-exchanger (2%, 3%, 5% and 7% using DBP as a solvent mediator) on the performance of the proposed sensors and the obtained calibration graphs are illustrated in Fig. 1. Moreover, Table 1 shows the obtained potentiometric characteristics of the CPSs prepared with varying compositions. It is clear from the obtained data that the best performance was obtained by using 2% of the ion-exchanger as it gives the nearest slope to the Nernstian value (29.49 ± 0.24 mV/decade), the widest linear range (5.96 × 10−6–1.00 × 10−2 mol/L) and the lowest LOD (5.25 × 10−6 mol/L). As a result, 2% TH-TPB was used as the best paste composition for the preparation of the sensors throughout the work procedures.
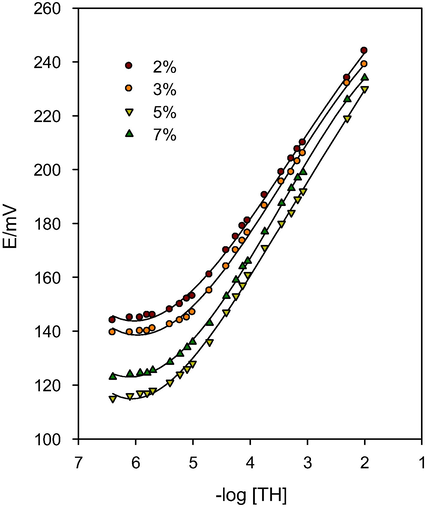
- Effect of variable ion-exchanger ratios on the potentiometric performance of the proposed carbon-paste sensors.
| Composition (w%) | Slope ± SEa | Linear range | LOD | LOQ | RSDb | r2 | ||
|---|---|---|---|---|---|---|---|---|
| TH-TPB | Plast. | C | (mV/decade) | (mol/L) | (%) | |||
| 2c | 49.0 DBP | 49.0 | 29.49 ± 0.24 | 5.96 × 10−6–1.0 × 10−2 | 5.25 × 10−6 | 1.73 × 10−5 | 1.43 | 0.9988 |
| 3 | 48.5 DBP | 48.5 | 34.61 ± 0.19 | 1.18 × 10−5–1.0 × 10−2 | 7.33 × 10−6 | 2.42 × 10−5 | 1.22 | 0.9985 |
| 5 | 47.5 DBP | 47.5 | 33.74 ± 0.17 | 6.31 × 10−6–1.0 × 10−2 | 8.18 × 10−6 | 2.70 × 10−5 | 0.61 | 0.9969 |
| 7 | 46.5 DBP | 46.5 | 33.81 ± 0.22 | 1.10 × 10−5–1.0 × 10−2 | 1.00 × 10−5 | 3.30 × 10−5 | 0.52 | 0.9960 |
| 2c | 49.0 NPOE | 49.0 | 29.60 ± 0.15 | 3.98 × 10−6–1.0 × 10−2 | 3.57 × 10−6 | 1.18 × 10−5 | 0.46 | 0.9998 |
| 2 | 49.0 TCP | 49.0 | 28.03 ± 0.28 | 3.98 × 10−6–1.0 × 10−2 | 3.74 × 10−6 | 1.31 × 10−5 | 1.26 | 0.9986 |
| 2 | 49.0 EHA | 49.0 | 24.84 ± 0.18 | 5.96 × 10−6–8.4 × 10−4 | 9.28 × 10−6 | 1.97 × 10−5 | 2.11 | 0.9929 |
| 2 | 49.0 DOP | 49.0 | 17.72 ± 0.59 | 3.98 × 10−6–8.4 × 10−4 | 3.89 × 10−6 | 1.31 × 10−5 | 2.35 | 0.9944 |
| 2 | 49.0 Paraffin Oil | 49.0 | 24.91 ± 0.91 | 7.94 × 10−6–9.09 × 10−5 | 8.38 × 10−6 | 2.62 × 10−5 | 2.09 | 0.9973 |
Plast.: plasticizer; C: graphite; SE: standard error; r2: correlation coefficient.
