Translate this page into:
Preparation and biological evaluation of coumarin amide derivatives bearing fluorine as potential fungicides and anticancer agents
⁎Corresponding authors. yangwude476@gzy.edu.cn (Wude Yang), yuxiang@gzy.edu.cn (Xiang Yu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
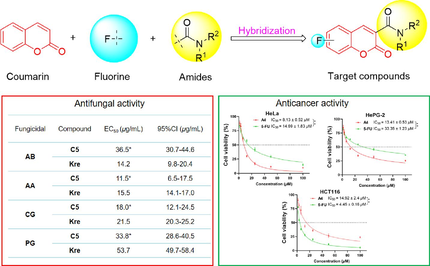
In order to find new potential fungicides and anticancer agents, a series of new coumarin amide derivatives bearing fluorine were synthesized and characterized spectroscopically. Compounds A6, B11, C2 and C7 were confirmed by X-ray diffraction further. The antifungal bioassays against five typical pathogenic fungi showed that compound C5 exhibited more remarkable fungicidal activities against Alternaria alternata (EC50 = 11.5 μg/mL), Colletotrichum gloeosporioides (EC50 = 18.0 μg/mL), Pyricularia grisea (EC50 = 33.8 μg/mL), surpassing kresoxim-methyl. Molecular docking result indicated that C5 displayed high binding affinity to chitinase, which plays crucial role in degradation and remodeling of fungal cell walls. In addition, the anticancer bioassays against three cancer cells demonstrated that compound A4 displayed excellent growth inhibitory effect against Hela cells with IC50 value of 8.13 μM, and low cytotoxicity against human normal cells BEAS-2B. Flow cytometric analysis further demonstrated that A4 significantly arrested cell cycle at the S phase and trigger apoptosis. With the above interesting biological profile, these coumarin derivatives could be used as anfungicides and anticancer candidates.
Keywords
Coumarin
Fluorine
Synthesis
Antifungal activities
Anticancer activity
1 Introduction
The natural compound coumarin, and its derivatives, can be found in many species of plants, particularly Rutaceae and Apiaceae (Santos Junior et al., 2023). They are classified into four groups: simple coumarins, furanocoumarins, pyranocoumarins, and pyrone-substituted coumarins (Fig. 1, I ∼ IV) (Küpeli Akkol et al., 2020). These compounds have been used for the benefit of humankind, including in medical and agrochemical applications (Akwu et al., 2023; Prusty and Kumar, 2020). In the field of medicinal chemistry, coumarin and its derivatives represent one of the most important skeletons of pharmaceutically active compounds, exhibiting a broad spectrum of medicinal activities. These activities include antitumor (Sakunpak et al., 2013; Maleki et al., 2020; Phutdhawong et al., 2021), antiviral activity (Tang et al., 2016; Liu et al., 2020), antibacterial (Zuo et al., 2016; Tan et al., 2017), antioxidant (Bizzarri et al., 2017; Kassim et al., 2013), neuroprotective (Huang et al., 2021; Yu et al., 2021), anti-inflammatory (Lv et al., 2015; Tuan Anh et al., 2017), Anticoagulant (Awaad et al., 2012; Gao et al., 2021), and more. In the agrochemical field, they also demonstrate a wide range of agro-activities, such as insecticidal (Siskos et al., 2008; Yang et al., 2022; Ma et al., 2024) and antifungal properties (Li et al., 2023; Song et al., 2017).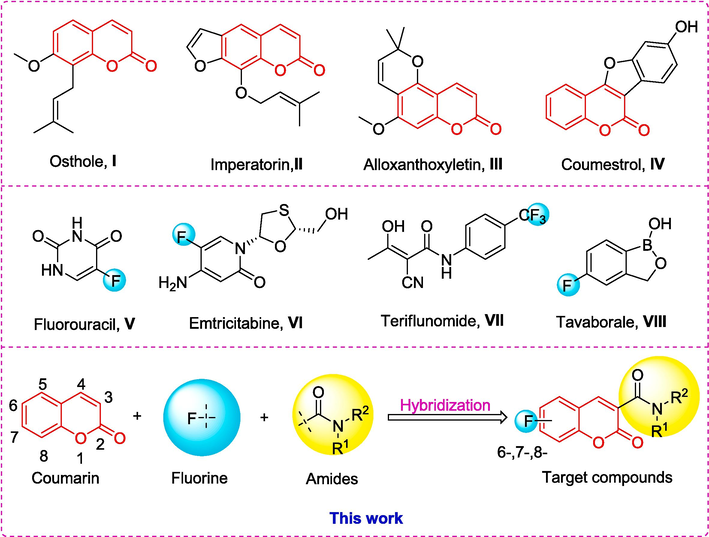
Design of the coumarin amide derivatives bearing fluorine.
Moreover, fluorine is a small atom with a van der Waals radius, positioned between hydrogen and oxygen, making it suitable to imitate a hydroxyl group and participate in hydrogen bonding interactions (Hunter, 2010; Ismail, 2002). Introducing fluorine into compounds can modulate physicochemical properties such as pharmacological properties, lipophilicity, metabolic stability, membrane permeability, and binding affinity (Purser et al., 2008). Previous studies have shown that organo-fluorine can be used not only in anticancer (Longley et al., 2003; Duan et al., 2013), anti-inflammatory (Luan et al., 2023; Deshpande and Pai, 2012) and antiviral agents (Han and Lu, 2023; Cavaliere et al., 2017), but also in the development of pesticides (Jeschke, 2017; Jeschke, 2004). Furthermore, many active drugs on the market contain fluorine, such as fluorouraci (Alzahrani et al., 2023), emtricitabine (Deeks, 2018), teriflunomide (Paik, 2021) and tavaborale (Poulakos et al., 2017) (Fig. 1, V ∼ VIII).
Molecular hybridization involves the fusion of the chemical structures of two drugs to create a single molecule, or the integration of the pharmacological components of both drugs into a hybrid molecule. These newly formed hybrid molecules, which combine the therapeutic properties of both drugs, enhance their pharmacological efficacy and mitigate their potential adverse effects. Alternatively, hybrid molecules can leverage the strengths and weaknesses of each drug to synergistically enhance their respective pharmacological activities and optimize the treatment outcome. It has become one of the current trends in the development of biologically active compounds (Zhao et al., 2023; Yang et al., 2024). Given the aforementioned facts, as shown in Fig. 1, in this study, we designed new fungicides and anticancer compounds by combining coumarin with fluorine and an amide moiety, and tested their in vitro antifungal activities against five phytopathogenic fungi, including Alternaria brassicae (AB), Alternaria alternata (AA), Colletotrichum gloeosporioides (CG), Pyricularia grisea (PG), Fusarium oxysporium f. sp.vasinfectum (FV) and antiproliferative effect against human cervical cancer cell line (HeLa), human hepatoma cancer cell line (HepG2), and human colorectal cancer cell line (HCT116). The preliminary anticancer mechanism of the powerful compounds was also examined using tests that evaluate the distribution of the cells in the phases of the cell cycle and the induction of apoptosis.
2 Materials and methods
2.1 General experimental
Thin-layer chromatography (TLC) was performed on silica gel plates using silica gel 60 GF254 (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). Melting points were determined on The X-5A micro melting point tester (Gongyi Kerui instrument Co., Ltd). NMR spectra were performed on a Bruker Avance neo 400 MHz instrument (Bruker, Bremerhaven, Germany). HRMS was performed on Xevo G2-SQTOF instrument (Waters, Milford, MA, USA).
2.1.1 Synthesis of the intermediates 5a ∼ c
The different coumarin-3-formyl chlorides 5a ∼ c were prepared as previous literature (Zhang et al., 2018). Firstly, fluorosalicylaldehydes compounds (1a ∼ c) (80 mmol), diethyl malonate (96 mmol) and catalytic amounts of piperidine (1 mL) were refluxed in ethanol for 2 h. After cooling to room temperature, the suspension was filtered off and 3a ∼ c was attained. Afterwards, compound 3a ∼ c was hydrolyzed in ethanolic solution with 2 N NaOH (aq.) at reflux for 30 min. After reaction, 2 N HCl (aq.) was added and acids 4a ∼ c were obtained by filtering the white precipitate, washing it with water, and then drying it. Finally, the solution of compounds 4a ∼ c (10 mmol) in dichloromethane (DCM, 100 mL) was added two drops dimethylformamide (DMF), and oxalyl chloride (13 mmol) slowly. The blend was agitated at ambient temperature for 8 h, then concentrated after completion of the reaction to yield compounds 5a ∼ c.
2.1.2 Synthesis of compounds A1 ∼ 9, B1 ∼ 14, C1 ∼ 11
A mixture of compounds 5a ∼ c (0.5 mmol), amines (0.5 mmol), triethylamine (6 mmol) in tetrahydrofuran (THF, 20 mL) was stirred at 50 °C overnight. The reaction mixture was diluted with 30 mL of ethyl acetate, followed by washing with saturated NaHCO3 solution (3 × 30 mL), saturated saline solution (50 mL), and drying with anhydrous Na2SO4. After evaporating the organic phase, the residues were purified using silica gel column chromatography (petroleum ether: ethyl acetate = 1: 1) to isolate the desired compounds A1 ∼ 9, B1 ∼ 14, C1 ∼ 11.
2.1.2.1 N-ethyl-6-fluoro-2-oxo-2H-chromene-3-carboxamide (A1)
Yield: 66 %, white solid, mp 183–184 °C; 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 1H), 8.75 (s, 1H), 7.47 – 7.30 (m, 3H), 3.51 (p, J = 6.9 Hz, 2H), 1.27 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 161.0, 160.8, 160.2 (d, J = 246.7 Hz), 150.5, 147.1, 121.6 (d, J = 24.7 Hz), 119.6, 119.4 (d, J = 9.6 Hz), 118.3 (d, J = 8.9 Hz), 114.7 (d, J = 23.6 Hz), 34.8, 14.6; HRMS (ESI) calcd for C12H11O3NF ([M + H]+) 236.0717, found 236.0713.
2.1.2.2 N-butyl-6-fluoro-2-oxo-2H-chromene-3-carboxamide (A2)
Yield: 77 %, yellow solid, mp 162–163 °C; 1H NMR (400 MHz, CDCl3) δ 8.86 (s, 1H), 8.78 (s, 1H), 7.47 – 7.31 (m, 3H), 3.47 (q, J = 6.8 Hz, 2H), 1.67 – 1.59 (m, 2H), 1.43 (h, J = 7.3 Hz, 2H), 0.96 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 161.0, 160.9, 160.2 (d, J = 247.3 Hz), 150.5 (d, J = 1.5 Hz), 147.1 (d, J = 2.9 Hz), 121.6 (d, J = 24.7 Hz), 119.6, 119.4 (d, J = 9.2 Hz), 118.3 (d, J = 8.2 Hz), 114.7 (d, J = 23.6 Hz), 39.7, 31.4, 20.1, 13.7; HRMS (ESI) calcd for C14H15O3NF ([M + H]+) 264.1030, found 264.1026.
