Translate this page into:
Preconcentration procedure trace amounts of palladium using modified multiwalled carbon nanotubes sorbent prior to flame atomic absorption spectrometry
*Corresponding author at: Environment Department, Research Institute of Environmental Sciences, International Center for Science, High Technology & Environmental Sciences, P.O. Box 76315-117, Kerman, Iran. Tel.: +98 3426226611–13, fax: +98 3426226617 darush_afzali@yahoo.com (Daryoush Afzali), daryoush_afzali@yahoo.com (Daryoush Afzali),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A method for preconcentration of palladium at trace level on modified multiwalled carbon nanotubes columns and determination by flame atomic absorption spectrometry (FAAS) has been developed. Multiwalled carbon nanotubes (MWCNTs) were oxidized with concentrated HNO3 and the oxidized multiwalled carbon nanotubes were modified with 5-(4′-dimethylamino benzyliden)-rhodanine, and then were used as a solid sorbent for preconcentration of Pd(II) ions. Factors influencing sorption and desorption of Pd(II) ions were investigated. The sorption of Pd(II) ions was quantitative in the pH range of 1.0–4.5, whereas quantitative desorption occurs with 3.0 mL 0.4 mol L−1 thiourea. The amount of eluted palladium was measured using flame atomic absorption spectrometry. The effects of experimental parameters, including sample flow rate, eluent flow rate, and eluent concentration were investigated. The effect of coexisting ions showed no interference from most ions tested. The proposed method permitted a large enrichment factor (about 200). The relative standard deviation of the method was ±2.73% (for eight replicate determination of 2.0 μg mL−1 of Pd(II)) and the limit of detection was 0.3 ng mL−1. The method was applied to the determination of Pd(II) in water, road dust, and standard samples.
Keywords
Carbon nanotubes application
Preconcentration
Palladium determination
Sorbent
1 Introduction
Recently, palladium is attracting a lot of attention in various fields, such as industry, technology, and medicine, due to its excellent chemical and physical characteristics. The abundance of palladium is extremely low in environments, mostly from pg g−1 to μg g−1 levels (Cotton and Wilkinson, 1998). Environmental or industrial pollutions by palladium have so far been hardly reported as compared with toxic heavy metals such as cadmium, mercury, and lead. However, the long-term exposure to palladium may affect the human health and the growth of living beings in the future (Nakajima et al., 2009). Therefore, development of analysis methods for the accurate and precise determination of traces of palladium is meaningful for quality control of industrial products, environmental monitoring, as well as palladium ore exploration (Liang et al., 2009). Palladium analysis requires analytical methods of high sensitivity, selectivity, and the control of interference effects. The most widely used methods for the determination of Pd in environmental samples include graphite furnace atomic absorption spectrometry (GFAAS) (Zylkiewicz, 2004) and inductive coupled plasma mass spectrometry (ICP-MS) (Gomez et al., 2000). In environmental samples, the low concentration of Pd (μg L−1 levels) together with the high concentration of interfering matrix components often requires an enrichment step combined with a matrix separation which allows an accurate and precise determination of Pd in samples with very low analyte content (Rauch et al., 2000). Liquid–liquid extraction has been widely used for separation and preconcentration of palladium (Schuster, 1992; Paiva, 2000). Because of some disadvantages of solvent extraction methods such as emulsion formation, different extracting efficiencies, and low sensitivity, much interest has been recently focused on repairing conventional solvent extraction methods for isolating environmental pollutants with solid phase extraction (SPE) techniques. SPE has been demonstrated to be a very effective preconcentration method when applied prior to spectrometric determination. The analytes are partitioned between a solid and liquid phase based on the affinity to the solid phase. Compared with liquid–liquid extraction, methods utilizing solid sorbents are simpler and faster, reduce organic solvent consumption and yield higher enrichment factors (Simpson, 2000). Different solid phase extractors such as Amberlite XAD resins (Tunceli and Turker, 2000), polyurethane foam (Kang and Lee, 1983), activated carbon (Chakrapani et al., 2001), and silica gel (Tokaliglu et al., 2004) with chelating groups have been the most widely used collectors.