3.2 Solvent mediator
Ethers and some fatty acid esters are usually used as solvent mediators in ISSs preparation because they improve electrical conductivity and mechanical properties of either the carbon-paste or the polymeric membrane. In addition, they can extend the lifetime of ISSs as reported by Zahran et al. (2014). The effect of different types of plasticizers (paraffin oil, DBP, DOP, TCP, EHA, NPOE) was studied and the obtained results can be found in Table 1. The calibration curves using the selected composition and solvent mediators are shown in Fig. S1 in the supporting information. The resulting calibration graphs were used to evaluate the potentiometric performance of the sensor in each case by following up the changes in their slopes, LODs and linear ranges. In addition, the lifetime of the sensors was taken into consideration during choosing the most suitable sensor. The obtained results reveal that DBP (slope of 29.49 ± 0.24 mV/decade) and NPOE (slope of 29.60 ± 0.15 mV/decade) are the most suitable sensors for potentiometric determination of TH in aqueous solutions. These results agree with the expected trend of sensitivity from the dielectric constant values of these solvent mediators (Table S1). The highest sensitivity is obtained using the NPOE (dielectric constant ∼24). Based on the above results, two sensors were prepared using DBP (sensor I) and NPOE (sensor II), characterized and applied to the determination of TH in aqueous media.
3.3 Effect of temperature
Changes in test solution temperature can affect the potentiometric performance of ISSs. These effects are mainly attributed to two reasons. Firstly, the solubility of the ion-exchanger in water is enhanced by temperature rise causing leaching of the ion-exchanger or the electro-active species from the carbon-paste. Secondly, since adsorption depends on weak chemical forces between the analyte molecules and the ion-exchanger molecules in the carbon-paste such, thermal energy which can overcome these chemical forces are able to inhibit the analyte adsorption on the surface of the sensor. Different test solution temperatures, 25, 30, 35, 40 and 45 °C for sensor (I) and 25, 30, 35 and 45 °C for sensor (II), were used to investigate the effect of temperature on the sensors behavior. The results (Table 2) show that sensor (I) does not show a significant change upon changing the test solution temperature as the slope values remain near Nernstian. However, the performance of sensor (II) is affected, to some extent, by temperature changes as the slope values increase by increasing test solution temperature. Calibration curves of the two sensors at different test solution temperatures are illustrated in Figs. S2 and S3. Both sensors can be used for TH determination at room temperature. Moreover, this conclusion can be supported by the calculated values of the thermal coefficients [
| Temperature | Slope | Linear range | LOD | LOQ | r2 |
|
|
|---|---|---|---|---|---|---|---|
| (°C) | (mV/decade) | (mol/L) | (mV) | ||||
| 2% + DBP | |||||||
| 25 | 31.06 | 3.98 × 10−6–10−2 | 3.73 × 10−6 | 12.31 × 10−6 | 0.998 | 256.5 | 544.7 |
| 30 | 26.8 | 9.90 × 10−6–10−2 | 1.17 × 10−6 | 3.86 × 10−6 | 0.996 | 228.7 | 511.6 |
| 35 | 32.95 | 5.96 × 10−6–10−2 | 4.20 × 10−6 | 13.86 × 10−6 | 0.998 | 247.5 | 526.9 |
| 40 | 29.57 | 5.96 × 10−6–10−2 | 5.88 × 10−6 | 19.40 × 10−6 | 0.999 | 228.3 | 504.2 |
| 45 | 36.98 | 5.96 × 10−6–10−2 | 6.42 × 10−6 | 21.19 × 10−6 | 0.998 | 255.2 | 527.5 |
| 2% + NPOE | |||||||
| 25 | 30.12 | 5.96 × 10−6–10−2 | 3.71 × 10−6 | 12.24 × 10−6 | 0.999 | 233.1 | 519.3 |
| 30 | 40.37 | 5.96 × 10−6–10−2 | 4.27 × 10−6 | 14.09 × 10−6 | 0.999 | 280.2 | 563.1 |
| 35 | 42.51 | 7.94 × 10−6–10−2 | 4.25 × 10−6 | 14.02 × 10−6 | 0.996 | 235.6 | 515.0 |
| 45 | 47.77 | 5.96 × 10−6–10−2 | 4.91 × 10−6 | 16.20 × 10−6 | 0.994 | 317.2 | 589.5 |
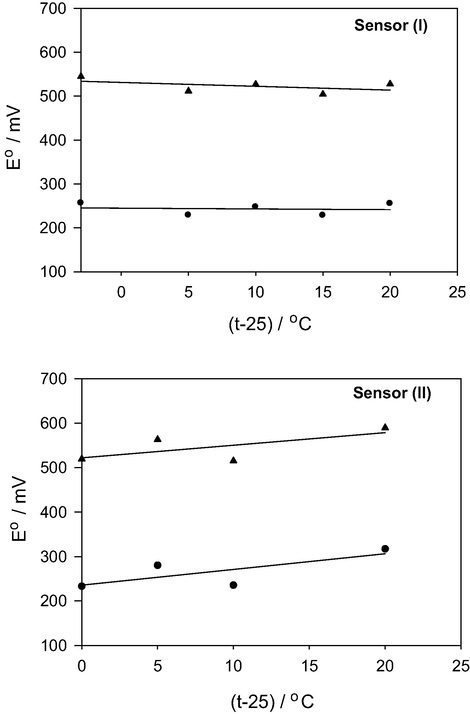
- Changing standard sensor potential (▴) and standard cell potential (●) with changing the test solution temperature.