2.1.2.3 6-fluoro-N-isopropyl-2-oxo-2H-chromene-3-carboxamide (A3)
Yield: 80 %, white solid, mp 169–170 °C; 1H NMR (400 MHz, CDCl3) δ 8.86 (s, 1H), 8.64 (s, 1H), 7.43 – 7.33 (m, 3H), 4.30 – 4.21 (m, 1H), 1.28 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 161.0, 160.2 (d, J = 247.2 Hz), 160.0, 150.5 (d, J = 2.1 Hz), 147.1 (d, J = 2.9 Hz), 121.6 (d, J = 24.8 Hz), 119.7, 119.4 (d, J = 9.4 Hz), 118.3 (d, J = 8.6 Hz), 114.7 (d, J = 23.9 Hz), 42.0, 22.5 × 2; HRMS (ESI) calcd for C13H13O3NF ([M + H]+) 250.0874, found 250.0869.
2.1.2.4 6-fluoro-2-oxo-N-(pentan-3-yl)–2H-chromene-3-carboxamide (A4)
Yield: 59 %, white solid, mp 141–142 °C; 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 1H), 8.59 (s, 1H), 7.51 – 7.33 (m, 3H), 4.04 – 3.95 (m, 1H), 1.73 – 1.52 (m, 4H), 0.96 (t, J = 7.4 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 161.1, 160.7, 160.2 (d, J = 246.9 Hz), 150.5 (d, J = 1.6 Hz), 147.2 (d, J = 2.9 Hz), 121.5 (d, J = 24.7 Hz), 119.7, 119.4 (d, J = 9.4 Hz), 118.2 (d, J = 8.9 Hz), 114.7 (d, J = 23.9 Hz), 52.7, 27.2 × 2, 10.2 × 2; HRMS (ESI) calcd for C15H17O3NF ([M + H]+) 278.1187, found 278.1179.
2.1.2.5 N-cyclopropyl-6-fluoro-2-oxo-2H-chromene-3-carboxamide (A5)
Yield: 65 %, white solid, mp 193–195 °C; 1H NMR (400 MHz, CDCl3) δ 8.88 (s, 1H), 8.78 (s, 1H), 7.45 – 7.33 (m, 3H), 2.99 – 2.93 (m, 1H), 0.91 – 0.86 (m, 2H), 0.72 – 0.60 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 162.3, 160.9, 160.2 (d, J = 247.2 Hz), 150.5 (d, J = 1.6 Hz), 147.1 (d, J = 2.9 Hz), 121.7 (d, J = 24.8 Hz), 119.4, 119.3 (d, J = 9.2 Hz), 118.3 (d, J = 8.2 Hz), 114.7 (d, J = 23.8 Hz), 23.0, 6.6 × 2; HRMS (ESI) calcd for C13H11O3NF ([M + H]+) 248.0717, found 248.0713.
2.1.2.6 N-cyclohexyl-6-fluoro-2-oxo-2H-chromene-3-carboxamide (A6)
Yield: 86 %, yellow solid, mp 217–218 °C; 1H NMR (400 MHz, CDCl3) δ 8.86 (s, 1H), 8.74 (s, 1H), 7.46 – 7.31 (m, 3H), 4.02 – 3.94 (m, 1H), 2.05 – 1.94 (m, 2H), 1.80 – 1.63 (m, 3H), 1.48 – 1.25 (m, 5H); 13C NMR (101 MHz, CDCl3) δ 161.0, 160.2 (d, J = 247.2 Hz), 159.9,150.5, 147.2 (d, J = 3.1 Hz), 121.5 (d, J = 24.8 Hz), 119.8, 119.4 (d, J = 9.5 Hz), 118.2 (d, J = 8.2 Hz), 114.7 (d, J = 23.7 Hz), 48.6, 32.7 × 2, 25.5, 24.6 × 2; HRMS (ESI) calcd for C16H17O3NF ([M + H]+) 290.1187, found 290.1181.
2.1.2.7 N-cycloheptyl-6-fluoro-2-oxo-2H-chromene-3-carboxamide (A7)
Yield: 69 %, yellow solid, mp 183–184 °C; 1H NMR (400 MHz, CDCl3) δ 8.85 (s, 1H), 8.78 (s, 1H), 7.50 – 7.32 (m, 3H), 4.20 – 4.12 (m, 1H), 2.07 – 1.94 (m, 2H), 1.71 – 1.56 (m, 10H); 13C NMR (101 MHz, CDCl3) δ 161.0, 160.2 (d, J = 246.9 Hz), 159.6, 150.5, 147.2 (d, J = 2.9 Hz), 121.5 (d, J = 24.7 Hz), 119.9, 119.4 (d, J = 9.3 Hz), 118.2 (d, J = 8.4 Hz), 114.7 (d, J = 23.5 Hz), 50.9, 34.7 × 2, 27.9 × 2, 24.1 × 2; HRMS (ESI) calcd for C17H19O3NF ([M + H]+) 304.1343, found 304.1337.
2.1.2.8 6-fluoro-N-methyl-2-oxo-N-phenyl-2H-chromene-3-carboxamide (A8)
Yield: 68 %, yellow solid, mp 219–220 °C; 1H NMR (400 MHz, CDCl3) δ 7.64 (s, 1H), 7.30 – 7.19 (m, 7H), 7.10 (d, J = 7.9 Hz, 1H), 3.48 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 164.4, 159.9 (d, J = 246.0 Hz), 157.1, 149.9, 142.8, 141.0, 129.4 × 2, 127.8, 127.2, 126.9 × 2, 120.0 (d, J = 24.7 Hz), 118.7 (d, J = 9.1 Hz), 118.3 (d, J = 8.3 Hz), 113.6 (d, J = 23.9 Hz), 37.5; HRMS (ESI) calcd for C17H13O3NF ([M + H]+) 298.0874, found 298.0873.
2.1.2.9 6-fluoro-N-(2-methoxyphenyl)-2-oxo-2H-chromene-3-carboxamide (A9)
Yield: 73 %, white solid, mp 234–235 °C; 1H NMR (400 MHz, CDCl3) δ 11.26 (s, 1H), 8.94 (s, 1H), 8.53 (d, J = 9.2 Hz, 1H), 7.46 – 7.36 (m, 3H), 7.12 (t, J = 7.1 Hz, 1H), 7.01 (t, J = 7.3 Hz, 1H), 6.95 (d, J = 8.1 Hz, 1H), 3.98 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 161.0, 160.3 (d, J = 247.5 Hz), 158.6, 150.6, 149.1, 147.5 (d, J = 2.9 Hz), 127.5, 124.7, 121.8 (d, J = 24.7 Hz), 121.0, 120.7, 120.2, 119.4 (d, J = 9.3 Hz), 118.4 (d, J = 8.4 Hz), 114.8 (d, J = 23.8 Hz), 110.2, 56.0; HRMS (ESI) calcd for C17H13O4NF ([M + H]+) 314.0823, found 314.0816.
2.1.2.10 N-ethyl-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B1)
Yield: 60 %, white solid, mp 171–173 °C; 1H NMR (400 MHz, CDCl3) δ 8.89 (s, 1H), 8.68 (s, 1H), 7.72 – 7.68 (m, 1H), 7.15 – 7.10 (m, 2H), 3.53 (p, J = 7.1 Hz, 2H), 1.27 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.2 (d, J = 258.8 Hz), 160.2 × 2, 154.8 (d, J = 13.7 Hz), 146.7, 130.8 (d, J = 10.7 Hz), 116.7 (d, J = 3.9 Hz), 114.6 (d, J = 2.7 Hz), 113.6 (d, J = 23.5 Hz), 103.6 (d, J = 25.8 Hz), 33.9, 13.8; HRMS (ESI) calcd for C12H11O3NF ([M + H]+) 236.0717, found 236.0713.
2.1.2.11 7-fluoro-2-oxo-N-propyl-2H-chromene-3-carboxamide (B2)
Yield: 59 %, white solid, mp 164–166 °C; 1H NMR (400 MHz, CDCl3) δ 8.89 (s, 1H), 8.72 (s, 1H), 7.72 – 7.68 (m, 1H), 7.22 – 7.01 (m, 2H), 3.51 – 3.38 (m, 2H), 1.66 (h, J = 7.3 Hz, 2H), 1.00 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 167.1 (d, J = 258.7 Hz), 161.2, 161.0, 155.7 (d, J = 13.7 Hz), 147.5, 131.6 (d, J = 10.4 Hz), 117.4 (d, J = 3.3 Hz), 115.4 (d, J = 2.7 Hz), 113.8 (d, J = 23.2 Hz), 104.4 (d, J = 25.9 Hz), 41.6, 22.6, 11.4; HRMS (ESI) calcd for C13H13O3NF ([M + H]+) 250.0874, found 250.0868.
2.1.2.12 N-butyl-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B3)
Yield: 71 %, white solid, mp 146–148 °C; 1H NMR (400 MHz, CDCl3) δ 8.89 (s, 1H), 8.73 (s, 1H), 7.77 – 7.68 (m, 1H), 7.17 – 7.09 (m, 2H), 3.52 – 3.43 (m, 2H), 1.62 (p, J = 7.4 Hz, 2H), 1.47 – 1.38 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 167.1 (d, J = 259.1 Hz), 161.2, 161.0, 155.7 (d, J = 13.3 Hz), 147.5, 131.6 (d, J = 10.8 Hz), 117.4 (d, J = 3.4 Hz), 115.4 (d, J = 1.8 Hz), 113.9 (d, J = 23.0 Hz), 104.4 (d, J = 25.7 Hz), 39.6, 31.4, 20.1, 13.7; HRMS (ESI) calcd for C14H15O3NF ([M + H]+) 264.1030, found 264.1025.
2.1.2.13 7-fluoro-N-hexyl-2-oxo-2H-chromene-3-carboxamide (B4)
Yield: 59 %, yellow solid, mp 132–134 °C; 1H NMR (400 MHz, CDCl3) δ 8.82 (s, 1H), 8.64 (s, 1H), 7.65 – 7.61 (m, 1H), 7.11 – 6.96 (m, 2H), 3.38 (q, J = 7.0 Hz, 2H), 1.55 (p, J = 7.2 Hz, 2H), 1.35 – 1.22 (m, 6H), 0.82 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 167.1 (d, J = 256.3 Hz), 161.1, 161.0, 155.7 (d, J = 12.9 Hz), 147.5, 131.6 (d, J = 11.2 Hz), 117.4 (d, J = 2.5 Hz), 115.4 (d, J = 2.5 Hz), 113.8 (d, J = 23.3 Hz), 104.4 (d, J = 26.5 Hz), 39.9, 31.4, 29.3, 26.6, 22.5, 13.9; HRMS (ESI) calcd for C16H19O3NF ([M + H]+) 292.1343, found 292.1339.
2.1.2.14 7-fluoro-N-isopropyl-2-oxo-2H-chromene-3-carboxamide (B5)
Yield: 76 %, yellow solid, mp 176–178 °C; 1H NMR (400 MHz, CDCl3) δ 8.88 (s, 1H), 8.56 (s, 1H), 7.72 – 7.68 (m, 1H), 7.17 – 7.05 (m, 2H), 4.31 – 4.20 (m, 1H), 1.28 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 167.0 (d, J = 258.3 Hz), 161.0, 160.2, 155.7 (d, J = 13.2 Hz), 147.5, 131.6 (d, J = 10.8 Hz), 117.6 (d, J = 3.0 Hz), 115.5 (d, J = 2.8 Hz), 113.8 (d, J = 23.2 Hz), 104.4 (d, J = 26.2 Hz), 41.9, 22.5 × 2; HRMS (ESI) calcd for C13H13O3NF ([M + H]+) 250.0874, found 250.0868.