Nowadays, carbon nanotubes (CNTs) have attracted much interest that was directed toward exploiting unique thermal, mechanical, electronic, and chemical properties (Popov, 2004) since they were first discovered. The extremely large surface area and the unique tubular structure make CNTs a promising adsorbent material. The highly developed hydrophobic surface of CNTs exhibits strong sorption properties toward various compounds. Due to their unique characteristic and strong adsorption ability (Saridara et al., 2005), they have been successfully used to remove many kinds of pollutants such as dioxin from air (Long and Yang, 2001), lead (Li et al., 2006), cadmium (Li et al., 2003a,b), zinc (Lu and Chiu, 2007), fluoride (Li et al., 2003a,b), 1,2-dichlorobenzene (Peng et al., 2003), and trihalomethanes (Lu et al., 2005) from different media. Also in our previous work we used MWCNTs as a solid sorbent for preconcentration trace amounts of Co(II) and Rh(III) ions (Afzali and Mostafavi, 2008; Ghaseminezhad et al., 2009).
The goal of this study is to modify MWCNTs by 5-(4′-dimethylamino benzyliden)-rhodanine. Moreover, the performances of modified multiwalled carbon nanotubes (MMWCNTs) are tested as a new sorbent for the preconcentration of trace Pd(II). Separation and preconcentration of Pd(II) are carried out in a glass column filled with MMWCNTs, and Pd(II) is determined by FAAS. Applications of the proposed method for the analysis of different samples are also described.
2 Experimental
2.1 Instrumentation
The palladium measurement was performed with a Varian SpectrAA 220 atomic absorption spectrometer (Australia). A palladium hollow cathode lamp, operated at 5 mA, was utilized as the radiation source. The analytical wavelength (363.5 nm) and slit width (0.1 nm) were used as recommended by manufacturers. The pH values were measured with a metrohm 827 pH meter (Switzerland) supplied with a combined glass electrode. A funnel-tipped glass tube (80 × 10 mm) was used as column for preconcentration. The vessels used for trace analysis were kept in 10% nitric acid for at least 24 h and subsequently washed three times with double distilled water.
2.2 Reagents and solutions
All reagents used were of the highest purity available and at least of analytical reagent grade. The stock solution of palladium (II) (100.0 μg mL−1) was prepared by dissolving the proper amount of Pd (CH3COO)2 from Merck (Darmstadt, Germany) in 2 mol L−1 hydrochloric acid solution. Standard solutions of palladium (II) were prepared daily by appropriate dilution of palladium stock solution. A 0.02% solution of 5-(4′-dimethylamino-benzyliden)-rhodanine (Merck) was prepared by dissolving it in ethanol. MWCNTs (95% purity) with an average outer diameter of 3–20 nm, length of 1–10 μm, number of walls 3–15 and surface area of 350 m2 g−1 were obtained from Plasma Chem. GmbH (Berlin, Germany). Raw MWCNTs were heated at 350 °C for 30 min to remove amorphous carbon. Prior to use, MWCNTs were oxidized with concentrated HNO3 according to the literature, in order to create binding sites onto the surface of MWCNTs (Xu et al., 2003). The treatment was carried out by the dispersion of 50 mL of concentrated HNO3 to 5.0 g of MWCNTs, and then refluxing for 5 h at 80 °C. Afterward, the oxidized MWCNTs were washed with distilled water until removing any excess nitric acid (neutral pH of solution), then 20 mL of a 0.02% solution of 5-(4′-dimethylamino-benzyliden)-rhodanine in ethanol was added to the 0.5 g of MWCNTs, and stirred for 24 h, producing MMWCNTs, which were dried at room temperature.
2.3 Preparation of column
Forty milligrams of MMWCNTs was slurred in water, and then poured into a funnel-tipped glass tube plugged with a small piece of glass wool at the ends. A glass column packed with 0.04 g of MMWCNTs sorbent (height of packing being about 15 mm) was used as the operational column. The column could be used repeatedly 30 times after washing with distilled water.
2.4 Procedures for wastewater samples
Wastewater samples were collected in acid leached polyethylene bottles. The wastewater samples were collected from copper factory in Sarchashmeh (Kerman, Iran). The only pretreatment was acidification to pH 2 with nitric acid, which was performed immediately after collection, in order to prevent adsorption of the palladium ions on the flask walls. The samples were filtered before analyses through a cellulose membrane of 0.45 μm pore size.
2.5 Procedures for road dust samples
The road dust samples were collected from different roadsides in Kerman province, Iran. The samples were dried at 90 °C for 2 h, ground, passed through a sieve of 120 meshes and homogenized. 1.0 g of each sample was weighed into a 100 mL of beaker. In order to decompose it, 10 mL of aqua regia was added to the beaker and the mixture was heated almost to dryness. Then, 5 mL of aqua regia and 2 mL H2O2 were added again to the residue and the mixture was evaporated to dryness. Finally, de-ionized water was added to beaker and then the insoluble part was filtered through a filter paper (blue band) and washed with de-ionized water. The pH was adjusted to 2.5 and the total volume was made up to 10.0 mL with de-ionized water in calibrated flaks.