3.4 Selectivity of the sensors
Complex samples may contain large number of interfering ions, especially metal ions. These ions are able to interfere with the analyte ions and give false results during analytical procedures. Furthermore, many multivitamin pharmaceuticals are combinations of vitamins and minerals especially those used as general tonics. Accordingly, TH may be found associated with different metal cations, sugars and amino acids when we are talking about pharmaceutical and/or biological samples. Selectivity of the proposed sensors toward TH is measured by calculating the selectivity coefficients in the presence of various interfering ions such as Na+, K+, Ca2+, Mg2+, Zn2+, D-alanine, DL-serine, fructose and lactose. The selectivity coefficients were calculated by applying the matched potential method (Horvai, 1997). The p-function of the selectivity coefficient values of sensors (I) and (II) is tabulated in Table S2. The higher these values are, the higher is the selectivity of the sensor toward the corresponding ion. The values in the table are small enough (2.2–3.8) indicating high selectivity of the sensors toward TH over the studied cations. The calibration curves of the interfering ions and TH using sensor (I) and sensor (II) are plotted in Fig. 3 from which we can notice that the sensors are nearly not sensitive toward the studied interfering ions.
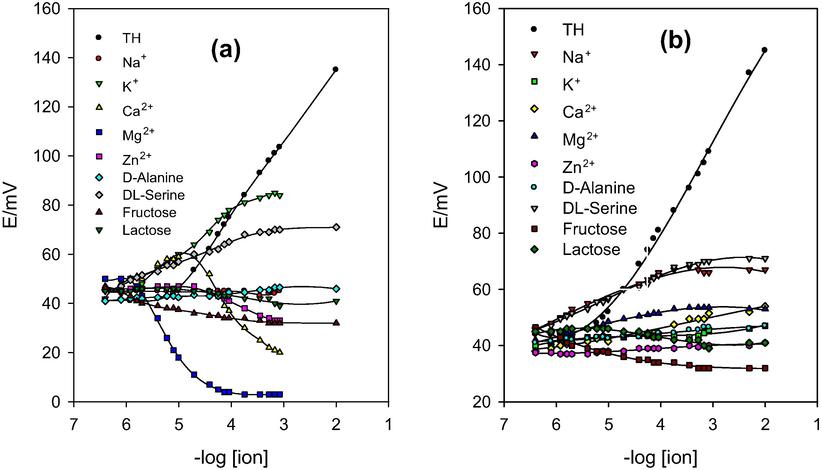
- Calibration graphs for TH and possible interfering metal ions, amino acids and sugars showing the response of the proposed CPSs towards TH over the interfering ions. (a) 2% TH-TPB + DBP; (b) 2% TH-TPB + NPOE.