2.1.2.15 N-cyclopropyl-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B6)
Yield: 82 %, white solid, mp 190–192 °C; 1H NMR (400 MHz, CDCl3) δ 8.90 (s, 1H), 8.71 (s, 1H), 7.72 – 7.68 (m, 1H), 7.21 – 7.07 (m, 2H), 2.99 – 2.92 (m, 1H), 0.90 – 0.85 (m, 2H), 0.69 – 0.63 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 167.1 (d, J = 259.5 Hz), 162.5, 161.0, 155.7 (d, J = 13.8 Hz), 147.5, 131.7 (d, J = 10.5 Hz), 117.2 (d, J = 3.4 Hz), 115.4 (d, J = 2.1 Hz), 113.8 (d, J = 22.8 Hz), 104.4 (d, J = 25.6 Hz), 22.9, 6.6 × 2; HRMS (ESI) calcd for C13H11O3NF ([M + H]+) 248.0717, found 248.0712.
2.1.2.16 N-cyclohexyl-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B7)
Yield: 64 %, yellow solid, mp 202–204 °C; 1H NMR (400 MHz, CDCl3) δ 8.88 (s, 1H), 8.65 (d, J = 8.2 Hz, 1H), 7.72 – 7.68 (m, 1H), 7.14 – 7.09 (m, 2H), 4.02 – 3.93 (m, 1H), 2.00 – 1.96 (m, 2H), 1.80 – 1.60 (m, 4H), 1.48 – 1.25 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 167.0 (d, J = 258.8 Hz), 161.0, 160.1, 155.7 (d, J = 13.3 Hz), 147.4, 131.6 (d, J = 10.9 Hz), 117.6 (d, J = 2.9 Hz), 115.4 (d, J = 2.8 Hz), 113.8 (d, J = 23.3 Hz), 104.4 (d, J = 25.9 Hz), 48.5, 32.7 × 2, 25.5, 24.6 × 2; HRMS (ESI) calcd for C16H17O3NF ([M + H]+) 290.1187, found 290.1179.
2.1.2.17 N-cycloheptyl-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B8)
Yield: 69 %, yellow solid, mp 190–192 °C; 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 1H), 8.71 (d, J = 8.0 Hz, 1H), 7.71 – 7.68 (m, 1H), 7.20 – 7.03 (m, 2H), 4.20 – 4.11 (m, 1H), 2.02 – 1.97 (m, 2H), 1.71 – 1.54 (m, 10H); 13C NMR (101 MHz, CDCl3) δ 167.0 (d, J = 258.7 Hz), 161.0, 159.9, 155.7 (d, J = 13.2 Hz), 147.4, 131.5 (d, J = 10.7 Hz), 117.7 (d, J = 3.0 Hz), 115.5 (d, J = 2.8 Hz), 113.8 (d, J = 23.1 Hz), 104.4 (d, J = 25.9 Hz), 50.8, 34.7 × 2, 28.0 × 2, 24.1 × 2; HRMS (ESI) calcd for C17H19O3NF ([M + H]+) 304.1343, found 304.1336.
2.1.2.18 7-fluoro-2-oxo-N-o-tolyl-2H-chromene-3-carboxamide (B9)
Yield: 71 %, white solid, mp 263–265 °C; 1H NMR (400 MHz, CDCl3) δ 10.66 (s, 1H), 9.02 (s, 1H), 8.23 (d, J = 8.1 Hz, 1H), 7.75 (t, J = 7.2 Hz, 1H), 7.28 – 7.08 (m, 5H), 2.42 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 167.3 (d, J = 259.6 Hz), 161.6, 159.1, 155.8 (d, J = 13.6 Hz), 148.3, 136.0, 131.8 (d, J = 10.9 Hz), 130.5, 128.6, 126.7, 125.0, 121.9, 117.7 (d, J = 3.0 Hz), 115.5 (d, J = 2.2 Hz), 114.1 (d, J = 23.2 Hz), 104.6 (d, J = 25.9 Hz), 18.0; HRMS (ESI) calcd for C17H13O3NF ([M + H]+) 298.0874, found 298.0867.
2.1.2.19 7-fluoro-2-oxo-N-m-tolyl-2H-chromene-3-carboxamide (B10)
Yield: 76 %, white solid, mp 222–224 °C; 1H NMR (400 MHz, CDCl3) δ 10.66 (s, 1H), 8.98 (s, 1H), 7.76 – 7.72 (m, 1H), 7.54 (d, J = 7.6 Hz, 2H), 7.26 (t, J = 8.0 Hz, 1H), 7.15 (t, J = 7.9 Hz, 2H), 6.98 (d, J = 7.5 Hz, 1H), 2.38 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 167.3 (d, J = 259.6 Hz), 161.4, 159.0, 155.8 (d, J = 13.8 Hz), 148.2, 139.0, 137.4, 131.8 (d, J = 10.6 Hz), 128.9, 125.7, 121.1, 117.7, 117.6 (d, J = 3.3 Hz), 115.5 (d, J = 2.5 Hz), 114.1 (d, J = 23.3 Hz), 104.5 (d, J = 25.9 Hz), 21.4; HRMS (ESI) calcd for C17H13O3NF ([M + H]+) 298.0874, found 298.0868.
2.1.2.20 N-(3,4-dimethylphenyl)-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B11)
Yield: 69 %, yellow solid, mp 200–202 °C; 1H NMR (400 MHz, CDCl3) δ 10.59 (s, 1H), 8.96 (s, 1H), 7.74 – 7.70 (m, 1H), 7.47 (d, J = 6.7 Hz, 2H), 7.17 – 7.10 (m, 3H), 2.28 (s, 3H), 2.24 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 167.3 (d, J = 259.3 Hz), 161.4, 158.9, 155.8 (d, J = 13.5 Hz), 148.0, 137.3, 135.2, 133.3, 131.8 (d, J = 10.9 Hz), 130.0, 121.8, 118.0, 117.6 (d, J = 2.9 Hz), 115.5 (d, J = 2.1 Hz), 114.1 (d, J = 23.0 Hz), 104.5 (d, J = 25.8 Hz), 19.8, 19.2; HRMS (ESI) calcd for C18H15O3NF ([M + H]+) 312.1030, found 312.1024.
2.1.2.21 N-(2,5-dimethylphenyl)-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B12)
Yield: 85 %, yellow solid, mp 216–218 °C; 1H NMR (400 MHz, CDCl3) δ 10.60 (s, 1H), 9.00 (s, 1H), 8.05 (s, 1H), 7.76 – 7.72 (m, 1H), 7.17 – 7.09 (m, 3H), 6.91 (d, J = 7.4 Hz, 1H), 2.36 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 167.3 (d, J = 259.6 Hz), 161.5, 159.0, 155.8 (d, J = 13.7 Hz), 148.2, 136.4, 135.7, 131.8 (d, J = 10.8 Hz), 130.2, 125.8, 125.5, 122.5, 117.7 (d, J = 3.3 Hz), 115.5 (d, J = 2.8 Hz), 114.1 (d, J = 22.9 Hz), 104.5 (d, J = 26.0 Hz), 21.2, 17.6; HRMS (ESI) calcd for C18H15O3NF ([M + H]+) 312.1030, found 312.1023.
2.1.2.22 N-(2,6-dimethylphenyl)-7-fluoro-2-oxo-2H-chromene-3-carboxamide (B13)
Yield: 84 %, yellow solid, mp 215–217 °C; 1H NMR (400 MHz, CDCl3) δ 10.06 (s, 1H), 9.01 (s, 1H), 7.74 – 7.71 (m, 1H), 7.19 – 7.10 (m, 5H), 2.28 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 167.3 (d, J = 259.6 Hz), 161.5, 159.5, 155.9 (d, J = 13.6 Hz), 148.5, 135.0 × 2, 133.7, 131.8 (d, J = 10.8 Hz), 128.2 × 2, 127.4, 117.3 (d, J = 2.9 Hz), 115.5 (d, J = 2.3 Hz), 114.1 (d, J = 23.2 Hz), 104.5 (d, J = 25.9 Hz), 18.6 × 2; HRMS (ESI) calcd for C18H15O3NF ([M + H]+) 312.1030, found 312.1024.
2.1.2.23 7-fluoro-N-(3-methoxyphenyl)-2-oxo-2H-chromene-3-carboxamide (B14)
Yield: 88 %, white solid, mp 226–228 °C; 1H NMR (400 MHz, CDCl3) δ 11.18 (s, 1H), 8.97 (s, 1H), 8.52 (d, J = 8.1 Hz, 1H), 7.74 – 7.67 (m, 1H), 7.19 – 7.09 (m, 3H), 7.00 (t, J = 7.6 Hz, 1H), 6.94 (d, J = 8.0 Hz, 1H), 3.97 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 167.2 (d, J = 253.5 Hz), 161.0, 158.9, 155.8 (d, J = 13.4 Hz), 149.1, 147.9, 131.7 (d, J = 10.9 Hz), 127.6, 124.6, 120.9, 120.6, 117.9 (d, J = 2.9 Hz), 115.5 (d, J = 2.6 Hz), 114.0 (d, J = 23.2 Hz), 110.2, 104.5 (d, J = 25.7 Hz), 55.9; HRMS (ESI) calcd for C17H13O4NF ([M + H]+) 314.0823, found 314.0816.
2.1.2.24 N-ethyl-8-fluoro-2-oxo-2H-chromene-3-carboxamide (C1)
Yield: 60 %, white solid, mp 191–192 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 8.70 (s, 1H), 7.49 – 7.41 (m, 2H), 7.35 – 7.30 (m, 1H), 3.51 (p, J = 6.9 Hz, 2H), 1.27 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 160.8, 160.1, 150.3 (d, J = 254.7 Hz), 147.6 (d, J = 2.9 Hz), 142.5 (d, J = 11.2 Hz), 125.1 (d, J = 6.6 Hz), 124.8 (d, J = 4.2 Hz), 120.4, 120.1 (d, J = 17.1 Hz), 119.4, 34.8, 14.6; HRMS (ESI) calcd for C12H11O3NF ([M + H]+) 236.0717, found 236.0712.
2.1.2.25 8-fluoro-2-oxo-N-propyl-2H-chromene-3-carboxamide (C2)
Yield: 55 %, white solid, mp 185–186 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 8.75 (s, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.47 – 7.41 (m, 1H), 7.33 (td, J = 8.0, 4.5 Hz, 1H), 3.47 – 3.41 (m, 2H), 1.71 – 1.62 (m, 2H), 1.00 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 160.9, 160.1, 150.3 (d, J = 254.6 Hz), 147.6 (d, J = 2.9 Hz), 142.5 (d, J = 11.6 Hz), 125.1 (d, J = 6.9 Hz), 124.8 (d, J = 4.2 Hz), 120.4, 120.1 (d, J = 16.7 Hz), 119.5, 41.6, 22.6, 11.4; HRMS (ESI) calcd for C13H13O3NF ([M + H]+) 250.0874, found 250.0869.
2.1.2.26 N-butyl-8-fluoro-2-oxo-2H-chromene-3-carboxamide (C3)
Yield: 77 %, white solid, mp 148–149 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (d, J = 1.6 Hz, 1H), 8.73 (s, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.46 – 7.41 (m, 1H), 7.33 (td, J = 8.0, 4.5 Hz, 1H), 3.51 – 3.43 (m, 2H), 1.63 (p, J = 7.6 Hz, 2H), 1.45 – 1.38 (m, 2H), 0.97 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 160.9, 160.1, 150.3 (d, J = 254.6 Hz), 147.6 (d, J = 2.8 Hz), 142.5 (d, J = 11.6 Hz), 125.1 (d, J = 6.7 Hz), 124.8 (d, J = 3.8 Hz), 120.4, 120.1 (d, J = 17.3 Hz), 119.5, 39.7, 31.4, 20.1, 13.7; HRMS (ESI) calcd for C14H15O3NF ([M + H]+) 264.1030, found 264.1024.