2.6 Procedures for platinum–iridium alloy samples
In order to test the applicability of the proposed method for the analysis of real samples, one platinum–iridium alloy was analyzed. To 5.0 mg of the alloy with known composition, 7 mL of aqua regia was added and the solution was evaporated. Five milliliters of concentrated hydrochloric acid was added to it and the solution was warmed, transferred to a 100 mL volumetric flask, and made up to the mark with distilled water.
2.7 General procedure
Initially, for column conditioning, distilled water was passed through the column. An aliquot of the solution containing 6.0 μg of Pd(II) was taken in a 100 mL beaker and it was added to it 5 mL of buffer solution with pH ∼2.5, then diluted to ∼50 mL with distilled water. This solution was passed through the column at a flow rate of 2 mL min−1. After passing this solution, the column was washed with 5 mL of distilled water. The adsorbed palladium on the column was eluted with 3.0 mL 0.4 mol L−1 thiourea, at a flow rate 1 mL min−1. Then, the eluted solution was aspirated directly into the flame of AAS and compared with a blank prepared in the same manner, but without the addition of Pd(II).
3 Results and discussions
Preliminary experiments showed that the oxidized MWCNTs have a low tendency for the retention of Pd(II) adsorption (36%). Recent work (Afzali and Mostafavi, 2008) indicated that the oxidized MWCNTs can adsorb organic material, and therefore, since 5-(4′-dimethylamino-benzyliden)-rhodanine is a good reagent complexing with Pd(II), it was added to MWCNTs. Modified multiwalled carbon nanotubes (MMWCNTs) were capable of retaining Pd(II) in the sample solution (97.6 adsorption of Pd(II) when the test solution contained 6.0 μg of Pd(II) in 50 mL water). Thus, the adsorption of 5-(4′-dimethylamino-benzyliden)-rhodanine on the surface of MWCNTs had an effective role in the sorption of PdII). In order to achieve the best performance from this system, different parameters were optimized. The pH of the sample, type, and concentration of the eluent, the effects of the sample and eluent flow rates on the extraction efficiency, the effect of foreign ions, the effect of the sample volume, and the maximum capacity of the sorbent for Pd(II) recovery were studied.
3.1 Effect of the sample pH
As such pH is one of the important factors which affects the efficiency of retention/elution of metal ions by solid phase extraction. The percent sorption of palladium ion on the sorbent surface as a function of the pH of the sample solution was examined in the range of 0.7–6.5. The pH was adjusted by using a buffer solution. As can be seen from Fig. 1, the percent sorption of Pd(II) depends on the pH of the sample solution, and is about 97% in the pH range 1.0–4.5. In subsequent studies, the pH was maintained at approximately 2.5.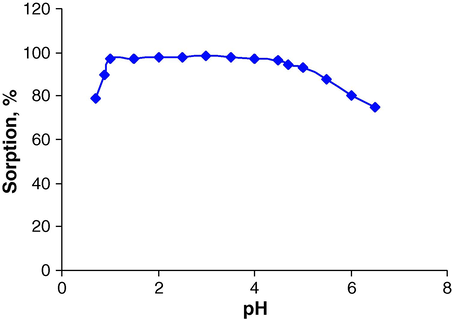
Effect of pH on the sorption of Pd(II). Conditions: Pd, 6.0 μg; flow rate of sample, 2 mL min−1; eluent solution, 3.0 mL of 0.4 mol L−1 thiourea with flow rate 1 mL min−1.
3.2 Effect of the sample flow rate
The flow rate of the sample solution is a measure of the contact time between the sample solution and the sorbent. The flow rate was adjusted simply by connecting the adsorbing column to a flask, which had a controllable vacuum. As a result of the investigation of this effect, valuable information about the adsorption rate of the palladium ions on the sorbent can be obtained. The flow rate of sample solution on the recovery of Pd(II) on MMWCNTs was also investigated in the range of 0.5–4 mL min−1. The results showed that flow rate variation in the range of 0.5–3 mL min−1 did not have a significant effect on the sorption of the Pd(II) ion. In order to achieve a good precision, a flow rate of 2 mL min−1 was chosen for further studies.