3.5 Hysteresis and response time
Response time of an ISS is defined as the length of time which elapses between the instant at which an ISS and a reference electrode are brought into contact with a sample solution (or at which the concentration of the ion of interest in a solution in contact with an ISS and a reference electrode is changed) and the first instant at which the potential of the cell becomes equal to its steady-state value within 1 mV (Buck and Lindner, 1994). However, hysteresis is known as the sensor memory. It is said to occur if there is a different potential reading is observed after the concentration has been changed and restored to its original value. The reproducibility of the sensor will consequently be poor. The systematic error is generally in the direction of the concentration of the solution in which the electrode was previously immersed (Buck and Lindner, 1994). Fig. 4 shows the response time and the memory of sensors (I) and (II). Sensor (I) responds to changes in analyte concentration within 10–12 s while the response time of sensor (II) is about 5 s. It is obvious from the figure that sensor (I) has almost no memory allowing the use of the sensor several times for analysis of different successive samples without need to renew the surface. On the other hand, sensor (II) shows some memory toward the concentration of the previous solution in which it was immersed. Actually, this is a non-issue because this problem can be resolved by renewing the surface of the carbon-paste between each two successive runs.
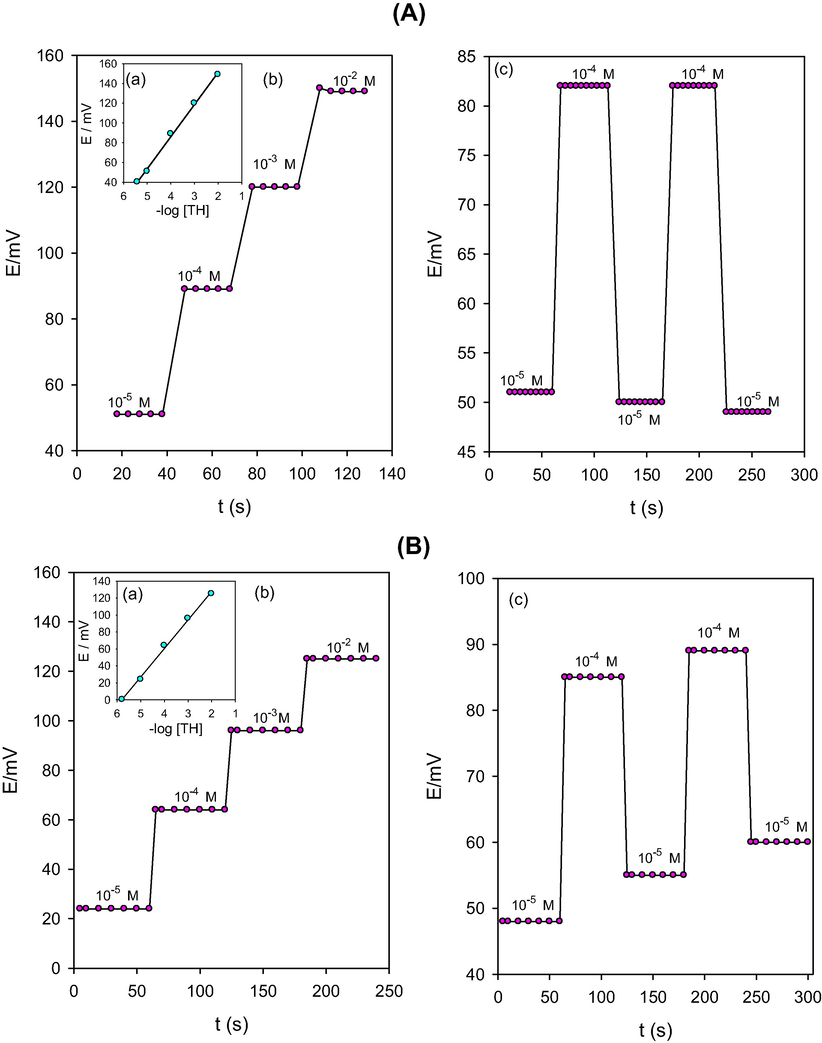
- Time-trace calibration plot (a), response time (b) and hysteresis (c) of the proposed CPSs. (A) 2% TH-TPB + DBP; (B) 2% TH-TPB + NPOE.
3.6 Lifetime
Following up the performance of the ISSs prepared using the same carbon-paste, it was found that the carbon-paste of sensor (I) has a lifetime of only 1–2 days. On the other hand, the lifetime of the carbon-paste of sensor (II) is about one month. These results are in line with the previously reported work concerning the influence of the type of solvent mediator on the lifetime of ISSs (Zahran et al., 2014).