2.1.2.27 8-fluoro-N-hexyl-2-oxo-2H-chromene-3-carboxamide (C4)
Yield: 66 %, yellow solid, mp 110–111 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (d, J = 1.6 Hz, 1H), 8.73 (s, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.46 – 7.41 (m, 1H), 7.32 (td, J = 8.0, 4.5 Hz, 1H), 3.48 – 3.42 (m, 2H), 1.63 (q, J = 7.6 Hz, 2H), 1.43 – 1.30 (m, 6H), 0.89 (t, J = 7.5 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 160.8, 160.1, 150.4 (d, J = 254.9 Hz), 147.6 (d, J = 2.7 Hz), 142.5 (d, J = 11.2 Hz), 125.1 (d, J = 6.6 Hz), 124.8 (d, J = 3.7 Hz), 120.4, 120.1 (d, J = 17.0 Hz), 119.5, 40.0, 31.4, 29.2, 26.6, 22.5, 13.9; HRMS (ESI) calcd for C16H19O3NF ([M + H]+) 292.1343, found 292.1337.
2.1.2.28 8-fluoro-2-oxo-N-(pentan-3-yl)–2H-chromene-3-carboxamide (C5)
Yield: 89 %, yellow solid, mp 115–116 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (d, J = 1.6 Hz, 1H), 8.53 (d, J = 8.8 Hz, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.46 – 7.41 (m, 1H), 7.32 (td, J = 8.0, 4.5 Hz, 1H), 4.04 – 3.95 (m, 1H), 1.72 – 1.61 (m, 2H), 1.60 – 1.51 (m, 2H), 0.96 (t, J = 7.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 160.6, 160.2, 150.3 (d, J = 254.7 Hz), 147.6 (d, J = 2.8 Hz), 142.5 (d, J = 11.6 Hz), 125.1 (d, J = 6.6 Hz), 124.8 (d, J = 3.8 Hz), 120.5, 120.0 (d, J = 16.8 Hz), 119.6, 52.7, 27.2 × 2, 10.2 × 2; HRMS (ESI) calcd for C15H17O3NF ([M + H]+) 278.1187, found 278.1179.
2.1.2.29 N-cyclopropyl-8-fluoro-2-oxo-2H-chromene-3-carboxamide (C6)
Yield: 80 %, white solid, mp 204–205 °C; 1H NMR (400 MHz, CDCl3) δ 8.93 (d, J = 1.6 Hz, 1H), 8.73 (s, 1H), 7.49 (d, J = 7.7 Hz, 1H), 7.46 – 7.41(m, 1H), 7.33 (td, J = 8.0, 4.5 Hz, 1H), 2.99 – 2.93 (m, 1H), 0.91 – 0.86 (m, 2H), 0.69 – 0.64 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 162.3, 160.0, 150.3 (d, J = 255.1 Hz), 147.5 (d, J = 2.8 Hz), 142.5 (d, J = 11.6 Hz), 125.2 (d, J = 6.7 Hz), 124.9 (d, J = 4.2 Hz), 120.4, 120.2 (d, J = 17.0 Hz), 119.2, 23.0, 6.6 × 2; HRMS (ESI) calcd for C13H11O3NF ([M + H]+) 248.0717, found 248.0710.
2.1.2.30 N-cyclohexyl-8-fluoro-2-oxo-2H-chromene-3-carboxamide (C7)
Yield: 69 %, yellow solid, mp 173–174 °C; 1H NMR (400 MHz, CDCl3) δ 8.91 (d, J = 1.6 Hz, 1H), 8.67 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.46 – 7.40 (m, 1H), 7.32 (td, J = 8.0, 4.5 Hz, 1H), 4.02 – 3.93 (m, 1H), 2.01 – 1.97 (m, 2H), 1.79 – 1.73 (m, 2H), 1.68 – 1.60 (m, 1H), 1.47 – 1.22 (m, 5H); 13C NMR (101 MHz, CDCl3) δ 160.1, 159.8, 150.3 (d, J = 254.8 Hz), 147.5 (d, J = 2.4 Hz), 142.5 (d, J = 11.6 Hz), 125.1 (d, J = 6.7 Hz), 124.8 (d, J = 4.2 Hz), 120.5, 120.0 (d, J = 17.0 Hz), 119.7, 48.7, 32.7 × 2, 25.5, 24.6 × 2; HRMS (ESI) calcd for C16H17O3NF ([M + H]+) 290.1187, found 290.1180.
2.1.2.31 N-cycloheptyl-8-fluoro-2-oxo-2H-chromene-3-carboxamide (C8)
Yield: 73 %, yellow solid, mp 138–139 °C; 1H NMR (400 MHz, CDCl3) δ 8.90 (d, J = 1.6 Hz, 1H), 8.73 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 7.9 Hz, 1H), 7.45 – 7.40 (m, 1H), 7.32 (td, J = 8.0, 4.4 Hz, 1H), 4.21 – 4.11 (m, 1H), 2.05 – 1.96 (m, 2H), 1.71 – 1.55 (m, 10H); 13C NMR (101 MHz, CDCl3) δ 160.1, 159.6, 150.3 (d, J = 255.0 Hz), 147.4 (d, J = 2.7 Hz), 142.5 (d, J = 11.7 Hz), 125.1 (d, J = 6.6 Hz), 124.8 (d, J = 4.1 Hz), 120.5, 120.0 (d, J = 17.4 Hz), 119.7, 50.9, 34.7 × 2, 27.9 × 2, 24.1 × 2; HRMS (ESI) calcd for C17H19O3NF ([M + H]+) 304.1343, found 304.1336.
2.1.2.32 8-fluoro-2-oxo-N-phenyl-2H-chromene-3-carboxamide (C9)
Yield: 73 %, white solid, mp 282–283 °C; 1H NMR (400 MHz, CDCl3) δ 10.97 (s, 1H), 9.20 (s, 1H), 7.64 – 7.53 (m, 4H), 7.48 – 7.39 (m, 3H), 7.29 (t, J = 7.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 160.4, 160.0, 150.4, 150.3 (d, J = 255.4 Hz), 142.4 (d, J = 11.6 Hz), 135.6, 129.3 × 2, 126.7, 126.0 (d, J = 6.4 Hz), 125.6 (d, J = 3.9 Hz), 122.1, 122.0 × 2, 121.4 (d, J = 17.3 Hz), 120.1; HRMS (ESI) calcd for C16H11O3NF ([M + H]+) 284.0717, found 284.0712.
2.1.2.33 N-(2,6-dimethylphenyl)-8-fluoro-2-oxo-2H-chromene-3-carboxamide (C10)
Yield: 87 %, white solid, mp 247–248 °C; 1H NMR (400 MHz, CDCl3) δ 10.08 (s, 1H), 9.04 (d, J = 1.6 Hz, 1H), 7.52 – 7.44 (m, 2H), 7.35 (td, J = 8.0, 4.4 Hz, 1H), 7.17 – 7.10 (m, 3H), 2.29 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 160.6, 159.2, 150.4 (d, J = 255.2 Hz), 148.5, 142.7 (d, J = 11.6 Hz), 135.0, 133.6 × 2, 128.2 × 2, 127.4, 125.3 (d, J = 6.5 Hz), 125.0 (d, J = 4.2 Hz), 120.5, 120.4 (d, J = 14.1 Hz), 119.4, 18.5 × 2; HRMS (ESI) calcd for C18H15O3NF ([M + H]+) 312.1030, found 312.1022.
2.1.2.34 8-fluoro-N-(2-methoxyphenyl)-2-oxo-2H-chromene-3-carboxamide (C11)
Yield: 76 %, white solid, mp 240–241 °C; 1H NMR (400 MHz, CDCl3) δ 11.22 (s, 1H), 9.00 (d, J = 1.6 Hz, 1H), 8.52 (dd, J = 8.0, 1.6 Hz, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.48 – 7.42 (m, 1H), 7.34 (td, J = 8.0, 4.5 Hz, 1H), 7.12 (td, J = 7.8, 1.6 Hz, 1H), 7.00 (td, J = 7.8, 1.4 Hz, 1H), 6.94 (dd, J = 8.2, 1.4 Hz, 1H), 3.97 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.1, 158.6, 150.4 (d, J = 254.8 Hz), 149.1, 147.9 (d, J = 11.4 Hz), 142.6 (d, J = 11.4 Hz), 127.5, 125.2 (d, J = 6.4 Hz), 124.9 (d, J = 3.9 Hz), 124.7, 120.9, 120.6, 120.4, 120.3 (d, J = 17.0 Hz), 119.6, 110.3, 56.0; HRMS (ESI) calcd for C17H13O4NF ([M + H]+) 314.0823, found 314.0816.
2.2 Antifungal assay
In vitro evaluations were conducted on compounds A1 ∼ 9, B1 ∼ 14, and C1 ∼ 11 to assess their impact on the growth of phytopathogenic fungi by inhibiting mycelial growth. Five phytopathogenic fungi, including Alternaria brassicae (AB), Alternaria alternata (AA), Colletotrichum gloeosporioides (CG), Pyricularia grisea (PG), Fusarium oxysporium f. sp.vasinfectum (FV) were used for the assays. The dissolved compounds in acetone were combined with the PDA at 50 μg/mL before being transferred to sterilized Petri dishes. Acetone served as blank controls, and kresoxim-methyl, a commercial fungicide, used as the positive control. Mycelia disks measuring 4 mm in diameter were inoculated onto PDA Petri dishes and incubated at 25 °C for a period of 4 days. Three replicates were performed for every treatment, measuring the radial growth of fungal colonies and analyzing the data statistically. Subsequently, the inhibition rates (%) versus seven concentrations of some compounds and kresoxim-methyl were obtained, and the median effective concentration (EC50) values were calculated.
2.3 Molecular docking studies
ChemDraw2019 was used to create a 2D structure of compound C5, which was saved as a cdx file. Next, the 3D structure of C5 was optimized using the MM2 force field in Chem3D 2019. Chitinase's 3D configurations were obtained from the PDB repository (https://www.rcsb.org, PDB: 1W9U). Subsequently, the proteins of interest were brought into AutoDock Tools for the processes of adding hydrogen atoms, calculating charges, and combining non-polar hydrogens, with the final outcome being saved in PDBQT format. Ultimately, execute AutoDock Vina to perform molecular docking, then utilize Discovery studio for visualizing the outcomes.
2.4 Anticancer assay
Three human cancer cells, including HeLa, HePG-2, HCT116, and normal human cells (BEAS-2B) were used to determine the cytotoxic effects of the prepared compounds (A1 ∼ 9, B1 ∼ 14, C1 ∼ 11), and CCK-8 assay was used to assess the impact on growth. Cells were placed in 96-well plates and exposed to the compounds at a concentration of 100 μmol/L for 48 h in triplicate. Each well was treated with CCK-8 solution at a concentration of 20 %, followed by incubation for 1 h and measurement using spectrophotometry at 450 nm. As a positive control, 5-fluorouracil (5-FU) was also used in identical conditions. The additional IC50 values for certain compounds was also tested. Each experiment was conducted thrice.