3.3 Selection of eluent solution
A series of selected eluents, such as HCl, HNO3, sodium thiosulfate, and thiourea, were used in order to find the best eluent for desorption of palladium ion from the sorbent surface. An aliquot of 0.2 μg mL−1 Pd(II) solution was passed at a flow rate of 2 mL min−1 through a series of columns containing 40 mg of sorbent at pH 2.5. The adsorbed Pd(II) ion was eluted by passing through the above-mentioned selected eluents by means of 3.0 mL of 0.4 mol L−1. The amount of palladium ion hence extracted into the liquid phase by each eluent was measured using FAAS, and the percent recoveries of Pd(II) were calculated in each case. The results showed that the best recovery was achieved when thiourea was used as the eluent. The effect of the concentration of thiourea on the recovery of Pd(II) was studied; the results are shown in Fig. 2.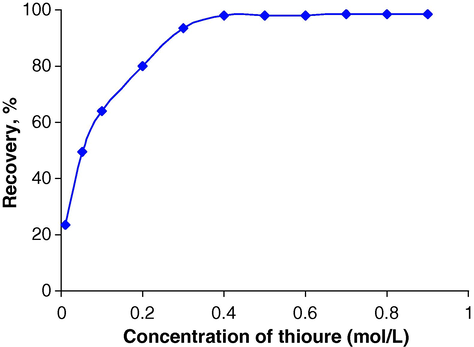
Effect of the concentration of thiourea on the recovery of Pd(II).
3.4 Effect of the flow rate of eluent solution
The effect of the flow rate of the eluent solution on the desorption of Pd(II), from the sorbent surface was studied in the range of 0.5–2.0 mL min−1. The Pd(II) ion was completely desorbed at an eluent flow rate of less than 1.5 mL min−1, for effective and quantitative elution. However, a flow rate of 1 mL min−1 was chosen for future studies.
3.5 Breakthrough volume
When solutions containing 1.0 μg of Pd(II) ion in 100, 200, 400, 500, and 600 mL of water were passed through the columns, the Pd(II) was quantitatively retained in all cases. We conclude that the breakthrough volume of the method under optimum conditions should be greater than 600 mL. The results showed that the necessary minimum concentration was 1.66 ng mL−1. Consequently, by considering the final elution volume of 3.0 mL of 0.4 mol L−1 thiourea, and a breakthrough volume of 600 mL, a preconcentration factor of 200 was easily achievable.
3.6 Evaluation of sorbent property
The sorption capacity of MMWCNTs was determined by passing 40 mL of 200 μg mL−1 Pd(II), followed by the determination of retained Pd(II) using FAAS. The maximum capacity of the sorbent was 2.34 mg of Pd(II) per gram of sorbent. The MMWCNTs sorbent was subjected to several loadings with the sample solution and subsequent elution. It was found that the adsorption properties of the sorbent remained constant after 20 cycles of sorption and desorption.
3.7 Interferences
The interferences of coexisting ions in binary mixtures of Pd(II) with foreign ions were studied on the percent recovery of palladium (2.0 μg mL−1). After introducing the binary solution into a column, the adsorbed palladium ion was eluted by a 0.4 mol L−1 thiourea solution. The content of palladium ions in effluents was determined using FAAS, and the recoveries were calculated. The results, summarized in Table 1, clearly indicate that most of the tested ions do not interfere with the determination of palladium. The tolerance limit was set as the amount of foreign ions required to cause a ±5% error. Conditions were same as Fig. 1.
Ion
Tolerance limit (mg)
Mg2+, Ca2+
60.0
Cu2+
15.0
Cd2+, Pb2+
2.9
Hg2+
0.6
Al3+, Fe3+
6.4
Zn2+, Ni2+, Mn2+
8.3
Rh3+, Au3+
2.7
Ir3+, Pt4+
2.1
Ag+, Co2+
1.4
3.8 Validation studies
Analytical figure of merit was evaluated for the determination of palladium according to the recommended procedure under the optimized conditions. Under optimum conditions, the calibration curve was linear from 1.7 ng mL−1 to 14.0 μg mL−1 in initial solution with a correlation coefficient of 0.9984 (R2). The recommended procedure was repeated eight times to find the relative standard deviation in the determination of 2.0 μg mL−1 of Pd(II) ion and relative standard deviation was found to be ±2.73%. The obtained detection limit was 0.3 ng mL−1, based on three times the standard deviation of the blank solution measurements (n = 8).