3.7 Surface topography
The amount of defects present in the surface of an adsorbent strongly affects the amount of the adsorbed species (Pan, 1992). As a result, it would be useful to study the surface topography of the proposed sensors to investigate the effect of chemical modification on the amount of defects and consequently on the adsorption capacity of the surface. Scanning electron micrographs of both sensors (I) and (II) are illustrated in Fig. 5. The topographies of the unmodified sensors are smoother and show fewer defects than the surfaces of the modified ones. In addition, the modified sensors have rough surfaces due to the presence of plate-like parts arranged in different elevations causing the appearance of many pores which act as active sites for TH adsorption (Atta et al., 2012). As a result, the active surface area available for adsorption of TH is also increased.
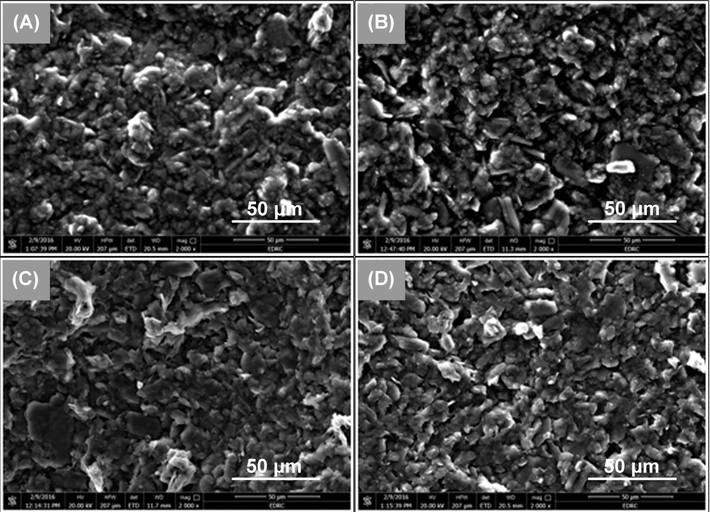
- SEM micrographs of CPSs. The magnification is 2000×. (A) bBare paste + DBP, (B) 2%TH-TPB + DBP, (C) bare paste + NPOE and (D) 2%TH-TPB + NPOE.
3.8 Assay of pure solutions and multivitamin ampoules
Different concentrations of TH were estimated in pure solution and a number of Egyptian multivitamin ampoules using the standard addition method and satisfactory results were obtained. The solutions of the ampoules were diluted as required and the appropriate volumes were taken and completed to 50 mL for standard addition measurements. Table 3 summarizes the recovery values obtained from the analytical application of sensor (I) and (II). The found recovery values are 97.92–103.7% and 97.21–102.2% for sensors (I) and (II), respectively. In addition, the relative standard deviation (RSD) values for five replicate measurements are low indicating the reproducibility and precision of the proposed sensors. Both pure solution and ampoule recovery values are excellent and acceptable. As a result, the proposed sensors can be used for accurate and precise determination of TH in aqueous solutions.
| CPS + DBP | CPS + NPOE | |||||
|---|---|---|---|---|---|---|
| Taken (mg) | Found | Recoverya | RSDb | Found | Recoverya | RSDb |
| (mg) | (%) | (%) | (mg) | (%) | (%) | |
| Pure solution | ||||||
| 0.1686 | 0.1730 | 102.63 | 1.23 | 0.1639 | 97.21 | 0.84 |
| 0.8432 | 0.8576 | 101.71 | 1.38 | 0.8494 | 100.74 | 1.29 |
| 1.686 | 1.717 | 101.85 | 0.96 | 1.649 | 97.81 | 0.74 |
| 8.432 | 8.456 | 100.29 | 1.19 | 8.518 | 101.02 | 0.62 |
| 16.86 | 17.26 | 102.37 | 1.20 | 16.69 | 98.99 | 1.73 |
| Neurovit® (ampoules 50 mg/mL) | ||||||
| 0.1686 | 0.1676 | 99.38 | 0.77 | 0.1676 | 99.43 | 0.85 |
| 0.8432 | 0.8486 | 100.64 | 0.37 | 0.8399 | 99.61 | 1.36 |
| 1.686 | 1.749 | 103.72 | 0.59 | 1.6671 | 98.88 | 0.81 |
| 8.432 | 8.284 | 98.24 | 0.36 | 8.459 | 100.32 | 0.42 |
| 16.86 | 17.05 | 101.13 | 0.94 | 17.23 | 102.19 | 1.49 |
| Neuroton® (ampoules 100 mg/mL) | ||||||
| 0.1686 | 0.1668 | 98.99 | 0.97 | 0.1714 | 101.67 | 1.21 |
| 0.8432 | 0.8670 | 102.82 | 1.19 | 0.8409 | 99.73 | 0.94 |
| 1.686 | 1.675 | 99.35 | 1.23 | 1.654 | 98.13 | 1.53 |
| 8.432 | 8.738 | 103.63 | 0.82 | 8.419 | 99.85 | 0.37 |
| 16.86 | 16.94 | 100.49 | 0.93 | 16.95 | 100.54 | 0.67 |
| Bécozyme® (ampoules 5 mg/mL) | ||||||
| 0.1686 | 0.1651 | 97.92 | 1.10 | 0.1694 | 100.48 | 0.33 |
| 0.8432 | 0.8419 | 99.84 | 0.78 | 0.8360 | 99.15 | 0.56 |