2.5 Cell cycle arrest assay
HeLa cells (2 × 106/well) were seeded in 6-well plates and incubated overnight, and then exposed to A4 (0, 4, 8, 16 μM) for 48 h. Afterward, PBS was rinsed over the cells and trypsin was added. After centrifugation, the liquid above the sediment was discarded and the remaining material was soaked in 70 % ethanol overnight. The cells were rinsed with PBS two times and then placed in a staining buffer with 190 µL volume, which included propidium iodide (1 mg/mL, 4 µL) and RNaseA (ribonuclease, 10 mg/mL, 4 µL), and Tritonx-100 (0.2 µL). Following a 20-minute staining period in darkness, the distribution of the cell cycle was analyzed with flow cytometry.
2.6 Cell apoptosis assay
The incubated HeLa cells (2 × 106/well) were exposed to A4 (0, 4, 8, 16 μM) for 48 h. Following the incubation period, PBS was rinsed over the cells and trypsin was added. Then the resulting cell was tmixed with Annexin binding buffer. Subsequently, annexin V/FITC (5 μL) and PI (10 μL) were introduced to the cell suspension and allowed to incubate at appropriate temperature for 15 min. Afterward, flow cytometry underwent analysis the stained cells.
2.7 Data analyses
The formula used to calculate the inhibitory effects on these fungi in vitro is Inhibition rate (%) = (C-T) × 100/(C-4 mm), with C representing the diameter in the blank control group and T representing the diameter in the treatments. The cell Inhibition rate (%) = (OD1-OD2)/(OD1-OD3) × 100 %. The cell viability rate (%) = (OD2-OD3) / (OD1-OD3) × 100 %. with OD1, OD2,OD3 representing the negative control, experiment and blank group. GraphPad Prism 8 software (GraphPad, Inc., San Diego, CA) was used to conduct statistical analysis.
3 Results and discussion
3.1 Chemistry
As shown in Scheme 1, initially, primary coumarin-3-formyl chlorides 5a ∼ c were synthesized through a four-step process beginning with readily available salicylaldehydes. Salicylaldehydes 1a ∼ c were subjected to Knoevenagle condensation with diethyl malonate (2) in ethanol by a catalytic amount of piperidine, forming coumarin-3-carboxylates 3a ∼ c. Next, the ethyl esters underwent hydrolysis using a NaOH solution to produce coumarin-3-carboxylic acids 4a ∼ c, which reacted with oxalyl chloride to give the intermediates 5a ∼ c. At last, a series of coumarin amide derivatives bearing fluorine (A1 ∼ 9, B1 ∼ 14, C1 ∼ 11) were prepared in 55 ∼ 89 % yields by reaction of 5a ∼ c with different amines in the presence of triethylamine at 50 °C. Fig. 2 summarizes all the structures. The chemical structures of these derivatives were verified using 1H NMR, 13C NMR and HRMS spectrometry (see Supporting Information). Moreover, X-ray diffraction was used to identify the stereo structures of A6, B11, C2 and C7, as shown in Fig. 3. The crystallographic data of these compounds were submitted to the CCDC under no. 2337042, 2337478, 2,337,043 and 2337044, respectively (see Supporting Information).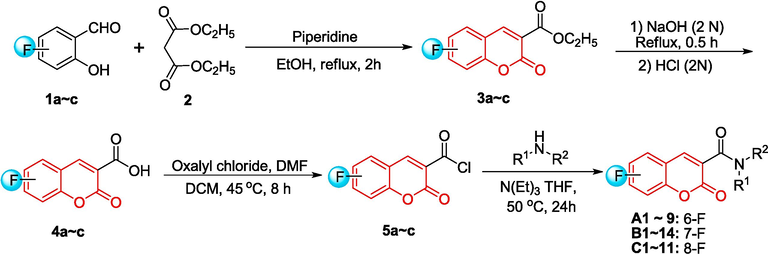
Synthetic route for coumarin amide derivatives bearing fluorine.
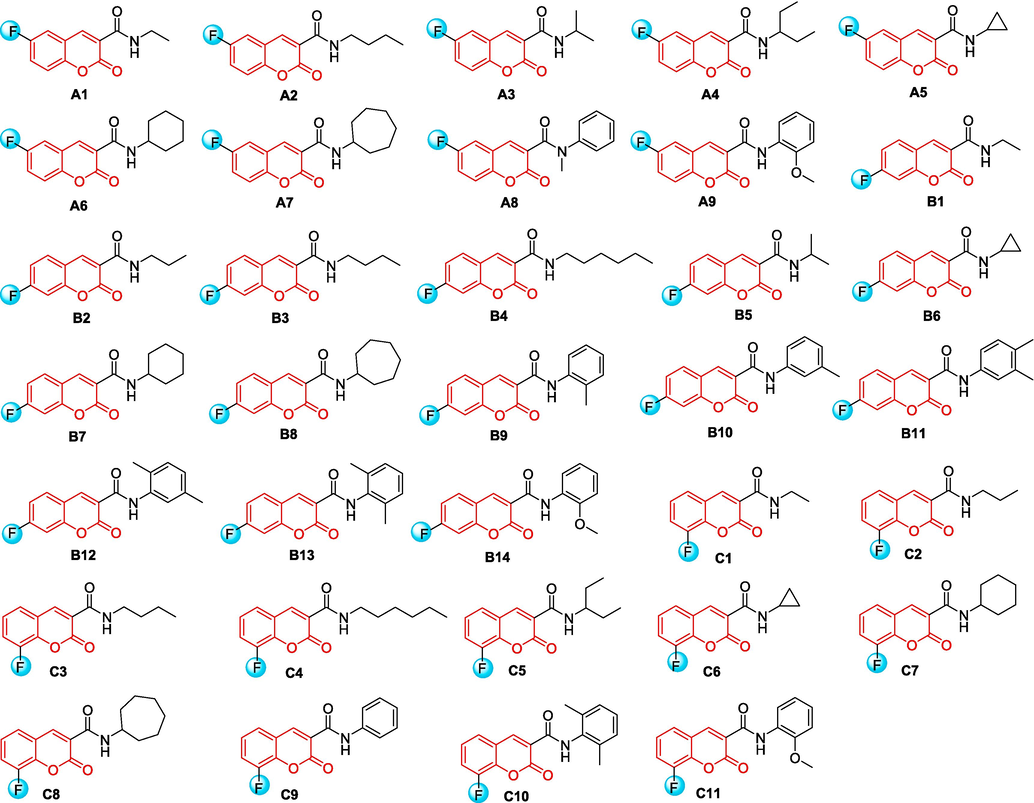
Chemical Structures of compounds A1-9, B1-14, C1-11.
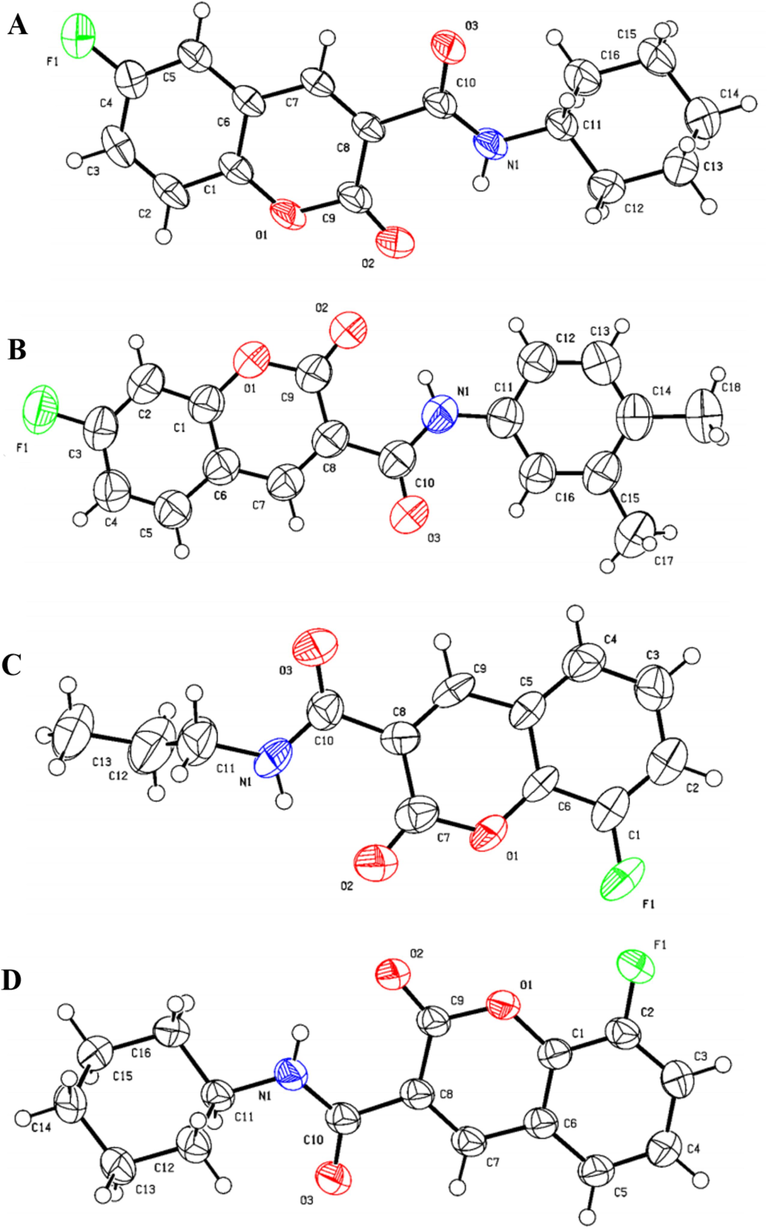
X-ray crystallographic structures of A6 (A), B11 (B), C2 (C) and C7 (D).
3.2 Antifungal activities
All the target compounds were screened their antifungal activities in vitro against five phytopathogenic fungi at 50 μg/mL using the mycelium linear growth rate method (Liu et al., 2022). Kresoxim-methyl (Kre) was used as positive control. As illustrated in Table 1, several compounds displayed significant inhibitory effects on specific fungi, which was consistent with the previous research findings that coumarin-3-carboxamide derivatives have good antibacterial activity (Yu et al., 2018). Among them, six compounds A4, A7, B2, B5, B6 and C5 inhibited AB with inhibition rates higher than 50 %. Compounds A4, A7, B2, and C5 exhibited better effectiveness with inhibition rates exceeding 67 % against the AA strain, surpassing Kre (64.3 %). For CG strain, compounds B2 (65.3 %), C2 (65.3 %), C3 (66.7 %), C5 (86.1 %), C6 (75.0 %) and C8 (59.7 %) displayed higher antifungal activities than Kre (53 %). Furthermore, three compounds A4 (58.3 %), B13 (56.35) and C5 (59.2 %) demonstrated slightly greater or comparable antifungal efficacy compared to Kre (53 %) for PG strain. Regrettably, nearly all of the desired substances showed weaker antifungal properties against the FV strain. In general, compound C5 demonstrated a more favorable and wide-ranging antifungal impact compared to the agricultural fungicides Kre.