3.9 Application of the proposed method
The developed method was applied to a platinum–iridium alloy for the determination of palladium. The result, based on the average of five replicates, is tabulated in Table 2. It is clear that the result is in good agreement with the certified value.
The proposed method was used for the determination of palladium in various wastewater samples from the Copper factory in Kerman region, and also in road dust samples. The results are given in Tables 3 and 4, and were calculated assuming 100% recovery.
Sample
Founda (ng mL−1)
Out flowing wastewater (copper factory, tinker 1)
2.14 ± 0.05
Out flowing wastewater from factory to dam (copper factory)
3.16 ± 0.09
Recycle water from dam to factory (copper factory)
1.82 ± 0.03
4 Conclusion
In this paper, a method for the application of MWCNTs for preconcentration trace amount of palladium ions is proposed. The modification of MWCNTs is simple and low cost, and the reagent remains in MMWCNTs that allows using the column several times. The procedure offers a useful, rapid, and reliable enrichment technique for preconcentration of Pd(II) in various samples with acceptable accuracy and precision. Under the optimum condition, palladium in aqueous samples was concentrated to about 200-fold. Comparison of the present methods for separation and preconcentration of trace amounts of palladium with other methods is shown in Table 5 (Tokaliglu et al., 2004; Jamali et al., 2007; Rojas et al., 2006; Praveen et al., 2006; Lesniewska et al., 2005; Farhadi and Teimouri, 2005). It shows that the proposed method has a low detection limit compared with previous studies (Tokaliglu et al., 2004; Rojas et al., 2006; Praveen et al., 2006; Farhadi and Teimouri, 2005) and it allows the determination of ng mL−1 levels of palladium. Also the preconcentration factor is higher than some of the other sorbents (Tokaliglu et al., 2004; Jamali et al., 2007; Praveen et al., 2006; Lesniewska et al., 2005; Farhadi and Teimouri, 2005).
References
Detection limit, ng ml−1
Preconcentration factor
Eluent
Analysis methods
Sorbent
Tokaliglu et al. (2004)
1.2
75
HCl in acetone
AAS
Silica gel
Jamali et al. (2007)
0.2
100
Thiourea
ICP-AES
Mesoporous silica
Rojas et al. (2006)
0.4
–
HCl
GFAAS
Silica gel
Praveen et al. (2006)
1.0
80
HCl in methanol
FIA–FAAS
Exfoliated graphite
Lesniewska et al. (2005)
0.04
66.5
FIA–FAAS
Fullerene C60
Farhadi and Teimouri (2005)
12
100
Na2SO3
AAS
Octadecyl silica
Present work
0.3
200
Thiourea
FAAS
Carbon nanotube
Acknowledgment
The authors gratefully acknowledge the financial support project by the Islamic Azad University of Bardsir Branch.
References
- Anal. Sci.. 2008;24:1135.
- Talanta. 2001;53:1139.
- Advanced Inorganic Chemistry (Fifth ed.). New York: John Wiley & Sons; 1998.
- Talanta. 2005;65:925.
- Talanat. 2009;80:168.
- Anal. Chim. Acta. 2000;404:258.
- Talanta. 2007;71:1524.
- J. Korean Chem. Soc.. 1983;27:268.
- Spectrochim. Acta B. 2005;60:377.
- Carbon. 2003;41:1057.
- Mater. Res. Bull.. 2003;38:469.
- Diamond. Relat. Mater.. 2006;15:90.
- Talanta. 2009;77:1854.
- J. Am. Chem. Soc.. 2001;123:2085.
- J. Nanosci. Nanotechnol.. 2007;7:1647.
- Water Res.. 2005;39:1183.
- Talanta. 2009;79:1050.
- Solvent Extr. Ion Exch.. 2000;18:223.
- Chem. Phys. Lett.. 2003;376:158.
- Mater. Sci. Eng.. 2004;43:61.
- Talanta. 2006;70:437.
- J. Anal. At. Spectrom.. 2000;15:329.
- Talanta. 2006;70:979.
- Anal. Chem.. 2005;77:1183.
- Fresenius J. Anal. Chem.. 1992;342:791.
- Solid Phase Extraction. Basel, Switzerland: Marcel Dekker, Inc.; 2000.
- Anal. Chim. Acta. 2004;511:255.
- Anal. Sci.. 2000;16:81.
- Talanta. 2003;60:1123.
- Microchim. Acta. 2004;147:189.