| 1.686 | 1.735 | 102.89 | 0.97 | 1.669 | 99.00 | 1.48 |
| 8.432 | 8.629 | 102.34 | 0.47 | 8.514 | 100.97 | 0.84 |
| 16.86 | 16.98 | 100.70 | 0.60 | 17.00 | 100.83 | 0.77 |
| B-Com® (ampoules 5 mg/mL) | ||||||
| 0.1686 | 0.1655 | 98.14 | 0.65 | 0.1689 | 100.18 | 0.45 |
| 0.8432 | 0.8414 | 99.79 | 0.46 | 0.8619 | 102.22 | 0.74 |
| 1.686 | 1.674 | 99.28 | 0.69 | 1.676 | 99.40 | 0.86 |
| 8.432 | 8.442 | 100.12 | 0.36 | 8.294 | 98.36 | 1.20 |
| 16.86 | 17.01 | 100.88 | 0.82 | 16.43 | 97.45 | 1.07 |
4 Conclusions
The present work presents the potentiometric and surface topography studies of two CPSs designed for the first time to estimate TH in aqueous solutions. Different characterization parameters were investigated to evaluate the sensors performance. The most worth noting advantages of the sensors are their ease of preparation, Nernstian behavior, low limits of detection, high accuracy and reproducibility. In addition, the sensors show fast response to changes in TH concentration and low interference from common interfering ions. Sensor (I) shows higher thermal stability than sensor (II). It was found that changing the type of plasticizer strongly affects the lifetime of CPSs as NPOE extends the lifetime of sensor (II) to one month instead of 1 or 2 days in case of sensor (I) which was prepared using DBP. SEM was used to characterize the prepared sensors in comparison with the chemically unmodified ones. The proposed sensors were successfully applied to the determination of TH in pure aqueous solution and four multivitamin ampoules obtained from the Egyptian market using the standard addition method. The results are rather better than or similar to the previously reported methods in the literature (Table 4). It is highly recommended to use the proposed sensors for TH determination in drug control laboratories.
| Method | Determination range (μg/mL) | LOD (μg/mL) | r2 | Recovery (%) | References |
|---|---|---|---|---|---|
| Sensor (I) | 2–3372 | 1.77 | 0.9988 | 97.92–103.72 | Current study |
| Sensor (II) | 1.34–3372 | 1.20 | 0.9998 | 97.21–102.19 | Current study |
| Micellar electrokinetic chromatography | 5–15 | 0.3–125 | >0.9990 | 99.0–101.2 | Okamoto et al. (2002) |
| Orion liquid membrane electrode (model 92) | 16.86–3372 | 1.0 | Not indicated | 96.5–99.2 | Hassan et al. (1985) |
| Fluorescent sensor | 3.37–16-86 | 0.094 | 0.9974 | 97.11–97.13 | Purbia and Paria (2016) |
| Reverse-phase HPLC | 489.9–1469.6 | Not indicated | 0.9997 | 99.8–100.2 | Poongothai et al. (2010) |
| Capillary zone electrophoresis | 5–200 | 0.9–9.0 | 0.9952 | 97.0–101.4 | Franco et al. (2012) |
| Thiamine-reineckate liquid membrane electrode | 0.337–337.27 | 0.3 | 0.9980 | 98.1 | Hassan and Elnemma (1989) |
| Whole cell immobilized amperometric biosensor | 0.0016–0.0337 | 0.0016 | 0.9956 | 97.2–102.5 | Akyilmaz et al. (2006) |
| Flow-through biparameter system | 2–30 | 0.10 | 0.9999 | 97.00–100.3 | Ortega Barrales et al. (2001) |
| UV photometric sensor | 2.0–33.0 | 0.16 | 0.9999 | 95.83–102.88 | Ortega Barrales et al. (1998) |
| CdSe quantum dots | 5.00–40.0 | 0.070 | 0.9963 | 99.35–99.85 | Sun et al. (2008) |
References
- 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-hydroxyethyl)-4-methyl thiazolium chloride hydrochloride as green corrosion inhibitor of copper in HNO3 solution and its adsorption characteristics. Green Chem. Lett. Rev.. 2011;4:273-279.