Compounds
Antifungal activities (inhibition % ± SE)a,b,c
AB
AA
CG
PG
FV
A1
43.2 ± 1.8
44.5 ± 0.0
46.7 ± 0.0
36.7 ± 2.0
41.1 ± 1.3
A2
27.9 ± 2.0
40.7 ± 3.5
34.7 ± 2.4
36.9 ± 1.7
24.4 ± 1.7
A3
46.5 ± 2.0
53.5 ± 2.3
33.3 ± 4.2
37.9 ± 1.7
24.4 ± 3.4
A4
55.8 ± 2.0
70.9 ± 1.8
41.7 ± 1.3
58.3 ± 1.7
26.4 ± 0.0
A5
48.8 ± 2.0
52.3 ± 4.0
30.6 ± 2.4
43.7 ± 1.7
21.4 ± 1.7
A6
34.9 ± 5.3
34.9 ± 5.3
31.9 ± 2.4
32.0 ± 1.7
21.4 ± 1.7
A7
50.0 ± 2.0
67.4 ± 2.0
29.2 ± 0.0
40.8 ± 1.7
23.4 ± 4.6
A8
20.9 ± 5.3
41.9 ± 2.0
18.1 ± 2.4
38.8 ± 0.0
24.4 ± 1.7
A9
17.9 ± 2.0
33.3 ± 0.0
24.6 ± 0.0
34.5 ± 2.0
34.5 ± 1.7
B1
31.5 ± 0.0
34.5 ± 0.0
35.8 ± 0.0
37.9 ± 2.0
31.1 ± 0.0
B2
61.6 ± 1.8
67.3 ± 3.9
65.3 ± 0.0
44.7 ± 0.0
42.3 ± 3.1
B3
46.5 ± 1.5
64.0 ± 1.8
55.6 ± 2.0
50.5 ± 2.4
37.3 ± 0.0
B4
24.4 ± 3.2
37.2 ± 0.0
22.2 ± 2.0
41.7 ± 0.0
25.4 ± 3.1
B5
53.4 ± 0.0
45.6 ± 1.2
43.4 ± 2.0
48.9 ± 0.0
38.0 ± 0.0
B6
51.2 ± 3.5
57.0 ± 3.4
45.8 ± 2.0
45.6 ± 0.0
27.4 ± 0.0
B7
36.0 ± 5.3
43.0 ± 4.0
47.2 ± 4.8
45.6 ± 1.7
31.3 ± 5.2
B8
43.0 ± 1.9
46.5 ± 0.0
45.8 ± 1.9
42.7 ± 1.7
37.3 ± 2.8
B9
29.6 ± 5.3
39.5 ± 5.3
27.8 ± 2.4
46.6 ± 4.4
25.4 ± 0.0
B10
31.4 ± 0.0
36.0 ± 0.0
29.2 ± 1.6
39.8 ± 1.7
25.4 ± 0.0
B11
32.6 ± 2.4
32.6 ± 2.0
33.3 ± 0.0
41.7 ± 0.0
25.4 ± 0.0
B12
32.6 ± 2.0
48.8 ± 0.0
31.9 ± 0.0
35.0 ± 0.0
25.4 ± 2.0
B13
39.5 ± 2.3
53.5 ± 2.0
29.2 ± 0.0
56.3 ± 0.0
25.4 ± 0.0
B14
33.7 ± 1.0
46.5 ± 3.8
31.9 ± 3.7
50.0 ± 2.0
29.0 ± 0.0
C1
40.4 ± 1.6
29.4 ± 1.3
30.2 ± 0.9
40.2 ± 4.2
30.3 ± 5.4
C2
38.4 ± 0.0
53.5 ± 2.0
65.3 ± 1.3
44.7 ± 0.0
25.4 ± 1.5
C3
45.3 ± 2.0
60.7 ± 2.0
66.7 ± 1.7
45.6 ± 1.7
29.4 ± 1.7
C4
46.5 ± 2.0
46.5 ± 2.0
41.7 ± 0.0
46.6 ± 1.7
41.3 ± 1.7
C5
54.7 ± 3.5
88.4 ± 2.0
86.1 ± 2.4
59.2 ± 2.9
46.3 ± 3.0
C6
47.7 ± 3.5
55.8 ± 5.3
75.0 ± 4.2
46.6 ± 1.7
33.3 ± 1.7
C7
46.5 ± 3.3
51.9 ± 0.0
44.4 ± 0.0
39.9 ± 0.0
46.3 ± 0.0
C8
46.5 ± 2.0
58.1 ± 3.5
59.7 ± 9.6
44.7 ± 2.9
38.3 ± 3.4
C9
36.0 ± 5.3
38.4 ± 5.3
22.2 ± 2.4
34.0 ± 3.4
28.4 ± 5.2
C10
34.9 ± 1.8
52.3 ± 2.2
23.6 ± 0.0
25.4 ± 2.0
26.4 ± 2.0
C11
31.4 ± 1.0
47.7 ± 3.5
29.2 ± 2.0
40.8 ± 3.4
25.4 ± 1.7
Kre
63.1 ± 2.5
64.3 ± 0.0
53.0 ± 8.1
53.0 ± 0.7
58.3 ± 0.0
After analyzing the bioassay results mentioned earlier, certain structure–activity relationships (SARs) were examined as follow: (1) generally, introducing fluorine atom at C-6 and C-8 of coumarin was more beneficial for the improvement of the fungicigal activities (A1 ∼ 9, C1 ∼ 11 vs. B1 ∼ 14). (2) For compounds A1 ∼ 9, the introduction of 1-ethylproyl group at R position led to a sharp increase in bioactivity, such as A4. (3) For compounds B1 ∼ 14, adding n-propyl (B2) at the R position typically enhanced the antifungal effectiveness compared to other alkyl groups. (4) When the fluorine atom at the C-8 of coumarin, introduction of 1-ethylproyl group at R position could also obtain the best compound (C5). (5) Unfortunately, it was discovered that adding aromatic groups to the R position resulted in decreased potency for compounds A8 ∼ 9, B9 ∼ 14 and C9 ∼ 11. The findings of the aforementioned structure–activity relationship will serve as a theoretical guide for future optimization of the structure.
To further investigate the antifungal capabilities, the EC50 values of compound C5 were examined against four phytopathogenic fungi (AB, AA, CG and PG) for more reliable results. As summarized in Table 2, this compound showed strong antifungal effects, with EC50 values ranging from 11.5 to 36.5 μg/mL. Particularly, compound C5 exhibited superior fungicidal activity against AA, CG, and PG strains, with EC50 values of 11.5, 18.0, and 33.8 μg/mL, significantly lower than Kre (AA: 15.5 μg/mL, BC: 21.5 μg/mL, PG: 53.7 μg/mL). Significant difference between the treatment group and Kre group (* p < 0.05).
Fungi
Compound
Regression equation
R2
EC50 (μg/mL)
95 % CI (μg/mL)
AB
C5
y = 0.8040x + 3.7436
0.9528
36.5*
30.7–44.6
Kre
y = 0.4424x + 4.4902
0.9527
14.2
9.8–20.4
AA
C5
y = 1.5709x + 3.3365
0.9526
11.5*
6.5–17.5
Kre
y = 2.3629x + 2.1090
0.9826
15.5
14.1–17.0
CG
C5
y = 1.7678x + 2.7826
0.9448
18.0*
12.1–24.5
Kre
y = 0.3288x + 4.5625
0.9702
21.5
20.3–25.2
PG
C5
y = 0.8422x + 3.7122
0.9314
33.8*
28.6–40.5
Kre
y = 0.7208x + 3.5630
0.9955
53.7
49.7–58.4
3.3 Molecular docking studies
Chitinase, a key enzyme in chitin production, plays a vital part in breaking down external chitin, as well as in degrading and restructuring fungal cell walls (Hartl et al., 2012). Literature studies have shown that amidocoumarins exhibit notable chitinase inhibitory effects and possess strong binding affinity to the receptor (Sharma et al., 2020). At present, molecular docking are commonly utilized in the study of natural products (López-López et al., 2021). Therefore, we employed molecular docking to investigate the binding interactions between compound C5 and chitinase models in order to explore potential antifungal mechanisms.
Docking studies were conducted with AutoDock Tools (ADT) to explain the inhibitory activity of chitinase, utilizing a well-classified chitinase (PDB code: 1W9U) as the reference model. The docking result displayed that C5 had good binding affinity to chitinase with the docking binding energies of −7.42 kcal/mol. As shown in Fig. 4, C5 displayed hydrogen bonds and van der Waals attractions with polar residues within the binding cavity (Fig. 4, A), and shared similar binding modes with argadin, a natural chitinase inhibitor (Fig. 4, B) (Arai et al., 2000). The two carbonyl groups of C5 formed hydrogen bonds with residues GLY221 and LYS224, respectively. The coumarin ring of C5 had pi-sulfur interaction with MET243, pi-pi T-shaped interaction with TYR178, pi-alkyl interaction with PRO219. The fluorine atom formed halogen bond with MET243 and SER218 with bond length of 3.49 and 3.45 Å, respectively. The outcome suggested that the specific compound C5 perfectly matched the active site and had a positive interaction with chitinase.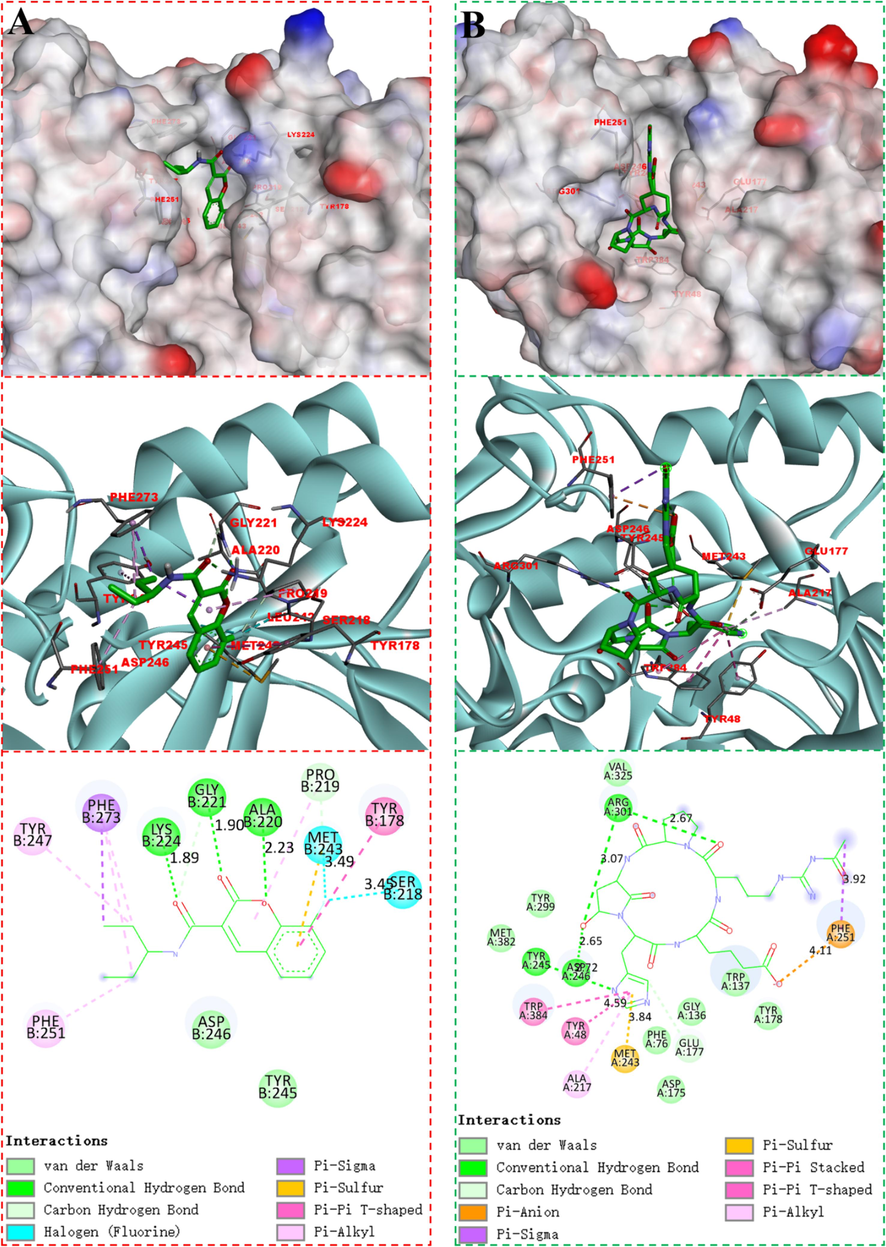
Molecular models of C5 (A) and argadin (B) binding to chitinase.
3.4 Anticancer activity
The synthesized compounds were evaluated for their anticancer properties at a concentration of 100 µmol/L on three human cancer cell lines: HeLa, HePG-2, and HCT116, and the results are collected in Table 3. Among these compounds, A4 demonstrated the highest effectiveness in inhibiting the growth of the three cancer cells, with rates of 90.28 %, 73.58 %, and 76.01 %, respectively. Additionally, several other compounds showed satisfactory cytotoxic effects on other cancer cells. For example, A2, A9, B2, B3 and C11 caused Hela cells growth inhibition rates exceeding 50 %. Compounds A2 and A7 showed moderate effectiveness in inhibiting cell growth in HePG-2 cells, with inhibition rates of 53.02 % and 50.14 %, respectively. On the other hand, all the target compounds exhibited poorer anticancer activities against HCT116 cells except A4. SARs indicated that introducing fluorine atom at C-6 of coumarin was more beneficial for the improvement of the anticancer activities. When the fluorine atom at the C-6 of coumarin, introducing of pentan-3-yl group at R position had positive influence on the anticancer activities. bNA means no action.
Compound
Growth Inhibition Rate (%)a
HeLa
HePG-2
HCT116
A1
48.50 ± 5.00
44.69 ± 0.17
40.02 ± 2.03
A2
51.98 ± 5.49
53.02 ± 5.11
41.91 ± 3.16
A3
43.13 ± 4.41
33.51 ± 0.20
NA
A4
90.28 ± 0.54
73.58 ± 1.34
76.01 ± 1.27
A5
48.82 ± 4.17
45.72 ± 3.71
NA
A6
45.99 ± 4.47
45.69 ± 0.61
18.05 ± 5.36
A7
46.31 ± 1.14
50.14 ± 4.34
22.9 ± 4.58
A8
32.23 ± 3.32
35.88 ± 4.05
16.18 ± 0.97
A9
61.48 ± 3.08
21.77 ± 4.97
NA
B1
29.80 ± 2.33
19.83 ± 1.52
27.55 ± 4.01
B2
50.42 ± 4.46
45.00 ± 3.11
39.25 ± 3.51
B3
53.57 ± 5.85
48.91 ± 5.08
37.32 ± 3.19
B4
38.30 ± 4.57
41.64 ± 3.76
16.19 ± 2.12
B5
26.08 ± 4.64
14.75 ± 1.44
20.97 ± 1.72
B6
12.00 ± 0.32
11.66 ± 0.8
15.57 ± 2.35
B7
38.27 ± 8.93
38.54 ± 5.41
18.38 ± 0.71
B8
33.07 ± 2.93
16.07 ± 0.04
15.65 ± 2.34
B9
22.27 ± 3.50
11.00 ± 2.09
5.43 ± 3.29
B10
29.88 ± 1.84
22.38 ± 3.28
15.73 ± 1.50
B11
43.62 ± 4.72
40.48 ± 3.10
31.90 ± 4.53
B12
27.58 ± 4.21
14.79 ± 2.88
18.60 ± 1.16
B13
17.59 ± 0.87
8.74 ± 2.11
5.28 ± 1.90
B14
46.19 ± 4.66
37.66 ± 3.70
35.00 ± 0.84
C1
21.83 ± 2.73
12.91 ± 1.61
NA
C2
19.90 ± 0.15
11.13 ± 1.37
NA
C3
21.00 ± 1.69
11.31 ± 1.82
NA
C4
24.57 ± 4.62
17.65 ± 1.23
NA
C5
16.98 ± 2.17
11.69 ± 4.31
NA
C6
21.81 ± 2.21
15.99 ± 0.61
NA
C7
17.76 ± 1.79
14.02 ± 0.84
NA
C8
21.34 ± 2.68
25.76 ± 5.87
NA
C9
49.82 ± 3.29
30.59 ± 0.77
NA
C10
28.54 ± 1.40
31.35 ± 4.30
10.72 ± 3.46
C11
52.66 ± 1.90
46.68 ± 5.01
34.08 ± 3.16
5-Fu
85.00 ± 2.01
64.65 ± 0.62
92.36 ± 3.64
In addition, as shown in Fig. 5, the compound A4 with the best anticancer activities were further examined to determine its IC50 against the three cancer cells, and the result delivered that it exhibited intriguing and remarkable profiles of inhibiting cell growth with IC50 values of 8.13 μM, 13.41 μM and 14.92 μM against Hela, HePG-2 and HCT116 cells, respectively, Approaching or exceeding the IC50 values of 5-FU (14.69 μM, 33.35 μM and 4.45 μM). Moreover, A4 was further evaluated for its cytotoxic activity against normal cells BEAS-2B. As depicted in Fig. 6, A4 showed relative low cytotoxicity to BEAS-2B cells, and the IC50 values was over 45 μM. This result indicated that A4 showed greater preference for cancer cells over human normal cells.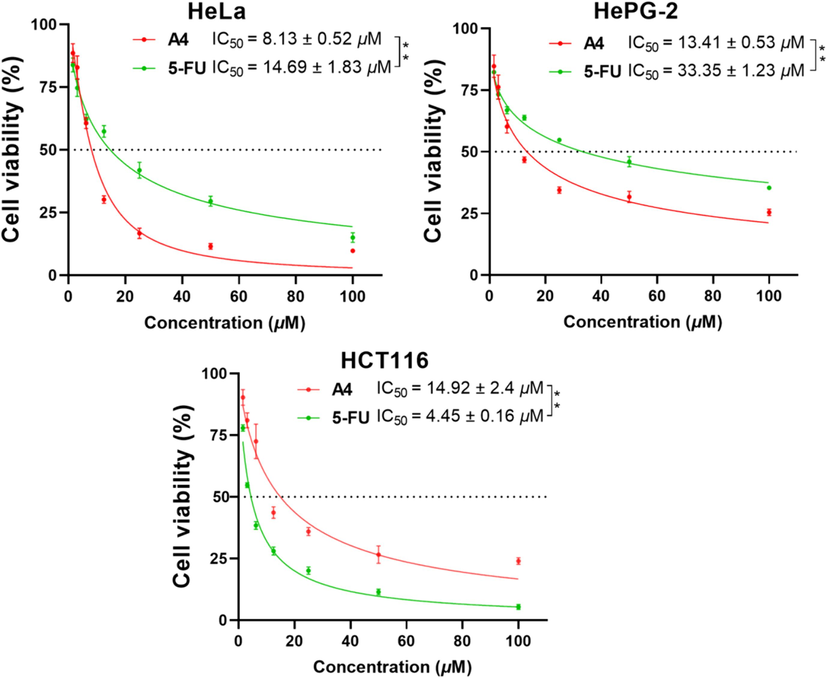
IC50 values of compound A4 and 5-FU against three cancer cells. (** p < 0.01).
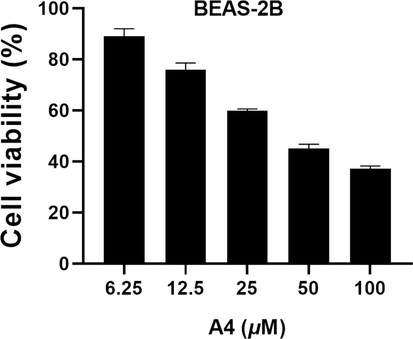
Cell viability of BEAS-2B treated with compound A4.
3.5 Cell cycle analysis
Building on the promising results from the initial in vitro antiproliferative tests, we delved deeper into the effects of A4 on the cell cycle advancement of Hela cells through flow cytometry analysis. During the study, Hela cells were exposed to different amounts of A4 (0, 4, 8, 16 μM) for 48 h. As shown in Fig. 7, In the presence of A4, the proportion of cancer cells in the S phase was 26 %, 29.45 %, and 36.21 %, compared to 22.21 % in the control group. The results showed that A4 caused a significant S-phase arrest in a concentration-dependent manner.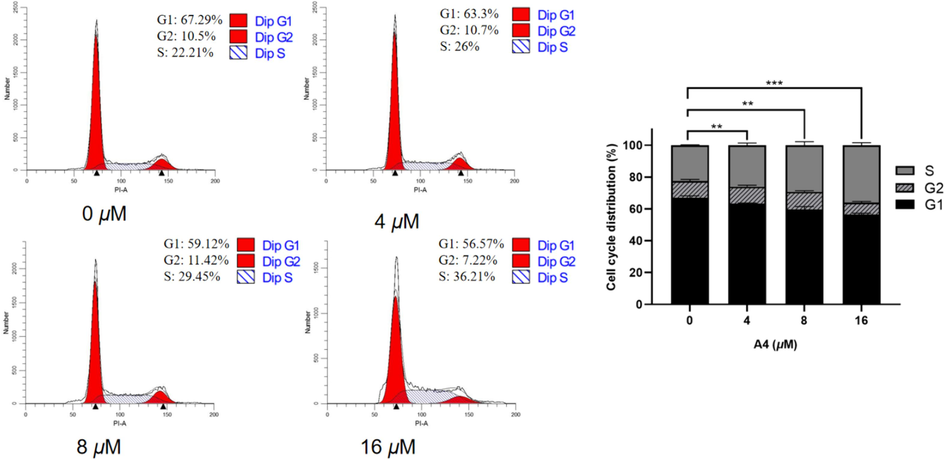
Effect of compound A4 on cell cycle in Hela cells. Flow cytometry of Hela cells treated with A4 for 48 h. (** p < 0.01, *** p < 0.001).