- [CrossRef] [Google Scholar]
- Determination of haloperidol drug in ampoules and in urine samples using a potentiometric modified carbon paste electrode. Measurement. 2016;78:180-186.
- [CrossRef] [Google Scholar]
- Determination of trihexyphenidyl hydrochloride drug in tablets and urine using a potentiometric carbon paste electrode. Sens. Actuators B: Chem.. 2016;235:18-26.
- [CrossRef] [Google Scholar]
- Whole cell immobilized amperometric biosensor based on Saccharomyces cerevisiae for selective determination of vitamin B1 (thiamine) Anal. Biochem.. 2006;354:78-84.
- [Google Scholar]
- A novel sensor of cysteine self-assembled monolayers over gold nanoparticles for the selective determination of epinephrine in presence of sodium dodecyl sulfate. Analyst. 2012;137:2658.
- [CrossRef] [Google Scholar]
- Computer approach to ion-selective electrode potentiometry by standard addition methods. Anal. Chem.. 1970;42:1172-1177.
- [CrossRef] [Google Scholar]
- Journal combat: initiating a publication, competing for visibility, and assuring ethical behavior. J. Evid. Based. Complement. Altern. Med.. 2012;17:4-8.
- [CrossRef] [Google Scholar]
- Recommendations for nomenclature of ion-selective electrodes. Pure Appl. Chem.. 1994;66:2527-2536.
- [Google Scholar]
- Palladium-catalyzed cross-coupling reactions of vinyl and aryl triflates with tetraarylborates. Tetrahedron Lett.. 1992;33:4815-4818.
- [CrossRef] [Google Scholar]
- A comparison of matrix resolution method, ratio spectra derivative spectrophotometry and HPLC method for the determination of thiamine HCl and pyridoxine HCl in pharmaceutical preparation. J. Pharm. Biomed. Anal.. 2000;22:915-923.
- [CrossRef] [Google Scholar]
- Application of CZE method in routine analysis for determination of B-complex vitamins in pharmaceutical and veterinary preparations. Int. J. Anal. Chem.. 2012;2012:1-7.
- [Google Scholar]
- Determination of thiamine by thiochrome reaction. Anal. Chem.. 1953;25:810-812.
- [CrossRef] [Google Scholar]
- Thiamine-reineckate liquid membrane electrode for the selective preparations. Analyst. 1989;114:735-737.
- [Google Scholar]
- Hassan, S.S.M., Iskander, M.L., Nashed, N.E., 1985. Papers A New Liquid Membrane Electrode for Determination of Vitamin in Multivitamin Preparations, 584–586.
- The matched potential method, a generic approach to characterize the differential selectivity of chemical sensors. Sens. Actuators B: Chem.. 1997;43:94-98.
- [CrossRef] [Google Scholar]
- Interaction of vitamin B1 with bovine serum albumin investigation using vitamin B1-selective electrode: potentiometric and molecular modeling study. J. Biomol. Struct. Dyn.. 2015;1102:1-24.
- [CrossRef] [Google Scholar]
- Using PVC ion-selective electrodes for the potentiometric flow injection analysis of distigmine in its pharmaceutical formulation and biological fluids. J. Adv. Res.. 2011;2:25-34.