3.6 Cell apoptosis analysis
Additionally, Hela cells were subjected to increasing doses of A4 (0, 4, 8, 16 μM) for cell apoptosis assessment. As shown in Fig. 8, after incubating with A4 for 48 h, the combined proportion of Q2 and Q4 apoptotic cells was 6.9 %, 9.7 %, and 16.5 %, respectively. On the other hand, the group that was not treated showed only 3.7 % of cells undergoing apoptosis. As a result, it was shown that compound A4 had the ability to inhibit cell growth by causing cellular apoptosis.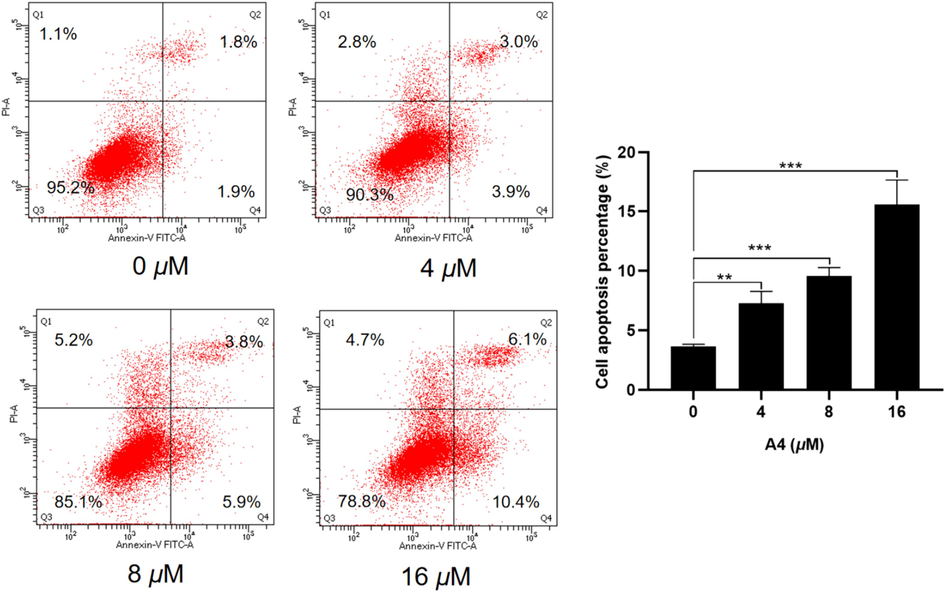
Effect of compound A4 on cell apoptosis in Hela cells. Flow cytometric analysis of apoptotic cells after treatment of Hela cells with A4 for 48 h. (** p < 0.01, *** p < 0.001).
4 Conclusion
In general, thirty-four novel coumarin amide derivatives bearing fluorine were prepared and their structures were elucidated through 1H NMR, 13C NMR and HRMS analyses. X-ray diffraction confirmed the structures of four compounds definitively. The antifungal assays showed that derivative C5 exhibited outstanding antifungal activities. Molecular docking studies displayed C5 had the good binding interactions with the target protein, chitinase. Moreover, three cancer cell lines were used to test the cytotoxic effects of the target compounds, and compound A4 display the best cytotoxic activity against Hela cells with an IC50 value of 8.13 μM, while demonstrating low cytotoxicity against BEAS-2B. Additional mechanistic investigations demonstrated that A4 could effectively halt the cell cycle during the S phase and trigger apoptosis. The findings indicate that these two new coumarin compounds may provide a promising option for fungicides and anticancer drugs.
CRediT authorship contribution statement
Xin Xiang: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Yafang Chen: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lang Wu: Investigation, Data curation, Conceptualization. Long Zhang: Investigation, Data curation. Yan Zhang: Methodology, Investigation, Formal analysis. Wude Yang: Writing – review & editing, Supervision, Project administration, Methodology. Xiang Yu: Writing – review & editing, Supervision, Project administration, Methodology, Formal analysis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82260833), Guizhou Provincial Natural Science Foundation [QKHJ-ZK(2022)YB503], Science and Technology Planning Project of Guizhou Province [QKHF-ZK(2023)G426]. The authors thank B.B. Chen for the chemical synthesis support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antidepressant effects of coumarins and their derivatives: a critical analysis of research advances. Eur. J. Pharmacol.. 2023;956:175958
- [CrossRef] [Google Scholar]
- 5–Fluorouracil and capecitabine therapies for the treatment of colorectal cancer (Review) Oncol. Rep.. 2023;50(4):175.
- [CrossRef] [Google Scholar]
- Argadin, a new chitinase inhibitor, produced by Clonostachys sp. FO-7314. Chem. Pharm. Bull. (tokyo). 2000;48(10):1442-1446.
- [CrossRef] [Google Scholar]
- New biological activities of Casimiroa edulis leaf extract and isolated compounds. Phytother Res.. 2012;26(3):452-457.
- [CrossRef] [Google Scholar]
- Regioselective IBX-mediated synthesis of coumarin derivatives with antioxidant and anti-influenza activities. J. Nat. Prod.. 2017;80(12):3247-3254.
- [CrossRef] [Google Scholar]
- Fluorinated nucleosides as an important class of anticancer and antiviral agents. Future Med. Chem.. 2017;9(15):1809-1833.
- [CrossRef] [Google Scholar]
- Bictegravir/emtricitabine/tenofovir alafenamide: a review in HIV-1 infection. Drugs. 2018;78(17):1817-1828.
- [CrossRef] [Google Scholar]
- Fluorine bearing sydnones with styryl ketone group: synthesis and their possible analgesic and anti-inflammatory activities. J. Enzyme Inhib. Med. Chem.. 2012;27(2):241-248.
- [CrossRef] [Google Scholar]
- Design and synthesis of novel 1,2,3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur. J. Med. Chem.. 2013;62:11-19.
- [CrossRef] [Google Scholar]
- The antithrombotic activity of natural and synthetic coumarins. Fitoterapia. 2021;154:104947
- [CrossRef] [Google Scholar]
- Fluorine in anti-HIV drugs approved by FDA from 1981 to 2023. Eur. J. Med. Chem.. 2023;258:115586
- [CrossRef] [Google Scholar]
- Fungal chitinases: diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol.. 2012;93(2):533-543.
- [CrossRef] [Google Scholar]
- (+/-)-Dievodialetins A-G: seven pairs of enantiomeric coumarin dimers with anti-acetylcholinesterase activity from the roots of Evodia lepta Merr. Phytochemistry. 2021;182:112597
- [CrossRef] [Google Scholar]
- The C-F bond as a conformational tool in organic and biological chemistry. Beilstein J. Org. Chem.. 2010;6:38.
- [CrossRef] [Google Scholar]
- Important fluorinated drugs in experimental and clinical use. J. Fluorine Chem.. 2002;118(1–2):27-33.
- [CrossRef] [Google Scholar]
- The unique role of fluorine in the design of active ingredients for modern crop protection. Chembiochem. 2004;5(5):571-589.
- [CrossRef] [Google Scholar]
- Latest generation of halogen-containing pesticides. Pest Manag. Sci.. 2017;73(6):1053-1066.
- [CrossRef] [Google Scholar]
- Antioxidant activity-guided separation of coumarins and lignan from Melicope glabra (Rutaceae) Food Chem.. 2013;139(1–4):87-92.
- [CrossRef] [Google Scholar]
- Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers (basel).. 2020;12(7):1959.
- [CrossRef] [Google Scholar]
- Design and synthesis of novel 2,2-dimethylchromene derivatives as potential antifungal agents. Mol. Divers.. 2023;27(2):589-601.
- [CrossRef] [Google Scholar]
- Natural products-based fungicides: synthesis and antifungal activity against plant pathogens of 2H-chromene derivatives. Chem. Biodivers.. 2022;19(11):e202200802
- [CrossRef] [Google Scholar]
- Bioactive prenylated coumarins as potential anti-inflammatory and anti-HIV agents from Clausena lenis. Bioorg. Chem.. 2020;97:103699
- [CrossRef] [Google Scholar]
- 5-Fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer.. 2003;3(5):330-338.
- [CrossRef] [Google Scholar]
- Informatics for chemistry, biology, and biomedical sciences. J. Chem. Inf. Model.. 2021;61(1):26-35.
- [Google Scholar]
- Anti-inflammatory activity of fluorine-substituted benzo[h]quinazoline-2-amine derivatives as NF-κB inhibitors. Bioorg. Chem.. 2023;132:106360
- [CrossRef] [Google Scholar]
- Anti-inflammatory coumarin and benzocoumarin derivatives from Murraya alata. J. Nat. Prod.. 2015;78(2):279-285.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological activities of novel coumarin derivatives as pesticide candidates. J. Agric. Food Chem.. 2024;72(9):4658-4668.
- [CrossRef] [Google Scholar]
- Discovering the structure-activity relationships of different O-prenylated coumarin derivatives as effective anticancer agents in human cervical cancer cells. Toxicol. In Vitro.. 2020;63:104745
- [CrossRef] [Google Scholar]
- Teriflunomide: pediatric first approval. Paediatr. Drugs.. 2021;23(6):609-613.
- [CrossRef] [Google Scholar]
- Synthesis and biological activity evaluation of coumarin-3-carboxamide derivatives. Molecules. 2021;26(6):1653.
- [CrossRef] [Google Scholar]
- Coumarins: antifungal effectiveness and future therapeutic scope. Mol. Divers.. 2020;24(4):1367-1383.
- [CrossRef] [Google Scholar]
- Isolation of new monoterpene coumarins from Micromelum minutum leaves and their cytotoxic activity against Leishmania major and cancer cells. Food Chem.. 2013;139(1–4):458-463.
- [CrossRef] [Google Scholar]
- Coumarins from Rutaceae: chemical diversity and biological activities. Fitoterapia. 2023;168:105489
- [CrossRef] [Google Scholar]
- Synthesis, antimicrobial and chitinase inhibitory activities of 3-amidocoumarins. Bioorg. Chem.. 2020;98:103700
- [CrossRef] [Google Scholar]
- Isolation and identification of insecticidal components from Citrus aurantium fruit peel extract. J. Agric. Food Chem.. 2008;56(14):5577-5581.
- [CrossRef] [Google Scholar]
- Evaluation of antifungal activities and structure-activity relationships of coumarin derivatives. Pest Manag. Sci.. 2017;3(1):94-101.
- [CrossRef] [Google Scholar]
- Antibacterial activities of pyrenylated coumarins from the roots of Prangos hulusii. Molecules. 2017;22(7):1098.
- [CrossRef] [Google Scholar]
- Antiviral spirotriscoumarins A and B: two pairs of oligomeric coumarin enantiomers with a spirodienone-sesquiterpene skeleton from Toddalia asiatica. Org. Lett.. 2016;18(19):5146-5149.
- [CrossRef] [Google Scholar]
- Anti-inflammatory coumarins from Paramignya trimera. Pharm. Biol.. 2017;55(1):1195-1201.
- [CrossRef] [Google Scholar]
- Preparation and biological evaluation of novel osthole-derived n-benzoylthioureas as insecticide candidates. J. Agric. Food Chem.. 2022;70(50):15737-15746.
- [CrossRef] [Google Scholar]
- Novel membrane-targeting isoxanthohumol-amine conjugates for combating methicillin-resistant Staphylococcus aureus (MRSA) infections. Eur. J. Med. Chem.. 2024;268:116274
- [CrossRef] [Google Scholar]
- Design, synthesis and antifungal activity evaluation of coumarin-3-carboxamide derivatives. Fitoterapia. 2018;127:387-395.
- [CrossRef] [Google Scholar]
- Design and synthesis of 7-diethylaminocoumarin-based 1,3,4-oxadiazole derivatives with anti-acetylcholinesterase activities. J. Asian Nat. Prod. Res.. 2021;23(9):866-876.
- [CrossRef] [Google Scholar]
- Design, synthesis and biological evaluation of novel furoxan-based coumarin derivatives as antitumor agents. Med. Chem. Res.. 2018;27:1198-1205.
- [CrossRef] [Google Scholar]
- Design, synthesis and biological activity determination of novel phenylpyrazole protoporphyrinogen oxidase inhibitor herbicides. Pestic. Biochem. Physiol.. 2023;196:105588
- [CrossRef] [Google Scholar]
- Synergism of coumarins from the Chinese drug Zanthoxylum nitidum with antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2016;23(14):1814-1820.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105872.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