- [CrossRef] [Google Scholar]
- Solubilities and solubility products of clomipramine hydrochloride ion-associates with tetraphenylborate and silicotungstate. Arab. J. Chem.. 2017;10:336-343.
- [Google Scholar]
- Extraction based on the flow-injection principle. Anal. Chim. Acta. 1980;114:129-136.
- [CrossRef] [Google Scholar]
- Modified carbon paste sensor for the potentiometric determination of neostigmine bromide in pharmaceutical formulations, human plasma and urine. Biosens. Bioelectron.. 2014;51:143-149.
- [CrossRef] [Google Scholar]
- Preparation and characterization of crystalline N-acylammonium salts. J. Org. Chem.. 1992;57:5136-5139.
- [CrossRef] [Google Scholar]
- Li, S., 2014. Handbook of Food Chemistry. In: Cheung, K.P.C., Mehta, M.B. (Eds.), Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 1–20. http://dx.doi.org/10.1007/978-3-642-41609-5_16-1.
- High-performance liquid chromatography method for the simultaneous determination of thiamine hydrochloride, pyridoxine hydrochloride and cyanocobalamin in pharmaceutical formulations using coulometric electrochemical and ultraviolet detection. J. Chromatogr. A. 2005;1094:91-98.
- [CrossRef] [Google Scholar]
- Simultaneous determination of ingredients in a vitamin enriched drink by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal.. 2002;30:815-822.
- [Google Scholar]
- Simultaneous determination of thiamine and pyridoxine in pharmaceuticals by using a single flow-through biparameter sensor. J. Pharm. Biomed. Anal.. 2001;25:619-630.
- [CrossRef] [Google Scholar]
- A selective optosensor for UV spectrophotometric determination of thiamine in the presence of other vitamins B. Anal. Chim. Acta. 1998;376:227-233.
- [CrossRef] [Google Scholar]
- Interaction of water, oxygen, and hydrogen with TiO2(110) surfaces having different defect densities. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 1992;10:2470.
- [CrossRef] [Google Scholar]
- Simultaneous and accurate determination of vitamins B1, B6, B12 and alphalipoic acid in multivitamin capsule by reverse-phase high performance liquid chromatographic method. Int. J. Pharm. Pharm. Sci.. 2010;2:133.
- [Google Scholar]
- A simple turn on fluorescent sensor for the selective detection of thiamine using coconut water derived luminescent carbon dots. Biosens. Bioelectron.. 2016;79:467-475.
- [Google Scholar]
- Preparation of Fe2O3-clorprenaline/tetraphenylborate nanospheres and their application as ion selective electrode for determination of clorprenaline in pork. Nanoscale Res. Lett.. 2016;11:1-8.
- [CrossRef] [Google Scholar]
- A feasible method for the sensitive and selective determination of vitamin B1 with CdSe quantum dots. Microchim. Acta. 2008;163:271-276.
- [CrossRef] [Google Scholar]
- A simplified approach to the determination of thiamine and riboflavin in meats using reverse phase HPLC. J. Food Compos. Anal.. 2006;19:831-837.
- [CrossRef] [Google Scholar]
- Determination of thiamine in human plasma and its pharmacokinetics. Eur. J. Clin. Pharmacol.. 1985;28:213-219.
- [CrossRef] [Google Scholar]
- A novel acetylcholine biosensor and its electrochemical behavior. J. Biomed. Nanotechnol.. 2013;9(5):736-740.
- [Google Scholar]
- Fabrication of an electrochemical biosensor array for simultaneous detection of l-glutamate and acetylcholine. J. Biomed. Nanotechnol.. 2013;9:1378-1382.
- [Google Scholar]
- Preparation and electrochemical behavior of l-glutamate electrochemical biosensor. J. Biomed. Nanotechnol.. 2013;9:318-321.
- [Google Scholar]
- Validation of HPLC method for determination of thiamin hydrochloride, riboflavine, nicotinamide, and pyridoxine hydrochloride in syrup preparation. Can. J. Sci. Ind. Res.. 2011;2(7):269-278.
- [Google Scholar]
- Polymeric plasticizer extends the lifetime of PVC-membrane ion-selective electrodes. Analyst. 2014;139:757-763.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2016.11.012.
Appendix A
Supplementary material







